Abstract
This work explored the activities of bergamot oil nano-emulsion (NBG) in modulating blood biochemical parameters, redox status, immunity indices, inflammation markers, semen quality, testicular changes and the expression of HSPs genes in stressed rabbit bucks. Twenty-four mature rabbit bucks (5 months) were randomly divided into three groups; control group (NBG0) received 1 ml of distilled water, while the other two groups received NBG orally at doses of 50 and 100 mg/kg (bw) twice a week. The present study's findings revealed that treated groups had lower values of total and direct bilirubin, triglyceride, lactate dehydrogenase, and creatinine compared with NBG0 group (p < 0.05). NBG100 group recorded the greatest of total protein, albumin, GPx, T3 and T4 values as well as the lowest values of uric acid, MDA, and indirect bilirubin. Both treated groups showed significantly reduced 8-OhDG, Amyloid A, TLR 4, while significantly increased nitric oxide, IgA, IgM, TAC, and SOD levels. Semen characteristics such as volume, sperm count, sperm motility, normal sperm, and vitality were significantly higher in the NBG100 group compared to the NBG50 and NBG0 groups, whereas sperm abnormalities and dead sperm were significantly reduced. HSP70, HSP72, and HSPA9 gene overexpression showed that testicular integrity was maintained after buck received oral doses of 50 or 100 mg/kg of NBG. Existing findings indicate that oral administration of NBG improves heat tolerance in rabbit bucks primarily as e result of its antioxidant and anti-inflammatory effects.
Keywords: Citrus bergamia, Bergamot oil nano-emulsion, Heat stress, Inflammation, Oxidative stress, Semen quality, Rabbit bucks
1. Introduction
Global climate change promotes the jeopardy of excessive heat actions and is categorized by temperatures transcending the long-term intermediates of duration, magnitude, and occurrence (Cheng et al., 2022, Tripathy, 2023), menacing animal health, and the reproductive competence (Abdelnour et al., 2020) as well as the economic capability of food-producing enterprises globally (Malik et al., 2022). As the high ambient temperature is progressively rising global, heat stress (HS) represents a perilous risk towered the livestock sector (Cheng et al., 2022). Conversely, the livestock division has adversative impacts on climate change, contributing around 14.5% of global greenhouse emissions (Scoones, 2023). Consequently, there is emergent concern in escalating the animal keeping as an economical and environmentally sustainable solution. However, HS triggered negative effects on rabbits' feed effectiveness, growth performance, immunity, and anti-oxidative system of rabbits (Sabry et al., 2021, Bashar et al., 2022, El-Gindy et al., 2022, Liang et al., 2022). The reduction in the previous aspects caused by HS might be associated with main economic losses, and extremely restrains the expansion and progress of the rabbit business (Madkour et al., 2021, Bashar et al., 2022), especially in the hot region (Sabry et al., 2021). Due mainly to malfunctioning or fewer sweat glands than normal in their epidermis, rabbits have a lot of difficulty releasing heat from their bodies (Abdelnour et al., 2020). Accumulative studies elucidated that severe HS is distinguished as an issue that strictly impacts the semen quality, and testicular development of rabbit bucks (El-Gindy et al., 2022, El-Kholy et al., 2021), instigating male infertility or sterility. HS can also increase the inflammatory cytokines execrations, testicular damage, metabolic dysfunction, reduce sexual behavior and generate oxidative stress and modify transcriptomic changes (Yasoob et al., 2022, Abdelnour et al., 2021). Recently, different nutritional strategies have been evidenced to mitigate the adverse consequences of HS in rabbit bucks including phytochemicals, and nano-emulsified vegetable oils in stressed broiler (Abudabos et al., 2021). Over the past two decades, growing evidence demonstrated that nano-emulsified essential oil components such as cinnamaldehyde, thymol and eugenol are an effective way in attenuating the oxidative stress and inflammation factors induced by pathogenesis or environmental issues in different animal species (Abudabos et al., 2021, Ibrahim et al., 2022, Ibrahim et al., 2021, Abd EL-Hamid et al., 2021). Nevertheless, our knowledge regarding their modes of action and aspects of their application in emaciating the undesirable influences of HS in rabbit bucks is still restricted.
Bergamot is prevalent term for Citrus bergamia Risso et Poit., a plant belonging to the Rutaceae family. The plants display large dark green ovate leaves similar to those of lemon, round yellow fruits and starshaped white flowers. During the last decades, C. bergamia juice has been raising attention because of its multi health promoting effects (Ilari et al., 2021, Scuteri et al., 2019). It is suggested that the beneficial properties of C. bergamia primarily derive from its superior amounts of polyphenols (Siano et al., 2023). Based on the previous studies, bergamot oil (BO) comprises considerable quantities of limonene, linalool, β- and α -pinene, linalyl acetate, γ-terpinene, myrcene, and sabinene as well as a wide range of vitamins (Cui et al., 2020, Siano et al., 2023). Moreover, BO has antioxidant, anti-inflammatory, antiulcerogenic, and hepatoprotective capabilities (Cui et al., 2020, Karaca et al., 2005, Kesbic et al., 2020). Gamma-aminobutyric acid (GABA) levels in the rat hippocampus were shown to be markedly increased in earlier studies, indicating a possible anxiolytic effect of BO (Saiyudthong and Marsden, 2011). Addition of BO (0.5 %) to Nile Tilapia foods enhanced the growth activity, feed efficiency and improved the blood metabolites in the fishes (Kesbiç et al., 2020). Moreover, BO attenuated aluminum-induced anxiety in rats (Cui et al., 2020), and showed a protective effect toward chemotherapy-induced neuropathic pains (Ilari et al., 2021). Based on the unique characteristic of BO as described in previous investigations, we hypothesized that the nano-emulsion of BO (NBG) would boost the immune system, and antioxidants capability, reduce the inflammation and improve the semen quality in stressed rabbit bucks. Thereafter, the intent of this work was to explore how oral NBG treatment during extremely difficult HS conditions affected bucks’ semen quality and testicular attributes possibly via reducing the oxidative stress and inflammation.
2. Materials and methods
2.1. Ethic al approval
The investigational fulfillments in this research were permitted by the IACUC (Institutional Animal Use and Care Committee), Zagazig University (Approval number, ZU IACUC/2/F/367/2022). According to the ARRIVE guidelines, the use and care of animals totally followed those rules.
2.2. Preparation of bergamot oil nano-emulsion (NBG)
Citrus essential oil (Citrus bergamia, bergamot) was purchased from the Horticulture Research Institute, Agricultural Research Centre (ARC), Egypt. According to the previous described method (Mohammadifar et al., 2021), a spontaneous emulsification method was applied to prepare the nano-emulsion of bergamot oil (NBG). Morphology of the freshly prepared NBG was visualized by transmission electron microscope (TEM); JEOL JEM-2100, JEOL Ltd., Tokyo, Japan) and presented in Fig. 1. The prepared NBG was preserved at 4˚C until further used and orally administered. The principal components of bergamot oil were examined using the gas chromatography-mass spectrometry (GC–MS) method as described by Meng et al. (2014). Mass spectrum similarities with a variety of commercially available libraries, such as the NIST/EPA/NIH Mass spectral Library and the Wiley Registry of Mass spectrum Data, was utilized to identify the constituents. The content of the essential oil is presented in Table 1.
