Abstract
Exposure to weightlessness causes severe osteopenia, resulting in raised fracture risk. The current study aimed to investigate whether nicotinamide mononucleotide (NMN) supplementation protected against the osteopenia in hindlimb unloading (HLU) rats in vivo and modeled microgravity-induced osteoblastic dysfunction in vitro. The 3-mo-old rats were exposed to HLU and intragastrically administered NMN every 3 days (500 mg/kg body weight) for 4 weeks. NMN supplementation mitigated HLU-induced bone loss, evidenced by greater bone mass and biomechanical properties and better trabecular bone structure. NMN supplementation mitigated HLU-induced oxidative stress, evidenced by greater levels of nicotinamide adenine dinucleotide and activities of superoxide dismutase 2 and lesser malondialdehyde levels. Modeled microgravity stimulation using rotary wall vessel bioreactor in MC3T3-E1 cells inhibited osteoblast differentiation, which was reversed by NMN treatment. Furthermore, NMN treatment mitigated microgravity-induced mitochondrial impairments, evidenced by lesser reactive oxygen species generation and greater adenosine triphosphate production, mtDNA copy number, and activities of superoxide dismutase 2 and Complex I and II. Additionally, NMN promoted activation of AMP-activated protein kinase (AMPK), evidenced by greater AMPKα phosphorylation. Our research suggested that NMN supplementation attenuated osteoblastic mitochondrial impairment and mitigated osteopenia induced by modeled microgravity.
Keywords: Rotary wall vessel bioreactor, Osteoblast, Oxidative stress, MC3T3-E1 cells, Hindlimb unloading
Introduction
Osteopenia is one of the well documented phenomena for crew members during a long spaceflight or patients subjected to long-term bed rest, which results in increased fracture risk (Coulombe et al. 2020; Frings-Meuthen et al. 2019). Numerous investigations have demonstrated that impaired osteoblastic function played an important role in weightlessness or microgravity‐induced osteopenia (Morey and Baylink 1978; Cao et al. 2021). Countermeasures including exercise regimes and supplementation of vitamin D, calcium and bisphosphonates developed to date are ineffective for prevention of bone loss following microgravity (Smith et al. 1999). Development of novel anti-osteoporotic drugs is required for space travelers and geriatric population.
As a crucial intermediate during biosynthesis of nicotinamide adenine dinucleotide (NAD+), accumulating evidence revealed that nicotinamide mononucleotide (NMN) was able to reverse defects in mitochondrial homeostasis, redox state, cell survival, as well as DNA repair caused by deficiency of NAD+ (Croteau et al. 2017). Recent preclinical studies have demonstrated NMN administration as a promising therapeutic compound to extend the lifespan and exert diverse pharmacological actions in various diseases (Covarrubias et al. 2021; Hong et al. 2020). Long-term NMN supplementation mitigated age-associated and aluminum-induced osteoporosis in animal experiments (Mills et al. 2016; Liang et al. 2019). In vitro, NMN treatment promoted osteogenesis and self-renewal of mesenchymal stromal cells (Song et al. 2019), and attenuated dexamethasone-induced osteogenic inhibition in bone mesenchymal stem cells (Huang and Tao 2020).
Our work aimed to test the therapeutic role of NMN supplementation on the osteopenia in hindlimb unloading (HLU) rats in vivo and osteogenic inhibition induced by stimulated microgravity in vitro and elucidate the possible mechanisms.
Material and methods
HLU model and NMN treatment
Three-month-old Wistar rats, obtained from Vital-River Animal Ltd (SCXK 2015–0002, Beijing, China), were fed with standard rodent chow and distilled tap water at an appropriate temperature (about 22 °C) with 12 h light/dark cycle. All experiments involving rats had been approved by the Institutional Animal Care and the Animal Ethics Committee of Xi'an Jiaotong University (No. 202104017).
Briefly, orthopedic adhesive tapes were applied along the proximal 1/3 of tails of animals and placed by using a metal ring, attaching with a metal bar on the top of the cage as previously described (Xin et al. 2015). Control animals were individually housed in same cage as HLU rats. NMN (every 3 days, 500 mg/kg) (Wan et al. 2021; Lee et al. 2016) or vehicle was intragastrically administered in both groups for 4 weeks. Animals were sacrificed with an intraperitoneal injection of 30 μg/g xylazine and 300 μg/g ketamine at the end of study. Then, blood and samples of femurs and tibiae were harvested for further measurements. The detail of methods was shown in the supplementary data.
