Abstract
BACKGROUND:
Beta-lactam therapeutic drug monitoring (BL TDM; drug level testing) can facilitate improved outcomes in critically ill patients. However, only 10–20% of hospitals have implemented BL TDM. This study aimed to characterize provider perceptions and key considerations for successfully implementing BL TDM.
METHODS:
This was a sequential mixed methods study from 2020–2021 of diverse stakeholders at three academic medical centers with varying degrees of BL TDM implementation (not implemented, partially implemented, and fully implemented). Stakeholders were surveyed, and a proportion of participants completed semi-structured interviews. Themes were identified, and findings were contextualized with implementation science frameworks.
RESULTS:
Most of the 138 survey respondents perceived that BL TDM was relevant to their practice and improved medication effectiveness and safety. Integrated with interview data from 30 individuals, two implementation themes were identified: individual internalization and organizational features. Individuals needed to internalize, make sense of, and agree to BL TDM implementation, which was positively influenced by repeated exposure to evidence and expertise. The process of internalization appeared more complex with BL TDM than with other antibiotics (i.e., vancomycin). Organizational considerations relevant to BL TDM implementation (e.g., infrastructure, personnel) were similar to those identified in other TDM settings.
CONCLUSION:
Broad enthusiasm for BL TDM among participants was found. Prior literature suggested that assay availability was the primary barrier to implementation; however, the data revealed many more individual and organizational attributes which impacted the BL TDM implementation. Internalization should particularly be focused upon to improve the adoption of this evidence-based practice.
Keywords: Pharmacokinetics, pharmacodynamics, critical illness, implementation, antibacterial agents
Introduction
Beta-lactam antibiotics (penicillins, cephalosporins, or carbapenems) are used in three-fourths of the patients treated with sepsis.1 These drugs exhibit high intra- and inter-individual variability in drug concentrations in the critically ill, rendering the therapeutic and toxic effects unpredictable.2–4 One strategy to better personalize beta-lactam therapy is the use of ‘therapeutic drug monitoring’ (TDM), an alternate term for drug level testing, to tailor dosage according to the patient’s needs.5–7
Beta-lactam TDM has reliably increased pharmacokinetic/pharmacodynamic (PK/PD) target attainment. Evidence suggests that low beta-lactam levels lead to a 1.5-fold increased risk of treatment failure, longer lengths of stay, escalation of tests or therapies, higher mortality, and antibiotic resistance.2,8–11 However, clinical trials have not consistently demonstrated that TDM-guided beta-lactam dosing could improve mortality or limit toxicity.11,12 Despite the limitations of the existing literature, the known pharmacokinetic variability in these patients prompted international guidelines to recommend the use of beta-lactam TDM to improve antimicrobial adequacy in critically ill patients.5–7 Despite the strong adoption of TDM for other antibiotics,13–15 TDM for beta-lactams is not yet considered mainstream. Multinational surveys suggest that only about 10–20% of practices use beta-lactam TDM in their ICUs.16–18 In the United States, the frequency is even less.19
One potential explanation for the limited adoption is the lack of attention to the complexity of implementing beta-lactam TDM, which is needed for the routinization of a new evidence-based practice. Key considerations for other antibiotic TDM programs have included a multiphase plan for roll-out, multidisciplinary buy-in, education and training of clinicians, and robust technical integration within the electronic health record.20–22 Previous implementation considerations with other antibiotics may be applicable to beta-lactam TDM; however, there is limited empirical evidence to support this assertion. Indeed, a fundamental difference exists between beta-lactam TDM and TDM for other drugs or drug classes. TDM for drugs like vancomycin and aminoglycosides has been justified on the basis of safety (i.e., to limit toxicity).13 In contrast, the primary argument for using TDM with beta-lactams is to improve effectiveness (i.e., enhance clinical and microbiologic cure). This may affect how clinicians perceive and approach the implementation. Within the limited available evidence,23 surveys suggest that a lack of knowledge and limited access to validated assays were the most significant drivers for the lag in beta-lactam TDM implementation.24 A detailed assessment of the key factors that drive beta-lactam TDM implementation is needed to overcome unacceptably low levels of adoption.
We hypothesized that (a) the implementation factors important in other TDM programs would continue to be relevant to beta-lactam TDM, and (b) among implementation factors, persuading end users of the importance of beta-lactam TDM would be the most salient challenge to implementation. To probe these hypotheses, we performed a mixed methods study to 1) determine ICU practitioners’ perspectives on implementing beta-lactam TDM and 2) comprehensively characterize barriers and facilitators for beta-lactam TDM implementation in real-world practice.
Methods
OVERALL DESIGN
This was a multicenter two-phase explanatory sequential mixed methods study (quant → QUAL)25 conducted between November 2020 and July 2021. The local Institutional Review Board approved the study (#20–007528), and all participants provided informed consent. The study was registered at clinicaltrials.gov (NCT04755777). A detailed description of the justification and methods have been previously published.26
QUANTITATIVE STRAND
A web-based questionnaire was developed by the investigating team based on published literature and with consultation from the local survey research center. The survey was pretested by diverse clinicians across the United States using a structured critique form; items and response options were revised based on feedback. The finalized questionnaire (Supplemental Digital Figure S1) was distributed over email to clinicians at three centers with different beta-lactam TDM practices at the time of the study: Center 1 – not implemented (NI), Center 2 – partially implemented (PI), and Center 3 – fully implemented (FI) (Supplemental Digital Table S1). Only a few centers in the United States used beta-lactam TDM as part of routine clinical practice at the time of the study.19 The practice was most prominent in Europe, China, Australia, and New Zealand.16–18 To capture a diverse group of clinical insights across the spectrum of experience (ranging from not implemented to fully implemented), we selected two centers in the United States and one in Australia for sampling. Non-responders were reminded twice, and the survey was closed after 4 weeks.
