Abstract
Background.
The drastic decline of Ukraine’s immunization coverage since 2009 led to concerns about potential resurgence diphtheria and tetanus, along with other vaccine-preventable diseases.
Methods.
To assess population immunity against diphtheria and tetanus, we tested specimens from the serosurvey conducted in 2017 among children born in 2006–2015, the birth cohorts targeted by the nationwide outbreak response immunization following a circulating vaccine-derived poliovirus type 1 outbreak in Zakarpattya province in 2015. We surveyed four regions of Ukraine, using cluster sampling in Zakarpattya, Sumy, and Odessa provinces and simple random sampling in Kyiv City. We tested serum specimens for IgG antibodies against diphtheria and tetanus, using microbead assays (MBA). We estimated seroprevalence and calculated 95% confidence intervals. We also obtained information on the immunization status of surveyed children.
Results:
Seroprevalence of ≥0.1 IU/mL diphtheria antibodies was <80% in all survey sites (50.0%–79.2%). Seroprevalence of ≥0.1 IU/mL tetanus antibodies was ≥80% in Sumy, Kyiv City, and Odessa (80.2%–89.1%) and 61.6% in Zakarpattya. Across the sites, the proportion of children vaccinated age-appropriately with diphtheria-tetanus-containing vaccines (DTCV) was 28.5%–57.4% among children born in 2006–2010 and 34.1%–54.3% among children born in 2011–2015. The proportion of recipients of <3 DTCV doses increased from 7.1%–16.7% among children born in 2006–2010 to 19.8%–38.6% among children born in 2011–2015, as did the proportion of recipients of zero-DTCV doses (2.6%–8.8% versus 8.0%–14.0%, respectively).
Conclusions:
Protection against diphtheria among children born in 2006–2015 was suboptimal (<80%), particularly in Zakarpattya. Protection against tetanus was adequate (≥80%) except in Zakarpattya. Diphtheria-tetanus immunization status was suboptimal across all sites. Catch-up vaccination of unvaccinated/under-vaccinated children and other efforts to increase immunization coverage would close these immunity gaps and prevent the resurgence of diphtheria and tetanus in Ukraine, particularly in Zakarpattya.
Keywords: Diphtheria, tetanus, seroprevalence, seroepidemiology, diphtheria antibodies, tetanus antibodies, diphtheria-tetanus multiplex bead assay (MBA), multiplex bead assay (MBA), diphtheria-tetanus vaccination coverage, Ukraine, WHO European Region
1. Background
Historically, Ukraine had a well-established national immunization program with high reported coverage that allowed a substantial reduction of vaccine-preventable diseases (VPDs) [1–5]. However, since 2009, vaccination coverage in Ukraine has declined to the lowest level among countries in the World Health Organization (WHO) European Region (Figure 1) [3]. By 2016, coverage with three doses of diphtheria-tetanus-pertussis vaccine (DTP3) in Ukraine reached a low of 19% (Figure 1). The reasons for this decline have been described previously [4–12], including insufficient funding and inadequate vaccine procurement practices that resulted in frequent shortages; widespread safety concerns and mistrust in vaccinations among the general population and healthcare providers, particularly after a failed national measles-rubella vaccination campaign in 2008 when the vaccine was incorrectly blamed for the death of a child [7, 13], and a strong anti-vaccine media environment. Political and economic instability and armed conflict, leading to large-scale population displacement in the east, further contributed to the collapse of the national immunization program [8–12]. The decline in vaccination coverage led to outbreaks of measles in 2012 and 2016–2019 [14, 15] and circulating vaccine-derived poliovirus type 1 (cVDPV1) in 2015 [4], and raised concerns about the resurgence of other VPDs, including diphtheria and tetanus.
Figure 1. Immunization coverage in Ukraine, 1990–2019.
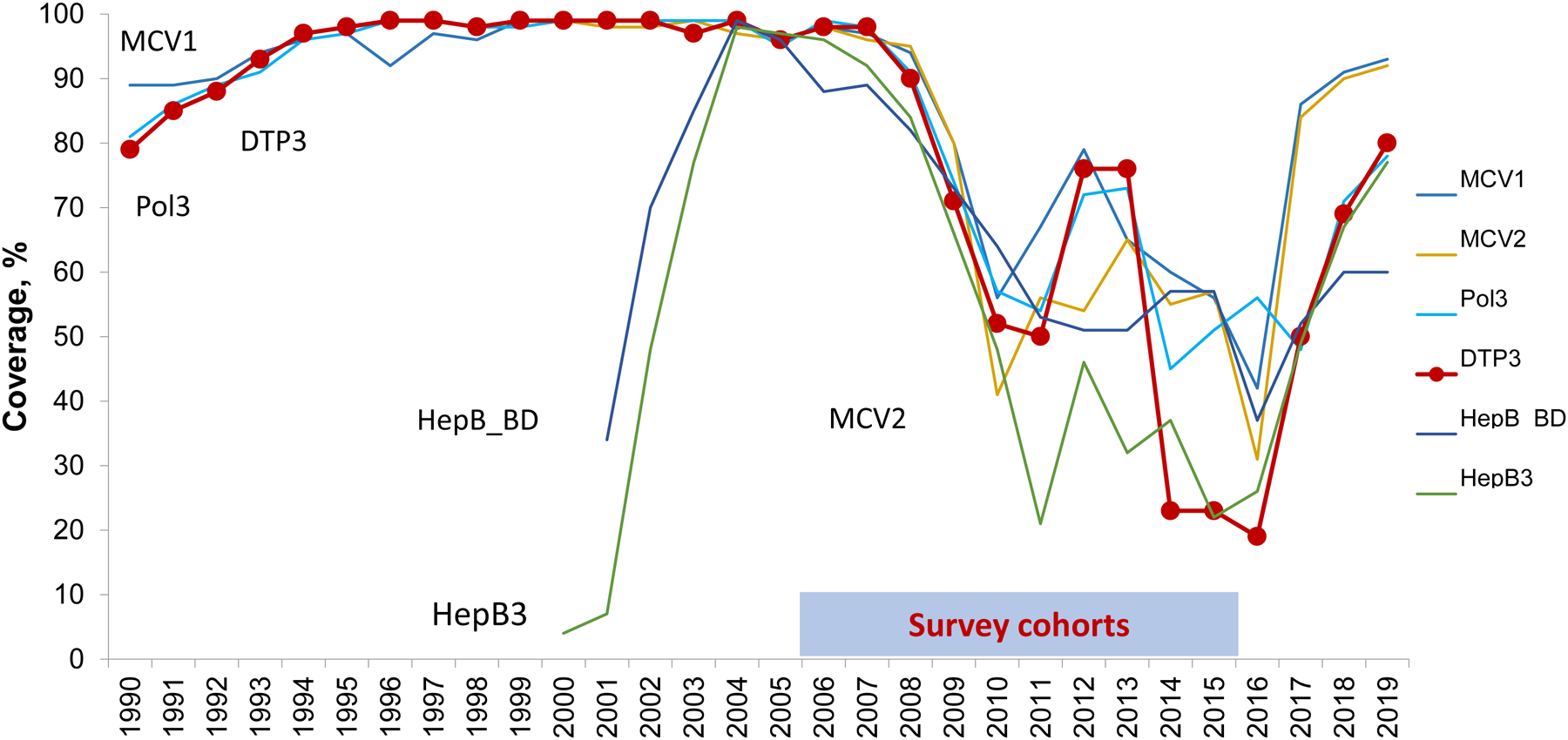
Source –Official country reports submitted to WHO, except for 2009, 2013, and 2014 [3]. No reports were submitted to WHO for those years. Therefore, WHO/UNICEF estimates are used. DTP3, third dose of diphtheria, tetanus, pertussis-containing vaccine; MCV1, first dose of measles-containing vaccine; MCV2, second dose of measles-containing vaccine; Pol3, third dose of polio vaccine; HepB3, third dose of hepatitis B vaccine; HepB_BD, birth dose of hepatitis B vaccine
The introduction of nationwide routine infant vaccination with diphtheria and tetanus-containing vaccines (DTCV) in 1958 resulted in the rapid decline of disease incidence in Ukraine [4, 16]. However, a large diphtheria outbreak with 19,141 reported cases occurred in Ukraine during 1991–1998 (Figure 2) [1, 4, 16]. This outbreak was part of a major resurgence of epidemic diphtheria in the former Soviet Union countries [4, 16]. Although catch-up immunizations among children since 1993 and nationwide vaccination of adults aged 16–60 years with DTCVs in 1995–1996 brought the outbreak under control [16, 17], a certain level of endemic transmission continued until the mid-2000s (Figure 2) [1, 18]. Very few cases were reported annually after 2008 [1]. Tetanus surveillance data, available since 1995, demonstrate generally low numbers of reported cases, particularly after the nationwide DTCV vaccination in response to the diphtheria outbreak (Figure 2) [2]. However, diphtheria and tetanus surveillance data are unavailable for some recent years (Figure 2) [1, 2], leading to uncertainty about the quality of surveillance. The childhood DTCV schedule in Ukraine includes a three-dose primary series given at 2, 4, and 6 months (3, 4, and 5 months before 2011), followed by booster doses at 18 months, 6 years, and 16 years of age1[19]. Suboptimal vaccination coverage and uncertainty regarding surveillance quality raised concerns about the risk of resurgence of diphtheria and tetanus in Ukraine.
Figure 2. Reported cases of diphtheria (1990–2019) and tetanus (1995–2019) in Ukraine.
