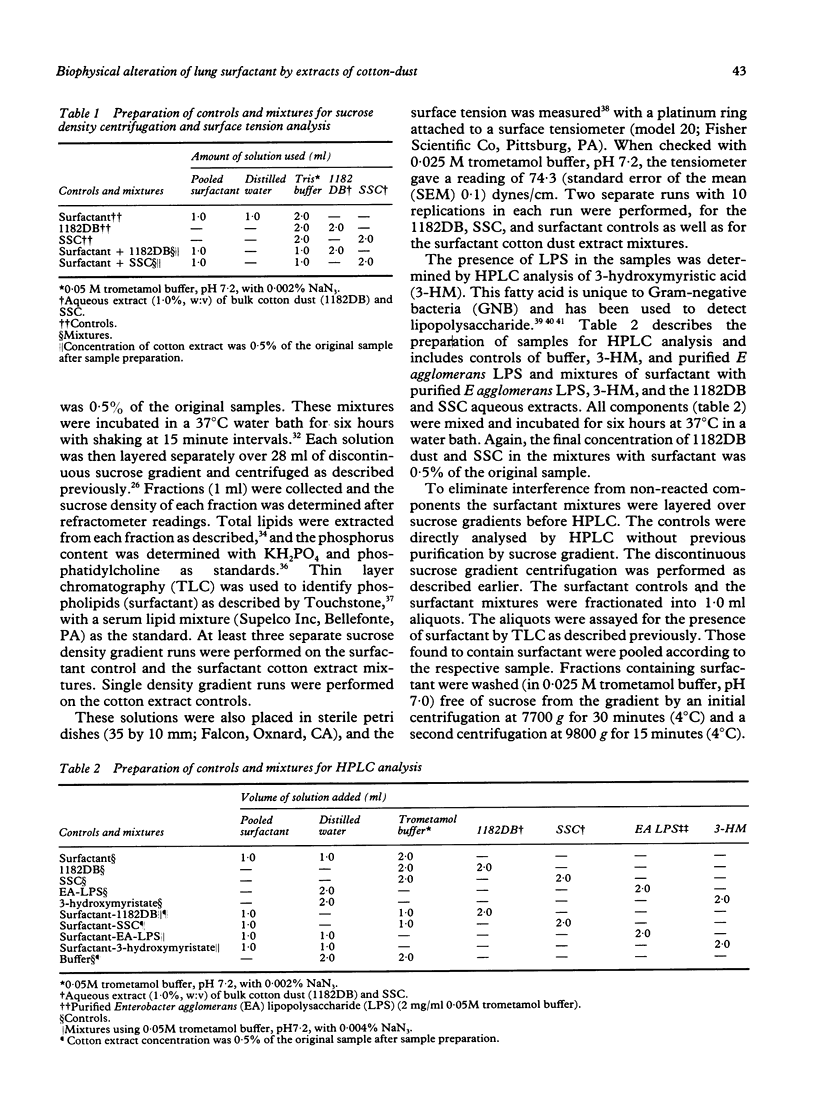
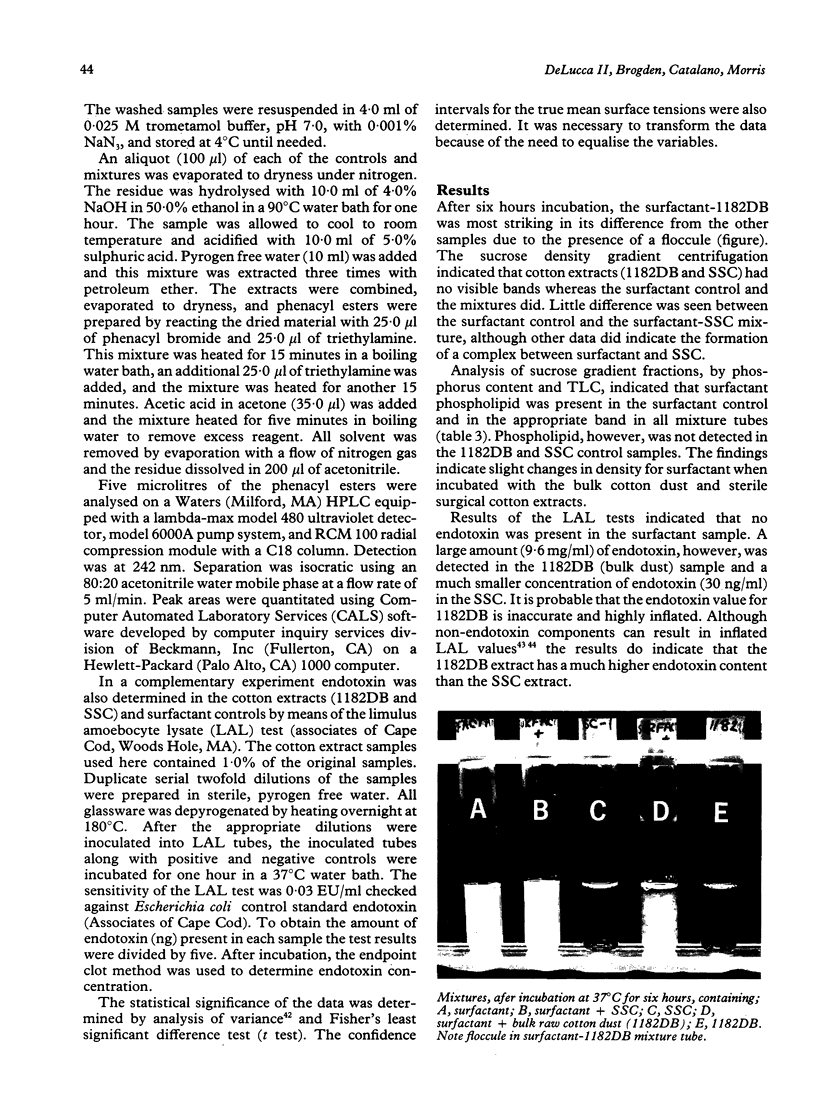
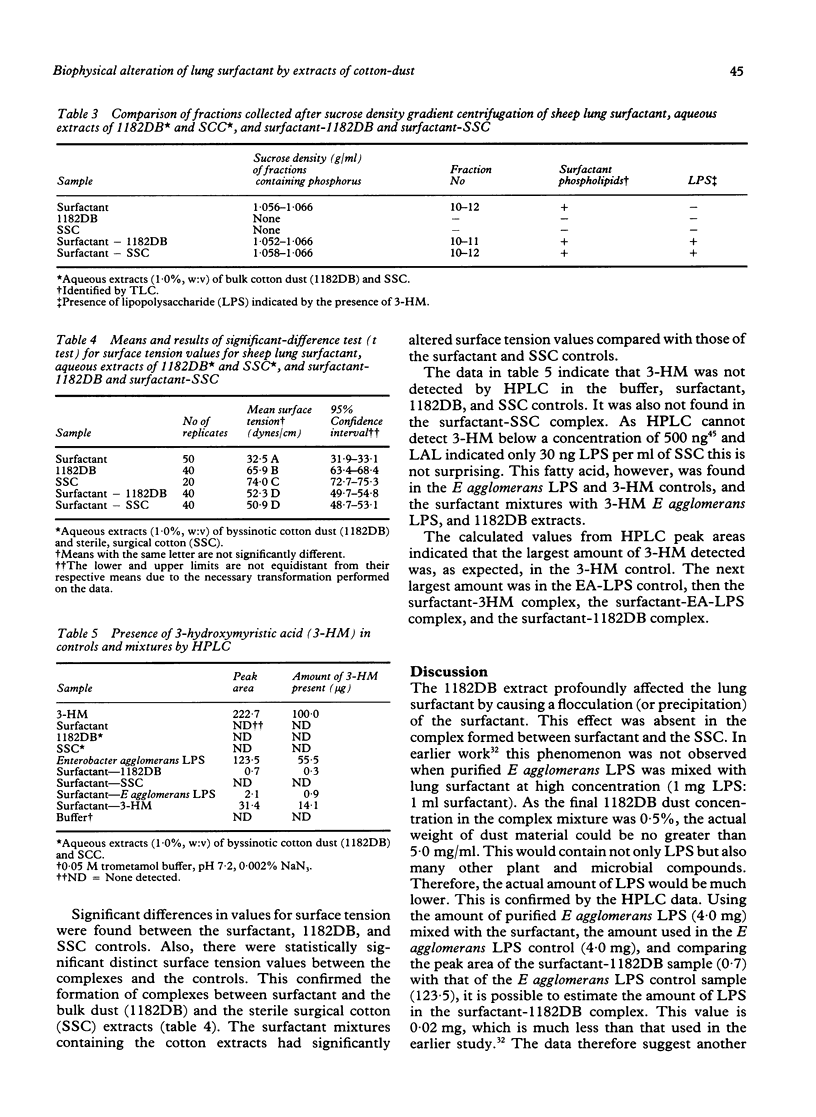
Abstract
Byssinosis, a lung disease that can affect cotton mill workers, may be caused in part by lipopolysaccharides (LPS) from Gram negative bacteria. In vitro, LPS complexes with sheep lung surfactant (SLS). To determine whether LPS in extracts of cotton dust alters the biophysical characteristics of lung surfactant, aqueous extracts (1.0% w:v) of sterile surgical cotton (SSC) and a bulk raw cotton dust (1182DB) were prepared. Aliquots of the soluble extracts were incubated with SLS and studied by sucrose gradient centrifugation, surface tension analysis, and high pressure liquid chromatography (HPLC). The chromatography was employed to analyse for 3-hydroxymyristate (3-HM), a fatty acid indicating LPS. Also, purified Enterobacter agglomerans LPS and 3-HM as controls and as mixtures with SLS, were studied by HPLC. Sucrose gradient centrifugation showed that SLS-SSC, SLS-1182DB, and the SLS control had similar densities that differed from the remaining controls. The SLS-1182DB exhibited a floccule absent in the other samples. Surface tension values of SLS-SSC and SLS-1182DB differed significantly from all controls but only slightly from one another. 3-Hydroxymyristate was detected by HPLC in the 3-HM control, EA-LPS, SLS-EA-LPS, and SLS-1182DB, but not in SLS-SSC or the remaining controls. Apparently, 3-HM was below the HPLC detection range in SSC. The data indicate that LPS in the 1182DB, SSC and EA-LPS samples complexed with SLS. Floccule development in SLS-1182DB but not in SLS-EA-LPS suggests a further component(s) present in the bulk raw cotton dust, as well as LPS, which complexes with SLS. The data suggest that biophysical alterations to lung surfactant may play a part in the pathogenesis of byssinosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINS E. L., PEARCE J. W. Mechanisms of the renal response to plasma volume expansion. Can J Biochem Physiol. 1959 Jan;37(1):91–102. [PubMed] [Google Scholar]
