Abstract
Background:
With the new highly active drugs available for people with multiple sclerosis (pwMS), vaccination becomes an essential part of the risk management strategy
Objective:
To develop a European evidence-based consensus for the vaccination strategy of pwMS who are candidates for disease-modifying therapies (DMTs).
Methods:
This work was conducted by a multidisciplinary working group using formal consensus methodology. Clinical questions (defined as population, interventions, and outcomes) considered all authorized DMTs and vaccines. A systematic literature search was conducted and quality of evidence was defined according to the Oxford Centre for Evidence-Based Medicine Levels of Evidence. The recommendations were formulated based on the quality of evidence and the risk–benefit balance.
Results:
Seven questions, encompassing vaccine safety, vaccine effectiveness, global vaccination strategy and vaccination in sub-populations (pediatric, pregnant women, elderly and international travelers) were considered. A narrative description of the evidence considering published studies, guidelines, and position statements is presented. A total of 53 recommendations were agreed by the working group after three rounds of consensus.
Conclusion:
This first European consensus on vaccination in pwMS proposes the best vaccination strategy according to current evidence and expert knowledge, with the goal of homogenizing the immunization practices in pwMS.
Keywords: Multiple sclerosis, vaccination, consensus, infections, disease-modifying therapy
Introduction
Over the last years, there has been a relevant change in the long-term prognosis of people with multiple sclerosis (pwMS), mainly due to the regulatory approval of a range of highly active immunotherapies with mechanisms of action that include alteration of lymphocyte trafficking, lymphocyte depletion and disruption of lymphocyte replication. PwMS receiving these drugs may be at risk of reactivation of latent pathogens, worsening of asymptomatic chronic infections, contracting de novo infections and experiencing a more severe course of common infections. 1 For this reason, individualized therapy must balance efficacy and side effects and should incorporate a set of preventive strategies to minimize risks.
An important part of the infectious risks for pwMS receiving highly active immunotherapies can be mitigated through vaccination. In the last few years, several national guidelines,2–4 consensus statements, 5 and review documents recommend vaccination in MS patients who are candidates for immunosuppressant drugs.6–8 However, questions remain in clinical practice as to when and whether to introduce a particular vaccine and which disease-modifying therapies (DMTs) can impact vaccine responses. In addition, vaccine coverage rates have been reported to be lower than desired for MS populations. 9
The purpose of this consensus document is to assist physicians, pwMS, healthcare providers, and health policymakers in making decisions about the vaccination as part of the global prevention strategy of pwMS. The recommendations represent an European expert consensus based on current knowledge and best available evidence.
Methods
This document has been developed under the auspices of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN), following a formal consensus methodology. It covers efficacy, safety, and vaccination strategy in untreated and treated pwMS and particular sub-populations (children, elderly, pregnant women, and international travelers).10,11
During a kick-off meeting in September 2020, an expert committee was set up, comprising a steering committee (involving six members with high expertise in MS and vaccines) and a multidisciplinary core working group composed of MS experts, vaccine advisors, and a patient representative. The committee identified the scope and topics formulating clinical questions according to the population, intervention, comparison, outcome mnemonic.
The clinical questions were informed according to a comprehensive literature search, summary, and grading of the evidence using standards from the Oxford Centre for Evidence-Based Medicine. 12 For questions 1 and 2, the search was updated based on the previous work in the French national guideline. 2 Searches in MEDLINE (accessed through PubMed), EMBASE (embase.com), and the Cochrane Central Register of Controlled Trials (The Cochrane Library) were performed up to April 2021. Complete search strings can be found in Supplemental Appendix 1. Citations to relevant studies were also tracked through the Web of Science (Clarivate Analytics). Reviews were only considered if they reported pooled analysis from original studies. For questions 3 to 7, the search also comprised relevant published guidelines on immunizations for MS and other autoimmune conditions treated with immunosuppressive drugs and pertinent information from the European Public Assessment Reports of the European Medicine Agency (EMA).
Study eligibility was pre-defined for each clinical question (Supplemental Appendix 2). DMTs and vaccines authorized by the EMA at the time of publication were considered. Due to the fast-changing developments on vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, this document does not include specific recommendations for these vaccines that can be found in recent documents.13–15 However, this recent evidence has expanded the overall knowledge on MS and immunizations and, therefore, is taken into account to indirectly support the recommendations for other vaccines. Finally, pwMS receiving hemopoietic stem cell transplantation (HSCT) were not considered in this consensus either, and specific guidance on immunization post-HSCT can be consulted elsewhere. 16
The formulation and agreement of the recommendations were done using the modified Nominal Group Technique, which is a highly structured procedure, based on iterative ratings with feedback, to reach consensus in a small group of experts on topics for which expert opinion is relevant. 11 The evidence was presented and discussed within the expert committee members and other invited discussants during the ECTRIMS focused workshop on “Risk of Infections in MS DMTs” held on April 2021. As a result, the first set of statements was circulated to the core working group members for a first round of voting through email, using a 9-point Likert-type scale, with a pre-defined 80% level of agreement. A follow-up virtual face-to-face meeting was held in June 2021 to discuss those statements for which consensus was not reached in the first round. The revised statements/recommendations were submitted for agreement in a further round of voting through email. The manuscript was submitted for external review and endorsement by eight ECTRIMS council members, the EAN scientific committee and representatives of the Multiple Sclerosis International Federation and the European Multiple Sclerosis Platform.
Results and recommendations
Safety and efficacy of vaccines
Question 1: Are vaccines associated with an increased risk of triggering exacerbations and/or disability worsening in pwMS?
Fifteen studies met the eligibility criteria, 1 of them investigated the risk of MS exacerbation following any vaccination, 17 and 14 studies addressed safety concerns related to individual vaccines (hepatitis B, tetanus, influenza, BCG, varicella, tick-borne encephalitis (TBE), rabies, and yellow fever).18–31 Evidence on the safety of TBE, rabies, and yellow fever vaccination will be reviewed in Question 7. Details on the methodology, level of the evidence and results of the included studies are available in Supplemental Appendix 3.
The Vaccines in Multiple Sclerosis study (level 4) 17 evaluated the relative risk (RR) of relapse associated with vaccination in 643 patients with MS and showed no risk of relapse after exposure to any vaccine of 0.71 (95% CI 0.40–1.26) or to individual vaccines such as influenza, hepatitis B or the combined diphtheria tetanus vaccine (RR 1.08; 95% CI 0.37–3.10, RR 0.67; 95% CI 1.20–2.17 and RR 0.22; 95% CI 0.05–0.99, respectively). 17 Six additional studies, two of them placebo-controlled trials, have evaluated vaccines against seasonal influenza and/or H1N1 strain.18–23 All but one 23 failed to show a link between seasonal and/or H1N1 influenza vaccination and MS relapses and changes in the expanded disability status scale (EDSS).
The safety of the BCG vaccine was evaluated in two different studies by Ristori et al: a single crossover MRI-monitored study (level 3),24,25 and a double-blind placebo-controlled trial (level 2). 25 Both studies reported a decrease in the frequency of gadolinium (Gd)-enhancing lesions and active lesions (new/enlarging T2-hyperintense lesions and total Gd-enhancing lesions) in the post-vaccination period and fewer cumulative number of relapses.
Only one study reported the absence of safety issues of the varicella-zoster vaccine administered in 50 treatment-naïve patients with progressive MS who were seropositive to varicella before vaccination. 32 However, the results are of limited value due to an insufficient description of the data in the manuscript.
Conclusion
Overall, the data indicate that commonly administered vaccines such as influenza, tetanus, or hepatitis B vaccines do not increase the risk of exacerbations and/or disability progression in MS. Similar results have been observed following BCG vaccination.
Vaccine safety
Statements
Statement 1. In MS patients with or without DMT, vaccines are not associated with an increased risk of relapses.
Statement 2. In MS patients with or without DMT, vaccines are not associated with an increased risk of disability.
Statement 3. In MS patients with or without DMT, the benefit of immunization greatly outweighs any potential risks.
Statement 4. Inactivated vaccines can be safely used in MS patients receiving DMTs.
Recommendations
Recommendation 1. Live attenuated vaccines can be safely used in MS patients without DMTs or in those receiving immunomodulatory treatments (interferons or glatiramer acetate (GA)) but should be avoided in patients receiving the following therapies: dimethyl fumarate (DMF), teriflunomide, sphingosine-1-phosphate modulators, natalizumab, cladribine, alemtuzumab, or anti-CD20 monoclonal antibodies.
Question 2a: Are vaccines as effective in treatment-naïve pwMS as in the general population?
Four studies evaluated the immunogenicity of vaccines in treatment-naïve pwMS; three of them focusing on influenza vaccination,20,33,34 and one on TBE 27 that will be reviewed in Question 7. These studies showed similar humoral responses to influenza vaccines with a significant increase in the mean antibody titers after vaccination in both pwMS and healthy individuals, indicating that pwMS not receiving immunotherapies can mount similar responses to those who do not have MS. In addition, pwMS responded to influenza antigens with higher proliferative responses of peripheral blood lymphocytes than healthy subjects.20,33 Details on the methodology, level of the evidence, and results of the included studies are available in Supplemental Appendix 3.
Question 2b: What is the effectiveness of vaccines in pwMS treated with DMTs?
Interferon-beta
The immunogenicity of vaccines in pwMS treated with interferon-beta (IFN-β) has been evaluated in six studies, all of them focusing on influenza vaccination.34–39 In two cohort studies, Olberg et al.34,35(level 3) showed no significant difference in the influenza seroprotection rates at 10 and 12-month post-vaccination between pwMS receiving IFN-β and healthy controls. More than 90% out of 46 pwMS treated with IFN-β achieved seroprotection for H1N1, H3N2, and B strains according to the Teriflunomide and Vaccination (TERIVA; level 3) study. 36 In addition, two nonrandomized, open-label studies (level 3) reported preserved humoral immune response in the IFN-β and control groups.37,38 The results of the five previous studies were meta-analyzed in a new study showing that pwMS receiving IFN-β therapy do not have a meaningful reduction in the likelihood of seroprotection to influenza vaccination (OR 1.51; 95% CI 0.79–2.90). 4 More recently, Metze et al. (level 3) found that following influenza vaccination, pwMS treated with IFN-β had high seroprotection rates (>84%) against H1N1, H3N2, and B strains and developed protective antibody titers to all three vaccine strains. 39 Furthermore, as IFN-β has potent in vivo antiviral effects, it may even exhibit a protective role against influenza infection.40,41
Glatiramer acetate
Three studies evaluated the immunogenicity of influenza vaccines in pwMS treated with GA.34,35,39 Olberg et al. 35 found lower protective antibody titers in the GA group than in the control group following seasonal influenza vaccination (58.3% vs. 71.2% for H1N1 and 41.7% vs. 79.5% for H3N2). This impaired response has not been confirmed in any of the later studies, in which no significant differences were observed for patients treated with GA as compared to controls, in the rates of protection against H1N1 strain at three, six and 12 months after vaccination 34 or against H1N1, H3N2, and B strains at 4 weeks after vaccination. 39
Teriflunomide
The efficacy of vaccines in individuals receiving teriflunomide has been evaluated in two studies. The multicenter, parallel-group TERIVA study (level 3) involving 128 pwMS in three arms (teriflunomide 7 mg, teriflunomide 14 mg, and IFN-β groups) showed that the proportion of pwMS meeting the European criterion for influenza vaccine efficacy ranged between 76.9% and 97.5% in both teriflunomide treatment groups. 36 A later randomized, double-blind, placebo-controlled study (level 2) evaluating responses to neoantigen (rabies vaccine) and recall antigens (Candida albicans, Trichophyton, and tuberculin) in 23 healthy subjects treated with teriflunomide, showed that all subjects achieved seroprotective titers following rabies vaccination, despite lower antibody levels in the teriflunomide group. 42 The responses to recall antigens did not differ notably between groups.
