Abstract
Major depressive disorder (MDD) is associated with immunologic and metabolic alterations linked to central processing dysfunctions, including attenuated reward processing. This study investigated the associations between inflammation, metabolic hormones (leptin, insulin, adiponectin), and reward-related brain processing in MDD patients with high (MDD-High) and low (MDD-Low) C-reactive protein (CRP) levels compared to healthy comparison subjects (HC). Participants completed a blood draw and a monetary incentive delay task during functional magnetic resonance imaging. Although groups did not differ in insulin or adiponectin concentrations, both MDD-High (Wilcoxon p = 0.004, d = 0.65) and MDD-Low (Wilcoxon p = 0.046, d = 0.53) showed higher leptin concentrations than HC but did not differ from each other. Across MDD participants, higher leptin levels were associated with lower brain activation during reward anticipation in the left insula (r = − 0.30, p = 0.004) and left dorsolateral putamen (r = -− 0.24, p = 0.025). In contrast, within HC, higher leptin concentrations were associated with higher activation during reward anticipation in the same regions (insula: r = 0.40, p = 0.007; putamen: r = 0.37, p = 0.014). Depression may be characterized by elevated pro-inflammatory signaling via leptin concentrations through alternate inflammatory pathways distinct to CRP.
Subject terms: Reward, Molecular neuroscience
Introduction
Major depressive disorder (MDD) affects approximately 163 million people and is the leading cause of years lived with disability worldwide1. Only one third of depressed individuals remit with their first antidepressant treatment2 and treatment response is known to be limited and difficult to predict3. Although anhedonia—inability to feel pleasure—is one of the core features of a major depressive episode and one of the most treatment-resistant symptoms of MDD4, little is known about mechanisms implicated in dysfunctional reward processing to inform treatment.
Depressive symptoms are linked to inflammation involving pro-inflammatory cytokines5–7 with meta-analyses indicating that interleukin-6 (IL-6), tumor necrosis factor (TNF), and C-reactive protein (CRP) are elevated in MDD patients8–10. Approximately half of depressed individuals have a CRP concentration of 3 mg/L or greater, indicating increased risk of future cardiovascular disease11. Furthermore, depressed individuals with elevated inflammation show heightened treatment resistance12,13 as well as attenuated brain reward processing within the dorsal and ventral striatum14–17. Thus, anti-inflammatory drugs may be a potential therapeutic target for a subset of depressed individuals with heightened inflammation18.
Complicating the clinical picture, however, is the fact that MDD has a high comorbidity with metabolic syndrome—a cluster of conditions that occur together (e.g., obesity, high blood pressure, cholesterol, hyperglycemia), with an approximate 41% overlap11. Although depression, inflammation, and metabolic disorders often co-occur and exacerbate each other11,19, mechanisms by which inflammation, metabolism, and impaired reward processing relate to depression remain unclear, thereby diminishing the ability to develop targeted treatment for individuals with MDD presenting with these issues.
Three metabolic hormones—insulin, leptin, and adiponectin—are integral to understanding relationships between inflammation, depression, and brain function. Insulin regulates glucose and energy20, while leptin regulates appetite, energy, and mood21–23; finally, adiponectin improves insulin sensitivity while increasing food intake and reducing energy24,25. Compared to healthy individuals, depressed patients are characterized by: (1) heightened insulin resistance not ameliorated by antidepressants26; (2) both increases and decreases in leptin levels27,28, with directionality of effects attributable to symptom heterogeneity, age, sex, and medical history29–31; and (3) attenuated adiponectin levels31,32. While insulin resistance and leptin signaling involve pro-inflammatory processes33,34, adiponectin is more closely aligned to anti-inflammatory mechanisms35–37. Crucially, all three hormones are thought to be important for dopaminergic reward signaling38. In sum, research indicates that insulin, leptin, and adiponectin are linked to inflammation, reward signaling, and depression, but it is still unclear how these hormones relate to altered brain reward processing in MDD as a function of inflammation.
To identify the relationship between metabolic markers, inflammation, and reward processing in MDD, we conducted secondary data analyses, focusing on neural responses to the monetary incentive delay (MID) task and serum metabolic hormone (insulin, leptin, and adiponectin) concentrations in a previously published subset of MDD patients varying in peripheral CRP inflammation14. The MID task39 reliably recruits the striatum, insula, and thalamus40, brain regions that are: (1) implicated in optimal prediction of future rewards40,41; (2) sensitized by metabolic hormones42,43; and (3) attenuated in depressed individuals44,45. We hypothesized that: (a) MDD with high CRP inflammation (MDD-High) would exhibit higher serum leptin and insulin and lower adiponectin concentrations than healthy controls (HC) and MDD patients with normative CRP inflammation (MDD-Low); (b) within MDD-High subjects, higher serum insulin/leptin and lower adiponectin concentrations would be correlated with lower blood oxygen level-dependent (BOLD) signal change within the striatum, insula, and thalamus during anticipation of rewards.
