Abstract
Inhibition of bacterial cell wall synthesis by antibiotics such as β-lactams is thought to cause explosive lysis through loss of cell wall integrity. However, recent studies on a wide range of bacteria have suggested that these antibiotics also perturb central carbon metabolism, contributing to death via oxidative damage. Here, we genetically dissect this connection in Bacillus subtilis perturbed for cell wall synthesis, and identify key enzymatic steps in upstream and downstream pathways that stimulate the generation of reactive oxygen species through cellular respiration. Our results also reveal the critical role of iron homeostasis for the oxidative damage-mediated lethal effects. We show that protection of cells from oxygen radicals via a recently discovered siderophore-like compound uncouples changes in cell morphology normally associated with cell death, from lysis as usually judged by a phase pale microscopic appearance. Phase paling appears to be closely associated with lipid peroxidation.
Subject terms: Antibacterial drug resistance, Antibiotics, Cellular microbiology, Pathogens, Cell death
Inhibition of bacterial cell wall synthesis by β-lactam antibiotics results in a loss of cell wall integrity, but cells die via a combination of downstream events involving metabolic perturbations and oxidative damage. Here, Kawai et al. identify key enzymatic steps that stimulate the generation of reactive oxygen species and highlight the role of iron homeostasis in the lethal effects mediated by oxidative damage.
Introduction
Peptidoglycan (PG) cell wall maintains characteristic shapes of bacteria and protects the cells from fluctuations in internal osmotic pressure. Expansion of the cell during growth requires the insertion of new PG by the action of glycosyltransferase (GTase) and transpeptidase (TPase) enzymes1,2. In most rod-shaped bacteria, cell elongation is governed indispensably by the “Rod system”, which involves a GTase called RodA3–5 and one or more TPases belonging to the family of class B penicillin-binding proteins (bPBPs)6,7. The Rod system is regulated spatially and temporally to achieve orderly cell extension by cytoskeletal proteins of the MreB (actin-like) family, working together with accessory proteins MreC and MreD2,8. Some organisms have more than one MreB paralogue: in Bacillus subtilis there are three, and MreB, Mbl (MreB-like) and MreBH, are essential for normal cell elongation9,10. Many bacteria have a second PG synthetic system based on bifunctional class A PBPs (aPBPs) that have both GTase and TPase activities1,11. This system seems to insert new PG in a dispersed manner leading to growth in a spherical form3, but its precise function is not clear, and all four aPBPs in B. subtilis can be deleted with only mild effects on cell growth4,12.
β-Lactams are one of the oldest and still most widely used clinical antibiotics. They prevent the insertion of new PG by binding to PBPs and irreversibly inactivating their TPase activity13. It is generally considered that this ultimately causes explosive cell lysis by loss of cell wall integrity. Hence, other essential factors of the Rod system (e.g. MreB) also have the potential as targets for new antibacterial agents14,15. Nevertheless, in B. subtilis, various suppressor mutations have been shown to rescue the growth of mreB and/or mbl mutants16,17. Some of the suppressor mutations lay in genes directly connected with cell wall synthesis. However, many of the others targeted carbon and amino acid metabolism, highlighting that the bacterial response to inhibition of wall synthesis that results in cell death is complex. Curiously, the growth deficiency of mreB, mbl and other elongation mutants, including rodA, can be rescued by addition of high concentrations of magnesium ions (Mg2+) to standard culture media16,18–20, for reasons not fully understood.
The cell lysis caused by loss of cell wall integrity in the presence of cell wall active antibiotics, or lytic enzymes such as lysozyme, can be prevented by use of osmoprotective (isotonic) media21–23. However, these cell wall-defective cells normally do not grow and they still die. Recent work examining the lethal effect in B. subtilis and other Gram-positive bacteria linked the non-lytic death during cell wall inhibition to an increase in glycolytic flux, resulting in the production of reactive oxygen species (ROS) from the respiratory chain (RC)24,25. Crucially, physiological compensation for the metabolic imbalance, by reducing glycolytic activity or the RC pathway, counteracts the toxicity24,25, and enables cell wall-independent proliferation in a state known as the L-form26,27.
This is consistent with previous work discovering toxic perturbations in the tricarboxylic acid (TCA) cycle and RC activity in response to β-lactam treatment in Escherichia coli28–30. It has been shown that inhibition of E. coli PBP2 (a cell elongation-specific bPBP) by the β-lactam mecillinam triggers a futile cycle of cell wall synthesis and degradation that contributes to the lethal activity31. Recent follow-up work using metabolomics led to a model that the lethality from PBP2 inhibition is a specific consequence of toxic metabolic shifts induced by energy demand from multiple catabolic and anabolic processes triggered by the PG futile cycling32. The metabolic shifts include central carbon oxidation pathways and ATP utilization, ultimately leading to a dysregulated cellular redox environment. Similarly, the lethality to Mycobacterium bovis of cell wall-active antibiotics depends on an increase in ATP production33. An increase in ROS production caused by β-lactams has been reported in a wide range of bacteria, including Pseudomonas species34, Enterococcus faecalis35 and Vibrio cholerae36. ROS-dependent cell death in L-forms is also evident in E. coli and Streptobacillus moniliformis25,37–39. These results highlight that dysregulation of cell wall metabolism in the target-proximal triggers toxic consequences from downstream metabolic pathways that contribute to bacterial cell death.
We recently identified and purified an actinomycete natural product compound called mirubactin C (MC) that rescues the growth of B. subtilis mreB and mbl mutants40. MC is a derivative of a known siderophore, mirubactin A (MA)41,42. Siderophores are iron-chelating metabolites that are synthesised and secreted by microorganisms to solubilize Fe3+ for its uptake in aerobic environments43. MC also binds iron and results in reduced cellular iron content in B. subtilis, consistent with a model in which it acts as an extracellular iron chelator that restricts iron bioavailability40. However, it remains unclear how iron utilization may have a role in the lethal effect of the cell wall mutants. Curiously, the growth-rescue effect is not induced by its larger relative MA40.
Here, we dissect the physiological connections between upstream perturbations of cell wall inhibition and downstream metabolic effects that contribute to ROS-mediated lethality of cell wall mutations in B. subtilis. We show that MC sequesters iron and prevents its uptake, thereby counteracting oxidative damage via lipid peroxidation (LPO), likely by avoiding the prooxidant effects of redox-active iron44. Remarkably, we found that when mutants are rescued by MC, morphological abnormalities such as bulging and twisting that are generally assumed to be a precursor to cell death by lysis, are largely unaffected. The main effect is on loss of the “pale” cellular appearance, often associated with the term lysis, which is presumably due to leakage of cell contents and reduced cytoplasmic density. We show that the pale cellular appearance is likely due to the perturbation of membrane integrity by LPO. These results provide insights into the mechanisms of bacterial cell death and antibiotic action. We also provide a view of the differentiated roles of the widely conserved Rod and aPBP mechanisms of bacterial cell wall synthesis.
Results
Mirubactins as probes for iron uptake and utilization in B. subtilis
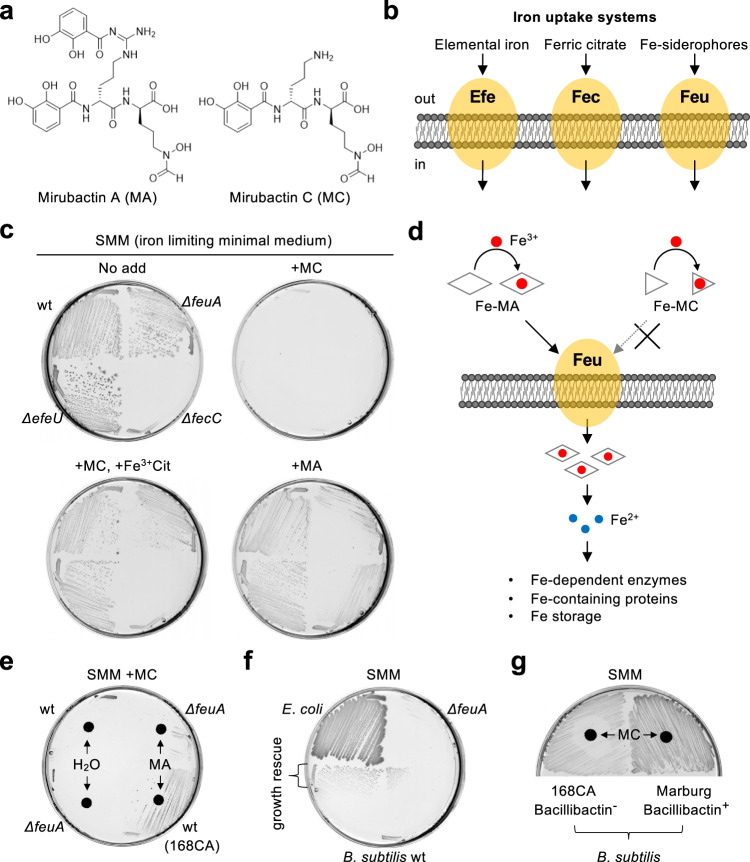
Our previous work showed that treatment of B. subtilis cells with MC substantially reduces cellular iron content (about 25% iron of untreated cells) and rescues the growth of both mbl and mreB mutants in complex-rich media40. The decreased cellular iron content suggested that MC may act extracellularly by sequestering iron (Fig. 1a). To understand the mechanism underlying this iron starvation, we cultured B. subtilis wild-type 168CA and various mutants impaired in known iron transporters (Fig. 1b)45 under iron-limiting conditions (Spizizen minimal medium; SMM46), with glucose and ammonium as carbon and nitrogen sources, but without added iron. (Note that B. subtilis 168CA and its derivatives are unable to synthesize the siderophore bacillibactin due to the presence of the sfp0 mutation45,47.) All of the strains grew well on a normal complex medium, i.e. iron-replete nutrient agar (NA, estimated to contain 0.274 mg/l Fe48), without obvious impairment (Supplementary Fig. 1a, no add). In the presence of iron-free synthetic MC, they showed a mild slow-growth phenotype (Supplementary Fig. 1a, +MC), as seen previously40. On iron-limiting SMM plates, growth occurred in each of the strains except the fecC mutant (Fig. 1c, no add), lacking the ferric citrate transporter (Fig. 1b), consistent with available iron (as a contaminant from the added chemicals and/or leached from glassware) being primarily complexed with citrate in this medium. In the presence of MC, growth was strongly inhibited in all of the tested B. subtilis strains (Fig. 1c, +MC). This inhibitory effect was largely alleviated by addition of ferric citrate to the medium, except for the ferric citrate transporter mutant (ΔfecC) (Fig. 1c, +MC +Fe3+Cit). These results were consistent with MC causing iron deficiency, likely by sequestering iron, thereby preventing its uptake and/or utilization (Fig. 1d).
