Abstract
Introduction
Bradykinesia (ie, slow movements) is one of the most prominent symptoms of Parkinson’s disease (PD) and has a negative impact on quality of life. Rhythmic auditory stimulation (RAS), a widely used and promising treatment technique, has been shown to effectively improve gait speed in patients with PD. The upper-limb movements, which also suffer from bradykinesia, are essential for daily life and directly impact quality of life. The term, patterned sensory enhancement (PSE) instead of RAS, is used when movement training targets the human body except lower limbs. Up until now, scarce studies have explored effects of training involving PSE on upper-limb movements. The purpose of this study is to investigate effects of movement training involving PSE on upper-limb movement speed and function in patients with PD.
Methods and analysis
A total of 138 patients with PD will be randomly assigned into two groups: the PSE group and the no-PSE group. A 21-day upper-limb training involving PSE (for the PSE group) or without PSE (for the no-PSE group) will be provided to the patients. An assessor will administer the box and block test and the Jebsen hand function test before and after training to assess upper-limb movement speed and function. The one-way analysis of covariance will be performed. This randomised controlled trial will provide evidence supporting effectiveness of upper-limb movement training involving PSE on reducing severity of bradykinesia in patients with PD.
Ethics and dissemination
Ethical approval has been obtained from the Institutional Review Board of the Hong Kong Polytechnic University with the reference number HSEARS20221027005. Informed consent forms will be gathered from all patients before their participation. Study results will be disseminated through conferences and peer-reviewed academic journals.
Trial registration number
Keywords: Acoustic stimulation, Parkinson’s disease, Arm, Movement, Bradykinesia
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a randomised controlled trial with one experimental group with Parkinson’s disease (PD) receiving 21-day upper-limb movement training with the aid of patterned sensory enhancement (PSE) and one control group with PD receiving the same training without the aid of PSE.
PSE is three tempi of metronome beat that are based on the participant’s fastest upper-limb movement speed before the training and gradually increase by each week.
Movement speed and quality are assessed using the box and block test and the Jebsen hand function test.
One possible concern of this training combining face-to-face sessions and home training sessions is whether participants will adhere to the home training protocol, which will be addressed via real-time video meetings during all home training sessions.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease caused by neurodegeneration of the substantia nigra, resulting in a decrease in dopamine.1 2 The incidence of PD is 14 per 100 000 people per year, and even reaches 160 per 100 000 people over the age of 65 years.3 Dopamine, a neurotransmitter, is important for the function of the basal ganglia in receiving, modulating and transmitting signals to various cortical areas, including those associated with movements.4 Therefore, in patients with PD, a decrease in dopamine leads to basal ganglia dysfunction in the cortico-striato-thalamo-cortical circuit, resulting in movement symptoms.5 6 Bradykinesia, meaning slowness of movements, is one of the most prominent symptoms of PD,7 extensively interferes with performances of daily activities such as eating, writing and walking,8 and substantially lowers the quality of life in patients.9 10
Pharmacotherapy has been shown to alleviate movement symptoms in patients with PD. Medications, such as levodopa, are able to adjust activities of the putamen and thalamus, modulate signals from basal ganglia to motor-related cortices, and thus enhance movements in patients.11 However, long-term use of medications increases medication resistance and thus reduces therapeutic effects, as well as increases side effects of medications such as dyskinesia.12 13 Therefore, developing non-pharmacological therapies is warranted and of clinical importance in order to tackle bradykinesia in patients with PD.
Rhythmic auditory stimulation (RAS) is repetitive, discrete sounds with a tempo.14 15 Because the tempo of human movements is naturally synchronised with the tempo of RAS,16 17 RAS has a high potential of being applied to movement training to guide human movement execution.18 Earlier studies19 have provided solid evidence that training involving RAS is effective in improving gait performance in patients with PD. Earlier classic research20 examining effects of training involving RAS on gaits in patients with PD provided 21-day training with 30 min per day, in which RAS with three different tempi (normal RAS, quick RAS, and fast RAS) was provided. Initially, researchers assessed the baseline walking tempo without the aid of RAS for each patient. For daily training, each patient was given normal RAS (100% of the baseline tempo), quick RAS (105% of the baseline tempo), and fast RAS (110% of the baseline tempo) to guide the gaits. Each RAS was increased by 5% when the time went to the next week.
