Abstract
Background
Helicobacter pylori (H. pylori) is recognized as a type I carcinogen in gastric cancer (GC). However, GC still occurs after H. pylori eradication, and its diagnosis is more complicated. This study aimed to summarize the characteristics of early GC (EGC) after H. pylori eradication to help accurately identify EGC and avoid missed diagnosis and misdiagnosis.
Methods
A total of 81 patients of EGC after H. pylori eradication (Hp-eradicated group), resected by endoscopic submucosal dissection (ESD), and 105 cases of H. pylori infection-related EGC (control group) were assessed. After propensity-score matching, the clinical characteristics, endoscopic manifestations, and histopathological features of the 62 matched patients in each group were analyzed. We also conducted specific analyses in combination with endoscopic and histopathological images.
Results
There were more patients in the Hp-eradicated group who received proton pump inhibitor (PPI) for >1 year compared to the control group (p < 0.001). More patients at OLGA stages I-II before the diagnosis of EGC were in the control group (p = 0.045), especially at stage II. The mucosa in the Hp-eradicated group showed more moderate-to-severe atrophy (p = 0.047), map-like redness (p < 0.001) and mild activity (p < 0.001). The predominant histopathological types differed between the two groups (p < 0.001), and the majority of cases in the Hp-eradicated group were high-grade intraepithelial neoplasia (HGIN). Ki-67 expression was lower in the Hp-eradicated group (p = 0.025). But different eradication intervals of H. pylori have little effect on the characteristics of EGC. Furthermore, PPI uses for >1 year (p = 0.005), mucosal map-like redness (p < 0.001), moderate mucosal atrophy (p = 0.017), and mild activity of gastric mucosa (p = 0.005) were independent characteristics of EGC after H. pylori eradication.
Conclusion
Our multicenter study revealed that EGC after H. pylori eradication was characterized by long-term PPI use, moderate mucosal atrophy, mucosal map-like redness, the mild activity of gastric mucosa, a higher proportion of HGIN cases, and lower levels of Ki-67.
Keywords: EGC after H. pylori eradication, H. pylori infection-related EGC, endoscopic characteristics, pathological characteristics, retrospective study
Key Messages
EGC after H. pylori eradication was characterized by long-term PPI use, moderate mucosal atrophy, mucosal map-like redness, mild activity of gastric mucosa, a higher proportion of HGIN cases, and lower levels of Ki-67.
H. pylori-positive patients at OLGA stages I-II are also more likely to progress to EGC.
According to the current data, different eradication intervals of H. pylori have little effect on the characteristics of EGC.
1. Introduction
Gastric cancer (GC) is one of the most common malignant tumors worldwide. According to cancer data released by the World Health Organization, approximately 1.089 million cases of GC were newly diagnosed worldwide in 2020, ranking fifth in cancer incidence and fourth in cancer deaths, and 43.9% of newly diagnosed GC cases and 48.6% of deaths due to GC occurred in China [1]. In view of the current status of GC, early detection, diagnosis, and treatment, as well as prevention, are of great significance.
GC is divided into early GC (EGC) and advanced GC (AGC) according to its course. EGC is confined to the gastric mucosa and submucosa, while AGC penetrates deeper than the submucosa. With the development of endoscopic submucosal dissection (ESD), EGC can undergo curative resection via endoscopy. Helicobacter pylori infection is an important risk factor for the development of GC [2,3]. H. pylori infection can cause chronic inflammation of the gastric mucosa, which gradually progresses into precancerous lesions, leading to atrophic gastritis and intestinal metaplasia, significantly increasing the risk of GC [4,5]. Although eradication of H. pylori could effectively reduce the risk of GC, GC can still occur after eradication [6]. Therefore, it is extremely important to analyze the features of GC detected after H. pylori eradication and perform appropriate diagnosis, treatment, and prevention.
Saka et al. proposed that GC, after H. pylori eradication refers to GC, detected and diagnosed at least one year after eradication therapy, including GC that occurred after eradication or before eradication but was discovered after eradication [7]. According to the Kyoto Classification of Gastritis, endoscopists can judge whether a patient has H. pylori infection and evaluate the risk of GC based on the appearance of the gastric mucosa under endoscopy [8]. It has been reported that patchy redness was also observed in the gastric mucosa after H. pylori eradication. Compared with the patchy redness of H. pylori infection, the patchy redness of H. pylori eradication had clearer borders and a concave shape [9].
Owing to the low incidence of GC after H. pylori eradication, few studies have explored its characteristics, and the number of patients in each study was limited. Therefore, this study aimed to comprehensively analyze the clinical, endoscopic, and histopathological characteristics of EGC after H. pylori eradication and H. pylori infection-related EGC and clarify the independent factors of EGC after H. pylori eradication to facilitate early detection and treatment of EGC after H. pylori eradication and ameliorate the quality of life of patients.
2. Methods
2.1. Study subjects
A total of 3760 cases of EGC after ESD were retrospectively analyzed from the Affiliated Hospital of Qingdao University, Shandong Second Provinical General Hospital, and People’s Hospital of Rizhao of Shandong Province from January 2015 to March 2022. The inclusion criteria for EGC after H. pylori eradication were as follows: (1) H. pylori infection was previously confirmed by gastroscopic pathology or 13C-urea breath test, and H. pylori eradication therapy was performed. (2) EGC was found at least one year after H. pylori eradication treatment, and ESD was performed concurrently. The histopathological diagnosis after ESD was adenocarcinoma, signet-ring cell carcinoma, or HGIN. (3) Histopathology after ESD showed that either H. pylori negative or not detected or the 13C-urea breath test after ESD was negative. Patients need to stop using PPI and antibiotics for at least 1 month before conducting the 13C-urea breath test. We excluded patients who remained positive for H. pylori after eradication therapy or who were diagnosed with EGC less than one year after H. pylori eradication therapy. Patients with gastric stumps, metachronous gastric cancer (MGC), metastatic cancer, or incomplete clinical data were also excluded. H. pylori infection-related EGC refers to adenocarcinoma, signet-ring cell carcinoma, or HGIN with positive H. pylori test results by gastroscopic pathological examination or 13C-urea breath test at least 1 year before ESD. And the H. pylori test results of H. pylori infection-related EGC after ESD was still positive.
Based on the inclusion and exclusion criteria, 81 cases of EGC diagnosed after H. pylori eradication (Hp-eradicated group) and 105 patients with H. pylori infection-related EGC (Control group) were screened by reviewing past medical records and telephone follow-up.
2.2. Clinical, endoscopic and pathological assessment of EGC
Patient clinical data and endoscopic and pathological characteristics were collected and summarized. Clinical data included age, sex, smoking history, drinking history, family history of gastric cancer, Charlson comorbidity index (CCI), number of clinical consultations before the diagnosis of EGC, number of endoscopic examinations before the diagnosis of EGC, duration of symptoms, clinical symptoms, duration of proton pump inhibitor (PPI) use, reasons for using PPI, and combined use of anti-platelet agents. The smoking history or drinking history in this article respectively refer to patients who have smoked or drunk for more than 1 year and have not quit smoking or drinking before being diagnosed with EGC. CCI was used to quantify the comorbidities [10]. Age score was not included in CCI in this study. GC was not included in the CCI total score because the subjects were EGC patients. The operative link on gastritis assessment (OLGA) and the operative link on gastric intestinal metaplasia assessment (OLGIM) are tools for risk stratification of chronic gastritis [11,12]. The OLGA and OLGIM scores were performed on the pathological results of patients who underwent gastroscopy at least one year prior to the diagnosis of EGC. Endoscopic features included background mucosal atrophy degree, lesion location, size, clear boundary, and Paris classification. Pathological features included histopathological type, degree of differentiation, and Ki-67 expression level.
