Abstract
Aims
Atrial fibrillation (AF) is associated with altered cAMP/PKA signaling and an AF-promoting reduction of L-type Ca2+-current (ICa,L), the mechanisms of which are poorly understood. Cyclic-nucleotide phosphodiesterases (PDEs) degrade cAMP and regulate PKA-dependent phosphorylation of key calcium-handling proteins, including the ICa,L-carrying Cav1.2α1C subunit. The aim was to assess whether altered function of PDE type-8 (PDE8) isoforms contributes to the reduction of ICa,L in persistent (chronic) AF (cAF) patients.
Methods and results
mRNA, protein levels, and localization of PDE8A and PDE8B isoforms were measured by RT-qPCR, western blot, co-immunoprecipitation and immunofluorescence. PDE8 function was assessed by FRET, patch-clamp and sharp-electrode recordings. PDE8A gene and protein levels were higher in paroxysmal AF (pAF) vs. sinus rhythm (SR) patients, whereas PDE8B was upregulated in cAF only. Cytosolic abundance of PDE8A was higher in atrial pAF myocytes, whereas PDE8B tended to be more abundant at the plasmalemma in cAF myocytes. In co-immunoprecipitation, only PDE8B2 showed binding to Cav1.2α1C subunit which was strongly increased in cAF. Accordingly, Cav1.2α1C showed a lower phosphorylation at Ser1928 in association with decreased ICa,L in cAF. Selective PDE8 inhibition increased Ser1928 phosphorylation of Cav1.2α1C, enhanced cAMP at the subsarcolemma and rescued the lower ICa,L in cAF, which was accompanied by a prolongation of action potential duration at 50% of repolarization.
Conclusion
Both PDE8A and PDE8B are expressed in human heart. Upregulation of PDE8B isoforms in cAF reduces ICa,L via direct interaction of PDE8B2 with the Cav1.2α1C subunit. Thus, upregulated PDE8B2 might serve as a novel molecular mechanism of the proarrhythmic reduction of ICa,L in cAF.
Keywords: Phosphodiesterases; PDE8; cAMP; Atrial fibrillation; Calcium handling; ICa,L
Structured Graphical Abstract
Structured Graphical Abstract.
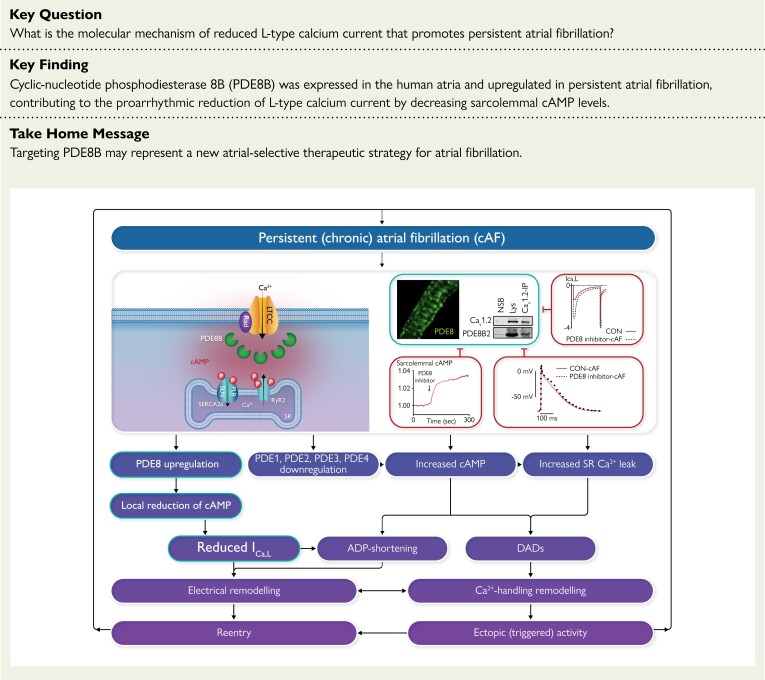
The reduction of L-type Ca2+-current in atrial myocytes promotes atrial fibrillation. Phosphodiesterase type 8B binds L-type Ca2+ channels and reduces local cAMP levels and PKA-dependent channel phosphorylation, thereby decreasing L-type Ca2+-current and abbreviating atrial action potential that promotes atrial fibrillation persistence. ADP, adenosine diphosphate; cAF, persistent (chronic) atrial fibrillation; cAMP, cyclic adenosine monophosphate; DAD, delayed afterdepolarization; ICa,L, L-type Ca2+ current; LTCC, L-type Ca2+ channel; PDE, phosphodiesterase; PLB, phospholamban; RyR2, ryanodine receptor type 2; SERCA2a, sarcoplasmic reticulum Ca2 + ATPase type 2a; SLN, sarcolipin; SR, sinus rhythm.
See the editorial comment for this article ‘Dysregulated Ca2+ cycling in atrial fibrillation’, by J.H. Rennison and D.R. Van Wagoner, https://doi.org/10.1093/eurheartj/ehad099.
Translational perspective.
The mechanism of the proarrhythmic reduction of L-type calcium current (ICa,L) in patients with persistent (chronic) atrial fibrillation (cAF) remains elusive. Here we show that patients with cAF exhibit a stronger binding of phosphodiesterase 8B2 (PDE8B2) to the pore-forming Cav1.2α1C subunit of ICa,L. Selective PDE8 inhibition enhanced Ser1928 phosphorylation of Cav1.2α1C, increased subsarcolemmal cAMP, and rescued the reduced ICa,L, leading to action potential prolongation in cAF atria. We thus discovered a new molecular mechanism for the proarrhythmic reduction of ICa,L in cAF and established PDE8B2 as a novel atrial-selective target for cAF treatment.
Introduction
Atrial fibrillation (AF) is a common disease with poorly understood pathophysiology.1,2 Remodeling of membrane receptors and alterations in 3′,5′-cyclic adenosine monophosphate (cAMP)-dependent regulation of Ca2+ handling mechanisms3,4 are considered important contributors to AF promotion. For instance, upregulated sarcoplasmic reticulum Ca2 + ATPase type 2a (SERCA2a)3,5 and PKA-dependent hyperphosphorylation of ryanodine receptor type 2 (RyR2),6–8 are hallmarks of AF-related remodeling. Indeed, cAMP levels are increased in long-standing persistent (chronic) AF (cAF),3 probably due to a reduction of cAMP-hydrolyzing phosphodiesterases (PDEs).9 Decreased L-type Ca2+ current (ICa,L) density3,10 linked to PKA-dependent dephosphorylation of L-type Ca2+ channel (LTCC) macromolecular complex11 is another feature of AF. Although type 2A protein phosphatase (PP2A) activity in AF is globally increased,12 the inhomogeneous AF-associated changes of protein phosphorylation point to a local regulation of PKA within distinct subcellular compartments.
cAMP levels, and thus the degree of PKA activation, are tightly regulated by PDEs that degrade cAMP in subcellular nanodomains.13 So far, most of our knowledge of cAMP compartmentation was predominantly obtained in the ventricular myocytes where PDE3 and PDE4 are considered to be the main contributors to total cAMP-hydrolysis.14,15 PDE3 is predominant in microsomal fractions in large mammals,16 with PDE4 accounting for only ≈20% of the total PDE activity in canine ventricles.17 Our previous studies demonstrated that although all the classical cardiac PDEs (PDE1–4) are expressed in human atria,9,18 PDE3 and PDE4 account for only 45% and 15% of total cAMP-hydrolysis, respectively, with a large portion of cAMP-hydrolysis remaining unexplained.9 In rodent19–21 and human20,22 ventricles, PDE4 was found to be associated with β-adrenoceptors, LTCC, RyR2 and phospholamban (PLN)-SERCA2 complexes. Previously, we could show that pharmacological inhibition of PDE4 increased the amplitude of ICa,L and frequency of potentially proarrhythmic Ca2+-sparks and Ca2+-waves in human atrial myocytes (HAMs) of sinus rhythm (SR) patients.7 PDE3 and PDE2 may also contribute to ICa,L regulation of HAMs.23 The total cAMP-hydrolytic activity in response to the unselective PDE inhibitor IBMX, which inhibits PDE1–4, is reduced in AF compared to SR patients,9 suggesting that a reduction in PDE1–4 activity may contribute to RyR2 hyperphosphorylation and the related RyR2-mediated Ca2+-dependent triggered activity in AF.6,7 However, this global reduction of PDE activity is inconsistent with the postulated cAF-associated reduction of LTCC,11,12 pointing to a contribution of additional PDE families. Since PDE8 is not inhibited by IBMX,24 an increase in PDE8 activity could underlie the decrease of ICa,L in cAF. The PDE8 family is encoded by the two genes, PDE8A and PDE8B, of which only PDE8A was found in human ventricles.25,26 We recently showed that PDE8A is also expressed in human atria.18 A previous study demonstrated that PDE8A is involved in the regulation of Ca2+ homeostasis in mouse ventricular myocytes.27 Whether PDE8B is expressed in human atria and how PDE8 isoforms may contribute to the proarrhythmic reduction of ICa,L in cAF is unknown and was the object of this study.
