Abstract
Background
Despite recognized improvements in obesity-related comorbidities, mounting evidence implicates surgical weight loss in the onset of skeletal fragility. Sleeve gastrectomy (SG) is the most commonly performed bariatric procedure and is associated with 3–7% axial bone loss in the year following surgery. Bisphosphonates are FDA-approved medications for the prevention and treatment of age-related bone loss and may represent a strategy to reduce bone loss following SG surgery.
Methods
The Strategies to Reduce the Onset of Sleeve Gastrectomy Associated Bone Loss (STRONG BONES) trial (NCT04922333) is designed to definitively test whether monthly administration of the bisphosphonate, risedronate, for six months can effectively counter SG-associated bone loss. Approximately 120 middle-aged and older (≥40 years) SG patients will be randomized to six months of risedronate or placebo treatment, with skeletal outcomes assessed at baseline, six, and 12-months post-surgery. The primary outcome of the trial is 12-month change in total hip areal bone mineral density (aBMD), measured by dual energy x-ray absorptiometry (DXA). This will be complemented by DXA-acquired aBMD assessment at other skeletal sites and quantitative computed tomography (QCT) derived changes in bone quality. Change in muscle mass and function will also be assessed, as well as biomarkers of bone health, turnover, and crosstalk, providing mechanistic insight into intervention-related changes to the bone-muscle unit.
Discussion
Results from the STRONG BONES trial have the potential to influence current clinical practice by determining the ability of bisphosphonate use to mitigate bone loss and concomitant fracture risk in middle-aged and older SG patients.
Keywords: Bone mineral density, Bariatric surgery, Older adults, Weight loss, Bisphosphonate
1. Introduction
Over the past decade, severe obesity [body mass index (BMI) ≥40 kg/m2] prevalence has steadily increased in the United States, with recent estimates showing 11% of middle-aged (40–59 years) and 6% of older (60+ years) adults affected. [1] Bariatric surgery is increasingly utilized to treat severe obesity, with the sleeve gastrectomy (SG) procedure accounting for ∼60% of all bariatric procedures. [2] Although SG is effective at reducing weight and comorbidities associated with obesity, evidence suggests a 3–7% concomitant reduction in areal bone mineral density (aBMD). [3] Reviews of fracture risk secondary to bariatric procedures indicate a higher likelihood of fracture. [[4], [5], [6]] In recognition of this clinical conundrum, the American Society for Metabolic and Bariatric Surgery (ASMBS) issued a position statement in 2020 calling for additional randomized data to better determine optimal interventions and treatments aimed at minimizing fracture risk in bariatric surgery patients. [7]
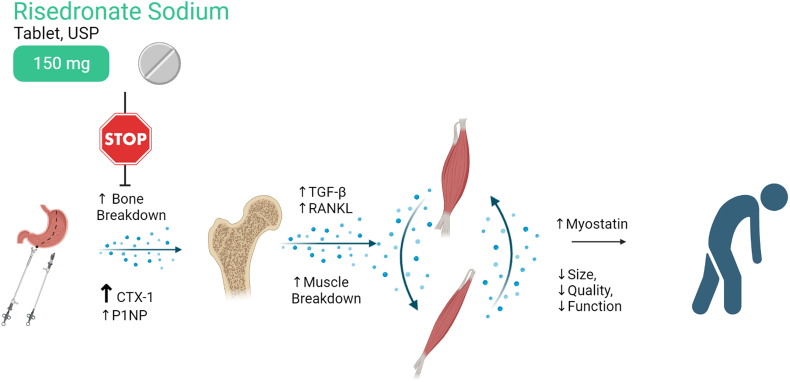
Once-monthly risedronate [8] is an oral bisphosphonate prescribed to prevent and treat bone loss [[8], [9], [10]] by decreasing osteoclast activity, thereby slowing bone resorption. [11,12] As bone loss following SG is driven by increases in resorption (versus declines in formation), [13] it is reasonable to hypothesize that SG-associated bone loss [14] could be countered by osteoclast inhibition with risedronate. Indeed, pilot data support this premise, demonstrating that 6-months of risedronate treatment is feasible and likely effective in reducing SG-associated bone loss and resorption, as compared to placebo. [15,16] Data from this pilot trial also signal a lean mass sparing effect with risedronate use. This novel finding aligns with data from murine models of clinical pathology [[17], [18], [19]] and limited observational data in humans, [20,21] which we hypothesize may be due to a blunting of SG-associated osteokine release (Fig. 1). The pilot trial makes use of short-term therapy during the most active weight loss phase to minimize patient exposure and the cost of therapy. An appropriately powered trial will support updates to postoperative care for SG patients, [22] while also providing a unique platform to investigate mechanisms of bone-muscle crosstalk. [7,22]
Fig. 1.
Hypothesized mechanism of lean tissue sparing effect from risedronate.
The main objective of the Strategies to Reduce the Onset of Sleeve Gastrectomy Associated Bone Loss (STRONG BONES) trial is to definitively test whether risedronate use can effectively counter SG-associated musculoskeletal tissue loss. To address this clinical question, approximately 120 middle aged and older (≥40 years) SG patients will be randomized to a six-month treatment of risedronate or placebo with outcomes assessments taken baseline, six, and 12-months. The primary outcome is total change in hip aBMD (measured by dual energy x-ray absorptiometry [DXA]) due to its robust change following surgery [3] and clinical utility in fracture risk assessment. [23] We hypothesize that patients assigned to risedronate will better preserve total hip aBMD than patients assigned to placebo. Secondarily, the trial will explore treatment-related change in DXA-acquired aBMD assessment at other skeletal sites; quantitative computed tomography (QCT) derived changes in bone quality; muscle mass and function; and biomarkers of bone health, turnover, and crosstalk.
