Abstract
Background:
Air pollution is linked to neurodevelopmental delays, but its association with longitudinal changes in brain network development has yet to be investigated. We aimed to characterize the effect of PM2.5, O3, and NO2 exposure at ages 9–10 years on changes in functional connectivity (FC) over a 2-year follow-up period, with a focus on the salience (SN), frontoparietal (FPN), and default-mode (DMN) brain networks as well as the amygdala and hippocampus given their importance in emotional and cognitive functioning.
Methods:
A sample of children (N = 9,497; with 1–2 scans each for a total of 13,824 scans; 45.6% with two brain scans) from the Adolescent Brain Cognitive Development (ABCD) Study® were included. Annual averages of pollutant concentrations were assigned to the child’s primary residential address using an ensemble-based exposure modeling approach. Resting-state functional MRI was collected on 3T MRI scanners. First, developmental linear mixed-effect models were performed to characterize typical FC development within our sample. Next, single- and multi-pollutant linear mixed-effect models were constructed to examine the association between exposure and intra-network, inter-network, and subcortical-to-network FC change over time, adjusting for sex, race/ethnicity, income, parental education, handedness, scanner type, and motion.
Results:
Developmental profiles of FC over the 2-year follow-up included intra-network integration within the DMN and FPN as well as inter-network integration between the SN-FPN; along with intra-network segregation in the SN as well as subcortical-to-network segregation more broadly. Higher PM2.5 exposure resulted in greater inter-network and subcortical-to-network FC over time. In contrast, higher O3 concentrations resulted in greater intra-network, but less subcortical-to-network FC over time. Lastly, higher NO2 exposure led to less inter-network and subcortical-to-network FC over the 2-year follow-up period.
Conclusion:
Taken together, PM2.5, O3, and NO2 exposure in childhood relate to distinct changes in patterns of network maturation over time. This is the first study to show outdoor ambient air pollution during childhood is linked to longitudinal changes in brain network connectivity development.
Keywords: Air pollution, Brain development, Functional magnetic resonance imaging, Resting-state, Functional connectivity, Longitudinal, Adolescence
1. Introduction
Ambient air pollutants are increasingly being recognized as consequential neurotoxicants, in addition to their link to adverse cardiovascular and pulmonary health (reviews by Castagna et al. 2022; Herting et al. 2019). The Environmental Protection Agency (EPA) tracks pollutants, including particulate matter (PM) of different size fractions, specifically particulate matter (PM) with diameter < 10 μm (PM10) and < 2.5 μm (PM2.5). Among these, the World Health Organization’s (WHO) Global Burden of Disease Project recognizes PM2.5 as a leading cause of adverse health outcomes; its small size allows for particles to be inhaled deeply into the lungs and enter the bloodstream, causing systemic inflammation and affecting multiple biological systems (Cohen et al. 2005). Outdoor PM2.5 comes primarily from combustion of gasoline, oil, diesel fuel, coal, or wood. Other pollutants of neurological concern tracked by the EPA include ground level ozone (O3), a key component of smog formed from the reaction between sunlight and nitrogen oxides, as well as nitrogen dioxide (NO2), an important fraction of PM2.5 and the main source of nitrate aerosols (WHO, 2022).
While the toxic neurological effects of ambient air pollution may impact individuals at all ages (Livingston et al. 2020; Russ, Reis, and van Tongeren 2019; Jayaraj et al. 2017), children are thought to be particularly vulnerable given their higher respiratory rates compared to adults (Buka, Koranteng, and Osornio-Vargas 2006) and the rapid and dynamic neural change that occurs during childhood (Sunyer 2008). Furthermore, the transition from childhood to adolescence represents a sensitive period of neurodevelopment, suggesting that exposures at this time may have an impact on long-term cognitive and emotional functioning (Kessler et al., 2005; Paus et al., 2008; Casey et al., 2008). Using magnetic resonance imaging (MRI), evidence suggests a number of neurodevelopmental consequences of outdoor air pollution exposure, including aberrations in gray and white matter volumes and microstructure, cortical thickness, and brain function (for review, see Herting et al. 2019; Peterson et al. 2015; Pujol, Martínez-Vilavella, et al. 2016; Pujol, Fenoll, et al. 2016; Mortamais et al. 2017; Guxens et al. 2018; Alemany et al. 2018; Cserbik et al. 2020; Lubczyńska et al. 2020; 2021; Burnor et al. 2021; Pérez-Crespo et al. 2022; Peterson et al. 2022; Sukumaran et al. 2023). Although initial MRI studies focused on prenatal exposure, emerging research suggests exposure to ambient PM2.5 and its constituents during childhood is also associated with differences in cortical thickness and subcortical volumes (Mortamais et al. 2017; Alemany et al. 2018; Cserbik et al. 2020; Lubczyńska et al. 2021) as well as altered white matter microstructural integrity (Lubczyńska et al. 2020; Burnor et al. 2021) and subcortical gray matter microarchitecture (Sukumaran et al. 2023). Potential mechanisms through which air pollution can cause neurotoxicity include systemic and neuro-inflammation, induced oxidative stress and the resulting increase in free radicals, as well as damage to neurovascular units, endothelial cells, and all tissue barriers in the body, including nasal, lung, gastrointestinal, and blood–brain barriers (for reviews, see Calderón-Garcidueñas et al. 2016 & You, Ho, and Chang 2022). However, the impact of air pollution exposure on functional brain network maturation during this critical period of early adolescence is not well understood.
One in vivo method that can be leveraged for studying air pollution and functional brain maturation is resting-state functional magnetic resonance imaging (rs-fMRI), which quantifies the temporal correlation of activity across different brain regions at rest, revealing robust large-scale resting-state functional networks (Beckmann et al. 2005; Yeo et al. 2011). These core brain networks continue to mature across childhood and adolescence (Grayson and Fair 2017), including the frontoparietal network (FPN), the default mode network (DMN), and the salience network (SN), which are involved in emotional regulation, acute stress response, and executive function (Seeley et al. 2007; Hamilton et al. 2011; Hermans et al. 2014). Specifically, these three networks are part of the triple-network model, with each network working in tandem to successfully produce a variety of cognitive tasks (Seeley et al. 2007; Sridharan, Levitin, and Menon 2008; V. Menon and Uddin 2010). The FPN is anatomically anchored in the dorsolateral prefrontal cortex (dlPFC) and the posterior parietal cortex (PPC) and is functionally described as a task-positive network involved in executive functions like attention, problem solving, and working memory (Marek and Dosenbach 2018). The DMN is anatomically anchored in the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), precuneus, and the angular gyrus and is functionally described as a task-negative network that is active in passive rest, mind-wandering, and day-dreaming (Raichle 2015). The SN is anatomically anchored in the insula and dorsal anterior cingulate cortex (dACC) and is theorized to mediate the switch between the FPN and DMN in response to salient stimuli (Seeley et al. 2007; Goulden et al. 2014). As Menon (2019) eloquently states, together these three networks consolidate past and present sensory, affective, and cognitive inputs (SN), integrate this information into an individualized narrative (DMN), and respond accordingly by executing emotional reactions and/or planned, goal-directed activities (FPN). These networks form the basis for a range of important cognitive and emotional functions integral to everyday life, with dysfunction of this triple network likely to contribute to an array of complex brain disorders, including psychopathologies that commonly emerge during adolescence.
Patterns of integration and segregation within and between intrinsic brain networks tend to mirror functional developmental milestones. For example, sensorimotor and visual networks reach maturation by early childhood, whereas networks involved in higher order cognitive and emotional functions (i.e., FPN, SN, DMN) experience dynamic restructuring in later childhood and into adulthood (Kiviniemi et al. 2000; Redcay, Kennedy, and Courchesne 2007; Lin et al. 2008; Xiao et al. 2016; Grayson and Fair 2017). In addition to network maturation, subcortical regions like the hippocampus and amygdala experience a period of significant plasticity during childhood and adolescence (Curlik, Difeo, and Shors 2014; DiFeo and Shors 2017; for reviews, see Scherf, Smyth, and Delgado 2013 & Tottenham and Galván 2016). Children exhibit increased subcortical-cortical connectivity compared to young adults, where there is more prominence among cortical connections (Grayson and Fair 2017). This neural maturation and increased subcortical-cortical connectivity is likely important for the development of executive functions, memory, and emotional regulation, and is thought to represent a period of particular vulnerability to insult from a myriad of exposures, including neurotoxicants like air pollution (Hedges et al. 2019). However, despite several existing studies on the topic, questions remain about the exact developmental profiles of intrinsic brain networks given inconsistencies in the literature. For example, while the intrinsic functional network hierarchy is largely in place as early as 1 year of age, important networks undergo differential patterns of between- and within-network integration and segregation (Gilmore, Santelli, and Gao 2018). In normative healthy neurodevelopment there is evidence that both the FPN and DMN have well-connected local (e.g., brain regions close together) within-network patterns early in development, with longer-range connections between more distal brain regions continuing to develop in late childhood (Long et al. 2017). In contrast, longitudinal reductions are seen in within-network SN connectivity during adolescence (Teeuw et al. 2019). Results from a more recent cross-sectional study by Sanders et al. (2023) further supports the tenet that age-related increases in within-network connectivity occur in DMN and FPN but decreases occur in within-network connectivity in the SN during childhood and into early adolescence. As for between-network connectivity, current evidence points to segregation for all canonical networks, namely those within the triple network model (FPN, SN, DMN) (DeSerisy et al. 2021; Thomson et al. 2022). For example, a recent study by DeSerisy et al. (2021) found connectivity between DMN and FPN brain regions were increasingly anticorrelated in older participants (age range: 7–25 years), suggesting between-network segregation in these networks. Additionally, Thomson et al. (2022) found decreased SN-FPN and SN-DMN network connectivity in children. As such, a tenet in the existing literature is that by adulthood brain regions within-network show stronger correlations with each other, whereas brain regions that are from different networks show weaker correlations, respectively. However, it is important to note that not all studies find the same degree of large-scale network reorganization across development (Sylvester et al. 2018) and accelerated cohort studies suggest that the transition from childhood to adolescence may in fact be a sensitive period hallmarked by greater, and possibly more dynamic, changes in functional connectivity (FC). For example, in a cross-sectional study conducted by Marek et al. (2015), both within- and between-network connectivity was lower during childhood to early adolescence (10–15 years of age); later in development, between-network integration was higher in young adulthood. The authors posit that the observed between-network increases may explain increased ability for cognitive control during the transition to young adulthood. However, Sanders et al. (2023) found no significant age-related differences in between-network dynamics involving our three networks of interest (i.e., SN-DMN, SN-FPN, or FPN-DMN). Discrepancies among the literature could likely be attributed to methodological differences, such as study design (cross-sectional vs longitudinal), analysis (i.e., pairwise correlations, graph theory, independent component analysis), scanner hardware and software differences, and differences in how head motion was handled. Thus, although patterns exist in children versus adults, few studies have examined longitudinal changes in these networks during the transition to early adolescence, highlighting the need for additional longitudinal neuroimaging studies aimed at characterizing intrinsic functional network connectivity (Stevens 2016; Grayson and Fair 2017; Thomson et al. 2022; Cao et al. 2016; Ernst et al. 2015, Supekar, Musen, and Menon 2009; Qin et al. 2012) as well as examining to what degree these patterns may be altered by outdoor air quality.