Gastric mucosal atrophy was classified as mild (C-1, C-2), moderate (C-3, O-1), or severe (O-2, O-3) according to the Kimura-Takemoto classification [13]. According to the 2005 Paris classification criteria, endoscopic lesions were divided into protruding (0-I), non-protruding and non-excavated (0-II), and excavated (0-III) lesions [14]. Type 0-II was further divided into slightly elevated (0-IIa), completely flat (0-IIb), slightly depressed (0-IIc), slightly elevated and depressed (0-IIa + IIc), or slightly depressed and elevated (0-IIc + IIa) types. To better describe the endoscopic manifestations after H. pylori infection or eradication, we referred to the 2018 edition of the Kyoto Classification of Gastritis and Saka’s description of GC after H. pylori eradication [7,15]. We also described the degree of inflammation and activity of gastric mucosa. GC resected by ESD is histologically classified into papillary adenocarcinoma, tubular adenocarcinoma, signet-ring cell carcinoma, undifferentiated carcinoma, and HGIN. According to the degree of differentiation, GC cells can be categorized as well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated cancers [16].
After ESD, histopathological specimens were stained with hematoxylin and eosin (HE), dehydrated, and mounted for microscopic observation. Furthermore, to analyze and evaluate the growth activity of tumors, Ki-67 immunostaining was performed. Other immunohistochemical indicators including CKpan, HER2, Syn, CgA, SMA, S100, CD31, D2-40, P53, MUC2, MUC5AC, MUC6, CEA were also explored.
Subgroup analysis of patients in Hp-eradicated group was also conducted according to the different times from eradication therapy to EGC diagnosis in order to explore the relationship between different H. pylori eradication times and characteristics of EGC.
2.3. Statistical analysis
Quantitative data were analyzed using Student’s t-test or Mann-Whitney U test. Qualitative data were analyzed using χ2 test or Fisher’s exact test and rank data were analyzed using the rank sum test. We used logistic regression for propensity scoring with a caliper value of 0.02, incorporating sex, age, smoking history, drinking history, family history of GC, CCI, and the number of endoscopic examination variables for one-to-one nearest-neighbor matching. Logistic regression analysis was also conducted to screen independent characteristics of EGC after H. pylori eradication. SPSS 26.0 was used to perform all the statistical analyses and p < 0.05 was significant.
3. Results
3.1. Comparison of General characteristics between the Hp-eradicated and control groups
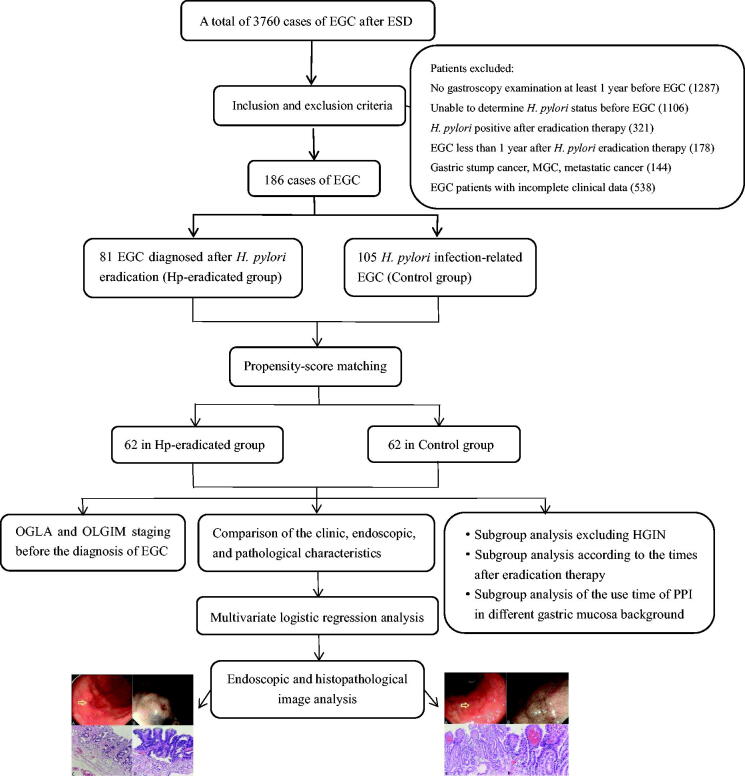
Figure 1 is the flow diagram of this study. Firstly, the clinical characteristics of the Hp-eradicated and control groups before and after propensity-score matching are summarized in Table 1. A total of 81 patients were included in the Hp-eradicated group in this study, with an average age of 61.81 ± 9.2 years, and 105 patients were included in the control group, with an average age of 61.14 ± 8.9 years. Patients in the Hp-eradicated group had more clinical consultations (p = 0.003) and endoscopic examinations (p = 0.022) before propensity-score matching. No statistically significant differences were found in smoking history, drinking history, family history of GC, CCI, clinical symptoms, and duration of symptoms between the two groups. We performed propensity-score matched analysis to remove the influence of confounding factors and improve the reliability of results. After propensity-score matching, 62 patients from each group were included in the analysis. There were more patients in the Hp-eradicated group who were administered PPIs for >1 year compared to in the control group before and after propensity-score matching (p < 0.001).
Figure 1.
The flow diagram of the study.
Table 1.
Comparison of clinical characteristics between the Hp-eradicated and control groups before and after propensity-score matching.
