Abstract
Purpose
To assess the utility of precise radiologic and pathologic correlation for establishing imaging-histologic concordance or discordance as a method to limit the number of patients requiring surgical excision when atypical lobular hyperplasia (ALH) or lobular carcinoma in situ (LCIS) is diagnosed at core biopsy.
Materials and Methods
This study was approved by the institutional review board, and the requirement to obtain informed consent was waived. The pathology database was searched from 2000 to 2010 for core biopsies yielding ALH or LCIS devoid of any additional lesion that independently necessitated excision. All cases had to have either subsequent surgical excision or a minimum of 2 years of imaging follow-up. This yielded 50 cases from 49 women aged 40–73 years (mean age, 59 years). The authors performed detailed radiologic-pathologic analysis while blinded to subsequent follow-up information, comparing all biopsy-related images with the histologic findings at core biopsy and then designating each core biopsy finding as concordant or discordant. Then, results of subsequent surgery or extended follow-up for each case were unblinded and compared with original concordant or discordant designations. Outcomes and confidence intervals (CIs) were calculated.
Results
Of the 43 benign concordant core biopsy findings, none were upgraded at surgery (n = 38) or extended follow-up (n = 5) (95% CI: 0%, 8%). Of the seven discordant biopsy findings, two were upgraded to ductal carcinoma in situ at surgery (n = 5); none of the cases were upgraded at follow-up (n = 2).
Conclusion
When careful radiologic-pathologic correlation is performed and concordance is achieved, women with ALH or LCIS at core biopsy can be observed.
© RSNA, 2013
Introduction
Atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS) are noninvasive proliferations of lobular cells known to be an indicator of future increased risk for breast cancer development in ipsi- and contralateral breasts. ALH and LCIS are usually incidental findings at core needle biopsy as there are no reliable imaging features attributable to them. The literature regarding the management of incidental ALH and/or LCIS at core biopsy is contradictory and ranges from ongoing clinical and imaging evaluation to obligatory surgical excision (1–32). Although florid LCIS and pleomorphic LCIS might indicate site-specific risk for the development of invasive lobular carcinoma, there is currently no evidence that the risk of subsequent invasive carcinoma is reduced by local excision of small-volume ALH or LCIS (4,26,32–35). Absent the presence of adjacent frank malignancy, there is no demonstrable benefit to excising classic type ALH or LCIS. Some studies have found a substantial upgrade rate in diagnoses at excisional biopsy following an antecedent diagnosis of ALH or LCIS at core biopsy (3,5,7,8,12,15,17,20,22,27,28). It is unclear whether this is secondary to an inherently greater risk of having an associated higher-grade lesion or simply inadequate sampling of the radiographic finding that initiated the biopsy (36). This study was conducted to assess the utility of precise radiologic and pathologic correlation for establishing imaging-histologic concordance or discordance as a method to potentially limit the number of patients requiring subsequent surgical excision when ALH or LCIS is diagnosed at core biopsy.
Materials and Methods
This retrospective study was approved by the investigational review board and was compliant with the Health Insurance Portability and Accountability Act. The requirement to obtain informed consent was waived. The radiology and pathology database systems were searched for all cases of LCIS or ALH found at core biopsy between 2000 and 2010. Overall, there were 141 cases in the database. All cases that had any additional lesion that would have independently necessitated an excision were excluded (eg, papilloma, atypical ductal hyperplasia, radial scar, ductal carcinoma in situ, flat epithelial atypia, invasive carcinoma). Of those remaining, 53 cases of ALH and LCIS in 51 women had sufficient prebiopsy and biopsy-related images to permit retrospective radiologic-pathologic correlation. A minimum follow-up of 2 years was required for cases not undergoing subsequent surgical excision. Three cases were excluded owing to insufficient follow-up, leaving a total of 50 cases in 49 women aged 40–73 years (mean age, 59 years). Two biopsies were performed in one woman, but these were not done synchronously (each individual biopsy was performed during a 2-year interval) and each was done on a different breast. We included cases of pleomorphic LCIS or florid LCIS at core biopsy. We defined florid LCIS as 10 or more terminal ductal lobular units with LCIS or 1.0 cm with LCIS throughout most of the region. Pleomorphic LCIS was defined as containing large cells with irregular nuclear contours and conspicuous nucleoli.
By consensus of the physicians involved in the diagnosis and treatment of breast disease at the University of Virginia, all cases of ALH or LCIS diagnosed at core needle biopsy receive a recommendation for surgical excision of the biopsy site.
All breast images available at the time of the initial core biopsy were reviewed contemporaneously with the prepared histologic slides from the core biopsy by two breast imagers (M.A.C., with 30 years of experience, and S.R., with 1 year of experience) and a breast pathologist (K.A.A., with 9 years of experience). At these review sessions, the original Breast Imaging Reporting and Data System (BI-RADS) designation was recorded and the imaging findings necessitating the biopsy reviewed. The biopsy images (ultrasonography [US]–guided versus stereotactic versus magnetic resonance [MR] imaging–guided biopsy) and the number of cores obtained were reviewed and recorded along with review of the specimen radiograph (when available) as well as the postbiopsy mammogram for a general estimate of sampling adequacy. In the case of calcifications, the number of calcifications removed and thus depicted on the specimen radiograph relative to the number of calcifications present in the initial target lesion depicted on the prebiopsy mammogram was subjectively evaluated to determine sample adequacy.
The adequacy of mass sampling was judged by using clip position and biopsy-associated imaging changes relative to the target lesion. The pathologist then comprehensively conveyed the findings in the core biopsy specimen, including all benign processes, and provided a general estimation of the number of calcifications (when present) and the specific processes associated with any definable microcalcifications (eg, sclerosing adenosis, columnar cell change). For mass lesions sampled, the pathologist provided substantive information with regard to whether the microscopic findings could provide a reasonable explanation for an imaging mass. The original hematoxylin-eosin (H-E)–stained core biopsy slides were reviewed in 46 of the 50 cases. New slides were generated from biopsy paraffin blocks in four cases because the original tissue slides were no longer available.
Then, with full knowledge of the imaging and histologic lesion features, the authors assigned the imaging features of the lesion as “concordant” or “discordant” with the histologic results of core biopsy. If the histologic findings adequately explained the imaging findings, the results were “concordant.” If, on the other hand, the histologic results did not fully explain the findings identified at imaging, the results were defined as “discordant.” Any BI-RADS category 5 lesions were deemed discordant because they would automatically necessitate surgical consultation as the standard of care—even in the setting of a benign core biopsy result. All participants in the radiologic-pathologic conferences were blinded to the results of the subsequent surgical excisions or long-term follow-up until the completion of the analysis of the entire set of study cases. One radiologist (M.A.C.) had been the original primary diagnostic radiologist in six cases, and another (S.R.) had participated in the care of two of the patients. At the core biopsy review sessions, the radiologist responsible for the final concordant or discordant assignation (M.A.C.) was blinded to any patient-specific identifiers. In addition, at minimum, 8 months had elapsed between the radiologic-pathologic review sessions and clinical participation in the care of any of the research subjects. Taken together, the possibility of recall bias was thus believed to be small. Finally, the results from the excisional biopsies were recorded and the number of upgrades to ductal carcinoma in situ (DCIS) or invasive carcinoma in the group classified as concordant and the group classified as discordant were tabulated to test the hypothesis that lesions at core biopsy that was determined to be concordant at the radiologic-pathologic sessions were true-negative findings and thus would not require subsequent surgical excision. The confidence interval (CI) was calculated at the 0.95 confidence level, and the P value between the concordant and discordant groups was calculated by using a two-tailed Fisher exact test. Some authors include discovery of atypical ductal hyperplasia and flat epithelial atypia in the surgical excision specimen as upgrades; however, we have elected to exclude these entities in the upgrade category because the subsequent treatment of the patient in terms of surgical margins, chemoprophylaxis, and future high-risk evaluation is unaffected by their discovery at surgery.
