Abstract
Objective(s):
Tracheostomy associated granulation tissue is a common, recurrent problem occurring secondary to chronic mucosal irritation. While granulation tissue is composed of predominantly innate immune cells, the phenotype of monocytes and macrophages in tracheostomy associated granulation tissue is unknown. This study aims to define the myeloid cell population in granulation tissue secondary to tracheostomy.
Methods:
Granulation tissue biopsies were obtained from 8 patients with tracheostomy secondary to laryngotracheal stenosis. Cell type analysis was performed by flow cytometry and gene expression was measured by quantitative real time PCR. These methods and immunohistochemistry were used to define the monocyte/macrophage population in granulation tissue and were compared to tracheal autopsy specimens.
Results:
Flow cytometry demonstrated macrophages (CD45+CD11b+) and monocytes (CD45+FSClowSSClow) represent 23.2±6% of the granulation tissue cell population. The M2 phenotype (CD206) is present in 77±11% of the macrophage population and increased compared to the M1 phenotype (p=0.012). Classical monocytes (CD45+ CD14highCD16low) were increased in granulation tissue compared to controls (61.2±7% and 30±8.5%, p=0.038). Eighty-five percent of macrophages expressed pro-inflammatory S100A8/A9 and 36±4% of macrophages co-localized CD169, associated with tissue-resident macrophages. M2 gene expression (Arg1/CD206) was increased in granulation tissue (3.7±0.4, p=0.035 and 3.5±0.5, p=0.047) while M1 gene expression (CD80/CD86) was similar to controls (p=0.64, p=0.3). Immunohistochemistry of granulation tissue demonstrated increased cells co-localizing CD11b and CD206.
Conclusions:
M2 macrophages are the dominant macrophage phenotype in tracheostomy associated granulation tissue. The role of this cell type in promoting ongoing inflammation warrants future investigation to identify potential treatments for granulation tissue secondary to tracheostomy.
Keywords: granulation tissue, macrophage polarization, monocytes, granuloma, laryngotracheal stenosis, M2 macrophage, tracheostomy
Lay Summary
In patients with tracheostomy, granulation tissue is a common, recurrent problem. We demonstrate that alternatively activated M2 macrophages are increased in tracheostomy associated granulation tissue, highlighting these inflammatory cells as therapeutic targets.
INTRODUCTION
Tracheostomy associated granulation tissue is a common and recurrent problem that occurs in tracheostomy dependent patients, attributed to chronic mucosal irritation secondary to the prosthesis. Tracheostomy associated granulation tissue occurs in 4% to 80% of all pediatric tracheostomies1 and can also develop in adult patients requiring tracheostomy for chronic ventilator dependence or laryngotracheal stenosis (LTS).2,3 Acutely, tracheostomy associated granulation tissue can lead to bleeding, upper airway obstruction, respiratory failure after decannulation, failure to wean from the ventilator, and failure to decannulate.4 In the long term, granulation tissue at the site of the tracheostoma promotes circumferential wound contracture and can lead to fibrosis and tracheal stenosis that ultimately requires intervention.4,5
Granulation tissue formation is part of the repair phase of wound healing, providing a scaffold for cell migration. Within granulation tissue, macrophages help to remove debris and release inflammatory cytokines that promote wound healing. However, in patients with tracheostomy and other airway prosthesis, persistent inflammation and constant movement of the prosthesis against the tracheal mucosa leads to granulation tissue formation.6 Additionally, tracheostomy tubes provide a potential surface for bacteria growth and biofilm formation, exacerbating the local inflammatory response and promoting immune cell recruitment.6,7 Granulation tissue is composed of a localized aggregate of host immune cells around a pathogen or site of persistent irritation. Histologically, granulation tissue displays an increased population of monocytes and macrophages recruited to the site of injury and/or inflammation.8
Current treatment strategies for tracheostomy associated granulation tissue rely on serial drug application and surgical debridement or excision in the operating room. Recurrence is common, requiring frequent surveillance, highlighting the poor understanding of disease pathogenesis and the need for targeted therapies.9–11 Prior studies have demonstrated that airway granulation tissue can develop in response to foreign bodies (nonabsorbable sutures, Teflon stents) and polymicrobial infections,12–14 however the molecular mechanism promoting granulation tissue and recurrence in patients with tracheostomy still remains largely unknown.
Granulation tissue has been extensively studied in the context of inflammatory diseases such as rheumatoid arthritis,15 tuberculosis,16 and sarcoidosis,17 but less is known about granulation tissue that occurs in patients with a tracheostomy. In sarcoidosis, monocytes and macrophages play a key role in granuloma formation. Monocytes and macrophages are innate immune cells observed in granulation tissue.20 Monocytes can be divided into 3 subsets: classical, non-classical, and intermediate, based on the differential expression of CD14 and CD16, with classical monocytes being the predecessors to monocyte-derived macrophages. Macrophages also exhibit distinct phenotypes dependent on local immune mediators.21 The most basic paradigm of macrophage phenotype includes M1 proinflammatory (classically activated) and M2 anti-inflammatory (alternatively activated) macrophages. These subpopulations arise in tissue environments due to polarization from surrounding cytokines, growth factors, and external molecular stimuli.20 There is evidence that indicates a shift towards M2 macrophage subsets in granulomas specimens.18 In granulomas related to mycobacterial infection, M1 macrophages are prevalent during early stages of the disease, while M2 macrophages predominate in the chronic stage of disease.19 The nature and role of the macrophage population in tracheostomy associated granulation tissue has not been delineated. The objective of this study is to define the macrophage population present in tracheostomy associated granulation tissue biopsies using qPCR, flow cytometry, and immunofluorescence. It was hypothesized that M2 macrophages are the predominant macrophage subtype in granulation tissue, along with increased monocyte infiltration and inflammatory cytokine expression.
METHODS
Patient Cohort
Tissue biopsies (approximately 2mm) from patients with non-infectious tracheostomy associated granulation tissue (n=12) and iLTS scar (n=5) were obtained during endoscopic dilation procedures following informed consent. Normal controls (n=3) were obtained from deidentified normal human tracheal autopsy specimens procured within 24 hours from subjects that did not have a history of intubation or tracheostomy. Tissue was procured from proximal tracheal mucosa, where a tracheostomy would typically be placed. All tissue samples were obtained in accordance with Johns Hopkins University Institutional Review Board (IRB00250531).
Gene Expression Measurement using qPCR
Human tissue biopsies from patients with tracheostomy associated granulation tissue (n=8), iLTS scar (n=5), and autopsy controls (n=3) were mechanically homogenized using metallic microbeads in RLT lysis buffer (1% ß-Mercaptoethanol). RNA was extracted and purified using RNeasy Mini Spin Column as specified in the RNeasy Mini-kit (Qiagen, Valencia, California) following the manufacturer’s protocol. cDNA was synthesized using Superscript III Reverse Transcriptase (Life Technologies, Thermo Fisher Scientific) following the manufacturer’s protocol. RNA was quantified with a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, Massachusetts) converted into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) (Supplemental material). Gene primers (Integrated DNA technologies, Coralville, Iowa) used included macrophage M1 markers (CD80 and CD86), macrophage M2 markers (Arginase 1 (ARG1) and MRC1 (also known as CD206)), and inflammatory cytokines: interleukin 1-beta (IL1B), interleukin 4 (IL4), interleukin 6 (IL6), tumor necrosis factor alpha (TNF), transforming growth factor beta (TGFB1) (Supplementary Table 1). Quantitative real-time PCR (qRT-PCR) was carried out in triplicate using SYBR Green primers and a StepOnePlus Real-time PCR System (Life Technologies, Thermo Fisher Scientific). Relative gene expression was calculated by the ΔΔCt method. Full details of gene expression analysis are detailed in Supplemental Material.
Flow Cytometry Characterization of Immune Cells in Dissociated Granulation Samples
Fresh biopsies from patients with tracheostomy associated granulation tissue (n=4) were collected during routine endoscopic procedures, along with normal controls (n=3), and immediately processed into single-cell suspensions. Biopsies were mechanically minced into 1–2 mm portions and subsequently digested with an enzyme solution [0.4 mg/ml DNA-ase (Sigma, St. Louis, MO), 1mg/ml Liberase (Sigma), RPMI with 10% FBS) for 20 minutes at 37 °C. Single cell suspensions were cryopreserved at −180°C, and later thawed for flow cytometry analysis, as previously described.22 Single cells were split into 106 cells/well (V-bottom 96-well plate) and stained for 30 minutes at 4°C with primary monoclonal antibodies (mAbs): CD3, CD45, CD15, CD14, CD16, CD11B, CD80, CD206, CD169, S100A8, and Siglec8 (Table 1). Figure 1 illustrates the gating scheme used to classify immune cells. The LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit was used to exclude dead cells. The samples were then subjected to fixation and permeabilization required for intracellular antibody staining. Detailed flow cytometry protocol is included as supplemental material. Macrophages were defined as CD45+CD11b+ positive cells. Further characterization of the macrophage population was performed using CD169 (tissue resident macrophage (TRM) marker), CD14 (infiltrating macrophage marker), and S100A8/A9 (pro-inflammatory marker) staining. In addition, subpopulations of monocytes were defined using differential expression of CD14 and CD16, including the classical subtype (CD14highCD16low), intermediate subtype (CD14highCD16high), and non-classical subtype (CD14lowCD16high). Granulation tissue samples were compared with normal tracheal samples used as controls, to calculate differences in antigen expression. All analyses were performed using FlowJo Flow Cytometry Analysis Software (v10.8.1, 2019, Ashland, OR).