Results
Demographics and clinical characteristics
Table 1 shows demographic and clinical characteristics comparing the combined MDD group and HC. There were no differences in age, sex, annual income, education, employment status, nor smoking status between MDD and HC; MDD group showed lower exercise status (p < 0.001), higher percent body fat (PBF) (p < 0.001), and higher body mass index (BMI) (p < 0.001) than HC. Regarding clinical ratings, MDD and HC differed on all PROMIS scores including alcohol use, anger, anxiety, depression, fatigue, physical function, sleep disturbance, and social isolation ratings (p ≤ 0.001), except nicotine dependence.
Table 1.
Sample demographics and clinical characteristics.
| MDD | HC | p value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| N | 88 | 44 | |
| Age (years) | 34.34 (11.06) | 30.91 (10.15) | 0.087a |
| Sex = male (%) | 27 (30.7) | 19 (43.18) | 0.220b |
| Annual income (US dollars) | 65,352 (115,668) | 51,686 (35,399) | 0.447a |
| Consolidated education | 6.20 (1.66) | 6.50 (1.42) | 0.300a |
| Employed = yes (%) | 53 (61.6) | 32 (78.0) | 0.102b |
| Smoke = yes (%) | 18 (20.5) | 5 (11.4) | 0.292b |
| Exercise = yes (%) | 32 (36.4) | 31 (70.5) | < 0.001b |
| IPAQ category (%) | 0.002 b | ||
| HEPA active | 28 (35.0) | 24 (64.9) | |
| Inactive | 33 (41.2) | 4 (10.8) | |
| Minimally active | 19 (23.8) | 9 (24.3) | |
| IPAQ minutes per week | 3021.66 (3550.23) | 5397.61 (4044.80) | 0.002a |
| Percent body fat | 39.18 (8.08) | 29.13 (10.84) | < 0.001a |
| Body mass index (kg/m2) | 30.56 (4.57) | 26.50 (5.22) | < 0.001a |
| Medication = un-medicated (%) | 26 (29.5) | NA | NA |
| PROMIS alcohol use | 48.91 (7.12) | 44.52 (6.78) | 0.001a |
| PROMIS anger | 58.11 (6.68) | 44.38 (5.56) | < 0.001a |
| PROMIS anxiety | 63.08 (6.50) | 45.60 (7.42) | < 0.001a |
| PROMIS depression | 61.02 (7.18) | 43.50 (6.38) | < 0.001a |
| PROMIS fatigue | 61.20 (7.58) | 43.61 (7.14) | < 0.001a |
| PROMIS nicotine dependence | 28.07 (10.84) | 25.32 (7.04) | 0.129a |
| PROMIS physical function | 50.04 (7.05) | 60.22 (6.64) | < 0.001a |
| PROMIS sleep disturbance | 57.31 (10.00) | 43.67 (7.67) | < 0.001a |
| PROMIS social isolation | 58.15 (5.43) | 42.69 (7.89) | < 0.001a |
| Current major depressive episode on MINI = yes (%) | 82 (93.2) | 0 (0) | < 0.001b |
Significant values are in bold.
IPAQ, International Physical Activity Questionnaire; PROMIS: Patient-Reported Outcomes Measurement Information System total score. MINI, Mini International Neuropsychiatric Inventory; NA = not applicable.
aTwo sample t test.
bχ2 test.
Immunoassay results
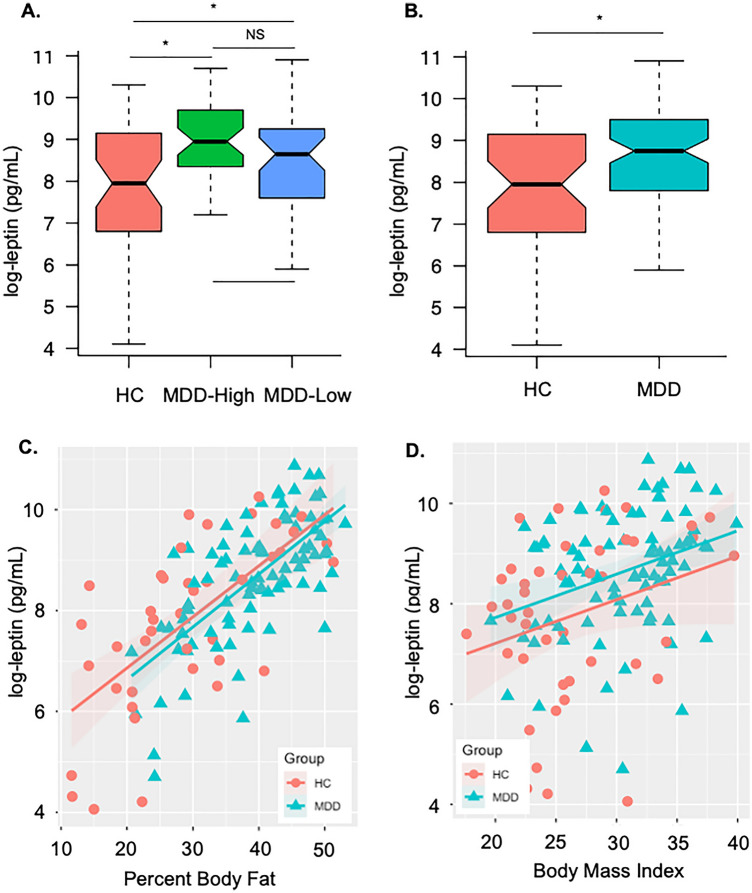
Although ANOVA tests indicated that MDD-High, MDD-Low, and HC groups did not differ on insulin or adiponectin concentrations, group differences were observed in serum leptin concentrations (Kruskal–Wallis χ2 = 8.87, p = 0.012). Specifically, both MDD-High (Wilcoxon p = 0.004, d = 0.65) and MDD-Low (Wilcoxon p = 0.046, d = 0.53) showed higher leptin concentrations than HC, while there was no difference between MDD-High and MDD-Low (Fig. 1A). Since no leptin differences were found between the two MDD groups, MDD-High and MDD-Low were combined as one MDD group and compared with HC on neuroimaging and leptin concentrations. A Mann–Whitney–Wilcoxon non-parametric test showed that the MDD group exhibited higher leptin levels than HC (p = 0.005, d = 0.62) (Fig. 1B).
