Abstract
Neurodegenerative diseases represent a growing burden on healthcare systems worldwide. Mesenchymal stem cells (MSCs) have shown promise as a potential therapy due to their neuroregenerative, neuroprotective, and immunomodulatory properties, which are, however, linked to the bioactive substances they release, collectively known as secretome. This paper provides an overview of the most recent research on the safety and efficacy of MSC-derived secretome and extracellular vesicles (EVs) in clinical (if available) and preclinical models of Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, multiple sclerosis, Huntington's disease, acute ischemic stroke, and spinal cord injury. The article explores the biologically active substances within MSC-secretome/EVs, the mechanisms responsible for the observed therapeutic effects, and the strategies that may be used to optimize MSC-secretome/EVs production based on specific therapeutic needs. The review concludes with a critical discussion of current clinical trials and a perspective on potential future directions in translating MSC-secretome and EVs into the clinic, specifically regarding how to address the challenges associated with their pharmaceutical manufacturing, including scalability, batch-to-batch consistency, adherence to Good Manufacturing Practices (GMP) guidelines, formulation, and storage, along with quality controls, access to the market and relative costs, value for money and impact on total expenditure.
Keywords: Exosomes, Microvesicles, Secretome, Mesenchymal stem cells, Secretome formulation, Brain regeneration
Graphical abstract
Highlights
-
•
Neurodegenerative diseases (NDs) are placing a significant and growing burden on healthcare systems worldwide, making the need for effective treatments urgent.
-
•
MSCs offer a promising therapy for NDs due to their neuroregenerative, neuroprotective, and immunomodulatory properties linked to the bioactive substances they release, known as secretome.
-
•
Clinical and pre-clinical studies have shown the potential of MSC-secretome as a therapeutic strategy for NDs.
-
•
Further research is necessary to optimize MSC-secretome efficacy and safety and to address challenges associated with its pharmaceutical manufacturing, formulation, storage, and quality controls.
-
•
An appropriate cost analysis must be performed to assess the positive cost-benefit ratio for MSC-secretome concerning current therapies for NDs; however, this will only be possible after defining the dosage, timing, and frequency of administration in phase III clinical trials for specific NDs.
1. Introduction
Neurodegenerative diseases (NDs) encompass many acute and chronic conditions that affect the central nervous system (CNS). These illnesses are characterized by the gradual deterioration of neurons and glial cells in the brain and/or spinal cord, resulting in significant declines in specific (brain) functions, like memory, movement, and cognition [1,2]. Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and Huntington's disease (HD) are all examples of neurodegenerative diseases where the degenerations extend over a decade, and for which the underlying mechanism remains elusive primarily. In acute ischemic stroke (AIS) and spinal cord injury (SCI), instead, neurodegeneration is a consequence of the disruption of blood and oxygen flow or a traumatic impact on the spine, respectively (Table 1).
Table 1.
Most common acute and chronic NDs, their clinical manifestations, and causes.
| Disease | Clinical manifestations | Cause |
|---|---|---|
| Alzheimer's disease (AD) | Memory loss and cognitive decline. | Neuronal death that involves a vast area of the CNS, promoted by the deposition of amyloid‐β (Aβ) peptides into plaques [3]. |
| Parkinson's disease (PD) | Motor and non-motor disorders, like tremors and delayed movement. Specifically, dyskinesia with resting tremors, muscle rigidity, and cognitive dysfunction [4]. | Loss of dopaminergic neurons in the substantia nigra, nigrostriatal pathway degeneration [5,6]. α-synuclein positive inclusions in cell bodies and neurites (Lewy bodies) of nigral and olfactory bulb neurons [[7], [8], [9]]. Further, neuroinflammation and oxidative stress are involved [10,11]. |
| Amyotrophic lateral sclerosis (ALS) | Muscles of the limbs, throat, tongue, and respiratory system gradually deteriorate and lose strength. | Degeneration of the upper motor neurons of the corticospinal tract and the lower motor neurons that control the skeletal muscles [12]. Mechanisms are still unclear, despite many studies demonstrating the involvement of several altered signaling pathways, such as mitochondrial dysfunction, glutamate excitotoxicity, oxidative stress, and neuroinflammation [13]. |
| Multiple sclerosis (MS) | Severe physical disabilities. | Demyelinating disease caused by CNS inflammation and immune dysfunction [14]. |
| Huntington's disease (HD) | Motor (involuntary movement disorder and impairment of voluntary movements), cognitive (problems of attention, cognitive slowing) and neuropsychiatric (depression, irritability, and apathy) disorders. | Autosomal dominant disorder caused by the elongation of CAG repeats on the short arm of chromosome 4p16.3 in the HTT gene. As per the proposed mechanisms, the degeneration of neurons in the putamen, caudate, and cerebral cortex, and the loss of substance-P-containing medium spiny neurons can be attributed to various factors. These include the accumulation of mutant protein aggregates, which impair the ubiquitin-proteosome pathway, transcriptional dysregulation, excitotoxicity caused by the increased release of glutamate and glutamate agonist, mitochondrial dysfunction, and altered energy metabolism, as well as changes in axonal transport and synaptic dysfunction [15,16]. |
| Acute ischemic stroke (AIS) | Aphasia, agraphia, acalculia, apraxia, visual field deficit, hemiparesis, and hemisensory loss [17]. | Neurodegeneration, cell death, changes in brain volume and cognitive performance consequent to the disruption of blood and oxygen flow [18]. |
| Spinal cord injury (SCI) | Flaccid paresis, exclusively diaphragmatic respiration, low blood pressure, bradycardia, and loss of control of the urinary outlet [19]. | Commonly results from a sudden, traumatic impact on the spine that fractures or dislocates vertebrae; following this primary injury, a secondary one starts and causes progressive damage to spinal cord tissue, impeding neurological recovery due to complex and interconnected cellular, molecular and biochemical phenomena [20]. |
Besides AIS and SCI, aging is a significant risk factor for most neurodegenerative disorders [21]; the number of people affected by NDs is therefore expected to rise due to the increasing population age. This also means that countries around the globe are facing a significant economic challenge. For example, according to some estimations, the US population aged ≥65 will increase from 53 million in 2018 to 88 million in 2050 [22]. Therefore, it is likely that the US$946 billion spent in 2016 caring for individuals with AD (which was already triple the expenditure in the year 2000 [23]) would enormously be expected to rise when the number of individuals with AD is likely to reach 115 million worldwide by 2050 [24]. A change in diagnostic and treatment methods is, therefore, crucial.
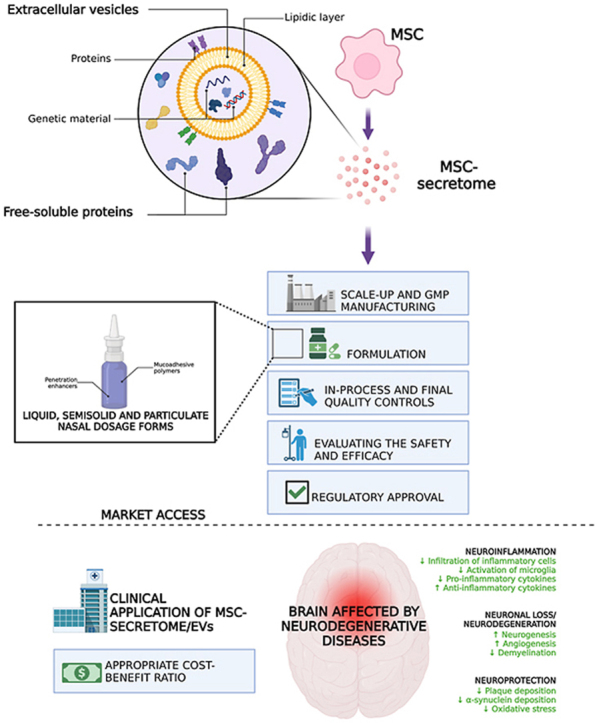
Where current treatments are mostly inadequate, stem cell therapies have emerged as a potential strategy to treat these disorders. In this regard, mesenchymal stem cells (MSCs) are particularly attractive for regenerative therapies due to their multipotentiality and pro-regenerative and immunomodulatory properties [25], which may also depend on the release of bioactive substances collectively known as secretome [26], and comprising free soluble factors and extracellular vesicles (EVs) [27].
This article provides an overview of the most recent research on the safety and efficacy of MSC-derived products, i.e., secretome and EVs, in preclinical models of NDs. The paper also explores the biologically active substances within MSC-secretome/EVs, the mechanisms responsible for the observed therapeutic effects, and the strategies that may be used to optimize MSC-secretome/EVs production based on specific therapeutic needs. The review concludes with a critical discussion of current clinical trials and a perspective on potential future directions in translating MSC-secretome and EVs into the clinic.
2. From MSCs to their secretome/EVs in NDs
As neuronal death, neurodegeneration, neuroinflammation, immune dysfunction, and oxidative stress are common features of NDs (see Table 1), it is not surprising that stem cells, particularly MSCs, have emerged as a promising treatment modality at the forefront of ND research [28,29]; this is especially significant considering the inadequacy of current treatments for most NDs [30]. Indeed, MSCs may mediate CNS repair thanks to their well-documented ability to migrate to injured tissue [31], differentiate into new cell types (multipotency) even after extended in vitro expansion [32], pro-regenerative capacity [33], and immunomodulation [34]. Multiple studies have evaluated the effects of MSCs in animal models of NDs administered by multiple delivery routes and over a wide range of doses. The safety and efficacy of MSCs undoubtedly emerge, as highlighted by recent systemic reviews and meta-analyses of the literature. For example, regarding the efficacy, transplantation of MSCs significantly improved memory loss and cognitive impairment in AD [35], rotational behavior and limb function in PD [36], improved neurobehavior in ALS [37], ameliorated the symptoms and delayed the progression of MS [38], promoted the recovery of nerve function and exerted a protective effect on the brain of cerebral AIS rats [39], and improved sensorimotor functions in SCI [40].
Due to this proven efficacy in pre-clinics, it is unsurprising that MSCs have been the broadly used adult stem cells in clinical trials on humans. However, although many clinical trials can be found when assessing the efficacy of MSCs in NDs on humans (source: http://www.clinicaltrials.org; last access 31/05/2023), only a few have been completed with published results (Table 2).
Table 2.
Summary of the clinical trials (completed and with results) involving MSCs in NDs. Source: http://www.clinicaltrials.org; last access 31/05/2023; search terms: mesenchymal stem cells and Alzheimer's disease or Parkinson's disease or amyotrophic lateral sclerosis or multiple sclerosis or Huntington's disease or acute ischemic stroke or spinal cord injury.
| Neurological disease | Clinical trial ID number | Completion date | Study design | Treatments | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| AD | NCT01297218 | 12/2011 | Phase I, interventional, open-label | UC-MSCs | Administration was safe and well tolerated. | [41] |
| NCT02054208 | 12/2019 | PhaseI/II, interventional, quadruple-blind | UC-MSCs vs saline | Administration was safe and well tolerated. | [42] | |
| ALS | NCT01771640 | 05/2012 | Phase I, interventional, open-label | BM-MSCs | Administration was safe and well tolerated. | [43] |
| NCT01759797 | 03/2014 | Phase I, interventional, open-label | BM-MSCs | Amelioration of the forced vital capacity and ALS functional rating scale-revised. | ||
| NCT01051882 | 03/2013 | Phase I/II, interventional, open-label | BM-MSCs secreting neurotrophic factors | Administration was safe and well tolerated. | [44] | |
| NCT01777646 | 09/2015 | Phase II, interventional, open-label | Indications of possible clinical benefits are to be confirmed in upcoming clinical trials. | |||
| NCT03828123 | 08/2019 | Phase I/II, interventional, open-label | Autologous MSCs | Administration was safe and well tolerated. | [45] | |
| The progression of the disease was slowed down. | ||||||
| NCT04821479 | 12/2020 | Phase I/II, interventional, open-label | MSCs | Administration was safe and well tolerated. | [46] | |
| Indications of medium-term clinical benefits. | ||||||
| MS | NCT00395200 | 12/2010 | Phase I/II, interventional, open-label | Autologous MSCs | Administration was safe and well tolerated. | [47] |
| Structural, functional, and physiological improvements suggestive of neuroprotection. | ||||||
| NCT00813969 | 05/2014 | Phase I, interventional, open-label | Autologous MSCs | Administration was safe and well tolerated. | [48] | |
| NCT02034188 | 03/2016 | Phase I/II, interventional, open-label | UC-MSCs | Administration was safe and well tolerated. | [49] | |
| NCT02166021 | 12/2018 | Phase II, interventional, double-blind | Autologous MSCs | Administration was safe and well tolerated. | [50] | |
| Short-term beneficial effects regarding the primary endpoints, especially in patients with active disease. | ||||||
| NCT00781872 | 12/2019 | Phase I/II, interventional, open-label | Autologous BM-MSCs | Administration was safe and well tolerated. | [51] | |
| Immediate immunomodulatory effects. | ||||||
| NCT04823000 | 04/2020 | Phase I/II, interventional, open-label | MSCs | Administration was safe and well tolerated. | [52] | |
| The presence of clinical benefits (especially in patients treated with more than two injections) lasted up to 4 years. | ||||||
| Short-term immunomodulatory effects. | ||||||
| SCI | NCT00816803 | 12/2008 | Phase I/II, interventional, single-blind | Autologous BM-MSCs | Administration was safe and well tolerated. | [53] |
| NCT01325103 | 12/2012 | Single-group assignment, interventional, open-label | Autologous BM-MSCs | Administration was safe and well tolerated. | [54] | |
| NCT02482194 | 03/2016 | Phase I, interventional, open-label | Autologous BM-MSCs | Administration was safe and well tolerated. | [55] | |
| NCT01909154 | 03/2019 | Phase I, interventional, open-label | Autologous BM-MSCs | Administration was safe and well tolerated. | [56] | |
| NCT03003364 | 02/2020 | Phase I/II, interventional, quadruple-blind | WJ-MSCs | Administration was safe and well tolerated. | [57] | |
| Sensory improvement in the segments adjacent to the injury site. | ||||||
| NCT03308565 | 10/2021 | Phase I, interventional, open-label | Autologous A-MSC | Administration was safe and well tolerated. | [58] | |
| Meaningful signs of improved, rather than stabilized, neurologic status. |
UC = umbilical cord; BM = bone marrow; A = adipose; WJ= Wharton Jelly.
Although there have been some promising indications [[43], [44], [45], [46], [47],[50], [51], [52],57,58], the efficacy of MSCs in treating NDs has yet to be confirmed through ongoing clinical trials. However, despite the relatively limited number of studies conducted thus far, there is a consensus that MSCs are safe for use in this context. In this regard, a recent meta-analysis reviewed the adverse events following MSC administration in all populations with different diseases; the pooled analysis demonstrated that the administration was safe and closely associated with only minor adverse effects, like short-term fever, adverse events at the administration site, constipation, fatigue, and insomnia [59].
Even if cell therapy with MSC continues to be a field of substantial interest, there has been, in recent years, a shift in the paradigm of MSC-based therapies: from MSC cell therapy to MSC-secretome therapy. Indeed, while initially, the therapeutic effects of MSCs were attributed to their ability to differentiate into various cell types and replace damaged or diseased tissues [60], recent research has revealed that their therapeutic effects are mediated mainly by their secreted products, known as MSC-secretome [61]. As mentioned above, the MSC-secretome comprises a diverse range of bioactive molecules, including cytokines, growth factors, and microRNAs [[62], [63], [64], [65], [66], [67], [68], [69], [70]], secreted as such or encapsulated into exosomes and other EVs. MSC-secretome and EVs have been shown to modulate various processes, including angiogenesis [66,[71], [72], [73], [74]], immunomodulation [64,75,76], and tissue repair [66,77,78]. Moreover, the MSC-secretome has been found to exhibit similar therapeutic effects as MSC cell therapy [79] without the potential risks and limitations associated with direct cell transplantation [27,[80], [81], [82]]. It is therefore not surprising that MSC-secretome therapy is also being explored as a potential treatment for NDs: the strategy is not anymore aimed at simply replacing the lost cells (e.g., neurons) by transplanting stem cells, but by utilizing MSCs as “drug stores” that secrete neuroprotective agents which are the actual effectors of the therapeutic effects observed (Fig. 1). The preclinical and clinical safety and efficacy evidence for MSC-secretome and EVs is discussed below for each neurodegenerative disease.