Here we show that both PDE8A and PDE8B subfamilies are present in HAMs. Patients with cAF exhibited a stronger binding of a specific PDE8B2 isoform to the pore-forming Cav1.2α1C subunit of LTCC. Selective PDE8 inhibition enhanced Ser1928 phosphorylation (pS1928) of Cav1.2α1C, increased subsarcolemmal cAMP, and rescued the lower ICa,L, leading to action potential (AP) prolongation in cAF atria. This provides a new molecular mechanism for the proarrhythmic reduction of ICa,L in cAF with PDE8B2 as a potential novel atria-selective target for cAF treatment.
Methods
Study design and human atrial samples
Right atrial (RA) tissues were obtained from patients in SR, with paroxysmal AF (pAF), or with cAF who had undergone open-heart surgery. All available RA appendages were used consecutively. Of the 165 RA samples obtained, 32 were used for qPCR, 69 for biochemical experiments, 47 for HAM isolation (thereof 19 for immunofluorescence, 18 for live cell imaging, and 10 for patch-clamp), and 17 for AP recordings (see Supplementary data online, Tables S1–S8). Experimental protocols were approved by ethical boards of University Hospital Essen (#12-5268-BO) and Medical Chamber Hamburg (WF-088/18) and were conducted in accordance with the Declaration of Helsinki. Each individual provided written informed consent. In addition, we included RA and left ventricular (LV) tissues from eight healthy (Ctl) and eight heart failure (HF) patients undergoing heart transplantation. This study was approved by the Scientific Board at the Hungarian Ministry of Health (ETT-TUKEB:4991-0/2010-1018EKU). Patient characteristics are provided for all samples, samples used for qPCR, immunoblotting, immunostaining, FRET, patch-clamp, and AP recordings (see Supplementary data online, Tables S1–S8).
RNA isolation, cDNA synthesis, real-time qPCR and quantification
Frozen tissue samples from 48 patients (16 SR, 8 pAF, 8 cAF, 8Ctl and 8 HF patients) were used for real-time PCR assays performed as previously described18 to study the expression of 2 target genes (PDE8A, PDE8B) related to specifically chosen28 reference genes (POLR2A, YWHAZ, GAPDH, IPO8, PPIA). Further experimental details are available in the online Supplement.
Western blot analysis and co-immunoprecipitation
54 snap-frozen atrial samples were used to perform western blot analysis. From those, 12 samples were, initially, incubated for 5 min with control buffer (CON), 30 nM PF-04957325 (PF), or 100 nM β-adrenergic agonist isoprenaline (ISO), and then snap-frozen. Another 15 snap-frozen samples were homogenized in immunoprecipitation buffer and used for co-immunoprecipitation experiments. Further experimental details are available in the online Supplement.
Isolation and culture of HAMs
After surgical excision, tissue samples were placed into Custodiol® solution (Dr. Franz Köhler Chemie GmbH, Bensheim, Germany) for the transport to the laboratory. Cell isolations from 47 patients were carried out as previously described.7 Please see also Supplementary material online.
Perforated patch-clamp in freshly-isolated and cultured HAMs
Whole-cell perforated patch-clamp configuration was used to record ICa,L in 16 HAMs from 10 patients as previously described.4,11
Live cell imaging of sarcolemmal cAMP in HAMs
Förster resonance energy transfer (FRET) based measurements of cAMP were performed on 38 living HAMs isolated from 18 patients and transduced with an adenovirus encoding for the pmEpac1-camps biosensor to measure cAMP at the membrane.29 See online Supplement.
Immunocytochemistry and confocal imaging
HAMs from 7 SR patients, 5 pAF patients and 7 cAF patients were used for immunostaining as described in online Supplement.
Sharp-electrode AP recordings
APs were recorded as previously described using standard intracellular microelectrodes in atrial trabeculae from 5 SR, 5 pAF and 7 cAF patients.30 Further experimental details are available in the online Supplement.
Data analysis and statistics
Results are presented as mean ± SEM. P < 0.05 was considered statistically significant. Normal distribution was tested by Kolmogorov–Smirnov or by Shapiro–Wilk tests. Statistical differences between groups with normally distributed data were analyzed using mixed ANOVA followed by Wald χ2 test, Student’s t-test for two-group comparison, or one-way ANOVA and Sidak multiple comparison test for multiple groups. Non-normally distributed continuous data or data for which normality could not be assessed were compared using Mann–Whitney tests or one-way ANOVA for multiple groups with a Kruskal–Wallis test. To investigate potential influences of individual clinical variables on specific parameters, two-way ANOVA sub-analyses with factors rhythm and either age, sex, bypass surgery, hyperlipidemia, use of AT1-blockers, ACE inhibitors, diuretics or amiodarone were performed (see Supplementary data online, Figures S4–S8).
Results
Patients
Patients with pAF had more often hyperlipidemia, diabetes and cardiopulmonary bypass surgery and were younger in some subgroups. pAF and cAF patients were more often taking digitalis, beta-blockers and diuretics, with cAF patients being more frequently treated with amiodarone (see Supplementary data online, Table S1). There were no significant differences between groups in sex, body mass index, comorbidities, degree of valvular regurgitation, left ventricular ejection fraction. Multivariate analysis for hyperlipidemia, cardiopulmonary bypass, digitalis, beta-blockers, and amiodarone showed no alterations in the results (see Supplementary data online, Figures S4–S8). Although use of diuretics was associated with higher mRNA levels of PDE8B in SR (see Supplementary data online, Figure S5), this association was not observed in any of the other data sets. Other medication was not different between groups.
Expression and localization of PDE8 isoforms and Cav1.2 in human atrium
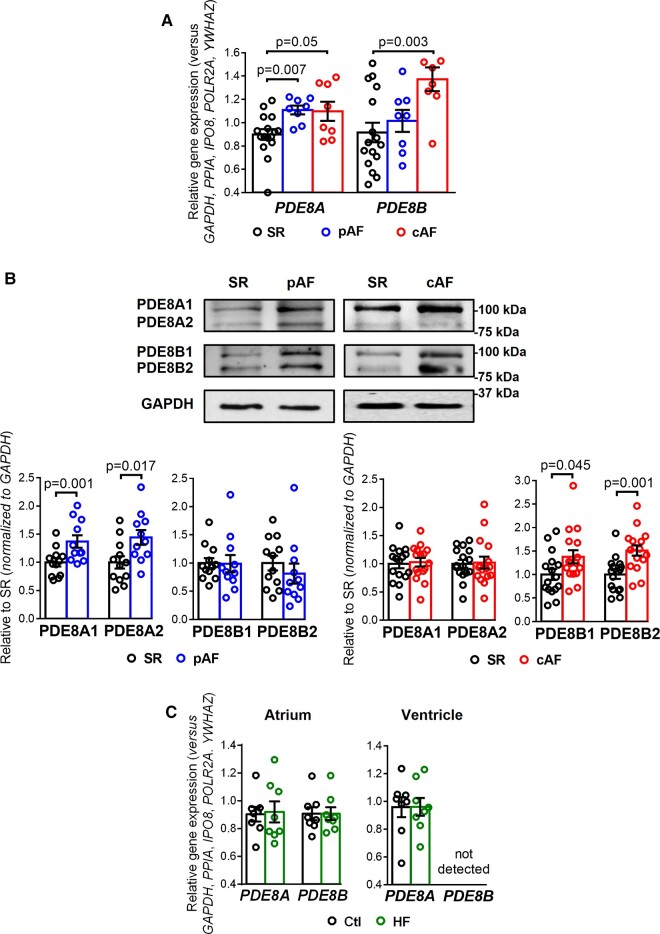
To determine if both PDE8 isoforms are present in human atria and altered in AF, we assessed gene expression (Figure 1A) and protein levels (Figure 1B) of PDE8A and PDE8B in atria from SR, pAF and cAF patients. PDE8A mRNA was present in human atria and increased in both AF groups, whereas PDE8B was increased in cAF only (Figure 1A).
