ABSTRACT
The biology of a cell, whether it is a unicellular organism or part of a multicellular network, is influenced by cell type, temporal changes in cell state, and the cell’s environment. Spatial cues play a critical role in the regulation of microbial pathogenesis strategies. Information about where the pathogen is—in a tissue or in proximity to a host cell—regulates gene expression and the compartmentalization of gene products in the microbe and the host. Our understanding of host and pathogen identity has bloomed with the accessibility of transcriptomics and proteomics techniques. A missing piece of the puzzle has been our ability to evaluate global transcript and protein expression in the context of the subcellular niche, primary cell, or native tissue environment during infection. This barrier is now lower with the advent of new spatial omics techniques to understand how location regulates cellular functions. This review will discuss how recent advances in spatial proteomics and transcriptomics approaches can address outstanding questions in microbial pathogenesis.
KEYWORDS: host-pathogen interactions, microbial pathogenesis, spatial proteomics, spatial transcriptomics
INTRODUCTION
Location is a critical regulatory node of cell biology during microbial pathogenesis at every “order of magnitude” from molecular interactions to tissue microenvironments. For instance, Gram-negative bacterial lipopolysaccharide induces a distinct Toll-like receptor 4 (TLR4) signaling complex at the cell surface compared to the endosome (1, 2). Apicomplexan parasites use unique secretory organelles to deliver virulence effectors, forming novel compartments for intracellular growth (3). Shigella and Listeria co-opt host actin, polymerizing “tails” that mediate host cell escape (4). In biofilms, bacterial gene expression is a function of their position within a colony (5). Moreover, many pathogens exploit specialized tissue environments with unique cell architectures that cannot be replicated in vitro, such as gastric infection by Helicobacter pylori or fibrotic remodeling of the lung during helminth infection (5, 6).
In the last decade, spatial proteomics tools have become accessible, followed closely by an expansion of spatial transcriptomics methods. These tools were primarily developed to study eukaryotic cell biology, and repurposing them to study microbial pathogenesis often requires unique adaptations, described here. Comparatively mature methods, including cell fractionation and proximity-labeling techniques are being creatively applied to address molecular, organellar, and cell type-specific changes during infection. However, most pathogenic microbes are not genetically tractable and infect non-model organisms or tissues that are difficult to access or reconstitute in vitro (Fig. 1). Exploring the biology of microbial pathogens with limited tools for experimental manipulation has required new tools to study microbes in their native environment, a need that has been met with the emergence of imaging-based transcriptomics and proteomics tools.
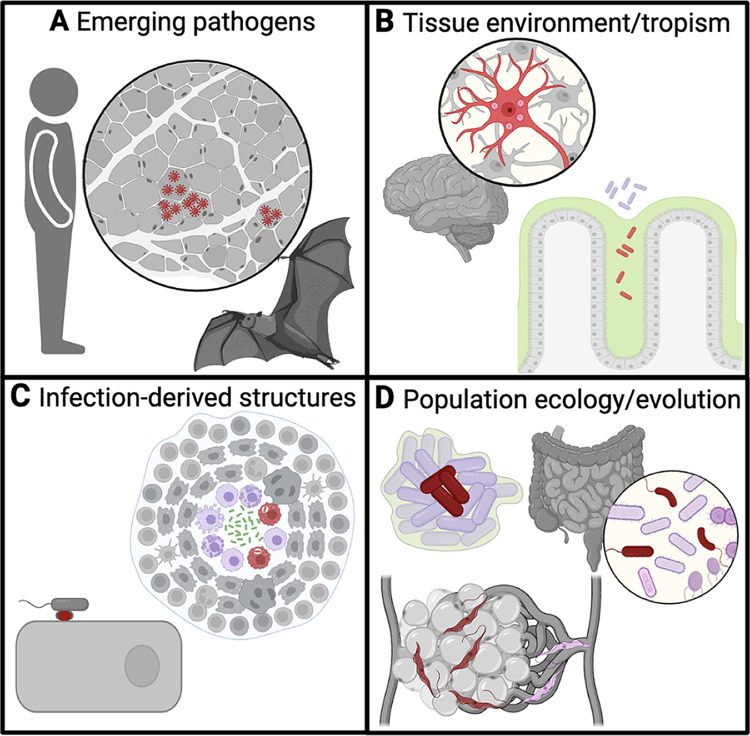
FIG 1.
Questions in microbial pathogenesis to approach with spatial omics tools. (A) Pathogens that lack model organisms or tools for experimental manipulation (e.g., a virus recently transferred to a zoonotic host); (B) studying the biology of infected primary cell types or tissues with complex architecture that are poorly modeled in vitro (e.g., infected neurons or bacteria encroaching on intestinal mucus [green]); (C) understanding the constituents of novel structures that form during infection like a bacterial pedestal or granuloma (green core); (D) evaluating subpopulations of microorganisms (e.g., bacteria deep within versus peripheral to a biofilm [green], a constituent of the gut commensal population, and tissue-resident versus circulating parasites). Red indicates a population of interest, and purple indicates nontargeted. The figure was generated by BioRender.
CELL FRACTIONATION APPROACHES TO STUDY PATHOGEN ORGANELLES AND MICROBIAL MANIPULATION OF HOST CELL COMPARTMENTS
Cell fractionation by differential centrifugation was introduced in the 1930s by Bensley and Hoerr, who first separated mitochondria and microsomes from insoluble cell debris (7). In the 1940s, pairing fractionation with electron microscopy allowed biochemists to associate the biochemical functions of organelles with the structure and location in the cell (8). These approaches continue to be important to define the unique organelle biology of parasitic protozoa (9), study bacterial outer membrane, inner membrane, cytoplasm, and periplasmic compartments (10, 11), and enrich stress granules as well as viral inclusion bodies from infected host cells (12). This approach coupled with liquid chromatography-mass spectrometry (LC-MS) has been applied to understand how macrophage and dendritic cell phagosomes are modified by Mycobacterium infection and how Legionella manipulates Dictyostelium phagocytosis (9, 13–15). However, the main drawbacks of differential centrifugation are that endolysosomal system components can elute across many density fractions, and it is impossible to separate small transport vesicles of similar density, which necessitates secondary validation of host protein recruitment.
Magnetic purification of microbe compartments.
Magnetic purification has been a notable solution to vesicle contamination issues inherent to density centrifugation. David Russell’s lab identified Mycobacterium effectors that arrest phagosome maturation by loading the phagolysosome pathway with iron dextran and magnetically isolating the vacuole after gentle permeabilization of the plasma membrane (16). Streptococcus pyrogens have been magnetically labeled prior to uptake by human neutrophils and used to isolate bacterium-containing phagosomes (17). Robust protocols have been developed to isolate magnetotactic bacteria based on their membrane-encased ferromagnetic nanoparticles, known as magnetosomes, and Plasmodium based on hemozoin in the feeding organelle (18–21). Hemozoin is sufficiently magnetic to separate infected and uninfected red blood cells. This approach was combined with phospho-proteomics analysis to identify 18 FIKK serine/threonine kinases used by Plasmodium falciparum but not by Plasmodium knowlesi to phosphorylate erythrocyte target proteins and secreted parasite virulence factors (22). Despite the robustness of magnetic isolation approaches, there has not been widespread use of these tools to isolate pathogen-containing vacuoles for omics analysis, an outstanding question for many obligate intracellular protozoan and bacterial pathogens.
LOPIT-mediated cell fractionation.
To circumvent fraction contamination issues, the Lilley Lab developed an experimental pipeline and informatics tool to infer high-likelihood organelle resident proteins. In LOPIT (localization of organelle proteins by isotope tagging) subcellular fractions are isotopically labeled and pooled into a single LC-MS sample, limiting run-to-run variability. Organelle proteomes are mapped by comparing the abundance of known organelle-resident proteins in each fraction to the abundance of every protein across each fraction in the sample using Bayesian analysis (23, 24). LOPIT has been used to quantify human cytomegalovirus (CMV) proteins localized to fibroblast organelles over a 120-h infection (25). However, the number of fractions that can be reliably measured by LOPIT is limited by the number of stable isotopes. As an alternative, “label-free” quantification approaches, where run-to-run MS variability is normalized informatically, have been developed (26–28).
In 2017, HyperLOPIT was released, where 10 fractions can be pooled and proteins are identified with higher-sensitivity MS and mapped to organelles using a multivariable machine learning tool (29). This technique is particularly well suited to understand the cellular architecture of apicomplexan parasites, which have a mixture of organelles used by euthermic eukaryotes (e.g., mitochondria, nucleus, endoplasmic reticulum [ER]), red algae (plastids), and evolutionarily distinct secretory organelles that deliver effectors into the host cytosol. For example, HyperLOPIT was used to map a high-coverage Toxoplasma gondii proteome to 25 functionally distinct parasite organelles or organelle regions (30). HyperLOPIT protein localization data sets are also proving exceptionally useful to predict whether proteins identified by coprecipitation or proximity labeling belong to a specific subcellular region, as described in the following section (29, 31).
ENZYMATIC PROXIMITY LABELING TOOLS FOR LOCAL PROTEIN AND TRANSCRIPT ENRICHMENT
Proximity labeling techniques use a label-targeting (bait) protein conjugated to an enzyme to decorate near-neighbor molecules with affinity purification tags. Decorated proteins or nucleic acids are then purified and identified by LC-MS or sequencing, respectively. These tools were initially developed to assess protein localization by immunofluorescence microscopy, enhance contrast in electron microscopy, and mediate click chemistry in live cells (32, 33), but are now proving robust techniques for spatial omics analysis. Each enzyme has unique considerations that necessitate evaluation during the experimental design phase (Table 1).