Results
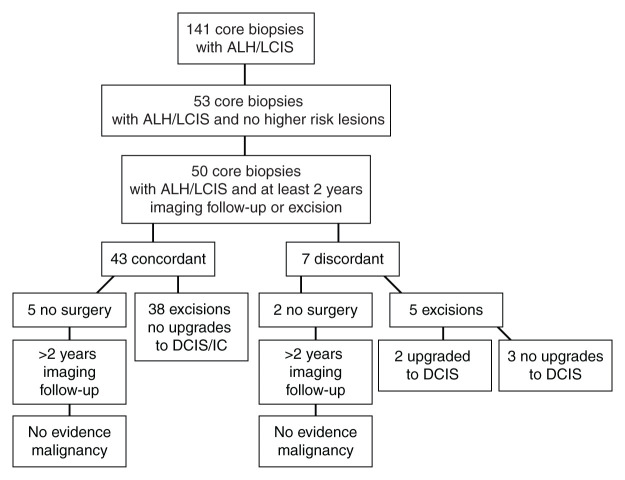
Fifty core biopsies from 49 patients had all radiologic and histologic images available for review and had an original core biopsy diagnosis of ALH or LCIS in an otherwise benign background. Excisions were performed in 43 of the 50 cases and are summarized in Figure 1. Those who did not undergo surgical excision underwent an imaging follow-up of 2–8 years.
Figure 1:
Flow diagram of total number of cases partitioned into radiologic and histologic concordance or discordance. IC = invasive carcinoma.
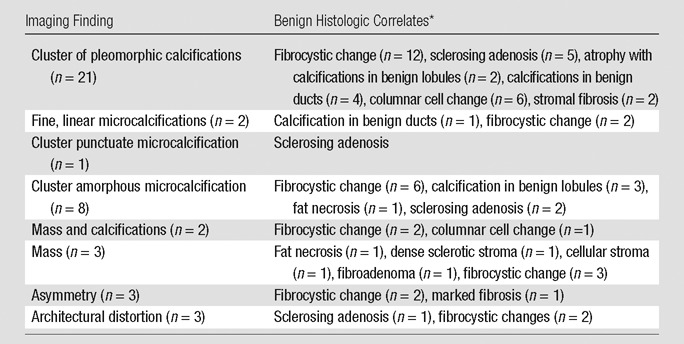
Imaging findings that originally prompted the core biopsies included mass (n = 8), asymmetry (n = 3), architectural distortion (n = 3), and calcifications (n = 36). The calcifications were described as pleomorphic (n = 24), amorphous (n = 9), fine linear (n = 2), and punctate (n = 1). Biopsy specimens were obtained with US guidance in 10 cases (average, 4.7 cores; range, 3–5 cores; median, five cores; eight with use of 14-gauge needles and two with use of 10-gauge needles), stereotactic biopsy in 39 cases (average, 8.2 cores; range, 4–12 cores; median, nine cores; 13 with use of 10-gauge needles, 24 with 11-gauge needles, and two with 12-gauge needles), and MR imaging–guided biopsy in one case (eight cores with use of an 11-gauge needle). One case was classified as BI-RADS category 3, 47 cases as BI-RADS category 4, and two cases as BI-RADS category 5. The 50 core biopsy specimens were classified as ALH (n = 11), LCIS (n = 33), florid LCIS (n = 4), and pleomorphic LCIS (n = 2) on the original core biopsy pathology report.
After radiologic-pathologic reviews were taken into consideration, seven of the 50 biopsies (14%) were classified as discordant (Table 1). The reason for the discrepancies included inadequate explanation for a mass in three cases (Fig 2) and insufficient calcifications in four. The calcification assessment broke down further into BI-RADS category 5 designation for microcalcifications in two cases (both were noted to have limited numbers of calcification on histologic slides), insufficient demonstration of calcifications on histologic slides in one case (Fig 3), and similarity of calcifications sampled near a biopsy site previously diagnosed as DCIS in one case. In two of the three masses, excisional biopsy showed fibrocystic changes and an intraductal papilloma. The remaining discordant case for a mass that did not have excisional biopsy underwent 5 years of follow-up imaging that revealed stability of the mass. Of the two cases classified as BI-RADS category 5 for microcalcifications and therefore discordant with benign histologic findings, the final diagnosis at surgical excisional biopsy was DCIS. For the case of insufficient demonstration of calcifications on histology slides, LCIS was diagnosed at excisional biopsy. Excisional biopsy was not performed in the calcifications sampled near a biopsy site previously diagnosed as DCIS; however, the calcifications have demonstrated 3 years of stability at imaging. Therefore, the upgrade rate to carcinoma in discordant biopsies was 29% (two of seven cases), with DCIS diagnosed in both cases (Table 1, Fig 1).
Table 1.
Results of Follow-up in Seven Discordant Cases
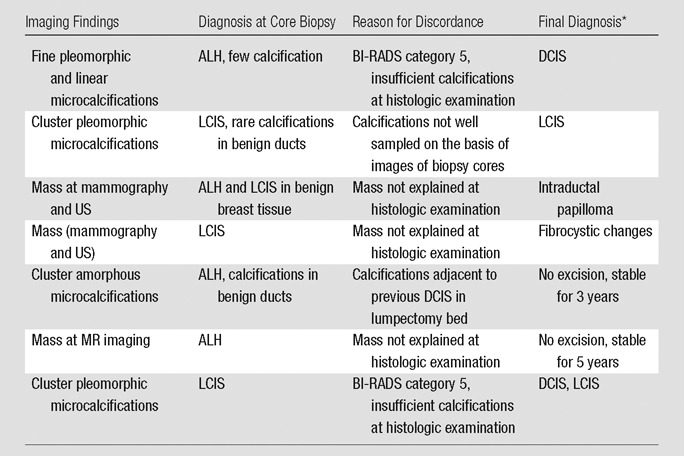
Final diagnosis was obtained at excision or follow-up.
Figure 2a:
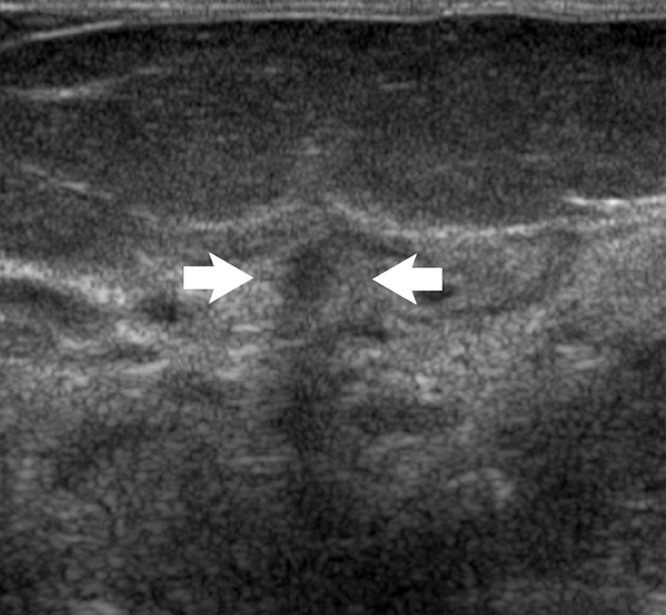
Images in 48-year-old woman with nipple discharge. (a) US scan shows small, irregular, hypoechoic mass (arrows), for which biopsy was recommended. (b) Low-power photomicrograph (H-E stain; original magnification, ×40) of core biopsy specimen reveals normal breast parenchyma with foci of ALH. This was discordant with imaging finding of mass, and surgical excision was recommended. (c) Photomicrograph of surgical specimen (H-E stain; original magnification, ×200) reveals small papilloma, which was fully excised.
