Abstract
Aims
A key treatment for patients with varying stages of heart failure with preserved ejection fraction (HFpEF) is exercise. Yet, despite a Class 1A recommendation, only one-third of patients exercise sufficiently. A huge treatment gap exists between guidelines and clinical practice. PRIORITY aims to establish the feasibility, clinical effectiveness and cost-effectiveness of a hybrid centre and home-based personalized exercise and physical activity intervention for patients along the HFpEF continuum.
Methods
An assessor-blinded, multicenter randomized controlled trial will be conducted among 312 patients along the HFpEF continuum. Participants will be randomized (1:1) to the PRIORITY intervention or a comparator group receiving only a written exercise prescription. Participants in the PRIORITY group will receive 18 supervised centre-based exercise sessions during one year, supplemented with a remotely guided home-based physical activity program. Outcomes will be assessed at baseline, 4 months, one and two years. The primary outcome is the peak oxygen uptake (pVO2) at 1-year. Secondary outcomes include physical activity, other physical fitness parameters, cardiovascular health, echocardiographic parameters, health-related quality of life and costs at 1-year FU. Machine learning algorithms will analyse big data on physical activity collected during the 1-year intervention to develop models that can predict physical activity uptake and adherence as well as changes in fitness and health. A cost-utility analysis will be performed to evaluate the cost-effectiveness of the PRIORITY intervention compared to the control condition.
Discussion
We anticipate that participants in the supervised home-based exercise intervention group will have a greater increase in pVO2 compared to those receiving a written exercise prescription.
Trial registration number
This trial is registered at ClinicalTrials.gov (NCT04745013) and is currently in the recruitment stage.
Keywords: exercise, personalized, heart failure, prevention, obesity, diabetes, cost-effectiveness (economics), randomized controlled trial
Background
Heart failure (HF) is a rapidly growing health problem with an estimated prevalence of 64.3 million people worldwide, which poses a major burden on public health and healthcare (1) Approximately half of the HF population has a preserved ejection fraction (HFpEF, left ventricular ejection fraction ≥50% (2). This syndrome is promoted by cardiovascular (CV) risk factors (stage A) such as obesity, exercise deficiency, hypertension and diabetes (2, 3). With increasing age these risk factors frequently result into structural and functional heart alterations without (stage B) or with (stage C) HF signs or symptoms such as exertional breathlessness, exercise intolerance and muscle fatigue (3, 4). With the extending life expectancy and increasing prevalence of CV risk factors, the prevalence of HFpEF as well as its burden on societal health are expected to increase over the next decades (5).
Thus, early and long-term preventive strategies are urgently needed to improve health-related quality of life and prognosis of patients along the continuum of HFpEF. Physical activity and exercise are recognized as effective interventions to prevent premature CV mortality and CV disease progression (6, 7). Therefore, exercise training gained a Class IA recommendation in cardiovascular disease (CVD) management and prevention, not only in patients but in all adults, especially in those with CV risk factors (8–10). How to provide and make it accessible to all? Given the rapidly expanding group of patients at risk for developing symptomatic HF in modern society, centre-based exercise programs are unlikely to gain acceptance as a cost-effective means of preventing progression towards overt HFpEF. Moreover, in current daily practice, participation and compliance rates are notoriously poor (11–13). The incorporation of exercise in an early comprehensive long-term care plan remains largely neglected and severely underused as confirmed by the European EUROASPIRE surveys (12). Also, the beneficial effect of a centre-based exercise intervention is often attenuated when the exercise intervention stops and the patient relapses into a sedentary lifestyle. As such, there is a critical need to facilitate the accessibility and affordability of structured, personalized exercise interventions in the home environment in order to increase uptake, effectiveness, and long-term adherence to exercise training in CV disease.
In this regard, home-based exercise may also be more promising from an health-economic point of view. To our knowledge, no studies yet examined the clinical and cost-effectiveness of remotely guided home-based exercise therapy in the prevention of overt HFpEF.
Aims and objectives
The PRIORITY trial will evaluate the feasibility, clinical and cost-effectiveness of a hybrid (centre and home-based) personalized exercise and physical activity intervention to prevent the deleterious effects of sedentary ageing on the heart and forestall the development and progression towards overt HFpEF.
The primary objective of the trial
The primary objective of the PRIORITY trial is to compare the effects of a hybrid personalized exercise intervention against an exercise prescription only in improving the peak exercise capacity (pVO2) of patients along the continuum of HFpEF. We hypothesize that the effect on pVO2 will be significantly larger in the experimental group (PRIORITY group) compared to the comparator group (written exercise prescription only) at one year.
Secondary objectives of the trial
-
•
To compare daily step count of patients at one year (key secondary outcome) and two years of follow-up (FU)
-
•
To compare weekly minutes of moderate to vigorous physical activity at one year (key secondary outcome) and two years of FU
-
•
To assess the safety of the PRIORITY intervention
-
•
To compare pVO2 at 4 months and two years of FU
-
•
To compare health-related fitness components (body composition, submaximal exercise capacity, muscle strength and muscle endurance) at 4 months, 1-year and 2 years of FU
-
•
To compare traditional CV risk factors at 1 year and 2-year of FU
-
•
To compare changes in echocardiographic parameters of left ventricular systolic and diastolic function at rest and during exercise at one year of FU
-
•
To compare changes in neurohumoral activation (NT-proBNP) at one year of FU
-
•
To compare psychosocial well-being and health-related quality of life at 1-year and 2-year of FU
-
•
To assess the implementation potential of the PRIORITY intervention in the Flemish healthcare setting
-
•
To evaluate the cost-effectiveness of the PRIORITY intervention compared to the control condition
-
•
To facilitate the creation of more personalized interventions and better-tailored exercise prescriptions to maximize their therapeutic effect by developing machine learning models which predict uptake of physical activity behaviour and changes in physical activity and cardiorespiratory fitness.
Methods
The PRIORITY trial protocol is written following recommendations from the Standard Protocol Items: recommendations for interventional trials (SPIRIT) checklist (14). This trial has been prospectively registered at ClinicalTrials.gov: (NCT04745013) on the 2nd of April 2021. Ethical approval has been obtained from the Ethics Committee of UZ/KU Leuven; Ethics Committee of UZA; Ethics Committee of Jessa Hospital and Ethics Committee of UHasselt.