To date, only three studies have described cross-sectional associations between exposure to air pollution and functional brain network connectivity (Pujol, Martínez-Vilavella, et al. 2016; Pujol, Fenoll, et al. 2016; Pérez-Crespo et al. 2022) and no studies have examined whether air pollution exposures affect longitudinal changes in FC development over time. In a sample of 236 children aged 8–12 years old from Barcelona, Spain, Pujol, Martínez-Vilavella, et al. (2016) found that air pollution exposure during childhood in the form of a combined estimate of NO2 and elemental carbon was significantly associated with decreased intra-DMN FC, indicative of lower within-network integration in the DMN. FC between the medial frontal cortex and the frontal operculum at the lateral boundary of the DMN was higher in more exposed children, suggestive of lower between-network segregation. A second study from the same group found that exposure to airborne copper, as measured via PM2.5, was related to decreased FC between the caudate and frontal lobe operculum in children aged 8–12 years (Pujol, Fenoll, et al. 2016). More recently, Pérez-Crespo et al. (2022) utilized a large longitudinal cohort of children (N = 2,197) from the Generation R Study to investigate the association between air pollution exposure during discrete developmental windows (i.e., pregnancy, birth to 3 years, 3 to 6 years, and 6 to 12 years of age) on network FC at age 12 years old. Higher levels of NO2, nitrogen oxides (NOx, and PM2.5 absorbance (a proxy for black carbon – also known as “soot”, a major component of PM (Cyrys et al. 2003)) were found to be related to greater between-network FC, albeit the associations between the exposure and the outcome varied for each pollutant depending on the timing of exposure. Specifically, PM2.5 absorbance had the most associations with brain network FC, demonstrating that higher exposure was associated with higher within- and between-network measures of FC and thus, higher within-network integration and lower between-network segregation. NO2 and NOx exposure demonstrated similar relationships - higher NO2 and NOx exposure and higher between-network FC (i.e., decreased between-network segregation) was observed. These studies found that pollutants interfered with expected between-network segregation but results regarding effects on within-network integration were mixed. While these three cross-sectional analyses of Western European children represent important advances in the investigation of ambient air pollution exposure and functional network outcomes during childhood and adolescence, additional large-scale and longitudinal studies from diverse geographical regions are needed.
The current exploratory longitudinal study aimed to understand how exposure to ambient air pollutants, including PM2.5, O3, and NO2, at ages 9–10 years are associated with changes in FC over a 2-year follow-up period from late childhood into early adolescence using data from the longitudinal Adolescent Brain Cognitive DevelopmentSM (ABCD) Study® cohort. By utilizing ABCD’s geographically diverse and large sample size, we are more likely to detect nuanced effects of air pollution xposure on pediatric brain maturation across the United States. We chose to focus on the potential effects of air pollution on the FC of the FPN, DMN, and SN, as well as subcortical regions of interest (ROI) due to their aforementioned development during this time period and their potential relative importance for emotional regulation and executive functioning. Considering most of the literature on resting-state brain maturation is based on cross-sectional studies, we first examined developmental changes in FC patterns over the 2-year period (in absence of any pollutant) to aid in the interpretation of our findings. We hypothesized that higher levels of outdoor air pollution exposure during late childhood would associate with altered FC development in the networks of interest over time.
2. Methods
2.1. Study population
The ABCD Study® is the largest long-term study of adolescent brain development from 21 communities throughout the United States, with 11,867 9–10-year-old children enrolled between years 2016 and 2018; these children are followed annually for over ten years with up to two brain imaging timepoints currently available (Volkow et al. 2018). ABCD Study inclusion criteria included age (≤10.99 years old at initial visit) and English language proficiency, whereas exclusion criteria included major medical or neurological conditions, history of traumatic brain injury, diagnosis of schizophrenia, moderate/severe autism spectrum disorder, intellectual disability, alcohol/substance use disorder, premature birth (gestational age <28 weeks), low birthweight (<1200 g), and contraindications to MRI scanning; detailed recruitment procedures can be found in Garavan et al. 2018. ABCD’s study procedures are approved under a centralized institutional review board from the University of California, San Diego; each study site also obtained approval from their own institutional review boards. All parents or caregivers provided written informed consent; children provided written assent.
For the current analyses, data were obtained from the ABCD’s 4.0 Data Release and included 9497 subjects across 21 sites with air pollution exposure estimates from baseline and good quality scans from baseline and/or year-two follow-up visit dates (9/2016 – 2/2020) (see below for quality control details). One subject from each family was randomly selected to reduce the number of hierarchical levels from three (subject, family, site) to two (subject, site), in that the number of both siblings and twins vary by site as part of the planned study design. Participants were 9–10 years old during the baseline assessment and 11–13 years old at the follow-up assessment approximately two years later. Of the total 9497 participants, 4327 (45.6%) had two time points of good quality imaging data, and the remainder (N = 5170, 54.4%) had one time point of good quality imaging data, either from the baseline or year-two follow-up visit (see additional details below and Table 1). Moreover, the final sample used here represents children with complete exposure and imaging data collected before March 1, 2020 to remove any potential confounding effects of stress inherent to the COVID-19 pandemic.
Table 1.
Cohort demographic and socioeconomic characteristics, baseline pollutant levels, and MRI information, including manufacturer and head motion as measured by average framewise displacement (FD).
| Cohort Characteristics | ||
|---|---|---|
|
| ||
| N total unique subjects | 9497 |
|
| Baseline | Year 2 | |
| N (%) subjects with one timepoint | 5170 (54.4%) | |
| N (%) subjects with one timepoint | 4292 (83%) | 878 (17%) |
| N (%) subjects with two timepoints | 4327 (45.6%) | |
| Mean Pollutant Levels in 2016, μg/m3 (SD) | ||
| PM2.5 | 7.65 (1.54) | 7.64 (1.52) |
| O3 | 81.3 (8.72) | 80.9 (8.58) |
| NO2 | 35.3 (10.9) | 34.8 (10.9) |
| Mean Age, months (SD) | 119 (7.52) | 143 (7.68) |
| Sex, N (%) Female | 4264 (49.5%) | 2449 (47.1%) |
| Race/Ethnicity, N (%) | ||
| White | 4662 (54.1%) | 2957 (56.8%) |
| Black | 1120 (13%) | 592 (11.4%) |
| Hispanic | 1752 (20.3%) | 1037 (19.9%) |
| Asian | 175 (2%) | 102 (2%) |
| Other | 910 (10.6%) | 517 (9.9%) |
| Handedness, N (%) | ||
| Right | 6943 (80.6%) | 4154 (79.8%) |
| Left | 590 (6.8%) | 356 (6.8%) |
| Mixed | 1086 (12.6%) | 695 (13.4%) |
| Highest Household Education, N (%) | ||
| Post Graduate Degree | 3068 (35.6%) | 1845 (35.4%) |
| Bachelor | 2249 (26.1%) | 1401 (26.9%) |
| Some College | 2198 (25.5%) | 1348 (25.9%) |
| HS Diploma/GED | 730 (8.5%) | 401 (7.7%) |
| < HS Diploma | 374 (4.3%) | 210 (4%) |
| Overall Income, N (%) | ||
| ≥100 K | 3465 (40.2%) | 2044 (39.3%) |
| ≥50 K & <100 K | 2291 (26.6%) | 1471 (28.3%) |
| <50 K | 2190 (25.4%) | 1304 (25.1%) |
| Don’t Know or Refuse | 673 (7.8%) | 386 (7.4%) |
| MRI Characteristics | ||
| N total MRI scans | 13,824 | |
| N (%) MRI scans per timepoint | 8619 (62.3%) | 5205 (37.7%) |
| MRI Manufacturer, N (%) | ||
| Siemens | 5616 (65.2%) | 3213 (61.7%) |
| GE | 2125 (24.7%) | 1419 (27.3%) |
| Philips | 878 (10.2%) | 573 (11%) |
| Mean FD, mm (SD) | 0.23 (0.22) | 0.17 (0.16) |
2.2. Ambient air pollution estimates
Annual ambient air pollution concentration for PM2.5, O3, and NO2 were assigned to primary residential addresses of each child as described in detail in Fan et al. (2021). Briefly, daily estimates of PM2.5 and NO2 as well as daily 8-hour maximums of ground-level O3 were derived at a 1-km2 resolution across the United States using hybrid spatiotemporal models, which utilize satellite-based aerosol optical depth models, land-use regression, meteorological data, and chemical transport models (Di et al. 2019; 2020; Requia et al. 2020). These daily estimates were averaged over the 2016 calendar year, corresponding to onset of ABCD Study enrollment. These concentrations were then assigned to the primary residential address at the baseline study visit when children were aged 9–10 years. The cross-validation of these models with EPA-monitored levels across the country were as follows: R2 Root Mean Square Error (RMSE) of 0.89 for PM2.5 annual averages (Di et al., 2019); 0.84 for NO2 annual averages (Di et al., 2020); 0.90 for daily 8-hour maximum O3 (Requia et al., 2020). PM2.5 was reported in μg/m3 and O3 and NO2 were originally reported in parts per billion (ppb). Prior to analysis, O3 and NO2 were converted from ppb to μg/m3 (O3: 1 ppb = 1.97 μg/m3; NO2: 1 ppb = 1.88 μg/m3).
2.3. Imaging
2.3.1. Image acquisition and Processing: rs-fMRI
Each scan was collected in accordance with harmonized procedures on Siemens Prisma, Philips, or GE 750 3T MRI scanners. Imaging acquisition protocols specific to ABCD have been described by Casey et al. (2018). Twenty cumulative minutes of resting-state data was collected across two sets of two five-minute acquisition periods, while subjects were instructed to keep their eyes open and fixed on a crosshair. This increased the probability of collecting enough data with low motion per ABCD’s standards (>12.5 min of data with framewise displacement (FD) < 0.2 mm) (Power et al. 2014). Resting-state scans were acquired using an echo-planar imaging sequence in the axial plane, with the following parameters: TR = 800 ms, TE = 30 ms, flip angle = 90°, voxel size = 2.4 mm3, 60 slices. Only images without clinically significant incidental findings (mrif_score = 1 or 2) that passed all ABCD quality-control parameters (imgincl_rsfmri_include = 1) were included in analysis. Image processing steps have been previously described by Hagler et al. (2019).
2.3.2. Gordon parcellation and functional connectivity analysis
Networks of interest included the SN, FPN, and DMN; subcortical ROIs included right and left amygdalae and hippocampi. Networks were functionally defined using resting-state FC patterns according to methods described by Gordon et al. (2016). Intra-network correlations were calculated by averaging the pairwise correlations for ROIs belonging to that network; inter-network correlations were calculated by averaging the pairwise correlations between ROIs within the first network and ROIs within the second network; subcortical-network correlations were calculated by averaging the pairwise correlations between ROIs within a network and a given subcortical ROI (Gordon et al. 2016).
2.4. Covariates
Covariates included demographic and socioeconomic variables, including race/ethnicity (White, Black, Hispanic, Asian, or Other), average household income in USD (≥100 K, ≥50<100 K, <50 K, or Don‘t Know/Refuse to Answer), and highest household education (Post-Graduate, Bachelor, Some College, High School Diploma/GED, or < High School Diploma), since pollution levels are higher in minority communities and those from disadvantaged social status backgrounds (Hajat, Hsia, and O’Neill 2015). We also included precision variables related to both the child and MRI collection, including the child’s sex at birth (male or female) and handedness (right, left, or mixed), as well as scanner manufacturer (Siemens, Philips, GE) to account for differences in both scanner hardware and software, and average framewise displacement (mm) due to fMRI’s sensitivity to head motion (Ciric et al. 2018).
2.5. Statistical model building approach
Given that linear mixed-effects (LME) modeling can handle correlated data (i.e., hierarchical structure of subjects within study sites and longitudinal data), as well as handle missing data, it has been widely used in the MRI literature to model neurodevelopmental trajectories using all available data (Mills et al. 2016; Tamnes et al. 2017; Herting et al. 2018). LME models were used to examine developmental changes in FC patterns as well as the effects of exposure to PM2.5, O3, and NO2 on these changes in FC over time. In each model, subjects (i.e., individuals) were nested within ABCD sites, and modeled as random effects. Age was z-scored using the scale() function in base R (R Core Team, 2020), resulting in a z-score of 0 equivalent to 10.69 years. Given the ability of LME models to handle a differing number of time points per subject data (Mills et al. 2016; Tamnes et al. 2017; Herting et al. 2018), all 9497 subjects with a total of 13,824 scans were included in the following analyses, without data imputation. All analyses were conducted using the R statistical software including the lmerTest::lmer() package in R (Version 4.1.2.) for LME models (Kuznetsova, Brockhoff, and Christensen 2017).
