Abstract
Background
Dementia is a neurological syndrome characterized by severe cognitive impairment with functional impact on everyday life. It can be classified as young onset dementia (EOD) in case of symptom onset before 65, and late onset dementia (LOD). The purpose of this study is to assess the risk of dementia due to light pollution, and specifically outdoor artificial light at night (LAN).
Methods
Using a case-control design, we enrolled dementia patients newly-diagnosed in the province of Modena in the period 2017–2019 and a referent population from their caregivers. We geo-referenced the address of residence on the date of recruitment, provided it was stable for the previous five years. We assessed LAN exposure through 2015 nighttime luminance satellite images from the Visible Infrared Imaging Radiometer Suite (VIIRS). Using a logistic regression model adjusted for age, sex, and education, we calculated the risk of dementia associated with increasing LAN exposure, namely using <10 nW/cm2/sr as reference and considering ≥10-<40 nW/cm2/sr intermediate and ≥40 nW/cm2/sr high exposure, respectively We also implemented non-linear assessment using a spline regression model.
Results
We recruited 58 EOD cases, 34 LOD cases and 54 controls. Average LAN exposure levels overlapped for EOD cases and controls, while LOD cases showed higher levels. Compared with the lowest exposure, the risk of EOD associated with LAN was higher in the intermediate exposure (OR = 1.36, 95% CI 0.54–3.39), but not in the high exposure category (OR = 1.04, 95% CI 0.32–3.34). In contrast, the risk of LOD was positively associated with LAN exposure, with ORs of 2.58 (95% CI 0.26–25.97) and 3.50 (95% CI 0.32–38.87) in the intermediate and high exposure categories, respectively. The spline regression analysis showed substantial lack of association between LAN and EOD, while almost linear although highly imprecise association emerged for LOD.
Conclusions
Although the precision of the estimates was affected by the limited sample size and the study design did not allow us to exclude the presence of residual confounding, these results suggest a possible role of LAN in the etiology of dementia, particularly of its late-onset form.
Keywords: Case-control study, Early-onset dementia, Late-onset dementia, Light at night, Light pollution, Risk
1. Introduction
Dementia is a clinical syndrome characterized by cognitive decline that interferes with an individual's home, social, and work life. It is estimated that in 2010 35.6 million people lived with dementia worldwide, and it is expected that the number of prevalent cases would double every 20 years [1]; it has also been estimated that the number of people with dementia would increase from 57.4 million cases globally in 2019 to 152.8 million cases in 2050 [2]. Despite its incidence increases with aging, dementia is not a pathological condition confined to the elderly, but it can also affect younger subjects. The term young onset dementia (EOD) identifies forms of cognitive impairment with onset of symptoms before the age of 65 years [3]. The main differences with the late onset (LOD) forms are the clinical presentation frequently with atypical syndrome [4,5] and the impact on subjects' family and working routine [[6], [7], [8]]. Recent epidemiological data indicate that 3.9 million people between the ages of 30 and 64 are living with EOD in the world [9]. Also in Italy, an increasing trend in EOD incidence has been reported with approximately 13 new cases per 100,000/year [4] and a prevalence ranging from 55 to 74 cases/100,000, the most common clinical diagnoses being Alzheimer's dementia (AD) and frontotemporal dementia (FTD) [4,10].
Dementia etiology is debated, and may include genetic, socioeconomic, and environmental factors [[11], [12], [13], [14], [15], [16]]. With regard to modifiable factors, several nutritional, lifestyle and environmental factors have been investigated, such as dietary habits, exposure to chemicals like heavy metals and metalloids with neurotoxic activity [[17], [18], [19], [20], [21]], and traumatic events, especially of the head [17,22]. In particular, the role of environmental factors is of increasing interest. A positive association between outdoor air pollutants from motorized traffic has also been reported [18,23,24]. More recently, a mediating effect of green space and artificial lighting during night hours, also referred as light at night (LAN), have been suggested for such association [25]. Increased LAN exposure has been associated with several detrimental effects on human health, including sleep [26,27], some type of cancers [28], cardiovascular and metabolic disorders [29,30], and mental disorders [31,32]. In particular, one previous investigation suggested a higher risk of mild cognitive impairment in Chinese veterans exposed to excessive outdoor LAN [32]. Similarly, another study suggested a positive relation between LAN exposure and all-cause mortality in China, with the strongest effect on neuron system disease, although no specific assessment of dementia was reported [33].
In this study, we aim at investigating the role of LAN exposure as a possible risk factor for dementia, including both the EOD and the LOD forms, in an Italian population.
2. Materials and methods
2.1. Study population
Following approval by the Modena Ethics Committee (no. 186/2016), we performed a case-control study in the province of Modena, Northern Italy. We recruited newly-diagnosed EOD and LOD cases according the age of symptom onset (<65 or ≥65 years) from Modena Hospital - Neurology clinics as previously described [17,34]. Each participant was evaluated by a neurologist of the Memory Center of Modena Neurology Clinic to have any dementia subtype, including Alzheimer's dementia [35], dementia with Lewy bodies [36], and frontotemporal dementia [37,38]. Exclusion criteria included coexisting diagnoses of pervasive developmental disorders, major psychiatric disorders, or cognitive impairment in the context of other neurological disorders characterized by noncognitive symptoms, e.g., multiple sclerosis or cerebrovascular disease with severe motor disability [17]. The controls were recruited from the caregivers of these patients. All participants signed a written consent form. The recruitment period lasted from 2017 to May 2019.