Trial design
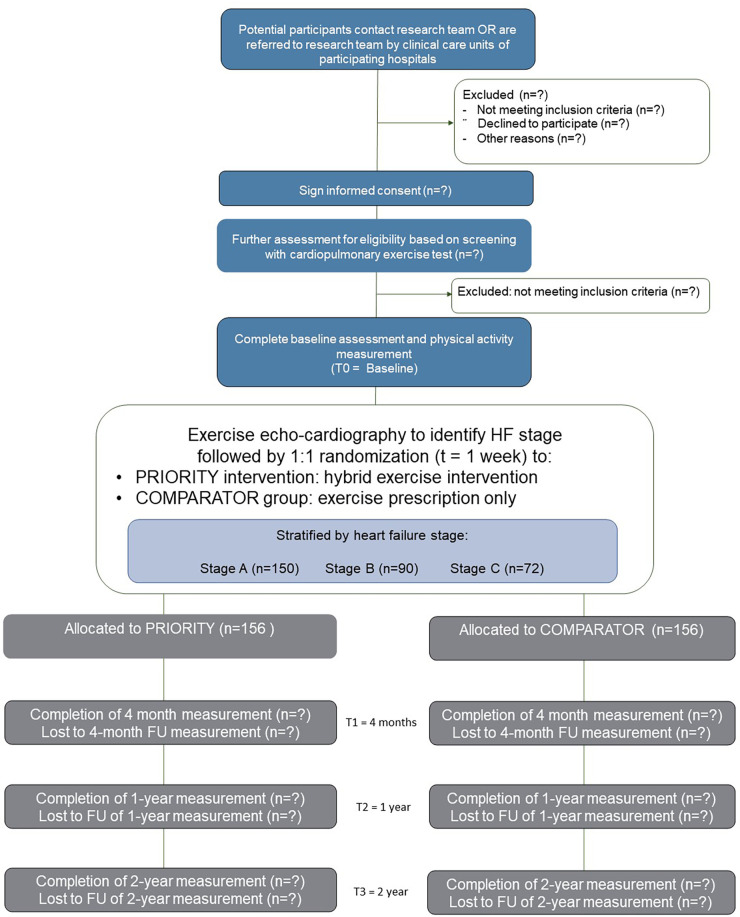
The PRIORITY trial is designed as a 2-year prospective randomized, controlled, assessor-blinded multicenter comparative trial with two parallel groups. The research approach has similarities to a hybrid type I effectiveness-implementation research design (15). The primary focus is to evaluate the effectiveness of a hybrid exercise and physical activity intervention, whilst concurrently gathering information on its potential for implementation in a real-world setting. Researchers performing the outcome analyses will be blinded to group allocation. Figure 1 shows the trial flow.
Figure 1.
Trial flow.
Trial setting
The PRIORITY trial will be conducted at three sites in Belgium: University Hospital Leuven—University of Leuven, Antwerp University Hospital, and Jessa Hospital Hasselt—Hasselt University. Recruitment will be performed via advertisements, flyers and social media and the different clinical units (e.g., hypertension clinic, obesity clinic and HF clinic) of the hospitals. In addition, eligible participants of the FLEMENGHO cohort (https://flemengho.eu/en/) visiting the University Hospital Leuven will be invited for participation. The University of Leuven will be the coordinating centre for the trial. Daily management of the trial will be performed by a local principal investigator (VC, DH, EVC) at each participating site.
Eligibility criteria and recruitment
The trial population will comprise 312 men and women (aged ≥30 years) along the continuum of HFpEF who are on optimal medical treatment and stable regarding symptoms and pharmacotherapy for at least 4 weeks before enrolment in the trial. All patients should have internet access at home. To represent the distribution of HFpEF stage A–C in the population, this will include 150 patients with HFpEF stage A, 90 patients with HFpEF stage B and 72 patients with HFpEF stage C. Participants will be recruited during a 2-year period which started in September 2021. Local investigators at each participating site will assess patient eligibility. A detailed description of the inclusion and exclusion criteria is shown in Table 1. Potentially eligible patients will then be contacted by a member of the research team and provided with a full oral explanation of the design and purpose of the trial, responsibilities of the participants, reasonably foreseeable inconveniences, and confidentiality of the information collected.
Table 1.
Inclusion and exclusion criteria for the PRIORITY trial.
| HFpEF stage | Inclusion criteria | Exclusion criteria |
|---|---|---|
| A “at risk” | History of treated or untreated hypertension (blood pressure 130/90–159/99 mmHg) AND/OR Prediabetes with either:
|
|
| B “pre-HFpEF” | CV risk factors as mentioned under stage A AND Subclinical signs of left ventricular diastolic dysfunction and/or raised left ventricular filling pressures (see Table 2.) | |
| C “symptomatic HF” | Criteria as mentioned under stage B with either:
|
HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; BMI, body mass index; CV, cardiovascular; HFA-PEFF, heart failure association—pretest assessment, echocardiographic and natriuretic peptide score, functional testing in case of uncertainty, final etiology.
Randomization and concealed allocation
Upon written informed consent and following the baseline measurements including a fasting blood draw, cardiopulmonary exercise test (CPET) and a rest and exercise echocardiography, patients will be randomized to either the PRIORITY group or the comparator group with a 1:1 allocation ratio stratified by HFpEF stage, as shown in Table 1. Asymptomatic patients with HF risk factors such as hypertension, (pre)diabetes, and obesity but free from echocardiographic abnormalities will be classified as stage A. Early HF phenotypes (stage B) will be defined as asymptomatic patients with HF risk factors and echocardiographic evidence of cardiac structural and functional abnormalities, consistent with the presence of left ventricular diastolic dysfunction and/or raised left ventricular filling pressures as defined by 2 out of 4 ESC criteria (Table 2) (16). Patients with symptomatic HF (stage C) will be characterized as symptomatic patients with an HFA-PEFF score of ≥5 (4). For patients with an intermediate HFA-PEFF score after resting echocardiography, the results of the diastolic stress test will be included in the score [i.e., average E/e′ ≥15 adds 2 points and an average E/e′ ratio ≥15 with a peak tricuspid regurgitation (TR) velocity >3.4 m/s adds 3 points to the previous score after the resting echocardiography]. Randomization schedules are generated using a computerized random number generator. Randomization is being performed by an independent designated member of the coordinating centre (KU Leuven) in response to a request from a local investigator, thus assuring concealed allocation and minimizing selection bias.
