ABSTRACT
Naegleria fowleri is an etiological agent that generates primary amoebic meningoencephalitis; unfortunately, no effective treatment or vaccine is available. The objective of this work was to determine the immunoprotective response of two vaccine antigens, as follows: (i) the polypeptide band of 19 kDa or (ii) a predicted immunogenic peptide from the membrane protein MP2CL5 (Smp145). Both antigens were administered intranasally in mice using cholera toxin (CT) as an adjuvant. The survival rate and immune response of immunized mice with both antigens and challenged with N. fowleri trophozoites were measured in the nose-associated lymphoid tissue (NALT) and nasal passages (NPs) by flow cytometry and enzyme-linked immunosorbent assay (ELISA). We also determined the immunolocalization of both antigens in N. fowleri trophozoites by confocal microscopy. Immunization with the polypeptide band of 19 kDa alone or coadministered with CT was able to confer 80% and 100% of protection, respectively. The immunization with both antigens (alone or coadministered with CT) showed an increase in T and B lymphocytes. In addition, there was an increase in the expression of integrin α4β1 and IgA in the nasal cavity of protected mice, and the IgA, IgG, and IgM levels were increased in serum and nasal washes. The immunolocalization of both antigens in N. fowleri trophozoites was observed in the plasma membrane, specifically in pseudopod-like structures. The MP2CL5 antigens evaluated in this work were capable of conferring protection which would lead us to consider them as potential candidates for vaccines against meningitis caused by N. fowleri.
KEYWORDS: Naegleria fowleri, membrane protein MP2CL5, vaccines, synthetic peptide, Smp145, protective immune responses
INTRODUCTION
Naegleria fowleri is a thermophilic free-living amoeba that generates the disease called primary amoebic meningoencephalitis (PAM), which is one of the most devastating and sudden infections that exist in humans (1). The most affected individuals are children and young adults with a recent history of recreational water activities, such as diving and swimming in lagoons, lakes, or some other sources of warm fresh water (2), with a higher and more likely infection rate occurring during the warm-weather seasons (3). N. fowleri enters by the nose and subsequently invades the brain, generating a necrotic meningoencephalitis that causes the death of the infected person once the clinical manifestations begin, generally within a week (1). Despite the therapeutic options available for this disease, there is only a survival rate of 5% of infected people, and there is also no validated vaccine available against N. fowleri for use in humans (4). Therefore, there is a need for vaccine candidates that confer protection against N. fowleri.
To obtain a vaccine, the study of the immune factors that are responsible for protection after immunization must be established; the protective immune responses elicited by mucosal vaccines are effective at the specific sites of pathogen-generated infection. Robust mucosal cellular and humoral immune responses may result in impaired attachment and thus absorption of pathogens via epithelial surfaces (5). In this regard, the study of nasopharynx-associated lymphoid tissue (NALT), which consists of macrophages, dendritic cells (DCs), B cells, and T cells, among other cells, is of great importance to understand the complex network of immune mechanisms (6), as it is a key component of mucosa-associated lymphoid tissue (MALT) and is capable of generating self-antigen immune responses (7). These antigens are recognized and transported by M cells through the epithelial barrier by phagocytosis. Once transported, they are delivered to the antigen-presenting cells to start the immune responses that generate the production of secreting IgA specific for foreign antigens (7, 8). At the mucosal site, antigen-induced lymphocytes migrate as effector cells to other mucosal sites in order to provide protection to all mucosal tissues (9).
Systems vaccinology includes the rational design of vaccines that require an understanding of the biology of the pathogen for the identification and obtention of the antigens of interest (10) and the host immune mechanisms as well as the new administration routes, such as mucosal immunization (11). In this regard, mucosal vaccines can induce immune responses that provide protection against pathogens in the specific sites where the infection is generated (5). Additionally, it is important to consider that systems of vaccinology are based on the use of genomics, proteomics, and bioinformatics tools to develop and obtain immunoprotective antigens (11).
Our working group recently identified N. fowleri antigenic proteins, such as the polypeptide band of 19 kDa which showed a different protein pattern expression than that of Naegleria lovaniensis (nonpathogenic species) (12). We also reported that the 19-kDa band is recognized by IgA, IgG, and IgM antibodies from serum and nasal washes of protected mice (survival, 100%) which were immunized with N. fowleri extracts plus CT (13).
Likewise, the polypeptide band and the spot corresponding to the 19-kDa band were identified by the mass spectrometry technique, obtaining the heat shock protein, Actin 1, and the membrane protein MP2CL5, among others (12). It is important to mention that the membrane protein MP2CL5 had already been reported previously as being present in N. fowleri but absent in nonpathogenic species, suggesting that this protein as a highly relevant pathogenicity factor (14) and an important candidate in the development of vaccines against N. fowleri (12).
In this work, two vaccine candidates were obtained, as follows: (i) a polypeptide band of 19 kDa or (ii) a predicted immunogenic peptide from the membrane protein MP2CL5 (Smp145). These vaccines alone or coadministered with cholera toxin (CT) as an adjuvant were immunized intranasally in BALB/c mice in the model of meningitis caused by Naegleria fowleri. Both vaccines induced an effector immune response against lethal challenge with N. fowleri trophozoites in immunized mice and were analyzed in NALT and nasal passages (NPs) by flow cytometry. In addition, the antibody response in serum and nasal washes was determined, and a comparative analysis of the proposed vaccines was performed.
RESULTS
Searching the synthetic peptide from the membrane protein (MP2CL5).
The sequence of 190 amino acids from the N. fowleri membrane protein MP2CL5 (19 kDa) was retrieved in the FASTA format from the Uniprot protein database (accession Q95UJ2) and is shown in Fig. 1A. The building of the three-dimensional (3D) structure of MP2CL5 was carried out using the program Robetta server (15). The Smp145 is exposed on the surface of the MP2CL5, which is a protein located on the lipidic membrane according to theoretical predictions (Fig. 1B).
FIG 1.
Immunoinformatic analysis of the sequence of the MP2CL5 protein. (A) Amino acid sequence of the MP2CL5 (Uniprot Q95UJ2). (B) Localization of Smp145 in MP2CL5. Orange color, Smp145; light gray, Mp2CL5. (C to E) Different regions of the Smp145 sequence that bind to mouse MHC-II proteins in the ligand binding pocket. (C) Smp145 (yellow) binds to the cavity of the H2-IAd allele. (D) Smp145 (green) binds to the pocket of the allele H2-IAb. (E) Smp145 (salmon) binds to the cavity of the H-2-IAk allele. (F to K) Different regions of the Smp145 sequence that interact with human MHC-II proteins in the binding pocket. (F) Smp145 (orange) binds to HLA-DRB1*0101. (G) Smp145 (lilac) couples to the HLA-DRB1*0401. (H) Smp145 (pink) binds to HLA-DRB*0701. (I) Smp145 (green) couples to HLA-DRB1*0801. (J) Smp145 (blue) binds to HLA-DRB1*1301. (K) Smp145 (purple) docks to HLA-DRB*1501.
Immunoinformatic analysis of the primary sequence of the MP2CL5 protein.
The results of the linear epitopes that bind to the universal major histocompatibility complex class II (MHC-II) were determined as we mentioned in the Materials and Methods section (“Immunoinformatic analysis of the primary sequence of the MP2CL5 protein”). According to these characteristics, the peptide 145 or Smp145 (IVFINFHNNGQDHLLKAVTNSLASI) was selected, which showed affinity for the following MHC II alleles: H-2-IAd in BALB/c (16), H-2-IAb in C57BL/6 (17), and H-2-IAk in A/J (18) and AKR/J mice (19). These alleles are found to be expressed by commonly used inbred mouse strains (Table 1). Likewise, it is important to mention that Smp145 also showed affinity for HLA-DRB1*0101, HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*0801, HLA-DRB1*1301, and HLA-DRB1*1501 (Table 2), and such alleles are distributed in the human population (20). Specifically, HLA-DRB1*0101 is more common in Caucasians, Native Americans, and Asians/Pacific Islanders, presenting higher frequencies of 68.3%, 78.7%, and 79.7%, respectively. It was also the second most common in Hispanics (38.3%) and African Americans (45.9%) (21). In the case of the HLA-DRB1*0701 and HLA-DRB1*1301 alleles, they occur in all populations, and on the other hand, the HLA-DRB1*0401 allele is more frequent in Eskimos and Caucasians, the HLA-DRB1*0802 is more frequent in Native Americans, and HLA-DRB1*1501 is more frequent in Caucasians (22) (Table 2). These HLA-DRB1 haplotypes were selected in this work for their distribution in different ethnic groups (21, 22). Furthermore, N. fowleri has been identified worldwide (23), and there are cases of people infected by this amoeba globally (24).
TABLE 1.
Affinity of Smp145 epitopes in different mice MHC-II haplotypesa
| Mouse strain | Reference | MHC-II allele | Peptide | Core | Affinity |
|---|---|---|---|---|---|
| BALB/c | 86 | H-2-IAd | IVFINFHNNGQDHLL | KAVTNSLAS | WBb |
| C57BL/6 | 80 | H-2-IAb | IVFINFHNNGQDHLL | KAVTNSLAS | WB |
| A/J and AKR/J | 81, 82 | H-2-IAk | IVFINFHNNGQDHLL | NFHNNGQDH | WB |
Affinity of linear Smp145 epitopes on MHC-II proteins (H-2-IAd, H-2-IAb, and H-2-IAk) from different mouse strains were identified.
WB, weak affinity.
TABLE 2.
Smp145 epitope affinity on different MHC-II haplotypes in the populationa
| DRB1 allele (human) | Distribution of DRB1 alleles in the population (ethnic group) | Reference | Peptide | Core | Affinityb |
|---|---|---|---|---|---|
| 0101 | Caucasians, Native Americans, Asian/Islanders, Hispanics, and in African Americans | 25 | IVFINFHNNGQDHLLKAVTNSLASI | LLKAVTNSL | WB |
| 0401 | Eskimos and Caucasians | 26 | IVFINFHNNGQDHLLKAVTNSLASI | LKAVTNSLA | SB |
| 0701 | All populations | 24 | IVFINFHNNGQDHLLKAVTNSLASI | LLKAVTNSL | WB |
| 0802 | Native Americans | 26 | IVFINFHNNGQDHLLKAVTNSLASI | LKAVTNSLA | WB |
| 1301 | All populations | 24 | IVFINFHNNGQDHLLKAVTNSLASI | LLKAVTNSL | WB |
| 1501 | Caucasians | 26 | IVFINFHNNGQDHLLKAVTNSLASI | LKAVTNSLA | WB |
The Smp145 epitopes that showed affinity for MHC-II proteins in humans (HLA-DRB1*0101, HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*0801, HLA-DRB1*1301, and HLA-DRB1*1501) in different ethnic groups of the population were identified.
WB, weak affinity; SB, strong affinity.
Molecular docking of the peptide 145 on the MHC-II alleles.
The 3D representation of molecular docking represented the binding affinity of the predicted epitope with MHC-II. The peptide 145 was subjected to molecular coupling with the different MHC-II alleles in either mice or humans (Fig. 1). We first observed the interaction of Smp145 with mouse MHC-II proteins (Fig. 1C to E). The peptide represented in yellow binds to the cavity of the H-2-IAd allele (Fig. 1C). We also observed that smp145 peptide (green) can bind to the pocket of allele H2-IAb (Fig. 1D). Finally, the peptide was observed binding to the cavity of the H-2-IAk allele (Fig. 1E, salmon).
We described some important details regarding molecular interactions. Smp145 with the H-2-IAd allele had an affinity given by Vander Waals force interactions and a hydrogen bond formed by the amino acid Ile1 (peptide) and Tyr8 (H-2-IAd) (Fig. 1C; supplemental material).
Likewise, the interaction between Smp145 and the H-2-IAb allele (Fig. 1D; supplemental material) showed that the affinity between the protein and the epitope is generated by Vander Waals force interactions, and different hydrogen bonds are formed between the alpha and beta chains of the H-2-IAb allele. In the case of the Alpha chain (chain C), 7 hydrogen bonds were formed, and for the beta chain (chain D), 7 hydrogen bonds were also formed, for which the peptide is very stable in this protein.
In the interaction of Smp145 with the H-2-IAk allele (Fig. 1E; supplemental material) was also given by Vander Waals force interactions, and different hydrogen bonds are formed between the alpha and beta chains of the H-2-IAk allele. In the case of the alpha chain (chain A), 2 hydrogen bonds were formed, and for the beta chain (chain B), 6 hydrogen bonds were formed, in addition to a salt bridge between His7 (epitope) and Asp57 (chain B). Therefore, the peptide is very stable in this protein.
