Abstract
Particular phases of the menstrual cycle may exacerbate affective symptoms for females with a diagnosed mental health disorder. However, there are mixed findings regarding whether affective symptoms change across the menstrual cycle in females without a clinical diagnosis. The window of vulnerability model proposes that natural increases in ovarian hormones in the mid-luteal phase of the menstrual cycle lead to systematic changes in brain networks associated with affective processing. Consequently, the model posits that females may experience stress more intensely and remember negative events more readily in the mid-luteal phase, increasing their risk for higher affective symptoms. Using a 35-day longitudinal study design, we tested the window of vulnerability model in a non-clinical sample. We tracked naturally cycling females’ daily stress and three types of affective symptoms: anxious apprehension, anxious arousal, and anhedonic depression. Using multilevel modeling, we simultaneously modeled within- and between-person associations among stress and menstrual phase for each affective symptom. We found increased anhedonic depression in the mid-luteal phase but not anxious apprehension or anxious arousal. Moreover, we detected a positive association between within- and between-person stress and anxious apprehension and anhedonic depression, but not anxious arousal. These associations were not stronger in the mid-luteal phase. Overall, we provide weak evidence for a window of vulnerability for affective symptoms in the mid-luteal phase of the menstrual cycle. Our findings suggest that stress is a better predictor of fluctuations in affective symptoms than the menstrual cycle. Moreover, our findings highlight the importance of measuring multiple negative affective symptoms because they may be differentially related to stress and the menstrual cycle.
Keywords: Menstrual cycle, Estradiol, Progesterone, Stress, Anxiety, Depression
1. Introduction
Affective disorders are more prevalent in females2 than males (McLean et al., 2011; Salk et al., 2017). Although the genesis of this asymmetry is not fully understood, evidence suggests that the menstrual cycle may play a role (Li and Graham, 2017). The menstrual cycle involves cyclical changes in hormone levels corresponding to ovum development (Hawkins and Matzuk, 2008). The average cycle lasts 28 days with ranges from 21 to 37 days (Schmalenberger et al., 2021). Ovarian hormones, estradiol and progesterone, fluctuate predictably during menstrual cycle phases. Schmalenberger et al. (2021) recommend that testing hypotheses about the menstrual cycle should use phases with discrete hormonal events rather than relative changes in hormone levels. They propose four menstrual cycle phases: mid-luteal, perimenstrual, mid-follicular, and periovulatory. The mid-luteal phase has high and stable estradiol and progesterone; in contrast, the perimenstrual phase has low estradiol and progesterone levels. The mid-follicular phase has slight increases in estradiol but low progesterone. The periovulatory phase has a steep rise and fall of estradiol with a slight increase in progesterone.
There is a commonly held belief that the mid-to-late luteal phase impacts females’ development and exacerbation of affective symptoms (Li and Graham, 2017). In clinical samples, females with anxiety (Van Veen et al., 2009), mood (Hartlage et al., 2004), eating (Klump et al., 2014), and other mental disorders experience an increase in affective symptoms during the mid-to-late luteal phase (Case and Reid, 2001). However, there is mixed evidence regarding the relationship between the menstrual cycle and affective symptoms for females not diagnosed with a mental disorder (Romans et al., 2012). Some studies find that females in their mid-to-late luteal phase report higher affective symptoms (Gonda et al., 2008; Kiesner et al., 2016), while others do not (Lorenz et al., 2017; Reynolds et al., 2018). Clarifying the relationship between the menstrual cycle and affective symptoms is critical because it may provide insights into sex-specific development, prevention, and treatment of affective disorders (Altemus et al., 2014; Kiesner, 2017).
Andreano et al. (2018) proposed the window of vulnerability model of affective disorders to provide a psychoneuroendocrinological account of how the menstrual cycle can impact affective symptoms. Ovarian hormones influence connectivity in the brain directly and indirectly. Estrogen and progesterone receptors are expressed throughout the brain, including in nodes of the default mode and salience networks. Allopregnanolone, synthesized from progesterone, is associated with increased amygdala activity and negative affect (Bäckström et al., 2014). Progesterone administration increases amygdala-medial prefrontal connectivity that increases perseverative negative thoughts (Makovac et al., 2016). Therefore, increases in progesterone in the mid-luteal phase may lead to stressors being experienced more intensely and remembered more readily. The window of vulnerability model proposes that these progesterone-induced changes in brain function, in turn, increase the chances of experiencing negative affective symptoms.
Elements of the window of vulnerability model may help explain the mixed findings in observational studies regarding the menstrual cycle and affective symptoms (Hengartner et al., 2017). The model specifies that stressors in the mid-luteal phase have a higher chance of increasing affective symptoms (Andreano et al., 2018). With some exceptions (Romans et al., 2013), these observational studies fail to account for daily stress levels. Null findings may be due to a lack of a strong stressor to elicit an increase in affective symptoms (Li et al., 2020a). In experimental studies, females show an increase in stress reactivity indexed by self-report (Dan et al., 2019), autonomic (Armbruster et al., 2018), and neural measures (Andreano and Cahill, 2010) in their mid-to-late luteal phase compared to other phases. This asymmetry in observational and experimental findings is exemplified by Lusk et al. (2017). In an experimental context, females exposed to a stressor in their mid-luteal phase experienced more distress than females in the early follicular phase. However, there were no phase differences in daily stress, anxiety, and depressed mood.
The mixed findings regarding associations between the menstrual cycle and affective symptoms may also be due to inconsistencies in operationalizing menstrual cycle phases (Schmalenberger et al., 2021). In one study, Gonda et al. (2008) operationalized seven days after menstruation as the early-follicular phase and seven days before as the late-luteal phase. In contrast, Li et al. (2020b) operationalized the early-follicular phase as 10–14 days before ovulation and the mid-luteal phase as 5–10 days after ovulation. A study observing 3.3 million females across 109 countries demonstrated a premenstrual negative mood increase (Pierson et al., 2021). However, they defined the premenstrual phase as two weeks before menstruation, which includes the early-, mid-, and late-luteal phases. This lack of standardization in defining phases produces less robust results that impede our ability to delineate the role of the menstrual cycle in impacting affective symptoms.