Table 2.
Objective evidence of cardiac structural or functional abnormalities consistent with the presence of left ventricular diastolic dysfunction/increased left ventricular filling pressures.
| Parameter | Threshold |
|---|---|
| LV mass index (LVMI) | ≥95 g/m2 (♀), ≥115 g/m2 (♂) |
| or Relative wall thickness (RWT) | >0.42 |
| LA volume index (LAVI) | >34 ml/m2 (SR), >40 ml/m2 (SR) |
| Average (septal and lateral) E/e′ ratio at rest | >9 |
| Estimated PA systolic pressure | >35 mmHg |
| TR velocity at rest | >2.8 m/s |
Sample size calculation
The power calculation is based on a constrained longitudinal data analysis (cLDA) model (17, 18) and calculated using an approach presented by Stroup (19). To have at least 90% power to detect a difference of 2.7 ml/kg/min in pVO2 after 12 months, 90 subjects per group (180 in total) are required based on a two-sided test and setting alpha equal to 0.05. The effect size of 2.7 is a weighted average of the expected effects in the three stages i.e., 0.20 × 1 ml/kg/min (Stage C) (20, 21) +0.30 × 2.5 ml/kg/min (Stage B) +0.50 × 3.5 ml/kg/min (Stage A) (22, 23). A common standard deviation of 5, a correlation between baseline and 12 months equal to 0.50 and a drop-out rate at 12 months as high as 40% were assumed (24). However, the sample size will be increased to 312 patients in total (156 per group) such that for two key secondary outcomes standardized effect sizes as small as 0.45 and 0.40 can be detected with more than 90% and 80% power, respectively (using a cLDA model assuming—as for the primary outcome—a baseline-year correlation of 0.50% and 40% dropout at 12 months). For example, for the key secondary outcome (moderate to vigorous physical activity, MVPA) a standardized mean difference equal to 0.42 corresponds to a difference of 1,235 steps (25). Note that for the power calculation of the key-secondary outcomes an alpha-level of 0.05/2 = 0.025 was used.
Trial intervention
Experimental group: PRIORITY intervention
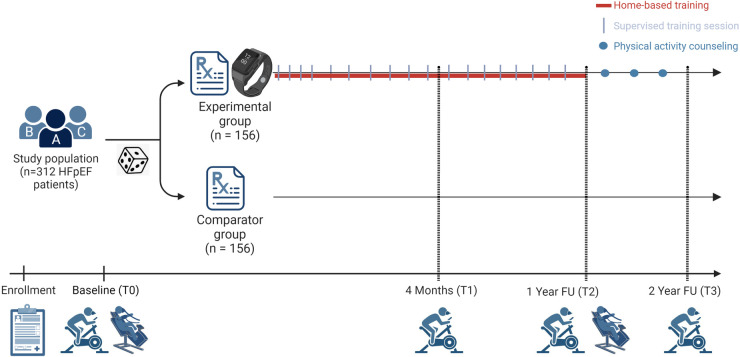
Patients in the PRIORITY group will participate in a 1-year hybrid exercise and physical activity intervention, consisting of 18 supervised exercise sessions and a remotely monitored home-based physical activity program. Following a CPET, patients randomized to PRIORITY will receive a personalized exercise prescription generated by the EXPERT tool (26), a Garmin sports watch and chest strap (Garmin Forerunner 45, Garmin Ltd. Kansas, USA) and access to a web-based exercise platform (www.inspanningstherapie.be). Patients with HFpEF stage C will also receive a home ergometer. Subsequently, the automatically generated exercise prescription will be fully person-tailored by one of the physiotherapists (CD, SN) or a physical therapist (ED) of the research team following the FITT-VP (frequency, intensity, time, type, volume, and progression) principle during the first one-on-one consultation and considering patients’ needs, barriers and goals. Over a period of one year, with gradually increasing time intervals between sessions, patients will be invited to participate in 18 supervised centre-based exercise sessions, using the checklist for intervention description and replication (TIDieR) (27) as described in more detail in Supplementary File S1. This number of sessions was chosen as this is the number of sessions patients get reimbursed for other injuries (e.g., shoulder pain, muscle injuries, etc.) when consulting a physiotherapist in Belgium. While providing supervised exercise, patients are acquainted with the use of the sports watch, the exercise platform, heart rate training zones and intensity levels, strength, and balance exercises. Additionally, facilitators and barriers will be discussed, including issues they encounter when (trying to be) being physically active at home. In this way adaptations to the exercise program could be made and follow-up will be regularly provided. Patients will be asked to register each exercise session (supervised and home-based) with sports watches and to upload their training data (including heart rate data, steps, distance travelled, floors climbed, speed, …) to the Garmin Connect software (Garmin Ltd. Kansas, USA). In addition, they will be requested to subjectively rate the intensity of the session using the BORG scale. Training sessions focus on aerobic endurance training and dynamic strength training and also include balance, coordination and flexibility when needed. As depicted in Figure 2, the number of supervised sessions gradually decreases over time to maximally encourage self-management and empowerment: month 1 (one weekly supervised session)—month 2–4 (one supervised session every 2 weeks)—month 5–12 (one supervised session per month). To enhance adherence to the intervention, patients will be contacted by the physiotherapist (via e-mail or phone according to preference) if six consecutive prescribed home-based sessions are missed.
Figure 2.
Intervention of the PRIORITY trial.
Comparator group
Patients randomized to the comparator group will be advised to be physically active and will receive a personalized exercise prescription as automatically generated by the EXPERT tool and orally explained to the patient (26). Subsequently, patients are free to participate in any form of physical activity or structured exercise. However, there will be no contact between the patients and the investigators to provide feedback or support during the 1-year FU period, except for the four-month FU assessment.
Concomitant care
All patients will continue to receive usual care which includes optimal medical and pharmacological treatment during the trial.
