This genetic association study analyzes data from 4 Alzheimer disease and cognitive aging cohorts to investigate whether sex and race modify associations of APOE ε4 and ε2 with cognition.
Key Points
Question
Does sex modify associations of APOE ε2 and ε4 with cognition, and do these sex effects differ in non-Hispanic Black and non-Hispanic White individuals?
Findings
This genetic association study found that among 32 427 older adults from different racial groups, females showed stronger negative APOE ε4 effects on memory, which did not statistically differ by race. Contrastingly, no sex effects were observed with APOE ε2, aside from an intersectional effect of race and sex, whereby the APOE ε2 protective effect on executive function was limited to non-Hispanic White females and non-Hispanic Black males.
Meaning
Sex differences in APOE associations with cognition are largely limited to ε4 and do not differ by race.
Abstract
Importance
Sex differences are established in associations between apolipoprotein E (APOE) ε4 and cognitive impairment in Alzheimer disease (AD). However, it is unclear whether sex-specific cognitive consequences of APOE are consistent across races and extend to the APOE ε2 allele.
Objective
To investigate whether sex and race modify APOE ε4 and ε2 associations with cognition.
Design, Setting, and Participants
This genetic association study included longitudinal cognitive data from 4 AD and cognitive aging cohorts. Participants were older than 60 years and self-identified as non-Hispanic White or non-Hispanic Black (hereafter, White and Black). Data were previously collected across multiple US locations from 1994 to 2018. Secondary analyses began December 2021 and ended September 2022.
Main Outcomes and Measures
Harmonized composite scores for memory, executive function, and language were generated using psychometric approaches. Linear regression assessed interactions between APOE ε4 or APOE ε2 and sex on baseline cognitive scores, while linear mixed-effect models assessed interactions on cognitive trajectories. The intersectional effect of race was modeled using an APOE × sex × race interaction term, assessing whether APOE × sex interactions differed by race. Models were adjusted for age at baseline and corrected for multiple comparisons.
Results
Of 32 427 participants who met inclusion criteria, there were 19 007 females (59%), 4453 Black individuals (14%), and 27 974 White individuals (86%); the mean (SD) age at baseline was 74 years (7.9). At baseline, 6048 individuals (19%) had AD, 4398 (14%) were APOE ε2 carriers, and 12 538 (38%) were APOE ε4 carriers. Participants missing APOE status were excluded (n = 9266). For APOE ε4, a robust sex interaction was observed on baseline memory (β = −0.071, SE = 0.014; P = 9.6 × 10−7), whereby the APOE ε4 negative effect was stronger in females compared with males and did not significantly differ among races. Contrastingly, despite the large sample size, no APOE ε2 × sex interactions on cognition were observed among all participants. When testing for intersectional effects of sex, APOE ε2, and race, an interaction was revealed on baseline executive function among individuals who were cognitively unimpaired (β = −0.165, SE = 0.066; P = .01), whereby the APOE ε2 protective effect was female-specific among White individuals but male-specific among Black individuals.
Conclusions and Relevance
In this study, while race did not modify sex differences in APOE ε4, the APOE ε2 protective effect could vary by race and sex. Although female sex enhanced ε4-associated risk, there was no comparable sex difference in ε2, suggesting biological pathways underlying ε4-associated risk are distinct from ε2 and likely intersect with age-related changes in sex biology.
Introduction
Apolipoprotein E (APOE) is the strongest genetic risk factor for late-onset Alzheimer disease (AD) with the ε2 allele reducing risk and ε4 increasing risk.1,2,3,4,5,6 Interestingly, associations between APOE ε4 and AD differ by sex, whereby associations are stronger among females compared with males.7,8,9,10,11,12,13,14 Likewise, APOE ε4 is associated with greater memory decline in females with both normal cognition and mild cognitive impairment (MCI),12,13 and although APOE ε2 effects on cognition have not been as thoroughly examined, 1 recent study showed female ε2 carriers had enhanced neuroprotection on memory performance compared with male ε2 carriers.14 Regarding racial differences, several studies have demonstrated that although ε4 frequency is higher in Black participants, associations between APOE ε4 and AD risk are weaker in non-Hispanic Black compared with non-Hispanic White individuals (hereafter, Black and White).15,16,17
Nevertheless, studies of APOE effects on cognition are inconsistent. Some studies suggest strong associations between APOE ε4 and rate of cognitive decline in Hispanic16,18,19 and African American populations,20 whereas other studies report no significant associations between APOE ε4 and cognition in these groups.21,22,23 As a result, relations among APOE (especially APOE ε2) and cognition in racially and ethnically diverse groups remain unclear. The sex- and race-specific effects have important implications for ongoing AD clinical trials, where APOE has been included as a screening criterion in some trials, and both outcomes and adverse events (such as amyloid-related imaging abnormalities) have been reported to differ by APOE genotype in previous trials.24,25,26,27,28
Progressive cognitive decline in AD is characterized by memory loss and impairments in executive functioning and language. These correlated domains have some unique and shared genetic architecture, which is important to uncover. Prior studies have assessed APOE effects on cognitive domains,29,30,31,32,33,34 with several demonstrating strong APOE effects on memory.29,32,33 In contrast, studies of APOE effects on executive function and language are inconsistent with some studies suggesting APOE ε4 carriers perform better on executive function tests,32,33 whereas others report APOE ε4 carriers have more rapid executive function decline.34 Regarding language function, 1 study suggested APOE ε4 carriers have greater rates of decline in language performance.34 Yet neither APOE ε2 effects nor intersectional effects of race, sex, and APOE on cognition have been thoroughly examined.
The goal of this study is to extend previous work by providing a comprehensive picture of the modifying effect of sex on APOE ε2 and APOE ε4 associations with domain-specific cognitive trajectories over the course of aging and AD. In 4 well-characterized cohorts of Black (n = 4453) and White (n = 27 974) older adults, we leverage 3 harmonized cognitive domains (memory, executive function, and language) using robust longitudinal data analysis to characterize the intersectional effects of race, sex, and APOE on cognitive performance along the spectrum of clinical disease. These results provide the most comprehensive picture to date of sex and racial differences in allele-specific associations of APOE with late-life cognitive performance.