The current study addressed these limitations in a longitudinal design that tracked naturally cycling females’ daily stress, menstrual cycle phase, and affective symptoms for 35 days. Guided by the window of vulnerability model, we tested the influence of daily stress, menstrual phase, and their interactions on three transdiagnostic affective symptoms: anxious apprehension, anxious arousal, and anhedonic depression (Clark and Watson, 1991; Meyer et al., 1990). Anxious apprehension or worry is characterized by repetitive, negative, and future-oriented verbal thought activity. Anxious arousal is characterized by somatic symptoms such as increased heart rate and sweating (Heller et al., 1997). Anhedonic depression is characterized by a lack of interest, low energy, and infrequent positive emotions (Pizzagalli, 2014). Assessing affective symptoms along a continuum of severity allows for a more nuanced examination of how the menstrual cycle can influence affective psychopathology (Kotov et al., 2017).
We tested two hypotheses from the window of vulnerability model. First, the model hypothesizes that females will experience more affective symptoms in the mid-luteal phase than in all other phases. Second, it predicts that on days where females experience more stress than their own average, they will experience higher affective symptoms and that this positive relationship is strongest in the mid-luteal phase.
2. Methods
2.1. Participants
One hundred and forty-four participants were recruited from the community through local and online advertising. We used a final sample of 96 female volunteers with usable data (ages 18–25 years old; M = 20.90, SD = 1.74). Of the 96 participants, 66.67 % were Caucasian/White, 21.88 % were African American/Black, 7.29 % were Asian American, and 4.16 % were of more than one race/ethnicity. Ninety-eight percent self-identified as women, while 2 % identified as non-binary. The sample was predominately heterosexual (77.08 %) with notable diversity (Bisexual: 13.54 %; Gay/Lesbian: 4.16 %; Asexual; ~ 1 %; Pansexual 3.13 %; and Queer; ~ 1 %). In terms of annual income, 26.04 % of participants reported earning less than $15,000, 11.46 % between $15,000 and $25,000, 12.5 % between $25,000 and $50,000, 9.38 % between $50,000 and $75,000, 10.42 % between $75,000 and $100,000, 20.83 % between $100,000 and $200,000%, and 9.38 % over $200,000. Most participants (72.91 %) reported being financially supported by someone else in the past year. A total of 80.91 % of the sample were students (76.04 % full-time; 4.17 % part-time), and 19.79 % were not students.
2.2. Overview of procedures
Interested volunteers were screened over the phone for eligibility. To be eligible, females had to report regular menstrual cycles (i.e., every 22–35 days) and not take hormonal contraceptives. Females could not be taking psychotropic or steroid medications within the past eight weeks, have no history of genetic or medical conditions such as thyroid and metabolic disorders, and no recent medications that can impact the endocrine system. Additional exclusion criteria included: pregnancy or lactated in the last year, schizophrenia, bipolar disorder, substance abuse disorder, epilepsy, physical or mental impairments that could interfere with data quality, head trauma that resulted in a loss of consciousness for greater than five minutes, and being a non-native English speaker.3
Eligible participants came to the lab for an intake visit to confirm eligibility criteria and receive study orientation information. Participants started at different phases of the menstrual cycle for the aims of the larger investigation. The study involved daily questionnaires for 35 days that measured affective symptoms, daily saliva sample collections to assay for estradiol and progesterone to better capture menstrual phases, four visits during which participants completed several cognitive tasks while their electroencephalography (EEG) activity was recorded, and a final visit for the administration of a structured diagnostic clinical interview. Cognitive visits occurred at different times of day as the saliva samples and affective assessments, so they were unlikely to have impacted these outcomes. Cognitive findings are reported in other published works (Gloe et al., 2021; Louis et al., 2021). We focus on menstrual phases, daily stress, and affective symptoms in the current study. Participants were compensated $280 for full participation with prorated compensation for partial data.
2.3. Saliva samples and menstrual phase coding
Participants provided daily saliva samples within thirty minutes of waking using the passive drool method for 35 consecutive days (Papacosta and Nassis, 2011). Participants stored their saliva samples in their home freezer immediately after collection. These samples were collected at each in-person visit and stored in a lab freezer at − 80 °F until they were shipped to Salimetrics Lab (State College, PA) for analysis. The saliva samples were analyzed using enzyme immunoassay kits to assay estradiol and progesterone and demonstrated excellent intra- and inter-assay coefficients of variation (estradiol = 7.1% and 7.5%; progesterone = 6.2% and 7.6%), assay sensitivity (measured by interpolating the mean optical density minus 2 SDs of 10–20 replicates at the 0-pg/ml level; estradiol = 0.10 pg/mL and progesterone = 5 pg/mL), and method accuracy (determined by spike recovery and linearity; estradiol = 104.2% and 99.4% and progesterone = 99.6% and 91.8%; Klump et al., 2016). Observations of estradiol and progesterone levels of more than three standard deviations from the participant’s own mean were excluded.
To test the window of vulnerability model, we focused on precisely coding the mid-luteal phase of the menstrual cycle. Coders were trained to use methods described by Klump et al. (2015). Each participant’s z-scored estradiol and progesterone data and menstruating days were aggregated onto a single graph, and each day was coded by two independent trained raters. Consistent with Schmalenberger et al.’ (2021) recommendations, the mid-luteal phase was coded as days when progesterone and estradiol levels were at their peak and relatively stable. Due to our study design in which participants started at different phases of the menstrual cycle, we frequently only captured partial cycles. For example, for one participant, the first day of their menstrual cycle was day 11 in the study, but we did not observe their subsequent cycle. Nevertheless, based on participants with sufficient cycle information, mid-luteal length ranged from 3 to 13 days (n = 78, M = 7.32, SD = 2.05) and averaged −3.84 days (SD = 1.75) to −10.37 (SD = 2.21) before the onset of the first day of menses,4 which is consistent with pre-existing definitions of mid-luteal phase (Schmalenberger et al., 2021).