Fig. 1. Mirubactin C inhibits iron uptake and its utilization.
a Schematic representation of structures of Mirubactin A (MA) and C (MC). b Schematic representation of iron uptake systems in B. subtilis. c Growth inhibition by MC in iron-limiting minimal medium (SMM). B. subtilis strains 168CA (wild-type), YK2739 (ΔefeU), YK2740 (ΔfecC) and YK2741 (ΔfeuA) were streaked on SMM plates with or without 20 μg/ml MA, 10 μg/ml MC and/or 100 μM ferric-citrate (Fe3+Cit), and incubated for 30–42 h at 37 °C. d Schematic representation of effects of Mirubactins on iron uptake and utilization. e Uptake and utilization of iron in B. subtilis mediated by MA. 168CA (wild-type) and YK2741 (ΔfeuA) were streaked on a SMM plate in the presence of 10 μg/ml MC, with paper disc containing 6 μl of H2O or 20 mg/ml MA, and incubated for 30–42 h at 37 °C. f Uptake and utilization of iron in B. subtilis mediated by E. coli. E. coli strain BW25113 and B. subtilis strains (wild-type 168CA and ΔfeuA) were streaked on a SMM plate containing 10 μg/ml MC, and incubated for 30–42 h at 37 °C. g Growth of B. subtilis Marburg strain in the presence of MC. 168CA (bacillibactin-) and Marburg (bacillibactin+) strains were streaked on a SMM plate with paper disc containing 6 μl of 5 mg/ml MC, and incubated for 30–42 h at 37 °C. The figures are representative of at least three independent experiments.
In contrast, in the presence of MA (a natural compound purified in its iron-free form40), the growth deficiency of the fecC mutant (ΔfecC) was ameliorated, whereas the growth of cells lacking the Fe-siderophore transporter (ΔfeuA) was impaired (Fig. 1c, +MA). This suggests that MA can be used as a source of iron for supporting B. subtilis growth and that uptake occurs as an Fe-MA complex via the Feu transporter (Fig. 1d). However, adding higher amounts of MA inhibited B. subtilis growth (Supplementary Fig. 1b, c). Since MA is known to spontaneously decompose into MC49, the growth inhibitory effect might be the consequence of an increased MC concentration under these conditions.
Consistent with the contrasting activities of MA and MC on iron utilization for the growth of B. subtilis, the growth deficiency caused by MC was counteracted in the presence of MA, provided that the Feu (iron-siderophore) transporter was functional (Fig. 1e).
Finally, we found that the Gram-negative bacterium E. coli did not show a growth defect in the presence of MC (Fig. 1f). This could be due to this organism being capable of taking up the Fe-MC complex as a source of iron. However, it seemed more likely that it was due to iron scavenging by the production of an endogenous E. coli siderophore, enterobactin, which is structurally similar to B. subtilis bacillibactin50,51. Consistent with this idea, the growth of wild-type B. subtilis, but not the feuA siderophore uptake mutant, was partially restored when grown adjacent to the E. coli strain (Fig. 1f). We assume that this growth restoration is due to B. subtilis taking up an iron complex of the enterobactin secreted by the E. coli strain. In support of this view, a bacillibactin-functional B. subtilis Marburg strain did not show a growth defect in the presence of MC (Fig. 1g). To conclude, it appears that MA acts as a classical iron siderophore, facilitating iron uptake via siderophore transporters, whereas MC also binds iron but is not taken up and can inhibit iron utilization.
MC rescues mbl mutant cell death but not morphological change
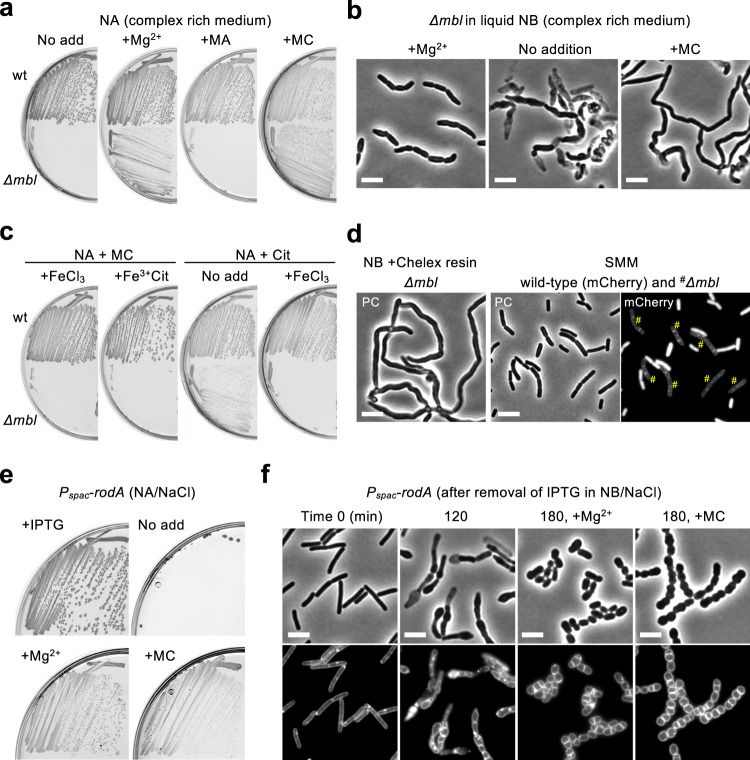
In light of the above experiments, it was interesting to revisit the question of how MC (and Mg2+) rescue the growth of an mbl mutant (Fig. 2a). In liquid nutrient broth (NB) with added Mg2+, mbl mutant cells grew well, although they were slightly shorter and wider than wild-type cells (Supplementary Fig. 2a), as described previously16. When the culture was diluted into fresh NB without added Mg2+, the cells took on abnormal bulging and twisted morphologies, and many cells became phase pale (Fig. 2b and Supplementary Fig. 2a). It is generally assumed that the phase pale or “ghost” appearance of cells treated with cell wall inhibitors is due to leakage of cell components through a damaged cell wall1,22,52,53. However, to our surprise, in NB without added Mg2+ but with MC, although the abnormal twisted-shape was often observed, growth was sustained without the appearance of significant numbers of phase pale cells (Fig. 2b and Supplementary Fig. 2a). It thus appears that the mbl mutant suffers from two distinct morphological abnormalities: shape changes, including bulging and twisting; and the phase pale appearance that may be the main signature of cell death. Furthermore, MC uncouples these effects and specifically rescues the phase pale (lysis) phenotype.
Fig. 2. Growth rescue of elongation mutants by restricting iron availability.
a Growth rescue of an mbl mutant by MC. B. subtilis strains 168CA (wild-type) and YK2638 (Δmbl) were streaked on NA plates with or without 10 mM MgSO4 (Mg2+), 10 μg/ml MA or MC, and incubated for 18 h at 37 °C. b Suppression of phase pale effect in an mbl mutant by MC. Phase contrast micrographs of exponentially growing mbl mutant cells in liquid NB containing 10 mM Mg2+. The cells were diluted into fresh NB with or without 10 μg/ml MC and incubated for 2–3 h. c Effects of iron availability on mbl mutant growth in complex rich medium. Wild-type and Δmbl strains were streaked on NA plates containing 10 μg/ml MC or 5 mM citrate (Cit), with or without 50 μM ferric chloride (FeCl3) or ferric citrate (Fe3+Cit) and incubated for 18 h at 37 °C. d Effects of iron availability on cell morphology in an mbl mutant. YK2265 (wild-type expressing mCherry) and YK2638 (Δmbl) strains were cultured in NB with added 10 mM Mg2+ at 37 °C. Δmbl cells were diluted into fresh NB pre-treated in the presence of 1% Chelex resin (no added Mg2+), and phase contrast (PC) micrographs were captured after 90 min incubation (left panel). Mixture of Δmbl and wild-type cells (expressing mCherry) were diluted into SMM, and phase contrast and the corresponding fluorescent micrographs were captured after 180 min incubation. e Growth rescue of a rodA mutant by MC. YK2245 (Pspac-rodA) was streaked on NA plates containing 100 mM NaCl (for osmoprotection), with or without 0.1 mM IPTG, 10 mM Mg2+ or 40 μg/ml MC, and incubated for 24 h at 37 °C. f Suppression of phase pale effect in a rodA mutant by MC. Phase contrast and the corresponding membrane staining images of exponentially growing Pspac-rodA strain (YK2245) in liquid NB containing 0.1 mM IPTG (Time 0). The cells were diluted into fresh NB with or without 10 mM Mg2+ or 10 μg/ml MC. Phase contrast and the corresponding membrane staining images of the cells were captured after 2–3 h incubation as indicated. Scale bars represent 5 μm. The figures are representative of at least three independent experiments.
mbl mutant cell death is dependent on iron utilization
The growth rescue effect of MC was abolished by the addition of ferric chloride (FeCl3) or ferric citrate (Fe3+Cit) to the culture medium (Fig. 2c, panels 1 and 2), suggesting that rescue was dependent on reduced iron availability or uptake. To test this we used a general iron chelator, citrate, and found indeed that this rescued mbl mutant growth (Fig. 2c, panel 3). This growth rescue was abolished by further addition of ferric chloride (Fig. 2c, panel 4). In addition, when available iron in NB was reduced by pre-treatment with Chelex resin, mbl mutant growth was sustained with the expected abnormal morphology but without the appearance of significant numbers of phase pale cells (Fig. 2d), just as was seen for MC treatment (Fig. 2b). Thus, the lethal effect of mbl mutation under normal culture conditions is dependent on the availability or utilization of iron.
Consistent with this, mbl mutant cells slowly but significantly grew on iron-limiting SMM plates supplemented with 4 mM Mg2+ (the lowest amount of Mg2+ required for robust growth of wild-type cells under these conditions) (Supplementary Fig. 1d, e). Increasing Mg2+ to 10 mM only slightly improved mbl mutant growth (Supplementary Fig. 1d, panel 2). The addition of MC abolished growth of both wild-type and mbl mutant strains (Supplementary Fig. 1d, panel 4), as described above (Fig. 1c), whereas the addition of MA slightly reduced mbl mutant growth likely by acting as a source of intracellular iron (Supplementary Fig. 1d, panel 3). However, the addition of ferric chloride improved mbl mutant growth (Supplementary Fig. 1e). We observed the cell morphology of an mbl mutant in liquid SMM (without added iron), and found that growth was sustained with no detectable morphological defects (Fig. 2d). Thus, factors other than iron could also contribute to the mbl rescue phenotype under these conditions.
Mirubactin C rescues growth of other elongation mutants
We previously showed that MC also rescues the growth of an mreB mutant, affected in the Rod elongation pathway, but not that of a mutant lacking aPBP activity – the alternative cell wall expansion system (“Δ4 mutant”)40. We hypothesized that MC would rescue mutants affected in other components of the Rod system (Supplementary Fig. 2b). Repression of rodA (using an IPTG-dependent promoter, Pspac) abolished growth on NA plates containing (osmoprotective) NaCl but, as expected, growth was rescued by addition of 10 mM Mg2+ (Fig. 2e)20. Importantly, growth was also rescued by addition of MC, without added Mg2+ (Fig. 2e). In liquid NB, as for the mbl mutant, rodA repression resulted in bulging and cell death (phase pale effect) (Fig. 2f). However, in the presence of added Mg2+ or MC, the cells remained phase dark, while switching to a spherical form of growth (Fig. 2f). Thus, cell death upon loss of RodA function is also probably associated with altered iron uptake or utilization, and MC uncouples this iron-mediated cell death from the morphological abnormalities, in this case a rounded shape, just as for the mbl mutant (Fig. 2b). The growth rescue effect, produced either by added Mg2+ or MC, was also observed in a strain lacking the mreBCD genes (Supplementary Fig. 2c).
We confirmed that the continued growth of rodA-depleted cells supported by MC is dependent on the aPBP system (Supplementary Fig. 2d), as expected based on the complementary functions of the Rod and aPBP systems during cell wall expansion3,4.