TABLE 1.
Current methods for spatial proteomics
| Tool (reference) | Isolation method | Labeling radius | Labeling time | Sample type | Applications |
|---|---|---|---|---|---|
| BioID (35–38, 41, 42) | Bait protein-conjugated BirA biotinylation | 40 nm | 18 h | Live cells | Protein-protein interactions, organelle proteomics |
| BioID2 (48, 49, 54, 55) | |||||
| TurboID (48, 53, 54) | 10 nm | 10 min | |||
| MiniTurbo (54, 55) | |||||
| Considerations | |||||
| Labeling kinetics and cytotoxicity: biotin-phenol labeling is less cytotoxic than H2O2 labeling used for APEX; BioID2 requires 10× less biotin-phenol than BioID, reducing cytotoxicity but is most efficient at 37°C; TurboID is 2× faster than miniTurbo, but associated with high background and endogenous biotin depletion toxicity | |||||
| Steric hinderance and stability: steric hinderance is an issue for BioID (36 kDa) and minimal for BioID2 (26 kDa), TurboID (35 kDa), and Miniturbo (28 kDa); miniTurbo is unstable | |||||
| Protein targeting: protein targeting requires genetic modification of a “bait” protein; best suited for cell lines | |||||
| APEX (56, 61) | Bait protein-conjugated ascorbate peroxidase monoavidin | 120 nm | 0.5–1 h | Live/fixed cells | Protein-protein interactions, organelle proteomics, in vivo proteomics |
| APEX2 (48, 51–53) | 20 nm | Live/fixed cells, in vivo labeling | |||
| Considerations | |||||
| Labeling kinetics and cytotoxicity: H2O2 labeling is more cytotoxic than biotin-phenol labeling (bioID); APEX2 has high activity at lower H2O2; APEX2 can be used for in vivo proteomics and transcriptomics | |||||
| Protein targeting: protein targeting requires genetic modification of “bait” protein; best suited for cell lines | |||||
| TSA-BAR (70, 71, 73) | Antibody-conjugated tyramide biotinylation | 500 nm | 10 min | Live/fixed cells, tissue | Protein-protein interactions, organelle proteomics |
| Considerations | |||||
| Pros: antibody-based tagging is ideal for studying primary cells, tissues, and organisms with limited genetic tools | |||||
| Cons: fixation and permeabilization required for intracellular structures | |||||
| HRP (62) | Bait protein-conjugated HRP | 300 nm | 60 min | In situ | Cell surface, in vivo proteomics |
| Considerations | |||||
| Pros: optimized for surface antigens, live-cell labeling, and in vivo labeling | |||||
| Cons: HRP labeling is incompatible with acidic or highly reducing organelle environments | |||||
| HyperLOPIT (29, 30) | Subcellular fractionation | NAa | NA | Lysed cells | Organelle proteomics |
| Considerations | |||||
| Pros: fractionation links protein sequence information with biochemical properties of organelle | |||||
| Cons: limited to 10 isotopically labeled fractions | |||||
| LCM (81, 83, 84, 89) | Image-targeted laser ablation/polymerization | 10 μm | 3 h | Live/fixed tissue | In situ cell proteomics |
| Considerations | |||||
| Pros: primary tissue compatible; user validates target region by imaging; compatible with fixed samples | |||||
| Cons: low protein yield/coverage; cannot resolve single-cell borders; labor- and time-intensive | |||||
| DVP (94) | AI image-targeted laser ablation | 10 μm | ≥3 h | Fixed tissue | In situ cell proteomics |
| Considerations | |||||
| Pros: utilizes AI to reduce human imaging bias; automation increases throughput | |||||
| Cons: labor-intensive; requires custom equipment | |||||
| autoSTOMP (95–97) | Imaging-guided UV biotinylation | ~1 μm | 72 h | Fixed cells and tissue | Organelle, cell, in situ proteomics |
| Considerations | |||||
| Pros: primary cell/tissue compatible; can resolve organelles; user validates target region by imaging | |||||
| Cons: requires fixation; low protein yield/coverage; time-intensive |
NA, not applicable.
Promiscuous biotin ligases: BioID, TurboID, and miniTurbo.
Biotin identification (BioID) evolved from a technique to evaluate protein-genome interactions using DNA adenine methyltransferase called DAM-ID (34). In BioID the “bait” protein of interest is fused to BirA, a promiscuous biotin ligase derived from Escherichia coli. BirA biotinylates proteins within a 10-nm radius upon the introduction of exogenous biotin (35). Fusing BirA to the host SNARE syntaxin-6 showed that the signal sequence YGRL, which regulates plasma membrane protein recycling to the trans-Golgi network, is manipulated by Chlamydia for vesicle delivery to the inclusion body (36) (Fig. 2A). The nuclear localization of the Chlamydia type II secretion system effector SINC (secreted inclusion protein-C) and its proximity to the nuclear lamin protein emirin were also determined by BioID and confirmed by coprecipitation (37) (Fig. 2B, panel i). In this study, 22 biotinylated proteins were conserved across BioID replicate experiments; however, over 260 proteins were unique to each replicate, consistent with high background labeling in this approach.
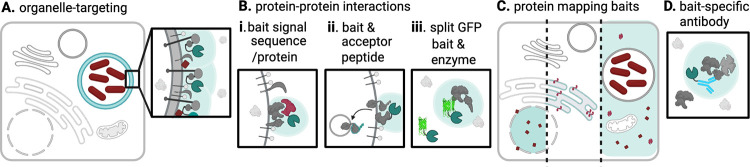
FIG 2.
Predicting molecular interactions and subcellular localization by enzyme-mediated proximity ligation with the APEX, BioID, and TSA-BAR approaches. (A) The labeling enzyme (teal Pac-Man) is conjugated to a bait protein (gray) or signal sequence that is abundant and robustly localized to a target organelle to label resident proteins or nucleic acids (light aqua region), here depicting a microbial (red) vacuole. (B) A bait protein is enzyme conjugated to label any nearby molecules (i), such as a target protein with an engineered acceptor peptide (ii), using split GFP (green) where the larger N terminus of GFP is conjugated to the enzyme under inducible control and the smaller C terminus is conjugated to the bait protein (iii). (C) In protein mapping approaches, cell lines are generated where each expresses a label-targeting protein (described in panel A) and comparative proteomics is used to map host and/or microbial protein abundance in each compartment. (D) In TSA-BAR and nanobody-APEX, a label-targeting enzyme is targeted via an antibody (blue) raised against a specific bait antigen. The figure was generated by BioRender.
BioID was used to understand the functional significance of evolutionarily divergent protozoan cytoskeletal structures. The hook complex (or bilobe) is a cytoskeletal structure at the distal flagellar pocket in kinetoplastids. By conjugating BirA to the Trypanosoma brucei protein TbMORN, 7 components of the hook complex were identified, including two flagellum attachment points (38). In apicomplexan parasites, the inner membrane complex (IMC) is a series of flattened membrane sacks between the inner leaflet of the parasite plasma membrane and a network of intermediate filaments, which is essential for motility, invasion, and division (39). Fusing BirA to the T. gondii IMC protein ISP3 identified 17 proteins partitioned to distinct regions of the IMC, including two apical cap proteins, AC1 and AC2 (40). BirA-AC2 was generated to identify a family of apical cap proteins that bridge the IMC with the plasma membrane and intersect with mitogen-activated-protein-kinase signaling, nicely demonstrating how proximity labeling can be used in iterative cycles to refine protein interaction maps (41–43).
BioID has helped elucidate transient interactions controlling viral entry and budding. Using the human immunodeficiency virus (HIV) protein Vpu as bait led to the discovery that Vpu promotes viral shedding by interacting with AP1 and the lipid raft protein tetherin (44). To assess whether the cooperation of herpes simplex virus glycoproteins was necessary for entry, researchers coexpressed gD-BirA with gH linked to a biotin acceptor peptide (45) (Fig. 2B, panel ii). The gH acceptor peptide was effectively biotinylated; however, a negative-control glycoprotein from Epstein-Bar virus (EBV) was too. This result highlights limitations of BioID related to the overexpression of the bait protein and 18-h labeling (Table 1), which can report artifact interaction at sites of protein maturation (ER) and turnover (lysosome). A third caveat of BioID is labeling steric hindrance imparted by the 321 amino acid BirA enzyme. For example, when BirA was fused to discrete sites on HIV group-specific antigen (Gag) or the Gag precursor, AEG-1 (astrocyte-elevated-gene-1) was the only protein conserved between experiments (46, 47).
Steric hindrance was addressed with BioID2, a 233-amino-acid BirA homolog from the thermophile Aquifex aeolicus. BioID2 requires a log less biotin but is limited to temperatures above 37°C and susceptible to background labeling from endogenous biotin (48). A split-green fluorescent protein (GFP)-BioID2 system (Fig. 2B, panel iii), in which an inducible BirA conjugated to the GFP N terminus is coexpressed with a C-terminal GFP-bait protein (49), has been used to discover innate immune signaling components activated by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protease nonstructural protein-5 (NSP5) in compared to an inactive NSP5 allele (50). In this report, over 2,000 labeled host proteins were prioritized by cross-referencing genome-wide-association studies’ data sets and applying Significance Analysis of INTeractome (SAINT) to calculate the probability that a candidate protein interacts with the bait based on confidence intervals generated from the abundance of known interaction partners in the data set (51). This approach may be useful to determine the localization and interaction partners of secreted microbial effectors.