From year one to year two
As shown in Figure 2, all patients will receive an overview of the change of their physical fitness test results one year after their baseline measurements. Patients in the PRIORITY group will then be invited for four physical activity consultations: immediately at month 12 and then subsequently at month 15, month 18 and month 21 to discuss their current physical activity behaviour and further co-design their physical activity program addressing barriers and enablers of physical activity. The key component of the physical activity intervention consists of motivational interviewing to evoke intrinsic motivation to support long-term behavioural change. Patients of the PRIORITY group will still be able to make use of the web-based exercise platform, their resistance training bands, their sports watch and chest strap. Patients with HFpEF stage C will also keep their home-ergometer. Patients in the comparator group will not be contacted during this year.
Monitoring and promoting adherence during a 2-year period
Strategies to improve adherence to an active lifestyle include a home-based mode of training, gradually decreasing in-person follow-up sessions, user-friendly training software (training diary + prescription with pre-recorded videos and pictures), periodized training volume, and personalized training intensities (relative training intensities based on CPET), co-development of an activity intervention adapted to the patient’s preferences and by the use of motivational interviewing techniques, SMART goal setting, self-monitoring of physical activity behaviour and gradually decreasing follow-up prompts.
Outcome measures and data collection
Assessments will be performed at baseline before randomization (T0), at 4 months (T1) as this is the median intervention duration in most supervised exercise studies, at the end of the 1-year intervention (T2) and after two years of FU (T3). The main goal is to assess the effectiveness of the intervention on different biopsychosocial outcomes at 1- and 2-year FU. A tabulated overview of the primary, secondary and other outcomes measured at the different time points is provided in Table 3.
Table 3.
Tabulated summary of trial schedule.
| Outcomes | Instrument | T0 | T1 | T2 | T3 |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Exercise capacity | Peak oxygen uptake via cardiopulmonary exercise test on cycle ergometer | X | X | X | X |
| Secondary outcomes | |||||
| Physical activity | ActiGraph GT9X Link | X | X | X | X |
| Health related physical fitness | |||||
| Body morphology/composition | Body mass (body weight scale), height (stadiometer)and waist circumference (stretch-resistant measuring tape). Bioelectrical impedance will be used to assess fat and fat-free mass. | X | X | X | X |
| Handgrip strength | Isometric handgrip grip strength of both hands using a Jamar Hydraulic Hand Dynamometer (Sammons Preston Inc.). | X | X | X | X |
| Quadriceps muscle strength | Three voluntary maximal isometric contractions (6 s) performed at a 60° angle of the knee, with a 60-second rest period between each test, on a dynamometer (Biodex Medical Systems Inc., 840–000 System 4, New York, USA). | X | X | X | X |
| Quadriceps muscle endurance | 25 repetitive maximal isokinetic knee extensions at 180°/s, performed on a dynamometer. Mean torque and percentage decrement score will be used as markers for muscle endurance. | X | X | X | X |
| Cardiovascular health | |||||
| Blood sampling | Glucose homeostasis, blood lipid profile, NT-proBNP | X | X | X | |
| Rest and stress echocardiography | Vivid E95 ultrasound scanner, semi-supine bicycle ergometer (Ergoline) is used for stress echocardiography. | X | X | X | |
| Health related QoL & psychosocial wellbeing | EQ-5D-5l Questionnaire | X | X | X | X |
| SF-36 Questionnaire | X | X | X | X | |
| Exercise Self-Efficacy Scale | X | X | X | X | |
| Exercise Barriers Questionnaire | X | X | X | X | |
| Social Support Questionnaire | X | X | X | ||
| Implementation potential | User experience Questionnaire | X | |||
| Adherence to exercise program | X | ||||
| Patient Debriefs: Self-reported and objective measures (exercise log, heart rate measurements) | X | ||||
| Evaluation towards cost-effectiveness | Productivity Cost Questionnaire | X | X | X | X |
| Medical Consumption Questionnaire | X | X | X | X | |
| Quality of life via EQ-5D-5l questionnaire | X | X | X | X | |
| Safety monitoring | Adverse events reporting by patients | X——————––X | |||
| Other outcomes | |||||
| Sociodemographic characteristics | General Questionnaire | X | X | X | X |
| Substudy: muscle metabolism | Device for muscle oxygenation measurements (PortaMon) | X | X | ||
| Substudy: vasoreactivity/vasomotor function | Flow mediated slowing via Vicorder | X | X | ||
T0, baseline; T1, measurement at 4 months; T2, measurements at 1 year; T3 measurements at 2 years; NIRS, near infrared spectroscopy; FMS, flow mediated slowing.
Primary outcome measure
Our primary outcome will be the cardiorespiratory fitness expressed as pVO2 during a graded maximal CPET on a bicycle until exhaustion (JAEGER Vyntus CPX, Vyaire medical, USA). pVO2 is determined as the highest attained peak VO2 during an average of 30 s of exercise. We choose pVO2 as our primary outcome because it has been shown to be the most important independent predictor of CV morbidity and mortality in patients with HFpEF stage A–C (28). A 5 + 5 W/min, 10 + 10 W/min, 20 + 20 W/min, or 50 + 25 W/min continuous ramp protocol will be used according to the participants’ estimated fitness level to ensure a CPET duration between the recommended 8–12 min (29). A 12-lead electrocardiogram will be monitored continuously, and gas exchange will be measured breath-by-breath. Blood pressure will be measured automatically every 2 min (SunTech Tango M2, SunTech Medical, USA). After reaching maximal exertion, the patients will cycle another 3 min to measure recovery heart rate, blood pressure, rating of perceived exertion and to document the reason for test termination. All raw CPET data will be forwarded for analysis to a blinded CPET core lab at KU Leuven to ensure reliable analysis of the data.
Secondary outcome measures
Physical activity
A validated tri-axial accelerometer (ActiGraph™ GT9X Link, ActiGraph LLC, Pensacola, Florida, USA) will be used to objectively measure the patient’s physical activity level (30). Participants will be asked to wear the ActiGraph GT9X Link on the non-dominant wrist for 24 h/day for 7 days. Measurements will be considered valid when at least 3 weekdays and 1 weekend-day of 10 h wear-time have been recorded (31). ActiLife software will be used to extract the raw data from the physical activity monitor which will then be transferred to the physical activity core lab at KU Leuven for offline analysis. The following parameters will be determined: number of steps (key secondary outcome), energy expenditure (total and active), time spent doing MVPA (key secondary outcome) and number of sedentary bouts.
