Abstract
Background: One of the most useful tools for identifying sleep disturbances is neuroimaging, especially magnetic resonance imaging (MRI). This review research was to look at the role of MRI findings in movement disorders and sleep disturbances. Methods: This review collects all MRI data on movement disorders and sleep disruptions. Between 2000 and 2022, PubMed and Google Scholar were utilized to find original English publications and reviews. According to the inclusion and exclusion criteria, around 100 publications were included. We only looked at research that explored MRI modality together with movement problems, sleep disorders, and brain area involvement. Most of the information focuses on movement irregularities and sleep interruptions. Results: Movement disorders such as Parkinson’s disease (PD), Huntington’s disease (HD), neuromuscular diseases, rapid eye movement (REM) sleep behavior movement disorder (RBD), cerebellar movement disorders, and brainstem movement disorders are assessed using MRI-based neuroimaging techniques. Some of the brain areas were associated with disorders in movement abnormalities and related sleep disturbances. This review found that many people with mobility disorders also have sleep problems. Some brain areas’ malfunctions may cause motor and sleep issues. Conclusion: Neuroimaging helps us understand the sleep difficulties associated with movement disorders by examining the structural and functional implications of movement disorders and sleep disturbances.
Keywords: Magnetic resonance imaging, Parkinson’s disease, REM sleep behavior disorder, movement disorders, sleep disturbance
Introduction
Movement disorders are a group of neurological conditions that affect the speed, fluency, quality of life [1,2]. They include Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and restless legs syndrome (RLS), among others [3-6]. Movement disorders are often accompanied by sleep disturbances, such as insomnia, excessive daytime sleepiness (EDS), rapid eye movement (REM) sleep behavior disorder (RBD), periodic limb movements during sleep (PLMS), and circadian rhythm disorders [3,7,8]. Sleep disturbances can have a significant impact on the quality of life, cognitive function, mood, and disease progression of patients with movement disorders [9-14].
Magnetic resonance imaging (MRI) is a non-invasive imaging technology that creates high-resolution three-dimensional (3D) pictures of organs throughout the body (< 1 mm) [15,16]. MRI has a basic advantage because it offers excellent soft-tissue contrast and deep penetration, allowing for cross-sectional anatomical information to be obtained from the organs [17-19]. This data may then be utilized to perform diagnostics. It’s possible that an MRI scan may provide details about tissue function, such as how well it functions and how much oxygen and other chemicals it contains [20]. MR neuroimaging has been widely used to investigate the structural and functional changes that occur in the brains of patients with movement disorders and associated sleep disturbances [21-23]. MRI can reveal abnormalities in brain regions that are involved in motor control, such as the basal ganglia, thalamus, cerebellum, and motor cortex, as well as in brain regions that are involved in sleep regulation, such as the hypothalamus, brainstem, amygdala, and hippocampus [24-29]. MRI can also assess the connectivity and network dynamics between these regions using techniques such as volumetric analyses, diffusion tensor imaging (DTI), quantitative susceptibility mapping (QSM), perfusion-weighted imaging (PWI), and resting-state fMRI (rs-fMRI) [23].
Many patients with movement disorders also have abnormal sleep and circadian rhythm disorders. The frequency and nature of sleep disruptions vary amongst movement disorders. For instance, the prevalence of sleep problems in Parkinson’s disease (PD) has been reported to be as high as 98 percent, with rapid eye movement (REM) sleep behavior disorder being well documented as predating the onset of impairment [30-33]. The quality of life is directly linked to the quality of one’s sleep, which means that sleep disorders are likely to result in severe morbidity. Early detection and treatment of sleep disorders are of significant therapeutic interest in order to improve quality of life, postpone institutionalization, and reduce healthcare costs [23]. Neuroimaging, particularly MRI, is a useful tool for detecting sleep abnormalities in a variety of movement disorders. The aim of this review study was to investigate the role of MRI findings in movement disorders and associated sleep disturbances.
Materials and methods
To conduct this review article, we searched PubMed and Google Scholar for original articles and reviews in English between 2000 and 2022 using the following keywords: structural and functional MRI; metabolic MRI; movement disorders; and sleep disorders. According to the inclusion and exclusion criteria, we selected approximately 100 publications that met our criterion of investigating MRI modality in conjunction with movement disorders, sleep disorders, and brain region involvement concurrently. The majority of the publications focused on abnormalities of movement and the resulting sleep disruptions they might cause. Finally, we classified the publications according to movement disorders, which included PD, HD, neuromuscular diseases, movement disorders linked with RBD, cerebellar movement disorders, and brainstem movement disorders.
Results
The PD, HD, neuromuscular disorders, movement disorders associated with RBD, and movement disorders associated with the cerebellum and brainstem were revealed in our study.
Figure 1 is a pie chart depicting the proportion of each movement disorder in the total number of studies surveyed by our search.
Figure 1.
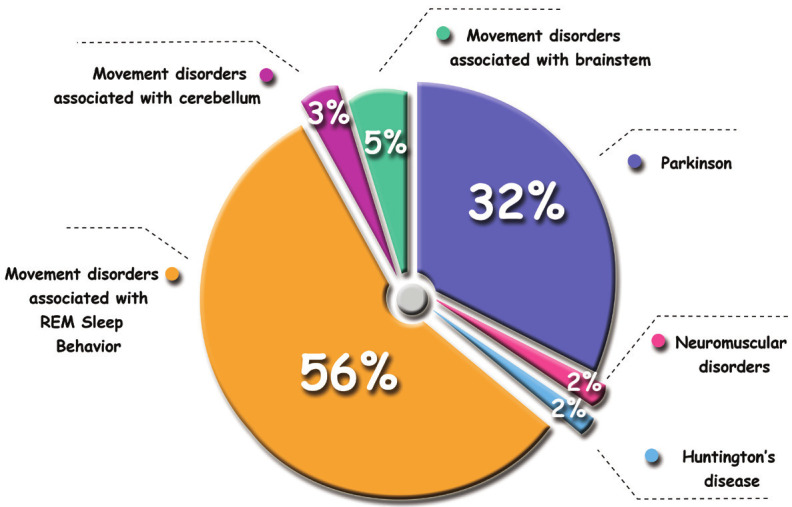
The contribution of each movement disorder from the total reviewed studies.
MRI-based neuroimaging studies are summarized in Supplementary Table 1. Articles reviewed in the area of MR neuroimaging, which includes all of the publications assessed. The number of patients, field strength, MR technique, and findings are all included in this table.
Shaking, stiffness, and problems with balance and coordination are just some of the side effects of PD, which is a condition of the brain [34]. HD is a lethal inherited neurological condition marked by chorea, psychiatric symptoms, and cognitive impairment [35]. Patients with neuromuscular diseases have a wide range of conditions that affect the motor nuclei of the neurons in the brain, spinal cord, peripheral nerves, and/or muscles [36]. Restless legs syndrome (RLS) is a prevalent sensorimotor disorder defined by a need to move that begins during rest or is increased by rest, appears in the evening or at night, and dissipates or improves with movement [37]. The sleep condition known as rapid eye movement sleep behavior disorder (RBD) falls under the category of parasomnia. Patients with idiopathic RBD had lower levels of normal skeletal muscle atonia during REM sleep, as well as higher levels of motor activity during the dream phase [38]. A vital element of the central nervous system (CNS), the cerebellum, or “small brain”, is engaged in a variety of brain activities, including motor planning, motor execution, learning, and breathing [39,40]. Many neurological illnesses, such as pain, sleep disturbances, autonomic disturbances, and neurodegenerative diseases, put the brainstem at risk [41].
As previously stated, MRI findings were gathered in the context of several disorders, such as PD and RBD. More significant results were seen in these findings, particularly in the PD and RBD parts, as compared to the neuromuscular disorders and movement disorders associated with the cerebellum and brainstem portions (see all findings in Supplementary Table 1). Tables 1 and 2 illustrate the valued outcomes of PD and RBD, respectively.
Table 1.