Dimethyl fumarate
A single open-label, multicenter study (level 3) assessed the ability of 38 DMF-treated pwMS to respond to different vaccines compared with non-pegylated IFN-treated pwMS. 43 Patients received: (1) tetanus-diphtheria toxoid (Td) to test T-cell–dependent recall response, (2) pneumococcal 23-polyvalent vaccine to test T-cell–independent humoral response, and (3) meningococcal oligosaccharide CRM197 conjugate vaccine (groups A, C, W-135, and Y) to test T-cell-dependent neoantigen response. The results demonstrated no statistically significant difference in the response rates between groups to Td vaccination (68% vs. 73%), pneumococcal serotype 3 (66% vs. 79%), pneumococcal serotype 8 (95% vs. 88%), and meningococcal serogroup C (53% vs. 53%). 43 Notably, no meaningful differences were observed between groups in the proportion of responders when stratified by lymphocyte count.
Fingolimod (and other sphingosine-1-phosphate–receptor modulators)
The efficacy of vaccines in pwMS treated with sphingosine-1-phosphate (S1 P) receptor modulators has been evaluated in six studies with Fingolimod,34,39,44–47 and one with siponimod. 48 In a small prospective observational study (level 3) patients receiving fingolimod, were able to mount similar cellular and antibody responses to influenza vaccine, regardless of lymphopenia (mean lymphocyte counts in fingolimod-treated pwMS were 64% of the lower normal range) as compared to controls. 44 The number of influenza-specific interferon-γ-secreting T cells was not significantly different between groups after vaccination. Similarly, the proportion of subjects fulfilling seroprotection criteria for influenza A and B was similar in both groups at 7, 14, and 28 days following vaccination. 44 Consistent results were observed in a randomized, placebo-controlled parallel-group study (level 2) with similar T-cell dependent and independent antibody responses in fingolimod and placebo healthy volunteers following immunization with neoantigens (keyhole limpet hemocyanin [KLH] and pneumococcal polysaccharides vaccine [PPV-23]) and a recall antigen (tetanus toxoid [TT]). 45 More recently, Mehling et al. 46 (level 3) evaluated the avidity of the immunoglobulin (Ig) G response targeting influenza A and B before and after influenza vaccination in 10 pwMS treated with fingolimod and compared it to 10 pwMS receiving IFN-β and 15 healthy controls. A significant vaccine-induced increase in the avidity of influenza-specific IgG was seen in pwMS treated with IFN-β and in healthy controls but not in fingolimod-treated pwMS, suggesting that despite comparative titers antibody responses are likely to be qualitatively influenced by fingolimod. 46 Further studies all showed reduced responses in patients treated with Fingolimod. In a randomized, multicenter, placebo-controlled study (level 2) the responder rates for influenza and TT booster vaccines in fingolimod-treated pwMS were significantly reduced compared to placebo at 3 weeks (OR 0.21; 95% CI 0.08–0.54 for influenza and OR 0.43; 95% CI 0.20–0.92 for TT) and at 6 weeks post-vaccination (OR 0.25; 95% CI 0.11–0.57 for influenza and OR 0.25; 95% CI 0.11–0.57 for TT). 47 Similarly, a prospective cohort study (level 3) 34 reported seroprotection rates of 22.2% against H1N1 at 12 months post-vaccination compared with 50% in untreated pwMS and 70.4% in healthy controls.
Only one study (level 2) has evaluated the effects of siponimod on influenza and PPV-23 vaccine responses in 120 healthy subjects. 48 The results showed that ⩾ 70% of participants achieved seroprotection H1N1 and H3N2 and, ⩾ 90% for PPV-23, concluding that siponimod had a limited effect on the immune response following influenza or PPV-23 vaccinations in healthy persons. 48
Natalizumab
Five studies evaluated the immunogenicity of influenza vaccines in pwMS treated with natalizumab with heterogenous results.34,35,39,49,50 The two studies by Olberg et al.34,35 showed that pwMS treated with natalizumab had an attenuated humoral response to influenza vaccination, compared to those exposed to INF-β or healthy controls. In line with these findings, Metze et al. 39 showed that pwMS receiving natalizumab had lower seroprotection rates (14.3%) against all three influenza strains (H1N1, H3N2, and B) than pwMS treated with IFN-β (73.3%). In contrast to the previous results, a small cohort study (level 3) showed similar humoral responses between 17 pwMS treated with natalizumab and 10 healthy controls at 4, 8, and 12 weeks following vaccination with trivalent influenza vaccine (A-H1N1/A-H3N2/B). 49 The proportion of responders to TT and KLH immunizations was also similar in the presence and absence of natalizumab according to a randomized, multicenter, open-label study (level 2). 50
Alemtuzumab
A single pilot case–control study (level 4) examined antibody responses to four common vaccines (diphtheria, tetanus, poliomyelitis vaccine, Haemophilus influenzae type b, meningococcal group C conjugate vaccine, and PPV-23) in 24 patients who received alemtuzumab between 1.8 and 86 months before vaccination (median 18). 51 All patients had seroprotective levels of antibodies to tetanus and diphtheria after vaccination, and ⩾ 95% against polio. Similarly, seroprotection rates to Haemophilus influenzae type b and meningococcal group C were also high (100% and 91%, respectively). 51 In addition, two-fold responses to pneumococcal 3 and 8 serotypes after alemtuzumab were similar to published rates. Although immune responses to common vaccines were preserved after alemtuzumab, vaccination within 6 months of treatment resulted in a smaller proportion of responders. 51 This study lacked a comparison group of untreated pwMS.
Cladribine
A single small study of 14 patients enrolled in MAGNIFY-MS trial provides preliminary evidence that patients taking cladribrine tablets are able to mount and maintain effective humoral responses against influenza and varicella vaccines, regardless of timing after treatment administration or total lymphocyte count. 52
Anti-CD20 therapy
One study specifically investigated the efficacy of vaccines in pwMS treated with anti-CD20 therapies. In the VELOCE study (level 2), Bar-Or et al. 53 evaluated antibody responses to influenza, TT, PPV-23, and KLH in pwMS treated with ocrelizumab. Response rates were assessed at 4- and 8 weeks post-vaccination, which corresponds to 16 and 20 weeks post-ocrelizumab dosing, respectively. Ocrelizumab-treated pwMS are approximately half as likely to mount an antibody response against tetanus toxoid vaccine (23.9% ocrelizumab vs. 54.5% controls) and about two thirds less likely to mount an antibody response to 12 or more pneumococcal serotypes (37.3% ocrelizumab vs. 97.1% controls). 53 Seroprotection rates at 4 weeks against 5 influenza strains ranged from 55.6% to 80% in the ocrelizumab group and 75% to 97% in the control group. 53
No studies evaluating the efficacy of vaccines in pwMS treated with rituximab or ofatumumab were found. Indirect evidence available for patients with rheumatoid arthritis resulted in decreased antibody responses to pneumococcal polysaccharide vaccine and KLH. 54 Similarly, a small study of 26 patients with neuromyelitis optica spectrum disorder (NMOSD) showed decreased responses to the H1N1 influenza vaccine in those receiving rituximab. 55 A systematic review of the literature on vaccine responsiveness in patients (including noncancer and cancer populations) receiving anti-CD20 therapy concluded that (1) vaccination appears safe in patients on anti-CD20 therapies; (2) the humoral response to vaccination in patients on active anti-CD20 therapy is low and approaches 0%; (3) anti-CD20 therapy lowers patients’ vaccine response beyond the impact of their disease or other treatments, and (4) response to vaccination improves incrementally over time but may not reach the level of healthy controls even 12 months after therapy. 56
Mitoxantrone and other DMTs
In the cohort study by Olberg et al., 35 none of the 11 mitoxantrone-treated pwMS vaccinated during the influenza pandemic in 2009 showed protective antibody titers to H1N1. There are no published studies investigating the efficacy of vaccines in pwMS treated with other DMTs such as, cyclophosphamide, methotrexate, azathioprine, and mycophenolate.
Further details on the methodology, level of the evidence, and results of the previous studies are available in Supplemental Appendix 3.
Conclusion and further data from COVID-19 vaccines
PwMS receiving IFN-β, GA, DMF, and teriflumomide mount an appropriate immune response to vaccines. Substantial evidence is available for all these DMTs and influenza vaccines, but also for other commonly used vaccines such as tetanus-diphtheria, pneumococcal, meningococcal for DMF, and rabies vaccine for teriflunomide. Recent data for COVID-19 vaccines confirms these results, showing no differences in post-vaccination seroconversion and antibody concentrations as compared to the untreated controls.57–61 For Teriflunomide, few studies involving a small number of patients also reported preserved humoral responses to COVID-19 vaccines.58,62,63
In PwMS, fingolimod treatment reduced immune responses to influenza and tetanus booster vaccines. In healthy subjects, siponimod has a limited effect on the efficacy of vaccinations with neoantigens. Consistently, evidence for COVID-19 vaccines confirms a significantly lower post-vaccination seroconversion, with significantly lower concentrations of antibodies in fingolimod-treated patients. 61 In addition, the interferon-gamma release assays in two studies suggested decreased odds of positive T-cell response.58,64
PwMS receiving natalizumab may have a reduced response to influenza vaccination. However, it does not seem to impair the humoral response to recall immunization with TT. Data on the immunogenicity to COVID-19 vaccine also support the presence of preserved humoral and T-cell responses. 61
In alemtuzumab-treated pwMS, humoral responses to vaccination with diphtheria, tetanus, poliomyelitis vaccine, Haemophilus influenzae type b, meningococcal group C conjugate vaccine and PPV-23 are preserved, but vaccination within 6 months of alemtuzumab infusion could compromise responses. For COVID-19 vaccines, studies based on a small number of patients have also reported preserved seroconversion rates.57,59,65 However, there was a significant correlation in the time from last treatment dosing to first vaccine dose on post-vaccination IgG titers, explained by the significant B- and T-cells depletion shortly after the infusion. 58 Similar preserved vaccine responses to influenza, varicella vaccines have also been reported for cladribine, according to limited evidence. This is consistent with the data for COVID-19 vaccines57–59 for which no impaired humoral responses were observed for patients treated with Cladribine, even in the small number of patients that were vaccinated within 4 weeks of their last cladribine dose. 60
PwMS treated with ocrelizumab have an attenuated, humoral response to tetanus, pneumococcus and seasonal influenza compared to those exposed to INF-β or no therapy. These observations have been largely confirmed by the recent experience with the COVID-19 vaccine. All studies consistently report a reduced humoral response to SARS-CoV-2 vaccination in patients treated with anti-CD20. 66 The response was dependent on the time since the last administration of anti- CD20 treatment and the number of repopulated B-cells at the time of vaccination. 67 Booster doses did not result in humoral immunization in the absence of seroconversion following priming vaccination, unless B-cells were reconstituted.68,69 Extending the time between the infusion of anti-CD20 monoclonal antibodies and vaccination may result in improved vaccine responses. Evidence also suggests that antigen- specific T cell responses after vaccination are adequate despite poor humoral responses, but whether T cell responses alone translate into long-term effective protection against SARS-CoV-2 remains unknown. 70
Vaccine effectiveness
Statements
Statement 1. In MS patients without DMT or those receiving interferons and GA, the achieved protection after vaccination is similar to the general population.
