Abstract
The monoamine hypothesis has significantly improved our understanding of mood disorders and their treatment by linking monoaminergic abnormalities to the pathophysiology of mood disorders. Even 50 years after the monoamine hypothesis was established, some patients do not respond to treatments for depression, including selective serotonin reuptake drugs. Accumulating evidence shows that patients with treatment-resistant depression (TRD) have severe abnormalities in the neuroplasticity and neurotrophic factor pathways, indicating that different treatment approaches may be necessary. Therefore, the glutamate hypothesis is gaining attention as a novel hypothesis that can overcome monoamine restrictions. Glutamate has been linked to structural and maladaptive morphological alterations in several brain areas associated with mood disorders. Recently, ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist, has shown efficacy in TRD treatment and has received the U.S. Food and Drug Administration approval, revitalizing psychiatry research. However, the mechanism by which ketamine improves TRD remains unclear. In this review, we re-examined the glutamate hypothesis, bringing the glutamate system onboard to join the modulation of the monoamine systems, emphasizing the most prominent ketamine antidepressant mechanisms, such as NMDAR inhibition and NMDAR disinhibition in GABAergic interneurons. Furthermore, we discuss the animal models used in preclinical studies and the sex differences in the effects of ketamine.
Keywords: Treatment-resistant depression; Ketamine; Glutamate hypothesis; Chronic stress; Models, animal; Sex cha-racteristics
INTRODUCTION
In the early 1900s, it was discovered that physiological mechanisms activated by stress could protect or harm the body [1]. There has been a long-standing interest in the kind of action in the brain that mediates mental disorders caused by stress [2]. Several studies using animal models of stress have shown that various forms of environmental stress have profound effects on the structure and morphology of the brain, resulting in dendritic remodeling, synaptic spine loss, glial loss, and volume loss [3]. It is noteworthy that functional (i.e., blood flow, metabolism) [4] and altered molecular function (i.e., gene expression, etc.) [5] as well as morphological changes in neurons and glia (cytoarchitectural) [3] are common in humans showing the depressive phenotypes. It has been observed that such functional and structural changes can be reversed by behavioral treatments (i.e., cognitive therapy), brain-stimulation, and pharmacological treatments [6]. Interestingly, these structural and morphological alterations occur in cortical limbic areas, where glutamate is mainly used as a neurotransmitter by the vast majority of neurons and synapses [3]. Various monoaminergic antidepressants and putative non-monoaminergic antidepressants have also been reported to reverse stress-induced morphological changes [7]. These results suggest that monoamine transport and glutamate release at synapses are essential for mediating the complex emotional/cognitive changes associated with stress-induced depressive symptoms [7].
The current pharmacological therapy for depression concentrates on monoamine-based targets as a result of the early accidental discovery that drugs that inhibit the reuptake or metabolism of monoaminergic neurotrans-mitters have antidepressant effects [8,9]. However, it typically takes several weeks or months for the full benefit of medication to manifest, and some individuals are often resistant to treatment. According to the National Institutes of Mental Health-funded Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, only 27% of patients with major depressive disorder (MDD) achieve remission within 12 weeks of starting treatment with citalopram, and despite trying other four types of monoaminergic antidepressants, 33% of patients failed to achieve remission [10]. Another STAR*D study reported that overall remission rates stabilize at 60−70% after more than three antidepressant treatments. It means that about 30% of MDD patients are resistant to antidepressant treatments [11]. Treatment-resistant depression (TRD) is generally defined as failure to respond to at least antidepres-sant treatments of adequate dose and duration [12]. It is quite surprising that more than 30% of patients with TRD attempt suicide at least once in their lifetime, which is about twice that of patients with MDD. It suggests an urgent need for TRD treatment [13]. Recent transcriptome- wide association studies (TWAS) have revealed that people with TRD have biological characteristics that distinguish them from treatment responders [14]. According to the TWAS study, TRD patients have more than twice as many neurotrophic pathway abnormalities as monoamine transmission, suggesting that TRD may be treated by enhancing synaptic plasticity and neurotrophic factor [14]. These findings indicate that, unlike patients who may be treated with monoamines antidepressants, TRD patients require another class of antidepressant treatments that involves neuroplasticity and neurotrophic factors, rather than monoamines.
Ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist, has recently attracted attention as a new treatment for TRD by reducing anhedonia and suicidal ideation within hours [15]. A recent animal study showed that ketamine can reverse the depressive behaviors that are induced by chronic treatment of corticosterone, the principal murine stress hormone by selectively rescuing lost spines and restoring prefrontal cortex (PFC) microcircuit dysfunction during chronic corticosterone exposure [16]. Consistently, a clinical study using magnetic resonance imaging study showed that a single ketamine injection can normalize brain volumes of inferior frontal gyrus, a part of frontal lobe which is correlated with depressive severity, in MDD patients [17]. Another imaging study utilizing the diffusion tensor imaging showed that ketamine can rapidly alter microstructural diffusion properties of white matter in individuals with TRD [18]. In addition, a recent systematic review revealed that ketamine can treat cognitive deficits observed in TRD populations [19]. How-ever, it is unclear how ketamine treats TRD although it was approved as a medicine for TRD in 2019 by the U.S. Food and Drug Administration (FDA).
In this review, we discuss recently hypothesized ketamine mechanisms, such as spontaneous NMDAR-mediated neurotransmission, inhibition of NMDAR on GABAergic interneurons, and the role of ketamine metabolites, and how these hypotheses are related to brain-derived neurotrophic factor (BDNF) signaling. In addition, we discuss the current animal models developed to investigate the differences in the neurobiological mechanisms of antidepressant responders and non-responders. Finally, we examine whether there were sex differences in ketamine action, highlighting the limitations of existing animal models and underlining the importance of developing additional animal models.
MAIN SUBJECTS
A Brief History of Antidepressants: Pharmacological Interventions Based on the “Monoamine Hypothesis”
In the 1930s, depression was initially treated using amphetamines, a subclass of central nervous system stimulants that increase synaptic concentrations of monoamine neurotransmitters [20]. However, serious antidepressant medication developments did not begin until the mid- twentieth century. The first important breakthrough in antidepressant treatment occurred serendipitously in the early 1950s (Fig. 1). While scientists were evaluating the new hydrazide drug iproniazid for tuberculosis therapy, they discovered that patients who took the drug gained energy, increased appetite, and slept better [21]. Subse-quent studies have shown that iproniazid suppresses monoamine oxidase (MAO), an enzyme that degrades monoamines or prevents their uptake, restores the monoaminergic system, maintains neurotransmitters at synapses, and enhances cell communication (Fig. 2) [9, 22]. Since reporting the first information on the effect of iproniazid on depression in 1957 at an American Psychiatric Association meeting, the function of MAO in depression has been established, and a new class of antidepressants called MAO inhibitors (MAOIs) has been developed, including isocarboxazid (MarplanⓇ) and tranylcypromine (ParnateⓇ) [23,24]. However, iproniazid, the first clinically effective MAOI, cannot be used as an antidepres-sant.
Fig. 1.
The main advances in antidepressant drugs since the 1950s. The monoamine oxidase inhibitor iproniazid was initially prescribed to treat tuberculosis. Iproniazid was broadened; however, it is now at the forefront of treating depression after a quick demonstration of its antidepressant effects in 1952. Around the same time, imipramine has also been shown to have antidepressant properties. Imipramine, the first TCA medicine to obtain FDA approval, is thought to help prevent monoamine reuptake, supporting the catecholamine hypothesis, notably the serotonin hypothesis. Evidence supporting the significance of serotonin in depression grew in the early 1970s as concerns about the side effects of MAOIs and TCAs grew. Fluoxetine was the first serotonin reuptake inhibitor, replacing previously used MAOIs and TCAs, and it was refined and FDA-approved as Prozac in 1985. Although these developments have become watersheds for the treatment of depression, monoamine-based medications can only be effective when taken continuously for several weeks, and many patients still do not fully recover. Therefore, new medications that function swiftly, particularly for patients who are resistant to traditional monoamine therapy, are required. As the limitations of the monoamine hypothesis became obvious, researchers began to notice that ketamine, found in 2000, dramatically relieved depressive symptoms within a few hours. Furthermore, when it was shown in 2006 that ketamine had antidepressant benefits in those who did not respond to standard monoamine drugs, the paradigm of depression research was radically altered. Because of this discovery, the FDA authorized the use of (S)-ketamine in 2019 for the treatment of treatment-resistant depression. Although the success of ketamine as an antidepressant has stimulated research into glutamate-based mechanisms of action, new NMDAR antagonists and glutamate-based drugs are yet to display the fast and long-lasting antidepressant effects of ketamine. Research on ketamine is underway to further understand its impact on treatment-resistant depression.
MAOIs, monoamine oxidase inhibitors; FDA, Food and Drug Administration; TCAs, tricyclic antidepressants; SSRIs, selective serotonin reuptake inhibitors; MDD, major depressive disorder; TRD, treatment-resistant depression.
Fig. 2.