- Albert R. K., Lakshminarayan S., Hildebrandt J., Kirk W., Butler J. Increased surface tension favors pulmonary edema formation in anesthetized dogs' lungs. J Clin Invest. 1979 May;63(5):1015–1018. doi: 10.1172/JCI109369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottoms G. D., Johnson M., Ward D., Fessler J., Lamar C., Turek J. Release of eicosanoids from white blood cells, platelets, smooth muscle cells, and endothelial cells in response to endotoxin and A23187. Circ Shock. 1986;20(1):25–34. [PubMed] [Google Scholar]
- Brogden K. A., Adlam C., Lehmkuhl H. D., Cutlip R. C., Knights J. M., Engen R. L. Effect of Pasteurella haemolytica (A1) capsular polysaccharide on sheep lung in vivo and on pulmonary surfactant in vitro. Am J Vet Res. 1989 Apr;50(4):555–559. [PubMed] [Google Scholar]
- Brogden K. A., Cutlip R. C., Lehmkuhl H. D. Complexing of bacterial lipopolysaccharide with lung surfactant. Infect Immun. 1986 Jun;52(3):644–649. doi: 10.1128/iai.52.3.644-649.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K. A., Rimler R. B., Cutlip R. C., Lehmkuhl H. D. Incubation of Pasteurella haemolytica and Pasteurella multocida lipopolysaccharide with sheep lung surfactant. Am J Vet Res. 1986 Apr;47(4):727–729. [PubMed] [Google Scholar]
- Brondz I., Olsen I. Determination of acids in whole lipopolysaccharide and in free lipid A from Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. J Chromatogr. 1984 Jun 8;308:19–29. doi: 10.1016/s0021-9673(01)87529-4. [DOI] [PubMed] [Google Scholar]
- Castellan R. M., Olenchock S. A., Hankinson J. L., Millner P. D., Cocke J. B., Bragg C. K., Perkins H. H., Jr, Jacobs R. R. Acute bronchoconstriction induced by cotton dust: dose-related responses to endotoxin and other dust factors. Ann Intern Med. 1984 Aug;101(2):157–163. doi: 10.7326/0003-4819-101-2-157. [DOI] [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Cinkotai F. F., Whitaker C. J. Airborne bacteria and the prevalence of byssinotic symptoms in 21 cotton spinning mills in Lancashire. Ann Occup Hyg. 1978 Dec;21(3):239–250. doi: 10.1093/annhyg/21.3.239. [DOI] [PubMed] [Google Scholar]