Cell culture and study design
The murine MC3T3-E1 cell (an osteoblastic cell line, Chinese Academy of Sciences Cell Bank, Shanghai, China) was cultured using α-MEM (gentamicin, 100 μg/ml; fetal bovine serum, 10%). Microgravity of 10−2G in vitro was modeled in cultured cells using the rotary wall vessel bioreactor (RWVB, Synthecon, Houston) as previously described (Zayzafoon et al. 2004).
Cells were cultured in osteogenic medium with 10 mM β-glycerophosphate under modeled microgravity or normal gravity and treated with NMN (0.1 or 1 mM) (Liang et al. 2019; Ryu et al. 2018) for 7 days. Activities of alkaline phosphatase (ALP) were determined using a kit (KA1642, Novus Biologicals, Littleton, CO, USA).
Cells were cultured under modeled microgravity with NMN or not for 96 h. The levels of osteopontin (OPN) and runt-related transcription factor 2 (Runx2) mRNA were determined using real-time polymerase chain reaction (RT-PCR).
Assessment of bone loss
The right femurs and tibiae were subjected to the dual energy X-ray test (Hologic, USA) to determine the bone mineral density (BMD) of the whole femurs and tibiae. Trabecular bone morphometry analysis in proximal tibiae (volume of interest: starting from the lowest point of the growth plate and extending toward the diaphysis for 1500 μm) was conducted by using μCT (Yang et al. 2015) with a 10.5-μm voxel size. The mechanical characteristics of midshaft of femurs were determined using a Bose Electro Force Testing System (ELF3510, Eden Prairie, USA).
Biochemical analysis
Urinary deoxypyridinoline (DPD) levels were determined with a kit (QUIDEL Corporation, CA, USA) to assess bone resorption and the final result of each sample was corrected for its creatinine concentration. Creatine levels in urine were assayed with a Creatinine Assay Kit (Colorimetric/Fluorometric, ab65339, Abcam, Cambridge, MA, USA). Serum levels of Ca2+ were assayed using the Hitachi 7170 autoanalyzer (Hitachi, Tokyo, Japan). Content of C-terminal cross-linked telopeptides of type I collagen (CTX, NBP2-82,444) and osteocalcin (NBP2-68,153) and activities of ALP (KA1642, Novus Biologicals) in serum and levels of malondialdehyde (MDA, KA1381) and activity of superoxide dismutase 2 (SOD2, DYC3419-2) in femurs were determined using kits obtained from Novus Biologicals (Littleton, CO, USA). The levels of NAD+ were assayed using an assay kit (K958, BioVision, USA).
Quantitative RT-PCR measurement
Femurs and cultured cells were harvested and total RNAs were then extracted using TRIzol reagent (R0016, Beyotime Biotechnology). The total RNAs concentrations were measured through the Spectrophotometer (NanoDrop 2000, Thermo Fisher, MA, USA). RT-PCR measurement was done using a RT-PCR Kit (Beyotime Biotechnology, D7268M). Transcription levels of genes of interest were quantified by 2−ΔΔCt method. The information of primers was shown in Table 1.
Table 1.
Primers sequences used in current study
| Genes | Sequence (5’-3’) | |
|---|---|---|
|
OPN (mouse) |
Sense | GACCACAGGACGACGATG |
| Antisense | TGGAACTTGCTTGACTATCGA | |
|
Runx2 (mouse) |
Sense | GACTGTGGTTACCGTCATGGC |
| Antisense | ACTTGGTTTTTCATAACAGCGGA | |
|
Osterix (mouse) |
Sense | CTGCGGAAAGGAGGCACAAAGAAG |
| Antisense | GGGTTAAGGGGAGCAAAGTCAGAT | |
|
Osteocalcin (mouse) |
Sense | ACGGTATCACTATTTAGGACCTGTG |
| Antisense | ACTTTATTTTGGAGCTGCTGTGAC | |
|
Col1a1 (mouse) |
Sense | ATGTTCAGCTTTGTGGACCTC |
| Antisense | CAGAAAGCACAGCACTCGC | |
|
COX-1 (mouse) |
Sense | ATTGCCCTCCCCTCTCTACGCA |
| Antisense | CGTAGCTTCAGTATCATTGGTGCCC | |
|
β-actin (mouse) |
Sense | CCATGTTCCAAAACCATTCC |
| Antisense | GGGCAACCTTCCCAATAAAT | |
| GAPDH | Sense | TATGTCGTGGAGTCTACTGGT |
| (mouse) | Antisense | GAGTTGTCATATTTCTCGTGG |
|
ALP (rat) |
Sense | GGGTCAAGGCCAACTACAAGA |
| Antisense | CACTGGTCTAATCGAGCAGC | |
| Osteocalcin (rat) | Sense | TCTCTGCTCACTCTGCTGG |
| Antisense | GTGGTGCCATAGATGCGCT | |
|
TRAP (rat) |
Sense | AATTGCCTACTCCAAGATCTCCAA |
| Antisense | GCGGAACTTTGAAACGCAAA | |
|
GAPDH (rat) |
Sense | TATCACTCTACCCACGGCAAG |
| Antisense | ATACTCAGCACCAGCATCACC |
Measurement of mitochondrial function
Lucigenin-enhanced chemiluminescence was applied for detection of mitochondrial reactive oxygen species (ROS). Mitochondrial adenosine triphosphate (ATP) was quantified using a kit (BioVision). The complex I and II activities were assayed as described previously (Maher et al. 2007). The intracellular NAD+ content was determined using the kit described above. The mitochondrial copy number was detected using PCR method as described previously (Zhai et al. 2019) using cyclooxygenase-1 (COX-1) and β-actin as marker of mitochondrial DNA and nuclear DNA, respectively. The primer pairs of COX-1 and β-actin were shown in Table 1.