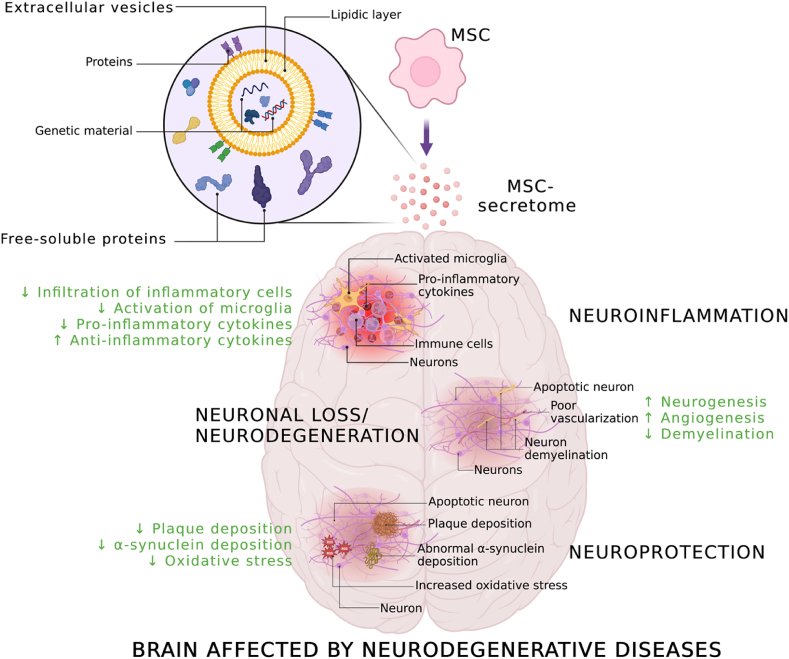
Fig. 1.
MSCs as “drug stores”. MSCs release secretome, which comprises free-soluble proteins and EVs enriched with genetic material. MSC-secretome and EVs exert multiple pharmacological effects; the mechanisms responsible for the anti-inflammatory, neuroregenerative, and neuroprotective effects are indicated. Created with BioRender.com.
3. MSC-secretome and EVs: pre-clinical and clinical safety and efficacy in neurodegenerative diseases
3.1. Alzheimer's disease (AD)
The effectiveness of MSC-secretome and EVs following intracerebral [[83], [84], [85], [86]], intravenous [[87], [88], [89], [90], [91]], or intranasal [[92], [93], [94], [95]] administration was assessed on transgenic animal models reporting mutations associated with AD [83,84,[87], [88], [89], [90], [91],[93], [94], [95]], or injected with Aβ1-42 oligomers [85,86] (Table 3).
Table 3.
Summary of therapeutic efficacy and safety of MSC-secretome and EVs in preclinical animal models of AD.
| Study | Animal model | Therapeutic agent | Route and dosage | Main outcomes |
|---|---|---|---|---|
| Wang et al. [83] | Adult male APP/PS1 double transgenic mice | EVs from mBM-MSCs | Intracerebral injection of EVs once per two days for two weeks | Improvement of cognitive behavior. |
| Suppression of Aβ induced iNOS expression. | ||||
| Rescue impairment of CA1 synaptic transmission and long-term potentiation. | ||||
| Cui et al. [87] | APP/PS1 double transgenic mice | EVs from normoxic or hypoxic mBM-MSCs | Injection via tail vein of 150 μg of EVs | Amelioration of cognitive decline. Better improvement of learning and memory capabilities by EVs released in hypoxic conditions. |
| Rescue of synaptic dysfunction. | ||||
| Regulation of inflammatory responses. | ||||
| Reduction of plaque deposition and Aβ levels in the brain. | ||||
| Decrease of astrocytes and microglia activation. | ||||
| Down-regulation of pro-inflammatory cytokines and up-regulation of anti-inflammatory ones. | ||||
| Ding et al. [88] | APP/PS1 double transgenic mice (7 months old) | EVs from hUC-MSCs | Injection via tail vein of 30 μg of EVs | Repair of cognitive dysfunctions. |
| Clear Aβ deposition. | ||||
| Modulation of the activation of microglia in brains. | ||||
| Inflammatory-regulating effects. | ||||
| Cui et al. [89] | APP/PS1 double transgenic mice (7 months old) | EVs from mBM-MSCs tagged or not with rabies viral glycoprotein (RVG) | Intravenous injection of 5 × 1011 EVs | Improvement of learning and memory capabilities. |
| Reduction of plaque deposition and Aβ levels. | ||||
| Normalization of inflammatory cytokines levels. | ||||
| EVs tagged with RVG were more effective than those not tagged. | ||||
| Elia et al. [84] | Adult male APP/PS1 double transgenic mice | EVs from mBM-MSCs | Intracerebral injection into the neocortex of 1 × 109 EVs | Reduction of Aβ plaque burden. Neprilysin into EVs had this action. |
| Reduction of dystrophic neurites in both the cortex and hippocampus. | ||||
| Reza-Zaldivar et al. [85] | C57BL/6 mice (7–8 weeks) injected with oligomer Aβ1-42 | EVs from hMSCs | Stereotaxic intracerebral injection of 10 μg of EVs | Alleviation of cognitive impairment. |
| Stimulation of neurogenesis in the subventricular zone. | ||||
| Losurdo et al. [92] | Female 3xTg-AD triple-transgenic mice | EVs from cytokine-preconditioned hBM-MSCs | Intranasal administration of 100 μL of EVs | Induction of immunomodulatory and neuroprotective effects. |
| Modulation of the activation of microglia cells. | ||||
| Increase of dendritic spine density. | ||||
| Ma et al. [93] | Female APP/PS1 double transgenic mice (9 months old) | EVs from A-MSCs | Intranasal administration of 1 mg of EV protein per kg | Induction of neuroprotective effects. |
| Increase of newborn neurons. | ||||
| Reduction of Aβ deposition. | ||||
| Decrease of microglia activation. | ||||
| Santamaria et al. [94] | Male APP/PS1 double transgenic mice | Secretome from mBM-MSCs exposed in vitro to AD mouse brain homogenates of different ages | Intranasal administration once a week | Induction of persistent memory recovery. |
| Reduction of plaques. | ||||
| Lower density of β-amyloid oligomers. | ||||
| Higher neuronal density in the cortex and hippocampus. | ||||
| Reduction in hippocampal shrinkage. | ||||
| Longer lifespan. | ||||
| Chen et al. [90] | JAX-006293 transgenic mice | EVs from hWJ-MSCs | Intravenous injection of 50 μg of EVs | Improvement in cognitive function. |
| Regulation of the neurons and astrocytes phases in the brain. | ||||
| Cone et al. [95] | Male and female 5XFAD transgenic mice (6 weeks) | EVs from hBM-MSCs | Intranasal administration of 20 × 108 EVs in 5 μL | Improvement of behavior in cognitive tests. |
| Lower Aβ plaque load in the hippocampus. | ||||
| Sha et al. [86] | Sprague-Dawley rats (5–6 weeks) injected with oligomer Aβ1-42 | EVs from rBM-MSCs | Stereotaxic intracerebral injection of 30 μg of EVs | Improvement in cognitive function. |
| Reduction of Aβ deposition area, plaques, and Aβ contents. | ||||
| Reduction of inflammatory cytokines (interleukin (IL)-1β, IL-6, and TNF-α). | ||||
| Effects were mediated by miR-29c-3p carried by EVs that activated the Wnt/β-catenin pathway. | ||||
| Wang et al. [91] | Male APP/PS1 double transgenic mice | EVs from hUC-MSCs | Injection via tail vein of 50 μg of EVs | Improvement of cognitive impairments. |
| Reduction of the hippocampal Aβ aggregation. | ||||
| Reduction of neuronal loss. | ||||
| Restoration of the calcium oscillations. EVs acted through the Nrf2 signaling pathway. |
m = mouse; h = human; r = rat.
BM = bone marrow; UC = umbilical cord; A = adipose; WJ = Wharton Jelly.
Researchers mainly observed amelioration in cognitive decline [83,[85], [86], [87], [88], [89], [90], [91],94,95] that resulted from many different mechanisms. For example, many studies reported reduced plaque deposition and Aβ levels [84,[86], [87], [88], [89],91,[93], [94], [95]]; interestingly, the primary mechanism for this effect was the presence of neprilysin on the surface of EVs [84]. Neprilysin is indeed a zinc metallopeptidase that previously showed capable of breaking down Aβ1-42 peptides [[96], [97], [98]]. Another important effect observed was regulating the inflammatory response [83,[86], [87], [88], [89],92], specifically the downregulation of pro-inflammatory cytokines and the upregulation of anti-inflammatory ones [86,87,89]. According to other studies, the anti-inflammatory effect of MSC-secretome/EVs happened through the decrease/modulation of activated microglia [87,88,92,93], which, when chronically activated, is an important mediator of inflammation [99]. Also, EVs have been found to reduce the expression of inducible nitric oxide synthase (iNOS), which is induced by Aβ, leading to the release of high levels of nitric oxide (NO) [83]; this high NO level can cause neurotoxicity by inhibiting mitochondrial respiration and leading to neuronal cell death [100]. Finally, limited evidence was also reported regarding the rescue of synaptic dysfunction [87] or, more generally, a reduction in neuronal degeneration that resulted from neurogenesis [85,91,94] and neuroprotection [92,93].
Many researchers reported how such activities are affected by the extracellular environment. For example, exposure of MSCs to hypoxia generated EVs that showed a better effect in rescuing learning memory impairments than EVs from MSCs grown under normoxic conditions [87]. Cytokine preconditioning with tumor necrosis factor (TNF)-α and interferon (IFN)-γ up-regulated the immunomodulatory markers cyclooxygenase-2 (COX2) and indoleamine-pyrrole 2,3-dioxygenase (IDO), allowing EVs to obtain increased immunomodulatory functions [92]. Again, exposure of MSCs to brain homogenates from young AD mice did not result in a reparative effect in the MSC-secretome, but it did have an impact on impaired memory when obtained from MSCs that had been exposed to AD brain homogenates from older mice. This suggests that inflammatory molecules, absent or present in minimal amounts in the young brain, are relevant for licensing the neuroprotective effect of MSCs [94]. Finally, it is possible to target EVs to the brain to increase their potency. According to a research study, EVs were modified using a targeted rabies viral glycoprotein (RVG) peptide, which interacts specifically with the acetylcholine receptor, allowing entry into neuronal cells. The results demonstrated that RVG-EVs had a higher targeting efficiency than unmodified EVs, with concentrated fluorescence observed in the cortex and hippocampus [89].
3.2. Parkinson's disease (PD)
Multiple research teams have focused on utilizing MSC-secretome/EVs to develop novel therapeutic strategies for PD (Table 4). The majority of studies have evaluated their efficacy in animal models of PD using 6-hydroxydopamine (6-OHDA) [[101], [102], [103], [104], [105]] and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTPT) [106], with only one study using transgenic mice expressing human α-synuclein [107]. There have been no studies on the efficacy of MSC-secretome or EVs in humans.
Table 4.
Summary of therapeutic efficacy and safety of MSC-secretome and EVs in preclinical animal models of PD.
| Study | Animal model | Therapeutic agent | Route and dosage | Main outcomes |
|---|---|---|---|---|
| Yao et al. [101] | Male Sprague-Dawley rats treated with 6-OHDA | rBM-MSCs secretome + neural stem cells | Implantation of 50 mg of secretome + 5 × 103 cells | Generation of dopaminergic neurons; promotion of cell survival, migration, and integration into damaged brain areas. |
| Amelioration of functional recovery, with significant reduction of apomorphine-induced rotational asymmetry and improved spatial learning ability. | ||||
| Teixeira et al. [102] | Male Wistar Han rats (10 weeks) treated with 6-OHDA | Secretome from hBM-MSCs | Injection into the substantia nigra and striatum | Increase of dopaminergic neurons and neuronal terminals. |
| Improvement of motor performance. | ||||
| Chen et al. [103] | Adult male Sprague-Dawley rats treated with 6-OHDA | EVs from hUC-MSCs | Injection via tail vein of 200 μg of EVs | Reduction of dopaminergic neuron loss and apoptosis in the substantia nigra; upregulation of the dopamine level in the striatum. |
| Relieved apomorphine-induced asymmetric rotation. | ||||
| Teixeira et al. [104] | Male Wistar Han rats (9 weeks) treated with 6-OHDA | Secretome from hBM-MSCs vs levodopa | Injection into the substantia nigra and striatum | Increase of tyrosine hydroxylase-positive cell expression and terminals. Significant amelioration of the motor outcomes. |
| Mahendru et al. [105] | Male Sprague-Dawley rats treated with 6-OHDA | Secretome from rBM-MSCs | Intravenous injection of 25 μg of secretome per kg | Significant modulation of inflammatory, oxidative stress and apoptotic markers. |
| Amelioration of impaired neurobehavioral parameters. | ||||
| Xue et al. [106] | BALB/c mice (8–10 weeks) treated with MTPT | EVs from hA-MSCs | Intraperitoneal injection of 200 μm/mL of EVs | Promotion of angiogenesis in the striatum and substantia nigra. |
| Increase in dopamine production. | ||||
| Yang et al. [107] | Male transgenic mice expressing A53T human α-synuclein | EVs from hBM-MSCs delivering antisense oligonucleotides targeting α-synuclein | Stereotaxic injection into the right lateral ventricle of 24 μg of EVs containing 20 μg of antisense nucleotides | Decrease of α-synuclein expression. |
| Decrease of dopaminergic neuron degeneration. | ||||
| Improvement of locomotor functions. | ||||
| Cai et al. [109] | Male C57BL/6 mice (6 weeks) treated with MTPT | EVs form BM-MSCs | Injection via tail vein of 200 μL of EVs | Decrease of neuron loss and injury. |
| Reduction of the inflammatory response by inhibiting Sp1 signaling. | ||||
| Li et al. [108] | Sprague-Dawley rats treated with 6-OHDA | EVs from quiescent rBM-MSCs vs EVs from rBM-MSCs during dopaminergic differentiation | Stereotaxic injection into the striatum of 15 μg of EVs | Downregulation of IL-6, IL-1β and TNF-α. |
| Reduction of the reactive oxygen species levels in the substantia nigra. | ||||
| Increased expression of tyrosine hydroxylase mRNA. | ||||
| Rescue of the rotation behavior and climbing speed. | ||||
| EVs from quiescent cells were more effective. | ||||
| Ma et al. [110] | Mice treated with 6-OHDA | hUC-MSCs loaded with miR-181a–2–3p | Injection via tail vein of 200 μg of EVs | Reduction of dopamine neurons apoptosis. |
| Reduction of oxidative stress. | ||||
| Peng et al. [111] | Male C57BL/6 mice (6–8 weeks) treated with MTPT | EVs from mBM-MSCs + curcumin | Intranasal administration | Reduction of α-synuclein aggregates. |
| Promotion of neuron function recovery. | ||||
| Alleviation of neuroinflammation. |
6-OHDA = 6-hydro-xydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
r = rat; h = human; m = mouse.
BM = bone marrow; UC = umbilical cord; A = adipose.
The studies consistently report improved functional recovery [102,104,105,107,108], including better motor performance, reduced rotational asymmetry induced by apomorphine [101,103], and improved spatial learning ability [101]. These effects are attributed to increased dopamine levels in the striatum [103,106,108], reduced loss and apoptosis of dopaminergic neurons [101,103,107,109,110] together with the generation of new dopaminergic neurons [101,104] and neuronal terminals [102,104], and improved neuron function [111]. Proteomic characterization reveals that the secretome and EVs of MSCs harbor crucial neuroregulatory molecules that can be held responsible for the aforementioned effects. Such molecules include cystatin C, glia-derived nexin, galectin-1, pigment epithelium-derived factor, vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor, interleukin (IL)-6, and glial cell line-derived neurotrophic factor [102]. Evidence also suggests that MSCs secretome/EVs may reduce neuroinflammation [105,108,109,111], oxidative stress [105,108,110], and abnormal deposition of α-synuclein [107,111]. Only Xue and colleagues evidenced angiogenesis following the administration of EVs from human MSCs [106], likely linked to the presence of VEGF [112]. A single study compared the effectiveness of MSCs versus traditional anti-Parkinson drugs, such as levodopa [104]: the two treatments were effective the same in ameliorating the animal behavior; however, the human bone marrow (BM)-MSCs secretome appeared to be a modulator of the dopaminergic system, enhancing tyrosine hydroxylase (a marker for dopamine)-positive cell expression and terminals both in the substantia nigra and striatum.