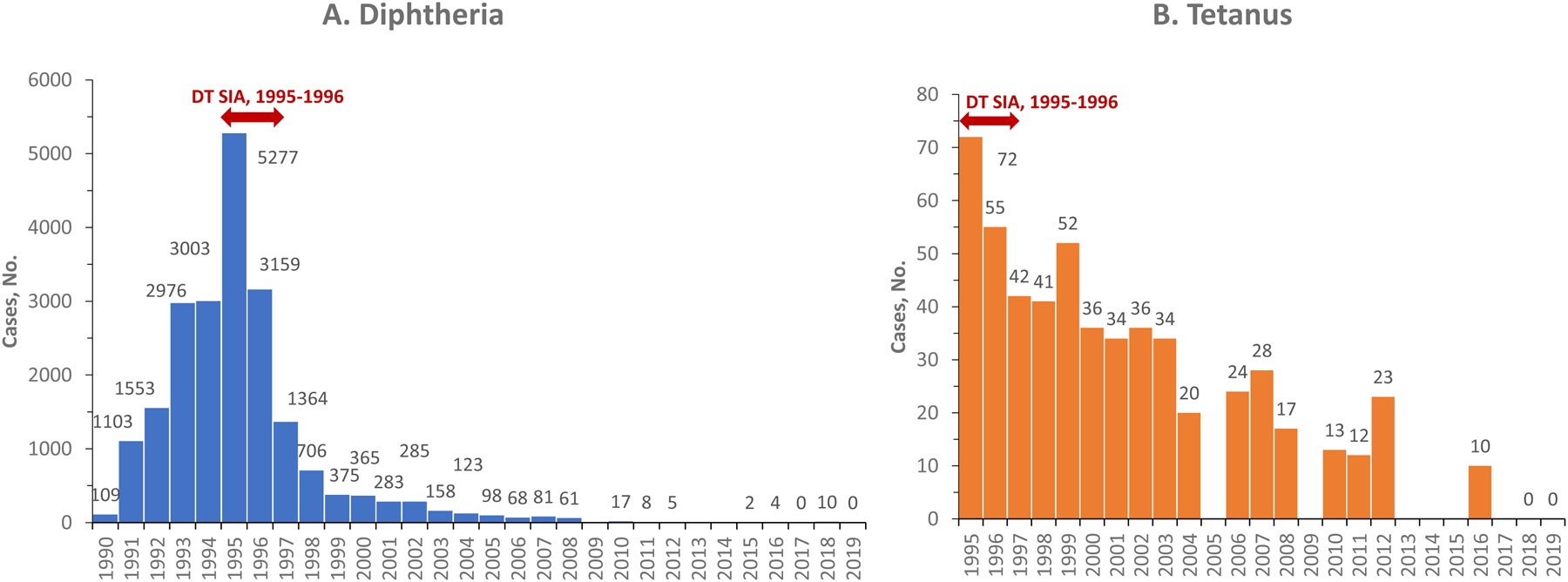
Source – Country reports submitted to WHO [1, 2]. No reports were submitted for diphtheria in 2009, 2013, and 2014; for tetanus in 2005, 2009, 2013 2014, 2015, and 2017
Population-based serologic surveys can provide information on population immunity to supplement coverage and surveillance data and help assess VPD outbreak risk and the effectiveness of vaccination programs. In 2017, a serosurvey was conducted in Ukraine to assess the immunity against polioviruses in the aftermath of the cVDPV1 outbreak and three nationwide rounds of polio outbreak response immunization among children born in 2006–2015 [6, 20]. We used the specimens and information collected through this serosurvey to determine the seroprevalence of antibodies against diphtheria and tetanus, as well as the DTCV vaccination status in these birth cohorts.
2. Methods
The survey design was based primarily on the needs of the polio serosurvey. Because logistical challenges prevented the implementation of a nationwide survey, we focused on four areas with problems related to cVDPVs and/or challenges with routine immunizations [4, 6]. These sites included Zakarpattya province in the west, Sumy province in the east, Odessa province in the south, and the capital, Kyiv City in the central part of the country (Figure 3). All these sites had underperforming immunization programs (Figure 1_supplementary). Zakarpattya was a site of the cVDPV1 outbreak in 2015. Single VDPVs without the evidence of their further circulation were detected in Sumy and Odessa (classified as ambiguous VDPVs and considered “VDPV events”) in 2016, indicating the potential for future emergence of circulating VDPVs. Finally, Kyiv City had additional challenges due to large population movement, strong anti-vaccine media environment and population resistance to vaccinations. These sites were located across all major geographic regions of Ukraine and accounted for 18% of the country’s total population2.
Figure 3. Sites included in the serosurvey for vaccine-preventable diseases — Ukraine, 2017.
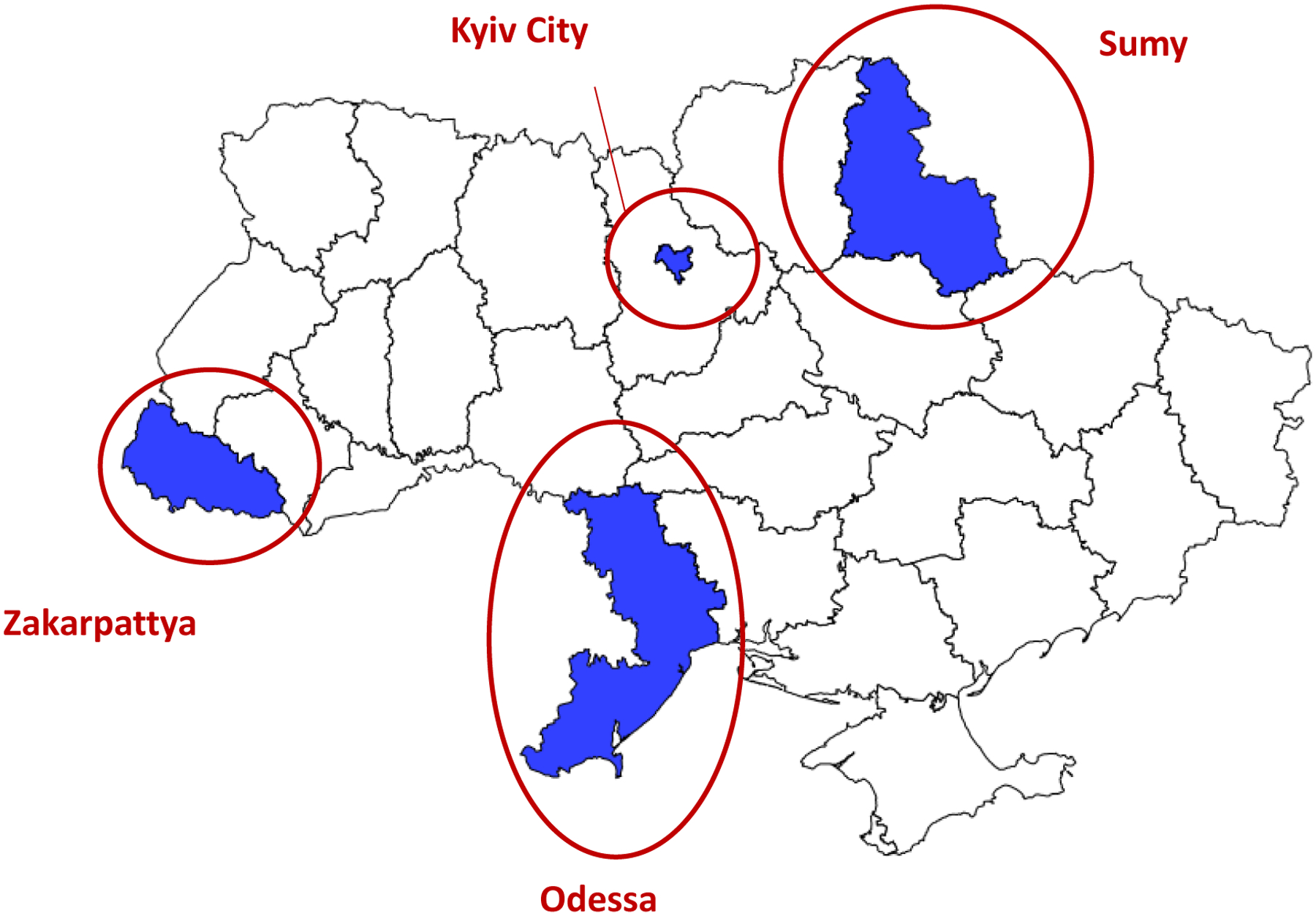
The designations used and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of WHO concerning the legal status of any country, territory, city or area, or of its authorities, nor concerning the delimitation of its frontiers or boundaries.
The serosurvey included two age strata of children targeted for the nationwide polio outbreak response immunization—children born during 2010–2015, after the decline in immunization coverage in Ukraine and eligible for all three polio immunization rounds, and children born during 2006–2009, eligible for only the third round. Sampling and participant selection were performed separately for the two age strata.
We used lists of children registered with healthcare facilities (HCF) as the sampling frames for the serosurvey because all persons in Ukraine are assigned at birth to an HCF based on their residence. We selected survey participants using cluster sampling in the three provinces and stratified simple random sampling (SRS) in Kyiv City.
We estimated the sample size, assuming a 70% expected prevalence of polio antibodies and a ±5% margin of error with 95% confidence. The estimate was inflated to allow for 20% non-response. In Kyiv, this resulted in 400 children per age stratum (800 children in total). In Zakarpattya, Sumy, and Odessa, the sample size was multiplied by an assumed design effect of 2.0 to account for cluster sampling, which resulted in 800 children per age stratum (1,600 children in total) in each province. A total of 5,600 children were targeted for inclusion across the four survey sites.