2. Methods
2.1. Overview
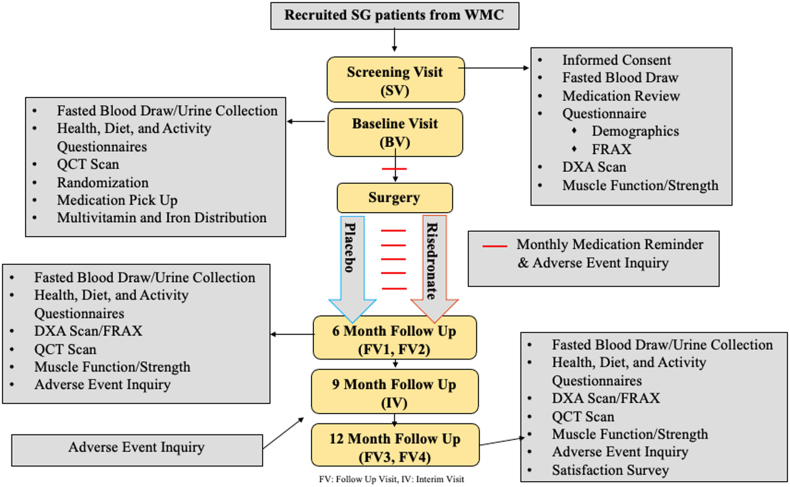
The STRONG BONES trial has been approved by the Wake Forest University Institutional Review Board (IRB00074763) and is registered on clinicaltrials.gov (NCT04922333) as a single site, interventional, placebo-controlled, double-blind clinical trial. An overview of the study design is provided in Fig. 2. Health Insurance Portability and Accountability Act (HIPAA) authorization and informed written consent will be obtained from all patients prior to enrollment and data collection.
Fig. 2.
Outline of study flow.
FRAX: Fracture Risk Assessment Tool; DXA: Dual-Energy X-Ray Absorptiometry; QCT: Quantitative Computed Tomography; FV: Follow Up Visit; IV: Interim Visit.
2.2. Patient population, recruitment, screening, and randomization
Potential patients will be recruited from the Atrium Health Wake Forest Baptist Weight Management Center prior to their surgery date and in accordance with the eligibility criteria outlined in Table 1, which serves to verify our target population and eliminate those who could be adversely affected by the intervention. Women of child-bearing potential are counseled about willingness to use effective contraceptives to prevent pregnancy during the 12-month observation period. After completing all screening assessments, eligible patients will be randomized to either risedronate or placebo treatment via computer-generated permuted block randomization scheme with blocks of random size (2, 4, 6, or 8) and stratified by sex.
Table 1.
Inclusion and exclusion criteria.
| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Written Consent | Sign and agree | Do not sign/agree |
| Age (years) | ≥40 | <40 |
| Obesity Stats | BMI ≥40 kg/m2 or BMI ≥35 kg/m2 | Weight >450 lbs [204 kg] (DXA/CT limit) |
| with ≥1 obesity related complication including: | ||
| Hypertension (>160/90 mm Hg); | ||
| Type II diabetes; Hyperlipidemia; Obstructive Sleep Apnea | ||
| Allergy | Known allergy to bisphosphonates | |
| Surgical Status | Cleared by attending physician for SG procedure | |
| Transportation | Able and willing to provide own transportation to screening and assessment visits | |
| Blood Markers | Serum calcium (8.6–10.2 mg/dL) | Serum calcium outside of normal range |
| PTH (15–65 pg/mL) | PTH outside of normal range | |
| eGFR ≥45 mL/min/1.73 m [2] | eGFR <45 mL/min/1.73 m [2] | |
| 25-hydroxyvitamin D levels ≥20 ng/mL | 25-hydroxyvitamin D levels <20 ng/mL | |
| Mobility | Dependent on cane/walker | |
| Medication use | Current or prior (within the past year) use of growth hormones; oral steroids for more than 6 consecutive days in the past 6 months or any use of prescription osteoporosis medications | |
| Unstable gastric reflux requiring two or more additional doses per month of anti-reflux medication | ||
| Research participation | Participation in another conflicting research study | |
| Unable to position on DXA or CT scanner independently | ||
| Medical Contraindications | Existing osteoporosis; Planned hip surgery or existing bilateral hip implants; Pregnancy or potential pregnancy (positive pregnancy test or unwilling to use a highly effective contraception method during active study phase); Esophageal abnormalities; Increased risk of ulceration; Paget's disease; Primary hyperparathyroidism; Hyper/hypothyroidism; Severe liver disease; History of malignancy within 5 years; Use of bone-active medications; Sensitivity to bisphosphonates; Extensive dental work involving extraction or dental implant within the past 2 months or planned upcoming 6 months; Existing risk of osteonecrosis of the jaw or atypical femur fracture; Deemed unfit for any reason by study physician/principal investigator |
BMI: Body Mass Index; kg: Kilogram; lb: Pound; DXA: Dual-Energy X-Ray Absorptiometry; CT: Computed Tomography; mm HG: millimeters mercury; SG: Sleeve Gastrectomy; mg: milligram; dL: Deciliter; PTH: Parathyroid Hormone; pg: picogram; eGFR: Estimated Glomerular Filtration Rate; min: minute; m: meters; ng: nanogram.
2.3. Surgery and interventions
2.3.1. Surgery requirements
All patients will adhere to this clinic visit schedule post-surgery: overnight hospital stay; 30-day nutrition and surgeon follow-up; three-month nutrition and blood draw follow-up; six-month surgeon, resting metabolic rate, and exercise follow-up; nine-month nutrition follow-up; and 12-month surgeon and resting metabolic rate follow-up. ASMBS recommendations for perioperative nutrition, metabolic, and nonsurgical support of bariatric surgery patients will be followed. [22] All patients will be provided a six-month supply of daily Celebrate Essential Multi 2-in-1 chewable tablet (providing ∼30% daily calcium and ∼95% daily vitamin D) and sex-dependent iron supplement to ensure micronutrient needs are met throughout the active study period.
2.3.2. Intervention description
Over-encapsulated tablets containing 150 mg risedronate or placebo will be dispensed from the Wake Forest University School of Medicine Investigational Drug Service to each participant post randomization at their baseline assessment visit. Patients will receive instructions to take the first dose 3–7 days prior to their procedure and will be instructed to follow label instructions (i.e., take medication with 6–8 oz of plain water; remain upright for the next 30 min; avoid taking vitamins, mineral supplements, or antacids at the same time). Monthly text or phone reminders for medication use, potential pregnancy (if applicable) and any new adverse events will be performed by the study team. A formal pill count will occur at six months based on the returned pill bottle. In the event of a missed dose (e.g., the participant was unable to take the medication on the planned date), the participant will be instructed to take the medication as near to the planned date as possible. However, if the next month's scheduled dose is within seven days, the participant will be instructed to wait until the next month's scheduled dose. In either scenario, the participant will resume the originally scheduled day of the month on the once-monthly administration schedule. Deviations from the planned date will be noted and missed doses will be corroborated with pill count assessments.