Figure 1.
PDE8A and PDE8B expression in human atrium. (A) Plot of the individual and mean ± SEM gene expression ratio of PDE8A and PDE8B normalized to a set of five housekeeping genes (POLR2A, YWHAZ, GAPDH, IPO8, PPIA) in atrial tissue homogenates from 16 sinus rhythm (SR), 8 paroxysmal atrial fibrillation (pAF) and 8 persistent (chronic) atrial fibrillation (cAF) patients. *P < 0.05 vs. sinus rhythm SR based on analysis of variance (ANOVA with a Kruskal–Wallis test). (B) Representative western blot (top) and protein expression quantification (bottom, mean ± SEM) of PDE8A and PDE8B in atrial tissue homogenates from 16 SR, 10 pAF and 16 cAF patients. GAPDH was used as loading control. *P < 0.05 vs. SR based on unpaired Student’s t-test analysis. (C) Plot of the individual and mean ± SEM gene expression ratio of PDE8A and PDE8B normalized to the set of five housekeeping genes in right atrial and left ventricular tissue homogenates from eight healthy control (Ctl) and eight end-stage heart failure (HF) patients. *P < 0.05 vs. Ctl based on ANOVA and Mann–Whitney test.
The full-length human PDE8A1 isoform has 829 amino acids with a calculated molecular weight of 93 kDa, whereas the PDE8A2 isoform consists of 783 amino acids with a calculated molecular weight of 88 kDA.31 Similarly, the full-length human PDE8B1 isoform has 885 amino acids and an expected molecular mass of approximately 99 kDa, whereas the PDE8B2 variant has 838 amino acids and an expected molecular mass of 87 kDa.32 Previous work could detect differential protein expression of different PDE8A and PDE8B isoforms in rat brain, mouse ovary and bovine testis.33,34 In human atria of SR, pAF, and cAF patients we could detect two specific bands between 75 and 100 kDa, which were in reasonable agreement with the expected molecular masses predicted for PDE8A1, PDE8A2, PDE8B1, and PDE8B2 (Figure 1B). The protein levels of PDE8A1 and PDE8A2 were significantly higher in pAF but unaltered in cAF. Conversely, PDE8B1 and PDE8B2 were upregulated in cAF only (Figure 1B). In contrast, human LV do not express PDE8B and expression of PDE8A was similar between control (Ctl) and HF patients (Figure 1C). Most important, although human atria express both PDE8A and PDE8B, their mRNA levels were not changed during HF, indicating that the PDE8B increases were AF-specific (Figure 1C).
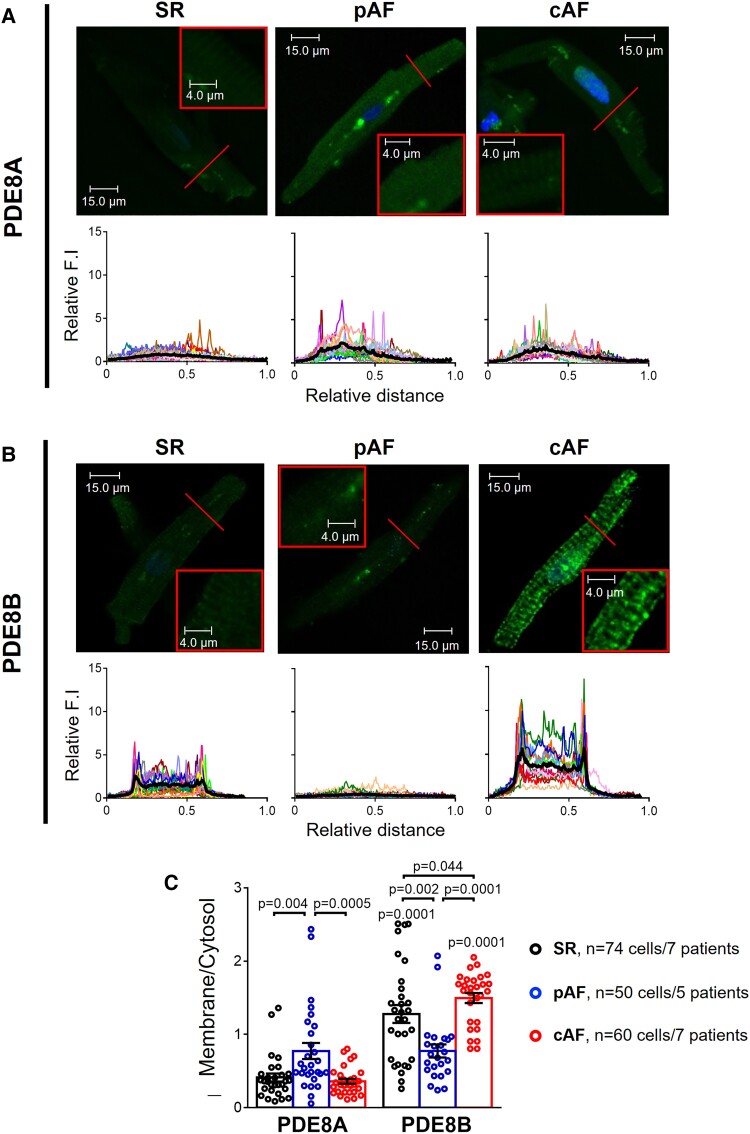
In addition, PDE8A was more abundant in the cytosol in HAMs and its abundance was increased in pAF vs. SR and cAF (Figure 2A and C and Supplementary data online, Figure S1). In contrast, PDE8B was preferentially localized at the plasma membrane, especially in cAF where its expression was increased compared to SR and pAF (Figure 2B and C and Supplementary data online, Figure S1).
Figure 2.
PDE8A and PDE8B localization in human atrial myocytes. (A) Representative immunocytochemistry confocal images of PDE8A showing its cytosolic distribution in 36 isolated human atrial myocytes (HAMs) from 7 sinus rhythm (SR), 25 HAMs from 5 paroxysmal atrial fibrillation (pAF) and 29 HAMs from 7 persistent (chronic) atrial fibrillation (cAF) patients. Lower panels show transversal line profiles of fluorescence intensity (F.I.) of PDE8A staining for all the myocytes analyzed (thin colored lines) and the average within all cells (thick black lines). (B) Similar representative immunocytochemistry confocal images and fluorescence intensity profiles of PDE8B showing mainly plasma membrane localization of this PDE isoform, in 38 HAMs from 7 SR, 25 HAMs from 5 pAF and 31 HAMs from 7 cAF patients. (C) Average F.I. of PDE8A and PDE8B at the plasma membrane (first and last 10% of the cell width) related to the F.I. in the cytosol (middle), in SR, pAF, and cAF. *P < 0.05 between groups of patients, # P < 0.05 vs. PDE8A, by mixed ANOVA followed by Wald χ2 test and Sidak multiple comparison test. All images were captured under the same conditions in order to obtain comparable fluorescence intensities.
