Abstract
Epithelial cells from mucociliary portions of the airways can be readily grown and expanded in vitro. When grown on a porous membrane at an air-liquid interface (ALI) the cells form a confluent, electrically resistive barrier separating the apical and basolateral compartments. ALI cultures replicate key morphological, molecular and functional features of the in vivo epithelium, including mucus secretion and mucociliary transport. Apical secretions contain secreted gel-forming mucins, shed cell-associated tethered mucins, and hundreds of additional molecules involved in host defense and homeostasis. The respiratory epithelial cell ALI model is a time-proven workhorse that has been employed in various studies elucidating the structure and function of the mucociliary apparatus and disease pathogenesis. It serves as a critical milestone test for small molecule and genetic therapies targeting airway diseases. To fully exploit the potential of this important tool, numerous technical variables must be thoughtfully considered and carefully executed.
Graphical Abstract

Introduction
Respiratory tract diseases are a leading cause of morbidity and mortality worldwide [1]. In vitro studies of respiratory tract epithelial cells have broadened our understanding of airway diseases and have served as a key translational step for therapeutic development. Respiratory tract cell culture has evolved over time, beginning first with lung cancer cells [2] followed by explant growth of healthy human bronchial epithelial cells [3]. Key events in the evolution of respiratory epithelial cell culture include the development of serum-free culture media for cell expansion on tissue culture plastic [4] and cell growth on thick collagen gels, which first enabled in vitro epithelial polarization and differentiation of secretory and multiciliated cells [5]. A watershed moment was the introduction of culture on porous membranes at an air-liquid interface (ALI), which allowed more systematic and reproducible production of electrically resistive, well-differentiated planar cultures [6].
For most studies, airway epithelial ALI cultures are created in two major steps. First, cells are procured from the pseudostratified portions of the respiratory tract, including nasal, tracheal, bronchial or bronchiolar regions via curettage or brushing, from excess surgical pathology specimens, or from lungs of organ donors unsuitable for transplantation. While the initial cell harvest (passage zero cells) can be cultured at an ALI, they are typically first expanded by culture on extracellular matrix-coated tissue culture plastic. This initial expansion phase can be performed using several different culture media options, both proprietary and non-proprietary. Second, the cells are differentiated by culture on an extracellular matrix-coated porous support of which there are several varieties and sizes. Again, there are both proprietary and non-proprietary choices of cell culture media for the differentiation phase. When the seeded cells become confluent, media is removed from the apical compartment, exposing the cells to air. These steps have been described in more detail in prior publications (e.g. [7]). The numerous technical choices and their impact are given in Table 1 and are discussed herein.
Table 1.
Airway epithelial air liquid interface (ALI) culture technical decisions, their potential impacts on the ALI model, and representative references. iPSC = induced pluripotent stem cell.
| Technical Considerations | Options | Impact on ALI model | Representative References |
|---|---|---|---|
| Cell model and cell source | 1. Airway epithelial cell lines 2. iPSC-derived airway models 3. Primary airway epithelial cells • Tissue procurement • Commercial source |
1. Epithelial composition 2. Ion transport properties 3. Mucus production/composition |
[142, 151] |
| Airway region | 1. Nasal epithelial cells 2. Large airway epithelial cells 3. Small airway epithelial cells |
1. Epithelial composition 2. Viral infectivity and host defense response 3. Mucus/secretion composition |
[152–154] |
| Donor status | 1. Disease status (CF, COPD, Asthma) 2. Model disease in healthy donor cells with IL13, IL4, etc. |
1. Epithelial composition 2. PCL composition 3. Mucus composition/concentration 4. Cilia beat frequency 5. Tight junctions/barrier function |
[67–72, 155] |
| Cell expansion | 1. Media choice • Conventional bronchial epithelial growth medium (BEGM) • Conditionally reprogrammed cell (CRC) method • Dual SMAD inhibition • Proprietary expansion media (e.g., PneumaCult EX) 2. Passage number/population doublings |
1. Number of cells and ALI cultures 2. Ion transport properties at late passage 3. Epithelial composition |
[113, 134] |
| Differentiation conditions | 1. Differentiation media • Non-proprietary ALI media • Proprietary (e.g., PneumaCult ALI) 2. Membrane choice • Polycarbonate membrane (Transwell®) • PET membrane (Transwell®) • PTFE membrane (Millicell®) |
1. Epithelial composition 2. Cilia beat frequency 3. Tight junctions/barrier function 4. Mucus composition |
[134, 139, 140, 156] |
| Culture maintenance | 1. Culture washing and media change schedule 2. Washing with PBS, TCEP, or other mucolytic |
1. Mucus secretions 2. Cilia beat frequency |
[157] |
The ALI culture model enables separate access to the apical and basolateral compartments and has several advantages including sided delivery of chemical agents and gene or molecular therapy vectors, the ability to measure transepithelial electrophysiologic properties, and examination of mucociliary clearance. They are highly amenable to the full repertoire of modern morphological and biochemical analyses. As such, the ALI model has been used in hundreds of published experiments and undoubtedly in many unpublished studies to support drug and device development in commercial laboratories. In support of our efforts to illustrate the major uses of ALI cultures and considerations for experimental success, the following NCBI PubMed search strategy was employed in December, 2022: ((epithelial[ti] AND cell*[ti]) OR epithelial cells[majr]) AND (respiratory system[majr] OR airway*[ti] OR trach*[ti] OR lung*[ti] OR respirat*[ti] OR pulmon*[ti] OR bronch*[ti]) OR hae[ti] OR beas[ti] AND (drug*[ti] OR anti-infect*[ti] OR toxicol*[ti] OR pharmacol*[ti] OR drug delivery systems[majr] OR drug effects[subheading] OR pharmacology[subheading] OR pharmacokin*[ti] OR pharmacokinetics[subheading] OR formulation*[ti] OR preparation*[ti]) AND (“air liquid”[ti] OR culture*[ti] OR 2d[ti] OR cell culture techniques[majr]) AND (humans[mesh] OR human)
This search strategy yielded 487 results. For practical reasons, the authors of the current review could not cite every reference but express their appreciation for excellent, uncited work. Furthermore, there have been recent advances in multicellular and microfluidic models of the respiratory tract such as the new “lung-on-a-chip” model that are beyond the current focus, but are presented in other articles in the current series [8–10]. Here, we focus on human respiratory epithelial cells and the intersection of the ALI model, mucus, mucins and drug development.
Structure and function of the airway surface layer
The airway surface layer (ASL) lies above the epithelium and presents the first protective barrier preventing uptake of inhaled and aspirated substances. The physiology and pathophysiology of mucins and mucus, key constituents of the ASL and apical airway epithelial cell surface, and the regulation of mucociliary clearance has been comprehensively reviewed recently [11]. The ASL is composed of two distinct gel layers, a transporting, superficial mucus layer and an underlying periciliary layer [12] (PCL; Figure 1). Superficial airway mucus is a complex viscoelastic secretion composed of water, salts, globular proteins, and polymeric mucins. It functions to trap inhaled particles and pathogens for subsequent removal by mucociliary transport (MCT) or cough clearance [11, 13]. MUC5AC and MUC5B, the two major secretory mucin components of airway mucus, are produced by secretory cells in the surface epithelium and submucosal glands. Strands of MUC5B along with antimicrobial molecules emanate from large airway submucosal glands where they mix with surface cell secretions [14, 15]. MUC5B is the dominant secretory mucin in healthy human airways [16, 17]. However, several hypersecretory diseases including cystic fibrosis (CF), non-CF bronchiectasis (NCFB), chronic obstructive pulmonary disease (COPD), and asthma are characterized by increased MUC5AC/MUC5B ratios [17–19].
Figure 1. An illustrated view of the multilayer human airway mucosal surface barrier.
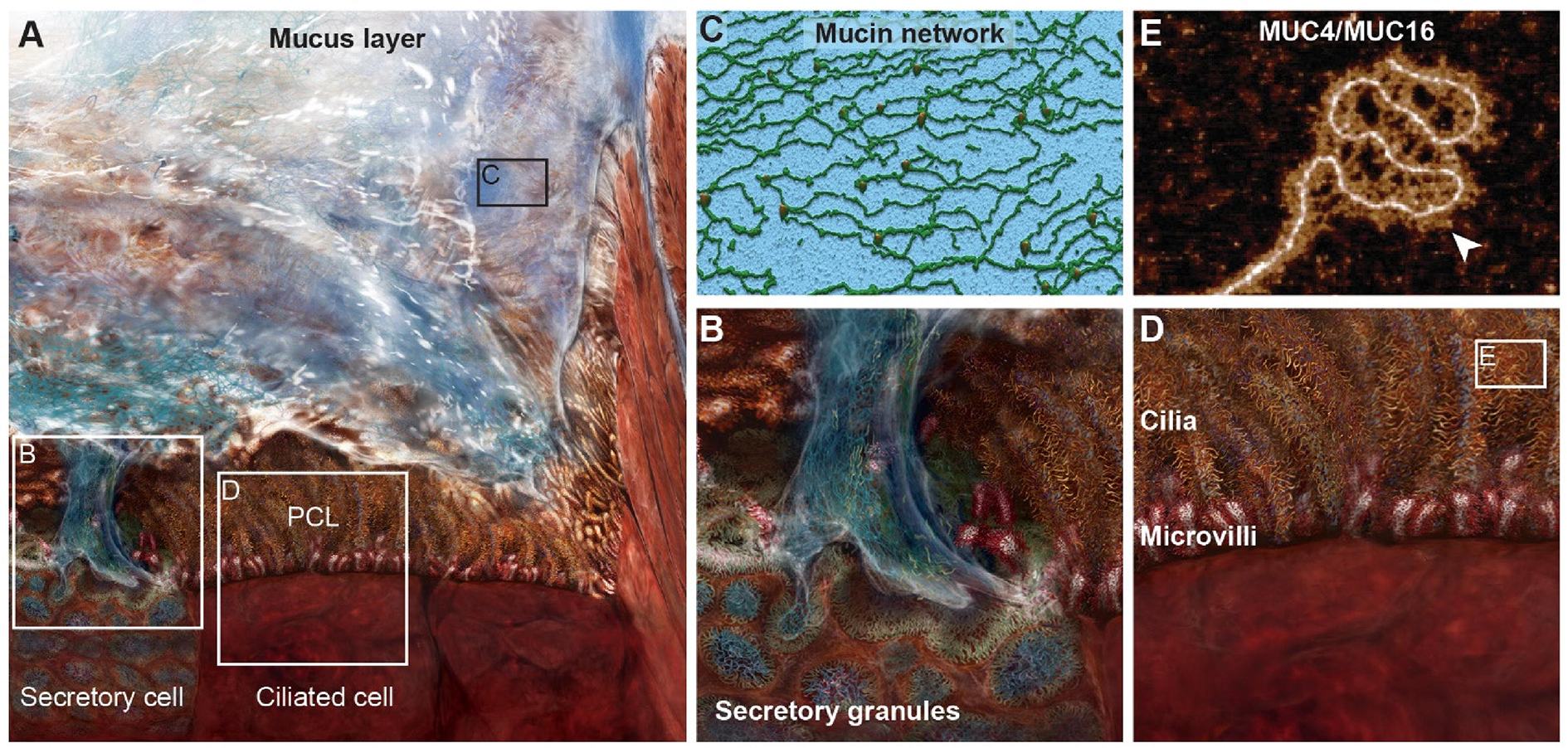
A) An overview of the mucociliary escalator system that is critical for innate airway defense. It consists of two essential layers, an overlying mucus layer and the periciliary layer (PCL) that is positioned between the mucus layer and the apical cell borders. B) A higher magnification view of a secretory cell highlighting stored mucins in secretory granules and their secretion contributing to the mucus layer. C) A 3D rendering of atomic force microscopy (AFM) topography of gel forming mucins, MUC5AC and MUC5B, the major macromolecules in the mucus layer showing their infrastructure to form a protective net-like gel layer. D) A magnified view of the PCL constituted by cilia and microvilli, both of which are densely decorated with membrane bound mucins, MUC1, MUC4, and MUC16. E) An AFM image of the membrane mucins, MUC4 and/or MUC16, isolated from airway epithelial cells, highlighting the presence of keratan sulfate decoration (white arrow). Figure is modified from [20] and [21].