Most important MRI findings in Parkinson’s Disease (PD)
| Technique | MR Imaging Findings |
|---|---|
| T1-w | ● The brain seed locations in both cortical and subcortical areas exhibited substantial structural pattern alterations [15] |
| ● Patients with sleep disorders exhibited regions of decreased GMV* and WM alterations in the cortex [16] | |
| ● PD-RBD patients revealed more nodal property alterations within the neocortex and limbic system [17] | |
| ● The pontomesencephalic tegmentum, medullary reticular formation, hypothalamus, putamen, and anterior cingulate cortex reduced volumes in PD patients [11] | |
| ● High GMV was found in the cerebellar vermis, whereas low GMV was seen in the right SOG of patients with PD-RBD [18] | |
| ● There was more GM atrophy in the PD with probable RBD group for men than for females [19] | |
| ● In PD patients with RBD, the right putamen had a lower volume. The greater volume of the right putamen, left hippocampus, and left thalamus shows the more significant the severity of RBD symptoms [20] | |
| ● There was a correlation between excessive daytime sleepiness and an increased OSA in individuals with PD and PSP, which indicates extensive degradation of brainstem sleep components [21] | |
| ● That patients with troubling nocturia had reduced cortical surface on the left pre-and postcentral regions, and total WM volume decreased bilaterally as well [22] | |
| ● PD Patients with RBD had significantly lower GM volume in the right thalamus [23] | |
| ● The PDRBD+ group had lower GM volume in the left posterior cingulate and hippocampus compared to the PDRBD-, as well as additional lower GM volume in the left precuneus, cuneus, medial frontal gyrus, postcentral gyrus, and both inferior parietal lobules compared to the HC group [24] | |
| ● There was substantial degeneration of GM in the frontal, temporal, and occipital regions of the brain in individuals with EDS compared to controls [25] | |
| ● There was increased regional GM volume in both the hippocampus and the parahippocampal gyri in the PD-EDS group [26] | |
| ● PD patients with impaired phonetic-fluency (a frontal lobe function) and those with iRBD or PDND who had lower levels of total-α-synuclein in their CSFτ were shown to have thinner frontal cortices, regardless of the presence of in their CSF [27] | |
| Diffusion-based | ● Pathways (CC, IFOF, CST, and MCP) with decreased anisotropy were found in PD patients with RBD compared to those without RBD [28] |
| ● The PD with sleep disruption group revealed extensive WM degeneration [29] | |
| ● ALFF values in the main motor cortex and premotor cortex were significantly lower in individuals with RBD than in those with PD without RBD [30] | |
| ● The right cingulum, left and right fornix, left ILF, right CST, right MCP, and genu of corpus callosum connections were considerably decreased in PD-RBD subjects with depressive symptoms [31] | |
| ● The PD-EDS group was shown to have higher AD values in both the left anterior thalamic radiation and CST, as well as bilaterally in superior corona radiata and SLF [26] | |
| fMRI | ● PD-RBD patients revealed more nodal property alterations within the neocortex and limbic system [17] |
| ● The ReHo values of the ACC varied considerably across the PD groups patients [18] | |
| ● Cortical FC in the default mode, central executive, and dorsal attention networks was less severely reduced in PD patients with sleep disturbances [29] | |
| ● Both RBD and PD were identified by connectivity measurements of basal ganglia network dysfunction with excellent specificity (74% for RBD disorder, and 78% for PD), highlighting its potential as an early indication of basal ganglia dysfunction [32] |
Abbreviations: GMV: Gray Matter Volume; WM: White Matter; PD: Parkinson’s Disease; RBD: Rapid Eye Movement (REM) Sleep Behavior Disorder (RBD); SOG: Superior Occipital Gyrus; OSA: Obstructive Sleep Apnea; PSP: Progressive Supranuclear Palsy; HC: Healthy Control; EDS: Excessive Daytime Sleepiness; iRBD: Idiopathic RBD; PDNP: PD Patients with No Dementia; CSF: Cerebrospinal Fluid; CC: Corpus Callosum; IFOF: Inferior fronto Occipital Fasciculi; CST: Corticospinal Tract; MCP: Middle Cerebellar Peduncle; ALFF: Amplitude of Low Frequency Fluctuation; ILF: Inferior Longitudinal Fasciculus; AD: Axial Diffusivity; SLF: Superior Longitudinal Fasciculus; ReHo: Regional Homogeneity; ACC: Anterior Cingulate Gyrus; FC: Functional Connectivity.
Table 2.
Most important MRI findings in REM sleep behavior disorder (RBD)
| Technique | MR Imaging Findings |
|---|---|
| T1-w | ● In patients with iRBD*, increases in GMV in the cerebellum, putamen, and thalamus may indicate a compensatory impact [33] |
| ● Patients with iRBD had considerably greater lateral ventricle sizes than HCs [34] | |
| ● Patients with RLS had higher GMVs in the left caudate and the left ventral rostral putamen [35] | |
| ● WM volume was considerably decreased in RLS patients compared to HCs, and these changes were linked to illness duration [36] | |
| ● People who suffer from RLS had a 7.5% lower than normal average thickness of the bilateral postcentral gyrus. A significant reduction in the corpus callosum posterior midbody was shown [37] | |
| ● A considerable loss in GM volume was seen in the anterior lobes of the right and left cerebellum and the tegmental area of the pons in individuals with iRBD compared to controls [38] | |
| ● There were substantial differences in the volume of the brains of patients with iRBD and HCs, with higher volumes of the frontal cortex, thalamus, and caudate nucleus [39] | |
| ● Individuals with iRBD had structural connectivity that was considerably different from that of HCs [39] | |
| ● There was a large amount of hub reorganization in patients diagnosed with iRBD, as measured by local network metrics [39] | |
| ● Patients diagnosed with iRBD exhibited an increase in the betweenness centrality of the caudate nucleus and frontal cortex [39] | |
| ● Both hippocampi of iRBD patients were shown to have increased GM density [40] | |
| ● Participants with iRBD had lower NM-sensitive volume and signal intensity compared to controls [41] | |
| ● GMV in the middle temporal gyrus and cerebellar posterior lobe was higher in iRBD patients than in controls, while it was lower in the Rolandic operculum, postcentral gyrus, insular lobe, gyrus of the cingulate, precuneus, and superior frontal gyrus [42] | |
| Diffusion-based | ● The WM of the brainstem, the right substantia nigra, the olfactory area, the left temporal lobe, the fornix, the internal capsule, the corona radiata, and the right visual stream were all reported to have considerable microstructural alteration in individuals with iRBD [43] |
| ● RLS/WED patients, had reduced AD in the leftposterior thalamic radiation. The intensity of RLS/WED symptoms was adversely linked with a decrease in AD in the left posterior corona radiata in those who had the symptoms. RLS/WED sufferers have higher RD in the superior cerebellar peduncles [44] | |
| ● FA was considerably decreased in RLS patients compared to HCs, and these changes were linked to illness duration [36] | |
| ● RLS patients had lower FA values in the CST and cingulum on the left side of the brain, and in the ATR and the IFOF on the right side of the brain. Patients’ attention/executive function and visual memory scores were linked positively with FA values in the right ATR, whereas anxiety levels correlated adversely with FA values in the right IFOF. FA levels in the left CST were also adversely associated with the frequency of periodic leg movements and the movement arousal score [10] | |
| ● In comparison to controls, RLS patients had lower FA in the corpus callosum’s genu and frontal WM near the inferior frontal gyrus. Both the AD and RD were greater in regions with lower FA than in the control participants [45] | |
| ● There were areas of changed FA in the subcortical WM on both sides, mostly in the temporal region but also the internal capsule on the right, the pons and the right cerebellum [46] | |
| ● In iRBD patients, FA reduced in the tegmentum of the midbrain and rostral pons, whereas MD increased in the pontine reticular formation, which overlapped with the cluster of decreased FA in the midbrain [40] | |
| ● Participants with iRBD had lower FA compared to controls [41] | |
| fMRI | ● In patients with iRBD, changed ALFF values in the parahippocampal gyrus and occipital cortices may contribute to the underlying neurodegenerative process in this condition [33] |
| ● In RLS patients, higher levels of ReHo were found in the striatum, thalamus, and limbic system, suggesting that emotional processing and motion regulation in the CSTC loop may be the source of dysfunction [47] | |
| ● Drug-treated RLS demonstrated considerably stronger thalamic connectivity for the left uvula, right tuber, left anterior insula, and right declive compared to drug-naïve RLS [48] | |
| ● Right precuneus, right precentral gyrus, right precentral gyrus, and bilateral lingual gyri all exhibited decreased connection with the thalamus in RLS patients; conversely, right superior temporal gyrus, bilateral middle temporal gyrus, and right medial frontal gyrus all showed improved connectivity with the thalamus [49] | |
| ● Severity of RLS was adversely linked with thalamic-right parahippocampal-gyrus connection [49] | |
| ● The rsFC patterns of putamen were found to be altered in RLS patients compared to those without the condition [35] | |
| ● Right pyramids in the RLS group had reduced SN FC, but bilateral orbitofrontal gyri and the right postcentral gyrus had increased SN FC [50] | |
| ● Idiopathic drug-naive RLS patients showed lower FCS in the cuneus and fusiform gyrus, as well as an increase in FCS in the superior frontal gyrus and thalamus, according to a hub analysis. Following FC investigations, it was shown that sensorimotor and visual processing networks had reduced functional connection, whereas the emotional cognitive network and cerebellar-thalamic circuit had enhanced FC [51] | |
| ● RLS patients had reduced ALFF in the sensorimotor and visual processing regions and greater ALFF in the insula, parahippocampal and hippocampal gyri, the left posterior parietal areas, and the brainstem compared to HC subjects [52] | |
| ● RLS patients had greater levels of activation in the dorsolateral prefrontal cortex [53] | |
| Other | ● Enhanced iron content was seen in the caudate and putamen of RLS patients compared to controls, indicating an increased R2* signal in these locations [54] |
| ● The relationship between neuronal origin-enriched extracellular vesicles (nEV) total ferritin and MRI measures of iron deposition in the substantia nigra was distinct between the two groups of participants with RLS and control participants [55] | |
| ● In patients with late-onset restless legs syndrome, the iron index in the substantia nigra was considerably lower than in controls [56] | |
| ● An incresed Glx/Cr value was found in patients with RLS when compared to controls [57] | |
| ● Magnetic sensitivity in the thalamus and dentate nucleus of individuals with restless leg syndrome was substantially lower than that of HCs [58] | |
| ● Comparing the RLS cohort to the control group revealed that the putamen, as well as the temporal and occipital compartments, had lower iron concentration when examined with R2* [36] | |
| ● In the substantia nigra pars compacta, RLS patients’ T2 relaxation times were longer than those of control subjects [53] |
Abbreviations: iRBD: Idiopathic rapid eye movement sleep behavior disorder; GMV: Gray matter volume; HC: Health control; RLS: Restless legs syndrome; WM: White matter; GM: Gray matter; NM: Neuromelanin; RLS/WED: Restless legs syndrome/Willis-Ekbom disease; AD: Axial diffusivity; RD: Radial diffusivity; MD: Mean diffusivity; FA: Fractional anisotropy; CST: Cortico-spinal tract; ATR: Anterior thalamic radiation; IFOF: Inferior fronto-occipital fasciculus; ALFF: Amplitude of low frequency fluctuation; ReHo: Regional homogeneity; CSTC: Cortico-striatal-thalamic-cortical; SN: Salience network; FC: Functional connectivity; rsFC: Resting-state functional connectivity; FCS: Functional connectivity strength; Glx: glutamate and glutamine; Cr: creatine.