In Zakarpattya, Sumy, and Odessa, a cluster was defined as an HCF precinct. In each province, we selected 40 clusters with probability proportionate to population size from the lists of all precincts. Then, 20 children per cluster were selected by SRS from the precinct’s lists of the catchment population of eligible age. In Kyiv City, where electronic line-lists were available at HCFs, we selected children directly by stratified SRS from the lists of all children in the targeted birth cohorts. The sample was allocated across the districts of Kyiv City proportionate to their population size.
The serosurvey enrollment took place in August–September 2017. After obtaining verbal consent from the caregivers, we collected 3–5 ml of whole venous blood from the participants and information about the participants’ sex, age, and residence, and the number of DTCV doses received (from HCF records). To avoid participation bias when assessing immunization status, we collected information on vaccine doses for all children selected for the serosurvey, including those who refused blood collection.
Serum was separated on the same day in provincial laboratories and the samples were shipped to the National Virology Laboratory of the Center for Public Health in Kyiv for aliquoting into four portions. Serum aliquots were stored at –20ʰC until shipment to the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA, where they were tested for antibodies against diphtheria and tetanus. Immunity against diphtheria and tetanus was assessed simultaneously by measuring IgG antibodies against diphtheria and tetanus toxoids, using a Luminex®-based multiplex bead assay (MBA). The MBA assay validation and methods have been described previously [21–25]. The details of MBA methodology are given in Supplement.
Levels of antibodies against diphtheria and tetanus were categorized according to the degree of protection conferred. Although no antibody level provides absolute protection against diphtheria or tetanus, higher levels are generally associated with greater duration and higher clinical protection [26, 27]. We used the following categories: <0.01 IU/mL (no protection), 0.01–0.09 IU/mL (minimal protection), and ≥0.1 IU/mL (full protection) further divided into 0.1–0.9 IU/mL, linked to shorter-term protection, and ≥1.0 IU/mL, associated with long-term protection [23, 26–31].
The main outcome measures were proportions [point estimates and 95% confidence intervals (CI)] of children with different levels of protection. We also calculated the median antibody levels. We calculated separate estimates for each survey site using SAS software (SAS Institute Inc., Cary, NC, USA). Crude estimates were adjusted to account for survey design, sampling weights, and non-response (due to refusal or not enough sample for testing). For each disease, we made comparisons of seroprevalence at ≥0.1 IU/mL antibody levels by age group, sex, and immunization status, using the chi-square test.
For the diphtheria-tetanus analysis, original age groups defined for polio purposes were slightly modified to align with diphtheria-tetanus immunization schedule and ensure that each age stratum included birth cohorts eligible to receipt of the same number of DTCV doses by the time of the serosurvey. In this analysis, the older age group included children born in 2006–2010 (eligible for five doses) and the younger age group included children born in 2011–2015 (eligible for four doses)3.
For this analysis, we considered 80% seroprevalence at the ≥0.1 IU/mL antibody level as the herd immunity threshold for diphtheria, though a range of 75%–90% has been reported in the literature [32–38]. Herd immunity does not apply to tetanus, which is transmitted by spores present in the environment, rather than person-to-person [39]; however, we similarly used ≥80% seroprevalence of antibody levels ≥0.1 IU/mL as the sufficient level of protection against tetanus in the population.
We assessed the immunization status of the surveyed population by calculating the percentage (point estimate and 95% CI) of children who had received a given number of vaccine doses by the time of the serosurvey. Children who received all age-appropriate DTCV doses (four doses for the younger age group and five doses for the older age group) were considered up-to-date on their vaccinations (fully vaccinated), while recipients of three doses were considered as having received the primary series.
The serosurvey was determined to be a public health program evaluation and not human subject research by the Human Research Protection Coordinator, Center for Global Health, CDC. The protocol was approved by the Ministry of Health (MOH) of Ukraine.
3. Results
3.1. Survey population
Among the 5,600 children initially selected for the serosurvey, 93 were found to have moved out of the area and were excluded, resulting in 5,507 eligible children. Of these, 5,078 (92.2%) children were enrolled and 429 (7.8%) refused participation. Sufficient serum for diphtheria and tetanus antibody testing was available for 4,729 (85.9%) of the eligible children. Across the survey sites, we tested 85.1%–93.1% of eligible participants born during 2006–2010 and 79.0%–86.6% of those born during 2011–2015 (Figure 2_supplementary). The proportion of children tested for diphtheria-tetanus antibodies was higher in the older age group (born 2006–2010) than in the younger age group (born in 2011–2015) in all sites, except Sumy (p-values, Zakarpattya and Kyiv City, 0.002; Odessa, 0.003; Sumy, 0.128) (Table 1). Across the sites, the proportion of children who had received <3 DTCV doses was lower among those who were tested compared with children not tested (p values, Zakarpattya, Sumy, and Odessa, <0.001; Kyiv City, 0.018). There were no significant differences by sex between tested and not tested groups at any site (p values, >0.05) (Table 1).
Table 1.
Comparison of demographic characteristics and vaccination status of children selected for the vaccine-preventable disease serosurvey by diphtheria-tetanus antibody testing status and survey site — Ukraine, 2017
| Characteristics | Tested for diphtheria and tetanus antibodies | Not tested (refused or not enough sample) | P value, tested versus not tested | ||
|---|---|---|---|---|---|
| No. | % of all children | No. | % of all children | ||
| Zakarpattya | |||||
| Total children | 1,306 | 100.0 | 275 | 100.0 | n/a |
| Born in 2006–2010 | 790 | 60.5 | 138 | 50.2 | 0.002 |
| Female | 613 | 46.9 | 129 | 46.9 | 0.994 |
| Received <3 doses of DTCV | 313 | 24.0 | 98 | 35.6 | <0.001 |
| Sumy | |||||
| Total children | 1,403 | 100.0 | 190 | 100.0 | n/a |
| Born in 2006–2010 | 835 | 59.5 | 102 | 53.7 | 0.128 |
| Female | 674 | 48.0 | 102 | 53.7 | 0.145 |
| Received <3 doses of DTCV | 261 | 18.6 | 118 | 62.1 | <0.001 |
| Odessa | |||||
| Total children | 1,298 | 100.0 | 236 | 100.0 | n/a |
| Born in 2006–2010 | 781 | 60.2 | 117 | 49.6 | 0.003 |
| Female | 628 | 48.4 | 124 | 52.5 | 0.240 |
| Received <3 doses of DTCV | 234 | 18.0 | 83 | 35.2 | <0.001 |
| Kyiv City | |||||
| Total children | 722 | 100.0 | 77 | 100.0 | n/a |
| Born in 2006–2010 | 442 | 61.2 | 33 | 42.9 | 0.002 |
| Female | 353 | 48.9 | 32 | 41.6 | 0.224 |
| Received <3 doses of DTCV | 86 | 11.9 | 17 | 22.1 | 0.018 |
Note: Doses of any diphtheria-tetanus-containing vaccines (DTCV) are included. Immunization information reflects the status as of July–August 2017, prior to the survey specimen collection in late August–September 2017; n/a – not applicable
3.2. Seroprevalence of antibodies against diphtheria and tetanus
In Sumy, Odessa, and Kyiv City, a small proportion of children (3.5%–7.9%) had no protection against diphtheria (antibodies <0.01 IU/mL), 17.3%–22.8% had minimal protection (0.01–0.09 IU/mL), and 69.3%–79.2% had full protection (≥0.1 IU/mL), including 25.1%–31.8% with long-term protection (≥1.0 IU/mL) (Table 2). In contrast, Zakarpattya had a substantial proportion of children with no protection against diphtheria (18.7%) and a low proportion of children with fully protective levels (50.0%, including only 16.2% with long-term protection). Median levels of diphtheria antibodies were 0.09 IU/mL in Zakarpattya and 0.29–0.42 IU/mL at other sites (Table 2).
Table 2.