The PCL lies between the superficial mucus layer and the epithelial cells. In healthy airways, the PCL provides a low viscosity environment in which cilia beat freely [12]. The PCL is replete with membrane-bound mucins including MUC4, MUC16, and MUC20 located on cilia and MUC16 located on the surface of secretory cells [20]. The next layer is the glycocalyx where glycolipids and glycoproteins, especially MUC1, densely decorate the microvilli and apical cell surface. Most membrane-bound mucins including MUC1, MUC4, and MUC16 can be decorated with keratan sulfate to further fortify the cell surface barrier [20, 21]. Collectively, the ASL creates a highly selective and tight barrier to inhaled pathogens, particulates, and other harmful materials (Figure 1).
Determinants of ASL permeability
As a whole, the ASL functions as a molecular sieve, with a meshwork of physical entanglements created by hydrophobic, electrostatic, and hydrogen bond interactions of polymeric mucin macromolecules (Figure 1C) [22, 23]. This highly connected network creates a mesh of heterogeneous pore sizes. A crucial determinant of ASL porosity is mucin/mucus concentration. Thickened, hyperconcentrated mucus alters penetration of particulate matter and migratory cells. Matsui et al. demonstrated that thickened mucus with a high percentage of solids decreases neutrophil mobility and migration, compromising bacterial capture and killing [24]. Secreted globular proteins can also interact with mucins, altering ASL biophysical properties [25]. For instance, secreted immunoglobulins can bind to mucus/mucins to effectively trap viruses and other pathogens in the mucus layer and prevent viral penetration to the airway epithelial surface [26, 27]. Several other groups have reported an inverse relationship between particle size and mobility through the ASL [28–30]. Beyond the mucus layer, the PCL also exhibits a size exclusion gradient. Button et al., showed that PCL penetration was dependent on particle size with particles ≥ 40 nm completely excluded from the PCL [12]. Mucus also traps particles through electrostatic or hydrophobic interactions, both of which are governed by epithelial ion transport and mucus pH. Anion secretion via epithelial ion channels mediates mucin polymer packaging and release by airway secretory cells and modulates mucin crosslinking and electrostatic charge [31]. Changes in pH regulate mucus filtration by promoting non-covalent mucin crosslinking and increasing overall mucus viscosity [32, 33]. Lieleg et al., studying the pH dependence of particle transport through porcine gastric mucins, demonstrated nearly free particle diffusion at neutral pH but particle mobility was lost when pH was lowered to 3.0 [33]. Finally, particle movement through the airway mucus layer is contingent on mucus quantity and composition, both of which are controlled by the underlying airway epithelium.
Mucus composition, especially the ratio of major secretory airway mucins, MUC5AC and MUC5B, is an important determinant of mucus layer biophysical properties including barrier penetration and viscoelasticity [17, 34]. MUC5AC creates a more branched/crosslinked network compared to MUC5B, which develops more linear and less branched networks [34]. The composition of mucin-interacting proteins also affects mucus layer barrier and biophysical properties by increasing crosslinking [25]. Extracellular DNA in purulent mucus is well known to alter mucus biophysical properties and is the basis for inhaled recombinant DNAse therapy in CF [35, 36]. First reported and initially shown to be present in vivo in the gastrointestinal tract by the group of Arturo Zychlinsky, neutrophils in mucus can release chromatin coated with antibacterial granule contents, forming net-like structures in a process currently termed NETosis [37, 38]. Neutrophil nets are also present in mucopurulent airway secretions, altering mucus gel viscoelasticity (reviewed in [39]). However, in the airway epithelial ALI culture apical environment, DNA from exfoliated and dying cells is typically minimal and experiments evaluating NETosis in vitro require co-culture with myeloid derived inflammatory cells and a stimulus such as viral infection [40].
The importance of airway mucus on agent diffusion to the cell layer cannot be understated. The major secreted mucin macromolecules, MUC5B and MUC5AC, are generally decorated with negatively charged glycoconjugates due to their sialic acid and sulfate content [18]. Additionally, mucus contains hundreds of other secreted, charged molecules, including small molecules with positive charges. Depending on its charge, the agent of interest may interact with and be trapped in the mucus and/or the PCL layer (reviewed in [31]). In one example, investigators used Quartz Crystal Microbalance with Dissipation (QCMD) to monitor interactions between a candidate peptide drug and mucins, finding that the peptide did not interact with mucus and penetrated through highly concentrated mucus as typically seen in CF [41].
Airway epithelial composition and barrier function
As well as generating a protective ASL, the airway epithelium itself serves as a barrier between the external environment and the body’s internal milieu. The nasal passages and the cartilaginous trachea and bronchi contain a tall pseudostratified epithelium composed of multiciliated cells, secretory cells, transitional cells, and basal cells overlying a network of submucosal glands. Proceeding distally to the bronchioles which lack cartilage and glands, the epithelium becomes shorter and eventually cuboidal before transitioning to the alveolar epithelium primarily composed of type I and type II cells. An updated landscape of the airway epithelium is detailed and beautifully illustrated in a recent review [42].
The integrity of the airway epithelium is maintained by apical junctional complexes (AJCs) composed of tight junctions, adherens junctions, gap junctions, and desmosomes, which fasten neighboring cells to one another and separate apical and basolateral membrane domains and subtended luminal and submucosal compartments [43]. The airway epithelium and AJCs create an electrically resistive barrier that prevents paracellular particle transport. Thus, transit across the airway epithelium is largely restricted to the controlled flow of ions across epithelial ion channels including the epithelial sodium channel (ENaC), the cystic fibrosis transmembrane regulator (CFTR), and the calcium activated chloride channel (CaCC) among others. Transepithelial electrical resistance (TEER) and ion transport dynamics can be measured in vitro in Ussing chambers, originally developed in the 1950s for measurements of native tissue [44]. Taken together, AJCs tightly knit the airway epithelium to create a formidable physical barrier to external particle uptake. Various factors can alter effectiveness of the epithelial barrier including allergens, cigarette smoke, bacteria, viruses and nanoparticles [45]. In this case, biomolecules and other innate defense factors can “leak” from the basal side to the apical surface or from the apical surface to the basal side to create a more robust response against these irritants/pathological factors. A particularly important example is illustrated by the response to house dust mites (HDM) to stimulate allergy and asthma. Dermatophagoides pteronyssinus is one of the ubiquitous HDM species that thrive in the domestic human environment. When inhaled as small particulates, this organism’s feces contain proteases that are both antigenic and capable of modifying airway epithelial AJCs thereby increasing transepithelial permeability and amplifying the host response [46].
Airway epithelial composition and cell polarity also determine the distribution of receptors lining the apical surface. Low rates of apical membrane uptake serve a vital role in protecting the inner sanctity of the body but often frustrates efforts for delivery of topical therapies. As illustrated by efforts for airway viral gene therapy and as discussed below [47], receptor distribution and rates of endocytosis differ dramatically between the apical and basolateral domains of airway epithelial cells. Strategies to chemically or physically disrupt the epithelial barrier to expose basolateral receptors are often used to increase cellular transduction and transfection efficacy [48].
Overall, the airway mucus layer, the underlying PCL, AJCs, and the airway epithelium itself with coordinated multiciliated cell beating collectively create effective and physiologically regulated mucociliary clearance, a vital innate defense mechanism. The barrier function and mucus clearance, with cough as a backup mechanism serve crucial protective roles to keep foreign particles out of the human body. Unfortunately, these mechanisms cannot distinguish between allergens, pathogens, and chemicals versus potentially beneficial particles or cells that are therapeutically delivered to the lungs. Therefore, understanding the components of airway host defense and modeling them accurately with appropriate ALI culture techniques is key for the design and evaluation of effective therapies.
From ALI culture to the clinic: examples of ALI cultures for drug development
ALI cultures play a critical role in the bench-to-bedside translation of airway-targeted therapeutics and drug delivery techniques. One of the great success stories of ALI culture drug development is the discovery of small molecule CFTR modulators to treat CF. CFTR modulators address the underlying cause of CF by directly acting on the CFTR protein to correct folding, trafficking, function, or stability [49]. In the early search for CFTR modulators, many candidate compounds produced false-positive “hits” in high throughput screens in heterologous cell types but failed to rescue CFTR function in the primary airway epithelial ALI culture model. Important reasons for the false hits likely include both higher levels of CFTR expression in the heterologous expression systems and differences in cell-type dependent mechanisms regulating CFTR translation and protein processing in cell lines versus primary cells. Since there are hundreds of pathogenic CFTR variants divided into different classes (reviewed in [50]), it is also important to highlight that the therapeutic approach is CFTR variant-specific. A “lower bar” for CFTR therapy is present in Class III CFTR variants, including the G551D mutation that is transcribed at normal levels. The resulting protein is normally translated and processed to the cell apical plasma membrane, but exhibits defective activation. VX-770 (ivacaftor) was the first compound to significantly rescue CFTR function in primary G551D cells in the ALI model [51] and ultimately progressed through clinical trials to achieve FDA approval in 2012 [52]. Preclinical testing in the ALI model using cells with the common F508del Class II CFTR variant has enabled the development of multidrug combinations and subsequent CFTR modulator approvals [53–56] such that in 2023, approximately 90% of people with CF are eligible for highly effective CFTR modulator therapy. Electrophysiology measurements in ALI cultures are now widely accepted as a reliable predictor of clinical benefit [57–59]. Indeed, this model has been so widely accepted that ALI culture electrophysiology has been used as a substitute for clinical trials to expand drug labels for CFTR modulators to diverse populations of people with CF either by personalized medicine approaches [60] or larger scale expansion to people with certain genotypes [61]. Overall, the ALI culture model serves as a critical milestone test separating the high throughput screen false-positive “hits” from compounds likely to make a functional difference in vivo.
Of particular note, the ALI culture model has been important to develop and test gene therapies. As in the case of CF pharmacotherapies, efficacy of potential gene therapy vectors including plasmid/lipid transfection agents, adenoviruses, retroviruses and adeno associated viruses are typically tested in the ALI model before proceeding to animal studies. In a particularly illuminating historical example, while primary bronchial epithelial cells grown on plastic are highly transducible with adenoviral vectors [62], apical application of adenovirus is less efficient in ALI cultures unless agents are used that permeabilize the apical cell border to provide access to the basolateral coxsackie/adenovirus receptor [63–65]. Extending in vitro studies of adenovirus vectors to mice and to explanted human lung tissue further illustrated the need for epithelial wounding to access transducible basal cells [66]. Essentially, the ALI model has predicted the minimal in vivo efficacy to date of gene therapies for CF (reviewed in [47]). Studies with evolving airway therapies such as lipid RNA or protein nanoparticles, exosomes, and genetically modified cells logically employ the ALI model in preparation for in vivo testing in animals and ultimate use in humans.
Asthma and chronic obstructive pulmonary disease (COPD) are major human worldwide health problems and the ALI model has been useful to better understand the biology of these diseases. For example, studies of cells from donors with asthma vs. control donors revealed altered cell “jamming” behavior [67], altered interactions with dendritic cells in response to particulates [68], and the role of microRNA 141 to regulate IL13 induced mucus production [69]. Studies of cells from donors with COPD vs. control donors showed differential effects of cigarette smoke on anti-microbial and inflammatory responses [70], effects of hypoxia and reoxygenation [71], and activation of NOTCH3 to promote goblet cell differentiation [72]. These are just a few examples of the many studies related to asthma and COPD performed with ALI cultures.
Finally, the utility of ALI cultures in drug development can also be seen in the ongoing Coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2). ALI cultures are an important model system to study SARS-CoV-2 infection, mirroring the viral tropism and epithelial cell host response seen in vivo [73]. The fidelity of the ALI model not only primes its use for mechanistic studies of viral infection, but also for the development of antiviral therapies. Changes in SARS-CoV-2 infectivity in the presence of candidate antivirals can be evaluated by measuring viral titers or the number of infected cells by immunofluorescent staining and virus localization [74–76].
Limitations of the ALI culture model
Protective airway innate and adaptive immunity in vivo is the product of multiple cells and systems dynamically interacting in a complex biophysical and mechanical environment. The inability to accurately and fully model in vivo complexity is an obvious limitation of ALI cultures solely containing airway epithelial cells. Multicellular “lung-on-a chip” models begin to address cell-type interactions, fluid and air flow effects and mechanical cues [9]. However, more complex models face challenges and limitations such as expense, replicate number, and media preferences of the different co-cultured cell types. In terms of drug delivery, this may limit examining a wide range of doses and time courses. Even with the relatively simple ALI model the important goal to study cells representing diverse populations of interest including age, sex, race, ethnicity and health status will be limited by supplies of cells from different donors. This issue is compounded by inevitable human donor-to-donor variability. Even with the ALI model, sufficient biological and technical replicates allowing statistical power may be difficult to achieve in view of effect magnitude, and donor and culture quality variability.