Methods
Participants
Data were obtained from 4 cohort studies of cognitive aging and AD: the Alzheimer’s Disease Neuroimaging Initiative (ADNI)35; the 3 harmonized cohorts of the Religious Orders Study, Rush Memory and Aging Project, and Minority Aging Research Study (ROS/MAP/MARS)36,37; the National Alzheimer Coordinating Center (NACC)38,39,40,41,42; and the Adult Changes in Thought (ACT) study.43 The ADNI and NACC studies enrolled individuals with normal cognition, MCI, or AD, while ROS/MAP/MARS and ACT enrolled participants without known dementia. Participants older than 60 years at baseline were included in this analysis. Sex and race were self-reported. Written informed consent was obtained from all participants in each study, and research was carried out with protocols approved by each site’s institutional review board. These secondary analyses were approved by the Vanderbilt University Medical Center institutional review board. The study followed the Strengthening the Reporting of Genetic Association Studies (STREGA) reporting guidelines.
Harmonization of Cognitive Measurements
Harmonization of cognitive data was previously described.44 We obtained granular data along with detailed documentation on each item in the neuropsychological protocol from each study, such as test versions, stimuli administered, and response coding. Then, a panel of neuropsychologists and neurologists (E.H.T., J.B.M., A.J.S., P.K.C.) assigned test items to a single primary domain: memory, executive function, language, visuospatial ability, or none of these. Differing versions and administration methods were noted at each time point. Next, quality control steps included parsing out missingness codes, recoding, and reverse coding data as needed. We selected the most recent visit for each participant as the reference for co-calibration. These choices enabled us to optimize the spread of cognitive abilities in the data set, which is desirable for ensuring parameters are valid over the entire range of ability levels45,46 and still including only a single observation per study participant.
We treated each cognitive item as an ordinal indicator of a domain. Common test items administered and scored the same way across ACT, ADNI, and ROS/MAP/MARS studies served as anchor items to facilitate co-calibration. We used confirmatory factor analysis models with robust maximum likelihood estimation that is robust to missing data in Mplus version 7.447 to co-calibrate separate scores for memory, executive function, and language. We extracted item parameters (loadings and thresholds) for all items, which were used to derive scores in each legacy study separately. These resulted in scores on the same metric.
APOE Genotyping
APOE haplotypes for NACC and ACT were determined from the single-nucleotide variants rs7412 and rs42935848 and from pyrosequencing of APOE codons 112 and 158 for ADNI and ROS/MAP/MARS.49
Statistical Analyses
Statistical analyses were performed using RStudio version 4.1.2. APOE ε2 and APOE ε4 were modeled separately using dominant models for APOE ε2 (because of the small ε2 homozygote sample size, especially when stratified by sex/race) and additive models for APOE ε4. We performed analyses in all participants and restricted to participants who were cognitively unimpaired at their first cognitive visit. We used linear regression for each domain to assess baseline cross-sectional APOE effects. We used linear mixed-effects regression restricted to those with at least 2 cognitive visits, with time (years from baseline) and the intercept as fixed and random effects. All models covaried for mean-centered age at first cognitive visit. Longitudinal models covaried for age at first visit × time. To test the modifying effect of sex, we included 2-way interactions (ie, sex × APOE) in cross-sectional models, and 3-way interactions (ie, sex × APOE × time) in longitudinal models. Sex-interaction models were also run stratified by race. The intersectional effects of both race and sex were assessed using a sex × APOE × race interaction in cross-sectional analysis and sex × APOE × race × time interaction in longitudinal models. Significant results were rerun in each cohort separately to assess consistency (eFigures 1 and 2 in Supplement 1). Corrections for multiple comparisons were performed using the Benjamini-Hochberg false discovery rate (FDR) procedure, accounting for all main effects and interactions modeled. Uncorrected P values are presented in the text, and P values that passed FDR correction are indicated in the tables.
Sensitivity analyses excluded ε2/ε4 individuals (n = 866), modeled APOE ε2 additively, stratified by age (≥75 or <75 years), covaried for education, or covaried for genetic ancestry via the first 5 principal components.
Results
Participant Characteristics
Participant characteristics are presented in Table 1 and by cohort in eTable 1 in Supplement 2. Participants missing APOE status were excluded (n = 9266). Of 32 427 participants who met inclusion criteria, there were 19 007 females (59%), 13 420 (41%) males, 4453 Black individuals (14%), and 27 974 White individuals (86%); the mean (SD) age at baseline was 74 years (7.9). At baseline, 6048 individuals (19%) had AD, 4398 (14%) were APOE ε2 carriers, and 12 538 (38%) were APOE ε4 carriers. Higher proportions of females were cognitively unimpaired at study entry, and APOE allele frequency differed by race.
Table 1. Participant Characteristics.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Non-Hispanic White participants (n = 27 974) | Non-Hispanic Black participants (n = 4453) | All participants (n = 32 427) | ||||
| Males | Females | Males | Females | Males | Females | |
| No. of participants | 12 265 (42.93) | 15 709 (56.16) | 1155 (25.94) | 3298 (74.06) | 13 420 (41.39) | 19 007 (58.61) |
| Age at first cognitive visit, mean (SD), y | 74.86 (7.72) | 74.95 (8.20) | 73.25 (7.28) | 73.66 (7.47) | 74.72 (7.70) | 74.73 (8.09) |
| Education, mean (SD), y | 16.29 (3.10) | 15.34 (2.90) | 14.29 (3.58) | 14.32 (3.17) | 16.12 (3.19) | 15.16 (2.97) |
| Clinical diagnosis at first cognitive visit | ||||||
| Normal cognition | 6119 (49.89) | 10 024 (63.81) | 594 (51.43) | 2000 (60.64) | 6713 (50.02) | 12 024 (63.26) |
| Impaired | 6146 (50.11) | 5685 (36.19) | 561 (48.57) | 1298 (39.36) | 6707 (49.98) | 6983 (26.74) |
| APOE ε2 count | ||||||
| 0 ε2 Alleles | 10 731 (87.49) | 13 664 (86.98) | 944 (81.73) | 2690 (81.56) | 11 675 (87.00) | 16 354 (86.04) |
| 1 ε2 Allele | 1482 (12.08) | 1983 (12.62) | 198 (17.14) | 585 (17.74) | 1680 (12.52) | 2568 (13.51) |
| 2 ε2 Alleles | 52 (0.42) | 62 (0.39) | 13 (1.13) | 23 (0.70) | 65 (0.48) | 85 (0.45) |
| APOE ε4 count | ||||||
| 0 ε4 Alleles | 7580 (61.80) | 9936 (63.25) | 626 (54.20) | 1927 (58.43) | 8206 (61.15) | 11 863 (62.41) |
| 1 ε4 Allele | 3882 (31.65) | 4949 (31.50) | 449 (38.87) | 1175 (35.63) | 4331 (32.27) | 6124 (32.22) |
| 2 ε4 Alleles | 803 (6.55) | 824 (5.25) | 80 (6.93) | 196 (5.94) | 883 (6.58) | 1020 (5.37) |
| No. of visits, mean (SD) | 4.51 (3.44) | 4.97 (3.86) | 4.28 (3.26) | 4.68 (3.67) | 4.49 (3.42) | 4.92 (3.83) |
| Follow-up, mean (SD), y | 3.67 (4.20) | 3.93 (4.26) | 3.38 (3.80) | 3.43 (3.79) | 3.64 (3.89) | 4.13 (4.19) |
| Memory score, mean (SD) | 0.20 (0.81) | 0.39 (0.86) | 0.07 (0.74) | 0.25 (0.77) | 0.19 (0.80) | 0.37 (0.86) |
| Executive function score, mean (SD) | 0.23 (0.70) | 0.34 (0.69) | −0.14 (0.70) | −0.05 (0.68) | 0.20 (0.71) | 0.27 (0.70) |
| Language score, mean (SD) | 0.28 (0.73) | 0.44 (0.78) | 0.07 (0.66) | 0.15 (0.66) | 0.26 (0.73) | 0.39 (0.77) |
Main Effects of APOE on Cognition
Results of all models are presented in eTable 2 (all diagnoses) and eTable 3 (cognitively normal) in Supplement 2. Among all participants, APOE ε4 was robustly associated with worse cognitive performance and faster rates of decline across all cognitive domains, and APOE ε2 was associated with better cognitive performance and slower rates of decline. Associations with memory had the largest effects. Results were similar when restricted to participants who were cognitively unimpaired at baseline, although the baseline APOE ε2 associations were attenuated.