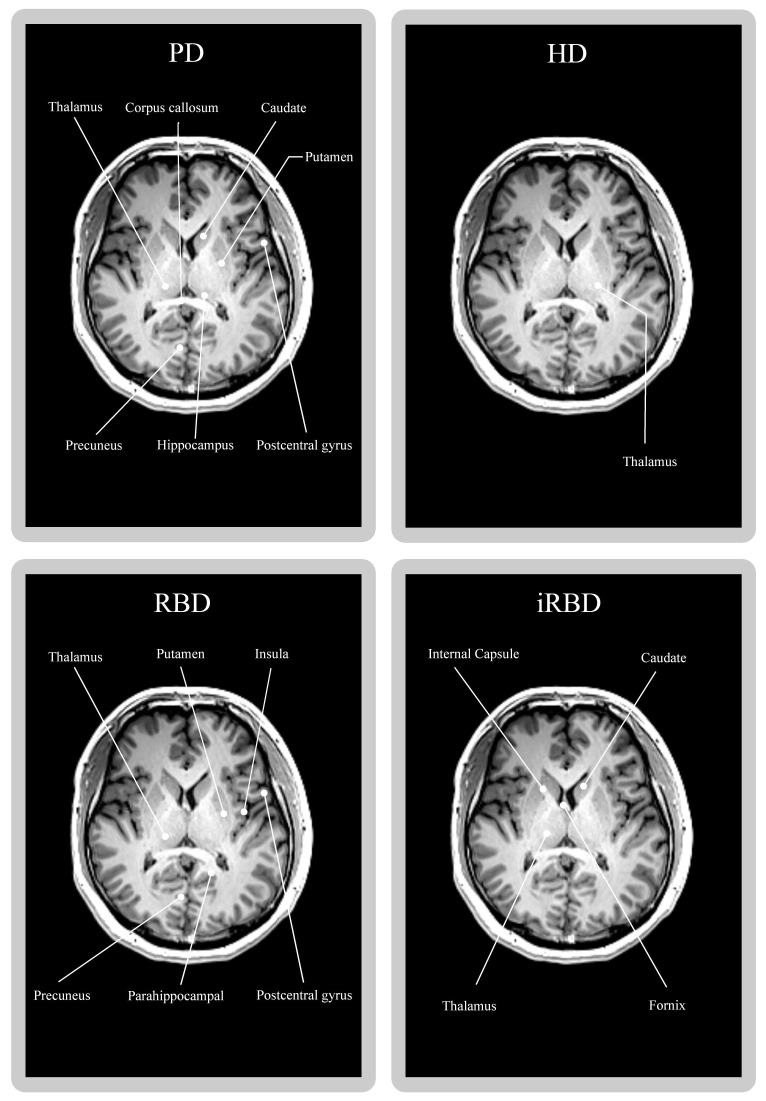
The association between the most prevalent distinct brain areas involved in movement disorders and accompanying sleep disturbances is shown in our research. Figure 2 shows that major parts of the thalamus, corpus callosum, caudate, putamen, precuneus, hippocampus, and postcentral gyrus were engaged in PD patients [38,47,50,51]. Even though RBD patients have more involvement in the thalamus, putamen, insula, precuneus, parahippocampal, and postcentral gyrus, iRBD patients only have more involvement in the internal capsule, caudate, thalamus, and fornix [58,62-64].
Figure 2.
The T1-weighted brain image of a healthy 29-year-old individual displays the possible brain regions implicated in movement disorders and associated sleep disturbances (PD, HD, RBD, and iRBD).
Discussion
In this review article, we investigated the role of MRI findings in movement disorders and associated sleep disturbances. We focused on three main aspects: 1) the structural and functional MRI findings in different movement disorders and their relation to sleep disturbances; 2) the quantitative imaging findings in movement disorders and associated sleep disturbances; and 3) the clinical implications and future directions of MRI research in this field.
Based on previous knowledge, We found that different MRI techniques can reveal different aspects of brain structure and function in patients with movement disorders and associated sleep disturbances [21-23]. For example, T1-weighted imaging can measure the volume of various brain regions and detect atrophy or hypertrophy of GM or WM [42,43,46-49,58,60]. T2-weighted imaging can identify pathological changes in brain tissues such as inflammation or edema [27,77]. DTI can assess the microstructural integrity of WM tracts and their connectivity [83,84]. SWI can measure the magnetic susceptibility of tissues and reflect their iron content [85,86]. DWI can measure the diffusion of water molecules in brain tissues and detect ischemia or necrosis [87,88]. QSM can quantify the magnetic susceptibility of tissues and provide information on iron deposition patterns [89-91]. MRS can measure the concentration of metabolites in brain tissues and reflect their biochemical status [92,93]. fMRI can measure the blood oxygen level-dependent (BOLD) signal changes in brain regions during tasks or at rest and reflect their functional activity [94,95].
Many movement disorders, including PD and RBD, have been shown to cause sleep disorders. Because sleep disorders reduce quality of life and cause complications such as short-term memory loss, increased blood pressure, and an increased risk of diabetes, it is critical to investigate movement disorders and the sleep disorders that result from them [96-99].
PD, RBD, HD, and other movement disorders related to different brain areas were reviewed in a total of 61 studies. There have been numerous studies in which MRI scanners of 1, 1.5, 3, and 7 tesla have been used. With 3 Tesla, there are nearly twice as many studies compared to 1.5 Tesla devices. Numerous studies have been conducted since 2014, which may be due to advances in MRI techniques and protocols, as well as newer devices with more consistent field characteristics. Field intensities above 1.5 tesla are recommended because of their superior accuracy and precision.
If you want the most accurate diagnosis, you need high-quality images, and both MRIs have that capability. The 3T MRI is the most powerful equipment available today, yet the 1.5T MRI remains the standard and most widely used technology for Mri machines, and for good reason. Studies have employed a varied set of cases, which might be attributed to differences in researchers’ access to individuals.
The use of 3D T1 weighting and VBM or SBM analysis is one of the most common ways to analyze and measure the volume of various parts of the brain. Different T1-weighted imaging methods, such as 3D SPGR, 3D MPRAGE, and T1 3D MPRAGE images, have been utilized to assess brain structural changes [22,27,28,37,38,42,43,45-53,55,56,58,59,61-67,70-72,75-78,80,100-107].
Cortical and subcortical alterations of white matter and gray matter were seen in the majority of the studies that were analyzed [42,48,53,55,60,70,71,103]. Patients with Parkinson’s disease and restless legs syndrome were shown to have a reduced amount of gray matter in deep GM locations such as the thalamus, putamen, and caudate. In regards to Parkinson’s disease, males have higher subcortical atrophy, and the volume of deep GM regions has decreased more in men than in women [100]. The cortex and gyrus areas of the frontal, parietal, and temporal lobes of Parkinson’s patients showed degeneration alterations. A higher rate of thalamic atrophy was seen in Huntington’s patients with sleep problems had more problems than those with only Huntington’s symptoms [101]. Atrophic alterations in WM and GM have been found in the setting of OSAS neuromyopathy using SBM analysis in the neuromuscular investigation [102]. The cerebellum, frontal cortex, putamen, thalamus, hippocampus, and caudate showed an increase in GM volume in iRBD patients [58]. The cerebellum, amygdala, hippocampus, and temporoparietal areas of the brain of these individuals and Parkinson’s patients were also shown to have GM volume loss and atrophy [106]. Patients with iRBD had higher GMV in the middle temporal gyrus and cerebellar posterior lobe than controls, but lower GMV in the Rolandic operculum, postcentral gyrus, insular lobe, cingulate gyrus, precuneus, and superior frontal gyrus [67].
T2-weighted images are used with various methods such as T2W GRE, T2W 3D GRE, and T2W FSE to investigate the structural pathologies of various brain regions. Only one study employed T2W images for pathological investigation of the cerebellum and brain stem regions; the findings of this study indicated that as the illness duration increased, so did atrophy and hyperintensity in the cerebellar peduncles and brain stem [27].
Advanced structural neuroimaging methods using MRI, including DTI, SWI, and DWI, were frequently employed in the research we evaluated. SWI is an MRI sequence that is very sensitive to molecules that modify the local magnetic field, making it effective in identifying blood products, calcium, and other substances [86]. SWI is a 3D high-resolution gradient-echo MRI sequence. Unlike most other standard sequences, SWI takes use of the influence on both phase and magnitude [108]. DWI is based on measuring the thermal Brownian motion of water molecules. Water molecules in cerebral white matter tend to diffuse more easily along axonal fascicles rather than across them. Anisotropy is the word used to describe this directional dependence of diffusivity. The final image shows the white-matter fibers’ highest diffusivity in the direction of this maximum [88].