Diphtheria and tetanus antibody levels among children born in 2006–2015 by survey site — Ukraine, 2017
| Antibody levels, IU/mL | Diphtheria | Tetanus | ||||
|---|---|---|---|---|---|---|
| No. | Adjusted % (95% CI) | Median, IU/mL | No. | Adjusted % (95% CI) | Median, IU/mL | |
| Zakarpattya (N = 1,306) | 0.09 | 0.26 | ||||
| <0.01 | 256 | 18.7 (13.0–26.1) | 292 | 21.5 (14.6–30.6) | ||
| 0.01–0.09 | 416 | 31.3 (27.7–35.2) | 23 | 16.9 (13.7–20.6) | ||
| 0.1–0.9 | 427 | 33.8 (28.4–39.6) | 347 | 27.1 (22.9–31.7) | ||
| ≥1.0 | 207 | 16.2 (12.7–20.5) | 435 | 34.5 (28.0–41.7) | ||
| Sumy (N = 1,403) | 0.42 | 1.56 | ||||
| <0.01 | 59 | 4.4 (3.2–6.2) | 59 | 4.4 (3.3–5.8) | ||
| 0.01–0.09 | 261 | 19.3 (16.3–22.7) | 90 | 6.5 (4.6–9.0) | ||
| 0.1–0.9 | 635 | 44.5 (41.1–47.8) | 373 | 26.6 (23.4–30.1) | ||
| >1.0 | 448 | 31.8 (28.6–35.2) | 881 | 62.5 (57.4–67.4) | ||
| Odessa (N = 1,298) | 0.29 | 0.94 | ||||
| <0.01 | 96 | 7.9 (5.8–10.8) | 93 | 7.9 (5.5–11.1) | ||
| 0.01–0.09 | 294 | 22.8 (19.8–26.1) | 145 | 11.5 (9.2–14.2) | ||
| 0.1–0.9 | 579 | 44.2 (40.7–47.9) | 435 | 32.9 (29.1–36.9) | ||
| ≥1.0 | 329 | 25.1 (21.9–28.4) | 625 | 47.8 (43.1–52.5) | ||
| Kyiv City (N = 722) | 0.42 | 1.23 | ||||
| <0.01 | 25 | 3.5 (2.3–5.2) | 30 | 4.3 (3.0–6.2) | ||
| 0.01–0.09 | 128 | 17.3 (15.3–19.5) | 53 | 6.8 (4.8–9.5) | ||
| 0.1–0.9 | 357 | 49.2 (43.7–54.7) | 239 | 32.4 (27.3–38.0) | ||
| ≥1.0 | 212 | 30.0 (27.1–33.1) | 400 | 56.4 (51.1–61.6) | ||
Antibody levels <0.01 IU/mL correspond to no protection, 0.01–0.09 IU/mL – to minimal protection, and ≥0.1 IU/mL to full protection, including 0.1–0.9 IU/mL, linked to shorter-term protection, and ≥1.0 IU/mL, associated with long-term protection
Immunity to tetanus generally followed similar trends, but overall, antibody levels were higher than those for diphtheria (Table 2). In Sumy, Odessa, and Kyiv City, a small proportion of survey participants (4.3%–7.9%) had no tetanus seroprotection (antibody levels <0.01 IU/mL); and the majority (80.7%–89.1%) of the children had antibodies in the ≥0.1 IU/mL range, including 47.8%–62.5% with ≥1.0 IU/mL. In contrast, a substantial proportion of the children surveyed in Zakarpattya (21.5%) lacked minimally protective levels of tetanus antibodies and only 61.6% had ≥0.1 IU/mL tetanus antibodies, including 34.5% with ≥1.0 IU/mL. Median levels of tetanus antibodies were >0.1 IU/mL in all survey sites, exceeding 1.0 IU/mL in Sumy and Kyiv City (Table 2).
For both diphtheria and tetanus, estimates of seroprevalence of ≥0.1 IU/mL were highest in Kyiv City and Sumy, followed by Odessa, and lowest in Zakarpattya (Table 3). There were no differences in ≥0.1 IU/mL seroprevalence by sex in any survey site for either disease. In Zakarpattya, Odessa, and Kyiv City, there were no significant differences in the distribution of full protection (≥0.1 IU/ml) against diphtheria or tetanus between age groups (Table 3). In Zakarpattya, Odessa and Kyiv City, distribution of different levels of seroprotection for both diseases was comparable between age groups (Figures 4A and 4B).
Table 3.
Seroprevalence of diphtheria and tetanus antibodies ≥0.1 IU/mL among children born in 2006–2015 by survey site, age group, and sex — Ukraine, 2017
| Variables | Children tested, n | Diphtheria antibodies ≥0.1 IU/mL | Tetanus antibodies ≥0.1 IU/mL | ||
|---|---|---|---|---|---|
| Adjusted % (95% CI) | p value | Adjusted % (95% CI) | p value | ||
| Zakarpattya | |||||
| Overall | 1,306 | 50.0 (41.9–58.1) | n/a | 61.6 (52.9–70.3) | n/a |
| Age group | |||||
| Born in 2006–2010 | 790 | 48.5 (39.8–57.1) | 61.0 (51.7–70.4) | 0.713 | |
| Born in 2011–2015 | 516 | 51.6 (42.7–60.4) | 62.2 (53.0–71.4) | ||
| Sex | |||||
| Male | 693 | 50.7 (42.1–59.4) | 61.8 (52.3–71.3) | 0.866 | |
| Female | 613 | 49.2 (40.9–57.5) | 61.4 (52.7–70.0) | ||
| Sumy | |||||
| Overall | 1,403 | 76.2 (72.2–80.3) | n/a | 89.1 (86.2–92.0) | n/a |
| Age group | |||||
| Born in 2006–2010 | 835 | 83.9 (80.8–87.0) | 93.3 (91.1–95.6) | <0.001 | |
| Born in 2011–2015 | 568 | 67.9 (61.3–74.5) | 84.6 (80.2–88.9) | ||
| Sex | |||||
| Male | 729 | 76.1 (71.4–80.7) | 89.6 (86.9–92.3) | ||
| Female | 674 | 76.5 (71.5–81.4) | 88.6 (84.9–92.2) | ||
| Odessa | |||||
| Overall | 1,298 | 69.3 (65.0–73.6) | n/a | 80.7 (76.5–84.9) | n/a |
| Age group | |||||
| Born in 2006–2010 | 781 | 71.9 (67.7–76.0) | 82.3 (78.3–86.3) | ||
| Born in 2011–2015 | 517 | 66.5 (60.6–72.4) | 79.0 (72.8–85.1) | ||
| Sex | |||||
| Male | 670 | 71.3 (66.5–76.1) | 81.0 (76.5–85.5) | ||
| Female | 628 | 67.1 (61.8–72.4) | 80.4 (75.3–85.4) | ||
| Kyiv City | |||||
| Overall | 722 | 79.2 (76.5–81.9) | n/a | 88.9 (86.3–91.4) | n/a |
| Age group | |||||
| Born in 2006–2010 | 442 | 80.8 (77.9–83.6) | 89.1 (84.6–93.7) | ||
| Born in 2011–2015 | 280 | 77.5 (72.6–82.4) | 88.6 (85.2–91.9) | ||
| Sex | |||||
| Male | 369 | 78.2 (75.7–80.7) | 87.8 (85.2–90.3) | ||
| Female | 353 | 80.2 (76.2–84.3) | 90.0 (86.0–94.0) | ||
Figure 4. Diphtheria and tetanus antibody levels among children born in 2006–2015 by age group and survey site — Ukraine, 2017.
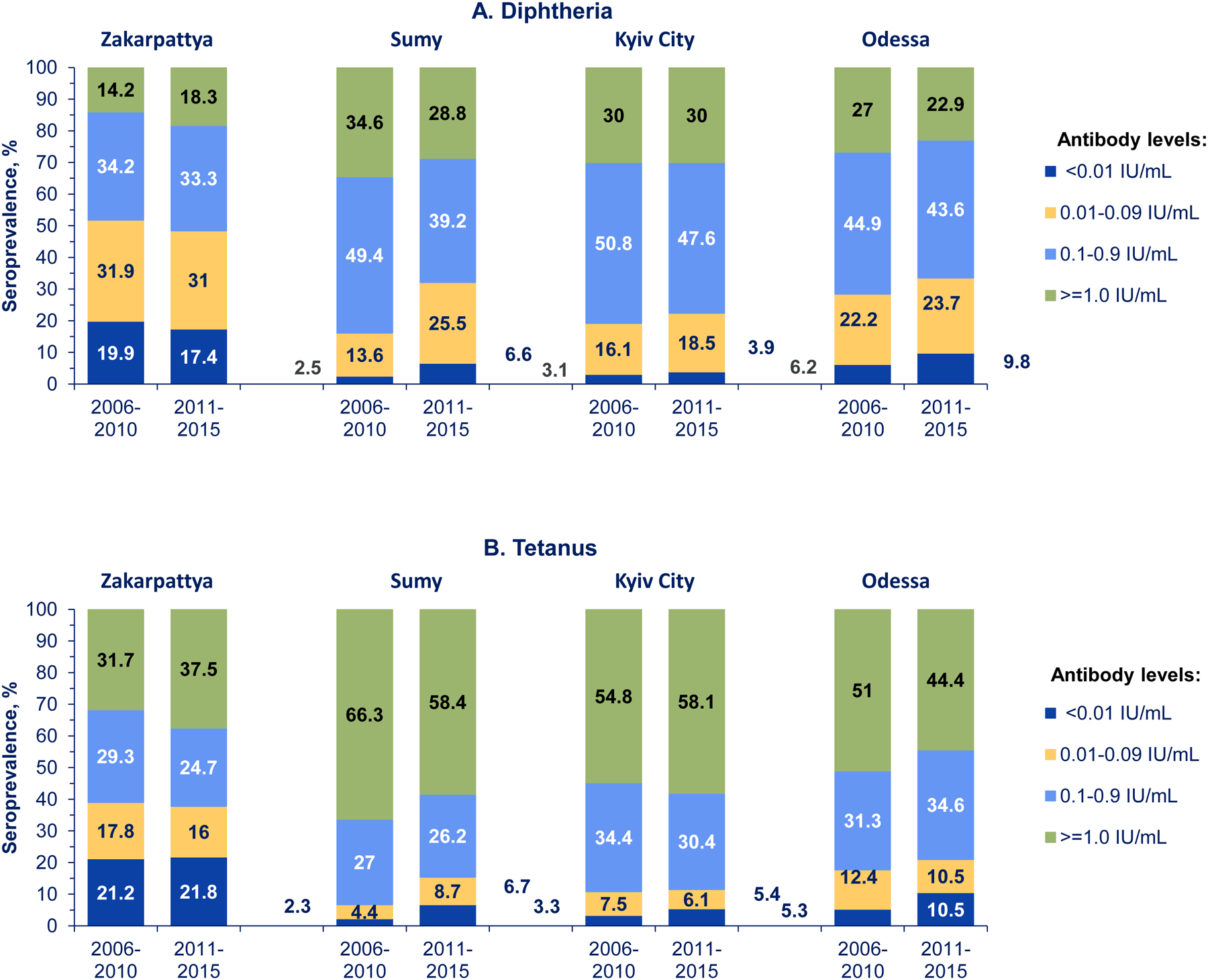
Antibody levels <0.01 IU/mL correspond to no protection, 0.01–0.09 IU/mL – to minimal protection, and ≥0.1 IU/mL to full protection, including 0.1–0.9 IU/mL, linked to shorter-term protection, and ≥1.0 IU/mL, associated with long-term protection.