| Clinical characteristics | All patients (n = 186) |
Propensity score-matched patients (n = 124) |
||||
|---|---|---|---|---|---|---|
| Hp-eradicated group n = 81 (%) |
Control group n = 105 (%) |
P value | Hp-eradicated group n = 62 (%) |
Control group n = 62 (%) |
P value | |
| Sex | 0.479 | 1.000 | ||||
| Male | 58 (71.6) | 80 (76.2) | 47 (75.8) | 47 (75.8) | ||
| Female | 23 (28.4) | 25 (23.8) | 15 (24.2) | 15 (24.2) | ||
| Age, yr | 61.81 ± 9.20 | 61.14 ± 8.90 | 0.616 | 60.97 ± 9.43 | 60.79 ± 9.09 | 0.915 |
| Age range | 0.863 | 0.719 | ||||
| ≤ 60 | 36 (44.4) | 48 (45.7) | 31 (50.0) | 29 (46.8) | ||
| >60 | 45 (55.6) | 57 (54.3) | 31 (50.0) | 33 (53.2) | ||
| Smoking history | 0.772 | 0.856 | ||||
| Yes | 33 (40.7) | 45 (42.9) | 27 (43.5) | 26 (41.9) | ||
| No | 48 (59.3) | 60 (57.1) | 35 (56.5) | 36 (58.1) | ||
| Drinking history | 0.771 | 1.000 | ||||
| Yes | 31 (38.3) | 38 (36.2) | 25 (40.3) | 25 (40.3) | ||
| No | 50 (61.7) | 67 (63.8) | 37 (59.7) | 37 (59.7) | ||
| Family history of GC | 0.284 | 0.717 | ||||
| Yes | 9 (11.1) | 7 (6.7) | 3 (4.8) | 5 (8.1) | ||
| No | 72 (88.9) | 98 (93.3) | 59 (95.2) | 57 (91.9) | ||
| CCI† | 0.93 ± 1.07 | 0.83 ± 1.03 | 0.531 | 0.85 ± 1.08 | 0.77 ± 0.97 | 0.663 |
| Number of clinical consultations | 3.98 ± 3.51 | 2.61 ± 2.31 | 0.003 | 3.81 ± 3.74 | 2.82 ± 2.46 | 0.086 |
| Number of endoscopic examinations | 1.98 ± 1.04 | 1.64 ± 0.95 | 0.022 | 1.82 ± 1.02 | 1.76 ± 0.80 | 0.696 |
| Clinical symptoms | 0.806 | 0.192 | ||||
| Abdominal pain | 22 (27.2) | 30 (28.6) | 20 (32.3) | 13 (21.0) | ||
| Bloating | 14 (17.3) | 14 (13.3) | 11 (17.7) | 8 (12.9) | ||
| Both pain and bloating | 10 (12.3) | 18 (17.1) | 6 (9.7) | 13 (21.0) | ||
| Others | 17 (21.0) | 18 (17.1) | 12 (19.3) | 9 (14.5) | ||
| None | 18 (22.2) | 25 (23.8) | 13 (21.0) | 19 (30.6) | ||
| Duration of symptoms (month) | 29.8 ± 61.37 | 17.3 ± 37.80 | 0.140 | 30.53 ± 68.13 | 14.16 ± 28.88 | 0.155 |
| Time of taking PPI† | <0.001 | <0.001 | ||||
| ≤ 1 year | 22 (27.2) | 83 (79.0) | 16 (25.8) | 47 (75.8) | ||
| >1 year | 59 (72.8) | 22 (21.0) | 46 (74.2) | 15 (24.2) | ||
†CCI: Charlson comorbidity index; PPI: proton pump inhibitor. Data are presented as the mean ± standard deviation or number (%).
PPIs are widely used to treat gastroesophageal reflux disease (GERD) and are often combined with anti-platelet agents. Therefore, we analyzed the long-term use of PPI due to GERD and the combined use of anti-platelet agents to know whether PPI administration is a risk in cases of GC after eradication of H. pylori with specific clinical background. More patients in the eradicated group underwent long-term PPI treatment due to GERD (p = 0.043) (Supplementary Table 1). But after matching, there was no statistical significance (p = 0.118). In addition, the combined use of anti-platelet agents showed no significant difference between the two groups.
We also explored the OGLA and OLGIM staging before the diagnosis of EGC in the Hp-eradicated group and control group (Table 2). There was no difference in the overall OLGA and OLGIM staging between Hp-eradicated group and H. pylori infection-related group. But more patients in the H. pylori infection-related group were at OLGA stages I-II at least one year before the diagnosis of GC (p = 0.045), especially at stage II.
Table 2.
The OGLA and OLGIM staging before the diagnosis of EGC in the Hp-eradicated and control groups before and after propensity-score matching.
| All patients (n = 186) |
Propensity score-matched patients (n = 124) |
|||||
|---|---|---|---|---|---|---|
| Hp-eradicated group n = 81 (%) |
Control group n = 105 (%) |
P value | Hp-eradicated group n = 62 (%) |
Control group n = 62 (%) |
P value | |
| OLGA | 0.375 | 0.346 | ||||
| I | 16 (19.8) | 15 (14.3) | 14 (22.6) | 8 (12.9) | ||
| II | 24 (29.6) | 49 (46.7) | 17 (27.4) | 34 (54.8) | ||
| III | 38 (46.9) | 39 (37.1) | 29 (46.8) | 19 (30.6) | ||
| IV | 3 (3.7) | 2 (1.9) | 2 (3.2) | 1 (1.6) | ||
| OLGA | 0.066 | 0.045 | ||||
| I-II | 40 (49.4) | 66 (62.9) | 31 (32.3) | 42 (67.7) | ||
| III-IV | 41 (50.6) | 39 (37.1) | 31 (50.0) | 20 (50.0) | ||
| OLGIM | 0.988 | 0.979 | ||||
| I | 20 (24.7) | 18 (17.1) | 18 (29.0) | 9 (14.5) | ||
| II | 24 (29.6) | 46 (43.8) | 15 (24.2) | 32 (51.6) | ||
| III | 35 (43.2) | 37 (35.2) | 27 (43.5) | 18 (29.0) | ||
| IV | 2 (2.5) | 4 (3.8) | 2 (3.2) | 3 (4.8) | ||
| OLGIM | 0.240 | 0.143 | ||||
| I-II | 44 (54.3) | 66 (62.9) | 33 (53.2) | 41 (66.1) | ||
| III-IV | 37 (45.7) | 39 (37.1) | 29 (46.8) | 21 (33.9) | ||
3.2. Comparison of the endoscopic and pathological characteristics between the Hp-eradicated and control groups
Table 3 shows the endoscopic and pathological characteristics of the Hp-eradicated and control groups before and after propensity-score matching. The mucosa in the Hp-eradicated group showed more moderate-to-severe atrophy (p = 0.047), map-like redness (p < 0.001), and mild activity (p < 0.001) than that in the control group. We found that the diameter of the lesions in the Hp-eradicated group was smaller than that in the control group (p = 0.042) before propensity-score matching. However, no difference was observed in the diameter of the lesions after propensity-score matching (p = 0.093). Most lesions in both groups were located in the lower part of the stomach (p = 0.419), and the boundary of most lesions was clear (p = 0.783). Twenty-seven cases of Paris type 0-IIc were in the Hp-eradicated group (27/62, 43.5%) and thirty-one cases were in the control group (31/62, 50.0%), indicating that most lesions were slightly depressed (p = 0.540).
Table 3.
Comparison of endoscopic and pathological characteristics between Hp-eradicated and control groups before and after propensity-score matching.