2.2. Exposure assessment
Through a questionnaire administered by one neurologist of the team of Memory Center, we collected the residential history. In case of difficulties to answer the questions, the caregivers were consulted to help in the completion. Then, we geocoded the address of residence at the date of recruitment for cases and for controls through the use of Google Earth Pro and OpenStreetMap. If there was a change in residence in the previous 5 years, the first address was considered.
LAN exposure was assessed through satellite imagery from the Visible Infrared Imaging Radiometer Suite (VIIRS), an instruments onboard the Suomi National Polar-Orbiting Partnership (Suomi NPP) spacecraft, launched on October 28, 2011 [39]. Global Nighttime light maps were provided by the Earth Observation Group (EOG) of the Colorado School of Mine's Payne Institute. For this study we used annual nighttime radiance maps from the Annual VNL (VIIRS Nighttime Light) V2 dataset [40,41]. The latter consists in a time series of annual global VIIRS nighttime lights produced from monthly cloud-free average radiance grids spanning from 2012 to 2020 with a spatial resolution of 15 arc second (RS WGS 84 lat/lon). As an indicator of average exposure for nighttime imagery during the 2012–2020 period, we extracted the median values of radiance (in nanowatt per steradian per square centimeter, nW/cm2/sr) at subjects' residence in 2015, through a spatial overlay in a Geographical Information System environment.
2.3. Statistical analysis
We used a multivariate unconditional logistic regression model to calculate odds ratios (ORs) and 95% confidence intervals (95% CI) of EOD and LOD according to increased exposure to LAN. We included in the model the following adjustment variables: sex, age (in years) and level of education (in years of study). The OR was computed considering the lowest exposure as reference in different exposure categories: above the median value, in the intermediate and upper tertiles based on the distribution on the control population, according to fixed exposure categories (<10; ≥10 and < 40; ≥40 nW/cm2/sr), and finally for continuous 1-unit linear increase of LAN exposure. We also explored the nonlinear relation between LAN and dementia risk using a cubic spline regression model with three knots at the fixed cutpoints (10th, 50th and 90th percentiles), and using either the median or the null value as reference points. We finally carried out subgroup analysis according to the most frequent dementia diagnosis, namely Alzheimer's dementia and frontotemporal dementia. For data analysis, we used the ‘logit’, 'mkspline' and 'xblc' routines of the program Stata-17.0 (Stata Corp., College Station, TX, USA, 2021) for data analysis.
3. Results
We recruited 146 subjects including 92 dementia cases (58 with EOD and 34 with LOD) and 54 controls. The characteristics of study participants are shown in Table 1. Most participants were females (57.4%). Mean age at recruitment was 63.8, 65.6 and 80.8 years for controls, EOD and LOD, respectively. LOD cases showed a lower educational attainment compared to both EOD and particularly controls.
Table 1.
Sociodemographic characteristics of controls and early-onset (EOD) and late-onset (LOD) dementia cases included in the study.
| Characteristics | Controls N (%) |
EOD Cases N (%) |
LOD Cases N (%) |
|---|---|---|---|
| Total population | 54 (100) | 58 (100) | 34 (100) |
| Age at completion of the questionnaire | |||
| Mean (DS) | 63.8 (9.6) | 65.6 (5.2) | 80.8 (6.3) |
| <65 years | 28 (51.9) | 22 (37.9) | – |
| ≥65 years | 26 (48.2) | 36 (62.1) | 34 (100) |
| Age at diagnosis of disease | |||
| Mean (DS) | – | 59.3 (4.7) | 73.8 (5.4) |
| Sex | |||
| Men | 23 (42.6) | 25 (43.1) | 15 (44.1) |
| Women | 31 (57.4) | 33 (56.9) | 19 (55.9) |
| Educational attainment | |||
| Primary or less | 11 (20.4) | 16 (27.6) | 15 (44.1) |
| Middle school | 11 (20.4) | 20 (34.5) | 10 (29.4) |
| High school | 21 (38.9) | 19 (32.8) | 6 (17.7) |
| College or more | 11 (20.4) | 3 (5.2) | 3 (8.8) |
| Marital status | |||
| Married | 48 (88.9) | 48 (82.8) | 25 (73.5) |
| Unmarried | 3 (5.6) | 1 (1.7) | – |
| Separated/divorced | 2 (3.7) | 3 (5.2) | – |
| Widowed | 1 (1.8) | 6 (10.3) | 9 (26.5) |
| Smoking habit | |||
| Ever smokers | 30 (57.7) | 35 (62.5) | 15 (44.1) |
| Never smokers | 22 (42.3) | 21 (37.5) | 18 (52.9) |
The most frequent type of dementia was Alzheimer's dementia for both EOD (n = 32) and LOD cases (n = 25), followed by FTD (19 with EOD; 2 with LOD) (Table 2).
Table 2.
Clinical diagnosis of early-onset (EOD) and late-onset (LOD) dementia cases.
| Diagnosis | EOD N (%) |
LOD N (%) |
|---|---|---|
| Total cases | 58 (100) | 34 (100) |
| Alzheimer's dementia | 32 (55.2) | 25 (73.5) |
| Spectrum of frontotemporal dementias | 19 (32.8) | 2 (5.9) |
| Frontotemporal dementia | 17 (29.3) | 2 (5.9) |
| Progressive Supranuclear Palsy | 2 (3.4) | – |
| Vascular dementia | 5 (8.6) | 1 (2.9) |
| Cerebral Amyloid Angiopathy | 1 (1.7) | 1 (2.9) |
| Lewy's body dementia | 1 (1.7) | 3 (8.8) |
| Parkinson disease with dementia | – | 1 (2.9) |
| Normal-pressure hydrocephalus | – | 1 (2.9) |
Annual average LAN exposure in the study area is presented in Fig. 1, namely Fig. 1A reports the map of light at night in the Emilia-Romagna region and Fig. 1B in the municipality of Modena. Controls were exposed to an average of 25.3 nW/cm2/sr (standard deviation−SD: 16.8) with median 25.8 nW/cm2/sr (interquartile range-IQR: 7.0–38.1). The EOD cases showed similar values with an average of 25.2 nW/cm2/sr (SD: 15.6) and median 25.8 nW/cm2/sr (IQR: 9.7–38.1). On the contrary, LOD cases exhibited a higher exposure, with an average of 31.5 nW/cm2/sr (SD: 13.9) and median 35.6 nW/cm2/sr (IQR: 26.8–41.7).