In Sumy, however, a higher proportion of older children born in 2006–2010 had fully protective levels (≥0.1 IU/ml) of diphtheria and tetanus antibodies (83.9% and 93.3%, respectively) than younger children born in 2011–2015 (67.9% and 84.6%, respectively) (Table 3). In Sumy, the estimated proportion of persons with no protection (<0.01 IU/mL) against diphtheria was 2.5% (95% CI, 1.4%–4.4%) among children born in 2006–2010 versus 6.6% (95% CI, 4.4%–9.8%) among children born in 2011–2015 (Figure 4A). The proportion with minimal protection (0.01–0.09 IU/mL) was 13.6% (95% CI, 11.2%–16.4%) among older children versus 25.5% (95% CI, 20.4%–31.3%) among younger children (Figure 4A). Similarly, the proportion of children unprotected (<0.01 IU/mL) against tetanus was lower among children born in 2006–2010 compared with those born in 2011–2015: 2.3% (95% CI, 1.4%–3.7%) versus 6.7% (95% CI, 4.9%–9.0%), respectively (Figure 4B).
Across the survey sites, the proportion of children with antibody levels ≥0.1 IU/mL for both diseases generally increased with the number of DTCV doses received (from <3 doses to 5 doses) but there was an overlap between the two age groups (Table 4). In Sumy, Odessa, and Kyiv City, the seroprevalence of ≥0.1 IU/mL diphtheria antibodies among recipients of three DTCV doses was 63.2%–75.3% for children born in 2006–2010 and 63.8%–69.8% for children born in 2011–2015; seroprevalence of protective levels of diphtheria antibodies among 4-dose recipients was 69.3%–85.6% among older children and 80.6%–89.1% among younger children. The ≥0.1 IU/mL diphtheria seroprevalence among 5-dose recipients who were born in 2006–2010 was 83.2%–89.4% as compared to the small group of the fifth DTCV dose recipients who were born in 2011 (86.5–94.7 percent) (Table 4).
Table 4.
Seroprevalence of diphtheria and tetanus antibody levels ≥0.1 IU/mL among children born in 2006–2015 by survey site, age group, and the number of diphtheria and tetanus-containing vaccine (DTCV) doses received — Ukraine, 2017
| Variables | DTCV doses received | Children tested, n | Diphtheria antibodies ≥0.1 IU/mL | Tetanus antibodies ≥0.1 IU/mL | ||
|---|---|---|---|---|---|---|
| Adjusted % (95% CI) | p value | Adjusted % (95% CI) | p value | |||
| Zakarpattya | ||||||
| <3 doses | 124 | 31.1 (15.5–46.8) | 44.4 (26.7–62.2) | |||
| 3 doses | 102 | 45.7 (33.7–57.7) | 58.6 (43.7–73.5) | |||
| 4 doses | 327 | 47.8 (38.7–56.9) | 60.5 (51.5–69.5) | |||
| 5 doses | 237 | 60.9 (46.7–75.1) | 72.7 (59.6–85.8) | |||
| <3 doses | 189 | 43.0 (32.0–53.9) | 56.5 (45.2–67.9) | |||
| 3 doses | 122 | 43.4 (30.7–56.1) | 53.2 (40.2–66.3) | |||
| 4 doses | 182 | 63.1 (52.3–74.0) | 71.5 (60.5–82.5) | |||
| 5 doses | 23 | 81.7 (64.0–99.4) | 87.9 (41.8–98.7) | |||
| Sumy | ||||||
| <3 doses | 59 | 66.6 (46.0–87.2) | 75.6 (62.5–88.8) | |||
| 3 doses | 65 | 75.3 (62.1–88.5) | 85.9 (77.0–94.8) | |||
| 4 doses | 234 | 79.3 (74.8–83.8) | 94.8 (91.3–98.3) | |||
| 5 doses | 477 | 89.4 (86.2–92.6) | 95.8 (93.5–98.0) | |||
| <3 doses | 202 | 53.3 (44.3–62.3) | 71.9 (64.2–79.6) | |||
| 3 doses | 174 | 69.8 (61.4–78.1) | 86.8 (80.9–92.7) | |||
| 4 doses | 173 | 80.6 (73.6–87.6) | 96.1 (93.5–98.7) | |||
| 5 doses | 19 | 91.2 (55.4–98.9) | 94.9 (85.9–95.0) | |||
| Odessa | ||||||
| <3 doses | 79 | 31.9 (16.2–47.6) | 45.2 (27.4–63.0) | |||
| 3 doses | 80 | 72.0 (61.5–82.5) | 81.0 (71.3–90.8) | |||
| 4 doses | 253 | 69.3 (62.8–75.9) | 85.2 (80.2–90.1) | |||
| 5 doses | 369 | 83.2 (79.2–87.3) | 89.4 (84.9–93.9) | |||
| <3 doses | 155 | 42.2 (31.5–52.8) | 58.0 (45.6–70.4) | |||
| 3 doses | 113 | 63.8 (52.5–75.0) | 81.6 (71.1–92.1) | |||
| 4 doses | 229 | 83.7 (77.7–89.6) | 91.3 (86.6–96.1) | |||
| 5 doses | 20 | 86.5 (71.5–89.6) | 93.7 (83.3–100.0) | |||
| Kyiv City | ||||||
| <3 doses | 33 | 41.9 (27.7–56.0) | 55.0 (41.4–68.5) | |||
| 3 doses | 47 | 63.2 (47.1–79.3) | 73.8 (55.3–92.4) | |||
| 4 doses | 183 | 85.6 (81.2–90.1) | 92.6 (88.9–96.2) | |||
| 5 doses | 179 | 87.6 (83.3–91.9) | 96.0 (92.4–99.5) | |||
| <3 doses | 53 | 54.8 (40.1–69.5) | 62.3 (48.0–76.6) | |||
| 3 doses | 70 | 67.2 (55.4–78.9) | 88.6 (80.1–97.1) | |||
| 4 doses | 138 | 89.1 (84.4–93.8) | 97.1 (91.9–97.5) | |||
| 5 doses | 19 | 94.7 (85.5–100.0) | 100.0 (100.0–100.0) | |||
In Zakarpattya, however, recipients of similar numbers of vaccine doses tended to have substantially lower proportions of seroprevalence of ≥0.1 IU/mL antibody levels compared with those in Sumy, Odessa, and Kyiv City (Table 4). In Zakarpattya, the seroprevalence of ≥0.1 IU/mL of diphtheria antibodies among recipients of three DTCV doses in both age groups was <50%. The seroprevalence among 4-dose recipients was 47.8% in the older age group and 63.1% in the younger age group. The seroprevalence among 5-dose recipients was 60.9% for children born in 2006–2010 and 81.7% in the small group of children born in 2011 who had received their fifth DTCV dose by the time of serosurvey4. The latter was the only subgroup in Zakarpattya in which ≥0.1 IU/mL diphtheria seroprevalence exceeded 80% (Table 4). Across all survey sites, the patterns observed for tetanus antibodies were similar to those for diphtheria but with higher seroprevalence levels for tetanus (Table 4).
3.3. Diphtheria-tetanus immunization status
Overall, a substantial proportion of children born during 2006–2015 had not received the full 3-dose primary series of DTCV by the time of the serosurvey (point estimates: Kyiv City, 13.5%; Zakarpattya, Sumy, and Odessa, 21.0%–27.6%), including 5.7%–11.4% of the children who received zero doses of DTCV (Table 5).
Table 5.