DTI is an MRI technique that assesses the axonal (white matter) architecture of the brain using anisotropic diffusion. An imaging method called fiber tractography (FT) uses diffusion tensor imaging data to create a 3D reconstruction of neural tracts [109]. It has been shown that RLS patients have microstructural or functional alterations in the CNS that can’t be detected by standard MRI, such as DTI and fMRI, according to research [61,68-70,73,76,77,110]. It is possible to detect degenerative parkinsonism in iRBD by looking for a lack of DNH on high-field SWI [111]. Although these alterations were broad, the mean diffusivity and fractional anisotropy of both PD groups increased in comparison to the HC [50].
Fornix, internal capsule, corona radiata, right visual stream and white matter of brain stem were all shown to have significant microstructural modification in persons with iRBD [29]. Reduced AD was found in the left posterior thalamic radiation by TBSS analysis in RLS/WED patients with RLS. RLS/WED patients show greater RD in the superior cerebellar peduncles [68]. White matter volume and FA were found to be significantly lower in RLS patients compared to healthy controls, and these alterations were connected to disease duration. For patients with RLS, the right FA signal from the frontopontine tract was shown to be inversely related to increases in the right primary motor cortex’s gray matter volume [61]. Patients with RLS reported reduced FA values in the CST and cingulum on the left side of the brain, as well as the ATR and IFO fasciculus on the right side. Patients’ attention/executive function and visual memory ratings were favorably connected to FA values in the right ATR, whereas anxiety levels were negatively related to FA values in the right IFO. FA levels in the left CST were also shown to be negatively related to the frequency of periodic leg movements as well as the movement arousal score [37].
A comparison of FA brainstem maps voxel by voxel showed significant microstructural abnormalities on both sides. According to Lindemann (2016) [110], a slice-wise study of FA maps of the cervical spinal cord revealed a trend for lower FA values in the second and third vertebral regions of the patient sample. This was seen in the cervical spinal cord.
RLS patients reported reduced FA in the corpus callosum’s genu and frontal WM near the inferior frontal gyrus compared to controls. AD and RD were also higher in regions with lower FA than in control subjects [69]. Subcortical white matter on both sides of the brain showed altered FA, primarily in the temporal region but also in the right internal capsule, pons, and right cerebellum. These modifications were made concurrently with the RD changes. A VBM assessment indicated no alterations in the gray matter of the brain [70].
FA was reduced in the tegmentum of the midbrain and rostral pons using the SPM method, whereas MD increased in the pontine reticular formation, which correlated with the cluster of decreased FA in the midbrain [65]. Participants with iRBD had reduced NM-sensitive volume and signal intensity, as well as lower FA when compared to controls [66].
The white matter of iRBD patients was shown to have severe microstructural abnormalities in the brainstem, right substantia nigra, olfactory area, left temporal lobe, fornix, and internal capsule [29]. The phase measurement of MRI data and the calculation of QSM in the thalamus and dentate nucleus (DN) show a link between the magnetic behavior of these areas and the frequency of limb movement when the person is sleeping [82].
The QSM technique is used to compute magnetic susceptibility. There was a significant difference between the two groups of people with RLS and control participants in the correlation between neuronal origin-enriched extracellular vesicles (nEV) total ferritin and MRI assessments of iron deposition in the substantia nigra [79]. RLS patients had higher iron concentrations in the caudate and putamen compared to controls, indicating an elevated R2* signal in these areas [78]. The RLS cohort showed substantially decreased iron levels in the putamen, as well as the temporal and occipital compartments, when compared to the control group [61]. According to Astrakas (2008) [77], patients in the substantia nigra pars compacta had considerably longer T2 relaxation times than control individuals.
Recently, there has been significant advancement in the field of neuroimaging known as MRS, which allows for noninvasive in vivo investigation of metabolites. According to our study, only a few studies have been undertaken in the field of MRS to investigate brain metabolites. In just one study, patients with RLS had a higher Glx/Cr ratio when compared to controls, and this value was directly related to the amount of time patients were awake during the sleep phase [81].
More research with field strengths higher than 1.5 Tesla is suggested to investigate and better distinguish brain metabolites such as N-acetylaspartate, creatine (Cr), choline (Cho), glutamate, glutamine, and myo-inositol (with different TEs) [81,112-114]. The fMRI, which is based on blood-oxygen level dependent (BOLD) techniques, has been widely used to study the functional activities and cognitive behaviors of the brain based on the induced stimulus by tasks, also known as task fMRI (tfMRI), or during task-free resting-state, also known as rsfMRI [115-118]. Multiple studies have indicated that combining multiple kinds of rs-fMRI features (such as ALFF, ReHo, and rsFC) improves classification ability. The combination of these qualities revealed this benefit [119-121].
The term “regional homogeneity”, or “ReHo”, refers to the sum of a node’s local FC with its closest neighbors. It may be thought of as a network centrality index that can be used to quantify the relevance of a node in the human functional connectome [122,123]. ACC ReHo levels are greater in TDPD-SD compared to HC and TDPD-SD compared to TDPD-NSD. ACC ReHo levels in TDPD-NSD were observed to be lower than in HC [45]. Higher levels of ReHo were found in the striatum, thalamus, and limbic system of people suffering from restless legs syndrome (RLS), suggesting that emotional processing and motion control in the cortico-striatal-thalamic-cortical (CSTC) loop may be the source of the problem [71].
It was revealed throughout the examination that the ReHo indices differed from one group to the next. In comparison to the PD group without RBD, the PD group with RBD had higher ReHo in the left cerebellar, right middle occipital, and left temporal areas, as well as lower ReHo in the occipital and frontal regions. The left cerebellum demonstrated a greater degree of functional connectivity with the bilateral occipital regions, the bilateral temporal region, and the bilateral supplementary motor area (SMA) in people with Parkinson’s disease who also had RBD than in those with Parkinson’s disease who did not have RBD. The areas of the right middle and occipital lobe on the right side of the skull of the PD group with RBD revealed an enhanced FC with the bilateral cerebellum when compared to the PD group that did not have RBD. The PD group with bilateral caudate exhibited lower functional activity in the left middle frontal areas than the PD group without RBD [107].
Using functional MRI, researchers were able to map functional-anatomical networks in the live human brain, characterize brain changes and differences in clinical populations, and explore comparative anatomy across species. This form of MRI is known as rsFC MRI [124].
Putamen rsFC patterns were shown to be different in people who had RLS compared to those who did not have the disease. As a result, correlation studies found that RLS patients had elevated GMVs in the left caudate as well as the left ventral rostral Putamen [60].
However, the values of the FC between L-OC and R-OC with ACC in TDPD-SD were significantly lower than in HC [45].
When compared to individuals with PD-nRBD, those with PD-RBD showed a lower FC between the right SOG and the posterior regions (left fusiform gyrus, left calcarine sulcus, and left superior parietal gyrus). This is about the FC. FC values in the right SOG-left superior parietal gyrus were shown to have positive associations with MoCA and visuospatial skills/executive function scores, as well as FC values in the right SOG-left calcarine sulcus and delayed memory scores [46].
The right pyramids in the RLS group exhibited decreased salience network (SN) FC, whereas the bilateral orbitofrontal gyri and right postcentral gyrus had greater SN FC. According to the findings of this study, SN FC in patients with RLS may be altered even when they are not experiencing symptoms. This finding is the result of research that indicates this possibility. It is probable that this will have an influence on sensory information processing as well as inhibitory systems [74].
In RLS patients, the right precuneus, right precentral gyrus, right precentral gyrus, and bilateral lingual gyri all had decreased connectivity with the thalamus; however, the right superior temporal gyrus, bilateral middle temporal gyrus, and right medial frontal gyrus all had improved connectivity with the thalamus [73].
When compared to non-treated (DN-RLS) mice, drug-treated (DT-RLS) animals had substantially larger thalamic connections for the left uvula, right tuber, left anterior insula, and right declive [72].
Chung (2017) [55] found that individuals with Parkinson’s disease who had sleep difficulties had a lower decrease in the severity of the loss of cortical functional connectivity in the default mode, central executive, and dorsal attention networks than those who did not.
Individuals with idiopathic RLS who were not taking any drugs exhibited lower functional connectivity strength (FCS) in the cuneus and fusiform gyrus, according to the results of a hub analysis. On the other hand, FCS increased in the superior frontal gyrus and thalamus. The networks responsible for visual processing and sensorimotor activity exhibited fewer functional connections, while the networks responsible for emotional cognition and the cerebellar-thalamic circuit had greater functional connections [75].
The ALFF is one approach for measuring the degree of regional spontaneous brain activity during an fMRI scan. This technique investigates low-frequency changes in blood oxygen levels that occur within a certain frequency band (0.01-0.08 Hz) [125].
ALFF values in the main motor cortex and premotor cortex of RBD patients were significantly lower than ALFF values in the main motor cortex and premotor cortex of PD patients who did not have RBD. Individuals with PD reported lower ALFF values in the putamen and caudate, but higher ALFF values in the prefrontal cortex [56].
Idiopathic RBD patients have much less gray matter in their brains than healthy controls. Although changes in ALFF values in the parahippocampal gyrus and occipital cortices may contribute to the disease’s underlying neurodegenerative process, increases in gray matter volume in the cerebellum, putamen, and thalamus may indicate a compensatory impact [58].
RLS patients showed lower ALFF in the sensorimotor and visual processing regions, while healthy controls had higher ALFF in the insula, parahippocampal and hippocampal gyri, left posterior parietal areas, and brainstem [76].