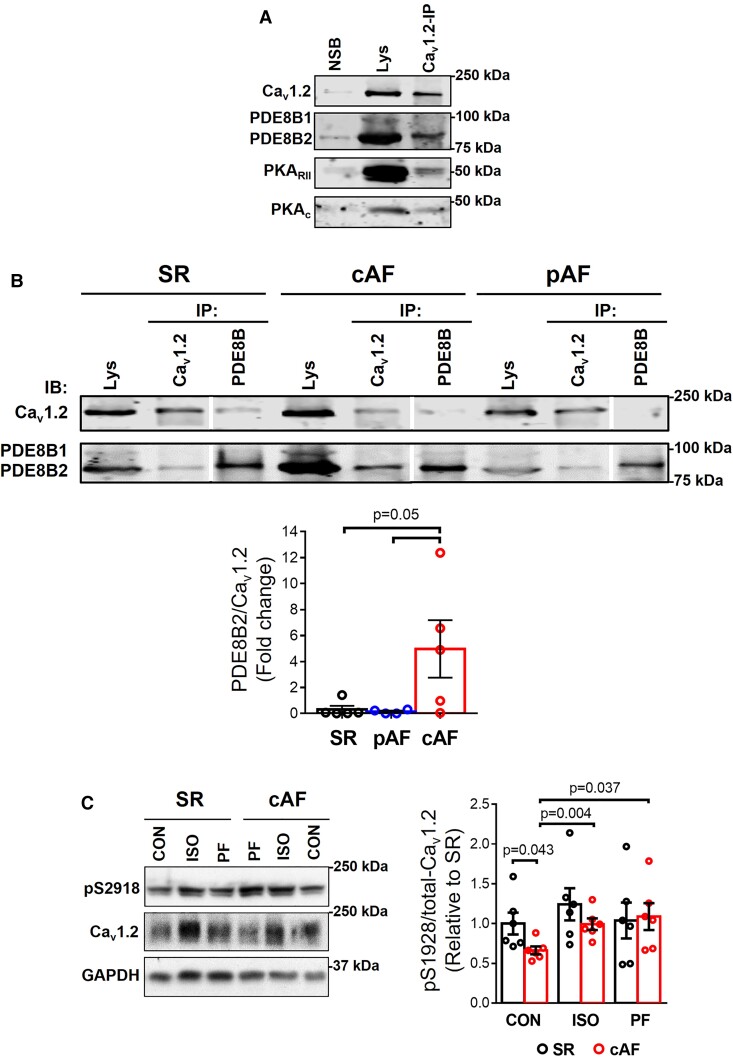
Since PDEs degrade cAMP13,15,22 and impaired PKA-dependent LTCC regulation was suggested to be the main mechanism for ICa,L reduction in cAF,11,35 we examined whether PDE8B, which we found upregulated in cAF, co-localizes with LTCC in the macromolecular complex (Figure 3A). In co-immunoprecipitations PDE8B was the only partner of Cav1.2, with PDE8B2 being the major isoform (Figure 3A). PDE8B2 showed a stronger binding to Cav1.2α1C subunit in cAF (Figure 3A).
Figure 3.
PDE8 co-localizes with LTCC. (A) Co-immunoprecipitation (Co-IP) assay followed by immunoblotting (IB) confirmed that with L-type Ca2+ channel (Cav1.2) binds to PDE8B, PKARII and PKAC in human atria from patients with persistent (chronic) atrial fibrillation (cAF). NSB, non-specific binding. (B) Top, representative Co-IP and IB of Cav1.2 binding to PDE8B in human atrial samples from patients in sinus rhythm (SR), paroxysmal AF (pAF) and cAF. Bottom, mean data of PDE8B binding to Cav1.2 for all groups (n = 6 SR, n = 4 pAF, and 5 cAF patients). Lys, Lysate. Vertical white lines separate non-adjacent lanes on the same blot. (C) Representative western blot (left) and quantification (right, mean ± SEM) of relative steady-state LTCC phosphorylation (pSer1928/total Cav1.2) in atrial tissue homogenates from 6 SR and 6 cAF patients, at baseline and after 5 min stimulation with the selective PDE8 inhibitor PF-04957325 (30 nM) or the β-adrenoceptor agonist isoprenaline (ISO, 100 nM). GAPDH was used as loading control. *P < 0.05 based on ANOVA with a Kruskal–Wallis test.
Subsequently, we studied the consequences of selective PDE8 inhibition on cAMP/PKA-regulation of LTCC by assessing the phosphorylation state of Cav1.2α1C at Ser1928 (Cav1.2-pS1928), a validated PKA phosphorylation site of Cav1.2α1C,36 in atrial tissue lysates incubated with control buffer (CON), the selective PDE8 inhibitor PF-04957325 (PF) or the β-adrenoceptor agonist isoprenaline (ISO) from SR and cAF patients. Although phosphorylation of Cav1.2-pS1928 is not required for β-adrenoceptor stimulation of LTCC in mouse ventricular myocytes and HEK293 cells,37–39 it is a well-established Cav1.2 PKA phosphorylation site36 and phosphorylation of its α1C subunit is relevant for channel function by increasing trafficking to the plasma membrane.40 In CON, the relative Cav1.2-pS1928 phosphorylation was significantly lower in cAF compared to SR and increased significantly after incubation with ISO or PF to levels seen in corresponding CONs (Figure 3C and Supplementary data online, Figure S2), pointing to significant contribution of PDE8 to LTCC dephosphorylation. In agreement with previous studies,12,41 no significant difference in total Cav1.2α1C protein expression was observed in cAF compared to SR (see Supplementary data online, Figure S2).
Altogether, these results show that PDE8B localizes at the plasma membrane of HAMs and accumulates in the LTCC complex of cAF patients, whereby it causes LTCC dephosphorylation. This positions PDE8B as a potential molecular mechanism of the reduced ICa,L that promotes AF.
PDE8 controls subsarcolemmal cAMP concentration in HAMs
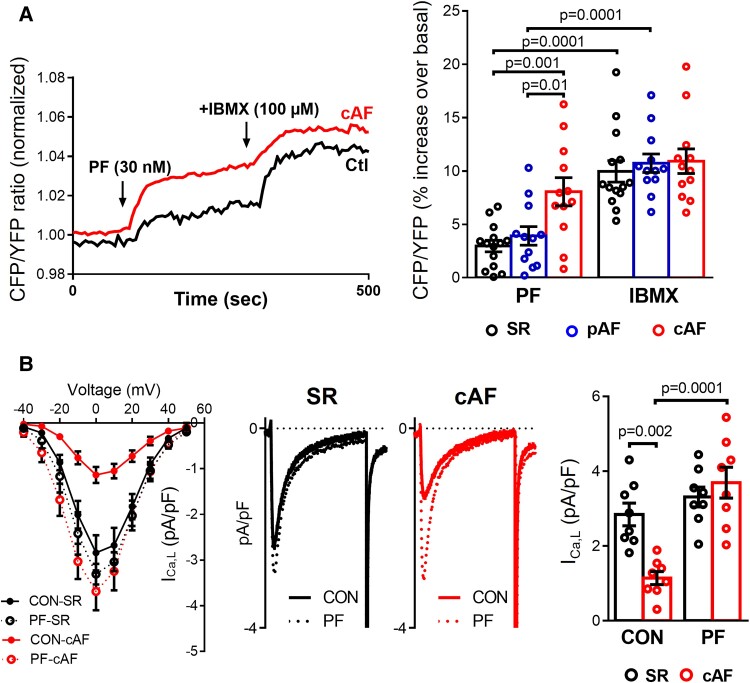
To directly test whether PDE8 controls cAMP levels near the LTCC, cAMP concentration at the plasma membrane was measure in living HAMs from SR, pAF and cAF patients using the FRET-based targeted biosensor pmEpac1-camps.9,29 Application of PF strongly increased basal levels of cAMP in the subsarcolemmal compartment in cAF compared to SR (Figure 4A), reaching approximately 74% of the respective maximal response elicited by the non-selective PDE inhibitor IBMX (100 µM). These results indicate that PDE8 controls basal cAMP levels at the plasma membrane of HAMs in cAF, proving that the observed increase of PDE8B2 in the LTCC complex could contribute to the dephosphorylation of LTCC and the reported reduction in ICa,L in cAF.
Figure 4.
Functional consequences of selective PDE8 inhibition in human atrial myocytes (HAMs). (A) Left panel, representative experiments showing the time course of the FRET signals indicating cAMP changes in HAMs from sinus rhythm (SR) and in persistent (chronic) atrial fibrillation (cAF) patients exposed to selective PDE8 inhibition with PF-04957325 (30 nM). Right panel, effects of the selective PDE8 inhibitor and the non-selective PDE blocker IBMX (100 mM) on the individual and mean values of cAMP levels expressed as percentage of increase of CFP/YFP over its control value measured in 14 HAMs from 6 SR, 12 HAMs from 6 paroxysmal AF (pAF), and 12 HAMs from 6 cAF patients. The changes in FRET ratio were expressed as a percentage change vs. basal. *P < 0.05 compared with SR, # P < 0.05 compared with PF. (B) Left panel, current-voltage (I-V) relationship for peak inward ICa,L density in HAMs from SR and cAF patients before (CON) and after exposure to PF. Middle panels, representative L-type Ca2+ current (ICa,L) recordings measured at 0 mV in HAMs from SR and cAF patients before (CON) and after exposure to PF. Right panel, average current densities before and after exposure to PF (n = 8 cells/5 patients SR and 8/5 cAF). *P < 0.05 vs. sinus rhythm SR, # P < 0.05 vs. CON. Statistical differences analyzed by mixed ANOVA followed by Wald χ2 test and Sidak multiple comparison test.