Fig. 1.
Map of light at night in the Emilia-Romagna region (A) and the municipality of Modena (B) obtained using satellites from the JPSS constellation in 2015. The points OSSGEO and DIEF indicate respectively the meteorological station of the University of Modena and Reggio Emilia, the Geophysical Observatory of Modena and the Department of Engineering “Enzo Ferrari”. Pixel size is 500 m × 500 m.
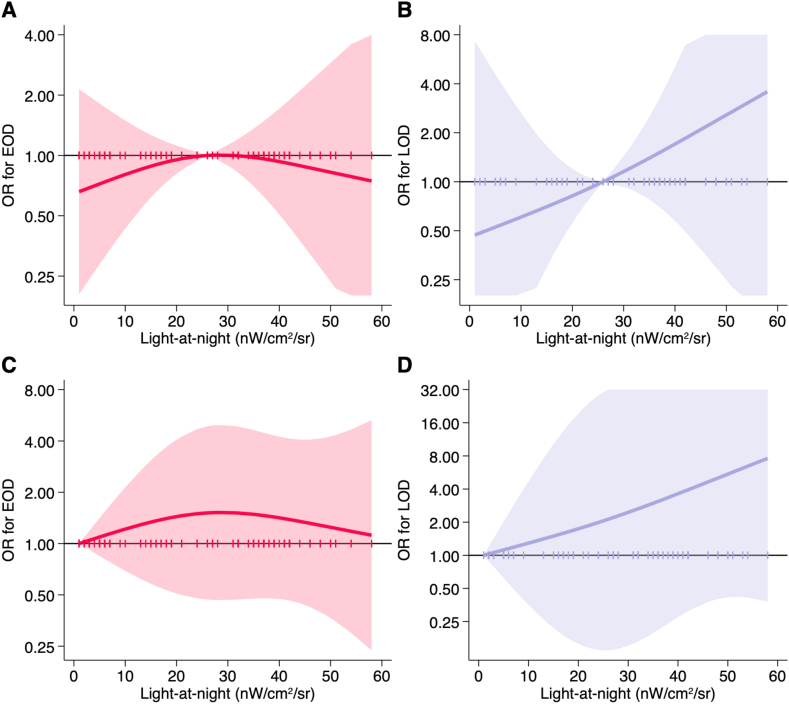
ORs of dementia according to LAN exposure are reported in (Table 3). For 1-unit continuous LAN increase, we found a small increased of both EOD and LOD, particularly for the latter. In the analysis using the median value (25.8 nW/cm2/sr) as cutpoint, we found a high though very imprecise excess risk for LOD in the highest exposure category (OR = 3.2, 95% CI 0.6–17.8) but not for EOD (OR = 1.1, 95% CI 0.5–2.4). Dementia risk by increasing categories of exposure, either tertiles or based on fixed cutpoints, showed an increased EOD risk particularly in the intermediate exposure category. Conversely, the risk of LOD smoothly increased with LAN exposure in both the intermediate and higher exposure categories, although the estimates were very imprecise. With regard to nonlinear risk analysis using the spline regressions, we found no change in EOD risk with increasing LAN exposure, while the increase in LOD risk was almost linear (Fig. 2).
Table 3.
Odds Ratio (OR) for early-onset dementia (EOD) and late-onset dementia (LOD) based on median (50th), tertiles, and fixed categories of nighttime luminance exposure (LAN) in nW/cm2/sr.
| LAN (nW/cm2/sr) | 50th | EOD |
LOD |
||||
|---|---|---|---|---|---|---|---|
| Cases/controls | OR | (95% CI) | Cases/controls | OR | (95% CI) | ||
| Linear trend (1-unit increase) | 1.01 | (0.98–1.03) | 1.04 | (0.98–1.09) | |||
| Linear trend (10-unit increase) | 1.05 | (0.82–1.35) | 1.41 | (0.84–2.37) | |||
| LAN-Median | |||||||
| Below the median | 6.7 | 28/26 | 1.00 | – | 8/26 | 1.00 | – |
| Above the median | 37.8 | 30/28 | 1.10 | (0.50–2.40) | 26/28 | 3.20 | (0.57–17.80) |
| LAN-Tertiles | |||||||
| 1st tertile | 5.2 | 19/18 | 1.00 | – | 6/18 | 1.00 | – |
| 2nd tertile | 25.1 | 21/18 | 1.25 | (0.49–3.20) | 11/18 | 1.32 | (0.14–12.10) |
| 3rd tertile | 42.1 | 18/18 | 1.10 | (0.42–2.90) | 17/18 | 3.31 | (0.43–25.15) |
| LAN-Fixed categories | |||||||
| <10 | 4.6 | 15/15 | 1.00 | – | 5/15 | 1.00 | – |
| ≥10; <40 | 26.8 | 32/27 | 1.36 | (0.54–3.39) | 18/27 | 2.58 | (0.26–25.97) |
| ≥40 | 47.1 | 11/12 | 1.04 | (0.32–3.34) | 11/12 | 3.50 | (0.32–38.87) |
Fig. 2.