On the other hand, Smp145 underwent molecular docking simulation with the different alleles of the human MHC-II (Fig. 1F to K). The Smp145 peptide is displayed in different colors where we clearly can observe the peptide interaction with the ligand-binding pocket of HLA-DRB1*0101, HLA-DRB1*0401, HLA-DRB1*0801, HLA-DRB1*0701, HLA-DRB1*1301, and HLA-DRB*1501, and for the human MHC-II alleles, we also describe some important interactions. For example, the nonbond interactions of the Smp145-DRB1*0101 complex (Fig. 1F; supplemental material) showed that Smp145 interacts with pocket 1 (Ala77A, Tyr112B) and Pocket 4 (Arg101B, Cys108B). The interactions between pocket 1 and Smp145 are mediated by electrostatic interactions, while for pocket 4, we observed electrostatic interactions and a hydrogen bond is formed between Arg101B and Ser21 from the peptide. On the other hand, the interactions of the Smp145 complex with HLA-DRB1*0401 (Fig. 1G; supplemental material) showed that Smp145 interacted with pocket 4 (Gln99B), pocket 6 (Ala89A, Val90A, and Asn94A), and pocket 9 (Ala93A, Asn94A, Arg101A, and Trp90B). In pockets 4, 6, and 9, the main interactions were electrostatic; however, in pocket 9, a hydrogen bond is formed between Arg101A (chain A DRB1*0401) and Ans8 (peptide 145). On the other hand, the interactions of the Smp145-DRB1*0701 complex (Fig. 1H; supplemental material) showed that Smp145 interacts with pocket 1 (Glu80A), pocket 4 (Asn87A, Asp99B, and Arg100B), pocket 6 (Ala89A, Val90A, Asn94A, Trp38B, Leu59B, and Arg100B), and pocket 9 (Ala93A, Asn94A, Ile97A, Met98A, Arg101A, Trp38B, Leu59B, Phe66B, Val86B, and Ser89B) through electrostatic interactions and hydrogen bonding. It is worth mentioning that interactions found for the complex Smp145-DRB1*0801 (Fig. 1I; supplemental material) showed that Smp145 interacts with pocket 1 (Ala79A, Val114B, and His10B), pocket 4 (Arg100B, Leu103B, Tyr107B, Phe55B, and Asn87A), pocket 6 (Asn87A, Val90A, Asn94A, Arg100B, Tyr59B, and Ser40B), and pocket 9 (Ala93A, Asn94A, Tyr59B, and Trp90B) predominantly by hydrogen bonds. The interactions of the Smp145 complex with HLA-DRB1*1301 (Fig. 1J; supplemental material) showed that Smp145 interacts with pocket 1 (Phe79A, Glu80A, and His110B), pocket 4 (Gln34A, Asn87A, Glu36A, Ser42B, Asp57B, and Glu100B), pocket 6 (Gln34A, Ala86A, Asn87A, Val90A, Asp91A, Asn94A, Glu38B, Ser42B, Asp57B, and Tyr59B), and pocket 9 (Ala93A, Asn94A, Glu38B, Tyr59B, Asp86B, and Trp90B). The predominant interactions are nonbonded contacts, but hydrogen bonds are also observed in pockets 4, 6, and 9. Finally, the interactions of the Smp145-DRB1*1501 complex (Fig. 1K; supplemental material) showed that Smp145 interacts with pocket 1 (Phe79A, Glu80A, and His110B), pocket 4 (Gln34A, Glu36A, Asn87A, Ser42B, and Tyr107B), pocket 6 (Gln34A, Glu36A, Ala86A, Asn87A, Val90A, Asp91A, Asn94A, Ser42B, Asp57B, and Glu100B), and pocket 9 (Ala93A, ASn94A, Tyr59B, Asp86B, and Trp90B) by hydrogen bonds.
The results described above are important since knowing the physicochemical properties of amino acids would allow us to determine the peptide-MHC binding force and, consequently, the peptide immune stimulation capacity for an effective response.
Analysis of the band with a relative molecular weight (rMW) of 19 kDa and Smp145 by Western blotting.
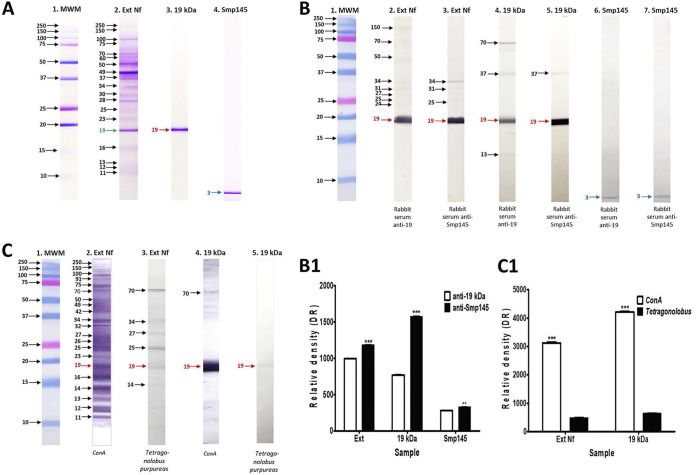
Total extracts of N. fowleri and the 19-kDa band purified by the electroelution technique were examined by 14% SDS-PAGE. In Fig. 2A, strip 2, the total extract of N. fowleri was examined, bands between 250 and 15 kDa were observed, and the 19-kDa band that was cut from the gels to be electroeluted is indicated by a green arrow. Finally, in Fig. 2A, strip 3, only the band obtained by the electroelution technique was observed, which is indicated by the red arrow at 19 kDa. The selected peptide Smp145 that was sent to be synthesized with a purity of 95% was examined by 14% SDS-PAGE. In Fig. 2A, strip 4, corresponding to Smp145, a single band with an rMW of 3 kDa was observed (blue arrow), and this molecular weight approximates to the theoretical molecular weight of this peptide.
FIG 2.
Gels, Western blot, and lectin blot of total extracts of N. fowleri, 19-kDa band, and Smp145 using specific antibodies. (A) The samples were separated on 1D SDS-PAGE. Strips are described as follows:1, molecular weight marker (MWM); 2, total extracts of N. fowleri; 3, band of 19 kDa purified by electroelution technique; 4, Smp145. Strips were stained with Coomassie. (B) Western blot. 1, MWM; 2 and 3, total extracts of N. fowleri; 4 and 5, band of 19 kDa purified by electroelution technique; 6 and 7, Smp145. Samples were electrophoresed on SDS-PAGE and transferred to a nitrocellulose membrane. The strips were then incubated with rabbit anti-19 serum (lanes 2, 4, and 6) or with rabbit anti-Smp145 serum (lanes 3, 5, and 7) and finally with peroxidized anti-rabbit IgG secondary antibody. (C) Lectin blot. 1, MWM; 2 and 3, total extracts of N. fowleri; 4 and 5, band of 19 kDa purified by electroelution technique. Samples were electrophoresed on SDS-PAGE and transferred to a nitrocellulose membrane. They were then incubated with Canavalia ensiformis “ConA” (strips 2 and 4) or with Tetragonolobus purpureas (strips 3 and 5) and incubated with SBHP (B1 and C1) The differences in the relative density of the bands were determined using ImageLab software. Data represent percentages of the mean ± SD of three independent experiments (spots from three independent replicates of each lectin blot or immunoblot). The data obtained were statistically analyzed using a Student’s t test (nonparametric tests) and then an unpaired t test. A level of significance with P < 0.05, P < 0.01, or P < 0.001 was considered to establish that there is a significant difference between the compared bands. **, P < 0.01; ***, P < 0.001.
To evaluate the reactivity and confirm the antibody specificity of the anti-19 kDa and anti-Smp145 serum obtained from rabbits, Western blotting was performed. The results obtained showed that for the total extracts of N. fowleri using the anti-19 kDa serum, bands from 150 to 19 kDa were recognized, identifying bands with an rMW of 150, 70, 50, 34, 31, 27, 25, and 24 kDa, which were lightly recognized; however, the 19-kDa band had the highest recognition by the IgG antibody (Fig. 2B, strip 2). It is worth mentioning that the 19-kDa band was also detected by the anti-Smp145 serum with great intensity. Other bands, such as those at 34 and 31 kDa, were also identified but with less intensity (Fig. 2B, strip 3). When we compared the band intensity, we observed that the band of 19 kDa was detected more intensely with the anti-Smp145 serum than with anti-19 kDa (Fig. 2B, strip 1). On the other hand, regarding the electroeluted 19-kDa band, it was clearly observed with the anti-19 serum. Other bands with less intensity were also identified, such as those with an rMW of 70, 37, and 13 kDa (Fig. 2B, strip 4). Likewise, the 19-kDa band was identified very intensely with the anti-145 serum. A band with an rMW of 37 kDa was also detected with less intensity (Fig. 2B, strip 5). The IgG from the serum that had the greatest recognition by the electroeluted band of 19 kDa was the anti-Smp-145 serum (Fig. 2B, strip 1). The Smp145 peptide was also immobilized, and it was detected with both serums, namely, the anti-19 kDa and anti Smp145 serum (Fig. 2B, strips 6 and 7, respectively) and an rMW of 3 kDa band was recognized, which is a molecular weight very close to the theoretical value reported for this peptide. When we compared the recognition given by both sera, we observed that the anti-Smp145 serum recognized the peptide slightly more than the anti-19-kDa serum (Fig. 2B, strip 1). Therefore, we confirm the immunoreactivity of anti-19 kDa and anti-Smp145 sera which recognized the 19-kDa bands and Smp145, respectively.
Analysis of the band with an rMW of 19 kDa by Lectin blotting.
To detect carbohydrates present in the band of interest, the total extracts of N. fowleri and the 19-kDa band were subjected to the lectin blot technique. The results showed that in the total extracts of N. fowleri using ConA, bands with an rMW of 250 to 11 kDa were obtained, identifying the 19-kDa band (Fig. 2C, strip 2). While when we used Tetragonolobus purpureas, the bands with an rMW of 70, 34, 27, 25, 19, and 14 kDa were detected (Fig. 2B, strip 3). On the other hand, using the same lectins but with the band of 19-kDa rMW purified, this band was detected very strongly with ConA; moreover, a slight band of 70-kDa rMW was also observed (Fig. 2B, strip 4). Tetragonolobus purpureas specifically detected the 19-kDa band (Fig. 2B, strip 5), finding this band less intense than that found with ConA.
Identification of glycan antigens present in proteins (glycoproteins) is important as they tend to be immunodominant in some infections caused by different pathogens (25). In addition, immunization with glycoprotein-rich materials has been shown to induce protective responses (26). Therefore, protective immune response given by the 19-kDa band could be correlated with the presence of residues of α-d-mannose, α-d-glucose, and fucose because antibodies against carbohydrates can be generated that can inhibit N. fowleri infection (27).
Immunolocalization of the band with a rMW of 19 kDa or Smp145 in the N. fowleri trophozoites.
The results obtained for the trophozoites that were incubated with the anti-19 serum (Fig. 3A, items A, B, C, D, and F) or with the anti-Smp145 serum (Fig. 3B, items A, B, C, D, and F), which were both made in rabbit and subsequently incubated with an Alexa Fluor 647 fluorescent anti-rabbit IgG secondary antibody, showed that, in the trophozoites, the anti-19 serum or the anti-145 serum, respectively, reacted very markedly with the plasma membrane of the amoeba. Likewise, it was possible to observe that both sera reacted with structures such as pseudopodia (Fig. 3A and B, item F, yellow arrows).
FIG 3.
Interaction of N. fowleri trophozoites with anti-19 or anti-145 serum. N. fowleri trophozoites were fixed with paraformaldehyde. (A) Trophozoites were incubated with rabbit anti-19 serum at a 1:1,000 dilution. (B) Trophozoites were incubated with rabbit anti-Smp145 serum at a 1:1,000 dilution. (A and B) Subsequently, Alexa Fluor 647 (red) fluorescent anti-rabbit IgG secondary antibody was applied at a 1:1,000 dilution. Finally, DAPI was added to visualize the slides in a confocal microscope. (A and D) N. fowleri trophozoites. (B and E) DAPI. (C and F) Merge.
Survival of mice immunized with the purified band of 19 kDa or with Smp145 alone or an adjuvant with CT.
The evaluation of the survival of mice immunized with the different antigens and later challenged with the lethal dose of virulent live N. fowleri is shown in Fig. 4. In the case of the mice that were immunized only with phosphate-buffered saline (PBS) and challenged with the trophozoites of N. fowleri, all of them died within a period of 5 to 8 days after the challenge (Fig. 4, red circle). On the other hand, of the mice that were immunized with the band of 19 kDa, only one mouse died after 10 days after the challenge and the other mice survived for a period of 60 days, obtaining a survival percentage of 80% (Fig. 4, pink square). With respect to the mice immunized with Smp145, two mice died, namely, the first at 5 days and the second at 9 days after the challenge, obtaining a survival percentage of 60% (Fig. 4, gray upward triangle). It is worth mentioning that the group of mice immunized with the 19-kDa band coadministered with CT had 100% survival (Fig. 4, dark-blue downward triangle). Finally, the mice that were immunized with Smp145 coadjuvanted with CT had 80% survival, with only one mouse dying 11 days after the challenge with N. fowleri trophozoites (Fig. 4, green diamond). In conclusion, immunization with the antigens (19 kDa, 19 kDa+CT, Smp145, and Smp145+CT) was capable of conferring protection against challenge with N. fowleri, and the group of mice immunized with the 19-kDa+CT band showed 100% protection.
FIG 4.

Survival of mice immunized with the 19-kDa band or Smp145 alone or coadministered with CT and subsequently challenged with the lethal dose of virulent live N. fowleri trophozoites by the intranasal route. BALB/c male mice (5 for each group) were challenged every 7 days on four occasions by the intranasal route with PBS (red circle), 19-kDa band (pink square), Smp145 (gray upward triangle), 19+CT band (triangle down, navy blue), or Smp145+CT (green diamond). After the last immunization, the animals were challenged with 1.2 × 104 live virulent N. fowleri trophozoites. Survival was evaluated for a period of 60 days. Data represent the results of three independent experiments for each group.
Phenotypic analysis of T and B lymphocytes in NALT and NPs in mice immunized with the 19-kDa band or Smp145 alone or coadministered with CT.