Immunization status with diphtheria and tetanus-containing vaccines among children born in 2006–2015 by survey site and age group — Ukraine, 2017
| Survey site | Diphtheria-tetanus-containing vaccine doses received | 2006–2015 cohorts, adjusted % (95% CI) | 2006–2010 cohorts, adjusted % (95% CI) | 2010–2015 cohorts, adjusted % (95% CI) |
|---|---|---|---|---|
| <3 doses | 27.6 (21.4–34.8) | 16.7 (11.4–23.8) | 38.6 (30.6–47.3) | |
| 0 doses | 11.4 (8.2–15.7) | 8.8 (5.8–13.0) | 14.0 (10.0–19.4) | |
| 1 dose | 6.2 (4.4–8.6) | 2.2 (1.4–3.5) | 10.2 (6.9–14.9) | |
| 2 doses | 10.0 (6.2–15.9) | 5.7 (2.8–11.3) | 14.4 (9.1–22.0) | |
| 3 doses | 17.8 (13.9–22.7) | 13.3 (9.4–18.4) | 22.4 (17.2–28.7) | |
| 4 doses | 38.1 (33.0–43.5) | 41.6 (35.6–47.8) | 34.7 (27.1–43.2) | |
| 5 doses | 16.4 (12.5–21.1) | 28.5 (21.8–36.4) | 4.2 (2.5–7.0) | |
| <3 doses | 21.0 (17.0–25.6) | 7.1 (3.8–12.9) | 35.8 (30.7–41.2) | |
| 0 doses | 5.9 (4.2–8.1) | 2.6 (1.2–5.6) | 9.4 (7.0–12.5) | |
| 1 dose | 7.1 (5.2–9.7) | 2.6 (1.2–5.6) | 12.0 (8.9–15.9) | |
| 2 doses | 8.0 (6.0–10.5) | 1.9 (0.9–4.1) | 14.4 (11.2–18.3) | |
| 3 doses | 18.5 (16.1–21.1) | 7.6 (5.3–10.8) | 30.1 (25.8–34.7) | |
| 4 doses | 29.3 (26.0–32.9) | 27.9 (23.4–32.9) | 30.8 (26.5–35.5) | |
| 5 doses | 31.2 (26.6–36.2) | 57.4 (49.7–64.7) | 3.3 (1.9–5.7) | |
| <3 doses | 22.6 (18.9–26.8) | 12.9 (9.4–17.4) | 32.4 (27.3–38.0) | |
| 0 doses | 9.7 (7.6–12.3) | 6.6 (4.9–8.9) | 12.8 (10.0–16.3) | |
| 1 dose | 6.2 (4.7–8.0) | 3.3 (1.8–5.8) | 9.1 (6.9–11.8) | |
| 2 doses | 6.7 (5.1–8.8) | 3.0 (1.9–4.7) | 10.5 (7.8–14.0) | |
| 3 doses | 16.8 (14.4–19.6) | 11.1 (8.3–14.5) | 22.6 (18.9–26.8) | |
| 4 doses | 36.8 (32.6–41.3) | 32.8 (28.0–37.9) | 40.9 (34.9–47.3) | |
| 5 doses | 23.8 (19.6–28.5) | 43.3 (35.5–51.4) | 4.1 (2.7–6.2) | |
| <3 doses | 13.5 (10.1–17.7) | 7.1 (5.1–9.8) | 19.8 (14.3–26.7) | |
| 0 doses | 5.7 (3.7–8.6) | 3.4 (1.8–6.1) | 8.0 (5.0–12.6) | |
| 1 dose | 1.9 (1.1–3.4) | 1.6 (0.8–3.0) | 2.2 (0.8–6.0) | |
| 2 doses | 5.9 (4.1–8.4) | 2.2 (1.2–3.8) | 9.6 (6.2–14.6) | |
| 3 doses | 19.0 (15.9–22.6) | 12.2 (8.4–17.4) | 25.9 (21.9–30.3) | |
| 4 doses | 44.4 (40.1–48.7) | 40.5 (35.2–46.0) | 48.2 (41.7–54.9) | |
| 5 doses | 23.1 (20.7–25.8) | 40.2 (34.8–45.9) | 6.1 (4.2–8.6) |
Note: Immunization data obtained from healthcare facility records; includes vaccine doses received by July-August 2017. All children selected for serosurvey are included, irrespective of their participation status. Recipients of ≥3 doses are considered to have received at least full primary series of diphtheria-tetanus-containing vaccines (DTCV); children in the older age group (those born in 2006–2010) are considered vaccinated age-appropriately if they have received five DTCV doses; children in the younger age group (those born in 2011–2015) are considered vaccinated age-appropriately if they have received ≥4 doses
The percentages of children who had received ≥3 doses in each of the regions ranged from 72.4%–86.5% with the lowest proportion observed in Zakarpattya (72.4%) and the highest in Kyiv City (86.5%). The proportion of recipients of the three DTCV doses accounted for 16.8%–19.0% of the children; there were no substantial differences across the survey sites. Greater proportion of children had received four doses in Kyiv City, Odessa and Zakarpattya (36.8%–44.4%) than in Sumy (29.3%). However, Sumy had the highest proportion of the recipients of five doses (31.2%) versus 16.4–23.8% in the other three sites (Table 5).
We noted a decline in DTCV immunizations among children born in 2011–2015 versus 2006–2010 across all sites (Table 5). Among children born in 2011–2015, the proportion who received <3 DTCV doses ranged from 32.4%–38.6% in Sumy, Zakarpattya, and Odessa; it was 19.8% in Kyiv City. We also noted an increase in zero-dose recipients among younger children, particularly in Sumy and Odessa (Table 5).
The proportion of children who were age-appropriately vaccinated (i.e., those born in 2011–2015 who had received four doses or those born 2005–2010 who had received five doses) was suboptimal in all subgroups and exceeded 50% only in Kyiv City among children born in 2011–2015 (54.3%), and in Sumy among children born in 2006–2010 (57.4%). The lowest proportions of age-appropriately vaccinated children were found in Zakarpattya (28.5%) among children born in 2006–2010 and in Sumy (34.1%) among children born in 2011–2015 (Table 5).
4. Discussion
The findings of this survey indicate that in 2017, most children in surveyed sites of Ukraine had at least a minimal level of protection against diphtheria and tetanus. However, point estimates of overall seroprevalence of fully protective (≥0.1 IU/mL) levels of diphtheria antibodies were below the 80% herd immunity threshold in all sites. For tetanus, ≥80% point estimates of seroprevalence ≥0.1 IU/mL antibodies were observed in all sites except Zakarpattya, suggesting an adequate level of protection in most sites. Seroprotection levels for both diseases were higher in Kyiv City, Sumy, and Odessa, while substantial immunity gaps were present in both age groups in Zakarpattya.
Diphtheria immunity in Zakarpattya was clearly below the herd immunity threshold in both age groups, indicating the potential for outbreaks and severe disease in case of diphtheria introduction. Only half of the children born in 2006–2015 had fully protective levels of antibodies and one in five lacked even minimally protective levels. Population immunity against tetanus was also suboptimal, with only ~60% overall seroprevalence of antibodies ≥0.1 IU/mL.
The serosurvey data provided insights into the performance of Ukraine’s immunization program. We observed high seroprevalence of ≥0.1 IU/mL antibody levels for both diphtheria and tetanus in Kyiv City, Sumy, and Odessa among age-appropriately vaccinated children (point estimates, 80.6%–89.1% for diphtheria and 89.4%–97.1% for tetanus) but not among the under-vaccinated children. This demonstrates the effectiveness of DTCV used in Ukraine and highlights the importance of receiving all the recommended vaccine doses. These findings confirm the high effectiveness of diphtheria toxoid in Ukraine as shown in the studies conducted during the diphtheria resurgence in the 1990s [33, 40, 41] and could help in addressing persistent mistrust and vaccine quality concerns in Ukraine.
However, the survey also demonstrated that, as of 2017, diphtheria-tetanus routine immunization levels, particularly among younger children, remained suboptimal in all four sites, consistent with reported official coverage at the national level and in the survey sites (Figure 1, Figure 1_supplementary). Approximately one in three children born in 2011–2015 in Zakarpattya, Sumy, and Odessa versus one in five children in Kyiv City had not completed the primary DTCV series; only one in two or three children across the survey sites were age-appropriately vaccinated with DTCV. A relatively low proportion of both zero-dose DTCV recipients and fully vaccinated children across the survey sites indicate that most children in Ukraine have access and utilize immunization services at least once but many receive vaccinations with delay or do not complete their immunization series. Widespread use of false contraindications and misguided perceptions about vaccine safety among both caregivers and healthcare providers [42, 43] likely contributed to high rates of immunization dropouts in Ukraine, along with frequent vaccine shortages in the past, particularly during 2012–2015 [4, 9, 10, 44].
In recent years, with international support, the Ukrainian government implemented efforts to strengthen the national immunization program. Transition to vaccine procurement via UNICEF since 2016 [45] has allowed more efficient use of funds and led to substantial improvements in vaccine supply. The revision of the list of immunization contraindications in 2019 to remove many conditions not recognized as valid contraindications by WHO [46] should reduce vaccination dropouts related to false contraindications. An increasing trend in officially reported immunization coverage since 2018 (Figure 1) suggests improvements in national immunization program performance. However, substantial immunity gaps among children who missed vaccinations in earlier years are still not adequately addressed. Before this survey, the enhanced immunization efforts implemented by the MOH had been primarily focused on cVDPV1 and measles outbreak responses [4, 47]. To improve population immunity against other VPDs, including diphtheria and tetanus, selective mop-up immunizations, involving identification and vaccination of unvaccinated and under-vaccinated children (i.e., recipients of less than the age-appropriate recommended number of vaccine doses) were initiated nationwide in 2019. The high levels of protection against diphtheria and tetanus antibody levels among the fully vaccinated children documented in most survey sites provide reassurance that focusing mop-up immunization efforts on unvaccinated and under-vaccinated groups should address most of the remaining diphtheria and tetanus susceptibility among children instead of implementing large-scale non-selective supplementary immunization with DCTV. Given the suboptimal protection levels in the younger age group and the continued suboptimal immunization coverage in Ukraine, children born after 2010, including those born after 2015 who were ineligible for this survey, warrant special focus during mop-up immunizations.
Diphtheria and tetanus seroprotection in Zakarpattya differ substantially from other survey sites. The low overall seroprevalence of diphtheria and tetanus antibodies in this province, suboptimal immunization levels, and low prevalence of full protection for both diseases among reportedly vaccinated children are cause for concern. Furthermore, in this serosurvey, Zakarpattya also had lower population immunity against polioviruses, measles, rubella [20], and a higher prevalence of chronic hepatitis B infection [6] than other survey sites. These findings confirm profound problems with the immunization system in Zakarpattya, resulting in a higher risk of VPD outbreaks.