We included clinicians from various disciplines (pharmacists, physicians, advanced practice providers), specialties (critical care, infectious diseases), levels of training (trainees, staff), and years of experience who would be/are involved with beta-lactam TDM. These individuals were believed to be familiar with antibiotic TDM in general but not necessarily for beta-lactams.
We analyzed survey data using descriptive statistics. Respondents that provided no additional answers beyond demographics were omitted from analyses. Missing data were reviewed for patterns but not imputed.
QUALITATIVE STRAND
Survey respondents were asked for consent to participate in a semi-structured interview. Among those who provided consent, we created a purposive sample for the qualitative strand based on center, clinical discipline, and expertise to ensure a diverse set of views. The target sample size was 30 individuals to achieve thematic saturation.27,28 If key disciplines or perspectives were insufficiently characterized, local study team members identified additional potential participants through a snowball sampling approach. The first three interviews were facilitated by a semi-structured interview guide and conducted jointly by the primary investigator (EFB), a pharmacist with expertise in TDM, and a trained health services researcher (PNC). After these interviews, the interview guide was refined (Supplemental Digital Figure S2), and the remaining interviews were conducted by the health services researcher. Interviews were recorded, transcribed verbatim, de-identified, and coded using Dedoose (version 9.0.46; Los Angeles, CA), a qualitative analysis software.
Using a structured codebook, the first three transcripts were deductively coded independently and in triplicate (EFB, PNC, KHP). The coding structure was based on the Consolidated Framework for Implementation Research (CFIR) and Normalization Process Theory (NPT).26 CFIR is a framework used to organize and interpret the determinants of implementation. The data were organized according to five constructs: intervention characteristics, outer setting (sociopolitical context), inner setting (institutional context), individual characteristics, and implementation processes.29 A preliminary codebook with operational definitions and coding guidance using the CFIR framework (https://cfirguide.org/) was adapted for this study. NPT is a framework that describes the ‘work’ done to implement evidence-based practices in healthcare and is grouped into four constructs: sense-making, enrolling and planning, enacting, and appraising work.30 Of these four constructs, the sense-making or ‘coherence’ construct was used to understand the meaning, use, and utility of beta-lactam TDM as a new evidence-based practice. After coding the first three transcripts, the codebook was refined, and the remaining interviews were divided and coded independently by PNC and KHP. EFB subsequently reviewed the coded data for completeness. Data were merged with quantitative findings to identify areas of complementarity, concordance, and discordance. Themes were characterized and supported by survey data and illustrative quotes.
Results
PARTICIPANTS
We contacted 633 individuals for study participation across the three sites, and 138 (22%) participants submitted complete or partial responses to the study questionnaire (Supplemental Digital Figure S3). Most of the survey respondents were attending physicians (38%) and pharmacists (33%) (Table 1), and most of them practiced in critical care (71%). Few individuals (5%) at Center 1-NI reported experience with beta-lactam TDM, while most individuals at Center 2-PI and Center 3-FI used beta-lactam TDM in the last 6 months (76% and 94%, respectively). Among those with experience using beta-lactam TDM, most (64%) reported use in 1–10 patients in the preceding month. A total of 89 individuals (64% of respondents) agreed to be contacted for an interview. Of these, 24 from the original cohort were included in the qualitative strand. Pharmacists were oversampled in the qualitative strand as they were identified by 96% of survey respondents as the healthcare team member who should be primarily responsible for managing beta-lactam TDM. Through interviews, we identified the need to obtain the perspective of the bedside nurse and the laboratory staff, which were not part of the original sample. Six additional individuals representing these groups were interviewed for a total of 30 interviewees.
Table 1.
Participant demographics and characteristics
| Quantitative strand | Total (N = 138) |
|---|---|
| Clinical role | |
| Attending physician | 53 (38) |
| Pharmacist | 45 (33) |
| In-training physician (fellow) | 20 (15) |
| Advanced practice provider (nurse practitioner/physician’s assistant) | 19 (14) |
| Other | 1 (1) |
| Years of experiencea | |
| 0 years (still in training) | 19 (15) |
| 1 to 5 years | 39 (31) |
| 6 to 10 years | 24 (19) |
| 11 to 20 years | 32 (25) |
| Greater than 20 years | 14 (11) |
| Practice environmenta, b | |
| Critical care | 90 (71) |
| Infectious diseases | 26 (21) |
| Other | 10 (8) |
| Average daily censusa | |
| 0 to 5 patients | 8 (6) |
| 6 to 10 patients | 21 (17) |
| 11 to 15 patients | 53 (42) |
| 16 to 20 patients | 15 (12) |
| 21 to 25 patients | 11 (9) |
| 26 to 30 patients | 5 (4) |
| Greater than 30 patients | 13 (10) |
| Qualitative strand | Total (N = 30) |
| Clinical role | |
| Attending physician | 7 (23) |
| Pharmacist | 12 (40) |
| In training physician | 1 (3) |
| Advanced practice provider (nurse practitioner/physician’s assistant) | 4 (13) |
| Bedside nurse | 4 (13) |
| Laboratory personnel | 2 (7) |
| Practice environment | |
| Critical care | 26 (87) |
| Infectious diseases | 2 (7) |
| Other | 2 (7) |
| Sex | |
| Woman | 15 (50) |
| Man | 15 (50) |
| Career stage | |
| Early (<5 years from terminal training) | 7 (23) |
| Middle (5–15 years from terminal training) | 9 (30) |
| Late (>15 years from terminal training) | 14 (47) |
Data available for 126 of 138 respondents, which is the denominator for calculated percentages
Survey was distributed to 544 individuals affiliated with critical care and 89 affiliated with infectious diseases
FINDINGS
We identified two major contributors to beta-lactam TDM implementation: individual internalization and organizational features (Figure 1). Individual internalization was uniquely important to beta-lactam TDM and differed across centers. In contrast, the organizational features identified seemed broadly generalizable to the implementation of a variety of evidence-based practices and were consistent across the sites.