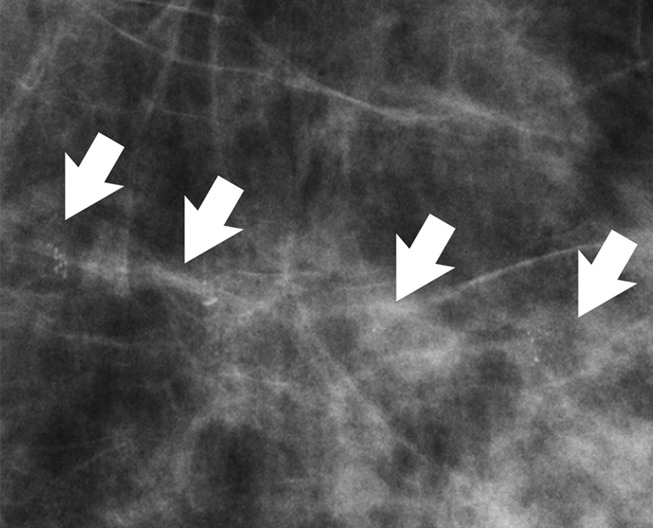
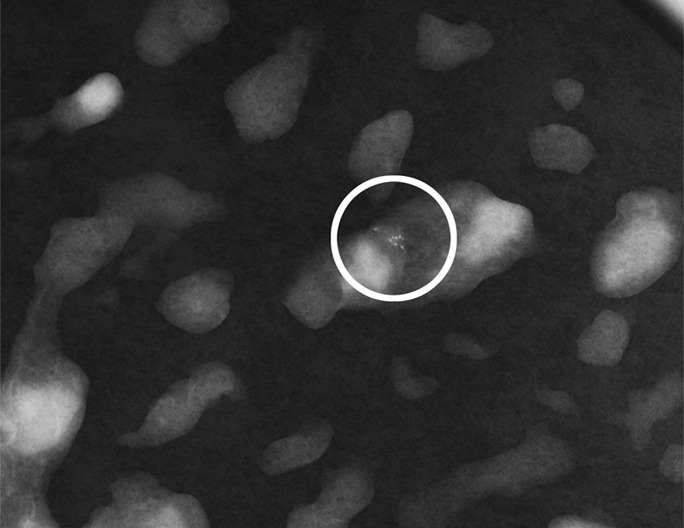
Figure 3a:
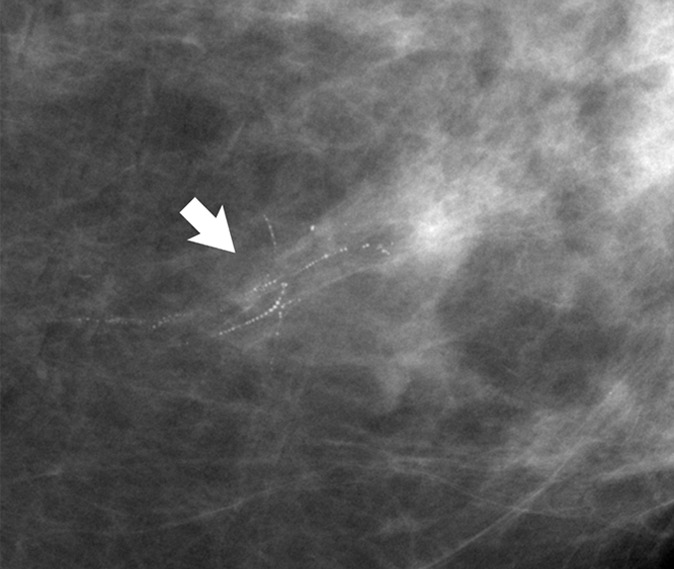
(a) Mammogram in 52-year-old woman shows suspicious fine linear branching microcalcifications (arrow), for which stereotaxic core biopsy was recommended and performed. (b) Radiograph of core specimen obtained at stereotaxic biopsy reveals numerous microcalcifications (circles). (c) Photomicrograph of core biopsy specimen (H-E stain; original magnification, ×200) reveals only scant benign calcification and foci of ALH (not pictured). The paucity of calcifications identified at histologic examination combined with the benign diagnosis was thought to be discordant, and surgical excision was recommended. (d) Photomicrograph of surgical specimen (H-E stain; original magnification, ×400) shows DCIS.
Figure 2b:
Images in 48-year-old woman with nipple discharge. (a) US scan shows small, irregular, hypoechoic mass (arrows), for which biopsy was recommended. (b) Low-power photomicrograph (H-E stain; original magnification, ×40) of core biopsy specimen reveals normal breast parenchyma with foci of ALH. This was discordant with imaging finding of mass, and surgical excision was recommended. (c) Photomicrograph of surgical specimen (H-E stain; original magnification, ×200) reveals small papilloma, which was fully excised.
Figure 2c:
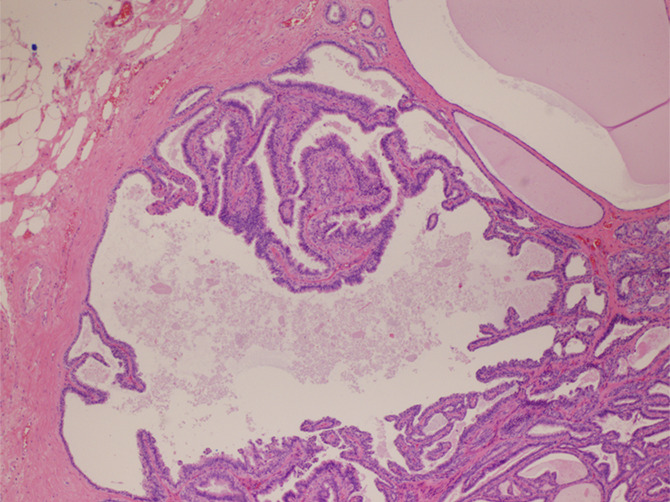
Images in 48-year-old woman with nipple discharge. (a) US scan shows small, irregular, hypoechoic mass (arrows), for which biopsy was recommended. (b) Low-power photomicrograph (H-E stain; original magnification, ×40) of core biopsy specimen reveals normal breast parenchyma with foci of ALH. This was discordant with imaging finding of mass, and surgical excision was recommended. (c) Photomicrograph of surgical specimen (H-E stain; original magnification, ×200) reveals small papilloma, which was fully excised.