| Characteristics | All patients (n = 186) |
Propensity score-matched patients (n = 124) |
||||
|---|---|---|---|---|---|---|
| Hp-eradicated group n = 81 (%) | Control group n = 105 (%) | P value | Hp-eradicated group n = 62 (%) | Control group n = 62 (%) | P value | |
| Mucosal atrophy | 0.010 | 0.047 | ||||
| Mild | 32 (39.5) | 61 (58.1) | 32 (39.5) | 47 (58.0) | ||
| Moderate | 40 (49.4) | 38 (36.2) | 40 (49.4) | 30 (37.0) | ||
| Severe | 9 (11.1) | 6 (5.7) | 9 (11.1) | 4 (5.0) | ||
| Mucosal map-like redness | <0.001 | <0.001 | ||||
| Yes | 69 (85.2) | 14 (13.3) | 53 (85.5) | 10 (16.1) | ||
| No | 12 (14.8) | 91 (86.7) | 9 (14.5) | 52 (83.9) | ||
| Lesion size (mm) | 15.00,10 | 15.00,13 | 0.042 | 15.00,10 | 15.00,8 | 0.093 |
| Lesion range | 0.039 | 0.524 | ||||
| ≤20 mm | 67 (82.7) | 73 (69.5) | 49 (79.0) | 46 (74.2) | ||
| >20mm | 14 (17.3) | 32 (30.5) | 13 (21.0) | 16 (25.8) | ||
| Lesion location | 0.730 | 0.419 | ||||
| Upper | 15 (18.5) | 15 (14.3) | 8 (12.9) | 5 (8.1) | ||
| Middle | 14 (17.3) | 20 (19.0) | 10 (16.1) | 15 (24.2) | ||
| Lower | 52 (64.2) | 70 (66.7) | 44 (71.0) | 42 (67.7) | ||
| Lesion border | 0.561 | 0.783 | ||||
| Clear | 72 (88.9) | 96 (91.4) | 54 (87.1) | 55 (88.7) | ||
| Unclear | 9 (11.1) | 9 (8.6) | 8 (12.9) | 7 (11.3) | ||
| Paris classification | 0.393 | 0.540 | ||||
| 0-IIa | 22 (27.2) | 29 (27.6) | 15 (24.2) | 11 (17.7) | ||
| 0-IIa + IIc | 21 (25.9) | 17 (16.2) | 17 (27.5) | 13 (21.0) | ||
| 0-IIb | 3 (3.7) | 5 (4.8) | 3 (4.8) | 4 (6.5) | ||
| 0-IIb + IIc | 0 | 3 (2.9) | 0 | 2 (3.2) | ||
| 0-IIc | 35 (43.2) | 50 (47.6) | 27 (43.5) | 31 (50.0) | ||
| 0-Ip | 0 | 1 (1.0) | 0 | 1 (1.6) | ||
| Inflammation | 0.243 | 0.207 | ||||
| Mild | 5 (6.2) | 2 (1.9) | 5 (8.1) | 1 (1.6) | ||
| Moderate | 76 (93.8) | 103 (98.1) | 57 (91.9) | 61 (98.4) | ||
| Activity | 0.001 | <0.001 | ||||
| Mild | 50 (61.7) | 39 (37.1) | 42 (67.7) | 19 (30.6) | ||
| Moderate | 31 (38.3) | 66 (62.9) | 20 (32.3) | 43 (69.4) | ||
| Intestinal metaplasia | 0.110 | 0.477 | ||||
| None | 3 (3.7) | 10 (9.5) | 3 (3.7) | 9 (11.1) | ||
| Mild | 22 (27.2) | 34 (32.4) | 22 (27.2) | 26 (32.1) | ||
| Moderate | 54 (66.7) | 58 (55.2) | 54 (66.7) | 43 (53.1) | ||
| Severe | 2 (2.5) | 3 (2.9) | 2 (2.5) | 3 (3.7) | ||
| Type† | 0.001 | <0.001 | ||||
| HGIN | 51 (63.0) | 36 (34.3) | 39 (62.9) | 16 (25.8) | ||
| WDA | 13 (16.0) | 24 (22.9) | 10 (16.1) | 16 (25.8) | ||
| MDA | 15 (18.5) | 28 (26.7) | 11 (17.8) | 18 (29.0) | ||
| PDA | 1 (1.2) | 13 (12.4) | 1 (1.6) | 9 (14.5) | ||
| SRCC | 1 (1.2) | 4 (3.8) | 1 (1.6) | 3 (4.8) | ||
| Differentiation | 0.037 | 0.058 | ||||
| Well | 28 (93.3) | 52 (75.4) | 23 (92.0) | 35 (74.5) | ||
| Poor | 2 (6.7) | 17 (24.6) | 2 (8.0) | 12 (25.5) | ||
| Ki-67 index | 0.014 | 0.025 | ||||
| 33 (51.6) | 33 (32.4) | 25 (53.2) | 19 (31.7) | |||
| ≥50% | 31 (48.4) | 69 (67.6) | 22 (46.8) | 41 (68.3) | ||
†HGIN: high-grade intraepithelial neoplasia; WDA: well-differentiated adenocarcinoma; MDA: moderately differentiated adenocarcinoma; PDA: poorly differentiated adenocarcinoma; SRCC: signet ring cell carcinoma.
Both the Hp-eradicated and control groups had a large proportion of mild and moderate intestinal metaplasia (p = 0.477). A significant difference existed between the two groups in the pathological type (p < 0.001), and a majority of patients in the Hp-eradicated group were HGIN (p < 0.001). It is generally believed that the differentiation degree of well-differentiated and moderately differentiated adenocarcinomas is good, while the differentiation degree of poorly differentiated and signet-ring cell carcinomas is poor. The degree of differentiation showed no significant difference between the two groups (p = 0.058). Ki-67 can be used as a reference index to evaluate the degree of tumor cell proliferation [17]. A total of 47 and 60 patients in the Hp-eradicated and control groups, respectively, underwent immunohistochemistry after matching. The proportion of patients with a Ki-67 index <50% in the Hp-eradicated group was larger than that in the control group, indicating that the expression level of Ki-67 in EGC after H. pylori eradication was lower (p = 0.025).
We also explored some other immunohistochemical markers, including CKpan, HER2, Syn, CgA, SMA, S100, CD31, D2-40, P53, MUC2, MUC5AC, MUC6, and CEA. We conducted an analysis of these markers between Hp-eradicated and control groups before and after propensity-score matching (Supplementary Table 2). We found that the positive expression rate of CgA in the Hp-eradicated group was higher than that in the control group (p = 0.033).
3.3. Subgroup analysis of characteristics excluding HGIN between Hp-eradicated and control groups
Due to HGIN being interpreted differently by pathologists, we conducted subgroup analysis of characteristics excluding HGIN between Hp-eradicated and control groups before and after propensity-score matching (Supplementary Table 3). After excluding HGIN, there were 69 patients remaining in the control group and 30 remaining in the Hp-eradicated group. We found that the time of taking PPI (p = 0.043), degree of mucosal atrophy (p = 0.009), and mucosal map-like redness (p < 0.001) were significantly different between Hp-eradicated and control groups after propensity-score matching. There were no significant differences in the activity of the gastric mucosa, pathological types, and Ki-67 index between the two groups, which were different from the characteristic analysis including HGIN.
3.4. Subgroup analysis of patients in Hp-eradicated group
We divided the patients in the Hp-eradicated group into three subgroups according to the different times from eradication therapy to EGC diagnosis, including 1 to 2 years (group A), 2 to 5 years (group B), and more than 5 years (group C). We found no significant differences between the three groups in terms of clinical, endoscopic, and pathological features (Table 4). Therefore, different H. pylori eradication times have little effect on the characteristics of EGC based on the current data.
Table 4.
