Abstract
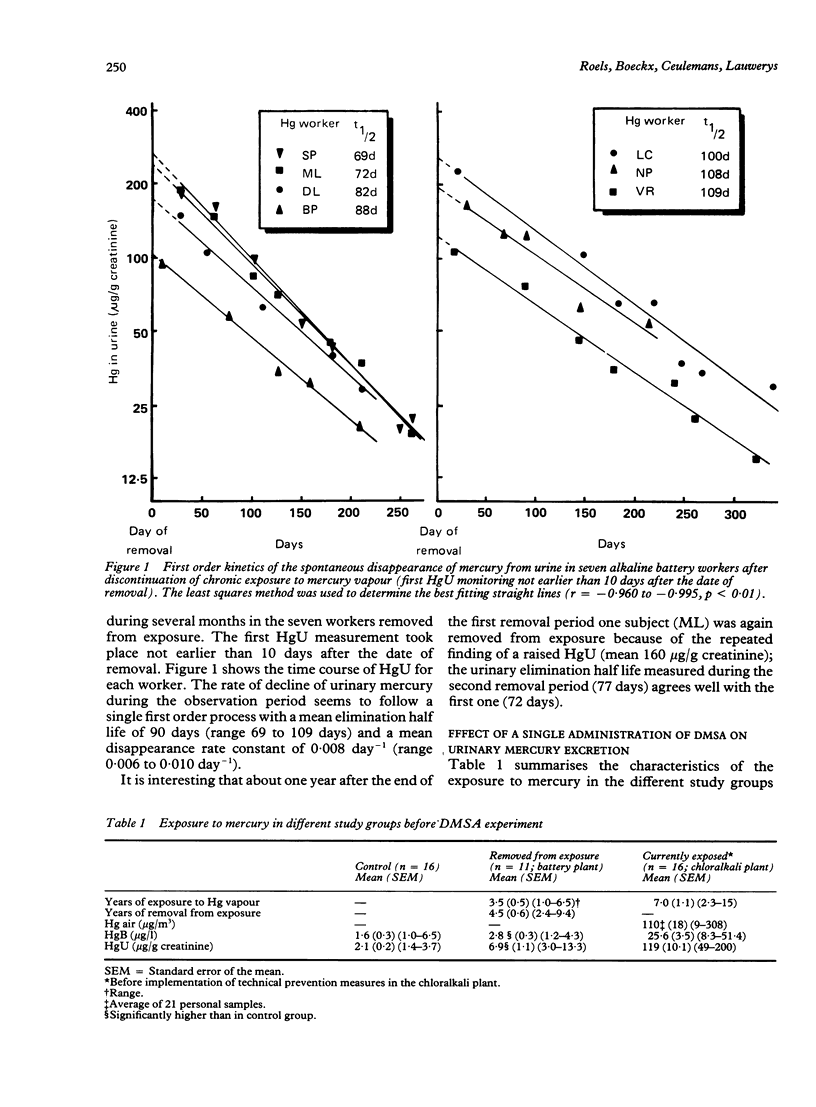
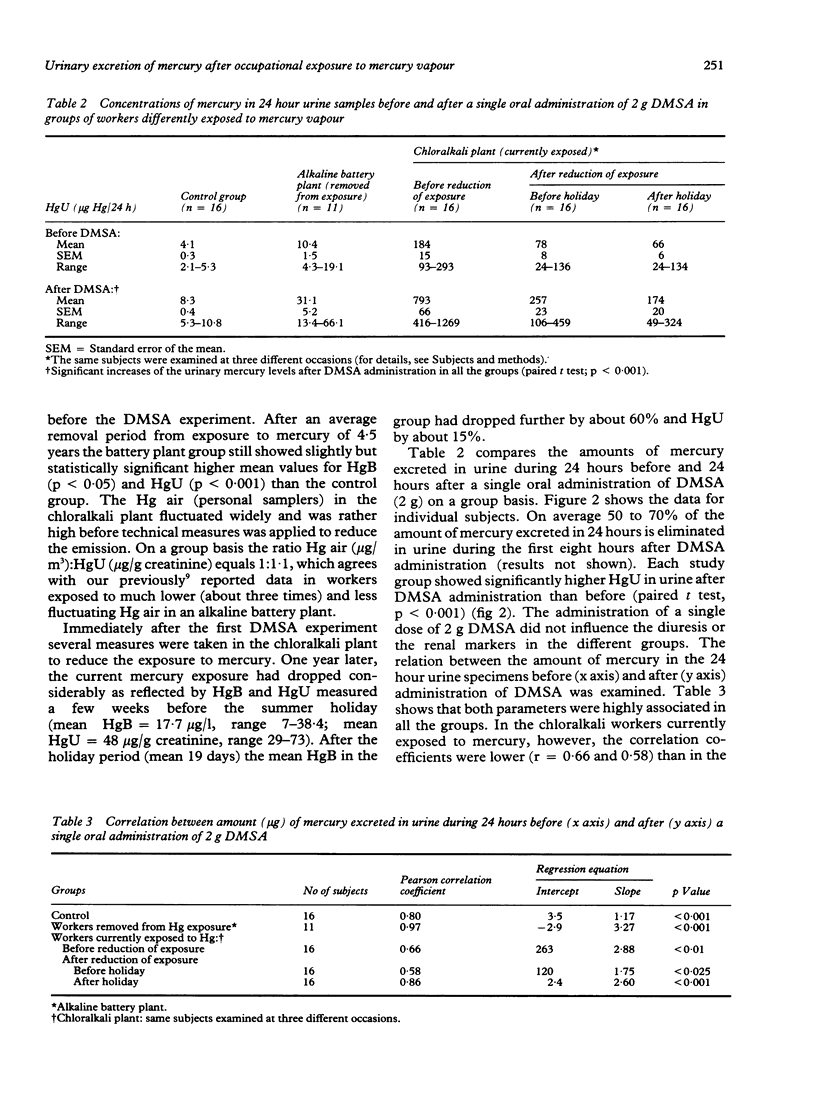
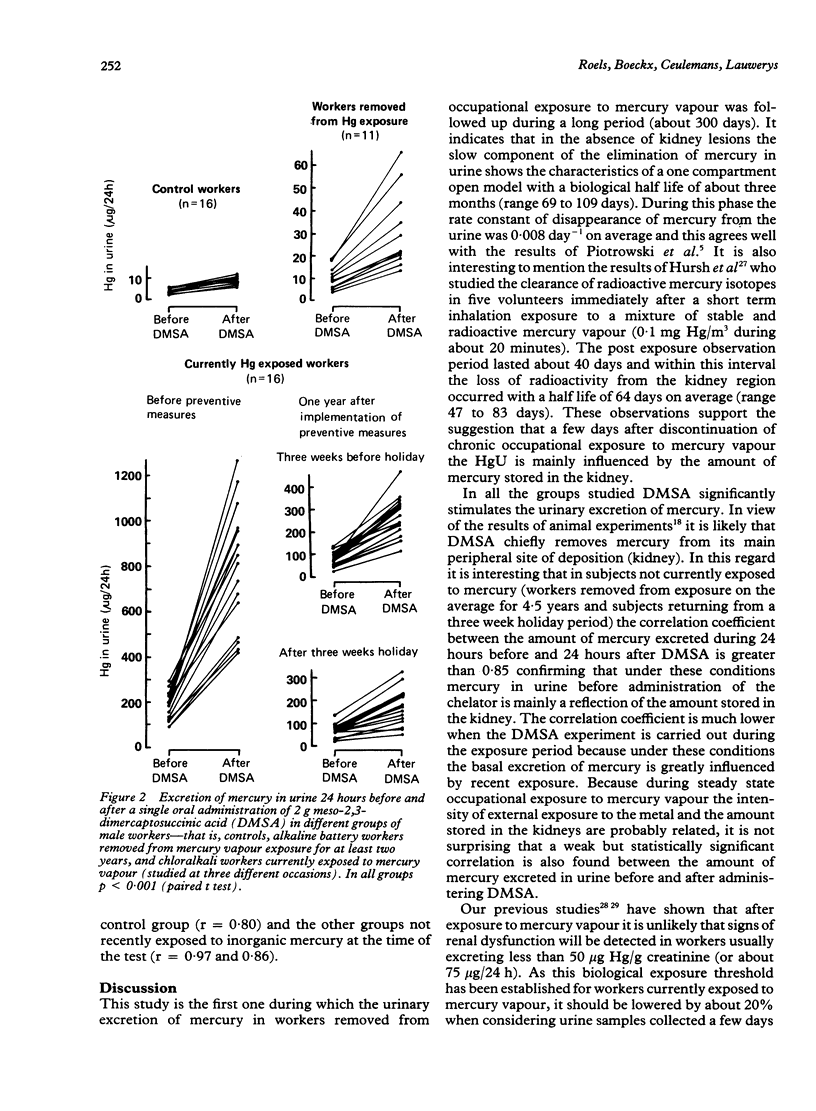
The spontaneous and chelator mediated excretion of mercury in urine was investigated in male subjects occupationally exposed to mercury vapour (alkaline battery and chloralkali plants) who did not exhibit any sign of kidney damage. The time course of the spontaneous elimination of mercury in urine was examined in seven workers (age 22-40) who had been removed from exposure to mercury vapour (average duration of exposure 4.4 years) because their urinary mercury concentrations repeatedly exceeded 100 micrograms/g creatinine. The post exposure observation period started 10 to 29 days after the date of removal and lasted about 300 days (slow HgU elimination phase). For each worker, the kinetics of the spontaneous HgU decline followed a first order process; the biological half life ranged from 69 to 109 days (mean 90 days). The increased urinary excretion of mercury after a single oral administration of 2 g meso-2,3-dimercaptosuccinic acid (DMSA) was investigated in 16 control workers (group A; age 23 to 49), in 11 workers removed from exposure for at least two years (group B; age 27 to 41), and in 16 workers currently exposed to mercury vapour (group C; age 21 to 58). In group C, the DMSA experiment was repeated twice (three weeks before and three weeks after a holiday) after measures had been taken to reduce the mercury emission. The urinary mercury excretion was significantly higher during the 24 hours after DMSA administration in all groups compared with that in the 24 hours before. The bulk (50-70%) of the DMSA