Figure 1.
Serum leptin concentrations between groups and their relationship with percent body fat and body mass index. (A) Both major depressive disorder (MDD) subjects with high CRP (MDD-High) and low CRP (MDD-Low) exhibited higher log-transformed serum leptin concentrations compared to healthy comparison subjects (HC). (B) The combined MDD group showed higher log-transformed serum leptin concentrations than HC. (C) Leptin concentrations were positively correlated with percent body fat in MDD and HC groups. (D) Leptin concentrations were positively correlated with body mass index in MDD group and partially correlated with BMI in HC group.
Higher PBF was associated with higher serum leptin concentrations within the MDD group (r = 0.68, p < 0.001) and the HC group (r = 0.67, p < 0.001); however, there was no slope difference on PBF and leptin concentration between MDD and HC (p = 0.99) (Fig. 1C). Similarly higher BMI was associated with higher serum leptin concentrations in the MDD group (r = 0.32, p = 0.002) and the HC group (r = 0.28, p = 0.07); again, there was no slope difference on BMI and leptin concentration between groups (p = 0.62) (Fig. 1D). In addition, leptin concentrations were negatively associated with PROMIS physical function scores within the HC group (r = − 0.53, p < 0.001), but not the MDD group (r = − 0.09, p = 0.39). No other correlations between leptin and PROMIS scores were observed. Moreover, higher PBF was associated with higher serum CRP concentrations within HC group (r = 0.58, p < 0.001) but not the MDD group (r = 0.15, p = 0.155), and there was a slope difference on PBF and CRP concentration between HC and MDD (p = 0.034); Similarly, higher BMI was associated with higher serum CRP concentrations within HC group (r = 0.57, p < 0.001) but not within the MDD group (r = 0.16, p = 0.139), and there was a slope difference on BMI and CRP concentration between HC and MDD (p = 0.018).
Neuroimaging results
Figure 2 shows that there was a significant slope difference between serum leptin and % fMRI signal change for the MID gain versus no gain contrast between MDD and HC within left mid-posterior insula (center-of-mass = 37.5, 11.3, 13.2; 87 voxels; peak t = -3.54) and left dorsolateral putamen (center-of-mass = 28.7, 13.5, − 0.9; 67 voxels; peak t = -4.10). Correlations within the MDD patients indicated that those individuals with the highest leptin concentrations also showed the lowest fMRI BOLD signal change for the MID gain versus no gain contrast in left insula (r = - − 0.30, p = 0.004) and left dorsolateral putamen (r = - − 0.24, p = 0.025). In contrast, within HC, those individuals with the highest leptin concentrations also showed the highest fMRI BOLD signal change for the MID gain versus no gain contrast in left insula (r = 0.40, p = 0.007) and left dorsolateral putamen (r = 0.37, p = 0.014). Fisher's r-to-z transformations were applied to these correlations for each group and then compared; results indicated that the relationship between serum leptin and gain versus non-gain BOLD signal change was significantly more negative in MDD than HC, within the left insula (z = − 3.88, p < 0.001) and the left dorsolateral putamen (z = − 3.31, p < 0.001).
Figure 2.
Correlations between serum leptin and % fMRI signal change for the MID gain versus no gain contrast between HC and MDD. Within MDD, higher leptin concentrations were associated with lower fMRI BOLD signal change for the MID gain versus no gain contrast in left insula and left dorsolateral putamen. Within HC, higher leptin concentrations were associated with higher fMRI BOLD signal change for the MID gain versus no gain contrast in left insula and left dorsolateral putamen.
In addition, partial correlations showed a significant negative relationship between leptin and gain versus non-gain BOLD signal change within the left insula (r = − 0.29, p = 0.006) and the left dorsolateral putamen (r = − 0.23, p = 0.033) in the MDD group after controlling for BMI. Similarly, partial correlations within HC subjects showed that the positive relationship between leptin and gain versus non-gain BOLD signal change within the left insula (r = 0.37, p = 0.014) and the left dorsolateral putamen (r = 0.38, p = 0.013) remained after controlling for BMI. Moreover, when CRP concentrations were used as a covariate, the significant negative correlation between leptin and gain versus non-gain BOLD signal change within the left insula (r = − 0.30, p = 0.005) and the left dorsolateral putamen (r = − 0.24, p = 0.025) remained the same within the MDD group, the positive relationship between leptin and gain versus non-gain BOLD signal change within the left insula (r = 0.38, p = 0.013) and the left dorsolateral putamen (r = 0.38, p = 0.011) in the HC group remained similar as well.