Subgroup analysis of patients in Hp-eradicated group according to the times after eradiaction therapy.
| Characteristics | Group A (1 to 2 years) n = 42 |
Group B (2 to 5 years) n = 24 |
Group C (>5 years) n = 15 |
P value |
|---|---|---|---|---|
| Sex | 0.434 | |||
| Male | 30 (71.4) | 19 (79.2) | 9 (60.0) | |
| Female | 12 (28.6) | 5 (20.8) | 6 (40.0) | |
| Age, yr | 62.38 ± 9.05 | 60.42 ± 8.97 | 62.47 ± 10.25 | 0.679 |
| Smoking history | 0.657 | |||
| Yes | 18 (42.9) | 8 (33.3) | 7 (46.7) | |
| No | 24 (57.1) | 16 (66.7) | 8 (53.3) | |
| Drinking history | 0.873 | |||
| Yes | 16 (38.1) | 10 (41.7) | 5 (33.3) | |
| No | 26 (61.9) | 14 (58.3) | 10 (66.7) | |
| Family history of GC | 0.830 | |||
| Yes | 5 (11.9) | 3 (12.5) | 1 (6.7) | |
| No | 37 (88.1) | 21 (87.5) | 14 (93.3) | |
| CCI | 0.95 ± 1.15 | 1.04 ± 1.00 | 0.67 ± 0.98 | 0.558 |
| Number of clinical consultations | 3.40 ± 3.61 | 4.67 ± 3.58 | 4.47 ± 3.04 | 0.316 |
| Number of endoscopic examinations | 1.76 ± 0.96 | 2.33 ± 0.96 | 2.00 ± 1.25 | 0.097 |
| Time of taking PPI | 0.406 | |||
| ≤1 year | 13 (31.0) | 7 (29.2) | 2 (13.3) | |
| >1 year | 29 (69.0) | 17 (70.8) | 13 (86.7) | |
| Mucosal atrophy | 0.251 | |||
| Mild | 14 (33.3) | 11 (45.8) | 7 (46.7) | |
| Moderate | 20 (47.6) | 12 (50.0) | 8 (53.3) | |
| Severe | 8 (19.0) | 1 (4.2) | 0 (0.0) | |
| Mucosal map-like redness | 0.100 | |||
| Yes | 39 (92.9) | 18 (75.0) | 12 (80.0) | |
| No | 3 (7.1) | 6 (25.0) | 3 (20.0) | |
| Lesion size (mm) | 18.26 ± 13.71 | 13.04 ± 6.56 | 17.07 ± 5.38 | 0.171 |
| Type | 0.366 | |||
| HGIN | 30 (71.4) | 14 (58.3) | 7 (46.7) | |
| WDA | 6 (14.3) | 4 (16.7) | 3 (20.0) | |
| MDA | 4 (9.5) | 6 (25.0) | 5 (33.3) | |
| PDA | 1 (2.4) | 0 (0.0) | 0 (0.0) | |
| SRCC | 1 (2.4) | 0 (0.0) | 0 (0.0) | |
| Ki-67 index | 0.393 | |||
| <50% | 20 (58.8) | 9 (47.4) | 4 (36.4) | |
| ≥50% | 14 (41.2) | 10 (52.6) | 7 (63.6) |
We also further explored the time of taking PPI in the different backgrounds of gastric mucosa of GC patients after H. pylori eradication. There was no significant difference in the use time of PPI in patients with different degrees of gastric mucosa atrophy, inflammation, activity, and intestinal metaplasia (Supplementary Table 4).
3.5. Multivariate logistic regression analysis of the characteristics of EGC after H. pylori eradication
Our multivariate logistic regression analysis included statistically significant risk factors in the univariate analysis, such as the duration of PPI use, degree of mucosal atrophy, mucosal map-like redness, and mild activity of gastric mucosa. Lesion size was also included in the multivariate logistic regression analysis for further judgment because some studies have suggested that GC patients have smaller lesions after H. pylori eradication. The results indicated that PPI use >1 year (odds ratio [OR] = 5.33, 95% confidence interval [CI]: 1.67–17.01, p = 0.005), mucosal map-like redness (OR = 32.18, 95% CI: 9.26–111.77, p < 0.001), moderate mucosal atrophy (OR = 5.20, 95% CI: 1.35–20.02, p = 0.017), and the activity of gastric mucosa (OR = 0.176, 95% CI: 0.05–0.60, p = 0.005) were independent characteristics of EGC after H. pylori eradication (Table 5). However, the significance of lesion size (OR = 0.48, 95% CI: 0.12–1.92, p = 0.301) needs to be further verified.
Table 5.
Logistic regression analysis of the characteristics of early gastric cancer after H. pylori eradication after propensity-score matching.
| Characteristics | OR | 95%CI | P value |
|---|---|---|---|
| Time of taking PPI | |||
| ≤1 year vs >1 year | 5.33 | 1.67–17.01 | 0.005 |
| Mucosal map-like redness | |||
| No vs Yes | 32.18 | 9.26–111.77 | <0.001 |
| Lesion range | |||
| ≤20 mm vs >20mm | 0.48 | 0.12–1.92 | 0.301 |
| Mucosal atrophy | 0.056 | ||
| Mild vs Moderate | 5.20 | 1.35–20.02 | 0.017 |
| Mild vs Severe | 1.98 | 0.26–14.80 | 0.507 |
| Activity | |||
| Mild vs Moderate | 0.176 | 0.05–0.60 | 0.005 |
OR: odds ratio; CI: confidence interval; PPI: proton pump inhibitor.
3.6. Endoscopic and histopathological analysis of EGC after H. pylori eradication and H. pylori infection-related EGC
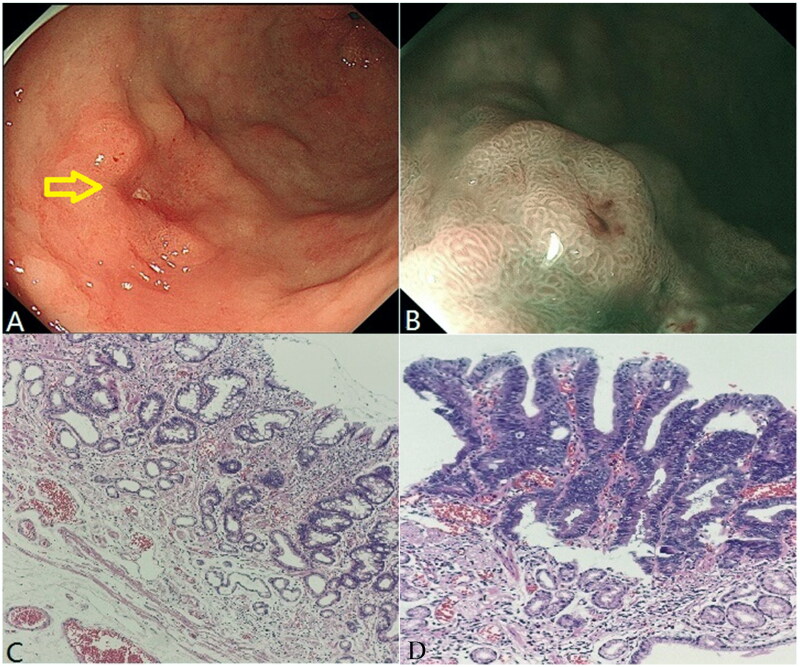
Figure 2 presents the endoscopic and histopathological images of a well-differentiated adenocarcinoma in a middle-aged male patient after H. pylori eradication. A 25 × 25-mm reddish depressed lesion was observed on the anterior wall of the upper part of the gastric antrum (Figure 2(A)). The surrounding mucosa showed map-like redness. The boundary of the lesion was clear on NBI magnifying endoscopy, with grid-like microvessels and disordered surface microstructures (Figure 2(B)). The gastric mucosa around the tumor was atrophied under low magnification, showing gastric pits, decreased glandular layers, and moderate intestinal metaplasia. Some glands were more proliferative, and lymphocytes and plasma cells infiltrated the interstitium (Figure 2(C)). When properly magnifying the adenocarcinoma area, the gastric mucosa was destroyed and the glands mainly showed a thick true papillary structure. The vascular axis of the papilla was visible, and the surface was covered with atypical epithelium, showing a well-differentiated papillary adenocarcinoma. Tumors in a small part of the area expanded in the form of tube glands, with dilated and irregular lumens, and enlarged glands were observed in the deep part of the tumor (Figure 2(D)).