Intersectional Effects of Sex, Race, and APOE on Cognition
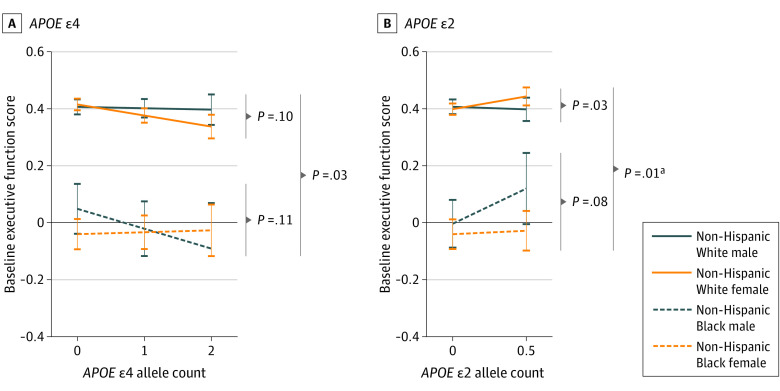
To assess whether the modifying effect of sex on APOE differed by race, we performed 3-way interactions of race, sex, and APOE (Table 2). One statistically significant intersectional effect was observed among cognitively normal participants, whereby APOE ε2 interacted with race and sex on baseline executive function (β = −0.165, SE = 0.066; uncorrected P = .01; FDR-corrected P = 4.48 × 10-2). APOE ε2 showed a female-specific protective effect among White participants but a male-specific protective effect among Black participants (Figure, B). A comparable intersectional effect was also observed for APOE ε4 on executive function (Figure, A), although this interaction did not survive correction for multiple comparisons. As no other significant intersectional effects of race and sex were observed, the remaining results focus on findings across racial groups.
Table 2. Sex × APOE × Race Interactions With Multiple Cognitive Domains.
| Outcome (diagnosis) | APOE allele | Sex × APOE × race interaction | |
|---|---|---|---|
| β (SE) | P value | ||
| Baseline results in participants with all diagnoses | |||
| Baseline memory (all diagnoses) | ε2 | −0.068 (0.077) | .38 |
| ε4 | 0.018 (0.046) | .69 | |
| Baseline executive function (all diagnoses) | ε2 | −0.132 (0.064) | .04a |
| ε4 | 0.018 (0.039) | .65 | |
| Baseline language (all diagnoses) | ε2 | −0.130 (0.067) | .05 |
| ε4 | 0.018 (0.041) | .67 | |
| Baseline results in participants with normal cognition at baseline | |||
| Baseline memory (normal cognition) | ε2 | −0.026 (0.060) | .66 |
| ε4 | 0.052 (0.044) | .24 | |
| Baseline executive function (normal cognition) | ε2 | −0.165 (0.066) | .01a,b |
| ε4 | 0.101 (0.048) | .03a | |
| Baseline language (normal cognition) | ε2 | −0.104 (0.066) | .12 |
| ε4 | −0.104 (0.052) | .28 | |
| Longitudinal results in participants with all diagnoses | |||
| Longitudinal memory (all diagnoses) | ε2 | −0.007 (0.014) | .61 |
| ε4 | −0.005 (0.009) | .61 | |
| Longitudinal executive function (all diagnoses) | ε2 | 0.009 (0.011) | .45 |
| ε4 | 0.005 (0.007) | .46 | |
| Longitudinal language (all diagnoses) | ε2 | 0.003 (0.012) | .83 |
| ε4 | −0.002 (0.008) | .84 | |
| Longitudinal results in participants with normal cognition at baseline | |||
| Longitudinal memory (normal cognition) | ε2 | 0.009 (0.014) | .50 |
| ε4 | 0.002 (0.010) | .86 | |
| Longitudinal executive function (normal cognition) | ε2 | 0.011 (0.010) | .29 |
| ε4 | 0.009 (0.008) | .23 | |
| Longitudinal language (normal cognition) | ε2 | 0.007 (0.011) | .55 |
| ε4 | 0.003 (0.008) | .72 | |
Association had uncorrected P < .05.
Association had false discovery rate–corrected P < .05.
Figure. APOE × Sex × Race Interactions on Executive Function Performance at Baseline Among Participants With Normal Cognition.
Sex- and race-specific associations between baseline executive function among cognitively unimpaired individuals in APOE ε4 and APOE ε2. P values are presented for both race-stratified sex × APOE interactions and intersectional APOE × sex × race interactions. APOE ε4 was coded additively, and APOE ε2 was coded dominantly.
aFalse discovery rate–corrected P < .05.