Mirubactin C prevents cell lysis during PG precursor depletion
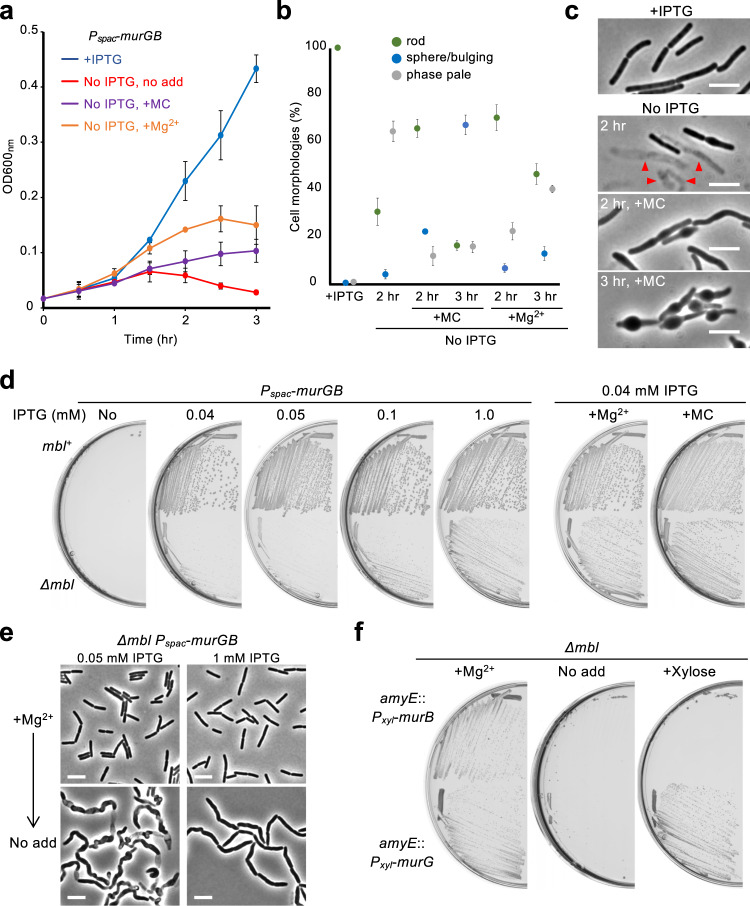
The functioning of the Rod system is dependent on the supply of PG precursors. We therefore tested the effects of MC on cell growth during the depletion of PG precursors by using a Pspac-fused murGB construct. MurG protein catalyses the final step in lipid II synthesis, while MurB acts earlier in the precursor pathway2 (see below). When growing cultures (IPTG supplemented) were diluted into fresh NB without IPTG, the cells stopped growing and lysed within 2 h after murGB repression (Fig. 3a). In the presence of MC, the cells again stopped growing but the cell lysis was clearly alleviated (Fig. 3a). Many cells had taken on a spherical form after 3 h of murGB repression (Fig. 3b, c, and Supplementary Fig. 3a). A similar rescue effect by MC for growth with reduced murGB expression was observed on solid NA plates (Supplementary Fig. 3b). Thus, MC can rescue the lethal effects of inhibiting not only Rod-complex function but also the supply of PG precursors, by uncoupling the cell death from the abonormal bulging. We have previously demonstrated that the morphological changes during depletion of PG precursors is due to dispersed PG synthesis by the aPBP system22. The switch to spherical growth upon murGB repression in the presence of MC suggests that the aPBP system is able to operate not only when the Rod system is perturbed, but also when precursor availability is reduced.
Fig. 3. Dysfunction of lipid II synthesis associates with an mbl mutant lethality.
a Growth curves during murGB repression. B. subtilis strain YK1540 (Pspac-murG-murB) was cultured in NB containing 0.1 mM IPTG (blue line) at 37 °C. The exponentially growing cells were diluted into fresh NB (without IPTG, red line) with 10 μg/ml MC (purple line) or 10 mM Mg2+ (orange line) and cultured at 37 °C for OD600 measurements. The means were obtained from three independent experiments. Error bars indicate standard deviation (SD). Source data are provided as a Source Data file. b Effects of MC on lethal effect and cell morphology during murGB repression. Cell morphologies of about 200 cells under each condition shown in panel a were classified into three types (rod, sphere/bulging or phase pale) based on the phase contrast images as shown in panel c and Suppremenratly a. The results were obtained from three independent experiments. Error bars indicate SD. Source data are provided as a Source Data file. c Phase contrast micrographs of Pspac-murG-murB (YK1540) cells were captured in the course of carrying out the growth curves shown in panel a. Scale bars represent 5 μm. d Effects of murGB expression levels on mbl mutant growth. YK1540 (Pspac-murG-murB) and YK2665 (Δmbl Pspac-murG-murB) strains were streaked on NA plates containing various concentrations of IPTG, with or without 10 μg/ml MC or 10 mM Mg2+. The plates were incubated for 18 h at 37 °C. e Effects of murGB expression levels on cell morphology of an mbl mutant. Phase contrast micrographs of strain YK2665 (Δmbl Pspac-murG-murB) in liquid NB containing 10 mM Mg2+, with 0.05 mM or 1 mM IPTG (+Mg2+). The exponentially growing cells were diluted into fresh NB (no added Mg2+) with 0.05 or 1 mM IPTG and incubated for 2–3 h (No add). Scale bars represent 5 μm. f Growth rescue of an mbl mutant by murG overexpression. YK2700 (Δmbl amyE::Pxyl- murB) and YK2701 (Δmbl amyE::Pxyl- murG) were streaked on NA plates with or without 10 mM Mg2+ or 0.5% xylose, and incubated for 18 h at 37 °C. The figures are representative of at least three independent experiments.
In the presence of added Mg2+, growth significantly continued for a while after murGB repression, and then the cells stopped growing and started to lyse (Fig. 3a, b). In this case, spherical cells were less evident (Fig. 3b and Supplementary Fig. 3a), suggesting that the growth rescue occurs by a mechanism distinct from that of MC, as previously discussed40.
mbl mutant lethality is rescued by overexpression of the murG gene
In the course of carrying out the Pspac-murGB experiments, we titrated the levels of IPTG needed for the growth of otherwise wild-type and mbl mutant strains. The mbl+ strain showed strong growth down to 0.04 mM IPTG (Fig. 3d). At this concentration of IPTG, the mbl mutant failed to grow but, as expected, growth was rescued by the addition of Mg2+ or MC (Fig. 3d, right panels), so presumably 0.04 mM IPTG provides sufficient PG precursor synthesis for growth in both wild-type and mbl mutant cells. Unexpectedly, however, at 1 mM IPTG, the mbl mutant was able to grow without added Mg2+ or MC. In liquid NB with added Mg2+, the mbl mutant grew, with a typical rod-shaped morphology, in the presence of either 0.05 or 1 mM IPTG (Fig. 3e). When the cultures were diluted into fresh NB without added Mg2+, the cells with 0.05 mM IPTG became phase pale but those with 1 mM IPTG did not (Fig. 3e). Thus, an increased level of murGB expression can overcome the lethal effects of an mbl mutation.
To test which of the genes, murG or murB, was responsible for the rescuing effect we constructed strains to overexpress each of them individually. As shown in Fig. 3f, rescue only required overexpression of murG. Our results suggest that disruption of the mbl gene may interfere with the function of MurG, plausibly by affecting the membrane localization or stability of the protein.
glmU expression levels impact lethality of the mbl mutant
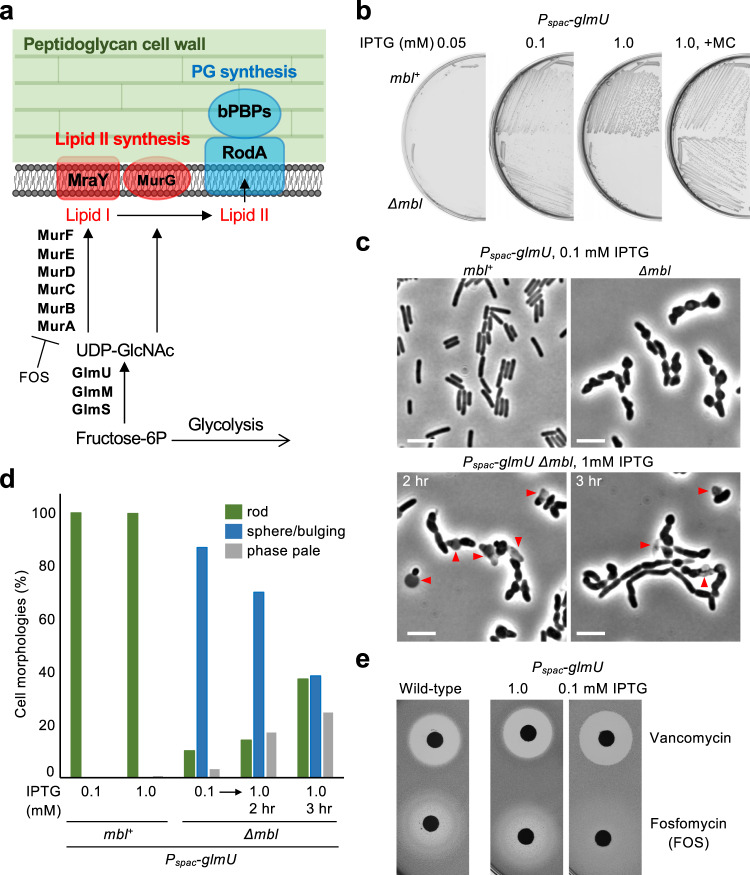
MurG catalyses the final step in lipid II synthesis by adding the nucleotide sugar UDP-GlcNAc to lipid I (Fig. 4a)2. The above rescue effect by murG overexpression could be due to decreased intracellular UDP-GlcNAc levels. To test this possibility we placed the glmU gene, required for UDP-GlcNAc synthesis (Fig. 4a)54, under Pspac control. Since glmU is essential for PG precursor synthesis, no growth occurred at low IPTG concentrations (at or below 0.05 mM) in either an mbl mutant or in its parental mbl+ strain (Fig. 4b, panel 1 and Supplementary Fig. 4a). A concentration of 0.1 mM provided sufficient expression to support at least partial growth of both the wild type and the mbl mutant strains (Fig. 4b, panel 2). However, whereas substantial growth occurred in the mbl+ background when more IPTG was added, to further increase glmU expression, growth of the mbl mutant was abolished above 0.5 mM IPTG (Fig. 4b, panel 3 and Supplementary Fig. 4a). Strikingly, this growth inhibition was again largely overcome in the presence of MC (Fig. 4b, panel 4). Thus, the mbl mutant does not appear to be able to tolerate levels of UDP-GlcNAc synthesis that support normal growth in wild-type cells and the toxicity effect is probably again dependent on iron availability.
Fig. 4. UDP-GlcNAc synthesis associates with mbl mutant lethality.
a Schematic representation of PG precursor synthesis in B. subtilis. b Growth rescue of an mbl mutant by reducing UDP-GlcNAc synthesis. B. subtilis strains YK1538 (Pspac-glmU) and YK2687 (Δmbl Pspac-glmU) were streaked on NA plates containing 0.1 or 1 mM IPTG, with or without 10 μg/ml MC, and incubated for 18 h at 37 °C. c Effects of glmU expression levels on cell morphology of an mbl mutant. Phase contrast micrographs of strains YK1538 (Pspac-glmU) and YK2687 (Δmbl Pspac-glmU) in liquid NB containing 0.1 mM IPTG (upper panels). In the culture of YK2687, IPTG was added to 1 mM, and phase contrast micrographs were captured after 2 and 3 h incubation (bottom panels). Scale bars represent 5 μm. d Cell morphologies shown in panel c were classified into three types (rod, sphere/bulging or phase pale). About 300 cells were examined for each condition. e Effects of glmU expression levels on fosfomycin sensitivity. Disc diffusion assay of B. subtilis strains (wild-type and Pspac-glmU) on NA plates with or without IPTG using paper discs with 6 μl of 1 mg/ml vancomycin or 50 mg/ml fosfomycin. The plates were incubated for 24 h at 37 °C. The figures are representative of at least three independent experiments.