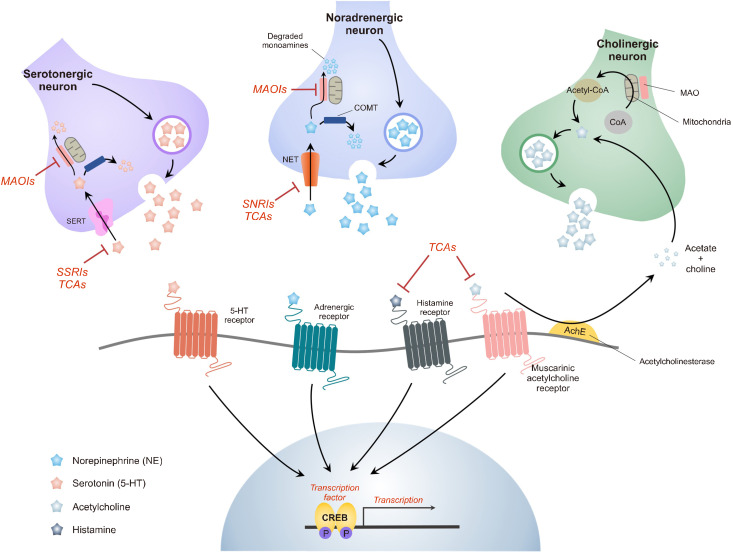
Key mechanisms of action for the antidepressants SSRI, TCA, and ketamine. MAOIs elevate brain amine levels by interfering with nerve-ending metabolism, resulting in increased vesicular storage of NE and 5-HT. Increased levels of amines are generated when nerve activity releases vesicles, boosting the activity of neurotransmitters. Tricyclic antidepressants have an immediate influence on neurotransmitter function at post-synaptic receptors by blocking the reuptake mechanism responsible for the synaptic termination of NE and 5-HT in the brain. The acute activity of SSRIs on the serotonin transporter is highly selective (SERT). SSRIs allosterically block the transporter by binding to sites other than serotonin. They may have little inhibition of the NE transporter or block adrenergic and cholinergic receptors. SNRI enhances the functions of both neurotransmitters by binding to SERT and NE transporters (NET). Unlike TCA, SNRI has no substantial blocking effect on peripheral receptors, such as histamine H1, muscarinic, or adrenergic receptors. This figure is adapted from Aswal et al. (J Formul Sci Bioavailab 2018;2:1000121) [134] and Martín-Hernández et al. (Monoaminergic system and antidepressants. 2021. p.345-355) [135] with original copyright holder’s permission.
SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; MAOIs, monoamine oxidase inhibitors; NE, norepinephrine; 5-HT, serotonin; SERT, serotonin transporter; SNRI, serotonin-norepinephrine reuptake inhibitor; COMT, catechol-o-methyltransferase; CoA, coenzyme A; CREB, cyclic adenosine monophosphate response element-binding protein.
Simultaneously, tricyclic antidepressants (TCA), such as imipramine, arose rapidly at the end of the revolutionary 1950s as the role of monoamine neurotransmitters such as serotonin, dopamine, and norepinephrine as mood regulators became understood [25,26]. TCAs influence approximately five different neurotransmitter pathways in a non-selective manner. They inhibit serotonin and norepinephrine absorption at presynaptic terminals by acting as competitive antagonists of post-synaptic alpha-cholinergic, muscarinic, and histaminergic receptors (Fig. 2) [27]. Similar to iproniazid, imipramine (TofranilⓇ) was originally intended to be used as a schizophrenia drug rather than an antidepressant. Although it was unable to demonstrate its usefulness in tests with schizophrenic individuals [28], it produced unforeseen effects in depres-sed patients, leading to its FDA approval as an antidepres-sant in 1959 [29]. Electroconvulsive therapy, the primary antidepressant therapy available in those days, was relegated by the accidental mood-raising effects of two antidepressants, iproniazid and imipramine [30]. As a result, TCA class medications have been swiftly developed as new, safe alternatives to MAOIs, which have limited effectiveness and serious side effects such as hypertension and liver damage [31].
The 1960s was a time of vigorous psychiatric controversy and rapid antidepressant success. The effectiveness of MAOIs and TCAs supports the monoamine hypothesis of depression, which contends that depression is caused by a deficiency in the monoaminergic system (Fig. 1) [8,9]. Serotonin and catecholamine were the two major concerns. The “catecholamine hypothesis” was proposed first based on the data given above about the mechanism of action of MAOIs and TCAs [32,33]. This hypothesis suggested that an absolute or relative deficit of catecholamines, particularly norepinephrine, causes depression [9,34]. Conversely, the “serotonin hypothesis” argued that the pathophysiology of depression is linked to a decrease in synaptic serotonin levels [35].
In the early 1970s, while concerns about the side effects of MAOIs and TCAs were being developed, evidence for the function of serotonin in depression was growing. This has sparked an era of novel antidepressants [36,37]. In this regard, researchers have attempted to discover compounds that may selectively inhibit serotonin reuptake without cardiotoxicity or anticholinergic effects of TCAs [38]. As a result, the selective serotonin reuptake inhibitors (SSRIs) family of antidepressants was developed. Eli Lilly and Company started to develop the most common SSRI, fluoxetine, in 1971. Fluoxetine began phase 1 clinical trials in 1976, followed by phase 2 and 3 studies in 1978 and 1981, respectively. Following unanimous approval by the FDA advisory panel in 1985, the U.S. FDA legally authorized fluoxetine in 1987 [39]. Soon after, it reached the market as ProzacⓇ. After it was released as the first SSRI, it has become the world’s most widely prescribed antidepressant. Since then, other SSRIs, such as sertraline (ZoloftⓇ) and citalopram (CelexaⓇ), have been developed [40,41]. Bupropion was presented as a slightly unusual antidepressant at the end of the 1980s, shortly after fluoxetine was released. The dopamine-norepineph-rine reuptake inhibitor bupropion (WellbutrinⓇ) is primarily more selective for dopamine transporters than for norepinephrine transporters [42]. It was approved by the FDA for the treatment of depression in 1985 [43] because of its superior efficacy and safety, including the lowest risk of sexual dysfunction, when compared to other types of depressants. However, no additional depression-related selective dopamine norepinephrine reuptake inhibitors were identified.
In the 1990s, another unique type of antidepressant called serotonin-norepinephrine reuptake inhibitors (SNRIs) was released. The prototypical drug, venlafaxine (EffexorⓇ), was approved by the FDA for the treatment of depression in 1993 [44]. Antidepressants in this FDA-approved class include duloxetine (CymbaltaⓇ) and levomilnacipran (FetzimaⓇ) [45,46]. Both SNRIs and TCAs block the serotonin and norepinephrine transporters. However, unlike TCAs, SNRIs show modest pharmacological activity on alpha cholinergic, muscarinic, histamine, dopamine, and post-synaptic serotonin receptors (Fig. 2) [27]. There have been reports that SNRIs may be more effective than SSRIs; however, the difference is minimal [47].
Despite these revolutions, several drawbacks remain unsolved. All antidepressants can cause side effects, including dry mouth, weight gain, nausea, dizziness, insomnia, and paraphilia [48]. It frequently takes too long to be effective, and up to one-third of patients with depression do not respond to these medications [10]. Since the discovery of antidepressants in the late 1950s, the hasty conclusion that all antidepressants function by correcting monoamine signaling deficiency has failed to offer novel therapeutic approaches. In fact, monoamine deficiency did not cause depression in healthy individuals and did not reliably worsen depression in drug-free patients [49]. When these aspects are considered together, disruption of monoamine signaling alone does not cause depression, and elucidating the etiology and pathophysiology beyond the monoamine system will be critical for developing novel treatments for depression.
Glutamate Hypothesis Sheds New Light on the Resistance to Monoamine-based Antidepressant Treatment
In 1921, Weston et al. [50] published a study on the mechanism of a drug that has now been identified as an N-methyl-D-aspartate (NMDA) antagonist used to alleviate agitation in people with depression. Since 1990, there has been a convincing theoretical foundation for the use of NMDAR antagonists in the treatment of depression, but they have had little impact on the development of antidepressants [9]. The glutamate hypothesis gained support when the clinical limitations of current monoamin-ergic approaches began to emerge at the beginning of the early 21st century, along with the recognition of the generally low efficacy of the available treatment methods. Although significant quantities of glutamate were discovered in the brain in the 1930s [51], it was not until the early 1980s that the amino acid glutamate was generally recognized as a neurotransmitter. Glutamate is recognized as the principal excitatory neurotransmitter in the neurological system [52]. According to accepted estimates, glutamate is used by 90% of all the synapses in the brain. In contrast, γ-aminobutyric acid (GABA), another amino acid neurotransmitter, mediates rapid inhibitory transmission. Glutamate is created from glutamine, which is produced only by astrocytes, and glutamate decarboxylase converts glutamate into GABA [53]. Glutamate receptors are divided into G protein-coupled metabotropic glutamate receptors (mGluRs) and ionotropic glutamate receptors (iGluRs). iGluRs, consisting of α-amino-3-hydroxy- 5-methyl-4-isoxazolepropionic acid receptor (AMPAR), NMDAR, and kainate receptors, allow rapid neurotrans-mission via glutamate-gated ion channels. Unlike iGluRs, mGluRs are grouped into three: Group I (1 and 5), Group II (2 and 3), and Group III (4, 6, 7, 8), based on distinct characteristics such as structure, synapse location, and pharmacological capabilities [54].