Table 1:
Macrophage Flow Cytometry Antibodies
| Antibody | Antibody Clone | Antibody Source | Fluorochrome | Marker Type |
|---|---|---|---|---|
| CD3 | OKT3 | Biolegend | BV 421 | Lymphocytes |
| CD15 | W6D3 | Biolegend | BV 421 | Neutrophils |
| Live/Death | N/A | Thermo Scientific | Aqua | Live Cells |
| CD86 | IT2.2 | Thermo Scientific | SB600 | M1 Macrophages |
| CD14 | M5E2 | Biolegend | BV 650 | Monocyte Subtypes |
| CD45 | HI30 | Thermo Scientific | Qdot800 | Leukocytes |
| CD68 | Y1/82A | Biolegend | FITC | Macrophages |
| Siglec 8 | 7C9 | Biolegend | PerCP-Cy5.5 | Eosinophils |
| S100A | NJ-4F3-D1 | Biolegend | PE | Pro-inflammatory Marker |
| CD169 | 7–239 | Biolegend | PE/CYN7 | Tissue-resident macrophages |
| CD206 | 15–2 | Biolegend | APC | M2 Macrophages |
| CD16 | B73.1 | Biolegend | AlexaFluor 700 | Monocyte Subtypes |
| CD11B | M1/70 | Thermo Scientific | APCefluor780 | Macrophage |
Figure 1: Flow cytometry gating scheme for monocytes and macrophages.
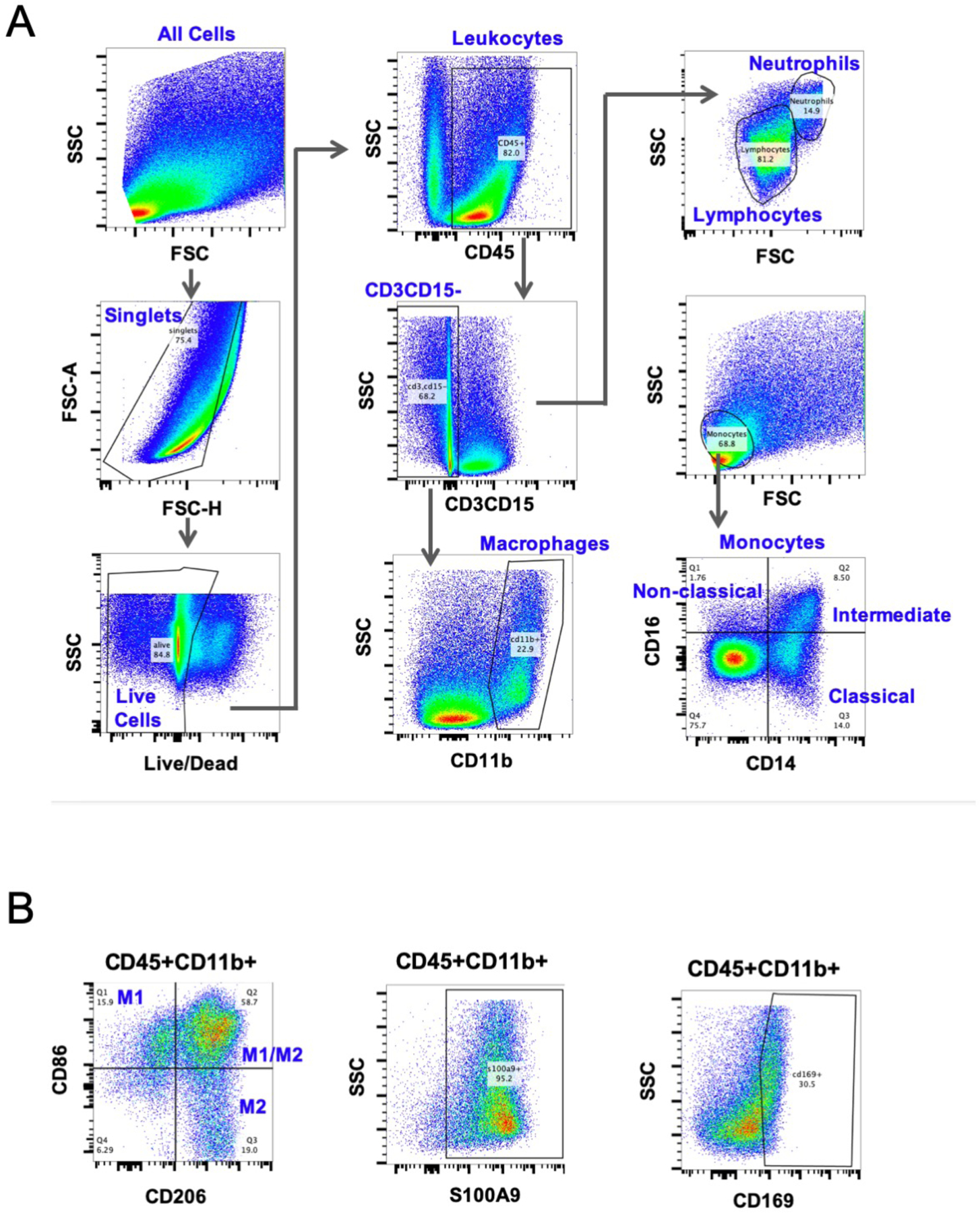
(A) CD45 was used to identify all hematopoietic cells, and macrophages were considered CD45 CD11b double positive. Other immune cells, including CD3+ T cells, CD15+ neutrophils, Siglec8+ eosinophils were excluded. After identifying monocytes (CD45+FSClowSSClow) using forward and side scatter, CD14 and CD16 expression was used to identify non-classical, classical, and intermediate subtypes. (B) Macrophages were further categorized into M1 or M2 using CD86 and CD206 respectively, as well as pro-inflammatory marker S100A9, and tissue resident marker CD169.
Immunofluorescence
Tissue sections from granulation tissue and normal tracheal autopsy controls were processed and stained with markers CD11b, CD86, and CD206 (Supplemental material). Briefly, all specimens were fixed in 10% formalin and embedded in paraffin. Slides were incubated overnight with both anti-CD11b (Rabbit anti-human, ab133357 Abcam) and either anti-CD86 (Mouse anti-human ab270719; Abcam) or anti-CD206 (Mouse anti-human ab117644; Abcam). After primary antibody incubation, slides were washed and then stained with a corresponding secondary antibody for 45 minutes. Finally, slides were mounted using Gold antifade DAPI (ThermoScientific, Fremont, CA). A set of 20 images of the entire tissue specimen were taken using Zeiss AxioObserver.Z2 inverted microscope (Zeiss, Oberkochen, Germany) at both 20x and 40x. Negative controls were used to establish background fluorescence and non-specific staining of the primary and secondary antibodies.
Statistical analysis
qPCR results were presented as a mean relative fold change (2-∆∆Ct) ± standard error of the mean (SEM). Flow cytometry results were presented as mean percent expressed ± standard deviation. Groups were compared using unpaired t-test to detect differences in gene expression and cell populations. Significance criterion for all analyses was set at p < 0.05. Data analysis and visualization was performed using Prism software (GraphPad Software Inc., CA, USA).
RESULTS
Experimental Cohort
Patient demographics are presented in Table 2 and were similar between groups. No significant differences were noted in age, sex, patient BMI, or comorbidities. Among the granulation tissue patients, the majority (92%) of patients were tracheostomy dependent secondary to iatrogenic laryngotracheal stenosis, while idiopathic subglottic stenosis was the cause of tracheostomy in 1 patient. All patients had a history of tracheostomy for greater than 6 months, with mean duration of 17.3 months and ranging from 9.8–21.2 months.