Western blot
The concentration of protein preparation was detected using a Beyotime bradford protein assay kit (Beyotime). Protein preparations of 25 μg were separated on SDS–polyacrylamide gels, and transferred to PVDF membranes (0.2 μm), and incubated with antibodies. Antibodies used in our work included anti-p-AMPKα (Thr172) (1:800; #50,081, CST, USA), anti-AMPKα (1:800; #5831, CST), and anti-GAPDH (1:5000; ab8245, Abcam).
Statistics
Two-way ANOVA followed by Tukey–Kramer test was applied for our statistical work using software of GraphPad Prism 9.0 (San Diego, CA). Differences were considered significant statistically at P-values < 0.05. All data of current investigation were expressed as mean ± SD.
Results
Benefits of NMN supplementation on bone loss in HLU rats
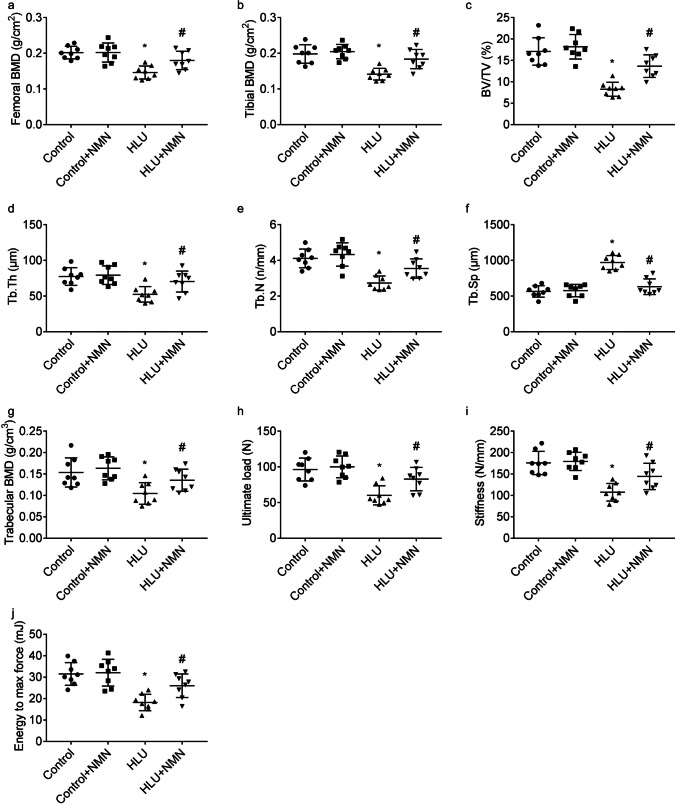
Adult rats were challenged with HLU exposure and intragastrically administered NMN or vehicle for 4 weeks. As compared to control rats, HLU rats displayed lower femoral and tibial BMD (Fig. 1a, b). NMN supplementation mitigated HLU-induced bone loss, evidenced by greater femoral and tibial BMD.
Fig. 1.
Benefits of NMN on the osteoporosis-like phenotype in HLU rats. Femoral (a) and tibial (b) BMD, trabecular bone morphometry including BV/TV, Tb.Th, Tb.N, Tb.Sp, and BMD (c-g) of proximal tibiae, and biomechanical properties including ultimate load (h), stiffness (i), and energy to max force (j) of femur midshaft were presented. * P < 0.05 vs. Control animals treated with vehicle; # P < 0.05 vs. HLU animals treated with vehicle. n = 8 or 5/group
As compared to control rats, HLU rats displayed impaired bone morphometry of proximal tibiae, evidenced by lesser bone volume/total volume (BV/TV, Fig. 1c), trabecular thickness (Tb.Th, Fig. 1d), trabecular number (Tb.N, Fig. 1e), and trabecular BMD (Fig. 1g), and greater trabecular separation (Tb.Sp, Fig. 1f), which was reversed by NMN supplementation, at least in part.