2.3.3. Assessments
All assessments will be conducted by trained and blinded assessors, with in-person visits occurring at baseline, six, and 12 months (see Table 2 timeline). Briefly, demographic data will be assessed at the screening visit. Questionnaires, physical function testing, safety assessment and biomarker specimens, and medical imaging will be obtained at the baseline, six, and 12-month visits.
Table 2.
Assessment chart indicating data collection and sampling for each visit.
| Week: |
−6 weeks to −3 days |
−1 to +23 |
25 |
38 |
52 |
|||
|---|---|---|---|---|---|---|---|---|
| Visit Window: | −3 to −7 days |
±15 days |
±15 days |
±15 days |
||||
| SV | BV | INT | FV1 | FV2 | IV | FV3 | FV4 | |
| Informed Consent | x | |||||||
| Demographic Characteristics | x | |||||||
| Medical History/Medication Use | x | x | x | x | ||||
| Vitals: Height/Weight/Blood Pressure | x | x | x | |||||
| BMD/FRAX Questionnaire | x | x | x | |||||
| SF-36 Questionnaire | x | x | x | |||||
| DXA Assessment (regional BMD, total body composition) | x | x | x | |||||
| QCT Assessment (hip/spine bone and muscle quantity and quality) | x | x | x | |||||
| Fasting Blood Draw for real-time safety (vitamin D, PTH, CMP, A1C, FSH) and storage (biomarkers of bone turnover and muscle bone crosstalk, and gut hormones) | x | x | x | x | ||||
| Fasted Urine Collection | x | x | x | |||||
| Pregnancy Testing (all pre-menopausal women who have not had a hysterectomy) | x | x | x | x | x | x | ||
| Muscle Function/Strength (400-m walk, stair climb, knee extension strength) | x | x | x | |||||
| Randomization, Drug, and Multivitamin Dispensation | x | |||||||
| Monthly Medication Compliance Reminders | x | |||||||
| Unused Drug Return, Pill Count for Compliance Assessment | x | |||||||
| Vioscreen FFQ | x | x | x | |||||
| CHAMPS Physical Activity Questionnaire | x | x | x | |||||
| Adverse Events | x | x | x | x | x | x | ||
| Exit Survey/Participant Satisfaction | x | |||||||
BMD: Bone Mineral Density; QCT: Quantitative Computed Tomography; PTH: Parathyroid Hormone; CMP: Complete Metabolic Panel; CHAMPS: Community Health Activities Model Program for Seniors; WFU: Wake Forest University; WFUSM: Wake Forest University School of Medicine; SV: Screening Visit; BV: Baseline Visit; INT: Intervention; IV: Interim Visit; FV: Follow Up Visit.
2.4. Primary outcome: DXA-derived total hip areal bone mineral density
This study is powered to detect significant group differences in change in total hip aBMD assessed by DXA over 12 months. The 12-month duration was selected to ensure adequate bone remodeling time in response to the intervention, [24] with inclusion of the six-month assessment to increase study power and allow for a midpoint safety assessment on all patients. DXA scans of the hip (as well as the lumbar spine, radius, and whole-body for secondary outcomes; see Section 2.4) will be acquired on an Hologic Horizon A device (Bedford, MA) and read by an International Society for Clinical Densitometry trained DXA technologist. Scans will be examined for proper patient positioning and exclusion of artifacts from the measured region; re-scanning will be performed as necessary. Daily quality control scans will be obtained with a calibration phantom, with repeat phantom scans if results are >2 standard deviations from baseline.
2.5. Secondary outcomes: DXA, QCT, and muscle function/strength assessments
To increase clinical utility and mechanistic understanding, we will assess intervention effectiveness on DXA-acquired metrics collected at different skeletal sites (aBMD of the femoral neck, lumbar spine and distal radius), as well as appendicular lean mass, total body fat, and visceral fat, using a mirroring protocol if necessary. [25] Secondary outcomes will also include QCT-acquired metrics of bone and muscle quantity and quality, as well as muscle function and strength assessments that are sensitive to intensive weight loss and predictive of fall risk.
2.5.1. Quantitative computed tomography (QCT) derived measures
Helical QCT scans of the L1-L5 vertebrae and femurs (proximal to mid-shaft) will be acquired at baseline, six, and 12 months on a GE 64-slice PET/CT Discovery MI scanner at 120 kV, 50-cm field of view, automatic exposure (target noise index 20 HU), 0.625-mm slice thickness, 1:1 pitch, and an abdomen reconstruction filter with secondary reconstruction to 2.5-mm (abdomen filter) and 0.625-mm (bone filter). The Mindways Model 3 CT calibration phantom and bolus bag (Mindways Software, Inc., Austin, TX) will be positioned under each participant and imaged in every scan to calibrate volumetric BMD (vBMD). Quality assurance scans will be performed monthly to monitor operational characteristics of the scanner and the phantom.