Sex Differences in APOE Effects by Allele
Among all participants, we observed a stronger association between APOE ε4 and baseline memory performance and baseline language performance among women compared with men (although the sex interaction on language was not FDR-significant) (Table 3). For APOE ε4, we observed a robust sex interaction on baseline memory (β = −0.071, SE = 0.014; uncorrected P = 9.6 × 10−7; FDR-corrected P = 1.87 × 10-6), whereby the APOE ε4 negative effect was stronger in females compared with males and did not significantly differ among races. Results were similar across sensitivity analyses. There were no significant sex differences in APOE ε4 associations with any of the longitudinal outcomes or when restricting to cognitively normal participants. Among all participants, APOE ε2 did not interact with sex on cognition (Table 3).
Table 3. Sex- and Race-Specific APOE Associations With Multiple Cognitive Domains.
| Outcome (diagnosis) | APOE allele | Model | Cross-ancestry | Non-Hispanic White | Non-Hispanic Black | |||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | |||
| Baseline results in participants with all diagnoses | ||||||||
| Baseline memory (all diagnoses) | ε2 | Sex-interaction | 0.011 (0.027) | .67 | 0.033 (0.030) | .26 | −0.032 (0.063) | .61 |
| Females | 0.215 (0.017) | 2.19 × 10-35a,b | 0.247 (0.020) | 4.18 × 10-35a,b | 0.154 (0.032) | 1.93 × 10-6a,b | ||
| Males | 0.201 (0.020) | 1.71 × 10-23a,b | 0.210 (0.022) | 1.63 × 10-22a,b | 0.187 (0.053) | 4.61 × 10-4a,b | ||
| ε4 | Sex-interaction | −0.071 (0.014) | 9.60 × 10-7a,b | −0.083 (0.016) | 1.40 × 10-7a,b | −0.066 (0.038) | .08 | |
| Females | −0.422 (0.010) | <2.23 × 10-308a,b | −0.441 (0.011) | <2.23 × 10-308a,b | −0.316 (0.020) | 1.81 × 10-54a,b | ||
| Males | −0.341 (0.011) | 7.68 × 10-217a,b | −0.347 (0.011) | 1.95 × 10-202a,b | −0.247 (0.032) | 4.63 × 10-14a,b | ||
| Baseline executive function (all diagnoses) | ε2 | Sex-interaction | 0.002 (0.023) | .98 | 0.036 (0.024) | .15 | −0.094 (0.057) | .10 |
| Females | 0.083 (0.014) | 6.85 × 10-9a,b | 0.126 (0.016) | 1.23 × 10-15a,b | 0.062 (0.029) | .03a,b | ||
| Males | 0.079 (0.018) | 1.18 × 10-5a,b | 0.088 (0.019) | 3.65 × 10-6a,b | 0.157 (0.051) | 2.24 × 10-3a,b | ||
| ε4 | Sex-interaction | −0.009 (0.013) | .49 | −0.015 (0.013) | .26 | 0.000 (0.036) | .99 | |
| Females | −0.200 (0.008) | 3.59 × 10-124a,b | −0.205 (0.009) | 4.65 × 10-113a,b | −0.131 (0.019) | 1.74 × 10-12a,b | ||
| Males | −0.018 (0.010) | 6.20 × 10-77a,b | −0.182 (0.010) | 1.23 × 10-70a,b | −0.129 (0.032) | 5.50 × 10-5a,b | ||
| Baseline language (all diagnoses) | ε2 | Sex-interaction | −0.023 (0.024) | .34 | 0.009 (0.026) | .74 | −0.121 (0.055) | .03a,b |
| Females | 0.095 (0.015) | 3.38 × 10-10a,b | 0.129 (0.017) | 1.10 × 10-13a,b | 0.080 (0.028) | 4.01 × 10-3a,b | ||
| Males | 0.114 (0.018) | 2.54 × 10-10a,b | 0.114 (0.019) | 2.84 × 10-9a,b | 0.201 (0.047) | 2.15 × 10-5a,b | ||
| ε4 | Sex-interaction | −0.027 (0.013) | .04a | −0.031 (0.014) | .03a,b | −0.014 (0.034) | .69 | |
| Females | −0.216 (0.009) | 1.68 × 10-131a,b | −0.220 (0.010) | 1.35 × 10-109a,b | −0.172 (0.018) | 4.19 × 10-22a,b | ||
| Males | −0.176 (0.010) | 6.87 × 10-72a,b | −0.174 (0.010) | 1.29 × 10-63a,b | −0.156 (0.029) | 9.53 × 10-8a,b | ||
| Baseline results in participants with normal cognition at baseline | ||||||||
| Baseline memory (normal cognition) | ε2 | Sex-interaction | −0.015 (0.021) | .49 | −0.004 (0.002) | .85 | −0.031 (0.052) | .56 |
| Females | −0.007 (0.012) | .60 | 0.014 (0.014) | .32 | −0.004 (0.025) | .88 | ||
| Males | 0.006 (0.017) | .71 | 0.015 (0.017) | .40 | 0.026 (0.045) | .56 | ||
| ε4 | Sex-interaction | −0.016 (0.015) | .27 | −0.024 (0.016) | .13 | 0.028 (0.038) | .47 | |
| Females | −0.049 (0.009) | 6.59 × 10-8a,b | −0.046 (0.010) | 3.49 × 10-6a,b | −0.020 (0.019) | .30 | ||
| Males | −0.025 (0.012) | .03a | −0.013 (0.012) | .30 | −0.049 (0.032) | .13 | ||
| Baseline executive function (normal cognition) | ε2 | Sex-interaction | 0.021 (0.023) | .38 | 0.052 (0.024) | .03a | −0.112 (0.064) | .08 |
| Females | 0.011 (0.014) | .44 | 0.045 (0.015) | 2.29 × 10-3a,b | 0.012 (0.030) | .68 | ||
| Males | −0.011 (0.019) | .56 | −0.009 (0.019) | .64 | 0.124 (0.060) | .04a | ||
| ε4 | Sex-interaction | −0.013 (0.017) | .44 | −0.028 (0.017) | .10 | 0.074 (0.046) | .11 | |
| Females | −0.044 (0.010) | 1.16 × 10-5a,b | −0.039 (0.011) | 2.20 × 10-4a,b | 0.007 (0.002) | .76 | ||
| Males | −0.027 (0.013) | .04a | −0.005 (0.014) | .72 | −0.070 (0.043) | .11 | ||
| Baseline language (normal cognition) | ε2 | Sex-interaction | −0.036 (0.023) | .12 | −0.012 (0.025) | .63 | −0.117 (0.057) | .04a |
