Abstract
The Myxococcus xanthus sglA1 spontaneous mutation was originally isolated because it allowed dispersed cell growth in liquid yet retained the ability to form fruiting bodies. Consequently, most of today’s laboratory strains either contain the sglA1 mutation or were derived from strains that carry it. Subsequent work showed that sglA was a gene for social gliding motility, a process which is mediated by type IV pili. Here sglA is shown to map to the major pil cluster and to encode a 901-amino-acid open reading frame (ORF) that is homologous to the secretin superfamily of proteins. Secretins form a channel in the outer membrane for the transport of macromolecules. The closest homologs found were PilQ proteins from Pseudomonas aeruginosa and Neisseria gonorrhoeae, which are required for type IV pili biogenesis and twitching motility. To signify these molecular and functional similarities, we have changed the name of sglA to pilQ. The hypomorphic pilQ1 (sglA1) allele was sequenced and found to contain two missense mutations at residues 741 (G→S) and 762 (N→G). In addition, 19 independent social (S)-motility mutations are shown to map to the pilQ locus. In-frame deletions of pilQ and its downstream gene, orfL, were constructed. pilQ is shown to be essential for pilus biogenesis, S-motility, rippling, and fruiting body formation, while orfL is dispensable for these processes. The pilQ1 allele, but not the ΔpilQ allele, was found to render cells hypersensitive to vancomycin, suggesting that PilQ1 alters the permeability properties of the outer membrane. Many differences between pilQ1 and pilQ+ strains have been noted in the literature. We discuss some of these observations and how they may be rationalized in the context of our molecular and functional findings.
In response to starvation, the gram-negative bacterium Myxococcus xanthus initiates a multicellular developmental program that culminates in cells aggregating and forming a fruiting body (19). Within this structure vegetative cells differentiate into spores. This process depends on gliding motility. Gliding is controlled by two distinct genetic systems called adventurous (A)-motility and social (S)-motility (22). S-motility, but not A-motility, depends on polar type IV pili (58, 61). Most of the M. xanthus pil genes are homologous to type IV pil genes found in Pseudomonas aeruginosa and Neisseria gonorrhoeae, which are also required for a type of motility called twitching and for pathogenesis (33, 53). These pili may retract, as well as polymerize, and they may provide the force for movement by pushing and pulling cells (2, 53). Many type IV pil genes are homologous to type II secretion genes (general secretion pathway) (42). In the type IV system, pilin (PilA) is the only known secretion product (33).
Pili and fibrils have been shown to mediate cohesion among cells and adhesion to substrates (4, 49, 61). Cohesive cells clump or aggregate in suspension, and their clumps stick to the walls of culture flasks. Native cultures of M. xanthus isolated from soil fail to suspend in liquid culture. To obtain dispersed growth in liquid medium, a spontaneous mutant which retained the ability to form fruiting bodies was isolated after continuous selection; it was named strain FB (15). This mutant of M. xanthus was amenable to necessary microbiological manipulations, such as dilutions, and as a result most laboratory strains were derived from strain FB. Later work showed that this mutation was in a social gliding motility gene, named sglA (22). Unlike other sgl mutations, the sglA1 mutant retains some S-motility and expresses pili at reduced levels, suggesting that sglA1 is a hypomorphic allele (23). Strains which carry sglA1 can form fruiting bodies on agar but not in submerged culture (27). This quality is associated with the decreased cohesiveness of sglA1 mutants, which consequently are unable to form a mat of cells (biofilm) within which fruiting bodies can develop. Strain DK1622 was constructed from strain FB; DK1622 is sglA+, fully S-motile, and capable of developing in submerged culture. Type IV pili are required by P. aeruginosa to form a biofilm (40), a process that resembles the formation of fruiting bodies (12).
Here we report the mapping and cloning and the sequence of the sglA locus. SglA is found to belong to a large family of proteins called secretins (33), which include PilQ proteins from P. aeruginosa and N. gonorrhoeae. To indicate the molecular nature of sglA, we have changed its name to pilQ, as has been done for other sgl genes in M. xanthus when their functions were recognized. In-frame deletions in pilQ and its downstream gene orfL were constructed. pilQ is shown to be essential for the biogenesis of pili and for S-motility, while orfL is not. The origin of the pilQ+ DK1622 strain and the role of PilQ in M. xanthus are discussed.
MATERIALS AND METHODS
Bacterial strains, phage, plasmids, and DNA manipulations.
Bacterial strains and plasmids are listed in Table 1. M. xanthus was cultured in CTT medium, CTT agar, or 1/2 CTT agar plates (21). DNA manipulations were done in Escherichia coli XL1-Blue cultured in Luria-Bertani medium (46). Antibiotics were added when appropriate (kanamycin at 20 μg/ml for M. xanthus and at 40 μg/ml for E. coli and ampicillin at 100 μg/ml). M. xanthus chromosomal DNA preparations, plasmid preparations, DNA manipulations, and Southern hybridizations were all performed as recommend by the manufacturers or as described previously (46, 58).
TABLE 1.
M. xanthus strains and plasmids
| Strain or plasmid | Genotype or relevant properties | Phenotype | Construction | Reference |
|---|---|---|---|---|
| M. xanthus | ||||
| DK101 | pilQ1 (sglA1) | A+ S− | M. xanthus FB | 15 |
| DK320 | aglB1 pilQ1 | A− S− | UV on DK101 | 21 |
| DK1217 | aglB1 | A− S+ | Mx8 (YS) × DK320→screen S+ | 22 |
| DK1241 | aglR4 pilQ1241 | A− S− | UV | 20a |
| DK1242 | cglB pilQ1242 | A− S− | UV | 20a |
| DK1243 | agl-12 pilQ1243 | A− S− | UV | 22 |
| DK1247 | aglK2 pilQ1247 | A− S− | UV | 20a |
| DK1287 | agl1 pilQ1287 | A− S− | UV | 20a |
| DK1291 | aglK1 pilQ1291 | A− S− | UV | 20a |
| DK1600 | pilG or pilH | A+ S− | Spontaneous; YS (MD2) | 56 |
| DK1609 | agl pilQ1609 | A− S− | ICR-191 | 37a |
| DK1622 | Wild type | A+ S+ | Mx8 (YS) × DK1217→screen A+ | 37a |
| DK1627 | cglC1 pilQ1627 | A− S− | ICR-191 | 37a |
| DK1633 | cglC1 pilQ1633 | A− S− | ICR-191 | 37a |
| DK1636 | cglB2 pilQ1636 | A− S− | ICR-191 | 37a |
| DK1675 | cglB2 pilQ1675 | A− S− | ICR-191 | 37a |
| DK1687 | agl pilQ1687 | A− S− | 37a | |
| DK2136 | aglB1 pilQ2136 | A− S− | ICR-191 | 37a |
| DK2140 | agl pilQ2140 | A− S− | ICR-191 | 37a |
| DK2148 | aglB1 pilQ2148 | A− S− | ICR-191 | 37a |
| DK2149 | aglB1 pilQ2149 | A− S− | ICR-191 | 37a |
| DK2150 | aglB1 pilQ2150 | A− S− | ICR-191 | 37a |
| DK2159 | aglB1 pilQ2159 | A− S− | ICR-191 | 37a |
| DK2167 | cglB2 pilQ10 rif-100 | A− S− | Spontaneous; YNS | 37a |
| DK2227 | cglB2 pilQΩ2227 | A− S− | 37a | |
| DK4293 | Ω4401 | A+ S+ | 26 | |
| DK4414 | Ω4414 | A+ S+ | 26 | |
| DK8610 | Ω3188 pilQ+ | A+ S+ | Mx4 (DK10389) × DK101→select Kmr; screen S+ | |
