Abstract
Background: Despite innovative advances in neonatal medicine, intraventricular hemorrhage (IVH) continues to be a significant complication in neonatal intensive care units globally.
Objective: The study aimed to discern the variables heightening the risk of severe IVH (Grade III and IV) in extremely premature infants weighing less than 750 grams. We postulated that a descending hematocrit (Hct) trend during the first week of life could serve as a predictive marker for the development of severe IVH in this vulnerable population.
Methods: This retrospective case-control study encompassed infants weighing less than 750 grams at birth, diagnosed with Grade III and/or IV IVH, and born in a tertiary center from 2009 to 2014. A group of 17 infants with severe IVH was compared with 14 gestational age-matched controls. Acid-base status, glucose, fluid goal, urine output, and nutrient (caloric and protein) intake during the first four days of life were meticulously evaluated. Statistically significant variables from baseline data were further analyzed via univariable and multivariable logistic regression analyses, ensuring control for potential confounding variables.
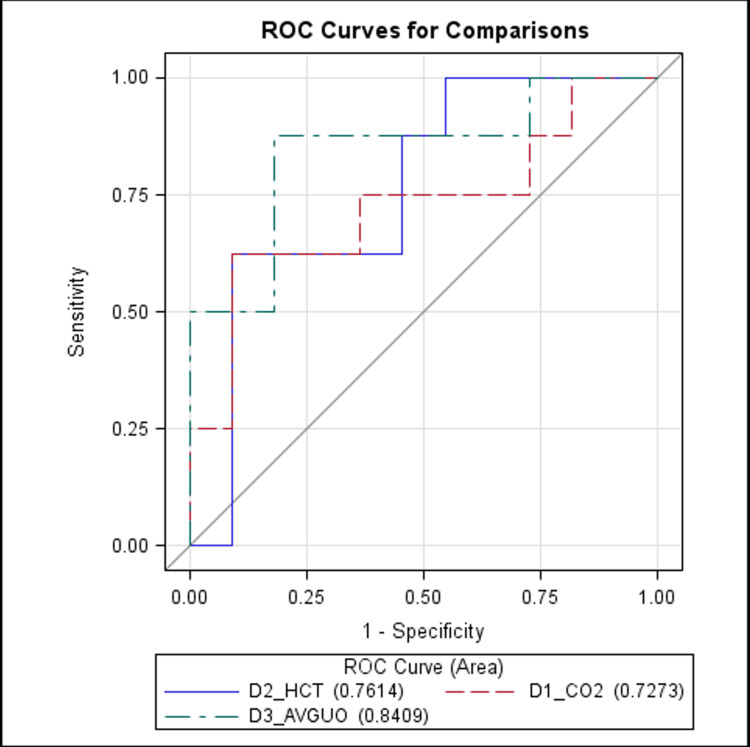
Results: The univariate logistic regression model delineated odds ratios (ORs) of 0.842 for day 2 average Hct (confidence interval [CI], 0.718-0.987) and 0.16 for urine output on day 3 (CI, 0.024-1.056), with the remaining six variables demonstrating no significant association. In the post-multivariable regression analysis, day 2 Hct was the only significant variable (OR, 0.731; 95% CI, 0.537-0.995; P=0.04). The receiver operating characteristic (ROC) curve analysis portrayed an area under the curve of 71% for the day 2 Hct variable.
Conclusion: The study revealed that a dip in Hct on day 2 of life augments the likelihood of Grade III and IV IVH among extremely premature infants with a birth weight of less than 750 grams. This insight amplifies our understanding of risk factors associated with severe IVH development in extremely preterm infants, potentially aiding in refining preventive strategies and optimizing clinical management and treatment of these affected infants.
Keywords: birth weight below 750 grams, neonates, retrospective study, gestational age, birth weight, hematocrit, intraventricular hemorrhage (ivh), extremely preterm infants
Introduction
Intraventricular hemorrhage (IVH), a prevalent complication linked with preterm birth, is of substantial concern due to its potential to engender profound neurodevelopmental deficits and permanent disabilities, such as deafness and blindness, in preterm infants [1-5]. It is estimated that approximately 20-25% of all preterm infants born before 28 weeks of gestational age suffer from IVH [2,6]. The occurrence of IVH is notably higher in infants with decreased birth weight or gestational age [7]. The late 20th century observed marked reductions in IVH incidence, owing to enhancements in obstetric, prenatal, and neonatal care [3,5,8]. However, parallel advancements have also improved survival rates in extremely low birth weight (ELBW) (<800 g) and very low gestational age (<26 weeks) infants, inadvertently escalating the risk of severe IVH in newborn infants [6,9,10].
IVH typically originates in the periventricular germinal matrix of preterm infants, a region rich in glial and neuronal precursor cells in the developing brain, within the first 48 hours of life [1,6]. The pathogenesis of IVH is multifactorial and complex, with numerous environmental factors possibly contributing to its development [1,6]. Specific predisposing factors include the innate fragility of germinal matrix vasculature, fluctuations in cerebral blood flow (CBF), and anomalies in platelet function and coagulation [1]. It has been observed that the severity of IVH is directly proportional to the risk of associated complications and long-term neonatal outcomes, with as many as 90% of infants with Grade IV IVH at risk [11]. The influence of shared biological pathways and risk factors on severe IVH is yet to be definitively excluded. Certain interventions like antenatal steroid therapy and cesarean birth have demonstrated efficacy in mitigating the risk of severe IVH [12-14]. Conversely, conditions like hypotension and hypercapnia could augment the risk of IVH by influencing CBF [15-17]. Similarly, the practice of delayed cord clamping which can increase blood volume is postulated to diminish IVH incidence [18]. Despite these findings, our understanding of the risk factors underpinning IVH and its clinical management remains limited.