| Females | −0.029 (0.014) | .04a | −0.003 (0.015) | .85 | −0.008 (0.027) | .77 | ||
| Males | 0.003 (0.018) | .88 | 0.002 (0.019) | .92 | 0.109 (0.051) | .03a | ||
| ε4 | Sex-interaction | −0.001 (0.016) | .95 | −0.002 (0.018) | .91 | 0.050 (0.041) | .23 | |
| Females | −0.017 (0.010) | .10 | −0.002 (0.011) | .86 | −0.021 (0.021) | .31 | ||
| Males | −0.001 (0.013) | .94 | 0.019 (0.018) | .17 | −0.069 (0.036) | .06 | ||
| Longitudinal results in participants with all diagnoses | ||||||||
| Longitudinal memory (all diagnoses) | ε2 | Sex-interaction | −0.004 (0.005) | .42 | −0.003 (0.005) | .58 | −0.010 (0.011) | .34 |
| Females | 0.029 (0.003) | 7.54 × 10-24a,b | 0.030 (0.003) | 1.35 × 10-18a,b | 0.020 (0.005) | 1.36 × 10-4a,b | ||
| Males | 0.036 (0.004) | 9.96 × 10-19a,b | 0.035 (0.004) | 1.11 × 10-15a,b | 0.032 (0.010) | 1.34 × 10-3a,b | ||
| ε4 | Sex-interaction | 0.006 (0.003) | .046a | 0.004 (0.003) | .21 | 0.000 (0.007) | .96 | |
| Females | −0.054 (0.002) | 7.02 × 10-190a,b | −0.060 (0.002) | 6.47 × 10-177a,b | −0.037 (0.004) | 1.13 × 10-24a,b | ||
| Males | −0.062 (0.002) | 2.08 × 10-157a,b | −0.065 (0.003) | 2.82 × 10-154a,b | −0.039 (0.006) | 7.03 × 10-10a,b | ||
| Longitudinal executive function (all diagnoses) | ε2 | Sex-interaction | −0.004 (0.004) | .30 | −0.005 (0.004) | .23 | 0.004 (0.009) | .68 |
| Females | 0.016 (0.002) | 4.17 × 10-12a,b | 0.015 (0.003) | 6.88 × 10-9a,b | 0.014 (0.004) | 6.17 × 10-4a,b | ||
| Males | 0.021 (0.003) | 2.72 × 10-11a,b | 0.022 (0.003) | 1.68 × 10-10a,b | 0.011 (0.008) | .16 | ||
| ε4 | Sex-interaction | 0.004 (0.002) | .07 | 0.002 (0.003) | .47 | 0.008 (0.006) | .16 | |
| Females | −0.032 (0.002) | 5.97 × 10-104a,b | −0.037 (0.002) | 1.99 × 10-105a,b | −0.016 (0.003) | 6.09 × 10-8a,b | ||
| Males | −0.038 (0.002) | 6.88 × 10-90a,b | −0.041 (0.002) | 1.61 × 10-88a,b | −0.024 (0.005) | 2.39 × 10-6a,b | ||
| Longitudinal language (all diagnoses) | ε2 | Sex-interaction | −0.007 (0.004) | .10 | −0.007 (0.005) | .13 | −0.004 (0.009) | .62 |
| Females | 0.022 (0.003) | 2.43 × 10-17a,b | 0.022 (0.003) | 2.61 × 10-13a,b | 0.014 (0.005) | 1.50 × 10-3a,b | ||
| Males | 0.030 (0.003) | 2.00 × 10-18a,b | 0.030 (0.004) | 7.71 × 10-16a,b | 0.019 (0.008) | .01a,b | ||
| ε4 | Sex-interaction | 0.004 (0.003) | .14 | 0.002 (0.003) | .58 | 0.002 (0.006) | .79 | |
| Females | −0.042 (0.002) | 8.52 × 10-148a,b | −0.048 (0.002) | 7.09 × 10-147a,b | −0.023 (0.003) | 6.55 × 10-13a,b | ||
| Males | −0.047 (0.002) | 2.64 × 10-124a,b | −0.051 (0.002) | 2.17 × 10-124a,b | −0.025 (0.005) | 5.98 × 10-7a,b | ||
| Longitudinal results in participants with normal cognition at baseline | ||||||||
| Longitudinal memory (normal cognition) | ε2 | Sex-interaction | −0.002 (0.005) | .67 | −0.004 (0.005) | .42 | 0.004 (0.011) | .69 |
| Females | 0.010 (0.003) | 2.62 × 10-4a,b | 0.008 (0.003) | .01a,b | 0.014 (0.005) | .01a,b | ||
| Males | 0.012 (0.004) | 6.20 × 10-4a,b | 0.012 (0.004) | 1.56 × 10-3a,b | 0.010 (0.009) | .29 | ||
| ε4 | Sex-interaction | −0.001 (0.003) | .89 | −0.002 (0.004) | .66 | 0.000 (0.008) | .96 | |
| Females | −0.024 (0.002) | 8.52 × 10-33a,b | −0.027 (0.002) | 3.16 × 10-32a,b | −0.014 (0.004) | 5.13 × 10-4a,b | ||
| Males | −0.022 (0.003) | 1.80 × 10-18a,b | −0.024 (0.003) | 2.34 × 10-18a,b | −0.013 (0.007) | .05 | ||
| Longitudinal executive function (normal cognition) | ε2 | Sex-interaction | −0.001 (0.003) | .76 | −0.003 (0.004) | .43 | 0.008 (0.009) | .36 |
| Females | 0.006 (0.002) | 2.20 × 10-3a,b | 0.005 (0.002) | .045a | 0.011 (0.004) | .01a,b | ||
| Males | 0.007 (0.003) | 7.76 × 10-3a,b | 0.008 (0.003) | 8.61 × 10-3a,b | 0.003 (0.008) | .72 | ||
| ε4 | Sex-interaction | −0.001 (0.003) | .76 | −0.002 (0.003) | .37 | 0.007 (0.007) | .34 | |
| Females | −0.013 (0.002) | 2.18 × 10-19a,b | −0.015 (0.002) | 5.86 × 10-21a,b | −0.005 (0.003) | .17 | ||
| Males | −0.013 (0.002) | 1.13 × 10-10a,b | −0.013 (0.002) | 3.44 × 10-10a,b | −0.010 (0.006) | .06 | ||
| Longitudinal language (normal cognition) | ε2 | Sex-interaction | −0.005 (0.004) | .15 | −0.007 (0.004) | .09 | −0.001 (0.009) | .88 |
| Females | 0.006 (0.002) | 4.74 × 10-3a,b | 0.005 (0.003) | .08 | 0.009 (0.004) | .04a | ||
| Males | 0.012 (0.003) | 3.12 × 10-5a,b | 0.011 (0.003) | 1.57 × 10-4a,b | 0.010 (0.007) | .15 | ||
| ε4 | Sex-interaction | −0.003 (0.003) | .31 | −0.004 (0.003) | .15 | −0.002 (0.007) | .78 | |
| Females | −0.016 (0.002) | 3.15 × 10-23a,b | −0.020 (0.002) | 9.84 × 10-27a,b | −0.004 (0.004) | .24 | ||
| Males | −0.013 (0.002) | 1.50 × 10-10a,b | −0.015 (0.002) | 1.40 × 10-11a,b | −0.003 (0.005) | .57 | ||
Association had uncorrected P < .05.