Fig. 1.
A TEM image of NBG reveals spherical particles of various sizes and few aggregations.
Table 1.
Identification and quantification of the main compounds in the essential oil of Citrus bergamia.
| No. | Compound | % | Rt |
|---|---|---|---|
| 1 | (+) – limonene | 51.32 | 4.36 |
| 2 | linalyl acetate | 21.34 | 10.23 |
| 3 | linalool | 7.2 | 6.47 |
| 4 | β-pinene | 6.81 | 4.78 |
| 5 | γ-terpinene | 6.38 | 6.85 |
| 6 | α-pinene | 1.18 | 3.97 |
| 7 | sabinene | 1.33 | 4.29 |
| 8 | myrcene | 1.17 | 5.47 |
| Total | 96.73 | ||
2.3. Animal and treatments
Twenty-four rabbit bucks aged 5 months, weighing 3.15 + 0.2 kg were picked out from the farm flock for this research. Animals were housed in natural environmental conditions with a natural ventilation system. Animals were kept individually in cages of galvanized wire net (60 × 60 × 40 cm, for width, length and height) and provided with a manual feeder and an automatic drinker. The bucks were kept under the same hygienic, managerial, and housing environments throughout the entire investigational period. Diets were formulated to comprise digestible energy 2718 kcal/kg, and the percentages of crude protein and fiber were 16.44, and 12.33%, respectively. The bucks (n = 24, 8 in each group) were alienated at random into three experimental treatments as follow: group 1 (control group); bucks fed with basic diet without any supplements (NBG0), groups 2 and 3 were treated with NBG (per oral) at doses of 50 mg (NBG50) and 100 mg/kg (NBG100) body weight per buck twice time weekly as previously recorded (Celia et al., 2013). The NBG administration was provided to the bucks by orally system in the morning between 8:00 a.m. and 9:00 a.m. To assess the severity of heat stress on rabbits, the temperature humidity index (THI) values were used for this propose according to previously published study (Marai et al., 2002). Utilizing a hygrothermograph (ST-50A, SEKONIC, Tokyo, Japan) fixed in the center of animal facility 30–50 cm above the cages, the daily ambient temperature (AT) and relative humidity (RH) were assessed. The following equation was used for identifying the THI values:
THI = db − [(0.31–––0.31(RH)] × [(db °C − 14.4)],
where db is the dry bulb temperature (AT) in °C. The THI readings were then into the following categories: < 27.8 = absence of heat stress; 27.8 to 28.9 = moderate heat stress; 28.9 to 30.0 = severe heat stress, and > 30.0 = extremely severe heat stress.
2.4. Biochemical analysis of blood parameters
After the treatment period of 3 months, six bucks form each group were selected for blood sample collection from the ear vein via sterilized heparinized tubes. Centrifugation (1700 × g for 15 min at 4 °C) was performed to blood samples for separating and collecting the plasma. Then, the plasma samples were kept at − 20 °C for more biochemical examination. Metabolites in the blood including total protein, albumin, globulins, total bilirubin, direct and indirect bilirubin, triglyceride, lactate dehydrogenase, uric acid and creatinine were estimated spectrophotometrically based on the constructer's procedure utilizing commercial ELISA kits procured from Biodiagnostic Company (Giza, Egypt).
2.5. Redox status and immunity assays
Utilizing ELISA kits supplied by Biodiagnostics company (Giza, Egypt), the redox status was evaluated using superoxide dismutase (SOD), reduced glutathione (GSH), and total antioxidant capacity (TAC), as determined by the methods of (Paglia and Valentine, 1967), (Durak, 1993), and (Abo-Elsoud et al., 2019),. The malondialdehyde (MDA) levels in plasma were evaluated using the method of Tsikas and Mikuteit (Tsikas and Mikuteit, 2022). According to the method of Abdulazeem and Jassim (Abdulazeem and Jassim, 2018), the immunoglobulins (IgA and IgM) were assessed by ELISA kit purchased from Elabscince company (Houston, Texas, 77079, USA), following the directions protocols.
2.6. Assays of inflammatory response, oxidative DNA marker and thyroid hormones
The plasma levels of TLR4 were calculated by sandwich ELISA (Rockland company, Limerick, PA 19468, USA) in a modified procedure suggested by the technique of Liu (Liu et al., 2012). Nitric oxide (NO) levels in the blood were determined using the Griess reagent, as described by Chen and his co-authors (Chen et al., 2022). The levels of Amyloid A (ng/ml) were measured with sandwich ELISA provided by (Biosource, Camarillo, CA) following the proceedings recommended and based on the method of Kepes (Képes et al., 2022). Additionally, the plasma levels of thyroid hormones including Triiodothyronine (T3) and Thyroxine (T4) were detected by commercial kits on radioimmunoassay (RIA) method. Moreover, the concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG; ng/ml) which is a valuable indicator for DNA injury, was assessed. Assessment of 8-OHdG levels from plasma was accomplished utilizing a competitive sandwich ELISA kit (Trevigen, Gaithersburg, MD, USA) following the manufacturer's directions based on the method of Kocak (Koçak et al., 2022).
2.7. Semen collection and assessments
In addition to the control group, ten bulks from each treated group were chosen for semen assessment after 3 months of oral administration of NBG. Semen samples (12 ejaculates from each bulk) were collected using artificial vagina method where a mature female was employed as a mount and introduced into the buck for the collection of semen. Immediately after semen collection, the samples were reserved in the water bath (37 °C) pending semen valuation. Semen attributes, counting volume of each ejaculate, sperm livability, progressive motility, sperm normality, abnormal sperm, and dead sperm of bucks were evaluated as described previously in our work (Abdelnour et al., 2021). Valuations of dead, live, and anomalous sperms were executed by tally 200 sperm cells using an Eosin–Nigrosin staining mixture. Complete or partial, purple-stained sperm cells were measured nonviable, while no stained sperm cells were measured viable. Proportions of movable sperms on a warm stage displaying continuous forward motion were visually assessed using a light microscope (X100).