Muscular fitness
A maximal voluntary isometric handgrip strength test will be performed using a JAMAR dynamometer (JLW Instruments, Chicago, Illinois, USA) (32). For each hand, the patient will perform three repetitions while sitting upright on a chair and with the elbow at 90° flexion. The maximum value (in kg) for each hand will be recorded. Additionally, muscle strength and endurance of the quadriceps muscle will be tested in the right leg using a Biodex system 3 Pro (Biodex Medical Systems Inc., Shirley, New York, USA) (33). Patients will be instructed to perform three voluntary maximal isometric quadriceps contractions for 6 s, interspersed with a 1 min rest period. The highest value will be used in the analysis as a measure of isometric power. This will be followed by two bouts of 25 repetitive maximal isokinetic knee extension-flexion movements interspersed with a 2 min recovery period.
Body Composition
Body composition will be measured in the supine position using Bodystat 1,500 (Bodystat Ltd, Douglas, Isle of Man, UK) (28). Anthropometric characteristics such as length (stadiometer) and body mass will be measured to the nearest 0.1 cm and 0.1 kg in fasting state and light clothing to calculate body mass index (kg/m²). Waist circumference is measured using non-elastic tape.
Blood biochemistry
A blood sample will be drawn with the patient in a fasting state and biochemical analysis includes fasting glucose, hemoglobin A1c, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, creatinine and NT-proBNP.
Rest transthoracic echocardiography
Comprehensive two- and three-dimensional echocardiography will be performed by experienced sonographers using a standardized protocol (see Supplementary File S2). All recordings will include at least 3 cardiac cycles and qualified readers will interpret the images offline using EchoPAC software (version 204, GE Vingmed, Horten, Norway). The standard protocol will include conventional cardiac dimensions, mass, and systolic and diastolic function in accordance with contemporary international guidelines (34, 35).
Exercise echocardiography combined with CPET (CPET-echo) will be performed following a standardized protocol as described before (see Supplementary File S2) (36–40). Exercise will be performed on a semi-supine bicycle ergometer (Ergoline GmbH, Bitz, Germany) with a continuous ramp protocol aiming for a total exercise duration of 10–12 min (60–65 rotations/min). Images will be acquired at rest, low intensity (HR between 90 and 100 beats per min, before fusion of E and A waves, or at an RER between 0.85 and 0.9 when chronotropic incompetence is present), and at peak exercise (RER >1.05). Loop registration of at least 10 beats will be made to overcome the expected decrease in acoustic quality caused by hyperventilation. At one year of follow-up, the CPET-echo will follow the same imaging protocol. The power output at low-intensity exercise will be identical to the low-intensity workload during the baseline CPET-echo at the time of inclusion. In contrast, the power output of the peak exercise stage will be determined based on the criterion of achieving RER 1.05. All analysis will be performed offline at the core lab of Leuven using EchoPAC software (version 204, GE Vingmed) in accordance with contemporary international guidelines (34, 35).
Health-related quality of life (HRQoL) will be assessed via the generic RAND-36 Questionnaire (SF-36), administered as an online survey via the web-based application REDCap (41). Here, different components are addressed: physical functioning (10 items), limitation due to physical health (4 items) or emotional problems (3 items), energy and fatigue (4 items), emotional well-being (5 items), social functioning (2 items), pain (2 items), general health (5 items) and perceived change in general health (1 item). All items are scored on a nominal or ordinal scale and transformed to a percentage of impairment (0% complete impairment, 100% no impairment). In addition, the EQ-5D-5l questionnaire will be administered covering five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The health status profile can be translated into a utility value between 0 and 1, serving as input for the health economic evaluation.
Safety
Adverse events will be monitored for 2 years after randomization. All adverse events will be recorded and reported in accordance with the Good Clinical Practice decision tree for adverse event reporting and will be reported to the central research ethics committee. Serious adverse events (SAE) are defined as all-cause mortality, hospitalization for CVD or serious atrial or ventricular arrhythmia. Other adverse events will include training-related adverse events such as muscle, tendon or joint problems that will preclude exercise participation or other diseases that require an interruption of the exercise intervention.
Implementation potential will be evaluated by measuring adherence [defined as a % of recommended duration of exercise (minutes)] and compliance [will be calculated as % of time at the recommended intensity (i.e., within correct heart rate zone)] to the exercise intervention. These data will be collected using the GARMIN sports watch or exercise diary for those patients encountering difficulties using the watch. In addition, the usability and feasibility of PRIORITY will further be assessed using the Users Experience Questionnaire (UEQ) and the System Usability Scale (SUS) (42).
Other exploratory outcome measures
In a subsample, continuous wave near-infrared spectroscopy (NIRS, PortaMon, Artinis Medical systems, Elst, The Netherlands) measurements will be performed during the CPET to evaluate muscle oxygen saturation. NIRS relies on the light absorption properties of chromophores in the tissue of interest (e.g., hemoglobin and myoglobin in muscle). As the underlying myoglobin concentration tends to remain constant during exercise, the changes in the oxy- or deoxygenation signals can be attributed to changes in hemoglobin content (43, 44). In the same sample, brachial artery vasoreactivity will be evaluated by means of flow-mediated slowing (FMS), a technique that measures brachial pulse wave velocity deceleration to reactive hyperaemia via the brachial-radial oscillometric technique (Vicorder device, SMT medical technology GmbH, Würzburg, Germany) (45).
Data management
Patient data will be anonymized using a personal identification code (PIC) on all case report forms and in the electronic database. Data will be recorded in hard copy at the time of the measurement and will subsequently be entered electronically in “REDCap (https://www.project-redcap.org)”, an open-source clinical trial software for electronic data capture and data management hosted at KU Leuven servers. Hard copies will be stored in a secured filing cabinet at the participating sites. Digital scans will be uploaded in the REDCap database and on the KU Leuven secure server. The type of activity that an individual user may undertake in the REDCap database is regulated by the privileges associated with his/her login credentials. Source data, randomization and pseudonymization lists are stored on a secure server of KU Leuven with password protection. All data analyses and reporting will be performed according to best practice and reported in agreement with Consolidated Standards of Reporting Trials guidelines (46). An independent researcher will regularly audit a randomly chosen subset of patients at each site to ensure adherence to the intervention program and the trial protocol. Any issues pertaining to nonadherence with the eligibility of a randomized participant, allocation of interventions, or concerns relating to adverse events are being discussed with and reviewed by the steering committee.