Association had false discovery rate–corrected P < .05.
Discussion
To our knowledge, this study is the largest investigation of the modifying effects of both sex and race on the association between APOE and AD-related cognitive decline. We provide strong evidence of a sex difference in the association between APOE ε4 and cross-sectional memory performance and, for the first time, also extend this sex difference to the language domain. Furthermore, these APOE ε4 effects were stronger in females, and there was no statistical evidence of race specificity. In contrast to APOE ε4, we did not observe evidence of sex differences in APOE ε2 associations with cognition across race. However, we did observe exciting novel evidence of intersectional effects for APOE ε2, whereby sex-specific associations on cognition may differ by racial group, particularly in the executive function domain. Together, our findings solidify current understandings of sex-specific effects of APOE ε4 while highlighting the pressing need to fully characterize intersectional effects of race, sex, and APOE ε2 on late-life cognition.
The strong association between APOE ε4 and cognition among women aligns with and extends past work. APOE ε4 showed stronger effects on baseline memory and language performance among females compared with males, and this effect was similar among Black and White individuals. Interestingly, these female-driven effects of APOE ε4 are only present when evaluating across clinical diagnoses and not among the cognitively unimpaired subset. Indeed, APOE ε4 is a stronger predictor of MCI and clinical AD risk among females compared with males, particularly among those aged 55 to 70 years.10,16 Likewise, past work has observed female ε4 carriers to have faster memory decline compared with male ε4 carriers.8 That said, there has not been much characterization of sex differences in APOE ε4 effects on language, making our findings of increased ε4 effects among females interesting. The mechanism underlying these female-specific findings, in both our research and that of other groups, remains unclear. Sex hormones may play a role, as studies have demonstrated estrogen levels affect cognition.50,51,52 Menopausal loss of estrogen may amplify negative APOE ε4 effects, resulting in greater cognitive impairment compared with males. Additionally, females tend to have steeper cognitive decline in the presence of high levels of AD neuropathology53; therefore, the effects of ε4 certainly could be driven by more advanced neuropathology. Tau pathology has been implicated as a potential pathway previously, as prior studies have highlighted stronger associations between APOE ε4 and biomarkers of tau pathology in females compared with males.7,54,55 Regardless of the underlying mechanism, the present results solidify the evidence of robust sex differences in the risk effect of APOE ε4.
Given the lower allele frequency and smaller effect size of ε2, few studies have explored sex- and race-specific APOE ε2 effects on cognitive decline. Given our large sample size, we were able to provide strong evidence that the sex difference in APOE ε4 risk does not extend to APOE ε2 protection, with strikingly consistent associations between APOE ε2 and cognition in males and females. Fascinatingly, among cognitive normal participants we do provide exciting new evidence of an intersectional effect of sex, race, and APOE ε2 on executive function. More specifically, the APOE ε2 protective effect was female-specific in White individuals but male-specific in Black individuals. Very few studies have evaluated such intersectional effects of APOE ε2 on cognition. In a study of 976 African American and 794 White middle-aged adults, Beydoun and colleagues22 investigated sex and race as potential modifiers of APOE on cognition. While the investigators noted a lack of consistency in associations between APOE ε2 and specific neuropsychological test scores across races, no race- or sex-specific associations were deemed significant.22 Studies are also mixed regarding differential APOE ε2 effects among the sexes, with one study demonstrating stronger effects among cognitively normal males,7 but another highlighting stronger effects among females.14 Additionally, a recent study found that the APOE ε2 association with cognitive decline in cognitively normal White men was stronger than in White women.56 In the present analysis, which included some of the same cohorts, we saw similar evidence of a stronger association between APOE ε2 and longitudinal decline in cognitively normal White men compared with cognitively normal White women, but the APOE ε2 × sex interaction did not reach statistical significance (Table 3). Continued reporting by sex and race will allow for larger studies to confirm whether the intersectional effects and the previous mixed findings in APOE ε2 carriers persist.
Because APOE ε2 and ε4 are known to have opposing effects, we performed sensitivity analyses excluding participants with the ε2/ε4 genotype. In our analyses, sex × APOE effects on cognition were generally strengthened after ε2/ε4 genotype removal. These results are in line with previous studies, which have reported as much as a 3-fold increase in the odds of developing AD for those with the ε2/ε4 genotype.57,58,59,60 We also conducted age-stratified sensitivity analyses to determine if significant sex × APOE interactions on cognition differed between participants younger than 75 years and participants 75 years or older at baseline. Consistent with past literature,10 we observed that sex × APOE ε4 interactions on cognition were primarily driven by the younger age group. In contrast to ε4, our results suggest that sex may modify the association between APOE ε2 and cognition among older participants. A previous study showed APOE ε2 protective effects against clinical dementia in the oldest old (≥90 years), so this will be an exciting avenue for future work.
Strengths and Limitations
The present study has several strengths. Longitudinal, harmonized cognitive data across multiple cognitive domains facilitated a large sample size, which allowed for investigation of the intersectional effects of race, sex, and APOE on cognition. However, our results should be interpreted taking weaknesses into consideration. While sex and race effects of amyloid-β and tau pathologies are important to consider in the context of APOE and cognition, harmonized biomarker data were not readily available; therefore, we could not integrate neuropathology and biomarker measures into our models. Additionally, only data for Black and White individuals were included in this study, so our results may not be generalizable to those in other racial and ethnic groups. Moreover, despite the large sample size, the number of ε2 homozygotes was small and warrants further exploration. To maximize statistical power in sex/race stratifications, APOE ε2 was modeled dominantly. Results from dominant and additive APOE ε2 models were, in general, similar (eTables 2 and 3 in Supplement 2); however, prior literature indicates White individuals with an APOE ε2/ε3 genotype have a significantly lower risk of AD compared with individuals with the APOE ε2/ε2 genotype.6 Whether this holds true among Black individuals is unclear, again highlighting the need for even larger sample sizes of racially diverse individuals.