Spline regression analysis of the risk of early-onset dementia (EOD) and late-onset dementia (LOD) by exposure to light at night (nW/cm2/sr) using as reference the median value of exposure (25.8 nW/cm2/sr, panels A and B for EOD and LOD, respectively) and the null value (0 nW/cm2/sr, panels C and D for EOD and LOD, respectively). OR: odds ratio. Solid line indicates the risk estimate and shaded area the 95% confidence interval. Vertical ticks on reference line indicates individual level of LAN exposure.
Stratified analysis by dementia diagnosis are presented in Table 4, Table 5. Considering continuous exposure, we found a positive association between LAN and both EO-AD and LO-AD, though higher for this latter form. Such positive relation was confirmed in the analysis using the median values as cutpoints. In the analyses assessing AD risk according to increasing exposure categories (based either on percentile or on fixed cutpoints), LAN exposure was positively associated with both EO-AD and LO-AD. Estimates for LO-AD the estimates were generally higher than for EO-AD, although they showed high statistical imprecision due to the low number of subjects. In nonlinear analysis, we observed a progressive increase in risk with increasing levels of LAN for both EO-AD and LO-AD, though somewhat steeper for the latter (Fig. 3).
Table 4.
Odds Ratio (OR) for early-onset Alzheimer's dementia (EOD) and late-onset Alzheimer's dementia (LOD) based on linear trend, median (50th), tertiles, and fixed categories of exposure to light at night (LAN) in nW/cm2/sr.
| LAN (nW/cm2/sr) | 50th | EOD |
LOD |
||||
|---|---|---|---|---|---|---|---|
| Cases/controls | OR | (95% CI) | Cases/Controls | OR | (95% CI) | ||
| Linear trend (1-unit increase) | 1.02 | (0.99–1.05) | 1.08 | (1.00–1.19) | |||
| Linear trend (10-unit increase) | 1.22 | (0.91–1.65) | 2.18 | (1.02–4.69) | |||
| LAN-Median | |||||||
| Below the median | 6.7 | 14/26 | 1.00 | – | 3/26 | 1.00 | – |
| Above the median | 37.8 | 18/28 | 1.30 | (0.52–3.29) | 22/28 | 5.87 | (0.59–58.14) |
| LAN-Tertiles | |||||||
| 1st tertile | 5.2 | 9/18 | 1.00 | – | 2/18 | 1.00 | – |
| 2nd tertile | 25.1 | 11/18 | 1.43 | (0.46–4.52) | 8/18 | 2.47 | (0.06–99.70) |
| 3rd tertile | 42.1 | 12/18 | 1.69 | (0.52–5.43) | 15/18 | 22.26 | (0.59–841.51) |
| LAN-Fixed categories | |||||||
| <10 | 4.6 | 7/15 | 1.00 | – | 2/15 | 1.00 | – |
| ≥10; <40 | 26.8 | 17/27 | 1.60 | (0.51–4.99) | 12/27 | 3.70 | (0.11–125.07) |
| ≥40 | 47.1 | 8/12 | 1.89 | (0.47–7.54) | 11/12 | 16.61 | (0.42-650.57) |
Table 5.
Odds Ratio (OR) for early-onset frontotemporal dementia (EOD) and late-onset frontotemporal dementia (LOD) based on linear trend, median (50th), tertiles, and fixed categories of exposure to light at night (LAN) in nW/cm2/sr.
| LAN (nW/cm2/sr) | 50th | EOD |
LOD |
||||
|---|---|---|---|---|---|---|---|
| Cases/controls | OR | (95% CI) | Cases/Controls | OR | (95% CI) | ||
| Linear trend (1-unit increase) | 1.00 | (0.96–1.03) | 1.03 | (0.92–1.15) | |||
| Linear trend (10-unit increase) | 0.98 | (0.68–1.40) | 1.34 | (0.45–3.98) | |||
| LAN-Median | |||||||
| Below the median | 6.7 | 9/26 | 1.00 | – | 0/26 | 1.00 | – |
| Above the median | 37.8 | 10/28 | 1.07 | (0.35–3.28) | 2/28 | – | |
| LAN-Tertiles | |||||||
| 1st tertile | 5.2 | 6/18 | 1.00 | – | 0/18 | – | |
| 2nd tertile | 25.1 | 7/18 | 1.20 | (0.32–4.52) | 2/18 | – | |
| 3rd tertile | 42.1 | 6/18 | 0.95 | (0.23–3.83) | 0/18 | – | |
| LAN-Fixed categories | |||||||
| <10 | 4.6 | 4/15 | 1.00 | – | 0/15 | – | |
| ≥10; <40 | 26.8 | 12/27 | 1.58 | (0.41–6.10) | 2/27 | – | |
| ≥40 | 47.1 | 3/12 | 0.77 | (0.13–4.69) | 0/12 | – | |
Fig. 3.
Spline regression analysis of the risk of early-onset Alzheimer's dementia (EOD) and late-onset Alzheimer's dementia (LOD) by exposure to luminance at night (light at night nW/cm2/sr) using as reference the median value of exposure (25.8 nW/cm2/sr, panels A and B for EOD and LOD, respectively) and the null value (0 nW/cm2/sr, panels C and D for EOD and LOD, respectively). OR: odds ratio. Solid line indicates the risk estimate and shaded area the 95% confidence interval. Vertical ticks on reference line indicates individual level of LAN exposure.
Considering LO-FTD, no results about dementia risk could be computed in categorical analysis due to low number of cases (n = 2), while the statistically unstable estimates yielded by the analysis based on continuous 1-unit LAN increase showed a positive association, substantially similar to that computed for the whole population of LOD cases (Table 5). For EO-FTD cases, we found substantial similar association with exposure to LAN than in the whole population, showing an increased risk the intermediate exposure category, not confirmed in the highest exposure category (Table 5). Due to the low sample size, it was not possible to perform a spline analysis for FTD.