The term, patterned sensory enhancement (PSE) instead of RAS, is used when movement training targets the human body except lower limbs.21 Most previous studies that investigated RAS effects in patients with PD focused on lower-limb movements such as gaits.19 However, upper-limb movements are also important for activities of daily living and directly affect quality of life in humans.22 In addition, it is commonly seen that rehabilitation training for upper-limb movements involves continuous movement repetition, which supports that PSE may be applicable to upper-limb movement therapy. It is worth investigating if upper-limb training involving PSE is effective for patients with PD. To date, only few studies23 24 have investigated effects of PSE on upper-limb movements in patients with PD. A case report23 indicated that movement training involving PSE may improve finger-tapping speed and finger dexterity in patients with PD. In addition, a study using the repeated measures design24 demonstrated that faster PSE immediately induced faster upper-limb movement speed in patients with PD. To date, randomised controlled trials have been needed to determine whether long-term training involving PSE is effective in improving upper-limb movement speed and function in patients with PD.
It has been suggested that training involving RAS/PSE establishes an internal sense of rhythm in humans because humans keep anticipating subsequent beats of RAS/PSE.15 The established sense of rhythms persists in humans and keeps affecting movement execution even after RAS/PSE disappears.15 25 In addition, powerful influences of RAS/PSE on movements may also be associated with plentiful neural connections between auditory and motor cortices, including the cortico-striato-cortical pathway, the cortico-cerebello-cortical pathway and auditory-motor neural connections directly in the cortex.26 Earlier research27–30 has reported that RAS/PSE not only activates neurons in the auditory cortex, but also induces neural firing in motor-related cortical regions (such as primary motor cortex, premotor and supplementary motor areas) even though examinees are stationary without moving. In patients with PD, RAS/PSE improves movements possibly by involving the cortico-cerebello-cortical pathway and auditory-motor cortical connections to modulate neural activity of the motor cortex and bypassing the damaged cortico-striato-cortical pathway.26 31 Additionally, RAS/PSE serves as external cues, can provide timing (tempo) information for patients with PD, and thus possibly reduces the dependence of patients’ movements on impaired modulation function of basal ganglia.15
To sum up, the purpose of this study is to investigate effects of movement training involving PSE on upper-limb movement speed and function in patients with PD. We hypothesise that movement training involving PSE improves upper-limb movement speed and function in patients with PD. Validation of this hypothesis will fill up the knowledge gap regarding whether PSE is applicable to upper-limb training in the PD population and provide clinicians with evidence of non-pharmacological therapy for upper-limb bradykinesia in patients with PD. The training programme will serve as a reference for clinical practitioners who are interested in using RAS/PSE in clinical training for patients with PD.
Methods and analysis
This study follows the Standard Protocol Items Recommendations for Interventional Trials guidelines for reporting.32
Study design
A randomised controlled trial will be used to validate the hypothesis. Patients with PD will be randomly assigned to two groups by using computer-generated random numbers: the PSE group and the no-PSE group. Each sealed envelope with an assigned group will be used. The PSE group will receive upper-limb movement training with the aid of PSE; the no-PSE group will receive upper-limb movement training without the aid of PSE. This study will provide 21-day training with a session per day and 40 min per session. Assessments will be completed face to face before and after the 21-day training by a senior therapist who is blinded to the group allocation. Blinding of patients and people who provide training is not feasible in this study because patients and training providers know group allocation. For all participants, training and assessments will be performed during the ‘on’ state of their anti-Parkinson medication. We will conduct weekly face-to-face meetings with patients and real-time video meetings during all home training sessions to ensure the adherence of participants to the training programme. We will monitor muscle fatigue during home training by using a daily training log. The study is expected to commence in August of 2023 and is anticipated to be completed within 2 years.