Figure 1. The conceptual framework for beta-lactam therapeutic drug monitoring implementation.
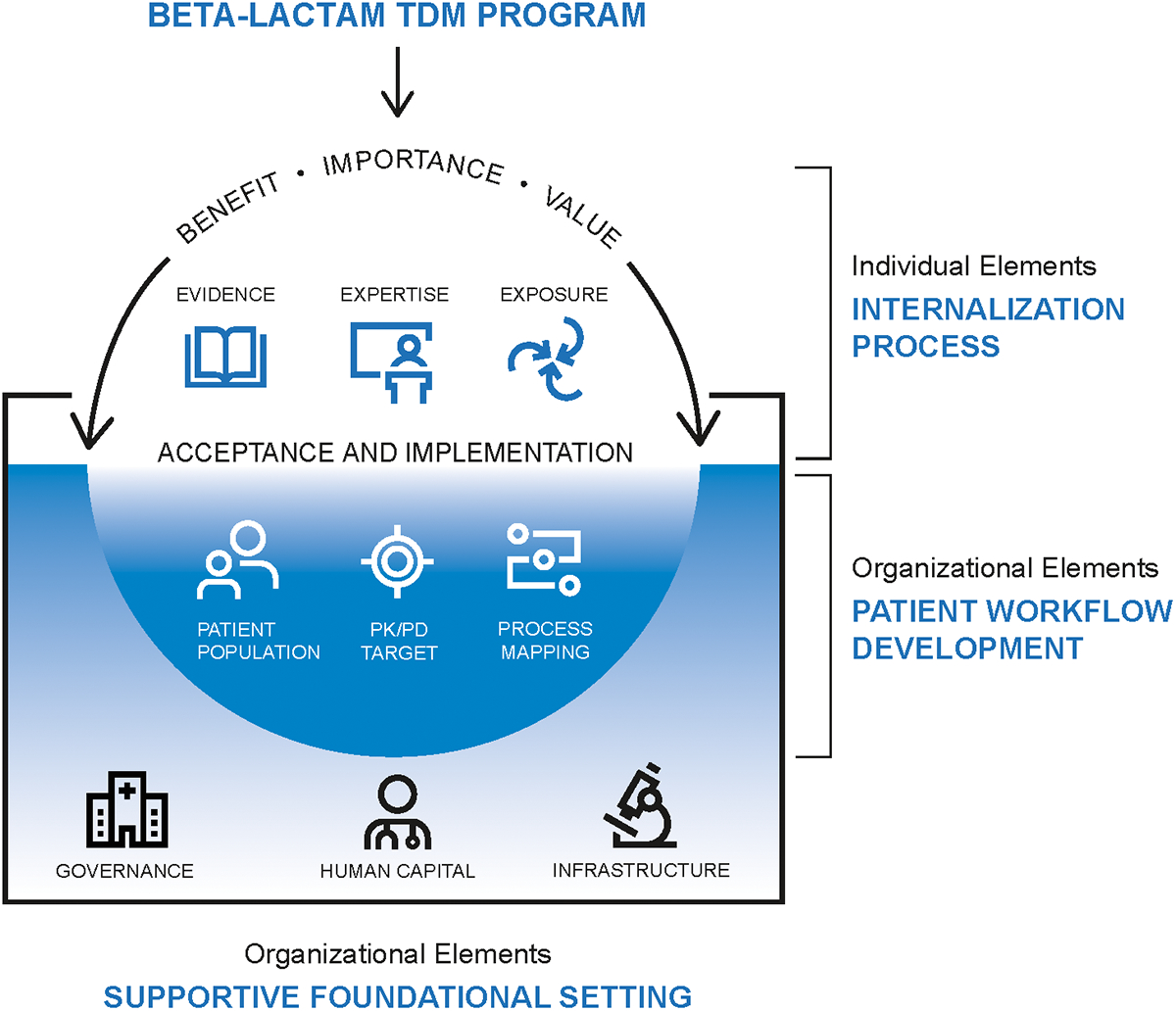
Successful implementation is determined by a supportive setting that serves as the foundation for internalization at the individual level and workflow development at the organizational level. Elements of an ideal setting to implement beta-lactam TDM include adequate, well-trained staff, access to validated tests with rapid turnaround time, information infrastructure such as an electronic health record and access to reference materials, robust communication networks, and deep leadership engagement. Individuals need the time to experience enough repeated exposure to credible evidence and expert insights to internalize, make sense of, and agree to embrace a beta-lactam TDM program. Finally, numerous decisions must be made to operationalize beta-lactam TDM, including the choice of patient candidates and pharmacokinetic/pharmacodynamic targets. Process mapping based on legacy TDM programs can facilitate the identification of gaps, inefficiencies, and responsible parties.
Abbreviations: TDM: Therapeutic drug monitoring; PK/PD: Pharmacokinetic/pharmacodynamic
Individual Internalization
In beta-lactam TDM implementation, it was critical that individuals internalize the importance of, make sense of, and agree to the future state of beta-lactam TDM (Table 2). This aspect of implementation is closely related to the coherence domain of NPT, the ‘sense-making’ construct.30 All individuals acknowledged routine use of TDM for other antibiotics (i.e., vancomycin), and the majority agreed that beta-lactam TDM was relevant to their practice (Figure 2). Nevertheless, at Center 1-NI, participants were skeptical about the evidence base. They acknowledged that beta-lactam TDM improved PK/PD target attainment but remained reluctant to embrace the practice due to concerns about the importance of this surrogate endpoint to patient outcomes. One intensivist said,
Table 2. Individual Internalization.