4. Discussion
The results of this study suggest an association between exposure to LAN and dementia risk, especially for the late onset form of the disease and for Alzheimer's dementia.
The relation between outdoor light at night with human health has been investigated in previous studies, since artificial lighting is widespread and increasing all over the world, especially but not only in developed countries [42]. Light impacts on the suprachiasmatic nucleus, which is influenced by the alternation of light and dark. Light is the most powerful regulator of the circadian rhythm and the synthesis of melatonin in the pineal gland occurs in general during the night hours in the absence of light, not only during the hours of sleep. Therefore, prolonged hours of artificial light induce a general deficiency of melatonin. Both experimental studies on animal models and epidemiological studies on humans have shown an association between circadian rhythm dysregulation and nocturnal inhibition of melatonin synthesis [43,44].
Some detrimental effects of LAN on humans are now well known: the reduction of melatonin production in a manner dependent on wavelength and intensity of light, the alteration of the circadian rhythm, the worsening of sleep quality [25,45,46]. Exposure to LAN induces variations in melatonin secretion, that leads to insomnia and other sleep disturbances, thus contributing to the onset of chronic diseases [47]. LAN exposure may also alter immune function since melatonin showed immune-dampening effects, reducing pro-inflammatory cytokine levels in animal models of high- or medium-grade inflammation [44,46]. Studies conducted in mice have shown that nighttime light stimulates biological pathways causing depressive-like behavior [48] and neuroimmune activation [49].
Interestingly, melatonin showed also neuroprotective effects in animal studies through stimulation of neuroplasticity [50]. A study conducted in a mouse model (Swiss albino mice) evaluated a consecutive exposure for three weeks to a light intensity of 5 lux, and reported alteration of cognitive functions and behavior [51]. In the neuronal cells, an increase in oxidative stress was observed, with increased of lipid peroxidation and reduced activity of superoxide dismutase and catalase. Specifically in the hippocampus, a reduction in brain neutrophic factor levels (namely synapsin II and doublecortin) was observed [51]. Another study carried out in mice showed that dim blue light (dLAN-BL) up regulates plasma corticosterone level and activates hippocampal microglia, causing neuroinflammation and oxidative stress thus leading to memory impairment [52].
Some studies carried out on Drosophila melanogaster investigated the mechanism underlying the correlation between circadian rhythm and neurodegeneration. A correlation between circadian rhythm, cell death pathways and toxicity induced by tau protein accumulation with the remodeling of neurons in the occipital lobe has been observed [53]. A study investigating the effect of dim light exposure with 3 lux intensity during the night cycle demonstrated a circadian disruption with accumulation of hTau in the fly brain, negatively affecting their lifespan [45]. Similarly, the dysregulation of the circadian clock alters the function of heat shock proteins in Drosophyla fly, thus impacting the regulatory mechanisms of huntingtin (a protein involved in another neurodegenerative disease, Huntington disease) aggregation [54].
In an experimental study carried out on zebra finches, neuronal cell proliferation and neuronal recruitment were quantified after 3 weeks of exposure to 1.5 or 5 lux of LAN in one group and 5 lux in the other. The study showed more resilience in males in the first group, since the cellular proliferation was higher in females due to higher suppression of their melatonin levels compared to males. In the second group both sexes had an increase of cellular proliferation around the ventricular zone [55]. A study conducted on Indian house crows showed that dim light (6 lux) at night negatively influences hippocampal expression of genes associated with depressive emotions, thus leading to alteration of bird daily activity, with sleep reduction and depressive responses [56].
In humans, low-level of light pollution can affect circadian rhythm and sleep-wake rhythm in healthy individuals and especially in those affected by neurodegenerative diseases, with symptom worsening [57]. It has been reported that there are two main areas that are involved in response to light at night: alertness-related subcortical areas (such as hypothalamus, brainstem and thalamus) and limbic areas (amygdala and hippocampus). This impacts on attention, executive functions and memory. Light is a stimulant for alertness and cognition and affects cognitive performance through its synchronizing and phase-shifting effects on the circadian clock, depending on the wavelength of the light [58]. In this regard, a cross-over design study has been conducted on a group of 16 young participants who were asked to perform tasks while continuously exposed to the same test light. Light of a wavelength between 461 nm and 589 nm increased executive responses in prefrontal areas and in the pulvinar. This study suggests a possible role of melanopsin in the cognitive function, due to the possible presence of a “photic memory” [59]. Another study conducted on university students in China and Japan has measured the amount of light exposure and its relation with bedtime and sleep onset. It was found that the blue-enriched white lighting has a greater impact on arousal level and melatonin suppression [60].
Growing evidence from the epidemiological studies has suggested that risk of dementia is associated with sleep disturbances, including insomnia, sleep-disordered breathing, disrupted circadian rhythms, and sleep-related movement disorders [61]. It has been shown that sleep problems in Alzheimer's disease are associated with cognitive impairment and behavioral problems [62]. However, other studies have also shown that dim light stimulation can benefit people already suffering from Alzheimer's disease. Bright light treatment should stabilize wake-sleep rhythms [63,64]. Another 9-week experimental study comparing bright light at 2500 lux in the treatment group and 114–307 lux in the control group showed an improvement in cognitive scores in the high-exposure group [65].
The only other human study investigating the relation between LAN exposure and cognition was carried out in Chinese veterans reported a positive association between excessive outdoor LAN exposure and risk of mild cognitive impairment [32].