Modeling complex cell-cell interactions in the ALI model in relationship to disease pathogenesis may nevertheless be possible with cell co-cultures. An example is Yonker et al.’s study of leukocyte epithelial transmigration, an important component of the inflammatory response. In this case, the airway epithelial cells were grown on the underside of a porous support with 3 μm pores, inverted, and allowed to differentiate before neutrophils were placed above the epithelial cells [77]. This system allowed real-time analysis of the regulation of neutrophil transmigration by lipid mediators and cell-cell interactions in response to a bacterial stimulus [78]. In another example, co-culture of primary human airway epithelial cells and macrophages illustrated macrophage differentiation and media dependent effects on respiratory syncytial virus (RSV) infection [79]. Finally, co-cultures of bronchial fibroblasts and airway epithelial cells can be used to study fibrotic remodeling of the airway wall, an important component of asthma. Indeed, in a study by Paw et al., fibroblast/epithelial co-cultures created from donors with or without asthma illustrated disease-dependent differences in both cellular compartments, and cell-cell interactions altering tissue remodeling responses [80].
A final limitation of the ALI culture model is the ability to deliver drugs in a clinically used format. Clinically, airway therapies are often delivered to the lungs in aerosolized form. Yet, this is challenging to model in the ALI culture system without specialized equipment. Conventional jet or vibrating mesh nebulizers can be modified for use in cell culture experiments, but the liquid output rates are low leading to long exposure times and poor delivery efficiency [81]. For this reason, most studies deliver test compounds and particulates in a liquid suspension. Not only does this represent an unphysiological scenario, but uptake kinetics are known to differ between aerosol and submerged delivery conditions [81]. Though some groups have been successful in modeling clinical nebulization rates [81, 82], the challenge of extending this technology for widespread use represents a limitation of the ALI culture system.
Technical choices that influence ALI culture experimental outcome
With the ALI culture system, therapeutic approaches can be assessed under well controlled conditions [83, 84]. However, numerous protocols and technical choices can alter results and obscure comparisons between studies. In this section, we outline the major technical decisions that influence experimental outcomes relevant to drug delivery (Table 1).
Cell model.
The first crucial decision for airway epithelial ALI culture experiments is the choice of the cells that will be used. Major options include immortalized or growth-enhanced cell lines, airway cells derived from induced pluripotent stem cells (iPSCs), or primary airway epithelial cells. Cell lines derived from non-respiratory tissue sources such as Fischer Rat Thyroid (FRT) cells [85] or Caco-2 human colonic cells [86] have been extensively used to study respiratory diseases. However, we focus here on cell lines derived from the human respiratory tract capable of forming a confluent, electrically resistive ALI culture. For a list of commonly used respiratory cell lines, advantages, and limitations, see a recent review by Gordon et al. [87].
Cell lines enable reproducibility, are amenable to high-throughput assays, and can be genetically manipulated while retaining their extended growth capacity. However, gains in cell lifespan can compromise the ability to recapitulate properties of the native parent tissue. Each cell line comes with unique caveats that must be weighed when designing ALI culture experiments. Cell lines can be generated from cancer cells, like the Calu-3 cell line, originally generated from a bronchial adenocarcinoma, or by transforming normal primary cells into cancerous cells through the introduction of proto-oncogenes. Expression of viral oncogenes such as SV40 early region or HPV E6/E7, sometimes in combination with human telomerase reverse transcriptase (hTERT), potently immortalizes cells, but can also create genetic instability and aneuploidy, dynamically shifting cell phenotype [88]. This effect appears to vary between cell lines and culture conditions, enabling some cell lines to maintain junctional integrity and mucociliary differentiation capacity for longer periods [89–91].
An interesting exception is the 16HBE14o- cell line in which the SV40 immortalizing gene became spontaneously incorporated into the CFTR gene locus, the gene causative of CF [92]. Unlike most SV40 cell lines, 16HBE14o- cells maintain their ability to polarize in ALI culture conditions likely because of the low expression of CFTR, and thus SV40 genes. The 16HBE14o- cell line has been further genetically engineered to carry a range of CFTR variants in an isogenic background [93], and these cells are being extensively used in drug discovery efforts. However, 16HBE14o- cells and their derivatives do not exhibit ENaC activity, a crucial regulator of ASL properties [93]. CFBE41o- is another SV40 human bronchial epithelial cell line generated from a CF donor homozygous for the F508del mutation. These cells form tight junctions to generate a polarized epithelium and display ion transport properties characteristic of CF, but they lack key functional features including mucus production [94, 95]. The Calu-3 cell line mentioned earlier also forms a functional tight epithelium when cultured at an ALI, but these cells do not differentiate into easily recognizable surface airway cell types.
Another class of “growth-enhanced” cell lines can be created by introduction of the B cell-specific Moloney murine leukemia virus integration site 1 (Bmi-1) proto-oncogene, sometimes in combination hTERT, to prolong cell lifespan without truly immortalizing cells [88, 96]. Unlike immortalized cell lines, Bmi-1/hTERT cells remain diploid and capable of polarization and mucociliary differentiation, depending on media choice. Recent studies illustrate similar responses between ALI cultures from Bmi-1/hTERT cell lines and parent primary cells to CFTR modulators in electrophysiology assays [97]. Another study compared transepithelial drug absorption in ALI cultures in a Bmi-1/hTERT cell line and the Calu-3 cell line, reporting that the former was “better able to model the effect of mucus on drug absorption” [98].
The breakthrough studies of Takahashi and Yamanaka ushered in the iPSC era [99]. Using a multiplicity of methods evolved from the original technique, iPSCs derived from many somatic cell types can undergo directed differentiation to a wide variety of cell types and organotypic structures. Capable of unlimited and clonal growth, iPSCs hold great promise and utility, particularly for selection of gene edited clones that can ultimately be expanded into large numbers of cells and still undergo directed differentiation. There have been multiple studies of airway epithelial directed differentiation of iPSCs, including from donors with genetic diseases [100–111]. Ion transport abnormalities are preserved in some examples of CF iPSC-derived ALI cultures [108, 112]. However, uniform differentiation to a resistive epithelial sheet may be less robust in the iPSC model compared to primary cells, and certain properties such as ENaC function may be reduced or absent [112].
Primary cell source.
Primary human airway epithelial cells have long served as the gold standard to model the airway in vitro [113–115]. However, one drawback of the primary cell model is donor-to-donor and culture-to-culture variability that may increase the number of biological or technical replicates needed to detect significant differences. In experiments using primary cells, the biological replicates are the number of donors while the technical replicates are the number of individual cultures per experimental group. Depending on the study goals, it may be important to consider including both male and female donors, and potentially hormonal treatments of cultures to replicate relevant sex dependent conditions [116]. In addition, other demographic factors such as ancestry and ethnicity need to be considered in the study design to be representative of the ultimate target population. Depending on the availability of donors, projects involving primary cell cultures as well as iPSC-derived cultures should aim to maximize donor diversity to cover the phenotypic and genotypic population spectrum. Commercial sources of primary airway epithelial cells suitable for ALI cultures (e.g., Lonza, Basel Switzerland and several others), as well as already well-differentiated ALI cultures are now available (e.g, Epithelix, Plan-les-Ouates Switzerland and MatTek, Ashland, MA USA). An alternative to commercial sources is investigator-based procurement of relevant human tissue biospecimens including excess pathology tissues from nasal surgery, nasal or bronchial brushings, curettage or biopsy, excess portions of lobectomy or pneumonectomy procedures, excess large and small airways from lungs donated for transplant, or airway and lung specimens from organ donors whose lungs were unsuitable for transplantation. Detailed non-proprietary protocols for cell harvest and culture from these types of specimens have been discussed in detail previously [7].
Besides the inertia barrier to investigator-based tissue procurement and the potentially daunting cost of commercial primary cells, a key point to consider is the proprietary nature of commercial products. There is often limited metadata on the tissues of origin and the procedures for obtaining and expanding commercial airway epithelial cells are not typically disclosed. As noted in sections below, the population doubling history of cells with finite lifespans may alter their differentiation capacity and behavior. Repeated purchase of specific donor cells through commercial sources may not be possible, preventing experiment replication. Proprietary expansion and differentiation media may contain unknown inhibitors or activators of molecular pathways of interest which would affect both baseline properties and agonist responses in the final ALI cultures. Finally, proprietary commercial products may be changed without notice or go on backorder for extended periods of time. While commercial sources often expend significant resources to optimize the production of cells and well-differentiated cultures, providing convenient quality products, there are advantages to investigator-based procurement and biobanking of cells and use of non-proprietary expansion and differentiation methods.
Airway region.
Nasal, bronchial, and bronchiolar airway regions are lined by an epithelium with unique cell type compositions in vivo [117]. Human bronchial and bronchiolar (i.e., large and small airway) cultures were recently characterized and compared [118]. Though ion transport function was comparable between these regions, differences in cell type frequency were noted with a higher proportion of ciliated cells and lower proportions of suprabasal cells and ionocytes in small airway cultures. Another study directly comparing paired CF bronchial and nasal cell samples demonstrated that ion transport function and mature airway cell markers were similar [119]. However, nasal and bronchial cells exhibit innate differences in viral infectivity [73] and antiviral host defense response [120]. Nasal cells also have stricter growth requirements and often originate from a small initial cell sample. In a recent study, optimal differentiation of nasal cells on the polyester (PET) Transwell culture membrane required PneumaCult ALI media versus other common media types [97]. These innate differences in cell growth capacity, epithelial composition, viral infectivity, and viral host defense can dramatically alter outcomes in ALI culture experiments unless planned for carefully.
Donor health status.
For primary cell models, another vital aspect of experimental design is considering the health status of the cell donor. A choice needs to be made regarding the acceptability of cells from donors with a history of smoking or vaping, which relies on patient or next of kin reporting and is known to often be inaccurate [121]. Information about occupational exposures may or may not be available and disease history may also be inaccurate. Airway disease phenotypes recapitulated by the ALI model can alter drug delivery outcomes by introducing added barriers. For example, ALI cultures from asthma, COPD, and CF are reported to exhibit goblet cell hyperplasia [122, 123] which in turn alters ASL composition and increases mucus secretion rates. ALI cultures from donors with a clinical history of asthma are reported to have increased inflammatory cytokine expression and secretion and reduced tight junctions [124] compared to control cells from previously healthy donors [125, 126]. Likewise, ALI cultures from COPD donors are reported to display higher baseline levels of cytokines and increased susceptibility to viral infection [127]. CF and primary ciliary dyskinesia (PCD) ALI cultures also have lower cilia beat frequency which changes the efficiency and distribution of delivered compounds [128, 129]. Despite these considerations, studying drug delivery in ALI cultures from donors with a history of airway disease allows investigators to evaluate phenotypic rescue as a readout.
Matrix coatings.
As noted previously there are two critical steps required to create ALI cultures. The first is the expansion phase, which is typically performed on cell culture treated plastic dishes. The second is the differentiation phase that is performed on porous supports, ultimately enabling culture at the air-liquid interface after cells reach confluence. Different extracellular matrix coatings variably enhance attachment, growth, and epithelial function. An early study using bovine bronchial cells compared bronchial cell-secreted matrix, type I collagen, type IV collagen, fibronectin, and laminin and found differences in attachment and RGD peptide-dependence of freshly harvested versus subcultured cells [130]. A more recent study using human cells included various ECM molecules, decellularized human dermis, decellularized rabbit trachea, chick chorioallantoic membrane, and collagen scaffolds, finding superior attachment to type IV collagen and variable restoration of mucociliary clearance and persistence after in vivo transplantation with different matrix constructs [131]. Culture on a collagen matrix with embedded fibroblasts in a device to minimize gel contraction is one approach to generate a more representative airway wall model [132]. Substratum models have become more complex and, in one case, the airway epithelium was cultured above a thermo-responsive hybrid scaffold containing either fibroblasts or mesenchymal stem cells [133]. There have been many studies of matrix effects beyond those mentioned here. An overall recommendation is that careful coating of tissue culture plastic with type I/III collagen and collagen type IV coating of porous supports, with removal of excess unattached collagen, is a minimally complex way to yield superior attachment, growth and differentiation [7].
Cell expansion.