2.8. Testis histological architecture
Three bucks form each treatment were selected for slaughtering (Islamic method) and the testis were removed from each buck. The testicular specimens were preserved in buffered neutral formalin solution (10% for 48 h). Then, the fixed specimens were administered for dehydration in alcohol, cleared in xylol, and embedded in paraffin. A thin section of samples (5 μm thicknesses) was prepared and picked up using microtome (Leica RM 2155, London, UK) and deparaffinised. After that, the sections were stained with H&E (eosin and haematoxylin) stains for histopathology evaluations (Suvarna et al., 2018). After staining, the alterations in testicular tissue sections were detected in certain fields under blindfold situations using standard light microscopy (Olympus BX 51, Japan) for exploring the pathological fluctuations. Then, the histopathological lesions grading was planned by explanation of histomorphology fluctuations in five pitches per segment conferring to (Gibson-Corley et al., 2013) protocol.
2.9. RNA isolation and real-time quantitative RT-PCR
Testicular tissues were picked up for studying the molecular changes induced by HS. RNA was extracted from testicular tissues utilizing Trizol reagent (Invitrogen, USA) following the instructions from the manufacturer. The RNA quantity and quality were evaluated using NanoDrop 1000 at 260/280 wavelength. The RNA was converted into cDNA via revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). A precise primer pair for the mRNA of the designated genes was intended via Primer-BLAST software and presented in Table 2. The reverse transcription was performed utilizing the PrimeScript RT reagent kit (Takara, China). The qRT-PCR analyses were carried out in QuantStudio 6 Flex system (Applied Biosystems, USA) utilizing SYBR Premix Ex Taq™ II reagents (No. RR820A, Takara, China) as designated earlier (Madkour et al., 2021, Liu et al., 2012). The fold changes in selected genes were determined by the equation, 2^-ΔΔCt (Livak and Schmittgen, 2001).
Table 2.
A list of the primer’s sequences, amplicon size, and annealing temperature used in the present study.
| Gene name | Primer | Molecular weight (bP) | Annealing temperature (℃) |
|---|---|---|---|
| HSP70 | F: CGTGGAGTCCTACACCTACAAC R: ACTCGTCTTTCTCGGCCATC |
152 | 58 |
| HSP72 | F: ATGGCCAAGCAATCAGCG R: TAATCCACCTGCACGATGGT |
122 | 59 |
| HSPA9 | F: CCACCAGGATGGCTGGAATGG R: CCAACAACTGCACCCTTAATTGCTT |
96 | 57.5 |
| b-actin | F: CTGGAACGGTGAAGGTGACA R: CGGCCACATTGCAGAACTTT |
73 | 56 |
2.10. Statistical model and analysis procedure
Results were expressed as means ± SE. The Levene and Shapiro–Wilk tests were conducted in order to check for normality and homogeneity of variance (Razali and Wah, 2011). One way ANOVA of statistical analysis system (Proc Anova; SAS., 2012 version 8, Cary, NC, USA) was used for assessing the studied variables. P-values<0.05 were recognized as statistically significant.
3. Results
3.1. Meteorological parameters
As depicted in Fig. 2, temperature-humidity index (THI) values were arranged between 74.78 and 87.51 during the whole experimental period. The first 6 weeks of experimental period, animals did not suffer from heat stress as reported in the material and methods (absence heat stress) depending on the THI values. The THI values were 81.69 after six weeks of treatment, indicating mild heat stress. The THI values for the remaining experiment weeks ranged from 84.39 to 87.51, indicating that the rabbits were under severe and extremely severe heat stress.
Fig. 2.
Calculated temperature-humidity index (THI) throughout the experimental period in Sharkia Province, Egypt.
3.2. Liver and kidney functions
As illustrated in Fig. 3, bucks administrated with 50 or 100 mg/kg of NBG had markedly (p < 0.05) lower levels of total bilirubin, direct and indirect bilirubin, ALT, AST, ALP, and LDH lactate. The lowest levels of liver parameters were noticed in NBG100. Total protein and albumin in the treated groups of bucks were significantly higher than in the control animal (p < 0.05). There were no statistical alterations in globulins and A/G ratio among the treated groups and the heat stressed group (p > 0.05). Bucks administrated with 50 or 100 mg/kg NBG had markedly (p < 0.05) lower levels of total bilirubin, direct and indirect bilirubin, triglyceride, lactate dehydrogenase, while the lowest levels of indirect bilirubin were noticed in NBG100 (p < 0.05). When compared to the NBG0 group, treated groups significantly reduced uric acids (p < 0.001) and creatinine (p < 0.05), with 100 mg/kg of NBG producing the best outcomes. Overall, the treatment of NBG (50 or 100 mg/kg) to heat-stressed rabbit bucks significantly reduced the damage caused by HS to the liver and kidney tissues. (Fig. 3, Fig. 4, Fig. 5).
Fig. 3.
Effects of orally administration of nano-emulsion of bergamot oil (NBG) on liver biomarkers of rabbit bucks during thermal stress. Data presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 compared to control. Rabbits received bergamot essential oil nano-emulsion at different concentrations: 0 (Control), 50 (NBG50) and 100 mg/kg (NBG100).
Fig. 4.
Effects of orally administration of nano-emulsion of bergamot oil (NBG) on total protein, albumin and globulin levels of rabbit bucks during thermal stress. Data presented as mean ± SEM, *P < 0.05, compared to control. Rabbits received bergamot essential oil nano-emulsion at different concentrations: 0 (Control), 50 (NBG50) and 100 mg/kg (NBG100).
Fig. 5.
Effects of orally administration of nano-emulsion of bergamot oil (NBG) on kidney biomarkers of rabbit bucks during thermal stress. Data presented as mean ± SEM, *P < 0.05, **P < 0.01 compared to control. Rabbits received bergamot essential oil nano-emulsion at different concentrations: 0 (Control), 50 (NBG50) and 100 mg/kg (NBG100).
3.3. DNA damage, inflammatory and immunity responses
The levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG; ng/ml), which is an indicator for oxidative destruction of DNA, were assessed. Both groups NBG50 and NBG100 significantly declined the 8-OHdG levels (ng/ml) as compared to the NBG0 group (p < 0.001) (Fig. 6). Amyloid A (p = 0.035) and TLR 4 (p = 0.005) were significantly inferior in all supplemented groups relative to the control. The lowest values were detected in bucks administrated with NBG at 100 mg/kg (Fig. 6). Moreover, bucks administrated with NBG (50 or 100 mg/kg) caused markedly an elevation in the nitric oxide levels in relation to untreated group (p = 0.028). Similarly, NBG50 and NBG100 groups had significantly higher IgM (an increase 458%, and 437%) and IgA (an increase 270% and 222%, respectively) levels than the control group (p < 0.001).
Fig. 6.
Effects of orally administration of nano-emulsion of bergamot essential oil (NBG) on DNA damage, inflammatory and immunity indices of rabbit bucks during thermal stress. Data presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 compared to control. Rabbits received bergamot essential oil nano-emulsion at different concentrations: 0 (Control), 50 (NBG50) and 100 mg/kg (NBG100).