Using a directed-evolution approach, the Ting lab has addressed the lengthy labeling kinetics of BirA with two additional tools. TurboID is a 35-kDa BirA protein with 15 amino acid modifications, and miniTurbo is an N-terminal truncation of TurboID (28 kDa) with 13 amino acid changes (52). TurboID and miniTurbo have 10-min minimal labeling times with 3- and 6-fold-higher labeling efficiency than BioID after 6 h. However, turboID is so efficient that it can cause cytotoxic sequestration of endogenous biotin, high background labeling, and bait protein instability (53) (Table 1). Some bait proteins are not equally amenable to tagging by both enzymes, as reported in a study that used miniTurbo or turboID to label all but three SARS-CoV-2 proteins, enabling network analysis of signaling cascades cooperatively regulated by multiple viral proteins (54). miniTurbo facilitated the first high-coverage proteome of the apicomplexan vacuole membrane, a novel compartment formed independently of the phago/endosomal system and which cannot be isolated by fractionation. To achieve this, miniTurbo was fused to the arginine-rich amphipathic helix (RAH) domain of the secreted T. gondii effector rop17. Remarkably, when ectopically expressed in human cells, BirA-RAH was selectively localized to the parasite vacuole membrane following infection, providing a high-coverage proteome of host and parasite proteins at the vacuole (3).
Ascorbate peroxidases: APEX and APEX2.
The second major genetically encoded proximity labeling technique uses ascorbate peroxidase (APEX). In the presence of hydrogen peroxide (H2O2), APEX oxidizes supplemented biotin-phenols to phenoxyl radicals, which label nearby nucleic acids or proteins until quenched. Biotin-phenol and H2O2 are both cytotoxic, but the APEX reaction time is brief (Table 1). H2O2 also inhibits APEX function; however, this was circumvented in APEX2 by two activity-enhancing mutations that also reduced the labeling radius to 20 nm (55, 56). APEX2 has revealed that SUMO regulates stress granule disassembly when conjugated to three bait proteins: G3BP1, HMR1, and FXR1 (57, 58). Repurposing this tool to study viral elementary bodies may be possible, given the structural similarities between these membrane-free organelles. For example, to identify proteins recruited to the poliovirus replication site the C terminus of enterovirus Golgi apparatus-specific-BFA-resistance-factor-1 tagged with APEX2 recruited to poliovirus inclusion bodies and labeled glycolytic enzymes that regulate de novo RNA synthesis, as well as RNA binding inhibitors of viral replication (59).
APEX2 labeling can be limited by protein tertiary and quaternary structures and transmembrane topology. However, membrane steric inhibition can be exploited to control spatial resolution (Fig. 2C) to identify structures like the inner mitochondrial membrane proteome. An analogous approach with horseradish peroxidase (HRP) chemistry identified the neuronal cell surface proteome (60, 61). Linking APEX2 to minimal localization motifs is proving a valuable tool to understand trafficking of secreted microbial effectors (Fig. 2A). APEX2 expressed in the host nucleus labeled a T. gondii modulator of the NCoR/SMRT repressor complex called TgNSM, which cooperates with a second effector, TgIST, to limit protein kinase R activation of cell death (62). The Seeliger lab adapted APEX2 labeling to visualize and isolate proteins enriched in the Mycobacterium tuberculosis cytoplasm or periplasmic space using click chemistry with tyramide-alkyne and tyramide-azide probes rather than biotin-phenol (63) (Fig. 2B, panel i).
Using a conceptually similar approach, RNA-GPS has been developed to isolate and sequence transcripts labeled by APEX2 targeted to discrete areas of the cell (64, 65) (Fig. 2C). This approach was used to evaluate regional amplification of the SARS-CoV-2 genome to eight regions, demonstrating an unexpected localization to the host mitochondria in addition to the nucleolus (66). The Ingolia lab paired APEX2-eIF4A1 RNA sequencing with LC-MS to show that this dead box helicase interacts with the ribosomal 43S preinitiation complex and stress granules when mTORC is inhibited (65).
In another advance of APEX, E. coli has been engineered to express APEX-nanobody fusion proteins with antigenic specificity for Ebola and Marburg viruses (Fig. 2D). A proof-of-concept study demonstrated that APEX-nanobodies can amplify the immunofluorescence signal from infected cells (67). This approach could be applied to a range of primary cells and non-model pathogens with limited tools for genetic manipulation, similar to TSA-BAR (tyramide signal amplification-biotinylation by antibody recognition) described below.
TSA-BAR.
In tyramide signal amplification-biotinylation by antibody recognition (TSA-BAR), a primary antibody specific to the target (molecule, organelle, or cell) is detected by a secondary antibody conjugated to HRP (Fig. 2D). In the presence of H2O2, tyramide-biotin covalently attaches to nucleic acids and proteins within a 0.5-μm radius of the target antigen (68, 69). Tyramide chemistry is reported to label nucleic acids with less steric hindrance than proteins. TSA sequencing approaches have identified chromosome and transcript sequences and calculated the distance of these targets from nuclear speckles (using SON as bait) or the inner nuclear envelope (using laminins as bait) (70–73). While this technique has not been applied to viral infection, there is potential to address long-standing questions regarding the location of viral integration and regulators of viral latency and reactivation.
Pros and cons of enzyme-mediated proximity labeling approaches.
There is tremendous potential for this suite of host labeling-targeting proteins to advance our understanding of microbial pathogenesis in a range of infection settings. APEX2, TurboID, and miniTurbo have a similar labeling range (10 to 20 nm) and the highest spatial resolution of all available tools (Table 1). A central limitation of BioID- and APEX-derived techniques is that they require molecular engineering of a label targeting protein and cell transduction. This is a significant barrier for many primary cell types, non-model host organisms, and pathogens that lack robust tools for genetic manipulation (Fig. 1A and B). The labeling times required for APEX, BioID1 and -2, and HRP approaches are too long to capture the dynamics of processes like microbial entry, vacuole escape, or cell-to-cell spread. APEX2 has addressed this issue by reducing the labeling time, followed by TurboID and miniTurbo, which can label in as few as 10 min. Protocols to conditionally express these tools in Drosophila and mice are opening the door to evaluating host-pathogen interactions in primary cells and native tissue environments (74–76). However, these experiments are extremely cost- and labor-intensive. TSA-BAR ameliorates this limitation at a cost of spatial resolution.
A caveat of these approaches is that bait proteins require robust targeting to the organelle of interest. Proteins with alternative pools in the cell or that relocalize in response to signals like cell stress, inflammatory cytokines, and secretion system expression can confound the interpretation of results because labeling will occur at all sites. Bulky enzyme adducts may be particularly problematic in host-pathogen interaction: for example, to identify effectors injected via secretion systems. Additional controls may be required to evaluate infection biology, such as comparison with attenuated microbes or host cells deficient in immune sensing or cell death pathways. Finally, these approaches pool thousands of cells so additional approaches to isolate infected from infected cells may be necessary to reduce the noise of irrelevant interactions.
MICROSCOPY-MEDIATED SPATIAL OMICS TOOLS TO STUDY ORGANELLES AND CELL BIOLOGY IN SITU
Single-cell RNA sequencing and proteomics techniques are rapidly changing our understanding of host and microbial cell type specificity and functional heterogeneity (77–79). However, these tools fail to capture important spatial information about cells within their native tissue or colony microenvironment. Moreover, harsh enzymatic and physical disruption methods used for single-cell omics can lose sensitive cell types and alter mechanical signaling and gene expression. To address these issues, new technology at the interface of microscopic tissue evaluation and transcription or protein expression is under development.
In situ proteomics and transcriptomics with laser capture microdissection and deep visual proteomics.
Laser capture microdissection (LCM) uses a fluorescence microscope to image cell types or structures of interest and isolate them from a larger sample (80). Ablative LCM employs a UV laser to remove unwanted cells, while enrichment LCM uses the infrared (IR) light to selectively activate a thermolabile polymer in target cells, after which the cell composite is lifted off the slide for genomic, transcriptomic, and proteomic analyses (81–84) (Fig. 3A). Depending on target abundance, LCM collection time can exceed 2 h per sample, limiting the number of cells that can be acquired from fresh tissue slices (85, 86). Fixation extends the sample collection window but reduces the number of identifiable peptides. For these reasons, read depth and proteome/transcriptome coverage remain major challenges in LCM.
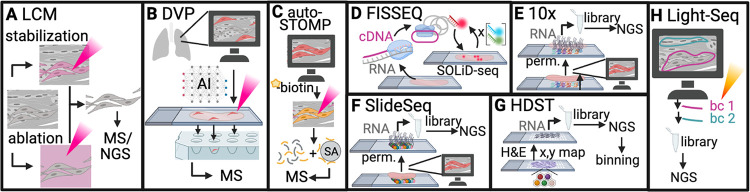
FIG 3.