Hematocrit (Hct), defined as the fraction of blood volume constituted by red blood cells, is often considered a surrogate marker for blood volume. Concordantly, several studies have probed into the direct relationship between Hct and IVH incidence [17,19-21]. A substantial recent study including ELBW infants (92 with IVH, 153 without IVH) highlighted that lower Hct (<45%) in the initial days of life (DOL) could potentially amplify the risk of IVH more than twofold (OR = 2.38, 95% CI = 1.19-4.76) [20]. The study further identified both C-section and gestational age as independent predictors of IVH. However, investigations into the impact of Hct in the initial DOL on the incidence of severe IVH (Grade III/IV) remain scant. The present study seeks to identify clinical variables that heighten the risk of severe IVH in preterm neonates. Our hypothesis posits that a decreased Hct during the first week of life augments the risk of severe IVH (Grade III/IV) in extremely premature infants (<750 g).
Materials and methods
Study design and participants
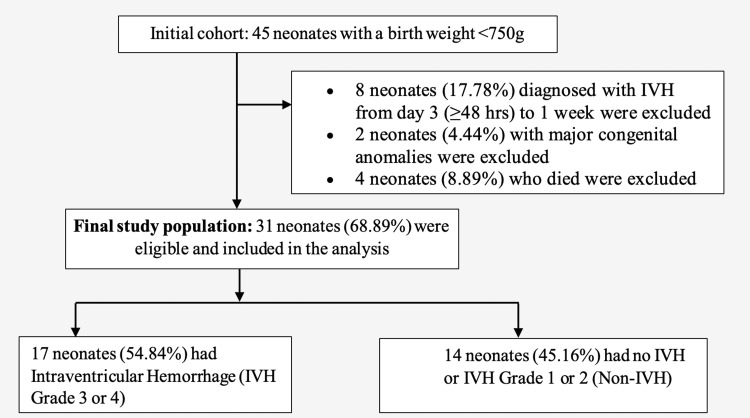
This investigation adopted a case-control study design, targeting very low birth weight (VLBW) infants diagnosed with Grade III and/or Grade IV IVH between 2009 and 2014 at a tertiary center. The study's subjects were juxtaposed against gestational age-matched control infants. Inclusion criteria for the infants were a birth weight of less than 750 grams and a diagnosis of Grade III and/or Grade IV IVH within the first week of life (Figure 1). Diagnosis and grading of IVH were performed by a certified pediatric radiologist through cranial sonography. The Papile classification was employed for grading IVH, where Grade III refers to more than 50% occupancy of lateral ventricle volume coupled with ventricular dilatation, and Grade IV denotes the existence of infarction and/or hemorrhage in the periventricular white matter [22].
Figure 1. Flowchart of study population selection.
Data collection
Baseline maternal data including age, ethnicity, prenatal care, antenatal steroid, antibiotic and magnesium sulfate use, mode of delivery, premature rupture of membranes (PROM), and parity were collated. Neonatal data comprising gestational age, gender, birth weight, admission temperature, and Apgar scores at one and five minutes post-delivery were also collected. Rigorous monitoring of the newborn infants facilitated the documentation of data pertaining to acid-base status, glucose levels, fluid goal, urine output, calorie intake, and protein intake during the first four days of hospitalization. The focus on data from the first four DOL stems from the average day of diagnosis for Grade III IVH in our sample being 3.6 DOL. We included infants who were diagnosed with IVH within the first 48 hours of life (equivalent to DOL 3 to DOL 4). Thus, this timeframe allowed us to evaluate how variable differences within this period may influence or predict the grade of IVH (the range of days when head ultrasound was performed extended from day 1 to day 9).
Inclusion and exclusion criteria
The inclusion criteria for the study were categorized into three distinct domains. Primarily, infants with a birth weight of up to 750 grams were considered eligible. Secondly, the timing of diagnosis was crucial; only those diagnosed with IVH within the first 48 hours of life were included. Finally, the study encompassed infants who survived beyond the immediate newborn period.
Conversely, certain conditions warranted participant exclusion. Infants diagnosed with IVH from day 3, or more than 48 hours after birth, through to the first week of life were excluded. Additionally, infants exceeding a birth weight of 750 grams were deemed ineligible. Neonates identified with any congenital anomalies were excluded from the study, and any infants not surviving the immediate newborn period were also omitted from the research.
Statistical analysis
The analytical plan comprised a comprehensive comparative assessment of clinical variables among case and control cohorts. Categorical data were delineated as proportions, while the distribution of continuous variables was appraised using the Shapiro-Wilk test. We presented parametric data as means and standard deviations (SDs), whereas non-parametric data were expressed as medians and interquartile ranges (IQRs).
The chi-square test or Fisher's exact test was utilized for the analysis of categorical variables, while the Student's t-test was applied for parametric variables. Non-parametric variables were examined using the Mann-Whitney U test or the Kruskal-Wallis test. Confidence intervals were set at a 95% significance level, and a p-value threshold of 0.05 was adopted to establish statistical significance.
For binary outcome variables, we computed odds ratios (ORs) to express effect estimates. Variables displaying significant correlation with severe IVH in the univariable analysis were advanced to the multivariable analysis phase. A binary logistic regression model was employed to assess the association between the binary outcome variable and the primary independent variable while adjusting for the effects of potential confounders.
Given that the diagnosis of IVH was ascertained on day 3, our logistic regression model included only those clinical variables from day 2 that demonstrated statistical significance. This approach facilitated the evaluation of the predictive capabilities of the independent variables based on measurements acquired prior to IVH diagnosis.
Subsequently, the Mantel-Haenszel (MH) method was implemented to detect variations in ORs across distinct subgroups of potential confounders. In the event of detecting effect modification, strata-specific ORs were additionally reported. All statistical analyses were carried out using the SAS 9.4 software (SAS Institute, Inc., Cary, North Carolina).