| DK8611 | Ω3188 pilQ1 | A+ S− | Mx4 (DK10389) × DK101→select Kmr; screen S− | |
| DK8612 | Wild type | A+ S+ | pDW140 × DK101→select Kmr; select Galr; screen Kms; screen by Southern blotting | |
| DK8613 | ΔorfL | A+ S+ | pDW146 × DK1622→select Kmr; select Galr; screen Kms; screen by Southern blotting | |
| DK8614 | aglB1 ΔorfL | A− S+ | pDW146 × DK1217→select Kmr; select Galr; screen Kms; screen by Southern blotting | |
| DK8615 | ΔpilQ | A+ S− | pDW131 × DK1622→select Kmr; select Galr; screen Kms; screen by Southern blotting | |
| DK8616 | aglB1 ΔpilQ | A− S− | pDW131 × DK1217→select Kmr; select Galr; screen Kms; screen by Southern blotting | |
| DK8617 | ΔpilQ Ω4401 | A+ S− | Mx4 (DK4293) × DK8615→select Kmr | |
| DK8618 | ΔpilQ Ω4414 | A+ S− | Mx4 (DK4414) × DK8615→select Kmr | |
| DK10389 | Ω3188 pilQ+ | A+ S+ | Mx4 (DK3188) × DK1622→select Kmr | |
| Plasmids | ||||
| pBluescript SK | Cloning vector | Apr | Stratagene (La Jolla, Calif.) | |
| pBGS18 | Cloning vector | Kmr | 51 | |
| pKG-2 | nptII galK | Apr Kmr | Kmr Gals cassette | 52 |
| pDW79 | Ω3188; pil region | Apr Kmr | 19-kb (Ω3188) ClaI-ClaI from DK10389 cloned in pBluescript (ClaI) | |
| pDW81 | pilQ fragment | Kmr | 2.1-kb MluI-MluI from pDW79 cloned in pBGS18 (SmaI) | |
| pDW83 | pilQ fragment | Kmr | Exonuclease III deletion of pDW81, leaving 1.0 kb | |
| pDW85 | pilQ fragment | Kmr | Exonuclease III deletion of pDW81, leaving 0.75 kb | |
| pDW92 | pil region | Kmr | 6.2-kb HindIII-KpnI from pDW79 cloned in pBGS18 (HindIII-KpnI) | |
| pDW94 | pil region | Kmr | 4.5-kb HindIII-KpnI from pDW79 cloned in pBGS18 (HindIII-KpnI) | |
| pDW105 | pil region | Apr | 6.2-kb HindIII-KpnI from pDW79 cloned in pBluescript (HindIII-KpnI) | |
| pDW131 | ΔpilQ orfL galK | Kmr Apr | PCR-generated ΔpilQ; nptII galK cassette cloned in pDW105 (BamHI-EcoRI) | |
| pDW137 | orfL | Apr | 2.9-kb BamHI-EcoRI from pDW105 in pBluescript | |
| pDW140 | pilQ fragment, galK | Kmr Apr | 2.0-kb SphI from pDW81 cloned in pBluescript (pDW96 EcoRV); nptII galK cassette cloned in EcoRI | |
| pDW146 | ΔorfL galK | Kmr Apr | PCR-generated ΔorfL cloned in pDW137 (EcoNI-EcoRI); nptII galK cloned in EcoRV | |
| pDW139 | orfL | Kmr | 5.0-kb KpnI-MscI (blunted and ligated) deletion of pDW92 | |
| pDW167 | pilQ orfL | Kmr | 2.3-kb KpnI-NotI (blunted and ligated) deletion of pDW92 | |
| pDW168 | pil region | Kmr | 11-kb HindIII from pDW79 cloned in pBGS18 (HindIII) | |
| pDW169 | pil region | Kmr | 3.3-kb PshAI-NotI from pDW79 cloned in pBGS18 (SmaI) | |
| pDW188 | pilQ orfL | Kmr | 2.1-kb KpnI-PstI (blunted and ligated) deletion of pDW92 |
Mx4 transductions were done as described previously (21). To score for S-motility, Kmr transductants were transferred with toothpicks to fresh CTT-kanamycin agar plates and visually scored for S-motility. Electroporation of plasmid DNA into M. xanthus was done as described previously (24, 43). To score for the rescue of S-motility, cells were plated on 0.5% agar CTT-kanamycin plates and visually checked after 7 days.
DNA sequencing and analysis.
Double-stranded plasmid DNA was sequenced with the Thermo Sequenase cycle-sequencing kit (Amersham Life Sciences). Restriction fragments were generated and cloned into pBluescript SK or pBGS18 (51) for sequencing. Additional deletion subclones were generated with exonuclease III (46). Primers were designed to cover gaps in the sequence. Both strands were completely sequenced at least once.
Sequence data was compiled and analyzed with DNA Strider and the Genetics Computer Group (Madison, Wis.) Sequence Analysis Software Package version 8.
Development.
Cells were grown overnight in CTT and placed at a calculated density of 1,000 Klett units on CF or TPM starvation agar plates (26). Fruiting body formation and rippling were monitored with a Leitz inverted microscope. Spore counts and β-galactosidase assays were performed as described previously (6, 26).
Immunoblotting and autoradiography.
Proteins were separated by sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon P membranes (Millipore) (46). For Western blotting the membrane was probed with rabbit anti-PilA serum diluted 1:4,000 (59), followed by peroxidase-conjugated goat anti-rabbit immunoglobulin G (Boehringer Mannheim) diluted 1:2,000. The blots were developed with Renaissance chemiluminescence reagent (NEN Life Science Products).
To label extracellular proteins, 1.8-ml cultures starting at 35 Klett units for CTT and 75 Klett units for A1 medium (3) were grown with 50 μCi of Trans35S-label (ICN Biochemicals)/ml for 6 h in an orbital shaker at 33°C. The cells were then pelleted by centrifugation (12,000 × g; 10 min; 4°C). Deoxycholate (0.01%) and 10% trichloroacetic acid were added to the supernatant, mixed, and stored overnight at −20°C. The insoluble proteins were pelleted by centrifugation (12,000 × g; 20 min; 4°C) and resuspended in SDS sample buffer (46). To neutralize the pH, a few microliters of sodium hydroxide (1 M) was added to the sample buffer (until it turned blue). The samples were boiled, separated by SDS-PAGE, and blotted as described above. The membranes were treated with En3hance spray (Dupont, NEN) for fluorography and developed overnight on Hyperfilm MP (Amersham Life Science).
Constructing in-frame deletions of pilQ and orfL.
A plasmid, pDW131, which deleted in frame 2,175 bp or 725 codons from the coding region of pilQ, was generated via PCR. To construct this pilQ in-frame deletion, two primers were designed, one for each end of the gene, oriented in opposite directions. These primers, pQ1 (5′-GCGAAGCTTGCCGGAGCCTGGGCGGCGAC-3′) and pQ2 (5′-GCGAAGCTTCATTGCGCAGACTCTGTAAGG-3′), had unique HindIII restriction sites (underlined) engineered in their 5′ tails. In separate PCRs, two fragments of pilQ DNA were amplified with pQ1 and pQ2, along with corresponding primers that were upstream and downstream of pQ1 and pQ2, respectively. These PCR products were cloned and subsequently ligated together via the HindIII restriction sites, generating an in-frame deletion with a HindIII restriction site inserted. The region across this deletion and insertion was verified by sequencing. To avoid PCR complications, a 0.45-kb BstEII-MluI cassette containing the deletion was swapped with the corresponding cassette of pDW105, generating pDW130. A Kmr-Gals cassette (52) was then cloned into the EcoRI-BamHI sites of pDW130, generating pDW131. pDW131 was electroporated into DK1622 and DK1217, and homologous recombination into the chromosomal locus was selected for by Kmr. Candidate transformants were screened for the expected tandem duplication of the pilQ+ and ΔpilQ alleles by Southern analysis. Recombinants with the expected duplication were then grown in CTT for 1 day to enrich for cells with a second recombination event that lost pDW131 and one of the pilQ alleles, thus leaving either a pilQ+ or ΔpilQ allele at the chromosomal locus. Such recombinants were selected for by galactose resistance. These Galr colonies were screened for Kms. Southern analysis was used to identify recombinants that had only the ΔpilQ allele left at the chromosomal locus.