Microscopy-mediated omics tools. (A) In LCM, structures of interest are imaged and stabilized or non-target structures are ablated in a photopolymer matrix. Cells are processed for RNA-seq or MS. (B) In DVP, an artificial intelligence platform identifies structures of interest and ablative LCM is used to isolate them for MS analysis. (C) In autoSTOMP, photo-biotin tags are conjugated to proteins in target structures using confocal microscopy and then purified for LC-MS. (D) In FISSEQ, cDNA synthesis and rolling circle amplification are used to identify transcripts in situ. (E and F) Slides containing a barcoded capture oligonucleotide array (10×/Visium [E]) or a bead array (SlideSeq [F]) are overlaid with tissue. Upon permeabilization, RNAs are captured for library prep in vitro and next-generation sequencing (NGS). (G) In HDST, a hexagonal well array containing capture beads is overlaid with tissue. RNAs are captured for in vitro library prep and NGS; however, the resolution is enhanced by binning reads from neighboring hexagonal wells. (H) In Light-seq, barcoded oligonucleotides are hybridized to RNAs for a cross-junctional synthesis reaction that facilitates cDNA amplification, library preparation and NGS. The figure was generated by BioRender.
Despite these caveats, LCM is an important approach to study host-microbe interactions, particularly for pathogens that infect non-model organisms or have tropisms that cannot be accurately recapitulated in vitro. For example, LCM-seq was used to determine that pig enterocytes infected by the swine pathogen Lawsonia intracellularis upregulate Rho GTPases compared to uninfected enterocytes in the same intestine (87). The Koshy lab employed LCM-seq to evaluate neurons that interacted directly with T. gondii by using a strain of parasite engineered to inject Cre-recombinase into zsGreen reporter mice (88). However, the dominant transcriptional signature identified belonged to CD8 T cells. This is in keeping with other reports showing LCM has insufficient resolution to capture complex cellular architecture and is best suited to study multicellular tissue structures (89–92).
Deep visual proteomics (DVP) combines LCM and machine learning to identify and isolate phenotypically distinct cells (Fig. 3B). This technique uses a custom scanning microscope with an LCM interface to image and excise cells of interest at a rate of 50 to 100 per hour. High-resolution MS and label-free quantitation are used to evaluate protein abundance (93). DVP has been used to analyze tumor cell heterogeneity in melanoma and salivary gland carcinoma, a methodology that could easily be extended to examine infected cell heterogeneity. Artificial intelligence (AI) image analysis has already expanded the capacity to discover and score infection-induced cell phenotypes beyond what a typical user can quantify (94). DVP could dramatically enhance spatial protein discovery, but the need for specialized microscopy hardware may hinder its rapid adoption for microbial pathogenesis research.
Organelle and cell enrichment in situ by autoSTOMP.
autoSTOMP (automated spatially targeted optical microproteomics) standard immunofluorescence staining and a confocal microscope have been used to visualize structures of interest and then selectively biotinylate proteins in those structures with a photochemical biotin tag for purification and identification by LC-MS (95) (Fig. 3C). The imaging and cross-linking can take 72 h per sample, but the process is automated. autoSTOMP has identified host and parasite proteins enriched on the T. gondii vacuole membrane and protein expression in tissue-resident immune cells (96, 97) (Table 1). Similar to LCM, image-based targeting means that autoSTOMP can be applied to primary cells and tissues. However, the throughput and resolution (~1 μm) are higher, so it is straightforward to modify the target region, based on colocalization stains, to capture subpopulations of a structure or control areas of the cell/tissue for comparative analysis. Although early in development, STOMP has the potential to evaluate difficult-to-isolate structures arising from microbial interactions, like viral inclusion bodies or actin tails used by Shigella and Listeria to escape host cells.
Sources of bias in spatial proteomics.
Spatial proteomics can be challenging to analyze with standard informatics tools. Differential enrichment pipelines generally assume full proteome coverage, but subcellular proteomics studies only capture a subset of the proteome by design. Moreover, low proteome coverage in LCM, DVP, and autoSTOMP can make it difficult to distinguish between proteins that are not expressed (true negatives) versus low-abundance proteins falling below the detection limit. For these reasons, imputing “missing values” with a standard value close to 0 is more likely to lead to false positives or invert fold change in spatial proteomic experiments compared to full-coverage proteomic experiments, and controls embedded in the experiment should be carefully evaluated early in the analysis.
Low coverage may be particularly problematic for the detection of pathogen proteins, which are often a minor fraction of a total host-pathogen proteome, and to resolve paralogous genes like the tandem repeat surface proteins used by Trypansosoma and Plasmodium species to evade antibody detection. Additionally, low-coverage compounds can have alignment errors due to posttranslational modifications, irreversible biochemical alterations (oxidative stress), and fixatives or detergents. It is likely that emerging enrichment strategies for single-cell proteomics (isotopically labeled carrier proteins) will also benefit the resolution of spatial proteomics.
Single-cell transcriptomics by FISSEQ.
Fluorescent in situ sequencing (FISSEQ) combines the spatial resolution of RNA-fluorescent in situ hybridization (FISH) with single-cell transcriptomics (98) (Fig. 3D). Samples are fixed and cDNAs are reverse transcribed in situ with rolling circle amplification. FISH probes are hybridized to the cDNA “nanoballs” to initiate SOLiD (sequencing by oligonucleotide ligation and detection) sequencing by ligation up to 27 times. Each sequencing reaction is aligned with the pixel coordinates of the FISH probe to map the transcriptional signature. When fibroblasts were targeted in a Drosophila embryo wound-healing model, 4,171 genes mapped to a 5-pixel area (pixel size in nanometers not reported) with 90% strand alignment. However, over 40% of reads aligned to rRNA. Most mRNA transcripts detected are highly expressed extracellular matrix proteins from fibroblasts, suggesting less abundant transcripts are likely missed due to limited read depth or signal crowding (99) (Table 2).
TABLE 2.
Current methods for spatial transcriptomics
| Method (reference) | RNA capture method | Labeling radius | Isolation time | Sample type | Application(s) |
|---|---|---|---|---|---|
| APEX2-seq (68) | In vitro RNA isolation and library prep | 20 nm | 1 h | Cell culture, in vivo labeling | Organelles, cells, in vivo transcriptomics |
| Considerations | |||||
| Pros: excellent spatial resolution, labeling time, and read depth | |||||
| Cons: requires genetic modification of “bait” protein; subcellular pools of bait protein cannot be resolved | |||||
| TSA-seq (78) | In vitro RNA isolation and library prep | 0.5 μm | 24 h | Live/fixed cells and tissue | Organelles, cells, in situ transcriptomics |
| Considerations | |||||
| Pros: good spatial resolution and excellent read depth; applicable to primary cells/tissues | |||||
| Cons: subcellular pools of bait cannot be resolved; steric hindrance from antibody-enzyme | |||||
| LCM (81, 83, 84, 89) | Image-targeted laser ablation/polymerization | 10 μm | 3 h | Live/fixed tissue | In situ cell proteomics |
| Considerations | |||||
| Pros: user validates target region by imaging | |||||
| Cons: low RNA yield/coverage; cannot resolve single cells borders; labor- and time-intensive | |||||
| FISSEQ (99) | In situ rolling circle amplification and SOLiD | 0.4 μm | 48 h | Fresh/frozen/fixed | In situ cell transcriptomics |
| Considerations | |||||
| Pros: user validates target region by imaging; good labeling radius with in situ cDNA synthesis | |||||
| Cons: very low mRNA coverage; reliably reports only most abundant transcripts | |||||
| 10×/Visium (100) | On-slide primer array and cDNA amplification | 55 μm | ≥2 h | Fresh/frozen tissue | In situ cell transcriptomics |
| Considerations | |||||
| Pros: user validates target region by imaging; commercial platform | |||||
| Cons: low coverage confounded by weak spatial resolution (5–20 cells) of on-slide barcode array; expensive | |||||
| Slide-seq (101) | On-slide bead array and SOLiD | 20 μm | ≥3 h | Fresh/frozen tissue | In situ cell transcriptomics |
| Considerations | |||||
| Pros: reasonable spatial resolution (1–2 cells); 62% of beads in array map to 1 cell; cost-effective | |||||
| Cons: no imaging; cell type position inferred from transcripts recovered; low mRNA coverage at 100–1,000 transcripts/bead | |||||
| HDST (102) | On-slide bead array and cDNA amplification | 13 μm | ≥3 h | Fresh/frozen tissue | In situ cell transcriptomics |
| Considerations | |||||
| Pros: good spatial resolution due to binning reads in hexagonal bead array (Lightseq > HDS > Slide-seq >> 10×/Visium); 86% of transcriptome identified across entire sample | |||||
| Cons: no imaging; cell type position inferred from transcripts recovered; low mRNA coverage at 1.3% of transcripts/bead | |||||
| Light-seq (103) | Light-directed barcoding and spatial indexing | 2 μm | ≥3 h | Fixed tissue | In situ cell transcriptomics |
| Considerations | |||||
| Pros: good spatial resolution due to light-conjugated barcoding (Light-seq > HDS > Slide-seq >> 10×/Visium); pooling 25 similar cells yields 85% coverage of transcriptome ~3,500 transcripts | |||||
| Cons: specialized microscopy and photolithography reagents needed |
Barcode and bead array sequencing using 10×/Visium, Slide-seq, and high-definition spatial transcriptomics.