Results
Participant profile and baseline characteristics
Throughout the five-year study duration, 17 infants diagnosed with severe IVH and 14 gestational age-matched control infants met the inclusion criteria. Upon evaluating the baseline demographics and clinical characteristics of both mothers and their infants, no statistically significant disparities emerged between the IVH and non-IVH infant groups (Table 1). However, several trends were discernible.
Table 1. Baseline demographics and clinical characteristics of mother and infant population.
*Birth weight <750 g. †Data were not available for all patients.
ANS: antenatal steroids; BW: birth weight; C-section: cesarean section; DOL: days of life; IVH: intraventricular hemorrhage; Mg: magnesium; GA: gestational age; PNC: postnatal care; PROM: prolonged rupture of membranes
| Characteristics | IVH (Grade III or IV); N=17 | Non-IVH; N=14 | p-value |
| Maternal age (years), mean (SD) | 25.12 (5.42) | 28.71 (7.79) | 0.140 |
| Ethnicity†, n (%) | |||
| - White | 0 (0.00) | 2 (14.29) | 0.500 |
| - Black | 10 (76.92) | 8 (57.14) | - |
| - Hispanic | 3 (23.08) | 4 (28.57) | - |
| Sex of infant, n (%) | |||
| - Female | 7 (41.18) | 9 (64.29) | 0.200 |
| PNC†, n (%) | |||
| - Yes | 12 (80.0) | 11 (78.57) | 1.000 |
| - No | 3 (20.0) | 3 (21.43) | - |
| ANS, n (%) | |||
| - Yes | 10 (58.82) | 12 (85.71) | 0.132 |
| - No | 7 (41.18) | 2 (14.29) | - |
| Number of doses, n (%) | |||
| - 0 | 7 (41.18) | 2 (16.67) | 0.288 |
| - 1 | 5 (29.41) | 3 (25.00) | - |
| - 2 | 5 (29.41) | 7 (58.33 ) | - |
| Chorioamnionitis, n (%) | |||
| - Yes | 3 (17.65) | 2 (14.29) | 1.000 |
| - No | 14 (82.35) | 12 (85.71) | - |
| Antibiotic treatment, n (%) | |||
| - Yes | 10 (66.67) | 8 (57.14) | 0.597 |
| - No | 5 (33.33) | 6 (42.86) | - |
| Mg treatment, n (%) | |||
| - Yes | 4 (28.57) | 7 (53.85) | 0.182 |
| - No | 10 (71.43) | 6 (46.15) | - |
| Outborn, n (%) | |||
| - Yes | 10 (58.82) | 3 (21.43) | 0.036 |
| - No | 7 (41.18) | 11 (78.57) | - |
| PROM duration (hours), median (range) | 0 (0-240) | 0 (0 – 360) | 0.574 |
| C-section, n (%) | |||
| - Yes | 9 (52.94) | 10 (71.43) | 0.293 |
| - No | 8 (47.06) | 4 (28.57) | - |
| Multiple births, n (%) | |||
| - Yes | 6 (35.29) | 2 (14.29) | 0.240 |
| - No | 11 (64.71) | 12 (85.71) | - |
| BW (g), mean (SD) | 588.9 (94.99) | 610.40 (76.83) | 0.500 |
| GA (weeks), mean (SD) | 24.06 (1.44) | 24.36 (0.93) | 0.720 |
| Apgar 1-minute, n (%) | |||
| - Poor | 12 (80.0) | 10 (71.43) | 0.680 |
| - Good | 3 (20.0) | 4 (28.57) | - |
| Apgar 5-minute, n (%) | |||
| - Poor | 8 (53.33) | 6 (42.86) | 0.570 |
| - Good | 7 (46.67) | 8 (57.14) | - |
| Admission temperature (°C), median (range) | 36.60 (33.00-37.20) | 36.60 (35.80-37.00) | 0.790 |
| Surfactant use | |||
| - Yes | 60 (99.77) | 29 (100.00) | 1.00 |
| - No | 2 (3.23) | 0 (0.00) | - |
| DOL at the start of treatment, mean (SD) | 4 (1-52) | 5 (2-44) | 0.40 |
Mothers delivering IVH infants exhibited a slightly lower mean age compared to those giving birth to non-IVH infants (25.1 vs 28.7 years) and were predominantly of Black ethnicity (76.9% vs 57.1%). It is noteworthy that the vast majority of mothers across both cohorts had received postnatal care (PNC). Regarding therapeutic measures, a substantial proportion of mothers giving birth to IVH infants had been administered magnesium and antenatal steroids. Intriguingly, a higher fraction of non-IVH infants were delivered via cesarean section.
In terms of neonatal characteristics, female infants constituted a majority within the non-IVH group (64.3% vs 41.2%). Infants diagnosed with IVH were slightly underweight and exhibited a marginally reduced average gestational age compared to non-IVH infants. Additionally, IVH infants demonstrated compromised Apgar scores at one and five minutes post-delivery.
Follow-up characteristics of infants
The univariate analysis of follow-up characteristics unveiled significant associations between distinct variables at specific stages (Table 2). On day 1, IVH infants demonstrated a significantly elevated mean PCO2 (partial pressure of carbon dioxide) level (45.15±12.00 vs. 37.93±5.06, P=0.03). On the second day, a higher calorie intake was observed among IVH infants. Notably, Hct levels on day 2 were significantly lower in IVH infants compared to non-IVH infants (35.42±5.08 vs. 40.82±6.74, P=0.02). This was succeeded by a notable decline in urine output among IVH infants on day 3 (3.45±1.18 vs. 4.83±0.83, P=0.01). On the final day of the observational period, the distribution of average base excess revealed a marked difference between the two groups (-11.31±3.47 vs. -6.44±4.00, P=0.02).