The percentage of T lymphocytes in NALT (Fig. 5B) and NPs (Fig. 5C) of the control or immunized mice was analyzed by flow cytometry. In the case of NALT, there was no statistically significant difference in the percentage of CD4+/CD3+ T cells in the groups immunized with the antigens alone (band of 19 kDa or Smp145) compared with the control group. However, in immunized mice with the antigens coadministered with CT (19 kDa+CT or Smp145+CT), there was a statistically significant increase of CD4+/CD3+ T cells compared with the control group (P < 0.001 and P < 0.001, respectively). We found more than 30% of CD4+/CD3+ T cells in the immunized group with 19 kDa+CT, while the immunized group with Smp145+CT, more than 19% of these cells were found. In contrast, no difference was found in the percentage of CD4+/CD3+ T cells between the immunized group with the band of 19 kDa with respect to the group immunized with Smp145. The group immunized with 19 kDa+CT showed a marked increase in the percentage of CD4+/CD3+ T cells compared with the group that was immunized with the 19-kDa band alone or with Smp145+CT (P < 0.01 or P < 0.05, respectively) (Fig. 5B).
FIG 5.
Phenotypic analysis of CD4/CD3, α4β1 T cells, and a proportion of B lymphocytes expressing the markers CD138/B22O, α4β1, and IgA and IgG antibodies in NALT and PNs. Different groups of male BALB/c mice were immunized four times at 7-day intervals by the i.n. route with the antigens 19-kDa band, Smp145, 19-kDa band+CT, and Smp145+CT or control to which only PBS was administered. Next, 24 h after the last immunization, the mice were challenged with N. fowleri trophozoites, 24 h later the animals were sacrificed, and the NALT and NP cells were suspended for analysis by flow cytometry. (A) Representative dot plots and histograms for the selection criteria of cell populations in NALT for the 19 kDa+CT group. Cells were selected based on size and granularity using the programs FSC-A and SSC-A to determine the region of lymphocytes; the same procedure was applied to all groups of mice. (B and D) NALT. (C and E) NP. Cells were stained with fluorochrome-conjugated monoclonal antibodies, as follows: anti-CD4PERCP, anti-α4β1FITC, and CD3e PE for T cells (C and D); and anti-B220PERCP, anti-α4β1FITC, anti-CD138APC, anti-IgAFITC, and anti-IgGFITC antibodies were used for B lymphocytes (C and E). (B to E) Data represent mean percentages ± SD of three independent experiments (using 5 mice/group). The data obtained were statistically analyzed by means of an analysis of variance (ANOVA) and then a Tukey post hoc test. A level of significance with P < 0.05, P < 0.01, or P < 0.001 was considered to establish that there is a significant difference between each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no statistically significant difference compared with the control group. a, P < 0.05; b, P < 0.01; c, P < 0.001; ns2, no statistically significant difference of the immunized groups alone compared with their respective immunized group with CT coadministered. +, P < 0.05; ++, P < 0.01; +++, P < 0.001; ns3, no statistically significant difference between the groups immunized with the 19-kDa band compared with those immunized with Smp145 or between these groups only coadministered with CT.
Some molecules, such as α4β1, can facilitate the recruitment of lymphocytes to inflamed mucosal tissues. Therefore, we also analyzed the CD4+/CD3+ T cell percentage expressing such a protein in NALT and NPs. In NALT, we observed an increase in immunized mice with the 19-kDa band or with the 19-kDa band plus CT compared with the control (P < 0.001). In the case of the immunized group with Smp145 or Smp145 coadministered with CT, no difference was found in cells that express α4β1 integrin with respect to the control. Likewise, no difference was found in cell percentages that express this integrin when we compare the groups immunized alone (19-kDa band or Smp145) with their respective group immunized with CT (band of 19 kDa+CT or Smp145+CT).
In contrast, a statistically significant increase in the percentage of T cells expressing α4β1 was observed in the group immunized only with the 19-kDa band compared with that of the group immunized with Smp145 (P < 0.001). We also observed an increase in the number of these cells in the group immunized with the 19-kDa band coadministered with CT compared with that of the group immunized with Smp145+CT (P < 0.05) (Fig. 5B).
On the other hand, in the NPs, an increased percentage of CD4+/CD3+ T cells was found for all immunized groups (band of 19 kDa, Smp145, band of 19 kDa+CT, or P145 + CT) with respect to the control (P < 0.001), finding an increase in the percentage of CD4+/CD3+ T cells above 26% for the immunized groups alone (19-kDa band or Smp145) and above 32% for the immunized groups coadministered with CT (19 kDa+CT or Smp145+CT). Regarding the groups immunized alone (19-kDa band or Smp145) and the groups adjuvanted with CT (band of 19 kDa+CT or Smp145+CT) there was no statistically significant difference in the percentage of CD4+/CD3+ T cells. Likewise, there was no difference in the percentage of these cells between the groups immunized alone (19-kDa band compared with Smp145) or coadministered with CT (19 kDa+CT compared with Smp145+CT) (Fig. 5C). On the other hand, with respect to the percentage of T cells that express α4β1+ in the NPs, an increase was observed in the groups immunized with the 19-kDa band and 19 kDa+CT with respect to the control group (P < 0.01 and P < 0.001, respectively). In addition, the immunized group with the 19-kDa band plus CT had a greater increase than the immunized group with the same 19-kDa band without CT (P < 0.01) (Fig. 5C). In contrast, the groups immunized with Smp145 or with Smp145+CT showed a slight upward trend in the percentage of positive T cells for α4β1+ integrin; however, we clearly did not observe a statistically significant difference compared with the control. When we compared the Smp145 plus CT immunized group with the Smp145 group, no significant differences were found.
Interestingly, as was observed for NALT, here in the NPs, a statistically significant increase in the percentage of T cells expressing α4β1+ was shown in the immunized groups with the 19-kDa band either administered with CT or alone with respect to Smp145 immunized groups alone or with CT (P < 0.001) (Fig. 5C).
To determine the percentage of B lymphocytes in NALT (Fig. 5D) and NPs (Fig. 5E) of the intranasally immunized groups, the proportions of lymphocytes expressing phenotypic markers CD138/BB20, α4β1, and IgA and IgG antibody-forming cells (IgA-CFA and IgG-CFA) were analyzed by flow cytometry. The percentage of B lymphocytes (CD138+BB20+) in NALT was higher in all immunized groups than that in the control group. This increase was higher for the group immunized with 19-kDa band plus CT (P < 0.001), followed by the immunized groups with Smp145 with CT, 19 kDa alone (P < 0.01), and finally with Smp145 (P < 0.05).
When we compared both immunized groups (19-Da band plus CT and 19 kDa alone), we observed a marked increase in the percentage of B lymphocytes (CD138+BB20+) when the 19-kDa antigen is administered with CT (P < 0.05). On the other hand, we did not find statistically significant differences between the group immunized with Smp145 and the group Smp145+CT or between the groups immunized with the antigens alone (19 kDa and Smp145). However, the group immunized with the band of 19 kDa+CT showed an increase in the percentage of B lymphocytes compared with the Smp145+CT group (P < 0.05) (Fig. 5D).
Regarding lymphocytes expressing α4β1+ in NALT, an increase was found in all immunized groups (19-kDa band and 19-kDa band+CT [P < 0.001]), Smp145 [P < 0.05], and (Smp145 plus CT [P < 0.01]) with respect to the control group. On the other hand, the groups immunized with the 19-kDa band coadministered with CT or Smp145 plus CT showed no statistically significant difference from the groups immunized with the 19-kDa band or with Smp145, respectively; the group immunized with the 19-kDa band showed an increase with respect to Smp145 (P < 0.05); and the group immunized with 19 kDa plus CT had no statistically significant difference with respect to the group immunized with Smp145 (Fig. 5D).
When IgA-AFC was observed, an increase in its proportion in the NALT was found in all the immunized groups (19-kDa band, 19 kDa band+CT, Smp145, and Smp145+CT) with respect to the control (P < 0.001). When we analyzed each immunized group, we found that there was no statistically significant difference in the percentage of IgA-AFC between the immunized groups either with the antigens alone (19-kDa band or Smp145) or between their respective groups coadministered with CT (19 kDa+CT or Smp145+CT) (Fig. 5D). In relation to the percentage of IgG-AFC in NALT, a statistically significant increase was found in all immunized groups with respect to the control. Interestingly, we observed that the overall increase in the percentage found for IgA-AFC in this tissue for all immunized groups was statistically greater (P < 0.001) than the IgG-AFC percentage (P < 0.01). Finally, when we compared the immunized groups, namely, those immunized with antigen alone or with CT coadministration, no statistically significant difference was found in the IgG-AFC percentage. A similar pattern result was found for IgA-AFC percentage where no significant differences were observed between all immunized groups (Fig. 5D).
Regarding the NPs, an increase in the percentage of B lymphocytes (CD138+BB20+) was found in all the immunized groups compared with that of the control group. The group that showed the greatest increase was the immunized group with the 19-kDa band plus CT compared with the rest of the immunized groups (19-kDa band, Smp145, and Smp145+CT) (P < 0.001). In contrast, no statistically significant differences were found between the immunized groups with the antigens alone (19 kDa and Smp145, both without CT), whereas the group immunized with the 19-kDa band coadministered with CT clearly showed a marked increase in B cell percentage, which was significantly different compared with the group immunized with Smp145 plus CT (P < 0.001) (Fig. 5E).
Additionally, when we measured the proportion of B lymphocytes that expressed α4β1+ in NPs, we also observed that all the immunized groups showed an increase with respect to the control group; in this case, the groups with the significantly higher proportion of these cells were those immunized with the 19-kDa band, 19 kDa+CT, and Smp145+CT (P < 0.001), followed by the Smp145 group (P < 0.01). When it was analyzed, the B lymphocyte percentage that expressed α4β1+ between the groups that were immunized with 19 kDa+CT or Smp145+CT and its respective groups immunized without CT, we observed again that such a percentage was significantly higher in the CT coadministered groups (P < 0.01). However, when we compared the type of antigen used, namely, the groups immunized with the 19-kDa band alone or Smp145 alone, there were no statistically significant differences between the groups immunized with the antigen and coadministered with CT (Fig. 5E).
We also analyzed the percentage of IgA-AFC in NPs (Fig. 5E), as we observed that an increase was shown in all the immunized groups (19-kDa band, band of 19 kDa+CT, Smp145, and Smp145+CT) compared with the control group (P < 0.001). We found again that the group immunized with the band of 19 kDa+CT had the higher percentage with respect to the 19-kDa band group (P < 0.001); while no statistically significant differences were found between the immunized groups with Smp145+CT and Smp145. However, for these cells, we found an increase in the group immunized with Smp145 compared with the group immunized with the 19-kDa band (P < 0.01), unlike the groups coadministered with CT, where an increase was found in the group immunized with the band of 19 kDa+CT compared with the Smp145+CT group (P < 0.05).
Finally, the IgG-AFC percentage in PNs was evaluated (Fig. 5E). We observed that only the groups that were immunized with the band with an rMW of 19 kDa or with the band with an rMW of 19 kDa coadministered with CT showed an increase with respect to the control group (P < 0.001). Also, the immunized groups with Smp145 or Smp145+CT showed no difference with respect to the control. In addition, it was observed that the group immunized with the band of 19 kDa+CT showed an increase in the percentage of IgG-AFC with respect to the group immunized only with the 19-kDa band (P < 0.001). We also observed a marked increase in the percentage of IgG-AFC in the group immunized with the 19-kDa band compared with that of the group immunized with Smp145 (P < 0.001). In the same way, an increase in the percentage of IgG-CFA was found in the group immunized with the 19-kDa band coadministered with CT compared with that of the group immunized with Smp145+CT (P < 0.001).
In summary, immunization with the 19-kDa band and Smp145 (alone or coadministered with CT) showed modifications in the populations of T and B lymphocytes, generally having an increase in these cells. Likewise, there was an increase in the expression of α4β1 integrin, IgA, and IgG in the nasal cavity of protected mice.
Antibody response in serum and nasal washes.
In general, in the immunized groups with all treatments, a statistically significant marked increase in IgA levels was observed both in nasal washes and serum with respect to the control group (P < 0.001) (Fig. 6A to D). In the case of nasal washes, the groups that were administered the antigen alone (19-kDa band or Smp145) did not show a statistically significant difference in the levels of IgA with respect to their corresponding groups coadministered with CT (19 kDa+CT or Smp145+CT) (Fig. 6A). Likewise, there was no statistically significant difference between the levels of this antibody when we compared the immunized group with the band 19 kDa with the Smp145 group or even when we compared the 19 kDa+CT with Smp+CT immunized groups (Fig. 6A).
FIG 6.
Anti-19, anti-145, anti-19+CT, or anti-Smp145+CT antibody response in nasal washes and serum. Groups of male BALB/c mice were immunized four times at 7-day intervals by the i.n. route and challenged with N. fowleri trophozoites. Next, 24 h after the challenge, they were sacrificed, and nasal washings and serum were obtained to measure the levels of IgA (A and D), IgG (B and E), and IgM (C and F) anti-19, anti-Smp145, anti-19+CT, or anti-Smp145+CT or control to which only PBS was administered. Antibody levels were determined using the ELISA technique. Data represent mean ± SD of three independent experiments (using 5 mice/group). The data obtained were statistically analyzed by means of an analysis of variance (ANOVA) and then a Tukey post hoc test. A level of significance with P < 0.05, P < 0.01, or P < 0.001 was considered to establish that there is a significant difference between each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no statistically significant difference compared with the control group. a, P < 0.05; b, P < 0.01; c, P < 0.001; ns2, no statistically significant difference of the immunized groups alone compared with their respective immunized group with CT coadministered. +, P < 0.05; ++, P < 0.01; +++, P < 0.001; ns3, no statistically significant difference between the immunized groups with the antigen alone (19-kDa band compared with Smp145) or between the antigens coadministered (19 kDa+CT compared with Smp145+CT).