According to the results of a single study that compared PD-RBD patients to NC patients, individuals with PD-RBD had more nodal property anomalies across the neocortex and limbic system. When compared to PD-nRBD, PD-RBD dramatically improved nodal efficiency in the bilateral thalamus and betweenness centrality in the left insula [44].
Interpretations, limitations, and recommendations
Many movement disorders, including PD and RBD, have been shown to cause sleep disorders. Because sleep disorders reduce quality of life and cause complications such as short-term memory loss, increased blood pressure, and an increased risk of diabetes, it is critical to investigate movement disorders and the sleep disorders that result from them [96-99].
This review provides an in-depth analysis of MRI findings in movement disorders and associated sleep disturbances based on 61 studies. A variety of MRI scanners and sequences have been utilized to assess structural and functional changes in the brains of patients with PD, RBD, HD and RLS. The use of 3 Tesla scanners and advanced MRI techniques is recommended for higher accuracy and precision.
Cortical and subcortical alterations of white matter and gray matter were commonly reported [42,48,53,55,60,70,71,103]. Patients with Parkinson’s disease and restless legs syndrome showed reduced gray matter volume in deep GM structures like the thalamus, putamen and caudate. Males with PD exhibited higher subcortical atrophy and greater deep GM volume loss [100]. The frontal, parietal and temporal cortices also showed degenerative changes in PD. Greater thalamic atrophy was found in HD patients with sleep problems [101]. These findings indicate that movement disorders may lead to loss of brain volume in both cortical and subcortical regions, especially in areas involved in motor control and cognition.
Advanced MRI techniques were frequently used to detect microstructural changes undetectable by standard MRI. DTI revealed altered white matter integrity in major white matter tracts connecting the cortex, basal ganglia and cerebellum [29,61,65,68-70,73,74]. SWI detected reduced NM-sensitive volume and signal intensity in the brainstem and substantia nigra of iRBD patients [66]. MRS found higher Glx/Cr ratios in the brains of RLS patients, suggesting excessive glutamatergic neurotransmission [81].
fMRI studies reported altered spontaneous brain activity and connectivity patterns in movement disorders. RLS and PD patients showed changed ALFF and ReHo in the sensorimotor cortex, visual cortex, insula, caudate, putamen, thalamus and cerebellum [44,56,58,71,73-76]. Disrupted resting-state functional connectivity was found within and between the default mode network, central executive network, salience network and cerebellar-thalamic circuit [45,46,55,72-74]. These findings suggest that movement disorders may lead to aberrant intrinsic brain activity and connectivity in motor, cognitive and emotion related networks.
Despite the detailed analysis, this review has some limitations. First, the heterogeneity of imaging sequences and analytical methods used in different studies makes it difficult to compare results and draw definite conclusions. Second, most studies had small sample sizes, limiting the generalizability of findings. Finally, the cross-sectional study design of most research precludes determining causality.
Future studies should attempt to standardize imaging protocols and analytical techniques. Larger sample sizes and longitudinal study designs are needed to confirm findings. Task-based fMRI studies are required to provide more insights into the neuropathological mechanisms underlying movement disorders and associated sleep disturbances. Additional advanced MRI techniques such as arterial spin labeling may help detect perfusion changes. Multimodal neuroimaging integrating structural, functional and metabolic information could provide a more comprehensive understanding of these disorders.
In summary, this review provides an overview of MRI findings in major movement disorders and related sleep disturbances. Both structural and functional changes have been detected in cortical and subcortical regions involved in motor control, cognition, emotion and the sleep-wake cycle. A better understanding of the neural substrates underlying these disorders may help guide diagnosis and treatment.
Conclusion
According to the findings of this review, a significant number of individuals who have movement disorders also exhibit symptoms associated with sleep disturbances. Some brain regions’ dysfunction may provide a similar underlying mechanism for both motor and sleep disorders. Further research is required to better understand the etiology of sleep disruptions across different movement disorders, in order to permit more focused and successful therapy options, and to determine if treating poor sleep leads to demonstrable changes in motor outcomes. This is important. Neuroimaging is a valuable tool for assessing the structural and functional correlates of movement disorders and associated sleep disruptions, helping us to shed light on the sleep issues associated with movement disorders.
Disclosure of conflict of interest
None.
Authors’ contribution
S.Ghaderi and M.Mohammadi contributed to the conception and design of the study; A.Karami, A.Gh-Langeroudi, N.Abdi, S.Sh.Sh.Jalali, M.Rezaei, P.K-Moghadam, Sh.Banisharif, M.Jalali contributed to the data collection; M.Mohammadi, S.Ghaderi, S.Mohammadi, A.Karami, and S.Sh.Sh.Jalali contributed to drafting the text or preparing the figure.
Supporting Information
References
- 1.Fahn S. Classification of movement disorders. Mov Disord. 2011;26:947–957. doi: 10.1002/mds.23759. [DOI] [PubMed] [Google Scholar]
- 2.Shipton EA. Movement disorders and neuromodulation. Neurol Res Int. 2012;2012:309431. doi: 10.1155/2012/309431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlino G, Gigli GL. Sleep-related movement disorders. Neurol Sci. 2012;33:491–513. doi: 10.1007/s10072-011-0905-9. [DOI] [PubMed] [Google Scholar]
- 4.Pandey S. Classification of movement disorders: the problem of terminology. Neurol India. 2018;66:S12–S14. doi: 10.4103/0028-3886.226446. [DOI] [PubMed] [Google Scholar]
- 5.Stoessl AJ, McKeown MJ. Movement disorders. Handb Clin Neurol. 2016;136:957–969. doi: 10.1016/B978-0-444-53486-6.00049-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XJ, Xu ZY, Wu YC, Tan EK. Paroxysmal movement disorders: recent advances and proposal of a classification system. Parkinsonism Relat Disord. 2019;59:131–139. doi: 10.1016/j.parkreldis.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Silber MH. Sleep-related movement disorders. Continuum (Minneap Minn) 2013;19:170–184. doi: 10.1212/01.CON.0000427207.13553.68. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Miyamoto M, Miyamoto T, Hirata K. [Sleep related movement disorders] Nihon Rinsho. 2015;73:954–964. [PubMed] [Google Scholar]
- 9.Anderson KN, Bradley AJ. Sleep disturbance in mental health problems and neurodegenerative disease. Nat Sci Sleep. 2013;5:61–75. doi: 10.2147/NSS.S34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishak WW, Bagot K, Thomas S, Magakian N, Bedwani D, Larson D, Brownstein A, Zaky C. Quality of life in patients suffering from insomnia. Innov Clin Neurosci. 2012;9:13–26. [PMC free article] [PubMed] [Google Scholar]
- 11.Kołtuniuk A, Kazimierska-Zając M, Pogłódek D, Chojdak-Łukasiewicz J. Sleep disturbances, degree of disability and the quality of life in multiple sclerosis patients. Int J Environ Res Public Health. 2022;19:3271. doi: 10.3390/ijerph19063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson O, Uglik-Marucha N, Miskowiak KW, Cairney SA, Rosenzweig I, Young AH, Stokes PRA. The relationship between sleep disturbance and cognitive impairment in mood disorders: a systematic review. J Affect Disord. 2023;327:207–216. doi: 10.1016/j.jad.2023.01.114. [DOI] [PubMed] [Google Scholar]
- 13.Stavitsky K, Neargarder S, Bogdanova Y, McNamara P, Cronin-Golomb A. The impact of sleep quality on cognitive functioning in Parkinson’s disease. J Int Neuropsychol Soc. 2012;18:108–117. doi: 10.1017/S1355617711001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wennberg AMV, Wu MN, Rosenberg PB, Spira AP. Sleep disturbance, cognitive decline, and dementia: a review. Semin Neurol. 2017;37:395–406. doi: 10.1055/s-0037-1604351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover VP, Tognarelli JM, Crossey MM, Cox IJ, Taylor-Robinson SD, McPhail MJ. Magnetic resonance imaging: principles and techniques: lessons for clinicians. J Clin Exp Hepatol. 2015;5:246–255. doi: 10.1016/j.jceh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karatas OH, Toy E. Three-dimensional imaging techniques: a literature review. Eur J Dent. 2014;8:132–140. doi: 10.4103/1305-7456.126269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruno F, Arrigoni F, Mariani S, Splendiani A, Di Cesare E, Masciocchi C, Barile A. Advanced magnetic resonance imaging (MRI) of soft tissue tumors: techniques and applications. Radiol Med. 2019;124:243–252. doi: 10.1007/s11547-019-01035-7. [DOI] [PubMed] [Google Scholar]
