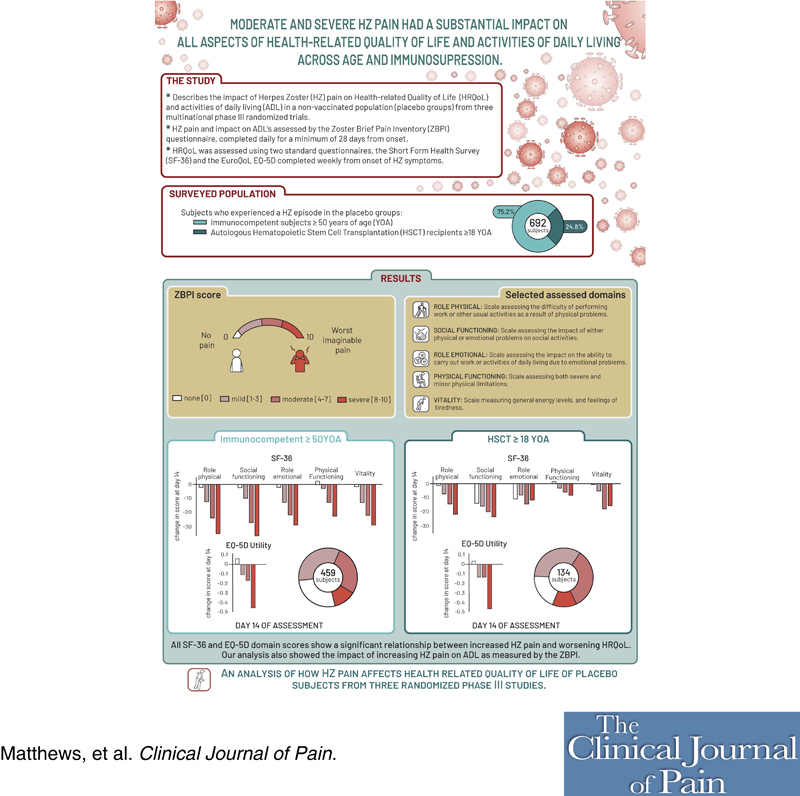
Objectives:
Herpes zoster (HZ) is a painful condition caused by the reactivation of the varicella-zoster virus, negatively affecting the lives of patients. In this post hoc analysis, we describe the impact of HZ pain on the health-related quality of life (HRQoL) and activities of daily living (ADL) of immunocompetent individuals 50 years of age and older and in hematopoietic stem cell transplantation (HSCT) recipients age 18 years of age and older.
Materials and Methods:
ZOE-50 (NCT01165177), ZOE-70 (NCT01165229), and ZOE-HSCT (NCT01610414) were phase III, randomized studies conducted in immunocompetent adults 50 years of age and older and 70 years of age and older and in HSCT recipients age 18 years of age and older, respectively. This analysis was performed on patients who experienced an HZ episode in the placebo groups. The impact of varying levels of HZ pain on HRQoL and ADL was analyzed using data from the Zoster Brief Pain Inventory (ZBPI) and the Short Form Health Survey 36 (SF-36) and EQ-5D questionnaires.
Results:
A total of 520 immunocompetent and 172 HSCT individuals with HZ were included. SF-36 and EQ-5D domain scores showed a significant relationship between increased HZ pain and worsening HRQoL. For every increase of 1 in the ZBPI pain score, the estimated mean decrease (worsening) in score in the ZOE-50/70 and ZOE-HSCT, respectively, was 2.0 and 2.4 for SF-36 Role Physical; 2.1 and 1.8 for SF-36 Social Functioning; and 0.041 and 0.045 for EQ-5D utility. Sleep and General activities were the ADL components most affected.
Discussion:
Moderate and severe HZ pain had a substantial negative impact on all aspects of HRQoL and ADL. This impact was independent of age and immunosuppression.
Key Words: HZ pain, quality of life, patient-reported outcomes, SF-36, EQ-5D
Herpes zoster (HZ) or shingles generally presents as a skin rash caused by reactivation of the varicella-zoster virus that blisters and scabs over a period of 7 to 10 days. The vast majority of people who get shingles will experience pain that typically lasts for 2 to 4 weeks; however, pain can persist beyond the acute phase (≤30 d post rash-onset) and subacute phase (30 to 90 d post rash-onset). Up to 30% of HZ patients develop postherpetic neuralgia (PHN, defined as pain persisting or appearing beyond 90 d post rash-onset), a chronic condition of debilitating pain that may last for months or even years and is very difficult to treat.1,2 One in 3 people in the United States will develop shingles in their lifetime, resulting in an estimated 1 million new cases annually.3
A few qualitative studies4,5 have described the patient experience of having HZ and PHN and the impact on health-related quality of life (HRQoL) at an individual level. Participants who developed HZ used words such as burning, sharp/shooting, sensitive to touch, and extreme/intense to describe their pain. These studies demonstrated that HZ has an impact on physical, emotional, social, and cognitive functioning, as well as sleep, hobbies, and work. Most publications of quantitative research describe HZ pain, but fewer publications describe how this pain impacts HRQoL. Schmader and colleagues detailed how increasing HZ pain and discomfort in older adults adversely affect a wide range of activities of daily living (ADL) as measured by the Zoster Brief Pain Inventory (ZBPI),6,7 whereas another study8 demonstrated that SF-36 Social Functioning and functional domains, Role Physical and Role Emotional, are substantially affected by HZ pain by comparing the scores to the normative values during the acute phase of the HZ episode.