- DeLucca A. J., 2nd, Brogden K. A., Engen R. Enterobacter agglomerans lipopolysaccharide-induced changes in pulmonary surfactant as a factor in the pathogenesis of byssinosis. J Clin Microbiol. 1988 Apr;26(4):778–780. doi: 10.1128/jcm.26.4.778-780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucca A. J., 2nd, Palmgren M. S. Mesophilic microorganisms and endotoxin levels on developing cotton plants. Am Ind Hyg Assoc J. 1986 Aug;47(8):437–442. doi: 10.1080/15298668691390016. [DOI] [PubMed] [Google Scholar]
- Elin R. J., Wolff S. M. Biology of endotoxin. Annu Rev Med. 1976;27:127–141. doi: 10.1146/annurev.me.27.020176.001015. [DOI] [PubMed] [Google Scholar]
- Ellakkani M. A., Alarie Y., Weyel D., Karol M. H. Chronic pulmonary effects in guinea pigs from prolonged inhalation of cotton dust. Toxicol Appl Pharmacol. 1987 May;88(3):354–369. doi: 10.1016/0041-008x(87)90211-0. [DOI] [PubMed] [Google Scholar]
- Emerson R. J., Davis G. S. Effect of alveolar lining material-coated silica on rat alveolar macrophages. Environ Health Perspect. 1983 Sep;51:81–84. doi: 10.1289/ehp.835181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerts G., Henricks P. A., Slootweg P. J., Nijkamp F. P. Endotoxin-induced inflammation and injury of the guinea pig respiratory airways cause bronchial hyporeactivity. Am Rev Respir Dis. 1988 Jun;137(6):1441–1448. doi: 10.1164/ajrccm/137.6.1441. [DOI] [PubMed] [Google Scholar]
- George G., Hook G. E. The pulmonary extracellular lining. Environ Health Perspect. 1984 Apr;55:227–237. doi: 10.1289/ehp.8455227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg M. Muscular endurance and surface electromyogram in isometric and dynamic exercise. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jul;51(1):1–7. doi: 10.1152/jappl.1981.51.1.1. [DOI] [PubMed] [Google Scholar]
- Hallman M., Spragg R., Harrell J. H., Moser K. M., Gluck L. Evidence of lung surfactant abnormality in respiratory failure. Study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J Clin Invest. 1982 Sep;70(3):673–683. doi: 10.1172/JCI110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm B. A., Notter R. H., Siegle J., Matalon S. Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J Appl Physiol (1985) 1985 Nov;59(5):1402–1409. doi: 10.1152/jappl.1985.59.5.1402. [DOI] [PubMed] [Google Scholar]