2.5.1. Developmental models
To put the putative air pollution exposure effects in the context of normal development, we first examined longitudinal changes in FC in a model that included age (in months) as the time variable as well as all covariates previously mentioned.
2.5.2. Single pollutant models
Single pollutant models included one pollutant (PM2.5, O3, or NO2) used as a continuous variable, age in months also used as a continuous variable, sex used as a binary variable (male or female), a three-way interaction term of age-by-sex-by-pollutant to allow testing for the impact of pollutants on changes over time as well as sex as a potential moderator of how each pollutant affects brain networks over time – previous research demonstrated both sex and age associations with brain network maturation (Satterthwaite et al. 2015; Schulz and Sisk 2016; Grayson and Fair 2017; Sanders et al. 2023) – plus all covariates previously mentioned. Nonsignificant interaction terms were then removed to achieve a more parsimonious model. Terms of interest in the final single-pollutant models included the fixed effects of age, pollutant, and the age-by-pollutant interaction term, respectively.
2.5.3. Multi-pollutant models
Finally, we built a multi-pollutant model to help address confounding effects of co-exposure to multiple pollutants on our outcomes which included age-by-PM2.5, age-by-O3, and age-by-NO2 interaction terms, the main effects of each pollutant (PM2.5, O3, and NO2) and age, and all previously mentioned covariates.
2.5.4. Sensitivity analyses
Due to the potential seasonality of pollutant concentrations, we performed an additional sensitivity analysis including meteorological season of the MRI scan as an additional time-varying covariate for each multi-pollutant model. Additionally, we performed a sensitivity analysis to examine effects in the subset of participants that had both waves of MRI data (N = 4327).
2.5.5. Correction for multiple comparisons
Given we conducted models for multiple brain outcomes, including 3 intra-network outcomes (SN, DMN, FPN), 3 inter-network outcomes (SN-DMN, SN-FPN, DMN-FPN), 6 amygdala (right and left) to network (SN, DMN, FPN) outcomes, and 6 hippocampus (right and left) to network (SN, DMN, FPN) outcomes, we corrected for multiple comparisons using false discovery rate (FDR) correction for the coefficients of interest across the 18 tests. We also denote in our tables which findings pass a more stringent Bonferroni-correction (i.e., 90 tests (18 tests * 5 models); p = 0.0005).
3. Results
Participant demographic and socioeconomic characteristics for the final study sample can be found in Table 1 and Supplemental Table 1. Data from 9497 participants with 1–2 waves of neuroimaging data were included in the final analyses. Excluding age at visit, subjects scanned at the baseline visit and the year-2 follow-up visit did not differ significantly on socioeconomic, demographic, or MRI variables. The discrepancy in N at the baseline visit compared to the year-2 follow-up visit can partially be explained by the onset of the COVID-19 pandemic, which interrupted data collection, and our decision to exclude scans collected after March 2020. The mean (SD) annual pollutant concentrations across all sites are as follows: PM2.5: 7.65 (1.53) μg/m3 (range, 1.72–15.9 μg/m3); O3: 81.2 (8.67) μg/m3 (range, 58.5 – 111 μg/m3); and NO2: 35.1 (10.9) μg/m3 (range, 1.16–69.7 μg/m3). PM2.5 and O3 were weakly negatively correlated (r = −0.17) and PM2.5 and NO2 were weakly positively correlated (r = 0.18); there was no correlation between O3 and NO2 (r = 0.003) (Supplemental Fig. 1).
Below we outline the results from the developmental models (Table 2) as well as the multi-pollutant models (Tables 3–4 and Figs. 1–2). Notably there were no significant age-by-sex-by-pollutant or sex-by-pollutant interactions across the initial single-pollutant models (see Supplemental Tables 2–4). Results of both sets of single-pollutant models were nearly identical to the multi-pollutant models and are therefore not discussed; however, results for the single pollutant models can be found in Supplemental Tables 2–7.
Table 2.
Results from developmental models examining the fixed effect of age over time, including Cohen’s f-squared, standardized betas, and 95% confidence intervals for each rs-fMRI outcome of interest.
| Main Effect of Age (over time) | f2 | β | CI (95%) | |
|---|---|---|---|---|
| Intra-Network | Intra-SN | 7.28E-03 | −0.06 * | −0.08, −0.05 |
| Intra-DMN | 4.81E-03 | 0.05 * | 0.03, 0.06 | |
| Intra-FPN | 0.01 | 0.07 * | 0.05, 0.08 | |
| Inter-Network | SN-DMN | 3.12E-05 | 0.004 | −0.01, 0.02 |
| SN-FPN | 0.01 | 0.08 * | 0.06, 0.09 | |
| FPN-DMN | 2.96E-08 | −0.0001 | −0.02, 0.02 | |
| Subcortical to Network | L amyg-DMN | 3.20E-05 | 0.01 | −0.01, 0.02 |
| R amyg-DMN | 1.77E-04 | −0.01 | −0.03, 0.005 | |
| L amyg-FPN | 7.52E-05 | −0.01 | −0.02, 0.009 | |
| R amyg-FPN | 6.70E-03 | −0.07 * | −0.09, −0.06 | |
| L amyg-SN | 0.01 | −0.10 * | −0.11, −0.08 | |
| R amyg-SN | 1.26E-03 | −0.03 * | −0.05, −0.01 | |
| L hippo-DMN | 7.72E-03 | −0.08 * | −0.09, −0.06 | |
| R hippo-DMN | 5.27E-03 | −0.07 * | −0.08, −0.05 | |
| L hippo-FPN | 1.27E-03 | −0.03 * | −0.05, −0.02 | |
| R hippo-FPN | 3.92E-04 | −0.02 | −0.03, −0.001 | |
| L hippo-SN | 7.37E-04 | −0.02 | −0.04, −0.007 | |
| R hippo-SN | 6.36E-04 | −0.02 | −0.04, −0.005 | |
Significant models are bolded (FDR-p < 0.05);
denotes passing a more stringent Bonferroni correction (p < 0.0005).
Abbreviations: salience network (SN), default-mode network (DMN), frontoparietal network (FPN), left (L), right (R), amygdala (amyg), hippocampus (hippo).
Table 3.
Results from multi-pollutant models, including Cohen’s f-squared, standardized betas, and 95% confidence intervals for each intra- and inter-network rs-fMRI outcome of interest.
| Multi-Pollutant Model | PM2.5 |
Age-by-PM2.5 |
O3 |
Age-by-O3 |
NO2 |
Age-by-NO2 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | ||
| Intra-Network | Intra-SN | 1.17E-03 | −0.01 | −0.04, 0.02 | 2.05E-04 | 0.01 | −0.005, 0.03 | 4.15E-05 | 0.004 | −0.02, 0.03 | 6.20E-05 | −0.01 | −0.02, 0.009 | 7.78E-04 | −0.01 | −0.04, 0.02 | 3.10E-04 | −0.01 | −0.03, 0.002 |
| Intra-DMN | 7.22E-05 | 0.003 | −0.03, 0.03 | 6.72E-06 | −0.002 | −0.02, 0.01 | 1.08E-05 | −0.002 | −0.02, 0.02 | 9.15E-04 | 0.02 | 0.006, 0.03 | 6.45E-04 | 0.01 | −0.02, 0.04 | 4.72E-09 | 0.00 | −0.01, 0.01 | |
| Intra-FPN | 0.01 | 0.02 | −0.009, 0.04 | 6.40E-06 | 0.002 | −0.01, 0.02 | 1.81E-04 | −0.003 | −0.03, 0.02 | 2.68E-04 | 0.01 | −0.003, 0.02 | 0.11 | −0.04 | −0.07, −0.02 | 6.37E-04 | −0.02 | −0.03, −0.002 | |
| Inter-Network | SN-DMN | 3.13E-05 | −0.001 | −0.03, 0.03 | 2.33E-03 | 0.04 * | 0.02, 0.05 | 6.41E-04 | 0.01 | −0.01, 0.04 | 1.05E-04 | −0.01 | −0.02, 0.007 | 4.99E-05 | 0.002 | −0.03, 0.03 | 2.36E-04 | −0.011 | −0.03, 0.004 |
| SN-FPN | 0.07 | 0.04 | 0.02, 0.06 | 3.74E-04 | 0.01 | −0.001, 0.03 | 4.95E-03 | 0.02 | −0.003, 0.04 | 7.40E-05 | −0.01 | −0.02, 0.008 | 8.75E-03 | −0.01 | −0.04, 0.01 | 1.14E-03 | −0.02 | −0.04, −0.009 | |
| FPN-DMN | 7.36E-04 | 0.01 | −0.02, 0.04 | 2.14E-03 | 0.03 * | 0.02, 0.05 | 1.51E-04 | 0.01 | −0.01, 0.04 | 4.30E-05 | −0.005 | −0.02, 0.01 | 2.14E-05 | 0.003 | −0.03, 0.03 | 2.33E-03 | −0.03 * | −0.05, −0.02 | |
Significant models are bolded (FDR-p < 0.05);
denotes passing a more stringent Bonferroni correction (p < 0.0005).
Abbreviations: salience network (SN), default-mode network (DMN), frontoparietal network (FPN).
Table 4.
Results from multi-pollutant models, including Cohen’s f-squared, standardized betas, and 95% confidence intervals for each subcortical-network rs-fMRI outcome of interest.
| Multi-Pollutant Model | PM2.5 |
Age-by-PM2.5 |
O3 |
Age-by-O3 |
NO2 |
Age-by-NO2 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | f2 | β | CI (95%) | ||
| Subcortical to Network | Lamyg-DMN | 2.83E-04 | 0.005 | −0.02, 0.03 | 4.86E-04 | −0.02 | −0.04, −0.004 | 4.94E-04 | −0.01 | −0.04, 0.01 | 8.04E-06 | −0.003 | −0.02, 0.01 | 9.15E-05 | 0.003 | −0.02, 0.03 | 2.05E-04 | 0.01 | −0.004, 0.03 |
| Ramyg-DMNL | 0.02 | −0.02 | −0.05, 0.006 | 1.32E-04 | 0.01 | −0.006, 0.03 | 5.36E-05 | 0.002 | −0.02, 0.02 | 1.45E-03 | −0.03 * | −0.05, −0.02 | 8.57E-04 | −0.005 | −0.03, 0.02 | 1.44E-04 | −0.01 | −0.03, 0.006 | |
| Lamyg-FPN | 7.53E-05 | 0.003 | −0.03, 0.03 | 1.21E-03 | 0.03 * | 0.01, 0.05 | 4.67E-04 | −0.01 | −0.04, 0.006 | 7.39E-04 | −0.02 | −0.04, −0.007 | 4.43E-03 | 0.03 | 0.0002, 0.05 | 1.03E-03 | −0.03 | −0.04, −0.01 | |
| Ramyg-FPN | 2.87E-03 | −0.01 | −0.04, 0.02 | 1.10E-03 | −0.03 * | −0.05, −0.01 | 3.61E-05 | −0.002 | −0.02, 0.02 | 3.73E-04 | 0.02 | 0.001, 0.03 | 1.78E-03 | 0.008 | −0.02, 0.03 | 6.20E-05 | 0.007 | −0.009, 0.02 | |
| Lamyg-SN | 0.02 | −0.03 | −0.06, −0.004 | 6.15E-04 | 0.02 | 0.005, 0.04 | 2.30E-04 | −0.01 | −0.03, 0.02 | 3.18E-05 | 0.005 | −0.01, 0.02 | 8.12E-04 | 0.01 | −0.02, 0.03 | 5.79E-07 | 0.00 | −0.02, 0.02 | |
| Ramyg-SN | 1.22E-04 | 0.003 | −0.02, 0.03 | 5.02E-04 | 0.02 | 0.003, 0.04 | 7.80E-04 | −0.02 | −0.04, 0.006 | 4.94E-05 | −0.01 | −0.02, 0.01 | 6.46E-05 | −0.003 | −0.03, 0.02 | 3.36E-04 | −0.02 | −0.03, 0.0005 | |
| Lhippo-DMN | 0.01 | −0.03 | −0.06, −0.007 | 7.HE-04 | 0.02 | 0.007, 0.04 | 2.60E-04 | 0.01 | −0.01, 0.03 | 9.65E-04 | −0.03 | −0.04, −0.01 | 1.62E-03 | 0.02 | −0.01, 0.04 | 7.20E-06 | −0.002 | −0.02, 0.01 | |
| Rhippo-DMN | 0.01 | 0.02 | −0.004, 0.05 | 2.73E-05 | −0.005 | −0.02, 0.01 | 7.07E-04 | 0.01 | −0.01, 0.03 | 1.83E-06 | 0.001 | −0.02, 0.02 | 2.32E-03 | 0.01 | −0.02, 0.03 | 1.84E-05 | 0.004 | −0.01, 0.02 | |
| Lhippo-FPN | 1.39E-03 | −0.02 | −0.05, 0.007 | 1.19E-03 | 0.03 * | 0.01, 0.05 | 1.59E-06 | −0.001 | −0.02, 0.02 | 4.32E-04 | −0.02 | −0.03, −0.002 | 1.80E-04 | 0.01 | −0.02, 0.04 | 5.74E-04 | −0.02 | −0.04, −0.005 | |
| Rhippo-FPN | 2.96E-05 | −0.01 | −0.04, 0.02 | 1.24E-03 | 0.03 * | 0.02, 0.05 | 5.08E-05 | −0.01 | −0.03, 0.02 | 2.16E-03 | −0.04 * | −0.06, −0.02 | 9.91E-06 | 0.003 | −0.02, 0.03 | 5.60E-04 | −0.02 | −0.04, −0.005 | |
| Lhippo-SN | 1.64E-04 | 0.01 | −0.02, 0.04 | 7.58E-04 | 0.02 | 0.007, 0.04 | 9.51E-05 | 0.01 | −0.01, 0.03 | 7.35E-04 | −0.02 | −0.04, −0.007 | 4.92E-04 | 0.01 | −0.02, 0.04 | 6.36E-04 | −0.02 | −0.04, −0.005 | |
| Rhippo-SN | 2.77E-03 | −0.01 | −0.04, 0.02 | 1.20E-03 | 0.03 * | 0.01, 0.05 | 9.88E-05 | 0.004 | −0.02, 0.03 | 2.55E-05 | 0.004 | −0.01, 0.02 | 1.91E-03 | 0.01 | −0.02, 0.03 | 2.05E-04 | −0.01 | −0.03, 0.004 | |
Significant models are bolded (FDR-p < 0.05);
denotes passing a more stringent Bonferroni correction (p < 0.0005).