Pickering et al9 carried out an extensive literature review in which they confirmed that acute HZ pain and PHN generate a significant impairment of HRQoL in the general population and more specifically in older persons, but also highlighted the scarcity of large-scale surveys such as analysis from clinical trials.
In this post hoc analysis, we describe the impact of HZ pain on HRQoL and ADL of both immunocompetent and immunocompromised participants during the acute phase using patient-reported outcomes (PROs) (see Graphical Abstract, Supplemental Digital Content, http://links.lww.com/CJP/B3).
MATERIALS AND METHODS
Study Design
The analysis is based on data from 3-multinational phase III randomized trials in which the primary endpoint assessed the vaccine efficacy of the recombinant zoster vaccine (RZV, Shingrix; GSK). The trials were conducted using similar methods in 2 adult populations: (1) the ZOE-50 study (NCT01165177), which included patients 50 years of age and older, and the ZOE-70 study (NCT01165229), which included patients 70 years of age and older; and (2) the ZOE-HSCT study (NCT01610414), which included an immunocompromised population of autologous hematopoietic stem cell transplantation (HSCT) recipients.10–12 Analysis of the characteristics of the HZ pain and the vaccine efficacy in reducing pain and the burden of illness are presented elsewhere.13,14
Our analysis includes only placebo recipients from the above clinical trials. The characteristics of the HZ episode in the same cohort of placebo participants are presented elsewhere.15
Outcome Measures
Patients with a suspected HZ episode were asked to complete the ZBPI daily for a minimum of 28 days after rash onset and then weekly until either the patient had been pain-free for 4 consecutive weeks or 90 days had elapsed after rash onset and for a maximum of 182 days. The ZBPI asks the participant to rate 4 categories of pain (least, worst, and average “in the last 24 hours,” and “right now”) on 11-point Likert-type scales (0 to 10, with 10 signifying the worst imaginable pain). The ZBPI questionnaire also assesses the degree to which the HZ pain interferes with 7 ADLs: general activity, mood, walking ability, work, relation with others, sleep, and enjoyment of life. These are all rated on 11-point Likert-type scales with 0 signifying ‘does not interfere’ and 10 ‘completely interferes’. A summary ADL score is calculated by averaging the scores for the 7 activities.13,14
HRQoL was assessed using 2 standard questionnaires, the Short Form Survey (SF-36)16 and the EuroQoL EQ-5D.17 Details of the SF-36 and EQ-5D can be found elsewhere but briefly, the SF-36 contains 36 questions from which 8 domain scores are calculated: Physical Functioning (PF), Role Physical (RP), Bodily Pain (BP), Social Functioning (SF), Vitality (VT), General Health (GH), Role Emotional (RE) and Mental Health (MH). Each domain is scored on a 0 to 100 scale in which a higher score indicates a higher level of functioning/quality of life (QoL). A brief explanation of each domain score can be found in the Supplementary Text (Supplemental Digital Content, http://links.lww.com/CJP/A964).
The EQ-5D-3L questionnaire is a generic measure of health status that defines health in terms of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. From these 5 categories, a single index or utility value is obtained. The United Kingdom time trade-off 18 was used to map the 5 responses to the corresponding utility value. The utility score ranges from −0.54 to 1, a higher score indicating a higher level of functioning/QoL.
A Visual Analog Scale (VAS) was also included as part of the EQ-5D in which a value in the range from 0 (worst imaginable health) to 100 (best imaginable health) is obtained. Both SF-36 and EQ-5D questionnaires were completed by all enrolled patients before the first vaccination and then at months 14, 26, and 38 in the ZOE-50 and 70 trials and at months 2 and 13 in the ZOE-HSCT trial. The latest SF-36 and EQ-5D questionnaires completed before the onset of the HZ episode served as the baseline values in our analysis.
During the HZ episode, the SF-36 and EQ-5D questionnaires were to be completed at the onset (day 0) and then on a weekly basis until the resolution of the episode.
To analyze the effect of HZ pain on HRQoL, we paired the ZBPI and SF-36/EQ-5D when both were completed on the same day. SF-36 and EQ-5D questionnaires that were not completed on the same day as the ZBPI were excluded from our analysis. Although the completion of questionnaires continued until the resolution of the HZ episode, we restricted our analysis to the time period between the day of onset of the episode and day 35.
Data from the ZOE-50 and ZOE-70 studies were combined for our analysis, whereas the ZOE-HSCT study was analyzed separately. This is justified as the ZOE-50 and ZOE-70 phase III randomized, placebo-controlled clinical trials were conducted concurrently at the same study sites using the same methods with patients 70 years of age and older randomly assigned to the ZOE-50 or ZOE-70 study.
Summary descriptive statistics of the domain scores and change from baseline in the SF-36 and EQ-5D domains were calculated by week (0, 1, 2, 3, 4, 5) and ZBPI Worst Pain Category (None [ZBPI Worst Pain Score=0], Mild [Score=1, 2, 3], Moderate [Score=4, 5, 6, 7] and Severe [Score=8, 9, 10]). This categorization of ZBPI worst pain follows exactly that used in the study by Schmader et al.6
We analyzed the change from the baseline of each SF-36/EQ-5D domain score over time using a repeated measures mixed effects model, adjusting for age and sex. Both the intercept and slope (ie, day of assessment) were allowed to vary per patient. The corresponding ZBPI worst pain score was included as a covariate.