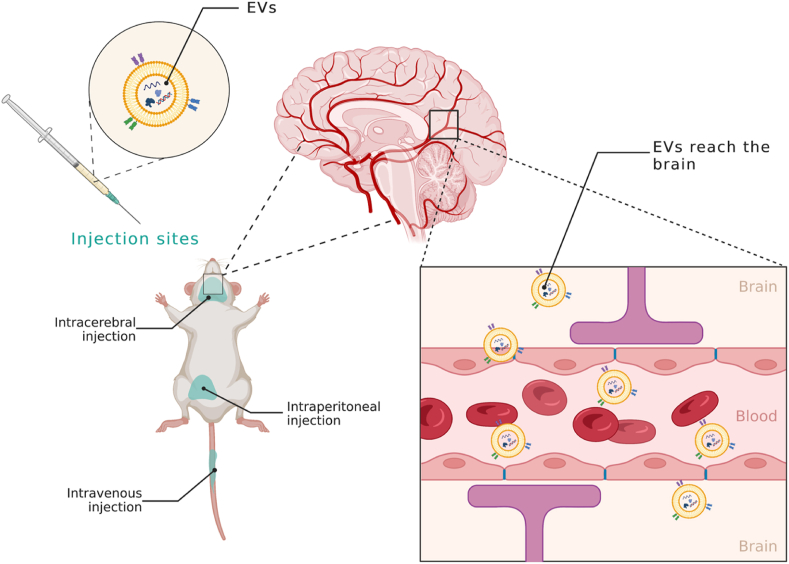
The therapeutic potential of MSC-secretome and EVs was observed when administered directly into the substantia nigra and striatum [101,102,104,107,108], as well as through intravenous [103,109,110] or intraperitoneal [106] injection, thus confirming the ability of EVs, even when administered systemically, to across the blood-brain-barrier reaching the substantia nigra [103] (Fig. 2). One study also investigated the possibility of administering EVs via intranasal administration [111].
Fig. 2.
Following intravenous, intracerebral, and intraperitoneal injection, MSC-EVs enter systemic circulation, cross the blood-brain barrier, and reach the brain. Created with BioRender.com.
3.3. Amyotrophic lateral sclerosis (ALS)
The application of MSC-secretome and EVs in ALS is recent, with only one study conducted on preclinical animal models [113] and two case reports after testing on humans [114,115] (Table 5).
Table 5.
Summary of therapeutic efficacy and safety of MSC-secretome and EVs in preclinical animal models of ALS and ALS patients.
| Study | Animal model | Therapeutic agent | Route and dosage | Main outcomes |
|---|---|---|---|---|
| Bonafede et al. [113] | SOD1(G93A) mice | EVs from mA-MSCs | Intravenous or intranasal administration of 1 μg of EVs | Improvement of motor performance following repeated administration of EVs. |
| Protection of lumbar motoneurons, neuromuscular junction, and muscle. | ||||
| Decrease of glial cell activation. | ||||
| Crose [114] | Human | EVs from hBM-MSCs | Intravenous injection | Improvement of motor performance: walking with minimal assistance for 25–50 feet after six months of no walking. |
| Improvement in speech and strength. | ||||
| Decrease in muscle spasms. | ||||
| Decrease of pain (from muscle spasms). | ||||
| Improvement in sleep. | ||||
| Ueda et al. [115] | Human | Secretome from exfoliated deciduous teeth hMSCs | Intranasal and intravenous administration | Improvement of range of motion in limbs. |
| Reduced deterioration of pulmonary function. | ||||
| No adverse effects. |
m = mouse; h = human.
A = adipose; BM = bone marrow.
However, all the reports consistently indicate an improvement in motor performance without any significant adverse effect [[113], [114], [115]]. Specifically, Crose's study reported that the patient, following the treatment with EVs from human BM-MSCs, could walk with minimal assistance for 25–50 feet after six months of no walking [114]. Similarly, Ueda and colleagues reported an improved range of motion in limbs [115]. Other improvements regarded speech and strength, the reduction of muscle spasms (and thus of the associated pain) [114], and reduced deterioration of pulmonary function [115]. These effects are likely linked to the protective effect exerted by MSC-secretome/EVs on limbal motoneurons, neuromuscular junction, and muscle and the protection from inflammation, as suggested by decreased glial cell activation [113]. Finally, improvement in sleep was also reported [114].
3.4. Multiple sclerosis (MS)
The primary experimental animal model of encephalitis used to assess the efficacy of MSC-secretome and EVs in treating MS was induced by administering oligodendrocyte glycoprotein peptide (MOG) 35–55 [[116], [117], [118], [119], [120], [121], [122], [123]]. At the same time, only one example reported the development of the disease model through immunization [124] (Table 6).
Table 6.
Summary of therapeutic efficacy and safety of MSC-secretome and EVs in preclinical animal models of MS.
| Study | Animal model | Therapeutic agent | Route and dosage | Main outcomes |
|---|---|---|---|---|
| Rajan et al. [116] | Male C57BL/6 mice (12 weeks old) treated with MOG35-55 | Secretome or EVs from hPDL-MSCs | Injection via tail vein | Immunosuppressive action. |
| NALP3 inflammasome inactivation. | ||||
| NF-κB reduction. | ||||
| These effects were exerted both by secretome and EVs. | ||||
| Farinazzo et al. [117] | C57BL/6 mice (6–8 weeks old) treated with MOG35-55 | EVs from mA-MSCs | Intravenous injection of 5 μg of EVs | Reduction of spinal cord inflammation and demyelination. |
| Marginal inhibition of antigen-specific T cell activation. | ||||
| Reduction of microglial activation. | ||||
| Reduction of demyelination in the spinal cord. | ||||
| Clark et al. [118] | Male and female C57BL/6J mice (3 months old) treated with MOG35-55 | EVs from hP- MSCs | Injection via tail vein of 1 × 107 or 1 × 1010 EVs | Improvement of motor function outcomes. |
| Improvement of symptoms. | ||||
| Reduction of DNA damage in oligodendroglia populations. | ||||
| Increase of myelination within the spinal cord. | ||||
| Li et al. [124] | Female rats immunized with guinea pig spinal cord homogenate + Freund's adjuvant containing Mycobacterium tuberculosis H37Ra | EVs from rBM-MSCs | Injection via tail vein of 100 μg or 400 μg of EVs | Decrease of neural behavioral scores. |
| Reduction of inflammatory cell infiltration into the CNS. | ||||
| Regulation of microglia polarization. | ||||
| Decrease of demyelination. | ||||
| Increase IL-10 and TGF-β levels. | ||||
| Reduction of TNF-α and IL-12 levels. | ||||
| Shamili et al. [119] | Female C57BL/6 mice (10–13 weeks) treated with MOG35-55 | EVs from mBM-MSCs conjugated with carboxylic acid-functionalized LJM-3064 aptamer | Intravenous injection of 200 μg of EVs | Reduction of the severity of the disease. |
| Suppression of the inflammatory response. | ||||
| Reduction of demyelination. | ||||
| Jafarinia et al. [120] | C57BL/6 mice (6–8 weeks old) treated with MOG35-55 | EVs from hA-MSCs | Intravenous injection of 60 μgof EVs | Decrease of the maximum mean clinical score. |
| Reduction of inflammation scores. | ||||
| Reduction of the percentage of demyelination areas. | ||||
| Fathollahi et al. [121] | Female C57BL/6 mice (6–8 weeks old) treated with MOG35-55 | EVs from mA-MSCs | Intranasal administration of 10 μg of EVs | Decrease the clinical symptoms. |
| Increase in immunomodulatory responses. | ||||
| Increase of TGF-β levels. | ||||
| Koohsari et al. [122] | Female C57BL/6 mice (6–8 weeks old) treated with MOG35-55 | EVs from hUC-MSCs | Intravenous injection | Reduction of pro-inflammatory cytokines (IL-17a, TNF-α, and IFN-γ). |
| Increase of the anti-inflammatory cytokines (IL-4 and IL-10). | ||||
| Decrease of leukocyte infiltration. | ||||
| Improvement of body weight. | ||||
| Zhang et al. [123] | Female C57BL/6 mice (6–8 weeks old) treated with MOG35-55 | EVs from moMSCs | Injection via tail vein of 5 × 1010 EVs | Improvement of neurological outcome. |
| Increase of myelin basic protein. | ||||
| Decrease of neuroinflammation. | ||||
| Modulation of microglia activation. | ||||
| Modulation of cytokine levels. |
MOG35-55 = oligodendrocyte glycoprotein peptide.
h = human; m = mouse; mo = monkey.
PDL = periodontal ligament; A = adipose; P = placenta; BM = bone marrow; UC = umbilical cord.
Following intravenous [[116], [117], [118], [119], [120],[122], [123], [124]] or intranasal [121] administration, secretome and EVs reduced the severity of the diseases, as improved motor function outcomes and symptoms were the main effects described by the researchers [[118], [119], [120], [121],123,124]. In this regard, a recent meta-analysis systematically reviewed the efficacy of MSC-EVs in preclinical rodent models of MS and reported an overall positive effect on the clinical score [125]. Also, MSC-secretome and EVs exerted anti-inflammatory and immunosuppressive properties [116,117,[119], [120], [121], [122], [123]], which markedly reduced the infiltration of inflammatory cells in the CNS [122,124] and modulated the cytokine levels [[122], [123], [124]]. This effect resulted from the presence in secretome and EVs of soluble immunomodulatory factors or their ability to inactivate NALP3 inflammasome and reduce NF-κB [116]. Furthermore, inhibition of antigen-specific T-cell activation [117] and reduction of microglial activation [117,123,124] were reported. More often, the administration of MSC-secretome or EVs reduced the demyelination process [117,119,120,124], while it was less evident an increase in myelination within the spinal cord [118,120,123]. Eventually, the immunomodulatory and remyelination effects of EVs may be increased by functionalizing their surface with LJM-3064 aptamer [119]. Finally, an increase in transforming growth factor (TGF)-β [121,124] and improvement in body weight were also reported [122].
3.5. Huntington's disease (HD)
Similarly to the previously described neurological conditions, MSC-secretome may help prevent the degeneration of neurons in the putamen, caudate and cerebral cortex observed in HD. However, to date, only Giampà and colleagues assessed the beneficial effects of the secretome from human amniotic fluid (AF)-MSC in an R6/2 mouse model of HD [126]. Following intraperitoneal injection, a protective effect in the CNS was observed. Specifically, the intervention led to a decrease in neurological dysfunction, a delay in hind paw clasping response during the tail suspension, an improvement in rotarod performance deficits, and a decrease in locomotor activity in an open field test. In addition, the study reported improvements in brain pathology, such as a reduction in striatal atrophy and the formation of striatal neuronal intranuclear inclusions, because of the intervention.
3.6. Acute ischemic stroke (AIS)
Many experimental studies investigated the effect of MSC-secretome and EVs in animal models of AIS obtained following transient [[127], [128], [129], [130], [131], [132], [133], [134]] or permanent [135,136] middle cerebral artery occlusion, subcortical infarct [137] or intracerebral hemorrhage [138] (Table 7).
Table 7.
Summary of therapeutic efficacy and safety of MSC-secretome and EVs in preclinical animal models of AIS.
| Study | Animal model | Therapeutic agent | Route and dosage | Main outcomes |
|---|---|---|---|---|
| Xin et al. [127] | Adult male Wistar rats were subjected to transient MCAO | EVs from rBM-MSCs | Intravenous injection of 100 μg of EVs | Improvement in functional recovery. |
| Increasing in axonal density and synaptophysin-positive areas. | ||||
| Increasing in the number of newly formed doublecortin (a marker of neuroblasts) and von Willebrand factor (a marker of endothelial cells) cells. | ||||
| Doeppner et al. [128] | C57BL6 mice subjected to transient MCAO | EVs from hBM-MSCs | Intravenous injection | Improvement in neurological impairment. |
| Long-term neuroprotection. | ||||
| Enhancement in angiogenesis and neurogenesis. | ||||
| Cerebral immune cell infiltration was not affected. | ||||
| Chen et al. [129] | Adult male Sprague-Dawley rats subjected to transient MCAO | EVs from mini-pig A-MSCs | Intravenous injection of 100 μg of EVs | Reduction of the brain-infarct zone. |
| Enhancement in neurological recovery. | ||||
| Lee et al. [135] | Male Sprague-Dawley rats subjected to permanent MCAO | EVs from hMSCs primed with normal/ischemic brain extract | Intravenous injection of 0.2 mg of EVs per kg | Enhancement of angiogenesis. |
| Enhancement of neurogenesis. | ||||
| Anti-inflammatory action. | ||||
| Enhancement of behavioral recovery. | ||||
| Reduction of the brain infarct zone. | ||||
| EVs from MSCs primed with normal or ischemic brain extract had significantly greater efficacy. | ||||
| Xin et al. [130] | Adult male Wistar rats subjected to transient MCAO | EVs from rBM-MSCs enriched or not with miR-17-92 | Intravenous injection of 100 μg of EVs | Improvement of functional recovery. |
| Improvement of neurological function. | ||||
| Enhancement of oligodendrogenesis, neurogenesis, and neurite remodeling/neuronal dendrite plasticity in the ischemic boundary zone. This effect was primarily observed for EVs enriched with miR-17-92. | ||||
| Otero-Ortega et al. [137] | Male Sprague-Dawley rats subjected to SCI | EVs from rA-MSCs | Intravenous injection of 100 μg of EVs | Improvement of functional recovery. |
| Enhancement of fiber tract integrity, axonal sprouting and white matter repair markers. | ||||
| Han et al. [138] | Adult male Wistar rats subjected to ICH | EVs from rBM-MSCs | Intravenous injection of 100 μg of EVs | Improvement of neurological function. |
| Improvement of motor recovery. | ||||
| Enhancement of angiogenesis. | ||||
| Enhancement of neurogenesis. | ||||
| Huang et al. [131] | Adult male Sprague-Dawley rats subjected to transient MCAO | EVs from rA-MSCs modified with pigment epithelial-derived factor | Intraventricular injection of 100 μg of EVs per kg | Amelioration of cerebral injury. |
| Activation of autophagy. | ||||
| Suppression of neuronal apoptosis. | ||||
| Jiang et al. [136] | Adult male Sprague-Dawley rats subjected to permanent MCAO | EVs from rA-MSCs enriched with miR-30d-5p | Intravenous injection of 80 μg of EVs | Reduction of the cerebral injury area of infarction. |
| Suppression of autophagy. | ||||
| Promotion of M2 microglia/macrophage polarization. | ||||
| Geng et al. [132] | Sprague-Dawley rats subjected to transient MCAO | EVs from miRNA-126-modified AD-MSCs | Intravenous injection | Improved functional recovery and significant increase of the expression of von Willebrand factor (an endothelial cell marker) and doublecortin (a neuroblasts marker). |
| Decrease of neuron cell death and increase of cell proliferation. | ||||
| Liu et al. [133] | Sprague Dawley rats (8–12 weeks) subjected to transient MCAO | EVs from rBM-MSCs loaded with enkephalin | Intravenous injection | EVs crossed the blood-brain barrier. |
| Reduction of LDH, p53, caspase 3, and NO levels. | ||||
| Improvement of brain neuron density. | ||||
| Improvement of the neurological score. | ||||
| Moon et al. [134] | Sprague-Dawley rats subjected to transient MCAO | EVs from rMSCs | Intravenous injection of 30 μg of EVs | Enhancement of neurogenesis. |
| Enhancement of angiogenesis. | ||||
| Improvement of behavior. |
MCAO = middle cerebral artery occlusion; ICH = intracerebral hemorrhage; SCI = subcortical infarct.
r = rat; h = human.
BM = bone marrow; A = adipose.
Following intravenous [[127], [128], [129], [130],[132], [133], [134], [135], [136], [137], [138]] or intraventricular injection [131], secretome/EVs were able to cross the blood-brain barrier [133] and exert their therapeutic effects, which ultimately led to improved functional recovery and neurological impairment [[127], [128], [129], [130],[132], [133], [134], [135],137,138]. Frequently, a reduction of the brain-infarct zone was observed [129,135,136], resulting from the enhanced neurogenesis [127,128,130,[133], [134], [135],137,138] and angiogenesis [127,128,134,135,138]. Autophagy seems involved, even if studies contradicted its activation [131] or suppression [136]. Beyond a reduction of lactate dehydrogenase (LDH), p53, caspase 3, and NO levels [133], also an anti-inflammatory effect was often reported [135], which, according to Jiang and colleagues, occurs through the promotion of M2 microglia/macrophage polarization [136]; conversely, no effect on immune cell infiltration was reported by another study [128]. According to Lee and colleagues, the therapeutic effects of MSCs were increased when primed with normal or ischemic brain extract [135]; the enrichment of EVs with miR-17-92, instead, significantly increased neurogenesis [130]. Finally, some EVs were modified with pigment epithelial-derived factor [131], enriched with miR-30d-5p [136], or loaded with enkephalin [133]. A recent meta-analysis of the literature confirmed that EV interventions improved lesion volume with respect to control groups [139].
3.7. Spinal cord injury (SCI)
In animal models with severe [140,141] or moderate [[142], [143], [144], [145], [146]] vertebral contusion/compression or severe transection [147], MSC-secretome/EVs have been administered intraspinallly [[140], [141], [142]], intrathecally [143,145,147], intramuscularly [144] or intravenously [146], showing improved motor function recovery [140,142,143,147] (Table 8), as also highlighted by a recent meta-analysis [148]. Other effects observed were enhanced angiogenesis [140], a tissue protective effect [140,143], an increased axonal regeneration [141,145,146], and the attenuation of inflammation [143,144,147]. According to the study of Chudickova and colleagues, these effects were common among MSCs and their secretome; however, only secretome treatment resulted in additional enhancements of axonal sprouting and a decrease in the number of reactive astrocytes in the injury site [145].