3.4. Redox status and thyroid hormones
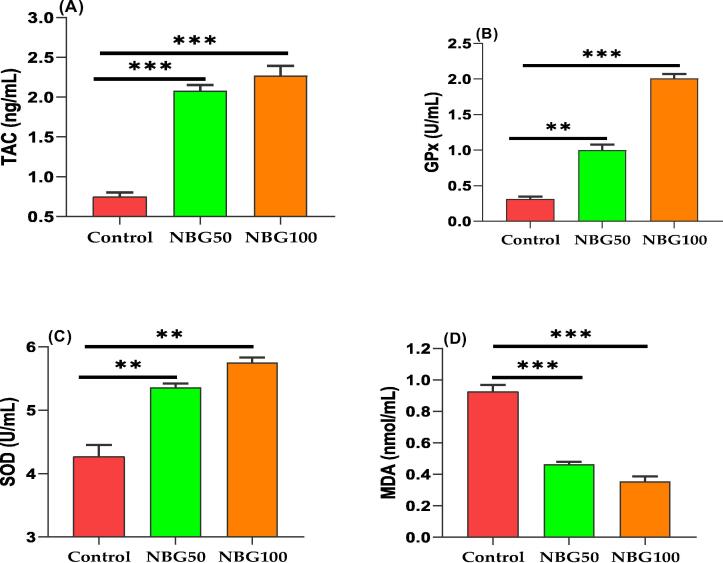
The values of TAC, GPx and SOD were very low in the untreated group, whereas the level of MDA was high. Administration of NBG at 50 and 100 mg/kg exhibited significantly higher levels of TAC, GPx and SOD in comparison to the control group (Fig. 7). Furthermore, the NBG100 group had the greatest levels of TAC and Gpx, while the NBG50 exhibited intermediate values for SOD activities. Heat stress induced significantly elevations in serum lipid peroxidation (MDA), while administration of NBG at dosage of 50 and 100 mg/kg markedly reduced this augmentation by 46.6% and 32.3% for NBG50 and NBG100, respectively (Fig. 7). T3 and T4 had the best results in the NBG100 group, with highly significant differences from the other groups. NBG50 showed intermediate values for T3 and T4 levels. Overall, T3 values were increased by 10.4 and 62.8%, while T4 increased by 10.9 and 17.5% in NBG50 and NBG100 groups, respectively (Fig. 8).
Fig. 7.
Effects of orally administration of nano-emulsion of bergamot oil (NBG) on antioxidative and oxidative biomarkers of rabbit bucks during thermal stress. Data presented as mean ± SEM, **P < 0.01, ***P < 0.001 compared to control. TAC; total antioxidant capacity, Gpx; Glutathione peroxidase, SOD; Superoxide dismutase, MDA; Malondialdehyde. Rabbits received bergamot essential oil nano-emulsion at different concentrations: 0 (Control), 50 (NBG50) and 100 mg/kg (NBG100).
Fig. 8.
Effects of orally administration of nano-emulsion of bergamot oil (NBG) on antioxidative and oxidative biomarkers of rabbit bucks during thermal stress. Data presented as mean ± SEM, **P < 0.01, ***P < 0.001 compared to control. T3; Triiodothyronine and T4; Thyroxine. Rabbits received bergamot essential oil nano-emulsion at different concentrations: 0 (Control), 50 (NBG50) and 100 mg/kg (NBG100).
3.5. Semen quality and testicular alterations
As depicted in Table 3, NBG administration significantly improved all semen parameters including semen volume, normal sperm, sperm concentration, progressive motility, sperm livability, while decreased significantly the dead sperm percentage and abnormality of heat stressed rabbit bucks. NBG100 exhibited the lowest values for dead sperm compared with the NBG50 and NBG0 groups. Moreover, the intermediate values for sperm concentration, and sperm livability were detected in NBG50 treatment. As shown in Fig. 9, rabbits received 100 mg/kg of NBG exhibited significantly higher levels of NSET, (numbers of seminiferous tubules), MDSET (mean diameters of seminiferous tubule, µm), HSET, (height of seminiferous, µm). Similarly, the NSP/SET (numbers of spermatogonia/ seminiferous tubule) and NSPC/SET (numbers of spermatocytes/ seminiferous tubule) measurements were also significantly higher.
Table 3.
Effects of orally administration of nano-emulsion of bergamot oil on semen quality and testicular attributes of rabbit bucks during thermal stress.
| Item1 | Treatments |
||
|---|---|---|---|
| Control | NBG50 | NBG100 | |
| Volume (ml) | 0.425 | 0.463 | 0.575* |
| Normal (%) | 75.25 | 90.12*** | 94.00*** |
| Sperm concentration (x106) | 72.25 | 87.7*** | 95.25*** |
| Progressive Motility (%) | 61.25 | 73.5** | 83.75*** |
| sperm livability (%) | 70.5 | 93.25*** | 96.00*** |
| Dead spermatozoa (%) | 29.5 | 6.75*** | 4.00*** |
| Abnormal spermatozoa (%) | 24.75 | 9.87*** | 6.00*** |
| NSET | 15 | 17* | 19* |
| MDSET | 200.66 | 233.3** | 279.33** |
| HSET | 60.66 | 69** | 81.66** |
| NSP/SET | 54 | 65.66** | 69.66** |
| NSPC/SET | 96.66 | 117*** | 132*** |
Results were presented as mean ± SEM, *P < 0.05, **P < 0.01 ***P < 0.001 compared to control. NSET, numbers of seminiferous tubules (SET); MDSET, mean diameters of SET (µm); HSET, Height of seminiferous (µm); NSP/SET, numbers of spermatogonia/SET, and NSPC/SET numbers of spermatocytes/SET. Rabbits received bergamot essential oil nano-emulsion at different concentrations: 0 (Control), 50 (NBG50) and 100 mg/kg (NBG100).
Fig. 9.
The lesions score of the severity extent within testicular tissues as affected by dietary supplement of NBG in rabbit buck diets. The represented scores are the mean lesion score. Histologic lesions were scored for severity (0 = No altrerations, 1 = Mild altrerations, 2 = Moderate altrerations, 3 = Severe altrerations).
3.6. Histological studies
Fig. 10 (A-F) demonstrated that some seminepherous tubules in the testes of heat-stressed rabbits (NGB0 group) had atrophied and were represented by necrotic germinal epithelial lining (Fig. 10A and 10B). Moreover, testicular tissues of those animals exhibited interstitial edema caused by heat stress. Rabbit bucks which had received 50 mg of NGB revealed moderate normal architectures of the majority of seminiferous tubules (Fig. 10C and D). However, less edema and depleted few numbers of germ cells in some tubules were encountered. Rabbit testicular tissues revealed normal histology of seminiferous tubules and Leyding cells after the treatment with 100 mg/kg of NGB (Fig. 10 E and F). Moreover, the seminiferous tubules exhibited normal spermatogonia, spermatocytes, spermatids and central impacted sperms.
Fig. 10.
