Abstract
Background
Over the past 30 years, numerous studies have focused on the treatment of cholangiocarcinoma (CCA), and these treatments have greatly evolved.
Objectives
To better understand the research trends, we evaluated the most influential publications and attempted to identify their characteristics using bibliometric methods.
Methods
The most influential publications were identified from the Clarivate Analytics Web of Science Core Collection database. The general characteristics of included papers were identified, and the research trends were explored via the bibliometric method.
Results
The average total number of citations for of the listed publications were 312 (range from 165 to 1922). The highest number of papers were published during period II (2001–2010, n = 50), followed by period III (2011–2020, n = 28), and period I (1991–2000, n = 22). The United States and Germany have made remarkable achievements in this field. Institutionally, Mayo Clinic and Memorial Sloan-Kettering Cancer Center were the leading institutions, with Blumgart and Zhu from the United States being the most influential authors. Close collaboration was established between the leading countries, institutions, and authors. The Annals of Surgery contributed the most to the papers with the highest total number of citations. Surgery predominated during period I (n = 14, 63.6%), with a gradual decline occurring during periods II (n = 19, 41.3%, P = 0.085) and period III (n = 3, 9.4%, P = 0.002). Contrastingly, the number of publications related to systemic therapy has increased significantly since period II and peaked in period III.
Conclusions
Surgery remains the most important treatment for CCA. However systemic therapy has become a research and clinical application hotspot. These findings will contribute to the translation of treatments for CCA and provide researchers with relevant research directions.
Keywords: Bibliometric analysis, Cholangiocarcinoma, Surgery, Systemic therapy, Targeted therapy
1. Introduction
Cholangiocarcinoma (CCA) is a highly lethal malignancy characterized by cholangiocellular differentiation, occurring anywhere along the biliary tree and liver parenchyma, with a five-year survival rate of <30% [1,2]. Although CCA remains a rare malignancy, its incidence has recently increased worldwide owing to several potential risk factors [3]. Surgery is the only potentially curative treatment for CCA patients, and the purpose of surgery is to achieve an R0 resection while preserving adequate liver function [4]. In patients with CCA, systematic chemotherapy has been found to improve prognosis, and for many years, gemcitabine and platinum have been considered the standard of care [5]. Additionally, because the combination of the three chemotherapies does not seem to lead to an improved prognosis or lower adverse effects than targeted therapy, targeted therapy has become increasingly popular in recent years [6]. Several potential targeted therapies for the treatment of CCA, such as vosidenib, pemigatinib, and selumetinib, are undergoing clinical trials and have shown clinical benefits in advanced CCA [[7], [8], [9]]. In recent years, several tumors have responded well to immunotherapy; however, in CCA, immune checkpoint inhibitors alone do not significantly improve prognosis, and more multicenter trials are necessary to demonstrate the effectiveness of immunotherapy in CCA [10].
With the prominent development of treatment regimens over the last three decades, it is a challenging task to identify the characteristics and research trends of publications focusing on the treatment of CCA. An effective way to resolve this problem is to conduct a bibliometric analysis of the existing literature. As a well-established statistical tool, bibliometric analysis based on total citations (TC) provides an overview of publications focused on treatments for CCA and allows scholars to obtain promising research directions from numerous papers published in that field. Bibliometric analysis has been extensively conducted to serve as a reference for clinicians and researchers in various diseases, such as gastric [11], colorectal [12], and gallbladder cancer [13]. Notably, prior bibliometric investigations of CCA focused on survival analysis and differential diagnosis, but did not provide a complete assessment of the field [14].
To identify the characteristics of publications and research trends over the past three decades, we utilized the bibliometric method to analyze the 100 most-cited articles in the field. This study summarizes the evolution of treatments for CCA for clinicians and researchers and provides meaningful perspectives for future research directions.
2. Results
2.1. The top-cited publications and publication period
Between January 1981 and December 2021, the Web of Science Core Collection (WOSCC) database identified 11,335 publications focusing on the treatment of CCA, of which 8570 were original articles (Fig. 1). The top 100 most influential papers are listed in descending order of TC in Table 2. The TC ranged from 1922 (Valle et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. New England Journal of Medicine. 2010) [15] to 165 (Berr et al. Photodynamic therapy for advanced bile duct cancer: Evidence for improved palliation and extended survival. Hepatology. 2000) [16]. The publication time trends for the included publications are shown in Fig. 2. A few publications can be found in period I (1991–2000, n = 22), wherein two studies published in 1991 that focused on combined portal vein resection and endoscopic treatment of CCA respectively was the earliest publications [17,18]. Period II (2001–2010; n = 46) had the highest growth rate in terms of the number of most-cited papers published, followed by period III (2011–2020; n = 32). There was a decline in the annual number of included publications after a peak in 2012 (n = 8), probably owing to the time-dependent accumulation pattern of TC. Additionally, the latest two articles were published in 2020; a phase 2 trial and a phase 3 trial, respectively, with pemigatinib in the treatment of advanced CCA and with ivosidenib in the treatment of isocitrate dehydrogenase 1 (IDH-1) mut [7,8].
Fig. 1.
Annual and cumulative number of publications on CCA management over the last 4 decades. CCA, Cholangiocarcinoma.
Table 2.
The top 100 papers in cholangiocarcinoma treatments.