Respondents revealed that internalization or the “understanding the value, benefits, and importance of a set of practices,” is critical to beta-lactam TDM implementation.30 Factors relevant to this node include evidence synthesis and interpretation, iterative exposure to the concept of beta-lactam TDM, and access to multidisciplinary clinical and methodologic experts in support of implementation (the ‘3E’s’).
| Factors | Representative quotes | Strategies |
|---|---|---|
Evidence synthesis and interpretation
|
“Unfortunately, none of the TDM data have demonstrated survival benefits. They do demonstrate the transition to achieving pharmacokinetic benefits.” [ID 19, Bedside nurse (critical care); Center 2-PI] “I think it makes it easier because the research is local, in a way, so people are familiar with the research that you’re doing. If they see a positive result from their research, they are probably more confident that it’s applicable.” [ID 24, Pharmacist (critical care); Center 3-FI] |
|
Iterative exposure to the concept of BL-TDM
|
“I would say, if you’re thinking about whether that is bringing some TDM practice to your own site prior to doing that, you need to test the waters a little bit, right? The only way to do that is to send out some concentrations…The best way to convince someone else that there is a need for something is to show them how it can be useful in improving antibiotic dosing, right?” [ID 25, Pharmacist (infectious diseases); Center 2-PI] “…Unfortunately, doctors, we are all very stuck in this is how I’ve always done it, and we’re probably the most resistant change population… I think if it’s more part of the undergraduate or graduate education, by the time you start your career, it’s already part of your thought pattern or part of your attitude.” [ID 23, Physician, trainee (infectious diseases); Center 3-FI] |
|
Multidisciplinary expertise
|
“Then when we all sit around together in the mornings and we talk about, “Well, why’d you pick that one,” and we discuss that with the information…from the pharmacist…we may say, “Well, what do you think about tryin’—and we can measure the levels, and this person weighs 800 pounds, or their GFR’s two, or they have no proteins, they’ve been malnourished for six months,” …I think it’s things like that when we pick it apart during the daylight hours that might make us wanna think about doing drug monitoring that’s more targeted instead of just throwing the spaghetti at the wall and using the standard recipe.” [ID 2, Physician, attending (critical care); Center 1-NI] |
|
Figure 2. Clinician perceptions about beta-lactam TDM (upper panel) and potential barriers to implementation of beta-lactam TDM (lower panel).

Abbreviations: Center 1-NI: Not implemented; Center 2-PI: Partially implemented; Center 3-FI: Fully implemented
“…I think I would have to have evidence of patient outcome benefit, which is, I’ve said, a little difficult to get. I’m not aware of a large multicentered, randomized control trial showing that.”
[ID 2, Physician, attending (critical care); Center 1-NI]
In contrast, at Centers 2-PI and 3-FI, individuals gauged the evidence for beta-lactam TDM as sufficient. Participants pointed out the discrepant rationales used by clinicians to support or refute various TDM programs. A pharmacist in infectious diseases said,
“It’s funny how we will often justify. We justify therapeutic drug monitoring for every other drug except the one drug that probably matters most in managing infection, which is beta-lactam, right? When it comes to a beta-lactam, then suddenly people feel like there isn’t sufficient evidence to support checking levels and adjusting doses.”
[ID 25, Pharmacist (infectious diseases); Center 2-PI]
An intensivist recalled,
“Well, you know, you can see my hair is gray. When I started in intensive care, the process was totally different…Even before 2008, TDM was an unknown quantity except for the toxicity of aminoglycosides and the toxicity of the glycopeptides…The TDM we are talking about is efficacy, and that’s a fundamentally different concept…Everybody wanted to not be toxic or to give their patients nontoxic doses, so it became a marketable commodity. Everybody believed in the worry of toxicity. Until you believe in the value of efficacy, you’re not gonna get buy-in.”
[ID 17, Physician, attending (critical care); Center 3-FI]
Access to influential local experts or local research, hands-on exposure prior to formal program implementation, and prevailing culture appeared to impact individual internalization. Participants clearly identified these ‘3 E’s’ of individual internalization: evidence, expertise, and exposure (Table 2).
Organizational Features
The other facet of implementation identified as important was organizational features which could be broadly categorized as elements of the setting or the workflow (Table 3). The setting included the people, resources, and leadership structure of the organization - the who and what. Workflow captured the decisions made when executing a beta-lactam TDM program - the how. There was a close connection between these elements and each of the CFIR constructs (intervention characteristics, outer setting, inner setting, individual characteristics, and implementation processes).29
Table 3. Organizational features.
Elements of the organization that impact beta-lactam TDM implementation include attributes of the setting itself and decisions made about the workflow.