Discussion
This study investigated whether two MDD groups varying in inflammation (low versus high CRP levels) and a HC group differed on three metabolic factors—insulin, leptin, and adiponectin—and if so, how variation in metabolism related to brain activation as a function of group while individuals were anticipating future reward. Contrary to our hypotheses, MDD groups with low versus high CRP levels (indicative of differences in peripheral inflammation levels) did not differ from each other on any of the three metabolic hormones, suggesting that peripheral inflammation does not influence metabolic-mediated outcomes in MDD. The combined MDD group also did not differ from the HC group on adiponectin and insulin levels. These findings align with prior work showing no significant relationship between adiponectin and depression46,47. Due to adiponectin’s role in both pro- and anti-inflammatory processing in depression, perhaps these competing effects cancel each other out46. Study results on insulin concentrations within MDD in other samples are also mixed26, suggesting that dysregulated inflammatory and metabolic profiles may not always co-occur in the same subtype of depression. As variation in metabolic profiles may represent different subtypes of depression (e.g., MDD with increased versus decreased appetite, weight change, or atypical vs typical depression)26,48,49, perhaps inflammatory and metabolic profiles of depression do not overlap.
Crucially, we demonstrate two important findings for the entire MDD group that are consistent with predictions for the MDD high inflammation group. First, MDD, regardless of inflammation status (as indicated by CRP level), showed greater serum leptin concentrations than healthy individuals, consistent with previous research27,28. The absence of leptin-mediated inflammatory function as measured by peripheral CRP concentrations suggests that alternate inflammatory pathways may be involved leptin’s role in reward processing that have yet to be explored50. Leptin binding to its receptor leads to canonical signaling via Janus kinases (JAK) and signal transducer and activator of transcription protein (STAT) pathways51, but also has the capability of extracellular signal-regulated kinases (ERK), p38 mitogen-activated protein kinases (MAPK), c-Jun N-terminal kinases (JNK), protein kinase C (PKC), and phosphatidylinositol 3-kinase (PI3K) activation50. These pathways, all of which have been implicated in depression, mediate leptin signaling on targets affecting neurotransmitter regulation, antidepressant activity, neuronal spine density, and glucocorticoid resistance52–56.
Second, left mid-posterior insula and left dorsolateral putamen responses during anticipation of reward were lowest in MDD patients with the highest leptin concentrations, but this pattern was reversed in healthy individuals (higher left insula and left putamen responses were linked to higher leptin levels). Taken together, these findings suggest that depression may be characterized by elevated pro-inflammatory signaling via leptin concentrations through alternate inflammatory pathways distinct to CRP, which relates to blunted mid-insula signals previously evident in MDD patients during the processing of bodily sensations57,58 as well as reduced insula and putamen responses during reward anticipation in girls at high risk for MDD59. As reduced striatal signal during reward processing may precede MDD onset60, it will be important for future research to determine whether high leptin levels are also present in individuals at high risk for future MDD. We speculate that perhaps elevated leptin and attenuated striatum activity might interact during adolescence to reduce positive mood and appetitive valuation of reward stimuli. Leptin regulates brain reward and motivation signals in striatal regions, including the caudate61; however elevated leptin levels may inhibit dopamine release and therefore reduce reward activation in the caudate, which may be associated with a high fat diet62. Prior work in our research group found that MDD patients had poor diet quality, including a high fat diet63. A healthy dietary pattern would be useful to reduce leptin concentrations, which may then increase reward activity in striatal regions64. Although leptin receptors were observed in the insular cortex in animal studies65, it is unclear whether neurons that contain leptin receptors can sense the peripheral leptin to regulate reward processing. Leptin signaling within the insular cortex may alter brain function66 through both JAK2-MAPK and JAK2-PI3K pathways, conceivably via elevated leptin concentrations (leptin resistance) leading to decreased reward processing in depressed individuals. Another pathway to reward processing could be the sympathetic activation that is modulated by leptin. It is possible that individuals with or at high risk for MDD lack these leptin-sensitive neurons67.
In our study, MDD differed in exercise, percent body fat (PBF), body mass index (BMI), and PROMIS scores compared to HC. MDD participants had lower exercise scores, as measured by the International Physical Activity Questionnaire, which may be attributed to depressive symptoms, higher BMI, and physical co-morbidity with other illnesses68. Despite the evidence that exercise/physical activity improves mental health parameters69,70, not all individuals with depression engage in physical activity or to levels that would be beneficial71,72. Also, MDD subjects showed higher PBF and BMI than HC and were both positively associated with leptin as previously reported73,74. Higher PBF and BMI are associated with increased risk for obesity, which is often comorbid with MDD and associated with a distinct set of depressive symptoms75–78. In our study, PBF and BMI are also highly correlated with CRP in HC group, but not in MDD group, which may explain elevated leptin concentrations regardless of CRP-mediated inflammatory status in MDD79. Leptin, which is synthesized in adipose tissue and influences body weight, may be linked to this bidirectional relationship between obesity and depression, due to higher levels of adipose in depressed individuals75,80. Lastly, PROMIS scores, except nicotine dependence, also differed in MDD versus HC. It is not surprising that PROMIS Anger, Anxiety, and Depression were all higher in MDD versus HC, which represents more negative affect81. While PROMIS Physical Function was different in MDD versus HC, it was only associated with leptin in HC (not MDD) suggesting that leptin may not affect physical function in depressed individuals.