Abbreviations: salience network (SN), default-mode network (DMN), frontoparietal network (FPN), left (L), right (R), amygdala (amyg), hippocampus (hippo).
Fig. 1.
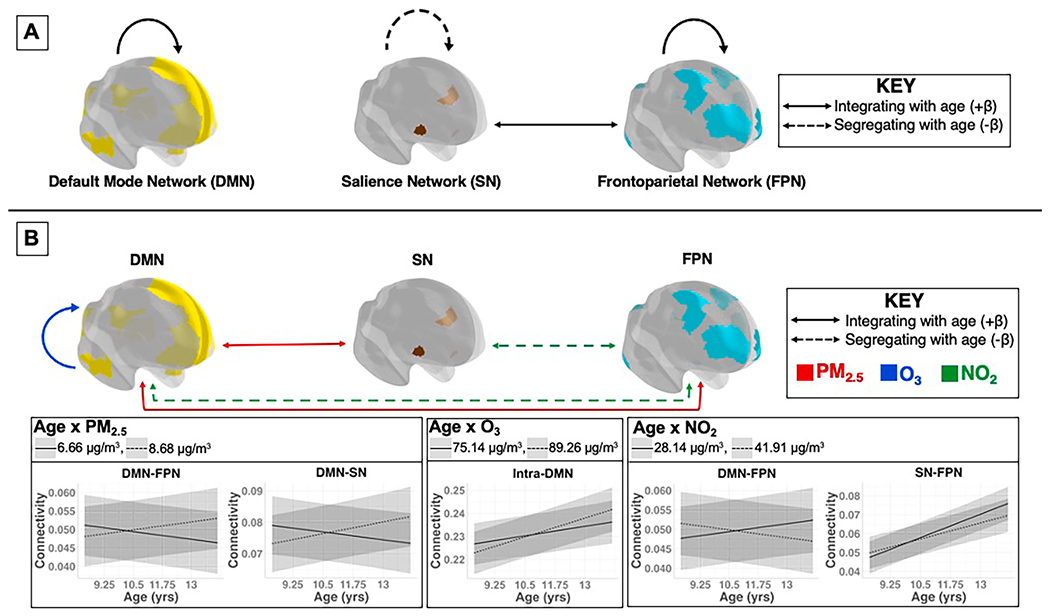
A) Longitudinal changes seen with age from 9 to 13 years in large-scaled cortical functional connectivity. B) Significant associations between the age-by-pollutant interaction term and intra- and inter-network rs-fMRI outcomes (FDR corrected). Red lines indicate the age-by-PM2.5 interaction term; blue lines indicate the age-by-O3 interaction term; green lines indicate the age-by-NO2 interaction term. Solid lines represent network integration, or increased functional connectivity as measured by BOLD rs-fMRI; dashed lines represent network segregation, or decreased functional connectivity as measured by BOLD rs-fMRI. For ease of interpretation, graphs depict significant interactions between pollutant and age per intra- and inter-network outcome for both the 0.75 quartile (dashed line) (PM2.5 = 8.68 μg/m3; O3 = 89.26 μg/m3; NO2 = 41.91 μg/m3) and 0.25 quartile (solid lines) (PM2.5 = 6.66 ϋg/m3; O3 = 75.14 μg/m3; NO2 = 28.14 μg/m3) of each pollutant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
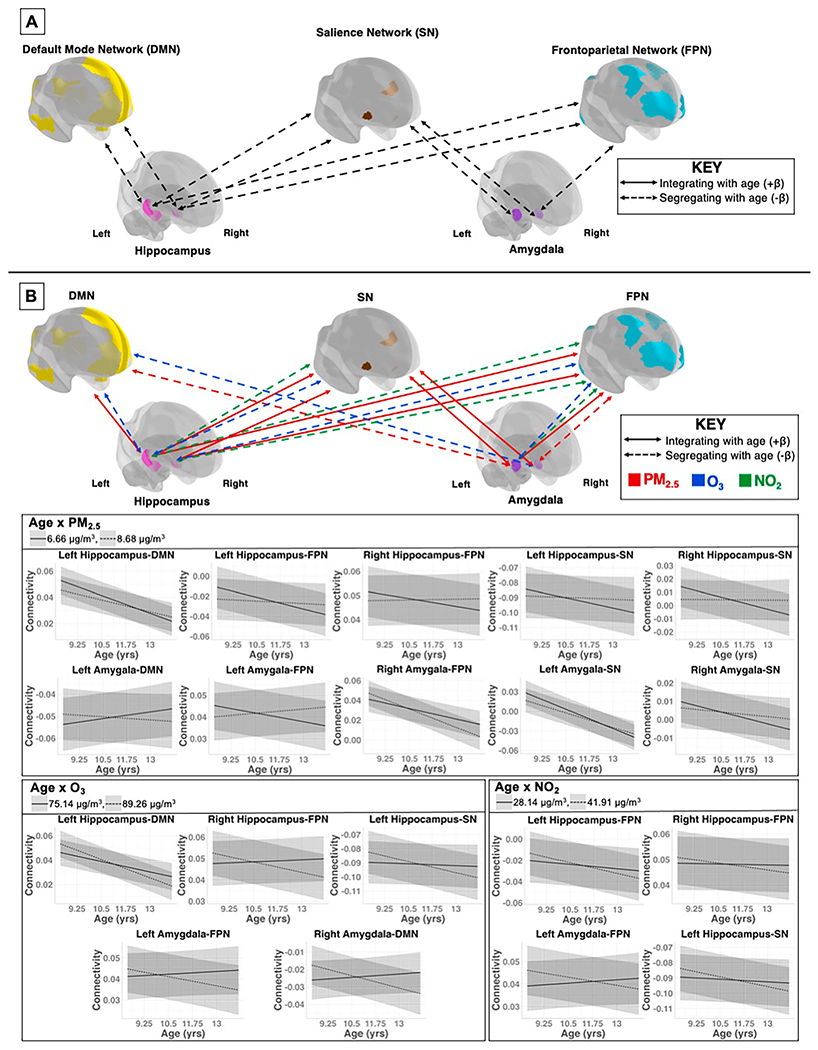
A) Longitudinal changes seen with age from 9 to 13 years in cortical-to-subcortical functional connectivity. B) Significant associations between the age-by-pollutant interaction term and subcortical-to-network rs-fMRI outcomes (FDR corrected). Red lines indicate the age-by-PM2.5 interaction term; blue lines indicate the age-by-O3 interaction term; green lines indicate the age-by-NO2 interaction term. Solid lines represent network integration, or increased functional connectivity as measured by BOLD rs-fMRI; dashed lines represent network segregation, or decreased functional connectivity as measured by BOLD rs-fMRI. Graphs depict significant interactions between pollutant and age per subcortical-to-network outcome. For ease of interpretation, graphs depict significant interactions between pollutant and age per subcortical-to-network outcome for both the 0.75 quartile (dashed line) (PM2.5 = 8.68 μg/m3; O3 = 89.26 μg/m3; NO2 = 41.91 μg/m3) and 0.25 quartile (solid lines) (PM2.5 = 6.66 μg/m3; O3 = 75.14 μg/m3; NO2 = 28.14 μg/m3) of each pollutant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1. Intra-network cortical functional connectivity
In developmental models, intra-SN FC decreased, whereas intra-DMN and intra-FPN FC increased over the 2-year follow-up period from age 9 - 13 (Table 2, Fig. 1a).
In fully adjusted multi-pollutant models, there was a significant age-by-O3 interaction, demonstrating that with higher O3 concentrations, greater intra-DMN integration was seen over the two-year follow up period from age 9 - 13 (Table 3, Fig. 1b). There were no significant age-by-PM2.5 or age-by-NO2 interactions on intra-network FC over the 2-year follow-up period. There was a main effect of NO2 on intra-FPN connectivity, such that higher NO2 concentrations were related to less intra-FPN FC at age 9 (Table 3).
3.2. Inter-network cortical functional connectivity
In developmental models, SN-FPN FC increased with age over time, demonstrating inter-network integration. There were no significant changes in SN-DMN or FPN-DMN FC over the 2-year follow-up period from age 9 - 13 (Table 2, Fig. 1a).
In fully adjusted multi-pollutant models, there were significant age-by-pollutant interactions for PM2.5 and NO2 (Table 3, Fig. 1b). Specifically, with higher PM2.5 concentrations, increases in SN-DMN and FPN-DMN inter-network FC were seen over the 2-year follow-up period from age 9 - 13 (Table 3, Fig. 1b). In contrast, higher NO2 exposure was associated with decreasing SN-FPN and FPN-DMN inter-network FC over the 2-year follow-up period from age 9 - 13 (Table 3, Fig. 1b). There were no significant main effects or interactions of O3 on inter-network FC.
3.3. Subcortical-network functional connectivity
3.3.1. Amygdala
In developmental models, right amygdala-FPN and bilateral amygdala-SN FC decreased with age over time, demonstrating amygdalae-to-network segregation (Table 2, Fig. 2a). There were no significant changes in amygdala-DMN FC over the 2-year follow-up period from age 9 - 13 (Table 2).
In fully adjusted multi-pollutant models, there were several significant age-by-pollutant interactions (Table 4, Fig. 2b). Higher PM2.5 concentrations were associated with decreasing left amygdala-DMN and right amygdala-FPN FC, but increasing left amygdala-FPN and bilateral amygdala-SN FC over time from age 9 - 13. On the other hand, higher O3 levels were related to decreasing right amygdala-DMN FC, whereas higher O3 and NO2 levels were associated with decreasing left amygdala-FPN FC over the 2-year follow-up period from age 9 - 13. No significant main effects of pollutants were seen for amygdala-to-network FC (Table 4).