We coded the perimenstrual phase as falling estradiol and progesterone that occurs three days before menstrual bleeding and the days during menstrual bleeding with low estradiol and progesterone. The mid-follicular phase was coded as a slight rise in estradiol with low progesterone. Lastly, the periovulatory phase consisted of a steep increase with a primary peak and a fall in estradiol levels with a slight increase in progesterone. Days that are not categorized into these four phases were removed from analyzes.5 Observations in the perimenstrual, mid-follicular, and periovulatory phases were labeled as “other phases” and used as the comparison days. Anovulatory observations, cycles that lacked the expected rise in progesterone and/or demonstrated a lack of patterning in ovarian hormones throughout the cycle, were removed from analyzes (n = 14). All graphs were compared across raters for consistency. Discrepancies in coding were resolved through discussion. Interrater reliability for phase coding was calculated for 25 % of coded participant plots and was satisfactory (Cohen’s Kappa = 0.79, 95% CI: 0.76, 0.82).
2.4. Daily questionnaires
Participants filled out daily questionnaires between 5:00 p.m. and 10:00 p.m. via Qualtrics for 35 days. If participants were unable to access the internet, paper copies were provided. The daily surveys consisted of several questionnaires to assess psychological health, including stress, affective symptoms, and eating behavior. We also asked if they were experiencing menstrual bleeding that day and used this information to determine the menstrual cycle phases. The daily stress measure was added later in the study, which resulted in missing values from the first 34 participants who had already completed data collection. After removing anovulatory participants (n = 14), the final sample size was N = 96.
We used a single-item measure of daily stress to ease the burden on participants. Participants were asked, “Considering all the events of the day, how would you rate the amount of stress in your life (at home and work)?” from 1 (No Stress) to 7 (Extreme Stress). Higher numbers indicated more stress (M = 3.29, SD = 1.58).
Anxious apprehension was assessed using a modified version of the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990). To measure daily anxious apprehension, the PSWQ instructions were modified to say, “Rate each of the following statements on a scale of 1 (not at all typical of me) to 5 (very typical of me) in relation to today.” Five of the 16 items were reversed coded. Items were summed to create a total score that ranges from 16 to 80, with higher numbers indicating more anxious apprehension (M = 40.16, SD = 15.18). Cronbach’s alpha was calculated using the average alpha value across the repeated assessments. The PSWQ demonstrated high internal consistency (α = 0.94) like other samples (Joos et al., 2012; van Rijsoort et al., 1999).
Anxious arousal and anhedonic depression were assessed with subscales from a modified Mood and Anxiety Symptom Questionnaire (MASQ; Clark and Watson, 1991; Watson et al., 1995). The MASQ instructions were modified to read “…Use the choice that best describes how much you have felt or experienced this way today” on a 1 (Not at all) to 5 (Extremely) scale. Daily anxious arousal items consisted of 17 items. They were summed to form a score ranging from 17 to 85, with higher numbers indicating greater anxious arousal (M = 26.58, SD = 8.12). Daily anhedonic depression questions consisted of 21 items6 with eight items reverse coded; items were summed to form a score ranging from 21 to 105, with higher numbers indicating more anhedonic depression (M = 67.84, SD = 13.56). The MASQ also demonstrated high reliability for the Anxious Arousal (α = 0.83) and Anhedonic Depression subscale (α = 0.88), similar to previous samples (Wardenaar et al., 2010).
2.5. Power analysis
We conducted a sensitivity analysis using G-power with a repeated measures design as a proxy for multilevel modeling (MLM) with a sample size of 96, MObservations = 25.97, and ICCs ranging from .42 to .76. Given the number of observations, 80 % power, and α = 0.05, we were sensitive to detecting a small effect size of f = 0.05 with 0.76 correlation among repeated measures and f = 0.09 with 0.07 correlation among repeated measures.
2.6. Data analytic plan
We used SPSS V26 to run separate multilevel models to examine the effects of daily stress, menstrual phase, and their interactions on anxious apprehension, anxious arousal, and anhedonic depression. MLMs help account for the nonindependence and repeated measurements in our data. We used restricted maximum likelihood (REML) for all our analyses. We used two levels: observations (Level 1) nested within individuals (Level 2). We used an autoregressive lag 1 covariance structure for the residuals to help account for stronger associations between the scores on the dependent variable on days closer in time. All models contained random slopes for within-centered person stress but not random slopes for the menstrual phase because the models would not converge.
We simultaneously modeled within- and between-person centering of stress to better understand how a person’s stress on a particular day differed deviation from their own mean stress levels (within) and how the person’s average stress, averaging over days, compared to other people’s average stress (between) are associated with each affective symptom. We calculated within-person predictors by computing the participant’s average stress and then subtracting their mean stress from their daily stress. Positive values from within-person centered stress reflect more stress than their average; therefore, a positive association between within-person centered stress and affective symptoms means that on days a person is experiencing more stress relative to their average, they also report experiencing more affective symptoms. We calculated between-person stress by computing the average of each participant’s stress, then grand mean centered those averages. Higher values from between-person centered stress reflect people who experience greater average stress and can be considered individual differences in average stress levels. A positive association with between-person centered stress and affective symptoms would indicate that females who experience more average stress report experiencing more affective symptoms.
Across all models, the menstrual phase was effects coded as 1, indicating the mid-luteal phase, and −1 indicating all other phases, to reflect our specific hypotheses about the mid-luteal phase. The menstrual phase for all models was a within-person variable since it reflects discrete changes in hormonal action occurring within a particular sequence and pattern. Any significant interactions between categorical and continuous variables were broken down by simple slopes analyses in which separate intercepts and slopes for the continuous variable were computed at each level of the categorical variable.
To test the robustness of some of the associations, we conducted two types of exploratory analyses in the Online Supplements: controlling for hormone levels and comparisons of distinct phases. We controlled for estradiol and progesterone in three ways in the Online Supplements for each affective symptom (Online Supplements 1–3): 1) we entered within-person centered estradiol and progesterone in the model and their interaction with phase, 2) we entered between-person centered estradiol and progesterone in the model and their interaction with phase, and 3) we entered both within- and between-person centered estradiol and progesterone as predictors in the model. For distinct phase comparisons, we compared the mid-luteal phase to three distinct phases Schmalenberger et al. (2021) recommended: perimenstrual, mid-follicular, and periovulatory (Online Supplements 1–3).
2.7. Data retention
Missing observations for stress and menstrual phase were dropped from analyses. Participants needed at least two observations to be included in the analyses. The total number of observations varied slightly by affective symptom (anxious apprehension = 2434, anxious arousal = 2435, anhedonic depression = 2536). We provide descriptive statistics for each affective symptom Table 1.
Table 1.