In Zakarpattya, almost 40% and 30% of the children with age-appropriate vaccination were not fully protected against diphtheria and tetanus, respectively. The low seroprevalence among vaccinated children is likely unrelated to vaccine quality problems because of the geographically limited scope of this finding and shared supply of vaccine with other provinces. In addition, in this survey, Zakarpattya had low seroprevalence among vaccinated children for other VPDs as well; however, this was not observed in other sites (data not shown). Other potential causes for suboptimal protection among vaccinated children in Zakarpattya include local problems with cold chain and vaccine handling, and/or unreliability of immunization records. Challenges with immunization records quality in Ukraine are well known and relate to the common practice of falsification [48, 49], particularly, when immunization requirement for school/daycare entry was enforced5. A comprehensive review of the status of the immunization program in Zakarpattya is necessary to gain a better insight into the causes of its suboptimal performance.
The selective mop-up strategy used in the rest of Ukraine might not be sufficient for addressing diphtheria and tetanus immunity gaps among children in Zakarpattya [17]. This approach would leave out large numbers of children who are reportedly vaccinated but are not serologically protected. Therefore, conducting a non-selective subnational diphtheria-tetanus immunization round with age-appropriate vaccines among children born since 2006 in Zakarpattya should be considered. Sustaining high-quality diphtheria surveillance and close monitoring of surveillance data in Ukraine, particularly in Zakarpattya and the surrounding regions, will be crucial for ensuring timely identification of potential diphtheria introductions and the prompt implementation of appropriate response.
The serosurvey had certain limitations. For logistical feasibility reasons, the survey was focused on four sites that were not selected by probability-based procedures; therefore, the data from different sites were not pooled in the analysis. The population-based estimates are representative of the survey sites but their generalizability to other regions is unclear. Additionally, given the predominance of adults in the diphtheria outbreak in the 1990s [16], data on adult susceptibility in Ukraine would help to assess diphtheria herd immunity status and outbreak risk across all age groups; however, these data are not available. Finally, small numbers in subgroups limited the power of statistical analysis by the number of vaccine doses and, in some cases, resulted in wide confidence intervals.
Overall, the findings of this survey demonstrate that despite the progress made in recent years, substantial challenges with Ukraine’s immunization program remain, particularly in Zakarpattya. Sustaining the current improvements and continued efforts to further strengthen routine immunization services and implement catch-up vaccination among children will be essential for reversing the impact of the decline in immunizations in the past decade and protecting the population from VPDs, including diphtheria and tetanus. The lessons learned from Ukraine’s efforts to rebuild the national immunization program in the context of substantial challenges can be helpful for other countries that also experienced a decline in their immunization programs.
Supplementary Material
Figure 1_supplementary. Officially reported immunization coverage across survey sites in Ukraine for DTP3 (2008–2017), DTP4 and DT5 (2012–2017)
DTP3, third dose of diphtheria-tetanus-pertussis-containing vaccines; DT4, fourth dose of diphtheria-tetanus-containing vaccines; DT5, fifth dose of diphtheria-tetanus-containing vaccines.
Source – Official coverage data from the Ministry of Health of Ukraine; province level data were available beginning with 2008 for DTP3 and beginning with 2012 for DTP4 and DT5.
Figure 2_supplementary. Serosurvey enrolment and diphtheria and tetanus antibody testing status by survey site and age group — Ukraine, 2017
Highlights.
Decline in immunizations raised diphtheria-tetanus resurgence concerns in Ukraine
Serosurvey conducted in Zakarpattya, Sumy, and Odessa provinces and Kyiv City
<80% of children were fully protected against diphtheria and ≥80% against tetanus (except 61% in Zakarpattya)
Diphtheria-tetanus vaccination coverage across all survey sites was suboptimal
Coverage should be increased to reduce the risk of diphtheria and tetanus in Ukraine
Acknowledgments
We would like to acknowledge contributions to the implementation of this survey by the public health officials and survey collaborators from Zakarpattya, Sumy, Odessa, and Kyiv City and the Ministry of Health of Ukraine; the staff from the World Health Organization (WHO) Country Office in Ukraine, WHO Regional Office for Europe, Global Immunization Division and Division of Parasitic Diseases and Malaria at the Centers for Disease Control and Prevention (CDC) and South Caucasus Field Epidemiology and Laboratory Training Program (FELTP) at CDC Office in Tbilisi, Georgia. We would also like to thank participants and their caregivers for participating in the serosurvey.
Funding sources:
Funding for this serosurvey was provided by the WHO Regional Office for Europe and Global Immunization Division, CDC. No external funding was received.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Some of the co-authors are staff members of the World Health Organization (WHO). The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of the WHO. The designations used and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of WHO concerning the legal status of any country, territory, city or area, or of its authorities, nor concerning the delimitation of its frontiers or boundaries.
According to the Decree of the Ministry of Health of Ukraine “On the procedure for preventive vaccinations in Ukraine and quality control and handling of medical immunobiological drugs” from September 16 2011, with subsequent updates [19], the national childhood immunization schedule includes DTCV doses at 2, 4, 6 (3, 4, and 5 months until 2011), and 18 months, and 6 and 16 years, administered as part of DTP or other combination vaccines. Adult booster doses every 10 years beginning at 26 years are also recommended. Immunizations against diphtheria and tetanus are recommended at the same time points, as vaccinations against polio for all doses, except at 16 years (polio booster is given at 14 years).
The administrative division of Ukraine consists of 24 provinces, two cities with special status (Kyiv City and Sevastopol), and the Autonomous Republic of Crimea. The Autonomous Republic of Crimea and Sevastopol are currently outside the Ukrainian government control.
Children born in 2011 became eligible for the fifth DTCV dose during 2017, as they turned 6 years old. By the time of serosurvey, only a small number of children born in 2011 had received their fifth DTCV dose. Thus, this birth cohort was included in the age group eligible for four doses.
Across the four survey sites, only 81 children born in 2011 had received the fifth dose of DTCV before enrolment.
The requirement was suspended by the Ministry of Health during widespread vaccine shortages; reinstated in 2018.
References
- 1. WHO.Immunization data portal. Diphtheria cases and incidence of diphtheria by year for Ukraine. Available at: https://immunizationdata.who.int/pages/incidence/diphtheria.html?CODE=UKR&YEAR=. Accessed June 14, 2021. [Google Scholar]
- 2.WHO. Immunization data portal. Tetanus cases and incidence by year for Ukraine. Available at: https://immunizationdata.who.int/pages/incidence/ttetanus.html?CODE=UKR&DISEASE=&YEAR=. Accessed June 14, 2021 [Google Scholar]
- 3.WHO. Immunization data portal. DTP3 coverage, Ukraine Available at: https://immunizationdata.who.int/pages/coverage/dtp.html?CODE=UKR&ANTIGEN=DTPCV3&YEAR= Accessed 14 June 2021
- 4.Khetsuriani N, Perehinets I, Nitzan D, Popovic D, Moran T, Allahverdiyeva V, et al. Responding to a cVDPV1 outbreak in Ukraine: Implications, challenges and opportunities. Vaccine 2017;35:4769–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitek CR, Wharton M. Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerg Infect Dis. 1998;4:539–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khetsuriani N, Zaika O, Chitadze N, Slobodianyk L, Allahverdiyeva V, O’Connor P, Huseynov S. Seroprevalence of hepatitis B virus infection markers among children in Ukraine, 2017. Vaccine 2021;39:1485–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khetsuriani N, Deshevoi S, Goel A, Spika J, Martin R, Emiroglu N. Supplementary immunization activities to achieve measles elimination: experience of the European Region. J Infect Dis 2011;2004 Suppl 1:S343–52 [DOI] [PubMed] [Google Scholar]
- 8.Twigg JL. Polio in Ukraine. Crisis, challenge and opportunity. March 2016. A report of the CSIS Global Health Policy Center. Center for Strategic and International Studies. Available at: https://csis-website-prod.s3.amazonaws.com/s3fs-public/publication/160329_Twigg_PolioUkraine_Web.pdf Accessed 14 June 2021 [Google Scholar]
- 9.WHO. Integrated assessment of immunization quality and safety, Ukraine. Report. Kyiv; 2008 [Google Scholar]
- 10.Ministry of Health of Ukraine, WHO, UNICEF, Bill & Melinda Gates Foundation, European Centre for Disease Prevention and Control, USAID/Maternal and Child Health Integrated Program, and US Centers for Disease Control and Prevention. Immunization Programme Management Review, Ukraine, 2012. Report 2012
- 11.Editorial. Measles, war, and health-care reforms in Ukraine. Lancet. 2018;392:711. [DOI] [PubMed] [Google Scholar]
- 12.Hadjipanayis A, van Esso D, Del Torso S, Dornbusch HJ, Michailidou K, Minicuci N, et al. Vaccine confidence among parents: Large scale study in eighteen European countries. Vaccine. 2020;38:1505–12 [DOI] [PubMed] [Google Scholar]
- 13.WHO. WHO, UNICEF and CDC regret the Government of Ukraine’s decision to suspend the National Measles and Rubella Vaccination Campaign. Available at: https://www.euro.who.int/en/media-centre/sections/press-releases/2008/05/who,-unicef-and-cdc-regret-the-government-of-ukraines-decision-to-suspend-the-national-measles-and-rubella-vaccination-campaign Accessed 14 June 2021
- 14.European Center for Disease Control. Rapid Risk Assessment. Outbreak of measles in Ukraine and potential for spread in the EU, 2012. Available at: https://www.ecdc.europa.eu/en/publications-data/outbreak-measles-ukraine-and-potential-spread-eu-2012 Accessed 14 June 2021 [Google Scholar]
- 15.Zimmerman LA, Muscat M, Singh S, Ben Mamou M, Jankovic D, Datta S, et al. Progress Toward Measles Elimination - European Region, 2009–2018. MMWR Morb Mortal Wkly Rep. 2019;68:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nekrassova LS, Chudnaya LM, Marievski VF, Oksiuk VG, Gladkaya E, Bortnitska II, et al. Epidemic diphtheria in Ukraine, 1991–1997. J Infect Dis. 2000;181 Suppl 1:S35–40. [DOI] [PubMed] [Google Scholar]
- 17.Dittmann S, Wharton M, Vitek C, Ciotti M, Galazka A, Guichard S. Successful control of epidemic diphtheria in the states of the Former Union of Soviet Socialist Republics: lessons learned. J Infect Dis. 2000;181(Suppl 1):S10–22 [DOI] [PubMed] [Google Scholar]
- 18.Wagner KS, White JM, Lucenko I, Mercer D, Crowcroft NS, Neal S, et al. Diphtheria Surveillance Network. Diphtheria in the postepidemic period, Europe, 2000–2009. Emerg Infect Dis. 2012;18:217–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health of Ukraine. Decree No. 595 “On the procedures for preventive vaccinations in Ukraine and quality control and handling of medical immunobiological drugs” from September 16 2011, with subsequent updates Available at: https://zakon.rada.gov.ua/laws/show/z1159-11/ed20110916#Text; accessed January 12, 2022.