(A-F). Photomicrograph of H&E-stained sections from testicular tissues of bucks. (A&B) Bucks reared under HS conditions (NBG), showing some atrophied seminepherous tubules (curved arrow) with necrotic germinal epithelial lining (arrowhead), in addition to interstitial edema (arrow). (C&D) Bucks received 50 mg/kg of NBG under hot conditions showing normal architectures of the majority of seminepherous tubules (thick arrow), less edema (arrow) and depleted few numbers of germ cells (arrowhead). (E&F) Bucks received 100 mg/kg of NBG showing regular histology of seminepherous tubules (thick arrow), spermatogonia (arrow), spermatocytes (arrowhead), central impacted sperms (star) and lyding cells (curved arrow).
3.7. Testicular heat stock proteins (HSPs) genes
The mRNA expression of HSPs including HSP70, HSP72 and HSPA9 were found to be downregulated in response to oral administration of NBG when compared with the control (Fig. 11).
Fig. 11.
Effect of nano-emulsion bergamot oil (NBG) on heat shock proteins (HSP72, HSP70, and HSPA9) related expressions in testicular tissues. Results are presented as mean ± SEM, **P < 0.01 ***P < 0.001 compared to control.
4. Discussion
High environmental temperature resulting from global warming has critical adverse effects on both animals and human, leading to impaired immune system, hormonal profile, dysregulated testicular development, reduced sperm function, and even infertility. The current data show that the liver function, immune system, and antioxidant status were all greatly improved, whereas DNA damage, inflammatory factors, and thyroid hormone synthesis were all dramatically decreased by the bergamot oil nano-emulsion. Additionally, NBG enhanced the reproductive capacity of buck under heat stress conditions via sustaining the testicular architecture, improving the semen quality and modulating the testicular heat shock protein families HSP70, HSP72 and HSPA9 genes.
Several studies have reported that HS could induce high damages in hepatic tissues of rabbits which resulted in significant levels of liver enzymes in the blood (Abdelnour et al., 2020, Bashar et al., 2022, Yasoob et al., 2022). In the present study, NGB attenuated liver injury by HS in rabbits via a significantly reduction in the levels of lactate dehydrogenase, triglycerides, total and direct bilirubin. Bergamot oil was found to have a hepatoprotective effect on rats in a prior study (Karaca et al., 2005), which agrees with the findings of our study. The use of medicinal plants in enhancing the blood metabolites in rabbits has been confirmed by many authors (Abdelnour et al., 2022, Abdelnour et al., 2020, Swelum et al., 2021). However, the application of nano-emulsion essential oils such NBG is a new strategy for reducing the negative effects of heat stroke in animal and is considered as an urgently needed safe and applicable method for more sustainable production. Previous investigations demonstrated that oral administration of fresh onion juice (El-Gindy et al., 2022) and orange peel extract (Morshedy et al., 2022) considerably improved blood parameters in male and female rabbits raised under severe heat stress. The earlier researchers observed that these phytochemicals improved reproductive efficiency and might lessen the adverse consequences of extreme heat exhaustion in rabbits. Additionally, (Perna et al., 2019) showed that bergamot oil (BO) has positive effects on hyperlipidemia by regulating triglycerides, LDL, HDL and total cholesterol. In a recent study on fish, dietary BO considerably increased total protein and reduced cholesterol and triglyceride levels. This is comparable to our current data in rabbit bucks exposed to HS. According to Saiyudthong and Marsden (2011), BO exhibits anxiolytic-like characteristics by weakening the HPA axis and reducing the corticosterone stress response (Saiyudthong and Marsden, 2011).
In comparison to rats treated with AlCl3, rats co-treated with bergamot oil significantly reduced oxidative stress by increasing antioxidative defenses like CAT and SOD in the hippocampus (p < 0.05) (Cui et al., 2020). MDA is the lipid peroxidation product. Heat stress meaningfully augmented the amounts of MDA in the serum of heat stressed rabbit bucks (p < 0.05) as compared to control group. Oral administration of NBG (50 or 100 mg/kg) attenuated the oxidative stress via diminishing the MDA in rabbit caused by HS. Several earlier efforts share similarities with our findings, regarding the potentiality of bergamot oil in boosting the antioxidative enzymes and reducing the lipid peroxidation (Cui et al., 2020, Ilari et al., 2021). According to Madkour and his co-authors, using BO in its nano-form may be able to prevent critical proteins from nitrating, protecting them from oxidative stress and maintaining the DNA's integrity from damage (Madkour et al., 2021). As reported by (Roberto et al., 2010), the antioxidant property of D-limonene may help to successfully protect lymphocytes from oxidative stress and mitochondrial malfunction. The presence of D-limonene maybe explains the improvement in the antioxidative system in heat stressed bucks.
Our results revealed that exposing rabbit bucks to high environmental temperature induced significantly the inflammatory responses including increased levels of amyloid A and TLR 4 levels, and DNA marker 8-OhDG (p < 0.001), while the nitric oxide was lower in NBG0 group compared with the other groups. With this sense, co-administration of NBG at different levels considerably lowered the inflammatory response by declining the amyloid A and TLR 4 levels in rabbit bucks given NBG (p < 0.05). According to the research by Cui and co-authors (Cui et al., 2020), bergamot oil reduced the inflammation markers (TNF-α and IL-1β) induced by AlCl3 in rats. However, additional markers of inflammation, such as amyloid A and TLR 4, were used in our investigation. Inflammation throughout the body may result from this process, which may be linked to the increase in oxidative grade. Otherwise, (Ríus et al., 2022) indicated that HS induced a significant higher level of serum amyloid A in calves, while the probiotics inclusion did not ameliorate this effect. Bergamot juice (20 mg/kg) is auspicious mediator in terms of pro-inflammatory mediators decrease (Perna et al., 2019).
High levels of inflammatory factors after a heat stroke may be a result of testicular dysfunction, which is often accompanied by higher sperm death, worse sperm quality, and thus decreased reproductive potential. Amyloid A displays substantial immunological action via inducing the secretions of various cytokines and by being chemotactic for mast cells and neutrophils (Eklund et al., 2012). Moreover, it was found that amyloid A could activate cell-surface receptors TLR4 for boosting the immune system in the body (Eklund et al., 2012). As shown in our research, NBG modifies the immune reactions of rabbit bucks under heat stress. We have for the first time discovered that NBG has immunomodulatory properties in rabbits, which may open up new opportunities for employing compounds that can reduce animal heat stress through immunomodulatory agents.
Thyroid hormones have important roles in thermoregulation system in animal. HS caused critical disturbances in T3 and T4 synthesis in rabbits (Liang et al., 2022). Presently, NBG at 100 mg showed the highest values of T3 and T4 compared with other groups. Reduction in feed utilization inhibits the releasing of T3 and T4 which decline the growth performance and other physiological process (Liang et al., 2022). In line with our data, (El-Kholy et al., 2021) found that the dietary phytochemicals addition in the buck diets enhanced the thyroid function and other blood metabolites including kidney and liver functions.