Figure 3b:
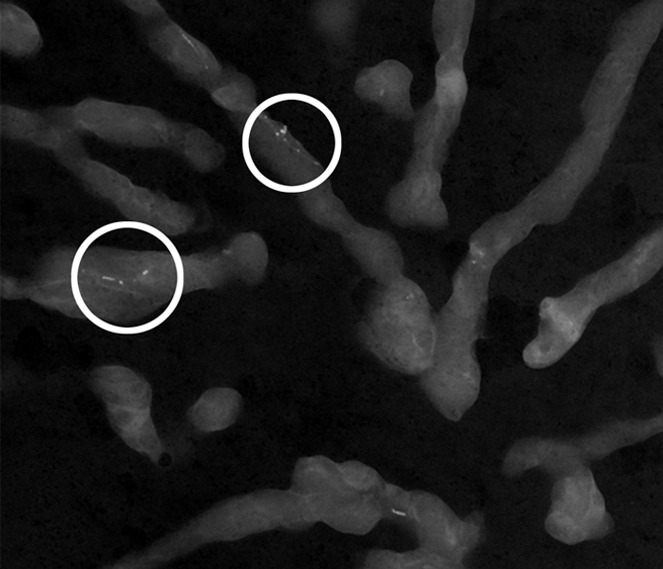
(a) Mammogram in 52-year-old woman shows suspicious fine linear branching microcalcifications (arrow), for which stereotaxic core biopsy was recommended and performed. (b) Radiograph of core specimen obtained at stereotaxic biopsy reveals numerous microcalcifications (circles). (c) Photomicrograph of core biopsy specimen (H-E stain; original magnification, ×200) reveals only scant benign calcification and foci of ALH (not pictured). The paucity of calcifications identified at histologic examination combined with the benign diagnosis was thought to be discordant, and surgical excision was recommended. (d) Photomicrograph of surgical specimen (H-E stain; original magnification, ×400) shows DCIS.
Figure 3c:
(a) Mammogram in 52-year-old woman shows suspicious fine linear branching microcalcifications (arrow), for which stereotaxic core biopsy was recommended and performed. (b) Radiograph of core specimen obtained at stereotaxic biopsy reveals numerous microcalcifications (circles). (c) Photomicrograph of core biopsy specimen (H-E stain; original magnification, ×200) reveals only scant benign calcification and foci of ALH (not pictured). The paucity of calcifications identified at histologic examination combined with the benign diagnosis was thought to be discordant, and surgical excision was recommended. (d) Photomicrograph of surgical specimen (H-E stain; original magnification, ×400) shows DCIS.
Figure 3d:
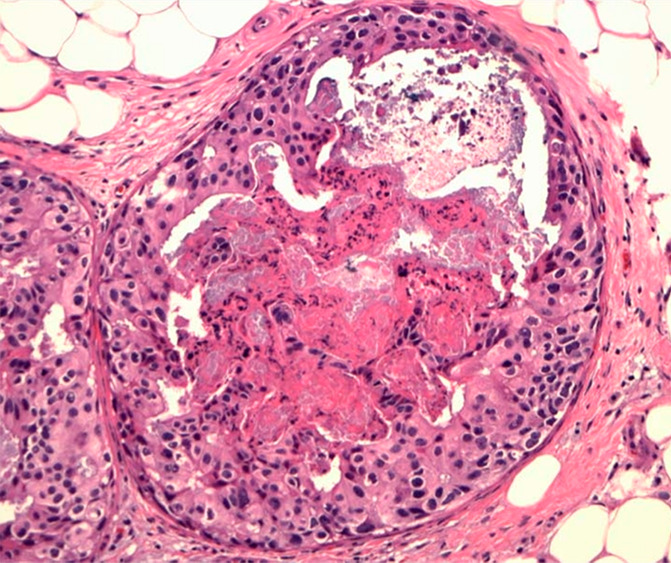
(a) Mammogram in 52-year-old woman shows suspicious fine linear branching microcalcifications (arrow), for which stereotaxic core biopsy was recommended and performed. (b) Radiograph of core specimen obtained at stereotaxic biopsy reveals numerous microcalcifications (circles). (c) Photomicrograph of core biopsy specimen (H-E stain; original magnification, ×200) reveals only scant benign calcification and foci of ALH (not pictured). The paucity of calcifications identified at histologic examination combined with the benign diagnosis was thought to be discordant, and surgical excision was recommended. (d) Photomicrograph of surgical specimen (H-E stain; original magnification, ×400) shows DCIS.
The four cases of florid LCIS and two cases of pleomorphic LCIS were all determined to be concordant at the radiologic-pathologic sessions (Fig 4). The results of excisional biopsy for the four cases of florid LCIS were invasive lobular carcinoma (diagnosed in another part of the breast, not where the diagnosis of LCIS was made) (n = 1), LCIS (n = 2), and atypical ductal hyperplasia and LCIS (n = 1). The excisions of the two cases of pleomorphic LCIS demonstrated the usual type of LCIS in one case and pleomorphic LCIS and atypical ductal hyperplasia in the other.
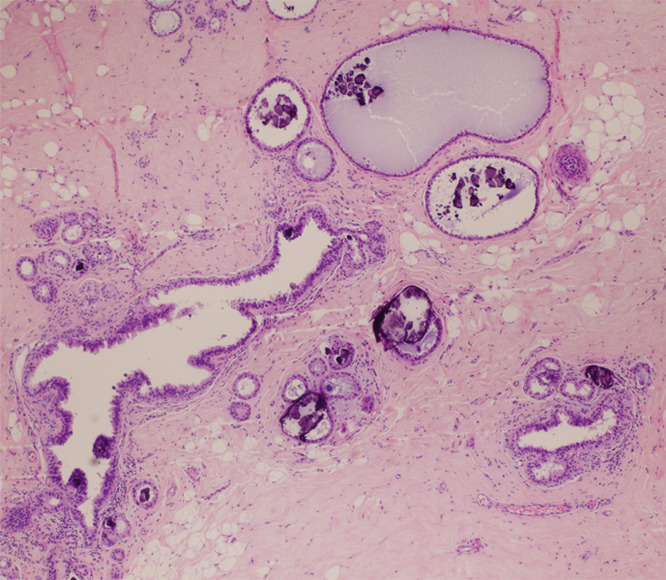
Figure 4a:
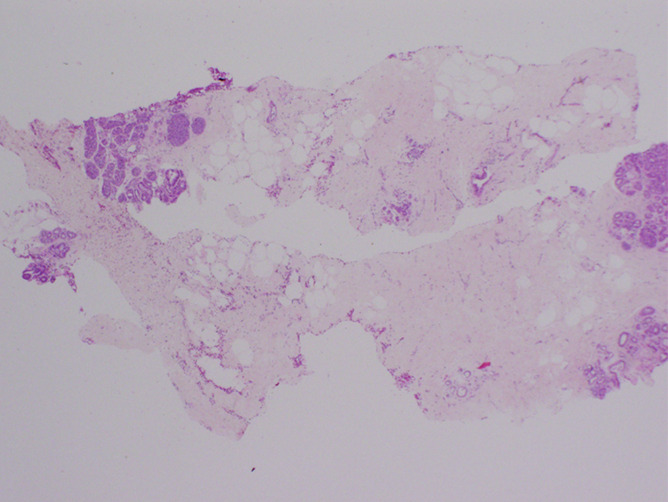
(a) Magnification view of left breast in 62-year-old woman reveals fine pleomorphic microcalcifications in segmental distribution (arrows), for which stereotaxic core biopsy was recommended. (b) Radiograph of specimen from stereotaxic biopsy reveals adequate sampling of suspicious microcalcifications (circle). (c) Photomicrograph of specimen (H-E stain; original magnification, ×400) reveals fibrocystic changes with scattered microcalcifications. (d) Although this histologic finding was concordant with imaging features, florid LCIS (H-E stain; original magnification, ×200) was also identified in core specimens and excision was recommended. Final excision showed noncalcified florid LCIS.
