Abstract
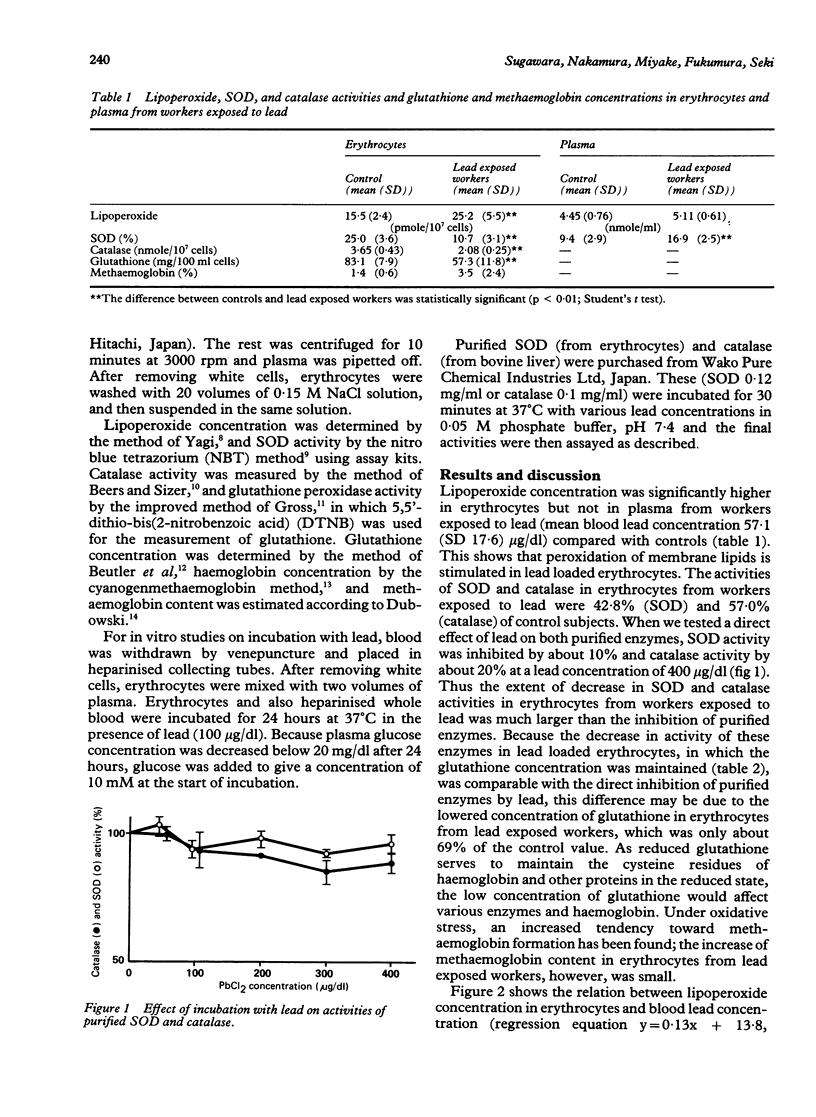
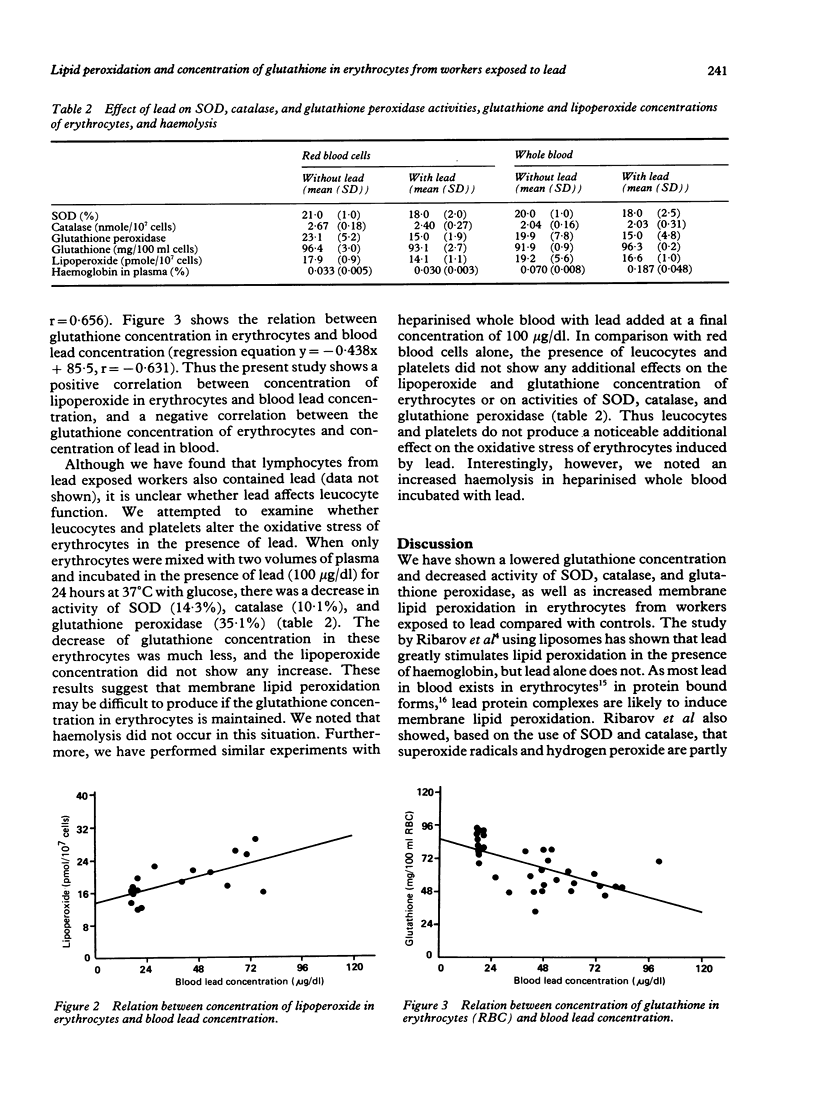
Lipoperoxide concentration in erythrocytes from workers occupationally exposed to lead (mean blood lead concentration 57.1 (SD 17.6) micrograms/dl) was significantly higher than that in controls. It was not different in plasma from the two groups. The activity of superoxide dismutase (SOD) and catalase in erythrocytes from workers exposed to lead was significantly lower than that of control subjects. The effect of lead was also seen in the glutathione concentration of erythrocytes from lead exposed workers, which was reduced to 69% of that found in erythrocytes from control workers. The increase in methaemoglobin content of erythrocytes from workers exposed to lead was less than expected and not significantly different from that of controls. A positive correlation between lipoperoxide concentration in erythrocytes and lead concentration in blood and a negative correlation between glutathione concentration in erythrocytes and blood lead concentration were found. Incubation of erythrocytes for 24 hours at 37 degrees C in the presence of lead (100 micrograms/dl) produced no changes in glutathione and lipoperoxide concentrations, although there was inhibition of activity of SOD (14.3%), catalase (10.1%), and glutathione peroxidase (35.1%). A similar experiment with heparinised whole blood showed increased haemolysis with no changes in membrane lipid peroxidation of erythrocytes. It is postulated that the lowered concentration of glutathione and decreased activity of SOD, catalase, and glutathione peroxidase in erythrocytes from workers exposed to lead may play a part in the increased membrane lipid peroxidation. Furthermore, the results suggest the possibility that leucocytes, or platelets, or both, may induce haemolysis in the presence of lead.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Gross R. T., Bracci R., Rudolph N., Schroeder E., Kochen J. A. Hydrogen peroxide toxicity and detoxification in the erythrocytes of newborn infants. Blood. 1967 Apr;29(4):481–493. [PubMed] [Google Scholar]