Table 2. Follow-up clinical characteristics of the infant population.
IVH: intraventricular hemorrhage; PCO2: partial pressure of carbon dioxide in blood; SD: standard deviation; Hct: hematocrit
| Clinical variable | IVH (Grade III or IV); N =17 | No IVH; N = 14 | p-value |
| Day 1 | |||
| PCO2, mean (SD) | 45.15 (12.00) | 37.93 (5.06) | 0.03 |
| Hct, mean (SD) | 37.26 (7.87) | 40.03 (9.25) | 0.375 |
| Calorie intake, mean (SD) | 2.86 (11.28) | 28.19 (6.71) | 0.222 |
| Protein intake, median (range) | 2.20 (1.40-3.20) | 2.40 (1.70-4.40) | 0.281 |
| Serum glucose, mean (SD) | 115.57 (44.16) | 92.19 (33.83) | 0.115 |
| Base excess, median (range) | -7.00 (-19.33--2.00) | -7.45 (-10.00--2.00) | 1.000 |
| Urine output, mean (SD) | 3.73 (1.77) | 2.98 (1.50) | 0.292 |
| Day 2 | |||
| PCO2, mean (SD) | 41.65 (10.07) | 39.97 (12.26) | 0.678 |
| Hct, mean (SD) | 35.42 (5.08) | 40.82 (6.74) | 0.02 |
| Calorie intake, mean (SD) | 44.32 (17.47) | 32.23 (7.20) | 0.03 |
| Protein intake, mean (SD) | 2.25 (0.68) | 2.51 (0.62) | 0.323 |
| Serum glucose, median (Range) | 123.50 (78.00-315.75) | 119.90 (44.33-193.00) | 0.311 |
| Base excess, median (Range) | -7.00 (-16.00--4.00) | -7.45 (-10.80--5.00) | 0.781 |
| Urine output, mean (SD) | 3.76 (1.64) | 3.47 (0.95) | 0.609 |
| Day 3 | |||
| PCO2, mean (SD) | 57.11 (17.56) | 55.28 (9.91) | 0.756 |
| Hct, median (Range) | 37.05 (28.33-602.83) | 38.40 (30.80-52.00) | 0.286 |
| Calorie intake, mean (SD) | 55.31 (14.89) | 40.95 (17.28) | 0.09 |
| Protein intake, mean (SD) | 2.09 (1.21) | 3.21 (1.36) | 0.08 |
| Serum glucose, median (Range) | 146.25 (81.63-195.33) | 108.20 (48.00-288.75) | 0.237 |
| Base excess, mean (SD) | -9.54 (4.67) | -7.74 (2.22) | 0.248 |
| Urine output, mean (SD) | 3.45 (1.18) | 4.83 (0.83) | 0.01 |
| Day 4 | |||
| PCO2, mean (SD) | 58.95 (5.46) | 41.49 (27.25) | 0.094 |
| Hct, median (range) | 34.50 (28.00-38.67) | 40.25 (0.00-50.50) | 0.340 |
| Calorie intake, mean (SD) | 45.58 (15.65) | 39.90 (23.04) | 0.670 |
| Protein intake, mean (SD) | 2.11 (1.04) | 2.98 (1.76) | 0.392 |
| Serum glucose, mean (SD) | 147.20 (54.92) | 90.46 (56.56) | 0.06 |
| Base excess, mean (SD) | -11.31 (3.47) | -6.44 (4.00) | 0.02 |
| Urine output, mean (SD) | 3.88 (1.39) | 3.38 (1.91) | 0.592 |
Multivariable predictors of severe IVH
Only significant baseline and follow-up variables were considered for the multivariable binary regression association analysis, employed for predicting severe IVH (Table 3). Following the multivariate regression analysis, day 2 average Hct emerged as the only significant variable (Table 3). Specifically, for every one-unit increase in Hct level on day 2, the risk of severe IVH decreased by 27% (OR=0.731, 95% CI = 0.537-0.995). The receiver operating characteristic (ROC) curve analysis of day 2 average Hct levels further corroborated these findings, presenting an area under the curve of 76.4% (Figure 2).
Table 3. Prediction of IVH using multivariable logistic regression analysis .
OR: odd's ratio; CI: confidence interval; Ref: benchmark condition for comparison; Hct: hematocrit
Models lacking an OR value are marked with a dash ("-")
| Clinical variable | Model 1 OR (95% CI) | Model 1 p-value | Model 2 OR (95% CI) | Model 2 p-value | Model 3 OR (95% CI) | Model 3 p-value | Model 4 OR (95% CI) | Model 4 p-value |
| JSH/outborn (Ref: Yes) | 1.007 (0.127-7.989) | 0.9950 | 0.695 (0.069-7.027) | 0.7581 | 1.101 (0.124-9.761) | 0.9313 | 0.911 (0.067-12.378) | 0.9442 |
| Day 2 Hct | 0.781 (0.607-1.005) | 0.0548 | 0.782 (0.607-1.006) | 0.0558 | 0.731 (0.542-0.986) | 0.0401 | 0.704 (0.514-0.963) | 0.0283 |
| Day 2 calorie intake | 1.100 (0.990-1.223) | 0.0748 | 1.098 (0.982-1.226) | 0.0996 | 1.098 (0.986-1.223) | 0.0892 | 1.091 (0.962-1.236) | 0.1737 |
| Birth weight (Ref: ≥550 g) | - | - | 5.732 (0.535-61.420) | 0.1491 | - | - | 9.540 (0.686-132.776) | 0.0932 |
| Gestational age (Ref: ≥25 weeks) | - | - | - | - | 0.243 (0.010-5.676) | 0.3792 | 0.092 (0.002-3.812) | 0.2092 |
Figure 2. ROC curve analysis for day 2 average Hct, day 1 average PCO2, and day 3 urine output.