As compared to control rats, HLU rats displayed impaired biomechanical properties of femur midshaft, evidenced by lesser ultimate load (Fig. 1h), stiffness (Fig. 1i), and energy to max force (Fig. 1j), which was also mitigated by NMN supplementation.
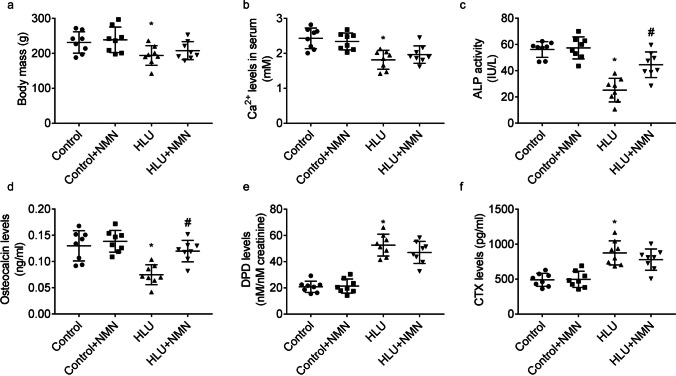
Effects of NMN supplementation on the biomarkers of bone metabolism
When compared to control animals, HLU animals displayed lesser body weight (Fig. 2a) and levels of Ca2+ (Fig. 2b), activities of ALP (Fig. 2c), and levels of osteocalcin (Fig. 2d) in serum and greater urinary DPD levels (Fig. 2e) and serum CTX levels (Fig. 2f). Body weight, serum levels of Ca2+ or CTX, and urinary levels of DPD in HLU + NMN animals were similar with that in HLU animals. Serum levels of ALP activities and osteocalcin in HLU + NMN animals were greater than that in HLU animals.
Fig. 2.
Benefits of NMN on the biomarkers of bone metabolism in vivo. Graphs showed the body mass (a), serum levels of Ca2+ (b), ALP activity (c), osteocalcin (d) and CTX (f), and urinary levels of DPD (e). * P < 0.05 vs. Control animals treated with vehicle; # P < 0.05 vs. HLU animals treated with vehicle. n = 8/group
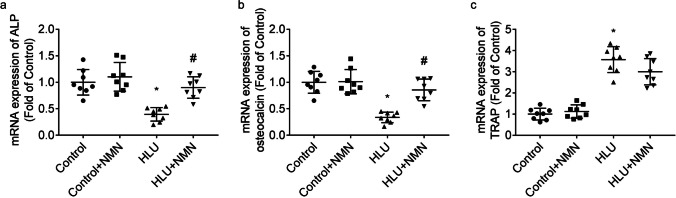
As compared to control rats, ALP (Fig. 3a) and osteocalcin (Fig. 3b) mRNA levels were lesser and tartrate-resistant acid phosphatase (TRAP) mRNA level was greater (Fig. 3c) in femurs of HLU rats. The femoral mRNA levels of ALP and osteocalcin in HLU + NMN animals were greater than that in HLU animals. The mRNA levels of TRAP in femurs were similar between HLU + NMN animals and HLU animals.
Fig. 3.
Modulation of NMN on the biomarkers involved in osteoclastogenesis and osteoblastogenesis. Graphs showed femoral mRNA levels of ALP (a), osteocalcin (b) and TARP (c). * P < 0.05 vs. Control animals treated with vehicle; # P < 0.05 vs. HLU animals treated with vehicle. n = 8/group
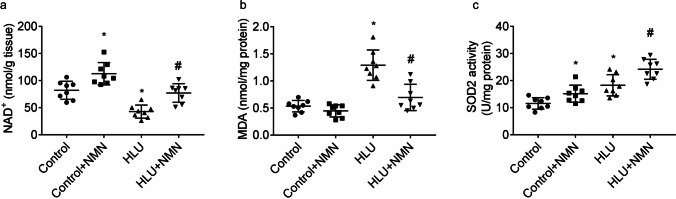
Antioxidant effects of NMN supplementation in HLU rats
As compared to control rats, levels of NAD+ (Fig. 4a) in femurs of HLU rats were lower. Femoral levels of NAD+ were greater in both control and HLU animals after NMN supplementation.
Fig. 4.
Modulation of NMN on oxidative stress and NAD+ levels. Graphs presented femoral NAD+ content (a) and MDA content (b) and SOD2 activity (c). * P < 0.05 vs. Control animals treated with vehicle; # P < 0.05 vs. HLU animals treated with vehicle. n = 8/group
As compared to control rats, MDA production (Fig. 4b) in femurs of HLU rats were higher, which was reversed by NMN partly.
