ABSTRACT
This review aimed to estimate the disease burden of herpes zoster (HZ) in China and explore the application of the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach in studies of disease burden. We searched for the literature of observational studies analyzing HZ incidence in populations of all ages in China. Meta-analysis models were constructed to calculate the pooled incidence of HZ and pooled risks of postherpetic neuralgia (PHN), HZ recurrence, and hospitalization. Subgroup analysis was performed according to gender, age, and quality assessment score. The quality of evidence for incidence was rated using the GRADE system. Twelve studies with a total of 25,928,408 participants were included in this review. The pooled incidence for all ages was 4.28/1000 person years (95% CI 1.22–7.35). It increased with the increasing in age especially for individuals aged ≥60 y, which was 11.69/1000 person years (95% CI 6.56–16.81). The pooled risks of PHN, recurrence, and hospitalization were 12.6% (95% CI 10.1–15.1), 9.7% (95% CI 3.2–16.2), and 6.0/100,000 population (95% CI 2.3–14.2), respectively. The quality of the evidence assessment of the pooled incidence by the GRADE for all ages was ‘low’; however, it was ‘moderate’ for the ≥60 yold subgroup. HZ is a serious public health problem in China and is more significant in individuals older than 60 y. Therefore, an immunization strategy for the zoster vaccine should be considered. The evidence quality assessment by the GRADE approach indicated that we had more confidence in the estimation of aged population.
KEYWORDS: Herpes zoster, incidence, disease burden, meta-analysis, GRADE
Introduction
Herpes zoster (HZ) is a prevalent and debilitating disease with typical characteristics of a painful blistering dermatomal rash, which results from the reactivation of varicella-zoster virus from latency.1,2 Although the rash usually heals within 2 to 4 weeks, postherpetic neuralgia (PHN), as the most common complication of HZ, can cause physical disability and emotional distress and interfere with daily activities.3,4 Both HZ and PHN can have a considerable negative impact on the quality of life of patients and result in a heavy burden on patients and healthcare systems worldwide.4 Healthcare providers and policymakers must be aware of the HZ burden in their country or region. At present, HZ is not commonly reported in China and a surveillance system for HZ has not been established.5 The data for the disease burden of HZ mainly come from several studies with different study designs, data sources, and survey methods, and their outcomes are inconsistent as well. Therefore, we conducted a systematic literature review and meta-analysis to provide a more accurate estimation for the disease burden of HZ.
Zoster vaccine has proven efficacy to attenuate the severity of HZ disease and significantly reduce the incidence of HZ and PHN.6 Currently, there are two types of HZ vaccines, Zoster Vaccine Live (ZVL, Zostavax) and Recombinant Zoster Vaccine (RZV, Shingrix), which were launched in 2006 and 2017, respectively.7,8 The Advisory Committee on Immunization Practices (ACIP) of the United States Centers for Disease Control and Prevention recommends RZV as the preferred vaccine for healthy adults aged 50 y or older and adults who have previously received ZVL.9 In May 2019, the National Medical Products Administration approved the use of RZV in China.10 Therefore, it is important to determine the HZ disease burden in China to provide more scientific evidence for subsequent recommendations of the HZ vaccine.
The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach was proposed by the GRADE Working Group in 2004 to rate the quality of evidence and grade the strength of recommendations.11 It has been adopted by 28 international organizations, including the Cochrane Collaboration and the World Health Organization. The framework offers a transparent and structured process for developing and presenting evidence summaries for systematic reviews and guidelines in healthcare and the steps involved in developing recommendations.12 However, the GRADE approach was usually used for the evaluation of therapeutic intervention research or clinical questions and rarely used for public health issues.13,14 Few studies that applied GRADE approach into estimating disease incidence or disease burden have been existed in the literature. A study about systematic reviews showed that only 4 in 235 systematic reviews of prevalence used GRADE approach for assessing the overall quality of evidence of prevalence.15 In this study, the GRADE approach was used in observational studies to estimate the incidence of disease in order to provide more reference for other researchers.
Methods
Inclusion criteria
The inclusion criteria were as follows: 1) observational studies, such as cross-sectional studies, epidemiological surveys, and surveillance studies; 2) Chinese study population; 3) the observed indicator included the HZ incidence; and 4) the outcome was the clinical diagnosis or self-diagnosis of HZ during the survey period. Studies on clinical characteristics, treatments, molecular biology, risk factors, seroprevalence, popular science lectures, newspaper articles, and literature reviews were excluded. For articles that were published repeatedly, we selected the one with the most complete information.