Figure 4b:
(a) Magnification view of left breast in 62-year-old woman reveals fine pleomorphic microcalcifications in segmental distribution (arrows), for which stereotaxic core biopsy was recommended. (b) Radiograph of specimen from stereotaxic biopsy reveals adequate sampling of suspicious microcalcifications (circle). (c) Photomicrograph of specimen (H-E stain; original magnification, ×400) reveals fibrocystic changes with scattered microcalcifications. (d) Although this histologic finding was concordant with imaging features, florid LCIS (H-E stain; original magnification, ×200) was also identified in core specimens and excision was recommended. Final excision showed noncalcified florid LCIS.
Figure 4c:
(a) Magnification view of left breast in 62-year-old woman reveals fine pleomorphic microcalcifications in segmental distribution (arrows), for which stereotaxic core biopsy was recommended. (b) Radiograph of specimen from stereotaxic biopsy reveals adequate sampling of suspicious microcalcifications (circle). (c) Photomicrograph of specimen (H-E stain; original magnification, ×400) reveals fibrocystic changes with scattered microcalcifications. (d) Although this histologic finding was concordant with imaging features, florid LCIS (H-E stain; original magnification, ×200) was also identified in core specimens and excision was recommended. Final excision showed noncalcified florid LCIS.
Figure 4d:
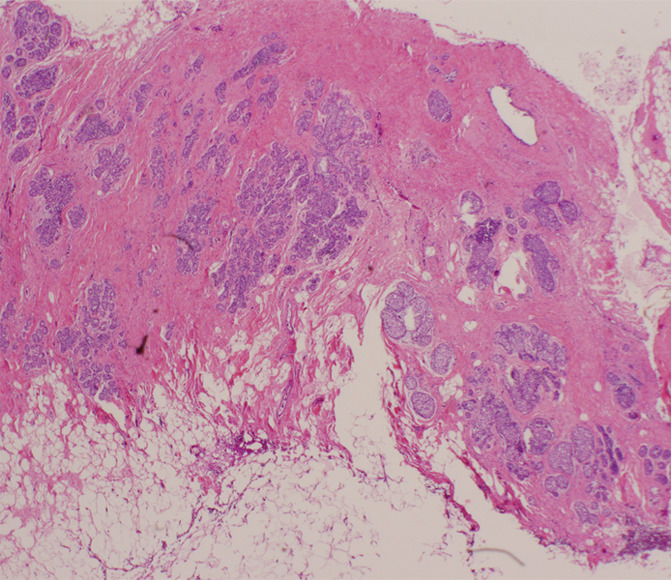
(a) Magnification view of left breast in 62-year-old woman reveals fine pleomorphic microcalcifications in segmental distribution (arrows), for which stereotaxic core biopsy was recommended. (b) Radiograph of specimen from stereotaxic biopsy reveals adequate sampling of suspicious microcalcifications (circle). (c) Photomicrograph of specimen (H-E stain; original magnification, ×400) reveals fibrocystic changes with scattered microcalcifications. (d) Although this histologic finding was concordant with imaging features, florid LCIS (H-E stain; original magnification, ×200) was also identified in core specimens and excision was recommended. Final excision showed noncalcified florid LCIS.
Of the 43 cases with concordant imaging and pathologic findings, 38 underwent subsequent excision. None of those 38 cases had any carcinoma (malignancy rate, 0%; 95% CI: 0%, 8%; Table 2), five showed atypical ductal hyperplasia, and 33 had no worse pathologic finding than that identified at the original core biopsy. In other words, none of the cases with concordant histologic and radiographic correlation were upgraded after excision (Figs 5, 6). All five cases without excision have had at least 3 years of follow-up imaging with no evidence of breast cancer (range, 3–8 years).
Table 2.
Histologic Findings in 43 Benign Concordant Core Biopsies according to Radiologic Finding
Some histologic diagnoses were used in multiple cases. In some cases, several histologic findings occurred in a single case.
Figure 5a:
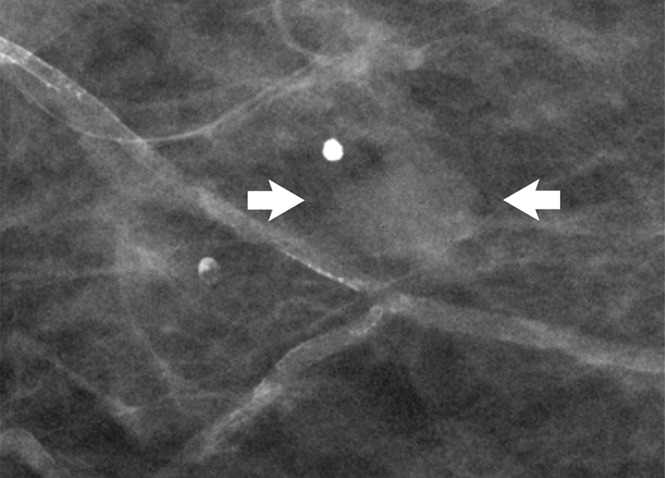
(a) Spot mammogram in 58-year-old woman reveals oval mass with indistinct margins (arrows), for which histologic sampling was recommended. (b) Photomicrograph of core biopsy specimen reveals sclerosed fibroadenoma (H-E stain; original magnification, ×200), which is concordant with imaging features.
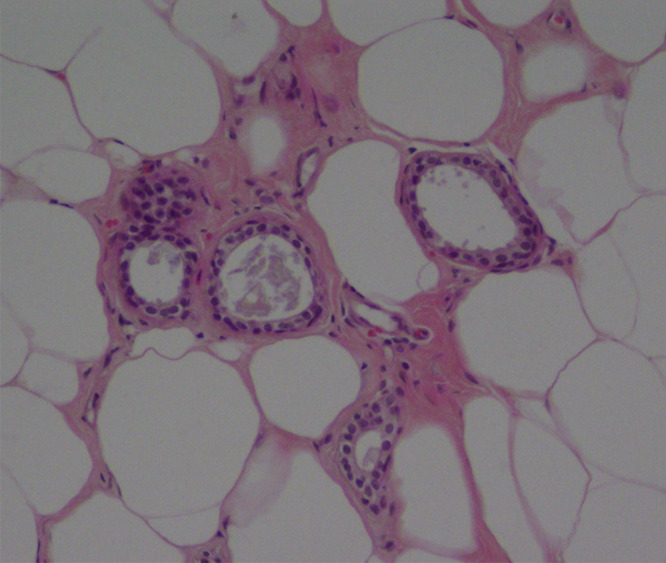
Figure 6a:
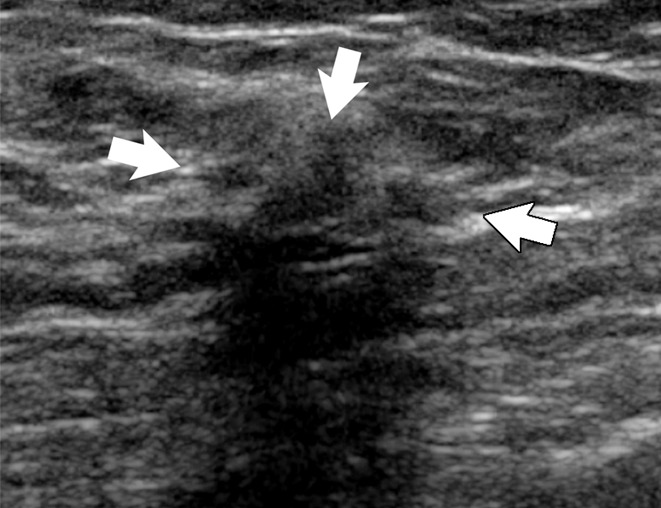
(a) Image in 54-year-old woman with irregular hypoechoic mass (arrows) with posterior acoustic shadowing, for which US-guided core biopsy was recommended. (b) Photomicrograph of core biopsy specimen (H-E stain; original magnification, ×40) shows extensive dense sclerosis in all cores (light pink stroma at left and top) and foci of ALH (right). Sclerosis was thought to be concordant with imaging features. Surgical excision revealed only ALH.