Table 2:
Patient Characteristics
| Granulation Tissue Biopsies (qPCR) | Granulation Tissue Dissociations (FC) | iLTS Biopsies (qPCR) | |
|---|---|---|---|
| Enrollment | n=8 | n=4 | n=5 |
| Mean age (SD) | 40.6 (10.2) | 40.3 (11.6) | 51 (10.2) |
| Sex, female (%) | 6 (75%) | 3 (75%) | 5 (100%) |
| BMI (SD) | 40.3 (11.2) | 37.4 (15.5) | 34.4 (8.6) |
| Race/Ethnicity (%) | |||
| Caucasian | 1 (12.5%) | 0 (0%) | 3 (60%) |
| African American | 5 (62.5%) | 4 (80%) | 1 (20%) |
| Asian | 1 (12.5%) | 1 (20%) | 1 (20%) |
| Other | 1 (12.5%) | 0 (0%) | 0 (0%) |
| History of tracheostomy (%) | 8 (100%) | 5 (100%) | 5 (100%) |
| Tracheostomy duration, months (SD) | 16 (12.7) | 21.2 (18.3) | 9.8 (13.8) |
| Intubation duration, days (SD) | 6.2 (2.7) | 5 (0) | 10 (6.1) |
| Charlson Comorbidity Index (SD) | 0.75 (1.4) | 0.75 (1.5) | 3 (2.2) |
| Comorbidities (%) | |||
| Hypertension | 1 (12.5%) | 1 (20%) | 4 (80%) |
| Diabetes | 1 (12.5%) | 1 (20%) | 2 (40%) |
| CAD | 0 (0%) | 1 (20%) | 1 (20%) |
| Months followed* (SD) | 16 (11.4) | 18 (14.6) | 9.8 (8.2) |
| Etiology (%) | |||
| Iatrogenic, post-intubation | 1 (12.5%) | 1 (20%) | 1 (20%) |
| Iatrogenic, post-tracheostomy | 0 (0%) | 1 (20%) | 1 (20%) |
| Iatrogenic, post-intubation and tracheostomy | 6 (75%) | 3 (60%) | 3 (60%) |
| Idiopathic | 1 (12.5%) | 0 (0%) | 0 (0%) |
SD=standard deviation
PCR=polymerase chain reaction
FC=flow cytometry
iLTS=iatrogenic laryngotracheal stenosis
months followed calculated from time of first clinic visit to procedure
Flow Cytometry Immune Cell Phenotype Characterization
Flow cytometry of granulation tissue demonstrated 48.5±13.7% of all live cells were leukocytes, defined as live cells staining positive for CD45. Monocytes (CD45+ CD14highCD16low, CD14lowCD16high, CD14highCD16high) represented 16±5% of the live cell population while macrophages (CD45+CD11B+) represent 7.3±2.8% of the live cell population. T-cells (CD3+) and neutrophils (CD15+) represent 4.5±2.3% and 10.5±3.1% of all live cells, respectively (Figure 2). A minority (12±4%) of neutrophils (CD15+) co-localized S100A8/A9, a pro-inflammatory marker. Eosinophils (Siglec 8+) were the least common cell type, representing 2±1% of the live cell population. Granuloma specimens had an increased combined macrophage/monocyte population compared to controls (23±.06% and 2±1%, p=0.03). There were no significant differences in total macrophage population compared to controls (7±2.8% and 1.6±1.0%, p=0.17), or total monocyte population compared to controls (16±.05% and 1±1%, p=0.07).
Figure 2: Immune cell profile in dissociated tracheal granulation tissue.
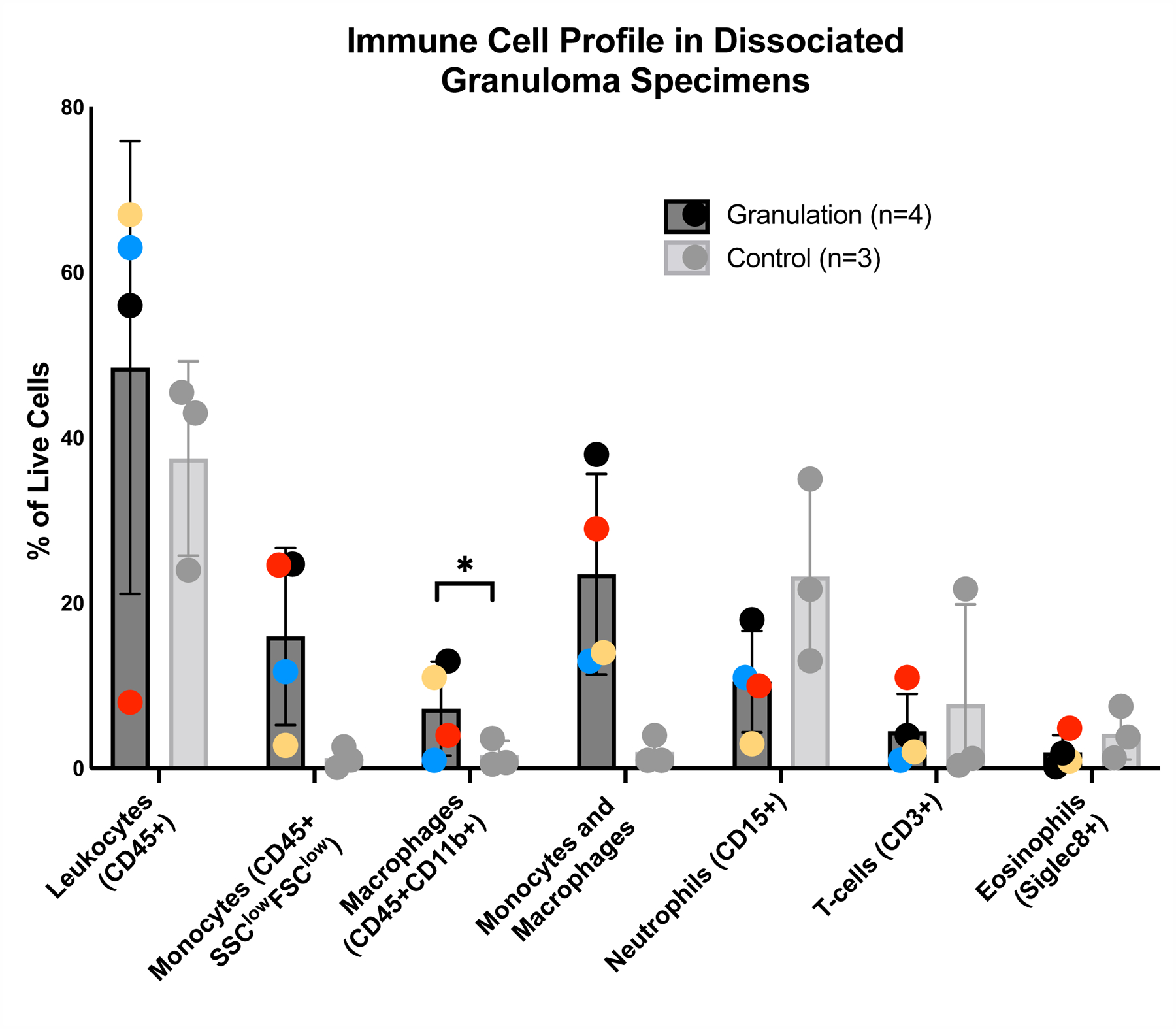
Dissociated granulation tissue and control samples were stained and analyzed using flow cytometry to identify leukocytes (CD45+), macrophages (CD45+CD11b+), monocytes (CD45+FSClowSCClow), including classical CD14highCD16low, intermediate CD14highCD16high, non-classical CD14lowCD16high) distinguished using forward and side scatter properties, T-cells (CD3), neutrophils (CD15), and eosinophils (Siglec 8). Error bars signify SEM.
Predominant Monocyte Populations in Granulation Tissue
Monocytes (CD45+FSClowSCClow), the precursors to monocyte-derived macrophages, are the most abundant cell type, making up 16±5% of the granulation tissue cell population. The relative CD14 and CD16 staining demonstrated that the monocyte population is comprised primarily of the classical subtype (CD14highCD16low) at 61.3±7.3%, followed by 27.6±11.6% intermediate subtype (CD14highCD16low), and 11.1±4.6% non-classical subtype (CD14lowCD16high). CD14highCD16low classical monocytes were increased in granulation tissue compared with normal controls (p=0.039), with no observed differences in CD14highCD16low intermediate (p=0.14) or CD14lowCD16high non-classical monocytes (p=0.75) (Figure 3A).
Figure 3: Monocyte and tissue resident macrophage subpopulations in granulation tissue.