An orfL in-frame deletion was also constructed by the galK counterselection method (52). This deletion removed 209 of the 220 codons in orfL. To generate this allele, two primers were designed, one at each end of the gene that were oriented in opposite directions. These primers, pL1 (5′-AGGCCTGAGATAGAAGTTCTTCATGAGCG-3′) and pL2 (5′-AGGCCTGAGCCCGAGGAAACGTAGTC-3′), had unique StuI restriction sites (underlined) engineered in their 5′ tails. The primers were used to amplify a 5.2-kb fragment which included pBluescript SK from pDW137. The amplified DNA was digested with StuI, gel purified, and self-ligated, generating pDW144. The region across the orfL in-frame deletion and StuI insertion was verified by sequencing. The wild-type orfL cassette in pDW137 was then swapped with the ΔorfL allele of pDW144 at unique EcoNI-EcoRI restriction sites (EcoRI is in pBluescript SK), generating pDW145. The Kmr-Gals cassette of pKG2 was then cloned into the EcoRV site of pDW145, generating pDW146. pDW146 was then electroporated into DK1622 and DK1217, and ΔorfL strains were subsequently isolated as described above for ΔpilQ.
Nucleotide sequence accession number.
The nucleotide sequence of pilQ and orfL has been deposited in GenBank under accession no. AF100157.
RESULTS
Mapping and cloning sglA.
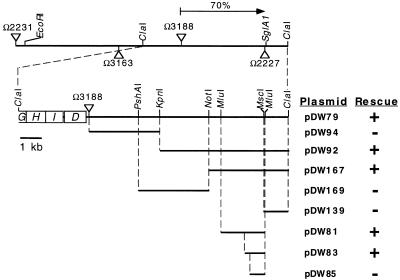
A Tn5 transposon, Ω3188, linked to the sglA locus had been isolated by David Morandi in this laboratory (unpublished). When Ω3188 was transduced into DK101 (sglA1), we observed some S+ transductants. However, Ω3188 is also linked to 10 other pil genes (58, 60, 61). To estimate the physical distance separating Ω3188 and the sglA locus, transductional crosses were made between DK1217 (A− S+) as the recipient and DK8611 (Ω3188 sglA1) as the donor. Of 231 Kmr transductants scored, 163 were S−, a cotransduction frequency of 70% (Fig. 1). Given that bacteriophage Mx4 packages 65 kb of DNA, Wu’s formula (62) suggests that the sglA1 mutation is 7 kb from Ω3188. Three point crosses between Ω2231, Ω3188, and sglA implied that the sglA locus lies to the right of Ω3188 relative to Ω2231, as depicted in Fig. 1.
FIG. 1.
Genetic map of the pil cluster. Triangles above the line represent Tn5 insertions that do not cause a S-motility defect, while those below the line inactivate S motility. The cotransduction frequency between Ω3188 and sglA (pilQ1) is given (arrow). The second line shows four pil genes that map to the left of Ω3188, and the relevant restriction sites are indicated. The abilities of plasmids to rescue the S-motility defect of sglA1 are indicated as follows: +, able to rescue; −, unable to rescue.
A restriction map for the neighborhood of Tn5 insertion Ω3188 was generated by using Tn5 DNA as a probe for Southern hybridization. A unique ClaI site was found to lie 9.6 kb to the right of Ω3188; according to the cotransduction frequencies, the Ω3188-to-ClaI fragment might include the sglA locus. There are no ClaI sites in Tn5, and there is a ClaI site 3.4 kb to the left of Ω3188 (Fig. 1) (60). A 19-kb ClaI-ClaI fragment (including Tn5) was cloned into the ClaI site of pBluescript SK by selecting for Kmr, generating pDW79. To test if pDW79 contained the sglA+ allele, the plasmid was electroporated into DK320 (sglA1), with selection for Kmr. Electroporants of DK320 regained S-motility from pDW79, demonstrating that the sglA+ gene is on this plasmid (Fig. 1). A series of subclones of pDW79 was constructed. As shown in Fig. 1, the sglA1 mutation could be rescued by a 1-kb subfragment cloned in pDW83.
A 3.6-kb region of DNA surrounding the sglA locus was sequenced. Two open reading frames (ORFs) on the same strand and reading frame were identified (Fig. 2). These ORFs had an 81 and a 77% third-codon-position GC bias, respectively, which is diagnostic of M. xanthus genes. The intergenic region between these ORFs is 39 bp. Both ORFs are oriented in the same direction as the 10 pil genes found immediately to the left of Ω3188 (58, 60, 61).
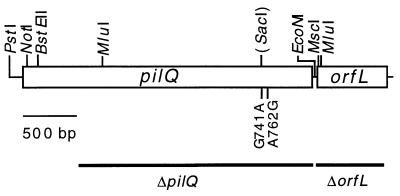
FIG. 2.
Genetic organization of pilQ and orfL. ORFs are read from left to right. The relevant restriction sites are indicated. The two pilQ1 mutations are shown, along with the new restriction site (SacI) generated by the mutation at codon 741. The black lines below these ORFs represent the regions removed by the in-frame deletions.
Since the sglA1 mutation could be rescued by DNA fragments to the left of the MscI site and not by pDW139 (Fig. 1), the sglA1 mutation must reside in the ORF labeled pilQ in Fig. 2. This ORF encodes a 901-amino-acid protein. A BLAST search of the GENEMBL database showed strong homology to a family of proteins called secretins. Secretins are known to transport macromolecules across the outer membranes of gram-negative bacteria (33). This diverse superfamily includes transporters for type IV pili, for filamentous phage, and for DNA; it has members that belong to type II and III secretion systems (20). Family members share homology in their C-terminal 440 amino acids, which can be subdivided into three parts. The C-terminal 180 amino acids are the most conserved, the middle 120 amino acids are moderately conserved, and the residues 460 to 600 are the least conserved (Fig. 3A). This conserved C-terminal domain includes a signature sequence, (V,I)PXL(S,G)XIPXXGXLF, present in all members of the family (Fig. 3B) (16). The M. xanthus sequence includes this signature. The closest matches identified with a BLAST search of the M. xanthus sequence were the PilQ proteins of P. aeruginosa (35) and N. gonorrhoeae (13), 37 and 34% identical over 434 and 430 amino acids, respectively, over the C-terminal region. The gap frequencies were 3.0 and 6.5. The N-terminal region of the secretin family is much less conserved (Fig. 3A) and for that reason is thought to regulate substrate recognition (9). The P. aeruginosa and N. gonorrhoeae PilQ proteins do contain blocks of similarities to M. xanthus PilQ in their N-terminal regions, including 23 and 26% identities over 192 and 128 amino acids (gap frequencies, 3.1 and 5.5%), respectively. In fact, the P. aeruginosa PilQ was found to be more similar to M. xanthus PilQ than it was to the N. gonorrhoeae PilQ.
FIG. 3.
(A) Modular representation of PilQ. The 29-amino-acid signal sequence (SS), the low-homology region, and the three conserved subdomains are shown. (B) Protein sequence of PilQ. The signal sequence is underlined. The two residues that are changed in PilQ1 are in boldface (residues 741 and 762), as is the signature sequence (V,I)PXL(S,G)XIPXXGXLF. (C) Protein sequence of OrfL. A putative type II signal sequence is underlined, and the signature sequence for a phosphopantetheine attachment site is in boldface.
A Shine-Dalgarno sequence (GAGG) was found 7 bp upstream from the proposed translational start ATG. As has been found for other secretin family members, a putative cleavable signal sequence was identified in the first 29 amino acids by PSORT (discrimination score, 4.33; signal score, 3.05 [Fig. 3]). The resulting mature PilQ protein would be 872 amino acids, making it the largest protein in the secretin family by over 150 amino acids.
pilQ mutants.