| Rank | Paper | TC |
|---|---|---|
| 1 | Valle, J. et al., Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med, 2010. 362(14): p. 1273-81. | 1922 |
| 2 | Livraghi, T. et al., Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology, 2003. 226(2): p. 441–451. | 959 |
| 3 | Jarnagin, W.R. et al., Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Annals of Surgery, 2001. 234(4): p. 507–517. | 905 |
| 4 | Nakeeb, A. et al., Cholangiocarcinoma - A spectrum of intrahepatic, perihilar, and distal tumors. Annals of Surgery, 1996. 224(4): p. 463–473. | 855 |
| 5 | DeOliveira, M.L. et al., Cholangiocarcinoma - Thirty-one-year experience with 564 patients at a single institution. Annals of Surgery, 2007. 245(5): p. 755–762. | 837 |
| 6 | Schnitzbauer, A.A. et al., Right Portal Vein Ligation Combined With In Situ Splitting Induces Rapid Left Lateral Liver Lobe Hypertrophy Enabling 2-Staged Extended Right Hepatic Resection in Small-for-Size Settings. Annals of Surgery, 2012. 255(3): p. 405–414. | 800 |
| 7 | Meng, F.Y. et al., Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology, 2006. 130(7): p. 2113–2129. | 778 |
| 8 | Wang, Y.Z. et al., Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. Journal of Clinical Oncology, 2013. 31(9): p. 1188–1195. | 660 |
| 9 | Nakamura, H. et al., Genomic spectra of biliary tract cancer. Nature Genetics, 2015. 47(9): p. 1003-+. | 591 |
| 10 | Endo, I. et al., Intrahepatic cholangiocardnoma - Rising frequency, improved survival, and determinants of outcome after resection. Annals of Surgery, 2008. 248(1): p. 84–96. | 550 |
| 11 | Bismuth, H., R. Nakache, and T. Diamond, Management strategies in resection for hilar cholangiocarcinoma. Annals of Surgery, 1992. 215(1): p. 31–38. | 546 |
| 12 | Farges, O. et al., Portal vein embolization before right hepatectomy - Prospective clinical trial. Annals of Surgery, 2003. 237(2): p. 208–217. | 518 |
| 13 | Borger, D.R. et al., Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified Through Broad-Based Tumor Genotyping. Oncologist, 2012. 17(1): p. 72–79. | 503 |
| 14 | Neuhaus, P. et al., Extended resections for hilar cholangiocarcinoma. Annals of Surgery, 1999. 230(6): p. 808–818. | 492 |
| 15 | de Jong, M.C. et al., Intrahepatic Cholangiocarcinoma: An International Multi-Institutional Analysis of Prognostic Factors and Lymph Node Assessment. Journal of Clinical Oncology, 2011. 29(23): p. 3140–3145. | 452 |
| 16 | Rea, D.J. et al., Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Annals of Surgery, 2005. 242(3): p. 451–461. | 415 |
| 17 | Ortner, M.E.J. et al., Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology, 2003. 125(5): p. 1355–1363. | 396 |
| 18 | Primrose, J.N. et al., Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncology, 2019. 20(5): p. 663–673. | 396 |
| 19 | Tse, R.V. et al., Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Journal of Clinical Oncology, 2008. 26(4): p. 657–664. | 390 |
| 20 | Abou-Alfa, G.K. et al., Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncology, 2020. 21(5): p. 671–684. | 383 |
| 21 | Nagino, M. et al., Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer - Surgical outcome and long-term follow-up. Annals of Surgery, 2006. 243(3): p. 364–372. | 370 |
| 22 | Hemming, A.W. et al., Preoperative portal vein embolization for extended hepatectomy. Annals of Surgery, 2003. 237(5): p. 686–691. | 334 |
| 23 | Andersen, J.B. et al., Genomic and Genetic Characterization of Cholangiocarcinoma Identifies Therapeutic Targets for Tyrosine Kinase Inhibitors. Gastroenterology, 2012. 142(4): p. 1021-U552. | 330 |
| 24 | Jarnagin, W.R. et al., Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma - Implications for adjuvant therapeutic strategies. Cancer, 2003. 98(8): p. 1689–1700. | 325 |
| 25 | Meyer, C.G., I. Penn, and L. James, Liver transplantation for cholangiocarcinoma: Results in 207 patients. Transplantation, 2000. 69(8): p. 1633–1637. | 325 |
| 26 | Burke, E.C. et al., Hilar cholangiocarcinoma - Patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Annals of Surgery, 1998. 228(3): p. 385–392. | 322 |
| 27 | Javle, M. et al., Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. Journal of Clinical Oncology, 2018. 36(3): p. 276-+. | 320 |
| 28 | Lee, J. et al., Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncology, 2012. 13(2): p. 181–188. | 311 |
| 29 | Sia, D. et al., Integrative Molecular Analysis of Intrahepatic Cholangiocarcinoma Reveals 2 Classes That Have Different Outcomes. Gastroenterology, 2013. 144(4): p. 829–840. | 306 |
| 30 | Murad, S.D. et al., Efficacy of Neoadjuvant Chemoradiation, Followed by Liver Transplantation, for Perihilar Cholangiocarcinoma at 12 US Centers. Gastroenterology, 2012. 143(1): p. 88-U610. | 292 |
| 31 | Ross, J.S. et al., New Routes to Targeted Therapy of Intrahepatic Cholangiocarcinomas Revealed by Next-Generation Sequencing. Oncologist, 2014. 19(3): p. 235–242. | 288 |
| 32 | Arai, Y. et al., Fibroblast Growth Factor Receptor 2 Tyrosine Kinase Fusions Define a Unique Molecular Subtype of Cholangiocarcinoma. Hepatology, 2014. 59(4): p. 1427–1434. | 287 |
| 33 | De Palma, G.D. et al., Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointestinal Endoscopy, 2001. 53(6): p. 547–553. | 277 |
| 34 | Weber, S.M. et al., Intrahepatic cholangiocarcinoma: Resectability, recurrence pattern, and outcomes. Journal of the American College of Surgeons, 2001. 193(4): p. 384–391. | 277 |
| 35 | Seyama, Y. et al., Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Annals of Surgery, 2003. 238(1): p. 73–83. | 270 |
| 36 | Malka, D. et al., Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncology, 2014. 15(8): p. 819–828. | 263 |
| 37 | Kosuge, T. et al., Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Annals of Surgery, 1999. 230(5): p. 663–671. | 261 |
| 38 | Ebata, T. et al., Hepatectomy with portal vein resection for hilar cholangiocarcinoma - Audit of 52 consecutive cases. Annals of Surgery, 2003. 238(5): p. 720–727. | 259 |
| 39 | Hemming, A.W. et al., Surgical management of hilar cholangiocarcinoma. Annals of Surgery, 2005. 241(5): p. 693–702. | 252 |
| 40 | Abou-Alfa, G.K. et al., Ivosidenib in IDH1-mutant, chemotherapy-refractory Croatia& cholangiocarcinoma (ClarlDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncology, 2020. 21(6): p. 796–807. | 251 |
| 41 | Farshidfar, F. et al., Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Reports, 2017. 18(11): p. 2780–2794. | 250 |
| 42 | Pichlmayr, R. et al., Surgical treatment in proximal bile duct cancer - A single-center experience. Annals of Surgery, 1996. 224(5): p. 628–638. | 249 |
| 43 | Philip, P.A. et al., Phase II study of erlotinib in patients with advanced biliary cancer. Journal of Clinical Oncology, 2006. 24(19): p. 3069–3074. | 248 |
| 44 | Knox, J.J. et al., Combining gemcitabine and capecitabine in patients with advanced biliary cancer: A phase II trial. Journal of Clinical Oncology, 2005. 23(10): p. 2332–2338. | 246 |
| 45 | Pitt, H.A. et al., Perihilar cholangiocarcinoma - postoperative radiotherapy does not improve survival. Annals of Surgery, 1995. 221(6): p. 788–798. | 236 |
| 46 | Hong, T.S. et al., Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Journal of Clinical Oncology, 2016. 34(5): p. 460-+. | 234 |
| 47 | De Vreede, I. et al., Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transplantation, 2000. 6(3): p. 309–316. | 232 |
| 48 | Vauthey, J.N. et al., Is extended hepatectomy for hepatobiliary malignancy justified? Annals of Surgery, 2004. 239(5): p. 722–730. | 232 |
| 49 | Anderson, C.D. et al., Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. Journal of Gastrointestinal Surgery, 2004. 8(1): p. 90–97. | 230 |
| 50 | Goyal, L. et al., Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discovery, 2017. 7(3): p. 252–263. | 230 |
| 51 | Borad, M.J. et al., Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma. Plos Genetics, 2014. 10(2). | 229 |
| 52 | Ohtsuka, M. et al., Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. British Journal of Surgery, 2002. 89(12): p. 1525–1531. | 229 |
