Abstract
Purpose:
Develop a simple phone questionnaire, without physical exam input, that predicts which patients calling with symptoms of a posterior vitreous detachment (PVD) have a retinal tear or rhegmatogenous retinal detachment (RT/RD).
Design:
Prospective cohort (quality improvement) study.
Participants:
All patients with symptoms consistent with a PVD calling a major academic ophthalmology department over a four-month period in 2020 and were seen on follow-up within 1.5 months (211 screened, 193 included).
Methods:
A comprehensive phone questionnaire assessing for RT/RD risk factors was administered by phone triage staff to all patients calling with symptoms of flashes, floaters, or curtain/veil in their vision. Multivariable logistic regression was used to determine risk factors most predictive of having a RT/RD during the add-on visit. Risk factor odds ratios were used to develop a RT/RD risk score.
Main Outcomes Measures:
Development of a clinical risk score for having a RT/RD at the add-on visit following phone triage.
Results:
Approximately 55% of patients were previously established in the retina clinic, 26% were new to the department, 19% were previously established in the comprehensive clinic, and 7% had a RT/RD at the add-on visit. Out of 23 questions and 70 pre-specified possible answers from the phone questionnaire, the final clinical risk score for RT/RDs is derived from 7 questions and 15 possible answers. The simplified questionnaire can be administered quickly by phone operators without any reference to physical exam or the patient’s chart. The receive-operator curve for our final multivariable logistic regression and clinical risk score models have an area under the curve of >0.90. Using a conservative clinical risk score, nearly 50% of all patients without a RT/RD can be safely seen non-urgently. Progressively higher scores can be used to determine relative urgency of an appointment.
Conclusions:
This is the first study to predict risk of a RT/RD in a patient calling with symptoms consistent with a PVD without reference to the patient’s physical exam or chart. Our clinical risk scoring system can be used to determine urgency of an add-on appointment and increase the number of low-risk patients with symptomatic PVDs that are scheduled routinely.
Précis
To accurately triage patients calling with posterior vitreous detachment symptoms, we develop a risk score for retinal tear/detachment based entirely on a simple phone questionnaire without reference to the patient’s chart or physical exam.
INTRODUCTION
Symptoms heralding a posterior vitreous detachment (PVD) are among the most common reasons for an urgent ophthalmologic examination1-6. However, demand for urgent appointments often outstrips the available supply of eye care providers7, making accurate triage of such patients over the phone by ancillary staff essential. If the patient’s risk of retinal tear (RT) or rhegmatogenous retinal detachment (RD) could be accurately determined entirely via questions by phone, many with symptoms of flashes or floaters could be seen on a non-urgent basis when appointment slots are more available. To date, no attempt has been made to develop a data-driven triage scoring system for symptomatic PVDs based entirely on phone screening.
Here, we conducted a prospective study of all patients calling a major academic ophthalmology department with symptoms of flashes, floaters, or curtain/veil over approximately four months to determine risk factors obtainable exclusively by phone that predict the presence of RT/RD on follow-up exam. Each patient was asked a detailed battery of standardized questions about their symptoms, ocular history, and medical history, and were then assessed for a RT or RD at the follow-up appointment scheduled by the triage technician. Risk factors predictive of a RT or RD in multivariable logistic regression analysis were converted into a predictive scoring system that allowed non-MD triage staff to determine which patients with symptoms of a PVD could be safely seen non-urgently.
METHODS
All research adhered to the tenets of the Declaration of Helsinki. As this was a quality improvement study, the Michigan Medicine Institutional Review Board designated the study exempt from further review. Over a four-month period (8/27/2020 through 12/10/2020), the Michigan Medicine Kellogg Eye Center’s triage call center personnel, split between retina and comprehensive clinics, utilized a question and limited-selection answer prompt if they felt an incoming patient call was primarily about symptoms of floaters, flashes, or a curtain or veil in the patient’s vision. All triage technicians had multiple years of experience with phone triage. Once the question-and-answer prompt was complete, the triage technician routed the triage note to the physician(s) responsible for determining when to bring the patient in for an exam, and the timeframe for first exam was left to the physician’s discretion. The triage note was simultaneously routed to the study’s principal investigator, who excluded any patients he felt did not describe symptoms consistent with a PVD. In total, 18 screened patients were excluded from the study for the following reasons: the principal investigator determined symptoms described in the phone triage note were inconsistent with a PVD or not the primary source of the phone complaint (e.g. age-related macular degeneration metamorphopsia or fixed scotoma from ischemic optic neuropathy (33%, N=6)); patients did not follow-up for their post-triage appointment at the Kellogg Eye Center within 1.5 months of the phone triage date (50%, N=9); patients had either an incomplete phone triage note or the follow-up exam was so clearly unrelated to a symptomatic PVD that the provider did not dilate the patient (e.g. - a keratoconus patient with long-standing fixed visual crescent after corneal cross-linking) (17%, N=3).
Upon completion of the phone triage period (12/10/2020), the first follow-up visit note was interrogated for whether a new RT or RD was present, along with a determination of whether the patient underwent scleral depression and/or ultrasound (B-scan). Additionally, all past ocular history and relevant past medical history documented in the triage note were verified in the first follow-up note or in prior outpatient visit notes. Each entry was verified by two graders (D.A.B. and J.M.L.M.).
Univariate logistic regression analysis was performed and Fisher’s exact test was used for certain variables when appropriate. Those risk factors from univariate analysis with p≤0.2 or that had strong theoretical reasons for being important in predicting RT/RDs were subsequently included in multivariable logistic regression models with Firth’s penalized maximum likelihood estimation8, 9. The full model contained the following risk factors: sex, symptoms in one eye vs. both eyes, duration of symptoms, number of floaters, description of floaters, number of flashes, blurred vision or curtain in vision, need for glasses to drive when young, history of RT/RD in either eye, history of retinal surgery in either eye, history of cataract surgery in the symptomatic eye, and presence of diabetes. Subsequent refinement of the full multivariable logistic regression model was based on a combination of those factors significant in multivariable analysis and those variables that were biologically complementary to each other. Out of eight further models queried, the final model included the following variables: symptoms in one eye vs. both eyes, duration of symptoms, constant blurred vision or curtain/veil in vision, need for glasses to drive when young, history of RT/RD in either eye, history of retinal surgery in either eye, and presence of diabetes. Predicted probability of developing the outcome (RT/RD) for each individual and the receiver operator curve (ROC) of the final model were generated. From this final multivariable model, the odds ratios for critical risk factors were used as the starting point for deriving a clinical risk scoring system for RT/RDs. Based on wanting to ensure certain patients were always seen urgently (for example, patients with new onset symptoms and blurred vision in one eye who were not diabetic), the starting point system was slightly modified. The final point system was then applied to the entire dataset to assess goodness of predicting the outcome (RT/RD) in a univariate logistic regression model where the derived clinical risk score was the only predictor. ROC curves from this point-system model were compared to the ROC curves derived from the calculated probabilities using the final refined multivariable model. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Graphs were created using Prism 8 (GraphPad by Dotmatics, San Diego, CA, USA).