stimulated mercury excretion appeared within the first eight hours. In each group, the amount of mercury (microgram Hg/24h) excreted after DMSA was significantly correlated with that before administration of DMSA. The groups whose exposure had ceased, however, exhibited much higher correlation for coefficients (r=0.97 for group B and 0.86 for group C after three weeks of holiday) than those currently exposed to mercury vapour (r-0.66 for group C before and 9.58 after reduction of exposure). The data suggest that after a few days of cessation of occupational exposure to mercury vapour the HgU before and after administration of DMSA mainly reflects the amount of mercury stored in the kidney, which represents a mercury pool with a slow turnover.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaseth J., Frieheim E. A. Treatment of methyl mercury poisoning in mice with 2,3-dimercaptosuccinic acid and other complexing thiols. Acta Pharmacol Toxicol (Copenh) 1978 Apr;42(4):248–252. doi: 10.1111/j.1600-0773.1978.tb02196.x. [DOI] [PubMed] [Google Scholar]
- Aposhian H. V. Biological chelation: 2,3-dimercapto-propanesulfonic acid and meso-dimercaptosuccinic acid. Adv Enzyme Regul. 1982;20:301–319. doi: 10.1016/0065-2571(82)90022-x. [DOI] [PubMed] [Google Scholar]
- Aposhian H. V. DMSA and DMPS--water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- Bell Z. G., Jr, Lovejoy H. B., Vizena T. R. Mercury exposure evaluations and their correlation with urine mercury excretions. 3. Time-weighted average (TWA) mercury exposures and urine mercury levels. J Occup Med. 1973 Jun;15(6):501–508. [PubMed] [Google Scholar]
