Abstract
Background:
Jansen de Vries Syndrome (JdVS) is a rare neurodevelopmental disorder (NDD) caused by gain-of-function (GOF) truncating mutations in PPM1D exons 5 or 6. PPM1D is a serine/threonine phosphatase that plays an important role in the DNA damage response (DDR) by negatively regulating TP53 (P53). JdVS-associated mutations lead to the formation of a truncated PPM1D protein that retains catalytic activity and has a GOF effect because of reduced degradation. Somatic PPM1D exons 5 and 6 truncating mutations are well-established factors in a number of cancers, due to excessive dephosphorylation and reduced function of P53 and other substrates involved in DDR. Children with JdVS have a variety of neurodevelopmental, psychiatric, and physical problems. In addition, a small fraction has acute neuropsychiatric decompensation apparently triggered by infection or severe non-infectious environmental stress factors.
Methods:
To understand the molecular basis of JdVS, we developed an induced pluripotent stem cell (iPSC) model system. iPSCs heterozygous for the truncating variant (PPM1D+/tr), were made from a patient, and control lines engineered using CRISPR-Cas9 gene editing. Proteomics and phosphoprotemics analyses were carried out on iPSC-derived glutamatergic neurons and microglia from three control and three PPM1D+/tr iPSC lines. We also analyzed the effect of the TLR4 agonist, lipopolysaccharide, to understand how activation of the innate immune system in microglia could account for acute behavioral decompensation.
Results:
One of the major findings was the downregulation of POGZ in unstimulated microglia. Since loss-of-function variants in the POGZ gene are well-known causes of autism spectrum disorder, the decrease in PPM1D+/tr microglia suggests this plays a role in the neurodevelopmental aspects of JdVS. In addition, neurons, baseline, and LPS-stimulated microglia show marked alterations in the expression of several E3 ubiquitin ligases, most notably UBR4, and regulators of innate immunity, chromatin structure, ErbB signaling, and splicing. In addition, pathway analysis points to overlap with neurodegenerative disorders.
Limitations:
Owing to the cost and labor-intensive nature of iPSC research, the sample size was small.
Conclusions:
Our findings provide insight into the molecular basis of JdVS and can be extrapolated to understand neuropsychiatric decompensation that occurs in subgroups of patients with ASD and other NDDs.
Keywords: pediatric acute-onset neuropsychiatric syndrome (PANS), autism, regression, POGZ, ubiquitin ligase, UBR4, SRRM1, NUCKS1, PPM1D, Jansen de Vries Syndrome
Introduction
Jansen de Vries Syndrome (JdVS) (OMIM 617450) is a recently discovered neurodevelopmental disorder (NDD) caused by truncating mutations in PPM1D exons 5 or 6 (1-4). It is characterized by mild to severe intellectual disability, anxiety disorder, attention deficit hyperactivity disorder (ADHD), obsessive behavior, hypotonia, sensory integration problems, and in some cases, autism spectrum disorder (ASD). In addition, feeding difficulties and gastrointestinal problems (e.g., constipation, esophageal reflux, and cyclic vomiting syndrome) are common. Approximately half of the reported cases have an increase in childhood infections, although this has not been systematically evaluated and the pathogenesis has not been established. PPM1D codes for a member of the PP2C serine/threonine phosphatase family. So far, every PPM1D mutation found in JdVS is predicted to translate into a truncated protein (e.g., nonsense mutations and frameshifts) because of the loss of C-terminal amino acids. The catalytic domain encoded largely by exons 1-4 is preserved, and an increase in PPM1D half-life occurs because truncated proteins lose a degradation signal that maps within the terminal 65 amino acids (5,6).
PPM1D is a well-known tumor suppressor gene, acting as a negative regulator of P53 and other proteins involved in the DNA damage response (DDR) pathway, such as MDM2, ATM, CHK1, CHK2, ATR, and H2AX (7-9). Somatic GOF truncating mutations in exons 5 or 6 have been found in a variety of cancers (7-14). Cancer risk in JdVS has not yet been established, although a normal P53 response to ionizing radiation was found in EB-transformed lymphocytes derived from children with JdVS (4).
In addition to the neurodevelopmental and psychiatric features of JdVS, a small subgroup of patients experience behavioral decompensation that appears to be linked to infection or severe physical stress. One patient we identified was diagnosed with pediatric acute-onset neuropsychiatric syndrome (PANS) as a child, several years prior to exome sequencing for NDD revealed a typical PPM1D truncating variant (15). PANS is an enigmatic, neuroinflammatory disorder characterized by the abrupt onset of severe neurological and psychiatric symptoms that includes obsessive-compulsive disorder (OCD), restricted eating, anxiety, cognitive deficits with academic regression, disrupted sleep, rage, mood disturbance, joint inflammation, and autonomic nervous system disturbances (e.g., enuresis, postural orthostatic tachycardia syndrome) (16-18). Subsequently, we identified two other JdVS case in which severe behavioral and motor regression occurred following infection and noninfectious triggers.
Mouse Ppm1d knockout (KO) models have been developed, which show effects on dendritic spine morphology and memory processes, a disturbance in T- and B-lymphocyte differentiation, proliferation, cytokine production, and an increase in phagocytosis and autophagy in peripheral macrophages (19-22). However, a mouse Ppm1d KO is not an appropriate model for JdVS GOF variants. Consequently, in order to understand the underlying molecular basis of truncated PPM1D on neuronal function and the apparent propensity a subgroup of JdVS patients has for acute neuropsychiatric decompensation, we developed an induced pluripotent stem cell (iPSC) model and analyzed glutamatergic neurons and microglia by proteomics and phosphoproteomics.
Methods
Subjects
The JdVS patient is a male who was born full-term following an uncomplicated pregnancy who was diagnosed as a child following whole exome sequencing (WES), which revealed a typical PPM1D truncating mutation in exon 5 (c.1210C>T; p.Q404X). All PPM1D heterozygotes, whether patient-derived or developed using CRISPR-Cas9 editing (see below), will be referred to as PPM1D+/tr. Analysis of parental DNA showed that the mutation was de novo, as is the case for >90% of JdVS cases. His typically developing brother was used as one of the controls. Another typically developing control male was used to develop isogenic iPSC PPM1D+/tr lines in which a truncating mutation was introduced in exon 5 using CRISPR-Cas9 gene editing (see Additional file 1: Expanded Methods for details). A third male was used as a typically developing control. These last two controls were characterized in another study (23).
Development of iPSCs from peripheral blood CD34+ cells
All methods used in this study are described briefly here; details can be found in Additional file 1: Expanded Methods. iPSC lines were generated from human peripheral blood CD34+ hematopoietic stem cells (HSC) with a CytoTune-iPS 2.0 Sendai Reprogramming Kit (Invitrogen) following the manufacturer’s protocol, as previously described (24). All lines were capable of differentiating into the three germ layers and showed no expression of reprogramming transcription factors. Cytogenetic analysis was negative.
CRISPR-Cas9 gene editing
A heterozygous truncating variant in PPM1D exon 5 was generated by CRISPR-Cas9 gene editing, using a protocol described by Ran et al (25). Briefly, a guide RNA (gRNA) sequence coding for a region in exon 5 adjacent to a PAM sequence 9 base pairs from the patient mutation was chosen (Figure 1: Additional file 1: Expanded Methods).
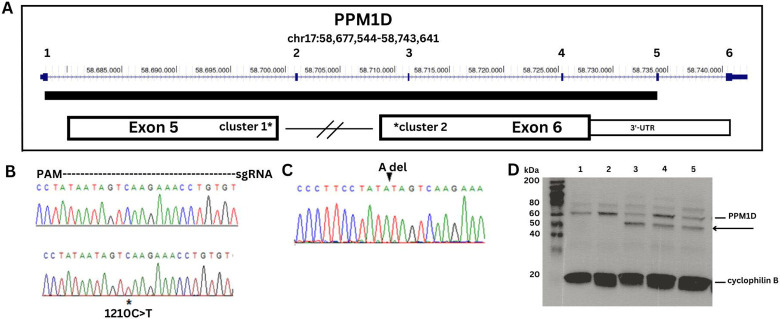
Figure 1. DNA sequence analysis and Western blot of PPM1D truncating variants.
A. Map of PPM1D showing the 6 exons with the catalytic domain depicted as a solid bar. The two clusters of JdVS mutations are in the 3'-end of exon 5 and the 5'-end of exon 6. B. DNA sequence strip of wild type allele on top and the patient sample showing the c.1210C>T; p.Q404X nonsense mutation on bottom. The region covered by the guide RNA used for CRISPR-Cas 9 engineering is shown. C. Two isogenic lines with an "A" deletion were generated with CRISPR. D. PPM1D Western blot showing wild-type protein and the truncated protein. Cyclophilin is a loading control. Lanes 1 and 2 are control samples, lane 3 is the patient sample, and lanes 4 and 5 are two CRISPR lines.
Differentiation of iPSCs into glutamatergic neurons
iPSCs were maintained as previously described (23). Glutamatergic neuronal differentiation was induced using a protocol developed by Zhang et al., in which differentiation is driven by overexpression of the transcription factor NGN2 (26). A tet-inducible expression system was introduced and lentivirus particles prepared from the plasmid vectors; pLV_TRET_hNgn2_UBC_Puro (plasmid #61474) and FUdeltaGW-rtTA (plasmid #19780), followed by treatment with doxycycline and selection with puromycin (see Additional file 1: Expanded Methods for details). The protocol routinely leads to the production of a nearly pure culture of excitatory cortical glutamatergic neurons.
Neurite outgrowth
Neurite outgrowth was assessed blind to genotype using the NeuronJ plugin (27). Glutamatergic neurons were stained with Map2 and Tuj1 antibodies and imaged at 10x resolution. Images of patient and control neurons were converted to 8-bit grayscale, and individual dendrites were traced and labeled using the semiautomatic manual tracing tool. Approximately 10 images with an average of 12 neurons per field were analyzed per sample. The “measure tracings” function was used to determine mean length (in pixels) of the dendritic branches.
Differentiation of iPSCs into microglia
To generate microglia, we used kits from STEMCELL™ Technologies (STEMdiff™ Hematopoietic Kit, catalog number 05310; STEMdiff™ Microglia Differentiation Kit, catalog number 100-0019; STEMdiff™ Microglia Maturation Kit, catalog number 100-0020) according to the manufacturer’s instructions, with minor modifications as described in Additional file 1: Expanded Methods. iPSCs are first differentiated into HSCs, followed by terminal differentiation into microglia. The microglia grow in suspension with the control and PPM1D+/tr showing a similar morphology. (Additional file 2: Fig. S1).
Fluorescence-activated cell sorting (FACS)
Single-cell suspensions were used for flow cytometry staining. We followed a protocol for Staining Cell Surface Targets for Flow Cytometry from ThermoFisher. All antibodies were obtained from Stemcell Technologies, except for TMEM119, which is from Novus Biologicals. For HSCs, we used CD45 FITC (Catalog number 60018FI.1) CD43 APC (Catalog number 60085AZ.1), and CD34 PE (Cat. 60013PE.1) antibodies. For microglia we used TMEM119 APC (Catalog FAB10313A) and CD11b PE (Catalog 60040PE.1) antibodies. Antibody concentrations were 5ul per 100ul for all Ab except TMEM119 for which 0.5ul per 100ul was used. Flow cytometry acquisition was obtained using a BD LSRII analyzer, and BD FlowJo software was used for data analysis.
Cytokine array
Microglia were seeded at 5 x 105 cells/well in a 12 well, Matrigel-coated plate in STEMdiff Microglia Maturation media 24 days post differentiation. After 5 days of maturation, cells were stimulated with 100ng/ml LPS (O111:B4 strain; Sigma catalog # L4391) for 24 hours at 37°C. Supernatants were collected and analyzed using the Proteome Profiler Array Human Cytokine Array (R&D Systems catalog # ARY005B) following the manufacturer’s instructions. Arrays were analyzed using Quick Spots Image Analysis Software. Each cytokine and chemokine on the array is measured in duplicate.
Western Blotting
Proteins were prepared with Pierce™ RIPA Buffer (Thermoscientific catalog # 89900) according to the manufacturer’s protocol, with a protease inhibitor cocktail mix (Sigma catalog # P8340). Protein concentrations were verified using the BCA assay. Western Blotting was essentially carried out as previously described, with modifications, as described in Additional file 1: Expanded Methods. Phosphorylation of CaMKII (CaMK2) was analyzed by comparing the phosphorylated and unphosphorylated proteins, which are also described in more detail in the expanded methods section.
Proteomics and phosphoproteomics
Proteomics and phosphoproteomics, and subsequent bioinformatics analyses were performed as previously described (28-35) (see Additional file 1: Expanded Methods for details)
Results
Development of iPSCs
A patient-specific line was developed from a male with JdVS who had a de novo nonsense mutation at codon 404 in exon 5 (c.1210C>T; p.Q404X) (Figure 1). The same mutation was found in one of the subjects in the original JdVS paper (individual 4) {{6152 Jansen,S. 2017}}. His typically developing brother was used as a control. We also used CRISPR-Cas9 gene editing on another control line to create truncating mutations in exon 5 near the patient’s variant. Two clones with an “A” deletion 5 bp from the patient mutation were obtained. The deletion causes a frameshift and premature termination after 6 additional amino acids are inserted (c.1209delA; N402Ifs*6). This is still within the boundaries of the most proximal truncating mutation described by Jansen et al., at cDNA position 1188 (4). Both the patient sample and the CRISPR-engineered lines show the truncated protein on a Western blot (Figure 1).
Proteomics: glutamatergic neurons
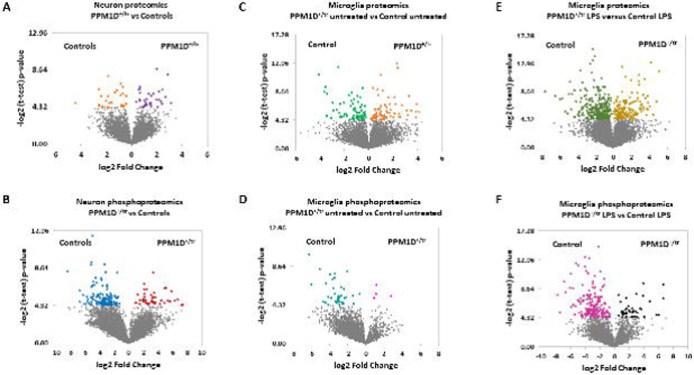
Proteomics and phosphoproteomics were carried out on glutamatergic neurons (day 21] differentiated from iPSCs. A total of four control and five PPM1D+/tr samples were analyzed. 4,948 proteins were detected among which 35 were significantly upregulated and 26 that were downregulated in the PPM1D+/tr neurons (p<0.05, corresponds to a p-value of 4.32, see Additional file 3: Table S1). The volcano plots for this analysis, as well as the subsequent proteomics and phosphoproteomics data described below are shown in Figure 2.
Figure 2. Volcano plots of differentially expressed proteins and phosphoproteins for all analyses.
−log2 value of 4.32 corresponds to p=0.05, which is the cutoff.
Gene Ontology (GO) analysis was carried out to characterize the pathways and processes affected by differentially expressed proteins (DEPs). The top GO pathway was, surprisingly, positive regulation of T cell differentiation (Table 1; Additional file 3: Table S1). This is probably due to expression of regulatory factors influenced by PPM1D that are expressed in both neurons and T-cells, an idea supported by the finding that DEPs contributing to the T cell differentiation GO term in neurons; CBFB, PNP, AP3D1, ANXA1, AP3B1, SART1, BAD, STAT5B, and ZMIZ1, are also expressed in peripheral blood mononuclear cell (PBMC) types and microglia (36). In addition, Ppm1d has been found to regulate Th9 cell development and T-cell differentiation in mice (37,38). Thus, the common regulation of these proteins in neurons and immune cells could be coincidental. The other top GO terms are related to the apparently novel effect of PPM1D on processing H/ACA snoRNAs, a class of small nucleolar RNAs (snoRNAs) that regulate ribosome biogenesis and alternative splicing (39).
Table 1. Gene Ontology (GO) analysis, neurons.