2.5.1.1. Volumetric BMD (vBMD)
Lumbar vertebra trabecular vBMD and proximal femur (femoral neck, trochanter, intertrochanter, and total hip) trabecular, cortical, and integral vBMD will be measured in QCT scans using QCT Pro™ software (Mindways Inc., Austin, TX). Mean vertebral, femoral neck, trochanter, intertrochanter, and total hip vBMD will be calibrated in terms of equivalent aqueous K2HPO [4] density values in the 5-port phantom. [26]
2.5.1.2. Cortical thickness
The proximal femur will be manually segmented from CT scans. Cortical thickness will be measured from the segmentations using a cortical density-based algorithm implemented in Stradview (University of Cambridge, UK). [[27], [28], [29]] Each participant's baseline, six, and 12-month cortical thickness maps will be rigidly registered using the iterative closest point algorithm to measure global and localized cortical thickness changes. [30]
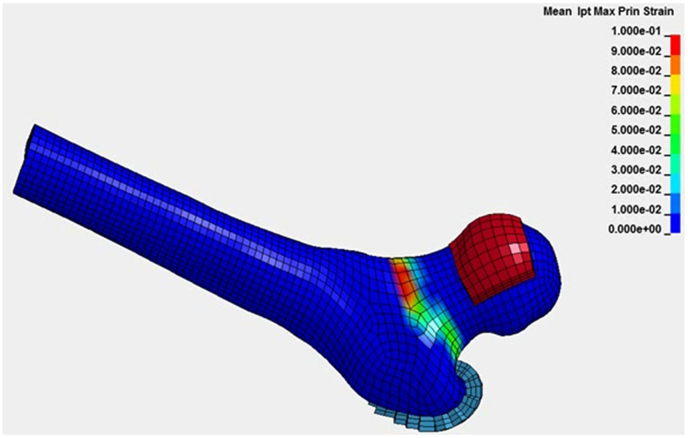
2.5.1.3. Finite-element (FE) modeling-derived bone sstrength
Subject-specific FE models of the proximal femur at baseline, six, and 12 months will be developed. [31,32] Subject-derived material properties will be implemented using vBMD for elasticity and variable cortical thickness to improve accuracy. [33,34] Bone strength and fracture risk will be estimated with simulated tests of a sideways fall (Fig. 3). [35] Simulations will be performed using the LS-Dyna implicit FE solver (LSTC, Livermore, CA) [32] where bone strength will be defined as the peak force between the impactor and the femoral head. Strain-based criteria have proved effective to predict bone fracture. [36]
Fig. 3.
Finite element hip bone strength (high femoral neck strains in red). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.5.2. Muscle area and quality
Mid-thigh muscle cross-sectional area (CSA), muscle density (measured by Hounsfield Unit [HU] attenuation), and intermuscular fat area of the mid-thigh and total trunk muscles at the L3 vertebra level will also be assessed. The mid-thigh position will be defined as the midpoint between the superior aspect of the greater trochanter and the inferior aspect of the lateral condyle measured on an anterior-posterior scout of the entire femur. All trunk muscles will be defined from a CT slice at the midpoint of the L3 vertebral body. Muscle and intermuscular fat areas will be segmented from CT by thresholding for fat (−190 to −30 HU) and muscle (−29 to 150 HU) and then manually refining the segmentation as needed. Abdominal visceral and subcutaneous fat area at the mid-L3 vertebra level will be measured and segmented from CT by thresholding (−190 to −30 HU) and then manually refining the segmentations.
2.5.3. Muscle function/strength
The fast 400-m walk test will be used to measure gait speed. [37] In addition, stair climbing ability will be assessed by the fastest time to climb a 12-step staircase (height 213.36 cm) in two trials. Knee extension strength (peak torque in Newton meters [Nm]) will be measured on the same side as the DXA scan (non-dominant unless there is hardware, fracture or non-weight-bearing >6 weeks in the last 12 months) via isokinetic dynamometry (Humac Norm, CSMi, Massachusetts). These tests are proven to be sensitive to intensive weight loss [38,39] and predictive of fall and future fracture risk. [40,41]
2.6. Tertiary outcomes: biomarkers
2.6.1. Biomarkers of bone health, bone turnover, bone-muscle crosstalk and gut hormones
To elucidate mechanism contributing to change in imaging parameters of bone and muscle, we propose to investigate the impact of SG alone and with risedronate use on select biomarkers of bone health, [42,43] bone turnover, [44] and bone-muscle crosstalk. [[45], [46], [47]] Additionally, we include biomarkers of select circulating gut hormones to explore the potential role of SG-associated gut hormone change on secondary bone resorption/loss. [[48], [49], [50]] Serum, plasma, whole blood in DNA/RNA stabilizing tubes, and urine will be collected and stored for use in future ancillary studies. After processing, aliquots will be stored at −70 °C or colder until analysis. Total blood volume collected over the course of the study is estimated to be 193.5 ml.
2.7. Covariate assessment
Demographic data will be ascertained based on participant self-report at baseline. Medical information on prior and existing co-morbidities, falls, and hospitalizations will be ascertained by self-report and querying the medical record at all visits. Similarly, the Fracture Risk Assessment Tool [FRAX (v 4.1)] will be used to assess 10-year major osteoporotic and hip fracture risk on all patients, [51] the Short Form Health Survey (SF-36), [52] the Vioscreen Dietary Assessment Tool, [53] and the Community Health Activities Model Programs for Seniors (CHAMPS) [54] will be administered as self-reported measures of health.
Protocol adherence will be measured using monthly self-reported pill counts, and a formal six-month pill count. A monthly health status questionnaire will facilitate standardized collection of AEs and concomitant medication usage, in accordance with our data and safety monitoring plan.
2.8. Data and statistics
2.8.1. Data management and statistical approach
In accordance with best practices, data will be retained in a secure, password-protected electronic database. Dynamic reports and statistical analyses will monitor data quality. A participant-based inventory system will track recruitment, retention, adherence, and missing data from entry through exit, close-out, and final lock-down. Primary analyses will use intention-to-treat principles per CONSORT guidelines, with secondary analysis following a per protocol approach (including patients who are >80% compliant with the medication protocol) to assess whether protocol compliance affects observed intent-to-treat (ITT) results. Serious AEs will be reported within 48 h to maintain up to-date safety information for reporting to the Data and Safety Monitoring Board. All data will undergo range checks at the time of data entry and will be examined monthly by histograms and bivariate scatterplots to check for inconsistencies, unusual data needing further verification, and outliers. Regression diagnostics and exploratory analyses will be performed to find appropriate transformations of variables if needed. Order of priority in choosing a transformation will be to satisfy: 1) linearity, 2) homogeneity, and 3) normality assumptions. We will attempt to identify baseline covariates that predict attrition and compliance; and if identified, secondary analyses may need to incorporate stratification by these factors to decrease bias. As recommended by the National Academy of Sciences, [55] if the outcome is missing at random, we will attempt to identify baseline covariates that predict attrition and use these covariates to impute missing data based on multiple imputation. Sensitivity analyses, using pattern mixture models, will explore the effect of missing outcomes on inference if not at random.