This project possesses several strengths, including: (1) the integration of blood-based inflammation and metabolic data with functional neuroimaging; (2) a sizable sample of MDD patients (n = 88); and (3) analysis of the MID paradigm that enables separation of reward anticipation and outcome phases. However, this project focused on secondary analysis of the Tulsa 1000 project data, which also includes multiple limitations. First, most of the insulin research in depression has specifically focused on insulin resistance as opposed to total insulin concentration as measured in the Tulsa 1000 project. Second, in contrast to prior work selecting MDD patients on particular characteristics relevant to appetite, weight, or atypical symptoms when investigating metabolic factors linked to depression26,48,49, individuals with MDD from the Tulsa 1000 project were not selected on the basis of these criteria. Third, as research indicates that body fat and leptin are positively correlated30, it is imperative to note that MDD patients in our sample had a higher BMI than HC that also positively scaled with leptin levels; however, within our MDD group, BMI was not significantly related with insula and putamen activation during reward anticipation and did not account for the negative correlation between leptin and reward-related brain activation. Fourth, although two-thirds of the MDD group were currently taking at least one type of psychotropic medication, medicated and unmedicated MDD participants did not differ on leptin levels, BMI, insula and striatum BOLD signal, or relationships between these metrics suggesting that medication was not accounting for the present results.
We show evidence that the association between leptin and anticipatory reward processing is different in depressed versus non-depressed individuals, which may be due to a metabolic re-regulation involving leptin-related pathways. More importantly, our findings suggest that leptin’s mechanism of action to reward processing is not dependent on inflammation as related to CRP peripheral concentrations, this may provide us with a new therapeutic window (e.g., increase leptin sensitivity to reward processing) for treatment of the most treatment-resistant depressive symptom—anhedonia.
Methods
Participants
A subset of participants varying on peripheral inflammation, as determined by serum CRP levels, was drawn from the first 500 subjects as part of the Tulsa 1000 study, a naturalistic longitudinal study of 1000 individuals with mental illness and HC82. The Tulsa 1000 study was approved by the Western Institutional Review Board and conducted in accordance with the Declaration of Helsinki; all participants provided written informed consent and received compensation for their participation. See Victor et al. for the complete Tulsa 1000 study protocol82.
See Burrows et al. for the study population used in the present analysis14. Briefly, three, age and sex matched groups of subjects were selected based on their serum CRP concentrations: (a) MDD subjects with CRP > 3 mg/L (MDD-High, n = 44, CRP range 3.12–22.99 mg/L); (b) MDD subjects with CRP concentrations between 0 and 3 mg/L (MDD-Low, n = 44, CRP range 0.07–2.89 mg/L); and (c) HC subjects regardless of their CRP concentrations (HC, n = 44, CRP range 0.05–9.47 mg/L). All MDD participants completed the Mini International Neuropsychiatric Inventory (MINI)83 and met either Diagnostic and Statistical Manual of Mental Disorders (DSM)–IV or DSM-5 criteria for a major depressive disorder. The three groups were matched on demographics including age, sex, income, education, employment status, and smoking status.
All participants provided blood samples and completed two runs of the functional magnetic resonance imaging (fMRI) scan with the MID task. Venous blood samples, collected in BD Vacutainer serum collection tubes, were centrifuged at 1300×g for 10 min at room temperature. The serum was then aliquoted and stored at − 80 °C until analysis.
Immunoassays
Serum leptin and insulin concentrations were both analyzed using the Human Leptin, Insulin Kit (Meso Scale Diagnostics, Maryland, USA). Adiponectin was measured with the Human Quantikine ELISA kit (R&D Systems, Minneapolis, USA). The Neuroinflammation Panel 1 Human Kit (Meso Scale Diagnostics, Maryland, USA) was used to measure CRP concentrations. All analytes were tested in duplicate. The intra- and inter-assay coefficients of variation (CV) were 6.7% and 8.9% (leptin), 6.9% and 8.5% (insulin), 2.8% and 6.9% (adiponectin), 2.2% and 10.0% (CRP), respectively.
fMRI MID task
Brain reward processing was measured using the MID task84 programmed in PsychoPy85. Each MID run included 45 trials and lasted 562 s. On each trial, a cue that indicated a potential win or loss (circle or square) was presented, then a target (white triangle) was presented after a short delay. Participants were instructed to press a button within a short response time to win or avoid losing the amount of money indicated by the cue. To make sure each participant succeeded on approximately 66% of trails, task difficulty was calibrated by each participant’s reaction time during a practice session and updated during the scan. Brain images were acquired with two identical GE MR750 3T scanners at Laureate institute for Brain Research, Tulsa, Oklahoma, USA. The scanning parameters were TR/TE = 2000/27 ms, FOV/slice = 240/2.9 mm, 128 × 128 matrix, 39 axial slices. High resolution structural T1-weighted images were acquired (TR/TE = 5/2.012 ms, FOV/slice = 240 × 192/0.9 mm, 186 axial slices).