Search strategy
We searched for articles published before September 2022 in the following databases: China National Knowledge Internet, Wan Fang Database, Chinese Biomedical Literature Service System (SinoMed), PubMed, EMBASE, Web of Science, and Cochrane Library. We used the search terms ‘herpes zoster’, ‘incidence’, ‘disease burden’, ‘prevalence’, ‘characteristics’, and ‘survey’. ‘Chinese’ and ‘China’ were used as search terms when searching for English articles to identify articles that presented data for the incidence of HZ in the Chinese population (Supplementary File 1). The reference lists of selected articles and key reviews published previously5,16,17 were also manually searched and reviewed. This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol.18
Study selection
NoteExpress (3.2.0.7629) was used to eliminate duplicates. Two reviewers (ZZ and XL) independently screened the studies based on the inclusion criteria described above. Disagreements were resolved by consensus or consultation with a third member of the team (LL).
Data extraction
Data extraction forms were developed for performing the methodological quality assessment of individual studies, subgroup analysis, and quality of evidence rating. Two reviewers (ZZ and XL) independently performed the data extraction. In the event of missing or inaccurate data, the authors of the original articles were contacted.
For each study included in the analysis, we extracted information on the characteristics of the study (authors, study year, study design, data sources, sample design, sampling frame, response situation, and conflicts of interest), patient definition, diagnosis and reports, PHN, recurrence and hospitalization counts, patient and population counts, and gender and age counts. The patient count or population was calculated by the reviewers when it was not reported directly, but there was enough data to make an estimate.
Methodological quality assessment
A quality assessment was performed for included studies based on the criteria developed from the guidelines for the evaluation of incidence studies.19,20 Studies were given a score of 0 to 8 based on the degree to which they fulfilled eight criteria related to the assessment, the quality of the statistical analysis, and the extent to which the sample population represented the population at large.
Meta-analysis
Stata (version 15.0) was used to perform all of the statistical calculations in this meta-analysis. The incidence of HZ refers to the number of new patients with HZ in the population during a certain period.21,22 Data were combined and estimated for the pooled incidence (per 1,000 person year), and the 95% confidence interval (CI) was calculated. The incidence and CIs of the subgroups were analyzed by sex, age, and quality assessment score. The pooled risks and CIs for PHN (%), HZ recurrence (%), and hospitalization (/100,000 population) were also calculated. The I2 value was used to assess the heterogeneity of the included studies.23,24 We considered I2 ≥50 to indicate high heterogeneity and chose random effects models, and I2 <50 to indicate low heterogeneity and chose fixed-effects models. Publication bias was assessed using Egger’s regression test, with p < .05 used to indicate evidence of publication bias.25
Evidence quality rating
We used GRADEpro (version 3.5) to rate the quality of evidence of the incidences using the GRADE system. Multiple researchers participated in the process according to the method described in a previous study.26 The quality of the evidence was divided into four categories: ‘high,’ ‘moderate,’ ‘low,’ and ‘very low.’ This reflects our confidence that the estimation of the incidence was close to the actual incidence of the population.27 Randomized trials were considered as high-quality evidence, and observational studies were considered as low-quality evidence.28 Five factors that can reduce the quality of evidence included limitations, inconsistency, indirectness, imprecision, and publication bias. Three factors that can increase the quality of evidence consisted of large effect, plausible residual confounding, and dose–response gradient.29
Results
Search results
A total of 2,488 articles were obtained from the literature search, and two additional articles were obtained from reference list articles or reviews; 386 duplicate articles were excluded. After reviewing the titles and abstracts, 2,069 articles were not original research, analyzed other topics, or had different populations. We read the full texts of the 35 related articles and determined that some were not original research or that incidence was not the main indicator. Finally, 12 original studies met our inclusion criteria for meta-analysis, including six Chinese articles and six English articles (Figure 1; references listed in Table 1).30–41
Figure 1.
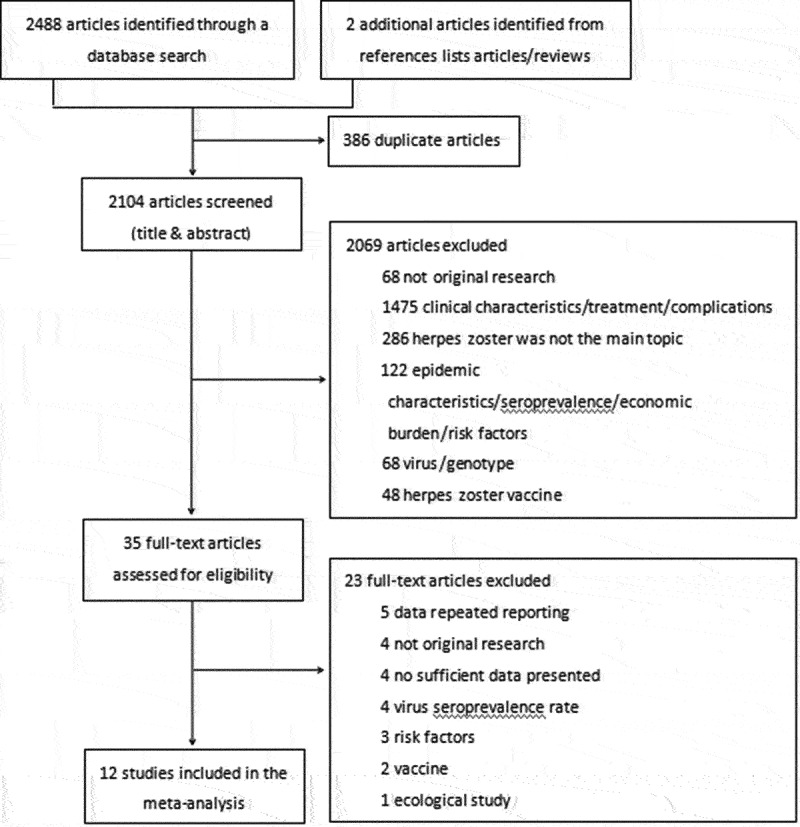
Flow diagram of the study selection: two reviewers independently selected the studies according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Disagreements were resolved by consulting with a third person.