RESULTS
Patient Demographics, Diagnosis Rates of Retinal Tears & Detachments, Phone Triage Timing, and Time to Clinic Follow-Up
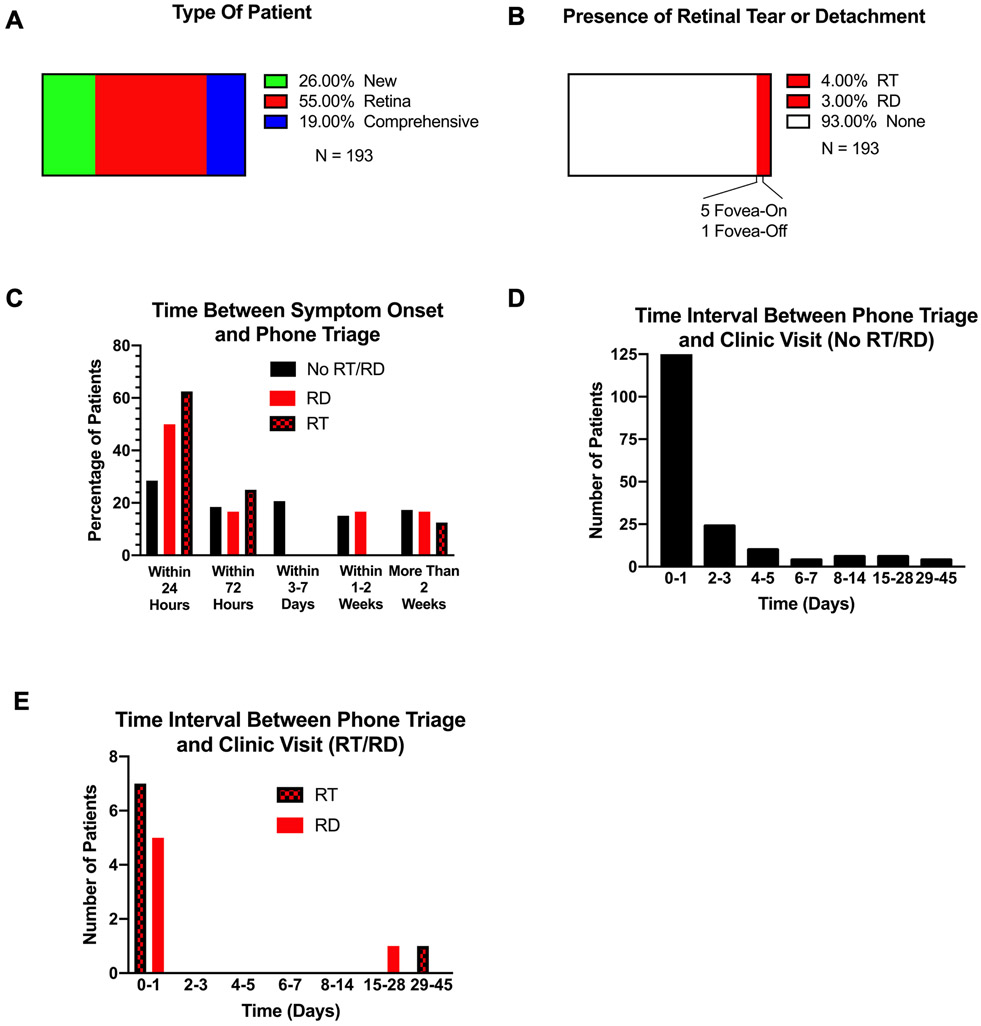
Each patient calling with symptoms consistent with a PVD was asked the template questions and provided limited-selection answer options listed in Table 1. Of the 193 patients who met inclusion criteria for the study, 55% had previously been seen in the retina clinic, 26% were new patients to the Kellogg Eye Center, and 19% had previously been seen by the comprehensive clinic (Figure 1A). The mean age and standard deviation of all patients was 55.49 ± 1.09 years (Figure S2A). When stratified, the mean age of patients without RT/RD was 55.22 ± 1.15 years and those with RT/RD was 59.00 ± 3.31 years. Of all patients in the study, 4% (8 patients) had a RT and 3% (6 patients) had a RD, with only one RD being fovea-off (Figure 1B). On follow-up exam, just over 60% of patients without a RT/RD underwent scleral depression or had a B-scan, whereas nearly all patients with a RT/RD had scleral depression or a B-scan (Figure S2B). Patients with a RT/RD were slightly more likely to be male (Figure S2C) and less likely to have concurrent diabetes or uveitis (Figure S2D).
Table 1.
Phone Triage Questions.