Berman et al. [55] found that a single subanesthetic dose of the NMDAR antagonist ketamine dramatically reduced patients’ standardized depression levels. Converging the line of evidence for ketamine as an antidepressant, Zarate et al. [56] showed a robust, rapid, and relatively sustained response to a single dose of ketamine in TRD patients. Additionally, clinical investigations have demonstrated that single-dose ketamine is effective in treating cases of bipolar depression (BD) that are resistant to treatment [57]. However, the neurobiological mechanism underlying the antidepressant effects of ketamine is more sophisticated than simple NMDAR inhibition. It is based on the duration of the therapeutic response and long-term influence of ketamine. Low single-doses of ketamine generate a moderate, temporary psychotomimetic, and dissociative effect that lasts approximately 40 minutes and then disappears. After the initial stage of psychotomimetics, antidepressant effects are detected after 110 minutes and peak between 24 and 72 hours; this effect lasts for approximately 7 days following ketamine therapy [58]. These preliminary findings suggest that ketamine has a complex neurobiological mechanism, that glutamate-related antidepressants are more effective than currently available monoamine drugs, and that they modulate depression through mechanisms different from those of monoamine drugs.
Development of a Chronic Stress Paradigm-based Animal Model for Treatment-resistant Depression
Preclinical animal modeling for depression studies has relied on several types of chronic stress paradigms based on epidemiological data, which show that early adversity, childhood trauma, and stressful life events are highly related to a high risk for depression [59,60]. Depressive-like animal models must fulfill three main criteria: (a) face validity (a reasonable degree of symptomatic homology), (b) construct or etiological validity (similar causative factors), and (c) pharmacological validity (which requires reversal of depressive symptoms by available antidepressants) [61]. Moreover, the following additional conditions must be met to develop a TRD model that is resistant to antidepressant treatment: (i) increased sensitivity to stress precipitation of a depressive behavioral phenotype, (ii) resistance to chronic treatment with conventional antidepressants, (iii) a good response to novel modes of antidepressant treatment (e.g., ketamine and deep brain stimulation) that are reported to be effective in TRD, and (iv) a parallel to a known clinical risk factor [62]. To develop a model that suits these criteria, researchers have attempted to discover and understand the mechanisms of responsiveness and resistance to long-term antidepressant treatment in chronic stress-based animal models that fulfill the requirements of conventional depression models [61].
Chronic mild stress or chronic unpredictable stress (CUS) was initially induced in rats using chronic stressors as a behavioral model of anhedonia that might be treated with TCA [63]. Subsequent studies have shown that CUS dramatically impairs hippocampal (HPC) neurogenesis and sucrose consumption in rats [64,65]. Converging lines of evidence have demonstrated that CUS mediates depression-like behavior and that long-term antidepressant treatment reverses depression-like behavior [66]. It is worth noting that CUS suits both criteria (i) and (ii) for developing a TRD animal model [67]. Importantly, previous studies have shown that reduced sucrose preference through CUS could be treated with antidepressants, such as escitalopram and sertraline, but approximately 40−50% of mice receiving CUS did not respond to antidepressant treatment [68]. They also found that CUS and escitalopram non-responders had a reduced number of bromodeoxyuridine-positive cells compared to escitalopram responders and controls in HPC [68]. These data suggest that antidepressant resistance is mediated by the antidepressant action on cell proliferation in HPC. Moreover, a previous study showed that GABA release was restored in SSRI responders after CUS, but not in SSRI non-responder mice, and this difference was thought to be due to differences in the expression of presynaptic proteins associated with neurotransmitter release [69,70]. Thus, CUS is a useful tool for investigating the mechanisms underlying antidepressant response and non-response. Interestingly, the CUS may also be suitable for criterion (iii). As in a previous study, adding the atypical antipsychotic quetiapine to fluoxetine-resistant mice significantly decreased depression-like behaviors and stimulated HPC neurogenesis [71]. Quetiapine increases BDNF mRNA expression in the HPC, suggesting that BDNF is involved in TRD [72]. It is noteworthy that CUS-mediated depressive-like behaviors can be reversed with vagus nerve stimulation (VNS), which is an FDA-approved noninvasive treatment for depression in clinical practice [73]. Recent research suggests that VNS intervention during CUS stimulates the afferent vagus nerve, which projects to the nucleus tractus solitarius, forms an indirect ascending projection into the HPC, and mediates antidepressant effects by upregulating the α7 nicotinic acetylcholine receptor in the HPC [74]. Besides VNS, deep-brain stimulation has also been shown to improve depression-like behaviors in CUS [75]. Although CUS has been proven to be a suitable model for TRD, no data have demonstrated that depressive-like behaviors can be treated by injecting ketamine into antidepres-sant-resistant CUS mice. Nonetheless, the CUS was the first chronic stress-based animal model used to study ketamine function in depression [76]. In an early study, Duman and Aghajanian [77] suggested that ketamine rapidly and transiently increased glutamate transmission in the PFC of mice that received CUS, possibly by inhibiting tonically active GABAergic intermediate neurons. They also suggested that the rapid antidepressant effect of ketamine, unlike conventional antidepressants, might be due to the activity-dependent release of BDNF. Taken together, these results suggest that CUS is a suitable animal model for TRD.
Similar to CUS, another animal model that allows for four criteria for studying TRD is chronic social defeat stress (CSDS). In contrast to the CUS paradigm, this model demonstrates that after 10 days of social defeat stress, some animals develop resistance to chronic stress without expressing depressed phenotypes, whereas others are CSDS-susceptible, and these phenotypes are reversible with antidepressant treatment [78]. Although fluoxetine or desipramine alleviated some depression-like behaviors in CSDS-susceptible mice, some mice still exhibited depression-like behaviors. They also found that higher expression of phosphorylated mammalian target of rapamycin (mTOR) in the ventral tegmental area decreased brain reward function, which was assessed using an intracranial self-stimulation procedure [78]. This finding supports previous studies suggesting that mTOR plays a vital role in treatment resistance. Furthermore, susceptible mice with the highest intracranial self-stimulation thresholds displayed lower antidepressant efficacy [78]. This is consistent with clinical findings that more severe anhedonia is a key negative prognostic indicator of TRD and may predict a poor antidepressant response [79].
It is noteworthy that comparative transcriptome analysis of ketamine and conventional antidepressants has yet to be conducted in the CUS model but is being actively pursued in the CSDS model. A recent study comparing the transcriptional profiles of imipramine and ketamine was conducted using the CSDS model [5]. Bagot et al. [5] analyzed the transcriptional profiles in the PFC, nucleus accumbens, HPC, and amygdala, according to antidepres-sant response versus non-response to imipramine or ketamine. They found that the PFC showed the most significant degree of overlap between ketamine and imipramine non-responders, and that ketamine significantly reduced the dual specificity phosphatase 1 (Dusp1) and activity-regulated cytoskeletal (Arc) gene expression in the PFC, which was found to be increased in postmortem tissues of depressed humans [5]. Associated with these studies, in the latest study, single-cell RNA-sequencing was used for transcriptional profiling of the ventral HPC (vHPC) of ketamine-treated mice [80]. They identified a gene encoding potassium voltage-gated channel subfamily Q member 2 as an important regulator of ketamine action in glutamatergic neurons of vHPC [80]. Another study examined the effect of ketamine isomers on mTOR regulation [81]. Interestingly, (S)-ketamine mediated the antidepressant effect by regulating mTOR, whereas (R)- ketamine mediated the antidepressant effect by regulating the extracellular signal-regulated kinase pathway [81]. This suggests that the isomers of ketamine may treat depression through their different biological properties. Taken together, animal studies using the chronic stress paradigm were able to delineate human depression and TRD, and elucidate the role of antidepressants. Neverthe-less, there are still limitations in TRD animal models of TRD. A major challenge in creating a TRD model is to demonstrate and validate that treatments that have been shown to be effective in TRD patients can reverse depression-like behaviors in animals that do not respond to classical antidepressants. Although ketamine or VNS, a therapy to help patients with TRD, has been demonstrated to be effective in chronic stress animal model [74], the effectiveness of ketamine or VNS treatment in antidepressant- resistant mice has not been eluci-dated. As a result, it is necessary to discover the mechanisms by which mice that do not respond to conventional therapies respond to novel treatments, such as ketamine and VNS, in addition to the factors that potentially predict TRD.