3.3.2. Hippocampus
In developmental models, FC decreased between the bilateral hippocampus-DMN, -FPN, and -SN over time, demonstrating hippocampal-to-network segregation from age 9 - 13 (Table 2, Fig. 2a).
In fully adjusted multi-pollutant models, higher PM2.5 concentrations were related to increasing left hippocampus-DMN, bilateral hippocampal-SN, and bilateral hippocampal-FPN FC over the 2-year follow-up period from age 9 - 13 (Table 4, Fig. 2b). Higher O3 concentrations were associated with decreasing left hippocampus-DMN, right hippocampus-FPN, and left hippocampus-SN FC over time (Table 4, Fig. 2b). Lastly, higher NO2 levels were associated with decreasing bilateral hippocampal-FPN and left hippocampus-SN FC over the follow-up period from age 9 - 13 (Table 4, Fig. 2b). No significant main effects of pollutants were detected in hippocampal-to-network FC (Table 4).
3.4. Sensitivity analyses
In models including the season in which the MRI was collected, the previously noted findings all remained significant (Supplemental Table 8). Similarly, magnitude and direction of effects (standardized ß’s) were nearly identical in the subset of participants with both waves of MRI data as compared to the full analytic sample that included all individuals with one or two waves of MRI data (Supplemental Table 9).
4. Discussion
In this study, we leveraged longitudinal LME modeling and resting-state fMRI data to assess how one year of exposure to PM2.5, O3, and NO2 during childhood changes large-scaled cortical network and subcortical-to-cortical FC over a 2-year follow-up period. PM2.5 was found to relate to a greater number of changes in FC (i.e., 2 inter-network and 10 subcortical-to-cortical changes) over the transition from late childhood into early adolescence as compared to O3 (i.e., 1 intra-network and 5 subcortical-to-cortical changes) and NO2 (i.e., 2 inter-network and 4 subcortical-to-cortical changes). The current findings of differential FC development in children exposed to higher levels of air pollution expand upon the previous cross-sectional studies to show that ambient air pollution exposure during late childhood contributes to differential, within-subject changes in FC development as measured over a 2-year period.
4.1. Longitudinal trajectories of intrinsic functional network development
In terms of large-scaled network dynamics, previous literature, largely focused on comparing children versus adults, suggests that individual networks segregate from each other, while also displaying increased connectivity within each network (i.e., integration) to increase efficiency across childhood and adolescence (Grayson and Fair 2017). However, Marek et al. (2015) found that both within- and between-network FC decreases in early adolescence (ages 10–15 years), followed by between-network integration increasing later as individuals transition into adulthood. Sanders et al. (2023), however, found linear and non-linear age-related differences in both intra- and inter-network connectivity in a cross-sectional analysis using child and adolescent data from the Human Connectome Project (HCP). Given that most of the developmental resting-state FC studies to date have been limited by cross-sectional study design, small N, and/or inclusion of a wide age-range (for review, see Stevens 2016), we first sought to characterize age-related intra-, inter-, and subcortical-to-network FC trajectories within the large longitudinal ABCD sample. We found increasing intra-network integration for both the FPN and DMN from ages 9–13 years-old, which suggests increased communication or connectivity between regions in the same network. This finding is consistent with previous literature demonstrating increased intra-network integration with age in developmental cohorts, even those with wider age ranges. However, we also observed decreased intra-network connectivity in the SN as well as increased inter-network connectivity between the SN-FPN, at odds with most of the literature to date but more consistent with the findings of Sanders et al. (2023) and Teeuw and colleagues (2019) as it relates to intra-network dynamics, as well as Marek et al. (2015) to an extent. We also did not find age-related changes in inter-network connectivity between the FPN-DMN or SN-DMN. Thus, longitudinal changes seen between the narrow developmental window of ages 9–13 years within our sample are incongruent with the simplistic theory of intra-network integration and inter-network segregation throughout development which has largely been based on group differences between children and adults (Supekar, Musen, and Menon 2009; Qin et al. 2012; DeSerisy et al. 2021; Stevens 2016). Our findings highlight more nuanced patterns of large-scaled FC development during the transition from childhood to adolescence.
Beyond cortical FC, we also found distinct segregation patterns between subcortical-to-cortical FC over the 2-year follow-up period. The amygdala was found to segregate from SN and FPN, whereas the hippocampus segregated from all three networks of interest. The current findings are inconsistent with others that have reported the PFC and hippocampus integrate over the course of development from ages 8 to 32 years (Calabro et al. 2020). Inconsistencies in the literature could be due to issues with head motion resulting in spurious results or subcortical signal dropout, both common issues in rs-fMRI methodology (Boubela et al. 2015; Grayson and Fair 2017). Additionally, these other studies have focused on smaller and more homogenous samples or used different study designs (i.e., cohort-sequential or cross-sectional).
Taken together, our findings in a large and diverse sample point to dynamic changes to SN connectivity, namely intra-SN segregation and SN-FPN integration; intra-network integration in FPN and DMN; and subcortical-to-cortical network segregation broadly from ages 9 to 13 years of age. These findings support previous studies suggesting that the transition from childhood into early adolescence may be a time characterized by more dynamic change in network FC, within the overall intra-network integration and inter-network segregation noted to occur by adulthood (Heyn et al. 2019; Jalbrzikowski et al. 2017; Peters et al. 2017; Wendelken et al. 2017).
4.2. Distinct changes in functional network organization during the transition to adolescence by pollutant type
Building from these developmental changes in FC, our results suggest that outdoor air pollution exposure is associated with distinct differences in functional organization during the transition from childhood to early adolescence depending on the pollutant type. For instance, PM2.5 had opposite effects as compared to O3 and NO2 on inter-network and subcortical-to-network changes over time. Importantly, we implemented a multi-level model analytic framework which accounted for study site differences, controlled for potential seasonality in sensitivity analyses, and examined both single and multi-pollutant models to try to disentangle these complex multiple exposure challenges. Thus, while the opposing effects of PM2.5 compared to O3 and NO2 may initially seem counterintuitive, it is feasible that these differences may reflect varying underlying mechanisms and/or be the result of compensatory restructuring of crosstalk between brain regions resulting from exposure. Moreover, our findings also suggest patterns of change for large-scaled networks during the transition from childhood to early adolescence may be exaggerated or diminished depending on the type of pollutant. For example, higher O3 exposure was related to greater increases in intra-network FC above increases seen with age alone, suggestive of exaggerated connectivity in this network over time. In contrast, higher PM2.5 exposure was linked to greater integration between cortical networks and subcortical regions, which may reflect impairments in the otherwise expected subcortical-to-network segregation seen in our sample during this developmental stage. This disruption of developmental change in functional network organization from ambient air pollution may have important implications, as an optimal balance in the synchrony within the triple network model is vital for various cognitive and emotional processes (for reviews, see Menon 2011; van Oort et al. 2017), with potential consequences related to the emergence of psychopathologies (for review, see Menon 2019). Therefore, additional research is warranted to determine whether these notable exposure-related changes in the functional balance of intrinsic brain networks during the transition to early adolescence may subsequently contribute to various mental health disorders that typically emerge during mid- to late adolescence (Kessler et al. 2005).
4.3. Potential effects of pollutant composition, dose, and timing of exposure
Similarities and differences between the current study and the three previous studies also suggest that examining the chemical composition, dose of pollutant, and timing of exposure are likely important to consider in future research. PM2.5 effects on FC development were most prominent in the current study, yet Pérez-Crespo et al. (2022) did not find any associations between PM2.5 exposure and functional brain connectivity outcomes in their study sample. Pérez-Crespo et al. (2022) also found that higher NO2 exposure from birth to age 3 was linked to lower between-network segregation at age 12 years, whereas we found higher NO2 exposure at ages 9–10 years was related to greater between-network segregation over time, up to age 13 years. It is possible that chemical composition or source differences in exposures as well as differential doses of exposure between the studies may account for such discrepancies. For example, PM2.5 is known to carry heavy metals as well as include sulfate, nitrate, ammonium, black and organic carbon, and other materials, with composition varying regionally (Hyslop 2009). In the Generation R project, local traffic emissions within Rotterdam, Netherlands were responsible for the vast majority of NO2 exposure, whereas NO2 sources in the ABCD project are likely more complex and vary as a function of regional differences across the U.S. Moreover, we utilized a multi-pollutant approach, and our average levels of exposure were relatively low as compared to the other studies to date that have focused either on single pollutant or summary exposure estimates. Thus, it is possible there may be differential dose dependent effects that warrant further investigation.
Moving forward, the timing of exposure is likely a key piece in fully characterizing how air pollution exposure affects FC development across childhood and adolescence. Although the exact biological mechanisms linking ambient air quality and neurodevelopmental outcomes remain unclear, animal studies suggest both prenatal and childhood exposure lead to changes in oxidative stress, neuroinflammation, microglial activation, and neuronal structure and function (Morgan et al. 2011; Yan et al. 2015; Levesque et al. 2013; 2011; Li et al. 2012). However, the routes and degree by which prenatal and childhood exposures impact these processes may be distinct. Specifically, prenatal exposure to air pollution is expected to activate maternal immune function, leading to systemic changes in oxidative stress and inflammation as well as impairment of placental function and epigenetic modification (Johnson et al. 2021; Ha 2021). In addition to systemic inflammation and oxidative stress, childhood exposure may also damage the blood–brain barrier (BBB), making the brain more vulnerable to many exogenous toxins (Ha 2021; Kang et al. 2021; Lilian Calderón-Garcidueúas et al. 2008). Moreover, regardless of the mechanism(s) at play, prenatal and childhood exposure may present unique neurodevelopmental deficits based on the differential timing of various neurodevelopmental processes (i.e., neurulation, proliferation, migration, differentiation, synaptogenesis, gliogenesis, and myelination) and known spatial differences in periods of plasticity across various brain regions (e.g., visual and sensory systems develop earlier than the prefrontal cortex) (Herting et al. 2019). Thus, it is reasonable to hypothesize that prenatal and childhood exposures may also have varying consequences on developmental trajectories of intrinsic brain network development. In this regard, the current findings, suggesting one-year annual average exposure during childhood is linked to changes in FC patterns as the brain matures into early adolescence, is congruent with those of Pujol et al. (2016a, 2016b). They reported similar associations as seen with PM2.5 in the current study – namely decreased within-network and increased between-network FC – when the timing of child’s exposure and the brain outcomes coincide at ages 8–12 years old (Pujol et al., 2016a,b). In contrast, however, the associations between air pollution exposure and differential FC at age 12 years reported by Pérez-Crespo et al. (2022) were only apparent when the exposure occurred in early life (i.e., birth to age 3 years and age 3 to 6 years), whereas no association was found between more recent exposures in relation to the time of the brain scanning. Interestingly, however, despite differences in the timing of exposure, both Pérez-Crespo et al. (2022) and the current study found air pollution exposure to relate more broadly to inter-network as compared to intra-network FC; which is also congruent with the between-network differences noted in both studies by Pujol et al. (2016a, 2016b). Taken together, it seems ambient air pollution during childhood development may directly interfere with the diverging activation and communication between functional networks, which is thought to be necessary to support increased ability of cognitive and emotional functioning (for reviews, see Menon 2011; van Oort et al. 2017). As such, more research is necessary to understand the nuances in the relative importance of both the potential concurrent, delayed, and/or cumulative effects of exposure periods on intra-network brain maturation. Again, given the transition from childhood to adolescence may in fact be a sensitive period with dynamic changes in FC development (Thomson et al. 2022; Long et al. 2017; Heyn et al. 2019; Jalbrzikowski et al. 2017; Peters et al. 2017; Wendelken et al. 2017), it will be important for future research to also consider how air pollution influences the underlying developmental profile of FC that may exist within the age ranges studied. Hence the current study aimed to examine developmental patterns of change in FC, as to better understand how air pollution may influence developmental processes captured from ages 9–13 years. Future cross-sectional and longitudinal air pollution and FC studies should consider examining age, especially in samples with wider age ranges of children (i.e., 8–12 years), as well as how air pollution may interact with those age-related patterns, to more fully characterize how air quality may influence the potential dynamic changes that likely occur during this period of early adolescent neurodevelopment in addition to the amount of change in pollution over time.