Among the limitations of our study, there is the limited sample size, which severely affected the precision of the estimates particularly in subgroup analyses, and in addition precluded some stratified analyses. In particular, we had only 2 LOD cases diagnosed with FTD, and therefore it was not possible to assess such relation. Another limitation relates to the observational design of the study, not allowing to rule out residual, uncontrolled confounding. In particular, we could not take into account levels of air pollution, a likely risk factor for dementia and AD in particular, which tends to be associated with light pollution [25]. Similarly, we could not take into account other environmental factors, like the residential availability of green space and noise [18,[66], [67], [68]]. We took into account light intensity, but we were unable to consider also its wavelength [69] although we implemented the LAN assessment with the highest spatial resolution available through the use of VIIRS data [70]. In addition, the methodology we used for control selection could have led to some overmatching, being composed by the caregivers of dementia cases. However, this should have decreased the effect, thus further supporting the associations between LAN and dementia risk that we identified.
With reference to the strengths of this study, the use of a Geographic Information System allowed performing an objective assessment of exposure to LAN, not based on a self-assessment of the subjects neither allowing for selection bias due to lack of participation of eligible subjects. Another strength is the ascertainment of the historical residence of participants without limiting the analysis to the current residence at diagnosis, and based on a stable residence for a few years. Finally, the comparison between LOD and EOD revealed a possible difference in risk for the two forms of dementia in relation to LAN as well as when AD and FTD are considered, although in this latter group the very limited number of cases suggests caution in the interpretation of the results.
5. Conclusions
This investigation suggests a possible contributing role of artificial light at night in the etiology of dementia, particularly for late-onset dementia and more specifically for AD. However, given the methodological limitations of the study and the small sample size, the results of this study should be evaluated with caution.
Ethics statement
This study complies with the Declaration of Helsinki and was approved by the Modena Ethics Committee (approval no. 186/2016).
Funding statement
This study was supported by the grant “Dipartimenti di Eccellenza 2018–2022” to the UNIMORE Department of Biomedical, Metabolic and Neural Sciences from the Italian Ministry of Education, University and Research. This study was also supported by the grants “UNIMORE FAR 2022 and 2023” from the University of Modena and Reggio Emilia and from Fondazione di Modena.
Author contribution statement
Elena Mazzoleni: Analyzed and interpreted the data; Wrote the paper.
Marco Vinceti: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sofia Costanzini: Performed the experiments; Analyzed and interpreted the data.
Caterina Garuti: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Giorgia Adani, Giulia Vinceti, Giovanna Zamboni, Manuela Tondelli, Chiara Galli, Simone Salemme: Contributed reagents, materials, analysis tools or data.
Sergio Teggi: Performed the experiments.
Annalisa Chiari: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Tommaso Filippini: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
The data presented in this study are available on reasonable request from the corresponding author. The data about subjects’ residence are not publicly available due to privacy issue while database used for exposure assessment are publicly available and reported in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to all the patients and their families for participating in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17837.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Prince M., Ali G.C., Guerchet M., et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer's Res. Ther. 2016;8:23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols E., Steinmetz J.D., Vollset S.E., et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieira R.T., Caixeta L., Machado S., et al. Epidemiology of early-onset dementia: a review of the literature. Clin. Pract. Epidemiol. Ment. Health. 2013;9:88–95. doi: 10.2174/1745017901309010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiari A., Vinceti G., Adani G., et al. Epidemiology of early onset dementia and its clinical presentations in the province of Modena, Italy. Alzheimers Dement. 2021;17:81–88. doi: 10.1002/alz.12177. [DOI] [PubMed] [Google Scholar]
- 5.Mendez M.F. Early-onset Alzheimer disease and its variants. Continuum. 2019;25:34–51. doi: 10.1212/CON.0000000000000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikes P., Hall M. The impact of parental young onset dementia on children and young people's educational careers. Br. Educ. Res. J. 2018;44:593–607. doi: 10.1002/berj.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakata N., Okumura Y. Job loss after diagnosis of early-onset dementia: a matched cohort study. J Alzheimers Dis. 2017;60:1231–1235. doi: 10.3233/JAD-170478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiari A., Pistoresi B., Galli C., et al. Determinants of caregiver burden in early-onset dementia. Dement. Geriatr. Cogn. Dis. Extra. 2021;11:189–197. doi: 10.1159/000516585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendriks S., Peetoom K., Bakker C., et al. Global prevalence of young-onset dementia: a systematic review and meta-analysis. JAMA Neurol. 2021;78:1080–1090. doi: 10.1001/jamaneurol.2021.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borroni B., Alberici A., Grassi M., et al. Prevalence and demographic features of early-onset neurodegenerative dementia in Brescia County, Italy. Alzheimer Dis. Assoc. Disord. 2011;25:341–344. doi: 10.1097/WAD.0b013e3182147f80. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez L.J., Veronese N., Vernuccio L., et al. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients. 2021;13:4080. doi: 10.3390/nu13114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanoiselee H.M., Nicolas G., Wallon D., et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedel B.C., Thompson P.M., Brinton R.D. Age, APOE and sex: triad of risk of Alzheimer's disease. J. Steroid Biochem. Mol. Biol. 2016;160:134–147. doi: 10.1016/j.jsbmb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosi M., Malavolti M., Garuti C., et al. Environmental and lifestyle risk factors for early-onset dementia: a systematic review. Acta Biomed. 