No changes in ICa,L density or cell capacitance were observed when comparing freshly-isolated and 48-h cultured HAMs from the same patients at baseline and after ISO stimulation (see Supplementary data online, Figure S3).
PDE8 controls ICa,L function
We then studied the direct consequences of increased PDE8B2 for ICa,L function with perforated patch-clamp recordings. ICa,L amplitude was lower in cAF, but was rescued by selective PDE8 inhibition with PF (Figure 4B) and this effect was fully reversible. These findings validate the novel concept that enhanced PDE8B is a major molecular mechanism for the reduction of ICa,L in cAF.
Effects of PDE8 inhibition on AP in cAF
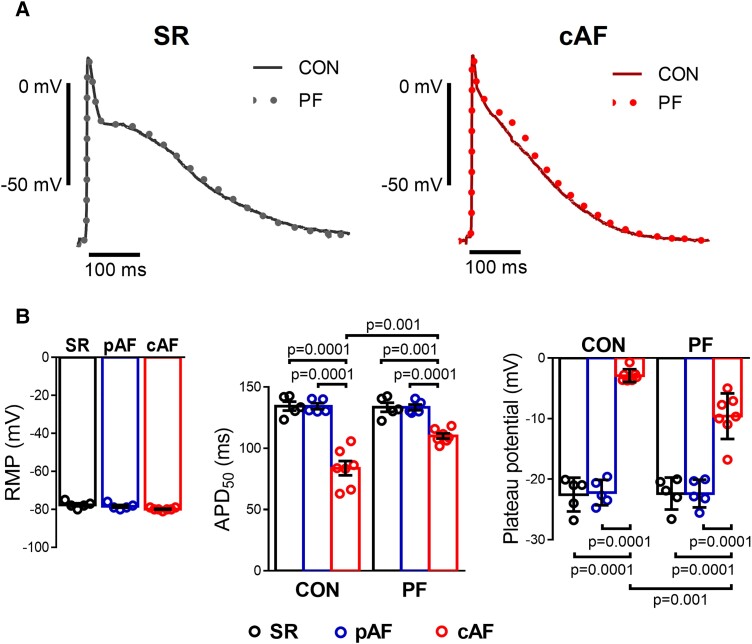
To assess the consequences of PDE8 inhibition and the related upregulation of ICa,L for AP properties, we performed sharp-electrode AP recordings in atrial trabeculae from SR, pAF and cAF patients. Resting membrane potentials were similar between all groups. In cAF PDE8 inhibition shifted the plateau potential, calculated as the average voltage during a time window of 20 to 90 ms after AP upstroke, to more positive values. This shift leads to a slight prolongation of the AP duration at 50% of repolarization (APD50) in cAF vs. SR and pAF (Figure 5), which is consistent with a stimulation of ICa,L. The PF effect was reversible on washout.
Figure 5.
Effect of selective PDE8 inhibition on action potential (AP) properties of human atrial trabeculae. (A) Representative traces of AP at baseline (CON) and after 20 min of exposure to the selective PDE8 inhibitor PF-04957325 (PF, 100 nM) recorded in atrial trabeculae from patients in sinus rhythm (SR) and in persistent (chronic) atrial fibrillation (cAF). (B) Left panel, average resting membrane potentials in trabeculae from five SR, five paroxysmal AF (pAF), and seven cAF patients. Middle panel, individual and mean values of the effects of the selective PDE8 inhibitor (PF) on AP duration at 50% of repolarization (APD50) in the three groups of patients. Right panel, plateau potential before (CON) and after exposure to the selective PDE8 inhibitor (PF). *P < 0.05 vs. SR, # P < 0.05 vs. CON based on ANOVA with a Kruskal–Wallis test.
Discussion
Our study demonstrates that PDE8A and PDE8B are expressed in human atria and HAMs. PDE8B preferentially localizes at the plasma membrane in close proximity to Cav1.2α1C, and the PDE8B2 isoform is enriched in the LTCC complex in cAF. Furthermore, PDE8B is central in controlling cAMP at the plasma membrane which provides a new molecular mechanism of the reduced ICa,L in cAF (Structured Graphical Abstract). These novel insights suggest that selective PDE8B inhibition may constitute a novel atrial-selective anti-AF strategy.
Although a reduction in ICa,L amplitude has been consistently reported in HAMs from cAF patients,3,10,11,42–44 the underlying molecular mechanism has been poorly understood. Reduced ICa,L is considered a main cause of the reentry-promoting APD shortening,45 and atrial contractile dysfunction in AF.46 Different mechanisms have been suggested to contribute to the reduced ICa,L in AF. Although expression of the accessory subunit α2δ seems to be reduced,47 protein expression of the pore-forming Cav1.2 subunits α1C or β2 are unchanged in AF,41 suggesting that the reduced ICa,L might be due to altered regulation of single-channel properties. In addition, we and others have demonstrated a higher sensitivity of ICa,L to β-adrenoceptor stimulation in AF compared to SR10,11,44 suggesting that, opposite to the hyperphosphorylation of RyR2 or PLN,3,7,8 basal phosphorylation of the LTCC complex appears reduced.12 The stronger stimulatory effect of isoprenaline on ICa,L in AF11 indicates that the alterations in Ca2+-handling in AF are likely due to a specific alteration of cAMP-dependent protein phosphorylation in discrete subcellular nanodomains. These results are in agreement with recent studies reporting atrial-specific cAMP nanodomains in the sarcolemmal axial tubule dyadic junctions with the SR in association with RyR2 hyperphosphorylation and clustering,35 and in the caveolar structures linked to extradyadic LTCC subpopulations.48 Since protein phosphorylation states depend on the dynamic balance between protein kinases and protein phosphatases, it has been suggested that a local regulation of PP2A activity may contribute to these compartmental differences in cAMP/PKA-dependent phosphorylation.12 However, although increased PP2A may contribute to the reduced ICa,L, the strong decrease in ICa,L in the face of hyperphosphorylated RyR2 and PLN in AF3,7,8 likely results from different molecular mechanisms. Since in ventricular cardiomyocytes local PKA activity highly depends on the local availability of cAMP, which in turn depends on its degradation by PDEs at each discrete nanodomain,13,15,22 PDEs could contribute to the unique LTCC complex dephosphorylation in AF. Although the classical cardiac PDE2–4 were associated with ICa,L,9,23 their activity decreases in AF,9 suggesting that the decreased ICa,L in AF cannot result from altered PDE2–4 activities. Here we show that a novel PDE8 isoform, PDE8B, is present in human atria, but unlike PDE8A,25 PDE8B is not present in human ventricles,26 and constitutes an atrial-selective PDE isoform. Importantly, our results demonstrate that PDE8B1 and PDEB2 proteins are upregulated in cAF. Furthermore, PDE8B localizes at the sarcolemma of HAMs, with PDE8B2 strongly binding to Cav1.2α1C in cAF. Functionally, PDE8B inhibition increases Cav1.2-pS1928 phosphorylation, cAMP levels in the subsarcolemma, and rescues ICa,L, thereby prolonging APD50 in cAF.