Table 1.
Basic information of studies included in this meta-analysis [30–41].
| Author | Study Year | Data Sources | Age of Population | Population of Study | Incidence(/1000 Person Years) | Quality Assessment Scorea |
|---|---|---|---|---|---|---|
| Suo Luodan | 2015 | Health Information System records | all ages | 3,688,000 | 8.76 | 5 |
| Qinghai Wang | 2019 | Health Information System records | all ages | 1,178,999 | 3.38 | 5 |
| D. Y. Chao | 2000–2008 | National Health Insurance (NHI) database | all ages | 2,055,589 | 5.79 | 6 |
| Jiang Wei | 2016–2017 | Yichang Healthcare Big Data Platform Center | all ages | 1,875,800 | 5.06 | 6 |
| Lin Zhao | 2013.1–2017.6 | Shandong Multi-Center Healthcare Big Data Platform | all ages | 15,296,994b | .70 | 7 |
| Li Lu | 2012.12–2013.3 | Community-based survey | all ages | 118,220 | 2.00 | 5 |
| Xiong Ding | 2018–2019 | Yichang Healthcare Big Data Platform Center | ≥18 y | 850,608 | 6.32 | 6 |
| Xiaohui Sun | 2015–2017 | The electronic health care record database |
≥50 y | 649,699 | 6.64 | 7 |
| Qi Zhu | 2011–2013 | Community-based survey | ≥50 y | 20,256 | 4.30 | 4 |
| Yan Li | 2013.5–2014.5 | Community-based survey | ≥50 y | 149,163 | 3.43 | 5 |
| Xia Zang | 2017.2–7 | Community-based survey | ≥45 y | 3580 | 6.42 | 4 |
| Weiyan Zhang | 2014–2018 | Community-based survey | ≥60 y | 41,500 | 9.49 | 6 |
aScore = Methodological strength of study (maximum 8).
bIt was calculated by incidence which was given in article.
Study characteristics
A total of 12 studies on the incidence of HZ were included, and all of those were retrospective studies. Of these, seven studies included data extracted from a relevant electronic healthcare database, such as the health information system of the hospital, Health Insurance database, and Healthcare Big Data Platform, whereas five studies included data based on community surveys. The present meta-analysis included 25,928,408 participants, and 79,272 of them were cases (Table 1).
There were six studies that included all age groups, seven studies that described populations of those aged ≥60 y, five studies with details on gender,30–33,35 six studies on age groups,30,31,33,37,39,41 eight studies on PHN,31,33,35,37–41 four studies on HZ recurrence,35,39–41 and four studies on hospitalization rates.30,35,39,41
The quality scores for the included studies ranged from 4 to 7 points out of a possible 8 points. The median quality score was 5.5 (4–7). The main reasons for score deductions were as follows: closed group-like patients in the hospital were used to substitute the total population, 95% CIs were not reported, nonrandom sampling was used, and the response rate was not reported. The details are shown in Supplementary File 2.
Meta-analysis of the incidence of HZ
Six studies have reported the incidence of the entire population (all ages). The meta-analysis indicated that the studies had statistically significant heterogeneity (I2 = 100%); thus, the pooled estimate of the incidence was calculated using a random effects model. The pooled HZ incidence was 4.28/1000 person years (95% CI: 1.22–7.35; Figure 2). There was evidence of publication bias indicated by the Egger’s regression test (p = .047; Figure 3). After one study with the lowest incidence (0.7/1000 person years) was removed,33 the pooled HZ incidence was 5.00/1000 person years (95% CI: 2.91–7.10) with no significant publication bias (Egger’s regression test, p = .432).
Figure 2.

Random effects model for the incidence of herpes zoster.
Figure 3.

Funnel plot of the incidence of herpes zoster.