| Factors | Representative quotes | Strategies |
|---|---|---|
Setting
|
“The people who are institutionalizing it are behind desks…They’re not thinking about the 90 other things that we have to complete at the same time. They’re not thinking about how I might be giving this medication, and the patient’s jerking, coming off sedation, trying to pull out the tube, and family members crying in the room.” (ID 20, Bedside nurse (critical care); Center 1-NI) “Right now, you’re dealin’ with a very burnt-out healthcare profession. We have nurses leaving the bedside in droves…We have a lot of fresh, fresh faces…That’s another barrier. Lack of experience.” [ID 28, Bedside nurse (critical care); Center 1-NI] “The one thing that comes across as a big-picture principle is communication. The crux of the matter is, if the individual organizations and specialties could actually talk to each other…in forums such as journal club, a conference, or in-house grand rounds.” [ID 18, Physician, attending (critical care); Center 3-FI] “I think having the lab availability more often, having that is a huge difference to actually making it be something meaningful that your team understands.” [ID 1, Pharmacist (critical care); Center 2-PI] “Well, we were working with the stewardship team, which includes physician and pharmacists, and we’re in discussions with them about how we would roll this out, and who would have access to it initially, and then how that would be broadened. Then a policy had to be written and approved within the Department of Pharmacy in the hospital, and then that had to be approved by the Pharmacy and Therapeutics Committee at the hospital. All of the in-house regulatory steps were put in place, and the approvals were acquired.”[ID 29, Laboratory personnel; Center 2-PI] |
|
Workflow
|
“…Meningitis, ventriculitis, intra-abdominal infections, patients post-trauma or post-burns…likely augmented renal clearance, especially in young patients. We would do TDM-guided therapy, and we have seen quite significant changes in dosing based on TDM in these patient groups” [ID 18, Physician, attending (critical care); Center 3-FI] “I think you need to have a really good understanding of what you’re gonna do with that result. You need to understand how the antibiotic works. What is the PK/PD of the antibiotic? What does that result mean? Are you looking at a trough? Are you looking at the area under the curve? Obviously, with beta-lactams, you’re looking for the time above MIC, but if you don’t have an understanding of what that means and how it relates to an MIC, or even what an MIC is, which a lot of people don’t really know ‘cause it is quite specialized, then it’s not very useful…I think it does require a fair amount of training and really good pharmacy support to really understand all aspects “ [ID 23, Physician, trainee (infectious diseases); Center 3-FI] “..one other thing is it would be nice to know the trends of what I’m doing. That’s something I’ve talked with our team about. I like to know how often am I decreasing the dose? How often am I increasing the dose? Even though I know this is an individualized regimen, and oftentimes the response I get is, “Get more levels. Then you’ll know.” [ID 1, Pharmacist (critical care), Center 2-PI] “I think it really depends on the differences between your peak and your trough. If you’re seeing something like accumulation by having a little variation between that peak and trough, that gets me a little bit more concerned about do I need to hold the dose? I’ve been in that situation before. Do I need to de-accumulate the drug first before I would administer more drug?” [ID 1, Pharmacist (critical care), Center 2-PI] |
|
Setting
Human Capital
Key individuals involved in beta-lactam TDM included nurses and licensed laboratory personnel to draw blood, technical personnel to assay the samples, and clinicians to interpret the data. Most participants (70%) envisioned the ICU pharmacist as the primary individual responsible for beta-lactam TDM for critically ill patients. The high patient census, the severity of illness, and the rapidity of practice change in the COVID-19 pandemic limited the capacity of these team members to perform new responsibilities such as launching and executing beta-lactam TDM. The individuals involved with beta-lactam TDM also need to be well-trained. A total of 90% of the participants said they needed more education on beta-lactam TDM. Even at Centers 2-PI and 3-FI, 80–84% of respondents indicated a desire for more education.
Physical and information infrastructure
Both physical and information infrastructure were important to beta-lactam TDM implementation. Access to on-site rapid turnaround validated assays compliant with regulatory standards was deemed necessary. While commercial laboratories could be used to process patient samples in the United States, the 2–5 day turnaround time (sample collection to results reporting) was generally seen as impractical. Even at Centers 2-PI and 3-FI with locally performed assays, 14–47% of respondents still felt that turnaround time was inadequate to drive clinical care meaningfully. In addition to access to on-site assays, the information infrastructure and communication networks of the organization were equally important facilitators of implementation. Simply having a timely drug level available was not enough to impact care. Participants relied on tertiary references (i.e., Micromedex, UpToDate), clinical decision support in the electronic health record (EHR), and dosing calculators to facilitate implementation. Even in Centers 2-PI and 3-FI, only 28–40% of respondents agreed that beta-lactam TDM was adequately integrated into the EHR.
Governance and leadership
Deep leadership engagement facilitated the implementation and adoption of beta-lactam TDM. For instance, formal approvals by Pharmacy and Therapeutics Committees to allow pharmacist ordering of beta-lactam levels facilitated implementation. Endorsements by antimicrobial stewardship groups, local ICU leadership, and infectious diseases subcommittees facilitated TDM implementation.
Workflow
Successful implementation of beta-lactam TDM requires practical decisions about the workflow. First, centers must establish clear criteria for beta-lactam TDM candidacy. More than 50% of survey respondents indicated that beta-lactam TDM should only be used in a few or some critically ill patients. Respondents cited “patients at risk for sub- or supratherapeutic dosing” as the preferred candidates. However, the lack of specificity in this criterion limited its operational salience. Specific suggested situations included acute kidney injury, augmented renal clearance, liver dysfunction, weight extremes, drug resistance, persistent culture positivity, cystic fibrosis, neurological illnesses, or the presence of extracorporeal support.
Second, a local consensus is needed on the preferred therapeutic target for beta-lactam TDM.31 In other words, this would be the drug level or range, which, if not achieved, would prompt a dose adjustment. Generally speaking, some multiplier of the minimum inhibitory concentration of the organism for part or all of the dosing interval was suggested. The justification for the preferred target should be provided to end users to facilitate implementation.
Third, process mapping can help identify gaps, inefficiencies, and responsible parties. At Centers 2-PI and 3-FI, one persistent process gap noted was poorly timed laboratory draws. Factors that potentiated this gap included nursing bandwidth and inadequate communication across the care team members. Another process gap was insufficient feedback on performance. Few respondents were aware of beta-lactam PK/PD target achievement in their unit or hospital. As one respondent reported, “seeing is believing” [ID 18, Physician, attending (critical care); Center 3-FI], and access to real-time feedback or performance dashboards facilitates implementation.32–34 To streamline processes, respondents suggested an adaptation of legacy workflows for routinely monitored drugs like vancomycin. Finally, proactive planning for difficult scenarios was necessary, including strategies on how to handle empiric vs. definitive therapy, dynamic end-organ function, introduction or withdrawal of extracorporeal devices, unexpected findings (i.e., concern for accumulation, contamination), and resampling.