We derived the area under the curve (AUC) during the acute phase (first 30 d) of the HZ episode of each SF-36 and EQ-5D domain (adjusted for baseline value) and ZBPI worst pain score using a trapezoidal approximation of the observed scores.19 The Spearman correlation coefficient between each domain score AUC and the ZBPI worst pain AUC was estimated.
RESULTS
In the ZOE-50, ZOE-70, and ZOE-HSCT studies, 280, 240, and 172 HZ cases occurred among the patients receiving placebo, respectively. The patient demographics by study group are presented in Table 1 (Supplemental Digital Content, http://links.lww.com/CJP/A965). The baseline (pre-HZ episode) SF-36 and EQ-5D domain scores are presented in Table 2 (Supplemental Digital Content, http://links.lww.com/CJP/A966) for ZOE-50/70 combined and ZOE-HSCT separately. The baseline mean scores were consistently lower across all domains for patients in the HSCT study compared with the ZOE-50/70 combined, notably for Role Physical (mean: 78.3 vs. 64.2) and Social Functioning (mean: 87.6 vs. 79.9).
Adherence to completion of questionnaires during the HZ episode was high (Table 3, Supplemental Digital Content, http://links.lww.com/CJP/A967). In the first 35 days of the HZ episode, 1257 SF-36 and ZBPI questionnaires were jointly completed on the same day by 277 (98.9%) patients in the ZOE-50, 1058 questionnaires by 237 patients (98.8%) in the ZOE-70 and 668 questionnaires by 163 (94.8%) patients in the ZOE-HSCT.
Figures 1 and 2 and Tables 4 (http://links.lww.com/CJP/A968) and 5 (http://links.lww.com/CJP/A969) of Supplemental Digital Content present the mean change from baseline for all SF-36 domain and EQ-5D utility and VAS scores across all timepoints categorized by ZBPI Worst Pain category for both the ZOE-50/70 and ZOE-HSCT. In the ZOE-50/70 analysis, in general, large changes from baseline (ie, worsening) were observed across all SF-36 and EQ-5D domains and across all timepoints for patients experiencing moderate and severe HZ pain. For example, on day 7, patients with severe pain reported a mean decrease of 32.7 in Role Physical score, a mean decrease of 19.5 in Mental health score, and a mean reduction of 0.4002 in EQ-5D utility score.
FIGURE 1.
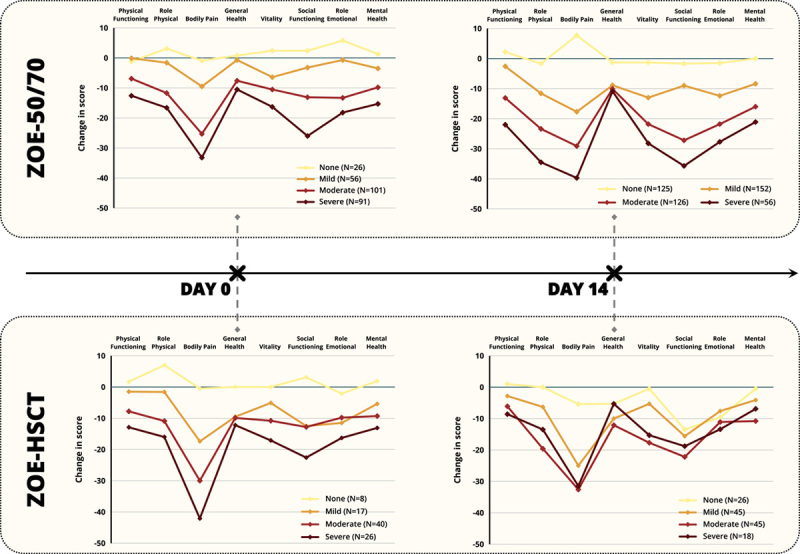
SF-36 Domain Scores by corresponding ZBPI Worst Pain Category at day 0 and day 14 of HZ episode. ZOE-50/70 and ZOE-HSCT. ZOE-50/70=Placebo participants from the ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) studies who had an HZ episode. ZOE-HSCT=Placebo participants from the ZOE-HSCT study (NCT01610414) who had an HZ episode. SF-36=Short Form Survey 36, ZBPI=Zoster Brief Pain Inventory. Pain categories: No Pain=ZBPI Worst pain score=0; Mild Pain=ZBPI pain score=1, 2, 3; Moderate Pain=ZBPI pain score=4, 5, 6, 7; Severe Pain=ZBPI pain score=8, 9, 10. N=Number of participants with a baseline (pre-HZ episode) SF-36 questionnaire and an SF-36 and ZBPI questionnaire at the relevant time point during the HZ episode. HSCT indicates hematopoietic stem cell transplantation; HZ, herpes zoster.
FIGURE 2.
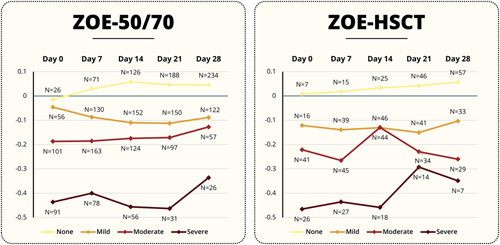
Change from baseline in EQ-5D Utility Score during HZ episode by corresponding ZBPI Worst Pain Category and time point. ZOE-50/70 ZOE-HSCT. ZOE-50/70=Placebo participants from the ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) studies who had an HZ episode. ZOE-HSCT=Placebo participants from the ZOE-HSCT study (NCT01610414) who had an HZ episode. EQ-5D=EuroQoL EQ-5D. Pain categories: None=ZBPI Worst pain score=0; Mild Pain=ZBPI pain score=1, 2, 3; Moderate Pain=ZBPI pain score=4, 5, 6, 7; Severe Pain=ZBPI pain score=8, 9, 10. N=Number of participants with a baseline (pre-HZ episode) EQ-5D utility assessment and both an EQ-5D utility and ZBPI worst pain score assessed at the relevant time point during the HZ episode. HSCT indicates hematopoietic stem cell transplantation; HZ, herpes zoster; ZBPI, Zoster Brief Pain Inventory.