Activities of SOD2 (Fig. 4c) in femurs of HLU group were higher when compared to that of control group. Femoral SOD2 activities were greater in both control and HLU animals after NMN supplementation.
Benefits of NMN on osteoblastic differentiation
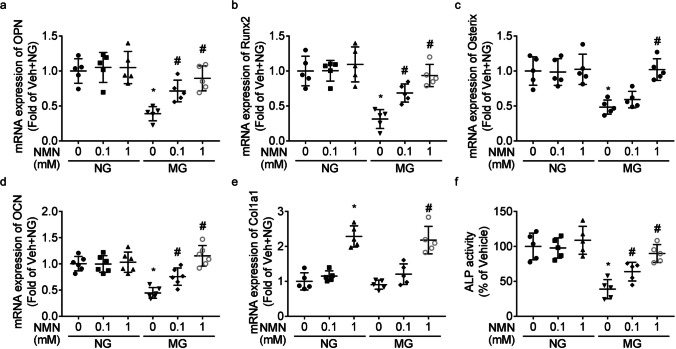
When compared to MC3T3-E1 cells cultured under normal gravity, mRNA levels of OPN (Fig. 5a), Runx2 (Fig. 5b), Osterix (Fig. 5c), and osteocalcin (Fig. 5d) and activities of ALP (Fig. 5f) were lesser under modeled microgravity, which was reversed by NMN treatment dose-dependently.
Fig. 5.
Benefit of NMN on osteoblastic differentiation. The mRNA levels of genes of OPN (a), Runx2 (b), Osterix (c), osteocalcin (OCN, d), and Col1a1 (e) and activities of ALP (f) were shown. * P < 0.05 vs. group cultured under normal gravity (NG) and treated with vehicle; # P < 0.05 vs. group cultured under microgravity (MG) and treated with vehicle
Modeled microgravity stimulation had no significant effect on Col1a1 mRNA expression (Fig. 5e). The mRNA expression of Col1a1 were higher in NMN-treated group than that in vehicle-treated group, under both modeled microgravity and normal gravity.
Benefits of NMN treatment on mitochondrial impairment
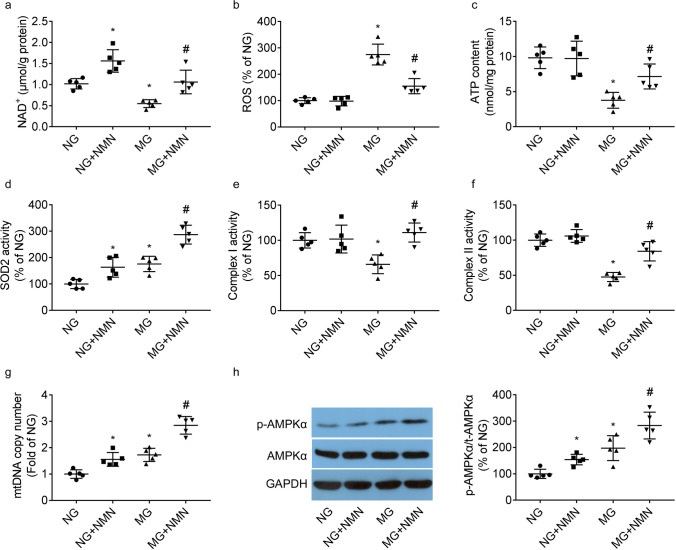
When compared to MC3T3-E1 cells cultured under normal gravity, intracellular NAD+ levels (Fig. 6a) were lesser under modeled microgravity. NAD+ levels were greater in NMN-treated group than that in vehicle-treated group, under both modeled microgravity and normal gravity.
Fig. 6.
Benefit of NMN on mitochondrial impairments. Graphs presented the intracellular levels of NAD+ (a), mitochondrial ROS production (b), ATP content (c), activities of SOD2 (d) and Complex I and II (e, f), and mtDNA copy number (g). Western blot of AMPKα phosphorylation and responding quantification results (h) were shown. * P < 0.05 vs. group cultured under normal gravity (NG) and treated with vehicle; # P < 0.05 vs. group cultured under microgravity (MG) and treated with vehicle
When compared to cells cultured under normal gravity, modeled microgravity stimulation led to a severe mitochondrial impairment, evidenced by greater mitochondrial ROS generation (Fig. 6b) and lesser ATP content (Fig. 6c) and Complex I and II activities (Fig. 6e, f), which was reversed by NMN treatment.
When compared to cells cultured under normal gravity, SOD2 activity (Fig. 6d), mtDNA copy number (Fig. 6g), and AMPKα phosphorylation (Fig. 6h) were greater under modeled microgravity. SOD2 activity, mtDNA copy number, and AMPKα phosphorylation were greater in NMN-treated group than that in vehicle-treated group, under both normal gravity and modeled microgravity.