Table 8.
Summary of therapeutic efficacy and safety of MSC-secretome and EVs in preclinical animal models of SCI.
| Study | Animal model | Therapeutic agent | Route and dosage | Main outcomes |
|---|---|---|---|---|
| Cantinieaux et al. [140] | Adult female Wistar rats subjected to severe contusion at T11 | Secretome from rBM-MSCs | Intraspinal injection | Improvement of motor recovery. |
| Enhancement of angiogenesis. | ||||
| Tissue protection. | ||||
| Asadi-Golshan et al. [142] | Adult male Sprague-Dawley rats subjected to moderate compression at T7 | Secretome from human exfoliated deciduous teeth hMSCs | Intraspinal injection | Improvement of functional recovery. |
| Cizkova et al. [143] | Adult male Wistar rats subjected to moderate compression at T8-T9 | Secretome from rBM-MSCs | Intrathecal injection | Improvement of motor function recovery. |
| Increasing in spared spinal cord tissue. | ||||
| Enhancement of GAP-43 expression. | ||||
| Attenuation of inflammation. | ||||
| Reduced levels of IL-2, IL-6, and TNF-α. | ||||
| Kanekiyo et al. [141] | Adult female Sprague-Dawley rats subjected to severe contusion at T8-T9 | Secretome from rBM-MSCs | Continuous injection through the cerebrospinal fluid via the fourth ventricle | Improvement of locomotion. |
| Tissue repair. | ||||
| Axonal regeneration. | ||||
| Khoshsirat et al. [144] | Female Wistar rats subjected to moderate contusion at T9-T10 | Secretome from BM-MSCs | Intramuscular injection | Reduction of inflammation. |
| Reduction of TGF-β1 expression. | ||||
| Increasing in mean spinal cord volume. | ||||
| Chen et al. [147] | Adult female Sprague-Dawley rats subjected to severe transection at T8 | Secretome from hBM-MSCs | Intrathecal injection | Reduction of inflammation. |
| Improvement of functional restoration. | ||||
| Chudickova et al. [145] | Male Wistar rats subjected to moderate compression at T8 | hWJ-MSCs vs secretome from hWJ-MSCs | Intrathecal injection | Increasing of spared grey and white matter. |
| Enhancement of expression of genes related to axonal growth. | ||||
| Only secretome treatment resulted in additional enhancements of axonal sprouting and decreased the number of reactive astrocytes in the injury site. | ||||
| Tsai et al. [146] | Adult female Sprague-Dawley rats subjected to moderate contusion at T10 | Secretome from rBM-MSCs | Intravenous administration of 150 μL | Improvement of behavioral recovery. |
| Increasing of axons in the lesion site. | ||||
| Upregulation of Olig 2 and Heat Shock Protein 70 (HSP70) levels and autophagy-related proteins in the injured spinal cords. |
r = rat; h = human.
BM = bone marrow; WJ= Wharton's Jelly.
4. Exploring the current landscape and future possibilities of clinical trials using MSC-secretome and EVs in NDs: a forward-looking perspective
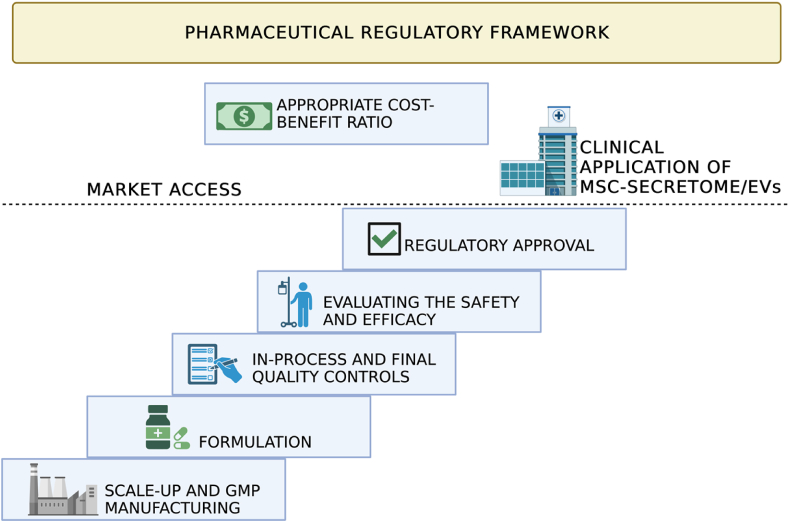
The studies described above constitute extensive evidence that supports the efficacy of MSC-secretome or MSC-EVs for treating different NDs, making them a new and promising cell-free alternative therapeutic agent. However, bringing a new medicinal product to the market involves a complex journey encompassing several stages, including molecular discovery, production process scale-up, dosage form formulation, preclinical and clinical trials, and, ultimately, regulatory approval. It is a process that takes between 10 and 15 years and requires a significant investment of hundreds of millions of dollars. These same principles also apply to MSC-secretome and EVs, but it is commonly evident to researchers active in this field that their translation into clinical practice has been slower than the standard path of medicines. It is, therefore, unsurprising that, despite the considerable evidence in preclinical animal models, clinical trial activation to evaluate the safety and efficacy of MSC-secretome and EVs in NDs is still limited (Table 9). Consequently, no regulatory-approved MSC-secretome or EV products are currently on the market.
Table 9.
MSC-secretome and EVs in clinical trials for neurological diseases. Source: http://clinicaltrials.gov, last search 31 May 2023; search terms: mesenchymal stem cells secretome or mesenchymal stem cell exosome or mesenchymal stem cell extracellular vesicles and Alzheimer's disease or Parkinson's disease or amyotrophic lateral sclerosis or multiple sclerosis or Huntington's disease or acute ischemic stroke or spinal cord injury.
| Disease | Clinical Trial ID Number | Study Design | Treatment | Year and Location | Status |
|---|---|---|---|---|---|
| AD | NCT04388982 | Phase I/II, interventional, open-label | MSCs-EVs | 2020, China | Completed, without results |
| PD/MSA | NCT04876326 | Single-group assignment, interventional, open-label | Autologous/allogenic UC-MSCs + A-MSC-secretome | 2021, Indonesia | Recruiting |
| AIS | NCT05008588 | Phase I/II, interventional, open-label | UC-MSCs + their secretome | 2022, Indonesia | Recruiting |
| NCT03384433 | Single-group assignment, interventional, open-label | MSCs-EVs | 2019, Islamic Republic of Iran | Unknown |
This delay likely comes from the lack of a comprehensive and efficient path for achieving regulatory compliance of MSC-secretome or EVs, thus delaying the arrival of new potentially effective therapies in the clinical practice and amplifying the risk of losing the invested resources. Therefore, there is a need to bridge the gap between the academic laboratory scale, industrial production, and possible market distribution of MSC-secretome and EVs, once they are developed and approved. In this regard, it is desirable that since early investigations in the laboratory, fundamental aspects for the regulatory approval are taken into consideration; these include the need to define a robust and Good Manufacturing Practices (GMP)-compliant manufacturing process, with proper in-process and end-process controls that ensure the final product's pharmaceutical quality, stability, and shelf-life; the need to formulate MSC-secretome/EVs as any other drug into a pharmaceutical dosage form, and then the need to demonstrate formulation's safety and efficacy through in vitro and in vivo animal models and, finally, human clinical trials. Below will be discussed in-depth considerations about manufacturing and characterization, appropriate quality control measures, and preclinical safety and efficacy testing to ensure straightforward translation of MSC-secretome and EVs into the clinic. To this end, a hypothetic “path”, reported in Fig. 3, will be taken into consideration; this scheme does not claim to be exhaustive but aims to put face-to-face with the high degrees of complexity in the clinical translation of MSC-secretome and EVs.
Fig. 3.
The “stair” to translate MSC-secretome and EV into the clinic (and the market) is made of many steep steps, all “under” the pharmaceutical regulatory framework. Created with BioRender.com.
4.1. Scale-up and GMP manufacturing of MSC-secretome/EVs
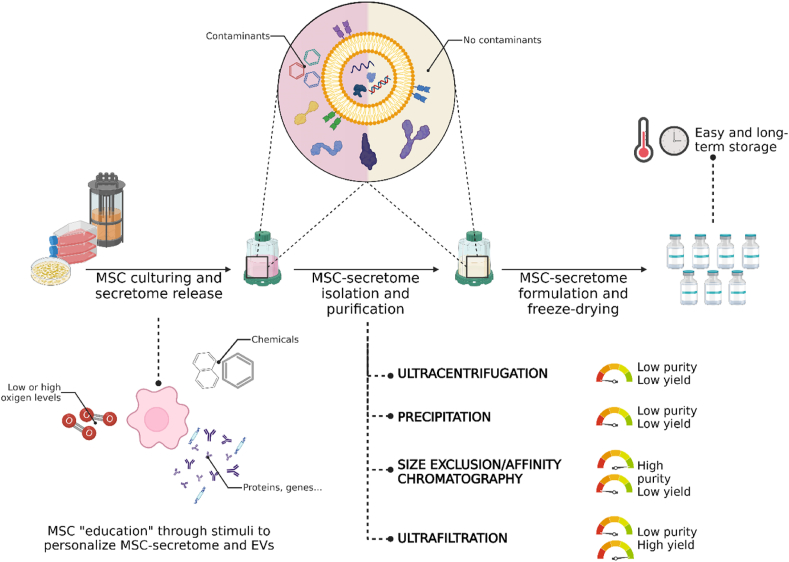
The manufacturing process of MSC-secretome and EVs must be designed to allow the production of large quantities (scalable), possibly economically, maintaining batch-to-batch consistency and following GMP guidelines. The manufacturing of MSC-secretome/EVs can benefit from the methods currently employed in producing classical biologics, such as antibodies, proteins, or cell therapy products, that assure scalability and compliance with GMP. However, being even MSC-secretome and EVs produced by living organisms, the risks of the production process are increased compared to purely synthetic drugs, as the intrinsic biological variability leads inevitably to the heterogenicity of the final product. In other words, assuring batch-to-batch consistency may be difficult, as creating a replica of a biological product with the same characteristics is nearly impossible. Indeed, a unique relationship exists between the production process and the characteristics of the resultant final product: “the product is the process”, meaning that the quality of the product, e.g., its yield, composition, and bioactivity, can be significantly influenced by both upstream and downstream processing choices [149] (Fig. 4). For instance, the culture conditions, including cell passage, cell density, and the frequency of secretome/EV harvesting, are critical upstream process steps. Therefore, these conditions must be defined early in the lab, and the upstream process should be appropriately designed to control all these parameters and the cell environment without forgetting the need to produce many cells for scalability reasons. This latter aspect may be achieved by culturing MSCs in multilayered culture flasks or, better, in bioreactors. Currently, there is no consensus on the optimal technology for MSCs production, but from a safety perspective, closed systems are preferred, even if monitoring can be more challenging than open systems [150]. Once MSC-secretome and EVs are released, technologies commonly used for biologics, such as (ultra)centrifugation, precipitation, size exclusion or affinity chromatography, depth filtration (adsorption), and tangential flow filtration (ultrafiltration), are used to collect them [151]. Even in this case, there is currently no consensus on the most appropriate isolation technique; some should be avoided as they are not scalable or may impact the integrity of EVs, such as ultracentrifugation [152]. Others may present challenges in obtaining simultaneously high yield and purity; for example, size exclusion or affinity chromatography allows highly pure preparations but with low yields, while ultrafiltration increases the final product yield but with lower purity [153].
Fig. 4.
Upstream and downstream processing choices when preparing medicinal products containing MSC-secretome and EVs. As “the product is the process”, any minimal changes introduced may affect the qualitative and quantitative composition and biological activity of the final product. However, this can be exploited to “educate” cells to produce personalized secretome/EVs. Created with BioRender.com.
To minimize batch-to-batch inconsistencies, numerous studies aim to develop synthetic EVs using various techniques such as top-down, bottom-up, or biohybrid approaches, as discussed in Ref. [154]. In a top-down strategy, the parent cells are dismantled using extrusion-based, filtration, or microfluidic methods, resulting in EVs that retain the membrane protein topology [155]. Conversely, a bottom-up approach requires an in-depth understanding of the composition of natural EVs, particularly their key components, which can be chemically synthesized and then used as the building blocks for constructing larger and more intricate artificial structures. In both cases, as EVs are no longer exclusively produced by living organisms, the inherent biological variability and consequent heterogeneity of the final product are diminished. Moreover, generating artificial EVs offers practical advantages for translation due to simpler production processes, and the yield can be improved by combining cell-derived lipids with synthetic ones, as long as the resulting hybrid EVs retain their targeting and uptake properties. Nevertheless, despite the progress made thus far, substantial further research is required to achieve artificial EVs with identical or fully comparable characteristics to those derived from cells.
4.2. Formulation of MSC-secretome and EVs
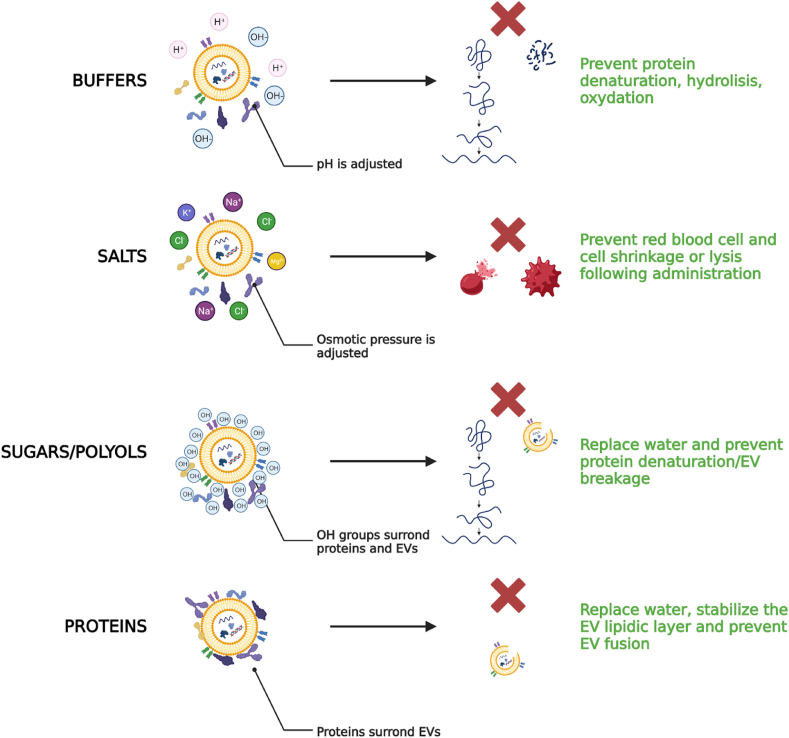
As with any other active ingredient, once produced, MSC-secretome and EVs must be formulated and stored at appropriate conditions until use (Fig. 4). Some studies investigated the most appropriate storage conditions of EVs (−80 °C vs 4 °C) by assessing the size, charge, and number of EVs and reported that the act of storing EVs can alter their size distribution, reduce their quantity and contents, and affect their cellular uptake and biodistribution [156]. Therefore, storage at −80 °C is generally recommended, even if it is the logistically most challenging and costly method. As an alternative for long-term storage, it has been proposed to freeze-drying of MSC-secretome and EVs [62,64,[157], [158], [159], [160]], and using cryo/lyoprotectants, it was demonstrated that the biological activity is preserved when tested both in vitro [64,66,68,[161], [162], [163]] and in vivo [66,164]. Commonly used excipients suitable for the formulation of MSC-secretome and EVs may include: (i) buffers, such as TRIS, histidine, and sodium acetate, to stabilize the pH at appropriate values that prevent protein/lipid degradation; (ii) salts, such as NaCl, KCl, MgCl2 to make the preparation isosmotic and thus suitable for injection; (iii) antioxidants, such as methionine, to prevent the protein/lipid oxidation; (iv) sugars/polyols, such as mannitol, trehalose, sucrose, glucose, that act as stabilizers or cryo/lyoprotectants and as such are helpful when the final product is frozen or freeze-dried; (v) for the only EV preparations, the addition of proteins, such as albumin, may be helpful to stabilize the lipidic layer [165] (Fig. 5).
Fig. 5.
Excipients suitable for the formulation of MSC-secretome and EVs. Buffers stabilize the pH to appropriate values compatible with the body administration and the product stability (e.g., to minimize hydrolysis and oxidation of proteins and lipids). Salts increase the ionic concentration making the preparation isosmotic with the body fluids. Sugars and polyols have many OH groups that surround and stabilize (by H-bonds) proteins and EVs even when the water in the formulation is subtracted following freezing or freeze-drying. Finally, proteins are adsorbed on the surface of EVs, creating a protein corona that prevents EV fusion and stabilizes the lipidic layer when the water in the formulation is subtracted following freezing or freeze-drying. Created with BioRender.com.