Discussion
SUMMARY OF FINDINGS
In this multicenter mixed methods study, we characterized clinician perceptions about beta-lactam TDM use in critically ill patients and identified determinants of successful implementation. Consistent with our hypothesis, individual internalization, the ‘sense-making’ work30 clinicians do to rationalize an evidence-based practice, differed across centers and distinguished beta-lactam TDM implementation from the implementation of other antibiotic TDM programs. The theme aligns with a sub-concept of the ‘coherence’ construct in the NPT framework.30 At Center 1-NI, beta-lactam TDM was deemed important in survey responses,; however, deeper probing revealed skepticism about the evidence sufficiency among interviewees. In contrast, clinicians at Centers 2-PI and 3-FI, who had access to the same published scientific literature as those at Center 1-NI, gauged the evidence as confirmatory to justify beta-lactam TDM implementation. Several potential reasons exist for this observation. We included three sites across the world, two in the US (Centers 1-NI and 2-PI) and one in Australia/New Zealand (Center 3-FI). Historically, much of the literature for precision dosing of beta-lactam TDM has emerged from Europe, China, Australia, and New Zealand. The non-United States site included in this study (Center 3-FI) could therefore have had greater access to local research, clinical experts, and practical experience with beta-lactam TDM, which were identified as facilitators of implementation. It is also possible that the differences between healthcare systems in the United States and Australia/New Zealand contributed to the findings (a component of the outer setting in the CFIR framework). The fee-for-service payment structure in the United States means that new innovations are scrutinized for cost implications and utility, which may limit or slow the adoption of this system. Comparatively, innovations may be more readily implemented in a universal healthcare system like Australia or the United Kingdom, where the hospital receives payment from the government irrespective of the number of patients seen or procedures performed. We identified that local evidence, access to experts, and frequent exposure (the ‘3E’s’) appeared to facilitate the internalization process.
Second, we hypothesized that some implementation practices deemed important in other settings would emerge as salient to beta-lactam TDM as well. As expected, we found key organizational features which facilitated beta-lactam TDM implementation included adequate and well-trained staff in the ICUs, access to on-site, rapid turnaround beta-lactam assays, electronic resources, including clinical decision support, and deep leadership engagement. These attributes are broadly generalizable to the implementation of a variety of evidence-based practices beyond beta-lactam TDM. The ‘who,’ ‘what,’ and ‘how,’ of beta-lactam TDM implementation mapped to core CFIR domains, including characteristics of the intervention, individuals involved in implementation, the inner setting, and the implementation processes. Proactive attention to the preferred patient population, PK/PD targets, processes of care, and how to handle difficult scenarios each facilitated implementation. One unique consideration explored in our study was the impact that size of the institution and the magnitude of resources had on implementation. Often these attributes are used to explain assay availability; however, our data suggested that large or well-resourced organizations also likely have access to other determinants of successful implementation, including multidisciplinary experts, adequate human capital, robust physical and information infrastructure, and supportive leadership. Center size is not the only factor that determines the likelihood or success of implementation. Indeed, among the three hospitals involved in this study, the smallest was the hospital that had fully implemented TDM. Smaller centers may be more agile when it comes to practice change as there may be fewer individuals to train and less layers of administrative review.
RELATIONSHIP TO OTHER LITERATURE
Therapeutic drug monitoring has been used to individualize pharmacotherapy since the 1970s;35 however, guidance on best practices for implementing such programs is sparse. Even for vancomycin and aminoglycosides, determinants of successful TDM program implementation have not been well studied. Recently, for example, updated guidelines recommended a new PK/PD target for vancomycin but did not address how to implement this change.15 Single-center experiences provided limited insights into implementation;20,21,36 however, mixed-methods studies indicated persistent poor adherence to practice guidelines.37–39 For vancomycin, this revised PK/PD target could be seen as an incremental change to an existing practice. In contrast, the implementation of beta-lactam TDM would be completely new for most institutions and, thus, even more complex.
The largest report that we are aware of that summarized beta-lactam TDM implementation considerations was a review of data from six cross-sectional surveys of more than 5,000 respondents, primarily conducted in Europe and China.24 The included studies characterized the frequency with which beta-lactam TDM was conducted across and within centers relative to other antibiotics, test turnaround time, assays used, PK/PD targets, and approach to dose adjustment. The authors suggested that key factors for implementation included knowledge (about the evidence-based practice from published literature, professional organizations, local experts, and multidisciplinary team members), accessibility to validated assays with adequate turnaround time, and adequate resources to develop the test, workflow, and guidelines. A single-center’s experience reinforced these core findings and underscored the need for multidisciplinary engagement and daily on-site testing.23 Our approach differed from these reports as we engaged and compared quantitative and qualitative insights from stakeholders across centers at various stages of implementation. Findings were analyzed through the theoretical lens of frameworks grounded in implementation science. This method facilitated the rich characterization of the full experience of implementation at both the individual and organizational levels. We identified similar themes to this previous work at the organizational level; however, the individual considerations found in our data were distinct.
LIMITATIONS AND STRENGTHS
This study is not without limitations. The study was performed at three referral centers with an active pharmacist presence in the critical care environment and general familiarity with TDM. These findings may not fully capture the experience of care providers at rural or community medical centers or in environments with an altered critical care team composition. The results of the study may have been affected by non-response error. The response rate of 22% is in line with our previous experience, and that reported in the literature for web-based surveys of clinicians.40–42 It remains possible that non-respondents were systematically different from those who responded. Findings were enriched through in-depth interviews, including the addition of key perspectives (nurse, laboratory director), which emerged as important. The study was also conducted during the COVID-19 pandemic, a period defined by resource limitations, staff shortages, overwhelming patient capacity, burnout and exhaustion among clinicians, and supply chain disruptions. Centers 2 (partially implemented) and 3 (fully implemented) each had launched their beta-lactam TDM program several years prior to the pandemic (2016 and 2009, respectively). Still, the timing of the research may have affected study participation or responses provided. Despite these limitations, these data richly characterized the implementation considerations surrounding beta-lactam TDM and identified a novel factor, individual internalization, which requires further exploration.