| Category | Question Asked | Answer Choices |
|---|---|---|
| Age | How old are you? | - Patient states age at time of call |
| Sex | Are you male or female? | - Male - Female |
| Established versus New Patient | Have you been seen at Kellogg Eye Center before? If so, have you been seen in retina or comprehensive clinics? | - No (new patient) - Yes (retina or comprehensive based on patient answer and electronic medical record) |
| Affected Eye | Which eye has symptoms? | - Left eye - Right eye - Both eyes |
| Symptom Onset | When did your symptoms start? | - Within 24 hours - Within 72 hours - 3-7 days ago - 1-2 weeks ago - More than 2 weeks ago |
| Evolution of Vision Change | Has your vision change remained stable, improved, or worsened? | - Stable - Improved - Worsened |
| Floater Presence | Have you noted new floaters in your vision? If so, how many? | - None - Less than 5 - More than 5 but less than 10 - More than 10 but less than 100 - More than 100 |
| Floater Characterization | What do your floaters look like? | - Cobweb - Tiny dots - Big blobs - Swirls - Other |
| Flashes Presence | Have you noted new flashes in your vision? If so, how many? | - None - Less than 5 per day - More than 5 per day |
| Blurred Vision Presence | Ignoring your floater(s), have you experienced blurred vision? If so, how would you characterize it? | - None - Intermittent - Constant |
| Symptoms of Curtain/Veil/Shadow | Have you experienced a non-moving curtain or veil or shadow in the side of your vision? | - Yes - No |
| Pain Presence and Characterization | Do you have pain in the affected eye? If so, how would you characterize it? | - No - Dull/Achy - Throbbing - Sharp - Pressure Sensation - Scratchy/Sandy/Foreign Body Sensation |
| Prior Glasses Use | When you were a young adult (before any procedures to your affected eye), did you need glasses to see to drive? | - Yes - No |
| Prior Retinal Tear or Retinal Detachment | Did you have a prior retinal tear or detachment in the affected eye? The unaffected eye? |
- Yes - No - Yes - No |
| Prior Retinal Tear or Retinal Detachment Characterization | Are your current symptoms similar to past tears/detachments? | - Yes - No - Not applicable (if patient did not have retinal tear or retinal detachment) |
| Diabetes Status | Are you a diabetic? | - Yes - No |
| Uveitis Status | Do you have uveitis? | - Yes - No |
| Trauma Status | Have you had a significant trauma to your head or affected eye? If yes, when? | - No - Within the past 2 months - More than 2 months ago |
| Retinal Surgery Status | Have you ever gone to the operating room for retinal surgery in either eye? | - No - Within the past 2 months - More than 2 months ago |
| Cataract Surgery Status | Have you ever gone to the operating room for cataract surgery in the affected eye? | - No - Within the past 2 months - More than 2 months ago |
| Intravitreal Injection Status | Have you had an intravitreal injection in the affected eye within the past 12 hours? | - Yes - No |
| Primary Care Provider | When was the last time you saw your primary care provider? | - Never - Within the past month - Within the past 3 months - Within the past 6 months - Within the past year - Within the past 2 years - More than 2 years ago |
Figure 1. Number of Patients with RT/RDs and Timeline Between Symptom Onset, Phone Triage, and Clinic Visit.
(A) Clinic source for patient, (B) Number of patients with RT/RDs, (C) Timeframe between symptom onset and phone triage, stratified by RT/RD status, (D) Interval between phone triage and add-on appointment for patients without RT/RDs, (E) Interval between phone triage and add-on appointment for patients with RT/RDs.
Patients with RT/RDs were much more likely to call within 24 hours of symptom onset (>50%) (Figure 1C). Regardless of RD/RT status, over 70% of patients (N=137) were seen within 24 hours of their phone triage call (Figure 1D-E).
Characterization of Symptoms Documented by Phone Triage
A significantly higher percentage of patients with RT/RD reported constant blurred vision or a curtain/veil in their field of view (Figure S3A). The phone triage questionnaire specifically asked patients with blurred vision if their symptoms were constant or intermittent. Interestingly, no patient with intermittent blurred vision had a RT/RD, suggesting the distinction between constant and intermittent blurred vision is important for triage.
While the presence of more floaters was associated with increased risk of a RT/RD, the presence of more flashes was not (Figure S3B-C). In describing the quality of their floaters, patients who reported “tiny dots” were significantly more likely to have a RT/RD than those who used other descriptors for their floaters (Figure S3D, 50% versus 21.78%). Patients who reported a combination of floaters and flashes of light were no more likely to have a RT/RD (Figure S3E).
History of Myopia, Trauma, Surgery, and Prior Retinal Tears or Detachments
While myopia is a risk factor for RT/RDs10, 11, patients may not be able to identify their specific type of refractive error over the phone. Furthermore, patients who previously underwent a refractive procedure or cataract surgery may not report they were previously myopic. To best capture the risk factor of myopia without reference to any physical exam results, we therefore asked patients if they needed glasses in order to drive when they were young, prior to any procedures on the eye. Using this framework as a proxy for myopia, those with myopia were significantly more likely to have an RT/RD compared to those without myopia (Figure S4A, 78.57% versus 48.60%). In contrast, past trauma did not predict the presence of RT/RDs (Figure S4B). RT/RDs were more common in patients with past history of retinal surgery in either eye (21.42% versus 3.35% for those with and without retinal surgery in past two months; 50.00% versus 17.32% for those with and without retinal surgery more than two months ago), along with history of cataract surgery in the symptomatic eye (42.86% versus 20.67% for those with and without cataract surgery in affected eye) (Figure S4C-D). A history of retinal tear in either the symptomatic eye or the contralateral eye also predicted the presence of a RT/RD on the exam following phone triage (Figure S4E, 28.57% versus 10.05% for a history of RT/RD versus no history in the symptomatic eye; 42.85% versus 15.64% for a history of RT/RD versus no history in the asymptomatic eye).
Univariate and Multivariable Analysis of Potential Risk Factors for RT/RD
Subjecting each variable collected during phone triage (Table 1) to univariate logistic regression analysis resulted in several statistically significant risk factors (Table S2). While we established “intravitreal injection in the last 12 hours” as a phone triage question meant to screen out patients unlikely to have experienced a RT/RD, no patients answered yes to this question in the study. The phone triage question asking “date of last visit to primary care provider” was designed as a proxy for how frequently a patient accesses the health care system. Our hypothesis was that those who rarely access health care but felt their eye symptoms were serious enough to call the eye clinic may be more likely to have a true RT/RD. However, our univariate analysis failed to validate this hypothesis.
For multivariable logistic regression, we started our model with risk factors from univariate analysis that had a p-value of 0.2 or lower. While the presence of diabetes was not a significant negative predictor of RT/RDs in univariate analysis, we retained this variable in multivariable analysis. Our personal experience is that the vast majority of diabetics without typical RT/RD risk factors who experience floaters are experiencing a vitreous hemorrhage from neovascularization. Thus, we hypothesized that when other RT/RD risk factors were accounted for in multivariable modeling, the presence of diabetes would be a strong negative predictor of a RT or RD. Two other variables we included from univariate analysis despite p>0.2 were the presence of flashes and whether symptoms were in one eye or both eyes. We included flashes in the multivariable model because of how common this symptom is in patients with PVDs. We included symptoms in one vs. both eyes because we hypothesized that patients with bilateral symptoms were very unlikely to be suffering from simultaneous bilateral PVDs, and this variable may become significant in multivariable analysis once other risk factors for RT/RDs were accounted for.