4.3. Quality controls in-process and on the final product
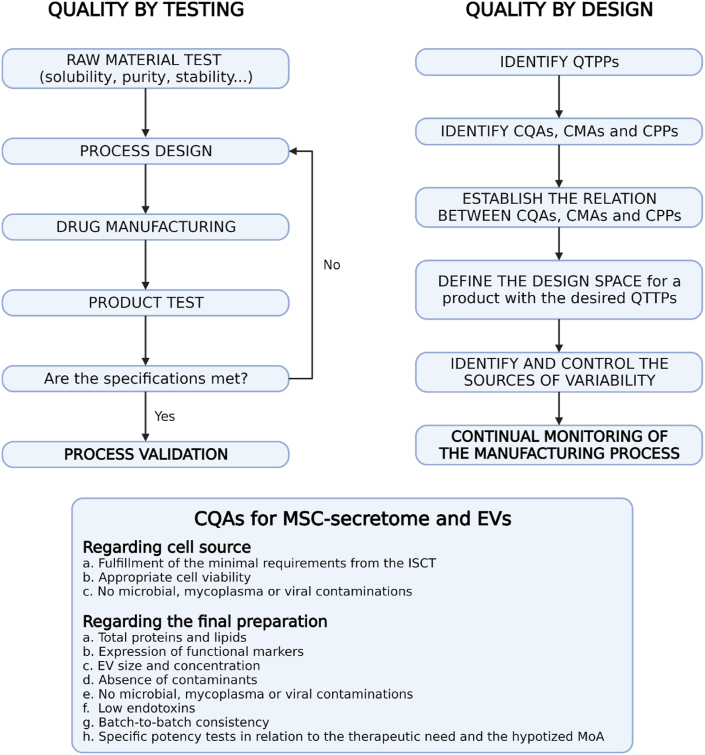
Quality-by-design is increasingly recommended by both Food and Drug Administration (FDA) and European Medicines Agency (EMA) when manufacturing a medicinal product, and it should also be applied for MSC-secretome and EVs (Fig. 6). According to this approach, quality should be built in by design, meaning that the Quality Target Product Profile (QTPP), i.e., the quality of the final product, relies on the identification of the Quality Attributes of the drug substances and excipients that should remain within a specific range throughout the entire manufacturing process, thus becoming Critical Quality Attributes (CQAs) [166]. In our opinion, CQAs for MSC-secretome and EVs products are the amount of proteins and lipids, expression of functional markers, EV size and concentration, the absence of contaminants, no microbial, mycoplasma or viral contamination, low endotoxins, and batch-to-batch consistency. CQAs should also include parent cell properties (e.g., MSCs should fulfill the minimal requirement adherence to the International Society for Cellular Therapy (ISCT) criteria [167], be viable and other minimal criteria [168]) and, significantly, potency tests. Indeed, functional aspects are necessary to demonstrate that the final product maintains the biological activity, which has to be tested through standardized potency assays that reflect, likely for MSCs, the hypothesized mechanism of action [169]. Then, an in-depth understanding of the process and the process parameters is needed to identify the Critical Process Parameters (CPPs) and Critical Material Attributes (CMAs) and evaluate for their association and the impact that they may have on CQAs. Practically, in-process controls must be implemented to ensure the final product meets the previously identified CQAs. Whenever the quality of the final product is hampered, for example, by an incomplete purification, a lack of stability, or insufficient reproducibility during a specific step in the manufacturing process, it is possible to set in and solve the situation.
Fig. 6.
Comparison between quality by testing and quality by design, and CQAs for MSC-secretome and EVs. Modified from Ref. [174]. Created with BioRender.com.
Unfortunately, detecting and evaluating changes in product quality for MSC-secretome and EVs is difficult due to their size and structural and compositional complexity. Indeed, the limitations of current analytical technologies hinder the detailed characterization of the physicochemical, immunochemical, or functional properties of MSC-secretome/EVs [170]. According to the most recent Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines, EVs must be characterized in size and yield, using, for example, the nanoparticle tracking analysis, morphology and presence of a bilayer by electron microscopy; at least one transmembrane or cytosolic protein should be detected to differentiate EVs from cell debris or other contaminants [171,172]. In addition, other technologies like mass spectrometry may contribute to a more in-depth characterization with proteomic, lipidomic, and transcriptomic studies [173]. Finally, as identifying all the components in a drug that trigger specific pharmacological, immunological, or metabolic effects is mandatory, the challenge in the characterization of MSC-secretome and EVs may further slow down their translational process.
4.4. Evaluating the safety and efficacy of MSC-secretome/EVs
Safety and efficacy of MSC-secretome and EVs must be ensured in their final formulation, at first in small and large animals and then in humans, evaluating immunotoxicology and inflammatory potential based on the dose, dosage form, and route of administration. Specifically, the investigations must include pharmacokinetics and pharmacodynamic assessment, immunogenicity, biocompatibility, and overall safety. Regarding biodistribution, many studies reported a short life of EVs administered systemically, following a non-specific accumulation of EVs in the liver, spleen, lung, and gastrointestinal tract [175]. As the large-scale pharmaceutical production and production costs impose the use of MSC-secretome and EVs in an allogeneic setting, continuous monitoring should be applied to assess immunologic and oncogenic effects, risk of microbial, mycoplasma or viral contamination, an adequate level of endotoxin and, for formulations administered intravenously, blood compatibility [170]. Once the animal model(s) pertinent to human disease has been identified, establishing a correlation between animal and human data becomes crucial. This can be accomplished by combining computational or theoretical modeling and experimental results or leveraging advanced technologies such as organs-on-chips. This approach allows for more precise predictions regarding the efficacy and safety of MSC-secretome and EVs in clinical trials, which must, in any case, be done with an appropriate design and under the control of the regulatory authorities. Even during the clinical trials, to comprehensively evaluate the efficacy and safety of MSC-secretome and EVs, it is crucial to establish their identity and purity, but achieving this goal requires conducting a systematic investigation that involves characterizing EVs and studying their interaction with the body's cells and tissues, which, again, can be a challenging task. Furthermore, guidelines from regulators regarding evaluating the safety and potency of MSC-secretome or EVs have not yet been released, with varying laboratories and companies utilizing different assays.
4.5. Moving toward regulatory approval and the market access
At the end of the path described above, all the data required to prepare the Common Technical Document (CTD) will be collected. The CTD consists of drug application dossiers for registering a medicinal product in the European Union, Japan, and the United States. It is specifically composed of four modules: module 2, which collects the summaries of the subsequent modules (i.e., the quality overall summary, the nonclinical overview and summary, and the clinical overview and summary); module 3, which is specifically dedicated to the quality of the final medicinal product; the module 4, which collects the non-clinical study reports; and module 5 which is dedicated to the clinical studies report (Table 10).
Table 10.
Composition of the CTD.
| Content | |
|---|---|
| Module 1 | Administrative information (not formally part of the CTD). |
| Module 2 | Quality overall summary; nonclinical overview; nonclinical summary; clinical overview; clinical summary. |
| Module 3 | Chemical, pharmaceutical, and biological information for medicinal products containing chemical and/or biological active substances (quality). |
| Module 4 | Non-clinical studies report (safety). |
| Module 5 | Clinical studies report (safety and efficacy). |
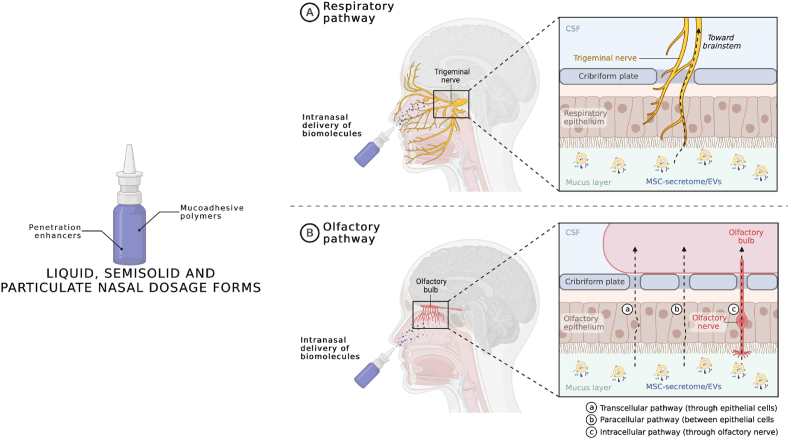
After carefully evaluating the CTD, if approved by regulatory authorities, MSC-secretome and EVs would finally have access to the market. At this point, the success of MSC-secretome and EVs in treating NDs would also depend on their relative costs, value for money, and impact on total expenditure. These domains, together with disease severity and the level of unmet clinical need, are usually considered by third payers (national health services, social insurance systems, private insurance) when they decide the reimbursement status of a new treatment [176,177]. MSC-secretome may have lower costs than stem cell therapies because appropriate facilities (e.g., the cell factories) have to be implemented only in the production site and not even in the administration site. Furthermore, pharmaceuticalizing MSC-secretome and EVs into freeze-dried and stable powder products would help make this therapy even less costly. In this regard, as MSC-secretome/EVs have been shown to reach the brain and exert their therapeutic effect also following intranasal administration [[92], [93], [94], [95],111,113,115,121], the possibility of formulating them in nasal dosage form, with reduced complexity for end-users (i.e., clinicians and health care organizations) with respect to parenteral formulations, could be relevant for market penetration. As pictured in Fig. 7, once administered intranasally, the proteins and EVs of MSC-secretome could reach the brain after being uptaken by the terminal axons of the trigeminal nerve or following the olfactory pathway, i.e., penetrating through the olfactory nerve, or the olfactory epithelium via the transcellular or paracellular pathway [178]. The residence time of the formulation in the nasal cavity should be increased using positively charged polymers that interact with the negative-charged mucus (e.g., chitosan, carbopol, polyacrylic acid, carboxymethylcellulose, and hypromellose); bile salts, fatty acids, cyclodextrins, and hydrophilic polymers, may instead be used as penetration enhancers to improve adsorption through the biological barriers [179,180]. Surfactants should be avoided to prevent the dissolution of the EV lipidic layer, with consequent loss and degradation of their cargo, and attention should be paid to the preservatives selected, as some of them may affect mucociliary clearance due to ciliostatic or ciliotoxic effects (these effects are typical of chlorbutol, hydroxybenzoate, methyl hydroxybenzoate, propyl hydroxybenzoate, chlorocresol, benzalkonium chloride, edetate, thiomersal, and phenylmercuric acetate). The typical liquid, semisolid or particulate formulations already extensively studied for intranasal delivery, as reviewed in Ref. [178], should be appropriate also for MSC-secretome and EVs, however considering that there is no need for nanoencapsulation, as EVs already act as natural nanocarriers.
Fig. 7.
Formulation of MSC-secretome/EVs as a nasal dosage form to enhance market penetration in treating NDs. Mucoadhesive excipients should be used to increase the residence time of the liquid, semisolid or particulate formulation in the nasal cavity, while penetration enhancers should be used to improve the passage of MSC-secretome/EVs through the biological barriers. CSF = cerebrospinal fluid. Created with BioRender.com.
The actual treatment costs are also contingent on dosage, timing, and frequency of administration of MSC-secretome and EVs for the relevant NDs, which will be determined during the clinical development processes in phase III clinical trials for that specific disease. More importantly, the value-for-money of treatments based on MSC-secretome and EVs will also depend on their relative risk-benefit profile. If they provide an added therapeutic value, are appropriately formulated to reduce complexity for end-users, and are less costly than competitors, they will be reimbursed. If not, payers will decide on the grounds of comparative cost-benefit analysis.
5. Conclusions
NDs are placing a significant and growing burden on healthcare systems worldwide, making the need for effective treatments urgent. According to the recent literature, MSCs offer a promising therapy due to their neuroregenerative, neuroprotective, and immunomodulatory properties, which are, however, linked to the bioactive substances they release, collectively known as secretome. In this regard, clinical and preclinical studies have shown that MSC-secretome and EVs can improve cognitive decline, plaque deposition, inflammatory response, and neuronal degeneration in AD, enhance dopamine levels, reduce loss and apoptosis of dopaminergic neurons, and improve motor performance in PD, boost motor performance, speech, strength, and sleep, and reduce glial cell activation in ALS, and decrease disease severity and improve motor function outcomes in MS. However despite, overall, MSC-secretome and EVs exhibit significant potential as a therapeutic strategy for the treatment of NDs, further research is necessary to optimize their efficacy and safety. Efforts should also be dedicated to addressing the challenges associated with their pharmaceutical manufacturing, including scalability, batch-to-batch consistency, and adherence to GMP guidelines. In addition, formulation and storage of MSC-secretome and EVs, along with quality controls, including the requirement for potency tests to demonstrate that the final product maintains its biological activity, should be considered. Finally, an appropriate cost analysis must be performed to assess the positive cost-benefit ratio for MSC-secretome and EVs with respect to the current therapies for NDs, but, unfortunately, this will be possible only after defining the dosage, timing, and frequency of administration of MSC-secretome and EVs in phase III clinical trials for that specific NDs.
Funding
This work was partially supported by Interreg V-A Italy-Switzerland 2014–2020, ATEx—Advanced Therapies Experiences. Project ID 3859153 and by the Italian Ministry of Health (Project MINSAL_INVITRO_TUMOR, CUP E85F21003590001).
Ethics approval and consent to participate
Not applicable, as it is a review.
Declaration of competing interest
Maria Luisa Torre is a co-founder and member of the advisory board of Pharmaexceed S.r.l.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Hong J.S. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Park. Relat. Disord. 2009;15:S28. doi: 10.1016/j.it.2008.05.002. -S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindvall O., Kokaia Z. Stem cells in human neurodegenerative disorders - time for clinical translation? J. Clin. Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elia C.A., Losurdo M., Malosio M.L., Coco S. Extracellular vesicles from mesenchymal stem cells exert pleiotropic effects on amyloid-beta, inflammation, and regeneration: a spark of hope for alzheimer's disease from tiny structures? Bioessays. 2019:41. doi: 10.1002/bies.201800199. [DOI] [PubMed] [Google Scholar]
- 4.Kalia L.V., Kalia S.K., McLean P.J., Lozano A.M., Lang A.E. alpha-Synuclein oligomers and clinical implications for Parkinson disease. Ann. Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelli V., Benedetti E., Antonosante A., Catanesi M., Pitari G., Ippoliti R., Cimini A., d'Angelo M. Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci. 2019;12 doi: 10.3389/fnmol.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cali T., Ottolini D., Brini M. Calcium signaling in Parkinson's disease. Cell Tissue Res. 2014;357:439–454. doi: 10.1007/s00441-014-1866-0. [DOI] [PubMed] [Google Scholar]
- 7.Braak H., Del Tredici K., Rub U., de Vos R.A.I., Steur E., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 8.Ubeda-Banon I., Saiz-Sanchez D., de la Rosa-Prieto C., Martinez-Marcos A. alpha-Synuclein in the olfactory system of a mouse model of Parkinson's disease: correlation with olfactory projections. Brain Struct. Funct. 2012;217:447–458. doi: 10.1007/s00429-011-0347-4. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan E., Chandrasekhar G., Chandrasekar P., Anbarasu K., Vickram A.S., Karunakaran R., Rajasekaran R., Srikumar P.S. Alpha-synuclein aggregation in Parkinson's disease. Front. Med. 2021;8 doi: 10.3389/fmed.2021.736978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castelli V., D'Angelo M., Lombardi F., Alfonsetti M., Antonosante A., Catanesi M., Benedetti E., Palumbo P., Cifone M.G., Giordano A., Desideri G., Cimini A. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson's disease models. Aging-Us. 2020;12:4641–4659. doi: 10.18632/aging.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caggiu E., Arru G., Hosseini S., Niegowska M., Sechi G., Zarbo I.R., Sechi L.A. Inflammation, infectious triggers, and Parkinson's disease. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longinetti E., Fang F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr. Opin. Neurol. 2019;32:771–776. doi: 10.1097/WCO.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonafede R., Mariotti R. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front. Cell. Neurosci. 2017;11 doi: 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attfield K.E., Jensen L.T., Kaufmann M., Friese M.A., Fugger L. The immunology of multiple sclerosis. Nat. Rev. Immunol. 2022;22:734–750. doi: 10.1038/s41577-022-00718-z. [DOI] [PubMed] [Google Scholar]
- 15.Gatto E.M., Rojas N.G., Persi G., Etcheverry J.L., Cesarini M.E., Perandones C. Huntington disease: advances in the understanding of its mechanisms. Clin.Parkinsonism Relat. Disord. 2020;3:100056. doi: 10.1016/j.prdoa.2020.100056. -100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A. Ajitkumar, O. De Jesus, Huntington Disease in: T.I.F.S. Publishing, Jan-. (Eds.) In: StatPearls [Internet]. , https://www.ncbi.nlm.nih.gov/books/NBK559166/, Updated 2022 Oct 7.