Patient and public involvement
Patients were involved in the design and dissemination of this research. We carefully designed the training duration and content to prevent fatigue in patients according to literature and our pilot study.24 In addition, we will share key findings of this study with participants at the end of the study.
Participants
Patients with PD will be recruited from hospitals through posters and physician referrals. At pretest, this study will collect demographic and clinical data, including age, gender, disease duration, the more-affected side and medication dosage. The more-affected side (left or right) refers to the side of the body exhibiting more severe bradykinesia, which will be determined visually by an experienced physician. In addition, we will calculate the levodopa equivalent dose33 to measure medication dosage. Criteria for selecting patients are as follows: (A) idiopathic PD diagnosed by a neurologist based on the Movement Disorders Society clinical diagnostic criteria34; (B) the Hoehn and Yahr stage is 2 or 3, meaning that bilateral movement problems or combination with mild postural instability35; (C) a score of Montreal Cognitive Assessment is equal to or higher than 21 to ensure that they understand experimental instructions36–38 (D) a score of Edinburgh Handedness Inventory is above 60 to ensure that they are right-handed39 and (E) Types and doses of medications remain unchanged in the past month right before participation. Exclusion criteria include the presence of medical conditions or diseases that may affect hand movements, vision or hearing based on self-report. Patients will sign an informed consent form prior to admission to this study.
Sample size estimation
Because of no existing PD studies testing effects of training involving PSE on upper-limb movements, we calculated the effect size of training involving PSE (f=0.255) according to data of the classic study20 examining effects of training involving RAS on gait speed in patients with PD. The G*Power software (V.3.1.9.7) was used to estimate the required sample size under the following conditions: analysis of covariance as the statistical test, an effect size f of 0.255, the power of 0.8, the alpha level of 0.05, 2 groups and 10 covariates (age, gender, the Hoehn and Yahr stage, disease duration, the more-affected side, medication dosage, the number of training sessions the participant completes, the score of the depression item in the first part of the Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS) at pretest, the score of the anxiety item in the first part of MDS-UPDRS at pretest, and a pretest score of an outcome variable, including the score of the box and block test (BBT), the error rate during executing BBT, the score of the Jebsen hand function test (JHFT) and the domain score of the third part of MDS-UPDRS). The estimated total sample size was 124. Considering the dropout rate of 10%, the final total sample size was 138 patients (69 patients per group).
Intervention
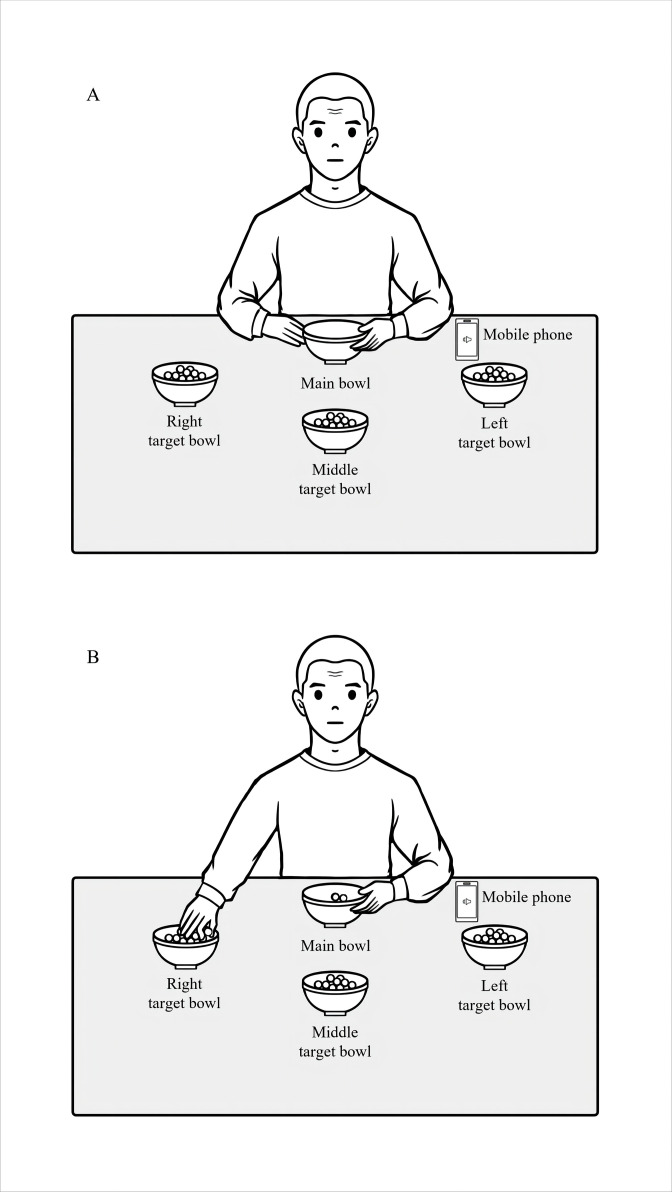
Patients with PD will be randomly assigned to two groups (the PSE group and the no-PSE group). Three-week training will be provided for both groups. The training protocol is mainly based on the classic study20 examining effects of RAS on gait speed in patients with PD and a recent study40 examining effects of PSE on upper-limb movements in the population exhibiting movement slowness. We increase the frequency of breaks during training per day because our pilot study24 observed that patients with PD had muscle fatigue easily when following PSE to execute upper-limb movements without frequent breaks. The training task of this study will be to use the right hand to move wooden beads one by one from one target bowl to the main bowl on the table (figure 