The studies were divided into subgroups according to sex, age, and quality assessment score and were subsequently analyzed. The heterogeneity of the subgroups was significant; therefore, random-effect models were used for the following analysis. The pooled incidence of HZ in females, which was 5.64/1000 person years (95% CI: 3.11–8.18), was higher than that of 4.46/1000 person years (95% CI: 2.63–6.28) for males. The pooled incidence estimates increased as the age group increased, from the 0–29 y age group (1.55/1000 person years [95% CI: 0.30–2.80]) to the ≥80 y age group (12.78/1000 person years [95% CI: 4.96–20.6]), and in the population aged ≥60 y, it was 11.69/1000 person years (95% CI: 6.56–16.81). Different quality assessment scores had different incidences, with a 5 score having an incidence of 4.72/1000 person years (95% CI: 4.6–8.97) and a 6–7 score having an incidence of 3.85/1000 person years (95% CI: 0.07–7.63) (Table 2).
Table 2.
The pooled incidence of herpes zoster after meta-analysis.
| Subgroup | No. of Study | Population of Study | Incidence (/1000 Person Years) | 95% CI | I2 Value (%) | Publication Bias |
|---|---|---|---|---|---|---|
| Totala | 6 | 24,213,602 | 4.28 | (1.22,7.35) | 100 | 0.047 |
| Gendera | ||||||
| Male | 5 | 4,462,846 | 4.46 | (2.63,6.28) | 99.9 | 0.358 |
| Female | 5 | 4,375,516 | 5.64 | (3.11,8.18) | 99.9 | 0.613 |
| Age group | ||||||
| 0–29 | 3 | 2,341,822 | 1.55 | (0.30,2.80) | 99.8 | 0.641 |
| 30–39 | 3 | 1,159,916 | 3.85 | (0.96,6.74) | 99.9 | 0.380 |
| 40–49 | 3 | 1,119,868 | 4.62 | (2.02,7.22) | 99.8 | 0.668 |
| 50–59 | 5 | 1,393,247 | 6.87 | (2.93,10.81) | 99.9 | 0.711 |
| 60–69 | 6 | 854,703 | 11.06 | (5.43,16.70) | 99.9 | 0.550 |
| 70–79 | 6 | 462,345 | 11.00 | (5.10,16.90) | 99.7 | 0.975 |
| 80+ | 6 | 235,123 | 12.78 | (4.96,20.60) | 99.7 | 0.245 |
| ≥60 y | 7 | 1,823,666 | 11.69 | (6.56,16.81) | 99.9 | 0.557 |
| Quality Assessment Score a | ||||||
| 5 score | 3 | 4,985,219 | 4.72 | (4.60,8.97) | 100 | 0.669 |
| 6–7 score | 3 | 19,228,383 | 3.85 | (0.07,7.63) | 100 | 0.042 |
| Study Design | ||||||
| Electronic healthcare database | 7 | 25,595,689 | 5.23 | (2.34,8.13) | 100 | 0.006 |
| Community survey | 5 | 332,719 | 5.01 | (3.15,6.86) | 100 | 0.190 |
| Clinical Characters of HZ | ||||||
| PHN | 8 | 19,517 | 12.6b | (10.1,15.1) | 95.7 | 0.053 |
| Recurrence | 4 | 1639 | 9.7b | (3.2,16.2) | 96.2 | 0.278 |
| Hospitalization | 4 | 33,996,883 | 6.0c | (2.3,14.2)b | 0.0 | 0.945 |
aOnly six studies with all age groups were included for data extraction.
bThe pooled proportion was calculated and the denominator was HZ cases.
cThe unit of pooled hospitalization was per 100 000 population.
Four studies did not provide a definition for PHN, two studies defined PHN as persistent pain for 30 d or more, and two studies defined PHN as pain persisting for 90 d or more after the HZ rash healed. The pooled risk for PHN development was 12.6% (95% CI: 10.1–15.1). HZ recurrence was defined as HZ reactivation after a 180-d HZ-free period since the previous HZ diagnosis in one study, and it was not reported in the other three studies. The pooled risk of HZ recurrence was 9.7% (95% CI: 3.2–16.2). All four studies investigated patients hospitalized for HZ or for whom HZ was the main diagnosis. The hospitalization rate was 6.0/100,000 population (95% CI: 2.3–14.2) (Table 2).
The quality of evidence assessment in GRADE framework
The indicator in our evidence quality assessment was the incidence of HZ, and incidence is important for evaluating disease burden. Therefore, the importance was rated as crucial (eight points). Because all of the included studies were observational studies, the initial quality of the evidence was ‘low.’