Discussion
Our work demonstrated that NMN supplementation mitigated osteopenia in HLU rats. The 19.5-day space flight in rats strikingly inhibited bone formation (Morey and Baylink 1978). It was also observed that simulated microgravity in vitro inhibited differentiation phenotypes of osteoblastic cells (Cao et al. 2021; Xin et al. 2015). Therefore, impairment of bone formation contributed to the osteopenia induced by microgravity. NMN treatment attenuated aluminum or dexamethasone-induced osteogenic inhibition in vitro (Liang et al. 2019; Huang and Tao 2020). In our investigation, serum levels of ALP activities and osteocalcin in HLU + NMN animals were greater than that in HLU animals. Furthermore, NMN treatment reversed the modeled microgravity-induced reduction of differentiation phenotypes in MC3T3-E1 cells. We demonstrated that NMN supplementation promoted osteoblastogenesis, which might explain its anti-osteoporotic action under modeled microgravity.
Oxidative stress is one of the most investigated events involved in bone tissues in response to the microgravity (Tian et al. 2017). Accumulating evidence reported that excessive ROS produced in bone intervened osteoblast differentiation and prevented osteoblast activity (Tao et al. 2020). NMN abated oxidative stress in various diseases involved in heart, aorta, tendon and brain (Yamaura et al. 2022; Wu et al. 2021; Tarantini et al. 2019; de Picciotto et al. 2016). The antioxidant property of NMN was linked to enhanced activities of antioxidant enzymes such as SOD (Wan et al. 2021). SOD was crucial for osteoblast differentiation and trabecular bone loss was reported in the mice of osteoblast lineage Sod2 deficiency (Schoppa et al. 2022). In current work, SOD2 activities were greater and MDA levels were lesser in femurs of HLU + NMN animals than that of HLU animals. Thus, the antioxidant function of NMN might contributed to its protective effects against bone loss induced by microgravity.
Recently, a comprehensive multi-omics analysis demonstrated that mitochondrial dysfunction including impaired respiratory chain and increased mitochondrial ROS formation was a consistent phenotype for spaceflight biology (da Silveira et al. 2020). In mesenchymal stem cells, simulated microgravity increased mtDNA copy number, but inhibited mitochondrial oxidative phosphorylation (Liu et al. 2020). In current study, Complex I and II activities were lesser in cells when stimulated with modeled microgravity. Active mitochondria were required for osteoblastic differentiation (Shares et al. 2018). NAD+ was required in glycolysis in mitochondria and NAD depletion resulted in mitochondrial dysfunction (Sims et al. 2018; Alano et al. 2010). In our work, NAD+ levels were lesser in bone tissues and cells when exposed to modeled microgravity. NMN treatment improved osteoblastic mitochondrial function, which contributed to its benefit against microgravity-induced osteopenia.
AMPK played a key role in bone formation and AMPKα deficiency inhibited osteoblastic differentiation (Kanazawa et al. 2018). The crosstalk between mitochondria and AMPK, such as mitochondrial impairment causing abnormal activation of AMPK and AMPK activation preserving mitochondrial homeostasis (Wu and Zou 2020; Jornayvaz and Shulman 2010), have been demonstrated. In our work, AMPKα phosphorylation were greater in NMN-treated group than that in vehicle-treated group, under both normal gravity and modeled microgravity. It might also contribute to its protection against microgravity-induced mitochondrial dysfunction and bone loss.
It should be noted that NMN supplementation had good safety. No increased mortality rate or obvious side effects was reported in mice after NMN administration (300 mg/kg/day) for one year (Mills et al. 2016). In rats, oral NMN supplementation for 90 days at doses up to 1500 mg/kg/d did not generate serious toxicity as seen from biochemical parameters in blood as well as histological analysis of liver and kidney (Cros et al. 2021). These reports provided hope for translating the findings of our work to human beings.
As a ground-based analog for microgravity in animals, HLU model had several weaknesses including inconsistencies of stress reaction in HLU animals and continued loading of forelimbs and upper spine (Globus and Morey-Holton 2016). Therefore, further investigations are needed to confirm the efficacy of NMN in animals in spaceflight and humans in bed rest and spaceflight.
In summary, NMN supplementation attenuated osteoblastic mitochondrial impairment and mitigated osteopenia induced by modeled microgravity in rats. NMN supplementation may be a promising nutritional strategy for anti-osteoporotic therapy of astronauts and geriatric population.