Figure 2.
Well-differentiated adenocarcinoma after Helicobacter pylori eradication of a middle-aged male patient. (A) White light endoscopy image. The yellow arrow indicates the lesion location. (B) NBI image. (C) Normal gastric mucosa surrounding the tumor (HE,×100). (D) Well-differentiated adenocarcinoma area (HE, ×400).
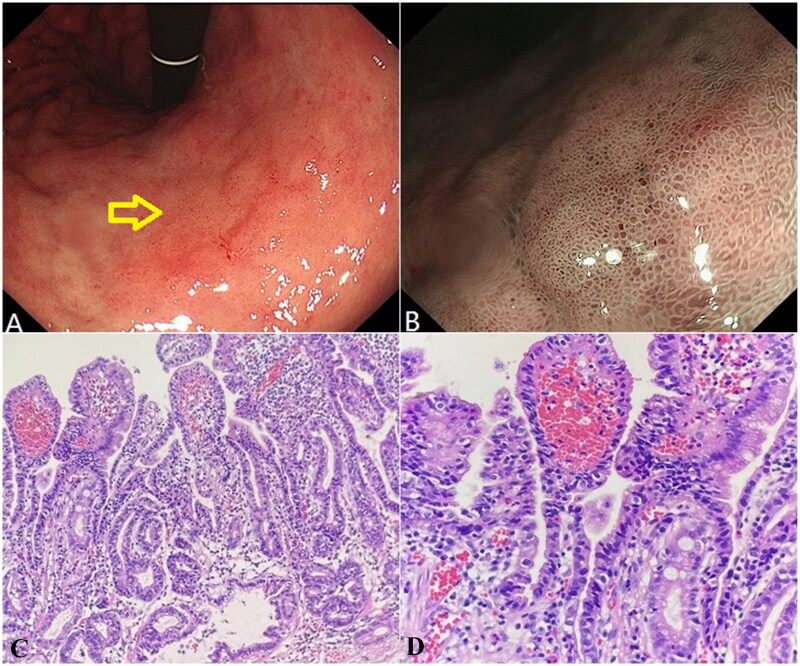
The endoscopic and histopathological characteristics of H. pylori infection-related well-differentiated adenocarcinoma are shown in Figure 3. There was a slightly reddish, transverse, patchy mucosa with a rough surface and slight depression in the center, approximately 28 × 18 mm in size on the side of the lesser curvature in the middle of the gastric body (Figure 3(A)). Under NBI, the boundary was clear, and most of the microstructures were caviar-like with dilated microvessels (Figure 3(B)). The glands in the adenocarcinoma area were mainly thick papillary structures covered with atypical epithelium (Figure 3(C)). Tumor cell atypia was remarkable and the nucleocytoplasmic ratio was significantly increased (Figure 3(D)). In the comparison of the two cases, the map-like redness and depressed features of GC after H. pylori eradication were more obvious, which was in line with the endoscopic characteristics. Intestinal metaplasia was observed in both cases and cell atypia was obvious.
Figure 3.
Helicobacter pylori infection-related well-differentiated adenocarcinoma of an Elderly male patient. (A) White light endoscopy image. The yellow arrow indicates the lesion location. (B) NBI image. (C) Well-differentiated adenocarcinoma area (HE, ×200). (D) Well-differentiated adenocarcinoma area (HE, ×400).
4. Discussion
The occurrence of GC is a complex process, and H. pylori infection is a key factor listed as a class I carcinogen of GC [18]. Due to persistent H. pylori infection, a series of gastric mucosal lesions develop, starting with an inflammatory response and eventually developing into GC. Furthermore, studies have shown that persistent H. pylori infection can lead to an increased risk of MGC [19,20]. Eradication of H. pylori can prevent further gastric mucosal atrophy and even reverse atrophy [21]. H. pylori eradication can decrease the incidence of GC, and H. pylori eradication treatment after endoscopic resection of EGC lesions can significantly decrease the incidence of MGC [22,23]. However, some studies have shown that GC still occurs even after successful eradication of H. pylori, although the incidence of GC is lower than that with persistent H. pylori infection [24,25].
By comparing the clinical characteristics of the Hp-eradicated and control groups, we found that most patients with GC after H. pylori eradication received PPI for >1 year (p < 0.001). Although we can’t claim that PPI use >1 year was the independent risk factor for GC after HP eradication, our result indirectly suggests that long-term use of PPI might promote the occurrence of GC after H. pylori eradication. Both groups of patients in this study ultimately developed gastric cancer. H. pylori was a recognized carcinogenic factor in the H. pylori infection-related group, but this important carcinogenic factor was missing in the Hp-eradicated group which still progressed to GC. Therefore, we want to explore other possible reasons by comparing the various characteristics of the two groups, providing reference for our further exploration. The association between PPI use and GC is biologically plausible and might be mediated by several factors. PPI can cause hypergastrinemia because gastrin secretion is restrained by acidity [26]. Gastrin is considered to be a potential growth factor that may lead to hyperplasia [27]. In addition, long-term use of PPI may induce changes in the gut microbiome, including a reduction in microbial diversity, which causes an increasing risk of GC [28,29]. These factors may result in the development of GC in PPI users, even if they have successfully eradicated H. pylori. Hagiwara et al. found that the long-term use of PPI increased the risk of developing GC after eradication [30]. Nevertheless, another study found that long-term PPI use increased the risk of GC independent of H. pylori eradication [6]. It is controversial whether long-term use of PPI has an explicit effect on the occurrence of GC after H. pylori eradication, and more prospective trials are needed to elucidate this. Unlike previous studies, this study did not include non-GC patients as control and did not directly explore the impact of long-term use of PPI on the occurrence of GC. This study selected a new perspective and focused on exploring the independent characteristic factors of GC after H. pylori eradication compared to H. pylori infection-related GC.
Long-term use of PPI due to GERD is a characteristic of GC patients after H. pylori eradication before propensity score matching, which suggests that we should pay attention to the long-term management of GERD, especially whether refractory GERD should prioritize other non-pharmacological treatment measures such as cardiac constriction surgery. Previous studies have shown that the use of anti-platelet drugs may reduce the risk of developing GC after H. pylori eradication, which may be related to its ability to eliminate inflammation [31,32]. However, we did not find a significant difference in the combined use of anti-platelet drugs in GC after H. pylori eradication and in H. pylori infection-related GC.
Patients at OLGA/OLGIM stages III-IV should receive regular endoscopic examinations regardless of H. pylori infection. It has been shown that H. pylori-positive patients at OLGIM stage II are also more likely to progress to EGC and have a higher risk of GC [33]. Our study has the similar finding in the OLGA stage II. For patients with high-risk staging, regular gastroscopic surveillance should be conducted even after successful H. pylori eradication, and high-resolution gastroscopy should be performed to avoid missing some microscopic tumor lesions. This study did not involve patients with non-gastric cancer as control, so we cannot further explore the independent risk factors for OLGA/OLGIM stages III-IV. However, a previous study has demonstrated that serum pepsinogen I (PGI) and H. pylori infection are independent risk factors for OLGA stages III-IV, while age and PGR (PGI and PGII ratio) are risk factors for OLGIM stages III-IV [33].