Overall, the evidence quality assessment of the pooled HZ incidence was ‘low.’ Limitations was not rated down as the study population could well represent the target population. Inconsistency was not rated down as we thought the reason for the significant heterogeneity was partly caused by the large sample size, which led to an increase in the I2 value.42,43 Different data sources were also one of the reasons for the observed heterogeneity. Indirectness was not rated down as the population, indicator, and outcome of the studies were consistent with what we studied in this meta-analysis. Imprecision was not rated down as the sample size of our study was sufficient and the CI was moderate. Publication bias was rated down by one category as Egger’s regression test indicated evidence of publication bias. Large effect was rated up as we considered the HZ incidence was very high. Because there was no publication bias, the quality of evidence assessment of the incidence rose to ‘moderate’ in the ≥60 y age subgroup.
Discussion
Meta-analysis
This study systematically estimated the incidence of HZ, as well as the risk of PHN, recurrence, and hospitalization, in China using the GRADE approach. Our analysis included approximately 25 million Chinese individuals from 12 studies, which was substantially more than that in a previous review,5 which only included studies published in English (three from mainland China and seven from Taiwan), and those without any pooled HZ incidence.
In our present study, the HZ incidence was 4.28/1000 person years, which was similar to that in multiple countries or regions of North America, Europe, and Asia-Pacific (3/1,000 to 5/1,000 person years).44 We also demonstrated that the incidence in females was higher than that in males, that the incidence increased with age, and that the severity increased sharply in those older than 60 y, all of those findings were consistent with the results of other studies.45–49 It was estimated that the annual number of HZ cases in China probably exceeds 6 million according to the population of 1.41 billion at the end of 2021,50 which is equivalent to the total of national legal infectious disease (6,233,537 cases) reported in 2021.51 Furthermore, as the population aging problem in China has entered a stage of rapid development, the proportion of individuals aged ≥65 y will increase from 6.8% in 2000 to 23.6% in 2050,52,53 and the number of HZ cases is expected to increase substantially.
It is generally believed that the occurrence of PHN is influenced by age and sex.54 Due to the varying prevalence of disability and other underlying comorbidities, the risk of PHN varies from 5% to >30%,44 and in our study, the risk of PHN in the patients with HZ was 12.6%. In addition to complications, the risks of hospitalization and recurrence were also indicators of HZ. The hospitalization rate was 6.0/100,000 population in our study, which was similar to that in other countries (4–13.4/100,000 population),55–57 and the risk of recurrence was 9.7%, which was slightly higher than that in some previous long-term follow-up studies in other countries (5–6%).58,59
In summary, HZ has become a major public health problem with a significant health burden in China. This problem is more serious among the elderly, as the elderly population is growing rapidly. In addition to the severity and duration of pain associated with HZ, vaccination substantially reduces the incidence of HZ and incidence of PHN. RZV has been used in the European Union, the United States, Japan, Canada, and Australia to prevent HZ or PHN in adults aged ≥50 y. Although RZV has been approved for use in China, it is not widely recommended, and both vaccination willingness and vaccination rate remain low.60,61 Therefore, implementation of the immunization strategy of the zoster vaccine should be considered to reduce the disease burden of HZ in China.
Evidence quality assessment
Our assessment of the GRADE rating results of the quality of evidence of HZ incidence for all ages was ‘low,’ which meant that our confidence in this estimated incidence was limited. However, a ‘low’ quality of evidence could still be strongly recommended,29 because our confidence has improved by findings of an incidence similar to that in other countries. We believe that the burden of HZ in China was serious. Because the quality of evidence in the population aged 60 y or older was relatively improved, we had more confidence in the estimated incidence of the aged population.
Nevertheless, in the process of assessing the quality of evidence using the GRADE approach, we demonstrated that there was limited experience with GRADE in the evaluation of public health questions, especially for estimating incidences. The GRADE rating method needs more detail regarding how to use it in the estimation of incidence. Second, for disease incidence studies, evidence was commonly derived from observational studies rather than randomized controlled trials. Even if the study was well designed and implemented, the evidence from observational studies remained generally initially ‘low,’28 which needed to be considered whether this was appropriate. Finally, we suggested that technical guidelines should be formulated for observational studies in general, or even for observational studies on estimating disease incidence, including study design, especially sample design, study implementation, article writing, etc., to improve the overall quality of evidence on a global scale.
In addition to the aforementioned problems of evidence quality, our study still has several limitations. Some of the included studies gave an incorrect definition or no definition of PHN, as the best option for defining PHN would be clinically meaningful pain lasted for more than 90 d after rash onset.62 We considered that PHN risk would be underestimated and that PHN risk in the actual situation was probably more serious. The approach of GRADE requires a high level of knowledge of researchers in related fields, and even so it was difficult to eliminate the researcher’s subjective judgment.12 Therefore, we reduced subjective influence through parallel evaluation by multiple researchers as much as possible.
Conclusions
The available data on HZ disease burden demonstrated that HZ is a serious public health problem in China, especially for individuals aged ≥60 y. Implementing the immunization strategy for the zoster vaccine should be considered in the elderly population. The evidence quality assessment by the GRADE approach indicated that we had more confidence in estimation for the aged population.