- 17.Hinkle J.L., Guanci M.M. Acute ischemic stroke review. J. Neurosci. Nurs. : J. Am. Associat.Neurosci. Nurse. 2007;39:285–310. doi: 10.1097/01376517-200710000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Cumming T.B., Brodtmann A. Can stroke cause neurodegenerative dementia? Int. J. Stroke. 2011;6:416–424. doi: 10.1111/j.1747-4949.2011.00666.x. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson A.K. Autonomic dysfunction in spinal cord injury: clinical presentation of symptoms and signs. Autonomic Dysfunct. Spinal Cord Injury. 2006;152:1–8. doi: 10.1016/S0079-6123(05)52034-X. [DOI] [PubMed] [Google Scholar]
- 20.Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Y.J., Dan X.L., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 22.Alzheimer's A. Alzheimer's disease facts and figures. Alzheimer's Dementia. 2018;14:367–425. 2018. [Google Scholar]
- 23.Xu J.F., Zhang Y.Q., Qiu C.X., Cheng F. Global and regional economic costs of dementia: a systematic review. Lancet. 2017;390:S47. -S47. [Google Scholar]
- 24.Myszczynska M.A., Ojamies P.N., Lacoste A.M.B., Neil D., Saffari A., Mead R., Hautbergue G.M., Holbrook J.D., Ferraiuolo L. Applications of machine learning to diagnosis and treatment of neurodegenerative diseases. Nat. Rev. Neurol. 2020;16:440–456. doi: 10.1038/s41582-020-0377-8. [DOI] [PubMed] [Google Scholar]
- 25.Dabrowska S., Andrzejewska A., Janowski M., Lukomska B. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.591065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A. Karimian, S.M. Khoshnazar, T. Kazemi, A. Asadi, A. Abdolmaleki, Role of secretomes in cell-free therapeutic strategies in regenerative medicine, Cell Tissue Bank.. [DOI] [PubMed]
- 27.Bari E., Ferrarotti I., Torre M.L., Corsico A.G., Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: the long way through "pharmaceuticalization" for the best formulation. J. Contr. Release. 2019;309:11–24. doi: 10.1016/j.jconrel.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Staff N.P., Jones D.T., Singer W. Mesenchymal stromal cell therapies for neurodegenerative diseases. Mayo Clin. Proc. 2019;94:892–905. doi: 10.1016/j.mayocp.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shariati A., Nemati R., Sadeghipour Y., Yaghoubi Y., Baghbani R., Javidi K., Zamani M., Hassanzadeh A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: a promising frontier. Eur. J. Cell Biol. 2020;99 doi: 10.1016/j.ejcb.2020.151097. [DOI] [PubMed] [Google Scholar]
- 30.Overcoming gaps in the treatment of neurodegenerative disease. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu X.R., Liu G., Halim A., Ju Y., Luo Q., Song G.B. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8 doi: 10.3390/cells8080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okolicsanyi R.K., Camilleri E.T., Oikari L.E., Yu C., Cool S.M., van Wijnen A.J., Griffiths L.R., Haupt L.M. Human mesenchymal stem cells retain multilineage differentiation capacity including neural marker expression after extended in vitro expansion. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasanthan J., Gurusamy N., Rajasingh S., Sigamani V., Kirankumar S., Thomas E.L., Rajasingh J. Role of human mesenchymal stem cells in regenerative therapy. Cells. 2021;10 doi: 10.3390/cells10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song N., Scholtemeijer M., Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol. Sci. 2020;41:653–664. doi: 10.1016/j.tips.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin C., Lu Y., Wang K., Bai L., Shi G., Huang Y., Li Y. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer's disease: a meta-analytic review on potential mechanisms. Transl. Neurodegener. 2020;9 doi: 10.1186/s40035-020-00199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riecke J., Johns K.M., Cai C.Y., Vahidy F.S., Parsha K., Furr-Stimming E., Schiess M., Savitz S.I. A meta-analysis of mesenchymal stem cells in animal models of Parkinson's disease. Stem Cell. Dev. 2015;24:2082–2090. doi: 10.1089/scd.2015.0127. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q., Yuan M., Qiu W., Cao W., Xu R. Preclinical studies of mesenchymal stem cells transplantation in amyotrophic lateral sclerosis: a systemic review and metaanalysis. Neurol. Sci. 2021;42:3637–3646. doi: 10.1007/s10072-020-05036-7. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Ge M., Zhang Y., Hao Q., Dong B. Mesenchymal stem cells in experimental autoimmune encephalomyelitis model of multiple sclerosis: a systematic review and meta-analysis. Multiple Sclerosis Relat. Disord. 2020:44. doi: 10.1016/j.msard.2020.102200. [DOI] [PubMed] [Google Scholar]
- 39.Li R., Su G., Liu J., Xu D., Gao X., Ma T., Zhang Z. Neuroprotective effect of stem cell transplantation on cerebral ischemia-reperfusion injury in rats: a systematic review. Chin. J.Tissue Eng. Res. 2023;27:1634–1640. [Google Scholar]
- 40.Muthu S., Jeyaraman M., Gulati A., Arora A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: systematic review and meta-analysis. Cytotherapy. 2021;23:186–197. doi: 10.1016/j.jcyt.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Kim H.J., Seo S.W., Chang J.W., Lee J.I., Kim C.H., Chin J., Choi S.J., Kwon H., Yun H.J., Lee J.M., Kim S.T., Choe Y.S., Lee K.-H., Na D.L. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase 1 clinical trial. Alzheimer's Dementia. 2015;1:95–102. doi: 10.1016/j.trci.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H.J., Seo S.W., Chang J.W., Lee J.I., Kim C.H., Chin J., Choi S.J., Kwon H., Yun H.J., Lee J.M., Kim S.T., Choe Y.S., Lee K.-H., Na D.L. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase I clinical trial. Alzheimer's Res. Ther. 2021;13 doi: 10.1186/s13195-021-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabavi S.M., Arab L., Jarooghi N., Bolurieh T., Abbasi F., Mardpour S., Azimyian V., Moeininia F., Maroufizadeh S., Sanjari L., Hosseini S.E., Aghdami N. Safety, feasibility of intravenous and intrathecal injection of autologous bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: an open label phase I clinical trial. Cell J. 2019;20:592–598. doi: 10.22074/cellj.2019.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrou P., Gothelf Y., Argov Z., Gotkine M., Levy Y.S., Kassis I., Vaknin-Dembinsky A., Ben-Hur T., Offen D., Abramsky O., Melamed E., Karussis D. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016;73:337–344. doi: 10.1001/jamaneurol.2015.4321. [DOI] [PubMed] [Google Scholar]
- 45.Sykova E., Rychrnach P., Drahoradova I., Konradova S., Ruzickova K., Vorisek I., Forostyak S., Homola A., Bojar M. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26:647–658. doi: 10.3727/096368916X693716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrou P., Kassis I., Yaghmour N.E., Ginzberg A., Karussis D. A phase II clinical trial with repeated intrathecal injections of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis. Front. Biosci.Landm. 2021;26:693–706. doi: 10.52586/4980. [DOI] [PubMed] [Google Scholar]
- 47.Connick P., Kolappan M., Crawley C., Webber D.J., Patani R., Michell A.W., Du M.-Q., Luan S.-L., Altmann D.R., Thompson A.J., Compston A., Scott M.A., Miller D.H., Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen J.A., Imrey P.B., Planchon S.M., Bermel R.A., Fisher E., Fox R.J., Bar-Or A., Sharp S.L., Skaramagas T.T., Jagodnik P., Karafa M., Morrison S., Koc J.R., Gerson S.L., Lazarus H.M. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Multip.Sclerosis J. 2018;24:501–511. doi: 10.1177/1352458517703802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riordan N.H., Morales I., Fernandez G., Allen N., Fearnot N.E., Leckrone M.E., Markovich D.J., Mansfield D., Avila D., Patel A.N., Kesari S., Rodriguez J.P. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis (vol 16, 57, 2018) J. Transl. Med. 2021;19 doi: 10.1186/s12967-018-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrou P., Kassis I., Levin N., Paul F., Backner Y., Benoliel T., Oertel F.C., Scheel M., Hallimi M., Yaghmour N., Ben Hur T., Ginzberg A., Levy Y., Abramsky O., Karussis D. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143:3574–3588. doi: 10.1093/brain/awaa333. [DOI] [PubMed] [Google Scholar]
- 51.Karussis D., Karageorgiou C., Vaknin-Dembinsky A., Gowda-Kurkalli B., Gomori J.M., Kassis I., Bulte J.W.M., Petrou P., Ben-Hur T., Abramsky O., Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrou P., Kassis I., Ginzberg A., Halimi M., Yaghmour N., Abramsky O., Karussis D. Long-term clinical and immunological effects of repeated mesenchymal stem cell injections in patients with progressive forms of multiple sclerosis. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.639315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-kheir W.A., Gabr H., Awad M.R., Ghannam O., Barakat Y., Farghali H.A.M.A., El Maadawi Z.M., Ewes I., Sabaawy H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 54.Pinheiro Mendonca M.V., Larocca T.F., de Freitas Souza B.S., Villarreal C.F., Maia Silva L.F., Matos A.C., Novaes M.A., Pinheiro Bahia C.M., de Oliveira Melo Martinez A.C., Kaneto C.M., Carneiro Furtado S.B., Sampaio G.P., Pereira Soares M.B., dos Santos R.R. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014;5 doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satti H.S., Waheed A., Ahmed P., Ahmed K., Akram Z., Aziz T., Satti T.M., Shahbaz N., Khan M.A., Malik S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a Phase I pilot study. Cytotherapy. 2016;18:518–522. doi: 10.1016/j.jcyt.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Geffner L.F., Santacruz P., Izurieta M., Flor L., Maldonado B., Auad A.H., Montenegro X., Gonzalez K., Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 2008;17:1277–1293. doi: 10.3727/096368908787648074. [DOI] [PubMed] [Google Scholar]
- 57.Albu S., Kumuru H., Coll R., Vives J., Valles M., Benito-Penalva J., Rodriguez L., Codinach M., Hernandez J., Navarro X., Vidal J. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. 2021;23:146–156. doi: 10.1016/j.jcyt.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Bydon M., Dietz A.B., Goncalves S., Moinuddin F.M., Alvi M.A., Goyal A., Yolcu Y., Hunt C.L., Garlanger K.L., Del Fabro A.S., Reeves R.K., Terzic A., Windebank A.J., Qu W. CELLTOP clinical trial: first report from a phase 1 trial of autologous adipose tissue-derived mesenchymal stem cells in the treatment of paralysis due to traumatic spinal cord injury. Mayo Clin. Proc. 2020;95:406–414. doi: 10.1016/j.mayocp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Yi H.X., Song Y.C. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res. Ther. 2021;12 doi: 10.1186/s13287-021-02609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caplan A.I. Mesenchymal stem-cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 61.Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bari E., Perteghella S., Di Silvestre D., Sorlini M., Catenacci L., Sorrenti M., Marrubini G., Rossi R., Tripodo G., Mauri P., Marazzi M., Torre M.L. Pilot production of mesenchymal stem/stromal freeze-dried secretome for cell-free regenerative nanomedicine: a validated GMP-compliant process. Cells. 2018;7 doi: 10.3390/cells7110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin S., Lee J., Kwon Y., Park K.S., Jeong J.H., Choi S.J., Bang S.I., Chang J.W., Lee C. Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and wharton's jelly. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bari E., Perteghella S., Catenacci L., Sorlini M., Croce S., Mantelli M., Avanzini M.A., Sorrenti M., Torre M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine. 2019;14:753–765. doi: 10.2217/nnm-2018-0240. [DOI] [PubMed] [Google Scholar]
- 65.Cruz-Barrera M., Florez-Zapata N., Lemus-Diaz N., Medina C., Galindo C.C., Gonzalez-Acero L.X., Correa L., Camacho B., Gruber J., Salguero G. Integrated analysis of transcriptome and secretome from umbilical cord mesenchymal stromal cells reveal new mechanisms for the modulation of inflammation and immune activation. Front. Immunol. 2020:11. doi: 10.3389/fimmu.2020.575488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bari E., Di Silvestre D., Mastracci L., Grillo F., Grisoli P., Marrubini G., Nardini M., Mastrogiacomo M., Sorlini M., Rossi R., Torre M.L., Mauri P., Sesana G., Perteghella S. GMP-compliant sponge-like dressing containing MSC lyo-secretome: proteomic network of healing in a murine wound model. Eur. J. Pharm. Biopharm. : Off.J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2020 doi: 10.1016/j.ejpb.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Agrelo I.S., Schira-Heinen J., Beyer F., Groh J., Butermann C., Estrada V., Poschmann G., Bribian A., Jadasz J.J., Lopez-Mascaraque L., Kremer D., Martini R., Muller H.W., Hartung H.P., Adjaye J., Stuhler K., Kury P. Secretome analysis of mesenchymal stem cell factors fostering oligodendroglial differentiation of neural stem cells in vivo. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21124350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bari E., Ferrarotti I., Di Silvestre D., Grisoli P., Barzon V., Balderacchi A., Torre M.L., Rossi R., Mauri P., Corsico A.G., Perteghella S. Adipose mesenchymal extracellular vesicles as alpha-1-antitrypsin physiological delivery systems for lung regeneration. Cells. 2019:8. doi: 10.3390/cells8090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zagoura D., Trohatou O., Makridakis M., Kollia A., Kokla N., Mokou M., Psaraki A., Eliopoulos A.G., Vlahou A., Roubelakis M.G. Functional secretome analysis reveals Annexin-A1 as important paracrine factor derived from fetal mesenchymal stem cells in hepatic regeneration. EBioMedicine. 2019;45:542–552. doi: 10.1016/j.ebiom.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mocchi M., Grolli S., Dotti S., Di Silvestre D., Villa R., Berni P., Conti V., Passignani G., Brambilla F., Bue M.D., Catenacci L., Sorrenti M., Segale L., Bari E., Mauri P., Torre M.L., Perteghella S. Equine mesenchymal stem/stromal cells freeze-dried secretome (lyosecretome) for the treatment of musculoskeletal diseases: production process validation and batch release test for clinical use. Pharmaceuticals. 2021;14:553. doi: 10.3390/ph14060553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Estrada R., Li N., Sarojini H., An J., Lee M.-J., Wang E. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J. Cell. Physiol. 2009;219:563–571. doi: 10.1002/jcp.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bronckaers A., Hilkens P., Martens W., Gervois P., Ratajczak J., Struys T., Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014;143:181–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Katagiri W., Kawai T., Osugi M., Sugimura-Wakayama Y., Sakaguchi K., Kojima T., Kobayashi T. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillof. Plast. Reconstruct. Surg. 2017;39 doi: 10.1186/s40902-017-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maacha S., Sidahmed H., Jacob S., Gentilcore G., Calzone R., Grivel J.C., Cugno C. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cell. Int. 2020;2020 doi: 10.1155/2020/4356359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruno S., Deregibus M.C., Camussi G. The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol. Lett. 2015;168:154–158. doi: 10.1016/j.imlet.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Lunyak V.V., Amaro-Ortiz A., Gaur M. Mesenchymal stem cells secretory responses: senescence messaging secretome and immunomodulation perspective. Front. Genet. 2017;8 doi: 10.3389/fgene.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gnecchi M., Danieli P., Malpasso G., Ciuffreda M.C. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Mesenchymal Stem Cells, 2 Ed.: Method. Protocol. 2016;1416:123–146. doi: 10.1007/978-1-4939-3584-0_7. [DOI] [PubMed] [Google Scholar]