At the minimal permissive concentration of IPTG (0.1 mM) for glmU expression, mbl+ cells had their normal rod-shaped phase dark appearance (Fig. 4c, d). The mbl mutant cells had a highly abnormal bulging and twisted appearance but notably the cells were virtually all phase dark, indicating a lack of cell lysis. The abnormal morphologies probably explained the poor growth on plates (Fig. 4b, panel 2). When glmU gene expression was increased (1 mM IPTG), the mbl mutant cells started to take on a more elongated form but they also began to undergo lysis (Fig. 4c, d), as shown above (Fig. 2b). Thus, it seems that the toxicity associated with UDP-GlcNAc synthesis can be separated from the morphological defects generated by the loss of the mbl gene.
Sensitivity to Fosfomycin is also influenced by UDP-GlcNAc synthesis
Fosfomycin (FOS) is a broad-spectrum bactericidal antibiotic that prevents the first committed step in lipid I synthesis by inhibiting the MurA enzyme (Fig. 4a)55. Inhibition of MurA should also result in decreased utilization of UDP-GlcNAc for PG precursor synthesis, which, on the basis of the above experiments, might contribute to FOS toxicity. We took wild-type background cells bearing the Pspac-glmU construct and tested to see whether titration of expression (via IPTG concentration) would affect sensitivity to FOS. Remarkably, reducing glmU expression with 0.1 mM IPTG conferred much more resistance (smaller zone of growth inhibition) compared with the wild-type, and fosfomycin sensitivity returned to the wild-type level when glmU expression was restored by the addition of 1 mM IPTG (Fig. 4e). In contrast, glmU expression levels had no detected effect on sensitivity to vancomycin (Fig. 4e), which inhibits the insertion of new lipid II into the PG wall56. These results again support the hypothesis that a reduction of UDP-GlcNAc utilization contributes to the toxic effect when cell wall synthesis is perturbed.
UDP-GlcNAc levels are affected by Mg2+ but not MC treatment
We then directly measured the intracellular levels of UDP-GlcNAc in wild-type cells cultured with added Mg2+ or MC. In the presence of Mg2+, UDP-GlcNAc levels were substantially reduced (Supplementary Fig. 4b), consistent with a role for UDP-GlcNAc concentration in the toxic effects of cell wall perturbations. Nevertheless, addition of MC did not lead to reduced UDP-GlcNAc levels (Supplementary Fig. 4b). Similar results were obtained in mbl mutant cells (Supplementary Fig. 4c). Thus, the growth rescue by Mg2+ could occur via a reduction of intracellular UDP-GlcNAc levels, whereas MC probably acts by a mechanism downstream from that of Mg2+.
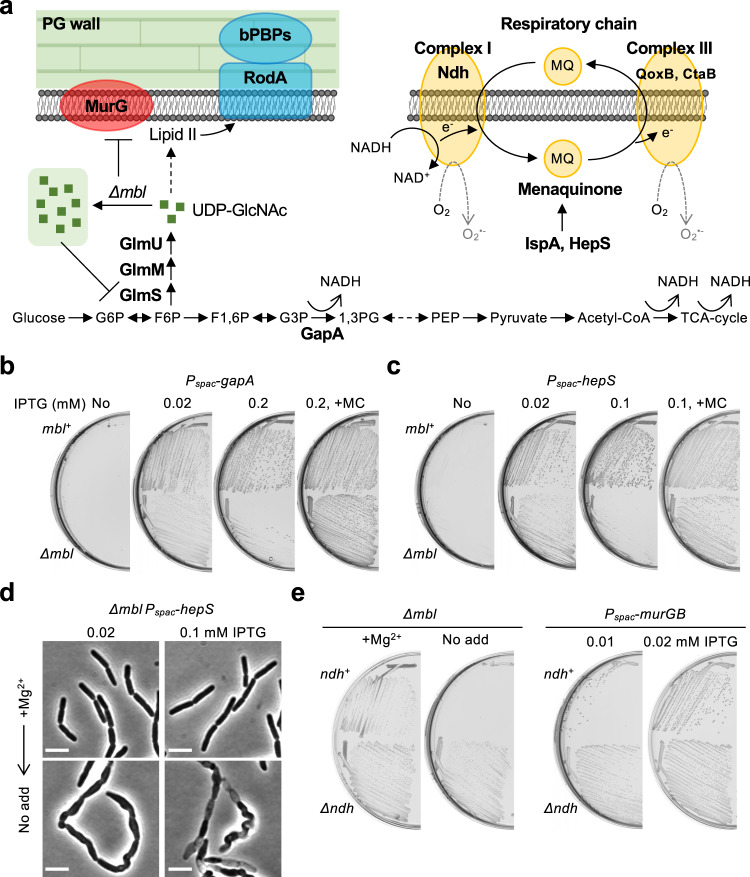
Lethality of the mbl mutation involves glycolysis
GlmS acts as an anabolic-catabolic (PG wall - glycolysis) checkpoint by modulating the flux of a sugar-phosphate intermediate, fructose-6-phosphate (Fig. 5a, F6P)57–61. When the intracellular concentration of UDP-GlcNAc is high, GlmS activity is reduced. We previously showed that blockage of the UDP-GlcNAc pathway by repression of glmS during cell wall inhibition results in the redirection of glucose metabolism towards glycolysis25. Importantly, the increased carbon flux through glycolysis acts as a serious impediment to the growth of cell wall-free L-forms. This correlates with an increased generation of toxic ROS from the RC pathway. Considering the above experiments, we wondered whether this might also be the case in mbl mutants. If so, slowing down glycolysis in an mbl mutant should rescue growth, as seen previously for L-forms25. We introduced Pspac-gapA, which encodes an essential glycolytic enzyme, NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (Fig. 5a)62, into an mbl mutant and titrated the levels of IPTG needed for growth. As shown in Fig. 5b, no growth occurred in either an mbl mutant or its isogenic mbl+ strain on NA plates without IPTG (panel 1), consistent with the expectation that the cells depend on generating energy through glycolysis under these conditions. At a low concentration of IPTG, just sufficient to allow growth of the wild-type (0.02 mM), the mbl mutant was able to grow (Fig. 5b, panel 2), whereas at higher IPTG concentrations (above 0.2 mM), growth was inhibited (Fig. 5b, panel 3 and Supplementary Fig. 5a). Just as in the experiments described earlier, growth inhibition was suppressed in the presence of MC (Fig. 5b, panel 4).
Fig. 5. Cellular respiration associates with mbl mutant lethality.
a Schematic representation of a link between PG precursor synthesis and central carbon metabolism in B. subtilis. b Effects of gapA expression levels on mbl mutant growth. YK1567 (Pspac-gapA) and YK2711 (Δmbl Pspac-gapA) were streaked on NA plates containing 0.02 or 0.2 mM IPTG, with or without 10 μg/ml MC, and incubated for 18 h at 37 °C. c Effects of menaquinone synthesis on mbl mutant growth. YK1450 (Pspac-hepS-menH-hepT) and YK2625 (Δmbl Pspac-hepS-menH-hepT) were streaked on NA plates containing 0.02 or 0.1 mM IPTG, with or without 10 μg/ml MC, and incubated for 18 h at 37 °C. d Effects of menaquenone synthesis on cell morphology of an mbl mutant. Phase contrast micrographs of a strain YK2625 (Δmbl Pspac-hepS-menH-hepT) in NB containing 10 mM Mg2+, with 0.02 mM or 0.1 mM IPTG (+Mg2+). The exponentially growing cells were diluted into fresh NB (no added Mg2+) with IPTG and incubated for 2–3 h (No add). Scale bars represent 5 μm. e Growth rescue of an mbl mutant and murGB repression by disrupting NADH dehydrogenase. YK2638 (Δmbl) and YK2646 (Δmbl Δndh) were streaked on NA plates with or without 10 mM Mg2+ and incubated for 18 h at 37 °C (left panels). YK1540 (Pspac-murG-murB) and YK2689 (Δndh Pspac-murG-murB) were streaked on NA plates with 0.01 or 0.02 mM IPTG and incubated for 18 h at 37 °C (right panels). The figures are representative of at least three independent experiments.
Consistent with the critical importance of glycolysis in the lethal effect, activating gluconeogenesis by supplying a gluconeogenic carbon source (malate) also rescued the growth of an mbl mutant (Supplementary Fig. 5b).
NADH oxidation in the RC pathway associates with the lethality in mbl mutant
These results suggested that the utilization of sugar-phosphate intermediates through glycolysis may cause an increased generation of ROS from the RC pathway in mbl mutants. We previously found that several mutations promoting robust L-form growth work by reducing RC activity, which reduces the generation of toxic ROS24. The hepS-menH-hepT operon is one of the previously identified suppressor loci and the products are required for the biosynthesis of menaquinone (Fig. 5a), which is normally essential in electron transport and ATP generation in many Gram-positive bacteria63. We introduced a Pspac-hepS operon construct into an mbl mutant and tested the effects of reducing menaquinone synthesis on mbl mutant growth. As expected, the partial repression of menaquinone synthesis with 0.02 mM IPTG rescued the growth of the mbl mutant (Fig. 5c, panel 2). Growth was inhibited at higher levels of expression (IPTG >0.1 mM) and again the growth inhibition was counteracted in the presence of MC (Fig. 5c, panels 3 and 4). In liquid NB with added Mg2+, the mbl mutant grew in a typical rod-shaped morphology in the presence of either 0.02 or 0.1 mM IPTG (Fig. 5d). When the cultures were diluted into the fresh NB but without added Mg2+, the cells with 0.1 mM IPTG became phase pale, but those with 0.02 mM IPTG did not (Fig. 5d).
We then tested the effects of various other L-form promoting mutations in the RC pathway (i.e. ndh, ispA, qoxB and ctaB24) to see whether they would also rescue mbl mutant growth. As shown in Fig. 5e and Supplementary Figure 6a, ndh (NADH dehydrogenase in complex I) and ispA (geranyltranferase for menaquinone synthesis) mutations rescued mbl mutant growth. However, no growth rescue occurred in the presence of qoxB (cytochrome quinol oxidase in complex III) or ctaB (heme O synthase in complex III) mutation. We also tested a mutation in sdhA, which encodes succinate dehydrogenase forming complex II in the RC pathway64, but again no growth rescue occurred (Supplementary Fig. 6a). Thus, electron transfer from NADH dehydrogenase to menaquinone in the NADH oxidation reaction, which generates superoxide as a by-product (Fig. 5a)65, seems to associate with the mbl toxicity effect. We also tested the ndh mutation on the toxic effect of murG repression, and found that the growth deficiency at 0.01 mM IPTG was largely overcome (Fig. 5e, right panels), just as for treatment with MC (Supplementary Fig. 3b).