Statement 2. In people with MS receiving DMF, teriflunomide, and natalizumab, the production of antibodies can be lower compared to non-treated patients or patients receiving interferons, but patients achieve sufficient seroprotection.
Statement 3. In people with MS receiving sphingosine-1-phosphate modulators and anti-CD20, the antibody production is lower than in non-treated patients or patients receiving interferons, and the achieved seroprotection after vaccination can be reduced.
Statement 4. There are limited data about the protection after vaccination in patients treated with alemtuzumab and cladribine. However, due to the drug’s mechanism of action, a reduced seroprotection could be expected until a complete immune reconstitution is achieved.
Recommendations
Recommendation 1. People with MS receiving some immunosuppressive therapies (sphingosine-1-phosphate modulators, or anti-CD20 monoclonal antibodies or alemtuzumab and cladribine before immune reconstitution) should receive counseling about the risk of diminished protection after vaccination and the need to follow other protective strategies against infections.
Immunization strategy
Question 3: What is the recommended immunization strategy in pwMS before, during, and after immunosuppressive therapies?
The first guidelines on immunizations in patients with MS published in 2002 were developed by the Immunization Panel of the MS Council for Clinical Practice Guidelines in the American Academy of Neurology (AAN). 7 These recommendations emphasized the importance of vaccination for the prevention of infections and highlighted the safety of the most commonly administered vaccines, thus recommending that patients with MS and their household contacts should follow the immunization schedule for the general adult population. 7 However, no specific recommendations were made on the use of vaccines with the available DMTs (i.e. injectable immunomodulatory treatments). Newer DMTs that have more broad immunosuppressive effects pose more challenges to vaccination. 6 Patients with MS who are receiving immunosuppressive therapies need to be risk assessed by adopting an individualized, case-by-case approach that differs significantly from the general population, providing the rationale for specific vaccination guidelines.
Currently, several guidelines and/or consensus, including the updated version of the aforementioned AAN guidelines, aim to provide recommendations regarding vaccines in patients with MS, including specific advice regarding vaccination safety and efficacy in patients receiving -or going to receive- DMTs. In the absence of solid evidence on the use of vaccines in pwMS, expert recommendations could help in the decision-making process. In this regard, expert groups from Italy, Spain, and France have published consensus statements on this topic.2,71,72 The authors of this European consensus statement have referred to all previously published guidelines/consensus and all data reviewed in questions 1, 2a, and 2b to generate recommendations for this review question. The overall experience with the use of biologic/immunosuppressant agents in patients with other autoimmune or autoinflammatory diseases was also considered, as well as vaccination guidelines for patients with immunosuppressive conditions (e.g. HIV and other immunodeficiencies).73–76
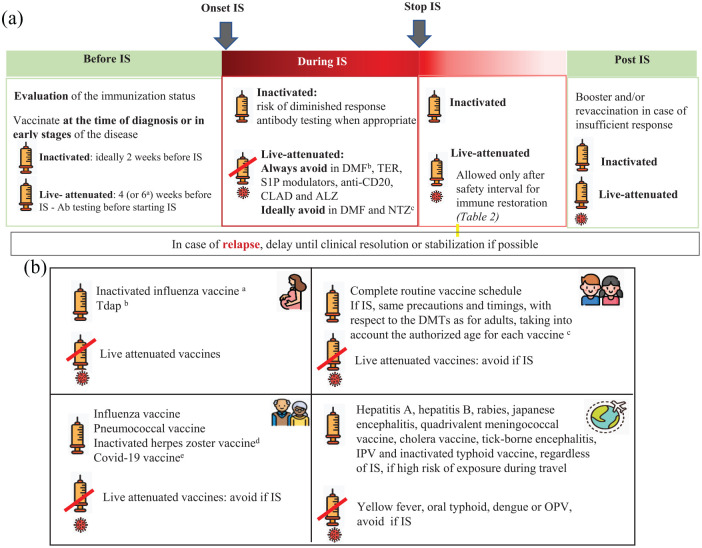
According to evidence reviewed in question 1, both inactivated and attenuated vaccines are safe biological products that can be administered in patients with MS taking into account the specific contraindications for live-attenuated vaccines in patients receiving immunosuppressive therapies. Patients should be appropriately immunized with routine vaccines, (included in the adult vaccination schedule) plus other specific ones, including those largely used in case of immunosuppression, such as influenza and pneumococcal and vaccines, and those with restricted indications depending on the treatment and clinical situation. It is also important to ensure a correct immunization of the household contacts against common infectious agents for which the patients cannot be immunized (i.e. live attenuated infections if immunosuppressive therapy) or might have a partial immune response (ie influenza).2,6 The recommended vaccines for pwMS, schemes, and indications are detailed in Table 1. The decisions about the optimal timing for vaccination should consider the patient’s clinical situation, the type of vaccine and DMT, the relative need for rapid protection, the risk for suboptimal response to vaccination, and the potential risk of vaccine-induced side effects. 6 Specific caution is needed when considering live attenuated vaccines in patients with planned initiation of immunosuppressive therapies. Details about the timing of live attenuated vaccines for the different DMTs are available in Figure 1(a) and Table 2.
Table 1.
Recommended vaccines in MS patients receiving disease-modifying drugs.
| Vaccine | Type | Schedule | Indications | |
|---|---|---|---|---|
| General MS population | Special MS sub-populations | |||
| Seasonal influenza | Inactivated. Fractioned or subunits |
Single IM/SC dose every year | Annually, especially in case of present/future IS and/or significant disability |
 During any trimester During any trimester Annually for all Annually for all From 6 months of age, in case of present/future IS From 6 months of age, in case of present/future IS |
| Pneumoccocal: 13-PCV 23-PPV 20-PCV |
Inactivated | 13-PCV and 23-PPV (at least 2 months apart)Or single-dose 20-PCV |
In case of present/future immunosuppression and/or significant disability a |
  PCV13 as age-appropriate and PPSV23 2 months apart, in case of present/future IS PCV13 as age-appropriate and PPSV23 2 months apart, in case of present/future IS |
| Tetanus-Diphtheria (dT) Tetanus-Diphtheria—pertussis (dTap) |
Inactivated; tetanus and diphtheria toxoids |
3 IM doses (0,1, 6 months) in naïve patients Single IM booster dose in first vaccinated |
Same indications as in the general population b |
 dTap during the end of the second or the third trimester. Repeat during each pregnancy
c dTap during the end of the second or the third trimester. Repeat during each pregnancy
c
|
| Measles, mumps, rubella (MMR) | Live attenuated | 2 IM/SC doses given 4 weeks apart | Recommended in seronegative patients Complete 4 weeks before immunosuppression d |
 In seronegative, vaccinate in the post-partum period before initiating DMT. In seronegative, vaccinate in the post-partum period before initiating DMT. |
| Varicella | Live attenuated | 2 IM/SC doses given 4 weeks apart | Recommended in VZV seronegative patients. Complete 4 weeks before immunosuppression d |
 In seronegative, vaccinate in the post-partum period before initiating DMT. In seronegative, vaccinate in the post-partum period before initiating DMT. |
| Human papillomavirus | Inactivated (recombinant) |
3 IM doses at months 0, 2, and 6 | Consider in women and men with MS who will receive treatment with ALZ, S1 P modulators, CLAD or anti-CD20 drugs, independently of their age e |
 Ensure complete immunization in all girls and boys
b Ensure complete immunization in all girls and boys
b
|
| Herpes zoster | Inactivated (recombinant) f |
2 IM doses separated by 2–6 months | Consider in patients aged over 18 years g if treatment with CLAD, ALZ, S1 P modulator, NTZ, and anti-CD20 drugs |
 Especially indicated in those receiving immunosuppressive therapies Especially indicated in those receiving immunosuppressive therapies From 18 years of age From 18 years of age |
| Hepatitis B virus | Inactivated (recombinant) |
Regular vaccines 3 IM doses at months 0,1,6 Enhanced immunity vaccines h 4 IM doses (0,1,2,6-12 months) for high load (40mcg) or adjuvanted (AS03) 2 IM doses (0,1 months) for adjuvanted (CpG 1018) |
Consider in high-risk i seronegative patients, especially if treatment with anti-CD20 therapies |
 Ensure complete immunization in all girls and boys
b Ensure complete immunization in all girls and boys
b
|
| COVID-19 vaccine | mRNA Adenoviral vector Inactivated (recombinant adyuvanted) |
Primovaccination with one or two-dose scheme
j
Additional booster doses k |
Recommended for all MS patients |
  During any trimester During any trimester mRNA vaccines, from 6 months of age, in case of present/future IS mRNA vaccines, from 6 months of age, in case of present/future IS |
 Patients under 18 years of age.
Patients under 18 years of age.  Patients of 60 years and older
Patients of 60 years and older
13-valent pneumococcal conjugate vaccine (13-PCV, Prevenar 13®); 20-valent pneumococcal conjugate vaccine (20-PCV, Appenxnar®) Pneumococcal polysaccharide vaccine (PPSV23, Pnaumovax®). Use following general recommendations for immunosuppression. Age and/or comorbidities should also be considered in the indication of pneumococcal vaccination following guidelines applicable in each country. For children: routine vaccination with PCV13 as age-appropriate and in children of at least 2 years of age administer PPSV23 2 months apart.
Following national immunization schedules.
Unless national recommendations state otherwise.
Always avoid in MS patients who are already receiving the following immunosuppressive therapies (sphingosine-1-phosphate (S1 P) modulators, anti-CD20 monoclonal antibodies and before immune restoration for cladribine and alemtuzumab). Ideally avoid in MS patients who are already receiving the following immunosuppressive therapies (natalizumab, DMF and teriflunomide without lymphopenia). In these patients and in very exceptional cases, such as high risk of infection, vaccination with live attenuated vaccines could be considered if the potential risk of acquiring the infection is superior to the risk of developing vaccine-related infections.
There can be limitations and variations regarding upper age limit depending on the country and the Summary of product characteristics.
A live, attenuated herpes zoster vaccine (Zostavax®) is also available, but not recommended for patients who are receiving immunosuppressants.
With a background of chickenpox disease or live-attenuated varicella vaccination (otherwise consider varicella immunization).
Enhanced Immunity Vaccines include High load (HBVaxpro® 40mcg) or adjuvanted (AS03-Fendrix®, CpG 1018-Heplisav®). Consider If onset of immunosuppressants in the following 6 months or in patients already immunosuppressed.
Risk of sexual exposure, patients on dialysis, parenteral drug users, healthcare workers with occupational risk, and patients with specific comorbidities (HIV or HCV infection, chronic liver or kidney disease, solid organ transplant/hemopoietic stem cell transplantation recipients and/or people receiving blood products.
EMA authorized COVID-19 vaccines: Comirnaty (0, 28 days), Spikevax (0, 28 days) Valneva (0,28 days), Nuvaxoid (0,21 days), Vaxzevria (0, 28 days), Jcovden (single-dose), VidPrevtyn Beta (single booster after mRNA) Available at: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#originally-authorised-covid-19-vaccines-section.
Follow most updated local/country guidance on COVID-19 vaccination for high-risk patients.
IS: immunosuppression; 13-PCV: 13-valent conjugate vaccine; 20-PCV: 20-valent conjugate vaccine; 23PPV: 23-valent polysaccharide vaccine; MS: multiple sclerosis; mRNA: messenger ribonucleic acid; S1 P: selective Sphingosin-1-phosphate-receptor-1 CLAD: cladribine; ALZ: alemtuzumab; DMF: dimethyl fumarate; TER: teriflunomide; NTZ: natalizumab; VZV: varicella-zoster virus.