10×/Visium has commercialized a competing technology in which a glass slide is arrayed with barcoded reverse transcription primers (100) (Fig. 3E). The tissue is overlaid, imaged, and permeabilized, and local RNAs hybridize to the primers. After cDNA synthesis, the tissue is removed, and a library is prepared in vitro for and next-generation sequencing (NGS). Despite its low spatial resolution, 55 μm in diameter or 5 to 20 cells, the MacLeod lab used the 10×/VisiumST (spatial transcriptomics) to show that brains chronically infected with T. brucei contained tissue-remodeling macrophages, follicular-like CD4+ T cells, and plasma cells in reticular network formations resembling tertiary lymphoid structures (101). Moreover, T. brucei expressing “slender” or “stumpy” phenotype transcripts were in discrete brain regions, supporting a model in which T. brucei exploits the circumventricular organs to enter the central nervous system. In Mycobacterium leprae infection, reversal reactions are associated with tuberculoid leprosy and bacterial clearance rather than lepromatous lesions, and the biology is poorly modeled in animals. 10×/VisiumST showed that skin biopsy specimens from patients undergoing reversal reactions contained granuloma macrophages expressing antimicrobial peptides, whereas biopsy specimens from disseminated lepromatous patients did not (102). In reovirus-infected hearts, this approach showed cytotoxic T cells undergoing pyroptotic cell death contribute to myocardial pathology (103). 10×/VisiumST has been used to identify tumor-resident bacterial families and develop a GeoMX spatial profiling antibody panel that showed that intratumor bacteria reside in a poorly vascularize microenvironment, containing immune cells with tissue repair markers and low T cell activation markers (104). These studies highlight the utility of spatial transcriptomics to evaluate host-pathogen interactions that depend on tissue architecture or occur in non-model organisms as well as the importance of secondary studies to validate the function of differentially enriched transcripts.
Bead arrays, initially developed to mediate single-cell sequencing, have emerged as a high-resolution solution for spatial transcriptomics at a near-single-cell level (Fig. 3F). In Slide-seq, 1.5 million barcoded 10-μm beads are arrayed on silicon-coated slides and then overlaid with a tissue section (105, 106). RNA capture, primer hybridization, and imaging are performed on the slide array using SOLiD chemistry like FISSEQ. In a pilot paper, 65.8% of beads were clearly identifiable as a single cell type, consistent with 10- to 20-μm resolution, where 100 to 1,000 transcripts mapped to beads, depending on the tissue assayed. This technique has been applied to assess immune cell identity and localization in damaged kidneys (107).
High-definition spatial transcriptomics (HDST) captures RNAs on ~3 million barcoded beads arrayed across 1.4 million hexagonal wells placed 2 μm apart on a silicon wafer (108) (Fig. 3G). Tissue sections are laid on the array, and RNAs are captured by barcoded primers on the bead surface. Sequential RNA hybridization with label decoder oligonucleotides generates a unique fluorescence “address” for each barcode prior to in vitro cDNA amplification and library preparation. While the coverage for each spatial barcode is low, at 1.3% of transcripts per bead, 86% of all genes were detected across the entire array (similar to 10×/Visium and FISSEQ). Each cluster of 24 wells containing the bead array is 13 μm (~one cell). However, the functional resolution of HDST is higher because transcripts belonging to every possible group of 24 wells are compared (binned) to informatically assess the transcriptionally similar and dissimilar wells representing a cell border (a concept borrowed from hexagonal confocal microscope detector arrays like Zeiss Airyscan). Vickovic and colleagues reported that HDST has a 1,400-fold-higher resolution than 10×/Visium but a 25-fold lower resolution than Slide-seq. This superior spatial resolution may be tremendously valuable for host interactions with extracellular pathogens.
Photoactivated barcoding for in situ transcriptomics by Light-seq.
Light-seq was developed to circumvent the limitations of bead array density and deconvolution by labeling cDNAs synthesized in situ with UV cross-linkable barcoded DNA oligonucleotides (109) (Fig. 3H). An advantage of this approach is that unique barcode sets can be used to study of multiple cell types in the same sample, a distinct advantage for microbial pathogenesis experiments comparing infected and uninfected cell types or even immune cells and extracellular eukaryotic pathogens in the same microenvironment. In mixed-cell-line experiments, as few as 25 cells per phenotype (barcode) could be differentially enriched at 85% coverage of the transcriptome, another advantage to study rare cell types in a mixed population or tissue. Cell-type-specific targeting in the retina led to the differential enrichment of 3,400 transcripts profiling as few as 91 to 1,112 cells per phenotype. Although this technique has not been directly compared to array approaches, it is four times more sensitive than single cell sequencing and has a resolution of 2 μm, approaching HDST.
Sources of bias in spatial transcriptomics.
Limited read depth is a central caveat for spatial transcriptomics that leads to a reporting bias toward abundant transcripts. APEX-seq and TSA-BAR are less susceptible to these issues because spatial transcripts from millions of cells are pooled. Confident evaluation of transcripts belonging to a specific cell type generally requires a median read depth of 1 million reads, depending on the frequency of a cell type in a population (110). Infection diversifies the cell phenotypes in the tissue niche, further compounding coverage per cell type and downstream analysis. Simply increasing read depth may not be sufficient to enhance spatial transcriptome coverage because “drop-out” events, where a transcript is not captured or amplified, can be caused by mRNAs or cDNAs competing for a limited number of hybridization events in a constrained space (111). The resolution of the hybridization array also influences transcriptome coverage. For example, the limited number of barcoded amplicons in FISSEQ is partially compensated for by the high spatial resolution of in situ rolling circle amplification, whereas the coverage of the 10×/Visium platform is impaired by the poor spatial resolution of the array. An additional consideration for bacterial pathogenesis is that these spatial transcriptomic tools have not been optimized to label and enrich prokaryotic transcripts.
CONCLUSIONS
Spatial omics techniques are opening the door to understanding molecular interactions between microbes and their host cells, novel structures formed during infection, cell complexity in situ, and pathogens with limited experimental tools. Adaptations to the proof-of-principle protocols, originally designed to study eukaryotic biology, will be necessary to isolate unique microbial structures (e.g., hyphal wall), resolve highly paralogous microbial gene products (e.g., Var genes), and detect low-abundance microbial sequences in infected samples. Moreover, dramatic changes in gene expression that occur during infection may require additional controls, such as attenuated microbial strains or host genetic controls, to narrow candidates to the relevant biology. The rapid advance of single-cell transcriptomics and proteomics will likely accelerate the resolution of spatial techniques that similarly suffer from limited material and coverage. Despite these caveats, spatial omics techniques have enormous potential to impact our understanding of the relationships between pathogens and their primary cell or native tissue environment. Addressing these questions in molecular pathogenesis will likely refine these emerging technologies as well.
ACKNOWLEDGMENTS
This work was funded by NIH R21AI156153 (S.E.E.), R35GM13831 (S.E.E.), 2T32AI007496-27A1 (S.L.), and 2T32AI007046-46 (D.M.).
We declare no conflict of interest.
Contributor Information
Sarah E. Ewald, Email: se2s@virginia.edu.