- 20.Khetsuriani N, Zaika O, Slobodyanik L, Demchishina I, Weldon W, Chitadze N, et al. Poliomyelitis, measles, rubella, diphtheria, tetanus, and hepatitis b seroepidemiology among children born in 2006–2015 in four regions of UKRAINE, 2017. Abstracts of the 39th Annual Meeting of the European Paediatric Infectious Disease Society (ESPID), online, May 24–29 2021, abstract No. 287 [Google Scholar]
- 21.Rogier E, Moss DM, Chard AN, Trinies V, Doumbia S, Freeman MC, et al. Evaluation of Immunoglobulin G Responses to Plasmodium falciparum and Plasmodium vivax in Malian School Children Using Multiplex Bead Assay. The American journal of tropical medicine and hygiene. 2017;96:312–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold BF, Scobie HM, Priest JW, Lammie PJ. Integrated Serologic Surveillance of Population Immunity and Disease Transmission. Emerg Infect Dis. 2018;24:1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scobie HM, Mao B, Buth S, Wannemuehler KA, Sorensen C, Kannarath C, et al. Tetanus Immunity among Women Aged 15 to 39 Years in Cambodia: a National Population-Based Serosurvey, 2012. Clinical and vaccine immunology 2016;23:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minta AA, Andre-Alboth J, Childs L, Nace D, Rey-Benito G, Boncy J, et al. Seroprevalence of Measles, Rubella, Tetanus, and Diphtheria Antibodies among Children in Haiti, 2017. Am J Trop Med Hyg 2020;103:1717–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scobie HM, Khetsuriani N, Efstratiou A, Priest JW. Validation of a diphtheria toxoid multiplex bead assay for serosurveys. Diagn Microbiol Infect Dis 2021;100(3):115371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Immunological Basis for Immunization Series Module 2: Diphtheria Update 2009. Available at: https://apps.who.int/iris/bitstream/handle/10665/44094/9789241597869_eng.pdf;jsessionid=1597129966E63842F00389633A5B1CB6?sequence=1 Accessed 14 June 2021
- 27.WHO Immunological Basis for Immunization Series Module 3: Tetanus Update 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/275340/9789241513616-eng.pdf?ua=1 Accessed 14 June 2021
- 28.Efstratiou A, Maple PAC. Diphtheria. Laboratory diagnosis of diphtheria. The expanded programme on immunization in the European region of WHO. 1994. WHO ICP/EPI 038. [Google Scholar]
- 29.Maple PA, Efstratiou A, George RC, Andrews NJ, Sesardic D. Diphtheria immunity in UK blood donors. Lancet. 1995;345:963–5. [DOI] [PubMed] [Google Scholar]
- 30.Basta NE, Borrow R, Berthe A, Onwuchekwa U, Traoré Eps Dembélé A, Almond R, et al. Higher Tetanus Toxoid Immunity 2 Years After PsA-TT Introduction in Mali. Clin Infect Dis 2015;61 Suppl 5:S578–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scobie HM, Patel M, Martin D, Mkocha H, Njenga SM, Odiere MR, et al. Tetanus immunity gaps in children 5–14 years and men ≥15 years of age revealed by integrated disease serosurveillance in Kenya, Tanzania, and Mozambique. Am J Trop Med Hyg 2017;96:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson RM. The concept of herd immunity and the design of community-based immunization programmes. Vaccine 1992;10:928–35 [DOI] [PubMed] [Google Scholar]
- 33.Sutter RW, Hardy IR, Kozlova IA, Tchoudnaia LM, Gluskevich TG, Marievsky V, et al. Immunogenicity of tetanus-diphtheria toxoids (Td) among Ukrainian adults: implications for diphtheria control in the Newly Independent States of the Former Soviet Union. J Infect Dis. 2000;181 Suppl 1:S197–202. [DOI] [PubMed] [Google Scholar]
- 34.Fine PE Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302 [DOI] [PubMed] [Google Scholar]
- 35.Plans-Rubió P Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum Vaccin Immunother. 2012;8:184–8. [DOI] [PubMed] [Google Scholar]
- 36.Begg N Manual for the management and control of diphtheria in the European Region. Copenhagen: WHO, 1994. Available at: https://apps.who.int/iris/bitstream/handle/10665/108107/ICP_EPI_038_%28B%29.pdf?sequence=1&isAllowed=y Accessed 14 June 2021 [Google Scholar]
- 37.de Melker HE, Berbers GA, Nagelkerke NJ, Conyn-van Spaendonck MA. Diphtheria antitoxin levels in the Netherlands: a population-based study. Emerg Infect Dis. 1999. Sep-Oct;5(5):694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. Diphtheria vaccine: WHO position paper – August 2017. Weekly Epidemiological Record 2017;92:417–3628776357 [Google Scholar]
- 39.WHO. Tetanus vaccine: WHO position paper – February 2017. Weekly Epidemiological Record 2017;92:53–7628185446 [Google Scholar]
- 40.Tsu V, Tyshchenko DK. Case-control evaluation of an adult diphtheria immunization program in Ukraine. J Infect Dis 2000;181 Suppl 1:S188–92 [DOI] [PubMed] [Google Scholar]
- 41.Chen RT, Hardy IR, Rhodes PH, Tyshchenko DK, Moiseeva AV, Marievsky VF. Ukraine, 1992: first assessment of diphtheria vaccine effectiveness during the recent resurgence of diphtheria in the Former Soviet Union. J Infect Dis. 2000. Feb;181 Suppl 1:S178–83. [DOI] [PubMed] [Google Scholar]
- 42.Oniskova OV. Vaccination: myths and facts (review of literature). Perinatologiya I Pediatriya. 2015;3:51–4; doi 10.15574/PP.2015.63.51 [DOI] [Google Scholar]
- 43.Tatochenko V, Mitjushin IL. Contraindications to vaccination in the Russian Federation. J Infect Dis. 2000;181(Suppl 1):S228–31. [DOI] [PubMed] [Google Scholar]
- 44.WHO. Ukraine Effective Vaccine Management assessment, 22 October–2 November 2018. Findings and recommendations: WHO Regional Office for Europe, 2019 [Google Scholar]
- 45.UNICEF. Ministry of Health signs agreements with international organizations for procurement of medicine for the 2018 budget. 27 April 2018 https://www.unicef.org/ukraine/en/press-releases/ministry-health-signs-agreements-international-organizations-procurement-medicine Accessed 14 June 2021
- 46.Ministry of Health of Ukraine. Decree No. 2070 October 11, 2019. On modifications to the schedule of prophylactic immunizations and to the list of medical contraindications to prophylactic immunizations in Ukraine (in Ukrainian) Available at: https://zakon.rada.gov.ua/laws/show/z1182-19#n15 Accessed 13 January 2021
- 47.WHO. 8th meeting of the European Regional Verification Commission for Measles and Rubella Elimination (RVC). 12–14 June 2019, Warsaw, Poland. Available at: http://www.euro.who.int/__data/assets/pdf_file/0019/413236/8th-RVC-Report.pdf. Accessed 14 June 2021 [Google Scholar]
- 48.UNICEF. Evaluation report based on the interviews: summary baseline KAPB survey (Knowledge, Attitude, Practice, Behaviour) on planned immunization and on measles/rubella immunization in Ukraine. Kyiv, 2008
- 49.Radio Liberty, Ukraine. May 14, 2018. UNICEF estimates that about 30% of immunization certificated are fake (in Ukrainian). Available at: https://www.radiosvoboda.org/a/news/29226039.html. Accessed 14 June 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1_supplementary. Officially reported immunization coverage across survey sites in Ukraine for DTP3 (2008–2017), DTP4 and DT5 (2012–2017)
DTP3, third dose of diphtheria-tetanus-pertussis-containing vaccines; DT4, fourth dose of diphtheria-tetanus-containing vaccines; DT5, fifth dose of diphtheria-tetanus-containing vaccines.
Source – Official coverage data from the Ministry of Health of Ukraine; province level data were available beginning with 2008 for DTP3 and beginning with 2012 for DTP4 and DT5.
Figure 2_supplementary. Serosurvey enrolment and diphtheria and tetanus antibody testing status by survey site and age group — Ukraine, 2017