1A). Three target bowls, labelled as the left, middle and right target bowl, will be placed on the table at an equal distance from the main bowl. The distance between a target bowl and the main bowl will be set at 30 cm, which is 50% of the upper-limb length (from the shoulder to the middle fingertip) of Hong Kong women,41 to ensure that beads in the target bowls are reachable for research participants. The angles between adjacent target bowls relative to the main bowl will be 30°. Wooden beads with a diameter of 2 cm will be put in the target bowls. The main bowl will be placed in front of the patient. Patients will be asked to use the right hand to take one bead at a time from the left target bowl to the main bowl, repeat this movement for the middle and right target bowls, and keep repeating this order (figure 1B).
Figure 1.
(A) The setup of the upper-limb training task. (B) The patient picks up one bead from one target bowl and is going to move the bead to the main bowl.
The training sessions will be conducted during the ‘ON’ period of medication. Specifically, the participant will be required to conduct daily training after 1 hour of taking medications. Each daily training will consist of three rounds separated by two 5 min breaks. Each round (about 10 min) will consist of four 2 min training sessions with a 30 s break between two adjacent sessions. On the first day after the pretest, patients will receive the first training session face to face in a hospital. After the first training session, patients will carry all training materials back home for subsequent 6-day home training sessions. Family members or caregivers will be only permitted to assist in setting up the training environment and not be allowed to assist the patient during training. On the first day of each subsequent training week, patients will be asked to return to the hospital to receive a face-to-face training session. We will conduct real-time video meetings during all home training sessions to ensure adherence to the treatment plan. We will also ask participants to complete daily training logs to record training completion and monitor the degrees of fatigue. In addition, we will calculate the number of training sessions the participant completes.
Before the first-day training, the baseline tempo of executing the training task will be assessed for each patient. The patient will be required to perform the aforementioned upper-limb movement task as fast as possible within 30 s for three times without listening to PSE. The obtained average number of wooden beads in the main bowl multiplied by two will be the baseline tempo (unit: beat per minute) for each patient.15 20
For the PSE group, each patient will receive a 45 min audio file, which includes briefing the patient about the daily training content (5 min) and providing PSE (10 min per round multiplied by three rounds, plus two 5 min break times; a total of 40 min). The normal PSE (100% of the baseline tempo), the quick PSE (105% of the baseline tempo) and the fast PSE (110% of the baseline tempo) will be provided in the first, second and third round of training (table 1). The patient will be asked to pick up a bead when she/he hears a beep sound of PSE. The tempo of the three PSE will be further increased by 5% of the baseline tempo when it goes to a new week. In the face-to-face session (the first day) of each week, the patient will obtain a new audio file and complete daily training face to face in the hospital. PSE will be metronome beep sounds generated by a metronome (SQ200, Seiko incorporated). The required tempo of PSE will be adjusted using the computer software Adobe Audition CC 2020. The audio file will be sent to the patient’s mobile phone, which will then be used to play the audio file during both face to face and home training.