Neuroimaging data preprocessing
Neuroimaging data preprocessing was conducted using the AFNI software package86. The preprocessing steps included discarding of first 3 TRs, despiking, slice timing correction, co-registration to anatomical volumes, motion correction, smoothing with a 4 mm Gaussian full width at half-max smoothing kernel, and normalization to Montreal Neurological Institute space. The blood oxygen level dependent (BOLD) response to each of the six anticipatory task conditions (three win and three loss) was modeled with four-second block regressors convolved with a canonical hemodynamic response function. Volumes with either a Euclidean norm of the derivatives of the six motion parameters greater than 0.3 or greater than 10% outlier voxels were removed from regression step. Regressors used in the model were the first 4 polynomial baseline terms, along with 6 motion parameters (roll/pitch/yaw/x/y/z translation), large loss (− 5), small loss (− 1), no loss (− 0), no win (+ 0), small win (+ 1), large win (+ 5).
Statistical analysis on immunoassays
Normality of leptin, insulin and adiponectin distributions were tested using Shapiro-Wilks test; all three analytes were log-transformed due to their non-Gaussian distributions. Group differences on insulin and adiponectin concentrations were assessed using Analysis of Variance (ANOVA). The distributions for leptin were found to be non-Gaussian even after log-transformation, therefore, Kruskal–Wallis and Mann–Whitney–Wilcoxon non-parametric tests were used to test group differences. Values with an absolute z greater than 3 for each analyte were defined as outliers, however, no outliers were found in this dataset. Effect size was computed with Cohen’s d. In addition, Pearson’s correlations were used to explore potential relationships between leptin and demographics/clinical characteristics including: PBF, BMI, exercise, and PROMIS ratings within the MDD or groups. ANOVA tests were used to evaluate leptin level slope differences between groups.
Statistical analysis on neuroimaging
The slope difference in the relationship between leptin and percent fMRI BOLD signal change on the MID gain versus non-gain contrast was evaluated using AFNI’s group analysis program 3dttest++ using the model beta ~ Group*log(leptin). Clusters with a significant interaction were selected based on a voxel wise p < 0.05. The family wise error rate was set to α < 0.05 using 3dClustsim to estimate probability of false positives and 3dFWHMx to measure the intrinsic smoothness of the residuals, both using the spatial autocorrelation function (acf) option. Small volume correction was performed by applying this cluster-wise correction separately for 10 different regions (left and right insula, thalamus, caudate, putamen, nucleus accumbens) that were selected a priori. Follow up regression analyses were conducted in R for significant clusters.
Acknowledgements
The blood processing and immunoassays were conducted in Integrative Immunology Center (IIC), School of Community Medicine, The University of Oklahoma, Tulsa, OK. The authors wish to thank the IIC staff Ashlee Rempel, Brenda Davis and Chibing Tan for their work and support involved in the data collection.
Author contributions
K.B. contributed to data collection and analysis, and manuscript writing and preparation; B.M. contributed to manuscript writing and preparation; P.A.S. contributed to manuscript writing and preparation; L.K.F. contributed to manuscript writing and preparation, and final edits; R.K. contributed to imaging data collection, review and editing the manuscript; T.A.V. contributed to data collection, review and editing of the manuscript; R.A. contributed to review and editing of the manuscript; S.S.K. contributed to review and editing of the manuscript; J.B.S. contributed to critical manuscript review; T.K.T. contributed to critical review of the manuscript; M.P.P. contributed to study design, and critical review of the manuscript; J.L.S. contributed to research design, supervision of data analysis, critical manuscript writing and preparation.
Funding
This work was supported by the National Institute of General Medical Sciences Center Grant Award (P20GM121312 to MPP), the National Institute of Mental Health (K99MH126950 to LKF), National Institute on Drug Abuse (R01DA050677 to JLS), and The William K. Warren Foundation.