Additionally, though this study leveraged harmonized cognitive scores from multiple cohorts, we observed some heterogeneity of effects across cohorts, as seen in eFigures 1 and 2 in Supplement 1. Most heterogeneity is likely driven by sample size differences, with the largest cohort (NACC) often driving significant results. Furthermore, while cohorts were of similar age and highly educated, recruitment schemes differed. Therefore, the presence of associations may be due to the relative frequency of AD cases and selection bias differences across study designs. The majority of our significant associations were observed when including all participants regardless of clinical diagnosis and thus are more likely reflective of the clinic-based sampling cohort from NACC. Moreover, APOE ε4 effects have been shown to vary with genetic ancestry, especially within African American subpopulations.61 While covarying for ancestry principal components did not affect our interpretation of results, evaluation of local ancestry at the APOE locus would allow for better dissociation of population effects.
Conclusion
The results of our study confirmed sex differences in the association between APOE ε4 and memory and provide strong evidence this sex difference does not differ among Black and White individuals. In contrast, we did not see robust sex differences in APOE ε2 effects on cognition, though we provide exciting new evidence of an intersectional effect of sex, race, and APOE ε2 on cognition. Our finding that APOE isoforms were differently modified by sex and race highlights the fact that ε2 and ε4 are not simply opposing sides of the same coin and underscores the need for a comprehensive precision-medicine approach in understanding AD progression.
eFigure 1. Forest plots of significant APOE-ε4*sex interactions
eFigure 2. Forest plots of significant APOE-ε2*sex interactions
eTable 1. Demographics by Cohort
eTable 2. APOE x Race x Sex Interactions on Cognition (All Diagnoses)
eTable 3. APOE x Race x Sex Interactions on Cognition (Normal Cognition)
Nonauthor collaborators
Data sharing statement
References
- 1.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180-184. doi: 10.1038/ng0694-180 [DOI] [PubMed] [Google Scholar]
- 2.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106-118. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65(12):1888-1893. doi: 10.1212/01.wnl.0000188871.74093.12 [DOI] [PubMed] [Google Scholar]
- 4.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2878-2886. doi: 10.1016/j.neubiorev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 5.Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J. 1996;10(13):1485-1494. doi: 10.1096/fasebj.10.13.8940294 [DOI] [PubMed] [Google Scholar]
- 6.Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. ; Alzheimer’s Disease Genetics Consortium . Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 2020;11(1):667. doi: 10.1038/s41467-019-14279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altmann A, Tian L, Henderson VW, Greicius MD; Alzheimer’s Disease Neuroimaging Initiative Investigators . Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563-573. doi: 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beydoun MA, Boueiz A, Abougergi MS, et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33(4):720-731.e4. doi: 10.1016/j.neurobiolaging.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland D, Desikan RS, Dale AM, McEvoy LK; Alzheimer’s Disease Neuroimaging Initiative . Higher rates of decline for women and apolipoprotein E ε4 carriers. AJNR Am J Neuroradiol. 2013;34(12):2287-2293. doi: 10.3174/ajnr.A3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74(10):1178-1189. doi: 10.1001/jamaneurol.2017.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ungar L, Altmann A, Greicius MD. Apolipoprotein E, gender, and Alzheimer’s disease: an overlooked, but potent and promising interaction. Brain Imaging Behav. 2014;8(2):262-273. doi: 10.1007/s11682-013-9272-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleisher A, Grundman M, Jack CR Jr, et al. ; Alzheimer’s Disease Cooperative Study . Sex, apolipoprotein E ε 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62(6):953-957. doi: 10.1001/archneur.62.6.953 [DOI] [PubMed] [Google Scholar]
- 13.Mortensen EL, Høgh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001;57(1):89-95. doi: 10.1212/WNL.57.1.89 [DOI] [PubMed] [Google Scholar]
- 14.Lamonja-Vicente N, Dacosta-Aguayo R, López-Olóriz J, et al. Sex-specific protective effects of APOE ε2 on cognitive performance. J Gerontol A Biol Sci Med Sci. 2021;76(1):41-49. doi: 10.1093/gerona/glaa247 [DOI] [PubMed] [Google Scholar]
- 15.Chan ML, Meyer OL, Farias ST, et al. APOE effects on late life cognitive trajectories in diverse racial/ethnic groups. J Int Neuropsychol Soc. 2023;29(2):126-135. doi: 10.1017/S1355617722000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer LA, Cupples LA, Haines JL, et al. ; APOE and Alzheimer Disease Meta Analysis Consortium . Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-1356. doi: 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- 17.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151-159. doi: 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olarte L, Schupf N, Lee JH, et al. Apolipoprotein E ε4 and age at onset of sporadic and familial Alzheimer disease in Caribbean Hispanics. Arch Neurol. 2006;63(11):1586-1590. doi: 10.1001/archneur.63.11.1586 [DOI] [PubMed] [Google Scholar]
- 19.Rippon GA, Tang MX, Lee JH, Lantigua R, Medrano M, Mayeux R. Familial Alzheimer disease in Latinos: interaction between APOE, stroke, and estrogen replacement. Neurology. 2006;66(1):35-40. doi: 10.1212/01.wnl.0000191300.38571.3e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murrell JR, Price B, Lane KA, et al. Association of apolipoprotein E genotype and Alzheimer disease in African Americans. Arch Neurol. 2006;63(3):431-434. doi: 10.1001/archneur.63.3.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosentino S, Scarmeas N, Helzner E, et al. APOE ε 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70(19 Pt 2):1842-1849. doi: 10.1212/01.wnl.0000304038.37421.cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beydoun MA, Weiss J, Beydoun HA, et al. Race, APOE genotypes, and cognitive decline among middle-aged urban adults. Alzheimers Res Ther. 2021;13(1):120. doi: 10.1186/s13195-021-00855-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss J, Hossain S, Maldonado AI, et al. Associations between race, APOE genotype, cognition, and mortality among urban middle-aged white and African American adults. Sci Rep. 2021;11(1):19849. doi: 10.1038/s41598-021-98117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pankiewicz JE, Sadowski MJ. APOE genotype and Alzheimer’s immunotherapy. Oncotarget. 2017;8(25):39941-39942. doi: 10.18632/oncotarget.17990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salloway S, Sperling R, Gilman S, et al. ; Bapineuzumab 201 Clinical Trial Investigators . A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061-2070. doi: 10.1212/WNL.0b013e3181c67808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. doi: 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- 27.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198-207. doi: 10.1001/archneurol.2011.1538 [DOI] [PubMed] [Google Scholar]