FUTURE DIRECTIONS
These findings could be used to select and test validated implementation strategies to increase beta-lactam TDM adoption. The CFIR-ERIC matching tool43 can help match identified barriers and facilitators with preferred implementation strategies. Based on an understanding of the key barriers to implementing an evidence-based practice, the tool can provide a prioritized list of strategies to facilitate implementation. Suggested implementation strategies to address the key barriers associated with individual internalization include identifying and preparing clinical champions, conducting educational meetings and outreach visits, developing educational materials, and informing key opinion leaders. Selected implementation strategies could then be formally evaluated in hybrid clinical trials. Hybrid trials blend effectiveness and implementation evaluations to facilitate more rapid future evidence translation.44
Conclusions
Although the use of beta-lactam TDM to personalize therapy for critically ill patients has been recommended, implementation into practice remains limited. In this multicenter explanatory mixed methods study, we found that individual internalization of beta-lactam TDM was key to successful implementation.
Supplementary Material
Acknowledgments
We extend our sincere gratitude to members of the local Survey Research Center for their assistance with operationalizing this study and the clinicians who provided their invaluable insights. We appreciate the valuable contributions of Drs. Omar M. Abu Saleh and Ognjen Gajic to this manuscript.
Conflicts of Interest and Source of Funding:
MHS reports a previous research contract with Allecra. EFB reports an ongoing consultancy agreement with Wolters-Kluwer. For the remaining authors, no conflicts of interest were declared. This project was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI143882 (PI; EFB). The funding organization had no role in study design; data collection, analysis, or interpretation; writing the report; or the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863. DOI: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083. DOI: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 3.Taccone FS, Laterre PF, Dugernier T, et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14(4):R126. DOI: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlier M, Carrette S, Stove V, Verstraete AG, De Waele JJ. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int J Antimicrob Agents. 2014;43(5):470–473. DOI: 10.1016/j.ijantimicag.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Evans L, Rhodes A, Alhazzani W, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):1974–1982. DOI: 10.1097/CCM.0000000000005357. [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46(6):1127–1153. DOI: 10.1007/s00134-020-06050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilhaumou R, Benaboud S, Bennis Y, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients – Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique – SFPT) and the French Society of Anaesthesia. Crit Care. 2019;23(1):1–20. DOI: 10.1186/s13054-019-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua NG, Loo L, Hee DKH, et al. Therapeutic drug monitoring of meropenem and piperacillin-tazobactam in the Singapore critically ill population – A prospective, multi-center, observational study (BLAST 1). J Crit Care. 2022;68:107–113. DOI: 10.1016/j.jcrc.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Thomas JK, Forrest A, Bhavnani SM, et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42(3):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rybak MJ. Pharmacodynamics: relation to antimicrobial resistance. Am J Infect Control. 2006;34(5)(suppl 1):S38–45; discussion S64. DOI: 10.1016/j.ajic.2006.05.227. [DOI] [PubMed] [Google Scholar]
- 11.Mangalore RP, Ashok A, Lee SJ, et al. Beta-lactam antibiotic therapeutic drug monitoring in critically ill patients: a systematic review and meta-analysis. Clin Infect Dis. Published online June 2022. DOI: 10.1093/cid/ciac506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagel S, Bach F, Brenner T, et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: a randomized controlled trial. Intensive Care Med. 2022;48(3):311–321. DOI: 10.1007/s00134-021-06609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg EJ, Barclay ML, Kirkpatrick CMJ. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 2001;52(suppl 1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imani S, Alffenaar JW, Cotta MO, et al. Therapeutic drug monitoring of commonly used anti-infective agents: A nationwide cross-sectional survey of Australian hospital practices. Int J Antimicrob Agents. 2020;56(6). Published online September 2020:106180. DOI: 10.1016/j.ijantimicag.2020.106180. [DOI] [PubMed] [Google Scholar]
- 15.Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health System Pharmacists, the Infectious Diseases Society of America, the Pediatr. Am J Heal Pharm. 2020;77(11):835–864. DOI: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 16.Tabah A, de Waele J, Lipman J, et al. The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J Antimicrob Chemother. 2015;70(9):2671–2677. DOI: 10.1093/jac/dkv165. [DOI] [PubMed] [Google Scholar]
- 17.Liebchen U, Paal M, Scharf C, et al. The ONTAI study – a survey on antimicrobial dosing and the practice of therapeutic drug monitoring in German intensive care units. J Crit Care. 2020;60:260–266. DOI: 10.1016/j.jcrc.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Fuentes YV, Blanco J, Díaz-Quijano DM, et al. Administration and Therapeutic Drug Monitoring of β-lactams and vancomycin in Critical Care Units in Colombia: the ANTIBIOCOL Study. Pharmaceutics. 2021;13(10). DOI: 10.3390/pharmaceutics13101577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Seabury RW, Steele JM, et al. Evaluation of β-lactam therapeutic drug monitoring among US health systems with postgraduate year 2 infectious diseases pharmacy residency programs. Am J Heal Pharm. 2022;XX(Xx):1–8. DOI: 10.1093/ajhp/zxac117. [DOI] [PubMed] [Google Scholar]