Morphological structure of seminiferous tubules reflects the reproductive health status of the male. The current investigation demonstrated that NBG treatment of NBG could maintain the testicular architecture of heat stressed rabbit bucks. It’s well known that HS caused damages in the spermatogonia, Leyding cell, reduced the testosterone levels, and led to sperm abnormalities and testicular dysfunction. Due to the powerful antioxidant capacity of NBG and its function in attenuating the oxidative stress in the cellular study, NBG significantly enhanced the volume, sperm motility, livability, and concentration, as well as reduced the abnormalities and the number of dead sperm (Table 3). Previously, it was found that D-limonene (30–40 mg/kg injection) protects sexual organs from oxidative stress induced by old age (Shereen, 2020).
D-limonene is the major compound of bergamot essential oil as shown in Table 1 and evidenced by other researchers (Cui et al., 2020, Furneri et al., 2012, Li et al., 2022). The findings of the current research showed that d-limonene accounts for 51.3% of all discovered compounds. This percentage is higher than the 33.7% found in the study conducted by Furneri et al., 2012, but it is lower than the 60.9% found in the study conducted by Cui et al., 2020. The percentages of other major compounds such as linalyl acetate (21.3%), linalool (7.2%), and γ-terpinene (6.4%) were lower than those reported by Furneri et al., 2012. In contrast to our findings, other researchers (Djenane, 2015, Salvino et al., 2022) revealed that bergamot essential oil primarily consists of linalool acetate and linalool. Indeed, the composition of essential oils could be influenced by fruit maturity, growth stage of the plant, storage conditions, extraction procedures. Additionally, it is likely that both genetic and geographical environmental factors contributed to the development of a chemical composition variation.
Similar to our findings, other investigations have demonstrated that the bergamot oil enhanced the thickness of the walls of seminiferous tubules and tubules with regular layers of various spermatogenic cell types in the testis of old rats (Roberto et al., 2010, Shereen et al., 2020).
As shown in Fig. 9 and Table 3, NBG (particularly 100 mg/kg) significantly improved bucks as seen by increased spermatogenic cell layer thickness. Other authors provided evidence of this (Cui et al., 2020, Roberto et al., 2010). The antioxidant impact of limonene may be also responsible for the improvement in testicular characteristics. Another main compound presented in bergamot oil is linalool, which exhibits antihyperalgesic and antinociceptive properties in various animal models in addition to anti‐inflammatory actions (Sakurada et al., 2009). Our findings show that NBG treatments improved buck sperm quality, including sperm count, motility, and normal sperm, as well as a clear decrease in the fraction of dead and abnormal sperm. Moreover, (El-Gindy et al., 2022) found that fresh onion juice (1.5, and 3 ml/kg) significantly enhanced sexual behavior such libido, and semen features viz. individual and mass sperm motility, semen concentration, sperm viability, and acrosome reaction in heat stressed rabbit bucks. HSPs are an intracellularly produced family of proteins that are tremendously conserved and prevalent and are triggered in response to numerous environmental issues (Abdelnour et al., 2019). In the current study, it was found that the NBG induced significant down regulation of the HSPs including HSP70, HSP72 and HSPA9 in testis tissues. Consequently, the reduction in the of HSP70, HSP72 and HSPA9 expressions in testicular tissues of treated NBG groups reflecting its antioxidant capability, which can affect the important enzymes that have a significant effect on maintaining the redox balance and homeostasis in the body (Yasoob et al., 2022, Abdelnour et al., 2019). As mentioned above, the oral administration of NBG can alleviate the adverse effects of HS in rabbit bucks via modulating the immune, inflammatory and HSFs genes due its powerful antioxidant, immunomodulatory, and anti-inflammatory properties. Nevertheless, to clarify its probable use as an anti-stressor agent in animal feeding and welfare, more molecular and transcriptome studies are required.
5. Conclusions
In summary, HS induced liver and kidney dysfunction, decreased antioxidant function, upregulated the protein expression of HSPs family, impaired the testicular tissues, caused inflammation, and declined the immune system of rabbit bucks. The HS-induced testicular injury is accompanied by reduced semen quality. Oral administration of bergamot oil nano-emulsion (50 or 100 mg/kg bw) protects against testicular damage and immune dysfunction in buck with HS via the action on associated HSPs (HSP70, HSP72, and HSPA9) genes.
Funding
This research was funded by the Researchers Supporting Project number (RSP2023R119), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to Researchers Supporting Project number (RSP2023R119), King Saud University, Riyadh, Saudi Arabia for funding this work.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sultan A.M. Saghir, Email: sultan_a1976@yahoo.com, sultan.s.ayesh@ahu.edu.jo.
Ramzi A. Mothana, Email: rmothana@ksu.edu.sa.
References
- Abd EL-Hamid, M. I., Ibrahim, S. M., Eldemery, F., EL-Mandrawy, S. A., Metwally, A. S., Khalifa, E., Elnahriry, S. S. & Ibrahim, D. 2021. Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 113, 96-105. [DOI] [PubMed]
- Abdelnour, S. A., Abd EL-Hack, M. E., Khafaga, A. F., Arif, M., Taha, A. E. Noreldin, A. E. 2019. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. Therm. Biol. 79, 120-134. [DOI] [PubMed]
- Abdelnour, S., EL-Saadony, M., Saghir, S., Abd EL-Hack, M., AL-Shargi, O., AL-Gabri, N., Salama, A. 2020. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 240, 104220.
- Abdelnour, S. A., AL-Gabri, N. A., Hashem, N. M., Gonzalez-Bulnes, A. 2021. Supplementation with proline improves haemato-biochemical and reproductive indicators in male rabbits affected by environmental heat-stress. Animals. 11, 373. [DOI] [PMC free article] [PubMed]
- Abdelnour, S. A., EL-Ratel, I. T., Peris, S. I., EL-Raghi, A. A., Fouda, S. F. 2022. Effects of dietary thyme essential oil on blood haematobiochemical, redox status, immunological and reproductive variables of rabbit does exposed to high environmental temperature. Ital. J. Anim. Sci. 21, 51-61.