NADH is produced via various enzymes in central carbon metabolism (Fig. 5a), any or all of which could act as a source for toxicity in the mbl mutant. GapA activity in glycolysis is coupled to the reduction of NAD+ to NADH (Fig. 5a)62 and reducing its activity can rescue the growth of the mbl mutant (Fig. 5b). We therefore tested mutations affecting pyruvate dehydrogenase (pdhA), 2-oxoglutarate dehydrogenase (odhA) and malate dehydrogenase (mdh), and found that inactivation of pdhA or odhA, but not mdh, rescued growth of the mbl mutant (Supplementary Fig. 6b). Thus, the mbl toxicity effect seems to associate with production of NADH from central carbon metabolism.
ROS-mediated cell death in the mbl mutant
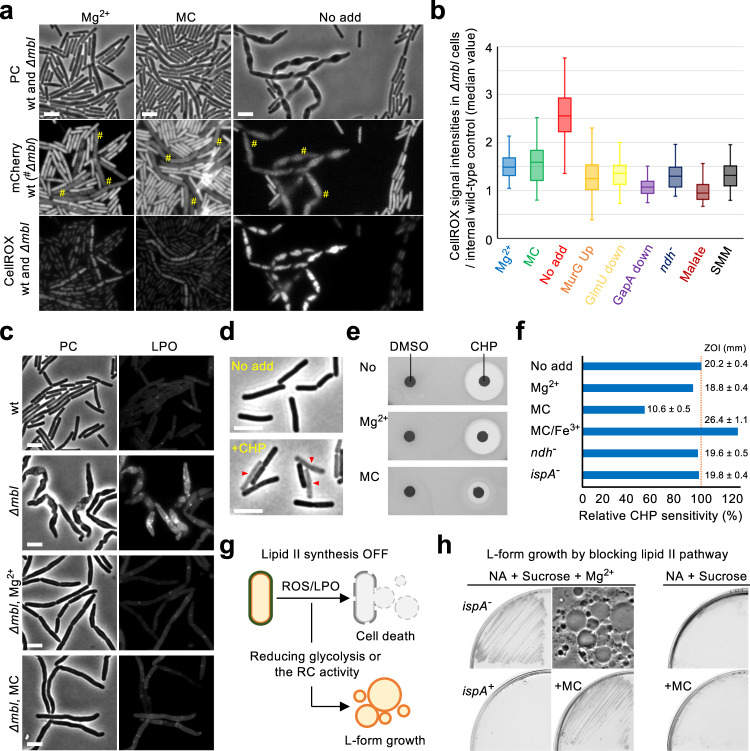
The above results suggested that the toxic effects of mbl mutation are due to increased ROS production through the RC pathway. We therefore measured intracellular ROS levels using the ROS-sensitive permeable dye CellROX Green, which becomes fluorescent upon binding DNA after being oxidized by superoxide (O2-) or hydroxyl radical (•OH)66. We cultured wild-type and mbl mutant strains in NB with added Mg2+, and the exponentially growing cells were aerobically incubated with CellROX for 30 min. Figure 6a shows a mixture of the wild-type (expressing mCherry) and mbl mutant cells (left panels). An obvious CellROX fluorescence was detected in both strains, but the fluorescence was clearly weaker in wild-type cells than in mbl mutant cells (Fig. 6a, b, Mg2+). Cultures (before treatment with CellROX) were then diluted in fresh NB with or without MC and incubated for 60 min, followed by CellROX treatment. In the presence of MC, the pattern of CellROX fluorescence was similar to the case for added Mg2+ (Fig. 6a, b, MC). Strikingly, in the absence of MC, strong fluorescence appeared in many mbl mutant cells but not in wild-type cells (Fig. 6a, b, No add). We confirmed that the growth rescue effects on mbl mutants under various conditions as described above (i.e. murG overexpression, glmU repression, gapA repression, ndh inactivation, addition of malate and SMM medium) all correlated with reduced ROS levels (Fig. 6b).
Fig. 6. MC treatment counteracts ROS-mediated cell death.
a ROS production in mbl mutant cells. YK2265 (wild-type expressing mCherry) and YK2638 (Δmbl) were cultured in NB with added 10 mM Mg2+ at 37 °C, followed by CellROX Green treatment. Phase contrast (PC) and the corresponding fluorescent images (mCherry and CellROX) of mixtures of the wild-type and Δmbl cells were captured (left panels). The cultures before CellROX treatment were diluted into fresh NB (no added Mg2+) with or without 10 μg/ml MC and incubated for 60 min, followed by CellROX reatment, before taking images. b YK2265 and mbl mutant strains (YK2638; Δmbl, YK2701; Δmbl amyE::Pxyl- murG, YK2687; Δmbl Pspac-glmU, YK2711; Δmbl Pspac-gapA, YK2646; Δmbl Δndh) were cultured in NB containing 10 mM Mg2+ with appropriate supplements. The cultures were diluted in fresh SMM, or NB (no added Mg2+) with or without 10 μg/ml MC, 0.5% xylose (for YK2701), 0.1 mM IPTG (for YK2687), 0.05 mM IPTG (for YK2711) or 0.2% malate, and incubated for 60–90 min, followed by CellROX treatment, before taking images. The relative signal intensity of green fluorescence in mbl mutant cells (n=100) over internal wild-type control (mean value, n=100) were represented by boxplots. Boxplots represent the upper and lower quartile values (boxes), median (horizontal lines in the boxes) and most extreme data points within 1.5 times interquartile ranges (whiskers). Source data are provided as a Source Data file. c Lipid peroxidation (LPO) in mbl mutant cells. Cultures of wild-type and mbl mutant cells in NB with 10 mM Mg2+ were diluted in fresh NB (no added Mg2+) with or without MC and incubated for 60 min, followed by C11-BODIPY581/591 treatment, before taking images. d Effects of CHP treatment on cell morphology. Phase contrast micrographs of wild-type (168CA) cells in liquid NB before or after treatment with 10 mM CHP. e Disc diffusion assay on NA plates with or without 10 mM Mg2+ or 10 μg/ml MC using paper discs with 6 μl of DMSO or 250 mM CHP. The plates were incubated for 24 h at 37 °C. f Zones of growth inhibition (ZOI) were measured from four independent disc diffusion assays. The means and SD were shown. Wild-type (168CA), YK1714 (Δndh) and YK1395 (ispA-) was cultured on NA plates with or without added Mg2+, 10 MC and 50 μM ferric chloride (Fe3+). g Schematic representation of L-form generation and ROS-mediated killing during inhibition of lipid II synthesis. h Effects of MC and Mg2+ on L-form growth. LR2 (ispA- Pxyl-murE) and BS115 (Pxyl-murE) strains were streaked on NA plates containing 0.5 M sucrose, with or without 20 mM MgSO4 (Mg2+) and/or 10 μg/ml MC, and incubated for 2–3 days at 30 °C. The phase contrast micrograph of L-form cells was obtained from the left plate adjacent to the micrograph. Scale bars represent 5 μm. The figures are representative of at least three independent experiments.
High levels of free radicals or ROS can damage cells by hitting a range of molecular targets67,68. Oxidative damage to lipids, lipid peroxidation (LPO), alters the physical properties of the cellular membrane, and generates a range of toxic effects in all cells44, such as ferroptosis in eukaryotic cells69. It seemed possible that LPO could be the source of the phase pale, lytic effect seen in the various mutants described above. To test this, we took advantage of a fluorescent fatty acid analog, C11-BODIPY581/591 24. In a control experiment with wild-type cells, no clear fluorescence was detectable (Fig. 6c, wt). In an mbl mutant, obvious fluorescence was not observed in the presence of added Mg2+ (Fig. 6c, Δmbl, Mg2+), whereas the fluorescence became readily detectable when the added Mg2+ was depleted (Fig. 6c, Δmbl). Crucially, the strong fluorescence in the mbl mutant was reduced in the presence of MC (Fig. 6c, Δmbl, MC). Thus, LPO seems to be a major cause for the phase pale effect in mbl mutants.
Mirubactin C reduces lipid peroxidation by restricting iron availability
To investigate the mechanism underlying the suppression of LPO by MC, we made use of an exogenous inducer, cumene hydroperoxide (CHP), which is a stable organic oxidizing agent that acts to initiate and propagate endogenous ROS-independent LPO44. When B. subtilis wild-type cells were treated with CHP, a lethal phase pale effect was induced (Fig. 6d, red arrowheads), as seen for elongation mutants (Fig. 2b, f) or following the repression of lipid II synthesis (Fig. 3b, c). Importantly, the toxic effect of CHP treatment was significantly suppressed in the presence of MC, as indicated by a much smaller zone of growth inhibition (Fig. 6e, f). Redox-active transition metals, especially iron, can trigger exacerbating rounds of free radical-mediated LPO by CHP44. Crucially, the resistant effect of MC was counteracted in the presence of added ferric iron (Fig. 6f). Thus, MC prevents LPO-mediated toxicity likely by restricting iron bioavailability. In contrast, Mg2+ did not confer significant resistance, and neither ndh nor ispA mutations influenced CHP toxicity (Fig. 6f).
SOD enzymes are an important antioxidant defense against superoxide70. They catalyse the dismutation of superoxide radical (O2-) (e.g. formed by electrons leaking from the RC) into molecular oxygen (O2) and hydrogen peroxide (H2O2). The latter then acts as a source of hydroxyl radical (•OH) formation via the Fenton/Haber-Weiss reaction cycle in the presence of redox-active iron67,71,72. We examined SOD activity in lysates of vegetative cells of wild type and mbl mutant cells in the presence of added Mg2+ or MC. In the presence of MC, SOD activity was substantially elevated in the mbl mutant compared with the wild-type (Supplementary Fig. 7), consistent with increased production of superoxide from the RC pathway in the mbl mutant. Strikingly, addition of Mg2+ did not lead to elevated SOD activity in either mutant or wild type cells (Supplementary Fig. 7). Thus, Mg2+ could work to rescue mbl viability by preventing excess superoxide production, whereas MC treatment acts downstream to prevent the formation of hydroxyl radical and LPO by restricting iron bioavailability.
Mirubactin C promotes L-form growth by avoiding ROS-dependent cell death
Our results suggest that iron starvation in the presence of MC works to rescue mbl mutant growth by preventing ROS-dependent cell death mediated through the RC pathway. If so, MC could also work to promote L-form growth by avoiding the LPO-dependent toxicity we previously reported (Fig. 6g)24,25. MC was not able to support the growth of cells when murGB expression was completely inhibited on NA plates (Supplementary Fig. 3b). (Prolonged culture without the supply of PG precursors ultimately results in osmotic lysis due to the formation of cell wall lesions via ongoing autolytic activity under standard conditions22). To induce an L-form state, lipid II synthesis was blocked on isotonic NA plates supplemented with 20 mM MgCl2 and 0.5 M sucrose21. As expected, based on our previous work, cells bearing an ispA mutation (inhibiting menaquinone synthesis) were able to switch into the L-form state and grow, whereas ispA+ cells were not viable (Fig. 6h). Strikingly, growth of the ispA+ strain was rescued in the presence of MC (Fig. 6h). Thus, MC appears also to prevent ROS-mediated toxicity when lipid II synthesis is completely inhibited, leading to growth in the L-form state, under osmoprotective conditions.
We also induced L-forms on isotonic NA plates with osmoprotective sucrose but without added Mg2+ to test for a possible role of the Mg2+ during L-form growth. In this case, no L-form growth occurred even in the presence of an ispA mutation, nor in wild-type cells with MC (Fig. 6h, NA + Sucrose on right panels). Thus, Mg2+ has a critical role to support L-form growth but via a different mechanism from that of MC.