Mechanism of Action of Ketamine Approved as a Novel Antidepressant
NMDARs, which occur as heterotetramers, are Ca2+- permeable ion channels that play crucial roles in synaptic plasticity, learning, and memory, as well as in nervous system damage and illness. Ketamine is a voltage-dependent NMDAR antagonist that blocks calcium and sodium transport when glutamate and glycine are present as co-agonists [82]. Since the 1960s, ketamine, which was initially developed from phencyclidine, has been used for surgical procedures in both veterinary and human medicine [83] after being first synthesized by the Parke-Davis Company in 1956 [84]. Domino and Corssen found evidence for the potential safety and efficacy of ketamine in a pioneering pharmacological study of the drug in 1965 [84]. They also discovered that when ketamine was administered, reflex and respiratory functions were unaffected, resulting in dissociative anesthesia, in which patients were awake but unresponsive to stimuli. They reported findings from early clinical trials using ketamine in 1966, showing that it had fewer adverse effects and no significant emergency delirium than phencyclidine [84]. Based on these preliminary studies, ketamine was approved by the FDA for human use under the name KetalarⓇ in 1970 [85]. Subsequent studies have found that ketamine is a racemic mixture of R (−) and S (+) enantiomers and has different affinities for NMDARs. S-ketamine produces 3−4 times more anesthetic potency than R-ketamine and has a greater affinity for the binding site on NMDARs [86].
Recently, a biologically potent (S)-ketamine nasal spray was developed to make drug administration more accessible, and its antidepressant effectiveness was assessed in individuals with depression who failed to respond to treatment [87]. This study used the Montgomery-Åsberg Depres-sion Rating Scale (MADRS) to evaluate the severity of depressive symptoms and found that depressive-like behavior rapidly decreased within 2 hours of intranasal (S)-ketamine treatment [87]. Given the promising results of these clinical studies, (S)-ketamine appears to significantly reduce the burden of depressive symptoms, prompting the FDA to approve (S)-ketamine nasal spray (SpravatoⓇ) as an adjunctive treatment for adults with TRD or major depression with suicidal thoughts or actions in 2019 [88]. Recently, a phase 2a trial was initiated in treatment-resistant patients utilizing arketamine (PCN-10), an R-ketamine formulation [89]. In preclinical animal models of depressive behavior, R-ketamine has shown the potential for longer endurance and possibly a more favorable safety and tolerability profile than S-ketamine, which might allow for at-home usage [90]. In particular, a previous study using animal models of depression suggested that arketamine can induce persistent antidepressant effects compared to S-ketamine, which appears to be mediated by increased BDNF-tropomyosin receptor kinase B (TrkB) signaling and synaptogenesis in the PFC, dentate gyrus, and CA3 [90]. In addition, an open pilot study of patients with TRD showed that the MADRS score was significantly reduced within 24 hours, and no particular dissociation was observed in the subjects [87]. Together, these data strongly suggest that glutamatergic neurotransmission plays a vital role in the pathogenesis of mood disorders, and that glutamate modulators, particularly ketamine, can be new treatment options for patients resistant to traditional anti-depressants.
Conflicting Hypotheses Regarding NMDAR Inhibition in the Antidepressant Action of Ketamine
Preliminary studies on the mechanisms of ketamine have shown that it decreases neuronal activation through NMDAR [91]. A growing body of evidence has shown that ketamine blocks excitatory postsynaptic potentials in cortical pyramidal cells and spinal cord neurons in a manner consistent with NMDAR antagonism [92]. Additional-ly, an animal study showed that NMDAR antagonists, such as AP-7 and MK-801 have antidepressant-like effects [93]. However, several studies have suggested that NMDAR antagonism may not fully account for the antidepressant effects of ketamine. Ketamine, an antidepressant, paradoxically enhances neuroplasticity while decreasing NMDARs, a key plasticity mediator [3]. Another study found that memantine, which blocks the NMDAR ion channel simultaneous with ketamine, is ineffective in treating depression in people [94]. These preliminary data suggest that the antagonistic effect of ketamine on NMDAR is not the only mechanism through which it exerts its antidepressant effects. Each new evidence suggests that ketamine and its metabolites may have effects that are both dependent on and independent of NMDAR antagonistic activity as well as the capacity to activate various neurotransmitter systems to elicit antidepressant effects.
Inhibition of NMDAR by ketamine in GABAergic interneurons
In 1997, it was shown that ketamine enhances cortical excitability and extrasynaptic glutamate levels in the medial PFC (mPFC) [95]. It has also been proposed that low dosages of ketamine might increase glutamatergic transmission by preferentially attenuating inhibitory tones that normally press on excitatory (pyramidal) neurons in this region [95]. These findings imply that because ketamine inhibits open/active NMDARs, it suppresses inhibitory interneurons that govern tonic activity more quickly. The following findings reveal that the NMDAR antagonist MK-801 reduces the firing rate of putative fast-spike inhibitory interneurons that disinhibit cortical pyramidal neuron activity [96]. This is assumed to occur because excitatory pyramidal neurons are generally in a functional state of quiescence, while inhibitory intermediate neurons are normally (tonically) active. Another study revealed low-dose ketamine-induced disinhibition of CA1 pyramidal cells and rapid antidepressant effectiveness [97]. In addition, it has also been suggested that ketamine may more effectively inhibit triheterometric NMDARs containing either GluN2C or GluN2D, which are widely expressed in forebrain inhibitory interneurons, than receptors containing only GluN1, GluN2A, and GluN2B sub-units [98]. Therefore, inhibition of NMDAR in GABAergic interneurons is expected to disinhibit pyramidal cells, enhance excitatory glutamatergic neurotransmission in the limbic system of the mPFC, and decrease overall inhi-bition in mood disorder-related brain regions (Fig. 3). Supporting research shows that administering partial inverse agonists, such as MRK-016, to the benzodiazepine-binding site of GABAA receptors with the α5-subunit, which is primarily expressed in the forebrain, quickly alleviates depression by preventing excitatory neurotransmission such as ketamine [99]. These findings support a role for NMDAR disinhibition of GABAergic interneurons in the forebrain and show that ketamine is similar to a negative allosteric modulator of α5-subunit-containing GABAA receptors. However, some studies have contradicted this hypothesis. A single subanesthetic dose of ketamine in GABAergic synaptic transmission defeats mice reversed depressive-like behaviors [100]. Similarly, disinhibition of somatostatin expressed in a subset of GABAergic interneurons can cause antidepressant-like behaviors [101]. These contradictory findings imply that NMDAR disinhibition of GABAergic interneurons is not the only mechanism by which ketamine acts.
Fig. 3.
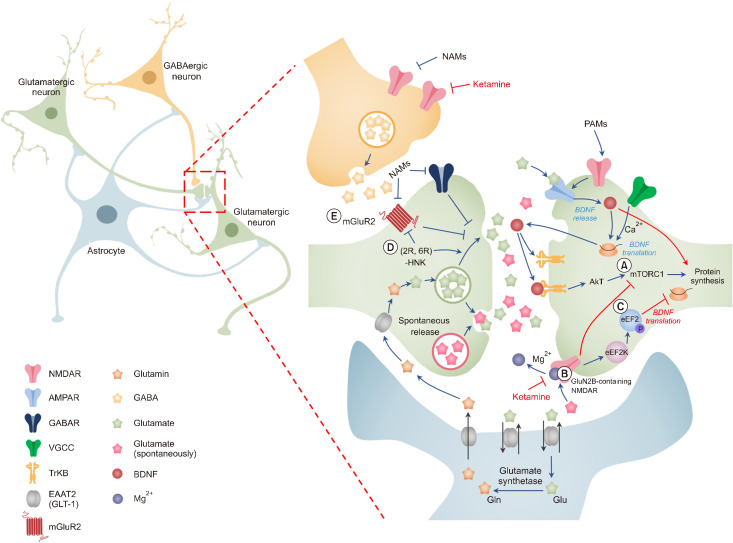
Major hypotheses regarding how ketamine acts as an antidepressant. Ketamine has been utilized as an anesthetic because it is an NMDAR antagonist. Following the discovery that ketamine has rapid antidepressant effects, numerous ideas have been proposed to explain how subanesthetic dosages of ketamine function as antidepressants. (A) According to the disinhibition hypothesis, ketamine selectively inhibits NMDARs expressed in GABAergic inhibitory interneurons, resulting in pyramidal neuron disinhibition and increased glutamatergic firing. Evoked glutamate binds to and activates post-synaptic AMPARs, resulting in enhanced BDNF release, TrkB receptor activation, and subsequent augmentation of protein synthesis via mTORC1. (B) It has been proposed that ketamine specifically inhibits extrasynaptic GluN2B-containing NMDARs, which are intensely stimulated by peripheral Glu levels controlled by EAAT2 in astrocytes, resulting in a decrease in mTORC1 activity. (C) It was predicted that ketamine would increase BDNF translation by decreasing NMDAR-mediated spontaneous neurotransmission, which would reduce eEF2K activity and prevent eEF2 substrate phosphorylation. (D, E) This hypothesis proposes that (2R,6R)-HNK serves as an antidepressant irrespective of NMDAR inhibition. Following treatment, ketamine may be metabolized to HNK, and these HNK metabolites may facilitate AMPAR-mediated synaptic enhancement or increase glutamate release by inhibiting mGluR2. This figure is adapted from Sanacora et al. [7], and Pham et al. [136] with permission. This figure is adapted from Sanacora et al. (Neuropharmacology 2012;62:63-77) [7] and Pham and Gardier (Pharmacol Ther 2019;199:58-90) [136] with original copyright holder’s permission.