- Abdulazeem L., Jassim Y. The effect of yolk immunoglobulin and heat killed Sallmonella typhi on rabbits. Res. J. Pharm. Technol. 2018;11:2503–2506. [Google Scholar]
- Abo-Elsoud M., Hashem N., El-Din A.N., Kamel K., Hassan G. Soybean isoflavone affects in rabbits: Effects on metabolism, antioxidant capacity, hormonal balance and reproductive performance. Anim. Reprod. Sci. 2019;203:52–60. doi: 10.1016/j.anireprosci.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Abudabos, A. M., Suliman, G. M., AL-Owaimer, A. N., Sulaiman, A. R. A., Alharthi, A. S. 2021. Effects of nano emulsified vegetable oil and betaine on growth traits and meat characteristics of broiler chickens reared under cyclic heat stress. Animals, 11, 1911. [DOI] [PMC free article] [PubMed]
- Bashar A.M., Abdelnour S.A., Abdelhalim A., Sheiha A.M. Effect of selenium nanoparticles and/or spirulina platensis on growth, hematobiochemical, antioxidant status, hormonal profile, immunity, and apoptosis of growing rabbits exposed to thermal stress. ERSA. 2022;32:77–103. [Google Scholar]
- Celia, C., Trapasso, E., Locatelli, M., Navarra, M., Ventura, C. A., Wolfram, J., Carafa, M., Morittu, V. M., Britti, D., DI Marzio, L. 2013. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids and Surfaces B: Biointerfaces, 112, 548-553. [DOI] [PubMed]
- Chen Q., Kao X., Gao Y., Chen J., Dong Z., Chen C. Nitric oxide-caused rabbit chondrocyte apoptosis is linked to cytoskeletal protein proteolysis anomaly through intracellular JNK and ERK signal pathways. Mol. Cell Toxicol. 2022:1–9. [Google Scholar]
- Cheng M., Mccarl B., Fei C. Climate change and livestock production: a literature review. Atmosphere. 2022;13:140. [Google Scholar]
- Cui Y., Che Y., Wang H. Bergamot essential oil attenuate aluminum-induced anxiety-like behavior through antioxidation, anti-inflammatory and GABA regulation in rats. Food Chem. Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111766. [DOI] [PubMed] [Google Scholar]
- Djenane D. Chemical profile, antibacterial and antioxidant activity of Algerian Citrus essential oils and their application in Sardina pilchardus. Foods. 2015;4:208–228. doi: 10.3390/foods4020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durak I. A methodological approach to superoxide dismutase (SOD) activity assay based on inhibition of nitroblue tetrazolium (NBT) reduction. Clin. Chim. Acta. 1993;214:103–104. doi: 10.1016/0009-8981(93)90307-p. [DOI] [PubMed] [Google Scholar]
- Eklund K.K., Niemi K., Kovanen P.T. Immune functions of serum amyloid A. Crit. Rev. Immunol. 2012;32:335–348. doi: 10.1615/critrevimmunol.v32.i4.40. [DOI] [PubMed] [Google Scholar]
- El-Gindy Y.M., Zahran S.M., Hassan M.A., Sabir S.A. Effect on physiological parameters and semen quality upon oral administration of fresh onion juice to V-line rabbit buck during severe heat stress. Animal Biotechnol. 2022:1–9. doi: 10.1080/10495398.2022.2070184. [DOI] [PubMed] [Google Scholar]
- El-Kholy, K. H., Wafa, W. M., EL-Nagar, H. A., Aboelmagd, A. M., El-Ratel, I. T. 2021. Physiological response, testicular function, and health indices of rabbit males fed diets containing phytochemicals extract under heat stress conditions. Journal of Advanced Res. Vet. Sci. 8, 256. [DOI] [PMC free article] [PubMed]
- Furneri P.M., Mondello L., Mandalari G., Paolino D., Dugo P., Garozzo A., Bisignano G. In vitro antimycoplasmal activity of Citrus bergamia essential oil and its major components. Eur. J. Med. Chem. 2012;52:66–69. doi: 10.1016/j.ejmech.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Gibson-Corley K.N., Olivier A.K., Meyerholz D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013;50:1007–1015. doi: 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, D., Abdelfattah-Hassan, A., Badawi, M., Ismail, T. A., Bendary, M. M., Abdelaziz, A. M., Mosbah, R. A., Mohamed, D. I., Arisha, A. H., EL-Hamid, M. I. A. 2021. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 11, 1-20. [DOI] [PMC free article] [PubMed]
- Ibrahim, D., Eldemery, F., Metwally, A. S., Abd-Allah, E. M., Mohamed, D. T., Ismail, T. A., Hamed, T. A., Al Sadik, G. M., Neamat-ALLAH, A. N., Abd El-Hamid, M. I. 2022. Dietary eugenol nanoemulsion potentiated performance of broiler chickens: Orchestration of digestive enzymes, intestinal barrier functions and cytokines related gene expression with a consequence of attenuating the severity of E. coli O78 infection. Front. Vet. Sci. 9. [DOI] [PMC free article] [PubMed]
- Ilari, S., Lauro, F., Giancotti, L. A., Malafoglia, V., Dagostino, C., Gliozzi, M., Condemi, A., Maiuolo, J., Oppedisano, F., PALMA, E. 2021. The protective effect of bergamot polyphenolic fraction (Bpf) on chemotherapy-induced neuropathic pain. Pharmaceuticals, 14, 975. [DOI] [PMC free article] [PubMed]
- Karaca M., Ilhan F., Altan H., Him A., Tutuncu M., Özbek H. Evaluation of hepatoprotective activity of Bergamot orange in rats. East. J. Med. 2005;10:1. [Google Scholar]
- Kepes, Z., Barkóczi, A., Szabó, J. P., Kálmán-Szabó, I., Arató, V., jószai, I., Deák, Á., Kertesz, I., Hajdu, I., Trencsenyi, G. 2022. In Vivo Preclinical Assessment of β-Amyloid–Affine [11C] C-PIB Accumulation in Aluminium-Induced Alzheimer’s Disease-Resembling Hypercholesterinaemic Rat Model. Int J Molec Sci. 23, 13950. [DOI] [PMC free article] [PubMed]
- Kesbic O.S., Acar Ü., Yilmaz S., Aydin Ö.D. Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus) Fish Physiol. Biochem. 2020;46:103–110. doi: 10.1007/s10695-019-00700-y. [DOI] [PubMed] [Google Scholar]
- Kocak N., Can E., Yeter V., Turunc M., Subaşi M., Niyaz L., Avci B. Aqueous humor and serum levels of 4-hydroxynonenal and 8-hydroxy-2′ deoxyguanosine in pseudoexfoliation syndrome and glaucoma. Int. Ophthalmol. 2022:1–10. doi: 10.1007/s10792-022-02539-4. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu S., Zhao C., Zhang Z., Nie D., Tang W., Li Y. The chemical composition and antibacterial and antioxidant activities of five Citrus essential oils. Molecules. 2022;27(20):7044. doi: 10.3390/molecules27207044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Z. L., Chen, F., Park, S., Balasubramanian, B., liu, W. C. 2022. Impacts of Heat Stress on Rabbit Immune Function, Endocrine, Blood Biochemical Changes, Antioxidant Capacity and Production Performance, and the Potential Mitigation Strategies of Nutritional Intervention. Front Vet Sci, 9. 906084. [DOI] [PMC free article] [PubMed]