- 18.Kalia V, Leung DG, Sneag DB, Del Grande F, Carrino JA. Advanced MRI techniques for muscle imaging. Semin Musculoskelet Radiol. 2017;21:459–469. doi: 10.1055/s-0037-1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaderi S, Olfati M, Ghaderi M, Hadizadeh H, Yazdanpanah G, Khodadadi Z, Karami A, Papi Z, Abdi N, Sharif Jalali SS, Khatyal R, Banisharif S, Bahari F, Zarasvandnia M, Mohammadi S, Mohammadi M. Neurological manifestation in COVID-19 disease with neuroimaging studies. Am J Neurodegener Dis. 2023;12:42–84. [PMC free article] [PubMed] [Google Scholar]
- 20.Mastrogiacomo S, Dou W, Jansen JA, Walboomers XF. Magnetic resonance imaging of Hard tissues and Hard tissue engineered bio-substitutes. Mol Imaging Biol. 2019;21:1003–1019. doi: 10.1007/s11307-019-01345-2. [DOI] [PubMed] [Google Scholar]
- 21.Desseilles M, Dang-Vu T, Schabus M, Sterpenich V, Maquet P, Schwartz S. Neuroimaging insights into the pathophysiology of sleep disorders. Sleep. 2008;31:777–794. doi: 10.1093/sleep/31.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gama RL, Távora DG, Bomfim RC, Silva CE, de Bruin VM, de Bruin PF. Sleep disturbances and brain MRI morphometry in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy - a comparative study. Parkinsonism Relat Disord. 2010;16:275–279. doi: 10.1016/j.parkreldis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Yousaf T, Pagano G, Wilson H, Politis M. Neuroimaging of sleep disturbances in movement disorders. Front Neurol. 2018;9:767. doi: 10.3389/fneur.2018.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippi M, Sarasso E, Agosta F. Resting-state functional MRI in Parkinsonian syndromes. Mov Disord Clin Pract. 2019;6:104–117. doi: 10.1002/mdc3.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper RM, Kumar R, Macey PM, Woo MA, Ogren JA. Affective brain areas and sleep-disordered breathing. Prog Brain Res. 2014;209:275–293. doi: 10.1016/B978-0-444-63274-6.00014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzaras R, Shi K, Artemiadis A, Zis P, Hadjigeorgiou G, Rominger A, Bassetti CLA, Bargiotas P. Brain neuroimaging of rapid eye movement sleep behavior disorder in Parkinson’s disease: a systematic review. J Parkinsons Dis. 2022;12:69–83. doi: 10.3233/JPD-212571. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz-Lopetegi A, Berenguer J, Iranzo A, Serradell M, Pujol T, Gaig C, Muñoz E, Tolosa E, Santamaría J. Magnetic resonance imaging abnormalities as a marker of multiple system atrophy in isolated rapid eye movement sleep behavior disorder. Sleep. 2021;44:zsaa089. doi: 10.1093/sleep/zsaa089. [DOI] [PubMed] [Google Scholar]
- 28.Rolinski M, Griffanti L, Piccini P, Roussakis AA, Szewczyk-Krolikowski K, Menke RA, Quinnell T, Zaiwalla Z, Klein JC, Mackay CE, Hu MT. Basal ganglia dysfunction in idiopathic REM sleep behaviour disorder parallels that in early Parkinson’s disease. Brain. 2016;139:2224–2234. doi: 10.1093/brain/aww124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unger MM, Belke M, Menzler K, Heverhagen JT, Keil B, Stiasny-Kolster K, Rosenow F, Diederich NJ, Mayer G, Möller JC, Oertel WH, Knake S. Diffusion tensor imaging in idiopathic REM sleep behavior disorder reveals microstructural changes in the brainstem, substantia nigra, olfactory region, and other brain regions. Sleep. 2010;33:767–773. doi: 10.1093/sleep/33.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postuma RB, Berg D. Advances in markers of prodromal Parkinson disease. Nat Rev Neurol. 2016;12:622–634. doi: 10.1038/nrneurol.2016.152. [DOI] [PubMed] [Google Scholar]
- 31.Eichenseer SR, Stebbins GT, Comella CL. Beyond a motor disorder: a prospective evaluation of sleep quality in cervical dystonia. Parkinsonism Relat Disord. 2014;20:405–408. doi: 10.1016/j.parkreldis.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Bailey GA, Hubbard EK, Fasano A, Tijssen MA, Lynch T, Anderson KN, Peall KJ. Sleep disturbance in movement disorders: insights, treatments and challenges. J Neurol Neurosurg Psychiatry. 2021;92:723–736. doi: 10.1136/jnnp-2020-325546. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, An H, Xi Q, Yang W, Xie H, Li Y, Huang D. Diffusion tensor imaging reveals deep brain structure changes in early Parkinson’s disease patients with various sleep disorders. Brain Sci. 2022;12:463. doi: 10.3390/brainsci12040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Błaszczyk JW. Parkinson’s disease and neurodegeneration: GABA-Collapse hypothesis. Front Neurosci. 2016;10:269. doi: 10.3389/fnins.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falup-Pecurariu C, Titova N, Ray Chaudhuri K. Editorial: movement disorders and sleep - underlying mechanisms, clinical aspects and treatment. Front Neurol. 2019;10:1034. doi: 10.3389/fneur.2019.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau D. Neuromuscular disease affecting the Larynx. Adv Otorhinolaryngol. 2020;85:144–157. doi: 10.1159/000456694. [DOI] [PubMed] [Google Scholar]
- 37.Park HR, Kim HR, Oh S, Seong JK, Joo EY. White matter tract-specific alterations in patients with primary restless legs syndrome. Sci Rep. 2021;11:16116. doi: 10.1038/s41598-021-95238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boucetta S, Salimi A, Dadar M, Jones BE, Collins DL, Dang-Vu TT. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson’s disease. Sci Rep. 2016;6:26782. doi: 10.1038/srep26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker MI, Person AL. Cerebellar control of reach Kinematics for endpoint precision. Neuron. 2019;103:335–348. e335. doi: 10.1016/j.neuron.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, Li N. A cortico-cerebellar loop for motor planning. Nature. 2018;563:113–116. doi: 10.1038/s41586-018-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Furst AJ. Brainstem diffusion tensor tractography and clinical applications in pain. Front Pain Res (Lausanne) 2022;3:840328. doi: 10.3389/fpain.2022.840328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Ye C, Sun J, Liang L, Lv H, Gao L, Fang J, Ma T, Wu T. Alteration of brain structural connectivity in progression of Parkinson’s disease: a connectome-wide network analysis. Neuroimage Clin. 2021;31:102715. doi: 10.1016/j.nicl.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ford AH, Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Burn DJ, O’Brien JT. Rapid eye movement sleep behavior disorder in Parkinson’s disease: magnetic resonance imaging study. Mov Disord. 2013;28:832–836. doi: 10.1002/mds.25367. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Zeng Q, Zhou W, Zhai X, Lai C, Zhu J, Dong S, Lin Z, Cheng G. Altered brain functional network in Parkinson disease with rapid eye movement sleep behavior disorder. Front Neurol. 2020;11:563624. doi: 10.3389/fneur.2020.563624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Hou X, Luo X, Zhou S, Liu X, Liu B, Chen J. Altered intra- and inter-regional functional connectivity of the Anterior cingulate gyrus in patients with tremor-dominant Parkinson’s disease complicated with sleep disorder. Front Aging Neurosci. 2019;11:319. doi: 10.3389/fnagi.2019.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Wu Z, Zhong M, Shen B, Zhu J, Pan Y, Yan J, Zhang W, Xu P, Xiao C, Zhang L. Abnormal gray matter volume and functional connectivity in Parkinson’s disease with rapid eye movement sleep behavior disorder. Parkinsons Dis. 2021;2021:8851027. doi: 10.1155/2021/8851027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamps S, van den Heuvel OA, van der Werf YD, Berendse HW, Weintraub D, Vriend C. Smaller subcortical volume in Parkinson patients with rapid eye movement sleep behavior disorder. Brain Imaging Behav. 2019;13:1352–1360. doi: 10.1007/s11682-018-9939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radziunas A, Deltuva VP, Tamasauskas A, Gleizniene R, Pranckeviciene A, Petrikonis K, Bunevicius A. Brain MRI morphometric analysis in Parkinson’s disease patients with sleep disturbances. BMC Neurol. 2018;18:88. doi: 10.1186/s12883-018-1092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salsone M, Cerasa A, Arabia G, Morelli M, Gambardella A, Mumoli L, Nisticò R, Vescio B, Quattrone A. Reduced thalamic volume in Parkinson disease with REM sleep behavior disorder: volumetric study. Parkinsonism Relat Disord. 2014;20:1004–1008. doi: 10.1016/j.parkreldis.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Lim JS, Shin SA, Lee JY, Nam H, Lee JY, Kim YK. Neural substrates of rapid eye movement sleep behavior disorder in Parkinson’s disease. Parkinsonism Relat Disord. 2016;23:31–36. doi: 10.1016/j.parkreldis.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 51.Kato S, Watanabe H, Senda J, Hirayama M, Ito M, Atsuta N, Kaga T, Katsuno M, Naganawa S, Sobue G. Widespread cortical and subcortical brain atrophy in Parkinson’s disease with excessive daytime sleepiness. J Neurol. 2012;259:318–326. doi: 10.1007/s00415-011-6187-6. [DOI] [PubMed] [Google Scholar]
- 52.Chondrogiorgi M, Tzarouchi LC, Zikou AK, Astrakas LG, Kosta P, Argyropoulou MI, Konitsiotis S. Multimodal imaging evaluation of excessive daytime sleepiness in Parkinson’s disease. Int J Neurosci. 2016;126:422–428. doi: 10.3109/00207454.2015.1023437. [DOI] [PubMed] [Google Scholar]