Figure 5b:
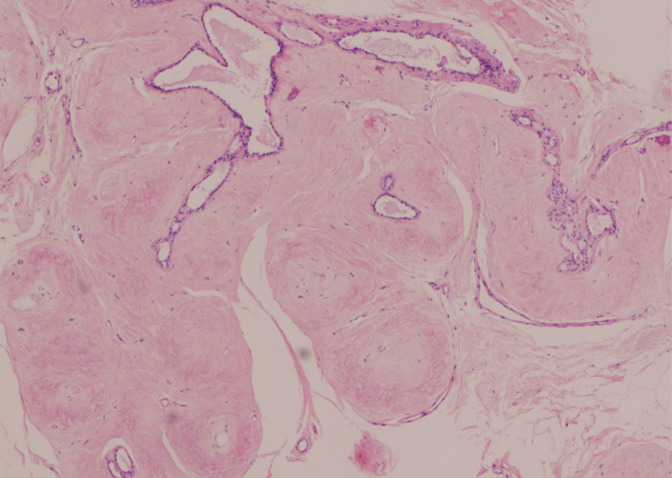
(a) Spot mammogram in 58-year-old woman reveals oval mass with indistinct margins (arrows), for which histologic sampling was recommended. (b) Photomicrograph of core biopsy specimen reveals sclerosed fibroadenoma (H-E stain; original magnification, ×200), which is concordant with imaging features.
Figure 6b:
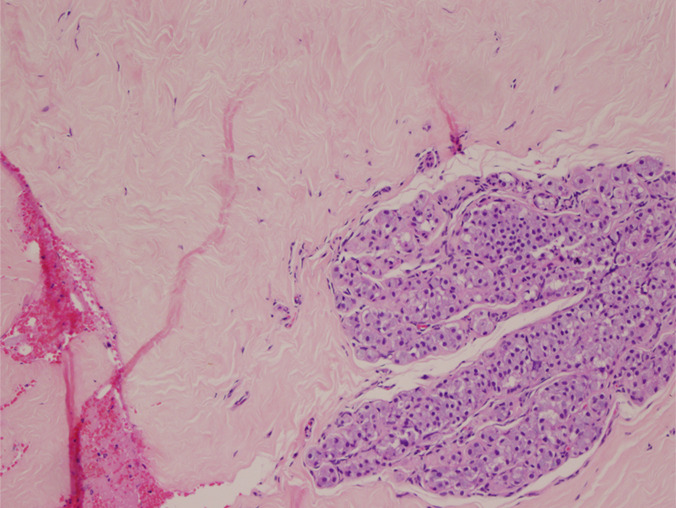
(a) Image in 54-year-old woman with irregular hypoechoic mass (arrows) with posterior acoustic shadowing, for which US-guided core biopsy was recommended. (b) Photomicrograph of core biopsy specimen (H-E stain; original magnification, ×40) shows extensive dense sclerosis in all cores (light pink stroma at left and top) and foci of ALH (right). Sclerosis was thought to be concordant with imaging features. Surgical excision revealed only ALH.
If we consider all original core biopsy results, the upgrade rate to DCIS was 4% (two of 50 cases). If atypical ductal hyperplasia is included, the upgrade rate was 14% (seven of 50 cases). With our radiologic-pathologic correlation, the upgrade rate to DCIS and/or invasive carcinoma for concordant cases was 0% (0 of 43 cases; 95% CI: 0%, 8%). Our ability to appropriately classify cases into concordant and discordant categories was statistically significant (P = .027). Forty-three of the 50 recommendations for surgical excision (86%) could have been avoided (95% CI: 76%, 95%). In our retrospective study, this would have translated to 32 actual excisions avoided (37 surgeries recommended minus the five surgeries deferred) (95% CI: 50%, 77%).
Discussion
In one of the first articles discussing the management of incidental LCIS at breast core biopsy, Liberman et al (9) suggested that if imaging-histologic concordance existed, excision was not necessary unless the LCIS was associated with another high-risk lesion or the lesion was noted to have features that overlapped with those of DCIS. However, several articles soon followed with the opposite conclusion, maintaining that upgrade rates for ALH and LCIS to DCIS and/or invasive carcinoma in up to 22% of cases mandated surgical excision in all cases where either diagnosis was made at core biopsy. Currently, many institutions, including ours, adhere to this guideline by recommending surgical excision of the biopsy site in all women with a core biopsy diagnosis of either ALH or LCIS. We questioned whether such a categoric approach to these lesions is necessary and whether, instead, an assiduous tandem review of the histologic specimen obtained at core biopsy together with the appropriate pre- and postbiopsy images would enable confident exclusion of a number of these cases from surgical excision. If this proved to be the case, the benefits of reducing the number of surgeries would be obvious from both an economic and patient perspective.
A caveat relating to the histologic diagnoses of florid and pleomorphic lobular carcinoma in situ diagnosed at core biopsy is in order. To minimize the possibility of selection bias, we included all consecutive cases of ALH and LCIS diagnosed at core biopsy. Thus, included were two cases of pleomorphic LCIS and four cases of florid LCIS, all benign concordant on study radiologic-pathologic review and none ultimately upgraded at surgery (malignancy rate, 0%; 95% CI: 0%, 8%). Existing data suggest that a pleomorphic histology or large volumes of LCIS may be predictive of a more focally aggressive natural history, leading to frank malignancy in a substantial number of cases (33,37–39). Obligatory surgical excision allows for increased volumetric analysis and removes a particular focus from the breast, thereby limiting the possibility for future progression to frank malignancy. The very small number of pleomorphic and florid LCIS cases analyzed in this study is insufficient to permit any management conclusions. Until more definitive data on the topic are published, we strongly suggest conservative management, with a recommendation to surgically excise all such cases diagnosed at core biopsy.
Of 32 recent articles looking at the surgical upgrade rates in core biopsies with lobular neoplasia, only nine have a comprehensive review of both histologic and imaging findings (2,5,6,9,10,16,29,31,32). Some articles give the overall upgrade rate of a core biopsy with only LCIS and then the excision diagnosis but neglect to comprehensively assess how many of the original core biopsies would have been considered discordant to the radiologist. In our series, when this consensus exercise was practiced, 43 of the 50 recommendations for surgical excision (86%) could have been safely avoided (95% CI: 76%, 95.6%). Even correcting for our recommendation to advise surgical excision in the six core biopsy cases with pleomorphic or florid LCIS, 37 of the 50 surgical excision recommendations (74%) could be avoided (95% CI: 61.8%, 86.1%). In our retrospective study, this would have translated to 32 (64%) actual excisions avoided (37 surgeries recommended minus the five surgeries deferred) (95% CI: 50%, 77%).