We speculate that the PDE8B2 upregulation in the LTCC complex, the subsequent Cav1.2α1C dephosphorylation at pS1928 and the related ICa,L reduction in cAF may be a protective mechanism to counteract the cytotoxic Ca2+ overload and related negative consequences for cardiac metabolic activity due to the high atrial rate and energy demand, although this occurs at the expense of reentry-promoting APD shortening.49 The PDE8B upregulation could promote Rad-mediated inhibition of LTCC37,50 and consequently reduce Ca2+ influx to compensate the increase in diastolic intracellular [Ca2+] that causes AF-promoting remodeling.1 Although Ca2+-channel blockers can be used to control the ventricular rate in AF, they could promote AF and cause ventricular dysfunction.51,52 Since PDE8B and particularly PDE8B2 occurrence appear atrial-selective, selective inhibition of PDE8B2 could constitute a viable approach to normalize the abnormal LTCC function and may constitute a novel atrial-selective anti-AF target. However, ICa,L is not downregulated in patients with pAF.53 Thus, the reduction of ICa,L in cAF could be a consequence of the persistency of the arrhythmia and/or the sustained high atrial rate during AF. The related shortening of ERP/APD, which promotes AF-maintaining reentry, promotes the progression and the stabilization of AF. Thus, restoration of physiological ICa,L by selective inhibition of PDE8B is expected to prevent the progression of AF to persistent forms rather than the initiation of AF and might also constitute a rhythm control strategy after cardioversion. These possibilities require direct verification in large animal models and suitable patient populations.
Our results clearly show that both PDE8A and PDE8B are expressed in human atrial tissue and myocytes. However, the immunoblot experiments showed multiple bands (Figure 1B and Figure 3A and B) and although the molecular weights of these bands were comparable to specific PDE8 splicing variants in previous studies,31–34 their identities as PDE8A and PDE8B isoforms should be further validated in future work.
Because PDE8 isoforms are important for the regulation of steroidogenesis,54–56 it remains to be determined whether selective PDE8B inhibition could be used for AF treatment without serious effects on adrenal gland or other organs.
Because of limited accessibility to human left atria at our centers, experiments were restricted to right atrial appendages. Different atrial regions may undergo distinct remodeling and further work should be directed to assess whether the observed changes in PDE8 in the right atria also apply to left atrial cells.
pAF patients had similar clinical parameters to SR patients except that they had more often hyperlipidemia and cardiopulmonary bypass surgery, were younger in some subgroups, and were more often taking digitalis, beta-blockers, and diuretics. cAF patients were more frequently treated with AT1-blockers, diuretics and amiodarone. The levels of PDE8B were higher in treated vs. non-treated SR patients with diuretics (see Supplementary data online, Figure S5). However, this was not observed at the protein level. Overall, controlling for individual comorbidities and medication did not alter the results of the individual sets of experiments.
A large number of atrial samples were collected, but each sample could only be used for a limited subset of experiments. Therefore, we performed extensive analyses to assess the influence of factors like patient age, sex, the influence of comorbidities and medications (see Supplementary data online, Figures S4–S8), we cannot fully exclude contributions from other unrecognized factors. The clinical profile of the patient subgroups used for biochemical experiments, FRET/patch-clamp, and multicellular AP recordings were comparable. The patient cohort used for qPCR and immunofluorescence showed differences in distribution of some clinical parameters; this needs to be considered when relating the differences that we noted in SR and AF patients to the other findings in our manuscript.
We observed an upregulation of PDE8A in pAF, but not cAF, and an upregulation of PDE8B in cAF, but not pAF. The underlying mechanisms for this differential regulation are still unclear. The increase of PDE8A in pAF could result from a specific atrial cardiomyopathy and might alter the regulation of targets other than ICa,L, although it might also constitute a transient event during the progression to persistent AF. Once persistent AF is established, the duration of the arrhythmia or/and the sustained high atrial rate could cause adaptative changes that may lead to a switch from PDE8A to PDE8B, along with a reduction in ICa,L, which is consistent with the selective increase in PDE8B in AF, but not in HF atria. Future work should directly address whether tachypacing upregulates atrial PDE8B.
Ser1928 is a well-established PKA phosphorylation site of Cav1.2α1C36 relevant for the trafficking of the channel to the membrane.40 In agreement, we found that both ISO and PDE8 inhibition increased Ser1928 phosphorylation. However, the phosphorylation of this residue is not required for ICa,L modulation in mouse ventricular myocytes and HEK293 cells.37–39 Previous works have identified Rad as the channel inhibitor responsible of the modulation of ICa,L via PKA phosphorylation in mice,37,50 but the functional role of Ser1928 and Rad in HAMs and in AF has not been addressed here and warrants additional studies. Finally, we studied the effects of PDE8 on ICa,L only. Whether and how PDE8 affects the function of other ionic currents that contribute to the atrial AP remains to be determined in future studies.
In summary, we establish PDE8B as a novel regulator of atrial Ca2+ handling and arrhythmogenesis and a new integral component of the LTCC macromolecular complex, which importantly contributes to the reentry-promoting reduction of ICa,L in cAF.
Author contribution
Istvan Baczko, Anne Garnier (Investigation: Supporting), Markus Kamler (Formal analysis: Supporting; Resources: Supporting), Renate B. Schnabel (Investigation: Supporting), Dobromir Dobrev (Formal analysis: Supporting; Funding acquisition: Supporting; Writing – original draft: Supporting), Cristina E. Molina, Ph.D. (Conceptualization: Lead; Data curation: Lead; Funding acquisition: Lead; Investigation: Supporting; Methodology: Lead; Supervision: Lead; Writing – original draft: Lead), Hermann Reichenspurner (Resources: Supporting), Viacheslav O. Nikolaev (Conceptualization: Supporting; Data curation: Supporting), Niels Voigt (Conceptualization: Supporting), Kira Beneke (Investigation: Lead; Writing – original draft: Supporting), Franziska Reinhardt (Formal analysis: Supporting), Nefeli Grammatika Pavlidou (Investigation: Lead; Writing – original draft: Supporting), Shokoufeh Dobrev (Formal analysis: Supporting; Investigation: Supporting), Issam H. Abu-Taha, Constanze Schmidt (Investigation: Supporting), Simon Pecha (Resources: Equal), and Eric Jacquet (Investigation: Supporting)
Supplementary Material
Acknowledgements
We thank Claudine Deloménie (ACTAGen platform of UMS-IPSIT) for her expertise in molecular biology, Ramona Löcker and the Imaging Center Campus Essen—University of Duisburg-Essen for technical support.
Contributor Information
Nefeli Grammatika Pavlidou, Institute of Experimental Cardiovascular Research and University Center of Cardiovascular Sciences, University Medical Center Hamburg Eppendorf (UKE), Martinistrasse 52, W23, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany.
Shokoufeh Dobrev, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstrasse 55, 45147 Essen, Germany.
Kira Beneke, Institute of Experimental Cardiovascular Research and University Center of Cardiovascular Sciences, University Medical Center Hamburg Eppendorf (UKE), Martinistrasse 52, W23, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany.
Franziska Reinhardt, Institute of Experimental Cardiovascular Research and University Center of Cardiovascular Sciences, University Medical Center Hamburg Eppendorf (UKE), Martinistrasse 52, W23, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany; Department of Cardiovascular Surgery, University Heart Center Hamburg, Martinistrasse 52, O70, 20246 Hamburg, Germany.
Simon Pecha, German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany; Department of Cardiovascular Surgery, University Heart Center Hamburg, Martinistrasse 52, O70, 20246 Hamburg, Germany.
Eric Jacquet, Université Paris-Saclay, CNRS, Institut de Chimie des Substances Naturelles, UPR 2301, 1 avenue de la Terrasse, 91198 Gif-sur-Yvette, France.
Issam H Abu-Taha, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstrasse 55, 45147 Essen, Germany.
Constanze Schmidt, Department of Cardiology, University Medical Center Heidelberg, Im Neuenheimer Feld 672, 69120 Heidelberg, Germany.
Niels Voigt, Institute of Pharmacology and Toxicology, University Medical Center Göttingen, Robert-Koch-Strasse 40, 37075 Göttingen, Germany; German Centre for Cardiovascular Research (DZHK), partner Site Göttingen, Potsdamer Strasse 58, 10785 Berlin, Germany; Cluster of Excellence ‘Multiscale Bioimaging: from Molecular Machines to Networks of Excitable Cells’ (MBExC), University of Göttingen, Robert-Koch- Strasse 40, 37075 Göttingen, Germany.
Markus Kamler, Department of Thoracic and Cardiovascular Surgery, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstrasse 55, 45147 Essen, Germany.
Renate B Schnabel, German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany; Cardiology Department, University Heart and Vascular Centre Hamburg-Eppendorf, Martinistrasse 52, O70, 20246 Hamburg, Germany.