- Bernard A. M., Lauwerys R. R. Continuous-flow system for automation of latex immunoassay by particle counting. Clin Chem. 1983 Jun;29(6):1007–1011. [PubMed] [Google Scholar]
- Bernard A. M., Roels H., Cardenas A., Lauwerys R. Assessment of urinary protein 1 and transferrin as early markers of cadmium nephrotoxicity. Br J Ind Med. 1990 Aug;47(8):559–565. doi: 10.1136/oem.47.8.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchet J. P., Lauwerys R. R. Influence of 2,3-dimercaptopropane-1-sulfonate and dimercaptosuccinic acid on the mobilization of mercury from tissues of rats pretreated with mercuric chloride, phenylmercury acetate or mercury vapors. Toxicology. 1989 Mar;54(3):323–333. doi: 10.1016/0300-483x(89)90067-x. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Roels H., Bernard A., Lauwerys R. Assessment of renal function of workers exposed to inorganic lead, calcium or mercury vapor. J Occup Med. 1980 Nov;22(11):741–750. [PubMed] [Google Scholar]
- Cherian M. G., Hursh J. B., Clarkson T. W., Allen J. Radioactive mercury distribution in biological fluids and excretion in human subjects after inhalation of mercury vapor. Arch Environ Health. 1978 May-Jun;33(3):109–114. doi: 10.1080/00039896.1978.10667318. [DOI] [PubMed] [Google Scholar]
- Cherian M. G., Miles E. F., Clarkson T. W., Cox C. Estimation of mercury burdens in rats by chelation with dimercaptopropane sulfonate. J Pharmacol Exp Ther. 1988 May;245(2):479–484. [PubMed] [Google Scholar]
- Friedheim E., Graziano J. H., Popovac D., Dragovic D., Kaul B. Treatment of lead poisoning by 2,3-dimercaptosuccinic acid. Lancet. 1978 Dec 9;2(8102):1234–1236. doi: 10.1016/s0140-6736(78)92103-7. [DOI] [PubMed] [Google Scholar]