2.8.2. Sample size and power
Our preliminary data suggests that, following SG, total hip aBMD declines by approximately 6.7% (−0.072 g/cm2) in 12 months from a baseline (SD) value of 1.06 (0.13) g/cm2. [16] We conservatively hypothesize that risedronate use will attenuate total hip aBMD change by ∼43% [−0.03 g/cm2, i.e., risedronate treatment difference (improvement) of 0.023 g/cm2 or 2.2% of baseline, which is considered a clinically meaningful (i.e., 1.4–3.2%) amount [23]]. With 120 patients, we will have 92% power to detect a 0.023 g/cm2 difference between groups, based on a two-tailed t-test at α = 0.05 using common group standard deviations of 0.033 g/cm2 assuming 80% retention (87.5% observed at six months in the pilot RCT [15]), or 48 evaluable observations per group (Table 3).
Table 3.
Detectable 12-month differences for 80% power for the primary outcome and key secondary outcomes.
| Outcome | Baseline Mean | SD of change | Detectable mean difference (% of BL) | Power |
|---|---|---|---|---|
| Total Hip aBMD (g/cm2) | 1.06 | 0.033 | 0.023 (2.2%) | 92% |
| Femoral Neck aBMD (g/cm2) | 0.903 | 0.040 | 0.023 (2.6%) | 80% |
| Appendicular Lean Mass (kg) | 23.6 | 1.57 | 0.907 (3.8%) | 80% |
| Trabecular Hip vBMD (g/cm3) | 0.283 | 0.02 | 0.012 (4.1%) | 80% |
| Femoral Bone Strength (kN) | 2.044 | 0.065 | 0.037 (1.8%) | 80% |
| Fast-paced gait speed (m/s) | 1.20 | 0.15 | 0.087 (7.2%) | 80% |
BL: Baseline. *Assumes comparisons at α = 0.05 and 80% retention at 12 months (n = 48/group evaluable).
Analyses at secondary skeletal sites include measurements procured using DXA and QCT and select physical performance measures. Based on the sample size used to justify the primary aims, we present the detectable mean differences for key secondary outcomes using 80% power assuming 80% retention at 12 months and each comparison using a two-tailed t-test at α = 0.05 (Table 3). Although DXA-acquired aBMD has the most clinical relevance, these secondary outcomes require modest changes to achieve statistical significance at 80% power and, more importantly, can help identify regions of interest or additional variables for future trials, as well as insights for understanding potential mechanisms for the consequences of SG with or without risedronate treatment on musculoskeletal health outcomes.
Tertiary aims will explore mechanisms underlying SG-associated bone loss with and without risedronate use; thus, it is not formally powered due to the exploratory nature of the analyses. However, a sample of 120 per combined treatment group (48/group evaluable, considering attrition) has 80% power to detect a medium biomarker effect size of 0.578, using a two-tailed test at α = 0.05. Exploratory comparisons will be based primarily on nominal p-values and will apply false discovery rate adjustments [56] to account for multiple outcomes.
2.8.3. Statistical analysis plan
The primary aim for comparisons of total hip aBMD at 12 months will be tested using a linear mixed model fit with total hip aBMD as the primary outcome and the following independent variables: visit (six and 12-months), treatment effect indicator (1 for risedronate; 0 for placebo), the treatment by visit interaction, participant sex (to account for randomization strata and sex as a relevant biologic variable), and baseline total hip aBMD value, assuming an unstructured covariance. The effect of the intervention at 12 months will be estimated using a contrast statement to test the 12-month treatment group difference using a 0.05 level of significance. Additional analyses will test the short-term effect by estimating the six-month treatment effect from the same model. Per protocol sensitivity analyses will be conducted similar to primary analyses, except using only the subset of patients who took at least five of the six prescribed pills (risedronate or placebo). The secondary aim outcome variables will be tested similarly as the first, separately modeling each outcome using six and 12-month outcomes as the dependent variables of the mixed linear model and all other covariates the same, except respective baseline values will be used in place of baseline total hip aBMD. Treatment effects at 12 months, the comparisons of primary interest, will be tested at a two-tailed α = 0.05 level using contrast statements. All models will use completers only, noting that mixed models with baseline covariate adjustments are fairly robust to missing data; [57,58] however, sensitivity analyses will be conducted to ensure results are not biased due to differential missing data. Tertiary aim treatment effects will use the same models as the first two aims changing only the outcome and baseline covariate and treatment effects will be tested at the α = 0.05 level. Additional analyses will explore whether changes in biomarkers mediate the relationship between risedronate and BMD. Our pilot data do not suggest that we will see clinically meaningful differences in treatment effects by race/ethnicity or sex. However, to explore the possibility of differential treatment effects, we will include sensitivity analyses using race/ethnicity or sex as an interaction variable with treatment assignment to determine whether treatment effects differ across race/ethnicity or sex. All analyses will be conducted using SAS v9.4 (Cary, NC) and R software.
3. Discussion
The STRONG BONES trial is designed to definitively test whether musculoskeletal tissue loss following SG surgery can be mitigated through transient bisphosphonate therapy. Increasing utilization of SG to treat severe obesity, alongside increasing awareness of the skeletal complications of bariatric surgery, underscores the timeliness of this research question. If confirmed, data from this trial will present risedronate as a potential surgical adjuvant, aimed at maximizing the cardiometabolic benefits of SG surgery, while minimizing potential harm to the musculoskeletal system.
Bisphosphonates are a class of drugs that have been used commonly for more than two decades for the treatment and prevention of osteoporosis. As a class, these medications are particularly well absorbed in the trabecular region of the femoral neck and spine, [12] and have a potent effect on bone remodeling by inhibiting the breakdown of hydroxyapatite (the primary structural component of bone). [59] We specifically selected risedronate due to its efficacy, safety, [60,61] once-monthly dosing regimen, and low cost. [62] Together, the few reported AEs (5 total; 3.7% AE rate), high degree of protocol adherence (100% adherence among completers; 92% when non-completers were included), [15] and signal for total hip aBMD sparing [risedronate: -0.028 g/cm2 (−0.049, −0.006) vs. placebo: -0.047 g/cm2 (−0.063, −0.030)] at six months in the pilot study [15,16] provided impetus for the STRONG BONES trial primary aim and hypothesis.