Figure 1.
Immunization strategy in pwMS. (a) Immunization strategy and immunosuppression: timings and precautions. a. For ocrelizumab and alemtuzumab according to the Summary of product characteristics. b. If absolute lymphocyte counts < 800/mm3 (grade 2 and 3 lymphopenia). c. In very exceptional cases, such as a high risk of infection, vaccination with live attenuated vaccines in patients treated with NTZ and DMF could be considered if the potential risk of acquiring the infection is superior to the risk of developing vaccine-related infections.
IS: immunosuppression; Ab: antibody; S1 P: selective sphingosin-1-phosphate-receptor-1; CLAD: cladribine; ALZ: alemtuzumab; DMF: dimethyl fumarate; TER: teriflunomide; NTZ: natalizumab.
(b) Recommended vaccines in special sub-populations (pregnancy, children, elderly and international travel). a. During any trimester at the beginning of the influenza season. b. During the third trimester of pregnancy (between week 20 and 36), unless national recommendations state otherwise. c. See Table 1. d. With a background of chickenpox disease or live-attenuated varicella vaccination (otherwise consider varicella immunization). e. Follow most updated local/country guidance on COVID-19 vaccination for high-risk patients.
dTap: diphtheria, tetanus and acellular pertussis; IS: immunosuppression; IPV: inactivated polio vaccine; OPV: oral polio vaccine.
Table 2.
Recommended safety interval between drug suspension and live-attenuated vaccine administration.
| Disease-modifying drug | Interval to live-attenuated vaccine |
|---|---|
| Interferon/glatiramer acetate | None |
| Dimethyl fumarate | Until normal lymphocyte count |
| Teriflunomide | 3.5 months–2 years (accelerated elimination: wait 1.5 months after the first result of plasma concentrations of the drug is below 0.02 mg/l). |
| Fingolimod | >2 months |
| Siponimod | 4 weeks |
| Ozanimod | 3 months |
| Ponesimod | 2 weeks |
| Natalizumab | >3 months |
| Alemtuzumab | Until normal lymphocyte count (approximately 12 months) |
| Cladribine | Until normal lymphocyte count (30–90 weeks after the last dose) |
| Rituximab | Until B-cell repletion (>12 months) |
| Ocrelizumab | Until B-cell repletion (>18 months) |
| Ofatumumab | Until B-cell repletion (approximately 40 weeks) |
| Corticosteriods a | 1 month |
| Plasma exchange | None |
| Intravenous immunoglobulin (IVIg) | 3 months b |
⩾ 20 mg/day or ⩾ 2 mg/Kg/day (if weight <10 kg) of prednisone or equivalent for at least 2 consecutive weeks.
Risk of diminished response to measles up to 1 year.
Based on:European public assessment reports (EPAR) // Rubin LG, et al. Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014 // Furer, V. et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Annals of the rheumatic diseases 79, 39-52, doi:10.1136/annrheumdis-2019-215882 (2020) // Ciotti, J. R., Valtcheva, M. V. & Cross, A. H. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Multiple sclerosis and related disorders 45, 102439, doi:10.1016/j.msard.2020.102439 (2020).
Recommendations
Recommendation 1. An evaluation of the immunization status is recommended for all MS patients, regardless of initial therapeutic plans, as part of the disease management strategy to minimize risks.
Recommendation 2. Care providers should inform patients about the importance of immunization and the risks of not vaccinating. Patients’ opinions, values, and preferences should be considered, including the possibility of declining vaccination, to define a personalized immunization plan for each patient.
Recommendation 3. Vaccination should be performed at the time of diagnosis or in the early stages of the disease to prevent future delays in the initiation of therapies.
- Recommendation 4. In order to define the vaccination plan, it is essential to:
- Document the patient’s past, current, and, if planned, future therapies.
- Establish vaccination needs based on the patient’s natural immunity, vaccine history, as well as the results of the pre-vaccine serologic tests: varicella, measles, mumps, rubella, tetanus, hepatitis B, and other infections according to the local epidemiological context.
Recommendation 5. The specific vaccination guidance according to the prescribing instructions for each of the DMTs should be followed, considering the treatment-specific infectious risks, the epidemiological context, and the local immunization requirements.
Recommendation 6. In MS patients who are experiencing a relapse, vaccination should ideally be delayed until clinical resolution or stabilization.
Recommendation 7. Physicians should reassess the vaccination status of patients with MS before prescribing any immunosuppressive therapy (DMF, teriflunomide, sphingosine-1-phosphate modulators, natalizumab, cladribine, alemtuzumab, or anti-CD20 monoclonal antibodies).
- Recommendation 8. For non-treated MS patients or those receiving immunomodulatory treatment (interferons or GA) who are planning to start any immunosuppressive therapy (DMF, teriflunomide, sphingosine-1-phosphate modulators, natalizumab, cladribine, alemtuzumab, or anti-CD20 monoclonal antibodies) timing of vaccination should be adjusted:
- Inactivated vaccines can be administered any time, but ideally at least 2 weeks before treatment onset to ensure a complete immune response.
- Live attenuated vaccines should be administered at least 4 weeks before treatment onset, six weeks for ocrelizumab and alemtuzumab.
Recommendation 9. For MS patients planning to start any immunosuppressive therapy, accelerated vaccination schedules can be proposed when available and if needed.
- Recommendation 10. Live attenuated vaccines:
- Can be safely used in MS patients without DMT or those receiving immunomodulatory treatments (interferons or GA).
- Should ideally be avoided in MS patients receiving DMF and natalizumab due to the potential risk of developing vaccine-related infections. In very exceptional cases, such as a high risk of infection, vaccination with live attenuated vaccines could be considered if the potential risk of acquiring the infection is superior to the risk of developing vaccine-related infections.
- Should be avoided in MS patients receiving DMF*, teriflunomide, sphingosine-1-phosphate modulators, anti-CD20 monoclonal antibodies, and before immune restoration for cladribine and alemtuzumab, due to the potential risk of developing vaccine-related infections.
- * If absolute lymphocyte counts < 800/mm 3 (grade 2 and 3 lymphopenia).
Recommendation 11. MS patients receiving immunosuppressive therapies that are non-immune against measles and/ or varicella-zoster virus (VZV) should be informed that, in case of a risk exposure to measles and/or chickenpox, they should seek medical advice immediately, and a post-exposure prophylaxis with immunoglobulin should be offered.
Recommendation 12. For MS patients who are treated with anti-CD20 immunosuppressive therapies every 6 months, inactivated vaccines should ideally be administered, if the clinical situation allows it, at least 3 months after the last anti-CD20 treatment and 4–6 weeks before the next infusion to optimize vaccine responses.
- Recommendation 13. For MS patients who receive vaccines before initiation or during treatment with immunosuppressive therapies:
- Measurement of vaccine-induced antibody titers in an optimal interval of 1 to 2 months after the last dose of the vaccine is suggested for hepatitis B, tetanus, measles, mumps, and varicella to check whether they have mounted a protective immune response, according to accepted cut-off levels.
- In the case of attenuated live vaccines, the serological response should be confirmed before starting the immunosuppressive therapy.
- In case of insufficient response, consider administering a booster dose of the vaccine. For hepatitis B, a complete revaccination with an adjuvanted or high antigenic load vaccine is recommended.
Recommendation 14. MS patients who do not mount a protective immune response to hepatitis B after two complete courses of vaccination should be informed that, in the situation of a risk exposure to the virus, they should seek medical advice immediately, and post-exposure prophylaxis with immunoglobulin should be offered.
Recommendation 15. In MS patients who receive a short-term pulse of high-dose steroid treatment, live attenuated vaccines should be postponed for 1 month. Ideally, inactivated vaccines should also be delayed for 1 month, but can be administered any time.
Recommendation 16. In MS patients who stop receiving immunosuppressive therapies, inactivated vaccines can be administered any time, but preferably after immune restoration to maximize vaccine responses.
Recommendation 17. In MS patients who stop receiving immunosuppressive therapies, live attenuated vaccines should only be administered after a safety interval that ensures immune restoration is met (Table 2).
Recommended vaccines
Recommendation 18. Adult patients with MS should receive those vaccines included in the routine vaccination schedule for the general population unless there is a specific contraindication
Recommendation 19. MS patients, especially those who are candidates for/or on immunosuppressive therapies or those with a significant disability should receive yearly influenza vaccination, following general recommendations.
Recommendation 20. MS patients who are candidates for/or on immunosuppressive therapies or those with a significant disability should receive pneumococcal vaccination, following general recommendations for immunosuppression (Following guidelines applicable in each country; age and/or comorbidities should also be considered in the indication of pneumococcal vaccination).
- Recommendation 21. In MS patients who are candidates for/or on immunosuppressive therapies, other vaccines with more restrictive indications should be considered:
- Human papillomavirus vaccine in women and men with MS who are scheduled to receive treatment with alemtuzumab, sphingosine-1-phosphate modulators, cladribine, or anti-CD20 monoclonal antibodies, and have not already received the vaccine previously, independently of their age (in some countries, there can be limitations regarding age).
- Herpes zoster recombinant vaccine in patients over 18 years of age* who are scheduled to receive any treatment with a high risk of herpes infections such as cladribine, alemtuzumab, sphingosine-1-phosphate modulators, natalizumab, and anti-CD20 monoclonal antibodies (in some countries, there can be limitations regarding age).
- Hepatitis B in non-immune high-risk patients, especially those who are scheduled to receive treatment with anti-CD20.
- * With a background of chickenpox disease or live-attenuated varicella vaccination (otherwise consider varicella immunization).
- Recommendation 22. In people with MS receiving immunosuppressive therapies vaccination for household and healthcare professional contacts should be recommended:
- With influenza vaccines for all.
- With MMR and/or varicella vaccines for those non-immune to measles and/or varicella (through vaccination or natural immunity) and if the patient is not adequately protected against these infections.
Immuniation in special populations
Question 4: What is the recommended vaccination strategy in pediatric pwMS?
Vaccines are one of the most cost-effective approaches for reducing childhood disease burden and mortality. 77 MS is a disease of young adults, and a small proportion of patients with MS are children. 78 There are no published data on the safety and efficacy of vaccines in pediatric patients with MS. Therefore, it is not surprising that no vaccination guidelines for children with MS are available in Europe or elsewhere. The lack of data on pediatric patients with MS is noteworthy as children may be more susceptible to vaccine-preventable infections. 75 Confronted with this lack of information and/or authoritative guidance, the authors of this European consensus statement have referred to indirect data reviewed in questions 1, 2a, and 2b and to vaccination guidelines for immunocompromised children to generate recommendations for this review question. These recommendations are in line with the immunization programs in the European Union. All vaccines applicable to a child/adolescent with MS (e.g. meningococcal conjugate—MenACWY—vaccine, meningococcal B vaccine, human papillomavirus (HPV) vaccine and combined tetanus, diphtheria, and acellular pertussis vaccine) should be provided as per local immunization schedules. Special attention should be given to HPV with is the most common sexually transmitted infection worldwide and the leading cause of cervical cancer. 79 HPV vaccination should be administered routinely to adolescents either in routine or catch-up programs.79,80 The multidose schedule of HPV vaccination may delay starting DMT, and, therefore, the potential risks and benefits must be considered on a case-by-case basis. Additional information about the routine immunization schemes for each EU country can be found in Vaccine Scheduler. 81
Vaccination in children with MS
Statements
Statement 1. In children with MS with or without disease-modifying treatments, the benefit of immunization greatly outweighs any potential risks.