Karen M. Ottemann, University of California at Santa Cruz Department of Microbiology and Environmental Toxicology
REFERENCES
- 1.Ohnishi T, Muroi M, Tanamoto KI. 2003. MD-2 is necessary for the Toll-like receptor 4 protein to undergo glycosylation essential for its translocation to the cell surface. Clin Diagn Lab Immunol 10:405–410. doi: 10.1128/cdli.10.3.405-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohnishi H, Tochio H, Kato Z, Orii KE, Li A, Kimura T, Hiroaki H, Kondo N, Shirakawa M. 2009. Structural basis for the multiple interactions of the MyD88 TIR domain in TLR4 signaling. Proc Natl Acad Sci USA 106:10260–10265. doi: 10.1073/pnas.0812956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cygan AM, Jean Beltran PM, Mendoza AG, Branon TC, Ting AY, Carr SA, Boothroyd JC. 2021. Proximity-labeling reveals novel host and parasite proteins at the Toxoplasma parasitophorous vacuole membrane. mBio 12:e00260-21. doi: 10.1128/mBio.00260-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti PJ, Cossart P. 1999. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci 112:1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- 5.Amieva M, Peek RM. 2016. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weatherhead JE, Gazzinelli-Guimaraes P, Knight JM, Fujiwara R, Hotez PJ, Bottazzi ME, Corry DB. 2020. Host immunity and inflammation to pulmonary helminth infections. Front Immunol 11:594520. doi: 10.3389/fimmu.2020.594520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensley RR, Hoerr NL. 1934. Studies on cell structure by the freezing-drying method. V. The chemical basis of the organization of the cell. Anat Rec 60:251–266. doi: 10.1002/ar.1090600302. [DOI] [Google Scholar]
- 8.Ord MG, Stocken LA (ed). 1995. Foundations of biochemistry. Early adventures in biochemistry. Elsevier, Rochester, NY. [Google Scholar]
- 9.de Souza W, da Cunha-e-Silva NL. 2003. Cell fractionation of parasitic protozoa: a review. Mem Inst Oswaldo Cruz 98:151–170. doi: 10.1590/s0074-02762003000200001. [DOI] [PubMed] [Google Scholar]
- 10.Malherbe G, Humphreys DP, Davé E. 2019. A robust fractionation method for protein subcellular localization studies in Escherichia coli. Biotechniques 66:171–178. doi: 10.2144/btn-2018-0135. [DOI] [PubMed] [Google Scholar]
- 11.Thein M, Sauer G, Paramasivam N, Grin I, Linke D. 2010. Efficient subfractionation of Gram-negative bacteria for proteomics studies. J Proteome Res 9:6135–6147. doi: 10.1021/pr1002438. [DOI] [PubMed] [Google Scholar]
- 12.Singh A, Upadhyay V, Upadhyay AK, Singh SM, Panda AK. 2015. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact 14:1–10. doi: 10.1186/s12934-015-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. 2009. The phagosomal proteome in interferon-γ-activated macrophages. Immunity 30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Lee BH, Hanna J, King RW, Finley D. 2011. Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Mol Cell Proteomics 10:R110.003871. doi: 10.1074/mcp.R110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Zhang B. 2016. Integrative omics analysis reveals post-transcriptionally enhanced protective host response in colorectal cancers with microsatellite instability. J Proteome Res 15:766–776. doi: 10.1021/acs.jproteome.5b00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Russell DG. 2004. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc Natl Acad Sci USA 101:13642–13647. doi: 10.1073/pnas.0401657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lönnbro P, Nordenfelt P, Tapper H. 2008. Isolation of bacteria-containing phagosomes by magnetic selection. BMC Cell Biol 9:35. doi: 10.1186/1471-2121-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhu NN, Kowshik M. 2016. Techniques for the isolation of magnetotactic bacteria. J Microb Biochem Technol 8:188–194. doi: 10.4172/1948-5948.1000284. [DOI] [Google Scholar]
- 19.Rosenfeldt S, Mickoleit F, Jörke C, Clement JH, Markert S, Jérôme V, Schwarzinger S, Freitag R, Schüler D, Uebe R, Schenk AS. 2021. Towards standardized purification of bacterial magnetic nanoparticles for future in vivo applications. Acta Biomater 120:293–303. doi: 10.1016/j.actbio.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 20.Paul F, Roath S, Melville D, Warhurst DC, Osisanya JOS. 1981. Separation of malaria-infected erythrocytes from whole blood: use of selective high-gradient magnetic separation technique. Lancet 318:70–71. doi: 10.1016/S0140-6736(81)90414-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim TS, Kim HH, Lee SS, Na BK, Lin K, Cho SH, Kang YJ, Kim DK, Sohn Y, Kim H, Lee HW. 2010. Prevalence of Plasmodium vivax VK210 and VK247 subtype in Myanmar. Malar J 9:195. doi: 10.1186/1475-2875-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies H, Belda H, Broncel M, Ye X, Bisson C, Introini V, Dorin-Semblat D, Semblat J-P, Tibúrcio M, Gamain B, Kaforou M, Treeck M. 2020. An exported kinase family mediates species-specific erythrocyte remodelling and virulence in human malaria. Nat Microbiol 5:848–863. doi: 10.1038/s41564-020-0702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunkley TPJ, Watson R, Griffin JL, Dupree P, Lilley KS. 2004. Localization of organelle proteins by isotope tagging (LOPIT). Mol Cell Proteomics 3:1128–1134. doi: 10.1074/mcp.T400009-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Sadowski PG, Dunkley TPJ, Shadforth IP, Dupree P, Bessant C, Griffin JL, Lilley KS. 2006. Quantitative proteomic approach to study subcellular localization of membrane proteins. Nat Protoc 1:1778–1789. doi: 10.1038/nprot.2006.254. [DOI] [PubMed] [Google Scholar]
- 25.Jean Beltran PM, Mathias RA, Cristea IM. 2016. A portrait of the human organelle proteome in space and time during cytomegalovirus infection. Cell Syst 3:361–373.e6. doi: 10.1016/j.cels.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster LJ, de Hoog CL, Zhang Y, Zhang Y, Xie X, Mootha VK, Mann M. 2006. A mammalian organelle map by protein correlation profiling. Cell 125:187–199. doi: 10.1016/j.cell.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Sessler N, Krug K, Nordheim A, Mordmüller B, MacEk B. 2012. Analysis of the Plasmodium falciparum proteasome using Blue Native PAGE and label-free quantitative mass spectrometry. Amino Acids 43:1119–1129. doi: 10.1007/s00726-012-1296-9. [DOI] [PubMed] [Google Scholar]
- 28.Mund A, Brunner AD, Mann M. 2022. Unbiased spatial proteomics with single-cell resolution in tissues. Mol Cell 82:2335–2349. doi: 10.1016/j.molcel.2022.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Mulvey CM, Breckels LM, Geladaki A, Britovšek NK, Nightingale DJH, Christoforou A, Elzek M, Deery MJ, Gatto L, Lilley KS. 2017. Using hyperLOPIT to perform high-resolution mapping of the spatial proteome. Nat Protoc 12:1110–1135. doi: 10.1038/nprot.2017.026. [DOI] [PubMed] [Google Scholar]
- 30.Barylyuk K, Koreny L, Ke H, Butterworth S, Crook OM, Lassadi I, Gupta V, Tromer E, Mourier T, Stevens TJ, Breckels LM, Pain A, Lilley KS, Waller RF. 2020. A comprehensive subcellular atlas of the Toxoplasma proteome via hyperLOPIT provides spatial context for protein functions. Cell Host Microbe 28:752–766.e9. doi: 10.1016/j.chom.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geladaki A, Kočevar Britovšek N, Breckels LM, Smith TS, Vennard OL, Mulvey CM, Crook OM, Gatto L, Lilley KS. 2019. Combining LOPIT with differential ultracentrifugation for high-resolution spatial proteomics. Nat Commun 10:331. doi: 10.1038/s41467-018-08191-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howarth M, Ting AY. 2008. Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat Protoc 3:534–545. doi: 10.1038/nprot.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-Suárez M, Baruah H, Martínez-Hernández L, Xie KT, Baskin JM, Bertozzi CR, Ting AY. 2007. Re-directing lipoic acid ligase for cell surface protein labeling with small-molecule probes. Nat Biotechnol 25:1483–1487. doi: 10.1038/nbt1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Steensel B, Henikoff S. 2000. Identification of in vivo DNA targets of chromatin proteins using tethered Dam methyltransferase. Nat Biotechnol 18:424–428. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- 35.Kim DI, Birendra KC, Zhu W, Motamedchaboki K, Doye V, Roux KJ. 2014. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc Natl Acad Sci USA 111:E2453–E2461. doi: 10.1073/pnas.1406459111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabeiseman EJ, Cichos KH, Moore ER. 2014. The eukaryotic signal sequence, YGRL, targets the chlamydial inclusion. Front Cell Infect Microbiol 4:129. doi: 10.3389/fcimb.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mojica SA, Hovis KM, Frieman MB, Tran B, Hsia RC, Ravel J, Jenkins-Houk C, Wilson KL, Bavoil PM. 2015. SINC, a type III secreted protein of Chlamydia psittaci, targets the inner nuclear membrane of infected cells and uninfected neighbors. Mol Biol Cell 26:1918–1934. doi: 10.1091/mbc.E14-11-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morriswood B, Havlicek K, Demmel L, Yavuz S, Sealey-Cardona M, Vidilaseris K, Anrather D, Kostan J, Djinović-Carugo K, Roux KJ, Warren G. 2013. Novel bilobe components in Trypanosoma brucei identified using proximity-dependent biotinylation. Eukaryot Cell 12:356–367. doi: 10.1128/EC.00326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishi M, Hu K, Murray JM, Roos DS. 2008. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci 121:1559–1568. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen AL, Kim EW, Toh JY, Vashisht AA, Rashoff AQ, Van C, Huang AS, Moon AS, Bell HN, Bentolila LA, Wohlschlegel JA, Bradley PJ. 2015. Novel components of the Toxoplasma inner membrane complex revealed by BioID. mBio 6:e02357-14. doi: 10.1128/mBio.02357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen AL, Moon AS, Bell HN, Huang AS, Vashisht AA, Toh JY, Lin AH, Nadipuram SM, Kim EW, Choi CP, Wohlschlegel JA, Bradley PJ. 2017. Novel insights into the composition and function of the Toxoplasma IMC sutures. Cell Microbiol 19. doi: 10.1111/cmi.