Similar reductions were also observed late in the acute phase. On day 28, 83 patients were still reporting moderate or severe worst pain with a corresponding mean worsening in Role Physical of 25.5 for patients with moderate pain and 29.3 for those with severe pain. Similarly, a mean worsening in Mental Health score of 23.6 was observed for the 26 patients with severe pain on day 28. Worsening of scores was also observed in patients with mild ZBPI pain, but negligible changes were observed across all timepoints and SF-36/EQ-5D domains for patients reporting no ZBPI pain.
Similar results were observed in the ZOE-HSCT patients regarding the Bodily Pain, Role Physical, and EQ-5D utility domains, but less effect on Mental Health and Role Emotional domains was observed compared with the ZOE-50/70. Only 7 patients had reported severe pain by day 28. The 28 patients with moderate pain had a mean decrease in Role Physical of 22.5 and mean decrease in Social Functioning of 26.8.
Table 1 presents the estimates of the coefficient of the ZBPI worst pain score from the repeated measures mixed model. The estimate of −3.6 for Bodily Pain (ZOE-50/70) indicates that, for every 1-point increase in the ZBPI worst pain score, the corresponding mean decrease (worsening) in SF-36 Bodily Pain from the baseline score (pre-HZ episode) was 3.6.
TABLE 1.
Estimates of the Mean Reduction in SF-36/EQ-5D Domain Scores for Every Increase of 1 Point in the ZBPI Worst Pain Score
| Estimate | SE | t value | P | |
|---|---|---|---|---|
| ZOE-50/70 | ||||
| SF-36 | ||||
| PF | −1.50 | 0.141 | −10.65 | <0.0001 |
| RP | −2.00 | 0.197 | −10.18 | <0.0001 |
| BP | −3.63 | 0.181 | −20.11 | <0.0001 |
| GH | −0.84 | 0.113 | −7.44 | <0.0001 |
| VT | −1.64 | 0.155 | −10.58 | <0.0001 |
| SF | −2.07 | 0.186 | −11.13 | <0.0001 |
| RE | −1.36 | 0.198 | −6.87 | <0.0001 |
| MH | −1.52 | 0.134 | −11.37 | <0.0001 |
| EQ-5D | ||||
| Utility | −0.041 | 0.002 | −20.87 | <0.0001 |
| VAS | −1.94 | 0.136 | −14.30 | <0.0001 |
| ZOE-HSCT | ||||
| SF-36 | ||||
| PF | −1.57 | 0.307 | −5.11 | <0.0001 |
| RP | −2.35 | 0.387 | −6.06 | <0.0001 |
| BP | −3.78 | 0.367 | −10.30 | <0.0001 |
| GH | −0.60 | 0.203 | −2.93 | 0.036 |
| VT | −1.56 | 0.298 | −5.24 | <0.0001 |
| SF | −1.84 | 0.376 | −4.89 | <0.0001 |
| RE | −1.02 | 0.361 | −2.84 | 0.0048 |
| MH | −0.97 | 0.254 | −3.81 | 0.0002 |
| EQ-5D | ||||
| Utility | −0.044 | 0.0040 | −11.12 | <0.0001 |
| VAS | −2.31 | 0.271 | −8.55 | <0.0001 |
ZOE-50/70 combines participants from the ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) studies. ZOE-HSCT=participants from the ZOE-HSCT study (NCT01610414).
Estimates, SE, t values, and P-values obtained from the repeated measures, mixed effects model, adjusting for age and sex with random intercept and slope (ie, day of assessment) and ZBPI worst pain score included as a covariate. SF-36 domains and EQ-5D VAS are scored on a 0 to 100 scale, the range of the EQ-5D utility is (−0.5, 1). A higher score indicates a higher level of functioning/quality of life.
BP indicates Bodily Pain; EQ-5D, EuroQoL EQ-5D; GH, General Health; HSCT, hematopoietic stem cell transplantation; MH, Mental Health; PF, Physical Functioning; RE, Role Emotional; RP, Role Physical; SF, Social Functioning; SF-36, Short Form Health Survey 36; Utility, Utility Score; VAS, Visual Analog Scale; VT, Vitality; ZBPI, Zoster Brief Pain Inventory.
All SF-36 and EQ-5D domain scores showed a significant relationship between increased HZ pain and worsening HRQoL (both ZOE-50/70 and ZOE-HSCT). The domain scores that showed the highest absolute impact from HZ pain were SF-36 Bodily Pain (3.6 in the ZOE-50/70, 3.8 in the ZOE-HSCT), Role Physical (2.0 and 2.4, respectively), Social Functioning (2.1 and 1.8, respectively) and EQ-5D utility (0.041 and 0.045, respectively). The domain score showing the smallest absolute impact was General Health (0.8 and 0.6, respectively).