- Jobe A., Ikegami M., Glatz T., Yoshida Y., Diakomanolis E., Padbury J. Duration and characteristics of treatment of premature lambs with natural surfactant. J Clin Invest. 1981 Feb;67(2):370–375. doi: 10.1172/JCI110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. M., Hanson M. N., Rohrbach M. S. Endothelial cell cytotoxicity of cotton bracts tannin and aqueous cotton bracts extract: tannin is the predominant cytotoxin present in aqueous cotton bracts extract. Environ Health Perspect. 1986 Apr;66:97–103. doi: 10.1289/ehp.866697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Harada K. Gelation of the amoebocyte lysate of Tachypleus tridentatus by cell wall digest of several gram-positive bacteria and synthetic peptidoglycan subunits of natural and unnatural configurations. Biken J. 1977 Mar;20(1):5–10. [PubMed] [Google Scholar]
- Maitra S. K., Nachum R., Pearson F. C. Establishment of beta-hydroxy fatty acids as chemical marker molecules for bacterial endotoxin by gas chromatography-mass spectrometry. Appl Environ Microbiol. 1986 Sep;52(3):510–514. doi: 10.1128/aem.52.3.510-514.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Brigham K. L. Acute effects of Escherichia coli endotoxin on the pulmonary microcirculation of anesthetized sheep structure:function relationships. Lab Invest. 1983 Apr;48(4):458–470. [PubMed] [Google Scholar]
- Morris N. M., Catalano E. A., Berni R. J. 3-Hydroxymyristic acid as a measure of endotoxin in cotton lint and dust. Am Ind Hyg Assoc J. 1988 Feb;49(2):81–88. doi: 10.1080/15298668891379431. [DOI] [PubMed] [Google Scholar]
- O'Neill S., Lesperance E., Klass D. J. Rat lung lavage surfactant enhances bacterial phagocytosis and intracellular killing by alveolar macrophages. Am Rev Respir Dis. 1984 Aug;130(2):225–230. doi: 10.1164/arrd.1984.130.2.225. [DOI] [PubMed] [Google Scholar]
- PERNIS B., VIGLIANI E. C., CAVAGNA C., FINULLI M. The role of bacterial endotoxins in occupational diseases caused by inhaling vegetable dusts. Br J Ind Med. 1961 Apr;18:120–129. doi: 10.1136/oem.18.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsonk E. L., Olenchock S. A., Castellan R. M., Banks D. E., Mull J. C., Hankinson J. L., Bragg K. C., Perkins H. H., Cocke J. B. Human ventilatory response to washed and unwashed cottons from different growing areas. Br J Ind Med. 1986 Mar;43(3):182–187. doi: 10.1136/oem.43.