Yamamoto et al. found that most GC specimens detected after H. pylori eradication were depressed and had smaller lesions [34]. The researchers speculated that the improvement in gastric acid secretion after H. pylori eradication might inhibit the expansion and growth of GC lesions, making the lesion morphology different from that of H. pylori infection-related GC [35]. However, our study found that GC lesions after H. pylori eradication were not significantly smaller than those associated with H. pylori infection (p = 0.093). The gastric mucosa infected with H. pylori had endoscopic features such as atrophy, swelling, and diffuse redness. After H. pylori eradication, the diffuse redness of the gastric mucosa disappeared, the atrophy boundary was unclear, and the map-like redness was prominent [9]. Furthermore, normal columnar epithelium and ‘gastritis-like’ appearance are often observed on the surface of GC lesions after H. pylori eradication [36]. It is speculated that the boundary of GC lesions after H. pylori eradication is less distinct than that of non-cancerous lesions and requires careful identification.
It has been reported that severe gastric mucosal atrophy and intestinal metaplasia increased the risk of GC after H. pylori eradication [37,38]. Nagata et al. proposed that map-like redness may be a predictor of GC after H. pylori eradication. We also found that moderate-to-severe mucosal atrophy (p = 0.047) and mucosal map-like redness (p < 0.001) were more common in EGC after H. pylori eradication. We speculate that GC is more likely to occur in cases of moderate to severe atrophy after H. pylori eradication, but in cases of current infection, GC may occur even if atrophy is mild. It is challenging to diagnose GC after H. pylori eradication based on the endoscopic characteristics of the lesions. It will be helpful to avoid missed diagnoses by analyzing the endoscopic manifestation of GC lesions after H. pylori eradication continuously and carefully. Furthermore, periodic endoscopic examination after H. pylori eradication is of great significance for the timely diagnosis of GC lesions.
The activity of gastric mucosa means the presence of neutrophils in the lamina propria or epithelium or both. This means that eradication of H. pylori could reduce the infiltration of neutrophils in gastric mucosa, providing inspiration for further exploring the pathogenesis of gastric cancer after H. pylori eradication.
Gastric intraepithelial neoplasia (GIN), previously known as atypical hyperplasia or dysplasia, belongs to precancerous lesions, including low-grade intraepithelial neoplasia (LGIN) and high-grade intraepithelial neoplasia (HGIN). However, HGIN is usually classified with EGC because its biological behavior and intervention measures are similar to those of EGC. ESD treatment is recommended for HGIN of the gastric mucosa in expert consensus. The histopathological type of EGC after H. pylori eradication was different from that of the control group, with a higher proportion of HGIN cases in the Hp-eradicated group (p < 0.001); however, the expression level of Ki-67 was lower in the H. pylori infection-related EGC group (p = 0.025). Some researchers found that the expression levels of MUC2, Wnt5a, and Ki-67 in GC after H. pylori eradication were lower than those in H. pylori infection-related GC [34,39]. Therefore, we hypothesized that the proliferation and invasive ability of GC after sterilization would be lower than that of H. pylori-infection-related GC. In the analysis of other immunohistochemical indicators, we noticed that the positive expression rate of chromogranin A (CgA) in the Hp-eradicated group was higher. CgA is produced by endocrine and neuroendocrine cells and can be used both as an immunohistochemical marker and serum marker of neuroendocrine tumors [40]. Our result can indicate that there were more neuroendocrine cells in the pathological tissue of GC patients after Hp-eradication. However, it cannot be further demonstrated that they have a tendency to develop into neuroendocrine tumors, as we already had a clear understanding of the pathological results of the included GC patients. After excluding HGIN, we still found that long term use of PPI (p = 0.001), moderate mucosal atrophy (p = 0.008), and mucosal map-like redness (p < 0.001) were more common in EGC after H. pylori eradication, which were basically consistent with the EGC results including HGIN.
Our study found that different H. pylori eradication times had little impact on the characteristics of EGC. This finding seems to weaken the significance of early H. pylori eradication therapy. However, this result needs further validation because of the small sample size.
A review indicates that there is currently no clear evidence that long-term use of PPIs can lead to or accelerate the progression of gastric atrophy or intestinal metaplasia [41]. Based on our current data, we found that there was no difference in the use time of PPI in patients with GC after H. pylori eradication under different gastric mucosa backgrounds. The impact of long-term use of PPI on the risk of GC in different gastric mucosa backgrounds still needs to be explored in a large sample prospective trial.
Our multicenter retrospective study comparatively analyzed the clinical, endoscopic, and histopathological features of GC after H. pylori eradication and H. pylori infection-related GC, providing a reference for the early detection and diagnosis of GC after H. pylori eradication. We matched the variables, including sex, age, smoking history, drinking history, family history of GC, CCI, and the number of endoscopic examinations, to eliminate the effect of confounding factors, especially since patients in HP-eradicated group might have received longer duration of follow-up after HP eradication with more clinic visit and endoscopic surveillance than those in the control group. Our study has several limitations. First, this was a retrospective study with no prognostic analysis, which was not as convincing as a prospective study. Second, the number of patients diagnosed with EGC after H. pylori eradication is indeed very small although this research was a multicenter study. In addition, our study has strict inclusion and exclusion criteria, so the sample size in Hp-eradicated group is small as well. In the near future, we will conduct a prospective cohort study, expand the sample size, and perform a comparative analysis of the survival rates.
In conclusion, our multicenter study indicated that EGC after H. pylori eradication is characterized by long-term use of PPI, moderate mucosal atrophy, mucosal map-like redness, the mild activity of gastric mucosa, a higher proportion of HGIN, and lower levels of Ki-67.
Supplementary Material
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. And we thank all the authors for helping with the writing and publication of this article.
Funding Statement
The study was supported by the National Natural Science Foundation of China (No. 82270676), 2021 Shandong Province Graduate Education and Teaching Reform Research Project (SDYJG21110), and Qingdao Chinese Medicine Technology Project (2021-zyym26).
Author contributions
X-Y Liu and X-YW were responsible for the collection and assembly of data, data analysis and interpretation, and manuscript writing. TM and X-YY were responsible for the conception, design, and revising of the manuscript. ZW, J-DF, and JW were responsible for the collection of data, statistic expertise, and final approval of the manuscript. X-Y Li was responsible for revising the manuscript, financial support, and final approval of the manuscript. All authors have read and approved the final version of the manuscript.