Data availability
The data that support the findings of this study are available on request from the corresponding author, KB. The data are not publicly available due to restrictions e.g., their containing information that could compromise the privacy of research participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi MH, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME. Using biomarkers to predict treatment response in major depressive disorder: Evidence from past and present studies. Dialogues Clin. Neurosci. 2014;16:539–544. doi: 10.31887/DCNS.2014.16.4/mthase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese JR, et al. Methodological approaches and magnitude of the clinical unmet need associated with amotivation in mood disorders. J. Affect. Disord. 2014;168:439–451. doi: 10.1016/j.jad.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 5.O'Donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci. Biobehav. Rev. 2013;37:96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohler CA, et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rethorst CD, Bernstein I, Trivedi MH. Inflammation, obesity, and metabolic syndrome in depression: Analysis of the 2009–2010 National Health and Nutrition Examination Survey (NHANES) J. Clin. Psychiatry. 2014;75:e1428–1432. doi: 10.4088/JCP.14m09009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: Double trouble. Neuron. 2020 doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arteaga-Henriquez G, et al. Low-grade inflammation as a predictor of antidepressant and anti-inflammatory therapy response in MDD patients: A systematic review of the literature in combination with an analysis of experimental data collected in the EU-MOODINFLAME consortium. Front. Psychiatry. 2019;10:458. doi: 10.3389/fpsyt.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows K, et al. Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain Behav. Immun. 2021;93:214–225. doi: 10.1016/j.bbi.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzagalli DA. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felger JC, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treadway MT, et al. Association between interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol. Psychiatry. 2017;82:570–577. doi: 10.1016/j.biopsych.2017.02.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry. 2015;172:1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes. 2014;63:3992–3997. doi: 10.2337/db14-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Rahilly S. 20 years of leptin: what we know and what the future holds. J. Endocrinol. 2014;223:E1–3. doi: 10.1530/JOE-14-0506. [DOI] [PubMed] [Google Scholar]
- 22.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 23.Allison MB, Myers MG., Jr 20 years of leptin: Connecting leptin signaling to biological function. J. Endocrinol. 2014;223:T25–35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota N, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes BS, et al. Insulin resistance in depression: A large meta-analysis of metabolic parameters and variation. Neurosci. Biobehav. Rev. 2022;139:104758. doi: 10.1016/j.neubiorev.2022.104758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gecici O, et al. High serum leptin levels in depressive disorders with atypical features. Psychiatry Clin. Neurosci. 2005;59:736–738. doi: 10.1111/j.1440-1819.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- 28.Milaneschi Y, et al. Leptin, abdominal obesity, and onset of depression in older men and women. J. Clin. Psychiatry. 2012;73:1205–1211. doi: 10.4088/JCP.11m07552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou X, et al. Role of leptin in mood disorder and neurodegenerative disease. Front. Neurosci. 2019;13:378. doi: 10.3389/fnins.2019.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge T, Fan J, Yang W, Cui R, Li B. Leptin in depression: A potential therapeutic target. Cell Death Dis. 2018;9:1096. doi: 10.1038/s41419-018-1129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao B, et al. Leptin and adiponectin levels in major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2018;238:101–110. doi: 10.1016/j.jad.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Dong X, Chen J. Adiponectin and depression: A meta-analysis. Biomed. Rep. 2015;3:38–42. doi: 10.3892/br.2014.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vancampfort D, et al. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: A meta-analysis of prevalences and moderating variables. Psychol. Med. 2014;44:2017–2028. doi: 10.1017/S0033291713002778. [DOI] [PubMed] [Google Scholar]
- 34.Sen ZD, et al. Linking atypical depression and insulin resistance-related disorders via low-grade chronic inflammation: Integrating the phenotypic, molecular and neuroanatomical dimensions. Brain Behav. Immun. 2021;93:335–352. doi: 10.1016/j.bbi.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 36.Park PH, et al. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J. Biol. Chem. 2008;283:26850–26858. doi: 10.1074/jbc.M802787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumada M, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 38.Stouffer MA, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat. Commun. 2015;6:8543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 40.Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komura Y, et al. Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature. 2001;412:546–549. doi: 10.1038/35087595. [DOI] [PubMed] [Google Scholar]
- 42.Kirson D, et al. Decreased excitability of leptin-sensitive anterior insula pyramidal neurons in a rat model of compulsive food demand. Neuropharmacology. 2022;208:108980. doi: 10.1016/j.neuropharm.2022.108980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werther GA, et al. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 44.Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Curr. Opin. Psychol. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knutson B, Heinz A. Probing psychiatric symptoms with the monetary incentive delay task. Biol. Psychiatry. 2015;77:418–420. doi: 10.1016/j.biopsych.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 46.van Andel M, van Schoor NM, Korten NC, Heijboer AC, Drent ML. Ghrelin, leptin and high-molecular-weight adiponectin in relation to depressive symptoms in older adults: Results from the Longitudinal Aging Study Amsterdam. J. Affect. Disord. 2022;296:103–110. doi: 10.1016/j.jad.2021.09.069. [DOI] [PubMed] [Google Scholar]