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Back PS, O'Shaughnessy WJ, Moon AS, Dewangan PS, Hu X, Sha J, Wohlschlegel JA, Bradley PJ, Reese ML. 2020. Ancient MAPK ERK7 is regulated by an unusual inhibitory scaffold required for Toxoplasma apical complex biogenesis. Proc Natl Acad Sci USA 117:12164–12173. doi: 10.1073/pnas.1921245117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres JA, Pasquarelli RR, Back PS, Moon AS, Bradley PJ. 2021. Identification and molecular dissection of IMC32, a conserved Toxoplasma inner membrane complex protein that is essential for parasite replication. mBio 12:e03622-20. doi: 10.1128/mBio.03622-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kueck T, Foster TL, Weinelt J, Sumner JC, Pickering S, Neil SJD. 2015. Serine phosphorylation of HIV-1 Vpu and its binding to tetherin regulates interaction with clathrin adaptors. PLoS Pathog 11:e1005141. doi: 10.1371/journal.ppat.1005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lajko M, Haddad AF, Robinson CA, Connolly SA. 2015. Using proximity biotinylation to detect herpesvirus entry glycoprotein interactions: limitations for integral membrane glycoproteins. J Virol Methods 221:81–89. doi: 10.1016/j.jviromet.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritchie C, Cylinder I, Platt EJ, Barklis E. 2015. Analysis of HIV-1 Gag protein interactions via biotin ligase tagging. J Virol 89:3988–4001. doi: 10.1128/JVI.03584-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Sage V, Cinti A, Valiente-Echeverría F, Mouland AJ. 2015. Proteomic analysis of HIV-1 Gag interacting partners using proximity-dependent biotinylation. Virol J 12:138. doi: 10.1186/s12985-015-0365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim DI, Jensen SC, Noble KA, Kc B, Roux KH, Motamedchaboki K, Roux KJ. 2016. An improved smaller biotin ligase for BioID proximity labeling. Mol Biol Cell 27:1188–1196. doi: 10.1091/mbc.E15-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesari AS, Aryal UK, Lacount DJ. 2020. A novel proximity biotinylation assay based on the self-associating split GFP1–10/11. Proteomes 8:37–14. doi: 10.3390/proteomes8040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyers JM, Ramanathan M, Shanderson RL, Beck A, Donohue L, Ferguson I, Guo MG, Rao DS, Miao W, Reynolds D, Yang X, Zhao Y, Yang YY, Blish C, Wang Y, Khavari PA. 2021. The proximal proteome of 17 SARS-CoV-2 proteins links to disrupted antiviral signaling and host translation. PLoS Pathog 17:e1009412. doi: 10.1371/journal.ppat.1009412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi H, Larsen B, Lin ZY, Breitkreutz A, Mellacheruvu D, Fermin D, Qin ZS, Tyers M, Gingras AC, Nesvizhskii AI. 2011. SAINT: probabilistic scoring of affinity purification-mass spectrometry data. Nat Methods 8:70–73. doi: 10.1038/nmeth.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, Ting AY. 2018. Efficient proximity labeling in living cells and organisms with TurboID. Nat Biotechnol 36:880–887. doi: 10.1038/nbt.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.May DG, Scott KL, Campos AR, Roux KJ. 2020. Comparative application of BioID and TurboID for protein-proximity biotinylation. Cells 9:1070. doi: 10.3390/cells9051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May DG, Martin-Sancho L, Anschau V, Liu S, Chrisopulos RJ, Scott KL, Halfmann CT, Peña RD, Pratt D, Campos AR, Roux KJ. 2022. A BioID-derived proximity interactome for SARS-CoV-2 proteins. Viruses 14:611. doi: 10.3390/v14030611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnao MB, Acosta M, del Rio JA, García-Cánovas F. 1990. Inactivation of peroxidase by hydrogen peroxide and its protection by a reductant agent. Biochim Biophys Acta 1038:85–89. doi: 10.1016/0167-4838(90)90014-7. [DOI] [PubMed] [Google Scholar]
- 56.Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY. 2015. Directed evolution of APEX2 for electron microscopy and proteomics. Nat Methods 12:51–54. doi: 10.1038/nmeth.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marmor-Kollet H, Siany A, Kedersha N, Knafo N, Rivkin N, Danino YM, Moens TG, Olender T, Sheban D, Cohen N, Dadosh T, Addadi Y, Ravid R, Eitan C, Toth Cohen B, Hofmann S, Riggs CL, Advani VM, Higginbottom A, Cooper-Knock J, Hanna JH, Merbl Y, van den Bosch L, Anderson P, Ivanov P, Geiger T, Hornstein E. 2020. Spatiotemporal proteomic analysis of stress granule disassembly using APEX reveals regulation by SUMOylation and links to ALS pathogenesis. Mol Cell 80:876–891.e6. doi: 10.1016/j.molcel.2020.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmsaouri S, Markmiller S, Yeo GW. 2022. APEX proximity labeling of stress granule proteins. Methods Mol Biol 2428:381–399. doi: 10.1007/978-1-0716-1975-9_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moghimi S, Viktorova EG, Gabaglio S, Zimina A, Budnik B, Wynn BG, Sztul E, Belov GA. 2022. A proximity biotinylation assay with a host protein bait reveals multiple factors modulating enterovirus replication. PLoS Pathog 18:e1010906. doi: 10.1371/journal.ppat.1010906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hung V, Zou P, Rhee HW, Udeshi ND, Cracan V, Svinkina T, Carr SA, Mootha VK, Ting AY. 2014. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol Cell 55:332–341. doi: 10.1016/j.molcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Han S, Li H, Udeshi ND, Svinkina T, Mani DR, Xu C, Guajardo R, Xie Q, Li T, Luginbuhl DJ, Wu B, McLaughlin CN, Xie A, Kaewsapsak P, Quake SR, Carr SA, Ting AY, Luo L. 2020. Cell-surface proteomic profiling in the fly brain uncovers wiring regulators. Cell 180:373–386.e15. doi: 10.1016/j.cell.2019.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg A, Sibley LD. 2021. Toxoplasma gondii secreted effectors co-opt host repressor complexes to inhibit necroptosis. Cell Host Microbe 29:1186–1198.e8. doi: 10.1016/j.chom.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganapathy US, Bai L, Wei L, Eckartt KA, Lett CM, Previti ML, Carrico IS, Seeliger JC. 2018. Compartment-specific labeling of bacterial periplasmic proteins by peroxidase-mediated biotinylation. ACS Infect Dis 4:918–925. doi: 10.1021/acsinfecdis.8b00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fazal FM, Han S, Parker KR, Kaewsapsak P, Xu J, Boettiger AN, Chang HY, Ting AY. 2019. Atlas of subcellular RNA localization revealed by APEX-Seq. Cell 178:473–490.e26. doi: 10.1016/j.cell.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padrón A, Iwasaki S, Ingolia NT. 2019. Proximity RNA labeling by APEX-Seq reveals the organization of translation initiation complexes and repressive RNA granules. Mol Cell 75:875–887.e5. doi: 10.1016/j.molcel.2019.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu KE, Fazal FM, Parker KR, Zou J, Chang HY. 2020. RNA-GPS predicts SARS-CoV-2 RNA residency to host mitochondria and nucleolus. Cell Syst 11:102–108.e3. doi: 10.1016/j.cels.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherwood LJ, Hayhurst A. 2022. Visualizing filoviral nucleoproteins using nanobodies fused to the ascorbate peroxidase derivatives APEX2 and dEAPX. Methods Mol Biol 2446:427–449. doi: 10.1007/978-1-0716-2075-5_22. [DOI] [PubMed] [Google Scholar]
- 68.Bar DZ, Atkatsh K, Tavarez U, Erdos MR, Gruenbaum Y, Collins FS. 2018. Biotinylation by antibody recognition—a method for proximity labeling. Nat Methods 15:127–133. doi: 10.1038/nmeth.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dopie J, Sweredoski MJ, Moradian A, Belmont AS. 2020. Tyramide signal amplification mass spectrometry (TSA-MS) ratio identifies nuclear speckle proteins. J Cell Biol 219:e201910207. doi: 10.1083/jcb.201910207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Zhang Y, Wang Y, Zhang L, Brinkman EK, Adam SA, Goldman R, van Steensel B, Ma J, Belmont AS. 2018. Mapping 3D genome organization relative to nuclear compartments using TSA-Seq as a cytological ruler. J Cell Biol 217:4025–4048. doi: 10.1083/jcb.201807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, Zhang Y, Chen Y, Gholamalamdari O, Wang Y, Ma J, Belmont AS. 2020. TSA-seq reveals a largely conserved genome organization relative to nuclear speckles with small position changes tightly correlated with gene expression changes. Genome Res 31:251–264. doi: 10.1101/gr.266239.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L, Chen Y, Belmont AS. 2022. Measuring cytological proximity of chromosomal loci to defined nuclear compartments with TSA-seq. Methods Mol Biol 2532:145–186. doi: 10.1007/978-1-0716-2497-5_8. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Zhang Y, Zhang R, van Schaik T, Zhang L, Sasaki T, Peric-Hupkes D, Chen Y, Gilbert DM, van Steensel B, Belmont AS, Ma J. 2021. SPIN reveals genome-wide landscape of nuclear compartmentalization. Genome Biol 22:36. doi: 10.1186/s13059-020-02253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dumrongprechachan V, Salisbury RB, Soto G, Kumar M, MacDonald ML, Kozorovitskiy Y. 2021. Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum. Nat Commun 12:4855. doi: 10.1038/s41467-021-25144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen CL, Hu Y, Udeshi ND, Lau TY, Wirtz-Peitz F, He L, Ting AY, Carr SA, Perrimon N. 2015. Proteomic mapping in live Drosophila tissues using an engineered ascorbate peroxidase. Proc Natl Acad Sci USA 112:12093–12098. doi: 10.1073/pnas.1515623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pronobis MI, Zheng S, Singh SP, Goldman JA, Poss KD. 2021. In vivo proximity labeling identifies cardiomyocyte protein networks during zebrafish heart regeneration. eLife 10:e66079. doi: 10.7554/eLife.66079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stuart T, Satija R. 2019. Integrative single-cell analysis. Nat Rev Genet 20:257–272. doi: 10.1038/s41576-019-0093-7. [DOI] [PubMed] [Google Scholar]