| 53 | Wulf, J. et al., Stereotactic radiotherapy of targets in the lung and liver. Strahlentherapie Und Onkologie, 2001. 177(12): p. 645-+. | 226 |
| 54 | Su, C.H. et al., Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Annals of Surgery, 1996. 223(4): p. 384–394. | 224 |
| 55 | Ortner, M. et al., Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology, 1998. 114(3): p. 536–542. | 221 |
| 56 | Klempnauer, J. et al., Resectional surgery of hilar cholangiocarcinoma: A multivariate analysis of prognostic factors. Journal of Clinical Oncology, 1997. 15(3): p. 947–954. | 220 |
| 57 | Bekaii-Saab, T. et al., Multi-Institutional Phase II Study of Selumetinib in Patients With Metastatic Biliary Cancers. Journal of Clinical Oncology, 2011. 29(17): p. 2357–2363. | 219 |
| 58 | Kitagawa, Y. et al., Lymph node metastasis from hilar cholangiocarcinoma: Audit of 110 patients who underwent regional and paraaortic node dissection. Annals of Surgery, 2001. 233(3): p. 385–392. | 218 |
| 59 | Kondo, S. et al., Forty consecutive resections of Hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins - Results of a prospective study. Annals of Surgery, 2004. 240(1): p. 95–101. | 217 |
| 60 | Choi, S.B. et al., The Prognosis and Survival Outcome of Intrahepatic Cholangiocarcinoma Following Surgical Resection: Association of Lymph Node Metastasis and Lymph Node Dissection with Survival. Annals of Surgical Oncology, 2009. 16(11): p. 3048–3056. | 216 |
| 61 | Nagino, M. et al., Hepatectomy With Simultaneous Resection of the Portal Vein and Hepatic Artery for Advanced Perihilar Cholangiocarcinoma An Audit of 50 Consecutive Cases. Annals of Surgery, 2010. 252(1): p. 115–123. | 213 |
| 62 | Zoepf, T. et al., Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy. American Journal of Gastroenterology, 2005. 100(11): p. 2426–2430. | 213 |
| 63 | Hyder, O. et al., A Nomogram to Predict Long-term Survival After Resection for Intrahepatic Cholangiocarcinoma An Eastern and Western Experience. Jama Surgery, 2014. 149(5): p. 432–438. | 212 |
| 64 | Madariaga, J.R. et al., Liver resection for hilar and peripheral cholangiocarcinomas: A study of 62 cases. Annals of Surgery, 1998. 227(1): p. 70–79. | 208 |
| 65 | Guglielmi, A. et al., Intrahepatic Cholangiocarcinoma: Prognostic Factors After Surgical Resection. World Journal of Surgery, 2009. 33(6): p. 1247–1254. | 206 |
| 66 | Ramanathan, R.K. et al., A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemotherapy and Pharmacology, 2009. 64(4): p. 777–783. | 206 |
| 67 | Witzigmann, H. et al., Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma - Palliative photodynamic therapy plus stenting is comparable to R1/R2 resection. Annals of Surgery, 2006. 244(2): p. 230–239. | 206 |
| 68 | Sudan, D. et al., Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. American Journal of Transplantation, 2002. 2(8): p. 774–779. | 204 |
| 69 | Robles, R. et al., Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Annals of Surgery, 2004. 239(2): p. 265–271. | 195 |
| 70 | Steel, A.W. et al., Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointestinal Endoscopy, 2011. 73(1): p. 149–153. | 193 |
| 71 | Valle, J.W. et al., Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumors: a multicentre randomised phase II study - The UK ABC-01 Study. British Journal of Cancer, 2009. 101(4): p. 621–627. | 193 |
| 72 | Polydorou, A.A. et al., Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut, 1991. 32(6): p. 685–689. | 193 |
| 73 | Ben-Josef, E. et al., SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. Journal of Clinical Oncology, 2015. 33(24): p. 2617-U57. | 184 |
| 74 | Ribero, D. et al., Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma A Multi-institutional Analysis of 434 Patients. Archives of Surgery, 2012. 147(12): p. 1107–1113. | 184 |
| 75 | Iwatsuki, S. et al., Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. Journal of the American College of Surgeons, 1998. 187(4): p. 358–364. | 184 |
| 76 | Hyder, O. et al., Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery, 2013. 153(6): p. 811–818. | 183 |
| 77 | Miyazaki, M. et al., Combined vascular resection in operative resection for hilar cholangiocarcinoma: Does it work or not? Surgery, 2007. 141(5): p. 581–588. | 182 |
| 78 | Isomoto, H. et al., Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholanglocarcinoma cells. Hepatology, 2005. 42(6): p. 1329–1338. | 181 |
| 79 | Lubner, S.J. et al., Report of a Multicenter Phase II Trial Testing a Combination of Biweekly Bevacizumab and Daily Erlotinib in Patients With Unresectable Biliary Cancer: A Phase II Consortium Study. Journal of Clinical Oncology, 2010. 28(21): p. 3491–3497. | 181 |
| 80 | Hezel, A.F. and A.X. Zhu, Systemic therapy for biliary tract cancers. Oncologist, 2008. 13(4): p. 415–423. | 180 |
| 81 | Lee, S.G. et al., Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. Journal of Hepato-Biliary-Pancreatic Sciences, 2010. 17(4): p. 476–489. | 180 |
| 82 | Nathan, H. et al., Trends in survival after surgery for cholangiocarcinoma: A 30-year population-based SEER database analysis. Journal of Gastrointestinal Surgery, 2007. 11(11): p. 1488–1496. | 178 |
| 83 | Hochwald, S.N. et al., Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Archives of Surgery, 1999. 134(3): p. 261–266. | 177 |
| 84 | Javle, M. et al., Biliary Cancer: Utility of Next-Generation Sequencing for Clinical Management. Cancer, 2016. 122(24): p. 3838–3847. | 177 |
| 85 | Lowery, M.A. et al., Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clinical Cancer Research, 2018. 24(17): p. 4154–4161. | 177 |
| 86 | Sano, T. et al., One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Annals of Surgery, 2006. 244(2): p. 240–247. | 176 |
| 87 | Gruenberger, B. et al., Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncology, 2010. 11(12): p. 1142–1148. | 175 |
| 88 | Nagorney, D.M. et al., Outcomes after curative resections of cholangiocarcinoma. Archives of Surgery, 1993. 128(8): p. 871–879. | 175 |
| 89 | Farges, O. et al., Influence of Surgical Margins on Outcome in Patients With Intrahepatic Cholangiocarcinoma A Multicenter Study by the AFC-IHCC-2009 Study Group. Annals of Surgery, 2011. 254(5): p. 824–830. | 172 |
| 90 | Nuzzo, G. et al., Improvement in Perioperative and Long-term Outcome After Surgical Treatment of Hilar Cholangiocarcinoma Results of an Italian Multicenter Analysis of 440 Patients. Archives of Surgery, 2012. 147(1): p. 26–34. | 172 |
| 91 | Graham, R.P. et al., Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Human Pathology, 2014. 45(8): p. 1630–1638. | 171 |
| 92 | Farley, D.R., A.L. Weaver, and D.M. Nagorney, Natural-history of unresected cholangiocarcinoma - patient outcome after noncurative intervention. Mayo Clinic Proceedings, 1995. 70(5): p. 425–429. | 170 |
| 93 | Bengala, C. et al., Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. British Journal of Cancer, 2010. 102(1): p. 68–72. | 169 |
| 94 | Nimura, Y. et al., Combined portal-vein and liver resection for carcinoma of the biliary-tract. British Journal of Surgery, 1991. 78(6): p. 727–731. | 169 |
| 95 | Bryant, R. et al., Laparoscopic Liver Resection-Understanding its Role in Current Practice The Henri Mondor Hospital Experience. Annals of Surgery, 2009. 250(1): p. 103–111. | 168 |
| 96 | Inoue, K. et al., Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery, 2000. 127(5): p. 498–505. | 168 |
| 97 | Neuhaus, P. et al., Oncological Superiority of Hilar En Bloc Resection for the Treatment of Hilar Cholangiocarcinoma. Annals of Surgical Oncology, 2012. 19(5): p. 1602–1608. | 168 |
| 98 | Wakai, T. et al., Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer, 2005. 103(6): p. 1210–1216. | 167 |
| 99 | Casavilla, F.A. et al., Hepatic resection and transplantation for peripheral cholangiocarcinoma. Journal of the American College of Surgeons, 1997. 185(5): p. 429–436. | 167 |
| 100 | Berr, F. et al., Photodynamic therapy for advanced bile duct cancer: Evidence for improved palliation and extended survival. Hepatology, 2000. 31(2): p. 291–298. | 165 |
Fig. 2.