Recommendations
Recommendation 1. Care providers must remain vigilant in maintaining children’s vaccination status following local vaccination guidelines and complete vaccinations ideally before the start of any immunosuppressive therapy. In case of non-vaccinated children or missed doses, a catch-up vaccination program following local guidelines should be performed.
Recommendation 2. The same general precautions and timings with respect to the DMTs for immunization in adults should be applied to pediatric patients, taking into account the authorized age for the administration of each vaccine, specified in Table 1.
Recommendation 3. The safety and timing of vaccination should be discussed with the infant’s physician/family doctor.
Question 5: What is the recommended vaccination strategy in pregnant women with MS?
As MS is a common disorder among women of childbearing age, special consideration needs to be given to meeting the vaccination needs of women planning pregnancy and pregnant women with MS. 6 Pregnant women are at increased risk of morbidity and mortality from vaccine-preventable infections and are recognized as a priority group for vaccination. Vaccination during pregnancy is specifically recommended to prevent both influenza and pertussis, while other vaccines may be considered in cases of high risk or specific exposure.6,82 Inactivated vaccines are generally considered safe during pregnancy. In contrast, live attenuated vaccines are contraindicated during pregnancy due to the theoretical risk of perinatal infection. 82
Pregnant women are particularly vulnerable to severe infection from influenza resulting in poor maternal and neonatal outcomes.83,84 Importantly and reassuringly, maternal influenza vaccination has been shown to decrease the risk of influenza and its complications among pregnant women and their infants under 6 months of age. 85 Pregnant women with MS should be routinely offered the inactivated influenza vaccine in any trimester. Pertussis—a respiratory infection caused by Bordetella pertussis—remains a significant cause of infant morbidity and mortality. Infants are usually infected after exposure to close contacts who are either asymptomatic or have symptoms of a common cold. 82 Pertussis vaccination in pregnancy may protect infants through a passive and active transfer of maternal antibodies until they receive their primary immunization series.82,86 The vaccine does not contain any live components, and it should be given during each pregnancy at 20–36 weeks’ gestation. Influenza and pertussis vaccinations are not included in the routine vaccination schedule for pregnant women in some EU countries. 81
The safety and immunogenicity of vaccines in the context of DMTs should be carefully considered when formulating immunization strategies in pregnant women with MS receiving immunotherapies. The recommendations regarding immunization strategies in patients with MS receiving DMTs have been detailed in Question 3.
Vaccination in pregnant women with MS
Statements
Statement 1. In pregnant women with MS, inactivated vaccines are safe and can be administered during the second and third trimester of pregnancy*.
* Influenza vaccine can be administered at any time during pregnancy
Statement 2. In pregnant women with MS, live attenuated vaccines are contraindicated due to the theoretical risk of vaccine-related infections in the fetus.
Recommendations
Recommendation 1. In women with MS with childbearing potential, a complete review of vaccination status should be performed. If needed, immunization with live attenuated vaccines should be completed at least 1 month before pregnancy, unless there is a specific contraindication).
Recommendation 2. In pregnant women with MS, vaccination is recommended, as in the general population, to prevent potential infections with a high impact on maternal and infant morbidity and mortality.
Recommendation 3. Pregnant women with MS should be vaccinated with an inactivated influenza vaccine in any trimester at the beginning of the influenza season.
Recommendation 4. Pregnant women with MS should be advised to receive vaccination against diphtheria, tetanus, and pertussis (Tdap) during the end of second or third trimester of pregnancy, preferably between weeks 20 and 36* to allow the greatest materno-fetal transfer of anti-pertussis antibodies. This vaccination should be performed during each pregnancy, regardless of whether the Tdap vaccine has been previously administered.
* Unless national recommendations state otherwise.
Recommendation 5. Pregnant women with MS should be evaluated for evidence of immunity to rubella and varicella and be tested for the presence of HBsAg. Women without evidence of immunity to rubella or varicella should be vaccinated in the post-partum period before initiating DMT.
- Recommendation 6. In women with MS, the timing of vaccines post-partum should be adjusted to treatment plans to obtain fast protection and adequate vaccine responses:
- - Immunizations with live attenuated vaccine should be completed after delivery, regardless of breastfeeding (except for yellow fever vaccine), and 4 to 6 weeks before initiation of immunosuppressive DMT.
- - Inactivated vaccines can be administered at any time after delivery and during immunosuppressive treatment but, ideally, should be completed at least 2 weeks before the start of immunosuppressive DMT.
Recommendation 7. In newborns who have been exposed to anti-CD20 therapies during pregnancy or for some time before pregnancy, CD19-positive B-cell levels should be measured, and live-attenuated vaccines (i.e. rotavirus) should be delayed until B-cell levels have recovered.
Recommendation 8. In women with MS who are breastfeeding, vaccines are considered safe except for the yellow fever vaccine.
Question 6: What is the recommended vaccination strategy for elderly pwMS?
Elderly patients are at risk of acquiring vaccine-preventable infections, either because of incomplete immunization or waning immunity. 87 Immunosenescence (i.e. the weakening of the immune system associated with natural aging) results in suboptimal vaccine efficacy and increased frequency of common infectious diseases. 87 Vaccination is highly recommended throughout life because vaccine-preventable infections can cause significant morbidity and mortality in aging people. 87 Some vaccines have specific indications in elderly individuals, such as the recombinant subunit herpes zoster virus vaccine, the pneumococcal vaccines, the adjuvanted or high-dose influenza vaccines, and booster vaccinations against tetanus and diphtheria, among others.87,88
The development of new DMTs and advances in treating comorbidities have contributed to an increasing prevalence of aging pwMS worldwide. It is, therefore, essential that elderly pwMS undergo an appropriate vaccination program. 89 However, to date, no data are available on the safety and efficacy of vaccines in elderly pwMS and, therefore, no guidelines have been established on vaccinating this group of patients. In this consensus statement, the authors have referred to indirect data reviewed in questions 1, 2a, and 2b and to vaccination guidelines for otherwise healthy older adults to generate recommendations for this review question.87,88 These recommendations are in line with the immunization programs in the European Union. Similar to recommendations for younger pwMS, an individualized risk assessment is needed when making DMT decisions in elderly pwMS.
Vaccination in elderly pwMS
Recommendations
Recommendation 1. Elderly people with MS, as the general elderly population, should be informed about the higher risk of severe infections and the altered immune response to vaccines (i.e. antibody titer, antibody diversity, protective immunity).
Recommendation 2. In elderly people with MS, the same general vaccination strategy as in the adult MS population should be applied in terms of timings, recommended vaccines, and precautions according to DMTs.
Recommendation 3. Elderly people with MS should receive the influenza vaccine annually as well as pneumococcal and inactivated herpes zoster vaccines.
Question 7: What is the recommended vaccination strategy for patients with MS planning to undertake international travel?
Patients with MS planning to undertake international travel may be at risk for various potentially severe and vaccine-preventable infections that are not endemic in their country of origin.6,90 The risk of such infections varies depending on the itinerary, pre-existing health factors, and unique behaviors of the traveler. 90 Therefore, patients with MS who plan overseas travel should undergo a risk assessment and guidance on vaccination by a healthcare professional, ideally at least 2 to 3 months before traveling. An immunization encounter before travel also provides an opportunity to update all age-appropriate immunizations. 6
Six studies have evaluated the efficacy and/or safety of travel vaccines in pwMS.26–31 Details on the methodology, level of the evidence, and results of these studies are available in Supplemental Appendix 3.
Rabies
A single self-controlled retrospective study (level 3) reported the risk of relapses in 55 patients with MS who underwent pre-exposure rabies vaccination. 26 The annualized relapse rate in the pre-exposure, exposure-risk, and post-risk periods were 0.44, 0.22, and 0.10, respectively (rate ratio for exposure-risk to pre-exposure periods, 0.51 [95% CI 0.10–1.68]).
Tick-borne encephalitis
A small cohort study (level 3) conducted in 15 pwMS living in TBE risk areas reported no association between TBE vaccination and clinical or radiological disease activity. 28 In addition, all patients had protective antibody titers at follow-up. 28 Similarly, Winkelmann et al. 27 (level 3) reported that (a) the annualized relapse rate decreased from 0.65 in the year before TBE vaccination to 0.21 in the following year, (b) EDSS remained stable throughout the study period, and (c) 78% of patients had protective antibody titers after vaccination.
Yellow fever
Three studies have investigated the effects of yellow fever vaccination (YFV) on MS disease activity.29–31 A self-controlled case series study (level 4) assessed the risk of relapse in seven patients with relapsing-remitting MS vaccinated against yellow fever before traveling to endemic regions. 29 Age- and sex-matched healthy individuals, unvaccinated patients with MS, and influenza-vaccinated patients with MS were included as control groups. The at-risk period (ARP) was defined as 1 to 5 weeks from vaccination, and total follow-up lasted 24 months. 29 The exacerbation rate was higher during the ARP compared to the remaining 23 months of follow-up (8.57 vs. 0.67; RR 12.78, 95% CI 4.28–38.13; p < 0.001) and a significant increase in new or enlarging T2-weighted lesions and gadolinium-enhancing lesions was reported. 29 More recently, a retrospective cohort study (level 3) including 23 patients with a similar design did not confirm these findings. Instead, a sharp decrease in the annualized relapse rate was observed from 0.52 in the PEP to 0.17 and 0.13 in the ERP and PRP, respectively. 30 Consistent with these findings, Papeix et al. 31 observed no increased relapse rate or disability worsening in a cohort of 128 pwMS following YFV (level 3). The 1-year ARR following YFV was 0,219 in exposed patients compared with 0.208 in the non-exposed group, and the difference was not statistically significant (p = 0.92). Time to first relapse (HR 1.33, 95% CI 0.53–3.30; p = 0.54) and EDSS score worsening during the first year after YFV (15.6% vs. 13.5%; p = 0.77) were also not different between groups. 31
Conclusion
No increased risk of MS exacerbation and/or progression has been observed following rabies vaccination and there is no compelling evidence that YFV or TBE vaccination increases the risk of relapse in MS.
Based on the best available evidence, there are some guidelines and/or consensus that aim to provide recommendations regarding travel vaccines in patients with MS. The Yellow Book (Health Information for International Travel) by the Centres for Disease Control and Prevention (CDC) 91 in the USA includes specific advice regarding vaccination strategies in patients with MS. According to CDC guidance, inactivated travel vaccines such as rabies, Japanese encephalitis, and TBE are generally considered safe for patients with MS. In contrast, live vaccines, such as YF, MMR, and oral typhoid should not be given to patients with MS during therapy with immunosuppressants due to the potential risk of vaccine-transmitted disease. 91 A multidisciplinary expert panel in the UK has issued similar recommendations regarding pretravel counseling in adults with MS. 6 The safety and immunogenicity of vaccines in the context of DMTs should be carefully considered when formulating immunization strategies in travelers with MS receiving immunotherapies. The recommendations regarding immunization strategies in patients with MS receiving DMTs have been detailed in Question 3.
Vaccination for international travel
Statements
Statement 1. MS patients with or without immunosuppressive therapies can receive specific travel inactivated vaccines such as hepatitis A, hepatitis B, rabies, Japanese encephalitis, quadrivalent meningococcal vaccine, cholera vaccine, TBE, polio (IPV), and inactivated typhoid vaccine regardless of DMTs, if high risk of exposure during travel.