Statistical analysis
The full analysis set (FAS) will, in accordance with the intent-to-treat principle, include all randomised patients according to their randomised treatment. The FAS will be used for the evaluation of all efficacy and safety endpoints. Patients from the FAS with major protocol deviations will be excluded from the per protocol set (PPS). A cLDA (18) model will be used to compare the pVO2 at 12 months between both groups. In this model, both the baseline pVO2 and post-baseline pVO2 values (4 months and at 12 months) are modelled as dependent variables, as opposed to a longitudinal ANCOVA model in which the baseline value is included as a covariate. The comparison at 12 months will be based on a two-sided test, setting alpha equal to 0.05. An unstructured covariance matrix will be used for the three longitudinal measurements. The HFpEF stage will be added as a factor in the model and the differences between the groups are allowed to differ between the stadia. The intervention effect will be calculated as a weighted average of the stage-specific effects. The estimation of the model will be likelihood based and results are therefore valid under the MAR (missing at random) assumption, i.e., subjects with a missing pVO2 value at a specific timepoint are assumed to be well represented by other subjects not having a missing value at that timepoint and having the same observed values at the other timepoints. Note that the comparison at 4 months, which will be derived from the same model, is a secondary outcome. The cLDA model (restricted to baseline and 12 months) will also be used for the comparison of the two key secondary outcomes, applying a Bonferroni-Holm correction.
Health economic evaluation
The health economic evaluation will evaluate health outcomes [expressed as quality-adjusted life years (QALY)] and costs of the PRIORITY intervention compared to usual care. A cost-utility analysis will be performed applying a decision-analytic Markov model to predict costs and health effects of the intervention vs. usual care. A lifetime time horizon will be considered. The analysis will be conducted from a societal perspective, meaning that both direct and indirect costs will be included. Direct costs will include direct medical costs (e.g., hospitalization, nursing care, medication) and direct non-medical costs (e.g., travel costs). Indirect costs are those related to lost human productivity (for example productivity losses due to morbidity or mortality). The cost of delivering the program will be collected alongside the RCT. Information on participants’ health care resource use and absence from work will be obtained by a researcher using a questionnaire at T0, T1, T2 and T3 as shown in Table 3. Subsequently, resource use unit costs will be attached to the above mentioned data using publicly available databases (47). The ratio of the incremental costs to the incremental health effects, i.e., the incremental cost-effectiveness ratio (ICER) will be calculated. The model will link changes in exercise capacity and cardiovascular risk profiles to recurrent cardiovascular events and CVD mortality to estimate the cost-per-QALY associated with the PRIORITY intervention compared to usual care (48). One-way sensitivity and probabilistic sensitivity analyses will be performed to address the uncertainty related to key input parameters of the Markov model. In the one-way sensitivity analysis, the impact of key input parameters (e.g., costs, transition probabilities) on the ICER will be examined by varying their values separately. A probabilistic sensitivity analysis, based on 5,000 iterations, will be performed to evaluate the uncertainty for key input parameters by varying them concurrently.
Discussion
According to the latest WHO data, about 80% of CV disease is potentially avoidable with better management of CV risk factors such as diet and lifestyle. Therefore, the existing evidence advocates for a more aggressive preventive strategy including exercise and physical activity. Also, in patients with (risk factors for) HFpEF, there is no doubt that exercise is an imperative therapy in primary and secondary prevention (8, 16). Though, the implementation and accessibility of guidelines in daily clinical practice remains a challenge. Therefore, we initiated one of the largest multi-centre RCT that aims to validate the clinical impact and cost-effectiveness of a hybrid strategy to deliver person-tailored exercise therapy to patients along the HFpEF continuum.
The first strength of this approach is that it starts from a person-tailored exercise prescription using the EXPERT-tool and combines regular in person interaction with the support of technology. This allows us to move away from a healthcare provider-centric system and to empower patients in self-managing their health which is expected to increase adherence and compliance to the exercise and physical activity program. PRIORITY achieves this goal by bringing structured personalized exercise therapy to the patient’s home, which considers patient’s goals, co-morbidities, barriers, and enablers to exercise. Compared to the current situation, PRIORITY is designed with the aim to result in (1) a higher uptake of exercise therapy and physical activity by increasing accessibility (2) a better-sustained adherence to an optimally dosed exercise therapy and (3) a better clinical effectiveness of exercise therapy by providing a 1-year intervention.
Second, the PRIORITY trial applies an assessor-blinded, multicenter RCT design with recruitment targets able to achieve the power needed to make sound comparisons for our primary and important secondary outcomes in a sample which is representative for the continuum of HFpEF. To enhance external validity, the eligibility criteria for our population are broad and our recruitment strategy targets people from the age of 30 via different clinical units of (university) hospitals as well as the overall Flemish population. But at the same time the PRIORITY trial applies a thorough group allocation based on the latest guidelines combining echocardiography in rest and during exercise with biochemical parameters (NT-proBNP) to differentiate between HFpEF stages A, B and C. This recruitment strategy will enable us to evaluate the implementation potential in the real world and the overall effectiveness of our intervention.
Third, the design with the 2-year follow-up measurements is unique and will enable a high level of evidence of the longer-term effects on CV health, quality of life and physical activity behaviour of this hybrid strategy of providing person-tailored exercise therapy.
Fourth, PRIORITY has as its core concept the large-scale collection of sensed physical activity data (and patient input) via the sports watches and chest strap which allows us to better calculate internal training load during a 1-year period. In combination with the comprehensive phenotyping and behavioural data of our patients, we will develop predictive models by machine learning, to identify patients who are most likely to adopt a physically active lifestyle, increase their physical fitness or experience health benefits. This will enable the development of better-personalized exercise interventions and/or implementation strategies in the future.