Annual and cumulative number of the 100 most cited papers over the last 3 decades.
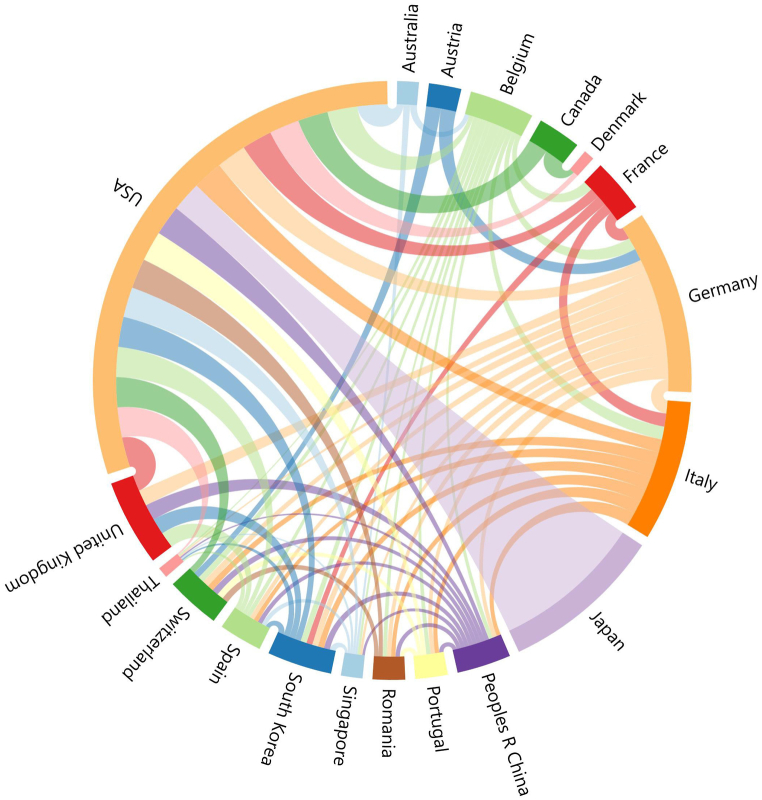
2.2. Countries, institutions, and cooperation
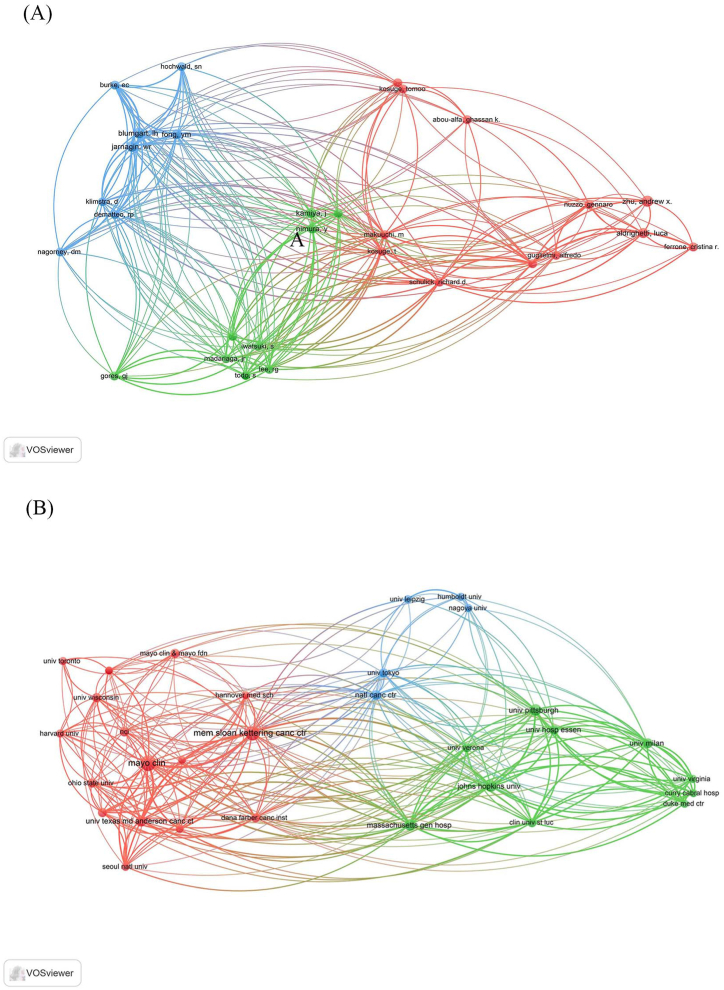
A world map was compiled based on the contributions of 19 countries and 303 institutions (Fig. 3). In Europe, the top 100 papers were relatively dispersed with close contributions from each country, whereas American and Japanese contributions accounted for a large proportion of the papers published in the Americas and Asia, respectively. Among the top 100 most influential articles (Table 3), the maximum number of studies was conducted in the United States (n = 51), followed by Germany (n = 17), and Japan (n = 15). As shown in Table 3, although Japan had the highest TC of 4,158, it shared the lowest ratio of TC to publication with South Korea (n = 277), whereas the United Kingdom had the highest ratio of 443. According to the analysis of the cooperation relationships between various countries, the United States, South Korea, and Belgium had strong international cooperation relationships, while other countries showed weak cooperation with each other (Fig. 4). As shown in Table 4, the leading institutions that published the most articles in the listed articles included Mayo Clinic, Memorial Sloan-Kettering Cancer Center, and Johns Hopkins University with 12, 11, and 7 papers, respectively, receiving 3238 citations, 3937 citations, and 2822 citations, respectively. Only two institutions had a TC-to-publication ratio of >400: Massachusetts General Hospital (n = 408) and Johns Hopkins University (n = 403), both from the United States. Analysis of the network map of institutions revealed 31 nodes and 271 links (Fig. 5A). As a result, 31 institutions were divided into three clusters. The institutions within each cluster actively collaborated, but the Japanese institution-dominated cluster had poor communication with the other two clusters.
Fig. 3.
National distribution of the 100 most cited papers.
Table 3.