- 47.Jeong HG, et al. Plasma adiponectin elevation in elderly individuals with subsyndromal depression. Psychoneuroendocrinology. 2012;37:948–955. doi: 10.1016/j.psyneuen.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Simmons WK, et al. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol. Psychiatry. 2018 doi: 10.1038/s41380-018-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ten Have M, et al. The identification of symptom-based subtypes of depression: A nationally representative cohort study. J. Affect. Disord. 2016;190:395–406. doi: 10.1016/j.jad.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 50.Francisco V, et al. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018;9:640. doi: 10.3389/fphys.2018.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006;393:7–20. doi: 10.1042/bj20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuda S, et al. Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases. 2019 doi: 10.3390/diseases7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandey GN, Sharma A, Rizavi HS, Ren X. Dysregulation of protein kinase C in adult depression and suicide: evidence from postmortem brain studies. Int. J. Neuropsychopharmacol. 2021;24:400–408. doi: 10.1093/ijnp/pyab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, et al. Stress-activated protein kinase JNK modulates depression-like behaviors in mice. Mol. Neurobiol. 2023 doi: 10.1007/s12035-023-03209-x. [DOI] [PubMed] [Google Scholar]
- 55.Miller AH, Raison CL. Cytokines, p38 MAP kinase and the pathophysiology of depression. Neuropsychopharmacology. 2006;31:2089–2090. doi: 10.1038/sj.npp.1301032. [DOI] [PubMed] [Google Scholar]
- 56.Wang JQ, Mao L. The ERK pathway: Molecular mechanisms and treatment of depression. Mol. Neurobiol. 2019;56:6197–6205. doi: 10.1007/s12035-019-1524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avery JA, et al. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeVille DC, et al. The neural bases of interoceptive encoding and recall in healthy adults and adults with depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018;3:546–554. doi: 10.1016/j.bpsc.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gotlib IH, et al. Neural processing of reward and loss in girls at risk for major depression. Arch. Gen. Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keren H, et al. Reward processing in depression: A conceptual and meta-analytic review across fMRI and EEG studies. Am. J. Psychiatry. 2018;175:1111–1120. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farooqi IS, et al. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.South T, Huang XF. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem. Res. 2008;33:598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- 63.Burrows K, et al. Association of poorer dietary quality and higher dietary inflammation with greater symptom severity in depressed individuals with appetite loss. J. Affect. Disord. 2020;263:99–106. doi: 10.1016/j.jad.2019.11.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendoza-Herrera K, et al. The leptin system and diet: A mini review of the current evidence. Front. Endocrinol. (Lausanne) 2021;12:749050. doi: 10.3389/fendo.2021.749050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott MM, et al. Leptin targets in the mouse brain. J. Comp. Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toyoda H. CB1 cannabinoid receptor-mediated plasticity of GABAergic synapses in the mouse insular cortex. Sci. Rep. 2020;10:7187. doi: 10.1038/s41598-020-64236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 2018;19:153–165. doi: 10.1038/nrn.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vancampfort D, et al. Could autonomous motivation hold the key to successfully implementing lifestyle changes in affective disorders? A multicentre cross sectional study. Psychiatry Res. 2015;228:100–106. doi: 10.1016/j.psychres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 69.Sylvia LG, et al. Nutrition, Exercise, and Wellness Treatment in bipolar disorder: Proof of concept for a consolidated intervention. Int. J. Bipolar Disord. 2013;1:24. doi: 10.1186/2194-7511-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chekroud SR, et al. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry. 2018;5:739–746. doi: 10.1016/s2215-0366(18)30227-x. [DOI] [PubMed] [Google Scholar]
- 71.Wielopolski J, et al. Physical activity and energy expenditure during depressive episodes of major depression. J. Affect. Disord. 2015;174:310–316. doi: 10.1016/j.jad.2014.11.060. [DOI] [PubMed] [Google Scholar]
- 72.Schuch F, et al. Physical activity and sedentary behavior in people with major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2017;210:139–150. doi: 10.1016/j.jad.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu H, et al. Serum leptin concentration is associated with total body fat mass, but not abdominal fat distribution. Int. J. Obes. Relat. Metab. Disord. 1997;21:536–541. doi: 10.1038/sj.ijo.0800437. [DOI] [PubMed] [Google Scholar]
- 74.Shah NR, Braverman ER. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luppino FS, et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 76.Mannan M, Mamun A, Doi S, Clavarino A. Prospective associations between depression and obesity for adolescent males and females: A systematic review and meta-analysis of longitudinal studies. PLoS ONE. 2016;11:e0157240. doi: 10.1371/journal.pone.0157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Badillo N, Khatib M, Kahar P, Khanna D. Correlation between body mass index and depression/depression-like symptoms among different genders and races. Cureus. 2022;14:e21841. doi: 10.7759/cureus.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharafi SE, et al. Prevalence of anxiety and depression in patients with overweight and obesity. Obes. Med. 2020;17:100169. doi: 10.1016/j.obmed.2019.100169. [DOI] [Google Scholar]
- 79.Figueroa-Hall LK, et al. Psychiatric symptoms are not associated with circulating CRP concentrations after controlling for medical, social, and demographic factors. Transl. Psychiatry. 2022;12:279. doi: 10.1038/s41398-022-02049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morris AA, et al. The association between depression and leptin is mediated by adiposity. Psychosom. Med. 2012;74:483–488. doi: 10.1097/PSY.0b013e31824f5de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schalet BD, et al. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J. Clin. Epidemiol. 2016;73:119–127. doi: 10.1016/j.jclinepi.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Victor TA, et al. Tulsa 1000: A naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open. 2018;8:e016620. doi: 10.1136/bmjopen-2017-016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- 84.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 85.Peirce JW. PsychoPy–Psychophysics software in Python. J. Neurosci. Methods. 2007;162:8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, KB. The data are not publicly available due to restrictions e.g., their containing information that could compromise the privacy of research participants.