NMDAR, N-methyl-D-aspartate receptor; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor kinase B; mTORC1, mammalian target of rapamycin complex 1; GluN2B, glutamate receptor subunit epsilon-2; EAAT2, excitatory amino acid transporter 2; eEF2K, eukaryotic elongation factor 2 kinase; HNK, hydroxynorketamine; NAMs, negative allosteric modulators; PAMs, positive allosteric modulators; VGCC, voltage-gated calcium channel.
Suppression of spontaneous NMDAR-mediated propagation
The release of neurotransmitters from presynaptic terminals and subsequent activation of post-synaptic receptors are necessary for information transfer at synapses. All synapses release neurotransmitters both evoked (calcium- dependent) and spontaneously (calcium-independent). In particular, spontaneous glutamate release is important for the brain to maintain synaptic strength and regulate spike timing [102]. Autry et al. [103] discovered that ketamine inhibits NMDAR-mediated spontaneous microexcitatory post-synaptic currents (mEPSC). They further proposed that a single anesthetic dosage of ketamine produces fast antidepressant-like behavioral effects by suppressing spontaneous NMDAR-mEPSCs and quickly enhancing BDNF translation while decreasing eukaryotic elongation factor 2 kinase (eEF2K) activity. In a subsequent study, ketamine and memantine effectively blocked NMDAR- mediated mEPSC in the absence of Mg2+. However, only ketamine inhibits NMDAR at rest when physiological quantities of Mg2+ are present in the extracellular solution. Memantine, unlike ketamine, does not inhibit eEF2 phosphorylation or boost BDNF expression, emphasizing the specific role of ketamine alone in increasing spontaneous glutamate release (Fig. 3) [104].
“Direct inhibition hypothesis”: extra-synaptic NMDARs
As previously stated, ketamine promotes the synthesis of proteins such as BDNF and enhances excitatory synaptic transmission in cortical neurons. However, it is unclear how blocking NMDAR signaling promotes protein synthesis. Miler et al. [105] proposed that when ambient glutamate tonically stimulates GluN2B-containing NMDARs to decrease protein production directly and basally in principal cortical neurons, the rapid antidepressant effects would begin by increasing protein synthesis and improving excitatory synaptic transmission in the PFC. The glutamate transporter EAAT2, which is expressed on glial cells, directly regulates tonic ambient glutamate levels [106]. Numerous studies have shown that stimulation of the mTOR signaling pathway in the cortical extrasynaptic GluN2B-selective NMDAR decreases protein synthesis, which preserves synaptic homeostasis [105]. Blocking extrasynaptic GluN2B-containing NMDARs inhibits protein synthesis and exerts an antidepressant effect via an mTOR- dependent mechanism (Fig. 3). GluN2C- and GluN2D- containing NMDARs are more efficiently inhibited by ketamine at physiological magnesium concentrations than GluN2B- and GluN2A-containing NMDARs, according to earlier research [98]. Although GluN2B-specific antagonists exhibited antidepressant-like effects in preclinical studies [107,108], clinical investigations have not shown antidepressant effects [109].
Independent mechanism hypothesis of NMDAR inhibition: metabolite
Despite numerous hypotheses, the main assumption of previous hypotheses is that NMDAR blockade is essential for the antidepressant action of ketamine. However, in 2006, findings that conflicted with this hypothesis were reported. Memantine was observed to be less effective than placebo [110] and had no impact as a clinical antidepressant, even after several weeks of continuous medication [111]. Furthermore, even high-affinity NMDAR channel blockers lack the preclinical antidepressant effects similar with that of ketamine [103,108,112]. In a clinical study, the low-capture, nonselective NMADR channel blocker AZD6765 temporarily produced an antidepressant response in patients with depression; however, this effect could not be sustained [113]. Therefore, these findings confirmed the hypothesis that ketamine may act as an antidepressant independent of NMDAR. In addition, interesting discoveries have been made regarding ketamine metabolites. Previous studies have shown that preventing ketamine from being metabolized to (2R,6R)-HNK blocks antidepressant-like effects, and the direct administration of (2R,6R)-hydroxynorketamine (HNK) has been shown to have ketamine-like antidepressant effects [113]. Subsequent studies have suggested that (2R, 6R)-HNK may have an antidepressant-like effect by promoting glutamatergic synaptic transmission and causing a sharp increase in BDNF release, a function similar to that of ketamine (Fig. 3) [114].
Independent mechanism hypothesis of NMDAR inhibition: AMPAR and mGLUR
According to the disinhibition hypothesis, the activation balance in these regions quickly shifts in favor of excitability, particularly in terms of AMPAR transmission, which may be the basis for antidepressant efficacy. Since AMPAR activation causes membrane depolarization and voltage-dependent NMDAR Mg2+ blocking, it is plausible that AMPAR inhibition selectively inhibits NMDAR activation [115]. Previous studies have shown that AMPAR activity is essential because AMPAR antagonist pretreatment prevents the preclinical antidepressant-like effect [108], and these results are supported by additional studies [116]. Consequently, the effects of ketamine might either affect AMPAR directly or be influenced by cellular functions that directly affect AMPA. Previous studies have shown that after ketamine injection, GluA1 and GluA2 of the membrane AMPAR subunits are upregulated [107]. Moreover, similar to ketamine, NMDAR antagonists mimic the effects of ketamine in inducing AMPAR-mediated synaptic potential [117]. Notably, previous studies have shown that the release of BDNF is caused by an activity-dependent increase in intracellular calcium mediated by AMPARs [118]. This is required for ketamine to function as an antidepressant [107]. BDNF binds to its receptor, TrkB, which promotes neuronal survival and contributes to the formation of new synaptic connections. According to several previous studies, ketamine exerts an antidepressant-like effect by initiating synapse generation in an AMPAR-BDNF-mTORC1-dependent manner (Fig. 3) [107]. Subsequent studies have suggested that this causes a sustained increase in the efficacy of synaptic transmission, including the recruitment of the mTORC1 effector, eukaryotic translation initiation factor 4E-binding protein 2 [119]. Following the AMPAR activation-dependent mechanism of ketamine’s antidepressant activity, (2R,6R)- HNK causes an increase in AMPAR-mediated excitatory post-synaptic potentials recorded in the CA1 of the HPC slice in response to the stimulation of Schaffer collateral axons [112]. More recently, mGlu2/3 has been suggested to regulate glutamate excitatory synaptic transmission, which is increased by ketamine and (2R,6R)-HNK (Fig. 3). Importantly, animals lacking Grm2, but not Grm3, lacked the behavioral effects of (2R,6R)-HNK, which are import-ant to antidepressants [120]. In addition, it was previously found that mGlu2/3 receptor antagonists activate AMPA receptors to promote 5-HT release from mPFC [121]. Therefore, we need to learn more about how mGluRs function in response to ketamine. Consequently, although the role of NMDAR inhibition in the antidepressant action of ketamine remains contro-versial, there is strong evidence that BDNF signaling is required for its antidepres-sant effect.
Sex Difference in Ketamine Effects
According to a recent clinical investigation, approximately 11% of over 177,000 people with depression treated with antidepressants developed TRD. Surprisingly, researchers discovered a higher prevalence of TRD in younger men hospitalized for depression and suffering from severe depression [122]. In contrast, another study found that 65% of patients with TRD were female [123]. It is not surprising that many women had TRD. According to accumulating evidence, MDD is about twice as common in women as in men, and this sex difference is one of the most conclusive discoveries in psychopathology research [124].