Author contributions
Conceptualization: Yunfei Huang, Yusheng Dou, Xiaobin Yang; Methodology: Yusheng Dou; Formal analysis and investigation: Yunfei Huang, Yusheng Dou, Bo Yang, Baorong He, Xuefang Zhang, Ke Zhang, Xiaobin Yang; Writing—original draft preparation: Yunfei Huang and Xiaobin Yang; Writing—review and editing: Yunfei Huang and Xiaobin Yang; Funding acquisition: Xiaobin Yang; Supervision: Xiaobin Yang.
Funding
The current study was funded by the Natural Science Foundation of Shaanxi Province (2021JM-575).
Data availability
The datasets analyzed or used in our investigation are available from the corresponding author without undue reservation.
Declarations
Ethics approval
All experiments involving animals were approved by the Institutional Animal Care and the Animal Ethics Committee of Xi'an Jiaotong University and were conducted with strict adherence to the American Association for the Accreditation of Laboratory Animal Care International and National Institutes of Health Guidelines embodied in the Guide for the Care and Use of Laboratory Animals.
Competing Interests
The authors declare that there are no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunfei Huang and Yusheng Dou equally contributed to the current study.
References
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Zhang Y, Wei S, Zhang X, Guo Y, Han B. Comprehensive circRNA expression profile and function network in osteoblast-like cells under simulated microgravity. Gene. 2021;764:145106. doi: 10.1016/j.gene.2020.145106. [DOI] [PubMed] [Google Scholar]
- Coulombe JC, Senwar B, Ferguson VL. Spaceflight-Induced Bone Tissue Changes that Affect Bone Quality and Increase Fracture Risk. Curr Osteoporos Rep. 2020;18:1–12. doi: 10.1007/s11914-019-00540-y. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros C, Cannelle H, Laganier L, Grozio A, Canault M. Safety evaluation after acute and sub-chronic oral administration of high purity nicotinamide mononucleotide (NMN-C®) in Sprague-Dawley rats. Food Chem Toxicol. 2021;150:112060. doi: 10.1016/j.fct.2021.112060. [DOI] [PubMed] [Google Scholar]
- Croteau DL, Fang EF, Nilsen H, Bohr VA. NAD+ in DNA repair and mitochondrial maintenance. Cell Cycle. 2017;16:491–492. doi: 10.1080/15384101.2017.1285631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira WA, Fazelinia H, Rosenthal SB, Laiakis EC, Kim MS, Meydan C, Kidane Y, Rathi KS, Smith SM, Stear B, Ying Y, Zhang Y, Foox J, Zanello S, Crucian B, Wang D, Nugent A, Costa HA, Zwart SR, Schrepfer S, Elworth RAL, Sapoval N, Treangen T, MacKay M, Gokhale NS, Horner SM, Singh LN, Wallace DC, Willey JS, Schisler JC, Meller R, McDonald JT, Fisch KM, Hardiman G, Taylor D, Mason CE, Costes SV, Beheshti A. Comprehensive Multi-omics Analysis Reveals Mitochondrial Stress as a Central Biological Hub for Spaceflight Impact. Cell. 2020;183:1185–1201.e20. doi: 10.1016/j.cell.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings-Meuthen P, Bernhardt G, Buehlmeier J, Baecker N, May F, Heer M. The negative effect of unloading exceeds the bone-sparing effect of alkaline supplementation: a bed rest study. Osteoporos Int. 2019;30:431–439. doi: 10.1007/s00198-018-4703-6. [DOI] [PubMed] [Google Scholar]
- Gao J, Feng Z, Wang X, Zeng M, Liu J, Han S, Xu J, Chen L, Cao K, Long J, Li Z, Shen W, Liu J. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25:229–240. doi: 10.1038/cdd.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus RK. Morey-Holton E (2016) Hindlimb unloading: rodent analog for microgravity. J Appl Physiol. 1985;120:1196–1206. doi: 10.1152/japplphysiol.00997.2015. [DOI] [PubMed] [Google Scholar]
- Hong W, Mo F, Zhang Z, Huang M, Wei X. Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism. Front Cell Dev Biol. 2020;8:246. doi: 10.3389/fcell.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RX, Tao J. Nicotinamide mononucleotide attenuates glucocorticoid-induced osteogenic inhibition by regulating the SIRT1/PGC-1α signaling pathway. Mol Med Rep. 2020;22:145–154. doi: 10.3892/mmr.2020.11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa I, Takeno A, Tanaka KI, Notsu M, Sugimoto T. Osteoblast AMP-Activated Protein Kinase Regulates Postnatal Skeletal Development in Male Mice. Endocrinology. 2018;159:597–608. doi: 10.1210/en.2017-00357. [DOI] [PubMed] [Google Scholar]
- Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, Tian R. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation. 2016;134:883–894. doi: 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Gao J, Zhang C, Li C, Wang Q, Fan J, Wu Z, Wang Q. Nicotinamide mononucleotide alleviates Aluminum induced bone loss by inhibiting the TXNIP-NLRP3 inflammasome. Toxicol Appl Pharmacol. 2019;362:20–27. doi: 10.1016/j.taap.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Liu L, Cheng Y, Wang J, Ding Z, Halim A, Luo Q, Song G. Simulated Microgravity Suppresses Osteogenic Differentiation of Mesenchymal Stem Cells by Inhibiting Oxidative Phosphorylation. Int J Mol Sci. 2020;21:9747. doi: 10.3390/ijms21249747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey ER, Baylink DJ. Inhibition of bone formation during space flight. Science. 1978;201:1138–1141. doi: 10.1126/science.150643. [DOI] [PubMed] [Google Scholar]
- Ryu KW, Nandu T, Kim J, Challa S, DeBerardinis RJ, Kraus WL. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science. 2018;360:aan5780. doi: 10.1126/science.aan5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa AM, Chen X, Ramge JM, Vikman A, Fischer V, Haffner-Luntzer M, Riegger J, Tuckermann J, Scharffetter-Kochanek K, Ignatius A. Osteoblast lineage Sod2 deficiency leads to an osteoporosis-like phenotype in mice. Dis Model Mech. 2022;15:dmm049392. doi: 10.1242/dmm.049392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shares BH, Busch M, White N, Shum L, Eliseev RA. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J Biol Chem. 2018;293:16019–16027. doi: 10.1074/jbc.RA118.004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims CA, Guan Y, Mukherjee S, Singh K, Botolin P, Davila A, Jr, Baur JA. Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI Insight. 2018;3:e120182. doi: 10.1172/jci.insight.120182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol. 1999;277:R1–10. doi: 10.1152/ajpregu.1999.277.1.r1. [DOI] [PubMed] [Google Scholar]
- Song J, Li J, Yang F, Ning G, Zhen L, Wu L, Zheng Y, Zhang Q, Lin D, Xie C, Peng L. Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death Dis. 2019;10:336. doi: 10.1038/s41419-019-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Ge G, Liang X, Zhang W, Sun H, Li M, Geng D. ROS signaling cascades: dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin (shanghai) 2020;52:1055–1062. doi: 10.1093/abbs/gmaa098. [DOI] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Ma X, Yang C, Su P, Yin C, Qian AR. The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. Int J Mol Sci. 2017;18:2132. doi: 10.3390/ijms18102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, He B, Zhu D, Wang L, Huang R, Zhu J, Wang C, Gao F. Nicotinamide mononucleotide attenuates doxorubicin-induced cardiotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Arch Biochem Biophys. 2021;712:109050. doi: 10.1016/j.abb.2021.109050. [DOI] [PubMed] [Google Scholar]
- Wu S, Zou MH. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int J Mol Sci. 2020;21:4987. doi: 10.3390/ijms21144987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Li B, Lin Q, Xu W, Zuo W, Li J, Liu N, Tu T, Zhang B, Xiao Y, Liu Q. Nicotinamide mononucleotide attenuates isoproterenol-induced cardiac fibrosis by regulating oxidative stress and Smad3 acetylation. Life Sci. 2021;274:119299. doi: 10.1016/j.lfs.2021.119299. [DOI] [PubMed] [Google Scholar]
- Xin M, Yang Y, Zhang D, Wang J, Chen S, Zhou D. Attenuation of hind-limb suspension-induced bone loss by curcumin is associated with reduced oxidative stress and increased vitamin D receptor expression. Osteoporos Int. 2015;26:2665–2676. doi: 10.1007/s00198-015-3153-7. [DOI] [PubMed] [Google Scholar]
- Yamaura K, Mifune Y, Inui A, Nishimoto H, Kurosawa T, Mukohara S, Hoshino Y, Niikura T, Kuroda R. Antioxidant effect of nicotinamide mononucleotide in tendinopathy. BMC Musculoskelet Disord. 2022;23:249. doi: 10.1186/s12891-022-05205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, He B, Liu P, Yan L, Yang M, Li D. Treatment with curcumin alleviates sublesional bone loss following spinal cord injury in rats. Eur J Pharmacol. 2015;765:209–216. doi: 10.1016/j.ejphar.2015.08.036. [DOI] [PubMed] [Google Scholar]
- Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Behera J, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates homocysteine-induced osteoblast dysfunction by inhibiting mitochondrial toxicity. J Cell Physiol. 2019;234:18602–18614. doi: 10.1002/jcp.28498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed or used in our investigation are available from the corresponding author without undue reservation.