Top 10 countries with the most publications.
| Country | Publication | TC | TC/Publication |
|---|---|---|---|
| USA | 51 | 16,107 | 316 |
| Germany | 17 | 5316 | 313 |
| Japan | 15 | 4158 | 277 |
| Italy | 13 | 3905 | 300 |
| United Kingdom | 8 | 3544 | 443 |
| South Korea | 6 | 1661 | 277 |
| Belgium | 6 | 1880 | 313 |
| France | 5 | 1504 | 301 |
| Switzerland | 5 | 1473 | 295 |
| China | 5 | 1667 | 333 |
TC, Total citations.
Fig. 4.
The cooperation relationships among countries that published the 100 most cited papers.
Table 4.
Top 10 institutions with the most publications.
| Institution | Country | Publication | TC | TC/Publication |
|---|---|---|---|---|
| Mayo Clinic | USA | 12 | 3238 | 270 |
| Memorial Sloan-Kettering Cancer Center | USA | 11 | 3937 | 358 |
| Johns Hopkins University | USA | 7 | 2822 | 403 |
| Massachusetts General Hospital | USA | 7 | 2859 | 408 |
| University of Pittsburgh | USA | 7 | 1612 | 230 |
| University of Texas MD Anderson Cancer Center | USA | 6 | 1520 | 253 |
| University Hospital Essen | Germany | 5 | 1323 | 265 |
| University of Milan | Italy | 5 | 1203 | 241 |
| National Cancer Centre | South Korea | 5 | 1492 | 298 |
| Cliniques Universitaires Saint-Luc | Belgium | 4 | 1167 | 292 |
TC, Total citations.
Fig. 5.
The cooperation relationships of (A) institutions and (B) authors that have co-published more than 3 top-cited articles.
2.3. Leading authors and the top-cited journals
The leading authors in the field are listed in Table 5, including Zhu, Blumgart and Jarnagin from the United States, with the same five papers, receiving TCs of 1,581, 2,006, and 2,006, respectively. Two researchers from the United States Bruke and DeMatteo, achieved the highest TC-to-publication ratio of 502 with only three articles. The network graph of productive authors (Fig. 5B) shows a positive collaborative relationship among the leading authors, among which Schulick, Blumgart, and Jarnagin had the strongest total link strength. The top 100 most influential papers were published in 34 different journals, and Table 6 presented the top 10 journals according to the number of publications. There were four journals with a TC-to-publications ratio >300, with Gastroenterology (impact factor = 33.883) having the highest ratio of 387 with six publications, followed by Annals of Surgery (TC-to-publications ratio of 369 with 29 publications), Oncologist (TC-to-publications ratio of 324 with three publications), and Journal of Clinical Oncology (TC-to-publications ratio of 305 with 11 publications).
Table 5.
Top 10 authors with the most publications.
| Author | Country | Publication | TC | TC/Publication |
|---|---|---|---|---|
| Zhu, AX | USA | 5 | 1581 | 316 |
| Blumgart, LH | USA | 5 | 2006 | 401 |
| Jarnagin, WR | USA | 5 | 2006 | 401 |
| Aldrighetti, L | Italy | 4 | 1020 | 255 |
| Fong, Y | USA | 4 | 1101 | 275 |
| Kamiya, J | Japan | 4 | 1016 | 254 |
| Nimura, Y | Japan | 4 | 1016 | 254 |
| Klimstra, D | USA | 3 | 1507 | 502 |
| Bruke, EC | USA | 3 | 1404 | 468 |
| DeMatteo, RP | USA | 3 | 1507 | 502 |
TC, Total citations.
Table 6.
Top 10 journals with the most publications.
| Journal | Publication | TC | TC/Publication | Impact Factor |
|---|---|---|---|---|
| Annals of Surgery | 29 | 10,700 | 369 | 13.787 |
| Journal of Clinical Oncology | 11 | 3354 | 305 | 50.717 |
| Lancet Oncology | 6 | 1779 | 297 | 54.433 |
| Gastroenterology | 6 | 2323 | 387 | 33.883 |
| Archives of Surgery | 4 | 669 | 167 | 2.895 |
| Surgery | 3 | 533 | 178 | 4.348 |
| Cancer | 3 | 669 | 223 | 6.921 |
| Journal of the American College of Surgeons | 3 | 628 | 209 | 6.532 |
| Hepatology | 3 | 633 | 211 | 17.298 |
| Oncologist | 3 | 971 | 324 | 5.837 |
TC, Total citations; IF, Impact factor.
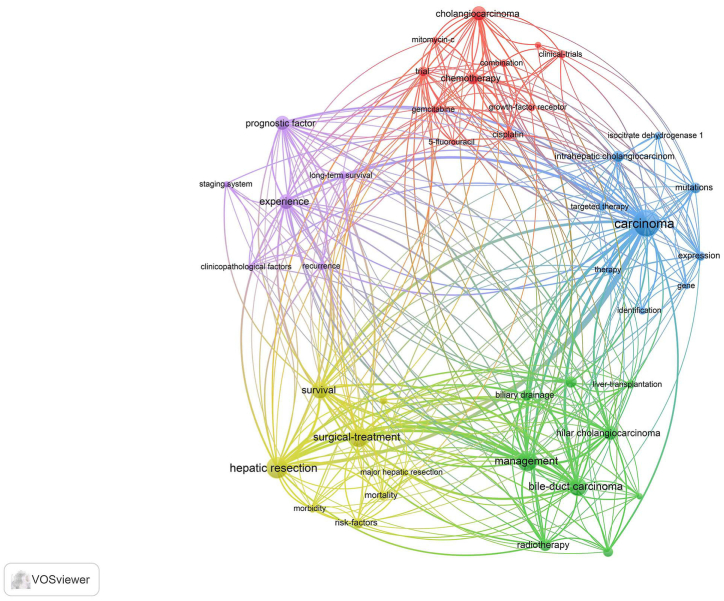
2.4. Keywords, main topic, subtopic, and article type
A panorama of several study subjects was uncovered by the term analysis algorithm of VOSviewer (Fig. 6). A total of five clusters of terms were identified, including those related to surgery, chemotherapy, targeted therapy, prognosis prediction, and other available regimens for treatment. The main topic, subtopic, and article type of the 100 most-cited papers were defined, and the relationships among them were established (Fig. 7). The most studied topic was systemic therapy (n = 39), followed by surgery (n = 37) and outcome prediction (n = 24). Targeted therapy was the most studied subtopic (n = 20), followed by clinicopathologic characteristics (n = 16), surgical strategy (n = 8), and liver resection (n = 8). Studies on surgery and outcome prediction have been primarily retrospective, and only a few prospective studies are available. Chemotherapy as adjuvant therapy or systemic therapy was mainly used in clinical trials and focused on phase 2 or 3 trials. Notably, targeted therapy is a promising research direction, and the research types are primarily basic research and early clinical.
Fig. 6.
The cluster analysis of keywords of the 100 most cited papers.
Fig. 7.
Main topic, subtopic, and article type of the 100 most cited papers.
2.5. Research trends of different main topics
The research trends for the various main topics were shown in Table 7 and Fig. 8. The most-cited publications that related to surgery dominated in the Period I (n = 14, 63.6%), began to decline in the period II (n = 19, 41.3%, P = 0.085), and almost disappeared in the period III (n = 3, 9.4%, P = 0.002). Our study also evaluated the clinical benefits of different surgical strategies over time, and the results showed that surgical intervention improved prognosis more significantly with technological advancements. In contrast, the publication percentages of systemic therapy grew memorably in period II, (32.6% vs. 9.1%, P = 0.036), and continued to maintain rapid growth in period III, (65.6% vs. 32.6%, P = 0.004). Interestingly, the percentages of publications focusing on outcome prediction has remained relatively stable over the last three decades.