That said, bariatric surgery patients are at heightened risk of developing hypocalcemia, [63] which may be elevated with concomitant bisphosphonate use. [64] Fortunately, hypocalcemia was not observed during the pilot study, however we still have planned safety measures to mitigate risk of this serious complication. All participants are screened at baseline to ensure serum calcium is within the normal range (8.6–10.2 mg/dL) and vitamin D levels are non-deficient (>20 ng/mL). Once enrolled, and in addition to nutrition counseling from their surgical team, all study participants receive a 6-month supply of the Celebrate Essential Multi 2-in-1 multivitamin, which provides ∼30% and ∼95% of the RDI for calcium and vitamin D, respectively (see Section 2.2.1). Additionally, routine post-operative clinical visits are scheduled at 1- and 3-months post procedure, where serologic markers are assessed; and, a fasting blood draw will be collected at the 6- and 12-month study visit to assess safety-related labs (including: calcium, vitamin D, eGFR, and parathyroid hormone). Finally, as a NIAMS-funded clinical trial, this study is overseen by an external, 5-member data safety monitoring board, which meets biannually to help the study team ensure participant safety and data integrity are prioritized throughout the trial.
This trial is also designed to offer additional insight into the biology of SG-associated lean mass loss, as well as potential counteractant effects of risedronate use. We specifically build off the appendicular lean mass sparing signal observed in the pilot study [risedronate: -1.2 kg (−2.3, −0.1) vs placebo: -2.1 kg (−3.0, −1.2)] to explore whether the blunting of SG-associated bone resorption can preserve muscle via bone-muscle crosstalk. As the field of bone-muscle crosstalk is rapidly evolving, our robust specimen storage will serve as a valuable resource for future work in this area. Ultimately, these data may also inform lean-mass research endeavors in related fields, such as exercise enhancement, sarcopenia prevention, and spaceflight safety.
Clinical recommendations to optimize bone outcomes following bariatric surgery include adequate consumption of calcium (1200–1500 mg/day), vitamin D (2000–3000 IU/day), and protein (60–75 g/day), along with regular weight-bearing exercise. [7,22] Increasingly, studies are being designed and implemented to test osteoprotective strategies, with many active trials registered on clinicaltrials.gov. Pertinent among these, are two exploring lifestyle-based therapies (NCT04193397, NCT04777305) studying either resistance training alone, or comparison of resistance training, aerobic training, or a combination of the two, and three (not including the STRONG BONES trial) are exploring pharmacotherapies (NCT04279392, NCT04087096, NCT04742010), using zoledronic acid or comparing zoledronic acid with denosumab. Two trials have been completed looking at mega-dosing vitamin D (NCT02092376) and the effects of tele-counseling behavioral counseling and a 12-week supervised exercise program (NCT03214471). As skeletal effects of bariatric surgery are multifactorial, future work in this area will likely aim to couple therapeutic strategies.
3.1. Strengths and limitations
Strengths of the STRONG BONES trial include the randomized controlled trial design and adequate power to examine treatment effects on the primary outcome, total hip aBMD. Pilot data informing active treatment selection (risedronate) and operational study flow are also strengths, as well as provision of robust biorepository specimen storage. However, limitations to the study design exist. Although DXA is the current clinical standard for diagnosis of osteoporosis ― with recent data supporting total hip aBMD as a surrogate outcome for fractures in future trials23 ― aBMD does not fully explain fracture risk prediction [65]. QCT provides additional bone quality information; however, both DXA and QCT have image resolution limitations. Ancillary work is planned to add high resolution peripheral quantitative computed tomography (HR-pQCT) scans to the study's bioimaging battery, providing state-of-the-art information on bone morphometry (e.g., trabecular number/spacing, cortical porosity) and remodeling. Finally, as free-living patients will be enrolled in this trial and bone turnover is influenced by multiple factors (including, but not limited to mechanical loading/unloading, hormonal perturbations, and nutritional intake), we cannot control for all potential confounders. That said, block randomization will be used to collect highly influential covariates.
4. Conclusion
While the benefits of bariatric surgery on weight and cardiometabolic health are significant and well-described, consideration of negative effects on lean mass including bone loss is important, particularly among older surgical patients. Results from this study will provide insight into the efficacy of risedronate use for attenuation of musculoskeletal tissue loss in this patient population. Along with the potential to influence clinical practice, mechanistic data exploring the counteractant effects of bisphosphonate therapy on SG-induced musculoskeletal loss will contribute to the growing field of bone-muscle crosstalk.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Institute of Arthritis and Musculoskeletal and Skin diseases (NIAMS) grant number U01 AR080969 (KMB, JDA) and the National Institute of Aging (NIA) grants K25 AG058804 (AAW) and P30 AG0021332 (Wake Forest Claude D. Pepper Older Americans Independence Center). The authors gratefully acknowledge use of the services and facilities of the Translational Imaging Program and Clinical Research Unit, funded by the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health, through grant award UL1 TR001420. Finally, the authors would like to thank Paige Rice for allowing them access to BioRender for figure licensing.