D1: day 1; D2: day 2; D3: day 3; Hct: hematocrit; PCO2: partial pressure of carbon dioxide; ROC: receiver operating characteristic; UO: urine output
Discussion
Principal findings
Our retrospective study explores the relationship between Hct and the occurrence of severe IVH (Grade III and Grade IV). This was done with the purpose of filling the current shortage of information on this clinical variable as a predictor of IVH. Current literature points to an abundance of information detailing the connection between clinical indicators PCO2 and calorie intake as predictors of IVH. Hct, however, is little explored and found through our work to be a more directly connected indicator of potential IVH risk.
The present understanding of the role of PCO2 as a predictor stems from findings regarding elevated PCO2 levels being significantly associated with IVH group preterm and low/ELBW infants in comparison to the control groups [23-25]. Vasodilation as a result of this hypercapnia leads to an increase in CBF that predisposes fragile blood vessels in the germinal matrix to rupturing [26]. In premature infants, autoregulatory mechanisms are underdeveloped and cannot compensate for these fluctuations in CBF-increasing the risk of IVH [27]. Further studies concluded that a PCO2>45 mmHg is also associated with loss of cerebral autoregulation and with the potential to induce IVH [28] while hypercarbia is associated with stronger PCO2 cerebral vasoreactivity in preterm infants [29]. Moving forward, a randomized trial conducted on ELBW infants concluded that poor neurodevelopmental outcomes related to elevated PCO2 are due to IVH (p<0.01) and birthweight (p<0.001) [25]. In our study, day 2 PCO2 was found to be significantly different among study and control populations from hypothesis testing. However, we did not find any association after univariate and multivariate analysis. Despite this, it trended toward significance which is consistent with previous work.
Results in the context of what is known
Multiple scientific studies have shown the importance of caloric intake within proper neurodevelopment. Caloric deprivation has been found to be a significant risk factor [30] as work has shown that infants with slower head growth often have lower caloric intake levels [31]. Continually, a positive association has been highlighted between nutrition and brain volume with nutritional intake having the largest statistically significant impact on brain volume [32]. In our study, cases and control groups had caloric intake within normal ranges, but controls had higher caloric intake levels compared to case group infants. Univariate and multivariate analysis did not show any significant association between IVH and caloric intake, unlike literature data.
With regards to Hct, our study shows after multivariate analysis that a day 2 Hct level of 35.42 is useful as a predictor for IVH in ELBW babies. In previous literature, low Hct is demonstrated to be a useful predictor for moderate-severe grade IVH [2,33,34]. The majority of these studies, however, were conducted on <1500 g and <1000 g babies while very little work was conducted on <750 g babies. In contrast to our study, none of this work discussed the accuracy (sensitivity and specificity) of using Hct as a test to predict IVH much before head ultrasound can predict it to point toward the potential earlier implementation of neuroprotective measures. As a result, our work looks to highlight the benefits of using Hct with regards to its application in screening for IVH in ELBW infants (<750 g) and toward earlier screening of IVH for preterm infants in general before day 3 as per current protocol.
Advantages of Hct
Hct provides several advantages as a diagnostic tool for IVH in comparison to the current gold standard-cranial ultrasounds. In ELBW babies, IVH occurs much earlier in comparison to VLBW babies. In a study comparing IVH timing between 500-700 g birth weight babies and 700-1500 g birth weight babies, 62% of cases occurred in the first 18 hours of life in the 500-700 gram birth weight babies group in comparison to 13% in the >700 g birth weight babies group [35]. Although the protocol of performing a head ultrasound on day 3 and day 7 can pick up the maximum number of IVH cases in >700 g birth weight babies, it can delay the diagnosis of IVH in <700 g birth weight babies.
From the standpoint of VLBW infants, it has been found that in <1500 gram birth weight babies, approximately 48% of IVH cases occurred in the first six hours after birth. This occurrence of early-stage IVH has pointed to this initial six-hour window as being valuable in diagnosing and preventing further progression of IVH [36], pointing to the use of Hct as a screening tool in this window much earlier than the current protocol of a day 3 cranial ultrasound. Further work has shown that in VLBW infants, low-grade (I and II) IVH is not detected with high sensitivity via cranial ultrasound. This bolsters the argument for the use of Hct in another scenario as a possible diagnostic tool for early-stage IVH [37].
Backed up by further literature showing that the cranial ultrasound has zero sensitivity in picking up Grade II IVH (no ventricular dilation) [38], progression toward Grade III and/or IV IVH is a concern in both ELBW and VLBW babies if proper neuroprotective measures are not taken. We found that a day 2 Hct of 35.42 has an 82.35% sensitivity and 14.29% specificity to detect IVH. Therefore, employing Hct as a predictor will not only enable us to detect IVH earlier but also to implement neuroprotective measures that prevent the further progression of IVH. This will aid in improving long-term neurodevelopmental outcomes. Some neuroprotective measures that can be implemented are a midline head position with head elevation alongside a low noise environment [23,39].
Comparison with earlier studies
There is a range of current literature that explores various other clinical risk factors and, if applicable, their sensitivity and specificity as a predictor of IVH. A study showed factors such as small for gestational age, lower platelet crit levels (PCT), and lower Apgar scores at 5 minutes were all significantly associated with occurrences of IVH in comparison to non-IVH group infants. Upon further multivariate analysis, it was found that low PCT levels were the most predictive indicator of IVH. This was simply one variable in their larger model analyzing the role of platelet-related factors in morbidity and mortality of preterm infants toward common diseases; thus, sensitivity and specificity were not calculated [40]. In general, however, our results agreed with these findings in part as we were able to observe an association between lower Apgar scores at 5 minutes for IVH group infants prior to univariate analysis.