The downstream differentiation characteristics and many phenotypic and functional endpoints are greatly impacted by the cell expansion method [113, 134]. Conventional methods (i.e., expansion with a non-proprietary bronchial epithelial growth media, BEGM) typically enables ~3 passages before primary cells reach senescence [114]. The newer conditionally reprogrammed cell (CRC) culture method (i.e., co-culture with irradiated 3T3J2 fibroblasts and a Rho kinase inhibitor) extends primary cell lifespan to >10 passages [113, 135]. However, as cells are further expanded they undergo a tendency towards squamous differentiation and lose ion transport function. CRC cells maintain differentiation potential better than cells expanded in BEGM [136] or with dual SMAD inhibition methods [137]. PneumaCult EX Plus proprietary expansion media also supports robust cell expansion [134].
Differentiation conditions and culture maintenance.
After cell expansion, investigators must define the optimal differentiation conditions for the experimental endpoints of interest. There are several choices of ALI cell culture inserts summarized in Table 1. Transwell hanging cell culture inserts (Corning) can be purchased with either a polycarbonate or polyester (PET) membrane. Millicell free standing cell culture inserts (MilliporeSigma) are made with a polytetrafluoroethylene (PTFE) membrane. A thicker, more stratified cell layer is routinely observed with culture on the PTFE membrane (Figure 2) [138]. Further, the convenience of certain experimental endpoints may differ for various cell culture inserts. For example, polycarbonate membranes are translucent rather than transparent, making it difficult to view cells by transmission light microscopy.
Figure 2. Insert membrane choice alters ALI culture morphology.
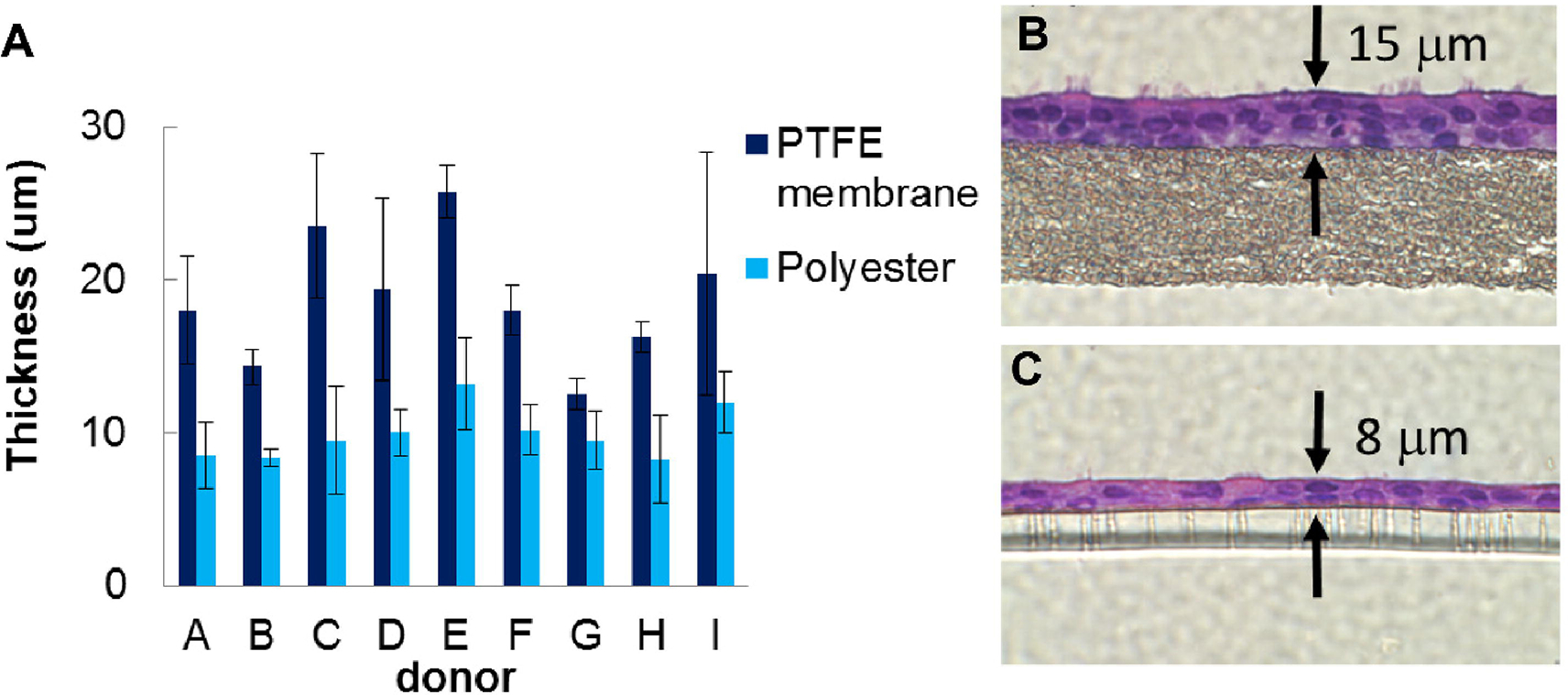
Human tracheobronchial airway epithelial cells grown on polyethylene terephthalate (PET; Snapwell and T-Clear Transwells; Corning) and polytetrafluoroethylene (PTFE; Millicell CM) membranes, demonstrating a thicker and more stratified cell layer on the PTFE membrane. A) Cell layer thickness obtained on both membrane types for samples from nine separate donors. B-C) Photomicrographs of representative histological sections of cells on PTFE (B) and PET (C) membranes. Figure is modified from [138].
Several studies have compared different ALI culture differentiation media [139–141]. We and others see dramatic differences in cell type composition and baseline electrophysiology in the final ALI culture (Figure 3) [139]. These effects have been observed on morphological, transcriptomic, and functional levels. Saint-Criq et al. have shown that the choice of differentiation media can affect functional responses to CFTR modulators in CF cells [141], indicating the importance of culture media conditions for specific questions and assays. Culture media can impact the barrier properties in the ALI culture model. For instance, the transepithelial electrical resistance (TEER) is reduced by half in cultures grown in PneumaCult ALI media compared to those grown in UNC ALI media (Figure 3I). The impact of culture conditions can also affect secretory phenotype of HBECs. Figure 4 shows a comparison of the proteomics profile of the apical secretions of ALI cultures grown in UNC ALI vs PneumaCult ALI media. Broadly speaking, the same donor cells differentiated and maintained in different media can have significantly different secretory phenotypes. This is likely due to the composition and additives (growth factors, cytokines, etc.) of culture media and can mimic abnormal and/or disease conditions rather than a baseline healthy state.
Figure 3. Media choice alters ALI culture morphology and electrophysiology.
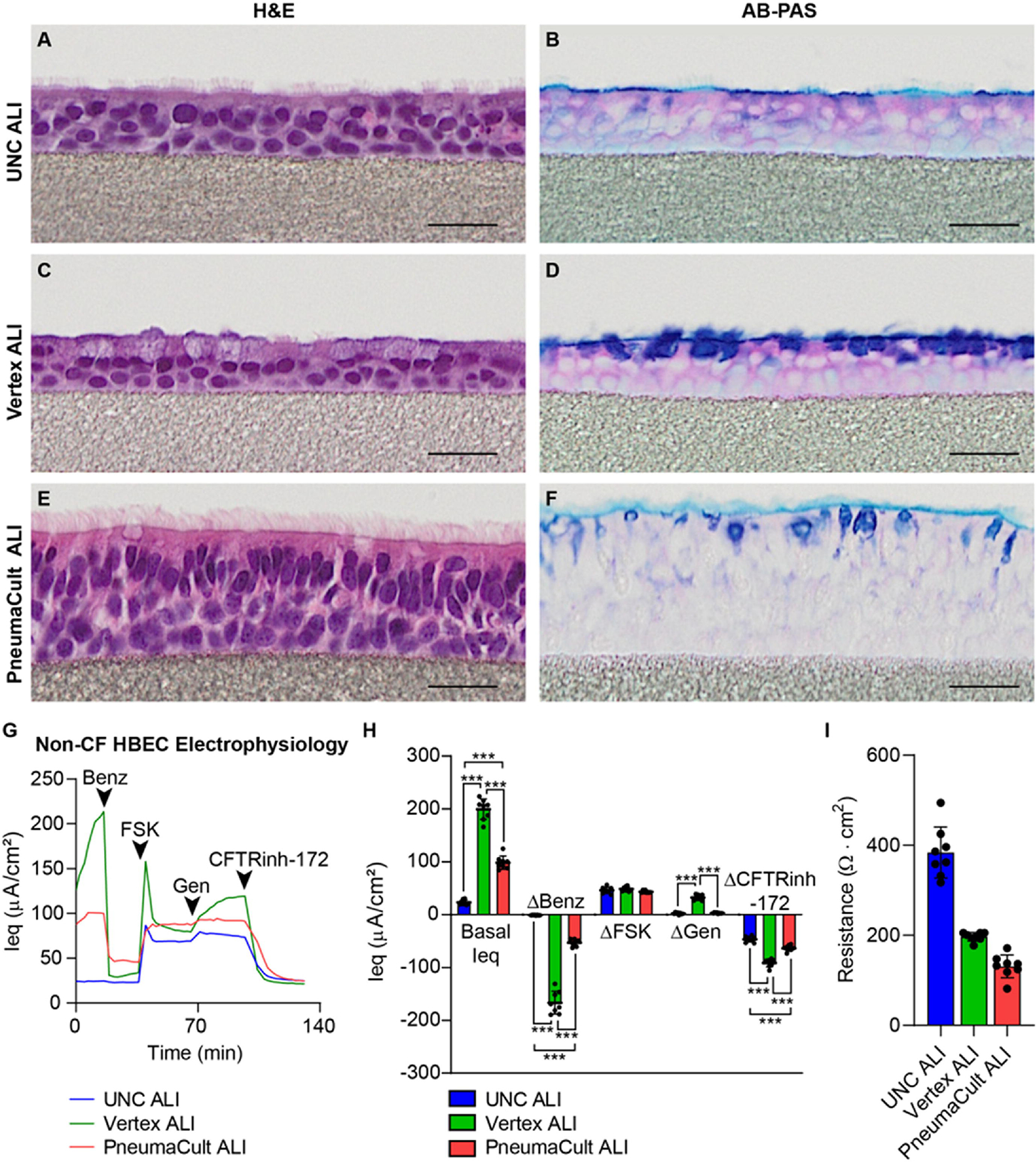
A-F) Representative histological sections stained with hematoxylin and eosin (H&E) or Alcian blue-Periodic acid Schiff (AB-PAS) of human tracheobronchial airway epithelial cells (HBECs) in various differentiation media choices as indicated. A-B) UNC ALI; C-D) Vertex ALI; E-F) PneumaCult ALI. Different proportions of characteristic basal, secretory and ciliated cell types, alterations in stored mucosubstances, and variable stratification and epithelial thickness are evident. HBECs were grown on PTFE membranes. All scale bars = 25 μM. G-H) TECC-24 electrophysiology measurements of non-CF HBEC ALI cultures grown in UNC ALI, Vertex ALI, or PneumaCult ALI differentiation media. G) TECC-24 tracing. Acute addition of benzamil (Benz), forskolin (FSK), genistein (Gen), and CFTR inhibitor-172 (CFTRinh-172) indicated by arrows. H) Basal equivalent current (Ieq) and change (Δ) in Ieq in response to Benz, FSK, Gen, and CFTRinh-172. One biological donor; 8 replicates.
Figure 4. Culture media choice impacts the composition of the tracheobronchial epithelial cell apical secretion proteome.