Table 7.
The publication of different main topics over time.
| Main Topic | Period I (1991–2000) (n = 22) | Period II (2001–2010) (n = 46) | P value | Period III (2011–2020) (n = 32) | P value |
|---|---|---|---|---|---|
| Surgery | 14 (63.6%) | 19 (41.3%) | 0.085 | 3 (9.4%) | 0.002* |
| Outcome Prediction | 6 (27.3%) | 12 (26.1%) | 0.917 | 8 (25.0%) | 0.914 |
| Systemic Therapy | 2 (9.1%) | 15 (32.6%) | 0.036* | 21 (65.6%) | 0.004* |
*P < 0.05.
Fig. 8.
Research trends of different main topics over the last 3 decades.
3. Discussion
In this study, we aimed to evaluate the publication characteristics of the 100 most-cited papers in the field over the past three decades using bibliometric methods. Based on keywords clustering and main topic trend analyses, it was determined that surgery remains the optimal treatment method, whereas systemic treatment is thriving and has become a hotspot for researchers. These findings have profound significance in that they provided clues for potential breakthrough directions in the research on CCA.
The annual number of publications on the treatment of CCA has fluctuated over the past 30 years. Most of the included papers emerged in period II and maintained rapid growth, indicating that the treatment of CCA flourished during this period. The annual number of included publications declined to a low level after peaking in 2012, probably because the accumulation pattern of TC was time-dependent. Although the 100 most influential papers were published in 19 countries on three continents, a significant imbalance existed in the geographical distribution of the listed papers.
According to an overview of prominent nations, the majority of the contributions were made by the United States, Germany, and Japan. Notably, the international distribution of articles related to the treatment of CCA was not associated with the frequency of CCA in any country but rather with the degree of development. Six of the top 10 institutions that published the most included articles were located in the United States, with Mayo Clinic having the most publications and Massachusetts General Hospital achieving the highest TC/Publication ratio of 408. Analysis of the authors found that Zhu, Blumgart, and Jarnagin from the United States published the most included papers reaching 5, while Klimstra and DeMatteo had the highest TC-to-publication ratio of 502.
Surgery-related research has mainly been conducted in retrospective studies, which illustrate that surgical treatment is a relatively experienced treatment protocol. As the most frequently occurring keyword in this cluster, which contained terms related to surgical management, hepatic resection is regarded as the only available curative option for patients with CCA [4]. To reduce postoperative mortality, R0 resection is the goal of surgery, while maintaining adequate future liver remnant function to improve prognosis [19]. Several volume optimization strategies are available and widely used in major liver resections to avoid postoperative liver dysfunction [20,21]. CCA typically requires hemihepatectomy or trisectionectomy to achieve R0 resection; if the tumor has extensive invasion, vascular resection and bile duct reconstruction are prescribed [22]. Although minimally invasive surgery is time-consuming and financially burdensome, it can lead to minimal invasiveness without compromising the overall survival in well-selected patients, making it the preferred treatment method [23,24]. Other alternative treatment protocols for CCA, including liver transplantation, have predominated the green cluster as potential breakthroughs in CCA management. Liver transplantation is not recommended as a routine treatment for CCA because of its high recurrence rate [25]. However, a follow-up multicenter study found that neoadjuvant chemoradiotherapy followed by liver transplantation resulted in a recurrence-free survival rate of 65% after 5 years [26].
To accurately predict the clinical benefits of liver transplantation, a novel liver transplant risk model, including indicators of vascular invasion, tumor size, and multiple tumors, was developed, which outperformed the Milan and University of California, San Francisco criteria in both the training and validation sets [27]. In response to the increasing popularity of intrahepatic cholangiocarcinoma, surgery-based systemic treatment has gradually become the preferred treatment method [28], so surgical research has gradually diminished from the Period II onward. Over the last 10 years, surgical methods have basically matured, the impact of surgery on prognosis has been largely established [4], and the number of citations to related literature has decreased. Therefore, papers related to surgery were excluded from the analysis after period II. Additionally, due to the upsurge in systemic therapies, such as immunotherapy and targeted therapy [29], the incidence of TC has increased over the past decade, which makes it more likely to be included in this study's analysis.
Due to the lack of a recognized therapeutic standard for patients with advanced CCA, an increasing number of systemic therapy-focused articles were published during period II. Chemotherapy is a subtopic of systemic therapy, and most clinical studies have been conducted, including phase 2 and 3 trials [6]. The results of a phase 3 trial demonstrated that gemcitabine combined with cisplatin improved median overall survival from 8.1 months to 11.7 months without increasing adverse events compared with gemcitabine alone [15]. A phase 2, single-arm trial presented at the 2022 annual meeting of the American Society of Clinical Oncology assessed efficacy and safety of perioperative neoadjuvant chemotherapy using gemcitabine, cisplatin, and nab-paclitaxel. The result showed that the combination achieved 23% partial response rate and 90% disease control rate [30]. In comparison to active symptom control alone, adjuvant folic acid, 5-fluorouracil, and oxaliplatin significantly increased median overall survival from 5.3 months to 6.2 months but were also associated with neutropenia, fatigue, or lethargy [31]. Hepatic artery infusion chemotherapy with a modified folic acid, 5-fluorouracil, and oxaliplatin regimen has also resulted in improved clinical outcomes. In patients treated with hepatic artery infusion chemotherapy, the median overall survival was 9.5 months, and the disease control rate was 61.8% [32]. A phase 3 randomized controlled multicenter study of capecitabine as the latest first-line treatment regimen also showed good clinical results, and prognosis significantly improved from the treatment group to the observation group (median overall survival, 36.4 vs. 51.5 months) [33]. As an adjuvant treatment for patients with CCA, radiotherapy is usually combined with chemotherapy and technological advances such as intensity-modulated radiotherapy, tomotherapy, and 3D conformal radiotherapy [28]. External beam radiation therapy is commonly used as an adjuvant therapy in cases of positive resection margins. Based on a retrospective study, external beam radiation therapy was associated with an improved prognosis in patients with R1/R2 resection (39.5 vs. 21.1 months; P = 0.052) [34]. Several large clinical cohort studies have demonstrated some benefit of radiotherapy in patients with locally unresectable perihilar or distal CCA; however, the true efficacy of radiotherapy remained to be determined [35]. Targeted therapy is currently a hotspot in CCA research, primarily in basic research and early clinical trials. A phase 3 randomized, double-blind controlled trial confirmed that the use of ivosidenib (targeted inhibitor of mutated IDH1) significantly improved progression-free survival and was well tolerated in subgrs with IDH mutations [7]. Pemigatinib, which inhibits fibroblast growth factor receptors 1–3, was approved by the US FDA as the first targeted drug for chemotherapy-refractory advanced CCA with fibroblast growth factor receptor aberrations [8,36]. In a muti-institutional phase 2 study of selumetinib, a selective inhibitor of mitogen-activated protein kinase, stable disease was observed in 17 patients (68%) and the median progression-free survival reached 3.7 months [9]. As targeted therapy has been applied in the clinic to cure CCA, identifying effective actionable molecular mutations and delaying the emergence of acquired resistance are important [30].