The rescue data shown in Fig. 1 narrowed the location of the pilQ1 (sglA1) mutation to an approximately 250-bp region. Accordingly, we cloned and sequenced this segment of the DNA from a pilQ1 mutant. Two closely linked missense mutations were found, one at codon 741 and a second at 762, which would generate Gly→Ser and Asn→Gly amino acid substitutions, respectively (Fig. 2 and 3). The missense mutation (G→A) in codon 741 creates a new SacI restriction site (Fig. 2). Both mutations lie in the most highly conserved one-third of the carboxyl end of the protein (Fig. 3). Residues that correspond to 741 and 762 show only limited conservation within the secretin family (9, 16, 35, 44). Some family members contain a Gly-741 and an Asn-762, like the pilQ+ allele, in these residues. Other members contain different residues, including the PilQ1 mutant alleles, Ser-741, and/or Gly-762. The natural occurrence of the mutant residues is consistent with the fact that the pilQ1 allele retains some biological activity. Whether both missense mutations are required to obtain the PilQ1 phenotype is not known.
Hodgkin and Kaiser (22) reported other S-motility mutations which seemed to map to the sglA locus by virtue of their transduction linkage to sglA1. With the pilQ gene in hand, we tested some of these mutants as well as mutants obtained from a more extensive screen for S-motility mutants carried out by D. Morandi (unpublished). Both sets of mutants were first tested for cotransduction with Ω2231 (Fig. 1). Mutations found to be linked to Ω2231 and whose cotransduction frequencies suggested that they were in the vicinity of pilQ were then tested for the ability of pilQ-containing plasmids to rescue their S-motilities. Table 2 summarizes the rescue results for 19 point mutants. Indeed, all of these mutants are rescued by pilQ minimal plasmids.
TABLE 2.
Rescue of S-motility mutants by pilQ
| Strain | Rescue by plasmida:
|
||||||
|---|---|---|---|---|---|---|---|
| pDW168 | pDW92 | pDW188 | pDW81 | pDW167 | pDW139 | pDW169 | |
| DK1241 | + | + | |||||
| DK1242 | + | + | − | − | − | − | |
| DK1243 | + | + | |||||
| DK1247 | + | + | − | − | − | − | |
| DK1287 | + | − | |||||
| DK1291 | + | + | |||||
| DK1609 | + | − | |||||
| DK1627 | + | + | − | − | − | − | |
| DK1633 | + | + | |||||
| DK1636 | + | + | |||||
| DK1675 | + | + | − | ||||
| DK1687 | + | + | |||||
| DK2136 | + | + | − | ||||
| DK2140 | + | + | − | ||||
| DK2148 | + | + | |||||
| DK2149 | + | + | |||||
| DK2150 | + | + | |||||
| DK2159 | + | + | − | ||||
| DK2167 | + | + | |||||
| DK8616 (ΔpilQ) | + | ||||||
Function of pilQ, as deduced from a null mutant.
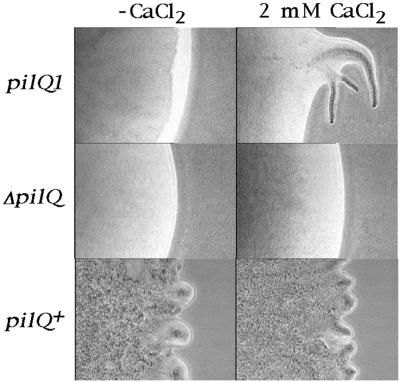
A deletion mutant of pilQ was constructed; the deletion was made in frame to avoid potential polar effects. Effects of the ΔpilQ mutation on S motility were monitored by constructing the double mutant aglB1 ΔpilQ (DK8616). DK8616 was plated on 1/2 CTT 0.5% agar plates, and as shown in Fig. 4, it failed to swarm. No flares were evident at any time over a 6-day period of observation. DK320 (aglB1 pilQ1) also failed to swarm in the absence of CaCl2. The active swarming and flare formation of DK1217 (aglB1 pilQ+) are shown at 6 h for comparison (Fig. 4; note the time difference). Even after prolonged incubation (>20 days) flares were never observed, implying a total loss of S motility in DK8616 (ΔpilQ). DK320 (pilQ1), by contrast, would produce some flares by 20 days (data not shown). Earlier studies have shown that Ca2+ is required for gliding motility (57). Although Ca salts are not added to the standard formulations of CTT or 1/2 CTT media, Ca2+ is nevertheless present in trace amounts in the agar, casitone, and water that are used for these media. We tested whether Ca2+ might be limiting by adding 2 mM CaCl2, and as shown in the bottom row of Fig. 4, the addition of CaCl2 did not dramatically change the swarming of DK1217. However, CaCl2 did enhance the swarming of DK320 (Fig. 4, top row). No swarming of the ΔpilQ strain DK8616 was evident with or without CaCl2 addition (Fig. 4, middle row). These results suggest that Ca2+ may be limiting in 1/2 CTT agar for a pilQ1 mutant.
FIG. 4.
Swarming in the presence (2 mM CaCl2) and absence (−CaCl2) of CaCl2 on 1/2 CTT 0.5% agar plates. The pictures were taken at 6 days for DK320 (aglB1 pilQ1) and DK8616 (aglB1 ΔpilQ) and at 6 h for DK1217 (aglB1 pilQ+).
The effect of the ΔpilQ mutation on cell movement in an A− motility background was examined by time lapse microscopy. Isolated cells and small groups of 10 to 100 cells were examined over 5-, 10-, and 30-min periods. No longitudinal movement greater than a cell’s length was detected in DK8616. Similar results were obtained with other A− Δpil mutants (61). Hence, in the S-motility system, pili are required not only for macroscopic swarming but also for movement at the cellular level.
The hypomorphic pilQ1 mutant produces fewer pili than do wild-type cells (23). No pili were evident on DK8616 cells as examined by electron microscopy. The pilQ deletion mutant did produce normal levels of pilin, the monomer unit and product of the pilA gene, as judged by Western blotting (Fig. 5A). Despite the abundance of pilin, no pili were detected by the sensitive shear assay (55, 59) (Fig. 5B). While ΔpilQ mutants make wild-type levels of pilin, they fail to assemble it into filamentous pili.
FIG. 5.
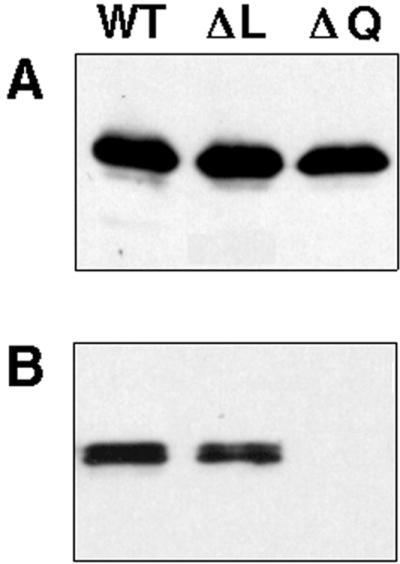
(A) Western blot for PilA expression from 5 × 106 whole cells. (B) Detection of extracellular pili (PilA) from 2 × 108 cells. The pili were sheared off the cells by passage through a 25-gauge 3.5-inch needle as previously described (59). The strains are DK1622 (WT), DK8613 (ΔL), and DK8615 (ΔQ).