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S. A. Lung surfactant. Environ Health Perspect. 1984 Apr;55:205–226. doi: 10.1289/ehp.8455205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S. A. The surfactant system and lung phospholipid biochemistry. Am Rev Respir Dis. 1985 Mar;131(3):439–460. doi: 10.1164/arrd.1985.131.3.439. [DOI] [PubMed] [Google Scholar]
- Rylander R., Beijer L. Inhalation of endotoxin stimulates alveolar macrophage production of platelet-activating factor. Am Rev Respir Dis. 1987 Jan;135(1):83–86. doi: 10.1164/arrd.1987.135.1.83. [DOI] [PubMed] [Google Scholar]
- Rylander R. Organic dusts and lung reactions--exposure characteristics and mechanisms for disease. Scand J Work Environ Health. 1985 Jun;11(3 Spec No):199–206. doi: 10.5271/sjweh.2234. [DOI] [PubMed] [Google Scholar]
- Rylander R., Schilling R. S., Pickering C. A., Rooke G. B., Dempsey A. N., Jacobs R. R. Effects after acute and chronic exposure to cotton dust: the Manchester criteria. Br J Ind Med. 1987 Sep;44(9):577–579. doi: 10.1136/oem.44.9.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTNICK A. I., SOLOFF L. A. ATELECTASIS WITH PNEUMONIA: A PATHOPHYSIOLOGIC STUDY. Ann Intern Med. 1964 Jan;60:39–46. doi: 10.7326/0003-4819-60-1-39. [DOI] [PubMed] [Google Scholar]
- Schwartz L. W., Christman C. A. Alveolar macrophage migration. Influence of lung lining material and acute lung insult. Am Rev Respir Dis. 1979 Aug;120(2):429–439. doi: 10.1164/arrd.1979.120.2.429. [DOI] [PubMed] [Google Scholar]
- Snella M. C., Rylander R. Endotoxin inhalation induces neutrophil chemotaxis by alveolar macrophages. Agents Actions. 1985 Sep;16(6):521–526. doi: 10.1007/BF01983657. [DOI] [PubMed] [Google Scholar]
- TUFFNELL P. The relationship of byssinosis to the bacteria and fungi in the air of textile mills. Br J Ind Med. 1960 Oct;17:304–306. doi: 10.1136/oem.17.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek T. J., Jr, Gundel R. H., Wegner C. D., Schachter E. N., Buck M. G. Acute pulmonary response to cotton bract extract in monkeys: lung function and effects of mediator modifying compounds. Lung. 1988;166(1):25–31. doi: 10.1007/BF02714026. [DOI] [PubMed] [Google Scholar]