From the initial full multivariable model (Table S3), we iteratively created more parsimonious models based on statistical significance from prior models. Of note, some iterations required the reversal of reference levels such that the higher odds ratios always indicated an increased chance of developing a RT/RD. After testing eight models, our final refined model representing the best balance between adequate explanatory power and parsimonious use of risk factors is shown in Table 4. While a few risk factors in the final refined model have marginal significance, they have strong biologic rationale for inclusion. For example, patients with simultaneous symptoms in both eyes are highly unlikely to be experiencing simultaneous PVDs. Furthermore, of the 14 patients with a RT/RD, none had bilateral symptoms, whereas 20 out of 179 (11.1%) patients without a RT/RD complained of bilateral symptoms. Patients with more recent onset of symptoms were more likely to have a RT/RD (Figure 1C). Finally, while a history of RT/RDs was not significantly predictive of a new RT/RD in the multivariable model in Table 4, this is entirely because of the concurrent presence of the “past retinal surgery in either eye” variable. When the retinal surgery variable is not present in the multivariable model, a history of RT/RD in either eye becomes highly significant (Table S5), consistent with prior associations between RT/RD history and risk for new RT/RDs12-14. As we intended to have this model broadly applicable to both comprehensive and retina clinic patients, and most comprehensive clinic patients are unlikely to have had retinal surgery, we decided to keep both the “history of retinal surgery” and “history of RT/RD” variables in the final multivariable model.
Table 4.
Final Multivariable Model for Phone Triage Data. Multivariable logistic regression analysis was performed on univariate analysis from Table S2 for select variables and associated levels with a p-value <0.2 unless otherwise specified for reasons detailed in the text.
| Variable | Level | OR | 95% OR CI Lower |
95% OR CI Upper |
P-value |
|---|---|---|---|---|---|
| Eye | OD/OS vs. OU | 18.63 | 0.36 | 975.51 | 0.1476 |
| Start of Symptoms | 24-72 hours vs. More than 72 hours | 2.63 | 0.51 | 13.70 | 0.2503 |
| Within 24 hours vs. More than 72 hours | 3.01 | 0.64 | 14.20 | 0.1641 | |
| Constant Blurred Vision or Curtain or Veil | Yes vs. No | 12.01 | 2.83 | 50.99 | 0.0008 |
| Use of Glasses to Drive While Young | Yes vs. No | 2.73 | 0.65 | 11.45 | 0.1705 |
| Prior Retinal Surgery in Either Eye | Yes vs. No | 14.93 | 2.75 | 81.07 | 0.0017 |
| Diabetic | Yes vs. No | 6.15 | 1.01 | 37.55 | 0.0493 |
| History of RT/RD in Either Eye | Yes vs. No | 1.53 | 0.33 | 7.19 | 0.5876 |
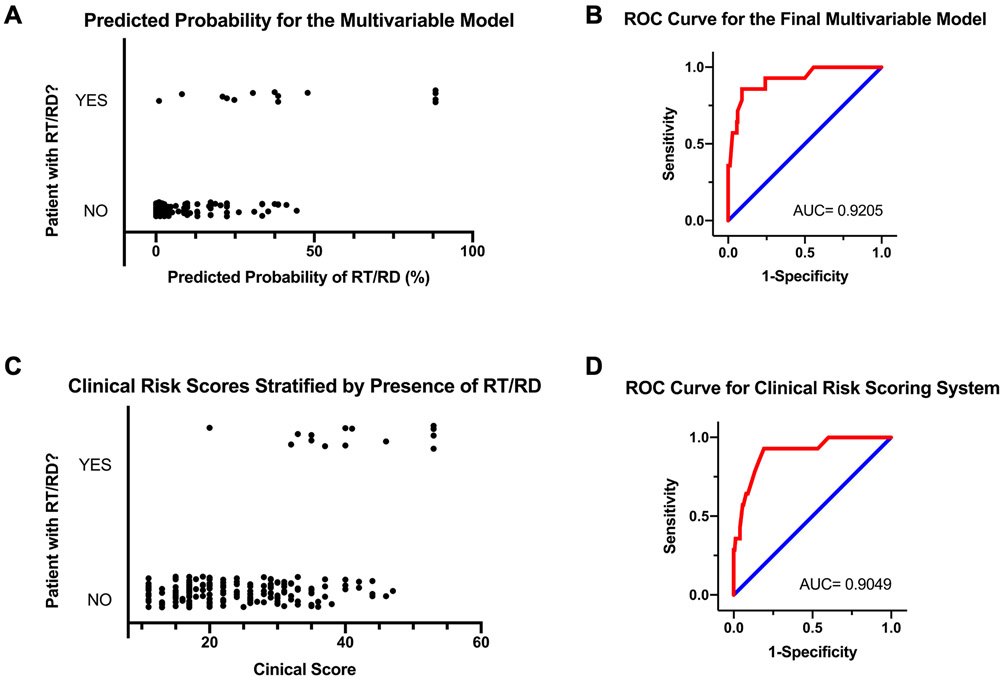
Utilizing the multivariable model from Table 4, we calculated the predicted probability of a RT/RD for each patient in our cohort. The graph in Figure 5A demonstrates strong separation in predicted probabilities between those with an actual RT/RD (top of Y-axis) and those without an actual RT/RD (bottom of Y-axis). The receiver operator curve (ROC) for the multivariable model demonstrates good sensitivity and specificity with an area under the curve (AUC) of 0.9205 (Figure 5B).
Figure 5. Performance of Final Multivariable and Clinical Risk Score Models in Predicting RT/RDs Among Phone Triage Patients.
(A) Predicted probability of RT/RD using final multivariable model (Table 2), (B) Receiver-Operator Curve (ROC) for final multivariable model (Table 2), (C) Distribution of clinical risk scores for patients with and without RT/RDs, (D) ROC for clinical risk score model.
To create a RT/RD risk scoring system for easy use during phone triage, we assigned each risk factor from the multivariable model in Table 4 a point score approximately in proportion to variable’s odds ratio (with small adjustments). The phone triage questions and associated points for generating the clinical risk score are displayed in Table 6. The graph in Figure 5C again shows good separation in the clinical risk scores between those with a RT/RD (top of Y-axis) and those without a RT/RD (bottom of Y-axis). Most RT/RD patients had a clinical score above 29 (13/14 patients). The ROC AUC for the clinical risk score is comparable to the ROC AUC for the multivariable model (compare Figure 5B vs. Figure 5D). Table 7 illustrates the effect of using different clinical risk score cutoffs for determining the urgency of a follow-up appointment during phone triage. A score of 20 captures all patients with a RT/RD at follow-up while excluding nearly half of patients without a RT/RD. A score of 29 captures 13 of 14 patients who had a RT/RD while excluding nearly 75% of patients without a RT/RD. These scores can help guide phone triage decisions about how urgently to schedule a patient for follow-up.