Studies of the PilQ homolog from E. coli bacteriophage f1, called GpIV, have suggested that some secretin point mutants increase the membrane permeability of the cells, so that they are more sensitive to small molecules such as vancomycin (molecular mass, about 1,400 Da) and deoxycholate (DOC), a mild detergent (44, 45). We found that the pilQ1 mutations did render M. xanthus 1,000-fold more sensitive to vancomycin, but the ΔpilQ allele did not (Table 3). However, no increase in sensitivity to DOC was found. (It should be noted that wild-type cells are extremely sensitive to DOC; 0.005% is lethal, so it may not be possible to observe a greater sensitivity.) These results show that the pilQ1 mutation increases the permeability of the outer membrane. Presumably this increased sensitivity results from changes in the multiprotein PilQ channel complex, such that molecules as small as vancomycin can enter the periplasmic space, where they presumably block peptidoglycan synthesis. At higher concentrations pilQ+ cells are sensitive to vancomycin, and this antibiotic induces sporulation genes as a consequence of interfering with the recycling of peptidoglycan components (39).
TABLE 3.
Sensitivity to vancomycin
| Strain | pil allele | EOPaon vancomycin (20 μg/ml) |
|---|---|---|
| DK1217 | pilQ+ | 1 |
| DK320 | pilQ1 | 6 × 10−4 |
| DK8611 | ΔpilQ | 1 |
| DK8601 | ΔpilA | 1 |
EOP, efficiency of plating.
Origin of DK1622.
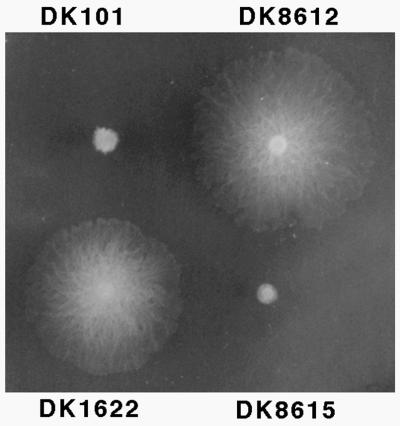
Despite its wide use, a detailed description of the origin of DK1622 has never been published. Although DK1622 is a descendent of DK101 (pilQ1), it has a pilQ+ allele, resulting in full S-motility, as shown in Fig. 6. DK1622 cells are able to form biofilms and to develop in submerged culture (27). Unlike DK101, DK1622 forms symmetric fruiting bodies, it ripples during development; and it forms fruiting bodies faster than DK101. For these reasons DK1622 is commonly used as a “wild-type” strain. It should be noted, however, that during the construction of DK1622 a 222-kbp deletion occurred that removed several tandem copies of a prophage-like element called Mx alpha without other structural rearrangements, since the physical maps are otherwise identical (5). This deletion may have occurred during the UV irradiation of DK101 used to generate DK320 (22).
FIG. 6.
Role of pilQ in swarming. Strains were inoculated on 1/2 CTT 0.5% agar plates and incubated for three days at 33°C. Genotypes: DK101, pilQ1; DK8612, pilQ+ (isogenic derivative of DK101); DK1622, pilQ+; and DK8615, ΔpilQ (isogenic derivative of DK1622).
DK320 (pilQ1) was rendered pilQ+ by Mx8 transduction from a YS (56) (DK1600) donor, thereby generating a strain with full S motility, DK1217 (22; Table 1). DK1217 then served as the recipient for a second Mx8 transduction, again using YS as the donor strain. Transductants were screened for full (A+ S+) motility, yielding DK1622 (37a). In the course of our studies we observed that YS (DK1600) was defective in swarming on 0.5% agar plates, where it swarmed slightly faster than DK101 but significantly slower than DK1622. The addition of 2 mM CaCl2 to the agar failed to improve the swarming rate of YS, in contrast to that of DK101. These observations argue that YS contains a mutation in the S-motility system that is different from pilQ1.
To identify the mutant locus in YS involved in its S-motility defect, and thereby to clarify the origin of DK1622, we sought to map the S− mutation in YS. YS was transformed with the overlapping plasmids pDW79 and pSWU257 (58), which together cover the entire known pil region. Both plasmids were found to rescue the S-motility defect of YS. These plasmids overlap in a 3.4-kbp region, which contains the pilG, -H, -I, and -D genes (Fig. 1). Additional transformants were made to map the YS mutation: plasmid pSWU449 was found to rescue the motility defect of YS, while pSWU402 (60) could not. Thus, the YS mutation can be in either the pilG or -H gene or both. As shown in Fig. 1, the minimum distance between a pilG or -H mutation and pilQ1 is 10 kbp, a distance sufficiently large that a pilQ+ transductant from YS would not necessarily receive the pilG or -H mutation at the same time.
Role of pilQ in development.
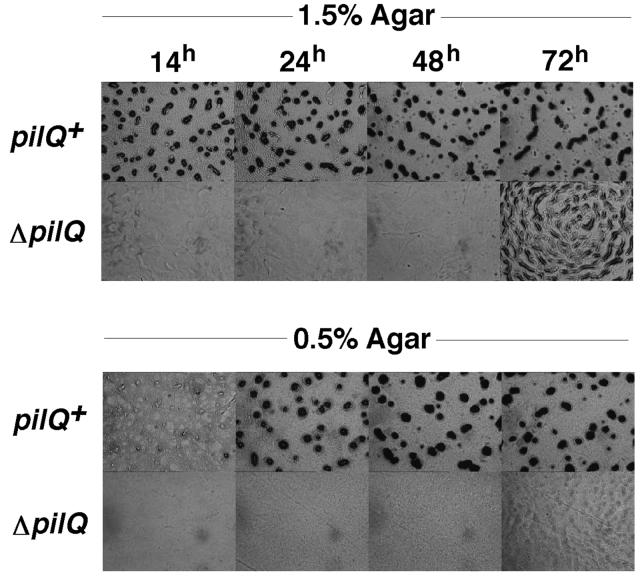
S-motility is necessary for rippling, and it plays an important role in fruiting body development (22, 50, 60). The specific effects of the ΔpilQ mutation on development were examined. Figure 7 shows that the aggregation stage of development was greatly delayed in the ΔpilQ mutant DK8615: At 72 h, aggregates appeared, with structures similar to those seen 60 h earlier (at 6 to 10 h) in wild-type cells. These aggregates never developed into dark fruiting bodies (Fig. 7). On hard (1.5%) agar, A-motility dominates (Fig. 7, top). On soft (0.5%) agar, S-motility dominates (48). Figure 7 (bottom) shows that aggregation and fruiting body formation were completely blocked in the ΔpilQ strain on soft agar, nor did ripples ever form. These results suggest that under certain conditions, i.e., hard agar, A-motility can partially substitute for the lack of S-motility.
FIG. 7.
Development on CF. DK1622 (pilQ+) and DK8615 (ΔpilQ) were placed on 1.5 or 0.5% CF agar plates, and development was monitored over three days as indicated.
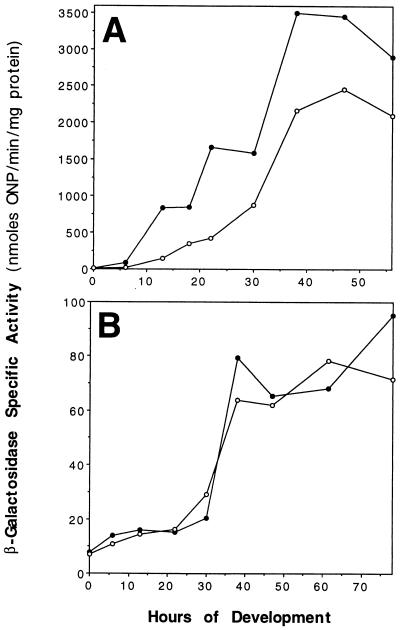
Previously the pilQ1 mutation was found to reduce the developmental expression of the myxobacterial hemagglutinin (MBHA) protein about eightfold (7). Here the effect of the ΔpilQ mutation on the expression of two other developmentally regulated reporter fusions, Ω4414 and Ω4401 (26), was examined. Figure 8 shows that the expression of Ω4414, whose expression normally begins at 6 h, was reduced two- to fivefold over the course of development in a ΔpilQ background. In contrast, the ΔpilQ mutation did not appreciably affect the expression of Ω4401, whose expression starts at the beginning of sporulation (24 h). Interestingly, the ΔpilQ mutant sporulated at slightly higher levels than the parental DK1622 strain. Other pil null mutants have also been shown to sporulate at ∼2-fold-higher levels than wild-type cells (60). This increase in sporulation may be artifactual, since spores are more easily dispersed from pil mutants than from pil+ cells, which could increase the titer of viable spores.