Istvan Baczkó, Department Pharmacology and Pharmacotherapy, University of Szeged, H-6721, Szeged, Dóm tér 12, Szeged, Hungary.
Anne Garnier, Inserm, UMR-S 1180, Université Paris-Saclay, Faculté de pharmacie, 17 avenue des Sciences, 91400 Orsay, France.
Hermann Reichenspurner, German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany; Department of Cardiovascular Surgery, University Heart Center Hamburg, Martinistrasse 52, O70, 20246 Hamburg, Germany.
Viacheslav O Nikolaev, Institute of Experimental Cardiovascular Research and University Center of Cardiovascular Sciences, University Medical Center Hamburg Eppendorf (UKE), Martinistrasse 52, W23, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany.
Dobromir Dobrev, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstrasse 55, 45147 Essen, Germany; Department of Medicine and Research Center, Montreal Heart Institute and Université de Montréal, 4100 Molson Street, Suite 340, H1Y 3N1 Montréal, Canada; Department of Molecular Physiology & Biophysics, Baylor College of Medicine, One Baylor Plaza, 77030 Houston, USA.
Cristina E Molina, Institute of Experimental Cardiovascular Research and University Center of Cardiovascular Sciences, University Medical Center Hamburg Eppendorf (UKE), Martinistrasse 52, W23, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site Hamburg/Kiel/Lübeck, Potsdamer Strasse 58, 10785 Berlin, Germany; Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Hufelandstrasse 55, 45147 Essen, Germany.
Supplementary data
Supplementary data is available at European Heart Journal online.
Data and materials availability
All data are available in the manuscript or the supplementary materials.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (ES 569/2-1 to CM), the German Centre for Cardiovascular Research (DZHK), the National Institutes of Health (R01HL136389, R01HL131517, R01HL089598, and R01HL163277 to D.D.), the European Union (large-scale integrative project MEASTRIA, No. 965286 to D.D.), and the Hungarian National Research, Development and Innovation Office (TKP2021-EGA-32, K-128851 and GINOP-2.3.2-15-2016-00006).
References
- 1. Larupa Santos J, Rodríguez I, Olesen MS, Hjorth Bentzen B, Schmitt N. Investigating gene-microRNA networks in atrial fibrillation patients with mitral valve regurgitation. PLoS One 2020;15:e0232719. 10.1371/journal.pone.0232719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res 2020;127:51–72. 10.1161/CIRCRESAHA.120.316363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, et al. Enhanced sarcoplasmic reticulum Ca2 + leak and increased na+-Ca2 + exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. 10.1161/CIRCULATIONAHA.111.067306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Llach A, Molina CE, Prat-Vidal C, Fernandes J, Casado V, Ciruela F, et al. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur Heart J 2011;32:721–729. 10.1093/eurheartj/ehq464 [DOI] [PubMed] [Google Scholar]
- 5. Shanmugam M, Molina CE, Gao S, Severac-Bastide R, Fischmeister R, Babu GJ. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2 + uptake in human atrial fibrillation. Biochem Biophys Res Commun 2011;410:97–101. 10.1016/j.bbrc.2011.05.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Font ER, Arís A, et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic Reticulum in human atrial myocytes. Circulation 2004;110:1358–1363. 10.1161/01.CIR.0000141296.59876.87 [DOI] [PubMed] [Google Scholar]
- 7. Vest JA, Wehrens XHT, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 2005;111:2025–2032. 10.1161/01.CIR.0000162461.67140.4C [DOI] [PubMed] [Google Scholar]
- 8. El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, et al. Molecular determinants of altered ca 2+ handling in human chronic atrial fibrillation. Circulation 2006;114:670–680. 10.1161/CIRCULATIONAHA.106.636845 [DOI] [PubMed] [Google Scholar]
- 9. Molina CE, Leroy J, Richter W, Xie M, Scheitrum C, Lee IO, et al. Cyclic adenosine monophosphate phosphodiesterase type 4 protects against atrial arrhythmias. J Am Coll Cardiol 2012;59:2182–2190. 10.1016/j.jacc.2012.01.060 [DOI] [PubMed] [Google Scholar]
- 10. Wagoner DRV, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type ca 2+ currents and human atrial fibrillation. Circ Res 1999;85:428–436. 10.1161/01.RES.85.5.428 [DOI] [PubMed] [Google Scholar]
- 11. Reinhardt F, Beneke K, Pavlidou NG, Conradi L, Reichenspurner H, Hove-Madsen L, et al. Abnormal calcium handling in atrial fibrillation is linked to changes in cyclic AMP dependent signaling. Cells 2021;10:3042. 10.3390/cells10113042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christ T, Boknik P, Wöhrl S, Wettwer E, Graf EM, Bosch RF, et al. L-Type ca 2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation 2004;110:2651–2657. 10.1161/01.CIR.0000145659.80212.6A [DOI] [PubMed] [Google Scholar]
- 13. Mika D, Leroy J, Vandecasteele G, Fischmeister R. PDEs create local domains of cAMP signaling. J Mol Cell Cardiol 2012;52:323–329. 10.1016/j.yjmcc.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 14. Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DMF, Conti M, et al. A specific pattern of phosphodiesterases controls the cAMP signals generated by different gs-coupled receptors in adult rat ventricular myocytes. Circ Res 2006;98:1081–1088. 10.1161/01.RES.0000218493.09370.8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molina CE, Johnson DM, Mehel H, Spätjens RLHMG, Mika D, Algalarrondo V, et al. Interventricular differences in β-adrenergic responses in the canine heart: role of phosphodiesterases. J Am Heart Assoc 2014;3:e000858. 10.1161/JAHA.114.000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weishaar RE, Kobylarz-Singer DC, Steffen RP, Kaplan HR. Subclasses of cyclic AMP-specific phosphodiesterase in left ventricular muscle and their involvement in regulating myocardial contractility. Circ Res 1987;61:539–547. 10.1161/01.RES.61.4.539 [DOI] [PubMed] [Google Scholar]
- 17. Lugnier C, Muller B, Bec AL, Beaudry C, Rousseau E. Characterization of indolidan- and rolipram-sensitive cyclic nucleotide phosphodiesterases in canine and human cardiac microsomal fractions. J Pharmacol Exp Ther 1993;265:1142–1151. [PubMed] [Google Scholar]
- 18. Garnier A, Bork NI, Jacquet E, Zipfel S, Muñoz-Guijosa C, Baczkó I, et al. Mapping genetic changes in the cAMP-signaling cascade in human atria. J Mol Cell Cardiol 2021;155:10–20. 10.1016/j.yjmcc.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 19. Leroy J, Richter W, Mika D, Castro LRV, Abi-Gerges A, Xie M, et al. Phosphodiesterase 4B in the cardiac L-type Ca2 + channel complex regulates Ca2 + current and protects against ventricular arrhythmias in mice. J Clin Invest 2011;121:2651–2661. 10.1172/JCI44747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lehnart SE, Wehrens XHT, Reiken S, Warrier S, Belevych AE, Harvey RD, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor Complex promotes heart failure and arrhythmias. Cell 2005;123:25–35. 10.1016/j.cell.2005.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sprenger JU, Perera RK, Steinbrecher JH, Lehnart SE, Maier LS, Hasenfuss G, et al. In vivo model with targeted cAMP biosensor reveals changes in receptor–microdomain communication in cardiac disease. Nat Commun 2015;6:6965. 10.1038/ncomms7965 [DOI] [PubMed] [Google Scholar]
- 22. Berisha F, Götz KR, Wegener JW, Brandenburg S, Subramanian H, Molina CE, et al. cAMP imaging at ryanodine receptors reveals β 2 -adrenoceptor driven arrhythmias. Circ Res 2021;129:81–94. 10.1161/CIRCRESAHA.120.318234 [DOI] [PubMed] [Google Scholar]
- 23. Rozmaritsa N, Christ T, Wagoner DRV, Haase H, Stasch JP, Matschke K, et al. Attenuated response of L-type calcium current to nitric oxide in atrial fibrillation. Cardiovasc Res 2014;101:533–542. 10.1093/cvr/cvt334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher DA, Smith JF, Pillar JS, Denis SHS, Cheng JB. Isolation and characterization of PDE8A, a novel human cAMP-specific phosphodiesterase. Biochem Biophys Res Commun 1998;246:570–577. 10.1006/bbrc.1998.8684 [DOI] [PubMed] [Google Scholar]