2022;93 doi: 10.23750/abm.v93i6.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannone M.E., Filippini T., Whelton P.K., et al. Atrial fibrillation and the risk of early-onset dementia: a systematic review and meta-analysis. J. Am. Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adani G., Filippini T., Garuti C., et al. Environmental risk factors for early-onset Alzheimer's dementia and frontotemporal dementia: a case-control study in Northern Italy. Int. J. Environ. Res. Publ. Health. 2020;17:7941. doi: 10.3390/ijerph17217941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killin L.O., Starr J.M., Shiue I.J., et al. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016;16:175. doi: 10.1186/s12877-016-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinceti M., Chiari A., Eichmuller M., et al. A selenium species in cerebrospinal fluid predicts conversion to Alzheimer's dementia in persons with mild cognitive impairment. Alzheimer's Res. Ther. 2017;9:100. doi: 10.1186/s13195-017-0323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippini T., Adani G., Malavolti M., et al. Dietary habits and risk of early-onset dementia in an Italian case-control study. Nutrients. 2020;12:12. doi: 10.3390/nu12123682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbano T., Vinceti M., Mandrioli J., et al. Selenoprotein P concentrations in the cerebrospinal fluid and serum of individuals affected by amyotrophic lateral sclerosis, mild cognitive impairment and Alzheimer's dementia. Int. J. Mol. Sci. 2022;23:9865. doi: 10.3390/ijms23179865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider A.L.C., Selvin E., Latour L., et al. Head injury and 25-year risk of dementia. Alzheimers Dement. 2021;17:1432–1441. doi: 10.1002/alz.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power M.C., Adar S.D., Yanosky J.D., et al. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 2016;56:235–253. doi: 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbano T., Chiari A., Malagoli C., et al. Particulate matter exposure from motorized traffic and risk of conversion from mild cognitive impairment to dementia: an Italian prospective cohort study. Environ. Res. 2023;222 doi: 10.1016/j.envres.2023.115425. [DOI] [PubMed] [Google Scholar]
- 25.Stanhope J., Liddicoat C., Weinstein P. Outdoor artificial light at night: a forgotten factor in green space and health research. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111012. [DOI] [PubMed] [Google Scholar]
- 26.Lunn R.M., Blask D.E., Coogan A.N., et al. Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program's workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017;607–608:1073–1084. doi: 10.1016/j.scitotenv.2017.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y.X., Zhang J.H., Tao F.B., et al. Association between exposure to light at night (LAN) and sleep problems: a systematic review and meta-analysis of observational studies. Sci. Total Environ. 2023;857 doi: 10.1016/j.scitotenv.2022.159303. [DOI] [PubMed] [Google Scholar]
- 28.Urbano T., Vinceti M., Wise L.A., et al. Light at night and risk of breast cancer: a systematic review and dose-response meta-analysis. Int. J. Health Geogr. 2021;20:44. doi: 10.1186/s12942-021-00297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason I.C., Grimaldi D., Reid K.J., et al. Light exposure during sleep impairs cardiometabolic function. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2113290119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai K.Y., Sarkar C., Ni M.Y., et al. Exposure to light at night (LAN) and risk of obesity: a systematic review and meta-analysis of observational studies. Environ. Res. 2020;187 doi: 10.1016/j.envres.2020.109637. [DOI] [PubMed] [Google Scholar]
- 31.Tancredi S., Urbano T., Vinceti M., et al. Artificial light at night and risk of mental disorders: a systematic review. Sci. Total Environ. 2022;833 doi: 10.1016/j.scitotenv.2022.155185. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Tan J., Liu Y., et al. Long-term exposure to outdoor light at night and mild cognitive impairment: a nationwide study in Chinese veterans. Sci. Total Environ. 2022;847 doi: 10.1016/j.scitotenv.2022.157441. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y., Yin P., Wang J., et al. Light at night and cause-specific mortality risk in Mainland China: a nationwide observational study. BMC Med. 2023;21:95. doi: 10.1186/s12916-023-02822-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinceti M., Michalke B., Malagoli C., et al. Selenium and selenium species in the etiology of Alzheimer's dementia: the potential for bias of the case-control study design. J. Trace Elem. Med. Biol. 2019;53:154–162. doi: 10.1016/j.jtemb.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Dubois B., Feldman H.H., Jacova C., et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 36.McKeith I.G., Dickson D.W., Lowe J., et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 37.Gorno-Tempini M.L., Hillis A.E., Weintraub S., et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rascovsky K., Hodges J.R., Knopman D., et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elvidge C.D., Zhizhin M., Ghosh T., et al. Annual time series of global VIIRS nighttime lights derived from monthly averages: 2012 to 2019. Rem. Sens. 2021;13:922. doi: 10.3390/rs13050922. [DOI] [Google Scholar]
- 40.Elvidge C.D., Baugh K., Zhizhin M., et al. VIIRS night-time lights. Int. J. Rem. Sens. 2017;38:5860–5879. doi: 10.1080/01431161.2017.1342050. [DOI] [Google Scholar]
- 41.Elvidge C., Baugh K., Zhizhin M., et al. Why VIIRS data are superior to DMSP for mapping nighttime lights. Proceedings of the Asia-Pacific Advanced Network. 2013;35:62–69. doi: 10.7125/APAN.35.7. [DOI] [Google Scholar]
- 42.Falchi F., Cinzano P., Duriscoe D., et al. The new world atlas of artificial night sky brightness. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Touitou Y., Reinberg A., Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94–106. doi: 10.1016/j.lfs.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Cho Y., Ryu S.H., Lee B.R., et al. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015;32:1294–1310. doi: 10.3109/07420528.2015.1073158. [DOI] [PubMed] [Google Scholar]