FIG. 8.
Developmental expression of Ω4414 (A) and Ω4401 (B) on TPM (1.5%) agar. Reporter β-galactosidase transcriptional fusions are in DK1622 (•) or DK8615 (○). ONP, o-nitrophenol.
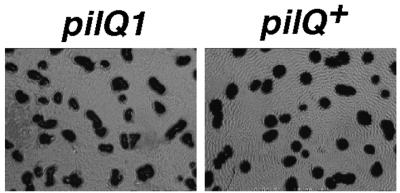
DK101 (pilQ1) forms fruiting bodies, though 1 day later than DK1622, and it ripples infrequently and less extensively. A pilQ+ isogenic derivative of DK101 was constructed by the galK counterselection method with a pilQ+ fragment from pDW140 (DK8612), and it was shown to be fully S-motile (Fig. 6). As illustrated in Fig. 9, DK8612 has a higher propensity to ripple than its parent, DK101. Though restored for S-motility and rippling, DK8612 remained unable to form fruiting bodies with the speed and proficiency of DK1622; it was slow, like DK101 (data not shown).
FIG. 9.
Rippling on CF agar. DK101 (pilQ1) and DK8612 (pilQ+) were placed on CF (1.5%) agar at a cell density of 1,000 Klett units and allowed to develop for 5 days at 27°C.
orfL.
Thirty-nine base pairs downstream of pilQ is the 220-amino-acid ORF orfL. A Shine-Dalgarno site (GGAG) was found 12 bp upstream from its putative translational start ATG. OrfL shows no significant sequence homology to any other protein in the GENEMBL database. However, orfL does contain a type II signal sequence (discrimination score, 4.3), suggesting that it may encode a lipoprotein. A lipid moiety would be predicted to be attached to Cys-17 (Fig. 3C). Near the C terminus of OrfL there is a signature sequence for a phosphopantetheine attachment site (Fig. 3C). Phosphopantothenate is the prosthetic group of acyl carrier proteins in some multienzyme complexes, where it functions as a “swing arm” for the attachment of activated fatty acids and amino acid groups. These enzymes produce diverse products, such as polyketide antibiotics and nodulation factor for Rhizobium species. In OrfL the putative pantetheine attachment site is Ser-170 (Fig. 3C).
To ascertain whether OrfL plays a role in S-motility, type IV pilus biogenesis, or fruiting body development, an in-frame deletion of orfL was constructed. When the ΔorfL allele was introduced into DK1217 (A− S+), the resulting strain, DK8614, was motile and displayed normal S-motility under a variety of environmental conditions (data not shown). DK8614 was checked for expression of PilA and pilus production; it was similar to the parental strain in both respects (Fig. 5). To test whether the ΔorfL allele had any effect on development, it was introduced in DK1622, generating DK8613. This strain exhibited normal fruiting body formation on TPM and CF agars; both rippling and sporulation were at wild-type levels.
Protein secretion.
M. xanthus secretes many proteins and is one of the most active secreters among gram-negative bacteria (17). Protein secretion appears to be required for (i) transporting catabolic enzymes into the medium for vegetative growth, (ii) intercellular signaling, and (iii) production and assembly of type IV pili. In gram-negative bacteria, type II and III secretion systems are major pathways for protein transport. Both of these pathways employ secretins. In P. aeruginosa, there is some overlap between type II secretion and type IV pilus production. The same protein, PilD/XcpA, is used by both systems as a signal peptidase, for example (38). In addition, secretion of type II-dependent proteins is decreased by a pilA mutation (31). To test whether a ΔpilQ mutation had an effect on protein secretion in M. xanthus, the spectrum of proteins secreted into the medium was examined. Figure 10 shows the protein composition of concentrated [35S]methionine-cysteine-labeled culture supernatants separated by PAGE. The ΔpilQ mutant had an amount and profile of proteins similar to those of the pilQ+ culture when grown in CTT-rich medium (casitone has low levels of methionine and cysteine). The ΔpilQ supernatant did contain a >100-kDa protein that was absent in the pilQ+ supernatant. When these identical cultures were shifted from CTT to A1 minimal medium (3) for 6 h, there were significant changes in the patterns of proteins found in the culture supernatants. Some proteins were more abundant in A1, e.g., those at 17, 31, 51, 62, and ∼120 kDa, while others decreased, e.g., those at 24, 36, 38, and 45 kDa. Compared to growth in CTT, greater differences between pilQ+ and ΔpilQ strains were seen in A1. Six proteins at 31, 32, 50, 55, 58, and 84 kDa were more abundant, while the protein(s) at ∼120 kDa was less abundant in ΔpilQ supernatants. In N. gonorrhoeae, pilQ mutations result in increased levels of PilC (∼105 kDa) and S-pilin (soluble truncated pilin, ∼16 kDa) in culture supernatants (13, 14). Perhaps some of these more abundant proteins from M. xanthus ΔpilQ supernatants are Pil proteins.
FIG. 10.
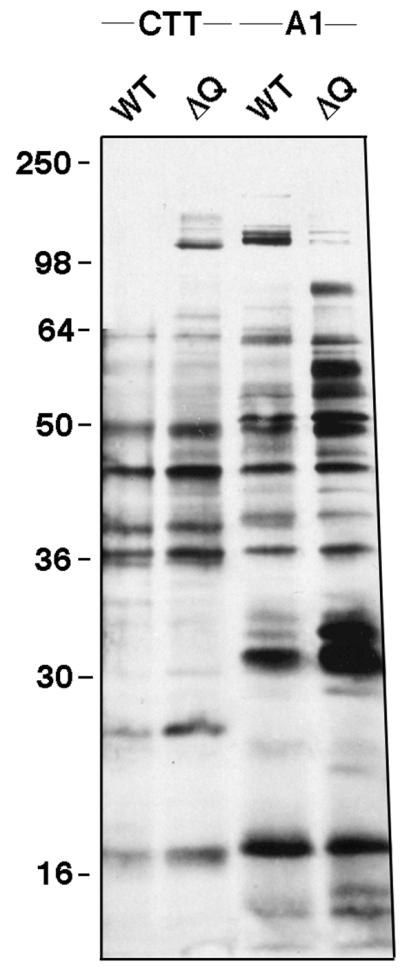
Profile of proteins found in culture supernatants. DK1622 (WT) and DK8615 (ΔQ) cells were grown in either CTT or A1 minimal medium with Tran35S-label. Culture supernatants were precipitated with trichloroacetic acid, and protein samples were separated by SDS–12% PAGE. The positions of molecular mass standards (in kilodaltons) are given.
DISCUSSION
We have shown that pilQ (sglA) encodes a secretin homolog. Secretins are an evolutionarily conserved superfamily of proteins involved in macromolecular transport across the outer membrane in three different secretion systems. Secretins are the only proteins common to type II (including type IV pili), type III, and filamentous phage secretion systems, suggesting that they play a fundamental role (33). In all three systems secretins are found in the outer membrane, where they form ring structures of 10 to 18 secretin subunits. These cylindrical structures, as visualized by electron microscopy, have central cavities ranging in size from 50 to 95 Å (1, 25, 30). Such cavities are large enough to accommodate the transport of folded proteins and assembled macromolecular complexes, such as filamentous phage (diameter, 65 Å) or type IV pili (diameter, ∼52 Å). Our evidence that ΔpilQ mutants lack pili and that pilQ1 mutants are hypersensitive to vancomycin also suggests that PilQ functions as a channel for type IV pilus export. In M. xanthus these polar pili have been observed to extend from “polar holes” (32). It is tempting to speculate that these polar holes are multimeric PilQ channels.