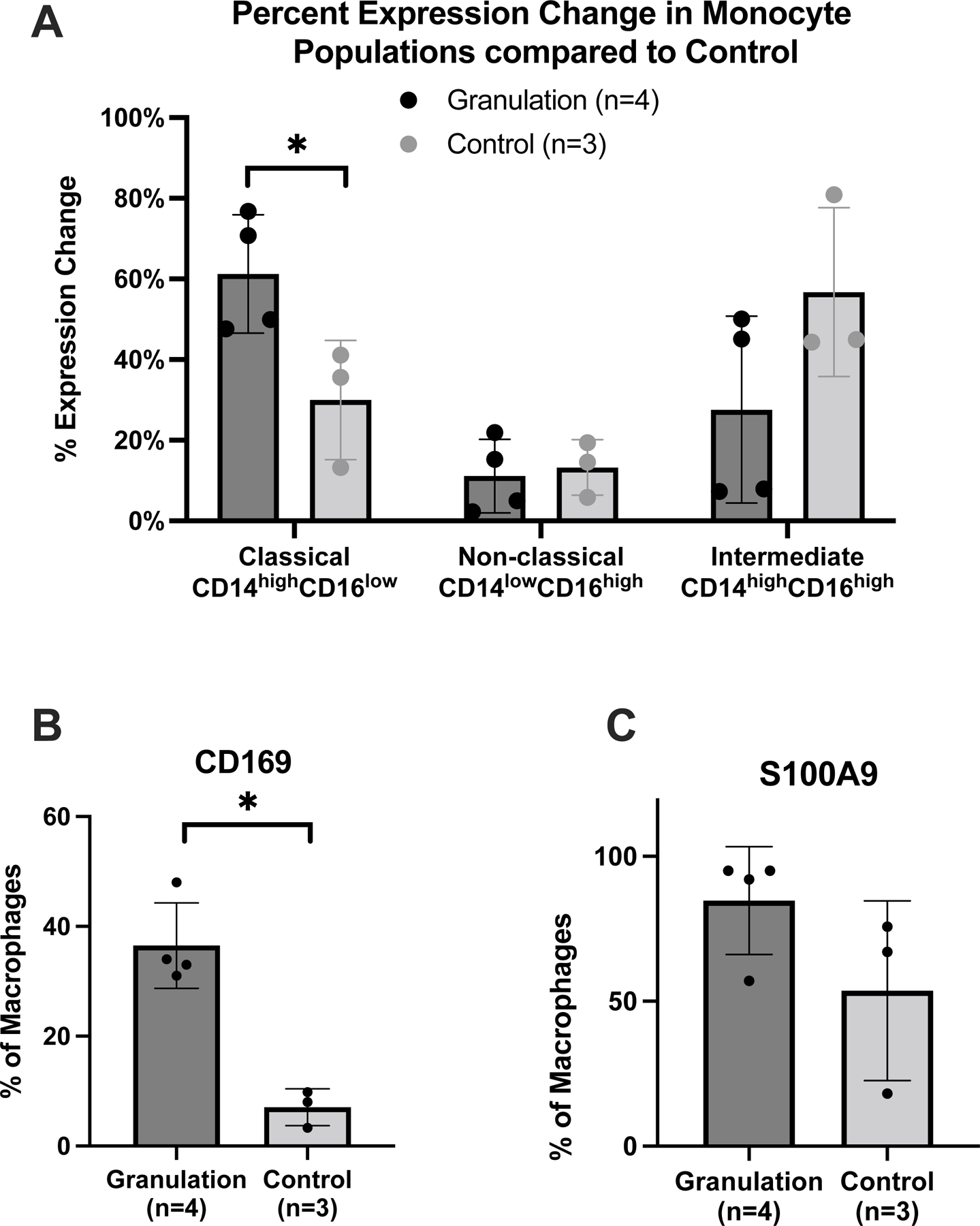
(A) Flow cytometry results designating intermediate, classical, and non-classical monocyte populations based on CD16 and CD14 antigen staining. (B) Tissue resident macrophages, defined by percentage of macrophages staining positive for CD169. (C) Percentage of macrophages staining positive for S100A8/A9. Values represent percent of monocytes or macrophages present in tissue population. Error bars signify standard deviation. (*) = p < 0.05.
n=number of samples
Predominant Macrophage Populations in Granulation Tissue
Analysis of the CD45+CD11B+ macrophage population in tracheostomy associated granulation tissue revealed that 36±4% of all CD45+CD11B+ macrophages co-localized with the tissue resident macrophage marker CD16923,24, compared to only 7±2% in control specimens (p=0.002) (Figure 3B). Additionally, 80±9% of the CD45+CD11B+ macrophages stained for the pro-inflammatory molecule S100A8/A9, compared to 54±18% in the normal controls (p=0.21) (Figure 3C). However, in the CD45+CD11B+CD169+ TRMs, S100A8/A9 was only expressed on 16±9% cells. There was no significant difference in CD14+ expression on CD45+CD11B+ macrophages in tracheostomy associated when compared to normal controls. Analysis of the CD45+CD11B+ macrophage population revealed that 77±11% were M2 macrophages co-localizing CD206. M1 macrophages which were CD86+ represented 23%±11% of the CD45+CD11B+ macrophage population. When the percentage of M1 macrophages was compared to M2 macrophages, there is a predominance of M2 macrophages (p=0.012) (Figure 4A). In normal controls, there was no difference in the percentage of M1 macrophages compared to the percentage of M2 macrophages (p=0.22). In addition, when the ratio of M1 macrophages to M2 macrophages in granulation tissue is compared to that of normal controls, there is no significant difference in expression (77±11% and 67±16%, p=0.603).
Figure 4: M2 macrophages are predominant in granulation tissue.
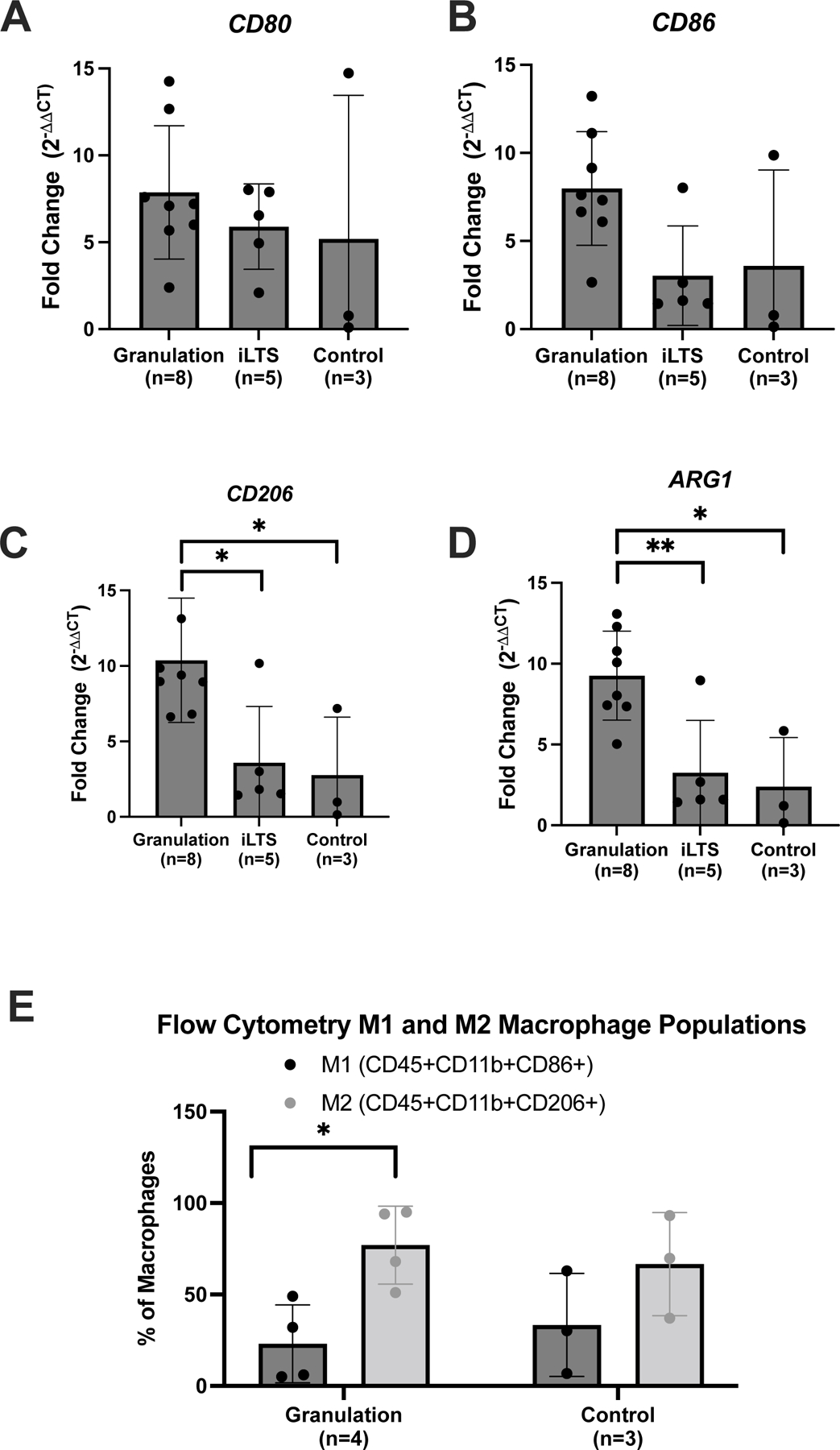
(A) Flow cytometry percentage of macrophages in M1 and M2 subpopulations, CD86 and CD206 (B and C) Mean gene expression of M1 (CD80 and CD86) and (D and E) M2 (CD206 and ARG1) macrophage biomarkers in granulation tissue and iLTS specimens compared to normal controls. (*) = p < 0.05; (**) = p < 0.01.