Table 1.
PSE tempi that are provided in daily upper-limb training
| Day | First round: normal PSE | Second round: quick PSE | Third round: fast PSE |
| 1st–7th | 100% of the baseline tempo | 105% of the baseline tempo | 110% of the baseline tempo |
| 8th–14th | 105% of the baseline tempo | 110% of the baseline tempo | 115% of the baseline tempo |
| 15th–21 | 110% of the baseline tempo | 115% of the baseline tempo | 120% of the baseline tempo |
PSE, patterned sensory enhancement.
The no-PSE group will receive a 45 min audio file, which briefs the patient about the daily training content (5 min) and instructs the patient to move beads as fast as possible in each round without PSE. The training protocol is the same in the PSE group and the no-PSE group, except no PSE is provided for the no-PSE group during training. Similarly, the no-PSE group will perform the training face to face on the first day of each week in the hospital and at home on the remaining 6 days of a week.
Our previous empirical study has reported immediate effects of PSE on inducing faster upper-limb movements in patients with PD.24 However, we observed that although PSE with the speed of 110% and 120% of the baseline tempo was effective, patients had muscle fatigue easily when following the beat of PSE on an upper-limb movement task. This study protocol provides daily 40 min upper-limb movement training at the fastest speed for 40 min, which is very intensive and easily causes muscle fatigue. To reduce muscle fatigue in patients, we will adopt PSE with the tempo speed starting from 100%, 105% and 110% of the baseline tempo in the first-week training instead of 100%, 110% and 120%, and will increase the frequency of training breaks.
Outcome measures
The MDS-UPDRS is a commonly used tool in clinical settings and research to assess influences of PD on multiple aspects in patients.35 It consists of four parts, including (the first part) non-motor subjective experiences of daily living, (the second part) motor-related subjective experiences of daily living, (the third part) the motor examination and (the fourth part) motor complications.35 We will calculate the domain score of the third part of MDS-UPDRS, which is used to reflect objective severity of movement symptoms in patients. Larger scores indicate more severe movement symptoms. We will also use the score of the depression item and that of the anxiety item separately in the first part of MDS-UPDRS to detect levels of depression and anxiety in patients.
The BBT is used to measure gross manual dexterity as well as upper-limb movement speed.42 It is a 53.7×25.4 cm box separated into two compartments by a 15.2 cm high erected partition, with 150 blocks in each compartment. Starting from the dominant hand, patients will be asked to move the blocks one by one from the compartment on the hand side to the opposite side (eg, move the blocks from the right compartment to the left compartment for the right-hand test). Patients should move the blocks with their arms raised and crossed over the partition. They have 1 min to move the blocks as fast as possible. The score of BBT for each hand is the number of blocks that are successfully transferred between compartments in 1 min. A higher BBT score indicates faster upper-limb movements and better dexterity. In addition, the number of dropping blocks during the blocks moving tasks of BBT in each hand will be recorded as the error score. We will calculate the error rate of executing BBT in each hand by dividing the error score by the sum of the error score and the BBT score to assess the accuracy of upper-limb movements. The higher error rate indicates less accurate upper-limb movements. For the elderly, the BBT has high test-retest reliability (intraclass correlation coefficient of 0.89–0.97) and construct validity.43
The JHFT is used to assess unimanual hand function when examinees perform daily activities. Seven items are included in JHFT: writing, turning cards, picking up small objects, simulated feeding, stacking checkers, moving large light objects and moving large heavy objects.44 Considering that the patients are Chinese speakers, it is not appropriate to do English writing. According to a previous study conducted in Chinese cultures,45 the JHFT could be modified by excluding the writing item to avoid cultural influences on scores. The score for each item is the completion time. The less time a patient takes, the better hand function she/he has. We will calculate the total score of these six items as one dependent variable. The JHFT has excellent test–retest reliability (intraclass correlation coefficients of 0.89–0.97) for patients with PD.46
Safety
To assess the data safety, scientific validity and integrity of clinical trials, a data monitoring committee will be formed by two senior researchers who are not involved in the group allocation and protocol implications. After the study is completed, the research data will be retained for 5 years and destroyed afterwards. In this study, adverse events, defined as any unfavourable medical occurrence in a patient, will be collected and reported to data monitoring committee for records. This study will provide movement training. If participants have muscle fatigue during training, research personnel will provide break time immediately.