What has emerged as the cornerstone of successful radiologic-pathologic correlation is the necessity for the pathologists to provide a complete and accurate analysis of the core biopsy material. Just as radiologists may defer imaging-histologic correlation under the institutional mandate that all ALH and LCIS diagnosed at core biopsy undergo surgery, many pathologists may realize that the identification of either ALH or LCIS at core biopsy will trigger a surgical excision and might truncate their report once they have identified this entity absent any other high-risk or malignant disease. For instance, although we found that the subjective quantification of calcifications was rarely noted in the original pathology reports, this information aided us greatly in assigning concordance and discordance in the radiologic-pathologic review. Our study suggests that by extending their analyses to include additional benign processes that might fully explain the imaging features, enhanced radiologic-pathologic analysis can be performed and subsequent surgical biopsy may be avoided in benign concordant cases.
One limitation of our study is the relatively small number of cases with ALH or LCIS (n = 50), which limits the conclusions that can be drawn from our data. A second limitation of potential recall bias by participants in the study radiologic-pathologic conferences was minimized by anonymizing the study cases and the minimum of 8 months that elapsed between clinical management and the actual study.
In summary, we found that, with careful pathologic-radiologic correlation, noninvasive ALH and LCIS were not independent risk factors for worse pathology on excision. None of the 43 (95% CI: 0%, 8%) benign concordant cases determined with careful radiologic-pathologic correlation were upgraded at subsequent surgical excision or extended imaging follow-up, which suggests that arbitrary excision in all cases of ALH or LCIS may not be necessary.
In essence, we have reaffirmed the work of Liberman et al (9), who suggested that LCIS (and we have added ALH) with concordant imaging-histologic analysis need not undergo surgical biopsy. We have found that if there is comprehensive communication between the radiologist and pathologist, triaging of the biopsy results works well and may save many patients from undergoing surgical excision. However, as with virtually all publications dealing with ALH and LCIS, the numbers presented herein are relatively small. Additional validation of this approach with use of some similar format of extended radiologic-pathologic analysis to establish imaging-histologic concordance or discordance by other investigators at different sites and with differing practice styles is required before this approach can be universally applied.
Advance in Knowledge
■ When careful radiologic-pathologic correlation is conducted in the setting of a breast core biopsy with atypical lobular hyperplasia or lobular carcinoma in situ, some women can be safely triaged to observation; of the 43 benign concordant cases, none were upgraded at surgery or extended follow-up (95% confidence interval: 0%, 8%).
Implication for Patient Care
■ Focused and complete radiologic-pathologic correlation may obviate excisional biopsy in patients with benign concordant biopsy findings.
Disclosures of Conflicts of Interest: K.A. No relevant conflicts of interest to disclose. M.A.C. No relevant conflicts of interest to disclose. B.N. No relevant conflicts of interest to disclose. S.R. No relevant conflicts of interest to disclose.
Received September 12, 2012; revision requested October 22; revision received February 22, 2013; accepted March 11; final version accepted May 15.
Abbreviations:
- ALH
- atypical lobular hyperplasia
- BI-RADS
- Breast Imaging Reporting and Data System
- CI
- confidence interval
- DCIS
- ductal carcinoma in situ
- HE
- hematoxylin-eosin
- LCIS
- lobular carcinoma in situ
References
- 1. Anderson BO , Calhoun KE , Rosen EL . Evolving concepts in the management of lobular neoplasia . J Natl Compr Canc Netw 2006. ; 4 ( 5 ): 511 – 522 . [DOI] [PubMed] [Google Scholar]
- 2. Berg WA , Mrose HE , Ioffe OB . Atypical lobular hyperplasia or lobular carcinoma in situ at core-needle breast biopsy . Radiology 2001. ; 218 ( 2 ): 503 – 509 . [DOI] [PubMed] [Google Scholar]
- 3. Brem RF , Lechner MC , Jackman RJ , et al. Lobular neoplasia at percutaneous breast biopsy: variables associated with carcinoma at surgical excision . AJR Am J Roentgenol 2008. ; 190 ( 3 ): 637 – 641 . [DOI] [PubMed] [Google Scholar]
- 4. Carder PJ , Shaaban A , Alizadeh Y , Kumarasuwamy V , Liston JC , Sharma N . Screen-detected pleomorphic lobular carcinoma in situ (PLCIS): risk of concurrent invasive malignancy following a core biopsy diagnosis . Histopathology 2010. ; 57 ( 3 ): 472 – 478 . [DOI] [PubMed] [Google Scholar]
- 5. Cangiarella J , Guth A , Axelrod D , et al. Is surgical excision necessary for the management of atypical lobular hyperplasia and lobular carcinoma in situ diagnosed on core needle biopsy? A report of 38 cases and review of the literature . Arch Pathol Lab Med 2008. ; 132 ( 6 ): 979 – 983 . [DOI] [PubMed] [Google Scholar]
- 6. Crisi GM , Mandavilli S , Cronin E , Ricci A Jr . Invasive mammary carcinoma after immediate and short-term follow-up for lobular neoplasia on core biopsy . Am J Surg Pathol 2003. ; 27 ( 3 ): 325 – 333 . [DOI] [PubMed] [Google Scholar]
- 7. Elsheikh TM , Silverman JF . Follow-up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: a correlative study of 33 patients with review of the literature . Am J Surg Pathol 2005. ; 29 ( 4 ): 534 – 543 . [DOI] [PubMed] [Google Scholar]
- 8. Foster MC , Helvie MA , Gregory NE , Rebner M , Nees AV , Paramagul C . Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology 2004. ; 231 ( 3 ): 813 – 819 . [DOI] [PubMed] [Google Scholar]
- 9. Liberman L , Sama M , Susnik B , et al. Lobular carcinoma in situ at percutaneous breast biopsy: surgical biopsy findings . AJR Am J Roentgenol 1999. ; 173 ( 2 ): 291 – 299 . [DOI] [PubMed] [Google Scholar]
- 10. Middleton LP , Grant S , Stephens T , Stelling CB , Sneige N , Sahin AA . Lobular carcinoma in situ diagnosed by core needle biopsy: when should it be excised? Mod Pathol 2003. ; 16 ( 2 ): 120 – 129 . [DOI] [PubMed] [Google Scholar]
- 11. O’Neil M , Madan R , Tawfik OW , Thomas PA , Fan F . Lobular carcinoma in situ/atypical lobular hyperplasia on breast needle biopsies: does it warrant surgical excisional biopsy? A study of 27 cases . Ann Diagn Pathol 2010. ; 14 ( 4 ): 251 – 255 . [DOI] [PubMed] [Google Scholar]