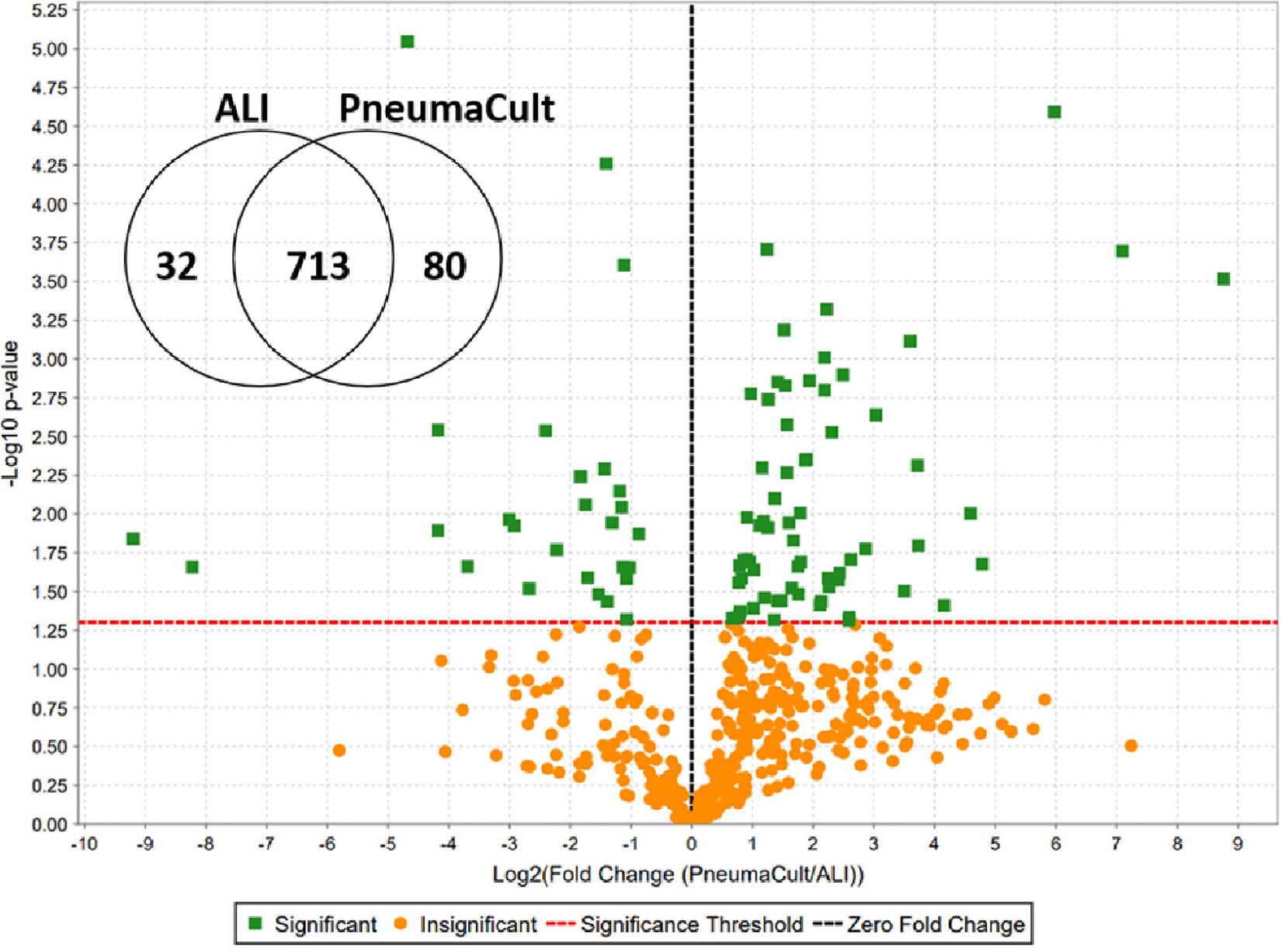
Among 825 identified apical secretome proteins, 80 and 32 were significantly increased and decreased, respectively in airway epithelial ALI cultures grown in PneumaCult ALI versus UNC ALI media. Each green dot represents a protein with significantly altered signal abundance in the volcano plot.
Factors in the culture conditions that determine mucin composition and their interacting proteins (e.g., donor prior health or disease status, smoking history, and cell differentiation conditions) will also affect mucus layer properties. For instance, in most cases cell cultures derived from a healthy non-smoker non-allergic/asthmatic donor grown in UNC ALI media will secrete little to no MUC5AC [142]. This agrees with what is observed in induced sputum from healthy individuals [17]. Some donor cells secrete MUC5AC significantly more when they are challenged with certain cytokines (e.g., IL1B, IL13) or grown in certain media (e.g., PneumaCult ALI media). Indeed, figure 4 shows that the abundance of over a hundred proteins were significantly affected by culture media choice. Pathway analysis of the proteins that were decreased in PneumaCult ALI conditions indicated that innate immune defense proteins were most affected while the sets of significantly increased proteins were part of insulin signaling and signal amplification pathways, pointing towards growth and metabolism increases in PneumaCult ALI culture conditions. Depending on the particular study focus, culture conditions may impact measured endpoints and thus the ability to detect treatment differences.
Finally, the culture maintenance schedule should be carefully planned. A combination of extracellular matrix coating, cell seeding density, incubator conditions, and media change frequency will alter the properties and potential of expanded cells to generate well-differentiated ALI cultures. In vivo, mucus clearance is directional, with cilia continuously propelling mucus from the nose towards the pharynx and up and out of the lungs via the tracheobronchial airways. Though cilia beat and mucociliary transport is well modeled by the ALI culture system, total mucus clearance cannot be achieved without culture washing. Given the importance of the mucus layer for drug delivery, investigators should maintain a regular washing schedule. Mucus can be removed from ALI cultures with regular PBS washes (~1/week) or treatment with mucolytics like tris(2-carboxyethyl)phosphine (TCEP) [143]. The washing strategy may be altered to more or less mimic the expected in vivo conditions. Overall, the choice of ALI culture methods impacts ASL composition, mucus secretion rates, tight junction formation, electrical resistance, and cell type differentiation, all of which influence topical therapeutic delivery and response.
Additional thoughts on ALI culture experimental design
A necessary question that must be considered when designing an experiment is ‘What in vivo state are you trying to model?’ Are you trying to model a healthy baseline state or an inflamed disease state? Careful consideration of this issue is imperative to guide model selection and decisions about culture technique. For example, increases in mucus secretion rates and, more specifically, upregulation of MUC5AC is a hallmark of major airway diseases [17]. However, upregulation of MUC5AC is observed in ALI cultures from previously healthy donors when cultured in PneumaCult ALI differentiation media versus other media choices (Figure 4) [139]. In addition, the main determinants of mucus properties, such as composition of mucins and their interacting proteins can be manipulated to mimic disease conditions of type 1 and type 2 inflammation by challenging the cell cultures with IL1B and IL-13, respectively [144, 145].
Studying a disease state may be challenging if well annotated diseased airway tissue cannot be readily obtained or if extended ALI culture results in a waning of the in vivo disease phenotype. For example, though the CF disease phenotype of reduced CFTR function is preserved in ALI cultures, Ribeiro and colleagues found that the hyperinflammatory state of the epithelium in CF lungs waned during in vitro culture. This group also found that this feature could be reproduced in vitro by treating ALI cultures derived from CF or previously healthy donors with supernatant from mucopurulent material (SMM) from CF airways [146]. Likewise, the clinical phenotype in diseases such as asthma and COPD with complex environmental and multigenic causes are less likely to be maintained in vitro. However, an option is mimicking disease states by treating ALI cultures from previously healthy or disease-state donors with cytokines. The application of Th2 cytokines such as IL-4 and IL-13 causes changes in bronchial epithelial cell differentiation reminiscent of asthma, leading to goblet cell hyperplasia, increased mucin production and hypersecretion [147–149]. Ultimately, successful in vitro assessment of drug delivery strategies begins and ends with the selection of an appropriate model system for the intended purpose.
Summary and outstanding questions
With daily exposures to pathogens, particulates, and other harmful materials, the airways and lungs employ numerous protective mechanisms to maintain respiratory tract homeostasis and health. Key among these defense systems is the production and maintenance of well-hydrated airway mucus and effective mucociliary clearance. Though vital for health, these protective mechanisms may limit the efficacy of inhaled therapies relying on cellular uptake of particulate moieties. The human airway epithelial ALI cell culture model has been instrumental for discovery of small molecule CFTR modulators [150] and also predicted the low clinical efficacy of luminally applied viral and non-viral CF gene therapy vectors [47]. It is a reasonable assumption that the model will similarly be useful to predict the efficacy of evolving inhaled airway-directed therapies such as nanoparticles, exosomes or cell therapy. An important remaining question is whether the human airway ALI epithelial culture model is relatively susceptible or resistant to the therapy of interest compared to the in vivo epithelium. No in vitro model will precisely replicate the in vivo state, and it is an open debate as to which of the many technical options for ALI cultures best achieves this goal. Despite the well-established utility of the human airway epithelial ALI culture model, it is important to understand its limitations and to be circumspect about the direct translation of ALI culture results to in vivo conditions. Animal model testing in vivo remains a vital translational link for the development of novel therapeutics.
Acknowledgements
We are grateful to Sarah Towner Wright for guidance in developing a PubMed strategy. We thank Eric Roe for assisting with reference formatting. Supported by Cystic Fibrosis Foundation grants RANDEL20XX2 and KESIME22G0 and NIH grants 1T32GM133364, 1F31HL158197, and 2P30DK065988.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].World Health Organization, Global Health Estimates: Life expectancy and leading causes of death and disability, The Global Health Observatory, World Health Organization, 2019. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed Aug. 26, 2022). [Google Scholar]
- [2].Frisch AW, Jentoft V, Barger R, Losli EJ, A human epithelium-like cell (maben) derived from an adenocarcinoma of lung; isolation, continuous propagation, and effects of selected viruses, Am. J. Clin. Pathol, 25 (1955) 1107–1112. [DOI] [PubMed] [Google Scholar]
- [3].Hoch-Ligeti C, Hobbs JP, Behavior of explants from human adult bronchial epithelium in vitro, Proc. Soc. Exp. Biol. Med, 97 (1958) 59–62. [DOI] [PubMed] [Google Scholar]
- [4].Lechner JF, LaVeck MA, A serum-free method for culturing normal human bronchial epithelial cells at clonal density, J. Tissue Cult. Methods, 9 (1985) 43–48. [Google Scholar]
- [5].Wu R, Yankaskas J, Cheng E, Knowles MR, Boucher R, Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis, Am. Rev. Respir. Dis, 132 (1985) 311–320. [DOI] [PubMed] [Google Scholar]
- [6].Whitcutt MJ, Adler KB, Wu R, A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells, In Vitro Cell. Dev. Biol, 24 (1988) 420–428. [DOI] [PubMed] [Google Scholar]
- [7].Fulcher ML, Randell SH, Human nasal and tracheo-bronchial respiratory epithelial cell culture, Methods Mol. Biol, 945 (2013) 109–121. [DOI] [PubMed] [Google Scholar]
- [8].Helena Macedo M, Baião A, Pinto S, Barros AS, Almeida H, Almeida A, das Neves J, Sarmento B, Mucus-producing 3D cell culture models, Adv. Drug Deliv. Rev, 178 (2021) 113993. [DOI] [PubMed] [Google Scholar]
- [9].Izadifar Z, Sontheimer-Phelps A, Lubamba BA, Bai H, Fadel C, Stejskalova A, Ozkan A, Dasgupta Q, Bein A, Junaid A, Gulati A, Mahajan G, Kim S, LoGrande NT, Naziripour A, Ingber DE, Modeling mucus physiology and pathophysiology in human organs-on-chips, Adv. Drug Deliv. Rev, 191 (2022) 114542. [DOI] [PubMed] [Google Scholar]
- [10].Huck BC, Murgia X, Frisch S, Hittinger M, Hidalgo A, Loretz B, Lehr CM, Models using native tracheobronchial mucus in the context of pulmonary drug delivery research: Composition, structure and barrier properties, Adv. Drug Deliv. Rev, 183 (2022) 114141. [DOI] [PubMed] [Google Scholar]
- [11].Hill DB, Button B, Rubinstein M, Boucher RC, Physiology and Pathophysiology of Human Airway Mucus, Physiol. Rev, 102 (2022) 1757–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M, A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia, Science, 337 (2012) 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boucher RC, Muco-Obstructive Lung Diseases, N. Engl. J. Med, 380 (2019) 1941–1953. [DOI] [PubMed] [Google Scholar]
- [14].Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, Powers LS, Gansemer ND, Bouzek DC, Cook DP, Meyerholz DK, Abou Alaiwa MH, Stoltz DA, Welsh MJ, Gel-forming mucins form distinct morphologic structures in airways, Proc. Natl. Acad. Sci. U. S. A, 114 (2017) 6842–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ermund A, Meiss LN, Rodriguez-Pineiro AM, Bahr A, Nilsson HE, Trillo-Muyo S, Ridley C, Thornton DJ, Wine JJ, Hebert H, Klymiuk N, Hansson GC, The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin, Biochem. Biophys. Res. Commun, 492 (2017) 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez F, Paine R 3rd, Woodruff PG, O’Neal WK, Boucher RC, Airway Mucin Concentration as a Marker of Chronic Bronchitis, N. Engl. J. Med, 377 (2017) 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Radicioni G, Ceppe A, Ford AA, Alexis NE, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez FJ, Ozkan E, Paine R 3rd, Woodruff PG, O’Neal WK, Boucher RC, Kesimer M, Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort, Lancet Respir. Med, 9 (2021) 1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Batson B, Zorn B, Radicioni G, Livengood S, Kumagai T, Dang H, Ceppe A, Clapp P, Tunney M, Elborn S, McElvaney G, Muhlebach M, Boucher RC, Tiemeyer M, Wolfgang M, Kesimer M, Cystic Fibrosis Airway Mucus Hyperconcentration Produces a Vicious Cycle of Mucin, Pathogen, and Inflammatory Interactions that Promote Disease Persistence, Am. J. Respir. Cell Mol. Biol, 67 (2022) 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ramsey KA, Chen ACH, Radicioni G, Lourie R, Martin M, Broomfield A, Sheng YH, Hasnain SZ, Radford-Smith G, Simms LA, Burr L, Thornton DJ, Bowler SD, Livengood S, Ceppe A, Knowles MR, Noone PGS, Donaldson SH, Hill DB, Ehre C, Button B, Alexis NE, Kesimer M, Boucher RC, McGuckin MA, Airway Mucus Hyperconcentration in Non-Cystic Fibrosis Bronchiectasis, Am. J. Respir. Crit. Care Med, 201 (2020) 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ, Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways, Mucosal Immunol, 6 (2013) 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carpenter J, Kesimer M, Membrane-bound mucins of the airway mucosal surfaces are densely decorated with keratan sulfate: revisiting their role in the Lung’s innate defense, Glycobiology, 31 (2021) 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lieleg O, Ribbeck K, Biological hydrogels as selective diffusion barriers, Trends Cell Biol, 21 (2011) 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sanders N, Rudolph C, Braeckmans K, De Smedt SC, Demeester J, Extracellular barriers in respiratory gene therapy, Adv. Drug Deliv. Rev, 61 (2009) 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, Grubb BR, Boucher RC, Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces, J. Immunol, 175 (2005) 1090–1099. [DOI] [PubMed] [Google Scholar]