Regarding serum IgA levels, a statistically significant increase was observed between the groups that were immunized alone (19 kDa band and Smp145) compared with their corresponding groups that were immunized with CT (19 kDa+CT or Smp145+CT) (P < 0.001 and P < 0.01, respectively). However, there was no statistically significant difference between the levels of IgA antibodies between the group immunized with the 19-kDa band and Smp145 or between the immunized groups with CT (Fig. 6D).
On the other hand, we also observed that there was a significant increase (P < 0.001) of IgG in nasal washes and serum from all immunized groups compared with that of the control group. When we compared the immunized groups with the antigen alone (19-kDa band or Smp145) with their coadministered CT group (band of 19 kDa+CT or Smp145+CT), there was no statistically significant difference. However, a statistically significant increase was found in the 19-kDa immunized group compared with mice immunized with Smp145 (Fig. 6B and E). Particularly in nasal washes, no statistically significant differences were found in IgG levels between these two groups coadministered with CT (Fig. 6B), while in serum, the statistically significant increase was found in the group immunized with the band of 19 kDa+CT (P < 0.001) (Fig. 6E).
Finally, IgM levels significantly increased in all immunized groups with respect to the control nasal washes or sera (P < 0.001). However, immunized groups with 19 kDa or Smp145, both coadministered with CT, did not elicit significant IgM antibody levels compared with their respective group with no CT coadministered. However, as with most of the results, we observed that there was a significant increased production of IgM in the immunized group with 19 kDa either alone or with CT coadministered compared with that of the Smp145 groups (P < 0.001 or P < 0.05) (Fig. 6C and F).
Therefore, we found that immunization with both antigens (alone or coadministered with CT) showed an increase in IgA, IgG, and IgM levels in serum and nasal washes.
DISCUSSION
To obtain vaccines that confer protection, it has been established that the proposed vaccine must be developed based on antigens of the pathogen or synthetic manufacturing, and such components of the pathogen can be represented in this way (10). Once a vaccine has been developed and tested, rational vaccine design allows us to understand the highly complex immune mechanisms involved in the protection conferred by vaccines. Consequently, in order to obtain vaccines that generate protection against PAM caused by N. fowleri, in the present work, we rely on systems vaccinology (11) using peptides contained in the 19-kDa band that were purified by the electroelution technique (Fig. 2A, strip 3), and in this way, we can compare its efficacy against a peptide designed “in silico” (Fig. 1) that we selected from the MP2CL5 protein with the aim of inducing protection against N. fowleri.
Although according to our objectives we first purified the 19-kDa band, it is important to mention that the peptide design was described at the beginning of the results in order to integrate both antigens, the 19 kDa band and the Smp145 peptide, in the same Western Blot results (Fig. 2). In this way, we first address the design of the peptide and then the results corresponding to the integrity and specificity given by the Western blot assay.
The in silico design was carried out once we obtained the linear sequence of the membrane protein MP2CL5 with its 3D structure (Fig. 1B). Smp145 was selected from the protein MP2CL5 (Q95UJ2) as it was found exposed on the surface of the 3D structure, a characteristic of great interest for the peptide to be recognized by cells of the immune system (28), and consequently it can induce an immunoprotective response against the challenge with N. fowleri trophozoites. We specifically focused on the presentation of exogenous antigens which are associated with MHC-II molecules (29). The Smp145 was recognized by several MHC-II haplotypes fulfilling the characteristic of promiscuity (30).
Therefore, Smp145 showed affinity for different MHC-II alleles, as follows: H-2-IAd in BALB/c (16), H-2-IAb in C57BL/6 (23), and H-2-IAk in A/J (18) and AKR/J mice (19) (Table 1). These different mouse strains were selected in this study since they have been widely used for experimental purposes and mainly BALB/c mice have been used in protection models against N. fowleri (27, 31, 32). In addition, Smp145 had affinity for universal MHC-II in humans (Table 2), a characteristic of great importance to obtain sequences that were promiscuous and found in different population groups (20). Although primary amoebic meningoencephalitis caused by N. fowleri is rare, there are cases reported worldwide (24), so the haplotypes we selected to carry out the in silico study showed that the Smp145 does show affinity for MHC-II molecules (Table 2) and also the interactions formed between the complex of MHC-II proteins with Smp145 (Fig. 1C to K), which is key for the activation and generation of the immune response (33, 34). In addition, since at present it has not been reported whether people infected with N. fowleri have specific HLA haplotypes, its selection in this work was carried out based on its geographical distribution since this amoeba has been identified with different genotypes mainly in America, Europe, the western Pacific (Japan and Oceania), and Asia (35). For this reason, we selected the haplotypes that occur most frequently in the entire population (HLA-DRB1*701 and HLA-DRB1*1301) or those that are more common in specific ethnic groups (HLA DRB1*0101 and HLA-DRB1*802), for example Native Americans (21, 22). These finding provides a fundamental basis for obtaining vaccine candidates that can be used universally in the future or in the population at greatest risk of being infected. It occurs in the United States where most reported cases of infection are caused by N. fowleri with 154 PAM infections from 1962 to 2021 and only four survivors (https://www.cdc.gov/parasites/naegleria/infection-sources.html, 19 January 2023). Also, in Mexico, there are around 11 cases reported from 1984 to 2007 due to infection with N. fowleri (3). It is worth mentioning that many cases around the world have been underestimated, which is due to the similarity of the clinical manifestations with other diseases, such as bacterial or viral meningitis, or some cases have been related to the nervous system or due to an unknown agent (3, 36). Therefore, true PAM incidence is unknown and there is great concern that MAP cases are not diagnosed (36).
In this work, we continue with the interactions that prevailed between peptide 145 and the different protein pockets in both mice and humans (Fig. 1C to K). In mice, these interactions were due mainly to both Vander Waals forces and hydrogen bonds with the MHC-II pocket proteins (H2-IAd, H2-IAb, and H2-IAk) (Fig. 1C to E). On the other hand, the cases of the different pockets (HLA-DRB1*0101, HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*0801, HLA-DRB1*1301, and HLA-DRB1*1501) of the MHC-II in humans were due to electrostatic interactions as well as the formation of hydrogen bonds (Fig. 1F to K). As it is known, these interactions, such as Van der Waals forces, are key to the formation of protein-ligand complexes (37). Likewise electrostatic interactions are of great importance as they generate a stable interaction between the peptides and proteins (38). Also, it has been reported that the complexes formed between the MHC-II and the peptides can achieve a high-affinity binding through hydrogen bonds that are generated energetically from the interactions that are formed with the different pockets (33). The formation of complexes of MHC-II proteins with peptides is key to being able to activate T helper lymphocytes through the TCR, which consequently release cytokines that are essential for cell-mediated responses and the production of antibodies and other factors of immune responses (33, 34). These results provided by this computational method allowed us to continue with the study of Smp145 and obtain this synthetic peptide, evaluate it, and compare it with the 19-kDa band.
The purification of the peptides contained in the 19-kDa band was carried out using the electroelution technique; this protein purification method had already been reported in other studies (39, 40). It has proven to be a simple, fast, reproducible, efficient, profitable technique that saves time (39), and protein bands can be obtained with a purity greater than 90% (41). In this work, this technique was successfully used, obtaining only the band of interest with an rMW of 19 kDa from the total extract of N. fowleri. The integrity of the synthetized Smp145 was also corroborated and it was also shown (Fig. 2A, strips 4). By Western blot technique, we detected with high intensity the 19-kDa band using serum from an immunized rabbit with polypeptides electroeluted from the 19-kDa band where it was possible to identify very intensely the 19-kDa band either in immobilized total extracts of N. fowleri and in an immobilized 19-kDa band (Fig. 2B, strips 2 and 4). Likewise, immobilized Smp145 was identified by the rabbit anti-Smp145 serum (Fig. 2B, strips 7).
Interestingly, the immobilized 19-kDa band was strongly identified with the serum of the rabbit immunized with Smp145 (Fig. 2B, strip 5). We analyzed this result with the knowledge that the 19-kDa antigenic band is made up of fragments of different proteins, such as heat shock protein, actin 1, and peptidyl-prolyl cis-trans isomerase, among others, and predominantly and abundantly including the membrane protein MP2CL5 (12). In the case of the Smp145 peptide that we designed and obtained in this work, it only contains a sequence of 25 amino acids (Table 2) of the MP2CL5 protein. Therefore, the greatest recognition of the anti-Smp145 serum toward the 19-kDa electroeluted band (Fig. 2B, strip 5) could be due to the fact that the sequence that these antibodies recognize is toward the antigens that are found in the native form of the protein and in a more abundant way. However, when we used the same serum to interact with the synthetic peptide Smp145 of 25 amino acids, we did not obtain a recognized band of the same intensity. This finding could be because this sample contains the peptide and it is not found in its native form, which is unlike the electroeluted band; therefore, the recognition of the peptide is of lower intensity than the 19-kDa band (Fig. 2B, strips 5 and 7).
In this regard, we know that for a molecule to be considered immunogenic, it must meet certain characteristics, such as being considered foreign to activate defense mechanisms, having a high molecular weight, and having chemical complexity to react with the different components of the immune system necessary to induce the immune response, mainly with antigen-presenting cells (APCs). These main features may confer an ability to stimulate the production of specific antibodies (42–44). In this regard, the 19-kDa band complies with the aforementioned characteristics. In the case of the antigens that make it up, they have a series of epitopes, which are different, so that each one can confer an ability to stimulate the activation of different clones of T and B lymphocytes that will obtain the induction of plasma cells capable of secreting specific antibodies (45) for different antigens of different peptides contained in the 19-kDa band (12).
On the other hand, in the case of the rabbit anti-Sm145 serum, the antibodies produced were formed from a single 25-amino acid sequence of the Smp145 peptide (Table 2), which we observed have the capacity to participate in the recognition of shared epitopic regions in the case of the 19-kDa band (Fig. 2B, strips 5) since it contains Smp145 epitopes (12). As we reported, this antibody response is focused predominantly on the MP2CL5 membrane protein contained in this band, which would increase its intensity.
It is also important to mention that the recognition of antibodies to specific antigen binding sites, as well as the amount of antigenic determinants in it, may be determining the types of antigen-antibody complexes that can be formed (46). Therefore, in the band of 19 kDa in which the membrane protein MP2CL5 predominates, which is antigenic (12) and is found in its native form, there are probably more antigenic repeat sites of this sequence or more exposed determinants within the antigen structure that contribute to a greater binding of the antibodies that were formed from the recognition of the Smp145 peptide by the immune system. This finding would explain why the antibodies synthesized in the serum of rabbits immunized with Smp145 have a greater binding to the 19-kDa band (Fig. 2B, strip 5). Conversely, immobilized Smp145 is recognized with low intensity by rabbit serum immunized with Smp145 (Fig. 2B, strip 7). This result is due to the amount of Smp145 that we loaded to carry out the assay since in other experiments (not shown) we observed that only by increasing the concentration of Smp145 can the intensity of recognition by anti-Smp145 rabbit serum increase. Therefore, the specificity and selectivity of antibodies are highly dependent on the particular context of the assay (47).
It is worth mentioning that in the Western blot in the lane where we loaded the 19-kDa band, the recognition of several bands by the rabbit anti-19 serum is observed (Fig. 2B, strip 4), which is explained because this electroeluted band is composed of different proteins, as mentioned previously, including heat shock protein (12). In this way, it can be explained why a band with an rMW of 70 kDa was identified corresponding to the molecular weight of the protein of heat shock (Fig. 2B, strip 4); however, we can clearly see that the 19-kDa band is the most immunogenic and the most abundant one (Fig. 2B, strip 4). It is important to consider that the additional bands may also represent protein degradation, posttranslational modification (PTM) cleavage, splice variants of the target protein, or other proteins that also contain the target epitope (48).
Using the lectin blot method, in total extracts of N. fowleri and the electroeluted 19-kDa band, we detected α-d-mannose and α-d-glucose residues that are recognized by the lectin Canavalia ensiformis (27) (Fig. 2C, lanes 2 and 4), as well as α-l-fucose residues that are recognized by the lectin Tetragonolobus purpureas (46) (Fig. 2C, lanes 3 and 5). In addition, the 19-kDa band was identified more intensely by the lectin Canavalia ensiformis than by Tetragonolobus purpureas (Fig. 2C), indicating that this band has more α-d-mannose and α-d-glucose residues than α-l-fucose residues. These glycoconjugates are of great importance since, for example, it has been reported that in N. fowleri trophozoites there are high levels of glycoconjugates on their surfaces, mainly α-d-glucose, α-d-mannose, N-acetylneuraminic acid, and terminal residues of α-l-fucose, by in vitro experiments (49–51). Also, the possibility that some of the glycoproteins found in N. fowleri represent an adherence factor to host cells has been demonstrated by in vivo experiments (27). In the 19-kDa band, our work group identified fragments of a heat shock protein with O-glycosylations within the posttranslational modifications (12), thus corroborating the intensity with which the 19-kDa band electroeluted with Canavalia ensiformis (Fig. 2C, strip 4). Based on the results obtained, we can suggest that this glycoprotein could represent an adhesion factor to the nasal epithelium and would probably be a key factor in the progression of the disease, as has been suggested for other pathogens, such as Acanthamoeba. Researchers have proposed that it can cause keratitis through a mannose-binding protein that is present in the superficial membranes of the amoeba and has the ability to interact through the corneal epithelium with mannose glycoproteins (52). Likewise, for Balamuthia mandrillaris, which is the etiological agent of infections in the lower respiratory tract, skin infections, and Balamutia amoebic encephalitis, it has been reported that the presence of galactose is essential for this pathogen to bind to target cells and generate infection (53).