Discussion
Previous work showed that treatment of B. subtilis cells with MC substantially reduces cellular iron content and rescues the growth of both mbl and mreB mutants40. Here, our results have established a mechanism for the protective effects of MC, involving the sequestration of iron outside the cell, restricting its intracellular availability.
The importance of iron homeostasis for β-lactam tolerance has been reported for several bacteria34,36,73. In this work, we have shown that iron chelation by MC is able to protect, not only cells deficient in the actin-like proteins Mbl and MreB, but also various other cell wall-deficient mutants, or cell wall-free L-forms that can be induced by treatment with antibiotics, from cell death. Taken together, these findings imply that this pathway to cell death is normally dependent on intracellular iron availability. In contrast, MC did not work to rescue the growth of a mutant lacking the alternate PG synthetic system based on aPBPs40. Therefore, the iron-dependent toxicity effect seems specifically to be induced when the Rod-based cell elongation system is affected. Given that the aPBP system uses the same PG precursors as the Rod system, it seems that the inactivation of aPBP activity must have a lesser impact on the metabolic re-routing that would otherwise lead to ROS toxicity. Here our data imply that the aPBP system can operate at lower PG precursor levels than can the Rod system. This is consistent with our previous finding that the cell wall expansion that occurs during the walled cell to L-form transition driven by inhibition of PG precursor synthesis depends on the aPBP system22. Our previous work also showed that the activity of autolytic enzymes is coupled to aPBP activity during the generation of L-forms22. Taken together, these findings suggest that a major role for the (non-essential) aPBP system is as a fail-safe system that protects the cells from catastrophic cell wall failure when synthesis or utilization of PG precursors is perturbed.
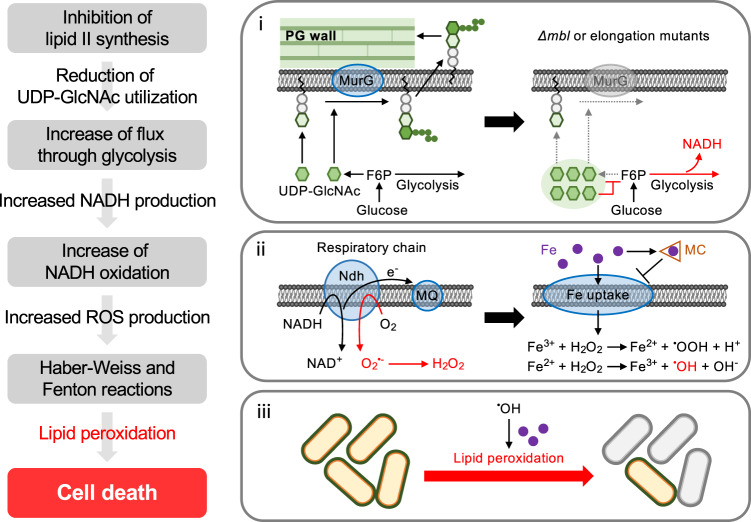
We went on to dissect the steps from perturbation of cell wall synthesis to cell death (Fig. 7). In line with previous findings for B. subtilis57–61 and several other organisms74,75, we found that UDP-GlcNAc is a key metabolite in the process. We showed that perturbations increasing or decreasing UDP-GlcNAc control whether or not cell death occurs, and that the death effect is dependent on excess NADH production from central carbon metabolism and RC activity. This is supported by a recent metabolomics study that the β-lactam mecillinam results in increased cellular levels of UDP-GlcNAc, glycolytic intermediates and NAD+ in E. coli32. Importantly, we showed here that intracellular levels of UDP-GlcNAc are reduced in the presence of excess Mg2+ (Supplementary Fig. 4). Elucidating how Mg2+ influences UDP-GlcNAc levels, thereby preventing cell death upon perturbation of cell wall synthesis, is an important challenge for future studies. Another intriguing question is why perturbation of cell wall synthesis has such a severe effect, compared to other metabolic disruptions, such as abrupt changes in nutrition. However, the Gram-positive cell wall constitutes a major proportion of the mass of the cell (about 10–20%), so perhaps blocking this pathway has a particularly severe effect on the flux pathways leading to ROS.
Fig. 7. Critical role for metabolic perturbations and ion homeostasis in the mbl lethality.
Schematic representation of key cellular functions that link metabolic perturbations in mbl mutants to bacterial cell death, and the critical connections between iron homeostasis and the killing activity. Disruption of an mbl gene or other elongation mutants infuences lipid II synthesis, plausibly by affecting the membrane localization or synthesis of MurG. This could cause the accumulation of UDP-GlcNAc, by preventing its utilization for lipid II synthesis (i). The increased intracellular concentration of UDP-GlcNAc is of critical importance in the downstream metabolic shifts. When the intracellular concentration of UDP-GlcNAc is high, GlmS activity is reduced, resulting in the rerouting of glucose metabolism towards glycolysis and increased NADH production (i). Oxidation of the excess NADH in the RC pathway increases ROS generation (ii), which drives the toxic effect of the mbl mutation in the presence of iron (iii). The sequestration of extracellular iron in the presence of MC could reduce the labile iron pool in the cytosol acts to catalyse the production of deleterious hydroxyl radical via the Fenton/Haber-Weiss reaction cycle under oxidative stress conditions, and thereby formation of hydroxyl radical through interaction with iron is prevented (ii). This, in turn, protects the mbl mutant cells from toxic oxidative damage.
When intracellular iron content is in excess, ROS production via cellular respiration stimulates the Fenton/Haber-Weiss reaction cycle, which catalyses the production of deleterious hydroxyl radical67,72. We showed that LPO, which is promoted by hydroxyl radical, also in the presence of iron44, is the main cause of death in cell wall mutants and that this is probably responsible for the phase pale (lytic) appearance. It is commonly assumed that the gross morphological changes that often accompany treatment with cell wall targeting antibiotics22,76,77, or mutations perturbing cell wall synthesis (typically twisting and or bulging)9,78,79, are the cause of subsequent lysis (phase pale appearance) and cell death. However, our studies with MC demonstrated a separation of the morphological changes and lysis, showing that there are at least two distinct pathways potentially leading to cell death upon antibiotic treatment, and support the idea that ROS can play a major role in cell killing. It also suggests that LPO, catalysed by redox-active iron, is a critical impediment to the growth of L-form bacteria.
In conclusion, our results highlight key enzymatic reactions that link the metabolic perturbations that occur upon cell wall inhibition to bacterial cell death, as well as critical connections between iron homeostasis and specific aspects of the killing activity. Further improvements in our understanding of the physiological changes occurring upon antibiotic treatment should prompt new strategies for antibacterial therapies.
Methods
Bacterial strains and growth conditions
The bacterial strains in this study are listed in Supplementary Table 1. DNA manipulations and transformations were carried out using standard methods. Nutrient agar and broth (Oxoid) were used as complex rich media for bacterial growth. For iron limiting conditions, Spizizen minimal medium (SMM)46, (0.2% (w/v) ammonium sulphate, 1.4% dipotassium phosphate, 0.6% potasium dihydrogen phosphate and 0.1% sodium citrate dihydrate), was used with supplementing 0.5% glucose, 0.02 mg/ml tryptophan, 0.02% casamino acids and 4 mM magnesium sulphate. L-form growth was induced on isotonic NA plates, composed of 2x magnesium-sucrose-maleic acid (MSM) pH7 (40 mM magnesium chloride, 1 M sucrose, and 40 mM maleic acid) mixed 1:1 with 2x NA, at 30 °C. 1 µg/ml FtsZ inhibitor, 8J80, was added to L-form medium to prevent the growth of walled cells when required. For selections of B. subtilis mutants, antibiotics were added to media at the following concentrations: 1 µg/ml erythromycin, 5 µg/ml chlroramphenicol, 60 µg/ml spectinomycin or 2.5 µg/ml kanamycin. The concentration of kanamycin was increased to 10 µg/ml in the presence of added Mg2+. IPTG, xylose and/or MgSO4 was supplemented, as appropriate. MA and MC used in this study were purified and/or synthesised previously40.
Construction of IPTG-inducible glmU, murG operon and pdhA, and disruption mutants of sdhA and mdh
To construct the IPTG-inducible glmU or murG mutant, the first 200–300 bp of the glmU, murG or pdhA gene containing Shine-Dalgarno sequence was amplified by PCR from genomic DNA of the B. subtilis strain 168CA, then introduced into the plasmid pMutin481, creating pM4-Pspac-glmU and pM4-Pspac-murG. The resulting plasmids were introduced into the B. subtilis strain 168CA to generate YK1538, YK1540 and YK2732 (Supplementary Table 1), respectively. The sequences of primers used for the strain construction are listed in Supplementary Table 2.
To disrupt sdhA or mdh, the first 200–300 bp of the sdhA or mdh gene was amplified by PCR from genomic DNA of the B. subtilis strain 168CA, then introduced into the plasmid pMutin481, creating pM4- ΔsdhA and pM4- Δmdh. The resulting plasmids were introduced into the B. subtilis strain 168CA to generate YK2718 and YK2720 (Supplementary Table 1), respectively. The sequences of primers are listed in Supplementary Table 2.
Construction of xylose-inducible murB and murG gene overexpression
To construct the xylose-inducible murB or murG mutants, full length of the murB or murG gene containing Shine-Dalgarno sequence was amplified by PCR, with MK537-MK538 or MK533-MK534 primer pairs, from genomic DNA of the B. subtilis strain 168CA, then assembled with the plasmid pSG172882, creating DNA fragments containing Pxyl-murB and Pxy-murG using MK117-MK536 and MK539-MK116 primers (for murB), or MK117-MK532 and MK535-MK116 primers (for murG). The DNA fragments were introduced into amyE locus of an mbl mutant (YK2638) to generate YK2700 or YK2701 (Supplementary Table 1), respectively. The sequences of primers are listed in Supplementary Table 2.
Microscopy and image analysis
For snapshot live cell imaging, walled cells were mounted on microscope slides covered with a thin film of 1.2% agarose in water. L-forms were mounted on microscope slides with isotonic NB containing MSM. All microscopy experiments were conducted using a Nikon Ti microsope equipped with a Nikon CFI Plan Apo DM Lambda x100 oil objective and a Photometrics Prime camera, using MetaMorph software (version 7.7, Molecular Devices). Images were analysed and processed using FIJI (version 2.9.0/1.53t, https://imagej.net/Fiji).