Multimerization of secretin subunits in the outer membrane requires assembly factors or chaperones. For example, the secretins PulD and OutD require their cognate assembly factors, PulS and OutS, for localization and assembly in the outer membrane (8, 18, 47). The PulS and OutS lipoproteins bind to the C-terminal 65 and 62 amino acids of PulD and OutD. M. xanthus PilQ protein does not have a C-terminal binding sequence, nor has a PulS or OutS homolog been identified in M. xanthus. Instead, multimerization of PilQ may be mediated by a PilP-like lipoprotein, as has been shown for N. gonorrhoeae (14). Upstream of the M. xanthus pilQ gene is an ORF that shows homologies to N. gonorrhoeae and P. aeruginosa pilP (unpublished data). Another candidate for a protein that interacts with PilQ is the Tgl lipoprotein (54), which contains six tandem tetratricopeptide repeats (43), motifs which are known to mediate protein-protein interactions (11). In M. xanthus we are interested in how these three proteins might interact.
Secretins can serve as signals for the induction of stress genes. During the course of filamentous bacteriophage (e.g., f1 or M13) infection in E. coli or overproduction of the phage secretin GpIV, it was discovered that an operon called pspABCE (for phage shock protein) is induced (reviewed in reference 37). This ς54-dependent operon is also specifically induced by the expression of other heterologous secretins, starvation, osmotic shock, heat stress, or ethanol stress (18, 37). Mutations in psp result in a loss of viability during stationary phase, and the mutants have defects in protein transport and maintaining membrane potential (37). Model and coworkers explain a diverse set of results by proposing that the secretin signal for psp induction is the process of insertion and assembly of a secretin in the outer membrane (37). Conditions which render this insertion process slow or inefficient or which mislocalize it lead to amplification of the signal for psp induction. In M. xanthus these findings are of interest because many differences have been found between pilQ+ and pilQ1 strains during development. For example, frz (che homolog) mutants are defective in sporulation in a pilQ+ background but sporulate at wild-type levels in a pilQ1 background (24). To explain this suppression, perhaps the PilQ1 mutant protein results in a psp-like induction of stress genes, which could compensate for the frz sporulation defect.
Fruiting body development depends on cell-cell signaling (6, 19, 28, 29) and on cell movement. Mutational defects in pilQ retard aggregation (Fig. 7) by eliminating S-motility. A decrease in the efficiency of C signaling is evident in the decreased expression of the Ω4414 reporter (Fig. 8A). The developmental defects of asg and dsg mutants worsen in a pilQ1 background (28). In that background (DK101) asgB and asgC mutants fruit poorly and produce only 10% as many viable spores as the parental strain (28). In a pilQ+ (DK1622) background, asgB and asgC mutants sporulate at 43 and 100% efficiencies relative to their parental strain and their morphological defects are less severe. A-factor, which requires asg genes, is a set of eight amino acids which are released by the action of extracellular proteases on extracellular proteins (28, 29, 41). Enhancement of the asg defect suggests that the PilQ secretin may be involved in releasing peptides, proteins, and proteases from the cell, and hence in A-factor production. If there were such a defect, then when the pilQ1 allele is combined with asgB or asgC mutations it could exaggerate their developmental defects.
In addition, dsg mutants fail to form fruiting bodies in a pilQ1 background and their ability to sporulate is reduced >10,000-fold (6). However, in a pilQ+ background dsg mutants can sporulate at wild-type levels, though aggregation is delayed. Recently, it has been suggested that the developmental block in dsg mutants is not related to a new signaling molecule but instead is a result of lower A-factor levels (6a). Thus, similarly to asg mutants, dsg would fail to develop due to the secretion defect of pilQ1.
Several caveats relating to genetic interactions with pilQ should be mentioned. First, pilQ1 mutants have pleotropic defects, including defects in piliation, S-motility, cell cohesiveness, and permeability properties. Any one or a combination of these defects could have indirect effects on other mutations. Second, the strains used, e.g., DK1622 and DK101 (or DZF1 and DZ2), are less isogenic (see “Origin of DK1622” in Results) than DK101 and DK8612 or DK1622 and DK8615. Third, the molecular natures of the pilQ alleles were not previously defined. Here we have constructed an in-frame deletion mutant, sequenced the pilQ1 mutations, and identified 19 additional pilQ alleles. Hopefully, these new alleles and the construction of DK8612 and DK8615 will facilitate understanding the genetic relationships between pilQ and other properties of the cell.
Our characterization of PilQ extends the striking similarities between proteins involved in S-motility and those required for twitching motility in P. aeruginosa and N. gonorrhoeae (53, 58). Additionally, we have found S-motility genes upstream of pilQ (downstream of pilD) which are homologous to the pilM, -N, -O, and -P genes from Pseudomonas sp. and N. gonorrhoeae (references 14 and 34 and unpublished data). No S-motility mutants or pil ORFs have been found downstream of pilQ, suggesting that pilQ is at the end of the pil cluster. Extensive screens for genes required for S-motility have yielded 160 mutants (22, 37a). About 100 of these mutations map to the pil cluster described here, and another 7 map to the tgl locus (reference 43 and unpublished data), which is also required for pilus assembly. This screen may be approaching saturation, since many of the new mutations are falling into known S-motility genes, e.g., 20 independent mutations map to pilQ, 4 map to pilA (58), 5 map to pilT (61), and the aforementioned 7 map to tgl. The genomes of P. aeruginosa and N. gonorrhoeae are sequenced, and at least in the case of P. aeruginosa, a near-saturation screen for twitching motility genes has been completed. Almost all of the twitching genes are either pil genes, transcriptional regulators, or, in the case of P. aeruginosa, signal transduction genes, i.e., frz and che homologs (10, 36) (N. gonorrhoeae has no obvious che homologs). Thus, type IV pilus genes are the major genetic determinant for S-motility and twitching motility. Future work with M. xanthus will be aimed at understanding how pil gene products interact and how they contribute to S-motility.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Science Foundation (MCB 9423182) to D.K. D.W. was a recipient of an American Cancer Society postdoctoral fellowship (PF-4138).
REFERENCES
- 1.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradley D E. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol. 1980;26:146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher A P, Kaiser D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978;133:763–768. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang B Y, Dworkin M. Isolated fibrils rescue cohesion and development in the Dsp mutant of Myxococcus xanthus. J Bacteriol. 1994;176:7190–7196. doi: 10.1128/jb.176.23.7190-7196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H W, Kuspa A, Keseler I M, Shimkets L J. Physical map of the Myxococcus xanthus chromosome. J Bacteriol. 1991;173:2109–2115. doi: 10.1128/jb.173.6.2109-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y, Kaiser D. dsg, a gene required for cell-cell interaction early in Myxococcus development. J Bacteriol. 1989;171:3719–3726. doi: 10.1128/jb.171.7.3719-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Cheng, Y., A. Kuspa, and D. Kaiser. Unpublished data.