- Liu, Y., Zhao, H., Zhang, Q., Tang, J., Li, K., Xia, X.-J., Wang, K.-N., LI, K., Lei, X. G. 2012. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J nutri. 142, 1410-1416. [DOI] [PMC free article] [PubMed]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madkour M., Aboelenin M.M., Shakweer W.M., Alfarraj S., Alharbi S.A., Abdel-Fattah S.A., Alagawany M. Early life thermal stress modulates hepatic expression of thermotolerance related genes and physiological responses in two rabbit breeds. Ital. J. Anim. Sci. 2021;20:736–748. [Google Scholar]
- Malik A., Li M., Lenzen M., Fry J., Liyanapathirana N., Beyer K., Boylan S., Lee A., Raubenheimer D., Geschke A. Impacts of climate change and extreme weather on food supply chains cascade across sectors and regions in Australia. Nature Food. 2022;3:631–643. doi: 10.1038/s43016-022-00570-3. [DOI] [PubMed] [Google Scholar]
- Marai I., Habeeb A., Gad A. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: a review. Livest. Prod. Sci. 2002;78:71–90. [Google Scholar]
- Meng J., Chen X., Yang W., Song J., Zhang Y., Li Z., Yang X., Yang Z. Gas chromatography-mass spectrometry analysis of essential oils from five parts of Chaihu (Radix Bupleuri Chinensis) J. Tradi. Chinese Med. 2014;34:741–748. doi: 10.1016/s0254-6272(15)30090-x. [DOI] [PubMed] [Google Scholar]
- Mohammadifar M., Aarabi M.H., Aghighi F., Kazemi M., Vakili Z., Memarzadeh M.R., Talaei S.A. Anti-osteoarthritis potential of peppermint and rosemary essential oils in a nanoemulsion form: behavioral, biochemical, and histopathological evidence. BMC Complement. Ther. Med. 2021;21:1–12. doi: 10.1186/s12906-021-03236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedy, S. A., Zahran, S. M., Sabir, S. A., EL-Gindy, Y. M. 2022. Effects of increasing levels of orange peel extract on kit growth, feed utilization, and some blood metabolites in the doe rabbits under heat stress conditions. Animal Biotechnol. 1-12. [DOI] [PubMed]
- Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Perna S., Spadaccini D., Botteri L., Girometta C., Riva A., Allegrini P., Petrangolini G., Infantino V., Rondanelli M. Efficacy of bergamot: From anti-inflammatory and anti-oxidative mechanisms to clinical applications as preventive agent for cardiovascular morbidity, skin diseases, and mood alterations. Food Sci. Nutr. 2019;7:369–384. doi: 10.1002/fsn3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razali N.M., Wah Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Comput. Simul. 2011;2:21–33. [Google Scholar]
- Ríus, A. G., Kaufman, J. D., li, M. M., Hanigan, M. D., Ipharraguerre, I. R. 2022. Physiological responses of Holstein calves to heat stress and dietary supplementation with a postbiotic from Aspergillus oryzae. Sci. Rep. 12, 1587. [DOI] [PMC free article] [PubMed]
- Roberto D., Micucci P., Sebastian T., Graciela F., Anesini C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010;106:38–44. doi: 10.1111/j.1742-7843.2009.00467.x. [DOI] [PubMed] [Google Scholar]
- Sabry M., Zaki M.M., Elgohary F.A., Helal M.M. Sustainable rabbit production under the global warming conditions in southern Mediterranean region. World's Vet. J. 2021;11:543–548. [Google Scholar]
- Saiyudthong S., Marsden C.A. Acute effects of bergamot oil on anxiety-related behavior and corticosterone level in rats. Phytother. Res. 2011;25:858–862. doi: 10.1002/ptr.3325. [DOI] [PubMed] [Google Scholar]
- Sakurada T., Kuwahata H., Katsuyama S., Komatsu T., Morrone L.A., Corasaniti M.T., Bagetta G., Sakurada S. Intraplantar injection of bergamot essential oil into the mouse hind paw: effects on capsaicin-induced nociceptive behaviors. Int. Rev. Neurobiol. 2009;85:237–248. doi: 10.1016/S0074-7742(09)85018-6. [DOI] [PubMed] [Google Scholar]
- Salvino R.A., Aroulanda C., De Filpo G., Celebre G., De Luca G. Composition and authenticity evaluation of bergamot essential oil assessed by nuclear magnetic resonance spectroscopy. Anal. Bioanal. Chem. 2022;414:2297–2313. doi: 10.1007/s00216-021-03869-5. [DOI] [PubMed] [Google Scholar]
- Scoones I. Livestock, methane, and climate change: The politics of global assessments. Wiley Interdiscip. Rev.: Clim. Change. 2023;14:e790. doi: 10.1002/wcc.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri D., Rombola L., Morrone L.A., Bagetta G., Sakurada S., Sakurada T., Tonin P., Corasaniti M.T. Neuropharmacology of the neuropsychiatric symptoms of dementia and role of pain: Essential oil of bergamot as a novel therapeutic approach. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20133327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.S., Mahmoud A., Mohamed Z.B., Mohamed A. Effect of D-limonene on the age-related androgenic changes in male rats. Med. J. Cairo Univ. 2020;88:599–609. [Google Scholar]
- Siano F., Picariello G., Castaldo D., Cautela D., Caruso T., Vasca E. Monitoring antioxidants by coulometry: Quantitative assessment of the strikingly high antioxidant capacity of bergamot (Citrus bergamia R.) by-products. Talanta. 2023;251 doi: 10.1016/j.talanta.2022.123765. [DOI] [PubMed] [Google Scholar]
- Suvarna, K. S., Layton, C., Bancroft, J. D. 2018. Bancroft's theory and practice of histological techniques E-Book, Elsevier health sciences.
- Swelum, A. A., Hashem, N. M., Abdelnour, S. A., Taha, A. E., Ohran, H., Khafaga, A. F., EL-Tarabily, K. A., Abd EL-Hack, M. E. 2021. Effects of phytogenic feed additives on the reproductive performance of animals. Saudi Journal of Biological Sciences, 28, 5816-5822. [DOI] [PMC free article] [PubMed]
- Tripathy, G. K. 2023. Climate Change and Policy Interventions for Livestock Sector. Impact of Climate Change on Livestock Health and Production. CRC Press.
- Tsikas D., Mikuteit M. N-Acetyl-L-cysteine in human rheumatoid arthritis and its effects on nitric oxide (NO) and malondialdehyde (MDA): Analytical and clinical considerations. Amino Acids. 2022;54:1251–1260. doi: 10.1007/s00726-022-03185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoob T.B., Khalid A.R., Zhang Z., Zhu X., Hang S. Liver transcriptome of rabbits supplemented with oral Moringa oleifera leaf powder under heat stress is associated with modulation of lipid metabolism and up-regulation of genes for thermo-tolerance, antioxidation, and immunity. Nutri. Rese. 2022;99:25–39. doi: 10.1016/j.nutres.2021.09.006. [DOI] [PubMed] [Google Scholar]