References
- 1.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. NCHS Data Brief; 2020. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018; p. 360. [PubMed] [Google Scholar]
- 2.ASBMS. Estimate of Bariatric Surgery Numbers. 2011-2020. ASBMS.org. Published online June 2022. [Google Scholar]
- 3.Beavers K.M., Greene K.A., Yu E.W. Bone complications of bariatric surgery: updates on sleeve gastrectomy, fractures, and interventions. Eur. J. Endocrinol. 2020;183(5) doi: 10.1530/EJE-20-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ablett A.D., Boyle B.R., Avenell A. Fractures in adults after weight loss from bariatric surgery and weight management programs for obesity: systematic review and meta-analysis. Obes. Surg. 2019;29(4) doi: 10.1007/s11695-018-03685-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Chen Y., Li J., et al. A meta-analysis of the effects of bariatric surgery on fracture risk. Obes. Rev. 2018;19(5) doi: 10.1111/obr.12665. [DOI] [PubMed] [Google Scholar]
- 6.Chaves Pereira de Holanda N., de Lima Carlos I., Chaves de Holanda Limeira C., et al. Fracture risk after bariatric surgery: a systematic literature review and meta-analysis. Endocr. Pract. 2022;28(1) doi: 10.1016/j.eprac.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Kim J., Nimeri A., Khorgami Z., El Chaar M., Lima A.G., Vosburg R.W. Metabolic bone changes after bariatric surgery: 2020 update, American society for metabolic and bariatric surgery clinical issues committee position statement. Surg. Obes. Relat. Dis. 2021;17(1) doi: 10.1016/j.soard.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 8.McClung M.R., Ebetino F.H. History of risedronate. Bone. 2020;137 doi: 10.1016/j.bone.2020.115407. [DOI] [PubMed] [Google Scholar]
- 9.Cranney A., Tugwell P., Adachi J., et al. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr. Rev. 2002;23(4) doi: 10.1210/er.2001-3002. [DOI] [PubMed] [Google Scholar]
- 10.Diab D.L., Watts N.B. Bisphosphonates in the treatment of osteoporosis. Endocrinol Metab. Clin. N. Am. 2012;41(3) doi: 10.1016/j.ecl.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83(9) doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cremers S., Drake M.T., Ebetino F.H., Bilezikian J.P., Russell R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019;85(6) doi: 10.1111/bcp.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivaska K.K., Huovinen V., Soinio M., et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95 doi: 10.1016/j.bone.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Bredella M.A., Greenblatt L.B., Eajazi A., Torriani M., Yu E.W. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95 doi: 10.1016/j.bone.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swafford A.A., Ard J.D., Beavers D.P., et al. Risedronate to prevent bone loss after sleeve gastrectomy: study design and feasibility report of a pilot randomized controlled trial. JBMR Plus. 2020;4(10) doi: 10.1002/jbm4.10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beavers K.M., Beavers D.P., Fernandez A.Z., et al. Risedronate use to attenuate bone loss following sleeve gastrectomy: results from a pilot randomized controlled trial. Clin Obes. 2021;11(6) doi: 10.1111/cob.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon S.H., Sugamori K.S., Grynpas M.D., Mitchell J. Positive effects of bisphosphonates on bone and muscle in a mouse model of Duchenne muscular dystrophy. Neuromuscul. Disord. 2016;26(1) doi: 10.1016/j.nmd.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe R., Fujita N., Takeda S., et al. Ibandronate concomitantly blocks immobilization-induced bone and muscle atrophy. Biochem. Biophys. Res. Commun. 2016;480(4) doi: 10.1016/j.bbrc.2016.10.112. [DOI] [PubMed] [Google Scholar]
- 19.Essex A.L., Pin F., Huot J.R., Bonewald L.F., Plotkin L.I., Bonetto A. Bisphosphonate treatment ameliorates chemotherapy-induced bone and muscle abnormalities in young mice. Front. Endocrinol. 2019;10 doi: 10.3389/fendo.2019.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada A., Ito S., Matsui Y., et al. Effect of alendronate on muscle mass: investigation in patients with osteoporosis. Osteoporos Sarcopenia. 2015;1(1) doi: 10.1016/j.afos.2015.07.005. [DOI] [Google Scholar]
- 21.Huang C.F., Shiao M.S., Mao T.Y. Retrospective study of the effects of zoledronic acid on muscle mass in osteoporosis patients. Drug Des. Dev. Ther. 2021;15 doi: 10.2147/DDDT.S328858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mechanick J.I., Apovian C., Brethauer S., et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by AMERICAN association of clinical endocrinologists/AMERICAN college of endocrinology, the obesity society, AMERICAN society for metabolic & bariatric surgery, obesity medicine association, and AMERICAN society of anesthesiologists - executive summary. Endocr. Pract. 2019;25(12) doi: 10.4158/GL-2019-0406. [DOI] [PubMed] [Google Scholar]
- 23.Black D.M., Bauer D.C., Vittinghoff E., et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8) doi: 10.1016/S2213-8587(20)30159-5. [DOI] [PubMed] [Google Scholar]
- 24.Heaney R.P. The bone‐remodeling transient: implications for the interpretation of clinical studies of bone mass change. J. Bone Miner. Res. 1994;9(10) doi: 10.1002/jbmr.5650091003. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd J.A., Ng B.K., Sommer M.J., Heymsfield S.B. Body composition by DXA. Bone. 2017;104 doi: 10.1016/j.bone.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witt R. 1973. Bone Standards for the Intercomparison and Calibration of Photon Absorptiometric Bone Mineral Measuring Systems. [Google Scholar]
- 27.Treece G.M., Gee A.H., Tonkin C., et al. Predicting hip fracture type with cortical bone mapping (CBM) in the osteoporotic fractures in men (MrOS) study. J. Bone Miner. Res. 2015;30(11) doi: 10.1002/jbmr.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treece G.M., Gee A.H. Independent measurement of femoral cortical thickness and cortical bone density using clinical CT. Med. Image Anal. 2015;20(1) doi: 10.1016/j.media.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Treece G.M., Gee A.H., Mayhew P.M., Poole K.E.S. High resolution cortical bone thickness measurement from clinical CT data. Med. Image Anal. 2010;14(3) doi: 10.1016/j.media.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besl P.J., McKay N.D. A method for registration of 3-D shapes. IEEE Trans. Pattern Anal. Mach. Intell. 1992;14(2) doi: 10.1109/34.121791. [DOI] [Google Scholar]