Ethics statement
This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Ethics code: QYFY WZLL 26810). Written informed consent was obtained from participants prior to the study.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
All data analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–13. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yamagata H, Kiyohara Y, Aoyagi K, et al. . Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: the Hisayama study. Arch Intern Med. 2000;160(13):1962–1968. doi: 10.1001/archinte.160.13.1962. [DOI] [PubMed] [Google Scholar]
- 3.Parsonnet J, Friedman GD, Vandersteen DP, et al. . Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela MA, Canales J, Corvalán AH, et al. . Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis. World J Gastroenterol. 2015;21(45):12742–12756. doi: 10.3748/wjg.v21.i45.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egi Y, Ito M, Tanaka S, et al. . Role of Helicobacter pylori infection and chronic inflammation in gastric cancer in the cardia. Jpn J Clin Oncol. 2007;37(5):365–369. doi: 10.1093/jjco/hym029. [DOI] [PubMed] [Google Scholar]
- 6.Fukase K, Kato M, Kikuchi S, et al. . Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 7.Saka A, Yagi K, Nimura S.. Endoscopic and histological features of gastric cancers after successful Helicobacter pylori eradication therapy. Gastric Cancer. 2016;19(2):524–530. doi: 10.1007/s10120-015-0479-y. [DOI] [PubMed] [Google Scholar]
- 8.Sugano K, Tack J, Kuipers EJ, et al. . Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe K, Nagata N, Nakashima R, et al. . Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol. 2013;19(27):4374–4379. doi: 10.3748/wjg.v19.i27.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Rugge M, Genta RM.. Staging and grading of chronic gastritis. Hum Pathol. 2005;36(3):228–233. doi: 10.1016/j.humpath.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Capelle LG, de Vries AC, Haringsma J, et al. . The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71(7):1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Miwata T, Quach DT, Hiyama T, et al. . Interobserver and intraobserver agreement for gastric mucosa atrophy. BMC Gastroenterol. 2015;15:95. doi: 10.1186/s12876-015-0327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. [DOI] [PubMed] [Google Scholar]
- 15.Ohno A, Miyoshi J, Kato A, et al. . Endoscopic severe mucosal atrophy indicates the presence of gastric cancer after Helicobacter pylori eradication analysis based on the Kyoto classification. BMC Gastroenterol. 2020;20(1):232. doi: 10.1186/s12876-020-01375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagtegaal ID, Odze RD, Klimstra D, et al. . The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asaka M, Mabe K, Matsushima R, et al. . Helicobacter pylori eradication to eliminate gastric cancer: the Japanese strategy. Gastroenterol Clin North Am. 2015;44(3):639–648. doi: 10.1016/j.gtc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–first American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- 19.Cho SJ, Choi IJ, Kook MC, et al. . Staging of intestinal- and diffuse-type gastric cancers with the OLGA and OLGIM staging systems. Aliment Pharmacol Ther. 2013;38(10):1292–1302. doi: 10.1111/apt.12515. [DOI] [PubMed] [Google Scholar]
- 20.Kim YI, Choi IJ, Kook MC, et al. . The association between Helicobacter pylori status and incidence of metachronous gastric cancer after endoscopic resection of early gastric cancer. Helicobacter. 2014;19(3):194–201. doi: 10.1111/hel.12116. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Haruma K, Kamada T, et al. . Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16(8):1449–1456. doi: 10.1046/j.1365-2036.2002.01311.x. [DOI] [PubMed] [Google Scholar]
- 22.Takenaka R, Okada H, Kato J, et al. . Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment Pharmacol Ther. 2007;25(7):805–812. doi: 10.1111/j.1365-2036.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- 23.Seo JY, Lee DH, Cho Y, et al. . Eradication of Helicobacter pylori reduces metachronous gastric cancer after endoscopic resection of early gastric cancer. Hepatogastroenterology. 2013;60:776–780. [DOI] [PubMed] [Google Scholar]
- 24.Choi J, Kim SG, Yoon H, et al. . Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014;12(5):793–800.e791. doi: 10.1016/j.cgh.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 25.Kwon YH, Heo J, Lee HS, et al. . Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther. 2014;39(6):609–618. doi: 10.1111/apt.12633. [DOI] [PubMed] [Google Scholar]
- 26.Dacha S, Razvi M, Massaad J, et al. . Hypergastrinemia. Gastroenterol Rep (Oxf). 2015;3(3):201–208. doi: 10.1093/gastro/gov004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundell L, Vieth M, Gibson F, et al. . Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42(6):649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 28.Seto CT, Jeraldo P, Orenstein R, et al. . Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42. doi: 10.1186/2049-2618-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imhann F, Bonder MJ, Vich Vila A, et al. . Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagiwara T, Mukaisho K, Nakayama T, et al. . Proton pump inhibitors and Helicobacter pylori-associated pathogenesis. Asian Pac J Cancer Prev. 2015;16(4):1315–1319. doi: 10.7314/apjcp.2015.16.4.1315. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Cheung KS, Wong IY, et al. . Nonaspirin nonsteroidal anti-inflammatory drugs and gastric cancer risk after Helicobacter pylori eradication: a territory-wide study. Cancer. 2021;127(11):1805–1815. doi: 10.1002/cncr.33412. [DOI] [PubMed] [Google Scholar]
- 32.Bai X, Ding SQ, Zhang XP, et al. . Exposure to commonly used drugs and the risk of gastric cancer: an umbrella review of meta-analyses. Cancers. 2023;15(2):372. doi: 10.3390/cancers15020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M, Feng S, Qian M, et al. . Helicobacter pylori infection combined with OLGA and OLGIM staging systems for risk assessment of gastric cancer: a retrospective study in Eastern China. Risk Manag Healthc Policy. 2022;15:2243–2255. doi: 10.2147/RMHP.S391386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto K, Kato M, Takahashi M, et al. . Clinicopathological analysis of early-stage gastric cancers detected after successful eradication of Helicobacter pylori. Helicobacter. 2011;16(3):210–216. doi: 10.1111/j.1523-5378.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- 35.Ito M, Tanaka S, Takata S, et al. . Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a short-term follow-up. Aliment Pharmacol Ther. 2005;21(5):559–566. doi: 10.1111/j.1365-2036.2005.02360.x. [DOI] [PubMed] [Google Scholar]
- 36.Tabata H, Fuchigami T, Kobayashi H, et al. . Helicobacter pylori and mucosal atrophy in patients with gastric cancer: a special study regarding the methods for detecting Helicobacter pylori. Dig Dis Sci. 1999;44(10):2027–2034. doi: 10.1023/a:1026622418625. [DOI] [PubMed] [Google Scholar]
- 37.Kodama M, Murakami K, Okimoto T, et al. . Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer. Scand J Gastroenterol. 2013;48(11):1249–1256. doi: 10.3109/00365521.2013.838994. [DOI] [PubMed] [Google Scholar]
- 38.Asonuma S, Imatani A, Asano N, et al. . Helicobacter pylori induces gastric mucosal intestinal metaplasia through the inhibition of interleukin-4-mediated HMG box protein Sox2 expression. Am J Physiol Gastrointest Liver Physiol. 2009;297(2):G312–322. doi: 10.1152/ajpgi.00518.2007. [DOI] [PubMed] [Google Scholar]
- 39.Matsuo T, Ito M, Tatsugami M, et al. . Gastric cancer development after Helicobacter pylori eradication therapy: a new form of gastric neoplasia. Digestion. 2012;85(1):61–67. doi: 10.1159/000335260. [DOI] [PubMed] [Google Scholar]
- 40.Louthan O. Chromogranin a in physiology and oncology. Folia Biol. 2011;57(5):173–181. [PubMed] [Google Scholar]
- 41.Song H, Zhu J, Lu D.. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev. 2014;2(12):Cd010623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.