4.4. Potential neurobiological mechanisms of pollutants’ effects on network connectivity
While mechanistically the neurobiology that contributes to patterns of resting-state FC remain unknown, experimental studies have quantified several immune- and cerebral vasculature-related effects of pollution exposure that may be relevant to how air pollution contributes to altered patterns of synchrony between large-scaled cortical networks and subcortical-to-cortical connectivity. For example, a study using human cerebral endothelial cells to create an in-vitro BBB model demonstrated that PM2.5 can cross the BBB and induce an upregulation of pro-inflammatory cytokines, illustrating a potential mechanism for neurotoxicity (Kang et al. 2021). Beyond PM2.5, O3 exposure has also been shown to increase the expression of pro-inflammatory cytokines near brain capillaries (Araneda et al. 2008). Animal studies of the effects of NO2 exposure reveal associated endothelial and inflammatory responses and a corresponding increased risk for ischemic stroke (Zhu et al. 2012) as well as weakened synaptic plasticity and increased risk for vascular dementia (Li and Xin 2013) in rodent stroke models. Additionally, the same group reported induced excitotoxicity in healthy rats (L ind Xin 2013). In terms of subcortical-to-cortical findings, changes in patterns of hippocampal and amygdalar connectivity development by ambient air pollution is supported by evidence from rodent models investigating the neurodevelopmental consequences of traffic-related air pollution. Patten et al. (2020) have shown traffic-related air pollution upregulates microglial expression in the CA1 region of the hippocampus, as well as impacts hippocampal neurogenesis in rodents. Additionally, the cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein and brain derived neurotrophic factor (BDNF) signaling pathway in the hippocampus has demonstrated vulnerability to the neurotoxic effects of PM2.5 (J. Liu et al. 2019; 2021; F. Liu et al. 2021). BDNF expression occurs in both the amygdala and the hippocampus, and is responsible for regulating synaptic plasticity, neurogenesis, proliferation, and dendritic spine morphology (Miranda et al. 2019). Thus, outdoor air pollution exposure may lead to dysregulation of various neural processes and pathways that could lead to aberrations in the maturation of brain networks across childhood and adolescence as captured by resting-state FC. Future translational work is needed that integrates human and experimental MRI imaging and histological approaches in animal models to clarify the mechanisms underlying air pollution effects on FC maturation.
4.5. Limitations, future directions, and conclusions
Limitations of our study include those inherent in rs-fMRI methodology, namely its extreme sensitivity to signal noise. To guard against this, we controlled for all available variables that could affect image quality, such as motion artifacts and incidental findings, and covaried for motion using framewise displacement. Another limitation is that the ABCD Study currently lacks air pollution exposure during the prenatal period, early life, and beyond the 9–10-year age period for participants, as well as more acute measures of air pollutant concentrations from the day of MRI scan. Recent evidence suggests acute effects of diesel exhaust exposure on intrinsic brain network FC (Gawryluk et al. 2023), albeit levels of exposure tested were very high as part of this randomized controlled cross-over design study. Nonetheless, it will be important for future studies to investigate potential acute versus chronic effects of ambient air pollution exposure on brain connectivity. In addition, the availability of up to only two neuroimaging time points, while a significant advance in the field and covering a relatively wide age range, limits the ability to fully examine the effects of air pollution on longitudinal brain health outcomes over a longer follow-up period, including potential non-linear exposure-outcome relationships that can only be accurately quantified with three or more time points of data per subject. Future studies are warranted in deciphering how lifetime pollution may impact brain development as the data becomes available in the ABCD Study cohort and other publicly available datasets. It will also be warranted for future studies to examine the effects of exposure during vulnerable windows of development, such as those identified by Pérez-Crespo et al. (2022), on longitudinal brain outcomes to evaluate the persistence of the observed altered connectivity. Lastly, this is the fourth study and the first longitudinal analysis to our knowledge examining effects of ambient air pollution and FC in childhood and adolescence, with low levels of exposures. Therefore, our findings warrant validation in other large, representative cohorts. Moreover, future studies should aim to connect cognitive and emotional functions to alterations in brain network maturation related to air pollution.
In conclusion, we find compelling evidence that exposure to ambient air pollution is associated with differences in the maturation of functional brain networks as measured by rs-fMRI in children as they transition into early adolescence. Of note, the level of exposures in the current study are well below EPA’s national standards (US EPA 2014), yet the current study shows even low level exposures are correlated with changes in longitudinal resting-state FC development in children across the U.S. Moreover, while the effect sizes seen are small – which could be in part because levels of exposure were low – it is also feasible that cumulative exposure to these low levels may have larger and/or persistent health effects on a population level (Funder et al., 2019). As such, our results may provide further support for new guidelines with more stringent recommendations, such as those recommended by the WHO in September 2021 (PM2.5 = 5 μg/m3; O3 = 100 μg/m3; NO2 = 10 μg/m3) (WHO 2021). Thus, the current findings should be taken into consideration by regulatory bodies as they set guidelines for acceptable levels of pollutants for the general population to optimize public health.
Supplementary Material
Acknowledgements
Research described in this article was supported by the National Institutes of Health [NIEHS R01ES032295, R01ES031074, P30ES007048-23S1, 3P30ES000002-55S1] and EPA grants [RD 83587201, RD 83544101].
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9-10 and follow them over 10years into early adulthood. The ABCD Study is supported by the National Institutes of Health Grants [ U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147]. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from 10.15154/1523041. Additional support for this work was made possible from NIEHS R01-ES032295 and R01-ES031074.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Megan Herting, PhD reports financial support was provided by National Institute of Environmental Health Sciences. Dr. Rob McConnell, MD reports financial support was provided by National Institute of Environmental Health Sciences. Dr. Joel D. Schwartz, PhD reports financial support was provided by United States Environmental Protection Agency. Dr. Rob McConnell, MD reports financial support was provided by United States Environmental Protection Agency.
Abbreviations:
- ABCD
Adolescent Brain Cognitive Development
- BDNF
Brain-Derived Neurotrophic Factor
- BBB
Blood-Brain Barrier
- BOLD
Blood Oxygen Level Dependent
- cAMP
Cyclic Adenosine Monophosphate
- CREB
cAMP Response Element-Binding Protein
- dACC
Dorsal Anterior Cingulate Cortex
- dlPFC
Dorsolateral Prefrontal Cortex
- DMN
Default Mode Network
- FDR
False Discovery Rate
- FC
Functional Connectivity
- FPN
Frontoparietal Network
- HCP
Human Connectome Project
- LME
Linear Mixed Effects
- mPFC
Medial Prefrontal Cortex
- NO2
Nitrogen Dioxide
- O3
Ozone
- PM
Particulate Matter
- PPC
Posterior Parietal Cortex
- ROI
Region of Interest
- rs-fMRI
Resting-State Functional Magnetic Resonance Imaging
- SD
Standard Deviation
- SN
Salience Network
- TE
Echo Time
- TR
Repetition Time
Footnotes
CRediT authorship contribution statement
Devyn L. Cotter: Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Claire E. Campbell: Writing – review & editing, Visualization. Kirthana Sukumaran: Project administration, Writing – review & editing. Rob McConnell: Methodology, Writing – review & editing. Kiros Berhane: Methodology, Writing – review & editing. Joel Schwartz: Methodology, Data curation, Resources, Writing – review & editing. Daniel A. Hackman: Methodology, Writing – review & editing. Hedyeh Ahmadi: Supervision, Writing – review & editing. Jiu-Chiuan Chen: Conceptualization, Methodology, Writing – review & editing. Megan M. Herring: Funding acquisition, Conceptualization, Methodology, Supervision, Project administration, Writing – review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2023.108001.
Data availability
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study, held in the NIMH Data Archive (NDA) and can be found at DOI https://doi.org/10.15154/1523041. The NDA study for this project can be found at https://dx.doi.org/10.15154/1528391.
References
- Alemany S, Vilor-Tejedor N, García-Esteban R, Bustamante M, Dadvand P, Esnaola M, Mortamais M, et al. , 2018. Traffic-Related Air Pollution, APOE E4 Status, and Neurodevelopmental Outcomes among School Children Enrolled in the BREATHE Project (Catalonia, Spain). Environ. Health Perspect 126 (8), 087001 10.1289/EHP2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Ambient (Outdoor) Air Pollution.” n.d. Accessed May 3, 2022. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health.