Intriguingly, the proportion of studies focusing on outcome prediction has remained essentially constant over the past three decades. The term analysis system of VOSviewer also identified a purple cluster at the top left, including six keywords mainly related to the survival and prognosis of CCA. Several clinicopathological factors have been shown to influence the prognosis of CCA, including non-R0 resection (hazard ratio [HR], 2.20; P < 0.001), multiple tumors (HR, 1.80; P = 0.001), and vascular invasion (HR, 1.59; P = 0.015). However, tumor size was not associated with survival outcomes [37]. Based on multiple retrospective studies, Yu et al. selected age at diagnosis, tumor size, tumor grade, lymph node metastasis, and T stage as predictive factors to propose a nomogram to predict prognosis, revealing promising predictive abilities [38]. Based on the Surveillance, Epidemiology, and End Results database, an Extreme gradient boosting (XGBoost) model was developed to predict the prognosis of elderly patients with CCA. Several adverse prognostic factors, including multiple (satellite) tumors/nodules, multiple invasions of the liver lobe and direct invasion of adjacent organs [39]. However, a large number of prospective studies to confirm the validity of these nomograms, since most researches on prognostic analysis were retrospective.
Using TC, we were able to evaluate the importance of the literature directly and effectively. Based on this, the top 100 articles can provide an overview of the current state of this research field, excluding the influence of low-quality studies on its conclusions and enriching and enhancing its development. Bibliometrics analysis based on TC has been performed in the field of colorectal cancer and has identified the most cited manuscripts related to gene mutation, immunologic, basic research, and surgical treatment [12]. Additionally, Powell et al. conducted a bibliometrics analysis focused on gastric cancer, which generalized its pathogenesis and served as the guideline for creating a reference list [11]. However, there has been a limited amount of bibliometric analysis of CCA in the past, and most of these studies have focused on survival analysis and differential diagnosis rather than providing a comprehensive assessment of the therapeutic field [14]. Through keyword clustering and trend analysis, we summarize several important aspects of treatment of CCA development over the last 30 years and provided clinicians and researchers with insights for future studies.
By utilizing mathematical techniques, this study objectively and comprehensively quantified and evolved research hotspots and trends in the field of the treatment of CCA based on bibliometrics. Additionally, we intend to contribute to research directions by summarizing the research over the past 30 years to assist researchers in identifying hotspots and trends. The treatment for CCA remains a dilemma. To determine the specific molecular characteristics of CCA and predict early recurrence postoperatively, a combination of advanced methods, including next-generation sequencing technology [40] and circulating tumor DNA detection technology [41], is needed to provide timely and effective interventions for patients.
However, our study has some limitations. It should be noted that only the data from the WOSCC database was retrieved, and only publications with the original article type were considered. Second, we only included the top 100 most-cited papers, there is a possibility that some important papers were not included. Therefore, it is important to download relevant data from multiple databases and merged them for further analysis in order to obtain a more comprehensive overview of the CCA therapeutic field. In addition, because TC accumulates over time, bibliometrics might suffer from a length-time-effect bias in citations, resulting in newer articles being at a disadvantage in obtaining citations, and many of the most important and recent studies in 2022 might be overlooked, such as immunotherapy, which has rarely been discussed. Finally, owing to the diversity of subject words and incomplete extraction of keywords by the software, the literature analysis may be incomplete, and the conclusion may be deemed less credible. To minimize these limitations, we manually searched for isolated keywords and new articles in 2022 and described them in the Discussion section, which were pooled with the results of the bibliometric analysis to provide the most comprehensive description possible.
4. Conclusion
In this study, we quantitatively generalized the published literature related to CCA and applied these metrics to predict the direction of research in the therapeutic field. Several scientific visualizations were constructed to provide a comprehensive bibliometric overview of publications. Furthermore, this study indicates that the application of targeted therapy against gene aberrations and the combination of immunotherapy and systemic therapy would be the potential breakthrough for the treatment of CCA in the future.
5. Materials and methods
5.1. Literature search and screening
A systematic literature search was conducted using the WOSCC database from January 1991 to December 2021 in June 2022, based on the search terms and strategies summarized in Table 1. Only original articles that focused on the treatment of CCA were included. To further filter the returned dataset, only English language and full manuscripts were included, and the data were sorted according to TC. Two independent investigators read and identified the top 100 most-cited articles to avoid irrelevant oes. A third investigator was consulted if necessary to resolve any disagreements between the two investigators. Finally, 11,335 studies that met the inclusion criteria were obtained for in-depth analysis.
Table 1.
The search strategy with a summary of keywords to identify the top 100 papers from Clarivate Analytics Web of Science Core Collection database.
| Search Sequence | Search Query | Result |
|---|---|---|
| Search #1 | TS=(Cholangiocarcinoma OR Cholangiocellular Carcinoma OR Cholangiocellular Cancer OR Cholangiocellular malignancy OR Cholangiocellular malignant tumor) | 19,207 |
| Search #2 | TS=(treatment OR therapy OR management OR surgery OR resection OR completion surgery OR curative resection OR hepatic resection OR liver resection OR portal lymphadenectomy OR lymph node dissection OR bile duct resection OR liver transplantation OR adjuvant therapy OR chemotherapy OR systemic chemotherapy OR locoregional therapy OR transarterial chemoembolization OR TACE OR hepatic arterial infusion OR HAI OR intraductal ablative procedures OR radiofrequency ablation OR photodynamic therapy OR PDT OR radiotherapy OR fractionated radiotherapy OR intensity-modulated radiation therapy OR stereotactic body radiation therapy OR stereotactic body radiotherapy OR SBRT OR targeted therapy OR immunotherapy) | 8,959,110 |
| Search #3 | #1 AND #2 | 11,335 |
Note: Indexes = SCI-EXPANDED, CPCI–S, Timespan = From January 1981 to December 2021.
SCI-EXPANDED, Science Citation Index Expanded; CPCI–S, Conference Proceedings Citation Index-Science.
5.2. Data analyses and visualization
Following the identification of the top 100 most cited papers that met the selection criteria, we downloaded all available data in the WOSCC database related to those articles. Next, VOS viewer Version 1.6.18 (Leiden University, Leiden, Netherlands) was chosen to convert and analyzed the raw data of the selected publications. Two investigators independently extracted the publication characteristics of the papers using VOSviewer, including the title, author, institution, country, TC number, publication year, and journal. In the event of disagreement, all issues were resolved through consultation, and if necessary, a third investigator was introduced. We obtained the impact factors of journals from the Journal Citation Reports for 2021. Finally, we read the title, abstract, the full text if necessary to determine the main topic, subtopic, and article type.
Prism Version 9.0.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the annual and cumulative numbers of publications and main topic research trends. Origin Version 9.8.0.200 (OriginLab Corporation, Northampton, MA, USA) was used to analyze the relationships between the main topics, subtopics, and research types. The pattern of contribution among the countries was created using Microsoft Excel 2019 Power Map (Microsoft Corp., Redmond, WA, USA). Chord charts for the 100 most-cited papers published in country or region partnerships were created with Microsoft Charticulator (https://charticulator.com/). Cooperative relationship among all countries, institutions, and authors was created and visualized by VOSviewer Version 1.6.18 (Leiden University, Leiden, Netherlands). Finally, utilizing the term analysis system of VOSviewer, a cluster analysis was conducted and a landscape of high-frequency research topics was presented.