- 53.Compta Y, Valente T, Saura J, Segura B, Iranzo Á, Serradell M, Junqué C, Tolosa E, Valldeoriola F, Muñoz E, Santamaria J, Cámara A, Fernández M, Fortea J, Buongiorno M, Molinuevo JL, Bargalló N, Martí MJ. Correlates of cerebrospinal fluid levels of oligomeric- and total-α-synuclein in premotor, motor and dementia stages of Parkinson’s disease. J Neurol. 2015;262:294–306. doi: 10.1007/s00415-014-7560-z. [DOI] [PubMed] [Google Scholar]
- 54.Ansari M, Rahmani F, Dolatshahi M, Pooyan A, Aarabi MH. Brain pathway differences between Parkinson’s disease patients with and without REM sleep behavior disorder. Sleep Breath. 2017;21:155–161. doi: 10.1007/s11325-016-1435-8. [DOI] [PubMed] [Google Scholar]
- 55.Chung SJ, Choi YH, Kwon H, Park YH, Yun HJ, Yoo HS, Moon SH, Ye BS, Sohn YH, Lee JM, Lee PH. Sleep disturbance may alter white matter and resting state functional connectivities in Parkinson’s disease. Sleep. 2017;40 doi: 10.1093/sleep/zsx009. [DOI] [PubMed] [Google Scholar]
- 56.Li D, Huang P, Zang Y, Lou Y, Cen Z, Gu Q, Xuan M, Xie F, Ouyang Z, Wang B, Zhang M, Luo W. Abnormal baseline brain activity in Parkinson’s disease with and without REM sleep behavior disorder: a resting-state functional MRI study. J Magn Reson Imaging. 2017;46:697–703. doi: 10.1002/jmri.25571. [DOI] [PubMed] [Google Scholar]
- 57.Ghazi Sherbaf F, Rahmani F, Jooyandeh SM, Aarabi MH. Microstructural changes in patients with Parkinson disease and REM sleep behavior disorder: depressive symptoms versus non-depressed. Acta Neurol Belg. 2018;118:415–421. doi: 10.1007/s13760-018-0896-x. [DOI] [PubMed] [Google Scholar]
- 58.Chen M, Li Y, Chen J, Gao L, Sun J, Gu Z, Wu T, Chan P. Structural and functional brain alterations in patients with idiopathic rapid eye movement sleep behavior disorder. J Neuroradiol. 2022;49:66–72. doi: 10.1016/j.neurad.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Han YH, Cho JW, Lee JS, Lee SJ, Kim DJ, Kim TH, Kang BM, Kim TH, Mun CW. Evaluation of brain iron content in idiopathic REM sleep behavior disorder using quantitative magnetic resonance imaging. Parkinsonism Relat Disord. 2014;20:776–778. doi: 10.1016/j.parkreldis.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Li T, Liu C, Lyu H, Xu Z, Hu Q, Xu B, Wang Y, Xu J. Alterations of sub-cortical gray matter volume and their associations with disease duration in patients with restless legs syndrome. Front Neurol. 2018;9:1098. doi: 10.3389/fneur.2018.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stefani A, Mitterling T, Heidbreder A, Steiger R, Kremser C, Frauscher B, Gizewski ER, Poewe W, Högl B, Scherfler C. Multimodal magnetic resonance imaging reveals alterations of sensorimotor circuits in restless legs syndrome. Sleep. 2019;42:zsz171. doi: 10.1093/sleep/zsz171. [DOI] [PubMed] [Google Scholar]
- 62.Lee BY, Kim J, Connor JR, Podskalny GD, Ryu Y, Yang QX. Involvement of the central somatosensory system in restless legs syndrome: a neuroimaging study. Neurology. 2018;90:e1834–e1841. doi: 10.1212/WNL.0000000000005562. [DOI] [PubMed] [Google Scholar]
- 63.Hanyu H, Inoue Y, Sakurai H, Kanetaka H, Nakamura M, Miyamoto T, Sasai T, Iwamoto T. Voxel-based magnetic resonance imaging study of structural brain changes in patients with idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2012;18:136–139. doi: 10.1016/j.parkreldis.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 64.Park KM, Lee HJ, Lee BI, Kim SE. Alterations of the brain network in idiopathic rapid eye movement sleep behavior disorder: structural connectivity analysis. Sleep Breath. 2019;23:587–593. doi: 10.1007/s11325-018-1737-0. [DOI] [PubMed] [Google Scholar]
- 65.Scherfler C, Frauscher B, Schocke M, Iranzo A, Gschliesser V, Seppi K, Santamaria J, Tolosa E, Högl B, Poewe W. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann Neurol. 2011;69:400–407. doi: 10.1002/ana.22245. [DOI] [PubMed] [Google Scholar]
- 66.Pyatigorskaya N, Gaurav R, Arnaldi D, Leu-Semenescu S, Yahia-Cherif L, Valabregue R, Vidailhet M, Arnulf I, Lehéricy S. Magnetic resonance imaging biomarkers to assess substantia Nigra damage in idiopathic rapid eye movement sleep behavior disorder. Sleep. 2017;40 doi: 10.1093/sleep/zsx149. [DOI] [PubMed] [Google Scholar]
- 67.Han XH, Li XM, Tang WJ, Yu H, Wu P, Ge JJ, Wang J, Zuo CT, Shi KY. Assessing gray matter volume in patients with idiopathic rapid eye movement sleep behavior disorder. Neural Regen Res. 2019;14:868–875. doi: 10.4103/1673-5374.249235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Paiva JPQ, Magalhães SC, Moura LM, Sato JR, Amaro E Jr, Sterr A, Schlaffke L, Eckeli AL, do Prado GF, Conforto AB. Sensorimotor white matter projections and disease severity in primary restless legs syndrome/Willis-Ekbom disease: a multimodal DTI analysis. Sleep Med. 2020;73:106–116. doi: 10.1016/j.sleep.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 69.Chang Y, Paik JS, Lee HJ, Chang HW, Moon HJ, Allen RP, Earley CJ, Cho YW. Altered white matter integrity in primary restless legs syndrome patients: diffusion tensor imaging study. Neurol Res. 2014;36:769–774. doi: 10.1179/1743132814Y.0000000336. [DOI] [PubMed] [Google Scholar]
- 70.Belke M, Heverhagen JT, Keil B, Rosenow F, Oertel WH, Stiasny-Kolster K, Knake S, Menzler K. DTI and VBM reveal white matter changes without associated gray matter changes in patients with idiopathic restless legs syndrome. Brain Behav. 2015;5:e00327. doi: 10.1002/brb3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhuo Y, Wu Y, Xu Y, Lu L, Li T, Wang X, Li K. Combined resting state functional magnetic resonance imaging and diffusion tensor imaging study in patients with idiopathic restless legs syndrome. Sleep Med. 2017;38:96–103. doi: 10.1016/j.sleep.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 72.Lee YS, Ku J, Kim KT, Chang H, Earley CJ, Allen RP, Cho YW. Resting-state connectivity and the effects of treatment in restless legs syndrome. Sleep Med. 2020;67:33–38. doi: 10.1016/j.sleep.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Ku J, Cho YW, Lee YS, Moon HJ, Chang H, Earley CJ, Allen RP. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting-state connectivity study using functional magnetic resonance imaging. Sleep Med. 2014;15:289–294. doi: 10.1016/j.sleep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 74.Ku J, Lee YS, Kim KT, Chang H, Cho YW. Alterations in salience network functional connectivity in individuals with restless legs syndrome. Sci Rep. 2020;10:7643. doi: 10.1038/s41598-020-64641-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu C, Wang J, Hou Y, Qi Z, Wang L, Zhan S, Wang R, Wang Y. Mapping the changed hubs and corresponding functional connectivity in idiopathic restless legs syndrome. Sleep Med. 2018;45:132–139. doi: 10.1016/j.sleep.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 76.Liu C, Dai Z, Zhang R, Zhang M, Hou Y, Qi Z, Huang Z, Lin Y, Zhan S, He Y, Wang Y. Mapping intrinsic functional brain changes and repetitive transcranial magnetic stimulation neuromodulation in idiopathic restless legs syndrome: a resting-state functional magnetic resonance imaging study. Sleep Med. 2015;16:785–791. doi: 10.1016/j.sleep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 77.Astrakas LG, Konitsiotis S, Margariti P, Tsouli S, Tzarouhi L, Argyropoulou MI. T2 relaxometry and fMRI of the brain in late-onset restless legs syndrome. Neurology. 2008;71:911–916. doi: 10.1212/01.wnl.0000325914.50764.a2. [DOI] [PubMed] [Google Scholar]
- 78.Beliveau V, Stefani A, Birkl C, Kremser C, Gizewski ER, Högl B, Scherfler C. Revisiting brain iron deficiency in restless legs syndrome using magnetic resonance imaging. Neuroimage Clin. 2022;34:103024. doi: 10.1016/j.nicl.2022.103024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chawla S, Gulyani S, Allen RP, Earley CJ, Li X, Van Zijl P, Kapogiannis D. Extracellular vesicles reveal abnormalities in neuronal iron metabolism in restless legs syndrome. Sleep. 2019;42:zsz079. doi: 10.1093/sleep/zsz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moon HJ, Chang Y, Lee YS, Song HJ, Chang HW, Ku J, Cho YW. T2 relaxometry using 3.0-tesla magnetic resonance imaging of the brain in early- and late-onset restless legs syndrome. J Clin Neurol. 2014;10:197–202. doi: 10.3988/jcn.2014.10.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allen RP, Barker PB, Horská A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology. 2013;80:2028–2034. doi: 10.1212/WNL.0b013e318294b3f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X, Allen RP, Earley CJ, Liu H, Cruz TE, Edden RAE, Barker PB, van Zijl PCM. Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 2016;22:75–82. doi: 10.1016/j.sleep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong YQ, Tan LK, Seow P, Tan MP, Abd Kadir KA, Vijayananthan A, Ramli N. Microstructural integrity of white matter tracts amongst older fallers: a DTI study. PLoS One. 2017;12:e0179895. doi: 10.1371/journal.pone.0179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halefoglu AM, Yousem DM. Susceptibility weighted imaging: clinical applications and future directions. World J Radiol. 2018;10:30–45. doi: 10.4329/wjr.v10.