All differentially expressed proteins and phosphoproteins were used to determine GO terms. Only the most significant terms are shown. For the complete list, see Additional file 3: Table S1. Tab1.
| GO term | Neuron Proteomics: Biological Processes | P-value |
|---|---|---|
| GO:0045582 | positive regulation of T cell differentiation | 7.36E-06 |
| GO:0000495 | box H/ACA snoRNA 3'-end processing | 1.30E-05 |
| GO:0034964 | box H/ACA snoRNA processing | 1.30E-05 |
| GO:0000375 | RNA splicing, via transesterification reactions | 2.09E-05 |
| GO:0031126 | snoRNA 3'-end processing | 2.31E-05 |
| GO:0006397 | mRNA processing | 2.77E-05 |
| GO:0000377 | RNA splicing, via transesterification with bulged adenosine as nucleophile | 3.12E-05 |
| GO:0000398 | mRNA splicing, via spliceosome | 3.12E-05 |
| GO:0043144 | snoRNA processing | 3.33E-05 |
| GO:0033979 | box H/ACA snoRNA metabolic process | 3.81E-05 |
| GO:0090669 | telomerase RNA stabilization | 3.81E-05 |
| GO:0045580 | regulation of T cell differentiation | 8.72E-05 |
| GO:0045621 | positive regulation of lymphocyte differentiation | 8.98E-05 |
| GO:0016074 | snoRNA metabolic process | 9.86E-05 |
| GO term | Neuron Phosphoproteomics: Biological Processes | P-value |
| GO:0007010 | cytoskeleton organization | 3.52E-07 |
| GO:0071840 | cellular component organization or biogenesis | 2.13E-06 |
| GO:0016043 | cellular component organization | 2.59E-06 |
| GO:0006397 | mRNA processing | 4.87E-06 |
| GO:0016071 | mRNA metabolic process | 7.23E-06 |
| GO:0050684 | regulation of mRNA processing | 6.40E-05 |
| GO:0008380 | RNA splicing | 7.29E-05 |
| GO:0009987 | cellular process | 7.29E-05 |
| GO:1903311 | regulation of mRNA metabolic process | 1.03E-04 |
| GO:1901879 | regulation of protein depolymerization | 1.18E-04 |
| GO:0006396 | RNA processing | 1.60E-04 |
We also analyzed neuronal DEPs by KEGG (Kyoto Encyclopedia of Genes and Genomes), which showed that the top pathway for upregulated proteins was spliceosome, consistent with the GO terms (Table 2). In addition, enrichment for proteins involved in several neurodegenerative disorders was also found (e.g., Amyotrophic Lateral Sclerosis [ALS], Huntington’s Disease (HD), Parkinson’s Disease (PD), Alzheimer’s Disease (AD), and prion disease). The top differentially expressed down-regulated KEGG pathways were metabolic pathways, ribosomes, and, similar to the up-regulated pathways, ALS, PD, and HD. These findings suggest that features underlying the pathogenesis of JdVS are shared with those involved in some neurodegenerative disorders
Table 2. KEGG analysis, neurons.
KEGG analysis based on differentially expressed up-regulated proteins and phosphoprotein, and differentially expressed down-regulated proteins and phosphoproteins. See Additional file 3: Table S1 for complete lists.
| KEGG pathway: up-regulated neuronal proteins | p-value | KEGG pathway: down-regulated neuronal proteins | p-value |
|---|---|---|---|
| Spliceosome | 1.56E-25 | Metabolic pathways | 1.69E-13 |
| Amyotrophic lateral sclerosis | 1.48E-14 | Ribosome | 3.29E-13 |
| Nucleocytoplasmic transport | 2.39E-14 | Amyotrophic lateral sclerosis | 2.01E-09 |
| Endocytosis | 1.87E-13 | Parkinson disease | 1.36E-08 |
| Huntington disease | 5.11E-13 | Pathways of neurodegeneration - multiple diseases | 4.91E-08 |
| Salmonella infection | 9.37E-13 | Thermogenesis | 1.33E-07 |
| Parkinson disease | 9.78E-13 | Huntington disease | 1.96E-07 |
| Alzheimer disease | 6.35E-11 | Oxidative phosphorylation | 4.27E-07 |
| Prion disease | 8.90E-11 | Valine, leucine and isoleucine degradation | 6.49E-07 |
| Pathways of neurodegeneration - multiple diseases | 9.66E-11 | Endocytosis | 6.74E-07 |
| KEGG pathway: up-regulated neuronal phosphoproteins | p-value | KEGG pathway: down-regulated neuronal phosphoproteins | p-value |
| ErbB signaling pathway | 3.18E-08 | Axon guidance | 1.40E-07 |
| Axon guidance | 5.53E-08 | Insulin signaling pathway | 1.49E-05 |
| Neurotrophin signaling pathway | 2.06E-06 | ErbB signaling pathway | 3.74E-05 |
| Regulation of actin cytoskeleton | 2.33E-06 | Regulation of actin cytoskeleton | 3.94E-05 |
| Pathogenic Escherichia coli infection | 6.54E-05 | Spliceosome | 1.76E-04 |
| Insulin signaling pathway | 8.58E-05 | Endocytosis | 6.98E-04 |
| Spliceosome | 4.57E-04 | Bacterial invasion of epithelial cells | 1.72E-03 |
| Focal adhesion | 4.57E-04 | Inositol phosphate metabolism | 2.19E-03 |
| Autophagy | 5.31E-04 | Focal adhesion | 2.19E-03 |
| Adherens junction | 6.21E-04 | Phosphatidylinositol signaling system | 1.09E-02 |
Analysis of the top individual up and down-regulated DEPs was particularly noteworthy for altered expression of proteins involved in ubiquitin signaling (Table 3). The top-upregulated protein, for example, was CUL4B, a scaffold protein of the CUL4B-Ring E3 ligase complex, which is expressed primarily in the nucleus where it plays a role in DNA repair and tumor progression (40-42). Loss of function (LOF) variants have been found in NDDs (43-46). CUL4B is also an immune regulator, and is involved in the degradation of SIN1, an mTORC2 component (40,47-49).
Table 3. Differentially expressed proteins and phosphoproteins, neurons.
Differentially expressed up and down-regulated proteins and phosphoproteins based on total scores (calculated by multiplying Fold Change by the p-value [−log 2 of 4.32 corresponds to p=0.05]). DEPs shown in descending order.
| Gene | Up-regulated Proteins PPM1D+/tr neurons | Score | Gene | Down-regulated Proteins PPM1D+/tr neurons | Score |
|---|---|---|---|---|---|
| CUL4B | Cullin-4B | 23.32 | PELI2 | E3 ubiquitin-protein ligase pellino homolog 2 | −9.9 |
| SMARCE1 | SWI/SNF-related matrix-associated actin-dependent chromatin regulator | 17.5 | EARS2 | Probable glutamate--tRNA ligase, mitochondrial | −9.97 |
| SLK | STE20-like serine/threonine-protein kinase | 15.6 | NUDT10 | Diphosphoinositol polyphosphate phosphohydrolase 3-alpha | −10.02 |
| POFUT1 | GDP-fucose protein O-fucosyltransferase 1 | 14.79 | C3orf49 | Putative uncharacterized protein C3orf49 | −10.07 |
| AUP1 | Lipid droplet-regulating VLDL assembly factor AUP1 | 14.62 | IBA57 | Putative transferase CAF17, mitochondrial | −10.12 |
| DKC1 | H/ACA ribonucleoprotein complex subunit | 13.41 | SLC30A1 | Zinc transporter 1 | −10.17 |
| NEUROG2 | Neurogenin-2 | 12.34 | LACTB | Serine beta-lactamase-like protein LACTB, mitochondrial | −10.72 |
| C5orf22 | UPF0489 | 11.57 | KRT1 | Keratin, type II cytoskeletal 1 | −10.88 |
| SVIL | Supervillin | 11.33 | RAB34 | Ras-related protein Rab-34 | −11.09 |
| PJA2 | E3 ubiquitin-protein ligase | 11.26 | STX4 | Syntaxin-4 | −11.53 |
| TTC28 | Tetratricopeptide repeat protein 28 | 10.5 | UAP1 | UDP-N-acetylhexosamine pyrophosphorylase | −11.61 |
| POLR2C | DNA-directed RNA polymerase II subunit | 10.23 | UPF2 | Regulator of nonsense transcripts 2 | −13.21 |
| PARN | Poly(A)-specific ribonuclease | 10.22 | NCAM2 | Neural cell adhesion molecule 2 | −14.11 |
| NUBP1 | Cytosolic Fe-S cluster assembly factor | 10.11 | TUB | Tubby protein homolog | −16.38 |
| EXOSC6 | Exosome complex component MTR3 | 9.89 | GSTZ1 | Maleylacetoacetate isomerase | −21.19 |
| Gene | Up-regulated phosphoproteins PPM1D+/tr neurons | Score | Gene | Down-regulated phosphoprotein PPM1D+/tr neurons | Score |
| SRRM1 | Serine/arginine repetitive matrix protein 1 | 34.88 | UBR4 | E3 ubiquitin-protein ligase | −30.52 |
| NUCKS1 | Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1 | 33.54 | RIF1 | Telomere-associated protein RIF | −30.80 |
| NUCKS1 | Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1 | 32.75 | CANX | Calnexin | −30.84 |
| MICAL3 | [F-actin]-monooxygenase MICAL3 | 32.61 | NEB | Nebulin | −31.28 |
| NUCKS1 | Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1 | 31.45 | MADD | MAP kinase-activating death domain protein | −32.21 |
| CWC22 | ubiquitin carboxyl-terminal hydrolase 8 | 30.88 | PLCL1 | Inactive phospholipase C-like protein 1 | −32.30 |
| DCX | Neuronal migration protein doublecortin | 30.56 | SVIL | Supervillin | −33.49 |
| MAP2 | Microtubule-associated protein 2 | 27.14 | DBN1 | Drebrin | −33.63 |
| SCG2 | Secretogranin-2 | 27.12 | ANK2 | Ankyrin-2 | −33.79 |
| RAP1GAP2 | Rap1 GTPase-activating protein 2 | 27.10 | ANK2 | Ankyrin-2 | −34.29 |
| DCX | Neuronal migration protein doublecortin | 26.11 | NAA10 | N-alpha-acetyltransferase 10 | −39.50 |
| AMER2 | APC membrane recruitment protein 2 | 22.99 | ARHGAP35 | Rho GTPase-activating protein 35 | −45.24 |
| CPNE1 | Copine-1 | 22.59 | PRKAR2B | cAMP-dependent protein kinase II-beta regulatory subunit | −46.80 |
| ADCY5 | Adenylate cyclase type 5 | 22.08 | SRSF7 | Serine/arginine-rich splicing factor 7 | −59.87 |
| TRAPPC14 | Trafficking protein particle complex subunit 14 | 21.89 | SRRM1 | Serine/arginine repetitive matrix protein 1 | −68.44 |
Other ubiquitin signaling proteins among the top 10 upregulated DEPs were PJA2, an E3 ubiquitin-protein ligase, and AUP1, which forms a complex with the ubiquitin-conjugating enzyme (E2), UBE2G2 (50-52). Among the top downregulated DEPs affecting ubiquitin signaling is PELI2, a member of the E3 ubiquitin ligase family that regulate the innate immune system by increasing NLRP3 inflammasome activation (53).
Other top upregulated DEPs of interest include SMARCE1, SLK, POFUT1, DKC1, and NEUROG2. SMARCE1 codes for an SWI/SNF chromatin remodeling complex component that regulates gene expression and can cause ASD when mutated (54-57).
Other proteins that were most downregulated in PPM1D+/tr neurons were UPF2, NCAM2, TUB, and GSTZ1. UPF2 is a regulator of nonsense-mediated decay (NMD) and low expression is a factor in resistance to ATR inhibitors: ATR is a DNA damage sensor and a PPM1D substrate (9,58). Disruption of NMD has been associated with neurodevelopmental disorders (59) GSTZ1 is a member of the glutathione S-transferase super-family that detoxifies products of oxidative stress, a process linked to PD, AD, and ALS (60-63).
Phosphoproteomics: glutamatergic neurons
Since PPM1D is a serine/threonine phosphatase, we also carried out a phosphoproteomics analysis on the samples used in the glutamatergic neuronal proteomics experiment. However, one sample was omitted for technical problems, so 4 control vs 4 PPM1D+/tr neuronal samples were analyzed. A total of 7,542 phosphosites were detected (Additional file 4: Table S2). At p < 0.05, 174 differentially expressed phosphosites (DEPP) differed significantly between control and PPM1D+/tr neurons; 46 were higher and 128 were lower. GO analysis showed that the most enriched phosphorylations are related to cytoskeleton organization, cellular component organization or biogenesis, and mRNA processing (Table 1). KEGG analysis of differentially expressed upregulated proteins showed that the top pathways were ErbB signaling, axon guidance, neurotrophin signaling, and regulation of the actin cytoskeleton (Table 2). Axon guidance and ErbB signaling were also among the top downregulated pathways, along with insulin signaling and spliceosome.
The top DEPP that increased in the PPM1D+/tr neurons was SRRM1, which is involved in RNA processing, as a component of pre- and post-splicing multiprotein mRNP complexes that play major roles in RNA metabolism (Table 3) (63). Altered expression affects prostate cancer aggression and invasion of hepatocellular carcinoma cells (64,65). Mutations in PPM1D mutations are associated with both, suggesting that altered SRRM1 phosphorylation plays a role in PPM1D-associated cancers (68, 69). Strikingly, two phosphosites on SRRM1 (Ser725 and Thr727) were also the top downregulated DEPPs. Predicted targets at Thr727 include HIPK1 and p38MAPK (http://www.phosphonet.ca/), which are PPM1D substrates. The findings suggest that regulation of SRRM1 is a novel feature of truncating PPM1D variants.
Interestingly, three of the top DEPPs were found in NUCKS1, a chromatin regulator that regulates DNA repair (66-68). NUCKS1 has been implicated in PD in genome wide association studies (GWAS) and is a known PPM1D substrate, although neither of the top three neuronal NUCKS1 phosphosites occurs at SQ/TQ motifs, which are canonical PPM1D targets (9,69,70). Another protein that scored multiple hits among the top upregulated phosphosites is DCX (doublecortin), a cytoskeletal protein that stabilizes microtubules and regulates neuronal migration and cortical layering during development (71).
Differential phosphorylation of proteins that are known PPM1D substrates, such as ATM, CHK1, CHK2, and P53, were not detected, perhaps because the neurons were postmitotic and not subjected to conditions that would most effectively induce their phosphorylation (e.g., ionizing radiation). In fact, among the DEPPs that showed a decrease in phosphorylation expected of a PPM1D GOF effect, only three, ENAH, AKAP12, and ANK2 occurred at SQ sites, suggesting that the majority of neuronal DEPPs are secondary to the downstream effects of PPM1D on other kinases and phosphatases, although novel, noncanonical targets are possible as well.
Overall, the neuronal proteomics and phosphoproteomics data showed differential expression of proteins and phosphoproteins coregulated in T-cells, splicing, DDR, chromatin regulation, neurodegeneration, ErbB signaling, and ubiquitin ligases.
Proteomics: Microglia
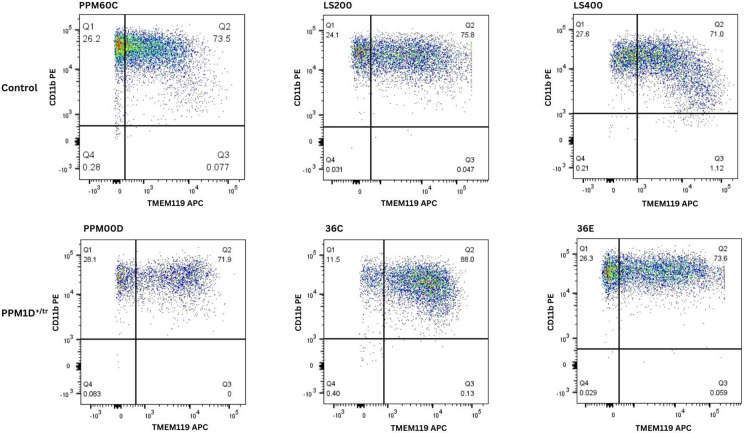
As described in the introduction, we identified several JdVS cases in whom severe motor and behavioral regression occurred following infections and non-infectious stressors. Although these examples of acute neuropsychiatric decompensation appear to be rare occurrences in JdVS, we extended the proteomics analysis to include microglia. An additional rationale is that microglia have been implicated in the pathogenesis of ASD and NDD (72-75). Microglia were developed from six iPSC lines (three control and three PPM1D+/tr). The differentiation protocol produced similar populations of TMEM119/CD11B double-positive cells; between 71.9 to 88% (Figure 3). 5,759 proteins were detected, which included 76 that were significantly upregulated and 76 that were downregulated (Additional file 5: Table S3; Figure 2). GO analysis showed enrichment of DEPs related to blood vessel development: aorta morphogenesis, blood vessel lumenization, and blood vessel morphogenesis, although the p-values are modest Table 4). Nevertheless, these findings are of interest. The upregulated proteins that contributed to these GO findings, DLL4, RBPJ, and LRP, are all involved in endothelial function that can affect the brain-blood barrier (BBB) suggesting that PPM1D truncating mutations increase BBB permeability (76,77). In fact, PPM1D has been shown to be a BBB regulator (78). The findings support the idea that patients with JdVS are prone to neuroinflammation in response to a peripheral immune challenge.
Figure 3. FACS analysis of microglia.
Microglia were developed from three control iPSC lines (PPM60C, LS200, and LS400), and three patient lines (PPMOOD, 36C, 36E). PPM6OC is the typically developing sibling control of PPMOOD, and LS200 is the isogenic control for 36C and 36E. LS400 is another typically developing control. Cells were sorted using conjugated antibodies against the microglia markers TMEM119 and CD11b, the latter of which also binds to macrophages. Microglia are positive for both double-positive cells.