- [25].Radicioni G, Cao R, Carpenter J, Ford AA, Wang T, Li L, Kesimer M, The innate immune properties of airway mucosal surfaces are regulated by dynamic interactions between mucins and interacting proteins: the mucin interactome, Mucosal Immunol., 9 (2016) 1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA, Diffusion of macromolecules and virus-like particles in human cervical mucus, Biophys. J, 81 (2001) 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang YY, Kannan A, Nunn KL, Murphy MA, Subramani DB, Moench T, Cone R, Lai SK, IgG in cervicovaginal mucus traps HSV and prevents vaginal herpes infections, Mucosal Immunol, 7 (2014) 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dawson M, Wirtz D, Hanes J, Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport, J. Biol. Chem, 278 (2003) 50393–50401. [DOI] [PubMed] [Google Scholar]
- [29].Murgia X, Pawelzyk P, Schaefer UF, Wagner C, Willenbacher N, Lehr CM, Size-Limited Penetration of Nanoparticles into Porcine Respiratory Mucus after Aerosol Deposition, Biomacromolecules, 17 (2016) 1536–1542. [DOI] [PubMed] [Google Scholar]
- [30].Saltzman WM, Radomsky ML, Whaley KJ, Cone RA, Antibody diffusion in human cervical mucus, Biophys. J, 66 (1994) 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Leal J, Smyth HDC, Ghosh D, Physicochemical properties of mucus and their impact on transmucosal drug delivery, Int. J. Pharm, 532 (2017) 555–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bhaskar KR, Gong DH, Bansil R, Pajevic S, Hamilton JA, Turner BS, LaMont JT, Profound increase in viscosity and aggregation of pig gastric mucin at low pH, Am. J. Physiol, 261 (1991) G827–832. [DOI] [PubMed] [Google Scholar]
- [33].Lieleg O, Vladescu I, Ribbeck K, Characterization of particle translocation through mucin hydrogels, Biophys. J, 98 (2010) 1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carpenter J, Wang Y, Gupta R, Li Y, Haridass P, Subramani DB, Reidel B, Morton L, Ridley C, O’Neal WK, Buisine MP, Ehre C, Thornton DJ, Kesimer M, Assembly and organization of the N-terminal region of mucin MUC5AC: Indications for structural and functional distinction from MUC5B, Proc. Natl. Acad. Sci. U. S. A, 118 (2021) e2104490118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL, Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum, Proc. Natl. Acad. Sci. U. S. A, 87 (1990) 9188–9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yang C, Montgomery M, Dornase alfa for cystic fibrosis, Cochrane Database Syst. Rev., 3 (2021) Cd001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A, Neutrophil extracellular traps kill bacteria, Science, 303 (2004) 1532–1535. [DOI] [PubMed] [Google Scholar]
- [38].Sollberger G, Tilley DO, Zychlinsky A, Neutrophil Extracellular Traps: The Biology of Chromatin Externalization, Dev. Cell, 44 (2018) 542–553. [DOI] [PubMed] [Google Scholar]
- [39].Morán G, Uberti B, Quiroga J, Role of Cellular Metabolism in the Formation of Neutrophil Extracellular Traps in Airway Diseases, Front. Immunol, 13 (2022) 850416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pandolfi L, Bozzini S, Frangipane V, Percivalle E, De Luigi A, Violatto MB, Lopez G, Gabanti E, Carsana L, D’Amato M, Morosini M, De Amici M, Nebuloni M, Fossali T, Colombo R, Saracino L, Codullo V, Gnecchi M, Bigini P, Baldanti F, Lilleri D, Meloni F, Neutrophil Extracellular Traps Induce the Epithelial-Mesenchymal Transition: Implications in Post-COVID-19 Fibrosis, Front. Immunol, 12 (2021) 663303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Terryah ST, Fellner RC, Ahmad S, Moore PJ, Reidel B, Sesma JI, Kim CS, Garland AL, Scott DW, Sabater JR, Carpenter J, Randell SH, Kesimer M, Abraham WM, Arendshorst WJ, Tarran R, Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease, Am. J. Physiol. Lung Cell Mol. Physiol, 314 (2018) L192–L205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hewitt RJ, Lloyd CM, Regulation of immune responses by the airway epithelial cell landscape, Nat. Rev. Immunol, 21 (2021) 347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Quiros M, Nusrat A, RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex, Semin. Cell Dev. Biol, 36 (2014) 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ramalho AS, Boon M, Proesmans M, Vermeulen F, Carlon MS, Boeck K, Assays of CFTR Function In Vitro, Ex Vivo and In Vivo, Int. J. Mol. Sci, 23 (2022) 1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Akdis CA, Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions?, Nat. Rev. Immunol, 21 (2021) 739–751. [DOI] [PubMed] [Google Scholar]
- [46].Abu Khweek A, Kim E, Joldrichsen MR, Amer AO, Boyaka PN, Insights Into Mucosal Innate Immune Responses in House Dust Mite-Mediated Allergic Asthma, Front. Immunol, 11 (2020) 534501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lee TW, Southern KW, Perry LA, Penny-Dimri JC, Aslam AA, Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease, Cochrane Database Syst. Rev., (2016) CD005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McCarron A, Cmielewski P, Drysdale V, Parsons D, Donnelley M, Effective viral-mediated lung gene therapy: is airway surface preparation necessary?, Gene Ther, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].King JA, Nichols AL, Bentley S, Carr SB, Davies JC, An Update on CFTR Modulators as New Therapies for Cystic Fibrosis, Paediatr. Drugs, 24 (2022) 321–333. [DOI] [PubMed] [Google Scholar]
- [50].Haq I, Almulhem M, Soars S, Poulton D, Brodlie M, Precision Medicine Based on CFTR Genotype for People with Cystic Fibrosis, Pharmgenomics Pers. Med., 15 (2022) 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P, Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770, Proc. Natl. Acad. Sci. U. S. A, 106 (2009) 18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS, A CFTR potentiator in patients with cystic fibrosis and the G551D mutation, N. Engl. J. Med, 365 (2011) 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian S, Waltz D, Xuan F, Rowe SM, Jain R, Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele, N. Engl. J. Med, 381 (2019) 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, Simard C, Han L, Ingenito EP, McKee C, Lekstrom-Himes J, Davies JC, Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis, N. Engl. J. Med, 377 (2017) 2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, Wang LT, Ingenito EP, McKee C, Lu Y, Lekstrom-Himes J, Elborn JS, Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del, N. Engl. J. Med, 377 (2017) 2013–2023. [DOI] [PubMed] [Google Scholar]
- [56].Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP, Traffic Study Group, Transport Study Group, Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR, N. Engl. J. Med, 373 (2015) 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Guerra L, Favia M, Di Gioia S, Laselva O, Bisogno A, Casavola V, Colombo C, Conese M, The preclinical discovery and development of the combination of ivacaftor + tezacaftor used to treat cystic fibrosis, Expert. Opin. Drug Discov, 15 (2020) 873–891. [DOI] [PubMed] [Google Scholar]
- [58].Silva IAL, Railean V, Duarte A, Amaral MD, Personalized Medicine Based on Nasal Epithelial Cells: Comparative Studies with Rectal Biopsies and Intestinal Organoids, J. Pers. Med., 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA, Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809, Proc. Natl. Acad. Sci. U. S. A, 108 (2011) 18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McCarthy C, Brewington JJ, Harkness B, Clancy JP, Trapnell BC, Personalised CFTR pharmacotherapeutic response testing and therapy of cystic fibrosis, Eur. Respir. J, 51 (2018). [DOI] [PubMed] [Google Scholar]
- [61].US Food & Drug Administration (FDA), Drugs@FDA: FDA-approved drugs, US Food & Drug Administration, 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=203188 (accessed June 7, 2022). [Google Scholar]
- [62].Pilewski JM, Sott DJ, Wilson JM, Albelda SM, ICAM-1 expression on bronchial epithelium after recombinant adenovirus infection, Am. J. Respir. Cell Mol. Biol, 12 (1995) 142–148. [DOI] [PubMed] [Google Scholar]
- [63].Coyne CB, Kelly MM, Boucher RC, Johnson LG, Enhanced epithelial gene transfer by modulation of tight junctions with sodium caprate, Am. J. Respir. Cell Mol. Biol, 23 (2000) 602–609. [DOI] [PubMed] [Google Scholar]
- [64].Coyne CB, Ribeiro CM, Boucher RC, Johnson LG, Acute mechanism of medium chain fatty acid-induced enhancement of airway epithelial permeability, J. Pharmacol. Exp. Ther, 305 (2003) 440–450. [DOI] [PubMed] [Google Scholar]
- [65].Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J, Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia, J. Biol. Chem, 274 (1999) 10219–10226. [DOI] [PubMed] [Google Scholar]
- [66].Pickles RJ, Barker PM, Ye H, Boucher RC, Efficient adenovirus-mediated gene transfer to basal but not columnar cells of cartilaginous airway epithelia, Hum. Gene Ther, 7 (1996) 921–931. [DOI] [PubMed] [Google Scholar]
- [67].Park JA, Kim JH, Bi D, Mitchel JA, Qazvini NT, Tantisira K, Park CY, McGill M, Kim SH, Gweon B, Notbohm J, Steward R Jr., Burger S, Randell SH, Kho AT, Tambe DT, Hardin C, Shore SA, Israel E, Weitz DA, Tschumperlin DJ, Henske EP, Weiss ST, Manning ML, Butler JP, Drazen JM, Fredberg JJ, Unjamming and cell shape in the asthmatic airway epithelium, Nat Mater, 14 (2015) 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gras D, Martinez-Anton A, Bourdin A, Garulli C, de Senneville L, Vachier I, Vitte J, Chanez P, Human bronchial epithelium orchestrates dendritic cell activation in severe asthma, Eur. Respir. J, 49 (2017). [DOI] [PubMed] [Google Scholar]