Measurement of intracellular UDP-GlcNAc levels
Overnight cultures of B. subtilis strains, 168CA (wild-type) and YK2638 (Δmbl), in LB (Luria-Bertani) liquid medium (with 10 mM Mg2+ for Δmbl) were diluted into 10 ml fresh LB with or without 10 mM Mg2+ or 10 μg/ml MC. The cultures were incubated at 37 °C to OD600nm = 0.5. The cells were harvested from 7 ml of culture by centrifugation and washed by resuspending the pellet in 1 ml of deionized water and subsequent centrifugation. The pellet was resuspended in 75 µl of 5% trichloroacetic acid and kept at room temperature for 20 min at 500 rpm shaking. The mixture was then centrifuged for 10 min at 15871 g. The supernatant was neutralized by the addition of 11.25 µl of KOH (2.5 M)/K2HPO4 (1.5 M) solution for LC-MS analysis. An Agilent Triple Quad 6460 linked to an Agilent LC 1290 Infinity with a CarboPac PA1 2 x 250 mm column (Thermo Fisher) was used for UDP-GlcNAc analysis. Mobile phases consisted of water (A) and 1 M ammonium acetate (B). A gradient of 25 to 30% of B over 37 min was employed at the fellow rate of 0.2 ml/min. Multiple reaction monitoring (MRM) technique with the following parameters was used for the quantitation of UDP-GlcNAc: negative polarity; precursor ion 606.07 Da; product ions 385.0 and 158.9 Da; dwell time 200 ms; collision energy for 385.0 Da was 29 V and for 158.0 was 49 V; fragmentation voltage for both product ions was 190 V and accelerating voltage was 4 V. Product ion 385.0 Da was used for quantitation. Source parameters were as follows: gas temperature 350 °C; gas fellow 12 l/min; nebulizer gas pressure 35 psi; sheath gas temperature 400 °C; sheath gas fellow 12 l/min; capillary voltage 4500 V; and nozzle voltage 500 V. Agilent MassHunter Quantitative Analysis 10.2 software was used to determine the concentration of UDP-GlcNAc on samples based on the standard curve generated by analysis of different concentration of UDP-GlcNAc (Sigma-Aldrich) on same LC-MS condition.
SOD activity in B. subtilis lysates
Overnight cultures of B. subtilis strains in 10 ml of PAB medium (Difco Antibiotic Medium 3) with 20 mM MgSO4 (Mg2+) were washed twice with PAB, and diluted (1:1000) into PAB with 20 mM Mg2+ or 10 μg/ml MC. The cultures were incubated at 37 °C to OD600nm= 0.6. The cells were harvested by centrifugation and washed with 20 mM HEPES buffer (pH 7.4), followed by a wash with 20 mM EDTA to remove surface-associated metals, then finally washed twice in PBS to remove trace EDTA.
Soluble cell extracts were prepared for SOD activity analysis by suspending cell pellets in 100 μl lysis buffer (20 mM Tris, pH 6.8, 0.1 mg/ml lysozyme, 10 μg/ml DNase, complete EDTA-free protease inhibitor tablet (Roche)), followed by incubation on ice for 1 h and subsequent sonication for 10 s at 4 °C. Soluble extracts were separated by centrifugation and total protein concentration was determined by Bradford assay. SOD activity was assessed quantitatively using an adjusted riboflavin-nitroblue tetrazolium (NBT) liquid assay method83. The assay was performed in 96-well plates where 20 μl of the soluble extract was mixed with 180 μl assay solution (10 mM methionine, 1.4 μM riboflavin, 66 μM NBT, and 10 μM EDTA in 50 mM potassium phosphate buffer, pH 7.8), immediately prior to 20 min incubation on a white light box followed by immediate measurement of absorbance at 560 nm using an Agilent BioTek ELx800 plate reader. Serial 2-fold dilutions of tested samples were assayed to identify sample concentrations within the linear range of the assay close to 1 U relative to a standard curve obtained using commercial Bovine SOD standard (S5639, Sigma). The fit of the linear range of the inhibition curve was generated using GraphPadPrism software (version 9.3.1) using a standard linear regression model. To calculate specific SOD activities, the assay was performed in triplicate using samples diluted to the concentrations within the assay’s linear range. Specific activity was expressed in terms of enzyme units per mg total protein in the soluble extracts (U.mg−1).
Detection of ROS
B. subtilis strains were cultured in NB at 37 °C. To detect ROS (superoxide and hydroxyl radical), 1 ml of the cultures were incubated with 5 μM CellROX Green (Invitrogen) for 30 min at 37 °. The cells were harvested by centrifugation and washed three times with fresh NB before being used for microscopic analysis. CellROX Green is a proprietary oxidation-sensitive dye whose fluorescence quantum yield at 500–550 nm after excitation at 488 nm increases dramatically on oxidation in the presence of dsDNA66.
Detection of lipid peroxidation
Lipid peroxidation was detected using a fluorescent probe (C11-BODIPY581/591; Molecular Probes) as described previously24. B. subtilis strains were cultured in NB at 37 °C. To detect Lipid peroxidation, 1 ml of the cultures were incubated with 5 μM C11-BODIPY581/591 for 60 min at 37°. The cells were harvested by centrifugation and washed three times with fresh NB before being used for microscopic analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Richard Daniel for the use of the B. subtilis strains, and the University of Sydney Mass Spectrometry Facility for access to instruments. This work was funded by grants from a European Research Council Advanced award (670980) to JE, a Wellcome Investigator Award (209500) to JE, an ARC Laureate Fellowship (FL210100071) to JE, and a BBSRC DTP studentship to KJW.
Source data
Author contributions
Y.K. and J.E. designed concepts. Y.K. designed and performed most experiments. M.K. contributed for constructions of B. subtilis strains. E.S.M. and B.K. performed the SOD assay. Y.D. purified Mirubactin A and performed UDP-GlcNAc measurements. Y.K., K.J.W. and J.E. analysed data with contributions from all other authors. Y.K. prepared figures, wrote the original draft of the manuscript with contributions from all other authors. K.J.W. and J.E. reviewed the manuscript and edited. The project was managed by J.E.
Peer review
Peer review information
Nature Communications thanks Yunrong Chai and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
Source data are provided with this paper. All data that generated in this study are provided in the Supplementary Information and Source Data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yoshikazu Kawai, Email: yoshikazu.kawai@sydney.edu.au.
Jeff Errington, Email: jeffery.errington@sydney.edu.au.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-39723-8.
References
- 1.Höltje JV. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol. Biol. Rev. 1998;62:181–203. doi: 10.1128/MMBR.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan AJF, Errington J, Vollmer W. Regulation of peptidoglycan synthesis and remodelling. Nat. Rev. Microbiol. 2020;18:446–460. doi: 10.1038/s41579-020-0366-3. [DOI] [PubMed] [Google Scholar]
- 3.Meeske AJ, et al. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature. 2016;537:634–638. doi: 10.1038/nature19331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emami K, et al. RodA as the missing glycosyltransferase in Bacillus subtilis and antibiotic discovery for the peptidoglycan polymerase pathway. Nat. Microbiol. 2017;2:16253. doi: 10.1038/nmicrobiol.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjodt M, et al. Structural coordination of polymerization and crosslinking by a SEDS-bPBP peptidoglycan synthase complex. Nat. Microbiol. 2020;5:813–820. doi: 10.1038/s41564-020-0687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi A, et al. FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol. 2019;4:587–594. doi: 10.1038/s41564-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichmann NT, et al. SEDS-bPBP pairs direct lateral and septal peptidoglycan synthesis in Staphylococcus aureus. Nat. Microbiol. 2019;4:1368–1377. doi: 10.1038/s41564-019-0437-2. [DOI] [PubMed] [Google Scholar]
- 8.Garner, E. C. Toward a Mechanistic Understanding of Bacterial Rod Shape Formation and Regulation. Annu Rev Cell Dev Biol10.1146/annurev-cellbio-010521-010834 (2021). [DOI] [PubMed]
- 9.Jones LJ, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. doi: 10.1016/S0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 10.Kawai Y, Asai K, Errington J. Partial functional redundancy of MreB isoforms, MreB, Mbl and MreBH, in cell morphogenesis of Bacillus subtilis. Mol. Microbiol. 2009;73:719–731. doi: 10.1111/j.1365-2958.2009.06805.x. [DOI] [PubMed] [Google Scholar]
- 11.Dion MF, et al. Bacillus subtilis cell diameter is determined by the opposing actions of two distinct cell wall synthetic systems. Nat. Microbiol. 2019;4:1294–1305. doi: 10.1038/s41564-019-0439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherson DC, Popham DL. Peptidoglycan synthesis in the absence of class A penicillin-binding proteins in Bacillus subtilis. J. Bacteriol. 2003;185:1423–1431. doi: 10.1128/JB.185.4.1423-1431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovering AL, Safadi SS, Strynadka NC. Structural perspective of peptidoglycan biosynthesis and assembly. Annu Rev. Biochem. 2012;81:451–478. doi: 10.1146/annurev-biochem-061809-112742. [DOI] [PubMed] [Google Scholar]
- 14.Iwai N, Nagai K, Wachi M. Novel S-benzylisothiourea compound that induces spherical cells in Escherichia coli probably by acting on a rod-shape-determining protein(s) other than penicillin-binding protein 2. Biosci. Biotechnol. Biochem. 2002;66:2658–2662. doi: 10.1271/bbb.66.2658. [DOI] [PubMed] [Google Scholar]
- 15.Gitai Z, Dye NA, Reisenauer A, Wachi M, Shapiro L. MreB actin-mediated segregation of a specific region of a bacterial chromosome. Cell. 2005;120:329–341. doi: 10.1016/j.cell.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Schirner K, Errington J. The cell wall regulator {sigma}I specifically suppresses the lethal phenotype of mbl mutants in Bacillus subtilis. J. Bacteriol. 2009;191:1404–1413. doi: 10.1128/JB.01497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai Y, Daniel RA, Errington J. Regulation of cell wall morphogenesis in Bacillus subtilis by recruitment of PBP1 to the MreB helix. Mol. Microbiol. 2009;71:1131–1144. doi: 10.1111/j.1365-2958.2009.06601.x. [DOI] [PubMed] [Google Scholar]
- 18.Formstone A, Errington J. A magnesium-dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis. Mol. Microbiol. 2005;55:1646–1657. doi: 10.1111/j.1365-2958.2005.04506.x. [DOI] [PubMed] [Google Scholar]
- 19.Leaver M, Errington J. Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis. Mol. Microbiol. 2005;57:1196–1209. doi: 10.1111/j.1365-2958.2005.04736.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawai Y, et al. A widespread family of bacterial cell wall assembly proteins. EMBO J. 2011;30:4931–4941. doi: 10.1038/emboj.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leaver M, Dominguez-Cuevas P, Coxhead JM, Daniel RA, Errington J. Life without a wall or division machine in Bacillus subtilis. Nature. 2009;457:849–853. doi: 10.1038/nature07742. [DOI] [PubMed] [Google Scholar]
- 22.Kawai Y, Mickiewicz K, Errington J. Lysozyme Counteracts beta-Lactam Antibiotics by Promoting the Emergence of L-Form Bacteria. Cell. 2018;172:1038–1049.e1010. doi: 10.1016/j.cell.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claessen D, Errington J. Cell Wall Deficiency as a Coping Strategy for Stress. Trends Microbiol. 2019;27:1025–1033. doi: 10.1016/j.tim.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Kawai Y, et al. Cell growth of wall-free L-form bacteria is limited by oxidative damage. Curr. Biol. 2015;25:1613–1618. doi: 10.1016/j.cub.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai, Y. et al. Crucial role for central carbon metabolism in the bacterial L-form switch and killing by β-lactam antibiotics. Nat Microbiol, 10.1038/s41564-019-0497-3 (2019). [DOI] [PMC free article] [PubMed]