Additionally, studies regarding plasma activin A have also suggested the presence of lesser-utilized clinical indicators of IVH. With regards to the detection of early-stage (I and II) IVH in preterm infants, a cutoff level of 0.8 μg/L activin A presented with a 100% specificity and sensitivity rate. This can point to neuroprotective measures being a beneficial intervention when concentration levels of plasma activin A in the infant's blood above that cutoff level are reached [41]. With respect to work done analyzing the role of Hct as a predictor, there are several studies detailing the high specificity and sensitivity of Hct as a predictor for other diseases such as ulcerative colitis [42], while others look into the sensitivities and specificities of mathematical models involving Hct as one of several variables [43]. As such, our work presents an opportunity to build upon Hct as an established predictor of disease which has shown use in being associated with a diagnosis of IVH prior as a group of variables.
Biological reasoning behind the association of Hct
The intricate relationship between Hct, blood flow, and viscosity is key to understanding the genesis of IVH, especially in ELBW infants. Hct is known to affect blood viscosity, which, in turn, influences CBF [44]. The immature germinal matrix, which is most abundant between 24 and 34 weeks of gestation, is susceptible to fluctuations in CBF. Our study identified that a day 2 Hct level of 35.42 is a significant predictor of IVH. A decrease in Hct, and thereby oxygen content, in the blood, necessitates an increase in CBF to maintain oxygen delivery [45]. This delicate equilibrium can be disturbed by changes in Hct levels, accelerating blood flow and increasing the risk of IVH, as suggested by Linder et al. and Mouqdad et al. [17,46]. Furthermore, this may lead to cerebral hypoperfusion, ischemia, and eventually, reperfusion-induced IVH [20]. Additionally, electrolyte levels in the blood, particularly serum sodium, can serve as another surrogate marker of blood flow and are implicated in the pathogenesis of IVH.
Prediction of IVH using multiple predictors
Anticipating IVH involves considering multiple predictors. Elevated PCO2 levels, indicative of hypercapnia, can predispose infants to IVH by causing vasodilation and a consequential increase in CBF [23-25]. Premature infants, lacking mature autoregulatory mechanisms, are particularly vulnerable to these fluctuations. Furthermore, outborn infants are found to be at a higher risk for IVH than their inborn counterparts. Antenatal steroid therapy, low 5-minute Apgar scores, and inadequate protein intake are also associated with heightened IVH risk. The administration of steroids to pregnant women serves to prevent RDS by inducing fetal lung maturity, potentially stabilizing CBF, and fortifying vascular structures in the germinal matrix [47,48]. However, surfactant treatment, while mitigating RDS, is another risk factor for IVH. Birth weight and serum sodium levels have also shown a significant correlation with IVH incidence. Hypernatremia-induced cerebral shrinkage, owing to increased osmolarity, can lead to vascular fragility and an elevated risk of IVH. Considering these variables in a multivariate model, accounting for potential confounding factors, can enhance the accuracy of IVH prediction [49,50].
Strengths and limitations
This case-control study offers novel insights into the association between Hct levels and severe IVH risk in extremely premature infants. The robust design, which matches cases and controls by gestational age, augments our understanding of IVH's potential mechanisms, highlighting Hct's potential as an early predictive biomarker. The rigorous use of both univariate and multivariate regression analyses also strengthens our findings.
However, inherent limitations of a retrospective case-control study, such as potential selection bias due to the single-center sample, restrict the generalizability of the findings. Additionally, the small sample size may limit statistical power and increase the chance of type II errors. Despite controlling for several confounding variables, unmeasured confounders may present residual confounding, necessitating these findings be confirmed in future prospective studies.
Future directions
To extend this research, larger multi-center studies are necessary for enhanced generalizability. These studies could elucidate further risk factors and underlying biological mechanisms. The integration of artificial intelligence (AI) could refine IVH prediction capabilities, using Hct and other variables to provide personalized risk scores. However, the application of AI tools necessitates rigorous validation and ethical considerations.
Moreover, investigating Hct-maintenance interventions could inform preventive strategies to reduce severe IVH incidence, thus improving clinical outcomes for extremely premature infants. Future research should, therefore, aim to explore, develop, and evaluate such interventions. While our study advances the understanding of severe IVH, further research is crucial to fully realize and leverage Hct's potential as a predictive marker.