Data collection and statistical analysis
The general information and results will be kept on a portable hard drive. Authors of this study will conduct an interim analysis to re-estimate the required sample size and determine if the study should continue or be modified. Only authors of this study will be allowed to get access to the dataset.
A one-way analysis of covariance will be conducted to examine effects of group (the PSE group vs the no-PSE group) on each dependent variable, including BBT scores, the error rate during executing BBT, JHFT scores and the domain score of the third part of MDS-UPDRS at posttest. The 10 potential confounding factors are age, gender, the Hoehn and Yahr stage, disease duration, the more-affected side, medication dosage, the number of training sessions the participant completes, the score of the depression item in the first part of MDS-UPDRS at pretest, the score of the anxiety item in the first part of MDS-UPDRS at pretest, and a pretest score of an outcome variable (including BBT scores, the error rate during executing BBT, JHFT scores and the domain score of the third part of MDS-UPDRS). The alpha level (two tailed) will be set at 0.05. It is hypothesised that after controlling for confounding influences, PSE increases scores of BBT and decreases JHFT scores, the domain score of the third part of MDS-UPDRS, and the error rate of BBT. Patients may drop out before the study is completed. The last data point of the patient will be used to handle the missing data. The SPSS package (V.25) will be used to conduct statistical analysis.
Supplementary Material
Footnotes
Contributors: WF: study conception and design, execution, writing of the first draft. KNKF: review and critique. S-MW: study conception and design, review and critique.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Jastrzębowska MA, Marquis R, Melie-García L, et al. Dopaminergic modulation of motor network compensatory mechanisms in parkinson’s disease. Hum Brain Mapp 2019;40:4397–416. 10.1002/hbm.24710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gale JT, Amirnovin R, Williams ZM, et al. From symphony to cacophony: pathophysiology of the human basal ganglia in parkinson disease. Neurosci Biobehav Rev 2008;32:378–87. 10.1016/j.neubiorev.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Schwarzschild MA. The epidemiology of parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15:1257–72. 10.1016/S1474-4422(16)30230-7 [DOI] [PubMed] [Google Scholar]
- 4.Haber SN, Adler A, Bergman H. 2012. The basal ganglia. In: The Human Nervous System: Elsevier;2012:678–738. [Google Scholar]
- 5.Sian J, Gerlach M, Youdim MB, et al. Parkinson's disease: a major hypokinetic basal ganglia disorder. J Neural Transm (Vienna) 1999;106:443–76. 10.1007/s007020050171 [DOI] [PubMed] [Google Scholar]
- 6.Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for parkinson's disease. Nat Rev Neurosci 2010;11:760–72. 10.1038/nrn2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhakrishnan DM, Goyal V. Parkinson's disease: a review. Neurol India 2018;66:S26–35. 10.4103/0028-3886.226451 [DOI] [PubMed] [Google Scholar]
- 8.Simões R, Litvan I. Bradykinesia. In: Encyclopedia of Mov Disord: Elsevier 2010:158–61. [Google Scholar]
- 9.Muslimovic D, Post B, Speelman JD, et al. Determinants of disability and quality of life in mild to moderate parkinson disease. Neurology 2008;70:2241–7. 10.1212/01.wnl.0000313835.33830.80 [DOI] [PubMed] [Google Scholar]
- 10.Rajiah K, Maharajan MK, Yeen SJ, et al. Quality of life and caregivers' burden of parkinson's disease. Neuroepidemiology 2017;48:131–7. 10.1159/000479031 [DOI] [PubMed] [Google Scholar]