The domain scores associated with Mental Health and Role Emotional functioning were also impacted, with scores of 1.5 and 1.4, respectively, reported in the ZOE-50/70; a smaller absolute impact of 1.0 in both domains was observed in the ZOE-HSCT.
Day of assessment was not a significant factor in each model apart from Bodily Pain, indicating that the effect of HZ pain on HRQoL was the same regardless of when in the acute phase the ZBPI pain was experienced. In the ZOE-50/70 studies, sex was only significant in the model of Role Emotional over time. By contrast, in the ZOE-HSCT study, sex was a significant factor in the decrease in Physical Functioning, General Health, Vitality, Role Emotional, and Mental Health, with females experiencing larger average reductions in HRQoL from baseline. Age was not a significant factor in any of the models apart from change in EQ-5D utility over time in the ZOE-50/70 analysis.
The AUC analysis provided similar results to the repeated measures mixed model. Table 6 (Supplemental Digital Content, http://links.lww.com/CJP/A970) presents the AUC for each domain score adjusted for baseline categorized by the corresponding ZBPI Worst Pain AUC and Table 2 presents the corresponding correlation coefficients. The ZBPI AUC is highly correlated with all SF-36 and EQ-5D domain AUC values.
TABLE 2.
Spearman Correlation Coefficients Between ZBPI Worst Pain Score AUC and Each Corresponding SF-36 Domain and EQ-5D Utility Score AUC During First 30 Days of Herpes Zoster Episode
| Correlation coefficient/P/(N) | |||
|---|---|---|---|
| Domain | ZOE-50/70 | ZOE-HSCT | |
| SF-36 | PF | −0.485/<0.0001/N=499 | −0.425/<0.0001/N=152 |
| RP | −0.571/<0.0001/N=499 | −0.491/<0.0001/N=151 | |
| BP | −0.785/<0.0001/N=498 | −0.757/<0.0001/N=152 | |
| GH | −0.372/<0.0001/N=499 | −0.319/<0.0001/N=152 | |
| VT | −0.536/<0.0001/N=499 | −0.428/<0.0001/N=151 | |
| SF | −0.596/<0.0001/N=498 | −0.421/<0.0001/N=152 | |
| MH | −0.483/<0.0001/N=499 | −0.357/<0.0001/N=151 | |
| RE | −0.510/<0.0001/N=499 | −0.374/<0.0001/N=151 | |
| EQ-5D | Utility | −0.701/<0.0001/N=498 | −0.615/<0.0001/N=151 |
| VAS | −0.557/<0.0001/N=498 | −0.486/<0.0001/N=151 | |
ZOE-50/70=Placebo participants from the ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) studies who had an herpes zoster episode.
ZOE-HSCT=Placebo participants from the ZOE-HSCT study (NCT01610414) who had an herpes zoster episode.
N=Number of participants with a nonmissing ZBPI AUC and domain AUC during the first 30 days of the herpes zoster episode.
AUC indicates area under curve; BP, Bodily Pain; EQ-5D, EuroQoL EQ-5D; GH, General Health; HSCT, hematopoietic stem cell transplantation; MH, Mental Health; PF, Physical Functioning; RE, Role Emotional; RP, Role Physical; SF, Social Functioning; SF-36, Short Form Health Survey 36; Utility, Utility Score; VAS, Visual Analog Scale; VT, Vitality; ZBPI, Zoster Brief Pain Inventory.
Effects of HZ Pain on ZBPI Components
Figure 3 shows the mean ADL component scores at the onset of the HZ episode (day 0) categorized by ZBPI worst pain score for both the ZOE-50/70 and ZOE-HSCT studies. The correlation between increasing pain and the negative impact on ADL was clear across both analyses. The components most affected were Sleep and General Activities, but the negative impact was seen in all components.
FIGURE 3.
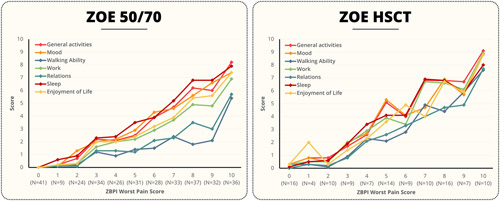
ZBPI ADL components at day 0 by Worst Pain Score. ZOE-50/70=Placebo participants from the ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) studies who had an HZ episode. ZOE-HSCT=Placebo participants from the ZOE-HSCT study (NCT01610414) who had an HZ episode. ADL indicates activities of daily living; HSCT, hematopoietic stem cell transplantation; HZ, herpes zoster; N, number of participants by ZBPI worst pain score at day 0; ZBPI, Zoster Brief Pain Inventory.
Table 3 presents the estimates of the coefficient of the ZBPI worst pain score from the repeated measures mixed model of the ADL components. The components with the highest coefficient were General Activities (0.57 in the ZOE-50/70, 0.76 in the ZOE-HSCT) and Sleep (0.57 in the ZOE-50/70, 0.68 in the ZOE-HSCT). It is worth noting that the estimates appear to be higher in the ZOE-HSCT, indicating that HZ pain appeared to have a larger impact on ADL in HSCT recipients.
TABLE 3.