- [69].Siddiqui S, Johansson K, Joo A, Bonser LR, Koh KD, Le Tonqueze O, Bolourchi S, Bautista RA, Zlock L, Roth TL, Marson A, Bhakta NR, Ansel KM, Finkbeiner WE, Erle DJ, Woodruff PG, Epithelial miR-141 regulates IL-13-induced airway mucus production, JCI Insight, 6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Amatngalim GD, Schrumpf JA, Henic A, Dronkers E, Verhoosel RM, Ordonez SR, Haagsman HP, Fuentes ME, Sridhar S, Aarbiou J, Janssen RAJ, Lekkerkerker AN, Hiemstra PS, Antibacterial Defense of Human Airway Epithelial Cells from Chronic Obstructive Pulmonary Disease Patients Induced by Acute Exposure to Nontypeable Haemophilus influenzae: Modulation by Cigarette Smoke, J. Innate Immun., 9 (2017) 359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang YY, Lin CJ, Wang CC, Chen CM, Kao WJ, Chen YH, Consecutive Hypoxia Decreases Expression of NOTCH3, HEY1, CC10, and FOXJ1 via NKX2–1 Downregulation and Intermittent Hypoxia-Reoxygenation Increases Expression of BMP4, NOTCH1, MKI67, OCT4, and MUC5AC via HIF1A Upregulation in Human Bronchial Epithelial Cells, Front. Cell Dev. Biol, 8 (2020) 572276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bodas M, Moore AR, Subramaniyan B, Georgescu C, Wren JD, Freeman WM, Brown BR, Metcalf JP, Walters MS, Cigarette Smoke Activates NOTCH3 to Promote Goblet Cell Differentiation in Human Airway Epithelial Cells, Am. J. Respir. Cell Mol. Biol, 64 (2021) 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Nakano S, Sun L, Fulcher ML, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC, Baric RS, SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract, Cell, 182 (2020) 429–446.e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Abdelnabi R, Foo CS, Jochmans D, Vangeel L, De Jonghe S, Augustijns P, Mols R, Weynand B, Wattanakul T, Hoglund RM, Tarning J, Mowbray CE, Sjö P, Escudié F, Scandale I, Chatelain E, Neyts J, The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern, Nat. Commun, 13 (2022) 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Guo W, Porter LM, Crozier TW, Coates M, Jha A, McKie M, Nathan JA, Lehner PJ, Greenwood EJ, McCaughan F, Topical TMPRSS2 inhibition prevents SARS-CoV-2 infection in differentiated human airway cultures, Life Sci. Alliance, 5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jonsdottir HR, Siegrist D, Julien T, Padey B, Bouveret M, Terrier O, Pizzorno A, Huang S, Samby K, Wells TNC, Boda B, Rosa-Calatrava M, Engler OB, Constant S, Molnupiravir combined with different repurposed drugs further inhibits SARS-CoV-2 infection in human nasal epithelium in vitro, Biomed. Pharmacother, 150 (2022) 113058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yonker LM, Mou H, Chu KK, Pazos MA, Leung H, Cui D, Ryu J, Hibbler RM, Eaton AD, Ford TN, Falck JR, Kinane TB, Tearney GJ, Rajagopal J, Hurley BP, Development of a Primary Human Co-Culture Model of Inflamed Airway Mucosa, Sci. Rep, 7 (2017) 8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pazos MA, Pirzai W, Yonker LM, Morisseau C, Gronert K, Hurley BP, Distinct cellular sources of hepoxilin A3 and leukotriene B4 are used to coordinate bacterial-induced neutrophil transepithelial migration, J. Immunol, 194 (2015) 1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ronaghan NJ, Soo M, Pena U, Tellis M, Duan W, Tabatabaei-Zavareh N, Kramer P, Hou J, Moraes TJ, M1-like, but not M0- or M2-like, macrophages, reduce RSV infection of primary bronchial epithelial cells in a media-dependent fashion, PLoS One, 17 (2022) e0276013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Paw M, Wnuk D, Jakieła B, Bochenek G, Sładek K, Madeja Z, Michalik M, Responsiveness of human bronchial fibroblasts and epithelial cells from asthmatic and non-asthmatic donors to the transforming growth factor-β(1) in epithelial-mesenchymal trophic unit model, BMC Mol. Cell. Biol, 22 (2021) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lenz AG, Stoeger T, Cei D, Schmidmeir M, Semren N, Burgstaller G, Lentner B, Eickelberg O, Meiners S, Schmid O, Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air-liquid interface conditions, Am. J. Respir. Cell Mol. Biol, 51 (2014) 526–535. [DOI] [PubMed] [Google Scholar]
- [82].Goralski JL, Wu D, Thelin WR, Boucher RC, Button B, The in vitro effect of nebulised hypertonic saline on human bronchial epithelium, Eur. Respir. J, 51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr., Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M, Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis, Sci. Transl. Med, 6 (2014) 246ra296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH, Well-differentiated human airway epithelial cell cultures, Methods Mol. Med, 107 (2005) 183–206. [DOI] [PubMed] [Google Scholar]
- [85].Saint-Criq V, Wang Y, Delpiano L, Lin J, Sheppard DN, Gray MA, Extracellular phosphate enhances the function of F508del-CFTR rescued by CFTR correctors, J. Cyst. Fibros, 20 (2021) 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hartwig O, Loretz B, Nougarede A, Jary D, Sulpice E, Gidrol X, Navarro F, Lehr CM, Leaky gut model of the human intestinal mucosa for testing siRNA-based nanomedicine targeting JAK1, J. Control. Release, 345 (2022) 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gordon S, Daneshian M, Bouwstra J, Caloni F, Constant S, Davies DE, Dandekar G, Guzman CA, Fabian E, Haltner E, Hartung T, Hasiwa N, Hayden P, Kandarova H, Khare S, Krug HF, Kneuer C, Leist M, Lian G, Marx U, Metzger M, Ott K, Prieto P, Roberts MS, Roggen EL, Tralau T, van den Braak C, Walles H, Lehr CM, Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology, Altex, 32 (2015) 327–378. [DOI] [PubMed] [Google Scholar]
- [88].Fulcher ML, Gabriel SE, Olsen JC, Tatreau JR, Gentzsch M, Livanos E, Saavedra MT, Salmon P, Randell SH, Novel human bronchial epithelial cell lines for cystic fibrosis research, Am. J. Physiol. Lung Cell Mol. Physiol, 296 (2009) L82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sheikh Z, Bradbury P, Pozzoli M, Young PM, Ong HX, Traini D, An in vitro model for assessing drug transport in cystic fibrosis treatment: Characterisation of the CuFi-1 cell line, Eur. J. Pharm. Biopharm, 156 (2020) 121–130. [DOI] [PubMed] [Google Scholar]
- [90].Walters MS, Gomi K, Ashbridge B, Moore MA, Arbelaez V, Heldrich J, Ding BS, Rafii S, Staudt MR, Crystal RG, Generation of a human airway epithelium derived basal cell line with multipotent differentiation capacity, Respir. Res., 14 (2013) 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, Welsh M, Klingelhutz AJ, Development of cystic fibrosis and noncystic fibrosis airway cell lines, Am. J. Physiol. Lung Cell Mol. Physiol, 284 (2003) L844–854. [DOI] [PubMed] [Google Scholar]
- [92].Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC, CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells, Am. J. Respir. Cell Mol. Biol, 10 (1994) 38–47. [DOI] [PubMed] [Google Scholar]
- [93].Valley HC, Bukis KM, Bell A, Cheng Y, Wong E, Jordan NJ, Allaire NE, Sivachenko A, Liang F, Bihler H, Thomas PJ, Mahiou J, Mense M, Isogenic cell models of cystic fibrosis-causing variants in natively expressing pulmonary epithelial cells, J. Cyst. Fibros., 18 (2019) 476–483. [DOI] [PubMed] [Google Scholar]
- [94].Ehrhardt C, Collnot EM, Baldes C, Becker U, Laue M, Kim KJ, Lehr CM, Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o, Cell Tissue Res, 323 (2006) 405–415. [DOI] [PubMed] [Google Scholar]
- [95].Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O’Toole GA, Stanton BA, The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability, Am. J. Physiol. Lung Cell Mol. Physiol, 295 (2008) L25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Munye MM, Shoemark A, Hirst RA, Delhove JM, Sharp TV, McKay TR, O’Callaghan C, Baines DL, Howe SJ, Hart SL, BMI-1 extends proliferative potential of human bronchial epithelial cells while retaining their mucociliary differentiation capacity, Am. J. Physiol. Lung Cell Mol. Physiol, 312 (2017) L258–l267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lee RE, Lewis CA, He L, Bulik-Sullivan EC, Gallant SC, Mascenik TM, Dang H, Cholon DM, Gentzsch M, Morton LC, Minges JT, Theile JW, Castle NA, Knowles MR, Kimple AJ, Randell SH, Small molecule eRF3a degraders rescue CFTR nonsense mutations by promoting premature termination codon readthrough, J. Clin. Invest, 132 (2022) e154571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lee DF, Lethem MI, Lansley AB, A comparison of three mucus-secreting airway cell lines (Calu-3, SPOC1 and UNCN3T) for use as biopharmaceutical models of the nose and lung, Eur. J. Pharm. Biopharm, 167 (2021) 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell, 126 (2006) 663–676. [DOI] [PubMed] [Google Scholar]
- [100].Chen YW, Huang SX, de Carvalho A, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, Liang AF, Palermo LM, Porotto M, Moscona A, Snoeck HW, A three-dimensional model of human lung development and disease from pluripotent stem cells, Nat. Cell Biol, 19 (2017) 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, White ES, Deutsch GH, Spence JR, In vitro generation of human pluripotent stem cell derived lung organoids, Elife, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, Verma IM, Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells, Proc. Natl. Acad. Sci. U. S. A, 111 (2014) E1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hawkins F, Kramer P, Jacob A, Driver I, Thomas DC, McCauley KB, Skvir N, Crane AM, Kurmann AA, Hollenberg AN, Nguyen S, Wong BG, Khalil AS, Huang SX, Guttentag S, Rock JR, Shannon JM, Davis BR, Kotton DN, Prospective isolation of NKX2–1-expressing human lung progenitors derived from pluripotent stem cells, J. Clin. Invest, 127 (2017) 2277–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hawkins FJ, Suzuki S, Beermann ML, Barillà C, Wang R, Villacorta-Martin C, Berical A, Jean JC, Le Suer J, Matte T, Simone-Roach C, Tang Y, Schlaeger TM, Crane AM, Matthias N, Huang SXL, Randell SH, Wu J, Spence JR, Carraro G, Stripp BR, Rab A, Sorsher EJ, Horani A, Brody SL, Davis BR, Kotton DN, Derivation of Airway Basal Stem Cells from Human Pluripotent Stem Cells, Cell Stem Cell, 28 (2021) 79–95.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW, Efficient generation of lung and airway epithelial cells from human pluripotent stem cells, Nat. Biotechnol, 32 (2014) 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Jacob A, Vedaie M, Roberts DA, Thomas DC, Villacorta-Martin C, Alysandratos KD, Hawkins F, Kotton DN, Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells, Nat. Protoc, 14 (2019) 3303–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Konishi S, Gotoh S, Tateishi K, Yamamoto Y, Korogi Y, Nagasaki T, Matsumoto H, Muro S, Hirai T, Ito I, Tsukita S, Mishima M, Directed Induction of Functional Multi-ciliated Cells in Proximal Airway Epithelial Spheroids from Human Pluripotent Stem Cells, Stem Cell Rep, 6 (2016) 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN, Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling, Cell Stem Cell, 20 (2017) 844–857 e846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Miller AJ, Hill DR, Nagy MS, Aoki Y, Dye BR, Chin AM, Huang S, Zhu F, White ES, Lama V, Spence JR, In Vitro Induction and In Vivo Engraftment of Lung Bud Tip Progenitor Cells Derived from Human Pluripotent Stem Cells, Stem Cell Rep, 10 (2018) 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, Rajagopal J, Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs, Cell Stem Cell, 10 (2012) 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J, Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein, Nat. Biotechnol, 30 (2012) 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Berical A, Lee RE, Lu J, Beermann ML, Le Suer JA, Mithal A, Thomas D, Ranallo N, Peasley M, Stuffer A, Bukis K, Seymour R, Harrington J, Coote K, Valley H, Hurley K, McNally P, Mostoslavsky G, Mahoney J, Randell SH, Hawkins FJ, A multimodal iPSC platform for cystic fibrosis drug testing, Nat. Commun, 13 (2022) 4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gentzsch M, Boyles SE, Cheluvaraju C, Chaudhry IG, Quinney NL, Cho C, Dang H, Liu X, Schlegel R, Randell SH, Pharmacological Rescue of Conditionally Reprogrammed Cystic Fibrosis Bronchial Epithelial Cells, Am. J. Respir. Cell Mol. Biol, 56 (2017) 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Randell SH, Fulcher ML, O’Neal W, Olsen JC, Primary epithelial cell models for cystic fibrosis research, Methods Mol. Biol, 742 (2011) 285–310. [DOI] [PubMed] [Google Scholar]