- 25. Soderling SH, Bayuga SJ, Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A 1998;95:8991–8996. 10.1073/pnas.95.15.8991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayashi M, Matsushima K, Ohashi H, Tsunoda H, Murase S, Kawarada Y, et al. Molecular cloning and characterization of human PDE8B, a novel thyroid-specific isozyme of 3’,5’-cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun 1998;250:751–756. 10.1006/bbrc.1998.9379 [DOI] [PubMed] [Google Scholar]
- 27. Patrucco E, Albergine MS, Santana LF, Beavo JA. Phosphodiesterase 8A (PDE8A) regulates excitation–contraction coupling in ventricular myocytes. J Mol Cell Cardiol 2010;49:330–333. 10.1016/j.yjmcc.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Molina CE, Jacquet E, Ponien P, Muñoz-Guijosa C, Baczkó I, Maier LS, et al. Identification of optimal reference genes for transcriptomic analyses in normal and diseased human heart. Cardiovasc Res 2018;114:247–258. 10.1093/cvr/cvx182 [DOI] [PubMed] [Google Scholar]
- 29. Perera RK, Sprenger JU, Steinbrecher JH, Hübscher D, Lehnart SE, Abesser M, et al. Microdomain switch of cGMP-regulated phosphodiesterases leads to ANP-induced augmentation of β-adrenoceptor-stimulated contractility in early cardiac hypertrophy. Circ Res 2015;116:1304–1311. 10.1161/CIRCRESAHA.116.306082 [DOI] [PubMed] [Google Scholar]
- 30. Wettwer E, Hála O, Christ T, Heubach JF, Dobrev D, Knaut M, et al. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation 2004;110:2299–2306. 10.1161/01.CIR.0000145155.60288.71 [DOI] [PubMed] [Google Scholar]
- 31. Wang P, Wu P, Egan RW, Billah MM. Human phosphodiesterase 8A splice variants: cloning, gene organization, and tissue distribution. Gene 2001;280:183–194. 10.1016/S0378-1119(01)00783-1 [DOI] [PubMed] [Google Scholar]
- 32. Hayashi M, Shimada Y, Nishimura Y, Hama T, Tanaka T. Genomic organization, chromosomal localization, and alternative splicing of the human phosphodiesterase 8B gene. Biochem Biophys Res Commun 2002;297:1253–1258. 10.1016/S0006-291X(02)02371-9 [DOI] [PubMed] [Google Scholar]
- 33. Sasseville M, Albuz FK, Côté N, Guillemette C, Gilchrist RB, Richard FJ. Characterization of novel phosphodiesterases in the bovine ovarian Follicle1. Biol Reprod 2009;81:415–425. 10.1095/biolreprod.108.074450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kobayashi T, Gamanuma M, Sasaki T, Yamashita Y, Yuasa K, Kotera J, et al. Molecular comparison of rat cyclic nucleotide phosphodiesterase 8 family: unique expression of PDE8B in rat brain. Gene 2003;319:21–31. 10.1016/S0378-1119(03)00809-6 [DOI] [PubMed] [Google Scholar]
- 35. Brandenburg S, Pawlowitz J, Steckmeister V, Subramanian H, Uhlenkamp D, Scardigli M, et al. A junctional cAMP compartment regulates rapid Ca2 + signaling in atrial myocytes. J Mol Cell Cardiol 2022;165:141–157. 10.1016/j.yjmcc.2022.01.003 [DOI] [PubMed] [Google Scholar]
- 36. Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci U S A 2006;103:16574–16579. 10.1073/pnas.0607294103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu G, Papa A, Katchman AN, Zakharov SI, Roybal D, Hennessey JA, et al. Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature 2020;577:695–700. 10.1038/s41586-020-1947-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang L, Katchman A, Kushner J, Kushnir A, Zakharov SI, Chen BX, et al. Cardiac CaV1.2 channels require β subunits for β-adrenergic-mediated modulation but not trafficking. J Clin Invest 2019;129:647–658. 10.1172/JCI123878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, et al. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem 2008;283:34738–34744. 10.1074/jbc.M804981200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ito DW, Hannigan KI, Ghosh D, Xu B, Villar SGD, Xiang YK, et al. β-adrenergic-mediated dynamic augmentation of sarcolemmal CaV 1.2 clustering and co-operativity in ventricular myocytes. J Physiol 2019;597:2139–2162. 10.1113/JP277283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schotten U, Haase H, Frechen D, Greiser M, Stellbrink C, Vazquez-Jimenez JF, et al. The L-type Ca2+-channel subunits alpha1C and beta2 are not downregulated in atrial myocardium of patients with chronic atrial fibrillation. J Mol Cell Cardiol 2003;35:437–443. 10.1016/S0022-2828(03)00012-9 [DOI] [PubMed] [Google Scholar]
- 42. Molina CE, Abu-Taha IH, Wang Q, Roselló-Díez E, Kamler M, Nattel S, et al. Profibrotic, electrical, and calcium-handling remodeling of the atria in heart failure patients with and without atrial fibrillation. Front Physiol 2018;9:1383. 10.3389/fphys.2018.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Llach A, Molina CE, Fernandes J, Padró J, Cinca J, Hove-Madsen L. Sarcoplasmic reticulum and L-type ca 2 + channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J Physiol 2011;589:3247–3262. 10.1113/jphysiol.2010.197715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dinanian S, Boixel C, Juin C, Hulot JS, Coulombe A, Rücker-Martin C, et al. Downregulation of the calcium current in human right atrial myocytes from patients in sinus rhythm but with a high risk of atrial fibrillation. Eu Heart J 2008;29:1190–1197. 10.1093/eurheartj/ehn140 [DOI] [PubMed] [Google Scholar]
- 45. Nattel S, Li D, Yue L. Basic mechanisms of atrial fibrillation–very new insights into very old ideas. Annu Rev Physiol 2000;62:51–77. 10.1146/annurev.physiol.62.1.51 [DOI] [PubMed] [Google Scholar]
- 46. Schotten U, Ausma J, Stellbrink C, Sabatschus I, Vogel M, Frechen D, et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation 2001;103:691–698. 10.1161/01.CIR.103.5.691 [DOI] [PubMed] [Google Scholar]
- 47. Grammer JB, Zeng X, Bosch RF, Kühlkamp V. Atrial L-type ca 2+ -channel, β-adrenoreceptor, and 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res Cardiol 2001;96:82–90. 10.1007/s003950170081 [DOI] [PubMed] [Google Scholar]
- 48. Glukhov AV, Balycheva M, Sanchez-Alonso JL, Ilkan Z, Alvarez-Laviada A, Bhogal N, et al. Direct evidence for microdomain-specific localization and remodeling of functional L-type calcium channels in rat and human atrial myocytes. Circulation 2015;132:2372–2384. 10.1161/CIRCULATIONAHA.115.018131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, et al. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res 2008;103:845–854. 10.1161/CIRCRESAHA.108.175463 [DOI] [PubMed] [Google Scholar]
- 50. Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the rem and rad GTPases. Proc Natl Acad Sci U S A 2003;100:14469–14474. 10.1073/pnas.2437756100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation 1995;92:1326–1331. 10.1161/01.CIR.92.5.1326 [DOI] [PubMed] [Google Scholar]
- 52. Multicenter Diltiazem Postinfarction Trial Research Group . The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med 1988;319:385–392. 10.1056/NEJM198808183190701 [DOI] [PubMed] [Google Scholar]
- 53. Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129:145–156. 10.1161/CIRCULATIONAHA.113.006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shimizu-Albergine M, Tsai LCL, Patrucco E, Beavo JA. CAMP-specific phosphodiesterases 8A and 8B, essential regulators of leydig cell steroidogenesis. Mol Pharmacol 2012;81:556–566. 10.1124/mol.111.076125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsai LCL, Shimizu-Albergine M, Beavo JA. The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland. Mol Pharmacol 2011;79:639–648. 10.1124/mol.110.069104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brown KM, Lee LCY, Findlay JE, Day JP, Baillie GS. Cyclic AMP-specific phosphodiesterase, PDE8A1, is activated by protein kinase A-mediated phosphorylation. FEBS Lett 2012;586:1631–1637. 10.1016/j.febslet.2012.04.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.