Table 4. Gene Ontology (GO) analysis, microglia.
All differentially expressed proteins and phosphoproteins were used to determine GO terms. Only the most significant terms are shown. For the complete list, see Additional file 5: Table S3.
| GO term | Microglia Proteomics: Biological Processes | P-value |
|---|---|---|
| GO:0035909 | aorta morphogenesis | 4.74E-05 |
| GO:0072554 | blood vessel lumenization | 5.37E-05 |
| GO:0048514 | blood vessel morphogenesis | 8.88E-05 |
| GO:0061314 | Notch signaling involved in heart development | 1.52E-04 |
| GO:0035912 | dorsal aorta morphogenesis | 1.52E-04 |
| GO:0009311 | oligosaccharide metabolic process | 1.59E-04 |
| GO:0044242 | cellular lipid catabolic process | 2.40E-04 |
| GO:0010885 | regulation of cholesterol storage | 3.11E-04 |
| GO:0032621 | Interleukin 18 production | 3.51E-04 |
| GO:0021983 | pituitary gland development | 4.44E-04 |
| GO:0090136 | epithelial cell cell adhesion | 4.96E-04 |
| GO:0048844 | artery morphogenesis | 5.95E-04 |
| GO:1900227 | positive regulation of NLRP3 inflammasome complex assembly | 5.98E-04 |
| GO term | Microglia Phosphoproteomics: Biological Processes | P-value |
| GO:0008380 | RNA splicing | 2.24E-20 |
| GO:0000398 | mRNA splicing, via spliceosome | 2.80E-19 |
| GO:0051056 | regulation of small GTPase mediated signal transduction | 5.03E-18 |
| GO:0006397 | mRNA processing | 1.32E-17 |
| GO:0035556 | intracellular signal transduction | 1.49E-17 |
| GO:0006338 | chromatin remodeling | 3.04E-13 |
| GO:0006325 | chromatin organization | 2.07E-12 |
| GO:0006468 | protein phosphorylation | 3.39E-11 |
| GO:0007010 | cytoskeleton organization | 1.19E-10 |
| GO:0006974 | cellular response to DNA damage stimulus | 2.12E-10 |
Also consistent with a neuroinflammatory phenomenon are the GO terms of enriched DEPs showing an effect on the production and positive regulation of NLRP3 inflammasome complex assembly. IL-18 is a proinflammatory cytokine produced, along with IL-1β, as a result of NLRP3 inflammasome activation (79-81). However, the level of significance for this GO term is modest.
The top pathway for upregulated proteins was lysosome, but similar to the KEGG analysis of neurons, among the top pathways for both up and downregulated proteins are neurodegenerative disorders. The lysosome pathway could indicate a predilection for disruption of autophagy, a process linked to neurodegenerative and neurodevelopmental disorders (Table 5) (82-85). Interestingly, the most upregulated DEPs proteins in PPM1D+/tr microglia are several regulators of ubiquitin signaling and innate immune pathways (Table 6). These included, CDC34, a Cullin-Ring E2 ubiquitin-conjugating enzyme, GBP5, a member of the GTPase subfamily induced by interferon-gamma (IFN-γ), CDBP2, a CD2 antigen cytoplasmic tail-binding protein that regulates T-cell activation and IL-2 production, KLHDC4, a member of the Kelch-like proteins that act as substrate adaptors for Cullin 3 ubiquitin ligases, PELI1, an E3 ubiquitin-protein ligase pellino homolog that regulates NLRP3-induced caspase-1 activation and IL-1β maturation, ZNFX1, which functions as a dsRNA sensor and regulator of antiviral responses, and TEP1, a telomerase protein component that can influence innate immune responses through cGAS/STING (cyclic GMP-AMP synthase-stimulator of interferon genes) activation, a cytosolic DNA sensor (86-92).
Table 5. KEGG analysis, microglia.
Shows lists of the most significant KEGG pathways for up and downregulated proteins and phosphoproteins in PPM1D+/tr microglia. See Additional file 5: Table S3 for complete lists.
| KEGG pathway up-regulated proteins microglia | p-value | KEGG pathway down-regulated proteins microglia | p-value |
|---|---|---|---|
| Lysosome | 6.94E-07 | Amyotrophic lateral sclerosis | 1.16E-08 |
| Parkinson disease | 4.53E-06 | Huntington disease | 8.41E-07 |
| Prion disease | 1.77E-05 | Phagosome | 1.35E-06 |
| RNA transport | 6.67E-05 | Salmonella infection | 1.71E-05 |
| Citrate cycle (TCA cycle) | 8.17E-05 | Prion disease | 6.06E-05 |
| Alzheimer disease | 1.52E-04 | Pathways of neurodegeneration | 6.77E-05 |
| Cholesterol metabolism | 2.22E-04 | Proteasome | 1.33E-04 |
| Aminoacyl-tRNA biosynthesis | 2.23E-04 | Pathogenic Escherichia coli infection | 1.33E-04 |
| Glycolysis/Gluconeogenesis | 2.48E-04 | Vibrio cholerae infection | 2.28E-04 |
| Phagosome | 4.26E-04 | RNA transport | 2.49E-04 |
| KEGG pathway up-regulated phosphoproteins microglia | p-value | KEGG pathway down-regulated phosphoproteins microglia | p-value |
| Spliceosome | 1.47E-07 | Spliceosome | 5.28E-08 |
| Yersinia infection | 3.92E-05 | Viral life cycle - HIV-1 | 7.29E-07 |
| Rap1 signaling pathway | 7.68E-05 | Regulation of actin cytoskeleton | 8.36E-07 |
| Shigellosis | 1.60E-04 | Thyroid hormone signaling pathway | 1.97E-06 |
| Insulin signaling pathway | 7.70E-03 | Platelet activation | 1.05E-05 |
| NOD-like receptor signaling pathway | 1.79E-02 | Fc gamma R-mediated phagocytosis | 2.77E-05 |
| Transcriptional misregulation in cancer | 2.41E-02 | Endocytosis | 4.57E-05 |
| Adherens junction | 3.31E-02 | Renal cell carcinoma | 5.48E-05 |
| Fc gamma R-mediated phagocytosis | 3.56E-02 | ErbB signaling pathway | 5.51E-05 |
| Platelet activation | 3.71E-02 | Insulin signaling pathway | 5.53E-05 |
Table 6. Differentially expressed proteins and phosphoproteins, uninduced microglia.
Differentially expressed up and down-regulated proteins and phosphoproteins in baseline, untreated microglia.
| Gene | Up-regulated proteins PPM1D+/tr microglia | Score | Gene | Down-regulated proteins PPM1D+/tr microglia | Score |
|---|---|---|---|---|---|
| CDC34 | Ubiquitin-conjugating enzyme E2 | 29.63 | ACOD1 | Cis-aconitate decarboxylase | −14.10 |
| GBP5 | Guanylate-binding protein 5 | 28.63 | DDX54 | ATP-dependent RNA helicase | −14.14 |
| CD2BP2 | CD2 antigen cytoplasmic tail-binding protein 2 | 26.46 | HERC5 | E3 ISG15--protein ligase | −14.51 |
| KLHDC4 | Kelch domain-containing protein 4 | 26.33 | VPS33A | Vacuolar protein sorting-associated protein 33A | −15.50 |
| SOX3 | Transcription factor SOX-3 | 22.47 | SLC38A2 | Sodium-coupled neutral amino acid transporter 2 | −16.01 |
| PELI1 | E3 ubiquitin-protein ligase pellino homolog 1 | 21.52 | MMP13 | Collagenase 3 | −16.11 |
| MAN1B1 | Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase | 21.44 | CDK1 | Cyclin-dependent kinase 1 | −16.67 |
| MYEF2 | Myelin expression factor 2 | 20.04 | GBP1 | Guanylate-binding protein 1 | −16.68 |
| TEP1 | Telomerase protein component 1 | 18.3 | PARP9 | Protein mono-ADP-ribosyltransferase PARP9 | −16.91 |
| CCNK | Cyclin-K | 17.87 | LIMD2 | LIM domain-containing protein 2 | −20.34 |
| DLL4 | Delta-like protein 4 | 17.7 | POGZ | Pogo transposable element with ZNF domain | −28.22 |
| AZI2 | 5-azacytidine-induced protein 2 | 17.28 | SLC38A10 | Putative sodium-coupled neutral amino acid transporter 10 | −30.52 |
| GSTM1 | Glutathione S-transferase Mu 1 | 16.66 | HERC6 | Probable E3 ubiquitin-protein ligase HERC6 | −31.45 |
| DHRSX | Dehydrogenase/reductase SDR family member on chromosome X | 16.03 | APOBEC3G | DNA dC->dU-editing enzyme APOBEC-3G | −33.14 |
| ANGEL2 | Protein angel homolog 2 | 14.85 | TANK | TRAF family member-associated NF-kappa-B activator | −46.18 |
| Gene | Up-regulated phosphoproteins PPM1D+/tr microglia | Score | Gene | Down-regulated phosphoproteins PPM1D+/tr icroglia | Score |
| PXN | Paxillin | 14.95 | CLASP2 | CLIP-associating protein 2 | −20.3 |
| DDX54 | ATP-dependent RNA helicase DDX54 | 8.97 | RETREG2 | Reticulophagy regulator 2 | −20.53 |
| TOP2B | DNA topoisomerase 2-beta | 8.85 | MAP4K4 | Mitogen-activated protein kinase kinase kinase kinase 4 | −20.92 |
| MACF1 | Microtubule-actin cross-linking factor 1 | 8.21 | TBC1D2BTBC1 | domain family member 2B | −21.38 |
| SRRM1 | Serine/arginine repetitive matrix protein 1 | 8.04 | RASAL3 | RAS protein activator like-3 | −21.7 |
| ATP6V0A2 | V-type proton ATPase 116 kDa subunit a2 | 7.9 | AMPD3 | AMP deaminase 3 | −21.94 |
| PARN | Poly(A)-specific ribonuclease PARN | 7.73 | CD2BP2 | CD2 antigen cytoplasmic tail-binding protein 2 | −22.21 |
| CCNY | Cyclin-Y | 7.72 | SNTB1 | Beta-1-syntrophin | −25.43 |
| TRAFD1 | TRAF-type zinc finger domain-containing protein 1 | 7.29 | PI4KB | Phosphatidylinositol 4-kinase beta | −26 |
| TRAFD1 | TRAF-type zinc finger domain-containing protein 1 | 7.08 | SUDS3 | Sin3 histone deacetylase corepressor complex component SDS3 | −26.29 |
| TMEM230 | Transmembrane protein 230 | 6.7 | VPS50 | Syndetin | −29.52 |
| SRRM1 | Serine/arginine repetitive matrix protein 1 | 6.65 | SNX5 | Sorting nexin-5 | −30.13 |
| STX11 | Syntaxin-11 | 6.36 | UFL1 | E3 UFM1-protein ligase 1 | −39.21 |
| ARHGEF2 | Rho guanine nucleotide exchange factor 2 | 6.3 | FLII | Protein flightless-1 homolog | −41.86 |
| DKC1 | H/ACA ribonucleoprotein complex subunit DKC1 | 6.01 | SERF2 | Small EDRK-rich factor 2 | −62.15 |
Many of the most downregulated proteins are also involved in innate immunity including TANK, which activates NF-κB and cGAS-STING signaling, APOBEC3G, a cytidine deaminase involved in anti-viral innate immunity, and HERC6, an E3 ligase for ISG15 that regulates ISGylation, a post-translational modification induced by interferon that has ubiquitin-like, protein modifying effects (93-103).
Another key downregulated protein is POGZ, a chromatin regulator that also promotes homology-directed DNA repair (104). LOF mutations are commonly found in ASD and NDD (105,106). POGZ binds to ADNP, and their deficiency in mice induces significant upregulation of genes enriched in neuroinflammation and altered microglial and glutamatergic neuronal function (105-108). LOF mutations in ADNP have been found in NDD and ASD (including regressive autism; see discussion) (109-111). Neither POGZ nor ADNP is significantly differentially expressed in glutamatergic neurons. These findings suggest that decreased POGZ expression in microglia is playing a role in the neurodevelopmental features of JdVS.
Phosphoproteomics: Microglia
The phosphoproteomics analysis detected 3458 phosphosites of which only 4 showed a significant increase in the PPM1D+/tr microglia, while 39 were significantly decreased (Additional file 6: Table S4; Figure 2). The top GO terms for differentially phosphorylated proteins were related to RNA splicing, regulation of small GTPase signaling, chromatin remodeling, protein phosphorylation, cytoskeleton organization, and cellular response to DNA damage stimulus, and the top KEGG pathway was spliceosome, similar to the neuronal proteomics and phosphoproteomics studies (Table 4; Table 5). The top upregulated phosphorylated proteins in PPM1D+/tr microglia were in PXN, DDX54, TOP2B, MACF1, CCNY, and SRRM1 (Table 6). Remarkably, this overlaps with the finding that SRRM1 was the top upregulated, as well as the top downregulated phosphorylated protein in PPM1D+/tr neurons, as described above, providing additional support for the idea that PPM1D truncating mutations disrupt SRRM1 function. An increase in phosphorylation at S429 in both neurons and microglia was detected. This is predicted to be a substrate for the PIM family of kinases, which promote tumorigenesis and immune escape by HIV (112,113).
Two phosphosites in TRAFD1 were among the top 10 DEPPs. TRAFD1 is a transcription factor that acts as a negative feedback regulator of the innate immune system to control excessive immune responses (114,115). A similar occurrence in microglia could perhaps be relevant to the acute neuropsychiatric decompensation that occurs in some JdVS patients.
The most downregulated DEPPs were found in SERF2, FLI1, UFL1, SNX5, and VPS50. SERF2 is small EDRK-rich factor 2 that modifies amyloid fiber assembly and promotes protein misfolding (116). FLI1 is a member of the ETS transcription factor family that is disrupted in Ewing Sarcoma and acute myelogenous leukemia (117). And UFL1 (UFM1-protein ligase 1; Ubiquitin-like modifier 1 ligating enzyme 1) is a regulator of UFM1 conjugation (UFMylation), a ubiquitin-like modification that plays a key role in maintaining cell homeostasis under cellular stress, including DDR (118-120). SNX5 is a component of an autophagosomal complex and VPS50 is an endosome-recycling protein (121,122).
In summary, the microglia proteomics and phosphoproteomics analyses suggest that reduced expression of POGZ is a candidate for the cognitive and behavioral aspects of JdVS, and that truncated PPM1D could disturb innate immune responses in the brain through altered regulation of ubiquitin signaling, DDR, splicing, altered expression or function of key genes, and perhaps BBB permeability.
Proteomics Analysis of lipopolysaccharide (LPS)-activated microglia
To test the hypothesis that PPM1D+/tr microglia have an altered response to an innate immune system challenge, the effect of LPS was analyzed by proteomics. One hundred and fifty-eight proteins were upregulated in the PPM1D+/tr samples, and 254 were downregulated. (Additional file 7: Table S5; Figure 2). The top GO term was negative regulation of interleukin-6 (IL-6), a proinflammatory cytokine implicated in neuroinflammation, maternal immune activation, ASD, schizophrenia, and depression (Table 7) (123-126). However, the p-value was modest. KEGG analysis showed that the top pathways for upregulated proteins were lysosome, metabolic pathways, and several neurodegenerative disorders, similar to the findings in uninduced microglia and glutamatergic neurons (Table 8). Downregulated proteins were enriched for spliceosome, nucleocytoplasmic transport, and ALS, overlapping with other proteomics findings. Proteins involved in the response to several infectious diseases were also detected in the KEGG analysis.
Table 7. Gene Ontology (GO) analysis, LPS induced microglia.
All differentially expressed proteins and phosphoproteins were used to determine GO terms. Only the most significant terms are shown. For the complete list, see Additional file 7: Table S5.
| GO term | LPS Microglia Proteomics: Biological Processes | P-value |
|---|---|---|
| GO:0032715 | negative regulation of interleukin-6 production | 3.23E-05 |
| GO:0072310 | glomerular epithelial cell development | 3.44E-05 |
| GO:0072015 | glomerular visceral epithelial cell development | 3.44E-05 |
| GO:0034446 | substrate adhesion-dependent cell spreading | 1.08E-04 |
| GO:0015850 | organic hydroxy compound transport | 1.39E-04 |
| GO:0031589 | cell-substrate adhesion | 1.63E-04 |
| GO:0072359 | circulatory system development | 1.90E-04 |
| GO:2000351 | regulation of endothelial cell apoptotic process | 2.08E-04 |
| GO:0003014 | renal system process | 2.51E-04 |
| GO:0006003 | fructose 2,6-bisphosphate metabolic process | 3.31E-04 |
| GO term | LPS Microglia Phosphoproteomics: Biological Processes | P-value |
| GO:0008380 | RNA splicing | 2.32E-20 |
| GO:0000398 | mRNA splicing, via spliceosome | 2.89E-19 |
| GO:0051056 | regulation of small GTPase mediated signal transduction | 5.17E-18 |
| GO:0006397 | mRNA processing | 1.37E-17 |
| GO:0035556 | intracellular signal transduction | 1.56E-17 |
| GO:0006338 | chromatin remodeling | 3.11E-13 |
| GO-0006325 | chromatin organization | 2.14E-12 |
| GO:0006468 | protein phosphorylation | 3.52E-11 |
| GO:0007010 | cytoskeleton organization | 1.22E-10 |
| GO:0006974 | cellular response to DNA damage stimulus | 2.18E-10 |
| GO:0045944 | positive regulation of transcription from RNA polymerase II promoter | 2.86E-10 |
| GO:0051301 | cell division | 4.59E-09 |
| GO:0030036 | actin cytoskeleton organization | 9.04E-09 |
| GO:0007165 | signal transduction | 1.01E-08 |
| GO:0090630 | activation of GTPase activity | 2.75E-08 |
Table 8. KEGG analysis, LPS treated microglia.