Supplementary Material
Acknowledgments
We would like to thank all the staff who had taken part in this study.
Funding Statement
This research was supported by Beijing Natural Science Foundation [L202008] in study design, in the collection, analysis and interpretation of data, and in writing the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors have read and approved the manuscript. Zhujiazi Zhang: Dr Zhang conceptualized the study, supervised data collection, carried out the initial analyses, interpreted results, drafted the manuscript, and submitted the final manuscript. Xinnong Liu: Dr Liu conceptualized the study, supervised data collection, carried out the initial analyses, interpreted results, reviewed the manuscript, and approved the final manuscript as submitted. Luodan Suo: Dr Suo conceptualized the study, supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Dan Zhao: Dr Zhao conceptualized the study, supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. Jingbin Pan: Dr Pan carried out data collection and the initial analyses and approved the final manuscript as submitted. Li Lu: Dr. Lu conceptualized the study, supervised data collection, interpreted the results, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed here. All of the articles included in this review are publicly available.
A preprint of this study has been published with a link https://www.researchsquare.com/article/rs-2256610/v1.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2228169.
Abbreviations
- HZ
Herpes zoster
- VZV
varicellazoster virus
- PHN
post-herpetic neuralgia
- ZVL
Zoster Vaccine Live
- RZV
Recombinant Zoster Vaccine
- ACIP
Advisory Committee on Immunization Practices
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- GRADE
Grades of Recommendation, Assessment, Development, and Evaluation
- CI
Confidence interval
References
- 1.Cohen JI, Brunell PA, Straus SE.. Recent advances in varicella-zoster virus infection. Annals of internal medicine. 1999;130(11):922–9. doi: 10.7326/0003-4819-130-11-199906010-00017. [DOI] [PubMed] [Google Scholar]
- 2.Gnann JJW, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347(5):340–6. doi: 10.1056/nejmcp013211. [DOI] [PubMed] [Google Scholar]
- 3.McElhaney JE. Herpes zoster: a common disease that can have a devastating impact on patients’ quality of life. Expert Rev Vaccines. 2010;9(sup3):27–30. doi: 10.1586/erv.10.31. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines. 2010;9(sup3):21–6. doi: 10.1586/erv.10.30. [DOI] [PubMed] [Google Scholar]
- 5.Yin D, Van Oorschot D, Jiang N, Marijam A, Saha D, Wu Z, Tang H, Diaz-Decaro J, Watson P, Xie X, et al. A systematic literature review to assess the burden of herpes zoster disease in China. Expert Rev Anti Infect Ther. 2021;19(2):165–79. doi: 10.1080/14787210.2020.1792290. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Kim E, Kong CL, Arnold BF, Porco TC, Acharya NR.. Effectiveness of the recombinant zoster vaccine in adults aged 50 and older in the United States: a claims-based cohort study. Clin Infect Dis. 2021;73(6):949–56. doi: 10.1093/cid/ciab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz SL. Varicella and herpes zoster vaccines WHO position paper,June 2014. Wkly Epidemiol Rec. 2014;89:265–87. [PubMed] [Google Scholar]
- 8.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 9.Miller ER, Lewis P, Shimabukuro TT, et al. Post-licensure safety surveillance of zoster vaccine live (Zostavax®) in the United States, Vaccine Adverse Event Reporting System (VAERS), 2006–2015. Hum Vaccin Immunother. 2018;14(8):1963–9. doi: 10.1080/21645515.2018.1456598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Medical Products Administration . Recombinant zoster vaccine are launched[EB/OL]. [accessed 2019 May 22]. https://www.nmpa.gov.cn/directory/web/nmpa/zhuanti/ypqxgg/gggzjzh/20190522150701437.html.