- 26.Klieneberger E. The natural occurrence of pleuropneumonia-like organisms in apparent symbiosis with Streptobacillus moniliformis and other bacteria. J. Pathol. Bacteriol. 1935;40:93–105. doi: 10.1002/path.1700400108. [DOI] [Google Scholar]
- 27.Errington, J., Mickiewicz, K., Kawai, Y. & Wu, L. J. L-form bacteria, chronic diseases and the origins of life. Philos. Trans. R Soc. Lond. B Biol. Sci. 371, 10.1098/rstb.2015.0494 (2016). [DOI] [PMC free article] [PubMed]
- 28.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 29.Dwyer DJ, Collins JJ, Walker GC. Unraveling the physiological complexities of antibiotic lethality. Annu Rev. Pharm. Toxicol. 2015;55:313–332. doi: 10.1146/annurev-pharmtox-010814-124712. [DOI] [PubMed] [Google Scholar]
- 30.Belenky P, et al. Bactericidal Antibiotics Induce Toxic Metabolic Perturbations that Lead to Cellular Damage. Cell Rep. 2015;13:968–980. doi: 10.1016/j.celrep.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell. 2014;159:1300–1311. doi: 10.1016/j.cell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobritz MA, et al. Increased energy demand from anabolic-catabolic processes drives beta-lactam antibiotic lethality. Cell Chem. Biol. 2022;29:276–286.e274. doi: 10.1016/j.chembiol.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shetty A, Dick T. Mycobacterial Cell Wall Synthesis Inhibitors Cause Lethal ATP Burst. Front Microbiol. 2018;9:1898. doi: 10.3389/fmicb.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeom J, Imlay JA, Park W. Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species. J. Biol. Chem. 2010;285:22689–22695. doi: 10.1074/jbc.M110.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leger L, et al. beta-Lactam Exposure Triggers Reactive Oxygen Species Formation in Enterococcus faecalis via the Respiratory Chain Component DMK. Cell Rep. 2019;29:2184–2191.e2183. doi: 10.1016/j.celrep.2019.10.080. [DOI] [PubMed] [Google Scholar]
- 36.Shin JH, et al. A multifaceted cellular damage repair and prevention pathway promotes high-level tolerance to beta-lactam antibiotics. EMBO Rep. 2021;22:e51790. doi: 10.15252/embr.202051790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber TW, Brinkley AW. Growth of cell wall-defective variants of Escherichia coli: comparison of aerobic and anaerobic induction frequencies. J. Clin. Microbiol. 1977;6:166–171. doi: 10.1128/jcm.6.2.166-171.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chikada T, et al. Direct Observation of Conversion From Walled Cells to Wall-Deficient L-Form and Vice Versa in Escherichia coli Indicates the Essentiality of the Outer Membrane for Proliferation of L-Form Cells. Front Microbiol. 2021;12:645965. doi: 10.3389/fmicb.2021.645965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts, D. M., Errington, J. & Kawai, Y. Characterization of the L-form switch in the Gram-negative pathogen Streptobacillus moniliformis. FEMS Microbiol Lett. 368, 10.1093/femsle/fnab156 (2021). [DOI] [PMC free article] [PubMed]
- 40.Kepplinger, B. et al. Mirubactin C rescues the lethal effect of cell wall biosynthesis mutations in Bacillus subtilis. Front Microbiol., 10.3389/fmicb.2022.1004737 (2022). [DOI] [PMC free article] [PubMed]
- 41.Giessen TW, et al. Isolation, structure elucidation, and biosynthesis of an unusual hydroxamic acid ester-containing siderophore from Actinosynnema mirum. J. Nat. Prod. 2012;75:905–914. doi: 10.1021/np300046k. [DOI] [PubMed] [Google Scholar]
- 42.Pu H, et al. Degradation of mirubactin to multiple siderophores with varying Fe(III) chelation properties. Org. Biomol. Chem. 2022;20:5066–5070. doi: 10.1039/D2OB00942K. [DOI] [PubMed] [Google Scholar]
- 43.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol. Biol. Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD. Role of the Fur regulon in iron transport in Bacillus subtilis. J. Bacteriol. 2006;188:3664–3673. doi: 10.1128/JB.188.10.3664-3673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young FE, Spizizen J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J. Bacteriol. 1961;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quadri LE, et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry. 1998;37:1585–1595. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 48.Theodore TS, Schade AL. Growth of Staphylococcus aureus in Media of Restricted and Unrestricted Inorganic Iron Availability. J. Gen. Microbiol. 1965;39:75–83. doi: 10.1099/00221287-39-1-75. [DOI] [PubMed] [Google Scholar]
- 49.Kishimoto S, Nishimura S, Hatano M, Igarashi M, Kakeya H. Total synthesis and antimicrobial activity of chlorocatechelin A. J. Org. Chem. 2015;80:6076–6082. doi: 10.1021/acs.joc.5b00532. [DOI] [PubMed] [Google Scholar]
- 50.Zawadzka AM, et al. Characterization of a Bacillus subtilis transporter for petrobactin, an anthrax stealth siderophore. Proc. Natl Acad. Sci. USA. 2009;106:21854–21859. doi: 10.1073/pnas.0904793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.May JJ, Wendrich TM, Marahiel MA. The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J. Biol. Chem. 2001;276:7209–7217. doi: 10.1074/jbc.M009140200. [DOI] [PubMed] [Google Scholar]
- 52.Reuter, M. et al. Mechanosensitive channels and bacterial cell wall integrity: does life end with a bang or a whimper? J R Soc Interface. 11, 10.1098/rsif.2013.0850 (2014). [DOI] [PMC free article] [PubMed]
- 53.Pospíšil, J. et al. Bacterial nanotubes as a manifestation of cell death. Nat Commun. 11, 10.1038/s41467-020-18800-2 (2020). [DOI] [PMC free article] [PubMed]
- 54.Olsen LR, Vetting MW, Roderick SL. Structure of the E. coli bifunctional GlmU acetyltransferase active site with substrates and products. Protein Sci. 2007;16:1230–1235. doi: 10.1110/ps.072779707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silver, L. L. Fosfomycin: Mechanism and Resistance. Cold Spring Harb Perspect Med. 7, 10.1101/cshperspect.a025262 (2017). [DOI] [PMC free article] [PubMed]
- 56.Wang X, et al. Towards new antibiotics targeting bacterial transglycosylase: Synthesis of a Lipid II analog as stable transition-state mimic inhibitor. Bioorg. Med Chem. Lett. 2018;28:2708–2712. doi: 10.1016/j.bmcl.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson PY, Fedor MJ. The glmS riboswitch integrates signals from activating and inhibitory metabolites in vivo. Nat. Struct. Mol. Biol. 2011;18:359–363. doi: 10.1038/nsmb.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel V, Wu Q, Chandrangsu P, Helmann JD. A metabolic checkpoint protein GlmR is important for diverting carbon into peptidoglycan biosynthesis in Bacillus subtilis. PLoS Genet. 2018;14:e1007689. doi: 10.1371/journal.pgen.1007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foulquier E, Pompeo F, Byrne D, Fierobe HP, Galinier A. Uridine diphosphate N-acetylglucosamine orchestrates the interaction of GlmR with either YvcJ or GlmS in Bacillus subtilis. Sci. Rep. 2020;10:15938. doi: 10.1038/s41598-020-72854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galinier A, Foulquier E, Pompeo F. Metabolic Control of Cell Elongation and Cell Division in Bacillus subtilis. Front Microbiol. 2021;12:697930. doi: 10.3389/fmicb.2021.697930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sachla AJ, Helmann JD. Resource sharing between central metabolism and cell envelope synthesis. Curr. Opin. Microbiol. 2021;60:34–43. doi: 10.1016/j.mib.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White MR, Garcin ED. D-Glyceraldehyde-3-Phosphate Dehydrogenase Structure and Function. Subcell. Biochem. 2017;83:413–453. doi: 10.1007/978-3-319-46503-6_15. [DOI] [PubMed] [Google Scholar]
- 63.Boersch M, Rudrawar S, Grant G, Zunk M. Menaquinone biosynthesis inhibition: a review of advancements toward a new antibiotic mechanism. RSC Adv. 2018;8:5099–5105. doi: 10.1039/C7RA12950E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hederstedt L. Succinate:quinone oxidoreductase in the bacteria Paracoccus denitrificans and Bacillus subtilis. Biochim Biophys. Acta. 2002;1553:74–83. doi: 10.1016/S0005-2728(01)00231-6. [DOI] [PubMed] [Google Scholar]
- 65.Larosa, V. & Remacle, C. Insights into the respiratory chain and oxidative stress. Biosci Rep. 38, 10.1042/BSR20171492 (2018). [DOI] [PMC free article] [PubMed]
- 66.Choi H, Yang Z, Weisshaar JC. Single-cell, real-time detection of oxidative stress induced in Escherichia coli by the antimicrobial peptide CM15. Proc. Natl Acad. Sci. USA. 2015;112:303–CM310. doi: 10.1073/pnas.1417703112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imlay JA. Pathways of oxidative damage. Ann. Rev. Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 68.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 69.Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheng Y, et al. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl Acad. Sci. USA. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradley JM, et al. Bacterial iron detoxification at the molecular level. J. Biol. Chem. 2020;295:17602–17623. doi: 10.1074/jbc.REV120.007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krawczyk-Balska A, Marchlewicz J, Dudek D, Wasiak K, Samluk A. Identification of a ferritin-like protein of Listeria monocytogenes as a mediator of beta-lactam tolerance and innate resistance to cephalosporins. BMC Microbiol. 2012;12:278. doi: 10.1186/1471-2180-12-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mir M, et al. Mycobacterial gene cuvA is required for optimal nutrient utilization and virulence. Infect. Immun. 2014;82:4104–4117. doi: 10.1128/IAI.02207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pensinger DA, et al. The Listeria monocytogenes PASTA Kinase PrkA and Its Substrate YvcK Are Required for Cell Wall Homeostasis, Metabolism, and Virulence. PLoS Pathog. 2016;12:e1006001. doi: 10.1371/journal.ppat.1006001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwarz U, Asmus A, Frank H. Autolytic enzymes and cell division of Escherichia coli. J. Mol. Biol. 1969;41:419–429. doi: 10.1016/0022-2836(69)90285-X. [DOI] [PubMed] [Google Scholar]
- 77.Klainer AS, Perkins RL. Antibiotic-induced alterations in the surface morphology of bacterial cells: a scanning-beam electron miscroscopy study. J. Infect. Dis. 1970;122:323–328. doi: 10.1093/infdis/122.4.323. [DOI] [PubMed] [Google Scholar]
- 78.Rogers HJ, Taylor C. Autolysins and shape change in rodA mutants of Bacillus subtilis. J. Bacteriol. 1978;135:1032–1042. doi: 10.1128/jb.135.3.1032-1042.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Botta GA, Buffa D. Murein synthesis and beta-lactam antibiotic susceptibility during rod-to-sphere transition in a pbpA(Ts) mutant of Escherichia coli. Antimicrob. Agents Chemother. 1981;19:891–900. doi: 10.1128/AAC.19.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams DW, Wu LJ, Czaplewski LG, Errington J. Multiple effects of benzamide antibiotics on FtsZ function. Mol. Microbiol. 2011;80:68–84. doi: 10.1111/j.1365-2958.2011.07559.x. [DOI] [PubMed] [Google Scholar]
- 81.Vagner V, Dervyn E, Ehrlich SD. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- 82.Lewis PJ, Marston AL. GFP vectors for controlled expression and dual labelling of protein fusions in Bacillus subtilis. Gene. 1999;227:101–110. doi: 10.1016/S0378-1119(98)00580-0. [DOI] [PubMed] [Google Scholar]
- 83.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are provided with this paper. All data that generated in this study are provided in the Supplementary Information and Source Data file. Source data are provided with this paper.