Descriptive statistics.
| Variable | M | SD | Observed range |
Possible range |
|---|---|---|---|---|
|
| ||||
| Stress | 3.19 | 1.579 | 1–7 | 1–7 |
| Stress: within-person centered | 0 | 1.28 | −3.38 to 5.46 | N/A |
| Stress: between-person centered | 0 | 0.93 | −1.92 to 2.71 | N/A |
| Anxious apprehension | 40.16 | 15.18 | 16–80 | 16–80 |
| Anxious arousal | 26.58 | 8.13 | 17–65 | 17–85 |
| Anhedonic depression | 67.83 | 13.54 | 21–103 | 21–105 |
3. Results
3.1. Anxious apprehension
Results of the model testing the association among within- and between-person centered stress, menstrual phase, and their interactions for anxious apprehension are presented in Table 2. Contrary to the window of vulnerability hypothesis, females did not report more anxious apprehension in their mid-luteal phase compared to all other phases when controlling for within- and between-person stress B = 0.204, SE = 0.315, t(1548.719) = 0.647, p = 0.518. As expected, on days females reported increases in their stress relative to their own average (within-person), they also reported increases in anxious apprehension, B = 5.374, SE = 0.294, t(105.827) = 18.248, p < 0.001. However, contrary to the window of vulnerability hypothesis, this within-person centered stress and anxious apprehension positive association was not stronger in the mid-luteal phase compared to all other phases (p = 0.717). Finally, females who reported more stress levels on average compared to other females (between-person) also tended to report higher levels of anxious apprehension, B = 8.272, SE = 0.441, t(381.594) = 18.743, p < 0.001. This between-person centered stress and anxious apprehension positive association was not moderated by the menstrual phase (p = 0.884). Please see Online Supplement 1 for exploratory analyses controlling for estradiol and progesterone and comparing the mid-luteal phase to the perimenstrual, mid-follicular, and periovulatory phases.7
Table 2.
Within-person stress, between-person stress, and phase predicting anxious apprehension.
| Variable | B | SE | P | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
|
| |||||
| Intercept | 40.276 | 0.407 | < 0.001 | 39.476 | 41.075 |
| Within Stress | 5.374 | 0.294 | < 0.001 | 4.790 | 5.958 |
| Between Stress | 8.272 | 0.441 | < 0.001 | 7.404 | 9.140 |
| Phase | 0.204 | 0.315 | 0.518 | −0.414 | 0.822 |
| Within Stress × Phase | −0.056 | 0.155 | 0.717 | −0.361 | 0.248 |
| Between Stress × Phase | −0.049 | 0.334 | 0.884 | −0.704 | 0.607 |
3.2. Anxious arousal
Results of the model testing the association among within- and between-person centered stress, menstrual phase, and their interactions for anxious arousal are presented in Table 3. Contrary to the window of vulnerability hypothesis, females did not report more anxious arousal in their mid-luteal phase than all other phases when controlling for within- and between-person stress, B = −0.351, SE = 0.199, t(2387.482) = −1.762, p = 0.078. Surprisingly, females did not report more anxious arousal on days when they experienced more stress than their own average (within-person), B = −0.203, SE = 0.120, t(113.725) = −1.692, p = 0.093. We did, however, detect a significant within-person stress by phase interaction, B = −0.192, SE = 0.081, t(2017.411) = −2.268, p = 0.018 (See Fig. 1). Contrary to the window of vulnerability hypothesis, this interaction revealed a negative association between within-person centered stress and anxious arousal in the mid-luteal phase, B = −0.394, SE = 0.162, t(325.709) = −2.439, p = 0.015. For all other phases, within-person centered stress was not associated with daily anxious arousal, B = −0.011, SE = 0.125, t(130.106) = −0.087, p = 0.931. Finally, females who tended to experience more stress than other females (between-person) did not report experiencing more anxious arousal (p = 0.062). This was also not moderated by the menstrual phase (p = 0.183). Please see Online Supplement 2 for exploratory analyses controlling for estradiol and progesterone and comparing the mid-luteal phase to the perimenstrual, mid-follicular, and periovulatory phases.
Table 3.
Within-person stress, between-person stress, and phase predicting anxious arousal.
| Variable | B | SE | P | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
|
| |||||
| Intercept | 26.606 | 0.426 | < 0.001 | 25.766 | 27.446 |
| Within Stress | −0.203 | 0.120 | 0.093 | −0.440 | −0.035 |
| Between Stress | 0.876 | 0.468 | 0.062 | −0.046 | 1.798 |
| Phase | −0.351 | 0.199 | 0.078 | −0.742 | 0.040 |
| Within Stress × Phase | −0.192 | 0.081 | 0.018 | −0.350 | −0.033 |
| Between Stress × Phase | −0.280 | 0.210 | 0.183 | −0.692 | 0.132 |
Fig. 1.