- 11.Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, O’Connell D, Oxman AD, Phillips B, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE working group. BMC Health Serv Res. 2004;4(1):1–7. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. Evid Based Med. 2008;13(6):162–3. doi: 10.1136/ebm.13.6.162-a. [DOI] [PubMed] [Google Scholar]
- 14.Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 9. Grading evidence and recommendations. Health Res Policy Sys. 2006;4(1):21. doi: 10.1186/1478-4505-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migliavaca CB, Stein C, Colpani V, Barker TH, Munn Z, Falavigna M.. How are systematic reviews of prevalence conducted? BMC Med Res Methodol. 2020. doi: 10.1186/s12874-020-00975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H, Liu F. Epidemiological characteristics of herpes zoster and research progress of vaccine immunization program in China. China Contin Med Educ. 2021;13(19):4. doi: 10.3969/j.issn.1674-9308.2021.19.039. [DOI] [Google Scholar]
- 17.Feng L, Yang T, Wang Q, Yang Y, Leng ZW, Chen SY, Jia MM, Zhang T, Chen FY, Zhang XX, et al. Prevent infectious diseases through vaccination, and protect health of the elderly. National Med J China. 2020;100(48):3821–6. doi: 10.3760/cma.j.cn112137-20201020-02882. [DOI] [PubMed] [Google Scholar]
- 18.Mark Vrabel MLS. Preferred reporting items for systematic reviews and meta-analyses[C]//Oncology nursing forum. Oncol Nurs Soc. 2015;42(5):552. doi: 10.1188/15.ONF.552-554. [DOI] [PubMed] [Google Scholar]
- 19.Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–6. [PubMed] [Google Scholar]
- 20.Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health. 1998;1(2):37–9. doi: 10.1136/ebmh.1.2.37. [DOI] [Google Scholar]
- 21.Jekel JF, Katz DL, Elmore JG. Epidemiology, biostatistics and preventive medicine. USA: Elsevier Health Sciences; 2007. [Google Scholar]
- 22.Streiner DL, Norman GR. PDQ epidemiology. USA: People's Medical Publishing House; 2009. [Google Scholar]
- 23.Deeks J, Higgins J, Altman D. Analyzing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of interventions: Cochrane book series. Wiley-Blackwell; 2008. doi: 10.1002/9780470712184. [DOI] [Google Scholar]
- 24.Wang D, Mou Z, Zhai J, Zong HX, Zhao XD.. Application of Stata software to heterogeneity test in meta-analysis. Chin J Epidemiol. 2008;29(7):4. [PubMed] [Google Scholar]
- 25.Wang D, Mou Z, Zhai J, Zong HX., Zhao XD.. Study on Stata software in investigating publication bias in meta-analysis. Mod Prev Med. 2008;35(15):4. doi:CNKI:SUN:XDYF.0.2008-15-002. [Google Scholar]
- 26.Zhang Z, Suo L, Pan J, Zhao D, Lu L. Two-dose varicella vaccine effectiveness in China: a meta-analysis and evidence quality assessment. BMC Infect Dis. 2021;21(1):543. doi: 10.1186/s12879-021-06217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori V, et al. GRADE guidelines. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–16. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Suo L, Wang Y, Pang X, Wang YC, Wang Q, Wang HH, Peng XH, Wang X, Zhu ZL, Wang YF, Pang XH.. Analysis of herpes zoster incidence and hospitalization in three areas of Beijing in 2015 based on health information system of medical institutions. Chin J Prev Med. 2019;53(5):503–7. doi: 10.3760/cma.j.issn.0253-9624.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Zhou M, Sun H. Epidemiological characteristics of herpes zoster in Xicheng District of Beijing in 2019: based on the health information system of medical institutions. Chin J Epidemiol. 2021;42(12):4. doi: 10.3760/cma.j.cn112338-20210706-00523. [DOI] [PubMed] [Google Scholar]
- 32.Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB.. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000–2008. Epidemiol Infect. 2012;140(6):1131–40. doi: 10.1017/S0950268811001786. [DOI] [PubMed] [Google Scholar]
- 33.Jiang W, Li G, Xu Y, Pei S, Yan Y, Tong H, Wang W, Xu C, Liu Y, Yin D.. Epidemiological characteristics of herpes zoster in urban areas of Yichang city during 2016-2017 based on the Yichang Big Data platform for health management. Chin J Vaccines Immun. 2019;25(4):4. [Google Scholar]
- 34.Zhao L, Wang HT, Ye RZ, Li Z-W, Wang W-J, Wei J-T, Du W-Y, Yin C-N, Wang S-S, Liu J-Y, et al. Profile and dynamics of infectious diseases: a population-based observational study using multi-source big data. BMC Infect Dis. 2022;22(1):1–12. doi: 10.1186/s12879-022-07313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Suo L, Li J, Pang, X.. A retrospective survey on herpes zoster disease burden and characteristics in Beijing, China. Hum Vaccin Immunother. 2018;14(11):2632–5. doi: 10.1080/21645515.2018.1489193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding X, Jiang W, Hu Y, Jiang J, Wu Y, Xu CZ, Wu ZZ, Yu YF, Liu XJ, Li GW, et al. Study on the incidence of adult herpes zoster in Yichang city and its association with early‑life famine exposure. Chin J Prev Med. 2021;55(11):4. doi: 10.3760/cma.j.cn112150-20201110-01350. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Wei Z, Lin H, Jit M, Li Z, Fu C.. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: data from an integrated health care network. J Infect. 2021;82(2):253–60. doi: 10.1016/j.jinf.