Conclusions
In conclusion, our study demonstrates the significance of decreasing Hct on the second day of life as a strong predictor for the occurrence of severe IVH (Grade III or IV) in extremely premature infants with a birth weight of less than 750 grams. The findings contribute to the growing body of knowledge surrounding the risk factors for IVH, potentially guiding advancements in prevention strategies and patient management. Future research should focus on validating these results in larger multicenter studies and developing advanced predictive models using machine learning to provide real-time, individualized risk assessments. Ultimately, such studies could lead to improved clinical outcomes for this vulnerable patient population.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Intraventricular hemorrhage in premature infants: mechanism of disease. Ballabh P. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elevated interleukin-6 concentration and alterations of the coagulation system are associated with the development of intraventricular hemorrhage in extremely preterm infants. Poralla C, Hertfelder HJ, Oldenburg J, Müller A, Bartmann P, Heep A. Neonatology. 2012;102:270–275. doi: 10.1159/000341266. [DOI] [PubMed] [Google Scholar]
- 3.The Vermont-Oxford Trials Network: very low birth weight outcomes for 1990. Investigators of the Vermont-Oxford Trials Network Database Project. https://pubmed.ncbi.nlm.nih.gov/8441556/ Pediatrics. 1993;91:540–545. [PubMed] [Google Scholar]
- 4.Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF. Neonatology. 2010;97:329–338. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Vohr BR, Wright LL, Dusick AM, et al. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 6.The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. McCrea HJ, Ment LR. Clin Perinatol. 2008;35:777-92, vii. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 8.Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Philip AG, Allan WC, Tito AM, Wheeler LR. https://pubmed.ncbi.nlm.nih.gov/2677959/ Pediatrics. 1989;84:797–801. [PubMed] [Google Scholar]
- 9.Outcomes of children of extremely low birthweight and gestational age in the 1990's. Hack M, Fanaroff AA. Early Hum Dev. 1999;53:193–218. doi: 10.1016/s0378-3782(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 10.Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. Jain NJ, Kruse LK, Demissie K, Khandelwal M. J Matern Fetal Neonatal Med. 2009;22:491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 11.Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Volpe JJ. Semin Pediatr Neurol. 1998;5:135–151. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 12.Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Mukerji A, Shah V, Shah PS. Pediatrics. 2015;136:1132–1143. doi: 10.1542/peds.2015-0944. [DOI] [PubMed] [Google Scholar]
- 13.Relationship between antenatal steroid administration and grades III and IV intracranial hemorrhage in low birth weight infants. Shankaran S, Bauer CR, Bain RP, et al. Am J Obstet Gynecol. 1995;173:305–312. doi: 10.1016/0002-9378(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 14.Method of delivery and intraventricular haemorrhage in extremely preterm infants. Dani C, Poggi C, Bertini G, Pratesi S, Di Tommaso M, Scarselli G, Rubaltelli FF. J Matern Fetal Neonatal Med. 2010;23:1419–1423. doi: 10.3109/14767051003678218. [DOI] [PubMed] [Google Scholar]
- 15.Both extremes of arterial carbon dioxide pressure and the magnitude of fluctuations in arterial carbon dioxide pressure are associated with severe intraventricular hemorrhage in preterm infants. Fabres J, Carlo WA, Phillips V, Howard G, Ambalavanan N. Pediatrics. 2007;119:299–305. doi: 10.1542/peds.2006-2434. [DOI] [PubMed] [Google Scholar]
- 16.Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. Kaiser JR, Gauss CH, Pont MM, Williams DK. J Perinatol. 2006;26:279–285. doi: 10.1038/sj.jp.7211492. [DOI] [PubMed] [Google Scholar]
- 17.Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Linder N, Haskin O, Levit O, et al. Pediatrics. 2003;111:0–5. doi: 10.1542/peds.111.5.e590. [DOI] [PubMed] [Google Scholar]
- 18.Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Rabe H, Gyte GM, Díaz-Rossello JL, Duley L. Cochrane Database Syst Rev. 2019;9:0. doi: 10.1002/14651858.CD003248.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The relationship between hematocrit and bleeding time in very low birth weight infants during the first week of life. Sola MC, del Vecchio A, Edwards TJ, Suttner D, Hutson AD, Christensen RD. J Perinatol. 2001;21:368–371. doi: 10.1038/sj.jp.7210546. [DOI] [PubMed] [Google Scholar]
- 20.Initial hematocrit values after birth and peri/intraventricular hemorrhage in extremely low birth weight infants. Dekom S, Vachhani A, Patel K, Barton L, Ramanathan R, Noori S. J Perinatol. 2018;38:1471–1475. doi: 10.1038/s41372-018-0224-6. [DOI] [PubMed] [Google Scholar]
- 21.Haemoglobin level at birth is associated with short term outcomes and mortality in preterm infants. Banerjee J, Asamoah FK, Singhvi D, Kwan AW, Morris JK, Aladangady N. BMC Med. 2015;13:16. doi: 10.1186/s12916-014-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. Papile LA, Burstein J, Burstein R, Koffler H. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 23.Elevated midline head positioning of extremely low birth weight infants: effects on cardiopulmonary function and the incidence of periventricular-intraventricular hemorrhage. Kochan M, Leonardi B, Firestine A, et al. J Perinatol. 2019;39:54–62. doi: 10.1038/s41372-018-0261-1. [DOI] [PubMed] [Google Scholar]
- 24.Perinatal factors and periventricular-intraventricular hemorrhage in preterm infants. Van de Bor M, Van Bel F, Lineman R, Ruys JH. Am J Dis Child. 1986;140:1125–1130. doi: 10.1001/archpedi.1986.02140250051035. [DOI] [PubMed] [Google Scholar]
- 25.Influence of PCO2 control on clinical and neurodevelopmental outcomes of extremely low birth weight infants. Thome UH, Dreyhaupt J, Genzel-Boroviczeny O, et al. Neonatology. 2018;113:221–230. doi: 10.1159/000485828. [DOI] [PubMed] [Google Scholar]