ARG1=Arginase 1
iLTS=iatrogenic laryngotracheal stenosis
Gene Expression of Immune Cell Biomarkers and Inflammatory Cytokines
M2 biomarker gene expression (Arg1 and CD206) was significantly increased in granulation tissue compared to controls (3.7±1.2, p=0.035 and 3.5±1.5, p=0.047). M1 biomarker gene expression (CD80 and CD86) was similar in granulation specimens and controls (p=0.64 and p=0.30). Gene expression of M1 markers (CD86) and M2 markers (Arg1 and CD206) of granulation tissue was increased compared to iLTS specimens (p=0.004, 0.01, 0.02, respectively) (Figure 4B and 4C). M1 or M2 biomarker gene expression in iLTS specimens were not significantly increased compared to controls. Finally, gene expression of inflammatory cytokines was not significantly elevated in tracheostomy associated granulation tissue specimens (Figure 5).
Figure 5: Inflammatory cytokine gene expression in granulation tissue.
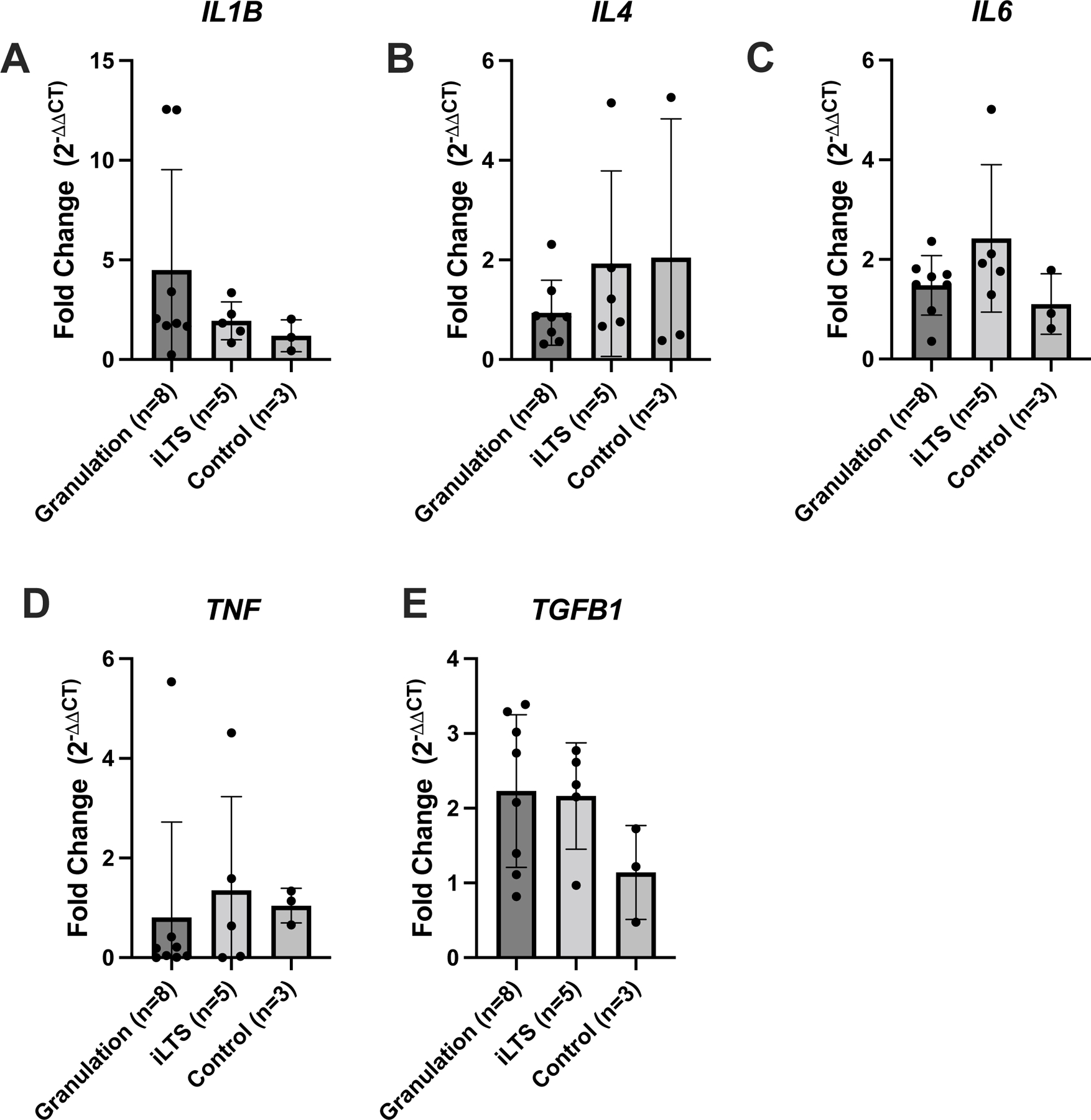
Gene expression analysis expressed in terms of relative fold change compared to normal control tissue for the genes (A) IL-1b, (B) IL-4, (C) IL-6, (D) TNF-alpha, and (E) TGF-beta. Error bars signify standard deviation.
IL=interleukin
n=number of samples
Immunohistochemistry
Tracheostomy associated granulation tissue specimens demonstrated increased macrophage infiltration compared to normal controls, as indicated by CD11b positive cells. CD86 and CD206 staining revealed a large population of macrophages with the M2 phenotype (CD206+) compared to the M1 (CD86+) phenotype (Figure 6).