Differentially expressed up and down-regulated proteins and phosphoproteins in baseline, untreated microglia. Additional file 7: Table S5.
| KEGG pathway up-regulated proteins LPS microglia | p-value | KEGG pathway down-regulated proteins LPS microglia | p-value |
|---|---|---|---|
| Lysosome | 2.38E-22 | Spliceosome | 6.00E-15 |
| Metabolic pathways | 1.22E-13 | Nucleocytoplasmic transport | 2.12E-13 |
| Huntington disease | 6.41E-12 | Amyotrophic lateral sclerosis | 1.68E-12 |
| Parkinson disease | 1.14E-11 | Salmonella infection | 1.86E-12 |
| Prion disease | 5.47E-11 | Ribosome | 1.15E-10 |
| Amyotrophic lateral sclerosis | 5.06E-10 | Protein processing in endoplasmic reticulum | 2.03E-10 |
| Oxidative phosphorylation | 1.25E-09 | Epstein-Barr virus infection | 2.74E-10 |
| Chemical carcinogenesis - reactive oxygen species | 4.44E-08 | Carbon metabolism | 1.08E-09 |
| Pathways of neurodegeneration - multiple diseases | 5.90E-08 | Shigellosis | 1.67E-09 |
| Neutrophil extracellular trap formation | 6.17E-08 | Leishmaniasis | 2.50E-08 |
| Proteasome | 1.43E-07 | Metabolic pathways | 2.64E-08 |
| Coronavirus disease - COVID-19 | 3.92E-08 | ||
| Influenza A | 7.27E-08 | ||
| KEGG pathway up-regulated phosphoproteins LPS microglia | p-value | KEGG pathway down-regulated phosphoproteins LPS microglia | p-value |
| Rap1 signaling pathway | 6.29E-06 | Spliceosome | 2.07E-09 |
| Yersinia infection | 7.87E-06 | Regulation of actin cytoskeleton | 7.01E-07 |
| Platelet activation | 8.81E-06 | Viral life cycle - HIV-1 | 7.27E-05 |
| Regulation of actin cytoskeleton | 7.58E-05 | Thyroid hormone signaling pathway | 9.62E-05 |
| Fc gamma R-mediated phagocytosis | 1.78E-04 | ErbB signaling pathway | 1.37E-04 |
| Adherens junction | 2.10E-04 | Shigellosis | 1.59E-04 |
| Shigellosis | 2.24E-04 | Insulin signaling pathway | 1.78E-04 |
| Pathogenic Escherichia coli infection | 2.59E-04 | Rap1 signaling pathway | 3.22E-04 |
| Thyroid hormone signaling pathway | 4.00E-04 | Leukocyte transendothelial migration | 4.17E-04 |
| Chemokine signaling pathway | 5.52E-04 | Yersinia infection | 5.00E-04 |
Examination of individual DEPs showed striking patterns consistent with innate immune dysregulation, in particular, ubiquitin signaling (Table 9). The top upregulated protein in PPM1D+/tr microglia was UHRF1BP1 (UHRF1-binding protein 1), which binds to UHRF1, a PPM1D+/tr microglia was UHRF1BP1 (UHRF1-binding protein 1), which binds to UHRF1, a RING-finger E3 ubiquitin ligase, a regulator of Treg cell proliferation (127,128). Non-synonymous variants have been found in systemic lupus erythematosus (129). However, the effects of UHRF1BP1 and UHRF1 on microglia are not known. GBA1 catalyzes the cleavage of glycosphingolipids–glucosylceramide and glucosyl sphingosine. Genetic variants are known risk factors for PD and Lewy Body Dementia, and biallelic LOF variants cause Gaucher disease (130-134). RAB11A is a member of the RAS family of small GTPases and is a regulator of toll receptor trafficking (134,135).
Table 9. Differentially expressed proteins and phosphoproteins, LPS-treated microglia.
Shows the top up and downregulated proteins and phosphoproteins in baseline, untreated microglia. See Additional file 7: Table S5 and Additional file 8: Table S6 for all proteins and phosphoproteins.
| Gene | Up-regulated proteins PPM1D+/tr LPS microglia | Score | Gene | Down-regulated proteins PPM1D+/tr LPS microglia | Score |
|---|---|---|---|---|---|
| UHRF1BP1 | UHRF1-binding protein 1 | 60.28 | PI4KA | Phosphatidylinositol 4-kinase alpha | −29.3389 |
| GBA1 | Lysosomal acid glucosylceramidase | 55.35 | RPL18 | 60S ribosomal protein L18 | −29.6675 |
| ETNK1 | Ethanolamine kinase 1 | 37.18 | DDX59 | Probable ATP-dependent RNA helicase DDX59 | −31.5494 |
| PYGL | Glycogen phosphorylase, liver form | 34.4 | SPRYD7 | SPRY domain-containing protein 7 | −32.4876 |
| UGP2 | UTP--glucose-1-phosphate uridylyltransferase | 32.4 | EPS8 | Epidermal growth factor receptor kinase substrate 8 | −33.9319 |
| RAB11A | Ras-related protein Rab-11A | 30.74 | CBR3 | Carbonyl reductase [NADPH] | −34.3485 |
| CSK | Tyrosine-protein kinase CSK | 29.54 | HSPBAP1 | HSPB1-associated protein 1 | −37.1573 |
| SLC25A35 | Solute carrier family 25 member 35 | 27.87 | MTA1 | Metastasis-associated protein MTA1 | −37.6405 |
| PAFAH2 | Platelet-activating factor acetylhydrolase 2, cytoplasmic | 27.23 | CPM | Carboxypeptidase M | −39.6778 |
| FGG | Fibrinogen gamma chain | 26.9 | KCTD5 | BTB/POZ domain-containing protein KCTD5 | −42.0408 |
| LAMB2 | Laminin subunit beta-2 | 25.49 | NUBP2 | Cytosolic Fe-S cluster assembly factor NUBP2 | −42.8254 |
| ZBTB33 | Transcriptional regulator Kaiso | 24.49 | TAX1BP3 | Tax1-binding protein 3 | −43.4695 |
| LYPLA2 | Acyl-protein thioesterase 2 | 22.79 | HSPB11 | Intraflagellar transport protein 25 homolog | −47.3325 |
| STARD3 | StAR-related lipid transfer protein 3 | 22.75 | HAS1 | Hyaluronan synthase 1 OS=Homo sapiens | −50.9275 |
| SIAE | Sialate O-acetylesterase | 22.49 | UBR4 | E3 ubiquitin-protein ligase UBR4 | −65.0923 |
| Gene | Up-reg. phosphoproteins PPM1D+/tr LPS microglia | Score | Gene | Down-reg. phosphoproteins PPM1D+/tr LPS microglia | Score |
| UBR4 | E3 ubiquitin-protein ligase | 62.42 | IRAG2 | Inositol 1,4,5-triphosphate receptor associated 2 | −37.17 |
| UBR4 | E3 ubiquitin-protein ligase | 47.723 | HSP90AB1 | Heat shock protein HSP 90-beta | −39.11 |
| IL16 | Pro-interleukin-16 | 38.099 | KIAA0930 | Uncharacterized protein KIAA0930 | −39.54 |
| LBR | Delta(14)-sterol reductase | 32.377 | BCLAF1 | Bcl-2-associated transcription factor 1 | −43.15 |
| TBC1D10B | TBC1 domain family member 10B | 30.032 | IRF2BP1 | Interferon regulatory factor 2-binding protein 1 | −45.51 |
| UBR4 | E3 ubiquitin-protein ligase | 29.951 | RPL23A | 60S ribosomal protein L23a | −46.79 |
| UBR4 | E3 ubiquitin-protein ligase UBR4 | 28.137 | PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 | −48.40 |
| PI4KA | Phosphatidylinositol 4-kinase alpha | 26.685 | PRRC2C | Protein PRRC2C | −52.17 |
| LTB4R | Leukotriene B4 receptor 1 | 23.842 | PRPF4B | Serine/threonine-protein kinase PRP4 homolog | −53.02 |
| PLXNC1 | Plexin-C1 | 21.067 | DVL3 | Segment polarity protein dishevelled homolog DVL-3 | −53.07 |
| SMARCA2 | Probable global transcription activator SNF2L2 | 20.538 | FNBP1 | Formin-binding protein 1 | −58.01 |
| RBM33 | RNA-binding protein 33 | 19.82 | LARP7 | La-related protein 7 | −58.89 |
| SIPA1L1 | Signal-induced proliferation-associated 1-like protein 1 | 17.973 | RBM25 | RNA-binding protein 25 | −63.53 |
| PLXNC1 | Plexin-C1 | 17.862 | HNRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 | −64.67 |
| HNRNPU | Heterogeneous nuclear ribonucleoprotein U | 17.634 | RASAL3 | RAS protein activator like-3 | −65.28 |
The most downregulated DEP was the ubiquitin protein ligase E3 component n-recognin 4 protein, UBR4, which regulates oxidative stress by promoting K27-linked-ubiquitylation of N-terminal oxidized cysteines leading to proteasomal degradation (136). It’s also a regulator of interferon signaling (137,138) and the proteasomal degradation of PINK1, which is involved in the pathogenesis of PD (see discussion) (138-140). UBR4 variants have been linked to early-onset dementia (140). Other top-downregulated proteins include HAS1, a regulator of the extracellular matrix that is induced by LPS and KCTD5, a BTB/POZ domain-containing protein that functions as substrate-specific adaptor for Cullin3-based E3 ligases (141-143).
Phosphoproteomics LPS treated microglia
Phosphoproteomics was carried out on the same samples used in the proteomics analysis. A total of 3,458 phosphosites were detected; 42 showed a significant increase in the LPS treated PPM1D+/tr microglia, and 182 showed a significant decrease (Additional file 8: Table S6; Figure 2). Strikingly, four of the top seven phosphosites that increased in the LPS-stimulated PPM1D+/tr microglia were in UBR4, although neither of the sites is a canonical PPM1D target motif (Table 9). The top phosphosite is at S2718, which is phosphorylated in UV irradiated cells, consistent with an effect of PPM1D or its substrates on DDR (144). This and the other top UBR4 phosphosites have an SS motif. As noted above, UBR4 is also the most downregulated DEP in LPS-treated microglia, suggesting that UBR4 phosphorylation is inversely correlated with UBR4 protein levels. There are also two enriched phosphosites in PLXNC1, a member of the plexin family of transmembrane receptors for semaphorins, which are involved in brain development and immune responses (145). IL16 had the most differentially increased phosphosite after UBR4. It is a CD4+ immune cell-specific chemoattractant cytokine that has been implicated in multiple sclerosis (146,147).
The most downregulated phosphosite was in RASAL3, a RasGAP that is highly expressed in neutrophils. Deficiency enhances immune activation in acute inflammatory conditions (148). GO analysis of all differential phosphosites showed enrichment of phosphoproteins involved in RNA splicing, regulation of small GTPase-mediated signal transduction, chromatin remodeling, cytoskeleton organization, and cellular response to DNA damage stimulus (Table 7). These findings overlap with the uninduced microglia phosphoproteomics analysis. Alterations in microglia’s cytoskeleton function could potentially cause problems with their migration or phagocytic potential.
The top KEGG pathways for phosphosites that increased in the PPM1D+/tr microglia were Rap1 signaling, Yesinia infection, platelet activation and regulation of cytoskeleton, while the terms spliceosome, regulation of actin cytoskeleton, viral life cycle (HIV-1) and thyroid hormone signaling pathway were pathways enriched with phosphosites that decreased in PPM1D+/tr microglia (Table 8). A modest enrichment of phosphosites involved in ErbB signaling was also seen, which by itself seems relatively minor. However, in view of the enrichment of phosphosites in this pathway in neurons and uninduced microglia, as well as LPS-treated cells, the findings suggest that PPM1D truncating mutations can disrupt ErbB signaling. PPM1D expression has been found to affect breast cancer growth (149,150). In the brain, ErbB signaling plays a role in synaptic plasticity and has been implicated in NDD (151-154).
Overall, the findings show that immune stimulation of PPM1D+/tr microglia results in altered expression of proteins involved in ubiquitin signaling, in particular, UBR4, actin cytoskeleton, RNA splicing, chromatin structure, and innate immune regulation, the latter of which could play a role in the decompensation some JdVS patients experience following infectious and non-infectious stressors.
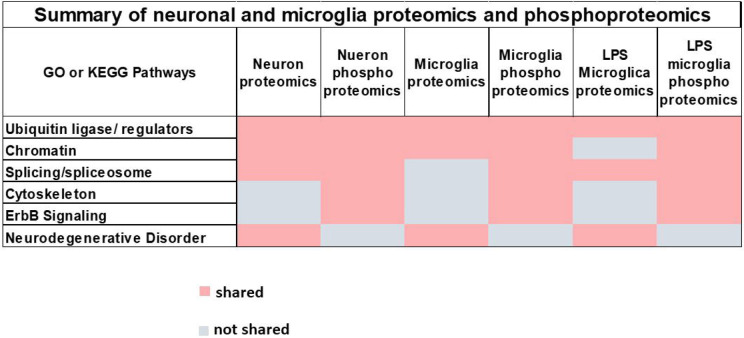
In summary, the three cell types in which proteomics and phosphoproteomics were carried out (glutamatergic neurons, uninduced microglia, and LPS-stimulated microglia) showed several overlapping pathways: splicing, ubiquitin ligase expression, neurodegenerative disorders, chromatin organization, cytoskeleton dynamics, and ErbB signaling (Figure 4).
Figure 4. Summary of neuronal and microglia proteomics and phosphoproteomics.
Six pathways (center of each block, light grey) were found by either GO or KEGG (up or downregulated) that were shared in three or more of the six different conditions analyzed in this study; proteomics and phosphoproteomics on glutamatergic neurons, untreated microglia, and LPS induced microglia (shared dark grey block; not shared, white block)
Functional analysis of glutamatergic neurons and microglia
Most of the differentially expressed phosphosites in neurons and microglia were not at canonical SQ or TQ motifs recognized by PPM1D, so the GOF effect of truncated PPM1D could not be unequivocally validated using the phosphoproteomics data we obtained. Consequently, we examined a known neuronal PPM1D target, CaMKII T287 to confirm a GOF effect in neuronal cells. As shown in Figure 5, there was a statistically significant, 2-fold decrease in the relative expression of phospho-CAMKII in PPM1D+/tr neuronal cells consistent with a GOF effect. Also shown in the figure, is a validation of the GO and KEGG findings that cytoskeleton function is disrupted in PPM1D+/tr glutamatergic neurons; a significant decrease in neurite outgrowth was found. Cytoskeleton function is critical for neurite outgrowth and synapse development, and many ASD and NDD candidate genes have an adverse effect on these processes (155-157).
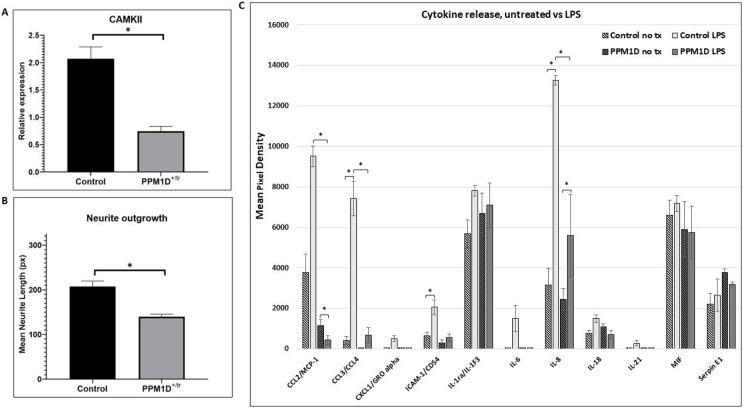
Figure 5. CaMKII, Neurite Outgrowth, Cytokine Release.