- [115].Van Goor F, Straley KS, Cao D, González J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PD, Negulescu P, Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules, Am. J. Physiol. Lung Cell Mol. Physiol, 290 (2006) L1117–1130. [DOI] [PubMed] [Google Scholar]
- [116].Sheridan JT, Gilmore RC, Watson MJ, Archer CB, Tarran R, 17beta-Estradiol inhibits phosphorylation of stromal interaction molecule 1 (STIM1) protein: implication for store-operated calcium entry and chronic lung diseases, J. Biol. Chem, 288 (2013) 33509–33518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hyde DM, Hamid Q, Irvin CG, Anatomy, pathology, and physiology of the tracheobronchial tree: emphasis on the distal airways, J. Allergy Clin. Immunol, 124 (2009) S72–77. [DOI] [PubMed] [Google Scholar]
- [118].Okuda K, Dang H, Kobayashi Y, Carraro G, Nakano S, Chen G, Kato T, Asakura T, Gilmore RC, Morton LC, Lee RE, Mascenik T, Yin WN, Barbosa Cardenas SM, O’Neal YK, Minnick CE, Chua M, Quinney NL, Gentzsch M, Anderson CW, Ghio A, Matsui H, Nagase T, Ostrowski LE, Grubb BR, Olsen JC, Randell SH, Stripp BR, Tata PR, O’Neal WK, Boucher RC, Secretory Cells Dominate Airway CFTR Expression and Function in Human Airway Superficial Epithelia, Am. J. Respir. Crit. Care Med, 203 (2021) 1275–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Brewington JJ, Filbrandt ET, LaRosa FJ 3rd, Moncivaiz JD, Ostmann AJ, Strecker LM, Clancy JP, Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies, JCI Insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mihaylova VT, Kong Y, Fedorova O, Sharma L, Dela Cruz CS, Pyle AM, Iwasaki A, Foxman EF, Regional Differences in Airway Epithelial Cells Reveal Tradeoff between Defense against Oxidative Stress and Defense against Rhinovirus, Cell Rep, 24 (2018) 3000–3007.e3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Stevens MA, Rabe KG, Boursi B, Kolluri A, Singh DP, Bamlet WR, Petersen GM, Accuracy of Smoking Status Reporting: Proxy Information in a Rapidly Fatal Cancer Setting, Mayo Clin. Proc. Innov. Qual. Outcomes, 4 (2020) 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Gohy S, Carlier FM, Fregimilicka C, Detry B, Lecocq M, Ladjemi MZ, Verleden S, Hoton D, Weynand B, Bouzin C, Pilette C, Altered generation of ciliated cells in chronic obstructive pulmonary disease, Sci. Rep, 9 (2019) 17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sajjan U, Keshavjee S, Forstner J, Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection, Infect. Immun, 72 (2004) 4188–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, Cremin C, Sones J, Djukanović R, Howarth PH, Collins JE, Holgate ST, Monk P, Davies DE, Defective epithelial barrier function in asthma, J. Allergy Clin. Immunol, 128 (2011) 549–556.e541–512. [DOI] [PubMed] [Google Scholar]
- [125].Cho JL, Ling MF, Adams DC, Faustino L, Islam SA, Afshar R, Griffith JW, Harris RS, Ng A, Radicioni G, Ford AA, Han AK, Xavier R, Kwok WW, Boucher R, Moon JJ, Hamilos DL, Kesimer M, Suter MJ, Medoff BD, Luster AD, Allergic asthma is distinguished by sensitivity of allergen-specific CD4+ T cells and airway structural cells to type 2 inflammation, Sci. Transl. Med, 8 (2016) 359ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hammad H, Lambrecht BN, The basic immunology of asthma, Cell, 184 (2021) 1469–1485. [DOI] [PubMed] [Google Scholar]
- [127].Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, Curtis JL, Martinez FJ, Hershenson MB, Sajjan U, Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease, Am. J. Respir. Crit. Care Med, 182 (2010) 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bayram H, Devalia JL, Khair OA, Abdelaziz MM, Sapsford RJ, Sagai M, Davies RJ, Comparison of ciliary activity and inflammatory mediator release from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients and the effect of diesel exhaust particles in vitro, J. Allergy Clin. Immunol, 102 (1998) 771–782. [DOI] [PubMed] [Google Scholar]
- [129].Devalia JL, Sapsford RJ, Rusznak C, Toumbis MJ, Davies RJ, The effects of salmeterol and salbutamol on ciliary beat frequency of cultured human bronchial epithelial cells, in vitro, Pulm. Pharmacol, 5 (1992) 257–263. [DOI] [PubMed] [Google Scholar]
- [130].Rickard KA, Shoji S, Spurzem JR, Rennard SI, Attachment characteristics of bovine bronchial epithelial cells to extracellular matrix components, Am. J. Respir. Cell Mol. Biol, 4 (1991) 440–448. [DOI] [PubMed] [Google Scholar]
- [131].Hamilton NJI, Lee DDH, Gowers KHC, Butler CR, Maughan EF, Jevans B, Orr JC, McCann CJ, Burns AJ, MacNeil S, Birchall MA, O’Callaghan C, Hynds RE, Janes SM, Bioengineered airway epithelial grafts with mucociliary function based on collagen IV- and laminin-containing extracellular matrix scaffolds, Eur. Respir. J, 55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Choe MM, Tomei AA, Swartz MA, Physiological 3D tissue model of the airway wall and mucosa, Nat. Protoc, 1 (2006) 357–362. [DOI] [PubMed] [Google Scholar]
- [133].Wu L, Magaz A, Huo S, Darbyshire A, Loizidou M, Emberton M, Birchall M, Song W, Human airway-like multilayered tissue on 3D-TIPS printed thermoresponsive elastomer/collagen hybrid scaffolds, Acta Biomater, 113 (2020) 177–195. [DOI] [PubMed] [Google Scholar]
- [134].Rayner RE, Makena P, Prasad GL, Cormet-Boyaka E, Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies, Sci. Rep, 9 (2019) 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, Yuan H, Cheluvaraju C, Clapp PW, Boucher RC Jr., Kamonjoh CM, Randell SH, Schlegel R, Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells, Proc. Natl. Acad. Sci. U. S. A, 109 (2012) 20035–20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Lee RE, Miller SM, Mascenik TM, Lewis CA, Dang H, Boggs ZH, Tarran R, Randell SH, Assessing Human Airway Epithelial Progenitor Cells for Cystic Fibrosis Cell Therapy, Am. J. Respir. Cell Mol. Biol, 63 (2020) 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Awatade NT, Wong SL, Capraro A, Pandzic E, Slapetova I, Zhong L, Turgutoglu N, Fawcett LK, Whan RM, Jaffe A, Waters SA, Significant functional differences in differentiated Conditionally Reprogrammed (CRC)- and Feeder-free Dual SMAD inhibited-expanded human nasal epithelial cells, J. Cyst. Fibros, 20 (2021) 364–371. [DOI] [PubMed] [Google Scholar]
- [138].Sellgren KL, Butala EJ, Gilmour BP, Randell SH, Grego S, A biomimetic multicellular model of the airways using primary human cells, Lab Chip, 14 (2014) 3349–3358. [DOI] [PubMed] [Google Scholar]
- [139].Lee DDH, Petris A, Hynds RE, O’Callaghan C, Ciliated Epithelial Cell Differentiation at Air-Liquid Interface Using Commercially Available Culture Media, Methods Mol. Biol., 2109 (2020) 275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Luengen AE, Kniebs C, Buhl EM, Cornelissen CG, Schmitz-Rode T, Jockenhoevel S, Thiebes AL, Choosing the Right Differentiation Medium to Develop Mucociliary Phenotype of Primary Nasal Epithelial Cells In Vitro, Sci. Rep, 10 (2020) 6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Saint-Criq V, Delpiano L, Casement J, Onuora JC, Lin J, Gray MA, Choice of Differentiation Media Significantly Impacts Cell Lineage and Response to CFTR Modulators in Fully Differentiated Primary Cultures of Cystic Fibrosis Human Airway Epithelial Cells, Cells, 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gupta R, Radicioni G, Abdelwahab S, Dang H, Carpenter J, Chua M, Mieczkowski PA, Sheridan JT, Randell SH, Kesimer M, Intercellular Communication between Airway Epithelial Cells Is Mediated by Exosome-Like Vesicles, Am. J. Respir. Cell Mol. Biol, 60 (2019) 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sears PR, Bustamante-Marin XM, Gong H, Markovetz MR, Superfine R, Hill DB, Ostrowski LE, Induction of ciliary orientation by matrix patterning and characterization of mucociliary transport, Biophys. J, 120 (2021) 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chen G, Sun L, Kato T, Okuda K, Martino MB, Abzhanova A, Lin JM, Gilmore RC, Batson BD, O’Neal YK, Volmer AS, Dang H, Deng Y, Randell SH, Button B, Livraghi-Butrico A, Kesimer M, Ribeiro CM, O’Neal WK, Boucher RC, IL-1beta dominates the promucin secretory cytokine profile in cystic fibrosis, J. Clin. Invest, 129 (2019) 4433–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Seibold MA, Interleukin-13 Stimulation Reveals the Cellular and Functional Plasticity of the Airway Epithelium, Ann. Am. Thorac. Soc., 15 (2018) S98–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O’Neal W, Boucher RC, Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia, J. Biol. Chem, 280 (2005) 17798–17806. [DOI] [PubMed] [Google Scholar]
- [147].Kanoh S, Tanabe T, Rubin BK, IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells, Clin. Exp. Allergy, 41 (2011) 1747–1756. [DOI] [PubMed] [Google Scholar]
- [148].Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Heaney LG, Shields MD, Effects of IL-13 on mucociliary differentiation of pediatric asthmatic bronchial epithelial cells, Pediatr. Res, 69 (2011) 95–100. [DOI] [PubMed] [Google Scholar]
- [149].Zuyderduyn S, Ninaber DK, Schrumpf JA, van Sterkenburg MA, Verhoosel RM, Prins FA, van Wetering S, Rabe KF, Hiemstra PS, IL-4 and IL-13 exposure during mucociliary differentiation of bronchial epithelial cells increases antimicrobial activity and expression of antimicrobial peptides, Respir. Res., 12 (2011) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Dumas MP, Xia S, Bear CE, Ratjen F, Perspectives on the translation of in-vitro studies to precision medicine in Cystic Fibrosis, EBioMedicine, 73 (2021) 103660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, DeMaria G, Knight D, Thornton DJ, Sheehan JK, Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways?, Am. J. Physiol. Lung Cell Mol. Physiol, 296 (2009) L92–L100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, Radicioni G, Kesimer M, Chua M, Dang H, Livraghi-Butrico A, Ehre C, Doerschuk CM, Randell SH, Matsui H, Nagase T, O’Neal WK, Boucher RC, Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways, Am. J. Respir. Crit. Care Med, 199 (2019) 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, Finkbeiner WE, Dolganov GM, Widdicombe JH, Boushey HA, Avila PC, In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects, J. Allergy Clin. Immunol, 123 (2009) 1384–1390.e1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Devalia JL, Sapsford RJ, Wells CW, Richman P, Davies RJ, Culture and comparison of human bronchial and nasal epithelial cells in vitro, Respir. Med., 84 (1990) 303–312. [DOI] [PubMed] [Google Scholar]
- [155].Jackson ND, Everman JL, Chioccioli M, Feriani L, Goldfarbmuren KC, Sajuthi SP, Rios CL, Powell R, Armstrong M, Gomez J, Michel C, Eng C, Oh SS, Rodriguez-Santana J, Cicuta P, Reisdorph N, Burchard EG, Seibold MA, Single-Cell and Population Transcriptomics Reveal Pan-epithelial Remodeling in Type 2-High Asthma, Cell Rep, 32 (2020) 107872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Leung C, Wadsworth SJ, Yang SJ, Dorscheid DR, Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media, Am. J. Physiol. Lung Cell Mol. Physiol, 318 (2020) L1063–l1073. [DOI] [PubMed] [Google Scholar]
- [157].Abdullah LH, Wolber C, Kesimer M, Sheehan JK, Davis CW, Studying mucin secretion from human bronchial epithelial cell primary cultures, Methods Mol. Biol, 842 (2012) 259–277. [DOI] [PMC free article] [PubMed] [Google Scholar]