Statement 2. In MS patients receiving immunosuppressive therapies, live attenuated vaccines such as yellow fever, oral typhoid, dengue, varicella, and/or measles-mumps-rubella are contraindicated.
Recommendations
Recommendation 1. Care providers should discuss potential travel plans with MS patients as early as possible, especially with those patients who will start immunosuppressive therapies.
Recommendation 2. MS patients planning to travel to a tropical or subtropical destination should be advised to consult a specialized Travel Clinic or a vaccination expert in coordination with the MS specialist for a specific evaluation and individualized indication of pretravel immunizations considering the risk–benefit balance.
Recommendation 3. Care providers should consider travel details about timing and destination to advise on the best immunization strategy before travel.
Recommendation 4. Immunizations needed to travel should ideally be started 2 to 3 months before departure. Accelerated vaccination schedules can be applied whenever available.
Recommendation 5. For MS patients receiving immunosuppressive therapies, post-vaccination serology for those vaccines with accepted antibody cut of levels, such as hepatitis A, hepatitis B, rabies, tetanus and/or polio should be verified, and additional booster doses may be required if negative responses.
Recommendation 6. Care providers should discuss the risks/benefits of stopping treatment for receiving a live attenuated vaccine for traveling.
Conclusions and future research
This is the first consensus statement on vaccination for MS patients with a European reach. The recommendations included in this consensus are intended to guide the best care according to currently available evidence for vaccination in MS and the experience of vaccination in patients with immunosuppressive treatment in other disciplines. Some key points of the recommendations have been highlighted in Table 3.
Table 3.
Key aspects about the immunization of pwMS.
|
1. In MS patients with or without disease-modifying treatment (DMT), vaccines are not associated with an increased risk of relapses or disability. 2. In pwMS receiving sphingosine-1-phosphate modulators and anti-CD20, the production of antibodies is lower as compared to non-treated patients or patients receiving interferons, and the achieved seroprotection after vaccination can be reduced. 3. There is limited data about the protection after vaccination in patients treated with alemtuzumab and cladribine. However, due to the drug’s mechanism of action, a reduced seroprotection could be expected until a complete immune reconstitution is achieved. 4. An evaluation of the immunization status is recommended for all pwMS, regardless of initial therapeutic plans to minimize risks. Ideally, vaccination should be performed at the time of diagnosis or in the early stages of the disease. 5. In pwMS experiencing a relapse, vaccination should ideally be delayed until clinical resolution or stabilization. 6. For non-treated pwMS or those receiving immunomodulatory treatment who are planning to start any immunosuppressive therapy: Inactivated vaccines can be administered any time, but ideally at least 2 weeks before treatment onset to ensure a complete immune response. Live attenuated vaccines should be administered at least 4 weeks before treatment onset, 6 weeks for ocrelizumab and alemtuzumab. 7. Live attenuated vaccines: Can be safely used in pwMS without DMT or in those receiving immunomodulatory treatments. Should ideally be avoided in pwMS who are receiving the following immunosuppressive therapies (dimethyl fumarate and natalizumab). Should be avoided in MS patients receiving dimethyl fumarate a , teriflunomide, sphingosine-1-phosphate (S1 P) modulators, anti-CD20 monoclonal antibodies, and before immune restoration for cladribine and alemtuzumab, due to the potential risk of developing vaccine-related infections 8. In pwMS who receive a short-term pulse of high-dose steroid treatment, live attenuated vaccines should be postponed for 1 month. Ideally, inactivated vaccines should also be delayed for 1 month, but can be administered any time. 9. Adult and pediatric pwMS should receive those vaccines included in the corresponding routine vaccination schedule for the general population. 10. In pregnant women with MS, vaccination is recommended, as in the general population, to prevent potential infections with a high impact on maternal and infant morbidity and mortality. 11. PwMS, specially those who are candidates for/or on immunosuppressive therapies or those with a significant disability should receive yearly influenza vaccination and pneumococcal vaccination (following guidelines applicable in each country) 12. In pwMS who are candidates for/or on immunosuppressive therapies, other vaccines with more restrictive indications should be considered: Human papillomavirus vaccine in women and men with MS b who are scheduled to receive treatment with alemtuzumab, fingolimod, cladribine, or anti-CD20, independently of their age. Herpes zoster inactivated vaccine in patients over 18 years of age c who are scheduled to receive any treatment with a high risk of herpes infections. Hepatitis B in non-immune high-risk patients, especially those who are scheduled to receive treatment with anti-CD20. |
If absolute lymphocyte counts < 800/mm3 (grade 2 and 3 lymphopenia).
There can be limitations and variations regarding upper age limit depending on the country and the Summary of product characteristics.
With a background of chickenpox disease or live-attenuated varicella vaccination (otherwise consider varicella immunization). pwMS, people with multiple sclerosis.
Immunization strategy
After a comprehensive analysis of the evidence on vaccination in MS patients, relevant knowledge gaps are worth mentioning. First, the limited evidence on vaccine effectiveness based on a small number of studies, with limited sample sizes and covering only a few vaccines (mainly influenza, tetanus, and pneumococcus) and a few DMTs. Moreover, all these studies are based on immunogenicity (antibody response) as a surrogate for vaccine response, and none consider “infection” as the main outcome. Therefore, it is difficult to conclude whether the observed humoral-based vaccination responses have their clinical correlates. This is especially relevant in the case of MS patients under immunosuppressive therapies, as the available correlates of protection (against infection and severity) following these vaccinations have been established mainly for immune-competent individuals. 92 In addition, the cellular immune responses that are closely correlated with vaccine efficacy have not been studied for the vaccinations covered in this consensus, with the exception of a few.38,42,44
Interestingly, in the context of the COVID-19 pandemic, a large amount of evidence on the effectiveness and safety of the different types of vaccines against SARS-CoV-2 in pwMS has been produced and may be adapted to other vaccinations in pwMS. The effectiveness correlates with the type of DMT received, as measured both by humoral and cellular responses.64,93–99 Preliminary data have been gathered on the protective effect of these vaccinations on the rate and severity of post-vaccination COVID-19 and will provide us with prospective information to better understand vaccination effectiveness.53,64,93–100 In addition, a few available case reports point to a potential increase in the risk of a first demyelinating event or disease exacerbation after SARS-CoV-2 vaccination, 101 also seen after natural infection. 102 However, self-controlled design analysis of larger cohorts concludes that the vaccine does not increase the short-term risk of clinical reactivation and that the benefits of vaccination outweigh the risks. 103
There were some outlined recommendations for which no consensus was reached in the first round, but only one that could not be adopted in the consensus. The statement suggested a strategy using treatment with natalizumab until immunization is completed to optimize vaccine responses in pwMS with highly active disease who are candidate to DMTs with higher potential interference with vaccine responses (anti CD20 monoclonal antibodies, S1 P modulators, cladribine, or alemtuzumab). In the absence of solid evidence to endorse such an approach, this statement did not reach a priority to become a recommendation. However, the lack of data has led to the development of several practice-based strategies that are likely to generate new evidence about their potential benefits in the future.
As more evidence becomes available regarding the long-term impact on the risk of infections of the new highly effective drugs available for treatment in patients with MS, changes in vaccination recommendations might occur. In addition, there are vaccines in advanced stages of development with a potential indication in these patients. Finally, the COVID-19 pandemic and the rapid development of different types of vaccines and information on their efficacy in pwMS who are treatment-naïve or under all kinds of DMTs have provided us with a large amount of data in a relatively short period. This information on the infection-vaccination-immunity triad will likely lead to more studies to update future guidelines for vaccinations in pwMS as more experience and evidence are built up.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231168043 for ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs by Susana Otero-Romero, Christine Lebrun-Frrénay, Saúl Reyes, Maria Pia Amato, Magda Campins, Mauricio Farez, Massimo Filippi, Yael Hacohen, Bernhard Hemmer, Rosa Juuti, Melinda Magyari, Celia Oreja-Guevara, Aksel Siva, Sandra Vukusic and Mar Tintoré in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585231168043 for ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs by Susana Otero-Romero, Christine Lebrun-Frrénay, Saúl Reyes, Maria Pia Amato, Magda Campins, Mauricio Farez, Massimo Filippi, Yael Hacohen, Bernhard Hemmer, Rosa Juuti, Melinda Magyari, Celia Oreja-Guevara, Aksel Siva, Sandra Vukusic and Mar Tintoré in Multiple Sclerosis Journal
Supplemental material, sj-docx-3-msj-10.1177_13524585231168043 for ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs by Susana Otero-Romero, Christine Lebrun-Frénay, Saúl Reyes, Maria Pia Amato, Magda Campins, Mauricio Farez, Massimo Filippi, Yael Hacohen, Bernhard Hemmer, Rosa Juuti, Melinda Magyari, Celia Oreja-Guevara, Aksel Siva, Sandra Vukusic and Mar Tintoré in Multiple Sclerosis Journal
Acknowledgments
The authors of the manuscript would like to express their gratitude to Ivan Solà and the Institute for Clinical Excellence INPECS team for their contribution in ensuring a sound and complete revision and analysis of the evidence easing the formulation of the recommendations. Special acknowledgments are also due to the external reviewers who have thoroughly reviewed the manuscript and provided valuable comments and insight. This manuscript has been externally reviewed by a group of appointed ECTRIMS council members (Cristina Granziera, Heinz Wiendl, Matilde Inglese, Eleni Leonidou, Joachim Burman, Emma Tallantyre, Jose Maria Prieto and Jan Lycke), as well as, patient representatives from the European MS Platform (Pedro Carrascal) and the Multiple Sclerosis International Federation (Peer Baneke). Finally, a very special commendation goes to Congrex Switzerland staff for their professional work and support.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.O.R. has received speaking and consulting honoraria from Genzyme, Biogen Idec, Novartis, Roche, Excemed and MSD; as well as research support from Novartis. S.R. has received speaking honoraria or scientific advisory fees from Merck, Novartis and Biogen. M.P.A. has served on Scientific Advisory Boards for Biogen, Novartis, Roche, Merck, Sanofi Genzyme, and Teva; has received speaker honoraria from Biogen, Merck, Sanofi Genzyme, Roche, Novartis, and Teva; has received research grants for her Institution from Biogen, Merck, Sanofi Genzyme, Novartis, and Roche. She is co-editor of the Multiple Sclerosis Journal and Associate Editor of Frontiers in Neurology. M.C. has received compensation for consulting services and speaking honoraria from GSK, Novartis, Sanofi Pasteur, MSD, Pfizer and Sequirus. M.F.F. has received a grant from Biogein Idec Argentina. M.F. is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services and/or speaking activities from Alexion, Almirall, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). B.H. has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Polpharma Sandoz and TG therapeutics; he or his institution have received speaker honoraria from Desitin; His institution received research grants from Regeneron for multiple sclerosis research. He holds part of two patents; one for the detection of antibodies against KIR4.1 in a sub-population of patients with multiple sclerosis and one for genetic determinants of neutralizing antibodies to interferon. All conflicts are not relevant to the topic of the study. R.J. has received advisory board honoraria from Bristol-Myers Squibb. M.M. has served on scientific advisory board, received support for congress participation or speaker honoraria from Biogen, Sanofi, Roche, Novartis, Merck, Abbvie, Alexion, BMS. The Danish MS Registry received research support from Biogen, Genzyme, Roche, Merck, Novartis. C.O.G. has received speaking and consulting honoraria from Biogen Idec, Sanofi Genzyme, Novartis, Merck, Teva, Roche, Jannsen and BMS. A.S. has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi İbrahim İlaç. S.V. received grants, personal fees and/or non-financial support from Biogen, BMS-Celgène, Sanofi Genzyme, Merck, Novartis, Roche, Teva. M.T. has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis Viela-Bio and Teva Pharmaceuticals M.T. has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis Viela-Bio and Teva Pharmaceuticals. C.L.F. and Y.H. report no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This consensus document has been possible thanks to a specific grant awarded by the ECTRIMS executive committee.