A. CaMKII phosphorylation was analyzed by quantifying Western blot signals for CaMKII, phospho-CaMKII, and cyclophilin as a loading control. The graph in 5A is a plot of the ratio of the normalized phospho-CaMKII signal (relative to cyclophilin) divided by the normalized CaMKII signal. A total of 4 control and 5 PPM1D+/tr neuronal samples were analyzed. The graph is the mean for the two groups, +/− SEM. The decrease in CaMKII phosphorylation was highly significant (p=0.0005, Student’s t-test, two-tailed). B. Neurite outgrowth was measured in day 21 glutamatergic neurons as described in the methods section. Tracings from two control and two PPM1D+/tr day 21 glutamatergic neurons were obtained, blind to genotype, for a total of 400 and 229 neurons analyzed, respectively. The difference between the two was highly significant (mean +/− SEM, 8.9E-07, Student’s t-test, two-tailed). C. Cytokine release was assayed using the Proteome Profiler Array Human Cytokine Array, as described in the methods. A total of 4 control and 3 PPM1D+/tr microglia samples were analyzed (the same samples that were analyzed in the proteomics and phosphoproteomics analyses plus an additional control that was not analyzed by proteomics. Culture supernatants were harvested after 24 hours of LPS treatment. Untreated (no tx) cells from the same differentiation were harvested simultaneously. The data are the means of 4 vs 3, with each spot on the array measured in duplicate. A Student’s t-test was used to calculate statistical significance. Those at p < 0.05 are indicated by asterisks: CCL2, untreated control LPS vs PPM1D+/tr LPS (p=0.01); untreated PPM1D+/tr vs PPM1D+/tr LPS (p=0.0009) (note CCL2 control vs control LPS had a p-value of 0.065); CCL3/CCL4, untreated control vs control LPS (p=0.02); CCL3/CCL4, control LPS vs PPM1D+/tr LPS (p=0.02); ICAM-1 untreated control vs control LPS (p=0.05); IL-8, untreated control vs control LPS (p=0.02), control LPS vs PPM1D+/tr LPS (p=0.05);
Finally, we measured the concentration of cytokines and chemokines in the supernatant following LPS treatment to assess microglia function following an innate immune challenge. The top GO term for LPS-treated PPM1D+/tr microglia was negative regulation of IL-6. This was based on DEPs that directly or indirectly affect IL-6 signaling (ZC3H12A, GBA, TNFAIP3, GAS6), rather than IL-6 levels per se, which was not detected in the proteomics analysis. As seen in Figure 5C, IL-6 was induced in LPS-treated control microglia, but not in the PPM1D+/tr cells as predicted. However, because of a large standard error and the small sample size, the induction was not statistically significant. In addition, we detected a slight increase in baseline IL-18 levels in PPM1D+/tr microglia compared with the controls, as predicted from the GO analysis, but that difference was also not statistically significant. There was also a decrease in IL-18 induction by LPS detected in the PPM1D+/tr microglia compared with LPS-treated controls, but the difference fell short of statistical significance (p=0.2). This is consistent with the proteomics data, which showed a statistical trend towards a decrease in IL-18 in the PPM1D+/tr LPS-treated microglia (−log2 p-value of 3.79 = 0.07, see Additional file 7: Table S5). In addition, there was, somewhat unexpectedly, a significant decrease in the induction of the chemokines CCL2 and CCL3/CCL4 by LPS between the control and PPM1D+/tr microglia (p=0.0009 and 0.02, respectively. In fact, while LPS induced an increase in CCL2 in the control sample that showed a trend towards statistical significance (p=0.065), a significant decrease was detected in the PPM1D+/tr cells (p=0.01). The findings suggest that truncated PPM1D causes deregulation of cytokine release.
Discussion
Although the sample size in this study was small, a number of interesting findings emerged that could explain the clinical features seen in JdVS. One is the significant decrease in the expression of the chromatin regulator and high-confidence ASD candidate gene POGZ in PPM1D+/tr microglia. This is consistent with the finding that Pogz KO mice show an upregulation of genes enriched in neuroinflammation and an increase in microglia phagocytosis in the prefrontal cortex (105). Similar and overlapping findings were found in Adnp KO mice. ADNP is another high-confidence ASD gene that forms a nuclear complex with POGZ and was also decreased in PPM1D+/tr microglia (108). Given the findings in mouse Pogz KO models, the significant decrease of POGZ in PPM1D+/tr suggests that microglia are playing a direct role in the neurodevelopmental aspects of JdVS. However, considering the neuronal proteomics analysis, neurons are likely also playing a causal role, as expected of a gene like PPM1D that is expressed ubiquitously throughout the CNS in neurons and non-neuronal cells. Considering that PPM1D is expressed at much higher levels in the cerebellum compared to other brain regions, as are both POGZ and ADNP, it will be particularly important to carry out the studies reported here in cerebellar organoids derived from our iPSC lines, or in mouse models. The cerebellum is now known to play a role in cognitive function and the development of ASD and NDD, in addition to its well-established effects on locomotor function and coordination (158-160).
Another interesting aspect of this study is related to the clinical observation that a small proportion of JdVS patients have acute neuropsychiatric decompensation, a phenomenon that has been described in genetic subgroups of NDD and ASD (109,161-164). One of the patients presented with symptoms consistent with PANS, as we previously reported (15), and two others with acute and subacute behavioral and motor regression following infection and noninfectious stressors (unpublished observations). PANS is an autoinflammatory/autoimmune disorder induced by group A beta-hemolytic Streptococcus and other infectious microbes, and behavioral regression in ASD has been hypothesized to have immune-based or infection-triggered underlying pathogenesis in some cases (162,165-167). Interestingly, ADNP is one of 11 ASD-associated candidate genes, most commonly found in regressive ASD (109). Our microglia proteomics and phosphoproteomics findings support the idea that PPM1D truncating mutations can affect the susceptibility to immune-based decompensation. For example, the microglia GO analysis showed that proteins involved in endothelial function that can affect the BBB are differentially expressed; disruption of the BBB resulting in increased permeability to peripheral inflammatory cytokines, chemokines, complement, and immune cells, has been viewed as a pathogenic feature of neuroinflammatory and neurodegenerative disorders (168-171). Also consistent with a neuroinflammatory vulnerability are the various connections to dysregulated innate immunity we detected in PPM1D+/− microglia, primarily through altered expression of ubiquitin ligases and ubiquitin-conjugating enzymes. Most notable is UBR4, which was the most downregulated DEP in LPS-stimulated microglia. UBR4 was also among the most down-regulated proteins detected in PPM1D+/tr microglia treated with poly I:C and IL-17 (unpublished observations, manuscript in preparation). As noted in the results section, UBR4 regulates oxidative stress and interferon signaling, and the degradation of PINK1, a mitochondrial serine/threonine kinase that recruits the E3 ligase PARKIN (PRKN) to induce mitophagy (136-138,172). Homozygous, LOF mutations in either PINK1 or PRKN are found in early onset, autosomal recessive forms of PD (139,140). Interestingly, we are aware of two cases of acute neuropsychiatric decompensation consistent with PANS who are heterozygous for LOF mutations in PRKN (manuscript in preparation). This connection between LPS-stimulated PPM1D+/tr microglia and PD suggests a common vulnerability to environmental stressors due to dysfunctional UBR4 signaling, with subsequent adverse effects on proteasomal degradation and mitophagy. A defect in mitophagy homeostasis has been implicated in the pathogenesis of PD, as well as AD (173,174). UBR4 variants have been found in some families with early-onset dementia (175). The effect of PPM1D truncating mutations on mitophagy has not yet been carried out. It should be noted that DEPs aside from UBR4 are connected to PD as seen in the KEGG pathway analyses showing that PD (and other neurodegenerative disorders) is among the top GO and KEGG pathways in both neurons and microglia.
In addition to UBR4, a number of other E3 ubiquitin ligases and their regulators, and ubiquitin-conjugating enzymes involved in innate immune responses were among the most differentially expressed proteins in untreated PPM1D+/tr microglia (CDC34, KLHDC4, and PELI1; upregulated: HERC6; downregulated), and in LPS stimulated microglia (UHRF1BP1, upregulated), as noted in the results section. In addition, the top-upregulated protein in PPM1D+/tr neurons was CUL4B, a component of the CUL4B-Ring E3 ligase. The specific substrates affected by these ubiquitin regulators in microglia and neurons, and how they might be involved in JdVS and neuroinflammation need to be investigated.
An important finding to consider regarding the neuroinflammatory potential of PPM1D+/tr microglia is the seemingly paradoxically lack of induction of IL-6 following LPS stimulation and the GO analysis that showed an enrichment of proteins that negatively regulate IL-6. This cytokine is one of the major proinflammatory cytokines implicated in neuroinflammation and maternal immune activation (123,176,177) In fact, in addition to IL-6, there was a generalized blunting of LPS-mediated cytokine induction in PPM1D+/tr microglia (Figure 5C). This was particularly the case for CCL2 and CCL3/CCL4, which showed significant decreases compared with LPS-induced control microglia. A blunted induction of ICAM-1 and IL-8 was also detected, but the control vs PPM1D+/tr difference was only significant for the latter (p=0.05). This suggests that TLR4 signaling is attenuated by truncated PPM1D. These findings need to be validated in a larger iPSC dataset, which is currently being carried out, and animal models. Reduced expression of CCL2 and CCL3/CCL4, which are potent monocyte attractants, in response to LPS activation, could affect the recruitment of transmigrating monocytes, a population of peripheral monocytes that can cross the BBB (178,179). Considering the dichotomy of monocytes and macrophages into pro- and anti-inflammatory subtypes, the effect of the blunted activation of CCL2 and CCL3/CCL4 on a neuroinflammatory process is difficult to predict and would need to be evaluated in an animal model. Furthermore, if the connection between PPM1D GOF variants and neuroinflammation is confirmed, then the idea that cytokines and chemokines affecting microglia and brain function are mediators may be too limiting. In the case of truncated PPM1D, a disturbance in microglia homeostasis and function may be at play, rather than an excessive release of proinflammatory cytokines and chemokines.
It is important to note that the iPSC lines used in these studies were not made from subjects with acute psychiatric decompensation, in either the JdVS subject we used or, especially, the CRISPR lines, which were made from a typically developing control. Thus, while connections to a neuroinflammatory process in this study are based on differences between control and PPM1D truncating mutations, they are occurring in the context of cellular stress conditions inherent in growing cultured cells in artificial media in an incubator. Therefore, replication in an animal model is essential.
Finally, it is important to consider the microglia findings in the context of the increased infections that occur in some JdVS children. In the original report of 14 cases, and a subsequent follow up of 33 cases, recurrent infections, in particular otitis media, were reported in approximately half (1,4). Although this has not been evaluated systematically, the increase in infections seen in some children has been sufficiently severe to warrant evaluations for immunodeficiencies by their physicians, which have been non-diagnostic so far. Considering the physiological and functional overlap between microglia and peripheral monocytes/macrophages, the differential expression of proteins involved in immune responses that were found in our proteomics analysis, as well as the blunted effect on cytokine and chemokine release could be having a similar effect in the periphery, reducing the effectiveness of an innate immune response to infections.
Limitations
The small sample size was a major limitation. Another is extrapolating data related to neuroinflammation using an in vitro microglia differentiation and cell culture system that is probably inducing cellular stress.
Conclusion
In summary, our findings show plausible mechanisms for the neurodevelopmental and cognitive features of JdVS, as well as the increased risk of neuroinflammatory-mediated decompensation, and perhaps the increased rate of infections seen in patients. The mechanistic links we identified to regression in NDD are also significant. Our findings provide additional support for the idea that a subgroup of NDD and ASD cases can experience neuropsychiatric decompensation caused by dysregulated innate immunity that is potentially treatable with immune modulators, as suggested by other investigators (162,180). In addition, the molecular connection to PD found with UBR4 and other DEPs (e.g., NUCKS1, GBA1) could also be significant in that unrecognized and under-treated neuroinflammatory processes could pose a future risk of PD and other neurodegenerative disorders. Thus, our analysis of JdVS, a rare disease with fewer than 100 reported cases, could be informative for disorders that have greater public health significance.
Supplementary Material
Additional file 1: Expanded Methods. This file contains a detailed description of the methods used in this study.
Additional file 2: Fig. S1. Microscopic image of unstained microglia grown in suspension. Two controls and two PPM1D+/tr samples are shown. The bar is 200 μMs. Two other samples not available.
Additional file 3: Table S1. Neuron proteomics, PPM1D+/tr vs controls. Tab 1. Gene Ontology (GO) analysis. Control sample names on top are shown in pastel; 1 and 4, 2 and 3 are biological duplicates. PPM1D+/tr samples are shown in light green. Samples 1 and 2, as well as 3 and 5, are biological duplicates. Differentially expressed proteins are arranged in descending order based on highest to lowest scores in a comparison of all PPM1D+/tr samples vs all controls. The score is calculated by multiplying Fold Change by the p-value (−log 2 of 4.32 corresponds to p=0.05). Tabs 2 and 3 (Neuron PPM1D+/tr Up KEGG and Neuron PPM1D+/tr Down KEGG, respectively) shows the KEGG analysis of all proteins that increased and decreased in the PPM1D+/− samples.
Additional file 4: Table S2. Neuron phosphoproteomics, PPM1D+/tr vs controls. Control sample names on top are shown in pastel. Controls 1 and 3, and 2 and 4 are biological duplicates. PPM1D+/− sample names are shown in green. Samples 2 and 4 are biological duplicates. Tab 1, DEPPs are differentially expressed phosphoproteins arranged in descending order based on highest to lowest scores, as described in the legend for Additional file 3: Table S1. Tab 1 (Phosphosites and GO) contains all phosphorylated proteins in descending order and the GO analysis. Tab 2 (normalize to protein value) show GO analysis and volcano plot. Tabs 3 and 4 are neuron phospho up 500 EnrichR and neuron phospho down 500 EnrichR, respectively, showing KEGG pathway analysis for phosphoproteins that increase or decrease in PPM1D+/− neurons. Additional GO analyses for up and down regulated phosphoproteins are shown, but were not described in the paper in order to avoid redundancy.
Additional file 5: Table S3. Microglia proteomics, PPM1D+/tr vs controls. Three control samples and three PPM1D+/− samples were analyzed. The microglia were in their baseline state – no treatment (no tx). Tab 1 (processing) shows the detailed steps of data processing starting from raw abundance to log2 transformation, to normalization and to imputation of missing values. Tab 2 is the differentially expressed protein list calculated as described in the neuron proteomics analysis (see figure legend, Additional file 3: Table S1), along with the GO analysis. Tab 3 is the DAVID KEGG analysis of up regulated proteins, which was not discussed in the paper since it overlapped with other findings. Tabs 4 and 5 are the KEGG pathways for the up and down regulated proteins, respectively, using 500 EnrichR. Tab 6 is the DAVID KEGG analysis of up regulated proteins.
Additional file 6: Table S4 Figure 2. Microglia phosphoproteomics, PPM1D+/tr vs controls. Phosphoproteomics was carried out on the same samples described in Additional file 5: Table S3. Tab 1 (PPM1D+tr untreated vs Control) contains the differentially expressed phosphoprotein list, along with the GO analysis. Tabs 2 and 3 (PPM1D+tr untreated Up KEGG and PPM1D+tr untreated Down KEGG) show KEGG pathways for up and downregulated phosphosites, respectively.
Additional file 7: Table S5. LPS treated microglia proteomics, PPM1D+/tr vs controls. Microglia derived from the same iPSC lines as described in the legend of Additional file 5: Table S3 were treated with LPS (see main text). Tab 1 (processing), as described in Additional file 5: Table S3. Tab 2 (microglia proteomics LPS) shows differentially expressed protein arranged by total score in descending order (PPM1D+/tr vs controls) and GO analysis. KEGG pathway analysis for up and downregulated proteins are shown in Tabs 3 and 4, respectively (Microglia LPS Up KEGG; Microglia LPS down KEGG).
Additional file 8: Table S6. LPS treated microglia phosphoproteomics, PPM1D+/tr vs controls. Phosphoproteomics was carried out on LPS treated microglia using the same samples described in Additional file 7: Table S5. Tab 1 (PPM1D+tr LPS vs Cont LPS) shows differentially phosphorylated phosphosites arranged by total score in descending order and the GO analysis. Tabs 2 and 3 (PPM1D+tr LPS enriched Up KEGG; PPM1D+tr LPS enriched Down KEGG) show the KEGG pathway analyses for phosphosites that increase or decreased, respectively.
Acknowledgments
We want to thank the Jansen de Vries Syndrome Foundation for their work and the families who provided samples for iPSC development. We also want to thank the Human Pluripotent Stem Cell Core (Director Eric Bouhassira, and Zi Yan) at the Albert Einsein College of Medicine for preparing and characterizing iPSC lines.