- 28.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11(3):241-249. doi: 10.1016/S1474-4422(12)70015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirono N, Hashimoto M, Yasuda M, Kazui H, Mori E. Accelerated memory decline in Alzheimer’s disease with apolipoprotein ϵ4 allele. J Neuropsychiatry Clin Neurosci. 2003;15(3):354-358. doi: 10.1176/jnp.15.3.354 [DOI] [PubMed] [Google Scholar]
- 30.Gharbi-Meliani A, Dugravot A, Sabia S, et al. The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimers Res Ther. 2021;13(1):5. doi: 10.1186/s13195-020-00740-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor WD, Boyd B, Turner R, et al. APOE ε4 associated with preserved executive function performance and maintenance of temporal and cingulate brain volumes in younger adults. Brain Imaging Behav. 2017;11(1):194-204. doi: 10.1007/s11682-016-9522-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolk DA, Dickerson BC; Alzheimer’s Disease Neuroimaging Initiative . Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional–executive network function in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107(22):10256-10261. doi: 10.1073/pnas.1001412107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Vlies AE, Pijnenburg YAL, Koene T, et al. Cognitive impairment in Alzheimer’s disease is modified by APOE genotype. Dement Geriatr Cogn Disord. 2007;24(2):98-103. doi: 10.1159/000104467 [DOI] [PubMed] [Google Scholar]
- 34.Li W, Qiu Q, Sun L, Li X, Xiao S. Short-term adverse effects of the apolipoprotein E ε4 allele over language function and executive function in healthy older adults. Neuropsychiatr Dis Treat. 2019;15:1855-1861. doi: 10.2147/NDT.S183064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alzheimer’s Disease Neuroimaging Initiative . Accessed June 6, 2023. http://www.adni-info.org
- 36.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Curr Alzheimer Res. 2012;9(6):628-645. doi: 10.2174/156720512801322573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9(6):734-745. doi: 10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beekly DL, Ramos EM, van Belle G, et al. ; NIA-Alzheimer’s Disease Centers . The National Alzheimer’s Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18(4):270-277. [PubMed] [Google Scholar]
- 39.Beekly DL, Ramos EM, Lee WW, et al. ; NIA Alzheimer’s Disease Centers . The National Alzheimer’s Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249-258. doi: 10.1097/WAD.0b013e318142774e [DOI] [PubMed] [Google Scholar]
- 40.Besser L, Kukull W, Knopman DS, et al. ; Neuropsychology Work Group, Directors, and Clinical Core leaders of the National Institute on Aging-funded US Alzheimer’s Disease Centers . Version 3 of the National Alzheimer’s Coordinating Center’s uniform data set. Alzheimer Dis Assoc Disord. 2018;32(4):351-358. doi: 10.1097/WAD.0000000000000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ uniform data set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91-101. doi: 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ neuropsychological test battery in the uniform data set (UDS). Alzheimer Dis Assoc Disord. 2018;32(1):10-17. doi: 10.1097/WAD.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737-1746. doi: 10.1001/archneur.59.11.1737 [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee S, Choi SE, Lee ML, et al. Cognitive domain harmonization and cocalibration in studies of older adults. Neuropsychology. 2023;37(4):409-423. doi: 10.1037/neu0000835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Embretson SE, Reise SP. Item Response Theory for Psychologists. Erlbaum; 2000:371. [Google Scholar]
- 46.Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of Item Response Theory. Sage Publications; 1991:174. [Google Scholar]
- 47.Muthén L, Muthén B. Mplus: Statistical Analysis With Latent Variables: User’s Guide (Version 8). Muthén & Muthén; 2017. [Google Scholar]
- 48.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436-441. doi: 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu L, Lutz MW, Farfel JM, et al. Neuropathologic features of TOMM40 '523 variant on late-life cognitive decline. Alzheimers Dement. 2017;13(12):1380-1388. doi: 10.1016/j.jalz.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci. 2003;15(2):161-167. doi: 10.1176/jnp.15.2.161 [DOI] [PubMed] [Google Scholar]
- 51.Ryan J, Scali J, Carrière I, et al. Impact of a premature menopause on cognitive function in later life. BJOG. 2014;121(13):1729-1739. doi: 10.1111/1471-0528.12828 [DOI] [PubMed] [Google Scholar]
- 52.Ryan J, Carrière I, Scali J, Ritchie K, Ancelin ML. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34(2):287-298. doi: 10.1016/j.psyneuen.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 53.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685-691. doi: 10.1001/archpsyc.62.6.685 [DOI] [PubMed] [Google Scholar]
- 54.Hohman TJ, Dumitrescu L, Barnes LL, et al. ; Alzheimer’s Disease Genetics Consortium and the Alzheimer’s Disease Neuroimaging Initiative . Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989-998. doi: 10.1001/jamaneurol.2018.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damoiseaux JS, Seeley WW, Zhou J, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32(24):8254-8262. doi: 10.1523/JNEUROSCI.0305-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood ME, Xiong LY, Wong YY, et al. Sex differences in associations between APOE ε2 and longitudinal cognitive decline. Alzheimers Dement. Published online March 30, 2023. doi: 10.1002/alz.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldberg TE, Huey ED, Devanand DP. Association of APOE e2 genotype with Alzheimer’s and non-Alzheimer’s neurodegenerative pathologies. Nat Commun. 2020;11(1):4727. doi: 10.1038/s41467-020-18198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H, Devanand DP, Carlson S, Goldberg TE. Apolipoprotein E genotype e2: neuroprotection and its limits. Front Aging Neurosci. 2022;14:919712. doi: 10.3389/fnagi.2022.919712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oveisgharan S, Buchman AS, Yu L, et al. APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology. 2018;90(24):e2127-e2134. doi: 10.1212/WNL.0000000000005677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren D, Lopez OL, Lingler JH, Conley Y. The effect of the APOE ε2ε4 genotype on the development of Alzheimer’s disease (AD) and mild cognitive impairment (MCI) in non-Latino Whites. J Am Geriatr Soc. 2020;68(5):1044-1049. doi: 10.1111/jgs.16337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendrie HC, Murrell J, Baiyewu O, et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int Psychogeriatr. 2014;26(6):977-985. doi: 10.1017/S1041610214000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Forest plots of significant APOE-ε4*sex interactions
eFigure 2. Forest plots of significant APOE-ε2*sex interactions
eTable 1. Demographics by Cohort
eTable 2. APOE x Race x Sex Interactions on Cognition (All Diagnoses)
eTable 3. APOE x Race x Sex Interactions on Cognition (Normal Cognition)
Nonauthor collaborators
Data sharing statement