ORCID iDs: Christine Lebrun-Frénay  https://orcid.org/0000-0002-3713-2416
https://orcid.org/0000-0002-3713-2416
Maria Pia Amato  https://orcid.org/0000-0003-3325-3760
https://orcid.org/0000-0003-3325-3760
Massimo Filippi  https://orcid.org/0000-0002-5485-0479
https://orcid.org/0000-0002-5485-0479
Bernhard Hemmer  https://orcid.org/0000-0001-5985-6784
https://orcid.org/0000-0001-5985-6784
Melinda Magyari  https://orcid.org/0000-0002-0972-5222
https://orcid.org/0000-0002-0972-5222
Celia Oreja-Guevara  https://orcid.org/0000-0002-9221-5716
https://orcid.org/0000-0002-9221-5716
Aksel Siva  https://orcid.org/0000-0002-8340-6641
https://orcid.org/0000-0002-8340-6641
Sandra Vukusic  https://orcid.org/0000-0001-7337-7122
https://orcid.org/0000-0001-7337-7122
Mar Tintoré  https://orcid.org/0000-0001-9999-5359
https://orcid.org/0000-0001-9999-5359
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Susana Otero-Romero, Department of Preventive Medicine and Epidemiology, Vall d’Hebron Barcelona Hospital, Barcelona, Spain Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Barcelona Hospital, Barcelona, Spain.
Christine Lebrun-Frénay, CRC-SEP Nice, CHU de Nice, Universite’ Nice Cote d’Azur UR2CA-URRIS, Nice, France.
Saúl Reyes, Fundación Santa Fe de Bogotá, Bogotá, Colombia School of Medicine, Universidad de los Andes, Bogotá, Colombia Blizard Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Maria Pia Amato, Department NEUROFARBA, University of Florence, Florence, Italy IRCCS Fondazione Don Carlo Gnocchi, Florence, Italy.
Magda Campins, Department of Preventive Medicine and Epidemiology, Vall d’Hebron Barcelona Hospital, Barcelona, Spain.
Mauricio Farez, Centro para la Investigación de Enfermedades Neuroinmunológicas (CIEN), FLENI, Buenos Aires, Argentina.
Massimo Filippi, Neuroimaging Research Unit, Division of Neuroscience, IRCCS San Raffaele Scientific Institute, Milan, Italy Neurology Unit, Neurorehabilitation Unit, and Neurophysiology Service, IRCCS San Raffaele Scientific Institute, Milan, Italy Vita-Salute San Raffaele University, Milan, Italy.
Yael Hacohen, Department of Paediatric Neurology, Great Ormond Street Hospital for Children, London, UK Department of Neuroinflammation, Queen Square Multiple Sclerosis Centre, UCL Institute of Neurology, London, UK.
Bernhard Hemmer, Department of Neurology, Klinikum Rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
Rosa Juuti, Multiple Sclerosis International Federation, London, UK.
Melinda Magyari, Department of Neurology, Danish Multiple Sclerosis Center and the Danish Multiple Sclerosis Registry, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark.
Celia Oreja-Guevara, Department of Neurology, Hospital Clínico San Carlos, IdISSC, Departamento de Medicina, Universidad Complutense, Madrid, Spain.
Aksel Siva, Department of Neurology, School of Medicine, Istanbul University Cerrahpasa, Cerrahpasa, Istanbul, Turkey.
Sandra Vukusic, Service de Neurologie, Sclérose en Plaques, Pathologies de la Myéline et Neuro-inflammation, Centre de Référence des Maladies Inflammatoires Rares du Cerveau et de la Moelle, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Lyon, France; Centre des Neurosciences de Lyon, Observatoire Français de la Sclérose en Plaques, INSERM 1028 et CNRS UMR5292, Lyon, France Université Claude Bernard Lyon 1, Faculté de Médecine Lyon Est, Lyon, France.
Mar Tintoré, Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Barcelona Hospital, Barcelona, Spain.
References
- 1.Levin SN, Kaplan TB. Infectious complications of novel multiple sclerosis therapies. Curr Infect Dis Rep 2017; 19(2): 7–8. [DOI] [PubMed] [Google Scholar]
- 2.Lebrun C, Vukusic S. Immunization and multiple sclerosis: Recommendations from the French multiple sclerosis society. Mult Scler Relat Disord 2019; 31: 173–188. [DOI] [PubMed] [Google Scholar]
- 3.Otero-Romero S, Rodríguez-García J, Vilella A, et al. Recommendations for vaccination in patients with multiple sclerosis who are eligible for immunosuppressive therapies: Spanish consensus statement. Neurologia 2021; 36(1): 50–60. [DOI] [PubMed] [Google Scholar]
- 4.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2019; 93(13): 584–594. [DOI] [PubMed] [Google Scholar]
- 5.Riva A, Barcella V, Benatti SV, et al. Vaccinations in patients with multiple sclerosis: A Delphi consensus statement. Mult Scler J 2020; 27: 0952310. [DOI] [PubMed] [Google Scholar]
- 6.Reyes S, Ramsay M, Ladhani S, et al. Protecting people with multiple sclerosis through vaccination. Pract Neurol 2020; 20: 435–445. [DOI] [PubMed] [Google Scholar]
- 7.Rutschmann OT, McCrory DC, Matchar DB, et al. Immunization and MS: A summary of published evidence and recommendations. Neurology 2002; 59(12): 1837–1843. [DOI] [PubMed] [Google Scholar]
- 8.Loebermann M, Winkelmann A, Hartung HP, et al. Vaccination against infection in patients with multiple sclerosis. Nat Rev Neurol 2012; 8(3): 143–151. [DOI] [PubMed] [Google Scholar]
- 9.Marrie RA, Walld R, Bolton JM, et al. Uptake of influenza vaccination among persons with inflammatory bowel disease, multiple sclerosis or rheumatoid arthritis: A population-based matched cohort study. Can Med Assoc Open Access J 2021; 9(2): E510–E521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waggoner J, Carline JD, Durning SJ. Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med 2016; 91(5): 663–668. [DOI] [PubMed] [Google Scholar]
- 11.McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016; 38(3): 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centre for Evidence-Based Medicine (CEBM), University of Oxford. Oxford Centre for Evidence-Based Medicine: Levels of evidence (March2009), https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed 27 November 2021).
- 13.Winkelmann A, Loebermann M, Barnett M, et al. Vaccination and immunotherapies in neuroimmunological diseases. Nat Rev Neurol 2022; 18(5): 289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS, https://www.nationalmssociety.org/coronavirus-covid-19-information/covid-19-vaccine-guidance/Timing-MS-Medications-with-COVID-19-Vaccines (accessed 22 June 2022).
- 15.Centonze D, Rocca MA, Gasperini C, et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: An expert consensus. J Neurol 2021; 268(11): 3961–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordonnier C, Einarsdottir S, Cesaro S, et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 2019; 19(6): e200–e212. [DOI] [PubMed] [Google Scholar]
- 17.Confavreux C, Suissa S, Saddier P, et al. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N Engl J Med 2001; 344(5): 319–326. [DOI] [PubMed] [Google Scholar]
- 18.Salvetti M, Pisani A, Bastianello S, et al. Clinical and MRI assessment of disease activity in patients with multiple sclerosis after influenza vaccination. J Neurol 1995; 242(3): 143–146. [DOI] [PubMed] [Google Scholar]
- 19.Miller AE, Morgante LA, Buchwald LY, et al. A multicenter, randomized, double-blind, placebo-controlled trial of influenza immunization in multiple sclerosis. Neurology 1997; 48(2): 312–314. [DOI] [PubMed] [Google Scholar]
- 20.Mokhtarian F, Shirazian D, Morgante L, et al. Influenza virus vaccination of patients with multiple sclerosis. Mult Scler 1997; 3(4): 243–247. [DOI] [PubMed] [Google Scholar]
- 21.Auriel E, Gadoth A, Regev K, et al. Seasonal and H1N1v influenza vaccines in MS: Safety and compliance. J Neurol Sci 2012; 314(1–2): 102–103. [DOI] [PubMed] [Google Scholar]
- 22.Farez M, Ysrraelit MC, Fiol M, et al. H1N1 vaccination does not increase risk of relapse in multiple sclerosis: A self-controlled case-series study. Mult Scler 2012; 18(2): 254–256. [DOI] [PubMed] [Google Scholar]
- 23.McNicholas N, Chataway J. Relapse risk in patients with multiple sclerosis after H1N1 vaccination, with or without seasonal influenza vaccination. J Neurol 2011; 258(8): 1545–1547. [DOI] [PubMed] [Google Scholar]
- 24.Ristori G, Buzzi MG, Sabatini U, et al. Use of Bacille Calmette-Guèrin (BCG) in multiple sclerosis. Neurology 1999; 53(7): 1588–1589. [DOI] [PubMed] [Google Scholar]
- 25.Ristori G, Romano S, Cannoni S, et al. Effects of Bacille Calmette-Guérin after the first demyelinating event in the CNS. Neurology 2014; 82(1): 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttner A, Lascano AM, Roth S, et al. Rabies vaccination and multiple sclerosis relapse: A retrospective cohort study. Mult Scler Relat Disord 2021; 51: 102906. [DOI] [PubMed] [Google Scholar]
- 27.Winkelmann A, Metze C, Frimmel S, et al. Tick-borne encephalitis vaccination in multiple sclerosis. Neurol Neuroimmunol Neuroinflam 2020; 7(2): e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumhackl U, Franta C, Retzl J, et al. A controlled trial of tick-borne encephalitis vaccination in patients with multiple sclerosis. Vaccines 2003; 21(Suppl. 1): S56–S61. [DOI] [PubMed] [Google Scholar]
- 29.Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol 2011; 68(10): 1267–1271. [DOI] [PubMed] [Google Scholar]
- 30.Huttner A, Eperon G, Lascano AM, et al. Risk of MS relapse after yellow fever vaccination. Neurol Neuroimmunol Neuroinflam 2020; 7(4): e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papeix C, Mazoyer J, Maillart E, et al. Multiple sclerosis: Is there a risk of worsening after yellow fever vaccination? Mult Scler 2021; 27: 2280–2283. [DOI] [PubMed] [Google Scholar]
- 32.Ross RT, Dawood MR, Cheang M, et al. Antibody response in seropositive multiple sclerosis patients vaccinated with attenuated live varicella zoster virus. Can J Infect Dis 1996; 7(5): 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriabadi NF, Niewiesk S, Kruse N, et al. Influenza vaccination in MS: Absence of T-cell response against white matter proteins. Neurology 2001; 56(7): 938–943. [DOI] [PubMed] [Google Scholar]
- 34.Olberg HK, Eide GE, Cox RJ, et al. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur J Neurol 2018; 25(3): 527–534. [DOI] [PubMed] [Google Scholar]
- 35.Olberg HK, Cox RJ, Nostbakken JK, et al. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: An explorative study. Mult Scler 2014; 20(8): 1074–1080. [DOI] [PubMed] [Google Scholar]
- 36.Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 2013; 81(6): 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwid SR, Decker MD, Lopez-Bresnahan M. Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. Neurology 2005; 65(12): 1964–1966. [DOI] [PubMed] [Google Scholar]
- 38.Mehling M, Fritz S, Hafner P, et al. Preserved antigen-specific immune response in patients with multiple sclerosis responding to IFNβ-therapy. PLoS ONE 2013; 8(11): e0078532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metze C, Winkelmann A, Loebermann M, et al. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. CNS Neurosci Ther 2019; 25(2): 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong J, Tejada-Simon MV, Rivera VM, et al. Anti-viral properties of interferon beta treatment in patients with multiple sclerosis. Mult Scler 2002; 8(3): 237–242. [DOI] [PubMed] [Google Scholar]
- 41.Koerner I, Kochs G, Kalinke U, et al. Protective role of beta interferon in host defense against influenza A virus. J Virol 2007; 81(4): 2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bar-Or A, Wiendl H, Miller B, et al. Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens. Neurol Neuroimmunol NeuroInflam 2015; 2(2): e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Hehn C, Howard J, Liu S, et al. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflam 2017; 5(1): e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehling M, Hilbert P, Fritz S, et al. Antigen-specific adaptive immune responses in fingolimod-treated multiple sclerosis patients. Ann Neurol 2011; 69(2): 408–413. [DOI] [PubMed] [Google Scholar]