- 26.Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Harrison AM, Lynch JM, Dean JM, Witte MK. Crit Care Med. 1997;25:1904–1908. doi: 10.1097/00003246-199711000-00032. [DOI] [PubMed] [Google Scholar]
- 27.Assessing key clinical parameters before and after intraventricular hemorrhage in very preterm infants. Lampe R, Rieger-Fackeldey E, Sidorenko I, et al. Eur J Pediatr. 2020;179:929–937. doi: 10.1007/s00431-020-03585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Kaiser JR, Gauss CH, Williams DK. Pediatr Res. 2005;58:931–935. doi: 10.1203/01.pdr.0000182180.80645.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Factors affecting cerebrovascular reactivity to CO2 in premature infants. Aly S, El-Dib M, Lu Z, El Tatawy S, Mohamed M, Aly H. J Perinat Med. 2019;47:979–985. doi: 10.1515/jpm-2019-0031. [DOI] [PubMed] [Google Scholar]
- 30.Early caloric deprivation in preterm infants affects Bayley-III scales performance at 18-24 months of corrected age. Lithoxopoulou M, Rallis D, Christou H, et al. Res Dev Disabil. 2019;91:103429. doi: 10.1016/j.ridd.2019.103429. [DOI] [PubMed] [Google Scholar]
- 31.The relationships of transcephalic impedance, head growth and caloric intake to outcome in preterm infants. Ellison P, Heimler R, Franklin S. Acta Paediatr Scand. 1984;73:820–827. doi: 10.1111/j.1651-2227.1984.tb17782.x. [DOI] [PubMed] [Google Scholar]
- 32.Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Coviello C, Keunen K, Kersbergen KJ, et al. Pediatr Res. 2018;83:102–110. doi: 10.1038/pr.2017.227. [DOI] [PubMed] [Google Scholar]
- 33.Frequent handling in the neonatal intensive care unit and intraventricular hemorrhage. Bada HS, Korones SB, Perry EH, et al. J Pediatr. 1990;117:126–131. doi: 10.1016/s0022-3476(05)72460-4. [DOI] [PubMed] [Google Scholar]
- 34.[Hematocrit values in newborns with intraventricular hemorrhage, periventricular hemorrhage and periventricular leukomalacia] Kornacka M, Krüger-Andrzejewska R. https://pubmed.ncbi.nlm.nih.gov/7721151/ Ginekol Pol. 1994;65:435–444. [PubMed] [Google Scholar]
- 35.Intraventricular hemorrhage in extremely small premature infants. Perlman JM, Volpe JJ. Am J Dis Child. 1986;140:1122–1124. doi: 10.1001/archpedi.1986.02140250048034. [DOI] [PubMed] [Google Scholar]
- 36.A systematic review and meta-analysis of the timing of early intraventricular hemorrhage in preterm neonates: clinical and research implications. Al-Abdi SY, Al-Aamri MA. J Clin Neonatol. 2014;3:76–88. doi: 10.4103/2249-4847.134674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Low-grade intraventricular hemorrhage: is ultrasound good enough? Parodi A, Morana G, Severino MS, et al. J Matern Fetal Neonatal Med. 2015;28 Suppl 1:2261–2264. doi: 10.3109/14767058.2013.796162. [DOI] [PubMed] [Google Scholar]
- 38.Accuracy of head ultrasound for the detection of intracranial hemorrhage in preterm neonates: comparison with brain MRI and susceptibility-weighted imaging. Intrapiromkul J, Northington F, Huisman TA, Izbudak I, Meoded A, Tekes A. J Neuroradiol. 2013;40:81–88. doi: 10.1016/j.neurad.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neutral head positioning in premature infants for intraventricular hemorrhage prevention: an evidence-based review. Malusky S, Donze A. Neonatal Netw. 2011;30:381–396. doi: 10.1891/0730-0832.30.6.381. [DOI] [PubMed] [Google Scholar]
- 40.Using platelet parameters to anticipate morbidity and mortality among preterm neonates: a retrospective study. Go H, Ohto H, Nollet KE, et al. Front Pediatr. 2020;8:90. doi: 10.3389/fped.2020.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Increased plasma concentrations of activin a predict intraventricular hemorrhage in preterm newborns. Florio P, Perrone S, Luisi S, et al. Clin Chem. 2006;52:1516–1521. doi: 10.1373/clinchem.2005.065979. [DOI] [PubMed] [Google Scholar]
- 42.[Sensitivity, specificity, and predictive values of the level of hemoglobin, hematocrit and platelet count as an activity index in ulcerative colitis] Ibarra-Rodríguez JJ, Santiago-Luna E, Velázquez-Ramírez GA, López-Ramírez MK, Fuentes-Orozco C, Cortés-Flores AO, González-Ojeda A. https://pubmed.ncbi.nlm.nih.gov/16336799/ Cir Cir. 2005;73:355–362. [PubMed] [Google Scholar]
- 43.Machine learning models for identifying preterm infants at risk of cerebral hemorrhage. Turova V, Sidorenko I, Eckardt L, Rieger-Fackeldey E, Felderhoff-Müser U, Alves-Pinto A, Lampe R. PLoS One. 2020;15:0. doi: 10.1371/journal.pone.0227419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerebral autoregulation. Paulson OB, Strandgaard S, Edvinsson L. https://pubmed.ncbi.nlm.nih.gov/2201348/ Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 45.The effect of hematocrit and systolic blood pressure on cerebral blood flow in newborn infants. Younkin DP, Reivich M, Jaggi JL, Obrist WD, Delivoria-Papadopoulos M. J Cereb Blood Flow Metab. 1987;7:295–299. doi: 10.1038/jcbfm.1987.66. [DOI] [PubMed] [Google Scholar]
- 46.Risk factors for intraventricular hemorrhage in premature infants in the central region of Saudi Arabia. Al-Mouqdad MM, Abdelrahim A, Abdalgader AT, Alyaseen N, Khalil TM, Taha MY, Asfour SS. Int J Pediatr Adolesc Med. 2021;8:76–81. doi: 10.1016/j.ijpam.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Effects of glucocorticoids on fetal and neonatal lung development. Grier DG, Halliday HL. Treat Respir Med. 2004;3:295–306. doi: 10.2165/00151829-200403050-00004. [DOI] [PubMed] [Google Scholar]
- 48.The effects of antenatal corticosteroid treatment on IVH-PVh of premature infants. Maksić H, Hadzagić-Catibusić F, Heljić S, Dizdarević J. Bosn J Basic Med Sci. 2008;8:58–62. doi: 10.17305/bjbms.2008.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hypernatremia. Adrogué HJ, Madias NE. N Engl J Med. 2000;342:1493–1499. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 50.Regulation of cell volume in health and disease. McManus ML, Churchwell KB, Strange K. N Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]