5.3. Statistical analysis
Categorical variables were reported as frequencies and percentages, and Pearson's chi-square test was used to compare the two groups using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). The P-value <0.05 was considered statistically significant.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author contribution statement
Kainan Lin, MD: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jiasheng Cao, MD: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Haibo Chen, MD: Performed the experiments.
Win Topatana, MD; Jingwei Cai, MD: Analyzed and interpreted the data.
Bin Zhang, MD; Jiahao Hu, MD: Contributed reagents, materials, analysis tools or data.
Renan Jin: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Brindley P.J., et al. cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021;7(1):65. doi: 10.1038/s41572-021-00300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato K., et al. Cholangiocarcinoma: novel therapeutic targets. Expert Opin. Ther. Targets. 2020;24(4):345–357. doi: 10.1080/14728222.2020.1733528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan S.A., Tavolari S., Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39(Suppl 1):19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 4.Cillo U., et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):143–155. doi: 10.1111/liv.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzo A., Brandi G. First-line chemotherapy in advanced biliary tract cancer ten years after the ABC-02 trial: "and yet it moves!". Cancer Treat Res Commun. 2021;27 doi: 10.1016/j.ctarc.2021.100335. [DOI] [PubMed] [Google Scholar]
- 6.Luvira V., et al. Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma. Cochrane Database Syst. Rev. 2021;9(9):Cd012814. doi: 10.1002/14651858.CD012814.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Alfa G.K., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory Croatia& cholangiocarcinoma (ClarlDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Alfa G.K., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekaii-Saab T., et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J. Clin. Oncol. 2011;29(17):2357–2363. doi: 10.1200/JCO.2010.33.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo A., Ricci A.D., Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expet Opin. Invest. Drugs. 2021;30(4):343–350. doi: 10.1080/13543784.2021.1897102. [DOI] [PubMed] [Google Scholar]
- 11.Powell A.G., et al. The 100 most influential manuscripts in gastric cancer: a bibliometric analysis. Int. J. Surg. 2016;28:83–90. doi: 10.1016/j.ijsu.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Wrafter P.F., et al. The 100 most influential manuscripts in colorectal cancer: a bibliometric analysis. Surgeon. 2016;14(6):327–336. doi: 10.1016/j.surge.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Cao J., et al. iLIVER; 2022. Identification of Publication Characteristics and Research Trends in the Management of Gallbladder Cancer. [Google Scholar]
- 14.Zhang Z., Wang Z., Huang Y. A bibliometric analysis of 8,276 publications during the past 25 Years on cholangiocarcinoma by machine learning. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.687904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valle J., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 16.Berr F., et al. Photodynamic therapy for advanced bile duct cancer: Evidence for improved palliation and extended survival. Hepatology. 2000;31(2):291–298. doi: 10.1002/hep.510310205. [DOI] [PubMed] [Google Scholar]
- 17.Nimura Y., et al. Combined portal-vein and liver resection for carcinoma of the biliary-tract. Br. J. Surg. 1991;78(6):727–731. doi: 10.1002/bjs.1800780629. [DOI] [PubMed] [Google Scholar]
- 18.Polydorou A.A., et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32(6):685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan A.S., et al. Assessment and optimization of liver volume before major hepatic resection: current guidelines and a narrative review. Int. J. Surg. 2018;52:74–81. doi: 10.1016/j.ijsu.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Hemming A.W., et al. Preoperative portal vein embolization for extended hepatectomy. Ann. Surg. 2003;237(5):686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chebaro A., et al. Liver venous deprivation or associating liver partition and portal vein ligation for staged hepatectomy?: a retrospective multicentric study. Ann. Surg. 2021;274(5):874–880. doi: 10.1097/SLA.0000000000005121. [DOI] [PubMed] [Google Scholar]
- 22.Nagino M., et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013;258(1):129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 23.Tang W., et al. Minimally invasive versus open radical resection surgery for hilar cholangiocarcinoma: comparable outcomes associated with advantages of minimal invasiveness. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi Sandri G.B., et al. The role of minimally invasive surgery in the treatment of cholangiocarcinoma. Eur. J. Surg. Oncol. 2017;43(9):1617–1621. doi: 10.1016/j.ejso.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Meyer C.G., Penn I., James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69(8):1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 26.Murad S.D., et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–U610. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X., et al. Promising role of liver transplantation in patients with combined hepatocellular-cholangiocarcinoma: a propensity score matching analysis. Ann. Transl. Med. 2022;10(8):434. doi: 10.21037/atm-21-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi S., et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krenzien F., et al. Treatment of intrahepatic cholangiocarcinoma-A multidisciplinary approach. Cancers. 2022;14(2) doi: 10.3390/cancers14020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maithel S.K., et al. 2022. NEO-GAP: A Phase II Single-Arm Prospective Feasibility Study of Neoadjuvant Gemcitabine/cisplatin/nab-Paclitaxel for Resectable High-Risk Intrahepatic Cholangiocarcinoma; p. 4097. 40(16_suppl) -4097. [Google Scholar]
- 31.Lamarca A., et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T., et al. Efficacy and safety of hepatic artery infusion chemotherapy with mFOLFOX in primary liver cancer patients with hyperbilirubinemia and ineffective drainage: a retrospective cohort study. Ann. Transl. Med. 2022;10(7):411. doi: 10.21037/atm-22-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Primrose J.N., et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 34.Hammad A.Y., et al. Is radiotherapy warranted following intrahepatic cholangiocarcinoma resection? The impact of surgical margins and lymph node status on survival. Ann. Surg Oncol. 2016;23(Suppl 5):912–920. doi: 10.1245/s10434-016-5560-1. [DOI] [PubMed] [Google Scholar]
- 35.Pollom E.L., et al. Does radiotherapy still have a role in unresected biliary tract cancer? Cancer Med. 2017;6(1):129–141. doi: 10.1002/cam4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goyal L., et al. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021;95 doi: 10.1016/j.ctrv.2021.102170. [DOI] [PubMed] [Google Scholar]
- 37.de Jong M.C., et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J. Clin. Oncol. 2011;29(23):3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 38.Yu Z., et al. Prognostic nomogram for predicting cancer-specific survival in patients with resected hilar cholangiocarcinoma: a large cohort study. J. Gastrointest. Oncol. 2022;13(2):833–846. doi: 10.21037/jgo-21-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Q., Lu X. Development and validation of an XGBoost model to predict 5-year survival in elderly patients with intrahepatic cholangiocarcinoma after surgery: a SEER-based study. J. Gastrointest. Oncol. 2022;13(6):3290–3299. doi: 10.21037/jgo-22-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikas I.P., et al. Evaluating pancreatic and biliary neoplasms with small biopsy-based next generation sequencing (NGS): doing more with less. Cancers. 2022;14(2) doi: 10.3390/cancers14020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter H., et al. Identification of circulating genomic and metabolic biomarkers in intrahepatic cholangiocarcinoma. Cancers. 2019;11(12) doi: 10.3390/cancers11121895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.