Estimates of the Mean Increase (Worsening) in ZBPI ADL Individual Component and Total Score for Every Increase of 1 Point in ZBPI Worst Pain Score
| ZBPI ADL component | Estimate | SE | t value | P | |
|---|---|---|---|---|---|
| ZOE-50/70 | General activities | 0.57 | 0.0137 | 41.13 | <0.0001 |
| Mood | 0.51 | 0.0137 | 37.22 | <0.0001 | |
| Walking ability | 0.27 | 0.0121 | 22.21 | <0.0001 | |
| Normal work | 0.47 | 0.0142 | 33.04 | <0.0001 | |
| Relations with other people | 0.33 | 0.0140 | 23.88 | <0.0001 | |
| Sleep | 0.57 | 0.0147 | 39.08 | <0.0001 | |
| Enjoyment of life | 0.49 | 0.0148 | 32.84 | <0.0001 | |
| ADL Total | 0.43 | 0.0098 | 44.11 | <0.0001 | |
| ZOE-HSCT | General activities | 0.76 | 0.0215 | 35.46 | <0.0001 |
| Mood | 0.65 | 0.0214 | 30.51 | <0.0001 | |
| Walking ability | 0.51 | 0.0225 | 22.84 | <0.0001 | |
| Normal work | 0.71 | 0.0237 | 29.78 | <0.0001 | |
| Relations with other people | 0.54 | 0.0235 | 22.99 | <0.0001 | |
| Sleep | 0.68 | 0.0227 | 29.93 | <0.0001 | |
| Enjoyment of life | 0.63 | 0.0241 | 26.16 | <0.0001 | |
| ADL Total | 0.64 | 0.0168 | 37.90 | <0.0001 |
ZOE-50/70=Placebo participants from the ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) studies who had an herpes zoster episode.
ZOE-HSCT=Placebo participants from the ZOE-HSCT study (NCT01610414) who had an herpes zoster episode.
The ZBPI Worst Pain score and ADL component and total scores all range from 0 to 10 with a higher score indicating increased pain/negative impact on quality of life.
Estimates, SE, t values, and P-values obtained from the repeated measures, mixed effects model, adjusting for age and sex with random intercept and slope (ie, day of assessment) and ZBPI worst pain score included as a covariate.
ADL indicates activities of daily living; HSCT, hematopoietic stem cell transplantation; ZBPI, Zoster Brief Pain Inventory.
DISCUSSION
In this study, we presented the PRO data from patients who experienced an HZ episode in the placebo groups of the ZOE-50, ZOE-70, and ZOE-HSCT studies. We have shown that increasing HZ pain is highly correlated with worsening HRQoL. A strength of our analysis is the large sample size and the quality of the data reflected in the high completion rate of questionnaires throughout the acute phase of the HZ episode. Another strength is the fact that each patient had a baseline PRO assessment before the HZ episode. This eliminates the need to compare domain scores during the HZ episode to population normative values. We were therefore able to run a repeated measures mixed model for the combined ZOE-50/70 analysis and ZOE-HSCT separately which gave robust estimates of the association between HZ pain and summary measures of HRQoL and ADL.
We have previously shown in another study that for this same cohort of placebo patients that pain was experienced by 491 of 520 patients (94.4%) in the combined ZOE-50/70 studies and by 163 of 172 of patients (93%) in the ZOE-HSCT study.15 The median duration of clinically significant pain (ie, a ZBPI worst pain score of at least 3) was estimated to be 17 days in the ZOE-50 study, 22 days in the ZOE-70 study, and 30 days in the HSCT study.
The impact of increasing HZ pain was observed across all SF-36 domains, but the Bodily Pain, Role Physical and Social Functioning domains showed the greatest impact in absolute terms of HZ pain in both the ZOE-50/70 and ZOE-HSCT. This is a similar finding to that observed in Lydick et al.8 The effect on the Bodily Pain domain may seem obvious at first, but it is nevertheless an important indication of the cross-validity of the ZBPI and SF-36. It is also relevant to highlight that SF-36 Bodily Pain covers both the magnitude of pain and also the interference of pain with normal work activities. The negative impact of increasing HZ pain on the EQ-5D utility score was also apparent. This is similar to the effects seen in patients with PHN in both Oster et al20 and Serpell et al.21
The impact of increasing HZ pain on participants’ ADL, as measured solely by the ZBPI, was also evident from our analysis. In particular, we observed a large negative effect on Sleep, Mood, General Activities, and ability to carry out normal work activities. We also observed that these negative effects were consistently higher for immunocompromised participants in the ZOE-HSCT analysis.
The absolute impact on HRQoL as assessed by the SF-36 and EQ-5D questionnaires was similar across both ZOE-50/70 and ZOE-HSCT analyses but it is worth mentioning that patients in the ZOE-HSCT study had lower baseline values for all domain scores, suggesting a lower level of functioning/QoL. Thus the relative impact of the worsening of QoL may have been greater for the immunocompromised patients.
We observed a minimal effect on HRQoL for patients who reported no HZ pain. We may have expected a negative impact on HRQoL or interference with ADL related to rash or itching, independent of pain. In the analysis by Schmader et al,6 such an association was described. It is important to mention, however, that in the ZOE-50/70 and ZOE-HSCT studies, patients with no pain were not required to complete the rest of the ZBPI questionnaire detailing ADL but were still nonetheless required to complete the SF-36 and EQ-5D.
We also observed that the changes in domain scores were independent of time, that is, given a certain level of pain (moderate or severe pain), the impact on HRQoL was similar at the beginning and the end of the acute phase.
It is well known that the majority of HRQoL domains decrease (worsen) with age but we observed that the mean reduction from baseline conditional on the level of HZ pain was independent of age. However, it should be mentioned that an absolute reduction in, for example, Physical Functioning of 20 points may have more of an impact (ie, greater relative reduction) in a frail older person with a lower normal/baseline value than the same absolute reduction in a healthier younger patient.