Later, we used the antibodies anti-19 kDa and anti-Smp145 to analyze by immunohistochemistry that regions of N. fowleri trophozoites were recognized with these antibodies, and it was observed that the 19-kDa band or Smp145 are expressed in the plasmatic membrane of N. fowleri trophozoites (Fig. 3A and B, respectively, section F). Both the band of 19 kDa and Smp145, instead of being identified uniformly organized in the plasmatic membrane, were organized in localized groups in pseudopod-like structures. This distinctive distribution pattern in N. fowleri trophozoites had already been reported by Réveiller et al. (14) which suggests that, for example, the native Mp2CL5 protein could be involved in sensing the environment for signals that lead to increased polarization, locomotion toward a substrate, or directional motility (chemotaxis) to a food source. N. fowleri has also been shown to respond chemotactically to nerve cell extracts (54). For example, in two bacterial pathogens, Mycoplasma pneumoniae and Mycoplasma genitalium, the aggregation of adhesins and accessory proteins related to cytoadherence have been observed in specialized spikes that allow a highly oriented surface of the bacteria to host target cells (55). On the other hand, we could also see in the case of the rabbit anti-19 serum that, in addition to reacting with the membrane, it recognizes the trophozoite cytoplasm, which was to be expected because the antibodies not only recognize MP2CL5 but also recognize other proteins that make up this 19-kDa band (12) with which the rabbit was immunized.
These results allowed us to continue the study of Smp145 and the 19-kDa band to test its efficacy in a PAM model in mice. It is worth mentioning that in this work, despite having carried out the molecular coupling with human MHC-II proteins, we decided to continue the experiments using the murine model, knowing that the basic organization of genes in both humans and mice of the MHC locus is similar (56) and that the mouse model used in this research was proposed by our work group had already been used previously, obtaining results of great importance (13, 31).
Regarding the results of the immunization of mice with the 19-kDa band or Smp145 or of these groups coadministered with CT, mice immunized with the 19-kDa band had a high percentage of survival (80%) than those immunized with Smp145, which had 60% survival. In contrast, mice immunized with the 19-kDa band coadjuvanted with CT had a survival percentage of 100% compared with those immunized with Smp145 coadministered with CT that showed 80% survival, and all these groups were compared with the control in which the mice died in a period of 5 to 8 days (Fig. 4). It is appropriate to mention that following this same protection model in mice when immunized only with CT, 60% survival is obtained (31, 57). CT is used in this work as it has been shown to be a powerful mucosal adjuvant in mice and induces a strong immune response (58).
The survival results obtained can be explained by the immune factors induced by this immunization schedule, namely, antibody secretion, activation of different populations of lymphocytes, and the compartmentalization of immune response induced by intranasal immunization. Therefore, knowledge about the mechanisms involved in the protection against N. fowleri of the different immunized groups, we were able to determine them with the phenotypic differences found in the cell populations of two different regional sites NALT and NP by flow cytometry, in the results we obtained that the BALB/c mice immunized by intranasal route (i.n.) route with the 19-kDa band or Smp145 or these groups coadministered with CT modify the CD4/CD3 and α4β1 populations of T lymphocytes and CD138/B220, α4β1 of B lymphocytes, and AFC. The first phenotypic difference that we found in these two analyzed compartments is that the two groups immunized alone (19 kDa or Smp145) had no difference with the control in the percentage of CD4+/CD3+ cells in NALT; however, the groups coadministered with CT (19 kDa +CT or Smp145+CT) did show a difference with respect to the control and with respect to the groups immunized alone (19 kDa or Smp145), showing a statistically significant increase in the group immunized with 19 kDa+CT with respect to the group with Smp145+CT (Fig. 5B and C). This finding could explain why the group immunized with the 19-kDa band coadministered with CT showed 100% protection of the immunized mice compared with the other immunized groups that had lower survival percentages (Fig. 4). Consequently, these results could be explained since the NALT, being considered an inductive site of great importance in the immune response (59), participated in the induction and regulation of the protective immune response (60). In this case, a greater protective immune response mediated by T cells was demonstrated in the group immunized with the 19-kDa band coadministered with CT than that of the other groups evaluated. In NPs, a difference was found in the percentage of CD4+/CD3+ cells of all the immunized groups with respect to the control group; however, there was no difference between the immunized groups, only a tendency to increase in the groups immunized with CT compared with the immunized groups alone (Fig. 5C). The presence and differences found in the percentages of CD4+/CD3+ cells in both NALT and NPs suggest that specific CD4+/CD3+ T cells of the immunized antigens primed in the NALT subsequently migrated to the NP, which is an effector site. In this regard, a part of this model had already been suggested previously by Carrasco-Yepez et al. (61) in which they reported that for mice immunized with the treatments N. fowleri lysates alone, N. fowleri lysates plus CT, and CT alone, the response of CD4+ cells for the immunized groups was higher than that for the control group. They further suggested that in NALT, N. fowleri-specific CD4 T cells migrated to NPs, and as they evaluated the presence of these cells in other tissues, they also suggested that these cells migrated to cervical ganglia and ultimately to the spleen.
On the other hand, in this work, in the immunized groups compared with the control group, they decided to determine the percentage of CD3+/CD4+ T lymphocytes that express the integrin α4β1 since it binds to the vascular cell adhesion molecule-1 (VCAM-1) or to an alternatively spliced segment of the extracellular matrix protein fibronectin (FN) termed connecting segment form (CS-1) (62). The mediation of cell adhesion and activation occurs through a variety of cell-cell and cell-extracellular matrix interactions that regulate leukocyte migration into tissues during inflammatory responses and lymphocyte trafficking, among others (63, 64).
This interaction with fibronectin seems to generate a firm adhesion within the extracellular matrix and has been related to multiple functional processes, such as the differentiation of progenitor lymphocytes, the proliferation of T cells, homing (63), and the triggering of intracellular signals, such as protein phosphorylation in T and B cells (62). All these mentioned processes could be involved especially in the group of mice immunized with the 19-kDa band coadministered with CT that had 100% protection of the animals (Fig. 4), since it presented a higher percentage of CD4+/CD3+ T cells than that of the integrin α4β1 (NALT and PNs) with respect to all other immunized groups (Fig. 5B and C, respectively). It is worth mentioning that using the same protection model as in this work and by immunizing mice only with CT, a statistically significant increase was obtained with respect to the control group of CD4/α4β1-positive cells in both NALT and PNs (57). Therefore, CT could be participating in an important way in the increase of positive CD3+/CD4+ T cells that express the integrin α4β1 when it is coadministered with the 19-kDa band. Likewise, Rojas-Hernández et al. (31) obtained this same protection (100%) with total extracts of N. fowleri coadjuvanted with CT as mentioned previously. Furthermore, it was proposed that the protection is due to the specific immune response against N. fowleri antigens, in which APCs have the capacity to capture and process these antigens that migrate and present the antigen to activated CD4+ T lymphocytes in NALT, NPs, and cervical nodules (CNs) and could significantly stimulate some inductive factors, such as chemokine receptors CXR4 and CCR10, and important adhesion molecules, such as α4β1 and l-selectin, thus strengthening nonspecific factors such as PMN (61). On the other hand, we analyzed the population of B lymphocytes, and in all the immunized mice, the percentage of CD138/B220 cells was higher than that of the control group in both NALT and PNs (Fig. 5D and E). We analyzed the expression of CD138 as this molecule within the hemopoietic system has been found to be limited mainly to the late stages of B cell differentiation, which is typically expressed at a high cell density in normal plasma cells (65) and CD45 isoform B220, which is a marker of B cells in mice. Its expression is found among the earliest precursor B cells and is retained by mature B cells after antigen exposure, transit through the center germ cells, and entry into the memory B cell pool (66).
It is worth mentioning that in this work we found an increase in the α4β1 integrin expressed by the CD138/B220 cells of the immunized groups with respect to the control both in the NALT and in the NPs. The groups that showed the greatest increase were those that had the highest percentage of survival (19 kDa band+CT [100% survival] and the Smp145+CT and the 19-kDa band alone [80% survival]). This finding could be explained since intranasal immunization is known to induce IgA-producing B cells to express the chemokine receptor CCR10 and the α4β1 integrin. Binding of CCR10 and α4β1 to the chemokine ligand CCL28 and vascular cell adhesion molecule 1 (VCAM-1), respectively, mediates trafficking of IgA-producing B cells to the genitourinary and respiratory tracts (67, 68). It is worth mentioning that in previous studies, Carrasco-Yepez et al. (61) analyzed the population of B lymphocytes and found that the percentage of CD45/CD19-positive cells increased in all immunized groups (lysates of N. fowleri alone, lysates of N. fowleri plus CT, and CT alone) compared with the control. Likewise, for the B lymphocyte population in the group immunized only with CT, the percentage of B220/α4β1-positive cells in NALT and NPs also increased statistically significantly with respect to the control group (57).
Also, it was reported that immunization with N. fowleri coadministered with CT or Cry1Ac adjuvants produces a response with a Th2 profile with an increase in the secretion of IgA and IgG antibodies (32, 69).
Those works agree with the present results, in which we found that immunization with the treatments generate an increase in the percentage of IgA antibody-forming cells in NALT and PNs (Fig. 5D and E). In the case of the IgG-CFA in the NALT, a percentage increase was found with respect to the control of the immunized groups (Fig. 5D). In NPs, in the animals immunized with the 19-kDa band coadministered with CT (100% survival), the greatest increase in the percentage of IgG-CFA was observed with respect to the control followed by the mice immunized with the 19-kDa band (80% survival) (Fig. 5E). Therefore, the high percentage of survival in these two immunized groups could be to a specific adaptive response would induce that reinforces the protective factors immune innates (31). In this regard, in this work, we found that all groups immunized alone (19 kDa or Smp145) or coadministered with CT (19 kDa+CT or Smp145+CT) were immunogenic, mainly inducing a high antibody response of IgA, IgG, and IgM both in nasal washes as well as in serum (Fig. 6A to F). This work agrees with what was reported by Rojas-Hernández et al. (31), in which the intranasal immunization of N. fowleri lysates coadministered with CT induces the production of IgA and IgG antibodies in mucosal samples that generate a protection against the infection caused by N. fowleri of 100% of the mice challenged with the virulent live amoeba. However, in the present work, this same percentage of survival was achieved only with the immunized mice with the band with 19 kDa plus CT (100% of survival) followed by the immunized mice with the 19-kDa band alone and the group immunized with the Smp145 plus CT which had 80% survival.
In this regard, the immune barrier function of the IgA antibody has been related to the content of specific IgA antibodies in mucosal secretions and resistance or protection against infection by a variety of microorganisms, such as bacteria, viruses, pathogenic protozoa, and helminths (70). In this aspect, a study by Jarillo-Luna et al. (71), in which it was reported that intranasal immunization in mice with N. fowleri lysates coadjuvanted with Cry1Ac generated an increased IgA antibody response in the nasal mucosa, suggests that these antibodies prevent both the adhesion and the subsequent invasion of the amoeba to the nasal epithelium. However, IgG and IgM antibodies seem to show a protective role in the mucosa against different mucosal infections. For example, in a mouse model using IgA knockout mice, it was shown that protective mucosal immunity against influenza appears to be mediated by both IgG and IgM antibodies (72). In another study, peptides ABC and PGV04 against HIV-1, administered by the intranasal route to mice, either alone or coadministered with the dendrimer G4-PAMAM, induced a high response of IgA antibodies in the vaginal mucosa and of IgG and IgM in serum (73). Also, the peptide-dendrimer complex G4-PAMAM generated the production of IgG and IgA antibodies in sera and nasal washes (73). In the case of PAM generated by N. fowleri, it has been proposed that to induce protection against this infection, the secretion of antibody production is key in the protection against N. fowleri (13, 31). In this study, the increase in IgA antibodies in the nasal cavity of protected mice could be due to the transport of these antibodies from the lamina propria to the lumen by the pIgR (32). Increased IgG antibodies from the nasal mucosa of protected mice could be derived from the plasma by a process of passive transudation along a concentration gradient (74). Then IgA and IgG in the lumen may be avoiding the adhesion of N. fowleri trophozoites to the nasal epithelium and therefore participating in the protection of the immunized mice.
Therefore, in this study, the 19-kDa band immunized to mice alone or coadministered with CT was able to confer a greater immunoprotective response than Smp145 alone or coadministered with CT, respectively. This result could be due to the fact that the 19-kDa band retains all its immunogenic characteristics, that is, within its chemical structure, it is made up of fragments of various proteins (e.g., MP2CL.5 membrane protein, heat shock protein, and actin 1) that in some cases present posttranslational modifications, such as O-glycosylation (12). Carbohydrates are very important since they could be related to the adhesion of the amoeba to the nasal epithelium (27) and could participate together with these protein fragments in the immune-protective response. Likewise, it is worth mentioning that obtaining in this study a survival percentage of 60% in the group of mice immunized with Smp145 is of great value since it, being classified within the subunit-based vaccines, has great advantages. The advantages have been described previously and include, for example, inducing immune responses toward specific targets of the microorganism. In addition, they are already characterized, and there is no risk of pathogenicity since it cannot replicate in the host (75).
Therefore, the design of the vaccines proposed in this study offers many advantages; not only are they able to produce immune responses but also these responses are immunoprotective. In addition, in our model, we were able to analyze several points, such as intranasal immunization, the use of antigens designed with bioinformatic tools or purified antigens that preserve their native characteristics (glycoproteins), and the understanding of the complex immunological networks that are modified by immunization.