Table 6.
Final Phone Triage Prompt and Associated Point Values to Generate a Clinical Risk Score.
| Question | Answer Choices |
|---|---|
| Which eye has symptoms? | - One eye (5 points) - Both eyes (1 point) |
| When did your symptoms start? | - Within 24 hours (6 points) - Between 24-72 hours ago (3 points) - More than 72 hours ago (1 point) |
| Do you have a non-moving curtain or veil or shadow in the side of your vision? | - Yes - No |
| Ignoring your floater(s), have you experienced blurred vision? If so, how would you characterize it? | - None - Intermittent - Constant |
| If “Yes” OR “Constant” = 14 points If “No” AND “None or Intermittent” = 1 point |
|
| When you were a young adult (before any procedures to your affected eye), did you need glasses to see to drive? | - Yes (3 points) - No (1 point) |
| Have you had a prior retinal tear or detachment in either eye? | - Yes (10 points) - No (1 point) |
| Are you diabetic? | - Yes (1 point) - No (5 points) |
| Have you ever gone to the operating room for retinal surgery in either eye? | - Yes (10 points) - No (1 point) |
Table 7.
Clinical Risk Scoring System Cut-Off Score Analysis. Yellow and brown highlighted scores are discussed in the text.
| Cut-off Score | Number of −RT/RD Patients | Number of +RT/RD Patients | Percent −RT/RD Included | Percent +RT/RD Included |
|---|---|---|---|---|
| 17 | 116 | 14 | 64.80% | 100.00% |
| 20 | 95 | 14 | 53.07% | 100.00% |
| 23 | 83 | 13 | 46.37% | 92.86% |
| 26 | 64 | 13 | 35.75% | 92.86% |
| 29 | 49 | 13 | 27.37% | 92.86% |
| 32 | 29 | 12 | 16.20% | 85.71% |
| 35 | 16 | 9 | 8.94% | 64.29% |
DISCUSSION
Given that a major percentage of urgent eye care visits are for symptomatic PVDs1-5, a reliable and efficient system for triaging which patients warrant urgent versus more routine follow-up is of high value. Numerous studies have demonstrated key risk factors that predict the presence of a RT/RD in symptomatic PVDs15-18. However, no study to date has attempted to judge RT/RD risk after symptomatic PVD entirely from questions that can be asked over the phone without knowledge of the patient’s history or exam. Here, we prospectively followed more than 200 consecutive patients calling to a major academic medical center with symptoms consistent with a symptomatic PVD, asked a standardized set of questions during the phone call, and then tracked which patients, on follow-up, had RT/RDs. From these data, we derived a clinical risk scoring system that allows non-physician personnel to appropriately triage patients calling with symptoms consistent with a PVD. The clinical risk score can be easily derived from a few questions to the patient (Table 6), and different clinical risk score cutoffs can be used to determine urgency of follow-up appointments (Table 7). Such a system could safely recategorize at least half of all patients with symptomatic PVDs to a non-urgent appointment slot.
One of the earliest attempts at PVD symptom triage was a prospective study conducted by Boldrey in 1983 looking at 589 patients. He found that patients who described their floaters as diffuse dots with lines, versus those without lines, were five times more likely to have a RT. The remainder of his analysis demonstrated no other triage screening variables prior to in-person examination accurately predicted the presence of a RT or RD. A subsequent meta-analysis of 17 studies from 1980 to 2009 suggested that only blurred vision predicted the presence of RT/RD on exam19. While several studies from the 2000s showed that a large number of floaters predicted the formation of a delayed RT/RD after an initial exam19-21, other studies found that physical examination findings (e.g. - vitreous pigment or hemorrhage) were the only data that reliably predicted the presence of RT/RDs22-25. Studies from the last decade have demonstrated that floaters are more predictive of RTs than flashes and have affirmed that short duration of symptoms (less than 24 hours), a large number of floaters, blurred vision, or a veil/cloud/curtain in the vision are all predictive of a RT15-18. However, no symptoms had enough discriminatory power to exclusively rely on for determining which patients should be seen urgently. Further, no attempts were made to combine symptoms into a risk score that could predict which patients should be seen urgently.
In the last two years, three studies have provided the most comprehensive look at how well patient symptoms predict a RT/RD. McCullagh, Higham, and Best created a scoring system (BERT Score) to determine if a patient had a complicated PVD (RT, RD, or dense vitreous hemorrhage)26. Their methodology resulted in 90% sensitivity, 80% specificity, 40% positive predictive value, and 98% negative predictive value. However, their scoring system included a combination of symptoms and physical examination findings like vitreous hemorrhage and vitreous pigment, precluding use of this scoring system for initial phone triage.
Seider, Conell, and Melles retrospectively examined 8305 patients in the Kaiser Permanente Northern California Healthcare System for variables available before examination that predicted RT/RDs27. They found that blurred vision, male gender, prior keratorefractive surgery, myopia, family history of RD, younger age, duration of symptoms for less than a week, and prior cataract surgery had higher odds ratios for patients with a RT/RD. The retrospective nature of the study, however, precluded more comprehensive and granular acquisition of patient symptoms and history prior to exam. Further, the study was not designed to determine which patients undergoing phone triage should be seen urgently versus routinely. Similar to the Kaiser Permanente study, we found that remote cataract surgery in the symptomatic eye marginally increased the risk for a RT/RD (p~0.07 in univariate analysis), but this risk disappeared in our multivariable analysis. The same pattern was also observed for history of myopia with significance in our univariate analysis (p~0.04) but not in our multivariable analysis.
More recently, Arevalo and colleagues implemented a written questionnaire for patients waiting in clinic prior to first-time evaluation for symptoms consistent with a PVD. The questionnaire covered some but not all of the questions asked in our study, and then linked which responses predicted the presence of a RT/RD28. Over a one-year period, they evaluated 237 patients and found that symptom-based predictive factors for patients having a RT/RD included subjective visual reduction or field loss. Similar to our study, there was no predictive value in the characteristics of flashes and floaters experienced by patients for having an increased likelihood of a RT/RD. The study covered almost exclusively retina clinic patients and did not have enough discriminatory power to generate a clinical risk score for RT/RDs that could be utilized for phone triage.