Notably, the efficacy of antidepressants may differ between women and men with depression. According to an earlier study by Kornstein et al. [125], men and women with chronic depression may respond to and tolerate SSRIs and TCA differently. They demonstrated that men were significantly more likely to respond favorably to imipramine than to sertraline, and women were signifi-cantly more likely to respond favorably to sertraline. A recent ketamine study showed that women exhibited 20% higher (S)-ketamine and (S)-norketamine clearance, resulting in higher plasma concentrations in men [126]. This suggests that the already approved drug SPRAVATOⓇ can be rapidly cleared from the plasma in women. In 2019, Freeman et al. [127] investigated sex differences in ketamine responsiveness as a treatment strategy for TRD in 99 patients. The participants received four different single ketamine doses (0.1 mg/kg, 0.2 mg/kg, 0.5 mg/kg, and 1.0 mg/kg). The six-item Hamilton Depression Rating Scale (HAMD6) was administered 24 hours after drug admini-stration. Interesting sex differences were observed in the 0.5 mg/kg and 1.0 mg/kg groups. Women fared considerably better on the HAMD6 test than males when given 0.5 mg/kg ketamine but worse when given 1.0 mg/kg ketamine. In another study, patients with treatment-resistant BD were divided into two groups based on ketamine usage. Each participant received a single intravenous infusion of 0.5 mg/kg ketamine. Men (77%) were found to be much more likely to have an antidepressant response to ketamine on day 7 than women (43%) [128]. These clinical findings imply that they may not function equally in both men and women. Preclinical research has helped validate this notion. Male mice exposed to isolation stress showed anhedonic behaviors together with a reduction in mPFC neuron spinal densities, postsynaptic density protein 95 expression, and synapsin expression, all of which improved 3 hours after a single ketamine infusion. In female mice, isolation stress reduced mPFC spine density, PSD-95, and synapsin, but had no effect on anhedonia behavior, and none of these effects were reversed by ketamine [129]. Another study found that ketamine did not reduce eEF2 in the HPC and PFC of female mice, whereas it decreased in male mice [130]. In another intriguing study, female mice required half the minimal dose to produce an antidepressant effect of ketamine, but this effect only occurred when both estradiol and progesterone were present [131], which is consistent with the findings of clinical research [127,128]. This suggests that ketamine has sex-specific effects and that ovarian hormones may alter how it works in the female brain. Estrogen has been shown to boost ketamine sensitivity in a mouse depression model [129,130]. However, to date, few studies have examined how ketamine affects women before and after menopause differently. Nevertheless, one study found that ketamine administration did not significantly differ between premenopausal and postmenopausal women [127]. In addition, some studies have found no differences between men and women in the ketamine response [132]. Therefore, although the results of studies suggesting that the action of ketamine is related to hormone levels and concentrations in women are attractive, additional in-depth preclinical studies are required.
CONCLUSION
TRD is one of the greatest preclinical and clinical challenges in the field of psychiatry. In particular, TRD is a life-threatening condition due to the high risk of suicide. Research on ketamine has provided new insights into the neuroscience of depression, leading to the development of a unique and unexpected class of antidepressants that have emerged as a viable choice for treating depression that has become resistant to therapy. Preclinical and clinical data have demonstrated that low-dose ketamine monotherapy can alleviate chronic stress and depression within 24 hours. This implies that ketamine initiates brain plasticity. Increased glutamate levels in the frontal lobes, AMPAR activation, and AMPAR circulation are likely to mediate these antidepressant effects. It is regulated by neuroplastic signaling pathways, specifically BDNF and mTORC1. Ketamine can also be used to inhibit putative extrasynaptic NMDARs while they are at rest, allowing for the formation of new synapses.
Future Direction
Although the effect of ketamine has been investigated in numerous preclinical studies, animal models are yet to be developed to elucidate how ketamine improves TRD, which is generally the outcome of a poor response to at least one traditional antidepressant therapy at the recommended dose and duration. CUS and CSDS have previously been used in preclinical investigations to identify depression-like symptoms and their underlying mechanisms in animals that do not respond to presently available medications. However, it is yet to be confirmed whether individuals who do not respond to traditional therapy genuinely recover when given ketamine, and if so, by what mechanisms.
Furthermore, despite the greater prevalence of depression in women, few studies have investigated sex differences and psychopharmacological response difficulties in MDD, specifically TRD [133-136]. As previously noted, some clinical and preclinical studies have suggested sex differences in ketamine-related features between indi-viduals with depression and TRD. Thus, placing men and women into the same model is incorrect, and studying the same behavioral results may not be acceptable in prac-tice. It may be more beneficial to focus on endophenotypes specific to each sex and to model depression in men and women separately. This methodology may identify novel sex-specific biomarkers and processes linked to depression and TRD as well as new sex-specific targets for antidepressant development.
Therefore, ketamine, which has been approved as a treatment for TRD, has reignited a dormant market, as resistance to conventional treatments has grown. Further research is needed to resolve critical issues, including TRD- specific preclinical models, nervous system, and sex differences, to develop the most effective therapies for TRD with minimal side effects.
Funding Statement
Funding This work was supported by the Korea Brain Research Institute Basic Research Program (Grant no. 22-BR-02-02, and 22-BR-02-18, and 22-BR-04-03).
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Jeongseop Kim, Tae-Eun Kim, and Ja Wook Koo. Funding: Ja Wook Koo. Supervision: Ja Wook Koo and Seung-Hwan Lee. Writing—original draft: Jeongseop Kim, Tae-Eun Kim, and Ja Wook Koo. Writing—review & editing: Jeongseop Kim, Tae-Eun Kim, Seung- Hwan Lee, and Ja Wook Koo.
References
- 1.Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi W, Kim JW, Kang HJ, Kim HK, Kang HC, Lee JY, et al. Interactive effects of serum leptin levels and physical comorbidity on the pharmacotherapeutic response of depressive disorders. Clin Psychopharmacol Neurosci. 2022;20:662–674. doi: 10.9758/cpn.2022.20.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry. 2017;81:285–295. doi: 10.1016/j.biopsych.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, et al. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272–288. doi: 10.1016/j.neubiorev.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsy-chopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunney WE, Jr, Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13:483–494. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 9.Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 10.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large- scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 11.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 12.Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: When enough is enough. JAMA Psychiatry. 2017;74:9–10. doi: 10.1001/jamapsychiatry.2016.2586. [DOI] [PubMed] [Google Scholar]
- 13.Corral R, Alessandria H, Agudelo Baena LM, Ferro E, Duque X, Quarantini L, et al. Suicidality and quality of life in treatment-resistant depression patients in Latin America: Secon-dary interim analysis of the TRAL study. Front Psychiatry. 2022;13:812938. doi: 10.3389/fpsyt.2022.812938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabbri C, Kasper S, Zohar J, Souery D, Montgomery S, Albani D, et al. Drug repositioning for treatment-resistant depression: Hypotheses from a pharmacogenomic study. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110050. doi: 10.1016/j.pnpbp.2020.110050. [DOI] [PubMed] [Google Scholar]
- 15.Wang SM, Kim NY, Na HR, Lim HK, Woo YS, Pae CU, et al. Rapid onset of intranasal esketamine in patients with treatment resistant depression and major depression with suicide ideation: A meta-analysis. Clin Psychopharmacol Neurosci. 2021;19:341–354. doi: 10.9758/cpn.2021.19.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:eaat8078. doi: 10.1126/science.aat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou YL, Wu FC, Liu WJ, Zheng W, Wang CY, Zhan YN, et al. Volumetric changes in subcortical structures following repeated ketamine treatment in patients with major depressive disorder: A longitudinal analysis. Transl Psychiatry. 2020;10:264. doi: 10.1038/s41398-020-00945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sydnor VJ, Lyall AE, Cetin-Karayumak S, Cheung JC, Felicione JM, Akeju O, et al. Studying pre-treatment and ketamine-induced changes in white matter microstructure in the context of ketamine's antidepressant effects. Transl Psychiatry. 2020;10:432. doi: 10.1038/s41398-020-01122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill H, Gill B, Rodrigues NB, Lipsitz O, Rosenblat JD, El-Halabi S, et al. The effects of ketamine on cognition in treatment-resistant depression: A systematic review and priority avenues for future research. Neurosci Biobehav Rev. 2021;120:78–85. doi: 10.1016/j.neubiorev.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen N. Making the first anti-depressant: Amphetamine in American medicine, 1929-1950. J Hist Med Allied Sci. 2006;61:288–323. doi: 10.1093/jhmas/jrj039. [DOI] [PubMed] [Google Scholar]