Figure 6: Immunohistochemistry of macrophage phenotypes:
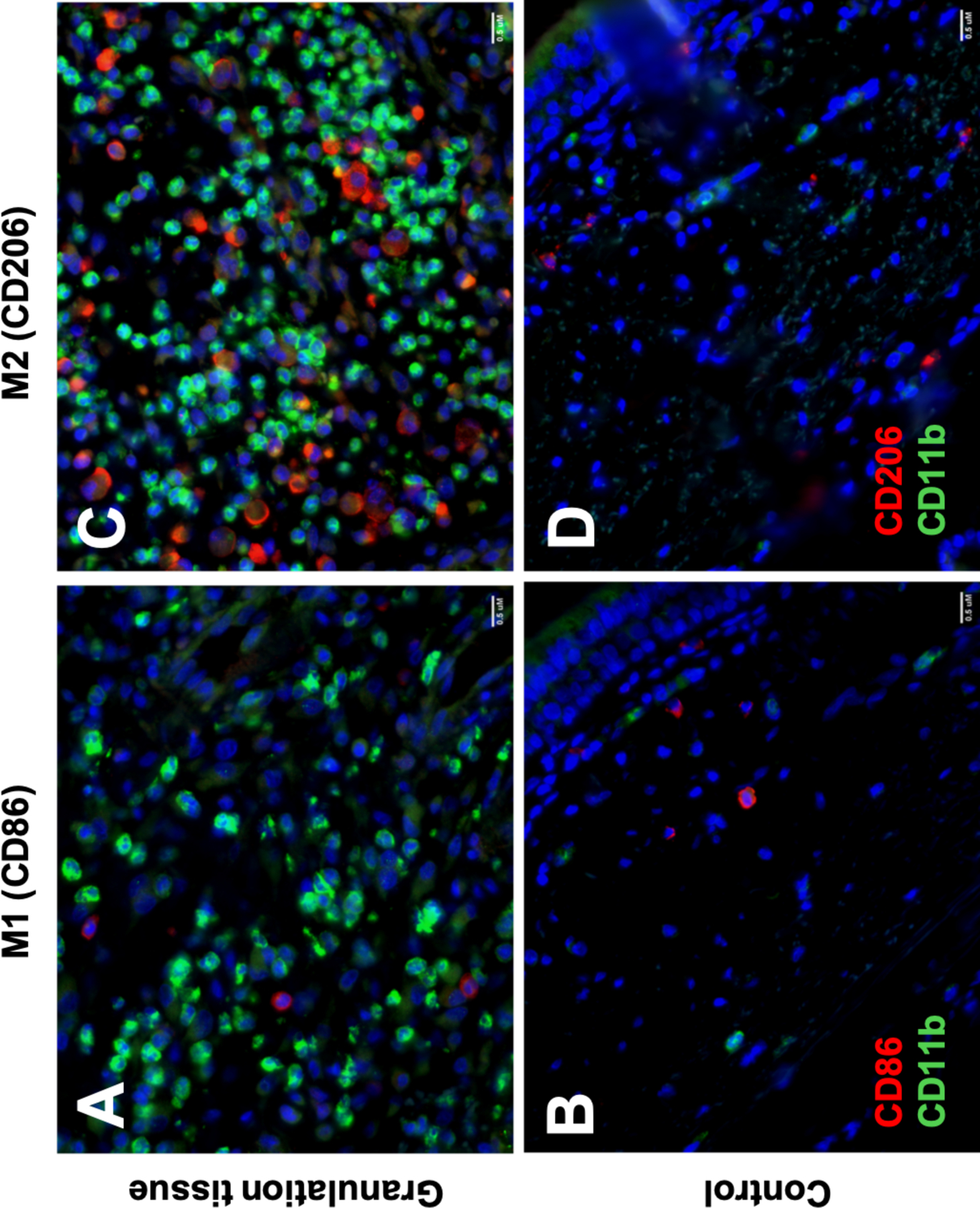
Immunohistochemistry comparing M1 (CD86+) and M2 (CD206+) macrophage presence in granuloma (A and C) and control specimens (B and D). Staining with CD11b (green fluorochrome) and CD86 or CD206 (red fluorochrome). Scale bar=0.5 uM
DISCUSSION
This study demonstrates that markers associated with alternatively activated M2 macrophages are increased in tracheostomy associated granulation tissue as determined by gene expression analysis of canonical biomarkers and cell surface antigens assessed by flow cytometry and immunofluorescence. Phenotypic assessment of the monocyte cell populations associated with granulation tissue reveals a predominant classical subtype. Collectively, these findings indicate that the monocyte and macrophage cell populations are increased in tracheostomy associated granulation tissue compared to controls. Further, the associated macrophage subpopulation is primarily M2 in nature, representing a potential target for future therapies. Ongoing investigation to identify key signaling nodes driving persistent immune cell recruitment and M2 differentiation in tracheostomy associated granulation tissue are warranted.
The data presented in this study demonstrates that tracheostomy associated granulation tissue has a disproportionate increase in M2 macrophages. Macrophage mediated immune responses have been linked to persistent pathologic inflammation and poor wound healing in human and murine models of chronic diabetic ulcers.25–27 Following acute kidney injury, a persistent M2 macrophage population, defined by F4/80 positivity along with M2 markers CD206 and Arginase 1, has been associated with persistent inflammation and the development of fibrosis in mouse models.28 Mechanistically, this M2 macrophage population leads to increased production of TGFB1 and growth factors that promote proliferation of myofibroblasts and subsequent fibrosis.29,30 These findings suggest that the M2 macrophage population associated with tracheostomy granulation tissue may be promoting granulation tissue recurrence as well as potentiating fibrosis near the tracheostomy; however, the mechanism for this remains to be elucidated.
Macrophages can originate from monocytes in the bloodstream that ultimately give rise to macrophages that reside in tissues (tissue resident macrophages).20 In contrast to monocyte-derived macrophages, which are actively recruited during inflammation,31 tissue-resident macrophages have the ability to self-renew, maintaining their populations within the tissues.32 Unlike monocyte-derived macrophages, TRMs originate from multipotent erythromyeloid progenitors that give rise to TRMs that carry out tissue-specific functions.33 Fifty-eight percent of macrophages in tracheostomy associated granulation tissue were monocyte-derived (CD14+). However, 36% of CD45+CD11b+ macrophage population stained positively for the cell surface marker CD169+, a marker associated with tissue-resident macrophages. Several studies have shown significant alteration in the number of CD169+ macrophages in diseased tissues, such as cancer and autoimmune disease, suggesting that this cell type may play a role in disease regulation.34,35 In particular, under inflammatory conditions such as systemic lupus erythematosus, atherosclerosis and transplant rejection, monocytes/macrophages may be induced to express surface marker CD169. Mouse models in CD169 deficient mice have demonstrated that CD169 may exacerbate autoimmune encephalomyelitis through inhibiting regulatory T cells.36 Unlike M1 and M2 macrophages, CD169+ macrophages can interact directly with T cells, B cells and dendritic cells and participate in immune regulation.37 Targeting this CD169+ cell population may impact downstream regulation of lymphocytes and antigen-presenting cells and ultimately prevent granulation tissue formation. The specific role of CD169+ myeloid cells in tracheostomy associated granulation tissue has not been well delineated and further studies are needed to define the role of this cell population in regulating the persistent inflammation driving pathologic granulation tissue formation.
Flow cytometry data demonstrates that monocytes (CD45+FSClowSSClow) are the dominant immune cell in tracheostomy associated granulation tissue. Subgroup analysis reveals that this monocyte population is primarily comprised of classical monocytes. Monocytes are a major component of human peripheral blood, accounting for approximately 10% of all circulating leukocytes, with three main phenotypes based on expression of CD14 and CD16, which encode for lipopolysaccharide receptor and low-affinity FC γ receptor, respectively. In human peripheral blood, classical (CD14highCD16low) monocytes are most prevalent (80–90%), followed by intermediate (CD14highCD16low) at 2–5% and non-classical (CD14lowCD16high) at 2–10%. Classical monocytes are the predecessors to monocyte-derived macrophages and are primarily involved in phagocytosis and immune-cell migration.38 In contrast, non-classical monocytes are involved in complement and Fc gamma-mediated phagocytosis and adhesion, while intermediate monocytes are the only subset to express CCR5 and express the highest level of antigen presentation related molecules.39,40 The extent to which different infiltrating classical monocyte subpopulations contribute to tracheostomy associated granulation tissue formation remains poorly understood. Changes in monocyte counts have been identified in the study of other chronic inflammatory disorders, including rheumatoid arthritis and chronic kidney disease, each demonstrating an increase in relative intermediate monocyte subpopulations.41,42 These differential changes in monocyte subpopulations may directly affect disease outcome, but also impact the development of granulation tissue and persistent inflammation through their differentiation into macrophages. Further work delineating the role of monocytes in the pathogenesis of granulation may lead to the development targeted therapies to limit monocyte infiltration and differentiation into monocyte-derived macrophages.
The majority of macrophages in tracheostomy associated granulation specimens were also S100A8/A9 positive. S100A8/A9 is a Ca+ binding protein constitutively expressed in neutrophils and monocytes, which is often released during inflammatory processes.43 In addition, S100A8/A9 is involved in myeloid differentiation and induces monocytes to secrete pro-inflammatory cytokines including TNFα and IL-1ß. During inflammation, S100A8/A9 stimulates leukocyte recruitment and induces cytokine secretion, and has been implicated as a prognostic biomarker and therapeutic target in several diseases involving chronic inflammation and fibrosis such as IPF,44 renal fibrosis,45 rheumatoid arthritis,46 and osteoarthritis.47 In addition, in the fibrotic disease adhesive capsulitis demonstrated that S100A8/A9 expression was primarily localized to CD68+ macrophages.48 In addition, in vitro studies have shown that stimulation of M2 macrophages with S100A8/A9 results in upregulation of pro-inflammatory cytokines typically produced by M1 macrophage (IL-1β, IL-6 and TNFα) without changing their surface protein M2 phenotype.49 Thus, the elevated expression of S100A8/A9 may represent a pro-inflammatory nidus in tracheostomy granulation tissue. However, while this study did not observe differences in inflammatory cytokine expression, gene expression analysis may not accurately reflect the true cytokine milieu as IL-1β and other cytokines are regulated post-translationally.50 Additionally, relatively low baseline expression levels of inflammatory cytokines may limit their detection threshold. As such, further investigation using protein-level assays is warranted.
Another distinct category of granulomatous lesions of the upper airway are laryngeal granulomas, which occur as contact and/or postintubation lesions in the posterior glottis commonly over the vocal process of the arytenoid cartilage.51 Laryngeal granulomas are benign lesions that are less vascular and more focally localized than tracheostomy-associated granulation tissue.52 Much like tracheostomy associated granulation tissue, the etiology of laryngeal granulomas is unclear, however the presumed pathogenesis involves irritation and possible underlying bacterial colonization.52 DNA microarray gene expression analysis of vocal fold granulomas show clusters of upregulated genes involved in wound repair and inflammation (HIF1A, PRG1, SCYA2, HPS, LAPTM5), along with genes that play a role in remodeling the extracellular matrix (COL1A2, COL3A1, LUM).22 While the inflammatory cascade is presumed to be integral to their formation, the specific immune phenotype and cell populations have not been extensively studied.53 As such, findings from this study may have relevance to vocal process granulomas but studies are warranted to define the immune cell population in this distinct entity.