- 45.Kim M., Subramanian M., Cho Y.H., et al. Short-term exposure to dim light at night disrupts rhythmic behaviors and causes neurodegeneration in fly models of tauopathy and Alzheimer's disease. Biochem. Biophys. Res. Commun. 2018;495:1722–1729. doi: 10.1016/j.bbrc.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Won E., Na K.S., Kim Y.K. Associations between melatonin, neuroinflammation, and brain alterations in depression. Int. J. Mol. Sci. 2021;23:305. doi: 10.3390/ijms23010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rumanova V.S., Okuliarova M., Zeman M. Differential effects of constant light and dim light at night on the circadian control of metabolism and behavior. Int. J. Mol. Sci. 2020;21:5478. doi: 10.3390/ijms21155478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An K., Zhao H., Miao Y., et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat. Neurosci. 2020;23:869–880. doi: 10.1038/s41593-020-0640-8. [DOI] [PubMed] [Google Scholar]
- 49.Chen R., Weitzner A.S., McKennon L.A., et al. Light at night during development in mice has modest effects on adulthood behavior and neuroimmune activation. Behav. Brain Res. 2021;405 doi: 10.1016/j.bbr.2021.113171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiter R.J., Cabrera J., Sainz R.M., et al. Melatonin as a pharmacological agent against neuronal loss in experimental models of Huntington's disease, Alzheimer's disease and parkinsonism. Ann. N. Y. Acad. Sci. 1999;890:471–485. doi: 10.1111/j.1749-6632.1999.tb08028.x. [DOI] [PubMed] [Google Scholar]
- 51.Namgyal D., Chandan K., Sultan A., et al. Dim light at night induced neurodegeneration and ameliorative effect of curcumin. Cells. 2020;9:2093. doi: 10.3390/cells9092093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q., Wang Z., Cao J., et al. Dim blue light at night induces spatial memory impairment in mice by hippocampal neuroinflammation and oxidative stress. Antioxidants. 2022;11:1218. doi: 10.3390/antiox11071218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Means J.C., Venkatesan A., Gerdes B., et al. Drosophila spaghetti and doubletime link the circadian clock and light to caspases, apoptosis and tauopathy. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu F., Kula-Eversole E., Iwanaszko M., et al. Circadian clocks function in concert with heat shock organizing protein to modulate mutant huntingtin aggregation and toxicity. Cell Rep. 2019;27:59–70. doi: 10.1016/j.celrep.2019.03.015. e54. 10.1016/j.celrep.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moaraf S., Heiblum R., Okuliarova M., et al. Evidence that artificial light at night induces structure-specific changes in brain plasticity in a diurnal bird. Biomolecules. 2021;11:1069. doi: 10.3390/biom11081069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taufique S.K.T., Prabhat A., Kumar V. Illuminated night alters hippocampal gene expressions and induces depressive-like responses in diurnal corvids. Eur. J. Neurosci. 2018;48:3005–3018. doi: 10.1111/ejn.14157. [DOI] [PubMed] [Google Scholar]
- 57.Helbich M., Browning M., Huss A. Outdoor light at night, air pollution and depressive symptoms: a cross-sectional study in The Netherlands. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140914. [DOI] [PubMed] [Google Scholar]
- 58.Vandewalle G., Maquet P., Dijk D.-J. Light as a modulator of cognitive brain function. Trends Cognit. Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Chellappa S.L., Ly J.Q.M., Meyer C., et al. Photic memory for executive brain responses. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6087–6091. doi: 10.1073/pnas.1320005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higuchi S., Lin Y., Qiu J., et al. Is the use of high correlated color temperature light at night related to delay of sleep timing in university students? A cross-country study in Japan and China. J. Physiol. Anthropol. 2021;40:7. doi: 10.1186/s40101-021-00257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sexton C.E., Sykara K., Karageorgiou E., et al. Connections between insomnia and cognitive aging. Neurosci. Bull. 2020;36:77–84. doi: 10.1007/s12264-019-00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brenowitz W.D., Xiang Y., McEvoy C.T., et al. Current Alzheimer disease research highlights: evidence for novel risk factors. Chin Med J (Engl) 2021;134:2150–2159. doi: 10.1097/CM9.0000000000001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dowling G.A., Mastick J., Hubbard E.M., et al. Effect of timed bright light treatment for rest-activity disruption in institutionalized patients with Alzheimer's disease. Int. J. Geriatr. Psychiatr. 2005;20:738–743. doi: 10.1002/gps.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S.J., Lee S.H., Suh I.B., et al. Positive effect of timed blue-enriched white light on sleep and cognition in patients with mild and moderate Alzheimer's disease. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-89521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C.R., Liou Y.M., Jou J.H. Pilot study of the effects of bright ambient therapy on dementia symptoms and cognitive function. Front. Psychol. 2021;12 doi: 10.3389/fpsyg.2021.782160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Besser L. Outdoor green space exposure and brain health measures related to Alzheimer's disease: a rapid review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-043456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zagnoli F., Filippini T., Jimenez M.P., et al. Is greenness associated with dementia? A systematic review and dose-response meta-analysis. Curr Environ Health Rep. 2022;9:574–590. doi: 10.1007/s40572-022-00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng L., Zhang Y., Zhang S., et al. Chronic noise exposure and risk of dementia: a systematic review and dose-response meta-analysis. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.832881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bozejko M., Tarski I., Malodobra-Mazur M. Outdoor artificial light at night and human health: a review of epidemiological studies. Environ. Res. 2023;218 doi: 10.1016/j.envres.2022.115049. [DOI] [PubMed] [Google Scholar]
- 70.Gibson J., Olivia S., Boe-Gibson G. Night lights in economics: sources and uses. J. Econ. Surv. 2020;34:955–980. doi: 10.1111/joes.12387. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data about subjects’ residence are not publicly available due to privacy issue while database used for exposure assessment are publicly available and reported in the article.