- 45.Boulton C, Meiser K, David OJ, et al. Pharmacodynamic effects of steady-state fingolimod on antibody response in healthy volunteers: A 4-week, randomized, placebo-controlled, parallel-group, multiple-dose study. J Clin Pharmacol 2012; 52(12): 1879–1890. [DOI] [PubMed] [Google Scholar]
- 46.Mehling M, Eichin D, Hafner P, et al. Avidity of vaccine-induced influenza IgG fails to increase in fingolimod-treated patients with MS. Neurol Neuroimmunol Neuroinflam 2014; 1(3): e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology 2015; 84(9): 872–879. [DOI] [PubMed] [Google Scholar]
- 48.Ufer M, Shakeri-Nejad K, Gardin A, et al. Impact of siponimod on vaccination response in a randomized, placebo-controlled study. Neurol Neuroimmunol Neuroinflam 2017; 4(6): e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vågberg M, Kumlin U, Svenningsson A. Humoral immune response to influenza vaccine in natalizumab-treated MS patients. Neurol Res 2012; 34(7): 730–733. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman M, Pardo G, Rossman H, et al. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J Neurol Sci 2014; 341(1–2): 22–27. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy CL, Tuohy O, Compston DAS, et al. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology 2013; 81(10): 872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmierer K, Wiendl H, Oreja-Guevara C, et al. Varicella zoster virus and influenza vaccine antibody titres in patients from MAGNIFY-MS who were treated with cladribine tablets for highly active relapsing multiple sclerosis. Mult Scler 2022; 28(13): 2151–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020; 95(14): e1999–e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bingham CO, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheum 2010; 62(1): 64–74. [DOI] [PubMed] [Google Scholar]
- 55.Kim W, Kim SH, Huh SY, et al. Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur J Neurol 2013; 20(6): 975–980. [DOI] [PubMed] [Google Scholar]
- 56.Vijenthira A, Gong I, Betschel SD, et al. Vaccine response following anti-CD20 therapy: A systematic review and meta-analysis of 905 patients. Blood Adv 2021; 5(12): 2624–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sormani MP, Schiavetti I, Carmisciano L, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. eBioMedicine 2021; 72: 103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J Neuroimmunol 2021; 361: 577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitzalis M, Idda ML, Lodde V, et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in Sardinian multiple sclerosis patients. Front Immunol 2021; 12: 781843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grothe C, Steffen F, Bittner S. Humoral immune response and lymphocyte levels after complete vaccination against COVID-19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J Cent Nerv Syst Dis 2021; 13: 1060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Etemadifar M, Nouri H, Pitzalis M, et al. Multiple sclerosis disease-modifying therapies and COVID-19 vaccines: A practical review and meta-analysis. J Neurol Neurosurg Psychiatry 2002; 93: 986–994. [DOI] [PubMed] [Google Scholar]
- 62.Tallantyre EC, Vickaryous N, Anderson V, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol 2022; 91(1): 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Disanto G, Sacco R, Bernasconi E, et al. Association of disease-modifying treatment and anti-CD20 infusion timing with humoral response to 2 SARS-CoV-2 vaccines in patients with multiple sclerosis. JAMA Neurol 2021; 78(12): 1529–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell–specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology 2022; 98(5): e541–e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capone F, Lucchini M, Ferraro E, et al. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics 2022; 19(1): 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cabreira V, Abreu P, Soares-dos-Reis R, et al. Multiple sclerosis, disease-modifying therapies and COVID-19: A systematic review on immune response and vaccination recommendations. Vaccines 2021; 9(7): 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.König M, Lorentzen ÅR, Torgauten HM, et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: The relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J Neurol Neurosurg Psychiatry 2023; 94: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brill L, Raposo C, Rechtman A, et al. SARS-CoV-2 third vaccine immune response in MS patients treated with ocrelizumab. Ann Neurol 2022; 91(6): 796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Achtnichts L, Jakopp B, Oberle M, et al. Humoral immune response after the third SARS-CoV-2 mRNA vaccination in CD20 depleted people with multiple sclerosis. Vaccines 2021; 9(12): 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gadani SP, Reyes-Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. eBioMedicine 2021; 73: 103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otero-Romero S, Rodríguez-García J, Vilella A, et al. Recomendaciones para la vacunación en pacientes con esclerosis múltiple candidatos a terapias inmunosupresoras: Documento de consenso español. Neurología 2021; 36(1): 50–60. [DOI] [PubMed] [Google Scholar]
- 72.Riva A, Barcella V, Benatti SV, et al. Vaccinations in patients with multiple sclerosis: A Delphi consensus statement. Mult Scler J 2020; 27(3): 347–359. [DOI] [PubMed] [Google Scholar]
- 73.Rubin LG, Levin MJ, Ljungman P, et al. Executive summary: 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58(3): 309–318. [DOI] [PubMed] [Google Scholar]
- 74.Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies—Executive summary. J Can Assoc Gastroenterol 2019; 2(4): 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Centers for Disease Control Prevention (CDC). ACIP general best guidance for immunization: Altered immunocompetence, 2019, https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html (accessed 24 October 2019).
- 76.Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020; 79(1): 39–52. [DOI] [PubMed] [Google Scholar]
- 77.Nandi A, Shet A. Why vaccines matter: Understanding the broader health, economic, and child development benefits of routine vaccination. Hum Vaccin Immunother 2020; 16(8): 1900–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belman AL, Krupp LB, Olsen CS, et al. Characteristics of children and adolescents with multiple sclerosis. Pediatrics 2016; 138(1): e20160120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walhart T. Parents, adolescents, children and the human papillomavirus vaccine: A review. Int Nurs Rev 2012; 59(3): 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salvadori MI. Human papillomavirus vaccine for children and adolescents. Paediatr Child Health 2018; 23(4): 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.European Centre for Disease Prevention and Control (ECDC). Vaccine scheduler, https://vaccine-schedule.ecdc.europa.eu/ (accessed 15 February 2022).
- 82.Swamy GK, Heine RP. Vaccinations for pregnant women. Obstet Gynecol 2015; 125(1): 212–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vousden N, Bunch K, Knight M, et al. Incidence, risk factors and impact of seasonal influenza in pregnancy: A national cohort study. PLoS ONE 2021; 16(1): e0244986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mertz D, Lo CKF, Lytvyn L, et al. Pregnancy as a risk factor for severe influenza infection: An individual participant data meta-analysis. BMC Infect Dis 2019; 19(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sperling RS, Riley LE. Influenza vaccination, pregnancy safety, and risk of early pregnancy loss. Obstet Gynecol 2018; 131(5): 799–802. [DOI] [PubMed] [Google Scholar]
- 86.Healy CM. Pertussis vaccination in pregnancy. Hum Vaccin Immunother 2016; 12(8): 1972–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cunningham AL, McIntyre P, Subbarao K, et al. Vaccines for older adults. BMJ 2021; 372: n188. [DOI] [PubMed] [Google Scholar]
- 88.Burke M, Rowe T. Vaccinations in older adults. Clin Geriatr Med 2018; 34(1): 131–143. [DOI] [PubMed] [Google Scholar]
- 89.Vaughn CB, Jakimovski D, Kavak KS, et al. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol 2019; 15(6): 329–342. [DOI] [PubMed] [Google Scholar]
- 90.Freedman DO, Chen LH. Vaccines for international travel. Mayo Clin Proc 2019; 94(11): 2314–2339. [DOI] [PubMed] [Google Scholar]
- 91.Centres for Disease Control and Prevention (CDC). Chapter 5: Travelers with additional considerations, 2020, https://wwwnc.cdc.gov/travel/yellowbook/2020/travelers-with-additional-considerations/immunocompromised-travelers (accessed 27 November 2021).
- 92.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccin Immunol 2010; 17(7): 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sabatino JJ, Wilson MR, Calabresi PA, et al. Anti-CD20 therapy depletes activated myelin-specific CD8+ T cells in multiple sclerosis. Proc Natl Acad Sci U S A 2019; 116(51): 25800–25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2021; 78(12): 1510–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iannetta M, Landi D, Cola G, et al. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult Scler Relat Disord 2021; 55: 103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kornek B, Leutmezer F, Rommer PS, et al. B cell depletion and SARS-CoV-2 vaccine responses in neuroimmunologic patients. Ann Neurol 2022; 91(3): 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021; 3(11): e789–e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27(11): 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berger T, Kornek B. T cells step up after SARS-CoV-2 vaccination with B cell depletion. Nat Rev Neurol 2021; 17(12): 729–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14: 1012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Havla J, Schultz Y, Zimmermann H, et al. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol 2022; 269(1): 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nabizadeh F, Ramezannezhad E, Kazemzadeh K, et al. Multiple sclerosis relapse after COVID-19 vaccination: A case report-based systematic review. J Clin Neurosci 2022; 104: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di Filippo M, Cordioli C, Malucchi S, et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry 2022; 93(4): 448–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231168043 for ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs by Susana Otero-Romero, Christine Lebrun-Frrénay, Saúl Reyes, Maria Pia Amato, Magda Campins, Mauricio Farez, Massimo Filippi, Yael Hacohen, Bernhard Hemmer, Rosa Juuti, Melinda Magyari, Celia Oreja-Guevara, Aksel Siva, Sandra Vukusic and Mar Tintoré in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585231168043 for ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs by Susana Otero-Romero, Christine Lebrun-Frrénay, Saúl Reyes, Maria Pia Amato, Magda Campins, Mauricio Farez, Massimo Filippi, Yael Hacohen, Bernhard Hemmer, Rosa Juuti, Melinda Magyari, Celia Oreja-Guevara, Aksel Siva, Sandra Vukusic and Mar Tintoré in Multiple Sclerosis Journal
Supplemental material, sj-docx-3-msj-10.1177_13524585231168043 for ECTRIMS/EAN consensus on vaccination in people with multiple sclerosis: Improving immunization strategies in the era of highly active immunotherapeutic drugs by Susana Otero-Romero, Christine Lebrun-Frénay, Saúl Reyes, Maria Pia Amato, Magda Campins, Mauricio Farez, Massimo Filippi, Yael Hacohen, Bernhard Hemmer, Rosa Juuti, Melinda Magyari, Celia Oreja-Guevara, Aksel Siva, Sandra Vukusic and Mar Tintoré in Multiple Sclerosis Journal