Finally, implementation of effective lifestyle measures for the primary prevention of HF in patients at high risk and long-term secondary prevention of HF stage C remains suboptimal. Moreover, important socio-economic differences regarding risk factor control have been reported (49). The health economic analysis within PRIORITY will provide valuable insight into cost-effectiveness and quality-adjusted life years. This highly needed evidence could further guide the healthcare system on deciding which interventions are ready for reimbursement to maximize a society’s health gain.
This trial also has limitations that must be underlined. First, there is a risk for selection bias with individuals volunteering to participate being potentially more motivated to initiate exercise therapy. From eligible patients being referred for participation by their physician, the reason for non-participation will be recorded. Furthermore, the written exercise prescription given to the usual care comparator group is already more tailored than just the advice to be physically active and might encourage some patients to start exercising regularly. However, given the class IA recommendation we felt that providing no advice is unethical in this group. Finally, during the 2-year FU new medications might be initiated which could impact outcome of the participants. Though, given the randomized design, we anticipate that this will be equally distributed among the two trial arms. Further, medication and its change will be documented during the 2-year period.
Conclusion
The PRIORITY trial aims to bridge the gap between guideline recommendations and the implementation into clinical practice by evaluating the effectiveness of a hybrid strategy for delivering personalized exercise therapy to the rapidly expanding population of patients with HFpEF stage A–C. The PRIORITY trial aims to provide the scientific evidence to support the use of remotely guided exercise therapy as an imperative preventive cost-effective treatment in the HFpEF continuum. The trial will focus on the prevention of progression of asymptomatic diastolic dysfunction towards symptomatic HFpEF (=primary prevention) and delaying progression of symptomatic HFpEF (=secondary prevention). This document provides a detailed description of the design, methodology and protocol of the PRIORITY trial. If the results of this trial are positive, this strategy of implementation of personalized exercise therapy can be easily extended to other patient populations with chronic diseases for whom exercise, and the adoption of a physically active heart healthy lifestyle are a core component in their disease management.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Commission Research UZ/KU Leuven; Ethical Commission Research—Jessa Hospital; Ethicial Commission University Hospital Antwerp; Ethical Commission Research University Hasselt. The patients/participants provided their written informed consent to participate in this study.
Funding Statement
This trial was peer-reviewed and received funding from the Scientific Research Foundation of Flanders (FWO—T004420N).
Author contributions
All authors read, gave final approval, and agreed to be accountable for all aspects of the work, ensuring integrity. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1194693/full#supplementary-material
References
- 1.Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. (2021) 28(15):1682–90. 10.1093/eurjpc/zwaa147 [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. (2014) 1(1):4–25. 10.1002/ehf2.12005 [DOI] [PubMed] [Google Scholar]
- 3.La Gerche A, Howden EJ, Haykowsky MJ, Lewis GD, Levine BD, Kovacic JC. Heart failure with preserved ejection fraction as an exercise deficiency syndrome: JACC focus seminar 2/4. J Am Coll Cardiol. (2022) 80(12):1177–91. 10.1016/j.jacc.2022.07.011 [DOI] [PubMed] [Google Scholar]
- 4.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European society of cardiology (ESC). Eur J Heart Fail. (2019) 40(40):3297–317. 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 5.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. (2023). [DOI] [PMC free article] [PubMed]
- 6.Warburton DER, Bredin SSD. Health benefits of physical activity: a strengths-based approach. J Clin Med. (2019) 8(12):2044. 10.3390/jcm8122044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. (2017) 32(5):541–56. 10.1097/HCO.0000000000000437 [DOI] [PubMed] [Google Scholar]
- 8.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. (2021) 42(1):17–96. 10.1093/eurheartj/ehaa605 [DOI] [PubMed] [Google Scholar]
- 9.Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur J Prev Cardiol. (2021) 28(5):460–95. 10.1177/2047487320913379 [DOI] [PubMed] [Google Scholar]
- 10.Hansen D, Niebauer J, Cornelissen V, Barna O, Neunhäuserer D, Stettler C, et al. Exercise prescription in patients with different combinations of cardiovascular disease risk factors: a consensus statement from the EXPERT working group. Sports Med. (2018) 48(8):1781–97. 10.1007/s40279-018-0930-4 [DOI] [PubMed] [Google Scholar]
- 11.De Bacquer D, Astin F, Kotseva K, Pogosova N, De Smedt D, De Backer G, et al. Poor adherence to lifestyle recommendations in patients with coronary heart disease: results from the EUROASPIRE surveys. Eur J Prev Cardiol. (2022) 29(2):383–95. 10.1093/eurjpc/zwab115 [DOI] [PubMed] [Google Scholar]
- 12.Kotseva K, De Backer G, De Bacquer D, Rydén L, Hoes A, Grobbee D, et al. Primary prevention efforts are poorly developed in people at high cardiovascular risk: a report from the European society of cardiology EURObservational research programme EUROASPIRE V survey in 16 European countries. Eur J Prev Cardiol. (2021) 28(4):370–9. 10.1177/2047487320908698 [DOI] [PubMed] [Google Scholar]
- 13.Kotseva K, De Backer G, De Bacquer D, Rydén L, Hoes A, Grobbee D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European society of cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol. (2019). 26(8):824–35. 10.1177/2047487318825350 [DOI] [PubMed] [Google Scholar]
- 14.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158(3):200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. (2012) 50(3):217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2022) 24(1):4–131. 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 17.Coffman CJ, Edelman D, Woolson RF. To condition or not condition? Analysing ‘change’ in longitudinal randomised controlled trials. BMJ Open. (2016) 6(12):e013096. 10.1136/bmjopen-2016-013096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang K, Zeger S. Longitudinal data analysis of continuous and discrete responses for pre-post designs. The Indian Journal of Statistics. (2000) 62(1):134–48. [Google Scholar]