While this study is the first to define the innate immune cell population present in tracheostomy associated granulation tissue and show M2 predominance among macrophages, there are several limitations. First, the dichotomous M1/M2 paradigm is not a full representation of the macrophage phenotypes, which has arisen in vitro and relies on stimulation of macrophages in culture with defined cytokines. To account for this, the gating strategy of this study includes double positive staining CD86 and CD206 in both M1 and M2 subpopulations. Further, this study used multiple methods, including flow cytometry and immunohistochemistry to support results of gene expression found using qPCR. Additionally, this study used immunohistochemistry from fresh biopsied tissue, which preserves the cell surface markers present in the granulation tissue microenvironment in vivo that may be cleaved during downstream tissue processing of flow cytometry. Our flow cytometry panel was limited by the number of antibodies permitted without fluorophore overlap. Further investigations with additional markers will elucidate the multifactorial role of dendritic cells and other immune cell phenotypes in the development of tracheostomy associated granulation tissue. Finally, we anticipated TGFB1 and inflammatory cytokines would be upregulated, but this was not observed in granulation tissue specimens. This could be related to gene expression assays not reflecting protein level expression. Additionally, assessing TGFB1 activity relies on quantification of TGFB1 active form which is not reflected in gene expression assessments.54 Future dedicated studies are warranted to assess TGFB1 and inflammatory cytokines in tracheostomy associated granulation tissue. Finally, studies involving RNA-sequencing would help to contextualize these findings using gene expression profiles and cell surface markers to subset macrophages and their role in the granulation tissue microenvironment.
CONCLUSION
This study demonstrates that alternatively activated M2 macrophages are elevated in tracheostomy associated granulation tissue. Additionally, monocytes represent the dominant immune cell population in granulation tissue with the majority being of a classical phenotype. The mechanisms promoting monocyte recruitment and macrophage differentiation represent critical signaling nodes that could be targeted to attenuate granulation tissue development. However, further investigation is needed to delineate the potential pathogenic mechanisms promoting this persistent cell population in tracheostomy associated granulation tissue. In addition to our findings, this work provides a validated flow cytometry platform that can be applied for the assessment of the macrophage and monocyte populations within inflammatory conditions.
Supplementary Material
Supplementary Methods
Detailed description of the methods for (1) gene expression measurement using qPCR, (2) flow cytometry characterization of immune cells in dissociated granulation samples, and (3) immunofluorescence.
Supplementary Table 1: qPCR Primer Sequences
Forward and reverse primer sequences used for qPCR with associated literature citations.
Acknowledgements:
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award numbers 1R01DC018567 and R21DC017225 and R01HL146401 and the Burroughs Wellcome Fund SCRIPS Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement:
The authors have no funding, financial relationships, or conflicts of interest to disclose.
The following manuscript is submitted in accordance with the American Laryngological Association requirement for accepted podium presentations (Combined Otolaryngology Spring Meetings in Dallas, TX; April 27 - May 1, 2022).
Level of Evidence: 3
REFERENCES
- 1.Gupta A, Cotton RT, Rutter MJ. Pediatric suprastomal granuloma: management and treatment. Otolaryngol Head Neck Surg 2004;131(1):21–25. doi: 10.1016/J.OTOHNS.2004.02.036 [DOI] [PubMed] [Google Scholar]
- 2.Ilan O, Gross M, Zaltzman Y, Sasson A, Marcus EL. Diagnosis and conservative management of late tracheotomy complications in chronic ventilator-dependent patients. Head Neck 2015;37(5):716–721. doi: 10.1002/HED.23665 [DOI] [PubMed] [Google Scholar]
- 3.Kelly EA, Badi AN, Blumin JH, Poetker DM. Subacute airway obstruction caused by a suprastomal tracheal granuloma following tracheotomy in an adult. Ear Nose Throat J 2011;90(9). doi: 10.1177/014556131109000919 [DOI] [PubMed] [Google Scholar]
- 4.Epstein S. Late complications of tracheostomy - PubMed https://pubmed.ncbi.nlm.nih.gov/15807919/. Accessed March 23, 2022.
- 5.Medrek SK, Lazarus DR, Zarrin-Khameh N, Mohyuddin N, Bandi V. Obstructive post-tracheotomy granulation tissue. Am J Respir Crit Care Med 2017;196(5):e12–e13. doi: 10.1164/RCCM.201703-0468IM/SUPPL_FILE/DISCLOSURES.PDF [DOI] [PubMed] [Google Scholar]
- 6.Brown MT, Montgomery WW. Microbiology of tracheal granulation tissue associated with silicone airway prostheses. Ann Otol Rhinol Laryngol 1996;105(8):624–627. doi: 10.1177/000348949610500807 [DOI] [PubMed] [Google Scholar]
- 7.Mazhar K, Gunawardana M, Webster P, et al. Bacterial biofilms and increased bacterial counts are associated with airway stenosis. Otolaryngol - Head Neck Surg (United States) 2014;150(5):834–840. doi: 10.1177/0194599814522765 [DOI] [PubMed] [Google Scholar]
- 8.Williams O, Fatima S. Granuloma. StatPearls September 2021. https://www.ncbi.nlm.nih.gov/books/NBK554586/. Accessed March 11, 2022. [Google Scholar]
- 9.Treatment of Tracheostomy Granulomas - Study Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02116608. Accessed March 11, 2022.
- 10.McShane DB, Bellet JS. Treatment of hypergranulation tissue with high potency topical corticosteroids in children. Pediatr Dermatol 2012;29(5):675–678. doi: 10.1111/J.1525-1470.2012.01724.X [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Bent JP, Parikh SR. Powered debridement of suprastomal granulation tissue to facilitate pediatric tracheotomy decannulation. Int J Pediatr Otorhinolaryngol 2011;75(12):1558–1561. doi: 10.1016/J.IJPORL.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 12.C P. Interposition cartilage graft laryngotracheoplasty. Ann Otol Rhinol Laryngol:97. [DOI] [PubMed] [Google Scholar]
- 13.Grillo HC. Development of tracheal surgery: a historical review. Part 1: techniques of tracheal surgery. Ann Thorac Surg 2003;75(2):610–619. doi: 10.1016/S0003-4975(02)04108-5 [DOI] [PubMed] [Google Scholar]
- 14.Matt BH, Myer CM, Harrison CJ, Reising SF, Cotton RT. Tracheal Granulation Tissue: A Study of Bacteriology. Arch Otolaryngol Neck Surg 1991;117(5):538–541. doi: 10.1001/ARCHOTOL.1991.01870170084019 [DOI] [PubMed] [Google Scholar]
- 15.Knöß M, Krukemeyer MG, Gehrke T, et al. [Differential diagnosis of rheumatoid granuloma]. Pathologe 2006;27(6):409–415. doi: 10.1007/S00292-006-0865-7 [DOI] [PubMed] [Google Scholar]
- 16.Pagán AJ, Ramakrishnan L. Immunity and Immunopathology in the Tuberculous Granuloma. Cold Spring Harb Perspect Med 2015;5(9). doi: 10.1101/CSHPERSPECT.A018499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164(10 Pt 1):1885–1889. doi: 10.1164/AJRCCM.164.10.2104046 [DOI] [PubMed] [Google Scholar]
- 18.Shamaei M, Mortaz E, Pourabdollah M, et al. Evidence for M2 macrophages in granulomas from pulmonary sarcoidosis: A new aspect of macrophage heterogeneity. Hum Immunol 2018;79(1):63–69. doi: 10.1016/J.HUMIMM.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 19.De Santis M, Locati M, Selmi C. The elegance of a macrophage. Nat Publ Gr 2017;15:196–198. doi: 10.1038/cmi.2017.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: Different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front Immunol 2019;10(MAY):1084. doi: 10.3389/FIMMU.2019.01084/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004;4(8):583–594. doi: 10.1038/NRI1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichard A, Asosingh K. Best Practices for Preparing a Single Cell Suspension from Solid Tissues for Flow Cytometry. Cytometry A 2019;95(2):219. doi: 10.1002/CYTO.A.23690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karasawa K, Asano K, Moriyama S, et al. Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. Am Soc NephrolSign 2015;26:896–906. doi: 10.1681/ASN.2014020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta P, Lai SM, Sheng J, et al. Tissue-Resident CD169 + Macrophages Form a Crucial Front Line against Plasmodium Infection. Cell Rep 2016;16(6):1749–1761. https://www.academia.edu/80493325/Tissue_Resident_CD169_Macrophages_Form_a_Crucial_Front_Line_against_Plasmodium_Infection. Accessed September 27, 2022. [DOI] [PubMed] [Google Scholar]