- 20.Gregory ER, Burgess DR, Cotner SE, et al. Vancomycin area under the curve dosing and monitoring at an Academic Medical Center: transition strategies and lessons learned. J Pharm Pract. Published online. 2019;33(6):774–778. DOI: 10.1177/0897190019834369. [DOI] [PubMed] [Google Scholar]
- 21.Gregory ER, Burgess DR, Cotner SE, et al. Pharmacist survey: pharmacist perception of vancomycin area under the curve therapeutic drug monitoring. J Pharm Pract. Published online. 2021;34(2):272–278. DOI: 10.1177/0897190019867494. [DOI] [PubMed] [Google Scholar]
- 22.Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: A cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889–894. DOI: 10.1093/ajhp/zxz070. [DOI] [PubMed] [Google Scholar]
- 23.Venugopalan V, Hamza M, Santevecchi B, et al. Implementation of a β-lactam therapeutic drug monitoring program: experience from a large academic medical center. Am J Health Syst Pharm. Published online June 2022;79(18):1586–1591. DOI: 10.1093/ajhp/zxac171. [DOI] [PubMed] [Google Scholar]
- 24.Abdulla A, van den Broek P, Ewoldt TMJJ, Muller AE, Endeman H, Koch BCPP. Barriers and facilitators in the clinical implementation of beta-lactam therapeutic drug monitoring in critically ill patients: A critical review. Ther Drug Monit. 2022;44(1):112–120. DOI: 10.1097/FTD.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 25.Creswell J, Plano Clark V. Designing and conducting mixed methods research. 3rd Revise. SAGE Publications Inc; 2017. [Google Scholar]
- 26.Barreto EF, Rule AD, Alshaer MH, et al. Provider perspectives on beta-lactam therapeutic drug monitoring programs in the critically ill: a protocol for a multicenter mixed-methods study. Implement Sci Commun. 2021;2(1):34. DOI: 10.1186/s43058-021-00134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leppin AL, Schaepe K, Egginton J, et al. Integrating community-based health promotion programs and primary care: a mixed methods analysis of feasibility. BMC Health Serv Res. 2018;18(1):72. DOI: 10.1186/s12913-018-2866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasileiou K, Barnett J, Thorpe S, Young T. Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Med Res Methodol. 2018;18(1):148. DOI: 10.1186/s12874-018-0594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. DOI: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology. 2009;43(3):535–554. DOI: 10.1177/0038038509103208. [DOI] [Google Scholar]
- 31.Barreto EF, Webb AJ, Pais GM, Rule AD, Jannetto PJ, Scheetz MH. Setting the beta-lactam therapeutic range for critically ill patients: is there a floor or even a ceiling? Crit Care Explor. 2021;3(6):e0446. DOI: 10.1097/CCE.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smalley CM, Willner MA, Muir MR, et al. Electronic medical record-based interventions to encourage opioid prescribing best practices in the emergency department. Am J Emerg Med. 2020;38(8):1647–1651. DOI: 10.1016/j.ajem.2019.158500. [DOI] [PubMed] [Google Scholar]
- 33.Taber P, Weir C, Butler JM, et al. Social dynamics of a population-level dashboard for antimicrobial stewardship: A qualitative analysis. Am J Infect Control. 2021;49(7):862–867. DOI: 10.1016/j.ajic.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rattray NA, Damush TM, Miech EJ, et al. Empowering implementation teams with a learning health system approach: leveraging data to improve quality of care for transient ischemic attack. J Gen Intern Med. 2020;35(Suppl 2)(suppl 2):823–831. DOI: 10.1007/s11606-020-06160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touw DJ, Neef C, Thomson AH, Vinks AA, Cost-Effectiveness of Therapeutic Drug Monitoring Committee of the International Association for Therapeutic Drug Monitoring and Clinical Toxicology. Cost-effectiveness of therapeutic drug monitoring: A systematic review. Ther Drug Monit. 2005;27(1):10–17. DOI: 10.1097/00007691-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Chan JD, Nguyen T, Jain R, Escobar ZK. Doing more with less: pragmatic implementation of vancomycin area-under-the-curve (AUC) monitoring. J Pharm Pract. Published online June 2021:089719002110272:8971900211027271. DOI: 10.1177/08971900211027271. [DOI] [PubMed] [Google Scholar]
- 37.Carland JE, Stocker SL, Baysari MT, et al. Are vancomycin dosing guidelines followed? A mixed methods study of vancomycin prescribing practices. Br J Clin Pharmacol. Published online April 2021:bcp.14834;87(11):4221–4229. DOI: 10.1111/bcp.14834. [DOI] [PubMed] [Google Scholar]
- 38.Bradley N, Lee Y, Sadeia M. Assessment of the implementation of AUC dosing and monitoring practices with vancomycin at hospitals across the United States. J Pharm Pract. Published online April 2021:089719002110123:8971900211012395. DOI: 10.1177/08971900211012395. [DOI] [PubMed] [Google Scholar]
- 39.Bland CM, Crosby CM, Orvin DL, Smith SE, Jones BM. Transitioning from guideline approval to practical implementation of AUC-based monitoring of vancomycin. Am J Health Syst Pharm. 2021;78(14):1270–1272. DOI: 10.1093/ajhp/zxab132. [DOI] [PubMed] [Google Scholar]
- 40.Frazee ENN, Personett HAA, Bauer SRR, et al. Intensive care nurses’ knowledge about use of neuromuscular blocking agents in patients with respiratory failure. Am J Crit Care. 2015;24(5):431–439. DOI: 10.4037/ajcc2015397. [DOI] [PubMed] [Google Scholar]
- 41.Torbic H, Bauer SRSR, Personett HA, et al. Perceived safety and efficacy of neuromuscular blockers for acute respiratory distress syndrome among medical intensive care unit practitioners: A multicenter survey. J Crit Care. 2017;38:278–283. DOI: 10.1016/j.jcrc.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 42.Weaver L, Beebe TJ, Rockwood T. The impact of survey mode on the response rate in a survey of the factors that influence Minnesota physicians’ disclosure practices. BMC Med Res Methodol. 2019;19(1):73. DOI: 10.1186/s12874-019-0719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waltz TJ, Powell BJ, Fernández ME, Abadie B, Damschroder LJ. Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement Sci. 2019;14(1):42. DOI: 10.1186/s13012-019-0892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–226. DOI: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