We designed our study to capture most of the risk factors previously associated with RT/RDs or that have a reasonable biological link to risk (Table 1). While many factors were associated (or trended towards association) with RT/RD risk in univariate analysis, most factors were no longer significant predictors in multivariable analysis. This included male sex (Figure S2C), number of floaters (Figure S3B), description of floaters as tiny dots (Figure S3D), and prior cataract surgery (Figure S4D). Other factors that had theoretical reasons for being predictive of a RT/RD were tested and rejected in univariate analysis. Increased number of flashes or the combination of flashes and floaters were not predictive, consistent with past studies (Figure S3C,E)15,28. Against our hypothesis, a history of trauma was not predictive of RT/RDs (Figure S4B), consistent with findings from another recent study28. However, our study is likely underpowered to detect the risk of a rare event, like serious recent ocular trauma, and we therefore advise such patients be seen urgently, regardless of their phone triage score. We also hypothesized that those with less contact with the health care system, using last primary care provider visit as a proxy, would be more likely to have a RT/RD as their symptoms were serious enough to warrant an uncommon engagement with a health care provider. Our data was unable to support this hypothesis (Table S2). Additionally, we hypothesized that patients complaining of pain accompanying their symptoms would be much less likely to have a RT/RD, but the data did not support any inverse relationship between pain and RT/RD risk (Table S2). Finally, whether symptoms were stable, worsening, or improving did not predict presence of RT/RDs (Table S2). Several variables with no statistical significance in univariate analysis increased in significance in multivariable analysis and had strong theoretical reasons for being included in our final clinical score system. For example, patients with bilateral simultaneous symptoms are highly unlikely to be suffering from completely synchronous bilateral PVDs (Table 4), and patients with diabetes and no other RT/RD risk factors are much more likely to experience floaters from neovascular-related vitreous hemorrhage than a RT/RD (Table 4).
How questions are phrased during phone triage plays a critical role in accurately assessing the clinical risk score we present here. For example, while myopia is a known risk factor for RT/RDs10, 29, we avoided using a patient’s refraction to analyze for myopia in this study as that necessitates a physical exam or prior knowledge of the patient’s medical record. At the same time, patients may be unable to verbalize that they are myopic over the phone, and pseudophakic or refractive surgery patients who were previously myopic no longer need glasses to see at distance. Thus, we assessed for myopia by asking whether patients needed glasses to drive when they were young, prior to any procedures on the symptomatic eye. Similarly, the presence of blurred vision has been shown to be a strong predictor of RT/RDs in both our study and previous studies26,28. However, patients frequently conflate a floater with blurred vision, so we asked patients to ignore their floater when evaluating if they had blurred vision. Additionally, with more nuanced questioning, we found that patients who stated that their blurred vision was intermittent were less likely to have a RT/RD (Figure S3A). Intermittent blurring is much more likely to derive from ocular surface issues. For any patients describing blurred vision during phone triage, follow-up questioning must ask if the blurred vision is constant or intermittent. Thus, to derive our clinical risk score, we suggest non-physicians performing phone triage structure their questions according to Table 6.
Our study carries notable strengths. This is the first study to risk-stratify patients with symptoms consistent with a PVD over the phone without reliance on a patient’s medical chart or physical exam. Our study structure allowed us to simultaneously interrogate the vast majority of risk factors previously associated with RT/RDs in symptomatic PVDs while also testing associations with novel theoretical risk factors. In addition, our study included a broad range of symptomatic patients, including those new to our department as well as both established comprehensive and retinal clinic patients (Figure 1A). The clinical risk score we established should therefore be universally implementable because all parts of the scoring system are obtainable for each patient and the type of clinic the patient is seen in doesn’t impact the risk calculations. Further, the phone triage questions needed to derive a clinical risk score are simple and short (Table 6), allowing for easy implementation by non-physician triage staff. Finally, practices utilizing this risk score can customize clinical score cutoffs to determine a time-frame for follow-up visit (Table 7). In short, we have created the first accessible clinical risk score system for triaging patients with symptomatic PVDs entirely by phone.
Our study also has limitations. The patient sample size of approximately 200 patients is significantly smaller than previously published big-data analyses of risk factors for symptomatic PVDs30, 31. However, the prospective nature of our study allowed us to establish a more comprehensive questionnaire for patients with no missing data compared to previously established analyses. This also allowed us to parse out very specific ways questions may need to be phrased to accurately assess a risk factor – see, for example, our discussion of intermittent vs. constant blurred vision above. Another limitation of our study is that it lacks independent validation. We are currently querying and prospectively following a second set of patients to independently validate our clinical risk score and anticipate having results in approximately one year. A third limitation of our study is that not all patients received a scleral depressed exam or B-scan, which is the gold-standard for determining the presence of a RT/RD. This raises the possibility that certain patients seen at their first post-phone-triage follow-up had an undetected RT/RD. To mitigate against this possibility, we carefully examined the long-term follow-up of our patients. Of the 84% of patients who were followed for at least four weeks after phone triage, only two had an RT/RD develop in delayed fashion (1.2% of all patients). Both of these patients had two scleral depressed exam visits prior to development of the RT/RD, suggesting these represented true delayed RT/RD development. Of the 16% of patients without at least four weeks post-phone-triage follow-up, only one patient with a PVD failed to undergo a scleral depressed exam. That patient was seen 17 days after symptom onset and had no risk characteristics for a RT/RD, such as Shafer’s sign or vitreous hemorrhage (Figure S6). Thus, follow-up data supports that it is highly unlikely any clinically impactful RT/RDs developed as a result of some of the lowest risk patients in our cohort (e.g. those with any of the following: PVD, Shafer’s sign, and vitreous hemorrhage) failing to undergo a scleral depressed exam or B-scan. A final limitation is that our study was carried out at a single academic medical center where the patient base engaging with our department may be different than in other practice settings. However, arguing for the generalizability of our study, we note that the age distribution of patients examined (Figure S2A) was consistent with the average age of onset for symptomatic PVDs from multiple prior studies31-34. Further, the percentage of patients calling with symptoms consistent with a PVD who had RT/RDs on their subsequent initial exam was in-line with large prior studies22, 35. Finally, the use of scleral depression during exam (Figure S2B) is consistent with rates reported in the literature36-38. Future studies will attempt to formally study extension of our findings to other institutions.