Funding
HML is supported by the NIH/NIMH (R21 MH131740) and the NIH/NICHD (P30 HD071593) to the Albert Einstein College of Medicine’s Rose F. Kennedy Intellectual and Developmental Disabilities Research Center. The Lachman lab also receives support from the Janice C. Blanchard Family Fund, The iPS Cell Research for Ryan Stearn fund. The Sidoli lab gratefully acknowledges funding from the Einstein-Mount Sinai Diabetes center, Merck, Relay Therapeutics, Deerfield (Xseed award), and the NIH Office of the Director (S10OD030286). The Cytek® Aurora System - Full Spectrum Flow Cytometry unit was supported by an NIH instrument grant, S10 OD026833. The Albert Einstein College of Medicine National Cancer Institute center grant, P30CA013330, provided support for the FACS facility
Abbreviations
- JdVS
Jansen de Vries Syndrome
- iPSC
induced pluripotent stem cell
- LOF
loss-of-function
- GOF
gain of function
- LPS
lipopolysaccharide
- NDD
neurodevelopmental disorder
- ADHD
attention deficit hyperactivity disorder
- ASD
autism spectrum disorder
- DDR
DNA damage response
- OCD
obsessive-compulsive disorder
- KO
knockout
- hSC
hematopoietic stem cells
- FACS
Fluorescence-activated cell sorting
- PANS
pediatric acute-onset neuropsychiatric syndrome
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- BBB
brain blood barrier
- ALS
Amyotrophic Lateral Sclerosis
- HD
Huntington Disease
- PD
Parkinson Disease
- AD
Alzheimer Disease
- IFN-γ
interferon-gamma
- cGAS/STING
cyclic GMP-AMP synthase-stimulator of interferon genes
Footnotes
Ethics approval and consent to participate
Informed consent was obtained by the corresponding author under an Albert Einstein College of Medicine, IRB-approved protocol.
Consent for publication
Consent for publication was obtained from participants and parents
Availability of data and materials
Proteomics and phosphoproteomics data were deposited at xxxxxxxxxx ().
Competing interests
The authors have no competing interests
References
- (1).Wojcik MH, Srivastava S, Agrawal PB, Balci TB, Callewaert B, Calvo PL, et al. Jansen-de Vries syndrome: Expansion of the PPM1D clinical and phenotypic spectrum in 34 families. Am J Med Genet A 2023. Jul;191(7):1900–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Porrmann J, Rump A, Hackmann K, Di Donato N, Kahlert AK, Wagner J, et al. Novel truncating PPM1D mutation in a patient with intellectual disability. Eur J Med Genet 2019. Jan;62(1):70–72. [DOI] [PubMed] [Google Scholar]
- (3).Lelieveld SH, Reijnders MR, Pfundt R, Yntema HG, Kamsteeg EJ, de Vries P, et al. Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat Neurosci 2016. Sep;19(9):1194–1196. [DOI] [PubMed] [Google Scholar]
- (4).Jansen S, Geuer S, Pfundt R, Brough R, Ghongane P, Herkert JC, et al. De Novo Truncating Mutations in the Last and Penultimate Exons of PPM1D Cause an Intellectual Disability Syndrome. Am J Hum Genet 2017. Apr 6;100(4):650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kleiblova P, Shaltiel IA, Benada J, Sevcik J, Pechackova S, Pohlreich P, et al. Gain-of-function mutations of PPM1D/Wip1 impair the p53-dependent G1 checkpoint. J Cell Biol 2013. May 13;201(4):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kahn JD, Miller PG, Silver AJ, Sellar RS, Bhatt S, Gibson C, et al. PPM1D-truncating mutations confer resistance to chemotherapy and sensitivity to PPM1D inhibition in hematopoietic cells. Blood 2018. Sep 13;132(11):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Dudgeon C, Shreeram S, Tanoue K, Mazur SJ, Sayadi A, Robinson RC, et al. Genetic variants and mutations of PPM1D control the response to DNA damage. Cell Cycle 2013. Aug 15;12(16):2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Bhattacharya D, Hiregange D, Rao BJ. ATR kinase regulates its attenuation via PPM1D phosphatase recruitment to chromatin during recovery from DNA replication stress signalling. J Biosci 2018. Mar;43(1):25–47. [PubMed] [Google Scholar]
- (9).Gräf JF, Mikicic I, Ping X, Scalera C, Mayr K, Stelzl LS, et al. Substrate spectrum of PPM1D in the cellular response to DNA double-strand breaks. iScience 2022. Aug 9;25(9):104892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Cardoso M, Paulo P, Maia S, Teixeira MR. Truncating and missense PPM1D mutations in early-onset and/or familial/hereditary prostate cancer patients. Genes Chromosomes Cancer 2016. Dec;55(12):954–961. [DOI] [PubMed] [Google Scholar]
- (11).Pechackova S, Burdova K, Macurek L. WIP1 phosphatase as pharmacological target in cancer therapy. J Mol Med (Berl) 2017. Jun;95(6):589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Li K, Liu Y, Xu S, Wang J. PPM1D Functions as Oncogene and is Associated with Poor Prognosis in Esophageal Squamous Cell Carcinoma. Pathol Oncol Res 2018. Oct 30. [DOI] [PubMed] [Google Scholar]
- (13).Alzahrani AS, Murugan AK, Qasem E, Alswailem MM, AlGhamdi B, Moria Y, et al. Absence of EIF1AX, PPM1D, and CHEK2 mutations reported in Thyroid Cancer Genome Atlas (TCGA) in a large series of thyroid cancer. Endocrine 2018. Sep 29. [DOI] [PubMed] [Google Scholar]
- (14).Nomura M, Mukasa A, Nagae G, Yamamoto S, Tatsuno K, Ueda H, et al. Distinct molecular profile of diffuse cerebellar gliomas. Acta Neuropathol 2017. Dec;134(6):941–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Trifiletti R, Lachman HM, Manusama O, Zheng D, Spalice A, Chiurazzi P, et al. Identification of ultra-rare genetic variants in pediatric acute onset neuropsychiatric syndrome (PANS) by exome and whole genome sequencing. Sci Rep 2022. Jun 30;12(1):11106–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Vreeland A, Thienemann M, Cunningham M, Muscal E, Pittenger C, Frankovich J. Neuroinflammation in Obsessive-Compulsive Disorder: Sydenham Chorea, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections, and Pediatric Acute Onset Neuropsychiatric Syndrome. Psychiatr Clin North Am 2023. Mar;46(1):69–88. [DOI] [PubMed] [Google Scholar]
- (17).Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol 2015. Feb;25(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chan A, Gao J, Houston M, Willett T, Farhadian B, Silverman M, et al. Children With PANS May Manifest POTS. Front Neurol 2022. Apr 26;13:819636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Fernandez F, Soon I, Li Z, Kuan TC, Min DH, Wong ES, et al. Wip1 phosphatase positively modulates dendritic spine morphology and memory processes through the p38MAPK signaling pathway. Cell Adh Migr 2012;6(4):333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Tang Y, Pan B, Zhou X, Xiong K, Gao Q, Huang L, et al. Wip1-dependent modulation of macrophage migration and phagocytosis. Redox Biol 2017. Oct;13:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Brichkina A, Bulavin DV. WIP-ing out atherosclerosis with autophagy. Autophagy 2012. Oct;8(10):1545–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sun B, Hu X, Liu G, Ma B, Xu Y, Yang T, et al. Phosphatase Wip1 negatively regulates neutrophil migration and inflammation. J Immunol 2014. Feb 1;192(3):1184–1195. [DOI] [PubMed] [Google Scholar]
- (23).Barnes J, Salas F, Mokhtari R, Dolstra H, Pedrosa E, Lachman HM. Modeling the neuropsychiatric manifestations of Lowe syndrome using induced pluripotent stem cells: defective F-actin polymerization and WAVE-1 expression in neuronal cells. Mol Autism 2018. Aug 15;9:44–3. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Olivier E, Qiu C, Bouhassira EE. Novel, high-yield red blood cell production methods from CD34-positive cells derived from human embryonic stem, yolk sac, fetal liver, cord blood, and peripheral blood. Stem Cells Transl Med 2012. Aug;1(8):604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013. Sep 12;154(6):1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 2013. Jun 5;78(5):785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 2004. Apr;58(2):167–176. [DOI] [PubMed] [Google Scholar]
- (28).Kulej K, Avgousti DC, Sidoli S, Herrmann C, Della Fera AN, Kim ET, et al. Time-resolved Global and Chromatin Proteomics during Herpes Simplex Virus Type 1 (HSV-1) Infection. Mol Cell Proteomics 2017. Apr;16(4 suppl 1):S92–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Aguilan JT, Kulej K, Sidoli S. Guide for protein fold change and p-value calculation for non-experts in proteomics. Mol Omics 2020. Dec 1;16(6):573–582. [DOI] [PubMed] [Google Scholar]
- (30).Engholm-Keller K, Birck P, Størling J, Pociot F, Mandrup-Poulsen T, Larsen MR. TiSH--a robust and sensitive global phosphoproteomics strategy employing a combination of TiO2, SIMAC, and HILIC. J Proteomics 2012. Oct 22;75(18):5749–5761. [DOI] [PubMed] [Google Scholar]
- (31).Thingholm TE, Larsen MR. The Use of Titanium Dioxide for Selective Enrichment of Phosphorylated Peptides. Methods Mol Biol 2016;1355:135–146. [DOI] [PubMed] [Google Scholar]
- (32).Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 2009. Feb 3;10:48–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chou MF, Schwartz D. Biological sequence motif discovery using motif-x. Curr Protoc Bioinformatics 2011. Sep;Chapter 13:Unit 13.15–24. [DOI] [PubMed] [Google Scholar]
- (34).Song C, Ye M, Liu Z, Cheng H, Jiang X, Han G, et al. Systematic analysis of protein phosphorylation networks from phosphoproteomic data. Mol Cell Proteomics 2012. Oct;11(10):1070–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Raaijmakers LM, Giansanti P, Possik PA, Mueller J, Peeper DS, Heck AJ, et al. PhosphoPath: Visualization of Phosphosite-centric Dynamics in Temporal Molecular Networks. J Proteome Res 2015. Oct 2;14(10):4332–4341. [DOI] [PubMed] [Google Scholar]
- (36).Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response to severe COVID-19. medRxiv 2020. Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wang P, Su H, Zhang L, Chen H, Hu X, Yang F, et al. Phosphatase wild-type p53-induced phosphatase 1 controls the development of T(H)9 cells and allergic airway inflammation. J Allergy Clin Immunol 2018. Jun;141(6):2168–2181. [DOI] [PubMed] [Google Scholar]
- (38).Schito ML, Demidov ON, Saito S, Ashwell JD, Appella E. Wip1 phosphatase-deficient mice exhibit defective T cell maturation due to sustained p53 activation. J Immunol 2006. Apr 15;176(8):4818–4825. [DOI] [PubMed] [Google Scholar]
- (39).Challakkara MF, Chhabra R. snoRNAs in hematopoiesis and blood malignancies: A comprehensive review. J Cell Physiol 2023. Jun;238(6):1207–1225. [DOI] [PubMed] [Google Scholar]
- (40).Dar AA, Sawada K, Dybas JM, Moser EK, Lewis EL, Park E, et al. The E3 ubiquitin ligase Cul4b promotes CD4+ T cell expansion by aiding the repair of damaged DNA. PLoS Biol 2021. Feb 1;19(2):e3001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Li T, Wu S, Jia L, Cao W, Yao Y, Zhao G, et al. CUL4 E3 ligase regulates the proliferation and apoptosis of lung squamous cell carcinoma and small cell lung carcinoma. Cancer Biol Med 2020. May 15;17(2):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Liu H, Lu W, He H, Wu J, Zhang C, Gong H, et al. Inflammation-dependent overexpression of c-Myc enhances CRL4(DCAF4) E3 ligase activity and promotes ubiquitination of ST7 in colitis-associated cancer. J Pathol 2019. Aug;248(4):464–475. [DOI] [PubMed] [Google Scholar]
- (43).Lopes F, Torres F, Soares G, Barbosa M, Silva J, Duque F, et al. Genomic imbalances defining novel intellectual disability associated loci. Orphanet J Rare Dis 2019. Jul 5;14(1):164–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Guan J, Liu X, Zhang H, Lv Y, Wang X, Yang X, et al. Generation of an iPSC line (SDQLCHi015-A) from peripheral blood mononuclear cells of a patient with mental retardation type 15 carrying c.1007_1011del, p.(Ile336fs) in CUL4B gene. Stem Cell Res 2019. Dec;41:101628. [DOI] [PubMed] [Google Scholar]
- (45).Kampmeier A, Leitão E, Parenti I, Beygo J, Depienne C, Bramswig NC, et al. PHIP-associated Chung-Jansen syndrome: Report of 23 new individuals. Front Cell Dev Biol 2023. Jan 16;10:1020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).López M, Pérez-Grijalba V, García-Cobaleda I, Domínguez-Garrido E. A 22.5 kb deletion in CUL4B causing Cabezas syndrome identified using CNV approach from WES data. Clin Case Rep 2020. Sep 29;8(12):3184–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Song Y, Li P, Qin L, Xu Z, Jiang B, Ma C, et al. CUL4B negatively regulates Toll-like receptor-triggered proinflammatory responses by repressing Pten transcription. Cell Mol Immunol 2021. Feb;18(2):339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Xu Z, Li L, Qian Y, Song Y, Qin L, Duan Y, et al. Upregulation of IL-6 in CUL4B-deficient myeloid-derived suppressive cells increases the aggressiveness of cancer cells. Oncogene 2019. Jul;38(30):5860–5872. [DOI] [PubMed] [Google Scholar]
- (49).Zhao Z, Wang H, Kang N, Wang Z, Hou X, Hu L, et al. Aurora kinase a promotes the progression of papillary thyroid carcinoma by activating the mTORC2-AKT signalling pathway. Cell Biosci 2022. Dec 5;12(1):195–z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Chiuso F, Delle Donne R, Giamundo G, Rinaldi L, Borzacchiello D, Moraca F, et al. Ubiquitylation of BBSome is required for ciliary assembly and signaling. EMBO Rep 2023. Feb 6:e55571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Kattan RE, Han H, Seo G, Yang B, Lin Y, Dotson M, et al. Interactome Analysis of Human Phospholipase D and Phosphatidic Acid-Associated Protein Network. Mol Cell Proteomics 2022. Feb;21(2):100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Smith CE, Tsai YC, Liang Y, Khago D, Mariano J, Li J, et al. A structurally conserved site in AUP1 binds the E2 enzyme UBE2G2 and is essential for ER-associated degradation. PLoS Biol 2021. Dec 8;19(12):e3001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Cristea I, Bruland O, Rødahl E, Bredrup C. K(+) regulates relocation of Pellino-2 to the site of NLRP3 inflammasome activation in macrophages. FEBS Lett 2021. Oct;595(19):2437–2446. [DOI] [PubMed] [Google Scholar]
- (54).Bögershausen N, Wollnik B. Mutational Landscapes and Phenotypic Spectrum of SWI/SNF-Related Intellectual Disability Disorders. Front Mol Neurosci 2018. Aug 3;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Sun X, Cheng L, Sun Y. Autism-associated protein POGZ controls ESCs and ESC neural induction by association with esBAF. Mol Autism 2022. Jun 1;13(1):24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Shillington A, Pedapati E, Hopkin R, Suhrie K. Early behavioral and developmental interventions in ADNP-syndrome: A case report of SWI/SNF-related neurodevelopmental syndrome. Mol Genet Genomic Med 2020. Jun;8(6):e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).D'Incal CP, Van Rossem KE, De Man K, Konings A, Van Dijck A, Rizzuti L, et al. Chromatin remodeler Activity-Dependent Neuroprotective Protein (ADNP) contributes to syndromic autism. Clin Epigenetics 2023. Mar 21;15(1):45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).O'Leary PC, Chen H, Doruk YU, Williamson T, Polacco B, McNeal AS, et al. Resistance to ATR Inhibitors Is Mediated by Loss of the Nonsense-Mediated Decay Factor UPF2. Cancer Res 2022. Nov 2;82(21):3950–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Johnson JL, Stoica L, Liu Y, Zhu PJ, Bhattacharya A, Buffington SA, et al. Inhibition of Upf2-Dependent Nonsense-Mediated Decay Leads to Behavioral and Neurophysiological Abnormalities by Activating the Immune Response. Neuron 2019. Nov 20;104(4):665–679.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).James MO, Jahn SC, Zhong G, Smeltz MG, Hu Z, Stacpoole PW. Therapeutic applications of dichloroacetate and the role of glutathione transferase zeta-1. Pharmacol Ther 2017. Feb;170:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Gao Q, Cheng B, Chen C, Lei C, Lin X, Nie D, et al. Dysregulated glucuronic acid metabolism exacerbates hepatocellular carcinoma progression and metastasis through the TGFβ signalling pathway. Clin Transl Med 2022. Aug;12(8):e995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Aborode AT, Pustake M, Awuah WA, Alwerdani M, Shah P, Yarlagadda R, et al. Targeting Oxidative Stress Mechanisms to Treat Alzheimer's and Parkinson's Disease: A Critical Review. Oxid Med Cell Longev 2022. Jul 31;2022:7934442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Alqahtani T, Deore SL, Kide AA, Shende BA, Sharma R, Chakole RD, et al. Mitochondrial dysfunction and oxidative stress in Alzheimer's disease, and Parkinson's disease, Huntington's disease and Amyotrophic Lateral Sclerosis -An updated review. Mitochondrion 2023. Jun 1. [DOI] [PubMed] [Google Scholar]