- 11.Kraft E, Loichinger W, Diepers M, et al. Levodopa-induced striatal activation in parkinson's disease: a functional MRI study. Parkinsonism Relat Disord 2009;15:558–63. 10.1016/j.parkreldis.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 12.Nonnekes J, Timmer MHM, de Vries NM, et al. Unmasking levodopa resistance in parkinson's disease. Mov Disord 2016;31:1602–9. 10.1002/mds.26712 [DOI] [PubMed] [Google Scholar]
- 13.Vorovenci RJ, Biundo R, Antonini A. Therapy-resistant symptoms in parkinson's disease. J Neural Transm (Vienna) 2016;123:19–30. 10.1007/s00702-015-1463-8 [DOI] [PubMed] [Google Scholar]
- 14.Ni M, Hazzard JB, Signorile JF, et al. Exercise guidelines for gait function in parkinson's disease: a systematic review and meta-analysis. Neurorehabil Neural Repair 2018;32:872–86. 10.1177/1545968318801558 [DOI] [PubMed] [Google Scholar]
- 15.Nombela C, Hughes LE, Owen AM, et al. Into the groove: can rhythm influence Parkinson's disease? Neurosci Biobehav Rev 2013;37:2564–70. 10.1016/j.neubiorev.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Thaut MH, Abiru M. Rhythmic auditory stimulation in rehabilitation of movement disorders: a review of current research. Music Percept 2010;27:263–9. 10.1525/mp.2010.27.4.263 [DOI] [Google Scholar]
- 17.Schaffert N, Janzen TB, Mattes K, et al. A review on the relationship between sound and movement in sports and rehabilitation. Front Psychol 2019;10:244. 10.3389/fpsyg.2019.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaut MH, McIntosh GC, Hoemberg V. Neurobiological foundations of neurologic music therapy: rhythmic entrainment and the motor system. Front Psychol 2014;5:1185. 10.3389/fpsyg.2014.01185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghai S, Ghai I, Schmitz G, et al. Effect of rhythmic auditory cueing on parkinsonian gait: a systematic review and meta-analysis. Sci Rep 2018;8:506. 10.1038/s41598-017-16232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaut MH, McIntosh GC, Rice RR, et al. Rhythmic auditory stimulation in gait training for parkinson's disease patients. Mov Disord 1996;11:193–200. 10.1002/mds.870110213 [DOI] [PubMed] [Google Scholar]
- 21.Thaut M, Hoemberg V. Handbook of neurologic music therapy. USA: Oxford University Press, 2014. [Google Scholar]
- 22.Cahn DA, Sullivan EV, Shear PK, et al. Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in parkinson's disease. Arch Clin Neuropsychol 1998;13:575–83. [PubMed] [Google Scholar]
- 23.Buard I, Dewispelaere WB, Thaut M, et al. Preliminary neurophysiological evidence of altered cortical activity and Connectivity with neurologic music therapy in Parkinson's disease. Front Neurosci 2019;13:105. 10.3389/fnins.2019.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan W, Li J, Wei W, et al. Effects of rhythmic auditory stimulation on upper-limb movements in patients with Parkinson's disease. Parkinsonism Relat Disord 2022;101:27–30. 10.1016/j.parkreldis.2022.06.020 [DOI] [PubMed] [Google Scholar]
- 25.Palmer C, Krumhansl CL. Mental representations for musical meter. J Exp Psychol Hum Percept Perform 1990;16:728–41. 10.1037//0096-1523.16.4.728 [DOI] [PubMed] [Google Scholar]
- 26.Leuk JSP, Low LLN, Teo W-P. An overview of acoustic-based interventions to improve motor symptoms in Parkinson's disease. Front Aging Neurosci 2020;12:243. 10.3389/fnagi.2020.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JL, Penhune VB, Zatorre RJ. Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex 2008;18:2844–54. 10.1093/cercor/bhn042 [DOI] [PubMed] [Google Scholar]