- 21.Kauffman GB. The discovery of iproniazid and its role in antidepressant therapy. J Chem Educ. 1979;56:35. doi: 10.1021/ed056p35.2. [DOI] [Google Scholar]
- 22.Axelrod J. Noradrenaline: Fate and control of its biosynthesis. Science. 1971;173:598–606. doi: 10.1126/science.173.3997.598. [DOI] [PubMed] [Google Scholar]
- 23.Yochelson S. Isocarboxazid in the treatment of depression. J Clin Exp Psychopathol Q Rev Psychiatry Neurol. 1960;21:196–204. doi: 10.1176/ajp.140.6.792. [DOI] [PubMed] [Google Scholar]
- 24.Agin HV. Tranylcypromine in depression: A clinical report. Am J Psychiatry. 1960;117:150–151. doi: 10.1176/ajp.117.2.150. [DOI] [PubMed] [Google Scholar]
- 25.Bogdanski DF, Weissbach H, Udenfriend S. The distribution of serotonin, 5-hydroxytryptophan decarboxylase, and monoamine oxidase in brain. J Neurochem. 1957;1:272–278. doi: 10.1111/j.1471-4159.1957.tb12082.x. [DOI] [PubMed] [Google Scholar]
- 26.Schayer RW, Smiley RL, Davis KJ, Kobayashi Y. Role of monoamine oxidase in nor-adrenaline metabolism. Am J Physiol. 1955;182:285–286. doi: 10.1152/ajplegacy.1955.182.2.285. [DOI] [PubMed] [Google Scholar]
- 27.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dua D, Agarwal AK, Dalal PK. Imipramine in schizophrenia with depressive symptomatology. Indian J Psychiatry. 1990;32:229–234. [PMC free article] [PubMed] [Google Scholar]
- 29.Pletscher A. The discovery of antidepressants: A winding path. Experientia. 1991;47:4–8. doi: 10.1007/BF02041242. [DOI] [PubMed] [Google Scholar]
- 30.Endler NS. The origins of electroconvulsive therapy (ECT) Convuls Ther. 1988;4:5–23. [PubMed] [Google Scholar]
- 31.Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2:849–850. doi: 10.1016/S0140-6736(63)92743-0. [DOI] [PubMed] [Google Scholar]
- 32.Zeller EA, Barsky J. In vivo inhibition of liver and brain monoamine oxidase by 1-Isonicotinyl-2-isopropyl hydrazine. Proc Soc Exp Biol Med. 1952;81:459–461. doi: 10.3181/00379727-81-19910. [DOI] [PubMed] [Google Scholar]
- 33.Glowinski J, Axelrod J. Inhibition of uptake of tritiated-noradrenaline in the intact rat brain by imipramine and structurally related compounds. Nature. 1964;204:1318–1319. doi: 10.1038/2041318a0. [DOI] [PubMed] [Google Scholar]
- 34.Schildkraut JJ, Schanberg SM, Breese GR, Kopin IJ. Norepine-phrine metabolism and drugs used in the affective disorders: A possible mechanism of action. Am J Psychiatry. 1967;124:600–608. doi: 10.1176/ajp.124.5.600. [DOI] [PubMed] [Google Scholar]
- 35.Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. Comparative cardiovascular toxicity of trazodone and imipramine in the rat. Arch Toxicol Suppl. 1978:169–172. doi: 10.1038/s41380-022-01661-0. [DOI] [PubMed] [Google Scholar]
- 36.Ashcroft GW, Crawford TB, Eccleston D, Sharman DF, MacDougall EJ, Stanton JB, et al. 5-hydroxyindole compounds in the cerebrospinal fluid of patients with psychiatric or neurological diseases. Lancet. 1966;2:1049–1052. doi: 10.1016/S0140-6736(66)92028-9. [DOI] [PubMed] [Google Scholar]
- 37.Lisciani R, Campana A, Scorza Barcellona P. Comparative cardiovascular toxicity of trazodone and imipramine in the rat. Arch Toxicol Suppl. 1978:169–172. doi: 10.1007/978-3-642-66896-8_23. [DOI] [PubMed] [Google Scholar]
- 38.Wong DT, Horng JS, Fuller RW. Kinetics of serotonin accumulation into synaptosomes of rat brain--effects of amphetamine and chloroamphetamines. Biochem Pharmacol. 1973;22:311–322. doi: 10.1016/0006-2952(73)90412-7. [DOI] [PubMed] [Google Scholar]
- 39.Wong DT, Perry KW, Bymaster FP. Case history: The discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- 40.Laporta M, Chouinard G, Goldbloom D, Beauclair L. Hypomania induced by sertraline, a new serotonin reuptake inhibitor. Am J Psychiatry. 1987;144:1513–1514. doi: 10.1176/ajp.144.11.1513. [DOI] [PubMed] [Google Scholar]
- 41.Gottlieb P, Wandall T, Overø KF. Initial, clinical trial of a new, specific 5-HT reuptake inhibitor, citalopram (Lu 10-171) Acta Psychiatr Scand. 1980;62:236–244. doi: 10.1111/j.1600-0447.1980.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 42.Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159–166. doi: 10.4088/PCC.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothmore J. Antidepressant-induced sexual dysfunction. Med J Aust. 2020;212:329–334. doi: 10.5694/mja2.50522. [DOI] [PubMed] [Google Scholar]
- 44.Khan A, Fabre LF, Rudolph R. Venlafaxine in depressed outpatients. Psychopharmacol Bull. 1991;27:141–144. doi: 10.4088/jcp.v59n1004. [DOI] [PubMed] [Google Scholar]
- 45.Asnis GM, Bose A, Gommoll CP, Chen C, Greenberg WM. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: A phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74:242–248. doi: 10.4088/JCP.12m08197. [DOI] [PubMed] [Google Scholar]
- 46.Fuller RW, Hemrick-Luecke SK, Snoddy HD. Effects of duloxetine, an antidepressant drug candidate, on concentrations of monoamines and their metabolites in rats and mice. J Pharmacol Exp Ther. 1994;269:132–136. [PubMed] [Google Scholar]
- 47.Machado M, Einarson TR. Comparison of SSRIs and SNRIs in major depressive disorder: A meta-analysis of head-to-head randomized clinical trials. J Clin Pharm Ther. 2010;35:177–188. doi: 10.1111/j.1365-2710.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 48.Ramic E, Prasko S, Gavran L, Spahic E. Assessment of the antidepressant side effects occurrence in patients treated in primary care. Mater Sociomed. 2020;32:131–134. doi: 10.5455/msm.2020.32.131-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: A paradigm shift for depression research and treatment. Neuron. 2019;101:774–778. doi: 10.1016/j.neuron.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weston PG. Magnesium as a sedative. Am J Psychiatry. 1922;78:637–638. doi: 10.1176/ajp.78.4.637. [DOI] [Google Scholar]
- 51.Watkins JC, Jane DE. The glutamate story. Br J Pharmacol. 2006;147(Suppl 1):S100–S108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orrego F, Villanueva S. The chemical nature of the main central excitatory transmitter: A critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience. 1993;56:539–555. doi: 10.1016/0306-4522(93)90355-J. [DOI] [PubMed] [Google Scholar]
- 53.Waagepetersen HS, Sonnerwald U, Schousboe A. Glutamine, glutamate and GABA: Metabolic aspects. In: Lajtha A, Oja SS, Schousboe A, Saransaari P, editors. Handbook of neurochemistry and molecular neurobiology: Amino acids and peptides in the nervous system. Springer US; Boston: 2007. pp. 1–21. [DOI] [Google Scholar]
- 54.Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-0152-y. Erratum in: Psychopharmacology (Berl) 2005;182:320. [DOI] [PubMed] [Google Scholar]
- 55.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 56.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major de-pression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 57.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine's antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: Targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry. 2006;59:1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Kang S, Choi TY, Chang KA, Koo JW. Metabotropic glutamate receptor 5 in amygdala target neurons regulates susceptibility to chronic social stress. Biol Psychiatry. 2022;92:104–115. doi: 10.1016/j.biopsych.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Koo JW, Chaudhury D, Han MH, Nestler EJ. Role of mesolimbic brain-derived neurotrophic factor in depression. Biol Psychiatry. 2019;86:738–748. doi: 10.1016/j.biopsych.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krishnan V, Nestler EJ. Animal models of depression: Mole-cular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Planchez B, Surget A, Belzung C. Animal models of major depression: Drawbacks and challenges. J Neural Transm (Vienna) 2019;126:1383–1408. doi: 10.1007/s00702-019-02084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katz RJ. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 1982;16:965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- 64.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Caldarone BJ, Zachariou V, King SL. Rodent models of treatment-resistant depression. Eur J Pharmacol. 2015;753:51–65. doi: 10.1016/j.ejphar.2014.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of de-pression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- 69.Bisgaard CF, Jayatissa MN, Enghild JJ, Sanchéz C, Artemychyn R, Wiborg O. Proteomic investigation of the ventral rat hippocampus links DRP-2 to escitalopram treatment resistance and SNAP to stress resilience in the chronic mild stress model of depression. J Mol Neurosci. 2007;32:132–144. doi: 10.1007/s12031-007-0025-4. [DOI] [PubMed] [Google Scholar]
- 70.Nieto-Gonzalez JL, Holm MM, Vardya I, Christensen T, Wiborg O, Jensen K. Presynaptic plasticity as a hallmark of rat stress susceptibility and antidepressant response. PLoS One. 2015;10:e0119993. doi: 10.1371/journal.pone.0119993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Chang T, Chen YC, Zhang RG, Wang HN, Wu WJ, et al. Quetiapine add-on therapy improves the depressive behaviors and hippocampal neurogenesis in fluoxetine treatment resistant depressive rats. Behav Brain Res. 2013;253:206–211. doi: 10.1016/j.bbr.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Fumagalli F, Molteni R, Bedogni F, Gennarelli M, Perez J, Racagni G, et al. Quetiapine regulates FGF-2 and BDNF expression in the hippocampus of animals treated with MK- 801. Neuroreport. 2004;15:2109–2112. doi: 10.1097/00001756-200409150-00022. [DOI] [PubMed] [Google Scholar]
- 73.Kamel LY, Xiong W, Gott BM, Kumar A, Conway CR. Vagus nerve stimulation: An update on a novel treatment for treatment-resistant depression. J Neurol Sci. 2022;434:120171. doi: 10.1016/j.jns.2022.120171. [DOI] [PubMed] [Google Scholar]