MATERIALS AND METHODS
N. fowleri cultures.
The Naegleria fowleri strain ATCC 30808 (American Type Culture Collection, Manassas, VA) was used and cultivated axenically in Bacto casitone medium (225930; Difco, Le Pont de Claix, France) supplemented with 10% fetal bovine serum (16170-078; Gibco, Grand Island, NY) at 37°C.
Animals.
Male BALB/c mice (8 to 10 weeks old and weighing 20 to 25 g) were obtained from the bioterium of the Escuela Superior de Medicina (IPN) (Mexico City, Mexico). All animals were handled in accordance with the Mexican federal regulation for animal experimentation and care (NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City, Mexico) and approved by the Institutional Committee for the Care and Use of Animals (ESM-CICUAL-ADEM-27-09-05/2019). Animals were kept in standard polypropylene cages with a 12-h light/dark cycle, at controlled temperature (22 ± 2°C), and were also provided with food (standard mouse chow) and water ad libitum. Mice were allowed to acclimatize to laboratory conditions for 1 week before undergoing the experiments.
Maintenance of virulence in N. fowleri.
The maintenance and reactivation of the virulence of N. fowleri was carried out according to what was reported by Rojas-Hernández et al. (31). A total of 105 N. fowleri trophozoites were inoculated intranasally in male BALB/c mice. After 4 days, the animals were sacrificed by cervical dislocation, and the amoeba was recovered from brain sections in axenic medium. The amoebas were passaged at least six times in mice.
Total extract of N. fowleri.
The amoebae cultured in 2% Bacto casitone medium in distilled water were harvested in the logarithmic growth phase (72 h) and washed on three occasions by centrifugation at 1,500 × g for 10 min with phosphate-buffered saline (PBS). Subsequently, 1 mL of 5 mM p-hydroxy-mercurybenzoic acid (H-0642; Sigma Chemical Co., St. Louis, MO) was added to the cell pellet as a protease inhibitor. The amoebae were lysed with a sonication cycle of 10 s at 100-W amplitude (ultrasonic processor). The resulting extract was stored at −70°C for later use. The protein concentration was determined by the Bradford technique, and the protein pattern was examined by SDS-PAGE electrophoresis.
Identification of the band with a rMW of 19 kDa in polyacrylamide gels.
Next, 20 μg of total extracts of N. fowleri were separated by SDS-PAGE (14%) under denaturing conditions at 100 V. Subsequently, they were stained with Coomassie blue until the appropriate contrast was achieved for the identification of the 19-kDa band.
Purification of the band with an rMW of 19 kDa by the electroelution technique.
The electroelution technique was carried out according to what was reported by Vázquez-Iglesias et al. (39), with some modifications. Briefly, the band of 19 kDa was identified, which was cut from each of the gels and placed in elution buffer (25 mM Tris base, 192 mM glycine, and 0.1% SDS) and stored at 4°C. The pieces of the cut gels (16 gels for each electro-elution) were electroeluted with the help of the electroeluter equipment (Model 422) at a voltage of 60 mA for 5 h. The samples obtained were placed on a dialysis membrane for 24 h, after which time they were centrifuged using 10-kDa molecular weight cut-off (MWCO) concentrator tubes at 2,000 rpm. The proteins obtained were quantified by the Bradford method and examined by SDS-PAGE electrophoresis on 14% gels.
Synthetic peptide design.
(i) Target protein. The primary sequence of the MP2CL5 protein was downloaded from the specialized Uniprot database (76). The protein crystal search was performed in the Protein Data Bank (PDB) database (77), but the 3D structure of the MP2CL5 protein was not found in the database. Therefore, the 3D structure of the MP2CL5 protein was determined, based on the homology methodology, using the Robetta Server program (15). The quality of the 3D structure of the MP2CL5 protein was evaluated with the SAVES v6.0 server (https://saves.mbi.ucla.edu/).
(ii) Immunoinformatic analysis of the primary sequence of the MP2CL5 protein. Linear epitopes of the Smp145 that bind to MHC-II proteins were identified. This affinity was determined using PREDBALB/c (78) for mice to predict the 15mer peptide (79) that might show affinity for MHC-II proteins, including H-2-IAd in BALB/c (16), H-2-IAb in C57BL/6 (23), and H-2-IAk in A/J (18) and AKR/J mice (19). In addition, the NetMHCII v2.3 server (https://services.healthtech.dtu.dk/services/NetMHCII-2.3/) was used to predict the linear 25-mer epitope of the Smp145 that might show HLA supertype affinity for the human universal MHC-II proteins (HLA-DRB1 *0101, HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*0801, HLA-DRB1*1301, and HLA-DRB1*1501) (26).
(iii) Molecular modeling of MHC-II proteins. The primary sequences of the MHC-II proteins were searched in the GenPet databases of NCBI and Uniprot. BLAST alignments were performed on the NCBI PDB database. From the results, in the PDB database, we searched for the three-dimensional structure of the MHC-II molecule; MHC-II alleles, including H-2-IAd (PDB 2IAD), H-2-IAb (PDB 4P23), and H-2-IAk (PDB 1IAK), were used in this study for peptide-mouse MHC docking analyses.
Additionally, in the PDB database, we searched for the three-dimensional structure of MHC-II molecules in humans; for HLA-DRB1*0101 (Uniprot identifier [ID], beta chain P04229), we found the crystal (PDB 6BIJ). In the case of the HLA-DRB1*0401 (Uniprot ID, beta chain P13760), HLA-DRB1*0701 (Uniprot ID, beta chain P13761), HLA-DRB1*0801 (Uniprot ID, beta chain Q30134), HLA-DRB1*1301 (Uniprot ID, beta chain Q5Y7A7), and HLA-DRB1*1501 (Uniprot ID, beta chain P01911) proteins, we found no crystals in the PDB, so we modeled the structure by homology modeling using Program Modeller 9.23 (https://salilab.org/modeller/9.23/release.html). We employed the crystal structure PDB 3PDO as the template. The quality of the 3D structure of the MHC-II protein was evaluated with the SAVES v6.0 server (https://saves.mbi.ucla.edu/).
(iv) Molecular modeling of selected epitopes. The three-dimensional structure of the selected epitopes was obtained using the PEP-FOLD 3 server (https://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD3/). This server employs a novo peptide prediction methodology based on a hidden Markov model (80).
(v) Protein-protein docking of MHC-II proteins and selected epitopes. The MHC-II-epitope complex was obtained by using the ClusPro Server (81). The various algorithms of the ClusPro server make it possible to evaluate millions of predictive complexes by taking into account their electrostatic energy and desolvation parameters (82). A peptide of interest named “Synthetic membrane peptide 145” (Smp145) was sent to Zhejiang Ontores Biotechnologies Co., Ltd. for synthesis with a purity of 95%. The Smp145 was examined by SDS-PAGE electrophoresis on 14% gels.
Obtaining anti-19 and anti-Smp145 sera.
Two male New Zealand White rabbits weighing 2.7 and 3 kg, respectively, were used for the production of antibodies according to Casais et al. (83) and Greenfield et (84), with some modifications. One rabbit was administered with the purified 19-kDa band (200 μg) and the other with the synthetic peptide (Smp145) (200 μg) by immunization. The immunization schedule used was as follows: as the first dose, the purified 19-kDa band or Smp145 was emulsified with 2 mL of Freud’s complete adjuvant (F5881; Sigma-Aldrich Co.) subcutaneously. After 14 days, the second dose with 2 mL of incomplete Freud’s adjuvant (F5506; Sigma-Aldrich Co.) was applied through the same route of administration. Fourteen days later, the 19-kDa band or Smp145 was inoculated intramuscularly with saline solution. Finally, 7 days after the last dose, sacrifice was performed by aortic puncture; the blood was centrifuged at 3,500 rpm for 20 min to obtain the serum; and the sample was stored at −20°C.
Western blot of the purified band with an rMW of 19 kDa and Smp145.
Next, 14% SDS-PAGE polyacrylamide gels were performed with total extracts of N. fowleri (20 μg/well) of the purified 19-kDa band (10 μg/well) and of Smp145 (10 μg/well). Subsequently, the proteins in the gel were transferred to nitrocellulose membranes, cut into approximately 4-mm strips, and blocked with 10% svelty milk overnight at 4°C. The strips were washed with PBS with Tween 20 (PBS-T) postincubation, and the following biotinylated lectins were added separately to each strip: Tetragonolobus purpureas (specific for α-l-fucose) and Canavalia ensiformis “ConA” (specific for α-d-mannose and α-d-glucose) (27). Rabbit sera were added separately to other strips as follows: rabbit anti-19 or anti-Smp145 serum at a dilution of 1:1,000. All the strips were incubated overnight at 4°C, and subsequently, they were washed three times with PBS-T. In the case of lectins, they were incubated with horseradish streptavidin peroxidase (SBHP: 1:1000), and in the case of rabbit sera, the strips were incubated with the peroxidized antibody (1:2,000) anti-rabbit IgG overnight at 4°C. Past this time, all strips were washed three times with PBS-T. To finish, the substrate (4-chloro-naphthol/methanol/H2O2) was added and washed with PBS-T three times.
Immunolocalization of the band with an rMW of 19 kDa or Smp145 in the N. fowleri trophozoites using the confocal fluorescence microscope.
Detection of the purified 19-kDa band and Smp145 in N. fowleri trophozoites was carried out by confocal fluorescence microscopy according to the method reported by Carrasco-Yepez et al. (85) with some modifications mentioned below. A total of 3.5 × 105 N. fowleri trophozoites were placed on a slide in 150 μL and incubated for 30 min at 37°C. A total of 2 mL of 2% paraformaldehyde was added, and two washes were performed with 1× PBS. Two groups (19 kDa band or Smp145) were subsequently added of 100 μL of 0.1% Triton at room temperature for 5 min and washed with PBS-T. Then rabbit anti-19 or anti-Smp145 serum was placed in a 1:1,000 dilution and incubated at 4°C overnight and washed with PBS-T. The fluorescent anti-rabbit IgG secondary antibody (goat anti-rabbit IgG, Alexa Fluor 647 [red]; Thermo Fisher Scientific) was added in a 1:1,000 dilution at 4°C for 24 h in the dark. Next, 4,6-diamidino-2-phenylindole (DAPI) was added and a coverslip was placed to visualize the slides under a confocal fluorescence microscope (Carl Zeiss, Mexico City, Mexico).
Immunization of mice.
The antigens were administered by the i.n. route to the male BALB/c mice (10 per group) according to our previously reported vaccination schedule (31, 32). The antigens were administered on days 1, 7, 14, and 21. The dose used for the groups, for each mouse immunization, was 10 μg/μL for the 19-kDa band or Smp145 and as adjuvant was 2 μg/μL for CT from Vibrio cholerae (C8052; Sigma-Aldrich Co.). The antigens administered to each group were as follows: (i) control (30 μL PBS), (ii) 19-kDa band, (iii) Smp145, (iv) 19-kDa band plus CT, and (v) Smp145 plus CT. After the last immunization, the animals were challenged with 1.2 × 104 live virulent N. fowleri trophozoites. Five animals from each group were sacrificed at 24 h after the challenge for the analysis of the immune response. For serum and nasal washes, the cells of the nasal passages (NPs) and cells of the lymphoid tissue associated with the nose (NALT) were obtained. The remaining five mice from each group of mice immunized with each of the aforementioned antigens were sacrificed 60 days after challenge to assess survival.
Analysis of the immune response.
(i) Cell isolation. The cell isolation of NALT and PNs and the flow cytometric assay were carried out as reported by Carrasco-Yépez et al. (61) with some modifications, which are described below.
(ii) NALT isolation. Briefly, mice that had already been bled by cardiac puncture and sacrificed by cervical dislocation were decapitated. The lower jaw and tongue were cut with sterile scissors. The heads were washed with cold, sterile PBS and immobilized on a dissection table with pins. With the help of fine forceps, the palates were excised. Subsequently, the tissue between the palates, the nasal septum, and the jaws was obtained with a scalpel blade. Palates were placed in 3.0 mL of ice-cold RPMI 1640 medium supplemented with 5% fetal bovine serum that had been heat inactivated previously, which was adjusted to pH 7.4, (Sigma Chemical Co., St. Louis, MO) in a petri dish, and the NALT was placed in RPMI 1640 medium to release cells. NALT suspensions from individual mice were pooled and cells were resuspended in RPMI 1640 medium.
(iii) Isolation of nasal passages. Briefly, nasal passage cells were obtained from the nasal cavity that was obtained after removal of the NALT (lateral walls, septum, and turbinates). The nasal passages were washed twice with RPMI 1640 to avoid contamination with blood cells. The tissue was cut into small pieces with sterile scissors, placed in RPMI 1640 medium containing 0.015% dithiothreitol (DTT) and 440 U/mL collagenase type IV (these reagents were purchased from Sigma Chemical Co.) in a conical centrifuge tube, and incubated at 37°C for 30 min in an incubator with a shaker at 180 rpm/min. Next, 100 μL of fetal bovine serum was added. All samples were filtered through a nylon mesh to remove tissue debris. The released cells were centrifuged for 10 min at 2,000 rpm and washed with RPMI 1640 medium.