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu C, Li W, Tong KA, Yeom KW, Kuzminski S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging. 2015;42:23–41. doi: 10.1002/jmri.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baliyan V, Das CJ, Sharma R, Gupta AK. Diffusion weighted imaging: technique and applications. World J Radiol. 2016;8:785–798. doi: 10.4329/wjr.v8.i9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fornasa F. Diffusion-weighted magnetic resonance imaging: what makes water run fast or slow? J Clin Imaging Sci. 2011;1:27. doi: 10.4103/2156-7514.81294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu C, Wei H, Gong NJ, Cronin M, Dibb R, Decker K. Quantitative susceptibility mapping: contrast mechanisms and clinical applications. Tomography. 2015;1:3–17. doi: 10.18383/j.tom.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ravanfar P, Loi SM, Syeda WT, Van Rheenen TE, Bush AI, Desmond P, Cropley VL, Lane DJR, Opazo CM, Moffat BA, Velakoulis D, Pantelis C. Systematic review: Quantitative Susceptibility Mapping (QSM) of brain iron profile in neurodegenerative diseases. Front Neurosci. 2021;15:618435. doi: 10.3389/fnins.2021.618435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: Interpreting positive susceptibility and the presence of iron. Magn Reson Med. 2015;74:564–570. doi: 10.1002/mrm.25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cecil KM. Proton magnetic resonance spectroscopy: technique for the neuroradiologist. Neuroimaging Clin N Am. 2013;23:381–392. doi: 10.1016/j.nic.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dhamala E, Abdelkefi I, Nguyen M, Hennessy TJ, Nadeau H, Near J. Validation of in vivo MRS measures of metabolite concentrations in the human brain. NMR Biomed. 2019;32:e4058. doi: 10.1002/nbm.4058. [DOI] [PubMed] [Google Scholar]
- 94.Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am. 2011;22:133–139. vii. doi: 10.1016/j.nec.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–349. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 97.Darchia N, Oniani N, Sakhelashvili I, Supatashvili M, Basishvili T, Eliozishvili M, Maisuradze L, Cervena K. Relationship between sleep disorders and health related quality of life-results from the Georgia SOMNUS study. Int J Environ Res Public Health. 2018;15:1588. doi: 10.3390/ijerph15081588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uchmanowicz I, Markiewicz K, Uchmanowicz B, Kołtuniuk A, Rosińczuk J. The relationship between sleep disturbances and quality of life in elderly patients with hypertension. Clin Interv Aging. 2019;14:155–165. doi: 10.2147/CIA.S188499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hashimoto Y, Sakai R, Ikeda K, Fukui M. Association between sleep disorder and quality of life in patients with type 2 diabetes: a cross-sectional study. BMC Endocr Disord. 2020;20:98. doi: 10.1186/s12902-020-00579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oltra J, Segura B, Uribe C, Monté-Rubio GC, Campabadal A, Inguanzo A, Pardo J, Marti MJ, Compta Y, Valldeoriola F, Iranzo A, Junque C. Sex differences in brain atrophy and cognitive impairment in Parkinson’s disease patients with and without probable rapid eye movement sleep behavior disorder. J Neurol. 2022;269:1591–1599. doi: 10.1007/s00415-021-10728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baker CR, Domínguez DJ, Stout JC, Gabery S, Churchyard A, Chua P, Egan GF, Petersén Å, Georgiou-Karistianis N, Poudel GR. Subjective sleep problems in Huntington’s disease: A pilot investigation of the relationship to brain structure, neurocognitive, and neuropsychiatric function. J Neurol Sci. 2016;364:148–153. doi: 10.1016/j.jns.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 102.Baima CB, Fim NC, Alves KF, Resende LAL, Fonseca RG, Betting LE. Analysis of patients with obstructive sleep apnea with and without pharyngeal myopathy using brain neuroimaging. Sleep. 2020;43:zsz216. doi: 10.1093/sleep/zsz216. [DOI] [PubMed] [Google Scholar]
- 103.Hermesdorf M, Sundermann B, Rawal R, Szentkirályi A, Dannlowski U, Berger K. Lack of association between shape and volume of subcortical brain structures and restless legs syndrome. Front Neurol. 2018;9:355. doi: 10.3389/fneur.2018.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Magalhães SC, Queiroz de Paiva JP, Kaelin-Lang A, Sterr A, Eckeli AL, Winkler AM, Fernandes do Prado G, Amaro E Jr, Conforto AB. Short-interval intracortical inhibition is decreased in restless legs syndrome across a range of severity. Sleep Med. 2019;62:34–42. doi: 10.1016/j.sleep.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 105.Rist PM, Tzourio C, Elbaz A, Soumaré A, Dufouil C, Mazoyer B, Kurth T. Structural brain lesions and restless legs syndrome: a cross-sectional population-based study. BMJ Open. 2014;4:e005938. doi: 10.1136/bmjopen-2014-005938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murray ME, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Liesinger AM, Senjem ML, Gunter JL, Preboske GM, Lowe VJ, Vemuri P, Dugger BN, Knopman DS, Smith GE, Parisi JE, Silber MH, Graff-Radford NR, Petersen RC, Jack CR Jr, Dickson DW, Kantarci K. MRI and pathology of REM sleep behavior disorder in dementia with Lewy bodies. Neurology. 2013;81:1681–1689. doi: 10.1212/01.wnl.0000435299.57153.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J, Shuai G, Fang W, Zhu Y, Chen H, Wang Y, Li Q, Han Y, Zou D, Cheng O. Altered regional homogeneity and connectivity in cerebellum and visual-motor relevant cortex in Parkinson’s disease with rapid eye movement sleep behavior disorder. Sleep Med. 2021;82:125–133. doi: 10.1016/j.sleep.2021.03.041. [DOI] [PubMed] [Google Scholar]
- 108.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kubicki M, Westin CF, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, Kikinis R, Jolesz FA, McCarley R, Shenton ME. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatry. 2002;10:324–336. doi: 10.1080/10673220216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lindemann K, Müller HP, Ludolph AC, Hornyak M, Kassubek J. Microstructure of the Midbrain and cervical spinal cord in idiopathic restless legs syndrome: a diffusion tensor imaging study. Sleep. 2016;39:423–428. doi: 10.5665/sleep.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Marzi R, Seppi K, Högl B, Müller C, Scherfler C, Stefani A, Iranzo A, Tolosa E, Santamarìa J, Gizewski E, Schocke M, Skalla E, Kremser C, Poewe W. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2016;79:1026–1030. doi: 10.1002/ana.24646. [DOI] [PubMed] [Google Scholar]
- 112.Hanoglu L, Ozer F, Meral H, Dincer A. Brainstem 1H-MR spectroscopy in patients with Parkinson’s disease with REM sleep behavior disorder and IPD patients without dream enactment behavior. Clin Neurol Neurosurg. 2006;108:129–134. doi: 10.1016/j.clineuro.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 113.Martin WR. MR spectroscopy in neurodegenerative disease. Mol Imaging Biol. 2007;9:196–203. doi: 10.1007/s11307-007-0087-2. [DOI] [PubMed] [Google Scholar]
- 114.Spiegelhalder K, Regen W, Nissen C, Feige B, Baglioni C, Riemann D, Hennig J, Lange T. Magnetic resonance spectroscopy in patients with Insomnia: a repeated measurement study. PLoS One. 2016;11:e0156771. doi: 10.1371/journal.pone.0156771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 116.Worsley KJ. An overview and some new developments in the statistical analysis of PET and fMRI data. Hum Brain Mapp. 1997;5:254–258. doi: 10.1002/(SICI)1097-0193(1997)5:4<254::AID-HBM9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 117.Linden DE, Prvulovic D, Formisano E, Völlinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- 118.Zhang S, Li X, Lv J, Jiang X, Guo L, Liu T. Characterizing and differentiating task-based and resting state fMRI signals via two-stage sparse representations. Brain Imaging Behav. 2016;10:21–32. doi: 10.1007/s11682-015-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Vos F, Koini M, Schouten TM, Seiler S, van der Grond J, Lechner A, Schmidt R, de Rooij M, Rombouts S. A comprehensive analysis of resting state fMRI measures to classify individual patients with Alzheimer’s disease. Neuroimage. 2018;167:62–72. doi: 10.1016/j.neuroimage.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 120.Gu L, Huang L, Yin F, Cheng Y. Classification of depressive disorder based on RS-fMRI using multivariate pattern analysis with multiple features. 2017 4th IAPR Asian Conference on Pattern Recognition (ACPR); 2017. pp. 61–66. [Google Scholar]
- 121.Zhang B, Liu S, Liu X, Chen S, Ke Y, Qi S, Wei X, Ming D. Discriminating subclinical depression from major depression using multi-scale brain functional features: a radiomics analysis. J Affect Disord. 2022;297:542–552. doi: 10.1016/j.jad.2021.10.122. [DOI] [PubMed] [Google Scholar]
- 122.Jiang L, Zuo XN. Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist. 2016;22:486–505. doi: 10.1177/1073858415595004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 124.Stevens WD, Spreng RN. Resting-state functional connectivity MRI reveals active processes central to cognition. Wiley Interdiscip Rev Cogn Sci. 2014;5:233–245. doi: 10.1002/wcs.1275. [DOI] [PubMed] [Google Scholar]
- 125.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.