- 78.Sharma PV, Thaiss CA. 2020. Host-microbiome interactions in the era of single-cell biology. Front Cell Infect Microbiol 10:536. doi: 10.3389/fcimb.2020.569070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie H, Ding X. 2022. The intriguing landscape of single-cell protein analysis. Adv Sci 9:2105932. doi: 10.1002/advs.202105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonner RF, Emmert-Buck M, Cole K, Pohida T, Chuaqui R, Goldstein S, Liotta LA. 1997. Laser capture microdissection: molecular analysis of tissue. Science 278:1481–1483. doi: 10.1126/science.278.5342.1481. [DOI] [PubMed] [Google Scholar]
- 81.Burgess JK, Hazelton RH. 2000. New developments in the analysis of gene expression. Redox Rep 5:63–73. doi: 10.1179/135100000101535348. [DOI] [PubMed] [Google Scholar]
- 82.Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, Geho DH, Petricoin EF, Liotta LA. 2006. Laser-capture microdissection. Nat Protoc 1:586–603. doi: 10.1038/nprot.2006.85. [DOI] [PubMed] [Google Scholar]
- 83.Golubeva Y, Salcedo R, Mueller C, Liotta LA, Espina V. 2013. Laser capture microdissection for protein and NanoString RNA analysis. Methods Mol Biol 931:213–257. doi: 10.1007/978-1-62703-056-4_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mikulowska-Mennis A, Taylor TB, Vishnu P, Michie SA, Raja R, Horner N, Kunitake ST. 2002. High-quality RNA from cells isolated by laser capture microdissection. Biotechniques 33:176–179. doi: 10.2144/02331md06. [DOI] [PubMed] [Google Scholar]
- 85.Chung SH, Shen W. 2015. Laser capture microdissection: from its principle to applications in research on neurodegeneration. Neural Regen Res 10:897–898. doi: 10.4103/1673-5374.158346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aguilar-Bravo B, Rodrigo-Torres D, Ariño S, Coll M, Pose E, Blaya D, Graupera I, Perea L, Vallverdú J, Rubio-Tomás T, Dubuquoy L, Armengol C, lo Nigro A, Stärkel P, Mathurin P, Bataller R, Caballería J, José Lozano J, Ginès P, Sancho-Bru P. 2019. Ductular reaction cells display an inflammatory profile and recruit neutrophils in alcoholic hepatitis. Hepatology 69:2180–2195. doi: 10.1002/hep.30472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vannucci FA, Foster DN, Gebhart CJ. 2013. Laser microdissection coupled with RNA-seq analysis of porcine enterocytes infected with an obligate intracellular pathogen (Lawsonia intracellularis). BMC Genomics 14:421. doi: 10.1186/1471-2164-14-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merritt EF, Johnson HJ, Wong ZS, Buntzman AS, Conklin AC, Cabral CM, Romanoski CE, Boyle JP, Koshy AA. 2020. Transcriptional profiling suggests T cells cluster around neurons injected with Toxoplasma gondii proteins. mSphere 5:e00538-20. doi: 10.1128/mSphere.00538-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clair G, Piehowski PD, Nicola T, Kitzmiller JA, Huang EL, Zink EM, Sontag RL, Orton DJ, Moore RJ, Carson JP, Smith RD, Whitsett JA, Corley RA, Ambalavanan N, Ansong C. 2016. Spatially-resolved proteomics: rapid quantitative analysis of laser capture microdissected alveolar tissue samples. Sci Rep 6:39223. doi: 10.1038/srep39223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griesser E, Wyatt H, ten Have S, Stierstorfer B, Lenter M, Lamond AI. 2020. Quantitative profiling of the human substantia nigra proteome from laser-capture microdissected FFPE tissue. Mol Cell Proteomics 19:839–851. doi: 10.1074/mcp.RA119.001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herrera JA, Mallikarjun V, Rosini S, Montero MA, Lawless C, Warwood S, O’Cualain R, Knight D, Schwartz MA, Swift J. 2020. Laser capture microdissection coupled mass spectrometry (LCM-MS) for spatially resolved analysis of formalin-fixed and stained human lung tissues. Clin Proteom 17:24. doi: 10.1186/s12014-020-09287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liotta LA, Pappalardo PA, Carpino A, Haymond A, Howard M, Espina V, Wulfkuhle J, Petricoin E. 2021. Laser capture proteomics: spatial tissue molecular profiling from the bench to personalized medicine. Expert Rev Proteomics 18:845–861. doi: 10.1080/14789450.2021.1984886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mund A, Coscia F, Kriston A, Hollandi R, Kovács F, Brunner AD, Migh E, Schweizer L, Santos A, Bzorek M, Naimy S, Rahbek-Gjerdrum LM, Dyring-Andersen B, Bulkescher J, Lukas C, Eckert MA, Lengyel E, Gnann C, Lundberg E, Horvath P, Mann M. 2022. Deep visual proteomics defines single-cell identity and heterogeneity. Nat Biotechnol 40:1231–1240. doi: 10.1038/s41587-022-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fisch D, Yakimovich A, Clough B, Wright J, Bunyan M, Howell M, Mercer J, Frickel E. 2019. Defining host-pathogen interactions employing an artificial intelligence workflow. eLife 8:e40560. doi: 10.7554/eLife.40560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hadley KC, Rakhit R, Guo H, Sun Y, Jonkman JEN, McLaurin J, Hazrati LN, Emili A, Chakrabartty A. 2015. Determining composition of micron-scale protein deposits in neurodegenerative disease by spatially targeted optical microproteomics. eLife 4:e09579. doi: 10.7554/eLife.09579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin B, Mendez R, Zhao XY, Rakhit R, Hsu KL, Ewald SE. 2020. Automated spatially targeted optical microproteomics (autoSTOMP) to determine protein complexity of subcellular structures. Anal Chem 92:2005–2010. doi: 10.1021/acs.analchem.9b04396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yin B, Caggiano LR, Li RC, McGowan E, Holmes JW, Ewald SE. 2021. Automated spatially targeted optical microproteomics investigates inflammatory lesions in situ. J Proteome Res 20:4543–4552. doi: 10.1021/acs.jproteome.1c00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Ferrante TC, Terry R, Turczyk BM, Yang JL, Lee HS, Aach J, Zhang K, Church GM. 2015. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat Protoc 10:442–458. doi: 10.1038/nprot.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, Terry R, Jeanty SSF, Li C, Amamoto R, Peters DT, Turczyk BM, Marblestone AH, Inverso SA, Bernard A, Mali P, Rios X, Aach J, Church GM. 2014. Highly multiplexed subcellular RNA sequencing in situ. Science 343:1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss M, Mollbrink A, Linnarsson S, Codeluppi S, Borg Å, Pontén F, Costea PI, Sahlén P, Mulder J, Bergmann O, Lundeberg J, Frisén J. 2016. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353:78–82. doi: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- 101.Quintana JF, Chandrasegaran P, Sinton MC, Briggs EM, Otto TD, Heslop R, Bentley-Abbot C, Loney C, de Lecea L, Mabbott NA, MacLeod A. 2022. Single cell and spatial transcriptomic analyses reveal microglia-plasma cell crosstalk in the brain during Trypanosoma brucei infection. Nat Commun 13:5752. doi: 10.1038/s41467-022-33542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma F, Hughes TK, Teles RMB, Andrade PR, de Andrade Silva BJ, Plazyo O, Tsoi LC, Do T, Wadsworth MH, Oulee A, Ochoa MT, Sarno EN, Luisa Iruela-Arispe M, Klechevsky E, Bryson B, Shalek AK, Bloom BR, Gudjonsson JE, Pellegrini M, Modlin RL. 2021. The cellular architecture of the antimicrobial response network in human leprosy granulomas. Nat Immunol 22:839–850. doi: 10.1038/s41590-021-00956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mantri M, Hinchman MM, McKellar DW, Wang MFZ, Cross ST, Parker JSL, de Vlaminck I. 2022. Spatiotemporal transcriptomics reveals pathogenesis of viral myocarditis. Nat Cardiovasc Res 1:946–960. doi: 10.1038/s44161-022-00138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galeano Niño JL, Wu H, LaCourse KD, Kempchinsky AG, Baryiames A, Barber B, Futran N, Houlton J, Sather C, Sicinska E, Taylor A, Minot SS, Johnston CD, Bullman S. 2022. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611:810–817. doi: 10.1038/s41586-022-05435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA. 2015. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, Macosko EZ. 2019. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363:1463–1467. doi: 10.1126/science.aaw1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marshall JL, Noel T, Wang QS, Chen H, Murray E, Subramanian A, Vernon KA, Bazua-Valenti S, Liguori K, Keller K, Stickels RR, McBean B, Heneghan RM, Weins A, Macosko EZ, Chen F, Greka A. 2022. High-resolution Slide-seqV2 spatial transcriptomics enables discovery of disease-specific cell neighborhoods and pathways. iScience 25:104097. doi: 10.1016/j.isci.2022.104097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vickovic S, Eraslan G, Salmén F, Klughammer J, Stenbeck L, Schapiro D, Äijö T, Bonneau R, Bergenstråhle L, Navarro JF, Gould J, Griffin GK, Borg Å, Ronaghi M, Frisén J, Lundeberg J, Regev A, Ståhl PL. 2019. High-definition spatial transcriptomics for in situ tissue profiling. Nat Methods 16:987–990. doi: 10.1038/s41592-019-0548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kishi JY, Liu N, West ER, Sheng K, Jordanides JJ, Serrata M, Cepko CL, Saka SK, Yin P. 2022. Light-Seq: light-directed in situ barcoding of biomolecules in fixed cells and tissues for spatially indexed sequencing. Nat Methods 19:1393–1402. doi: 10.1038/s41592-022-01604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haque A, Engel J, Teichmann SA, Lönnberg T. 2017. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med 9:75. doi: 10.1186/s13073-017-0467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Adil A, Kumar V, Jan AT, Asger M. 2021. Single-cell transcriptomics: current methods and challenges in data acquisition and analysis. Front Neurosci 15:398. doi: 10.3389/fnins.2021.591122. [DOI] [PMC free article] [PubMed] [Google Scholar]