- 19.Stroup W. Mixed model procedures to assess power, precision, and sample size in the design of experiments. In: Proceedings of the Biopharmaceutical Section. Alexandria, VA: American Statistical Association; (1999). p. 15–24. [Google Scholar]
- 20.Pandey A, Shah SJ, Butler J, Kellogg DL, Lewis GD, Forman DE, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 78(11):1166–87. 10.1016/j.jacc.2021.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller S, Winzer EB, Duvinage A, Gevaert AB, Edelmann F, Haller B, et al. Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. (2021) 325(6):542–51. 10.1001/jama.2020.26812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. (2009) 301(19):2024–35. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 23.van Baak MA, Pramono A, Battista F, Beaulieu K, Blundell JE, Busetto L, et al. Effect of different types of regular exercise on physical fitness in adults with overweight or obesity: systematic review and meta-analyses. Obes Rev. (2021) 22(Suppl 4):e13239. 10.1111/obr.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundqvist S, Cider Å, Larsson MEH, Hagberg L, Björk MP, Börjesson M. The effects of a 5-year physical activity on prescription (PAP) intervention in patients with metabolic risk factors. PLoS One. (2022) 17(10):e0276868. 10.1371/journal.pone.0276868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen RT, Wagner V, Korfitsen CB, Keller C, Juhl CB, Langberg H, et al. Effectiveness of physical activity monitors in adults: systematic review and meta-analysis. Br Med J. (2022) 376:e068047. 10.1136/bmj-2021-068047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen D, Dendale P, Coninx K, Vanhees L, Piepoli MF, Niebauer J, et al. The European association of preventive cardiology exercise prescription in everyday practice and rehabilitative training (EXPERT) tool: a digital training and decision support system for optimized exercise prescription in cardiovascular disease. Concept, definitions and construction methodology. Eur J Prev Cardiol. (2017) 24(10):1017–31. 10.1177/2047487317702042 [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Br Med J. (2014) 348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 28.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. (2004) 19(5):433–46. 10.1177/0115426504019005433 [DOI] [PubMed] [Google Scholar]
- 29.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. (2010) 122(2):191–225. 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 30.Valkenet K, Veenhof C. Validity of three accelerometers to investigate lying, sitting, standing and walking. PLoS One. (2019) 14(5):e0217545. 10.1371/journal.pone.0217545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nyström C, Mora-Gonzalez J, Löf M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. (2017) 47(9):1821–45. 10.1007/s40279-017-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benton MJ, Spicher JM, Silva-Smith AL. Validity and reliability of handgrip dynamometry in older adults: a comparison of two widely used dynamometers. PLoS One. (2022) 17(6):e0270132. 10.1371/journal.pone.0270132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drouin JM, Valovich-mcLeod TC, Shultz SJ, Gansneder BM, Perrin DH. Reliability and validity of the biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur J Appl Physiol. (2004) 91(1):22–9. 10.1007/s00421-003-0933-0 [DOI] [PubMed] [Google Scholar]
- 34.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 35.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2016) 17(12):1321–60. 10.1093/ehjci/jew082 [DOI] [PubMed] [Google Scholar]
- 36.Martens P, Claessen G, Van De Bruaene A, Verbrugge FH, Herbots L, Dendale P, et al. Iron deficiency is associated with impaired biventricular reserve and reduced exercise capacity in patients with unexplained dyspnea. J Card Fail. (2021) 27(7):766–76. 10.1016/j.cardfail.2021.03.010 [DOI] [PubMed] [Google Scholar]
- 37.Verwerft J, Bertrand PB, Claessen G, Herbots L, Verbrugge FH. Cardiopulmonary exercise testing with simultaneous echocardiography: blueprints of a dyspnea clinic for suspected HFpEF. JACC Heart Fail. (2023) 11(2):243–9. 10.1016/j.jchf.2022.11.004 [DOI] [PubMed] [Google Scholar]
- 38.Gojevic T, Van Ryckeghem L, Jogani S, Frederix I, Bakelants E, Petit T, et al. Pulmonary hypertension during exercise underlies unexplained exertional dyspnea in patients with type 2 diabetes. Eur J Prev Cardiol. (203) 30(1):37–45. 10.1093/eurjpc/zwac153 [DOI] [PubMed] [Google Scholar]
- 39.Claessen G, La Gerche A, Voigt JU, Dymarkowski S, Schnell F, Petit T, et al. Accuracy of echocardiography to evaluate pulmonary vascular and RV function during exercise. JACC Cardiovasc Imaging. (2016) 9(5):532–43. 10.1016/j.jcmg.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 40.Guazzi M, Wilhelm M, Halle M, Van Craenenbroeck E, Kemps H, de Boer RA, et al. Exercise testing in heart failure with preserved ejection fraction: an appraisal through diagnosis, pathophysiology and therapy—a clinical consensus statement of the heart failure association and European association of preventive cardiology of the European society of cardiology. Eur J Heart Fail. (2022) 24(8):1327–45. 10.1002/ejhf.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz LN, Camey SA, Fleck MP, Polanczyk CA. World health organization quality of life instrument-brief and short form-36 in patients with coronary artery disease: do they measure similar quality of life concepts? Psychol Health Med. (2009) 14(5):619–28. 10.1080/13548500903111814 [DOI] [PubMed] [Google Scholar]
- 42.Hyzy M, Bond R, Mulvenna M, Bai L, Dix A, Leigh S, et al. System usability scale benchmarking for digital health apps: meta-analysis. JMIR Mhealth Uhealth. (2022) 10(8):e37290. 10.2196/37290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barstow TJ. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol. (2019) 126(5):1360–76. 10.1152/japplphysiol.00166.2018 [DOI] [PubMed] [Google Scholar]
- 44.Grassi B, Quaresima V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt. (2016) 21(9):091313. 10.1117/1.JBO.21.9.091313 [DOI] [PubMed] [Google Scholar]
- 45.Ellins EA, New KJ, Datta DB, Watkins S, Haralambos K, Rees A, et al. Validation of a new method for non-invasive assessment of vasomotor function. Eur J Prev Cardiol. (2016) 23(6):577–83. 10.1177/2047487315597210 [DOI] [PubMed] [Google Scholar]
- 46.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. (2012);10(1):28–55. 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 47.https://www.riziv.fgov.be/nl/statistieken/uitkeringen/Paginas/default.aspx.
- 48.https://euroqol.org/eq-5d-instruments/.
- 49.Hermans MP, De Bacquer D, De Block C, Truyers C, Vankeirsbilck A, De Backer G. Cardiovascular risk factors: belgian target achievement. Acta Cardiol. (2014) 69(5):473–81. 10.1080/AC.69.5.3044873 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.