(iv) Flow cytometry. To determine the percentages of double-positive populations for CD4, CD3e, and α4β1 T cells and CD138, B220, and α4β1 B cells, samples coupled to fluorochromes were stained. In this case, anti-CD4PERCP, anti-α4β1FITC, and anti-CD3e PE were used for T lymphocytes, while anti-B220PERCP, anti-α4β1FITC, and anti-CD138APC were used for B lymphocytes. After this time, the samples were incubated for 20 min at room temperature in the dark and 1× PBS was added. They were centrifuged at 1,500 rpm for 5 min at 4°C, and the cells were resuspended in cytofix/cytoperm solution and incubated for 20 min at room temperature in the dark. After that time, the samples were centrifuged at 1,500 rpm for 5 min at 4°C, the supernatants were removed, and the cell buds were resuspended in 1× perm/wash solution. They were centrifuged at 1,500 rpm for 5 min at 4°C, the supernatants were removed, and the cell buttons were resuspended. For the case of antibody determination, anti–IgA+ (anti-IgAFITC) or IgG+ (anti-IgGFITC) antibodies were added and incubated for 20 min at room temperature in the dark. Next, 1× perm/wash solution was added, the samples were centrifuged at 1,500 rpm for 5 min at 4°C, the supernatants were removed, and the cell buds were resuspended in 1× perm/wash solution. Each one of the samples was filtered with a 0.1-mm-opening mesh and refrigerated protected from light for later analysis. The acquisition of the percentages of positive cells and antibodies was performed by means of flow cytometry in a FASC Canto II cytometer, and the analysis was performed with the FASC Diva v6.1.3 flow cytometry software. The counted events were 20,000.
(v) Antibodies by the ELISA method. The levels of anti-19 or anti-Smp145 antibodies in serum samples and nasal washes were evaluated using the ELISA technique (73). Specifically, 96-well plates were covered with 100 μL of carbonate buffer (0.1 M Na2CO3 [pH 9.6]) mixed with the purified 19-kDa band (10 μg/well) or with Smp145 (10 μg/well), and the entire plate was incubated overnight at 4°C. After incubation, they were washed three times with PBS-T and blocked with 6% skim milk in PBS-T for 4 h at 37°C. Plates were washed with PBS-T three times and incubated overnight at 4°C with anti-19kDa or anti-19kDa+CT mouse serum for wells covered with the purified 19-kDa band or anti-Smp145 for Smp145-coated wells; all sera were used at 1:100 dilution and nasal washes were used undiluted. Subsequently, the plates were washed with PBS-T, and peroxidated mouse anti-IgA, anti-IgG, and anti-IgM antibodies were added at a dilution of 1:500, 1:6,000, and 1:3,000, respectively. Antibodies were incubated overnight at 4°C. Plates were then washed with PBS-T, and developer solution was added (0.4 mg/mL O-phenylenediamine and 0.04% H2O2 in 50 mM phosphate-citrate buffer [pH 5.2]), and then the reaction was stopped with 2.5 M sulfuric acid. Absorbance was measured at 490 nm in a microplate reader (Thermo Labsystems).
(vi) Densitometric and statistical analysis. A GS900 calibrated densitometer was used to scan the lectin blots and Western blots in order to obtain the changes in the densitometry of the bands using the Image Lab program. For all the graphs reported in this work, the data represent the mean ± SD of three independent experiments. In the case of the graphs corresponding to the lectin blots and Western blots, the data were statistically analyzed using the t test (nonparametric tests) and subsequently by an unpaired t test. For the other graphs reported in this work, the data were statistically analyzed by means of an analysis of variance (ANOVA) and then a Tukey post hoc test. To establish significant differences between the compared data, a significance level of P < 0.05, P < 0.01, or P < 0.001 was considered.
Ethical approval.
This study was conducted under license according to the Mexican federal regulations for animal experimentation and care (ESM, CICUAL-ADEM-05/27-09-2019, NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City, Mexico).
ACKNOWLEDGMENTS
The study was funded by the following grants: CIENCIA DE FRONTERA CONACYT 265230, PAPIIT-UNAM IN224519, and IPN-SIP 20221248.
We declare that we have no conflict of interest.
S.R.-H., M.M.C.-Y., and M.G.-S. conceived and planned the research. M.G.-S. carried out the experiments. M.G.-S., S.R.-H., and M.M.C.-Y. steered the experiments. J.C.-B. and G.L.R.-S. carried out the bioinformatics studies. M.G.-S., S.R.-H., and M.M.C.-Y. analyzed data and conducted the statistical analyses. M.G.-S., M.M.C.-Y., and S.R.-H. wrote the manuscript.
All authors read and approved the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Saúl Rojas-Hernández, Email: saulrohe@yahoo.com.mx.
Guy H. Palmer, Washington State University
REFERENCES
- 1.Baig AM. 2016. Primary amoebic meningoencephalitis: neurochemotaxis and neurotropic preferences of Naegleria fowleri. ACS Chem Neurosci 7:1026–1029. doi: 10.1021/acschemneuro.6b00197. [DOI] [PubMed] [Google Scholar]
- 2.Cope JR, Ali IK. 2016. Primary amebic meningoencephalitis: what have we learned in the last 5 years? Curr Infect Dis Rep 18:31. doi: 10.1007/s11908-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonilla-Lemus P, Rojas-Hernández S, Ramírez-Flores E, Castillo-Ramírez DA, Monsalvo-Reyes AC, Ramírez-Flores MA, Barrón-Graciano K, Reyes-Batlle M, Lorenzo-Morales J, Carrasco-Yépez MM. 2020. Isolation and identification of Naegleria species in irrigation channels for recreational use in Mexicali Valley, Mexico. Pathogens 9:820. doi: 10.3390/pathogens9100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellini NK, Santos TM, da Silva MTA, Thiemann OH. 2018. The therapeutic strategies against Naegleria fowleri. Exp Parasitol 187:1–11. doi: 10.1016/j.exppara.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Lavelle EC, Ward RW. 2022. Mucosal vaccines—fortifying the frontiers. Nat Rev Immunol 22:236–250. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takaki H, Ichimiya S, Matsumoto M, Seya T. 2018. Mucosal immune response in nasal-associated lymphoid tissue upon intranasal administration by adjuvants. J Innate Immun 10:515–521. doi: 10.1159/000489405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Date Y, Ebisawa M, Fukuda S, Shima H, Obata Y, Takahashi D, Kato T, Hanazato M, Nakato G, Williams IR, Hase K, Ohno H. 2017. NALT M cells are important for immune induction for the common mucosal immune system. Int Immunol 29:471–478. doi: 10.1093/intimm/dxx064. [DOI] [PubMed] [Google Scholar]
- 8.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. 2013. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu HY, Russell MW. 1997. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res 16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 10.Pollard AJ, Bijker EM. 2021. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giese M. 2016. Introduction to molecular vaccinology. Anticancer Res 36:3224. [PubMed] [Google Scholar]
- 12.Gutiérrez-Sánchez M, Carrasco-Yepez MM, Herrera-Díaz J, Rojas-Hernández S. 2020. Identification of differential protein recognition pattern between Naegleria fowleri and Naegleria lovaniensis. Parasite Immunol 42:e12715. doi: 10.1111/pim.12715. [DOI] [PubMed] [Google Scholar]
- 13.Rojas-Hernández S, Gutiérrez-Sánchez M, Rojas-Ortega DA, Bonilla-Lemus P, Contis-Montes de Oca A, Herrera-Díaz J, López-Reyes I, Carrasco-Yépez MM. 2020. Identification of immunogenic antigens of Naegleria fowleri adjuvanted by cholera toxin. Pathogens 9:460. doi: 10.3390/pathogens9060460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Réveiller FL, Suh SJ, Sullivan K, Cabanes PA, Marciano-Cabral F. 2001. Isolation of a unique membrane protein from Naegleria fowleri. J Eukaryot Microbiol 48:676–682. doi: 10.1111/j.1550-7408.2001.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim DE, Chivian D, Baker D. 2004. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res 32:W526–W531. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XW, Zhang N, Li ZL, Dibo N, Ma ZR, Lu B, Huang YH, Chang YF, Chen HZ, Wu X. 2022. Epitope vaccine design for Toxoplasma gondii based on a genome-wide database of membrane proteins. Parasit Vectors 15:364. doi: 10.1186/s13071-022-05497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandersarren L, Bosteels C, Vanheerswynghels M, Moon JJ, Easton AJ, Van Isterdael G, Janssens S, Lambrecht BN, van Helden MJ. 2017. Epitope mapping and kinetics of CD4 T cell immunity to pneumonia virus of mice in the C57BL/6 strain. Sci Rep 7:3472. doi: 10.1038/s41598-017-03042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basavalingappa RH, Massilamany C, Krishnan B, Gangaplara A, Rajasekaran RA, Afzal MZ, Riethoven JJ, Strande JL, Steffen D, Reddy J. 2017. β1-Adrenergic receptor contains multiple IA(k) and IE(k) binding epitopes that induce t cell responses with varying degrees of autoimmune myocarditis in A/J mice. Front Immunol 8:1567. doi: 10.3389/fimmu.2017.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould KA, Dove WF. 1997. Localized gene action controlling intestinal neoplasia in mice. Proc Natl Acad Sci USA 94:5848–5853. doi: 10.1073/pnas.94.11.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Groot AS, Ardito M, McClaine EM, Moise L, Martin WD. 2009. Immunoinformatic comparison of T-cell epitopes contained in novel swine-origin influenza A (H1N1) virus with epitopes in 2008–2009 conventional influenza vaccine. Vaccine 27:5740–5747. doi: 10.1016/j.vaccine.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Collins MM, Tang T, Slack R, Sintasath D, Hartzman RJ, Ng J, Hurley CK, Ng J, Hurley CK. 2000. The relative frequencies of HLA-DRB1*01 alleles in the major US populations. Tissue Antigens 55:48–52. doi: 10.1034/j.1399-0039.2000.550108.x. [DOI] [PubMed] [Google Scholar]
- 22.Reveille JD, Bruce GS, Lahita RG. 2004. MHC class II and non-MHC genes in the pathogenesis of systemic lupus erythematosus, p 109–151. In Systemic lupus erythematosus (fourth edition). Academic Press, San Diego, CA. doi: 10.1016/B978-012433901-9/50007-7. [DOI] [Google Scholar]
- 23.Martínez-Castillo M, Cárdenas-Zúñiga R, Coronado-Velázquez D, Debnath A, Serrano-Luna J, Shibayama M. 2016. Naegleria fowleri after 50 years: is it a neglected pathogen? J Med Microbiol 65:885–896. doi: 10.1099/jmm.0.000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Jonckheere JF. 2011. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect Genet Evol 11:1520–1528. doi: 10.1016/j.meegid.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Nyame AK, Kawar ZS, Cummings RD. 2004. Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch Biochem Biophys 426:182–200. doi: 10.1016/j.abb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Ada G, Isaacs D. 2003. Carbohydrate-protein conjugate vaccines. Clin Microbiol Infect 9:79–85. doi: 10.1046/j.1469-0691.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- 27.Carrasco-Yepez M, Campos-Rodriguez R, Godinez-Victoria M, Rodriguez-Monroy MA, Jarillo-Luna A, Bonilla-Lemus P, De Oca AC, Rojas-Hernandez S. 2013. Naegleria fowleri glycoconjugates with residues of α-d-mannose are involved in adherence of trophozoites to mouse nasal mucosa. Parasitol Res 112:3615–3625. doi: 10.1007/s00436-013-3549-2. [DOI] [PubMed] [Google Scholar]
- 28.Korber B, LaBute M, Yusim K. 2006. Immunoinformatics comes of age. PLoS Comput Biol 2:e71. doi: 10.1371/journal.pcbi.0020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadegh-Nasseri S, Kim A. 2015. Exogenous antigens bind MHC class II first, and are processed by cathepsins later. Mol Immunol 68:81–84. doi: 10.1016/j.molimm.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraav I, Pandey K, Sharma M, Singh S, Dutta P, Bhardwaj A, Sharma S. 2016. Predicting promiscuous antigenic T cell epitopes of Mycobacterium tuberculosis mymA operon proteins binding to MHC class I and class II molecules. Infect Genet Evol 44:182–189. doi: 10.1016/j.meegid.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Rojas-Hernández S, Rodríguez-Monroy MA, López-Revilla R, Reséndiz-Albor AA, Moreno-Fierros L. 2004. Intranasal coadministration of the Cry1Ac protoxin with amoebal lysates increases protection against Naegleria fowleri meningoencephalitis. Infect Immun 72:4368–4375. doi: 10.1128/IAI.72.8.4368-4375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrasco-Yepez M, Campos-Rodriguez R, Lopez-Reyes I, Bonilla-Lemus P, Rodriguez-Cortes AY, Contis-Montes de Oca A, Jarillo-Luna A, Miliar-Garcia A, Rojas-Hernandez S. 2014. Intranasal coadministration of Cholera toxin with amoeba lysates modulates the secretion of IgA and IgG antibodies, production of cytokines and expression of pIgR in the nasal cavity of mice in the model of Naegleria fowleri meningoencephalitis. Exp Parasitol 145:S84–S92. doi: 10.1016/j.exppara.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 33.McFarland BJ, Beeson C. 2002. Binding interactions between peptides and proteins of the class II major histocompatibility complex. Med Res Rev 22:168–203. doi: 10.1002/med.10006. [DOI] [PubMed] [Google Scholar]