- 12. Polom K , Murawa D , Pawelska A , Murawa P . Atypical lobular hyperplasia and lobular carcinoma in situ without other high-risk lesions diagnosed on vacuum-assisted core needle biopsy: the problem of excisional biopsy . Tumori 2009. ; 95 ( 1 ): 32 – 35 . [DOI] [PubMed] [Google Scholar]
- 13. Nagi CS , O’Donnell JE , Bleiweiss IJ , Jaffer SM . Lobular neoplasia on core needle biopsy does not require excision . Cancer 2008. ; 112 ( 10 ): 2152 – 2158 . [DOI] [PubMed] [Google Scholar]
- 14. Renshaw AA , Cartagena N , Derhagopian RP , Gould EW . Lobular neoplasia in breast core needle biopsy specimens is not associated with an increased risk of ductal carcinoma in situ or invasive carcinoma . Am J Clin Pathol 2002. ; 117 ( 5 ): 797 – 799 . [DOI] [PubMed] [Google Scholar]
- 15. Shin SJ , Rosen PP . Excisional biopsy should be performed if lobular carcinoma in situ is seen on needle core biopsy . Arch Pathol Lab Med 2002. ; 126 ( 6 ): 697 – 701 . [DOI] [PubMed] [Google Scholar]
- 16. Subhawong AP , Subhawong TK , Khouri N , Tsangaris T , Nassar H . Incidental minimal atypical lobular hyperplasia on core needle biopsy: correlation with findings on follow-up excision . Am J Surg Pathol 2010. ; 34 ( 6 ): 822 – 828 . [DOI] [PubMed] [Google Scholar]
- 17. Arpino G , Laucirica R , Elledge RM . Premalignant and in situ breast disease: biology and clinical implications . Ann Intern Med 2005. ; 143 ( 6 ): 446 – 457 . [DOI] [PubMed] [Google Scholar]
- 18. Bowman K , Munoz A , Mahvi DM , Breslin TM . Lobular neoplasia diagnosed at core biopsy does not mandate surgical excision . J Surg Res 2007. ; 142 ( 2 ): 275 – 280 . [DOI] [PubMed] [Google Scholar]
- 19. Bauer VP , Ditkoff BA , Schnabel F , Brenin D , El-Tamer M , Smith S . The management of lobular neoplasia identified on percutaneous core breast biopsy . Breast J 2003. ; 9 ( 1 ): 4 – 9 . [DOI] [PubMed] [Google Scholar]
- 20. Dmytrasz K , Tartter PI , Mizrachy H , Chinitz L , Rosenbaum Smith S , Estabrook A . The significance of atypical lobular hyperplasia at percutaneous breast biopsy . Breast J 2003. ; 9 ( 1 ): 10 – 12 . [DOI] [PubMed] [Google Scholar]
- 21. Osborne MP , Hoda SA . Current management of lobular carcinoma in situ of the breast . Oncology (Williston Park) 1994. ; 8 ( 2 ): 45 – 49; discussion 49, 53–54 . [PubMed] [Google Scholar]
- 22. Irfan K , Brem RF . Surgical and mammographic follow-up of papillary lesions and atypical lobular hyperplasia diagnosed with stereotactic vacuum-assisted biopsy . Breast J 2002. ; 8 ( 4 ): 230 – 233 . [DOI] [PubMed] [Google Scholar]
- 23. Jacobs TW , Byrne C , Colditz G , Connolly JL , Schnitt SJ . Pathologic features of breast cancers in women with previous benign breast disease . Am J Clin Pathol 2001. ; 115 ( 3 ): 362 – 369 . [DOI] [PubMed] [Google Scholar]
- 24. Karabakhtsian RG , Johnson R , Sumkin J , Dabbs DJ . The clinical significance of lobular neoplasia on breast core biopsy . Am J Surg Pathol 2007. ; 31 ( 5 ): 717 – 723 . [DOI] [PubMed] [Google Scholar]
- 25. Lakhani SR , Audretsch W , Cleton-Jensen AM , et al. The management of lobular carcinoma in situ (LCIS): is LCIS the same as ductal carcinoma in situ (DCIS)? Eur J Cancer 2006. ; 42 ( 14 ): 2205 – 2211 . [DOI] [PubMed] [Google Scholar]
- 26. Esserman LE , Lamea L , Tanev S , Poppiti R . Should the extent of lobular neoplasia on core biopsy influence the decision for excision? Breast J 2007. ; 13 ( 1 ): 55 – 61 . [DOI] [PubMed] [Google Scholar]
- 27. Mahoney MC , Robinson-Smith TM , Shaughnessy EA . Lobular neoplasia at 11-gauge vacuum-assisted stereotactic biopsy: correlation with surgical excisional biopsy and mammographic follow-up . AJR Am J Roentgenol 2006. ; 187 ( 4 ): 949 – 954 . [DOI] [PubMed] [Google Scholar]
- 28. Margenthaler JA , Duke D , Monsees BS , Barton PT , Clark C , Dietz JR . Correlation between core biopsy and excisional biopsy in breast high-risk lesions . Am J Surg 2006. ; 192 ( 4 ): 534 – 537 . [DOI] [PubMed] [Google Scholar]
- 29. O’Driscoll D , Britton P , Bobrow L , Wishart GC , Sinnatamby R , Warren R . Lobular carcinoma in situ on core biopsy: what is the clinical significance? Clin Radiol 2001. ; 56 ( 3 ): 216 – 220 . [DOI] [PubMed] [Google Scholar]
- 30. Renshaw AA , Derhagopian RP , Martinez P , Gould EW . Lobular neoplasia in breast core needle biopsy specimens is associated with a low risk of ductal carcinoma in situ or invasive carcinoma on subsequent excision . Am J Clin Pathol 2006. ; 126 ( 2 ): 310 – 313 . [DOI] [PubMed] [Google Scholar]
- 31. Murray MP , Luedtke C , Liberman L , Nehhozina T , Akram M , Brogi E . Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision . Cancer 2013. ; 119 ( 5 ): 1073 – 1079 . [DOI] [PubMed] [Google Scholar]
- 32. Rendi MH , Dintzis SM , Lehman CD , Calhoun KE , Allison KH . Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy . Ann Surg Oncol 2012. ; 19 ( 3 ): 914 – 921 . [DOI] [PubMed] [Google Scholar]
- 33. Chivukula M , Haynik DM , Brufsky A , Carter G , Dabbs DJ . Pleomorphic lobular carcinoma in situ (PLCIS) on breast core needle biopsies: clinical significance and immunoprofile . Am J Surg Pathol 2008. ; 32 ( 11 ): 1721 – 1726 . [DOI] [PubMed] [Google Scholar]
- 34. Contreras A , Sattar H . Lobular neoplasia of the breast: an update . Arch Pathol Lab Med 2009. ; 133 ( 7 ): 1116 – 1120 . [DOI] [PubMed] [Google Scholar]
- 35. Page DL , Schuyler PA , Dupont WD , Jensen RA , Plummer WD Jr , Simpson JF . Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study . Lancet 2003. ; 361 ( 9352 ): 125 – 129 . [DOI] [PubMed] [Google Scholar]
- 36. Cohen MA . Cancer upgrades at excisional biopsy after diagnosis of atypical lobular hyperplasia or lobular carcinoma in situ at core-needle biopsy: some reasons why . Radiology 2004. ; 231 ( 3 ): 617 – 621 . [DOI] [PubMed] [Google Scholar]
- 37. Mihalik JE , Krupka L , Davenport R , Tucker L , Toevs C , Smith RS . The rate of imaging-histologic discordance of benign breast disease: a multidisciplinary approach to the management of discordance at a large university-based hospital . Am J Surg 2010. ; 199 ( 3 ): 319 – 323; discussion 323 . [DOI] [PubMed] [Google Scholar]
- 38. de Mascarel I , Brouste V , Asad-Syed M , Hurtevent G , Macgrogan G . All atypia diagnosed at stereotactic vacuum-assisted breast biopsy do not need surgical excision . Mod Pathol 2011. ; 24 ( 9 ): 1198 – 1206 . [DOI] [PubMed] [Google Scholar]
- 39. Carson W , Sanchez-Forgach E , Stomper P , Penetrante R , Tsangaris TN , Edge SB . Lobular carcinoma in situ: observation without surgery as an appropriate therapy . Ann Surg Oncol 1994. ; 1 ( 2 ): 141 – 146 . [DOI] [PubMed] [Google Scholar]