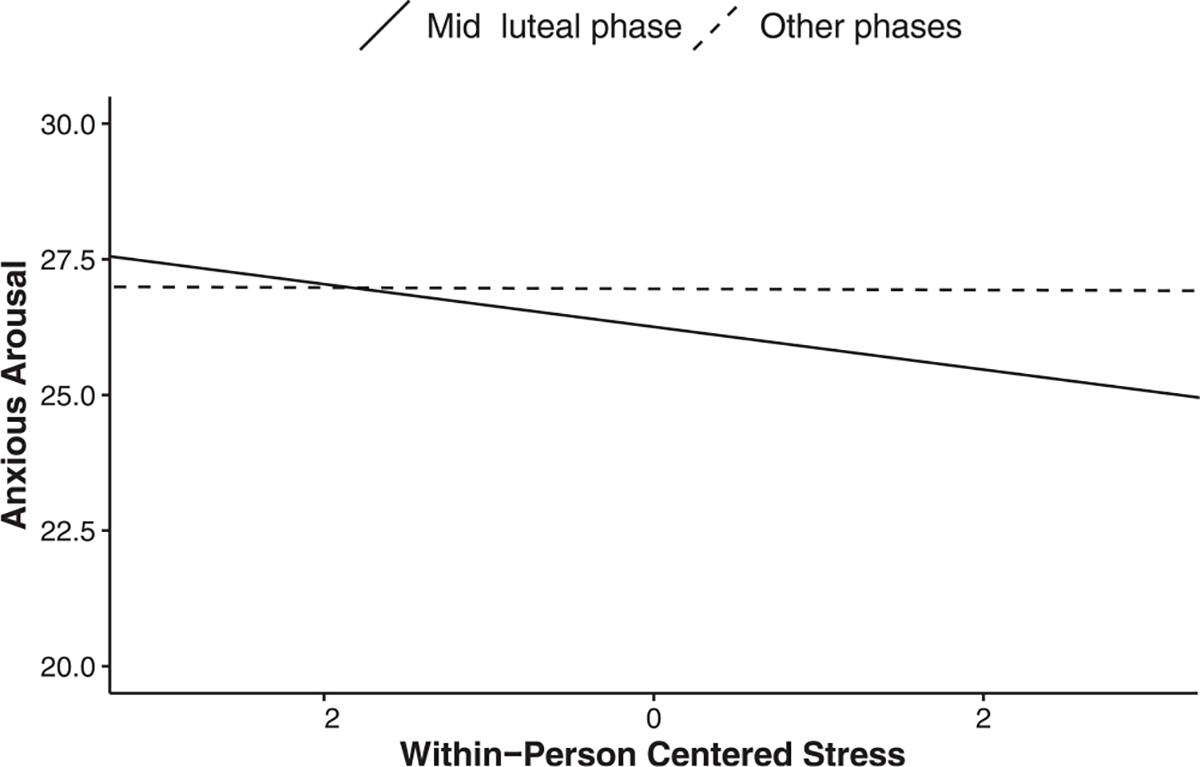
Breakdown of a two-way within-person stress by phase interaction for anxious arousal. For the mid-luteal phase, within-person centered stress predicted lower anxious arousal. For all other phases, higher within-person centered stress was not associated with anxious arousal.
3.3. Anhedonic depression
Results of the model testing the association among within- and between-person centered stress, menstrual phase, and their interactions for anhedonic depression are presented in Table 4. Consistent with the window of vulnerability hypothesis, females reported experiencing more anhedonic depression in their mid-luteal phase compared to all other phases, B = 0.737, SE = 0.329, t(2307.530) = 2.239, p = 0.025. To aid in the interpretation, we also dummy coded the phase variable providing an intercept of 67.493 with an increase of 1.474 units in anhedonic depression for a total average of 68.967 in the mid-luteal phase compared to all other phases.
Table 4.
Within-person stress, between-person stress, and phase predicting anhedonic depression.
| Variable | B | SE | p | Lower 95 % CI |
Upper 95 % CI |
|---|---|---|---|---|---|
|
| |||||
| Intercept | 68.230 | 0.632 | < 0.001 | 66.985 | 69.475 |
| Within Stress | 1.374 | 0.358 | < 0.001 | 0.662 | 2.085 |
| Between Stress | 2.953 | 0.693 | < 0.001 | 1.588 | 4.318 |
| Phase | 0.737 | 0.329 | 0.025 | 0.091 | 1.383 |
| Within | −0.059 | 0.139 | 0.675 | −0.332 | 0.215 |
| Stress × Phase | |||||
| Between | −0.176 | 0.348 | 0.613 | −0.858 | 0.506 |
| Stress × Phase | |||||
Moreover, on days females experienced more stress relative to their own average (within-person), they also experienced increases in daily anhedonic depression, B = 1.374, SE = 0.358, t(96.282) = 3.833, p < 0.001. However, contrary to the window of vulnerability hypothesis, this within-person stress and anhedonic depression positive association was not stronger in the mid-luteal phase than in all other phases (p = 0.675). Finally, females who reported more stress on average compared to other females (between-person) also tended to report higher levels of anhedonic depression, B = 2.953, SE = 0.693, t(241.012) = 4.262, p < 0.001. The menstrual phase did not moderate this relationship (p = 0.613). Please see Online Supplement 3 for exploratory analyses controlling for estradiol and progesterone and comparing the mid-luteal phase to the perimenstrual, mid-follicular, and periovulatory phases.
4. Discussion
4.1. Summary of results
We tested Andreano et al.’ (2018) window of vulnerability model of affective disorders by examining whether females not selected based on diagnostic status experienced more affective symptoms in the mid-luteal phase. We tracked daily stress, menstrual phase, and affective symptoms for 35 days. We used gold-standard hormonal methods for determining phase robust statistical modeling techniques for repeated measures of affective symptoms. Overall, we found weak support for the hypothesis that females will experience more affective symptoms in the mid-luteal phase of the menstrual cycle. The only finding partially supportive of this hypothesis was that females reported more anhedonic depression in the mid-luteal phase than in all other phases. However, we did not find a mid-luteal increase for anxious apprehension or anxious arousal.
When females experienced more stress than their average, they also experienced more anxious apprehension and anhedonic depression. However, we did not find support for the window of vulnerability hypothesis that this positive relationship would be stronger in the mid-luteal phase of the menstrual cycle. Surprisingly, we did not detect a positive relationship between within-person centered stress and anxious arousal, illustrating that these three affective outcomes are phenomenologically distinct. Lastly, we found that females who generally experienced more stress across the menstrual cycle reported more anxious apprehension and anhedonic depression, but not anxious arousal. Our findings are broadly consistent with the findings that perceived stress is a stronger predictor of affective symptoms than the menstrual cycle (Romans et al., 2013).
4.2. A mid-luteal increase in anhedonic depression but not anxiety-related symptoms
We found some support for a window of vulnerability in the mid-luteal phase for anhedonic depression but not anxious apprehension or arousal. When menstrual phase was dummy coded, the intercept for anhedonic depression was 67.493 with a mid-luteal increase of 1.474 for an average total expected value of 68. Although this increase is below the proposed cut-off of 76 for the clinical presentation of a mood disorder, it is significant because this suggests a monthly recurring risk factor for females (Buckby et al., 2007). We also likely underestimate the total anhedonic depression scores in our sample. Our measure of anhedonic depression consisted of 21 items compared to the 22 that are generally administered. We removed the item on “thoughts about death or suicide” since we would not be able to respond effectively if participants endorsed this item. Any interpretation of anhedonic depression compared to other samples that used all 22 items should bear this in mind.