- 34.Engelhard VH. 1994. Structure of peptides associated with MHC class I molecules. Curr Opin Immunol 6:13–23. doi: 10.1016/0952-7915(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 35.De Jonckheere JF. 2014. What do we know by now about the genus Naegleria? Exp Parasitol 145:S2–S9. doi: 10.1016/j.exppara.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Matanock A, Mehal JM, Liu L, Blau DM, Cope JR. 2018. Estimation of undiagnosed Naegleria fowleri primary amebic meningoencephalitis, United States. Emerg Infect Dis 24:162–164. doi: 10.3201/eid2401.170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bitencourt-Ferreira G, de Azevedo WF, Jr. 2019. How docking programs work. Methods Mol Biol 2053:35–50. doi: 10.1007/978-1-4939-9752-7_3. [DOI] [PubMed] [Google Scholar]
- 38.André I, Kesvatera T, Jönsson B, Akerfeldt KS, Linse S. 2004. The role of electrostatic interactions in calmodulin-peptide complex formation. Biophys J 87:1929–1938. doi: 10.1529/biophysj.104.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vázquez-Iglesias L, Estefanell-Ucha B, Barcia-Castro L, Páez de la Cadena M, Álvarez-Chaver P, Ayude-Vázquez D, Rodríguez-Berrocal FJ. 2017. A simple electroelution method for rapid protein purification: isolation and antibody production of alpha toxin from Clostridium septicum. PeerJ 5:e3407. doi: 10.7717/peerj.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borowiec M, Gorzkiewicz M, Grzesik J, Walczak-Drzewiecka A, Salkowska A, Rodakowska E, Steczkiewicz K, Rychlewski L, Dastych J, Ginalski K. 2016. Towards engineering novel PE-based immunotoxins by targeting them to the nucleus. Toxins 8:321. doi: 10.3390/toxins8110321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon KY, Tan WS, Tey BT, Lee KW, Ho KL. 2013. Native agarose gel electrophoresis and electroelution: a fast and cost-effective method to separate the small and large hepatitis B capsids. Electrophoresis 34:244–253. doi: 10.1002/elps.201200257. [DOI] [PubMed] [Google Scholar]
- 42.Dintzis HM, Dintzis RZ, Vogelstein B. 1976. Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci USA 73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahanty S, Prigent A, Garraud O. 2015. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunol 16:31. doi: 10.1186/s12865-015-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Tao A. 2015. Antigenicity, immunogenicity, allergenicity. Allergy Bioinformatics 8:175–186. doi: 10.1007/978-94-017-7444-4_11. [DOI] [Google Scholar]
- 45.McHeyzer-Williams MG, McHeyzer-Williams LJ, Fanelli Panus J, Bikah G, Pogue-Caley RR, Driver DJ, Eisenbraun MD. 2000. Antigen-specific immunity. Th cell-dependent B cell responses. Immunol Res 22:223–236. doi: 10.1385/IR:22:2-3:223. [DOI] [PubMed] [Google Scholar]
- 46.Orczyk-Pawiłowicz M, Augustyniak D, Hirnle L, Kątnik-Prastowska I. 2013. Lectin-based analysis of fucose and sialic acid expressions on human amniotic IgA during normal pregnancy. Glycoconj J 30:599–608. doi: 10.1007/s10719-012-9460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sela-Culang I, Kunik V, Ofran Y. 2013. The structural basis of antibody-antigen recognition. Front Immunol 4:302. doi: 10.3389/fimmu.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pillai-Kastoori L, Heaton S, Shiflett SD, Roberts AC, Solache A, Schutz-Geschwender AR. 2020. Antibody validation for Western blot: by the user, for the user. J Biol Chem 295:926–939. doi: 10.1074/jbc.RA119.010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Josephson SL, Weik RR, John DT. 1977. Concanavalin A-induced agglutination of Naegleria. Am J Trop Med Hyg 26:856–858. doi: 10.4269/ajtmh.1977.26.856. [DOI] [PubMed] [Google Scholar]
- 50.Cervantes-Sandoval I, Jesús Serrano-Luna J, Pacheco-Yépez J, Silva-Olivares A, Tsutsumi V, Shibayama M. 2010. Differences between Naegleria fowleri and Naegleria gruberi in expression of mannose and fucose glycoconjugates. Parasitol Res 106:695–701. doi: 10.1007/s00436-010-1727-z. [DOI] [PubMed] [Google Scholar]
- 51.Bose K, Ghosh DK, Bhattacharya A. 1989. Membrane carbohydrate characterization of Acanthamoeba astronyxis, A. castellanii and Naegleria fowleri by fluorescein-conjugated lectins. Int J Parasitol 19:737–741. doi: 10.1016/0020-7519(89)90060-x. [DOI] [PubMed] [Google Scholar]
- 52.Yang Z, Cao Z, Panjwani N. 1997. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect Immun 65:439–445. doi: 10.1128/iai.65.2.439-445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guzmán-Téllez P, Martínez-Castillo M, Flores-Huerta N, Rosales-Morgan G, Pacheco-Yépez J, la Garza M, Serrano-Luna J, Shibayama M. 2020. Lectins as virulence factors in Entamoeba histolytica and free-living amoebae. Future Microbiol 15:919–936. doi: 10.2217/fmb-2019-0275. [DOI] [PubMed] [Google Scholar]
- 54.Cline M, Carchman R, Marciano-Cabral F. 1986. Movement of Naegleria fowleri stimulated by mammalian cells in vitro. J Protozool 33:10–13. doi: 10.1111/j.1550-7408.1986.tb05547.x. [DOI] [PubMed] [Google Scholar]
- 55.Reddy SP, Rasmussen WG, Baseman JB. 1996. Isolation and characterization of transposon Tn4001-generated, cytadherence-deficient transformants of Mycoplasma pneumoniae and Mycoplasma genitalium. FEMS Immunol Med Microbiol 15:199–211. doi: 10.1111/j.1574-695X.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 56.Yuhki N, Beck T, Stephens RM, Nishigaki Y, Newmann K, O'Brien SJ. 2003. Comparative genome organization of human, murine, and feline MHC class II region. Genome Res 13:1169–1179. doi: 10.1101/gr.976103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castillo-Ramírez DA, Carrasco-Yépez MM, Rodríguez-Mera IB, Reséndiz-Albor AA, Rosales-Cruz É, Rojas-Hernández S. 2021. A 250-kDa glycoprotein of Naegleria fowleri induces protection and modifies the expression of α4β1 and LFA-1 on T and B lymphocytes in mouse meningitis model. Parasite Immunol 43:e12882. doi: 10.1111/pim.12882. [DOI] [PubMed] [Google Scholar]
- 58.Fukuyama Y, Okada K, Yamaguchi M, Kiyono H, Mori K, Yuki Y. 2015. Nasal administration of cholera toxin as a mucosal adjuvant damages the olfactory system in mice. PLoS One 10:e0139368. doi: 10.1371/journal.pone.0139368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lycke N. 2012. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 60.Kiyono H, Fukuyama S. 2004. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol 4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrasco-Yepez MM, Campos-Rodríguez R, Reséndiz-Albor AA, Peña-Juárez C, Contis-Montes de Oca A, Arciniega-Martínez IM, Bonilla-Lemus P, Rojas-Hernandez S. 2018. Naegleria fowleri immunization modifies lymphocytes and APC of nasal mucosa. Parasite Immunol 40:e12508. doi: 10.1111/pim.12508. [DOI] [PubMed] [Google Scholar]
- 62.Yang GX, Hagmann WK. 2003. VLA-4 antagonists: potent inhibitors of lymphocyte migration. Med Res Rev 23:369–392. doi: 10.1002/med.10044. [DOI] [PubMed] [Google Scholar]
- 63.Imai Y, Shimaoka M, Kurokawa M. 2010. Essential roles of VLA-4 in the hematopoietic system. Int J Hematol 91:569–575. doi: 10.1007/s12185-010-0555-3. [DOI] [PubMed] [Google Scholar]
- 64.Lin KC, Castro AC. 1998. Very late antigen 4 (VLA4) antagonists as anti-inflammatory agents. Curr Opin Chem Biol 2:453–457. doi: 10.1016/s1367-5931(98)80120-8. [DOI] [PubMed] [Google Scholar]
- 65.Gattei V, Godeas C, Degan M, Rossi FM, Aldinucci D, Pinto A. 1999. Characterization of anti-CD138 monoclonal antibodies as tools for investigating the molecular polymorphism of syndecan-1 in human lymphoma cells. Br J Haematol 104:152–162. doi: 10.1046/j.1365-2141.1999.01132.x. [DOI] [PubMed] [Google Scholar]
- 66.Rodig SJ, Shahsafaei A, Li B, Dorfman DM. 2005. The CD45 isoform B220 identifies select subsets of human B cells and B-cell lymphoproliferative disorders. Hum Pathol 36:51–57. doi: 10.1016/j.humpath.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. 2003. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol 170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 68.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. 2003. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest 111:1001–1010. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrasco-Yepez M, Rojas-Hernandez S, Rodriguez-Monroy MA, Terrazas LI, Moreno-Fierros L. 2010. Protection against Naegleria fowleri infection in mice immunized with Cry1Ac plus amoebic lysates is dependent on the STAT6 Th2 response. Parasite Immunol 32:664–670. doi: 10.1111/j.1365-3024.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 70.Lamm ME. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol 51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 71.Jarillo-Luna A, Moreno-Fierros L, Campos-Rodríguez R, Rodríguez-Monroy MA, Lara-Padilla E, Rojas-Hernández S. 2008. Intranasal immunization with Naegleria fowleri lysates and Cry1Ac induces metaplasia in the olfactory epithelium and increases IgA secretion. Parasite Immunol 30:31–38. doi: 10.1111/j.1365-3024.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 72.Mbawuike IN, Pacheco S, Acuna CL, Switzer KC, Zhang Y, Harriman GR. 1999. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J Immunol 162:2530–2537. doi: 10.4049/jimmunol.162.5.2530. [DOI] [PubMed] [Google Scholar]
- 73.Rodríguez-Fonseca RA, Bello M, de Los Muñoz-Fernández M, Luis Jiménez J, Rojas-Hernández S, Fragoso-Vázquez MJ, Gutiérrez-Sánchez M, Rodrigues J, Cayetano-Castro N, Borja-Urby R, Rodríguez-Cortés O, García-Machorro J, Correa-Basurto J. 2019. In silico search, chemical characterization and immunogenic evaluation of amino-terminated G4-PAMAM-HIV peptide complexes using three-dimensional models of the HIV-1 gp120 protein. Colloids Surf B Biointerfaces 177:77–93. doi: 10.1016/j.colsurfb.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 74.Gianchecchi E, Manenti A, Kistner O, Trombetta C, Manini I, Montomoli E. 2019. How to assess the effectiveness of nasal influenza vaccines? Role and measurement of sIgA in mucosal secretions. Influenza Other Respir Viruses 13:429–437. doi: 10.1111/irv.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma J, Bruce TJ, Jones EM, Cain KD. 2019. A review of fish vaccine development strategies: conventional methods and modern biotechnological approaches. Microorganisms 7:569. doi: 10.3390/microorganisms7110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.UniProt Consortium. 2020. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res 49:D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burley SK, Bhikadiya C, Bi C, Bittrich S, Chen L, Crichlow GV, Christie CH, Dalenberg K, Di Costanzo L, Duarte JM, Dutta S, Feng Z, Ganesan S, Goodsell DS, Ghosh S, Green RK, Guranović V, Guzenko D, Hudson BP, Lawson Catherine L, Liang Y, Lowe R, Namkoong H, Peisach E, Persikova I, Randle C, Rose A, Rose Y, Sali A, Segura J, Sekharan M, Shao C, Tao Y-P, Voigt M, Westbrook JD, Young JY, Zardecki C, Zhuravleva M. 2021. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res 49:D437–D451. doi: 10.1093/nar/gkaa1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang GL, Srinivasan KN, Veeramani A, August JT, Brusic V. 2005. PREDBALB/c: a system for the prediction of peptide binding to H2d molecules, a haplotype of the BALB/c mouse. Nucleic Acids Res 33:W180–W183. doi: 10.1093/nar/gki479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jensen KK, Andreatta M, Marcatili P, Buus S, Greenbaum JA, Yan Z, Sette A, Peters B, Nielsen M. 2018. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 154:394–406. doi: 10.1111/imm.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamiable A, Thévenet P, Rey J, Vavrusa M, Derreumaux P, Tufféry P. 2016. PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res 44:W449–W454. doi: 10.1093/nar/gkw329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vajda S, Yueh C, Beglov D, Bohnuud T, Mottarella SE, Xia B, Hall DR, Kozakov D. 2017. New additions to the ClusPro server motivated by CAPRI. Proteins 85:435–444. doi: 10.1002/prot.25219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 83.Casais R, Granda V, Balseiro A, Del Cerro A, Dalton KP, González R, Bravo P, Prieto JM, Montoya M. 2016. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors 9:435. doi: 10.1186/s13071-016-1717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greenfield EA. 2020. Standard immunization of rabbits. Cold Spring Harb Protoc 2020:100305. doi: 10.1101/pdb.prot100305. [DOI] [PubMed] [Google Scholar]
- 85.Carrasco-Yepez MM, Contis-Montes de Oca A, Campos-Rodriguez R, Falcon-Acosta D, Pacheco-Yepez J, Rodriguez-Mera IB, Bonilla-Lemus P, Rosales-Cruz E, Lopez-Reyes I, Rojas-Hernandez S. 2019. Mouse neutrophils release extracellular traps in response to Naegleria fowleri. Parasite Immunol 41:e12610. doi: 10.1111/pim.12610. [DOI] [PubMed] [Google Scholar]
- 86.Lizbeth RG, Jazmín GM, José CB, Marlet MA. 2021. Immunoinformatics study to search epitopes of spike glycoprotein from SARS-CoV-2 as potential vaccine. J Biomol Struct Dyn 39:4878–4892. doi: 10.1080/07391102.2020.1780944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2. Download iai.00181-23-s0001.docx, DOCX file, 1.4 MB (1.4MB, docx)