- 74.Wang JY, Zhang Y, Chen Y, Wang Y, Li SY, Wang YF, et al. Mechanisms underlying antidepressant effect of transcutaneous auricular vagus nerve stimulation on CUMS model rats based on hippocampal a7nAchR/NF-kB signal pathway. J Neuroinflammation. 2021;18:291. doi: 10.1186/s12974-021-02341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bambico FR, Bregman T, Diwan M, Li J, Darvish-Ghane S, Li Z, et al. Neuroplasticity-dependent and -independent mechanisms of chronic deep brain stimulation in stressed rats. Transl Psychiatry. 2015;5:e674. doi: 10.1038/tp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: Susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51:404–411. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopez JP, Lücken MD, Brivio E, Karamihalev S, Kos A, De Donno C, et al. Ketamine exerts its sustained antidepressant effects via cell-type-specific regulation of Kcnq2. Neuron. 2022;110:2283–2298.e9. doi: 10.1016/j.neuron.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, et al. Mechanistic target of rapamycin-independent antidepres-sant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83:18–28. doi: 10.1016/j.biopsych.2017.05.016. Erratum in: Biol Psychiatry 2020;87:686-687. [DOI] [PubMed] [Google Scholar]
- 82.Davies SN, Alford ST, Coan EJ, Lester RA, Collingridge GL. Ketamine blocks an NMDA receptor-mediated component of synaptic transmission in rat hippocampus in a voltage-dependent manner. Neurosci Lett. 1988;92:213–217. doi: 10.1016/0304-3940(88)90063-8. [DOI] [PubMed] [Google Scholar]
- 83.Domino EF. History and pharmacology of PCP and PCP-related analogs. J Psychedelic Drugs. 1980;12:223–227. doi: 10.1080/02791072.1980.10471430. [DOI] [PubMed] [Google Scholar]
- 84.Denomme N. The Domino effect: Ed Domino's early studies of psychoactive drugs. J Psychoactive Drugs. 2018;50:298–305. doi: 10.1080/02791072.2018.1506599. [DOI] [PubMed] [Google Scholar]
- 85.Parke Davis, author. Ketamine hydrochloride (Ketalar) Clin Pharmacol Ther. 1970;11:777–780. doi: 10.1002/cpt1970115777. [DOI] [PubMed] [Google Scholar]
- 86.White PF, Schüttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth. 1985;57:197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- 87.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry. 2018;75:139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cristea IA, Naudet F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry. 2019;6:975–977. doi: 10.1016/S2215-0366(19)30292-5. [DOI] [PubMed] [Google Scholar]
- 89.GlobeNewswire, Inc, author. Perception Neuroscience initiates Phase 2a study of PCN-101 (R-ketamine) for treatment resistant depression [Internet] GlobeNewswire, Inc.; Los Angeles: 2021. Sep 14, [cited at 2021 Sep 14]. Perception Neuroscience initiates Phase 2a study of PCN-101 (R-ketamine) for treatment resistant depression [Internet] https://www.globenewswire.com/news-release/2021/09/14/2296596/0/en/Perception-Neuroscience-initiates-Phase-2a-study-of-PCN-101-R-ketamine-for-treatment-resistant-depression.html. [Google Scholar]
- 90.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. Erratum in: Transl Psychiatry 2020;10:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: A site of action of ketamine? Nature. 1985;313:479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- 93.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-J. [DOI] [PubMed] [Google Scholar]
- 94.Omranifard V, Shirzadi E, Samandari S, Afshar H, Maracy MR. Memantine add on to citalopram in elderly patients with depression: A double-blind placebo-controlled study. J Res Med Sci. 2014;19:525–530. [PMC free article] [PubMed] [Google Scholar]
- 95.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xi D, Zhang W, Wang HX, Stradtman GG, Gao WJ. Dizocilpine (MK-801) induces distinct changes of N-methyl-D-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12:1395–1408. doi: 10.1017/S146114570900042X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A. 2018;115:E3007–E3016. doi: 10.1073/pnas.1718883115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khlestova E, Johnson JW, Krystal JH, Lisman J. The role of GluN2C-containing NMDA receptors in ketamine's psychotogenic action and in schizophrenia models. J Neurosci. 2016;36:11151–11157. doi: 10.1523/JNEUROSCI.1203-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, et al. A negative allosteric modulator for a5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro. 2017;4:ENEURO.0285-16.2017. doi: 10.1523/ENEURO.0285-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry. 2016;80:457–468. doi: 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2017;22:920–930. doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kavalali ET, Monteggia LM. Targeting homeostatic synaptic plasticity for treatment of mood disorders. Neuron. 2020;106:715–726. doi: 10.1016/j.neuron.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, et al. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet. 2003;12:2519–2532. doi: 10.1093/hmg/ddg267. [DOI] [PubMed] [Google Scholar]
- 107.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy- 5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 109.Gould TD, Zarate CA, Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol. 2019;59:213–236. doi: 10.1146/annurev-pharmtox-010617-052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 111.Kishi T, Matsunaga S, Iwata N. A meta-analysis of memantine for depression. J Alzheimers Dis. 2017;57:113–121. doi: 10.3233/JAD-161251. [DOI] [PubMed] [Google Scholar]
- 112.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–660. doi: 10.1124/pr.117.015198. Erratum in: Pharmacol Rev 2018;70: 879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–S203. doi: 10.33549/physiolres.932678. [DOI] [PubMed] [Google Scholar]
- 116.Machado-Vieira R, Henter ID, Zarate CA., Jr New targets for rapid antidepressant action. Prog Neurobiol. 2017;152:21–37. doi: 10.1016/j.pneurobio.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nosyreva E, Autry AE, Kavalali ET, Monteggia LM. Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade. Front Mol Neurosci. 2014;7:94. doi: 10.3389/fnmol.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, et al. Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature. 2021;590:315–319. doi: 10.1038/s41586-020-03047-0. [DOI] [PubMed] [Google Scholar]
- 120.Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, et al. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci U S A. 2019;116:6441–6450. doi: 10.1073/pnas.1819540116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fukumoto K, Iijima M, Chaki S. The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychophar-macology. 2016;41:1046–1056. doi: 10.1038/npp.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lähteenvuo M, Taipale H, Tanskanen A, Rannanpää S, Tiihonen J. Courses of treatment and risk factors for treatment-resistant depression in Finnish primary and special healthcare: A nationwide cohort study. J Affect Disord. 2022;308:236–242. doi: 10.1016/j.jad.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 123.Gronemann FH, Petersen J, Alulis S, Jensen KJ, Riise J, Ankarfeldt MZ, et al. Treatment patterns in patients with treatment-resistant depression in Danish patients with major depressive disorder. J Affect Disord. 2021;287:204–213. doi: 10.1016/j.jad.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 124.Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull. 2017;143:783–822. doi: 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 126.Sigtermans M, Dahan A, Mooren R, Bauer M, Kest B, Sarton E, et al. S(+)-ketamine effect on experimental pain and cardiac output: A population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111:892–903. doi: 10.1097/ALN.0b013e3181b437b1. [DOI] [PubMed] [Google Scholar]
- 127.Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, et al. Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J Psychiatr Res. 2019;110:166–171. doi: 10.1016/j.jpsychires.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rybakowski JK, Permoda-Osip A, Bartkowska-Sniatkowska A. Ketamine augmentation rapidly improves depression scores in inpatients with treatment-resistant bipolar depression. Int J Psychiatry Clin Pract. 2017;21:99–103. doi: 10.1080/13651501.2017.1297834. [DOI] [PubMed] [Google Scholar]
- 129.Sarkar A, Kabbaj M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry. 2016;80:448–456. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carrier N, Kabbaj M. Sex differences in the antidepressant- like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 131.Saland SK, Schoepfer KJ, Kabbaj M. Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci Rep. 2016;6:21322. doi: 10.1038/srep21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: A randomized controlled trial. Am J Psychiatry. 2019;176:401–409. doi: 10.1176/appi.ajp.2018.18070834. [DOI] [PubMed] [Google Scholar]
- 133.Bartova L, Dold M, Fugger G, Kautzky A, Mitschek MMM, Weidenauer A, et al. Sex-related effects in major depressive disorder: Results of the European Group for the Study of Resistant Depression. Depress Anxiety. 2021;38:896–906. doi: 10.1002/da.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aswal N, Singh SK, Kamarapu P. Study on antidepressant drug to cure depression. J Formul Sci Bioavailab. 2018;2:1000121. doi: 10.4172/2577-0543.1000121. [DOI] [Google Scholar]
- 135.Martín-Hernández D, Ulecia-Morón C, Bris AG, Pereira MP, Caso JR. Monoaminergic system and antidepressants. In: Martin CR, Hunter LA, Patel VB, Preedy VR, editors. The neuroscience of depression. Academic Press; Cambridge: 2021. pp. 345–355. [DOI] [Google Scholar]
- 136.Pham TH, Gardier AM. Fast-acting antidepressant activity of ketamine: Highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol Ther. 2019;199:58–90. doi: 10.1016/j.pharmthera.2019.02.017. [DOI] [PubMed] [Google Scholar]