In conclusion, we have established the first phone triage questionnaire and associated clinical risk scoring system that can be quickly executed by non-physician personnel and accurately predicts which patients with symptoms of a PVD should be seen urgently versus routinely. We anticipate this tool will simplify scheduling decisions about one of the most common triage questions ophthalmology practices face.
Supplementary Material
Figure S2. Additional Demographics and Physical Exam Characteristics for Screened Patients. (A) Age distribution of patients with and without RT/RDs, (B) Follow-up physical exam, (C) Gender in patients with and without RT/RDs, (D) Presence of diabetes or uveitis in patients with or without RT/RDs.
Figure S3. Symptoms in Patients With and Without RT/RDs. (A) Blurred vision or curtain/veil, (B) Number of floaters, (C) Number of flashes, (D) Description of floaters, (E) Patients with both flashes and floaters.
Figure S4. Ophthalmic History in Patients With and Without RT/RDs. (A) Use of glasses for driving when young (proxy for myopia), (B) Past eye trauma, (C) Past retinal surgery in either eye, (D) Past cataract surgery in symptomatic eye, (E) History of retinal tear or detachment in symptomatic or fellow eye.
Figure S6. Demographic Features, Physical Exam Characteristics, and Diagnoses of Patients Without a Scleral Depressed Exam (SDE) or B-scan on First Follow-Up Visit and Who Did Not Have a Follow-Up Visit at Least Four Weeks After Phone Triage. 15 patients (7.7% of all patients included in the study) that did not have an SDE or B-scan at their first clinical examination did not have follow-up four weeks or more from their phone triage date. Of these 15 patients, 13 did not have a PVD (and no Shafer’s sign or vitreous hemorrhage either), making them extremely low risk for a missed RT/RD. Two patients did have a PVD. However, one of these patients had a pre-existing PVD and their symptoms were attributed to ocular migraine. That leaves a single patient who failed to undergo SDE or B-scan and actually had a symptomatic PVD. This patient was seen 17 days after their phone triage, but not seen again, and again did not have Shafer’s sign or vitreous hemorrhage.
ACKNOWLEDGEMENTS
The authors thank Lesley Everett, MD, PhD, and David Musch, PhD, MPH, for advice and assistance in the early stages of this project.
Financial Support:
J.M.L.M. was supported by a Heed fellowship during parts of the data acquisition phase. The funding organization had no role in the design or conduct of this research.
Acronyms:
- PVD
Posterior vitreous detachment
- RT
Retinal tear
- RD
Retinal detachment
- ROC
Receiver operator curve
- AUC
Area under the curve
Footnotes
Conflict of Interest: No conflicting relationship exist for any of the authors.
References
- 1.Alangh M, Chaudhary V, McLaughlin C, et al. Ophthalmic referrals from emergency wards-a study of cases referred for urgent eye care (The R.E.S.C.U.E Study). Can J Ophthalmol 2016;51(3):174–9. [DOI] [PubMed] [Google Scholar]
- 2.Sridhar J, Isom RF, Schiffman JC, et al. Utilization of Ophthalmology-Specific Emergency Department Services. Semin Ophthalmol 2018;33(2):185–90. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin CR, Biehl M, Chan BJ, et al. Ophthalmology referrals from optometry: a comparative study (the R.O.C.S study). Can J Ophthalmol 2018;53(5):491–6. [DOI] [PubMed] [Google Scholar]
- 4.Docherty G, Hwang J, Yang M, et al. Prospective analysis of emergency ophthalmic referrals in a Canadian tertiary teaching hospital. Can J Ophthalmol 2018;53(5):497–502. [DOI] [PubMed] [Google Scholar]
- 5.Oliver C, Tadrous C, Docherty G, Warner S. Retrospective analysis of ophthalmology consults to a tertiary academic teaching centre. Can J Ophthalmol 2019;54(4):484–8. [DOI] [PubMed] [Google Scholar]
- 6.Deaner JD, Amarasekera DC, Ozzello DJ, et al. Accuracy of Referral and Phone-Triage Diagnoses in an Eye Emergency Department. Ophthalmology 2021;128(3):471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedekind L, Sainani K, Pershing S. Supply and Perceived Demand for Teleophthalmology in Triage and Consultations in California Emergency Departments. JAMA Ophthalmol 2016;134(5):537–43. [DOI] [PubMed] [Google Scholar]
- 8.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002;21(16):2409–19. [DOI] [PubMed] [Google Scholar]
- 9.Firth D. Bias Reduction of Maximum Likelihood Estimates. Biometrika 1993;80(1):27–38. [Google Scholar]
- 10.van Leeuwen R, Haarman AEG, van de Put MAJ, et al. Association of Rhegmatogenous Retinal Detachment Incidence With Myopia Prevalence in the Netherlands. JAMA Ophthalmol 2021;139(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crim N, Esposito E, Monti R, et al. Myopia as a risk factor for subsequent retinal tears in the course of a symptomatic posterior vitreous detachment. BMC Ophthalmol 2017;17(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia LY, Sun YX, Zhang YP, Ma K. Risk Factors of Recurrent Retinal Detachment Following Surgical Treatment for Rhegmatogenous Retinal Detachment: A Retrospective Study. Risk Manag Healthc Policy 2020;13:3165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster RE, Meyers SM. Recurrent retinal detachment more than 1 year after reattachment. Ophthalmology 2002;109(10):1821–7. [DOI] [PubMed] [Google Scholar]
- 14.Kapran Z, Uyar OM, Kaya V, Eltutar K. Recurrences of retinal detachment after vitreoretinal surgery, and surgical approach. Eur J Ophthalmol 2001;11(2):166–70. [DOI] [PubMed] [Google Scholar]
- 15.Gishti O, van den Nieuwenhof R, Verhoekx J, van Overdam K. Symptoms related to posterior vitreous detachment and the risk of developing retinal tears: a systematic review. Acta Ophthalmol 2019;97(4):347–52. [DOI] [PubMed] [Google Scholar]
- 16.Bond-Taylor M, Jakobsson G, Zetterberg M. Posterior vitreous detachment - prevalence of and risk factors for retinal tears. Clin Ophthalmol 2017;11:1689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst J, Johnson D, Law C, et al. Value of subjective visual reduction in patients with acute-onset floaters and/or flashes. Can J Ophthalmol 2015;50(4):265–8. [DOI] [PubMed] [Google Scholar]