To put our results into context, it is worthwhile examining minimally important differences (MID) in HRQoL measures across other conditions/disease areas. Several authors have suggested using Cohen d “medium” effect size (ie, half an SD) as a threshold for defining MIDs in HRQoL instruments.22–24 A threshold of 0.3 times the SD has also been proposed by others25 who commented that, although this method may be flawed, it does give us an indication of what may be considered an important change. Our observed changes are, for patients with moderate and severe HZ pain, almost always much greater than 0.5 times the SD across every SF-36 and EQ-5D domain at every time point.
Spiegel et al26 proposed a MID of 4.2 points (range from 3 to 5) for the SF-36 Vitality domain in individuals with compensated hepatitis C virus, based on an expert panel assessment using a modified Delphi technique. The vitality domain was also the focus of an analysis performed on data across multiple conditions from a medical outcomes study.27 MID of 5 points was estimated in patients with anemia, and of 6 points in patients with either congestive heart failure or chronic obstructive pulmonary disorder. Based on these values, the vitality score was impacted overall by an important difference in patients with HZ, in particular on day 0 and day 7, and for most weeks when patients had even just mild HZ pain. HZ patients with moderate or severe pain reported values that were on average consistently much higher than MID values of 5 to 6 points (Tables 4, Supplemental Digital Content, http://links.lww.com/CJP/A968 and 5, Supplemental Digital Content, http://links.lww.com/CJP/A969).
MIDs of 5.3 in Physical Functioning and 7.2 in the Bodily Pain domains were estimated for patients with worsening symptoms of osteoarthritis of the lower extremities.28 Mean MIDs of 9.3 for Social Functioning, 7.8 for Vitality, and 15.6 for Role Physical domains were observed in patients reporting an improvement in symptoms of rheumatoid arthritis.29
In a retrospective analysis of patients receiving chemotherapy for different types of cancers, MIDs for the EQ-5D UK utility scores ranging from 0.10 to 0.12 and VAS scores ranging from 8 to 12 were estimated.30 MIDs of 14.4 for the VAS score and 0.109 for the EQ-5D utility were estimated in patients with deteriorating health from inflammatory bowel disease.31 It is notable that the differences we observed across all domains in our analysis were consistently higher than the MIDs detailed above not only in patients with severe HZ pain but also in those patients who report moderate pain.
Qualitative research can help contextualize the findings of this quantitative research. Few studies examine the qualitative impact of HZ pain on HRQoL. In a recent study,4 a total of 32 patients with a mean age of 61 years with HZ participated in concept elicitation interviews by telephone and were asked to describe in their own words the impact of HZ. Overall, 97% of patients described an effect on Emotional Functioning, using words such as “self-conscious/embarrassed” and “stress/anxiety.” Effects on Social Functioning were reported by 63% of patients, with the majority citing social isolation and an inability to carry out normal hobbies. In the study of Weinke et al,5 280 patients with HZ with a mean age of 63.5 years were interviewed by telephone. A total of 80% of patients cited feelings of stress, 68% cited the impact on moderate physical efforts and 37% cited a lack of enjoyment in leisure activities caused by HZ.
The effect of vaccination with RZV in reducing the impact on HRQoL of HZ pain was evaluated in both the ZOE-50/70 pooled and ZOE-HSCT phase III clinical trials. In the ZOE-HSCT analysis, significant differences between the vaccinated and placebo groups were observed at week 1 of the HZ episode in the SF-36 Bodily Pain, Social Functioning, Role Emotional, Mental Health, and EQ-5D utility domains (see table 4 of Curran et al13). Similar trends were observed in the ZOE-50/70 studies14; however, as there were few breakthrough HZ episodes among the vaccinated cohort, none of the comparisons resulted in statistical significance. However, the data suggest a clear trend, that vaccination with RZV not only maintains QoL by preventing HZ episodes, but it also reduces the burden of HZ pain and consequently the negative impact of this pain on HRQoL in individuals who develop disease despite vaccination.
CONCLUSIONS
The pain associated with an HZ episode has a large impact across all aspects of HRQoL. The magnitude of impact increases with increasing pain.
DATA SHARING STATEMENT
GSK makes available the anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To request access to patient-level data and documents for this study, please submit an enquiry via HYPERLINK “http://www.clinicalstudydatarequest.com/”www.clinicalstudydatarequest.com. Information on GSK's data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at HYPERLINK “http://www.clinicalstudydatarequest.com/”www.clinicalstudydatarequest.com.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the investigators of the ZOE studies for their support in the conception of the studies. They also thank the Business and Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK.
Footnotes
D.C., K.S., and N.L. were involved in the conception and/or the design of the study. A.L.C., C.B., D.C., K.S., and N.L. participated in the collection/generation of the study data. A.L.C., C.B., D.C., E.S.C., K.S., N.L., and S.M. were involved in the analysis and/or the interpretation of data. All authors had full access to the data and gave approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with the recommendations of the International Committee of Medical Journal Editors for conducting, reporting, editing, and publication of scholarly work in medical journals.
Some of the data in this study have been presented as a poster at IDWeek 2021 (from September 29 to October 3, 2021, virtual event).