- 78.Varderidou-Minasian S., Lorenowicz M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979–5997. doi: 10.7150/thno.40122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar L.P., Kandoi S., Misra R., Vijayalakshmi S., Rajagopal K., Verma R.S. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Teixeira F.G., Salgado A.J. Mesenchymal stem cells secretome: current trends and future challenges. Neural Regenerat. Res. 2020;15:75–77. doi: 10.4103/1673-5374.264455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bari E., Ferrarotti I., Saracino L., Perteghella S., Torre M.L., Corsico A.G. Mesenchymal stromal cell secretome for severe COVID-19 infections: premises for the therapeutic use. Cells. 2020:9. doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bari E., Ferrarotti I., Saracino L., Perteghella S., Torre M.L., Richeldi L., Corsico A.G. Mesenchymal stromal cell secretome for post-COVID-19 pulmonary fibrosis: a new therapy to treat the long-term lung sequelae? Cells. 2021:10. doi: 10.3390/cells10051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S.S., Jia J.J., Wang Z.F. Mesenchymal stem cell-derived extracellular vesicles suppresses iNOS expression and ameliorates neural impairment in alzheimer's disease mice. J. Alzheim. Dis. 2018;61:1005–1013. doi: 10.3233/JAD-170848. [DOI] [PubMed] [Google Scholar]
- 84.Elia C.A., Tamborini M., Rasile M., Desiato G., Marchetti S., Swuec P., Mazzitelli S., Clemente F., Anselmo A., Matteoli M., Malosio M.L., Coco S. Intracerebral injection of extracellular vesicles from mesenchymal stem cells exerts reduced A beta plaque burden in early stages of a preclinical model of alzheimer's disease. Cells. 2019:8. doi: 10.3390/cells8091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reza-Zaldivar E.E., Hernandez-Sapiens M.A., Gutierrez-Mercado Y.K., Sandoval-Avila S., Gomez-Pinedo U., Marquez-Aguirre A.L., Vazquez-Mendez E., Padilla-Camberos E., Canales-Aguirre A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regenerat. Res. 2019;14:1626–1634. doi: 10.4103/1673-5374.255978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sha S., Shen X.L., Cao Y.P., Qu L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer's disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/beta-catenin pathway. Aging-Us. 2021;13:15285–15306. doi: 10.18632/aging.203088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui G.H., Wu J., Mou F.F., Xie W.H., Wang F.B., Wang Q.L., Fang J., Xu Y.W., Dong Y.R., Liu J.R., Guo H.D. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. Faseb. J. 2018;32:654–668. doi: 10.1096/fj.201700600R. [DOI] [PubMed] [Google Scholar]
- 88.Ding M., Shen Y., Wang P., Xie Z.H., Xu S.L., Zhu Z.Y., Wang Y., Lyu Y.T., Wang D.W., Xu L.L., Bi J.Z., Yang H. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in alzheimer's disease. Neurochem. Res. 2018;43:2165–2177. doi: 10.1007/s11064-018-2641-5. [DOI] [PubMed] [Google Scholar]
- 89.Cui G.H., Guo H.D., Li H., Zhai Y., Gong Z.B., Wu J., Liu J.S., Dong Y.R., Hou S.X., Liu J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease. Immun. Ageing. 2019;16 doi: 10.1186/s12979-019-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y.A., Lu C.H., Ke C.C., Chiu S.J., Jeng F.S., Chang C.W., Yang B.H., Liu R.S. Mesenchymal stem cell-derived exosomes ameliorate alzheimer's disease pathology and improve cognitive deficits. Biomedicines. 2021;9 doi: 10.3390/biomedicines9060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang H., Liu Y.Q., Li J.C., Wang T., Hei Y., Li H.M., Wang X., Wang L.N., Zhao R.J., Liu W.P., Long Q.F. Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP/PS1 mice. Cell Death Discover. 2021;7 doi: 10.1038/s41420-021-00620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Losurdo M., Pedrazzoli M., D'Agostino C., Elia C.A., Massenzio F., Lonati E., Mauri M., Rizzi L., Molteni L., Bresciani E., Dander E., D'Amico G., Bulbarelli A., Torsello A., Matteoli M., Buffelli M., Coco S. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Translat. Med. 2020;9:1068–1084. doi: 10.1002/sctm.19-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma X.Y., Huang M., Zheng M.N., Dai C.X., Song Q.X., Zhang Q., Li Q., Gu X., Chen H., Jiang G., Yu Y., Liu X.S., Li S.K., Wang G., Chen H.Z., Lu L.J., Gao X.L. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease. J. Contr. Release. 2020;327:688–702. doi: 10.1016/j.jconrel.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Santamaria G., Brandi E., Vitola P.L., Grandi F., Ferrara G., Pischiutta F., Vegliante G., Zanier E.R., Re F., Uccelli A., Forloni G., de Rosbo N.K., Balducci C. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer's mice. Cell Death Differ. 2021;28:203–218. doi: 10.1038/s41418-020-0592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cone A.S., Yuan X.G., Sun L., Duke L.C., Vreones M.P., Carrier A.N., Kenyon S.M., Carver S.R., Benthem S.D., Stimmell A.C., Moseley S.C., Hike D., Grant S.C., Wilber A.A., Olcese J.M., Meckes D.G. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11:8129–8142. doi: 10.7150/thno.62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nalivaeva N.N., Belyaev N.D., Zhuravin I.A., Turner A.J. The Alzheimer's amyloid-degrading peptidase, neprilysin: can we control it? Int. J. Alzheimer's Dis. 2012;2012:383796. doi: 10.1155/2012/383796. -383796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Dios C., Bartolessis I., Roca-Agujetas V., Barbero-Camps E., Mari M., Morales A., Colell A. Oxidative inactivation of amyloid beta-degrading proteases by cholesterol-enhanced mitochondrial stress. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., Kawashima-Morishima M., Lee H.J., Hama E., Sekine-Aizawa Y., Saido T.C. Identification of the major A beta(1-42)-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat. Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 99.Dheen S.T., Kaur C., Ling E.-A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 100.Togo T., Katsuse O., Iseki E. Nitric oxide pathways in Alzheimer's disease and other neurodegenerative dementias. Neurol. Res. 2004;26:563–566. doi: 10.1179/016164104225016236. [DOI] [PubMed] [Google Scholar]
- 101.Yao Y., Huang C., Gu P., Wen T.Q. Combined MSC-secreted factors and neural stem cell transplantation promote functional recovery of PD rats. Cell Transplant. 2016;25:1101–1113. doi: 10.3727/096368915X689938. [DOI] [PubMed] [Google Scholar]
- 102.Teixeira F.G., Carvalho M.M., Panchalingam K.M., Rodrigues A.J., Mendes-Pinheiro B., Anjo S., Manadas B., Behie L.A., Sousa N., Salgado A.J. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson's disease. Stem Cells Translat. Med. 2017;6:634–646. doi: 10.5966/sctm.2016-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen H.X., Liang F.C., Gu P., Xu B.L., Xu H.J., Wang W.T., Hou J.Y., Xie D.X., Chai X.Q., An S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson's disease model by inducing autophagy. Cell Death Dis. 2020:11. doi: 10.1038/s41419-020-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teixeira F.G., Vilaca-Faria H., Domingues A.V., Campos J., Salgado A.J. Preclinical comparison of stem cells secretome and levodopa application in a 6-hydroxydopamine rat model of Parkinson's disease. Cells. 2020:9. doi: 10.3390/cells9020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahendru D., Jain A., Bansal S., Malik D., Dhir N., Sharma A.R., Sarma P., Prakash A., Nahar U., Bhatia A., Bhattacharyya S., Medhi B. Neuroprotective effect of bone marrow derived mesenchymal stem cell secretome in 6-OHDA-induced Parkinson's disease. Regen. Med. 2021;16:915–930. doi: 10.2217/rme-2021-0018. [DOI] [PubMed] [Google Scholar]
- 106.Xue C.L., Li X.C., Ba L., Zhang M.J., Yang Y., Gao Y., Sun Z., Han Q., Zhao R.C.H. MSC-derived exosomes can enhance the angiogenesis of human brain MECs and show therapeutic potential in a mouse model of Parkinson's disease. Aging Dis. 2021;12:1211–1222. doi: 10.14336/AD.2020.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang J.L., Luo S.L., Zhang J.C., Yu T., Fu Z.H., Zheng Y.F., Xu X.M., Liu C.Y., Fan M.X., Zhang Z.T. Exosome-mediated delivery of antisense oligonucleotides targeting alpha-synuclein ameliorates the pathology in a mouse model of Parkinson's disease. Neurobiol. Dis. 2021;148 doi: 10.1016/j.nbd.2020.105218. [DOI] [PubMed] [Google Scholar]
- 108.Li Y.G., Li Z.S., Gu J.C., Xu X.M., Chen H.M., Gui Y.X. Exosomes isolated during dopaminergic neuron differentiation suppressed neuronal inflammation in a rodent model of Parkinson's disease. Neurosci. Lett. 2022:771. doi: 10.1016/j.neulet.2021.136414. [DOI] [PubMed] [Google Scholar]
- 109.Cai Y., Zhang M.M., Wang M., Jiang Z.H., Tan Z.G. Bone marrow-derived mesenchymal stem cell-derived exosomes containing Gli1 alleviate microglial activation and neuronal apoptosis in vitro and in a mouse Parkinson disease model by direct inhibition of Sp1 signaling. JNEN (J. Neuropathol. Exp. Neurol.) 2022;81:522–534. doi: 10.1093/jnen/nlac037. [DOI] [PubMed] [Google Scholar]
- 110.Ma J.J., Shi X.X., Li M.J., Chen S.Y., Gu Q., Zheng J.H., Li D.S., Wu S.P., Yang H.Q., Li X. MicroRNA-181a-2-3p shuttled by mesenchymal stem cell-secreted extracellular vesicles inhibits oxidative stress in Parkinson's disease by inhibiting EGR1 and NOX4. Cell Death Discover. 2022;8 doi: 10.1038/s41420-022-00823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng H., Li Y., Ji W.H., Zhao R.C., Lu Z.G., Shen J., Wu Y.Y., Wang J.Z., Hao Q.L., Wang J.W., Wang W.L., Yang J., Zhang X. Intranasal administration of self-oriented nanocarriers based on therapeutic exosomes for synergistic treatment of Parkinson's disease. ACS Nano. 2022;16:869–884. doi: 10.1021/acsnano.1c08473. [DOI] [PubMed] [Google Scholar]
- 112.Koh S.H., Kim K.S., Choi M.R., Jung K.H., Park K.S., Chai Y.G., Roh W., Hwang S.J., Ko H.J., Huh Y.M., Kim H.T., Kim S.H. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 113.Bonafede R., Turano E., Scambi I., Busato A., Bontempi P., Virla F., Schiaffino L., Marzola P., Bonetti B., Mariotti R. ASC-exosomes ameliorate the disease progression in SOD1(g93a) murine model underlining their potential therapeutic use in human ALS. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crose J.J. Treating amyotrophic lateral sclerosis with a bone marrow derived mesenchymal stem cell extracellular vesicles - a case report. Int. J.Sci.Res. Archiv. 2021;2:167–171. [Google Scholar]
- 115.Ueda M., Seta Y. The first in human case of amyotrophic lateral sclerosis treated with stem CellDerived conditioned medium: a 1-year- follow up. Neurol. Neurorehabilit. 2022;4 [Google Scholar]
- 116.Rajan T.S., Giacoppo S., Diomede F., Bramanti P., Trubiani O., Mazzon E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int. J. Immunopathol. Pharmacol. 2017;30:238–252. doi: 10.1177/0394632017722332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farinazzo A., Angiari S., Turano E., Bistaffa E., Dusi S., Ruggieri S., Bonafede R., Mariotti R., Constantin G., Bonetti B. Nanovesicles from adipose-derived mesenchymal stem cells inhibit T lymphocyte trafficking and ameliorate chronic experimental autoimmune encephalomyelitis. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-25676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clark K., Zhang S., Barthe S., Kumar P., Pivetti C., Kreutzberg N., Reed C., Wang Y., Paxton Z., Farmer D., Guo F.Z., Wang A.J. Placental mesenchymal stem cell-derived extracellular vesicles promote myelin regeneration in an animal model of multiple sclerosis. Cells. 2019:8. doi: 10.3390/cells8121497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shamili F.H., Alibolandi M., Rafatpanah H., Abnous K., Mahmoudi M., Kalantari M., Taghdisi S.M., Ramezani M. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J. Contr. Release. 2019;299:149–164. doi: 10.1016/j.jconrel.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 120.Jafarinia M., Alsahebfosoul F., Salehi H., Eskandari N., Azimzadeh M., Mahmoodi M., Asgary S., Hakemi M.G. Therapeutic effects of extracellular vesicles from human adipose-derived mesenchymal stem cells on chronic experimental autoimmune encephalomyelitis. J. Cell. Physiol. 2020;235:8779–8790. doi: 10.1002/jcp.29721. [DOI] [PubMed] [Google Scholar]
- 121.Fathollahi A., Hashemi S.M., Hoseini M.H.M., Tavakoli S., Farahani E., Yeganeh F. Intranasal administration of small extracellular vesicles derived from mesenchymal stem cells ameliorated the experimental autoimmune encephalomyelitis. Int. Immunopharm. 2021;90 doi: 10.1016/j.intimp.2020.107207. [DOI] [PubMed] [Google Scholar]
- 122.Koohsari S.A., Absalan A., Azadi D. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles attenuate experimental autoimmune encephalomyelitis via regulating pro and anti-inflammatory cytokines. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-91291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang J., Buller B.A., Zhang Z.G., Zhang Y., Lu M., Rosene D.L., Medalla M., Moore T.L., Chopp M. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 2022;347 doi: 10.1016/j.expneurol.2021.113895. [DOI] [PubMed] [Google Scholar]
- 124.Li Z.J., Liu F., He X., Yang X., Shan F.P., Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharm. 2019;67:268–280. doi: 10.1016/j.intimp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 125.Xun C.F., Deng H.Y., Zhao J., Ge L.T., Hu Z.P. Mesenchymal stromal cell extracellular vesicles for multiple sclerosis in preclinical rodent models: a meta-analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.972247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Giampa C., Alvino A., Magatti M., Silini A.R., Cardinale A., Paldino E., Fusco F.R., Parolini O. Conditioned medium from amniotic cells protects striatal degeneration and ameliorates motor deficits in the R6/2 mouse model of Huntington's disease. J. Cell Mol. Med. 2019;23:1581–1592. doi: 10.1111/jcmm.14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xin H.Q., Li Y., Cui Y.S., Yang J.J., Zhang Z.G., Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cerebr. Blood Flow Metabol. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Doeppner T.R., Herz J., Gorgens A., Schlechter J., Ludwig A.K., Radtke S., de Miroschedji K., Horn P.A., Giebel B., Hermann D.M. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Translat. Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen K.H., Chen C.H., Wallace C.G., Yuen C.M., Kao G.S., Chen Y.L., Shao P.L., Chai H.T., Lin K.C., Liu C.F., Chang H.W., Lee M.S., Yip H.K. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537–74556. doi: 10.18632/oncotarget.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xin H.Q., Katakowski M., Wang F.J., Qian J.Y., Liu X.S., Ali M.M., Buller B., Zhang Z.G., Chopp M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang X., Ding J., Li Y.F., Liu W.J., Ji J.L., Wang H., Wang X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp. Cell Res. 2018;371:269–277. doi: 10.1016/j.yexcr.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 132.Geng W.J., Tang H.L., Luo S., Lv Y., Liang D.D., Kang X.H., Hong W.D. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Tourism Res. 2019;11:780–792. [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y., Fu N.S., Su J.L., Wang X.M., Li X.H. Rapid enkephalin delivery using exosomes to promote neurons recovery in ischemic stroke by inhibiting neuronal p53/caspase-3. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/4273290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moon G.J., Sung J.H., Kim D.H., Kim E.H., Cho Y.H., Son J.P., Cha J.M., Bang O.Y. Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and MicroRNA study. Translat. Stroke Res. 2019;10:509–521. doi: 10.1007/s12975-018-0668-1. [DOI] [PubMed] [Google Scholar]
- 135.Lee J.Y., Kim E., Choi S.M., Kim D.W., Kim K.P., Lee I., Kim H.S. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci. Rep. 2016;6 doi: 10.1038/srep33038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiang M., Wang H.R., Zin M.M., Yang X.L., Ji H.F., Jiang Y.F., Zhang H.W., Wu F.F., Wu G.L., Lai X.Y., Cai L.Y., Hu R.G., Xu L.M., Li L.X. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell. Physiol. Biochem. 2018;47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 137.Otero-Ortega L., Laso-Garcia F., Gomez-de Frutos M.D., Rodriguez-Frutos B., Pascual-Guerra J., Fuentes B., Diez-Tejedor E., Gutierrez-Fernandez M. White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke. Sci. Rep. 2017;7 doi: 10.1038/srep44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Han Y.X., Seyfried D., Meng Y.L., Yang D.M., Schultz L., Chopp M. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J. Neurosurg. 2019;131:290–300. doi: 10.3171/2018.2.JNS171475. [DOI] [PubMed] [Google Scholar]