- 18.Jarocki A, Durrani A, Zhou Y, Miller JML. On-Call Examinations for Acute Onset of Flashes, Floaters, or Curtain by Junior Ophthalmology Residents: Outcomes, Safety, and Resource Use. Ophthalmol Retina 2021;5(4):330–6. [DOI] [PubMed] [Google Scholar]
- 19.Hollands H, Johnson D, Brox AC, et al. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA 2009;302(20):2243–9. [DOI] [PubMed] [Google Scholar]
- 20.van Overdam KA, Bettink-Remeijer MW, Mulder PG, van Meurs JC. Symptoms predictive for the later development of retinal breaks. Arch Ophthalmol 2001;119(10):1483–6. [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer KD, Eneh AA, Hurst J, et al. Predicting retinal tears in posterior vitreous detachment. Can J Ophthalmol 2011;46(6):481–5. [DOI] [PubMed] [Google Scholar]
- 22.Coffee RE, Westfall AC, Davis GH, et al. Symptomatic posterior vitreous detachment and the incidence of delayed retinal breaks: case series and meta-analysis. Am J Ophthalmol 2007;144(3):409–13. [DOI] [PubMed] [Google Scholar]
- 23.van Overdam KA, Bettink-Remeijer MW, Klaver CC, et al. Symptoms and findings predictive for the development of new retinal breaks. Arch Ophthalmol 2005;123(4):479–84. [DOI] [PubMed] [Google Scholar]
- 24.Tanner V, Harle D, Tan J, et al. Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomatology. Br J Ophthalmol 2000;84(11):1264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goh YW, Ehrlich R, Stewart J, Polkinghorne P. The incidence of retinal breaks in the presenting and fellow eyes in patients with acute symptomatic posterior vitreous detachment and their associated risk factors. Asia Pac J Ophthalmol (Phila) 2015;4(1):5–8. [DOI] [PubMed] [Google Scholar]
- 26.McCullagh D, Higham A, Best R. The BElfast Retinal Tear and detachment Score (BERT Score). Eye (Lond) 2021;35(5):1427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seider MI, Conell C, Melles RB. Complications of Acute Posterior Vitreous Detachment. Ophthalmology 2022;129(1):67–72. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad M, Sein J, Wang J, et al. Symptom-Based Risk Factors for Retinal Tears and Detachments in Suspected Posterior Vitreous Detachment. Ophthalmologica 2022. [DOI] [PubMed] [Google Scholar]
- 29.Chandra A, Banerjee P, Davis D, Charteris D. Ethnic variation in rhegmatogenous retinal detachments. Eye (Lond) 2015;29(6):803–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JH, Yang H, Kwon H, Jeon S. Risk Factors for Onset or Progression of Posterior Vitreous Detachment at the Vitreomacular Interface after Cataract Surgery. Ophthalmol Retina 2021;5(3):270–8. [DOI] [PubMed] [Google Scholar]
- 31.Shen Z, Duan X, Wang F, et al. Prevalence and risk factors of posterior vitreous detachment in a Chinese adult population: the Handan eye study. BMC Ophthalmol 2013;13(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hikichi T, Hirokawa H, Kado M, et al. Comparison of the prevalence of posterior vitreous detachment in whites and Japanese. Ophthalmic Surg 1995;26(1):39–43. [PubMed] [Google Scholar]
- 33.Hikichi T, Trempe CL. Relationship between floaters, light flashes, or both, and complications of posterior vitreous detachment. Am J Ophthalmol 1994;117(5):593–8. [DOI] [PubMed] [Google Scholar]
- 34.Yonemoto J, Ideta H, Sasaki K, et al. The age of onset of posterior vitreous detachment. Graefes Arch Clin Exp Ophthalmol 1994;232(2):67–70. [DOI] [PubMed] [Google Scholar]
- 35.Mitry D, Charteris DG, Fleck BW, et al. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol 2010;94(6):678–84. [DOI] [PubMed] [Google Scholar]
- 36.Shukla SY, Batra NN, Ittiara ST, Hariprasad SM. Reassessment of Scleral Depression in the Clinical Setting. Ophthalmology 2015;122(11):2360–1. [DOI] [PubMed] [Google Scholar]
- 37.Arjmand P, Hurley B. Flashes and floaters: a survey of Canadian ophthalmology residents' practice patterns. Can J Ophthalmol 2017;52(5):453–7. [DOI] [PubMed] [Google Scholar]
- 38.Patel PR, Minkowski J, Dajani O, et al. Analysis of Posterior Vitreous Detachment and Development of Complications Using a Large Database of Retina Specialists. Ophthalmol Retina 2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2. Additional Demographics and Physical Exam Characteristics for Screened Patients. (A) Age distribution of patients with and without RT/RDs, (B) Follow-up physical exam, (C) Gender in patients with and without RT/RDs, (D) Presence of diabetes or uveitis in patients with or without RT/RDs.
Figure S3. Symptoms in Patients With and Without RT/RDs. (A) Blurred vision or curtain/veil, (B) Number of floaters, (C) Number of flashes, (D) Description of floaters, (E) Patients with both flashes and floaters.
Figure S4. Ophthalmic History in Patients With and Without RT/RDs. (A) Use of glasses for driving when young (proxy for myopia), (B) Past eye trauma, (C) Past retinal surgery in either eye, (D) Past cataract surgery in symptomatic eye, (E) History of retinal tear or detachment in symptomatic or fellow eye.
Figure S6. Demographic Features, Physical Exam Characteristics, and Diagnoses of Patients Without a Scleral Depressed Exam (SDE) or B-scan on First Follow-Up Visit and Who Did Not Have a Follow-Up Visit at Least Four Weeks After Phone Triage. 15 patients (7.7% of all patients included in the study) that did not have an SDE or B-scan at their first clinical examination did not have follow-up four weeks or more from their phone triage date. Of these 15 patients, 13 did not have a PVD (and no Shafer’s sign or vitreous hemorrhage either), making them extremely low risk for a missed RT/RD. Two patients did have a PVD. However, one of these patients had a pre-existing PVD and their symptoms were attributed to ocular migraine. That leaves a single patient who failed to undergo SDE or B-scan and actually had a symptomatic PVD. This patient was seen 17 days after their phone triage, but not seen again, and again did not have Shafer’s sign or vitreous hemorrhage.