GlaxoSmithKline Biologicals SA (Brentford, United Kingdom) funded this study (GSK study identifiers: 110390, 113077) and was involved in all stages of study conduct, including the analysis of the data. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and publication of this article. C.B., D.C., E.S.C., and N.L. are employed by/hold shares in GSK. A.L.C. reports a grant from GSK and received honoraria paid to his institution Merck Serono (Merck, Darmstadt, Germany), and BioCSL/Sequirus (Melbourne, Australia) outside the submitted work. S.M. reports personal fees from GSK during the conduct of the study and outside the submitted work. N.L. reports patents planned, issued, or pending outside the submitted work. The remaining authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.clinicalpain.com.
Contributor Information
Sean Matthews, Email: sean.f.matthews@gsk.com.
Desmond Curran, Email: desmond.x.curran@gsk.com.
Eliazar Sabater Cabrera, Email: ELIAZAR.X.SABATERCABRERA@GSK.COM.
Céline Boutry, Email: celine.x.boutry@gsk.com.
Nicolas Lecrenier, Email: nicolas.lecrenier@gsk.com.
Anthony L. Cunningham, Email: tony.cunningham@sydney.edu.au.
Kenneth Schmader, Email: kenneth.schmader@duke.edu.
REFERENCES
- 1. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen KR, Salbu RL, Frank J, et al. Presentation and management of herpes zoster (shingles) in the geriatric population. P T. 2013;38:217–227. [PMC free article] [PubMed] [Google Scholar]
- 3. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57:1–30. [PubMed] [Google Scholar]
- 4. Van Oorschot D, McGirr A, Goulet P, et al. A cross-sectional concept elicitation study to understand the impact of herpes zoster on patients’ health-related quality of life. Infect Dis Ther. 2022;11:501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinke T, Edte A, Schmitt S, et al. Impact of herpes zoster and post-herpetic neuralgia on patients’ quality of life: a patient-reported outcomes survey. J Public Health (Bangkok). 2010;18:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmader KE, Sloane R, Pieper C, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–496. [DOI] [PubMed] [Google Scholar]
- 7. Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the Brief Pain Inventory. J Pain. 2004;5:344–356. [DOI] [PubMed] [Google Scholar]
- 8. Lydick E, Epstein RS, Himmelberger D, et al. Herpes zoster and quality of life: a self-limited disease with severe impact. Neurology. 1995;45:S52. [DOI] [PubMed] [Google Scholar]
- 9. Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Pract. 2011;11:397–402. [DOI] [PubMed] [Google Scholar]
- 10. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. [DOI] [PubMed] [Google Scholar]
- 11. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. [DOI] [PubMed] [Google Scholar]
- 12. Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curran D, Matthews S, Rowley SD, et al. Recombinant zoster vaccine significantly reduces the impact on quality of life caused by herpes zoster in adult autologous hematopoietic stem cell transplant recipients: a randomized placebo-controlled trial (ZOE-HSCT). Biol Blood Marrow Transplant. 2019;25:2474–2481. [DOI] [PubMed] [Google Scholar]
- 14. Curran D, Oostvogels L, Heineman T, et al. Quality of life impact of an adjuvanted recombinant zoster vaccine in adults aged 50 years and older. J Gerontol A Biol Sci Med Sci. 2019;74:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curran D, Matthews S, Boutry C, et al. Natural history of herpes zoster in the placebo groups of three randomized phase III clinical trials. Infect Dis Ther. 2022;11:2265–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ware J, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. QualityMetric Incorporated; 1993. [Google Scholar]
- 17. Kind P. Spilker B. The EuroQoL instrument: an index of health-related quality of life. Quality of Life and Pharmacoeconomics in Clinical Trials. Lippincott-Raven Publishers; 1996:191–201. [Google Scholar]
- 18. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 19. Cox DR, Fitzpatrick R, Fletcher AE, et al. Quality-of-life assessment: can we keep it simple? J R Stat Soc Ser A Stat Soc. 1992;155:353–393. [Google Scholar]
- 20. Oster G, Harding G, Dukes E, et al. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6:356–363. [DOI] [PubMed] [Google Scholar]
- 21. Serpell M, Gater A, Carroll S, et al. Burden of post-herpetic neuralgia in a sample of UK residents aged 50 years or older: findings from the zoster quality of life (ZQOL) study. Health Qual Life Outcomes. 2014;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sawilowski S. New effect size rules of thumb. J Mod Appl Stat Meth. 2009;8:597–599. [Google Scholar]
- 23. Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4:581–585. [DOI] [PubMed] [Google Scholar]
- 24. Farivar SS, Liu H, Hays RD. Half standard deviation estimate of the minimally important difference in HRQOL scores? Expert Rev Pharmacoecon Outcomes Res. 2004;4:515–523. [DOI] [PubMed] [Google Scholar]
- 25. Haim Erder M, Santanello N, Hays RD. Assessing the clinical significance of patient-reported outcomes: examples drawn from a recent meeting of the drug information association (DIA). Clin Ther. 2003;25:D12–D13. [Google Scholar]
- 26. Spiegel BMR, Younossi ZM, Hays RD, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800. [DOI] [PubMed] [Google Scholar]
- 27. Bjorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 Vitality Scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23:731–739. [DOI] [PubMed] [Google Scholar]
- 28. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–391. [DOI] [PubMed] [Google Scholar]
- 29. Kosinski M, Zhao SZ, Dedhiya S, et al. Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43:1478–1487. [DOI] [PubMed] [Google Scholar]
- 30. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stark RG, Reitmeir P, Leidl R, et al. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis. 2010;16:42–51. [DOI] [PubMed] [Google Scholar]