- 28.Bengtsson SL, Ullén F, Ehrsson HH, et al. Listening to rhythms activates motor and premotor cortices. Cortex 2009;45:62–71. 10.1016/j.cortex.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Koshimori Y, Thaut MH. Future perspectives on neural mechanisms underlying rhythm and music based Neurorehabilitation in Parkinson's disease. Ageing Res Rev 2018;47:133–9. 10.1016/j.arr.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 30.Grahn JA, Rowe JB. Feeling the beat: premotor and striatal interactions in musicians and nonmusicians during beat perception. J Neurosci 2009;29:7540–8. 10.1523/JNEUROSCI.2018-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braunlich K, Seger CA, Jentink KG, et al. Rhythmic auditory cues shape neural network recruitment in parkinson's disease during repetitive motor behavior. Eur J Neurosci 2019;49:849–58. 10.1111/ejn.14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyholm D, Jost WH. An updated Calculator for determining levodopa-equivalent dose. Neurol Res Pract 2021;3:58. 10.1186/s42466-021-00157-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for parkinson's disease. Mov Disord 2015;30:1591–601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 35.Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–70. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 36.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The moca: well-suited screen for cognitive impairment in parkinson disease. Neurology 2010;75:1717–25. 10.1212/WNL.0b013e3181fc29c9 [DOI] [PubMed] [Google Scholar]
- 37.Wang YP, Xu GL, Yang SQ, et al. Value of montreal cognitive assessment in identifying patients with mild vascular cognitive impairment after first stroke. Chin J Neuromed 2010;9:503–7. [Google Scholar]
- 38.Vitorio R, Stuart S, Gobbi LTB, et al. Reduced gait variability and enhanced brain activity in older adults with auditory cues: a functional near-infrared spectroscopy study. Neurorehabil Neural Repair 2018;32:976–87. 10.1177/1545968318805159 [DOI] [PubMed] [Google Scholar]
- 39.Yang N, Waddington G, Adams R, et al. Translation, cultural adaption, and test-retest reliability of Chinese versions of the edinburgh handedness inventory and Waterloo footedness questionnaire. Laterality 2018;23:255–73. 10.1080/1357650X.2017.1357728 [DOI] [PubMed] [Google Scholar]
- 40.Wang S-M, Chan S-T, Wong Y-L, et al. Rhythmic auditory stimulation incorporated in training improved movements in individuals with psychotic-like experiences. Eur Arch Psychiatry Clin Neurosci 2023;273:995–1005. 10.1007/s00406-022-01524-3 [DOI] [PubMed] [Google Scholar]
- 41.Alan HS. Chan. upper limb length. n.d. Available: http://personal.cityu.edu.hk/meachan/Online%20Anthropometry/Chapter2/Ch2-24.htm
- 42.Mathiowetz V, Volland G, Kashman N, et al. Adult norms for the box and block test of manual dexterity. Am J Occup Ther 1985;39:386–91. 10.5014/ajot.39.6.386 [DOI] [PubMed] [Google Scholar]
- 43.Desrosiers J, Bravo G, Hébert R, et al. Validation of the box and block test as a measure of dexterity of elderly people: reliability, validity, and norms studies. Arch Phys Med Rehabil 1994;75:751–5. [PubMed] [Google Scholar]
- 44.Jebsen RH, Taylor N, Trieschmann RB, et al. An objective and standardized test of hand function. Arch Phys Med Rehabil 1969;50:311–9. [PubMed] [Google Scholar]
- 45.Dong VA, Fong KNK, Chen Y-F, et al. 'Remind-to-move' treatment versus constraint-induced movement therapy for children with hemiplegic cerebral palsy: a randomized controlled trial. Dev Med Child Neurol 2017;59:160–7. 10.1111/dmcn.13216 [DOI] [PubMed] [Google Scholar]
- 46.Mak MKY, Lau ETL, Tam VWK, et al. Use of Jebsen Taylor hand function test in evaluating the hand dexterity in people with Parkinson's disease. J Hand Ther 2015;28:389–94. 10.1016/j.jht.2015.05.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.