- 139.Thomas J.M., Cunningham C.J., Lawrence C.B., Pinteaux E., Allan S.M. Therapeutic potential of extracellular vesicles in preclinical stroke models: a systematic review and meta-analysis. BMJ Open Sci. 2020;4 doi: 10.1136/bmjos-2019-100047. -e100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cantinieaux D., Quertainmont R., Blacher S., Rossi L., Wanet T., Noel A., Brook G., Schoenen J., Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kanekiyo K., Wakabayashi T., Nakano N., Yamada Y., Tamachi M., Suzuki Y., Fukushima M., Saito F., Abe S., Tsukagoshi C., Miyamoto C., Ide C. Effects of intrathecal injection of the conditioned medium from bone marrow stromal cells on spinal cord injury in rats. J. Neurotrauma. 2018;35:521–532. doi: 10.1089/neu.2017.5201. [DOI] [PubMed] [Google Scholar]
- 142.Asadi-Golshan R., Razban V., Mirzaei E., Rahrnanian A., Khajeh S., Mostafavi-Pour Z., Dehghani F. Sensory and motor behavior evidences supporting the usefulness of conditioned medium from dental pulp-derived stem cells in spinal cord injury in rats. Asian Spine J. 2018;12:785–793. doi: 10.31616/asj.2018.12.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cizkova D., Cubinkova V., Smolek T., Murgoci A.N., Danko J., Vdoviakova K., Humenik F., Cizek M., Quanico J., Fournier I., Salzet M. Localized intrathecal delivery of mesenchymal stromal cells conditioned medium improves functional recovery in a rat model of spinal cord injury. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19030870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khoshsirat S., Abbaszadeh H.A., Ahrabi B., Bahrami M., Abdollahi F.M.A., Khoramgah M.S., Roozbahany N.A., Darabi S. Evaluation of the effect of BMSCs condition media and methylprednisolone in TGF-beta expression and functional recovery after an acute spinal cord injury. Bratislava Med. J.Bratislavske Lekarske Listy. 2018;119:684–691. doi: 10.4149/BLL_2018_123. [DOI] [PubMed] [Google Scholar]
- 145.Chudickova M., Vackova I., Urdzikova L.M., Jancova P., Kekulova K., Rehorova M., Turnovcova K., Jendelova P., Kubinova S. The effect of Wharton jelly-derived mesenchymal stromal cells and their conditioned media in the treatment of a rat spinal cord injury. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tsai M.J., Liou D.Y., Lin Y.R., Weng C.F., Huang M.C., Huang W.C., Tseng F.W., Cheng H. Attenuating spinal cord injury by conditioned medium from bone marrow mesenchymal stem cells. J. Clin. Med. 2019;8 doi: 10.3390/jcm8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen Y.T., Tsai M.J., Hsieh N., Lo M.J., Lee M.J., Cheng H.R., Huang W.C. The superiority of conditioned medium derived from rapidly expanded mesenchymal stem cells for neural repair. Stem Cell Res. Ther. 2019;10 doi: 10.1186/s13287-019-1491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarveazad A., Toloui A., Moarrefzadeh A., Nafchi H.G., Neishaboori A.M., Yousefifard M. Mesenchymal stem cell-conditioned medium promotes functional recovery following spinal cord injury: a systematic review and meta-analysis. Spine Surg. Relat. Res. 2022;6:433–442. doi: 10.22603/ssrr.2022-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vulto A.G., Jaquez O.A. The process defines the product: what really matters in biosimilar design and production? Rheumatology. 2017;56:14–29. doi: 10.1093/rheumatology/kex278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dwarshuis N.J., Parratt K., Santiago-Miranda A., Roy K. Cells as advanced therapeutics: state-of-the-art, challenges, and opportunities in large scale biomanufacturing of high-quality cells for adoptive immunotherapies. Adv. Drug Deliv. Rev. 2017;114:222–239. doi: 10.1016/j.addr.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 151.Akbar A., Malekian F., Baghban N., Kodam S.P., Ullah M. Methodologies to isolate and purify clinical grade extracellular vesicles for medical applications. Cells. 2022:11. doi: 10.3390/cells11020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mol E.A., Goumans M.-J., Doevendans P.A., Sluijter J.P.G., Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017;13:2061–2065. doi: 10.1016/j.nano.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 153.Sidhom K., Obi P.O., Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21186466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Y.J., Wu J.Y., Liu J.H., Xu W.J., Qiu X.H., Huang S., Hu X.B., Xiang D.X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021;19 doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jang S.C., Kim O.Y., Yoon C.M., Choi D.S., Roh T.Y., Park J., Nilsson J., Lotvall J., Kim Y.K., Gho Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 156.Wu J.Y., Li Y.J., Hu X.B., Huang S., Xiang D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: a comparative evaluation of storage conditions. Drug Deliv. 2021;28:162–170. doi: 10.1080/10717544.2020.1869866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mocchi M., Bari E., Marrubini G., Bonda A.F., Perteghella S., Tartara F., Cofano F., Perna G.d., Giovannelli L., Mandracchia D., Sorlini M., Garbossa D., Torre M.L., Segale L. Freeze-dried mesenchymal stem cell-secretome pharmaceuticalization: optimization of formulation and manufacturing process robustness. Pharmaceutics. 2021;13:1129. doi: 10.3390/pharmaceutics13081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.El Baradie K.B.Y., Nouh M., O'Brien F., Liu Y.T., Fulzele S., Eroglu A., Hamrick M.W. Freeze-dried extracellular vesicles from adipose-derived stem cells prevent hypoxia-induced muscle cell injury. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guarro M., Suner F., Lecina M., Borros S., Fornaguera C. Efficient extracellular vesicles freeze-dry method for direct formulations preparation and use. Colloids Surf. B Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112745. [DOI] [PubMed] [Google Scholar]
- 160.Trenkenschuh E., Richter M., Heinrich E., Koch M., Fuhrmann G., Friess W. Enhancing the stabilization potential of lyophilization for extracellular vesicles. Adv. Healthc. Mater. 2022;11 doi: 10.1002/adhm.202100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bari E., Scocozza F., Perteghella S., Segale L., Sorlini M., Auricchio F., Conti M., Torre M.L. Three-dimensional bioprinted controlled release scaffold containing mesenchymal stem/stromal lyosecretome for bone regeneration: sterile manufacturing and in vitro biological efficacy. Biomedicines. 2022;10 doi: 10.3390/biomedicines10051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bari E., Roato I., Perale G., Rossi F., Genova T., Mussano F., Ferracini R., Sorlini M., Torre M.L., Perteghella S. Biohybrid bovine bone matrix for controlled release of mesenchymal stem/stromal cell lyosecretome: a device for bone regeneration. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22084064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bari E., Tartara F., Cofano F., Di Perna G., Garbossa D., Perteghella S., Sorlini M., Mandracchia D., Giovannelli L., Gaetani P., Torre M.L., Segale L. Freeze-dried secretome (lyosecretome) from mesenchymal stem/stromal cells promotes the osteoinductive and osteoconductive properties of titanium cages. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22168445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mocchi M., Bari E., Dotti S., Villa R., Berni P., Conti V., Del Bue M., Squassino G.P., Segale L., Ramoni R., Torre M.L., Perteghella S., Grolli S. Canine mesenchymal cell lyosecretome production and safety evaluation after allogenic intraarticular injection in osteoarthritic dogs. Animals. 2021;11 doi: 10.3390/ani11113271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Taguchi K., Okamoto Y., Matsumoto K., Otagiri M., Chuang V.T.G. When albumin meets liposomes: a feasible drug carrier for biomedical applications. Pharmaceuticals. 2021;14 doi: 10.3390/ph14040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu L.X., Amidon G., Khan M.A., Hoag S.W., Polli J., Raju G.K., Woodcock J. Understanding pharmaceutical quality by design. AAPS J. 2014;16:771–783. doi: 10.1208/s12248-014-9598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. Int. Soc. Cell Ther. Position Statement, Cytother. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 168.Torre M.L., Lucarelli E., Guidi S., Ferrari M., Alessandri G., De Girolamo L., Pessina A., Ferrero I. Gism, ex vivo expanded mesenchymal stromal cell minimal quality requirements for clinical application. Stem Cell. Dev. 2015;24:677–685. doi: 10.1089/scd.2014.0299. [DOI] [PubMed] [Google Scholar]
- 169.Galipeau J., Krampera M., Barrett J., Dazzi F., Deans R.J., Debruijn J., Dominici M., Fibbe W.E., Gee A.P., Gimble J.M., Hematti P., Koh M.B.C., Leblanc K., Martin I., McNiece I.K., Mendicino M., Oh S., Ortiz L., Phinney D.G., Planat V., Shi Y.F., Stroncek D.F., Viswanathan S., Weiss D.J., Sensebe L. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rohde E., Pachler K., Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21:581–592. doi: 10.1016/j.jcyt.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 171.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., Ayre D.C., Bach J.M., Bachurski D., Baharvand H., Balaj L., Baldacchino S., Bauer N.N., Baxter A.A., Bebawy M., Beckham C., Zavec A.B., Benmoussa A., Berardi A.C., Bergese P., Bielska E., Blenkiron C., Bobis-Wozowicz S., Boilard E., Boireau W., Bongiovanni A., Borras F.E., Bosch S., Boulanger C.M., Breakefield X., Breglio A.M., Brennan M.A., Brigstock D.R., Brisson A., Broekman M.L.D., Bromberg J.F., Bryl-Gorecka P., Buch S., Buck A.H., Burger D., Busatto S., Buschmann D., Bussolati B., Buzas E.I., Byrd J.B., Camussi G., Carter D.R.F., Caruso S., Chamley L.W., Chang Y.T., Chen C.C., Chen S., Cheng L., Chin A.R., Clayton A., Clerici S.P., Cocks A., Cocucci E., Coffey R.J., Cordeiro-da-Silva A., Couch Y., Coumans F.A.W., Coyle B., Crescitelli R., Criado M.F., D'Souza-Schorey C., Das S., Chaudhuri A.D., de Candia P., De Santana E.F., De Wever O., del Portillo H.A., Demaret T., Deville S., Devitt A., Dhondt B., Di Vizio D., Dieterich L.C., Dolo V., Rubio A.P.D., Dominici M., Dourado M.R., Driedonks T.A.P., Duarte F.V., Duncan H.M., Eichenberger R.M., Ekstrom K., Andaloussi S.E.L., Elie-Caille C., Erdbrugger U., Falcon-Perez J.M., Fatima F., Fish J.E., Flores-Bellver M., Forsonits A., Frelet-Barrand A., Fricke F., Fuhrmann G., Gabrielsson S., Gamez-Valero A., Gardiner C., Gartner K., Gaudin R., Gho Y.S., Giebel B., Gilbert C., Gimona M., Giusti I., Goberdhan D.C.I., Gorgens A., Gorski S.M., Greening D.W., Gross J.C., Gualerzi A., Gupta G.N., Gustafson D., Handberg A., Haraszti R.A., Harrison P., Hegyesi H., Hendrix A., Hill A.F., Hochberg F.H., Hoffmann K.F., Holder B., Holthofer H., Hosseinkhani B., Hu G.K., Huang Y.Y., Huber V., Hunt S., Ibrahim A.G.E., Ikezu T., Inal J.M., Isin M., Ivanova A., Jackson H.K., Jacobsen S., Jay S.M., Jayachandran M., Jenster G., Jiang L.Z., Johnson S.M., Jones J.C., Jong A., Jovanovic-Talisman T., Jung S., Kalluri R., Kano S., Kaur S., Kawamura Y., Keller E.T., Khamari D., Khomyakova E., Khvorova A., Kierulf P., Kim K.P., Kislinger T., Klingeborn M., Klinke D.J., Kornek M., Kosanovic M.M., Kovacs A.F., Kramer-Albers E.M., Krasemann S., Krause M., Kurochkin I.V., Kusuma G.D., Kuypers S., Laitinen S., Langevin S.M., Languino L.R., Lannigan J., Lasser C., Laurent L.C., Lavieu G., Lazaro-Ibanez E., Le Lay S., Lee M.S., Lee Y.X.F., Lemos D.S., Lenassi M., Leszczynska A., Li I.T.S., Liao K., Libregts S.F., Ligeti E., Lim R., Lim S.K., Line A., Linnemannstons K., Llorente A., Lombard C.A., Lorenowicz M.J., Lorincz A.M., Lotvall J., Lovett J., Lowry M.C., Loyer X., Lu Q., Lukomska B., Lunavat T.R., Maas S.L.N., Malhi H., Marcilla A., Mariani J., Mariscal J., Martens-Uzunova E.S., Martin-Jaular L., Martinez M.C., Martins V.R., Mathieu M., Mathivanan S., Maugeri M., McGinnis L.K., McVey M.J., Meckes D.G., Meehan K.L., Mertens I., Minciacchi V.R., Moller A., Jorgensen M.M., Morales-Kastresana A., Morhayim J., Mullier F., Muraca M., Musante L., Mussack V., Muth D.C., Myburgh K.H., Najrana T., Nawaz M., Nazarenko I., Nejsum P., Neri C., Neri T., Nieuwland R., Nimrichter L., Nolan J.P., Nolte-'t Hoen E.N.M., Noren Hooten N., O'Driscoll L., O'Grady T., O'Loghlen A., Ochiya T., Olivier M., Ortiz A., Ortiz L.A., Osteikoetxea X., Ostegaard O., Ostrowski M., Park J., Pegtel D.M., Peinado H., Perut F., Pfaffl M.W., Phinney D.G., Pieters B.C.H., Pink R.C., Pisetsky D.S., von Strandmann E.P., Polakovicova I., Poon I.K.H., Powell B.H., Prada I., Pulliam L., Quesenberry P., Radeghieri A., Raffai R.L., Raimondo S., Rak J., Ramirez M.I., Raposo G., Rayyan M.S., Regev-Rudzki N., Ricklefs F.L., Robbins P.D., Roberts D.D., Rodrigues S.C., Rohde E., Rome S., Rouschop K.M.A., Rughetti A., Russell A.E., Saa P., Sahoo S., Salas-Huenuleo E., Sanchez C., Saugstad J.A., Saul M.J., Schiffelers R.M., Schneider R., Schoyen T.H., Scott A., Shahaj E., Sharma S., Shatnyeva O., Shekari F., Shelke G.V., Shetty A.K., Shiba K., Siljander P.R.M., Silva A.M., Skowronek A., Snyder O.L., Soares R.P., Sodar B.W., Soekmadji C., Sotillo J., Stahl P.D., Stoorvogel W., Stott S.L., Strasser E.F., Swift S., Tahara H., Tewari M., Timms K., Tiwari S., Tixeira R., Tkach M., Toh W.S., Tomasini R., Torrecilhas A.C., Tosar J.P., Toxavidis V., Urbanelli L., Vader P., van Balkom B.W.M., van der Grein S.G., Van Deun J., van Herwijnen M.J.C., Van Keuren-Jensen K., van Niel G., van Royen M.E., van Wijnen A.J., Vasconcelos M.H., Vechetti I.J., Veit T.D., Vella L.J., Velot E., Verweij F.J., Vestad B., Vinas J.L., Visnovitz T., Vukman K.V., Wahlgren J., Watson D.C., Wauben M.H.M., Weaver A., Webber J.P., Weber V., Wehman A.M., Weiss D.J., Welsh J.A., Wendt S., Wheelock A.M., Wiener Z., Witte L., Wolfram J., Xagorari A., Xander P., Xu J., Yan X.M., Yanez-Mo M., Yin H., Yuana Y., Zappulli V., Zarubova J., Zekas V., Zhang J.Y., Zhao Z.Z., Zheng L., Zheutlin A.R., Zickler A.M., Zimmermann P., Zivkovic A.M., Zocco D., Zuba-Surma E.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Witwer K.W., Goberdhan D.C.I., O'Driscoll L., Thery C., Welsh J.A., Blenkiron C., Buzas E.I., Di Vizio D., Erdbrugger U., Falcon-Perez J.M., Fu Q.L., Hill A.F., Lenassi M., Lotvall J., Nieuwland R., Ochiya T., Rome S., Sahoo S., Zheng L. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choi D.S., Kim D.K., Kim Y.K., Gho Y.S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 174.Zhang L., Mao S.R. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017;12:1–8. doi: 10.1016/j.ajps.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kang M., Jordan V., Blenkiron C., Chamley L.W. Biodistribution of extracellular vesicles following administration into animals: a systematic review. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eichler H.G., Bloechl-Daum B., Abadie E., Barnett D., Konig F., Pearson S. OUTLOOK Relative efficacy of drugs: an emerging issue between regulatory agencies and third-party payers. Nat. Rev. Drug Discov. 2010;9:277–291. doi: 10.1038/nrd3079. [DOI] [PubMed] [Google Scholar]
- 177.van Nooten F., Holmstrom S., Green J., Wiklund I., Odeyemi I.A.O., Wilcox T.K. Health economics and outcomes research wiihin drug development challenges and opportunities for reimbursement and market access within biopharma research. Drug Discov. Today. 2012;17:615–622. doi: 10.1016/j.drudis.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 178.Ganger S., Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018;10 doi: 10.3390/pharmaceutics10030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bourganis V., Kammona O., Alexopoulos A., Kiparissides C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018;128:337–362. doi: 10.1016/j.ejpb.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 180.Mitragotri S., Burke P.A., Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]