- Araneda S, Cornmin L, Atlagich M, Kitahama K, Parraguez VH, Pequignot J-M, Dalmaz Y, 2008. VEGF Overexpression in the Astroglial Cells of Rat Brainstem Following Ozone Exposure. Neurotoxicology 29 (6), 920–927. 10.1016/j.neuro.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM, 2005. Investigations into Resting-State Connectivity Using Independent Component Analysis. Philos. Trans. R. Soc., B 360 (1457), 1001–1013. 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubela RN, Kalcher K, Huf W, Seidel E-M, Derntl B, Pezawas L, Našel C, Moser E, 2015. FMRI Measurements of Amygdala Activation Are Confounded by Stimulus Correlated Signal Fluctuation in Nearby Veins Draining Distant Brain Regions. Sci. Rep 5 (1), 10499. 10.1038/srepl0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka I, Koranteng S, Osornio-Vargas AR, 2006. The Effects of Air Pollution on the Health of Children. Paediatr. Child Health 11 (8), 513–516. [PMC free article] [PubMed] [Google Scholar]
- Burnor E, Cserbik D, Cotter DL, Palmer CE, Ahmadi H, Eckel SP, Berhane K, et al. , 2021. Association of Outdoor Ambient Fine Particulate Matter With Intracellular White Matter Microstructural Properties Among Children. JAMA Netw. Open 4 (12), e2138300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro FJ, Murty VP, Jalbrzikowski M, Tervo-Clemmens B, Luna B, 2020. Development of Hippocampal-Prefrontal Cortex Interactions through Adolescence. Cereb. Cortex 30 (3), 1548–1558. 10.1093/cercor/bhzl86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueúas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, et al. , 2008. Long-Term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid Beta-42 and Alpha-Synuclein in Children and Young Adults. Toxicol. Pathol 36 (2), 289–310. 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueúas L, Leray E, Heydarpour P, Torres-Jardón R, Reis J, 2016. Air Pollution, a Rising Environmental Risk Factor for Cognition, Neuroinflammation and Neurodegeneration: The Clinical Impact on Children and Beyond. Rev. Neurol., Neuroepidemiology 172 (1), 69–80. 10.1016/j.neurol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Cao M, Huang H, Peng Y, Dong Q.i., He Y, 2016. Toward Developmental Connectomics of the Human Brain. Front. Neuroanat 10, 25. 10.3389/fhana.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones Rebecca M., and Hare Todd A.. 2008. “The Adolescent Brain.” Annals of the New York Academy of Sciences 1124 (March): 111–26. 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, et al. , 2018. The Adolescent Brain Cognitive Development (ABCD) Study: Imaging Acquisition across 21 Sites. Dev. Cogn. Neurosci 32 (August), 43–54. 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna A, Mascheroni E, Fustinoni S, Montirosso R, 2022. Air Pollution and Neurodevelopmental Skills in Preschool- and School-Aged Children: A Systematic Review. Neurosci. Biobehav. Rev 136 (May), 104623 10.1016/j.neubiorev.2022.104623. [DOI] [PubMed] [Google Scholar]
- Ciric R, Rosen AFG, Erus G, Cieslak M, Adebimpe A, Cook PA, Bassett DS, Davatzikos C, Wolf DH, Satterthwaite TD, 2018. Mitigating Head Motion Artifact in Functional Connectivity MRI. Nat. Protoc 13 (12), 2801–2826. 10.1038/S41596-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Ross Anderson H, Ostro B, Pandey KD, Krzyzanowski M, Künzli N, Gutschmidt K, et al. , 2005. The Global Burden of Disease Due to Outdoor Air Pollution. J. Toxic. Environ. Health A 68 (13–14), 1301–1307. 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- Cserbik D, Chen J-C, McConnell R, Berhane K, Sowell ER, Schwartz J, Hackman DA, Kan E, Fan CC, Herting MM, 2020. Fine Particulate Matter Exposure during Childhood Relates to Hemispheric-Specific Differences in Brain Structure. Environ. Int 143 (October), 105933 10.1016/j.envint.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik DM, Difeo G, Shors TJ, 2014. Preparing for Adulthood: Thousands upon Thousands of New Cells Are Born in the Hippocampus during Puberty, and Most Survive with Effortful Learning. Front. Neurosci 8, 70. 10.3389/fnins.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrys J, Heinrich J, Hoek G, Meliefste K, Lewné M, Gehring U, Bellander T, et al. , 2003. Comparison between Different Traffic-Related Particle Indicators: Elemental Carbon (EC), PM2.5 Mass, and Absorbance. J. Eposure Sci. Environ. Epidemiol 13 (2), 134–143. 10.1038/sj.jea.7500262. [DOI] [PubMed] [Google Scholar]
- DeSerisy M, Ramphal B, Pagliaccio D, Raffanello E, Tau G, Marsh R, Posner J, Margolis AE, 2021. Frontoparietal and Default Mode Network Connectivity Varies with Age and Intelligence. Dev. Cogn. Neurosci 48 (January), 100928 10.1016/j.den.2021.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, James Kelly M, Sabath B, et al. , 2019. An Ensemble-Based Model of PM2.5 Concentration across the Contiguous United States with High Spatiotemporal Resolution. Environ. Int 130 (September), 104909 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, James Kelly M, Sabath B, et al. , 2020. Assessing NO2 Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. Environ. Sci. Tech 54 (3), 1372–1384. 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFeo G, Shors TJ, 2017. Mental and Physical Skill Training Increases Neurogenesis via Cell Survival in the Adolescent Hippocampus. Brain Res. 1654 (Pt B), 95–101. 10.1016/j.brainres.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA, 2015. FMRI Functional Connectivity Applied to Adolescent Neurodevelopment. Annu. Rev. Clin. Psychol 11, 361–377. 10.1146/annurev-clinpsy-032814-112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CC, Marshall A, Smolker H, Gonzalez MR, Tapert SF, Barch DM, Sowell E, et al. , 2021. Adolescent Brain Cognitive Development (ABCD) Study Linked External Data (LED): Protocol and Practices for Geocoding and Assignment of Environmental Data. Dev. Cogn. Neurosci 52 (December), 101030 10.1016/j.dcn.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder, David C, Ozer DJ, 2019. Evaluating Effect Size in Psychological Research: Sense and Nonsense. Adv. Methods Pract. Psychol. Sci 2 (2), 156–168. 10.1177/2515245919847202. [DOI] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, Zahs D, 2018. Recruiting the ABCD Sample: Design Considerations and Procedures. Developmental Cognitive Neuroscience, The Adolescent Brain Cognitive Development (ABCD) Consortium: Rationale, Aims, and Assessment Strategy 32 (August), 16–22. 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JR, Palombo DJ, Curran J, Parker A, Carlsten C, 2023. Brief Diesel Exhaust Exposure Acutely Impairs Functional Brain Connectivity in Humans: A Randomized Controlled Crossover Study. Environ. Health 22 (1), 7. 10.1186/sl2940-023-00961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Santelli RK, Gao W, 2018. Imaging Structural and Functional Brain Development in Early Childhood. Nat. Rev. Neurosci 19 (3), 123–137. 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Evan M., Laumann Timothy O., Adeyemo Babatunde, Huckins Jeremy F., Kelley William M., Petersen Steven E., 2016. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cerebral Cortex (New York, N.Y.: 1991) 26 (1), 288–303. 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG, 2014. The Salience Network Is Responsible for Switching between the Default Mode Network and the Central Executive Network: Replication from DCM. Neuroimage 99 (October), 180–190. 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Fair DA, 2017. Development of Large-Scale Functional Networks from Birth to Adulthood: A Guide to Neuroimaging Literature. Neuroimage 160 (October), 15–31. 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Lubczyńska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G, van der Lugt A.d., et al. , 2018. Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biol Psychiatry, Genetic and Environmental Factors Influencing Developmental Cognitive Impairments 84 (4), 295–303. 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Ha S, 2021. Air Pollution and Neurological Development in Children. Dev. Med. Child Neurol 63 (4), 374–381. 10.1111/dmcn.14758. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, SeanN Hatton M, Cornejo D, Makowski C, Fair DA, Dick AS, Sutherland MT, et al. , 2019. Image Processing and Analysis Methods for the Adolescent Brain Cognitive Development Study. Neuroimage 202 (November), 116091. 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Hsia C, O’Neill MS, 2015. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Current Environ. Health Reports 2 (4), 440–450. 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH, 2011. Default-Mode and Task-Positive Network Activity in Major Depressive Disorder: Implications for Adaptive and Maladaptive Rumination. Biol. Psychiatry, Neural Connectivity, Ruminations, and Suicide 70 (4), 327–333. 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DW, Erickson LD, Kunzelman J, Brown BL, Gale SD, 2019. Association between Exposure to Air Pollution and Hippocampal Volume in Adults in the UK Biobank. Neurotoxicology 74 (September), 108–120. 10.1016/j.neuro.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJAG, Joëls M, Fernández G, 2014. Dynamic Adaptation of Large-Scale Brain Networks in Response to Acute Stressors. Trends Neurosci. 37 (6), 304–314. 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Herting MM, Johnson C, Mills KL, Vijayakumar N, Dennison M, Liu C, Goddings A-L, et al. , 2018. Development of Subcortical Volumes across Adolescence in Males and Females: A Multisample Study of Longitudinal Changes. Neuroimage 172 (May), 194–205. 10.1016/j.neuroimage.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Younan D, Campbell CE, Chen J-C, 2019. Outdoor Air Pollution and Brain Structure and Function From Across Childhood to Young Adulthood: A Methodological Review of Brain MRI Studies. Front. Public Health 7, 332. 10.3389/fpubh.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn SA, Keding TJ, Ross MC, Cisler JM, Mumford JA, Herringa RJ, 2019. Abnormal Prefrontal Development in Pediatric Posttraumatic Stress Disorder: A Longitudinal Structural and Functional Magnetic Resonance Imaging Study. Biol. Psychiatry: Cognitive Neuroscience Neuroimaging 4 (2), 171–179. 10.1016/j.bpsc.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop NP, 2009. Impaired Visibility: The Air Pollution People See. Atmos. Environ., Atmospheric Environment - Fifty Years of Endeavour 43 (1), 182–195. 10.1016/j.atmosenv.2008.09.067. [DOI] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B, 2017. Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biol. Psychiatry 82 (7), 511–521. 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj RL, Rodriguez EA, Wang Y.i., Block ML, 2017. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Current Environmental Health Reports 4 (2), 166–179. 10.1007/S40572-017-0142-3. [DOI] [PubMed] [Google Scholar]
- Johnson NM, Hoffmann AR, Behlen JC, Lau C, Pendleton D, Harvey N, Shore R, et al. , 2021. Air Pollution and Children’s Health—a Review of Adverse Effects Associated with Prenatal Exposure from Fine to Ultrafine Particulate Matter. Environ. Health Prev. Med 26 (1), 72. 10.1186/sl2199-021-00995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Tan H-Y, Lee CY, Cho H, 2021. An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv. Sci 8 (21), 2101251. 10.1002/advs.202101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62 (6), 593–602. 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Pääkkö E, Oikarinen J, Vainionpää V, Rantala H, Biswal B, 2000. Slow Vasomotor Fluctuation in FMRI of Anesthetized Child Brain. Magn. Reson. Med 44 (3), 373–378. . [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB, 2017. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw 82 (December), 1–26. 10.18637/jss.v082.il3. [DOI] [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, et al. , 2011. Diesel Exhaust Activates and Primes Microglia: Air Pollution, Neuroinflammation, and Regulation of Dopaminergic Neurotoxicity. Environ. Health Perspect 119 (8), 1149–1155. 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, Johnson JA, McGraw C, Block ML, 2013. The Role of MAC1 in Diesel Exhaust Particle-Induced Microglial Activation and Loss of Dopaminergic Neuron Function. J. Neurochem 125 (5), 756–765. 10.1111/jnc.l2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xin X, 2013. Nitrogen Dioxide (NO2) Pollution as a Potential Risk Factor for Developing Vascular Dementia and Its Synaptic Mechanisms. Chemosphere 92 (1), 52–58. 10.1016/j.chemosphere.2013.02.061. [DOI] [PubMed] [Google Scholar]
- Li H, Chen L, Guo Z, Sang N, Li G, 2012. In Vivo Screening to Determine Neurological Hazards of Nitrogen Dioxide (NO2) Using Wistar Rats. J. Hazard. Mater 225–226 (July), 46–53. 10.1016/j.jhazmat.2012.04.063. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhu Q, Gao W, Chen Y, Toh C-H, Styner M, Gerig G, Smith JK, Biswal B, Gilmore JH, 2008. Functional Connectivity MR Imaging Reveals Cortical Functional Connectivity in the Developing Brain. AJNR Am. J. Neuroradiol 29 (10), 1883–1889. 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang C, Yang J, Song X, Han W, Xie M, Cheng L.i., Xie L, Chen H, Jiang L.i., 2019. Effects of Early Postnatal Exposure to Fine Particulate Matter on Emotional and Cognitive Development and Structural Synaptic Plasticity in Immature and Mature Rats. Brain and Behavior 9 (12), e01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu B, Yuan P, Cheng L.i., Sun H, Gui J, Pan Y, Huang D, Chen H, Jiang L.i., 2021a. Role of PKA/CREB/BDNF Signaling in PM2.5-Induced Neurodevelopmental Damage to the Hippocampal Neurons of Rats. Ecotoxicol. Environ. Saf 214 (May), 112005 10.1016/j.ecoenv.2021.112005. [DOI] [PubMed] [Google Scholar]