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Q, Zheng H, Qu H, Deng H, Zhang J, Ma W, Lin Y, Xie X, Qiu Q, Huang Z, et al. Epidemiology of herpes zoster among adults aged 50 and above in Guangdong, China. Hum Vaccin Immunother. 2015;11(8):2113–8. doi: 10.1080/21645515.2015.1016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, An Z, Yin D, Liu Y, Huang Z, Xu J, Ma Y, Tu Q, Li Q, Wang H, et al. Disease burden due to herpes zoster among population aged ≥50 years old in China: a community based retrospective survey. PLoS One. 2016;11(4):e0152660. doi: 10.1371/journal.pone.0152660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zang X, Li YF, Sun Y, Pan J, Zhao D, Lu L.. Cross-sectional study on herpes zoster among adults aged 45 and above in Xiamen and Dongtai. Chin Primary Health Care. 2019;33(9):59–62. doi: 10.3969/j.issn.1001-568X.2019.09.0021. [DOI] [Google Scholar]
- 41.Zhang W, Liu S, Sun H, Wang HY, Luan GJ, Sun L, Xu AQ.. Study of incidence and economic burden of herpes zoster based on community investigation among the aged in Laiwu district, Jinan city, Shandong Province of China. Chin J Prev Med. 2022;56(2):6. doi: 10.3760/cma.j.cn112150-20211125-01085. [DOI] [PubMed] [Google Scholar]
- 42.Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8(1):1–9. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yawn BP, Saddier P, Wollan PC, Sauver JLS, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 46.Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post⁃herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137(1):38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 47.Coplan P, Black S, Rojas C, Shinefield H, Ray P, Lewis E, Guess H. Incidence and hospitalization rates of varicella and herpes zoster before varicella vaccine introduction: a baseline assessment of the shifting epidemiology of varicella disease. Pediatr Infect Dis J. 2001;20(7):641–5. doi: 10.1097/00006454-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez CS, Sarazin M, Turbelin C, Lasserre A, Pelat C, Bonmarin I, Chosidow O, Blanchon T, Hanslik T. Herpes zoster: burden of disease in France. Vaccine. 2010;28(50):7933–8. doi: 10.1016/j.vaccine.2010.09.074. [DOI] [PubMed] [Google Scholar]
- 49.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward J. Incidence of herpes zoster, before and after varicella-vaccination–associated decreases in the incidence of varicella, 1992–2002. J Infect Dis. 2005;191(12):2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 50.National Bureau of Statistics . Total population at the end of the year[EB/OL]. [accessed 2021 Dec 31]. https://data.stats.gov.cn/easyquery.htm?cn=C01.
- 51.National Health Commission of the People’s Republic of China . Overview of national notifiable infectious diseases, 2021. [accessed 2022 Apr 22]. http://www.nhc.gov.cn/jkj/s3578/202204/4fd88a291d914abf8f7a91f6333567e1.shtml.
- 52.Chen F, Liu G. Population aging in China. International handbook of population aging. Springer Science & Business Media; 2009. p. 157–72. doi: 10.1007/978-1-4020-8356-3_8. [DOI] [Google Scholar]
- 53.Luo Y, Su B, Zheng X. Trends and challenges for population and health during population aging—China, 2015–2050. China CDC Weekly. 2021;3(28):593. doi: 10.46234/ccdcw2021.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choo PW, Galil K, Donahue JG, Walker AM, Spiegelman D, Platt R. Risk factors for postherpetic neuralgia. Arch Intern Med. 1997;157(11):1217–24. doi: 10.1001/archinte.1997.00440320117011. [DOI] [PubMed] [Google Scholar]
- 55.Bayas JM, Gil R, Llupià A, Díez C, Conesa A, Ariza C, Gil A, San Martín M. Hospitalizaciones por herpes zoster y neuralgia postherpética en Cataluña, 1998–2003. Vacunas. 2011;12(4):122–8. doi: 10.1016/S1576-9887(11)70019-X. [DOI] [Google Scholar]
- 56.Gil A, Gil R, Alvaro A, San Martín M, González A. Burden of herpes zoster requiring hospitalization in Spain during a seven-year period (1998–2004). BMC Infect Dis. 2009;9(1):55. doi: 10.1186/1471-2334-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brisson M, Edmunds WJ. Epidemiology of varicella zoster virus in England and Wales. J Med Virol. 2003;70(1):S9 14. doi: 10.1002/jmv.10313. [DOI] [PubMed] [Google Scholar]
- 58.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Med. 1982;61(5):310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Yawn BP, Wollan PC, Kurland MJ, St. Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J, Sun X, Hu J. Willingness to receive herpes zoster vaccine and factors influencing willingness among≥50-year-old adults of Shanghai in May-June 2020. Chin J Vaccines Immun. 2021;27(3):5. doi: 10.19914/j.CJVI.2021057. [DOI] [Google Scholar]
- 61.Huang SY, Song J-H, Xing LY. Willingness and influencing factors of herpes zoster vaccination among residents ≥50 years old in Zhengzhou. South China J Prev Med. 2022;48:4. [Google Scholar]
- 62.Coplan PM, Schmader K, Nikas A, Chan ISF, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–56. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed here. All of the articles included in this review are publicly available.
A preprint of this study has been published with a link https://www.researchsquare.com/article/rs-2256610/v1.