- 31.Sl S., Aa W., Dp B., et al. Development of subject-specific proximal femur finite element models of older adults with obesity to evaluate the effects of weight loss on bone strength. J Osteoporos Phys Act. 2018;6(1) doi: 10.4172/2329-9509.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beavers K.M., Walkup M.P., Weaver A.A., et al. Effect of exercise modality during weight loss on bone health in older adults with obesity and cardiovascular disease or metabolic syndrome: a randomized controlled trial. J. Bone Miner. Res. 2018;33(12) doi: 10.1002/jbmr.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schileo E., Dall'Ara E., Taddei F., et al. An accurate estimation of bone density improves the accuracy of subject-specific finite element models. J. Biomech. 2008;41(11) doi: 10.1016/j.jbiomech.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Morgan E.F., Bayraktar H.H., Keaveny T.M. Trabecular bone modulus-density relationships depend on anatomic site. J. Biomech. 2003;36(7) doi: 10.1016/S0021-9290(03)00071-X. [DOI] [PubMed] [Google Scholar]
- 35.Keyak J.H., Rossi S.A., Jones K.A., Skinner H.B. Prediction of femoral fracture load using automated finite element modeling. J. Biomech. 1997;31(2) doi: 10.1016/S0021-9290(97)00123-1. [DOI] [PubMed] [Google Scholar]
- 36.Schileo E., Taddei F., Cristofolini L., Viceconti M. Subject-specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro. J. Biomech. 2008;41(2) doi: 10.1016/j.jbiomech.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Simonsick E.M., Montgomery P.S., Newman A.B., Bauer D.C., Harris T. Measuring fitness in healthy older adults: the health ABC long distance corridor walk. J. Am. Geriatr. Soc. 2001;49(11) doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller G.D., Nicklas B.J., You T., Fernandez A. Physical function improvements after laparoscopic Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 2009;5(5) doi: 10.1016/j.soard.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller G.D., Nicklas B.J., Davis C., Loeser R.F., Lenchik L., Messier S.P. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity. 2006;14(7) doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs J.V. A review of stairway falls and stair negotiation: lessons learned and future needs to reduce injury. Gait Posture. 2016;49 doi: 10.1016/j.gaitpost.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 41.Kyrdalen I.L., Thingstad P., Sandvik L., Ormstad H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. 2019;24(1) doi: 10.1002/pri.1743. [DOI] [PubMed] [Google Scholar]
- 42.Pizzorno L. Bariatric surgery: bad to the bone, part 2. Integr. Med. 2016;15(2) [PMC free article] [PubMed] [Google Scholar]
- 43.Carrasco F., Basfi-Fer K., Rojas P., et al. Changes in bone mineral density after sleeve Gastrectomy or gastric bypass: relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes. Surg. 2014;24(6) doi: 10.1007/s11695-014-1179-0. [DOI] [PubMed] [Google Scholar]
- 44.Vasikaran S., Eastell R., Bruyère O., et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporosis Int. 2011;22(2) doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 45.Bonewald L. Use it or lose it to age: a review of bone and muscle communication. Bone. 2019;120 doi: 10.1016/j.bone.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brotto M., Bonewald L. Bone and muscle: interactions beyond mechanical. Bone. 2015;80 doi: 10.1016/j.bone.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonewald L.F., Kiel D.P., Clemens T.L., et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J. Bone Miner. Res. 2013;28(9) doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brzozowska M.M., Sainsbury A., Eisman J.A., Baldock P.A., Center J.R. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes. Rev. 2013;14(1) doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 49.Saad R., Habli D., El Sabbagh R., Chakhtoura M. Bone health following bariatric surgery: an update. J. Clin. Densitom. 2020;23(2) doi: 10.1016/j.jocd.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Paccou J., Caiazzo R., Lespessailles E., Cortet B. Bariatric surgery and osteoporosis. Calcif. Tissue Int. 2022;110(5) doi: 10.1007/s00223-020-00798-w. [DOI] [PubMed] [Google Scholar]
- 51.Watts N.B., Lewiecki E.M., Miller P.D., Baim S. National osteoporosis foundation 2008 clinician's guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J. Clin. Densitom. 2008;11(4) doi: 10.1016/j.jocd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Ware J.E., Sherbourne C.D. The MOS 36-item short-form health survey (Sf-36): I. conceptual framework and item selection. Med. Care. 1992;30(6) doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Kristal A.R., Kolar A.S., Fisher J.L., et al. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J. Acad. Nutr. Diet. 2014;114(4) doi: 10.1016/j.jand.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart A.L., Mills K.M., King A.C., Haskell W.L., Gillis D., Ritter P.L. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med. Sci. Sports Exerc. 2001;33(7) doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 55.The Prevention and Treatment of Missing Data in Clinical Trials. 2010. [DOI] [Google Scholar]
- 56.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57(1) doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 57.Twisk J., De Boer M., De Vente W., Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J. Clin. Epidemiol. 2013;66(9) doi: 10.1016/j.jclinepi.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Peters S.A.E., Bots M.L., Den Ruijter H.M., et al. Multiple imputation of missing repeated outcome measurements did not add to linear mixed-effects models. J. Clin. Epidemiol. 2012;65(6) doi: 10.1016/j.jclinepi.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Russell R.G.G., Mühlbauer R.C., Bisaz S., Williams D.A., Fleisch H. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif. Tissue Res. 1970;6(1) doi: 10.1007/BF02196199. [DOI] [PubMed] [Google Scholar]
- 60.Delmas P.D., McClung M.R., Zanchetta J.R., et al. Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis. Bone. 2008;42(1) doi: 10.1016/j.bone.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 61.McClung M.R., Zanchetta J.R., Racewicz A., et al. Efficacy and safety of risedronate 150-mg once a month in the treatment of postmenopausal osteoporosis: 2-year data. Osteoporosis Int. 2013;24(1) doi: 10.1007/s00198-012-2056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borgström F., Carlsson Å., Sintonen H., et al. The cost-effectiveness of risedronate in the treatment of osteoporosis: an international perspective. Osteoporosis Int. 2006;17(7) doi: 10.1007/s00198-006-0094-1. [DOI] [PubMed] [Google Scholar]
- 63.Shah M., Sharma A., Wermers R.A., Kennel K.A., Kellogg T.A., Mundi M.S. Hypocalcemia after bariatric surgery: prevalence and associated risk factors. Obes. Surg. 2017;27(11) doi: 10.1007/s11695-017-2705-7. [DOI] [PubMed] [Google Scholar]
- 64.Papapetrou P.D. Bisphosphonate-associated adverse events. Hormones (Basel) 2009;8(2) doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen N.D., Eisman J.A., Center J.R., Nguyen T.V. Risk factors for fracture in nonosteoporotic men and women. J. Clin. Endocrinol. Metab. 2007;92(3) doi: 10.1210/jc.2006-1476. [DOI] [PubMed] [Google Scholar]