- (64).Jiménez-Vacas JM, Herrero-Aguayo V, Montero-Hidalgo AJ, Gómez-Gómez E, Fuentes-Fayos AC, León-González AJ, et al. Dysregulation of the splicing machinery is directly associated to aggressiveness of prostate cancer. EBioMedicine 2020. Jan;51:102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Song X, Ma J. SRRM1 promotes the proliferation, migration, and invasion of hepatocellular carcinoma cells by regulating the JAK/STAT signaling pathway. Tissue Cell 2022. Dec;79:101954. [DOI] [PubMed] [Google Scholar]
- (66).Østvold AC, Grundt K, Wiese C. NUCKS1 is a highly modified, chromatin-associated protein involved in a diverse set of biological and pathophysiological processes. Biochem J 2022. Jun 17;479(11):1205–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Maranon DG, Sharma N, Huang Y, Selemenakis P, Wang M, Altina N, et al. NUCKS1 promotes RAD54 activity in homologous recombination DNA repair. J Cell Biol 2020. Oct 5;219(10):e201911049. doi: 10.1083/jcb.201911049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Yue Y, Leung SG, Liu Y, Huang Y, Grundt K, Østvold A, et al. Nucks1 synergizes with Trp53 to promote radiation lymphomagenesis in mice. Oncotarget 2016. Sep 20;7(38):61874–61889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Pan H, Liu Z, Ma J, Li Y, Zhao Y, Zhou X, et al. Genome-wide association study using whole-genome sequencing identifies risk loci for Parkinson's disease in Chinese population. NPJ Parkinsons Dis 2023. Feb 9;9(1):22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Yamaguchi H, Durell SR, Chatterjee DK, Anderson CW, Appella E. The Wip1 phosphatase PPM1D dephosphorylates SQ/TQ motifs in checkpoint substrates phosphorylated by PI3K-like kinases. Biochemistry 2007. Nov 6;46(44):12594–12603. [DOI] [PubMed] [Google Scholar]
- (71).Hehr U, Uyanik G, Aigner L, Couillard-Despres S, Winkler J. DCX-Related Disorders. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. GeneReviews(®) Seattle (WA): University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle; 1993. [Google Scholar]
- (72).Hughes HK, Moreno R J, Ashwood P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav Immun 2023. Feb;108:245–254. [DOI] [PubMed] [Google Scholar]
- (73).Lampiasi N, Bonaventura R, Deidda I, Zito F, Russo R. Inflammation and the Potential Implication of Macrophage-Microglia Polarization in Human ASD: An Overview. Int J Mol Sci 2023. Jan 31;24(3):2703. doi: 10.3390/ijms24032703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Zhao D, Mokhtari R, Pedrosa E, Birnbaum R, Zheng D, Lachman HM. Transcriptome analysis of microglia in a mouse model of Rett syndrome: differential expression of genes associated with microglia/macrophage activation and cellular stress. Mol Autism 2017. Mar 29;8:17–z. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 2012. Apr 5;484(7392):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Verma N, Velmurugan GV, Winford E, Coburn H, Kotiya D, Leibold N, et al. Aβ efflux impairment and inflammation linked to cerebrovascular accumulation of amyloid-forming amylin secreted from pancreas. Commun Biol 2023. Jan 3;6(1):2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Martinez-Lozada Z, Robinson MB. Reciprocal communication between astrocytes and endothelial cells is required for astrocytic glutamate transporter 1 (GLT-1) expression. Neurochem Int 2020. Oct;139:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Zhen H, Zhao L, Ling Z, Kuo L, Xue X, Feng J. Wip1 regulates blood-brain barrier function and neuro-inflammation induced by lipopolysaccharide via the sonic hedgehog signaling signaling pathway. Mol Immunol 2018. Jan;93:31–37. [DOI] [PubMed] [Google Scholar]
- (79).Olcum M, Tastan B, Kiser C, Genc S, Genc K. Microglial NLRP3 inflammasome activation in multiple sclerosis. Adv Protein Chem Struct Biol 2020;119:247–308. [DOI] [PubMed] [Google Scholar]
- (80).Anderson FL, Biggs KE, Rankin BE, Havrda MC. NLRP3 inflammasome in neurodegenerative disease. Transl Res 2022. Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Braatz C, Komes MP, Ravichandran KA, de Fragas MG, Griep A, Schwartz S, et al. NLRP3-directed antisense oligonucleotides reduce microglial immunoactivities in vitro. J Neurochem 2023. Feb 17. [DOI] [PubMed] [Google Scholar]
- (82).Deng Z, Zhou X, Lu J, Yue Z. Autophagy deficiency in neurodevelopmental disorders. Cell Biosci 2021. Dec 17;11(1):214–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Zhang Q, Sterling K, Xu L, Xing M, Cai F, Yu S, et al. CNTNAP2 Protein Is Degraded by the Ubiquitin-Proteasome System and the Macroautophagy-Lysosome Pathway. Mol Neurobiol 2023. May;60(5):2455–2469. [DOI] [PubMed] [Google Scholar]
- (84).Zapata-Muñoz J, Villarejo-Zori B, Largo-Barrientos P, Boya P. Towards a better understanding of the neuro-developmental role of autophagy in sickness and in health. Cell Stress 2021. Jun 29;5(7):99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Choi I, Heaton GR, Lee YK, Yue Z. Regulation of α-synuclein homeostasis and inflammasome activation by microglial autophagy. Sci Adv 2022. Oct 28;8(43):eabn1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Lv N, Zhao Y, Liu X, Ye L, Liang Z, Kang Y, et al. Dysfunctional telomeres through mitostress-induced cGAS/STING activation to aggravate immune senescence and viral pneumonia. Aging Cell 2022. Apr;21(4):e13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Harper JW, Schulman BA. Cullin-RING Ubiquitin Ligase Regulatory Circuits: A Quarter Century Beyond the F-Box Hypothesis. Annu Rev Biochem 2021. Jun 20;90:403–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Hill S, Reichermeier K, Scott DC, Samentar L, Coulombe-Huntington J, Izzi L, et al. Robust cullin-RING ligase function is established by a multiplicity of poly-ubiquitylation pathways. Elife 2019. Dec 23;8: 10.7554/eLife.51163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Gan Z, Wang B, Lu Y, Cai S, Cai J, Jian J, et al. Molecular characterization and expression of CD2BP2 in Nile tilapia (Oreochromis niloticus) in response to Streptococcus agalactiae stimulus. Gene 2014. Sep 10;548(1):126–133. [DOI] [PubMed] [Google Scholar]
- (90).Blasi G, Bortoletto E, Gasparotto M, Filippini F, Bai C, Rosani U, et al. A glimpse on metazoan ZNFX1 helicases, ancient players of antiviral innate immunity. Fish Shellfish Immunol 2022. Feb;121:456–466. [DOI] [PubMed] [Google Scholar]
- (91).Zhang L, Ko C, Li Y, Jie Z, Zhu L, Zhou X, et al. Peli1 facilitates NLRP3 inflammasome activation by mediating ASC ubiquitination. Cell Rep 2021. Oct 26;37(4):109904. [DOI] [PubMed] [Google Scholar]
- (92).Xu J, Yu T, Pietronigro EC, Yuan J, Arioli J, Pei Y, et al. Peli1 impairs microglial Aβ phagocytosis through promoting C/EBPβ degradation. PLoS Biol 2020. Oct 5;18(10):e3000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Zhou W, Wang J, Wang X, Wang B, Zhao Z, Fu J, et al. Degradation of HDAC10 by autophagy promotes IRF3-mediated antiviral innate immune responses. Sci Signal 2022. Dec 20;15(765):eabo4356. [DOI] [PubMed] [Google Scholar]
- (94).Pan M, Yin Y, Hu T, Wang X, Jia T, Sun J, et al. UXT attenuates the CGAS-STING1 signaling by targeting STING1 for autophagic degradation. Autophagy 2023. Feb;19(2):440–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Oduro PK, Zheng X, Wei J, Yang Y, Wang Y, Zhang H, et al. The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy. Acta Pharm Sin B 2022. Jan;12(1):50–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Al Hamrashdi M, Brady G. Regulation of IRF3 activation in human antiviral signaling pathways. Biochem Pharmacol 2022. Jun;200:115026. [DOI] [PubMed] [Google Scholar]
- (97).Stupfler B, Verriez C, Gallois-Montbrun S, Marquet R, Paillart J. Degradation-Independent Inhibition of APOBEC3G by the HIV-1 Vif Protein. Viruses 2021. Apr 3;13(4):617. doi: 10.3390/v13040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Bao Q, Zhou J. Various strategies for developing APOBEC3G protectors to circumvent human immunodeficiency virus type 1. Eur J Med Chem 2023. Feb 6;250:115188. [DOI] [PubMed] [Google Scholar]
- (99).Sharma S, Wang J, Alqassim E, Portwood S, Cortes Gomez E, Maguire O, et al. Mitochondrial hypoxic stress induces widespread RNA editing by APOBEC3G in natural killer cells. Genome Biol 2019. Feb 21;20(1):37–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Salter JD, Polevoda B, Bennett RP, Smith HC. Regulation of Antiviral Innate Immunity Through APOBEC Ribonucleoprotein Complexes. Subcell Biochem 2019;93:193–219. [DOI] [PubMed] [Google Scholar]
- (101).Yuan Y, Qin H, Li H, Shi W, Bao L, Xu S, et al. The Functional Roles of ISG15/ISGylation in Cancer. Molecules 2023. Jan 31;28(3):1337. doi: 10.3390/molecules28031337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Paparisto E, Woods MW, Coleman MD, Moghadasi SA, Kochar DS, Tom SK, et al. Evolution-Guided Structural and Functional Analyses of the HERC Family Reveal an Ancient Marine Origin and Determinants of Antiviral Activity. J Virol 2018. Jun 13;92(13):e00528–18. Print 2018 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Jacquet S, Pontier D, Etienne L. Rapid Evolution of HERC6 and Duplication of a Chimeric HERC5/6 Gene in Rodents and Bats Suggest an Overlooked Role of HERCs in Mammalian Immunity. Front Immunol 2020. Dec 18;11:605270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Heath J, Cheyou ES, Findlay S, Luo VM, Carpio EP, Lee J, et al. POGZ promotes homology-directed DNA repair in an HP1-dependent manner. EMBO Rep 2022. Jan 5;23(1):e51041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Conrow-Graham M, Williams JB, Martin J, Zhong P, Cao Q, Rein B, et al. A convergent mechanism of high risk factors ADNP and POGZ in neurodevelopmental disorders. Brain 2022. Sep 14;145(9):3250–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Bruno LP, Doddato G, Valentino F, Baldassarri M, Tita R, Fallerini C, et al. New Candidates for Autism/Intellectual Disability Identified by Whole-Exome Sequencing. Int J Mol Sci 2021. Dec 14;22(24):13439. doi: 10.3390/ijms222413439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Merriweather A, Murdock DR, Rosenfeld JA, Dai H, Ketkar S, Emrick L, et al. A novel, de novo intronic variant in POGZ causes White-Sutton syndrome. Am J Med Genet A 2022. Jul;188(7):2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Markenscoff-Papadimitriou E, Binyameen F, Whalen S, Price J, Lim K, Ypsilanti AR, et al. Autism risk gene POGZ promotes chromatin accessibility and expression of clustered synaptic genes. Cell Rep 2021. Dec 7;37(10):110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Tammimies K. Genetic mechanisms of regression in autism spectrum disorder. Neurosci Biobehav Rev 2019. Jul;102:208–220. [DOI] [PubMed] [Google Scholar]
- (110).Van Dijck A, Vandeweyer G, Kooy F. ADNP-Related Disorder. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al. , editors. GeneReviews(®) Seattle (WA): University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved; 1993. [Google Scholar]
- (111).Ganaiem M, Karmon G, Ivashko-Pachima Y, Gozes I. Distinct Impairments Characterizing Different ADNP Mutants Reveal Aberrant Cytoplasmic-Nuclear Crosstalk. Cells 2022. Sep 26;11(19):2994. doi: 10.3390/cells11192994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Bellon M, Nicot C. Feedback Loop Regulation between Pim Kinases and Tax Keeps Human T-Cell Leukemia Virus Type 1 Viral Replication in Check. J Virol 2022. Feb 9;96(3):e0196021–21. Epub 2021 Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Clements AN, Warfel NA. Targeting PIM Kinases to Improve the Efficacy of Immunotherapy. Cells 2022. Nov 21;11(22):3700. doi: 10.3390/cells11223700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).van der Graaf A, Zorro MM, Claringbould A, Võsa U, Aguirre-Gamboa R, Li C, et al. Systematic Prioritization of Candidate Genes in Disease Loci Identifies TRAFD1 as a Master Regulator of IFNγ Signaling in Celiac Disease. Front Genet 2021. Jan 25;11:562434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Sanada T, Takaesu G, Mashima R, Yoshida R, Kobayashi T, Yoshimura A. FLN29 deficiency reveals its negative regulatory role in the Toll-like receptor (TLR) and retinoic acid-inducible gene I (RIG-I)-like helicase signaling pathway. J Biol Chem 2008. Dec 5;283(49):33858–33864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Cleverley K, Lee WC, Mumford P, Collins T, Rickman M, Cunningham TJ, et al. A novel knockout mouse for the small EDRK-rich factor 2 (Serf2) showing developmental and other deficits. Mamm Genome 2021. Apr;32(2):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Chen B, Sheng D, Wang C, Liu W, Hu A, Xiao X, et al. FLI1 regulates inflammation-associated genes to accelerate leukemogenesis. Cell Signal 2022. Apr;92:110269. [DOI] [PubMed] [Google Scholar]
- (118).Jiang Q, Wang Y, Xiang M, Hua J, Zhou T, Chen F, et al. UFL1, a UFMylation E3 ligase, plays a crucial role in multiple cellular stress responses. Front Endocrinol (Lausanne) 2023. Feb 10;14:1123124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Li J, Tang X, Tu X, Jin Z, Dong H, Yang Q, et al. UFL1 alleviates ER stress and apoptosis stimulated by LPS via blocking the ferroptosis pathway in human granulosa-like cells. Cell Stress Chaperones 2022. Sep;27(5):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Fang Z, Pan Z. Essential Role of Ubiquitin-Fold Modifier 1 Conjugation in DNA Damage Response. DNA Cell Biol 2019. Oct;38(10):1030–1039. [DOI] [PubMed] [Google Scholar]
- (121).Wang Y, Que H, Rong Y. Autophagosomal components recycling on autolysosomes. Trends Cell Biol 2022. Nov;32(11):897–899. [DOI] [PubMed] [Google Scholar]
- (122).Shi Z, Chen S, Han X, Peng R, Luo J, Yang L, et al. The rare mutation in the endosome-associated recycling protein gene VPS50 is associated with human neural tube defects. Mol Cytogenet 2019. Feb 20;12:8–9. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Majerczyk D, Ayad EG, Brewton KL, Saing P, Hart PC. Systemic maternal inflammation promotes ASD via IL-6 and IFN-γ. Biosci Rep 2022. Nov 30;42(11):BSR20220713. doi: 10.1042/BSR20220713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Ma Y, Wang J, Guo S, Meng Z, Ren Y, Xie Y, et al. Cytokine/chemokine levels in the CSF and serum of anti-NMDAR encephalitis: A systematic review and meta-analysis. Front Immunol 2023. Jan 23;13:1064007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Mousten IV, Sørensen NV, Christensen RHB, Benros ME. Cerebrospinal Fluid Biomarkers in Patients With Unipolar Depression Compared With Healthy Control Individuals: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022. Jun 1;79(6):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Kwon J, Suessmilch M, McColl A, Cavanagh J, Morris BJ. Distinct trans-placental effects of maternal immune activation by TLR3 and TLR7 agonists: implications for schizophrenia risk. Sci Rep 2021. Dec 13;11(1):23841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Poli A, Abdul-Hamid S, Zaurito AE, Campagnoli F, Bevilacqua V, Sheth B, et al. PIP4Ks impact on PI3K, FOXP3, and UHRF1 signaling and modulate human regulatory T cell proliferation and immunosuppressive activity. Proc Natl Acad Sci U S A 2021. Aug 3;118(31):e2010053118. doi: 10.1073/pnas.2010053118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Wu J, Wang M, Chen H, Xu J, Zhang G, Gu C, et al. The Rare Variant rs35356162 in UHRF1BP1 Increases Bladder Cancer Risk in Han Chinese Population. Front Oncol 2020. Feb 11;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Grünblatt E, Oneda B, Ekici AB, Ball J, Geissler J, Uebe S, et al. High resolution chromosomal microarray analysis in paediatric obsessive-compulsive disorder. BMC Med Genomics 2017. Nov 28;10(1):68–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Granek Z, Barczuk J, Siwecka N, Rozpędek-Kamińska W, Kucharska E, Majsterek I. GBA1 Gene Mutations in α-Synucleinopathies-Molecular Mechanisms Underlying Pathology and Their Clinical Significance. Int J Mol Sci 2023. Jan 20;24(3):2044. doi: 10.3390/ijms24032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Parlar SC, Grenn FP, Kim JJ, Baluwendraat C, Gan-Or Z. Classification of GBA1 Variants in Parkinson's Disease: The GBA1-PD Browser. Mov Disord 2023. Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Vitner EB, Farfel-Becker T, Ferreira NS, Leshkowitz D, Sharma P, Lang KS, et al. Induction of the type I interferon response in neurological forms of Gaucher disease. J Neuroinflammation 2016. May 12;13(1):104–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Liu Q, Shen Z, Pan H, Ma S, Xiong F, He F. The molecular mechanism of Gaucher disease caused by compound heterozygous mutations in GBA1 gene. Front Pediatr 2023. Jan 26;11:1092645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Xu Y, Li Y, Wang C, Han T, Liu H, Sun L, et al. The reciprocal interactions between microglia and T cells in Parkinson's disease: a double-edged sword. J Neuroinflammation 2023. Feb 12;20(1):33–y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell 2014. Jul 31;158(3):506–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Heo AJ, Kim SB, Ji CH, Han D, Lee SJ, Lee SH, et al. The N-terminal cysteine is a dual sensor of oxygen and oxidative stress. Proc Natl Acad Sci U S A 2021. Dec 14;118(50):e2107993118. doi: 10.1073/pnas.2107993118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Tripathi S, Pohl MO, Zhou Y, Rodriguez-Frandsen A, Wang G, Stein DA, et al. Meta- and Orthogonal Integration of Influenza "OMICs" Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe 2015. Dec 9;18(6):723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Morrison J, Laurent-Rolle M, Maestre AM, Rajsbaum R, Pisanelli G, Simon V, et al. Dengue virus co-opts UBR4 to degrade STAT2 and antagonize type I interferon signaling. PLoS Pathog 2013. Mar;9(3):e1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Cavallieri F, Cury RG, Guimarães T, Fioravanti V, Grisanti S, Rossi J, et al. Recent Advances in the Treatment of Genetic Forms of Parkinson's Disease: Hype or Hope? Cells 2023. Feb 27;12(5):764. doi: 10.3390/cells12050764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Jia F, Fellner A, Kumar KR. Monogenic Parkinson's Disease: Genotype, Phenotype, Pathophysiology, and Genetic Testing. Genes (Basel) 2022. Mar 7;13(3):471. doi: 10.3390/genes13030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Bayón Y, Trinidad AG, de la Puerta ML, Del Carmen Rodríguez M, Bogetz J, Rojas A, et al. KCTD5, a putative substrate adaptor for cullin3 ubiquitin ligases. FEBS J 2008. Aug;275(15):3900–3910. [DOI] [PubMed] [Google Scholar]
- (142).Young BD, Sha J, Vashisht AA, Wohlschlegel JA. Human Multisubunit E3 Ubiquitin Ligase Required for Heterotrimeric G-Protein β-Subunit Ubiquitination and Downstream Signaling. J Proteome Res 2021. Sep 3;20(9):4318–4330. [DOI] [PubMed] [Google Scholar]
- (143).Chang MY, Kang I, Gale MJ, Manicone AM, Kinsella MG, Braun KR, et al. Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am J Physiol Lung Cell Mol Physiol 2017. Dec 1;313(6):L1069–L1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Boeing S, Williamson L, Encheva V, Gori I, Saunders RE, Instrell R, et al. Multiomic Analysis of the UV-Induced DNA Damage Response. Cell Rep 2016. May 17;15(7):1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Uesaka N, Uchigashima M, Mikuni T, Hirai H, Watanabe M, Kano M. Retrograde signaling for climbing fiber synapse elimination. Cerebellum 2015. Feb;14(1):4–7. [DOI] [PubMed] [Google Scholar]
- (146).Hridi SU, Barbour M, Wilson C, Franssen AJ, Harte T, Bushell TJ, et al. Increased Levels of IL-16 in the Central Nervous System during Neuroinflammation Are Associated with Infiltrating Immune Cells and Resident Glial Cells. Biology (Basel) 2021. May 27;10(6):472. doi: 10.3390/biology10060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Kouchaki E, Akbari H, Mahmoudi F, Salehi M, Naimi E, Nikoueinejad H. Correlation of Serum Levels of Interleukine-16, CCL27, Tumor Necrosis Factor-related Apoptosis-inducing Ligand, and B-cell Activating Factor with Multiple Sclerosis Severity. Iran J Allergy Asthma Immunol 2022. Feb 6;21(1):27–34. [DOI] [PubMed] [Google Scholar]
- (148).Saito S, Cao D, Victor AR, Peng Z, Wu H, Okwan-Duodu D. RASAL3 Is a Putative RasGAP Modulating Inflammatory Response by Neutrophils. Front Immunol 2021. Oct 27;12:744300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Wang J, Wang G, Cheng D, Huang S, Chang A, Tan X, et al. Her2 promotes early dissemination of breast cancer by suppressing the p38-MK2-Hsp27 pathway that is targetable by Wip1 inhibition. Oncogene 2020. Oct;39(40):6313–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Demidov ON, Kek C, Shreeram S, Timofeev O, Fornace AJ, Appella E, et al. The role of the MKK6/p38 MAPK pathway in Wip1-dependent regulation of ErbB2-driven mammary gland tumorigenesis. Oncogene 2007. Apr 12;26(17):2502–2506. [DOI] [PubMed] [Google Scholar]
- (151).Ledonne A, Mercuri NB. On the Modulatory Roles of Neuregulins/ErbB Signaling on Synaptic Plasticity. Int J Mol Sci 2019. Dec 31;21(1):275. doi: 10.3390/ijms21010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Spahic H, Parmar P, Miller S, Emerson PC, Lechner C, St Pierre M, et al. Dysregulation of ErbB4 Signaling Pathway in the Dorsal Hippocampus after Neonatal Hypoxia-Ischemia and Late Deficits in PV(+) Interneurons, Synaptic Plasticity and Working Memory. Int J Mol Sci 2022. Dec 28;24(1):508. doi: 10.3390/ijms24010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Chen Y, Hu N, Wu D, Bi L, Luo Z, Huang L, et al. PV network plasticity mediated by neuregulin1-ErbB4 signalling controls fear extinction. Mol Psychiatry 2022. Feb;27(2):896–906. [DOI] [PubMed] [Google Scholar]
- (154).Shi L, Bergson CM. Neuregulin 1: an intriguing therapeutic target for neurodevelopmental disorders. Transl Psychiatry 2020. Jun 16;10(1):190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Meldolesi J. Post-Synapses in the Brain: Role of Dendritic and Spine Structures. Biomedicines 2022. Aug 2;10(8):1859. doi: 10.3390/biomedicines10081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Hori K, Shimaoka K, Hoshino M. AUTS2 Gene: Keys to Understanding the Pathogenesis of Neurodevelopmental Disorders. Cells 2021. Dec 21;11(1):11. doi: 10.3390/cells11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).DeGiosio RA, Grubisha MJ, MacDonald ML, McKinney BC, Camacho CJ, Sweet RA. More than a marker: potential pathogenic functions of MAP2. Front Mol Neurosci 2022. Sep 16;15:974890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Goodwill AM, Low LT, Fox PT, Fox PM, Poon KK, Bhowmick SS, et al. Meta-analytic connectivity modelling of functional magnetic resonance imaging studies in autism spectrum disorders. Brain Imaging Behav 2023. Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Fabozzi F, Margoni S, Andreozzi B, Musci MS, Del Baldo G, Boccuto L, et al. Cerebellar mutism syndrome: From pathophysiology to rehabilitation. Front Cell Dev Biol 2022. Dec 2;10:1082947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Pang EW, Hammill C, Taylor MJ, Near J, Schachar R, Crosbie J, et al. Cerebellar gamma-aminobutyric acid: Investigation of group effects in neurodevelopmental disorders. Autism Res 2023. Mar;16(3):535–542. [DOI] [PubMed] [Google Scholar]
- (161).Whiteley P, Marlow B, Kapoor RR, Blagojevic-Stokic N, Sala R. Autoimmune Encephalitis and Autism Spectrum Disorder. Front Psychiatry 2021. Dec 17;12:775017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Malek M, Ashraf-Ganjouei A, Moradi K, Bagheri S, Mohammadi M, Akhondzadeh S. Prednisolone as Adjunctive Treatment to Risperidone in Children With Regressive Type of Autism Spectrum Disorder: A Randomized, Placebo-Controlled Trial. Clin Neuropharmacol 2020;43(2):39–45. [DOI] [PubMed] [Google Scholar]
- (163).Harutyunyan AA, Harutyunyan HA, Yenkoyan KB. Novel Probable Glance at Inflammatory Scenario Development in Autistic Pathology. Front Psychiatry 2021. Dec 22;12:788779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Santoro JD, Partridge R, Tanna R, Pagarkar D, Khoshnood M, Rehmani M, et al. Evidence of neuroinflammation and immunotherapy responsiveness in individuals with down syndrome regression disorder. J Neurodev Disord 2022. Jun 3;14(1):35–w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Golla S, Sweeney JA. Corticosteroid therapy in regressive autism: Preliminary findings from a retrospective study. BMC Med 2014. May 15;12:79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Bey AL, Gorman MP, Gallentine W, Kohlenberg TM, Frankovich J, Jiang YH, et al. Subacute Neuropsychiatric Syndrome in Girls With SHANK3 Mutations Responds to Immunomodulation. Pediatrics 2020. Feb;145(2):e20191490. doi: 10.1542/peds.2019-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Ferguson BJ, Marler S, Altstein LL, Lee EB, Mazurek MO, McLaughlin A, et al. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brain Behav Immun 2016. Nov;58:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Lee R, Funk KE. Imaging blood-brain barrier disruption in neuroinflammation and Alzheimer's disease. Front Aging Neurosci 2023. Mar 17;15:1144036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Park JS, Choe K, Khan A, Jo MH, Park HY, Kang MH, et al. Establishing Co-Culture Blood-Brain Barrier Models for Different Neurodegeneration Conditions to Understand Its Effect on BBB Integrity. Int J Mol Sci 2023. Mar 9;24(6):5283. doi: 10.3390/ijms24065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Jasiak-Zatońska M, Pietrzak A, Wyciszkiewicz A, Więsik-Szewczyk E, Pawlak-Buś K, Leszczyński P, et al. Different blood-brain-barrier disruption profiles in multiple sclerosis, neuromyelitis optica spectrum disorders, and neuropsychiatric systemic lupus erythematosus. Neurol Neurochir Pol 2022;56(3):246–255. [DOI] [PubMed] [Google Scholar]
- (171).Ju J, Su Y, Zhou Y, Wei H, Xu Q. The SARS-CoV-2 envelope protein disrupts barrier function in an in vitro human blood-brain barrier model. Front Cell Neurosci 2022. Aug 23;16:897564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Yamano K, Youle RJ. PINK1 is degraded through the N-end rule pathway. Autophagy 2013. Nov 1;9(11):1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Olagunju AS, Ahammad F, Alagbe AA, Otenaike TA, Teibo JO, Mohammad F, et al. Mitochondrial dysfunction: A notable contributor to the progression of Alzheimer's and Parkinson's disease. Heliyon 2023. Mar 11;9(3):e14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Han R, Liu Y, Li S, Li X, Yang W. PINK1-PRKN mediated mitophagy: differences between in vitro and in vivo models. Autophagy 2023. May;19(5):1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Monies D, Abouelhoda M, AlSayed M, Alhassnan Z, Alotaibi M, Kayyali H, et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum Genet 2017. Aug;136(8):921–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Yang J, Ran M, Li H, Lin Y, Ma K, Yang Y, et al. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front Mol Neurosci 2022. Oct 24;15:1013933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Soltani Khaboushan A, Pahlevan-Fallahy M, Shobeiri P, Teixeira AL, Rezaei N. Cytokines and chemokines profile in encephalitis patients: A meta-analysis. PLoS One 2022. Sep 1;17(9):e0273920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Kunz N, Kemper C. Complement Has Brains-Do Intracellular Complement and Immunometabolism Cooperate in Tissue Homeostasis and Behavior? Front Immunol 2021. Feb 25;12:629986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Dodd WS, Patel D, Lucke-Wold B, Hosaka K, Chalouhi N, Hoh BL. Adropin decreases endothelial monolayer permeability after cell-free hemoglobin exposure and reduces MCP-1-induced macrophage transmigration. Biochem Biophys Res Commun 2021. Dec 10;582:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Tammimies K. Genetic mechanisms of regression in autism spectrum disorder. Neurosci Biobehav Rev 2019. Jul;102:208–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Expanded Methods. This file contains a detailed description of the methods used in this study.
Additional file 2: Fig. S1. Microscopic image of unstained microglia grown in suspension. Two controls and two PPM1D+/tr samples are shown. The bar is 200 μMs. Two other samples not available.
Additional file 3: Table S1. Neuron proteomics, PPM1D+/tr vs controls. Tab 1. Gene Ontology (GO) analysis. Control sample names on top are shown in pastel; 1 and 4, 2 and 3 are biological duplicates. PPM1D+/tr samples are shown in light green. Samples 1 and 2, as well as 3 and 5, are biological duplicates. Differentially expressed proteins are arranged in descending order based on highest to lowest scores in a comparison of all PPM1D+/tr samples vs all controls. The score is calculated by multiplying Fold Change by the p-value (−log 2 of 4.32 corresponds to p=0.05). Tabs 2 and 3 (Neuron PPM1D+/tr Up KEGG and Neuron PPM1D+/tr Down KEGG, respectively) shows the KEGG analysis of all proteins that increased and decreased in the PPM1D+/− samples.
Additional file 4: Table S2. Neuron phosphoproteomics, PPM1D+/tr vs controls. Control sample names on top are shown in pastel. Controls 1 and 3, and 2 and 4 are biological duplicates. PPM1D+/− sample names are shown in green. Samples 2 and 4 are biological duplicates. Tab 1, DEPPs are differentially expressed phosphoproteins arranged in descending order based on highest to lowest scores, as described in the legend for Additional file 3: Table S1. Tab 1 (Phosphosites and GO) contains all phosphorylated proteins in descending order and the GO analysis. Tab 2 (normalize to protein value) show GO analysis and volcano plot. Tabs 3 and 4 are neuron phospho up 500 EnrichR and neuron phospho down 500 EnrichR, respectively, showing KEGG pathway analysis for phosphoproteins that increase or decrease in PPM1D+/− neurons. Additional GO analyses for up and down regulated phosphoproteins are shown, but were not described in the paper in order to avoid redundancy.
Additional file 5: Table S3. Microglia proteomics, PPM1D+/tr vs controls. Three control samples and three PPM1D+/− samples were analyzed. The microglia were in their baseline state – no treatment (no tx). Tab 1 (processing) shows the detailed steps of data processing starting from raw abundance to log2 transformation, to normalization and to imputation of missing values. Tab 2 is the differentially expressed protein list calculated as described in the neuron proteomics analysis (see figure legend, Additional file 3: Table S1), along with the GO analysis. Tab 3 is the DAVID KEGG analysis of up regulated proteins, which was not discussed in the paper since it overlapped with other findings. Tabs 4 and 5 are the KEGG pathways for the up and down regulated proteins, respectively, using 500 EnrichR. Tab 6 is the DAVID KEGG analysis of up regulated proteins.
Additional file 6: Table S4 Figure 2. Microglia phosphoproteomics, PPM1D+/tr vs controls. Phosphoproteomics was carried out on the same samples described in Additional file 5: Table S3. Tab 1 (PPM1D+tr untreated vs Control) contains the differentially expressed phosphoprotein list, along with the GO analysis. Tabs 2 and 3 (PPM1D+tr untreated Up KEGG and PPM1D+tr untreated Down KEGG) show KEGG pathways for up and downregulated phosphosites, respectively.
Additional file 7: Table S5. LPS treated microglia proteomics, PPM1D+/tr vs controls. Microglia derived from the same iPSC lines as described in the legend of Additional file 5: Table S3 were treated with LPS (see main text). Tab 1 (processing), as described in Additional file 5: Table S3. Tab 2 (microglia proteomics LPS) shows differentially expressed protein arranged by total score in descending order (PPM1D+/tr vs controls) and GO analysis. KEGG pathway analysis for up and downregulated proteins are shown in Tabs 3 and 4, respectively (Microglia LPS Up KEGG; Microglia LPS down KEGG).
Additional file 8: Table S6. LPS treated microglia phosphoproteomics, PPM1D+/tr vs controls. Phosphoproteomics was carried out on LPS treated microglia using the same samples described in Additional file 7: Table S5. Tab 1 (PPM1D+tr LPS vs Cont LPS) shows differentially phosphorylated phosphosites arranged by total score in descending order and the GO analysis. Tabs 2 and 3 (PPM1D+tr LPS enriched Up KEGG; PPM1D+tr LPS enriched Down KEGG) show the KEGG pathway analyses for phosphosites that increase or decreased, respectively.