Unfortunately, it is difficult to compare our findings with previous menstrual cycle studies that compared the mid-luteal phase with other phases since many only relied on counting methods (Gonda et al., 2008; Lorenz et al., 2017; Romans et al., 2013) and did not specifically use the mid-luteal phase as a comparison group (Petersen et al., 2016; Reynolds et al., 2018). The lack of increase in anxiety symptoms is consistent with one study that used an ovulation test with a counting method (Li et al., 2020a). In terms of the increase in anhedonic depression in the mid-luteal phase, no other study has looked at this specific affective symptom that used a similar coding methodology.
An explanation for this asymmetry may have to do with the type of stressors associated with these affective symptoms, their phenomenological features, and how these features may help facilitate or hinder regulation processes (Kendler et al., 2003; Sheppes et al., 2015). Symptoms of anxious apprehension and arousal typically occur because of uncertainties regarding current or future threatening situations (Grupe and Nitschke, 2013). Both cognitive and physiological features of anxiety prepare a person for managing immediate or future threats. These preparedness features may help people manage daily stressors and maintain anxiety levels to reduce stress. Anhedonic depression typically has a past orientation and occurs after losing something personally meaningful such as social status, a goal, a relationship, or a loved one (Kendler et al., 2003). The phenomenological experience of anhedonic depression reduces positive affect, increases sadness, and reduces the motivation to regulate (Spielberg et al., 2011). A study found evidence that increases in anhedonic depression are associated with decreased attempts to upregulate positive emotions (Werner-Seidler et al., 2013). Future studies using experience sampling methods can help better examine how different types of stressors influence affective symptoms.
4.3. Menstrual phase does not moderate the relationship between stress and affective symptoms
Contrary to the window of vulnerability model, we did not find that the positive relationship between within-person stress and affective symptoms was stronger in the mid-luteal phase compared to other phases. This is consistent with previous findings that the menstrual phase is a less influential predictor of affective symptoms than other factors such as stress (Romans et al., 2012, 2013). The lack of mid-luteal exacerbation may be due to affect regulation processes that buffer females from experiencing sub-clinical and clinical levels of affective symptoms even when they experience stress more intensely. One implication of this interpretation is that affective symptoms may not increase; however, mental fatigue may increase as the affect regulation system works harder to manage different stressors. A recent study supports this interpretation where females experienced more mental fatigue in their mid-luteal phase than their follicular phase, indicating more cognitive resources being used to manage different stressors (Li et al., 2020b). Li and Graham (2017) hypothesize that there may also be deficiencies regarding affect regulation processes on top of increases in stress reactivity in the mid-luteal phase. This is consistent with an affective symptom compensatory model in which affective symptoms in the mid-luteal phase do not increase due to affect regulation processes. Future studies can test this affective compensatory model in the mid-luteal phase of the menstrual cycle.
Another reason for the lack of exacerbation in the mid-luteal phase between within-person stress and affective symptoms may be due to our measures’ methodological features. For one, we only used a global, single-item measure of perceived stress to reduce the burden on participants. This may not fully encapsulate a person’s stress experience throughout the day, such as the source and frequency of the stressor (Epel et al., 2018). Future studies may benefit from using a more comprehensive measure of daily stress that distinguishes stressors that tend to be associated with different affective symptoms (Crosswell and Lockwood, 2020). Another reason for the lack of a mid-luteal exacerbation may have to do with how we measured our affective symptoms. Although our affective symptoms were anchored to the day instead of several days or weeks, this is still susceptible to retrospective bias (Mestdagh and Dejonckheere, 2021). Therefore, future studies may benefit from using experience sampling methods to assess and examine how different affective symptoms fluctuate throughout the day.
It is worth noting that we detected a significant interaction between within-person stress and phase for anxious arousal. However, contrary to the window of vulnerability model, higher levels of within-person stress predicted less anxious arousal in the mid-luteal phase, while there was no stress and anxious arousal association in all other phases. As we have pointed out, anxious arousal seems to be very different from anxious apprehension and anhedonic depression. Perhaps this is because it is primarily a measure of physical responding while the other anxious apprehension and anhedonic depression are more cognitive and affective based. Again, future studies may benefit from using experience sampling to determine how anxious arousal symptoms manifest throughout the day and their relationship to daily stressors.
4.4. Strengths and limitations
Our study has several strengths. First, we collected daily hormones and affective symptoms data for 35 days, allowing us to accurately code phases of interest and test their associations (Schmalenberger et al., 2021). Previous studies relied primarily on self-report methods to determine the menstrual phases, which are not the most accurate. Second, we measured affective symptoms in three distinct ways: anxious apprehension, anxious arousal, and anhedonic depression. These symptoms allowed us to distinguish which features of affective disorders are primarily influenced by the menstrual cycle. Although the window of vulnerability model specifies a general susceptibility to affective disturbances, affective symptoms vary in their phenomenology, neurophysiological underpinnings, and receptiveness to regulation processes (Sharp et al., 2015). In our study, we only found a mid-luteal increase in anhedonic depression and not in anxious apprehension or arousal. Additional theoretical and empirical work is needed to examine the type of affective symptoms influenced by the menstrual cycle and why. Another strength of our data is that we controlled for stress levels in our model. The mixed findings regarding observational studies may be due to not controlling for stress. We recommend that future studies examine how daily stressors manifest themselves into affective symptoms across the menstrual cycle. Based on our findings, we suspect the role of regulatory processes in maintaining affective homeostasis after experiencing a stressor.
One limitation of our analyses is that we did not factor in affective regulatory processes. Stressors activate homeostatic regulation processes immediately (Sheppes et al., 2015). Affect regulation is a core feature of many affective experiences and is a critical mechanism in which stress can develop into a risk for different affective symptoms. Many affective disorders have affective dysregulation as part of their symptomology (Sheppes et al., 2015). Perhaps the association between stressors and affective symptom are stronger for those not adept at using adaptive regulation strategies. The lack of increased anxious apprehension and anxious arousal in the mid-luteal phase may be a product of hidden or unmeasured affect regulation processes. Future studies should determine the role of affect regulation in modulating the relationship between stress and affective symptoms.