- Liu F, Wang Z, Wei Y, Liu R, Jiang C, Gong C, Liu Y, Yan B, 2021b. The Leading Role of Adsorbed Lead in PM2.5-Induced Hippocampal Neuronal Apoptosis and Synaptic Damage. J. Hazard. Mater 416 (August), 125867 10.1016/j.jhazmat.2021.125867. [DOI] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, et al. , 2020. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 396 (10248), 413–446. 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Benischek A, Dewey D, Lebel C, 2017. Age-Related Functional Brain Changes in Young Children. Neuroimage 155 (July), 322–330. 10.1016/j.neuroimage.2017.04.059. [DOI] [PubMed] [Google Scholar]
- Lubczyńska MJ, Muetzel RL, El Marroun H, Basagaña X, Strak M, Denault W, Jaddoe VWV, et al. , 2020. Exposure to Air Pollution during Pregnancy and Childhood, and White Matter Microstructure in Preadolescents. Environ. Health Perspect 128 (2), 27005. 10.1289/EHP4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubczyńska MJ, Muetzel RL, El Marroun H, Hoek G, Kooter IM, Thomson EM, Hillegers M, et al. , 2021. Air Pollution Exposure during Pregnancy and Childhood and Brain Morphology in Preadolescents. Environ. Res 198 (July), 110446 10.1016/j.envres.2020.110446. [DOI] [PubMed] [Google Scholar]
- Marek S, Dosenbach NUF, 2018. The Frontoparietal Network: Function, Electrophysiology, and Importance of Individual Precision Mapping. Dialogues Clin. Neurosci 20 (2), 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B, 2015. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol. 13 (12), e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct 214 (5–6), 655–667. 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, 2011. Large-Scale Brain Networks and Psychopathology: A Unifying Triple Network Model. Trends Cogn. Sci 15 (10), 483–506. 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon B, 2019. Towards a New Model of Understanding – The Triple Network, Psychopathology and the Structure of the Mind. Med. Hypotheses 133 (December), 109385. 10.1016/j.mehy.2019.109385. [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Herting MM, Meuwese R, Blakemore S-J, Crone EA, Dahl RE, et al. , 2016. Structural Brain Development between Childhood and Adulthood: Convergence across Four Longitudinal Samples. Neuroimage 141 (November), 273–281. 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Morici JF, Zanoni MB, Bekinschtein P, 2019. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci 13. https://www.frontiersin.org/article/10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TE, Davis DA, Iwata N, Tanner JA, Snyder D, Ning Z, Kam W, et al. , 2011. Glutamatergic Neurons in Rodent Models Respond to Nanoscale Particulate Urban Air Pollutants in Vivo and in Vitro. Environ. Health Perspect 119 (7), 1003–1009. 10.1289/ehp.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, van Drooge BL, Macià D, Martínez-Vilavella G, Reynes C, Sabatier R, et al. , 2017. Effect of Exposure to Polycyclic Aromatic Hydrocarbons on Basal Ganglia and Attention-Deficit Hyperactivity Disorder Symptoms in Primary School Children. Environ. Int 105 (August), 12–19. 10.1016/j.envint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Patten KT, González EA, Valenzuela A, Berg E, Wallis C, Garbow JR, Silverman JL, Bein KJ, Wexler AS, Lein PJ, 2020. Effects of Early Life Exposure to Traffic-Related Air Pollution on Brain Development in Juvenile Sprague-Dawley Rats. Transl. Psychiatry 10 (1), 1–12. 10.1038/s41398-020-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus Tomáš, Keshavan Matcheri, and Giedd Jay N.. 2008. “Why Do Many Psychiatric Disorders Emerge during Adolescence?” Nature Reviews Neuroscience 9 (12): 947–57. 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Peper JS, Van Duijvenvoorde ACK, Braams BR, Crone EA, 2017. Amygdala–Orbitofrontal Connectivity Predicts Alcohol Use Two Years Later: A Longitudinal Neuroimaging Study on Alcohol Use in Adolescence. Dev. Sci 20 (4), e12448. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, et al. , 2015. Effects of Prenatal Exposure to Air Pollutants (Polycyclic Aromatic Hydrocarbons) on the Development of Brain White Matter, Cognition, and Behavior in Later Childhood. JAMA Psychiat. 72 (6), 531–540. 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Bansal R, Sawardekar S, Nati C, Elgabalawy ER, Hoepner LA, Garcia W, et al. , 2022. Prenatal Exposure to Air Pollution Is Associated with Altered Brain Structure, Function, and Metabolism in Childhood. J. Child Psychol. Psychiatry 63 (11), 1316–1331. 10.1111/jcpp.13578. [DOI] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE, 2014. Methods to Detect, Characterize, and Remove Motion Artifact in Resting State FMRI. Neuroimage 84 (January), 320–341. 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Fenoll R, Macià D, Martínez-Vilavella G, Alvarez-Pedrerol M, Rivas I, Forns J, et al. , 2016a. Airborne Copper Exposure in School Environments Associated with Poorer Motor Performance and Altered Basal Ganglia. Brain and Behavior 6 (6), e00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Martínez-Vilavella G, Macià D, Fenoll R, Alvarez-Pedrerol M, Rivas I, Forns J, et al. , 2016b. Traffic Pollution Exposure Is Associated with Altered Brain Connectivity in School Children. Neuroimage 129 (April), 175–184. 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Pérez-Crespo L, Kusters MSW, López-Vicente M, Lubczyńska MJ, Foraster M, White T, Hoek G, Tiemeier H, Muetzel RL, Guxens M, 2022. Exposure to Traffic-Related Air Pollution and Noise during Pregnancy and Childhood, and Functional Brain Connectivity in Preadolescents. Environ. Int 164 (June), 107275 10.1016/j.envint.2022.107275. [DOI] [PubMed] [Google Scholar]
- Qin Shaozheng, Young Christina B., Supekar Kaustubh, Uddin Lucina Q., Menon Vinod, 2012. Immature Integration and Segregation of Emotion-Related Brain Circuitry in Young Children. Proc. Nat. Acad. Sci 109 (20), 7941–7946. 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “R Core Team (2020). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL Https://Www.R-Project.Org/” n.d. [Google Scholar]
- Raichle ME, 2015. The Brain’s Default Mode Network. Annu. Rev. Neurosci 38 (1), 433–447. 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Redcay E, Kennedy DP, Courchesne E, 2007. FMRI during Natural Sleep as a Method to Study Brain Function during Early Childhood. Neuroimage 38 (4), 696–707. 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Requia WJ, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, Sulprizio MP, Amini H, Shi L, Schwartz J, 2020. An Ensemble Learning Approach for Estimating High Spatiotemporal Resolution of Ground-Level Ozone in the Contiguous United States. Environ. Sci. Tech 54 (18), 11037–11047. 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ TC, Reis S, van Tongeren M, 2019. Air Pollution and Brain Health: Defining the Research Agenda. Curr. Opin. Psychiatry 32 (2), 97–104. 10.1097/YCO.0000000000000480. [DOI] [PubMed] [Google Scholar]
- Sanders Ashley F.P., Harms Michael P., Kandala Sridhar, Marek Scott, Somerville Leah H., Bookheimer Susan Y., Dapretto Mirella, et al. , 2023. Age-Related Differences in Resting-State Functional Connectivity from Childhood to Adolescence. Cerebral Cortex February, bhad011. 10.1093/cercor/bhadoll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite Theodore D., Wolf Daniel H., Roalf David R., Ruparel Kosha, Eras Guray, Vandekar Simon, Gennatas Efstathios D., et al. , 2015. Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cerebral Cortex (New York, N.Y.: 1991) 25 (9), 2383–2394. 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Smyth JM, Delgado MR, 2013. The Amygdala: An Agent of Change in Adolescent Neural Networks. Horm. Behav., Puberty and Adolescence 64 (2), 298–313. 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL, 2016. The Organizing Actions of Adolescent Gonadal Steroid Hormones on Brain and Behavioral Development. Neurosci. Biobehav. Rev., The Adolescent Brain 70 (November), 148–158. 10.1016/j.neubiorev.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci 27 (9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V, 2008. A Critical Role for the Right Fronto-Insular Cortex in Switching between Central-Executive and Default-Mode Networks. PNAS 105 (34), 12569–12574. 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, 2016. The Contributions of Resting State and Task-Based Functional Connectivity Studies to Our Understanding of Adolescent Brain Network Maturation. Neurosci. Biobehav. Rev., The Adolescent Brain 70 (November), 13–32. 10.1016/j.neubiorev.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Sukumaran Kirthana, Cardenas-Iniguez Carlos, Burnor Elisabeth, Bottenhorn Katherine L., Hackman Daniel A., McConnell Rob, Berhane Kiros, Schwartz Joel, Chen Jiu-Chiuan, Herting Megan M., 2023. Ambient Fine Particulate Exposure and Subcortical Gray Matter Microarchitecture in 9- and 10-Year-Olds Children across the United States. IScience January, 106087. 10.1016/j.isci.2023.106087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J, 2008. The Neurological Effects of Air Pollution in Children. Eur. Respir. J 32 (3), 535–537. 10.1183/09031936.00073708. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V, 2009. Development of Large-Scale Functional Brain Networks in Children. PLoS Biol. 7 (7), e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester ChadM., Smyser Christopher D., Smyser Tara, Kenley Jeanette, Ackerman Joseph J. Jr, Shimony Joshua S., Petersen Steve E., Rogers Cynthia E., 2018. Cortical Functional Connectivity Evident After Birth and Behavioral Inhibition at Age Two Years. American J. Psychiatry 175 (2), 180. 10.1176/appi.ajp.2017.17010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, Güroğlu B, et al. , 2017. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J. Neurosci 37 (12), 3402–3412. 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeuw J, Brouwer RM, Guimarães JPOFT, Brandner P, Koenis MMG, Swagerman SC, Verwoert M, Boomsma DI, Hulshoff Pol HE, 2019. Genetic and Environmental Influences on Functional Connectivity within and between Canonical Cortical Resting-State Networks throughout Adolescent Development in Boys and Girls. Neuroimage 202 (November), 116073. 10.1016/j.neuroimage.2019.116073. [DOI] [PubMed] [Google Scholar]
- Thomson P, Malpas CB, Vijayakumar N, Johnson KA, Anderson V, Efron D, Hazell P, Silk TJ, 2022. Longitudinal Maturation of Resting State Networks: Relevance to Sustained Attention and Attention Deficit/Hyperactivity Disorder. Cogn. Affect. Behav. Neurosci 22 (6), 1432–1446. 10.3758/sl3415-022-01017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Galván A, 2016. Stress and the Adolescent Brain: Amygdala-Prefrontal Cortex Circuitry and Ventral Striatum as Developmental Targets. Neurosci. Biobehav. Rev., The Adolescent Brain 70 (November), 217–227. 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA, OAR. 2014. “NAAQS Table.” Other Policies and Guidance. April 10, 2014. https://www.epa.gov/criteria-air-pollutants/naaqs-table.
- van Oort J, Tendolkar I, Hermans EJ, Mulders PC, Beckmann CF, Schene AH, Fernández G, van Eijndhoven PF, 2017. How the Brain Connects in Response to Acute Stress: A Review at the Human Brain Systems Level. Neurosci. Biobehav. Rev 83 (December), 281–297. 10.1016/j.neubiorev.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Volkow Nora D., Koob George F., Croyle Robert T., Bianchi Diana W., Gordon Joshua A., Koroshetz Walter J., Pérez-Stable Eliseo J., et al. , 2018. The Conception of the ABCD Study: From Substance Use to a Broad NIH Collaboration. Developmental Cognitive Neuroscience, The Adolescent Brain Cognitive Development (ABCD) Consortium: Rationale, Aims, and Assessment Strategy 32 (August), 4–7. 10.1016/j.den.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Ferrer E, Ghetti S, Bailey SK, Cutting L, Bunge SA, 2017. Frontoparietal Structural Connectivity in Childhood Predicts Development of Functional Connectivity and Reasoning Ability: A Large-Scale Longitudinal Investigation. J. Neurosci 37 (35), 8549–8558. 10.1523/JNEUROSCI.3726-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide.” n.d. Accessed August 30, 2022. https://www.who.int/publications/i/item/9789240034228. [PubMed]
- Xiao Y, Friederici AD, Margulies DS, Brauer J, 2016. Longitudinal Changes in Resting-State FMRI from Age 5 to Age 6years Covary with Language Development. Neuroimage 128 (March), 116–124. 10.1016/j.neuroimage.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Ji X, Shi J, Li G, Sang N, 2015. Acute Nitrogen Dioxide Inhalation Induces Mitochondrial Dysfunction in Rat Brain. Environ. Res 138 (April), 416–424. 10.1016/j.envres.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Thomas FM, Krienen JS, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, et al. , 2011. The Organization of the Human Cerebral Cortex Estimated by Intrinsic Functional Connectivity. J. Neurophysiol 106 (3), 1125–1165. 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You R, Ho Y-S, Chang R-C, 2022. The Pathogenic Effects of Particulate Matter on Neurodegeneration: A Review. J. Biomed. Sci 29 (1), 15. 10.1186/si2929-022-00799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N.a., Li H, Han M, Guo L, Chen L, Yun Y, Guo Z, Li G, Sang N, 2012. Environmental Nitrogen Dioxide (NO2) Exposure Influences Development and Progression of Ischemic Stroke. Toxicol. Lett 214 (2), 120–130. 10.1016/j.toxlet.2012.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study, held in the NIMH Data Archive (NDA) and can be found at DOI https://doi.org/10.15154/1523041. The NDA study for this project can be found at https://dx.doi.org/10.15154/1528391.