- 7.Cumsky M, Zusman D R. Myxobacterial hemagglutinin: a development-specific lectin of Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5505–5509. doi: 10.1073/pnas.76.11.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daefler S, Guilvout I, Hardie K R, Pugsley A P, Russel M. The C-terminal domain of the secretin PulD contains the binding site for its cognate chaperone, PulS, and confers PulS dependence on pIVf1 function. Mol Microbiol. 1997;24:465–475. doi: 10.1046/j.1365-2958.1997.3531727.x. [DOI] [PubMed] [Google Scholar]
- 9.Daefler S, Russel M, Model P. Module swaps between related translocator proteins pIV(f1), pIV(IKe) and PulD: identification of a specificity domain. J Mol Biol. 1997;266:978–992. doi: 10.1006/jmbi.1996.0866. [DOI] [PubMed] [Google Scholar]
- 10.Darzins A, Russell M A. Molecular genetic analysis of type-4 pilus biogenesis and twitching motility using Pseudomonas aeruginosa as a model system—a review. Gene. 1997;192:109–115. doi: 10.1016/s0378-1119(97)00037-1. [DOI] [PubMed] [Google Scholar]
- 11.Das A K, Cohen P T W, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 13.Drake S L, Koomey M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol Microbiol. 1995;18:975–986. doi: 10.1111/j.1365-2958.1995.18050975.x. [DOI] [PubMed] [Google Scholar]
- 14.Drake S L, Sandstedt S A, Koomey M. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol Microbiol. 1997;23:657–668. doi: 10.1046/j.1365-2958.1997.2511618.x. [DOI] [PubMed] [Google Scholar]
- 15.Dworkin M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962;84:250. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genin S, Boucher C A. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 17.Guespin-Michel J F, Letouvet-Pawlak B, Petit F. Protein secretion in Myxobacteria. In: Dworkin M, Kaiser D, editors. Myxobacteria. II. Washington, D.C: American Society for Microbiology; 1993. pp. 235–255. [Google Scholar]
- 18.Hardie K R, Lory S, Pugsley A P. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 1996;15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 19.Hartzell P L, Youderian P. Genetics of gliding motility and development in Myxococcus xanthus. Arch Microbiol. 1995;164:309–323. doi: 10.1007/BF02529977. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage, and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 20a.Hodgkin, J. Unpublished data.
- 21.Hodgkin J, Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci USA. 1977;74:2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol Gen Genet. 1979;171:177–191. [Google Scholar]
- 23.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashefi K, Hartzell P L. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF− defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 25.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 26.Kroos L, Kuspa A, Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986;117:252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- 27.Kuner J M, Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982;151:458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuspa A, Kaiser D. Genes required for developmental signalling in Myxococcus xanthus: three asg loci. J Bacteriol. 1989;171:2762–2772. doi: 10.1128/jb.171.5.2762-2772.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaRossa R, Kuner J, Hagen D, Manoil C, Kaiser D. Developmental cell interactions of Myxococcus xanthus: analysis of mutants. J Bacteriol. 1983;153:1394–1404. doi: 10.1128/jb.153.3.1394-1404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linderoth N A, Simon M N, Russel M. The filamentous phage pIV multimer visualized by scanning transmission electron microscopy. Science. 1997;278:1635–1638. doi: 10.1126/science.278.5343.1635. [DOI] [PubMed] [Google Scholar]
- 31.Lu H M, Motley S T, Lory S. Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol Microbiol. 1997;25:247–259. doi: 10.1046/j.1365-2958.1997.4561818.x. [DOI] [PubMed] [Google Scholar]
- 32.MacRae T H, Dobson W J, McCurdy H D. Fimbriation in gliding bacteria. Can J Microbiol. 1977;23:1096–1108. doi: 10.1139/m77-165. [DOI] [PubMed] [Google Scholar]
- 33.Manning P A, Meyer T F. Type-4 pili: biogenesis, adhesins, protein export and DNA import. Proceedings of a workshop. Gene. 1997;192:1–198. [PubMed] [Google Scholar]
- 34.Martin P R, Watson A A, McCaul T F, Mattick J S. Characterization of a five-gene cluster required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1995;16:497–508. doi: 10.1111/j.1365-2958.1995.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 35.Martin P R, Hobbs M, Free P D, Jeske Y, Mattick J S. Characterization of pilQ, a new gene required for the biogenesis of type IV fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1993;9:857–868. doi: 10.1111/j.1365-2958.1993.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 36.Mattick J S, Whitchurch C B, Alm R A. The molecular genetics of type-4 fimbriae in Pseudomonas aeruginosa—a review. Gene. 1996;179:147–155. doi: 10.1016/s0378-1119(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 37.Model P, Jovanovic G, Dworkin J. The Escherichia coli phage-shock-protein (psp) operon. Mol Microbiol. 1997;24:255–261. doi: 10.1046/j.1365-2958.1997.3481712.x. [DOI] [PubMed] [Google Scholar]
- 37a.Morandi, D. Unpublished data.
- 38.Nunn D N, Lory S. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc Natl Acad Sci USA. 1992;89:47–51. doi: 10.1073/pnas.89.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connor K A, Zusman D R. Starvation-independent sporulation in Myxococcus xanthus involves the pathway for beta-lactamase induction and provides a mechanism for competitive cell survival. Mol Microbiol. 1997;24:839–850. doi: 10.1046/j.1365-2958.1997.3931757.x. [DOI] [PubMed] [Google Scholar]
- 40.O’Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 41.Plamann L, Kuspa A, Kaiser D. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J Bacteriol. 1992;174:3311–3318. doi: 10.1128/jb.174.10.3311-3318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Soto J P, Kaiser D. The tgl gene: social motility and stimulation in Myxococcus xanthus. J Bacteriol. 1997;179:4361–4371. doi: 10.1128/jb.179.13.4361-4371.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russel M. Mutants at conserved positions in gene IV, a gene required for assembly and secretion of filamentous phages. Mol Microbiol. 1994;14:357–369. doi: 10.1111/j.1365-2958.1994.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 45.Russel M, Linderoth N A, Sali A. Filamentous phage assembly: variation on a protein export theme. Gene. 1997;192:23–32. doi: 10.1016/s0378-1119(96)00801-3. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 47.Shevchik V E, Robert-Baudouy J, Condemine G. Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi W, Zusman D R. The two motility systems of Myxococcus xanthus show different selective advantages on various surfaces. Proc Natl Acad Sci USA. 1993;90:3378–3382. doi: 10.1073/pnas.90.8.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimkets L J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986;166:837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimkets L J, Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982;152:451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spratt B G, Hedge P J, te Heesen S, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 52.Ueki T, Inouye S, Inouye M. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene. 1996;183:153–157. doi: 10.1016/s0378-1119(96)00546-x. [DOI] [PubMed] [Google Scholar]
- 53.Wall, D., and D. Kaiser. Type IV pili and cell motility. Mol. Microbiol., in press. [DOI] [PubMed]
- 54.Wall D, Kaiser D. Alignment enhances the cell-to-cell transfer of pilus phenotype. Proc Natl Acad Sci USA. 1998;95:3054–3058. doi: 10.1073/pnas.95.6.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wall D, Wu S S, Kaiser D. Contact stimulation of Tgl and type IV pili in Myxococcus xanthus. J Bacteriol. 1998;180:759–761. doi: 10.1128/jb.180.3.759-761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wireman J W, Dworkin M. Morphogenesis and developmental interactions in myxobacteria. Science. 1975;189:516–523. doi: 10.1126/science.806967. [DOI] [PubMed] [Google Scholar]
- 57.Womack B J, Gilmore D F, White D. Calcium requirement for gliding motility in myxobacteria. J Bacteriol. 1989;171:6093–6096. doi: 10.1128/jb.171.11.6093-6096.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu S S, Kaiser D. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]
- 59.Wu S S, Kaiser D. Regulation of expression of the pilA gene of Myxococcus xanthus. J Bacteriol. 1997;179:7748–7758. doi: 10.1128/jb.179.24.7748-7758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu S S, Wu J, Cheng Y L, Kaiser D. The pilH gene encodes an ABC transporter homologue required for type IV pilus biogenesis and social motility in Myxococcus xanthus. Mol Microbiol. 1998;29:1249–1261. doi: 10.1046/j.1365-2958.1998.01013.x. [DOI] [PubMed] [Google Scholar]
- 61.Wu S S, Wu J, Kaiser D. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol Microbiol. 1997;23:109–121. doi: 10.1046/j.1365-2958.1997.1791550.x. [DOI] [PubMed] [Google Scholar]
- 62.Wu T T. A model for three-point analysis of random general transduction. Genetics. 1966;54:405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]