Another limitation is that our primary measures of affective symptoms were not originally designed and validated ways to measure momentary or daily affect. We asked participants to anchor their answers to the past day. It is unclear how changing these measures’ time frames would affect their validity. However, the face validity of the questions such as “Felt sad” or “Felt cheerful” makes the time frame adjustment from the past week to the day less concerning. Still, future research should investigate the validity of daily measures of affective symptoms and their relationship with more validated measures of longer duration. Another limitation is that we did not use the LH test to determine ovulation accurately. Nevertheless, our coding scheme using the hormonal patterns allowed us to accurately code for the mid-luteal phase, the phase of primary interest. Lastly, we did not assess for conditions like PMDD or PMS that may mask detection of a mid-luteal rise in symptoms. We did, however, screen for broad medical conditions such as metabolic disorders or other medical conditions.
4.5. Conclusion
Using an intensive 35-day longitudinal design that tracked stress, menstrual phase, and affective symptoms daily, we provide weak evidence for a window of vulnerability for affective symptoms in the mid-luteal phase of the menstrual cycle. We find a mid-luteal increase in anhedonic depression but not for anxious apprehension and anxious arousal. Anhedonic depression may be the primary affective symptom affected by the mid-luteal phase of the menstrual cycle. Our findings highlight the importance of measuring different facets of affective symptoms across the menstrual cycle. Additional theoretical and empirical work is needed to better understand a mid-luteal increase in anhedonic depression but not anxious apprehension and anxious arousal across the range of symptom severity. We also find a robust positive relationship between within-person stress, anxious apprehension, and anhedonic depression. Overall, our findings support the notion that the menstrual cycle is a less influential predictor of affective symptoms than other factors such as stress.
Supplementary Material
Funding
The Brain Cycle Study is funded by the National Institute of Mental Health (NIMH, United States; Grant no.: 1R01MH108511-01).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.psyneuen.2022.105958.
We understand that sex assigned at birth and gender are distinct terms. Throughout this paper, the term “female” is used to refer to individuals who were assigned female sex at birth and experience menstrual cycles, which may apply to all gender identities.
Some criteria such as no head trauma that resulted in a loss of consciousness were included for the in-person EEG lab portion of the study.
Mid-luteal days were calculated by counting the days when progesterone and estradiol levels where at its peak and relatively stable that were between other concrete phases. For example, if a participant started data collection at their mid-luteal phase (Day 1), this would be considered insufficient information since we do not know if the day before they started would also be part of their mid-luteal phase.
The transition from mid-luteal phase to perimenstrual phase was not included.
We removed one item from the MASQ-Anhedonic Depression subscale on “thoughts about death or suicide” since we would not be able to respond effectively if participants endorsed this item.
There were slight variations in means and standard deviations based on centering for anxious apprehension, anxious arousal, and anhedonic depression. See Table 1 for full details.
References
- Altemus M, Sarvaiya N, Epperson CN, 2014. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 35, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L, 2010. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage 53, 1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Touroutoglou A, Dickerson B, Barrett LF, 2018. Hormonal cycles, brain network connectivity, and windows of vulnerability to affective disorder. Trends Neurosci. 41, 660–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster D, Grage T, Kirschbaum C, Strobel A, 2018. Processing emotions: effects of menstrual cycle phase and premenstrual symptoms on the startle reflex, facial EMG and heart rate. Behav. Brain Res. 351, 178–187. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Strömberg J, Timby E, van Broekhoven F, van Wingen GA, 2014. Allopregnanolone and mood disorders. Prog. Neurobiol. 113, 88–94. [DOI] [PubMed] [Google Scholar]
- Buckby JA, Yung AR, Cosgrave EM, Killackey EJ, 2007. Clinical utility of the Mood and Anxiety Symptom Questionnaire (MASQ) in a sample of young help-seekers. BMC Psychiatry 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case AM, Reid RL, 2001. Menstrual cycle effects on common medical conditions. Compr. Ther. 27, 65–71. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, 1991. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 100, 316–336. [DOI] [PubMed] [Google Scholar]
- Crosswell AD, Lockwood KG, 2020. Best practices for stress measurement: how to measure psychological stress in health research. Health Psychol. Open 7, 2055102920933072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan R, Canetti L, Keadan T, Segman R, Weinstock M, Bonne O, Reuveni I, Goelman G, 2019. Sex differences during emotion processing are dependent on the menstrual cycle phase. Psychoneuroendocrinology 100, 85–95. [DOI] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB, 2018. More than a feeling: a unified view of stress measurement for population science. Front. Neuroendocrinol. 49, 146–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloe LM, Kashy DA, Jacobs EG, Klump KL, Moser JS, 2021. Examining the role of ovarian hormones in the association between worry and working memory across the menstrual cycle. Psychoneuroendocrinology 131, 105285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda X, Telek T, Juhasz G, Lazary J, Vargha A, Bagdy G, 2008. Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 32, 1782–1788. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB, 2013. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 14, 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlage SA, Brandenburg DL, Kravitz HM, 2004. Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosom. Med. 66, 698–706. [DOI] [PubMed] [Google Scholar]
- Hawkins SM, Matzuk MM, 2008. The menstrual cycle: basic biology. Ann. N. Y. Acad. Sci. 1135, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA, 1997. Patterns of regional brain activity differentiate types of anxiety. J. Abnorm. Psychol. 106, 376–385. [DOI] [PubMed] [Google Scholar]
- Hengartner MP, Kruger THC, Geraedts K, Tronci E, Mancini T, Ille F, Egli M, Röblitz S, Ehrig R, Saleh L, 2017. Negative affect is unrelated to fluctuations in¨ hormone levels across the menstrual cycle: evidence from a multisite observational study across two successive cycles. J. Psychosom. Res. 99, 21–27. [DOI] [PubMed] [Google Scholar]