- 25.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Denis Alexander H, Ross OA. Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol 2018;9(APR). doi: 10.3389/FIMMU.2018.00586/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goren I, Müller E, Schiefelbein D, … UC-J of I, 2007 undefined. Systemic anti-TNFα treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. ElsevierSign in https://www.sciencedirect.com/science/article/pii/S0022202X15335727. Accessed March 30, 2022. [DOI] [PubMed] [Google Scholar]
- 27.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23(6):594–608. doi: 10.1111/J.1464-5491.2006.01773.X [DOI] [PubMed] [Google Scholar]
- 28.Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W. The Role of M2 Macrophages in the Progression of Chronic Kidney Disease following Acute Kidney Injury. PLoS One 2015;10(12):e0143961. doi: 10.1371/JOURNAL.PONE.0143961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motz K, Lina I, Murphy MK, et al. M2 Macrophages Promote Collagen Expression and Synthesis in Laryngotracheal Stenosis Fibroblasts. Laryngoscope 2021;131(2):E346–E353. doi: 10.1002/LARY.28980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillel AT, Samad I, Ma G, et al. Dysregulated Macrophages Are Present in Bleomycin-Induced Murine Laryngotracheal Stenosis. Otolaryngol Head Neck Surg 2015;153(2):244–250. doi: 10.1177/0194599815589106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginhoux F, Immunology SJ-NR, 2014 undefined. Monocytes and macrophages: developmental pathways and tissue homeostasis. nature.comSign in. 2014. doi: 10.1038/nri3671 [DOI] [PubMed] [Google Scholar]
- 32.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 2016;17(1):2–8. doi: 10.1038/NI.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Hirschi KK. Tissue-Resident Macrophage Development and Function. Front Cell Dev Biol 2020;8:617879. doi: 10.3389/FCELL.2020.617879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strömvall K, Kristoffer Sundkvist, Ljungberg B, Sofia |, Bergström H, Anders Bergh. Reduced number of CD169+ macrophages in pre‐metastatic regional lymph nodes is associated with subsequent metastatic disease in an animal model and with. Wiley Online Libr 2017;77(15):1468–1477. doi: 10.1002/pros.23407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asano K, Takahashi N, Ushiki M, et al. Intestinal CD169 + macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun 2015;6. doi: 10.1038/NCOMMS8802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohnishi K, Komohara Y, Saito Y, et al. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci 2013;104(9):1237–1244. doi: 10.1111/CAS.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Front Immunol. 2015;6(MAY). doi: 10.3389/FIMMU.2015.00263/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tak T, Drylewicz J, Conemans L, et al. Circulatory and maturation kinetics of human monocyte subsets in vivo. Blood 2017;130(12):1474–1477. doi: 10.1182/BLOOD-2017-03-771261 [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Tam H, Adler L, Ilstad-Minnihan A, Macaubas C, Mellins ED. The MHC class II antigen presentation pathway in human monocytes differs by subset and is regulated by cytokines. PLoS One 2017;12(8):e0183594. doi: 10.1371/JOURNAL.PONE.0183594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong KL, Tai JJY, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011;118(5):e16–e31. doi: 10.1182/BLOOD-2010-12-326355 [DOI] [PubMed] [Google Scholar]
- 41.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 2012;64(3):671–677. doi: 10.1002/ART.33418 [DOI] [PubMed] [Google Scholar]
- 42.Rogacev KS, Seiler S, Zawada AM, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J 2011;32(1):84–92. doi: 10.1093/EURHEARTJ/EHQ371 [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol 2018;9(JUN):1298. doi: 10.3389/FIMMU.2018.01298/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araki K, Kinoshita R, Tomonobu N, et al. The heterodimer S100A8/A9 is a potent therapeutic target for idiopathic pulmonary fibrosis. J Mol Med (Berl) 2021;99(1):131–145. doi: 10.1007/S00109-020-02001-X [DOI] [PubMed] [Google Scholar]
- 45.Tammaro A, Florquin S, Brok M, et al. S100A8/A9 promotes parenchymal damage and renal fibrosis in obstructive nephropathy. Clin Exp Immunol 2018;193(3):361. doi: 10.1111/CEI.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang KY, Woo JW, Park SH. S100A8/A9 as a biomarker for synovial inflammation and joint damage in patients with rheumatoid arthritis. Korean J Intern Med 2014;29(1):12. doi: 10.3904/KJIM.2014.29.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cremers NAJ, van den Bosch MHJ, van Dalen S, et al. S100A8/A9 increases the mobilization of pro-inflammatory Ly6Chigh monocytes to the synovium during experimental osteoarthritis. Arthritis Res Ther 2017;19(1):1–15. doi: 10.1186/S13075-017-1426-6/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowe LAN, McLean M, Kitson SM, et al. S100A8 & S100A9: Alarmin mediated inflammation in tendinopathy. Sci Reports 2019 91 2019;9(1):1–12. doi: 10.1038/s41598-018-37684-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rheum A, Schelbergen RF, Blom AB, et al. Alarmins S100A8 and S100A9 stimulate production of pro-inflammatory cytokines in M2 macrophages without changing their M2 membrane phenotype. Ann Rheum Dis 2012;71(Suppl 1):A76–A76. doi: 10.1136/ANNRHEUMDIS-2011-201238.10 [DOI] [Google Scholar]
- 50.Petrilli V, Papin S, Biology JT-C, 2005 undefined. The inflammasome. cell.comSign in. https://www.cell.com/current-biology/pdf/S0960-9822(05)00832-8.pdf. Accessed September 25, 2022. [Google Scholar]
- 51.Vocal Fold Granulomas: A Case Series https://www.alliedacademies.org/articles/vocal-fold-granulomas-a-case-series-9720.html. Accessed May 26, 2022.
- 52.Djukić V, Krejović-Trivić S, Vukašinović M, et al. Laryngeal Granuloma – Benefit in Treatment with Zinc Supplementation? J Med Biochem 2015;34(2):228. doi: 10.2478/JOMB-2014-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yumoto E, Sanuki T, Miyamaru S, Kumai Y. Does subepithelial hemorrhage cause persistence of laryngeal granuloma? Laryngoscope 2008;118(5):932–937. doi: 10.1097/MLG.0B013E318163819B [DOI] [PubMed] [Google Scholar]
- 54.Hayashi H, Sakai T. Biological significance of local TGF-β activation in liver diseases. Front Physiol 2012;3 FEB:12. doi: 10.3389/FPHYS.2012.00012/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Guo Z, Chen W, et al. Supplemental Information M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alhajj M, Bansal P, Goyal A. Physiology, Granulation Tissue. StatPearls October 2021. https://www.ncbi.nlm.nih.gov/books/NBK554402/. Accessed May 26, 2022. [Google Scholar]
- 57.Xi Z, Jones PS, Mikamoto M, et al. The Upregulation of Molecules Related to Tumor Immune Escape in Human Pituitary Adenomas. Front Endocrinol (Lausanne) 2021;12:1175. doi: 10.3389/FENDO.2021.726448/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alegre F, Martí-Rodrigo A, Polo M, et al. Macrophages Modulate Hepatic Injury Involving NLRP3 Inflammasome: The Example of Efavirenz. Biomedicines 2022;10(1). doi: 10.3390/BIOMEDICINES10010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trabanelli S, Ercolano G, Wyss T, et al. c-Maf enforces cytokine production and promotes memory-like responses in mouse and human type 2 innate lymphoid cells. EMBO J April 2022:e109300. doi: 10.15252/EMBJ.2021109300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. Non-cell autonomous tumor-growth driving supports sub-clonal heterogeneity. Nature 2014;514(7520):54. doi: 10.1038/NATURE13556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu XF, Xiang L, Zhou Q, et al. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc Natl Acad Sci U S A 2016;113(38):10666. doi: 10.1073/PNAS.1611481113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao CG, He XJ, Lu B, Li HP, Kang AJ. Increased expression of collagens, transforming growth factor-β1, and -β3 in gluteal muscle contracture. BMC Musculoskelet Disord 2010 111 2010;11(1):1–8. doi: 10.1186/1471-2474-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Detailed description of the methods for (1) gene expression measurement using qPCR, (2) flow cytometry characterization of immune cells in dissociated granulation samples, and (3) immunofluorescence.
Supplementary Table 1: qPCR Primer Sequences
Forward and reverse primer sequences used for qPCR with associated literature citations.