- Hernberg S., Nurminen M., Hasan J. Nonrandom shortening of red cell survival times in men exposed to lead. Environ Res. 1967 Nov;1(3):247–261. doi: 10.1016/0013-9351(67)90017-5. [DOI] [PubMed] [Google Scholar]
- JENSEN W. N., MORENO G. D., BESSIS M. C. AN ELECTRON MICROSCOPIC DESCRIPTION OF BASOPHILIC STIPPLING IN RED CELLS. Blood. 1965 Jun;25:933–943. [PubMed] [Google Scholar]
- Løvstad R. A. The protective action of ceruloplasmin on copper ion stimulated lysis of rat erythrocytes. Int J Biochem. 1982;14(7):585–589. doi: 10.1016/0020-711x(82)90041-6. [DOI] [PubMed] [Google Scholar]
- MILLS G. C., RANDALL H. P. Hemoglobin catabolism. II. The protection of hemoglobin from oxidative breakdown in the intact erythrocyte. J Biol Chem. 1958 Jun;232(2):589–598. [PubMed] [Google Scholar]
- Ribarov S. R., Benov L. C., Benchev I. C. The effect of lead on hemoglobin-catalyzed lipid peroxidation. Biochim Biophys Acta. 1981 Jun 23;664(3):453–459. doi: 10.1016/0005-2760(81)90123-5. [DOI] [PubMed] [Google Scholar]
- Ribarov S. R., Benov L. C. Relationship between the hemolytic action of heavy metals and lipid peroxidation. Biochim Biophys Acta. 1981 Feb 6;640(3):721–726. doi: 10.1016/0005-2736(81)90102-4. [DOI] [PubMed] [Google Scholar]
- Sakai T., Yanagihara S., Kunugi Y., Ushio K. Relationships between distribution of lead in erythrocytes in vivo and in vitro and inhibition of ALA-D. Br J Ind Med. 1982 Nov;39(4):382–387. doi: 10.1136/oem.39.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. G., Stern A. Interdependence of hemoglobin, catalase and the hexose monophosphate shunt in red blood cells exposed to oxidative agents. Biochem Pharmacol. 1980 Sep 1;29(17):2351–2359. doi: 10.1016/0006-2952(80)90269-5. [DOI] [PubMed] [Google Scholar]
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976 Apr;15(2):212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- deSilva P. E. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Br J Ind Med. 1981 Aug;38(3):209–217. doi: 10.1136/oem.38.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]