- Joos E, Vansteenwegen D, Brunfaut E, Bastiaens T, Demyttenaere K, Pieters G, Hermans D, 2012. The Penn State Worry Questionnaire—past day: development and validation of a measure assessing daily levels of worry. J. Psychopathol. Behav. Assess. 34, 35–47. [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA, 2003. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch. Gen. Psychiatry 60, 789–796. [DOI] [PubMed] [Google Scholar]
- Kiesner J, 2017. The menstrual cycle-response and developmental affective-risk model: a multilevel and integrative model of influence. Psychol. Rev. 124, 215–244. [DOI] [PubMed] [Google Scholar]
- Kiesner J, Mendle J, Eisenlohr-Moul TA, Pastore M, 2016. Cyclical symptom change across the menstrual cycle: attributional, affective, and physical symptoms. Clin. Psychol. Sci. 4, 882–894. [Google Scholar]
- Klump KL, Hildebrandt BA, O’Connor SM, Keel PK, Neale M, Sisk CL, Boker S, Burt SA, 2015. Changes in genetic risk for emotional eating across the menstrual cycle: a longitudinal study. Psychol. Med. 45, 3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, O’Connor SM, Hildebrandt BA, Keel PK, Neale M, Sisk CL, Boker S, Alexandra Burt S, 2016. Differential effects of estrogen and progesterone on genetic and environmental risk for emotional eating in women. Clin. Psychol. Sci. 4, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S, Keel PK, 2014. Influences of ovarian hormones on dysregulated eating: a comparison of associations in women with versus women without binge episodes. Clin. Psychol. Sci. 2, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, Brown TA, Carpenter WT, Caspi A, Clark LA, 2017. The Hierarchical Taxonomy of Psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J. Abnorm. Psychol. 126, 454. [DOI] [PubMed] [Google Scholar]
- Li SH, Denson TF, Graham BM, 2020a. Women with generalized anxiety disorder show increased repetitive negative thinking during the luteal phase of the menstrual cycle. Clin. Psychol. Sci. [Google Scholar]
- Li SH, Graham BM, 2017. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 4, 73–82. [DOI] [PubMed] [Google Scholar]
- Li SH, Lloyd AR, Graham BM, 2020b. Physical and mental fatigue across the menstrual cycle in women with and without generalised anxiety disorder. Horm. Behav. 118, 104667. [DOI] [PubMed] [Google Scholar]
- Lorenz TK, Gesselman AN, Vitzthum VJ, 2017. Variance in mood symptoms across menstrual cycles: implications for premenstrual dysphoric disorder. Women’s Reprod. Health 4, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis CC, D’Esposito M, Moser JS, 2021. Investigating interactive effects of worry and the catechol-o-methyltransferase gene (COMT) on working memory performance. Cogn. Affect. Behav. Neurosci. 21, 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk BR, Carr AR, Ranson VA, Felmingham KL, 2017. Women in the midluteal phase of the menstrual cycle have difficulty suppressing the processing of negative emotional stimuli: An event-related potential study. Cogn. Affect. Behav. Neurosci. 17, 886–903. [DOI] [PubMed] [Google Scholar]
- Makovac E, Meeten F, Watson DR, Herman A, Garfinkel SN, Critchley HD, Ottaviani C, 2016. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol. Psychiatry 80, 786–795. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG, 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 45, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestdagh M, Dejonckheere E, 2021. Ambulatory assessment in psychopathology research: current achievements and future ambitions. Curr. Opin. Psychol. 41, 1–8. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD, 1990. Development and validation of the Penn State Worry Questionnaire. Behav. Res. Ther. 28, 487–495. [DOI] [PubMed] [Google Scholar]
- Papacosta E, Nassis GP, 2011. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J. Sci. Med. Sport 14, 424–434. [DOI] [PubMed] [Google Scholar]
- Petersen N, London ED, Liang L, Ghahremani DG, Gerards R, Goldman L, Rapkin AJ, 2016. Emotion regulation in women with premenstrual dysphoric disorder. Arch. Women’s Ment. Health 19, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson E, Althoff T, Thomas D, Hillard P, Leskovec J, 2021. Daily, weekly, seasonal and menstrual cycles in women’s mood, behaviour and vital signs. Nat. Hum. Beh. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, 2014. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds TA, Makhanova A, Marcinkowska UM, Jasienska G, McNulty JK, Eckel LA, Nikonova L, Maner JK, 2018. Progesterone and women’s anxiety across the menstrual cycle. Horm. Behav. 102, 34–40. [DOI] [PubMed] [Google Scholar]
- Romans S, Clarkson R, Einstein G, Petrovic M, Stewart D, 2012. Mood and the menstrual cycle: a review of prospective data studies. Gend. Med. 9, 361–384. [DOI] [PubMed] [Google Scholar]
- Romans SE, Kreindler D, Asllani E, Einstein G, Laredo S, Levitt A, Morgan K, Petrovic M, Toner B, Stewart DE, 2013. Mood and the menstrual cycle. Psychother. Psychosom. 82, 53–60. [DOI] [PubMed] [Google Scholar]
- Salk RH, Hyde JS, Abramson LY, 2017. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 143, 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, Girdler SS, Kiesner J, Ditzen B, Eisenlohr-Moul TA, 2021. How to study the menstrual cycle: practical tools and recommendations. Psychoneuroendocrinology, 104895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PB, Miller GA, Heller W, 2015. Transdiagnostic dimensions of anxiety: neural mechanisms, executive functions, and new directions. Int. J. Psychophysiol. 98, 365–377. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Suri G, Gross JJ, 2015. Emotion regulation and psychopathology. Annu. Rev. Clin. Psychol. 11, 379–405. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Heller W, Silton RL, Stewart JL, Miller GA, 2011. Approach and avoidance profiles distinguish dimensions of anxiety and depression. Cogn. Ther. Res. 35, 359–371. [Google Scholar]
- van Rijsoort S, Emmelkamp P, Vervaeke G, 1999. The Penn state worry questionnaire and the worry domains questionnaire: structure, reliability and validity. Clin. Psychol. Psychother.: Int. J. Theory Pract. 6, 297–307. [Google Scholar]
- Van Veen JF, Jonker BW, Van Vliet IM, Zitman FG, 2009. The effects of female reproductive hormones in generalized social anxiety disorder. Int. J. Psychiatry Med. 39, 283–295. [DOI] [PubMed] [Google Scholar]
- Wardenaar KJ, van Veen T, Giltay EJ, de Beurs E, Penninx BWJH, Zitman FG, 2010. Development and validation of a 30-item short adaptation of the Mood and Anxiety Symptoms Questionnaire (MASQ). Psychiatry Res. 179, 101–106. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA, 1995. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J. Abnorm. Psychol. 104, 15–25. [DOI] [PubMed] [Google Scholar]
- Werner-Seidler A, Banks R, Dunn BD, Moulds ML, 2013. An investigation of the relationship between positive affect regulation and depression. Behav. Res. Ther. 51, 46–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