- Graziano J. H., Siris E. S., LoIacono N., Silverberg S. J., Turgeon L. 2,3-Dimercaptosuccinic acid as an antidote for lead intoxication. Clin Pharmacol Ther. 1985 Apr;37(4):431–438. doi: 10.1038/clpt.1985.67. [DOI] [PubMed] [Google Scholar]
- Hursh J. B., Cherian M. G., Clarkson T. W., Vostal J. J., Mallie R. V. Clearance of mercury (HG-197, HG-203) vapor inhaled by human subjects. Arch Environ Health. 1976 Nov-Dec;31(6):302–309. doi: 10.1080/00039896.1976.10667240. [DOI] [PubMed] [Google Scholar]
- Lenz K., Hruby K., Druml W., Eder A., Gaszner A., Kleinberger G., Pichler M., Weiser M. 2,3-Dimercaptosuccinic acid in human arsenic poisoning. Arch Toxicol. 1981 Jun;47(3):241–243. doi: 10.1007/BF00368684. [DOI] [PubMed] [Google Scholar]
- Magos L., Clarkson T. W. Atomic absorption determination of total, inorganic, and organic mercury in blood. J Assoc Off Anal Chem. 1972 Sep;55(5):966–971. [PubMed] [Google Scholar]
- Magos L. Mercury--blood interaction and mercury uptake by the brain after vapor exposure. Environ Res. 1967 Dec;1(4):323–337. doi: 10.1016/0013-9351(67)90023-0. [DOI] [PubMed] [Google Scholar]
- Magos L. The effects of dimercaptosuccinic acid on the excretion and distribution of mercury in rats and mice treated with mercuric chloride and methylmercury chloride. Br J Pharmacol. 1976 Apr;56(4):479–484. doi: 10.1111/j.1476-5381.1976.tb07460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiussi R., Armeli G., Bareggi V. Statistical study of the correlation between mercury exposure (TWA) and urinary mercury concentrations in chloralkali workers. Am J Ind Med. 1982;3(3):335–339. doi: 10.1002/ajim.4700030308. [DOI] [PubMed] [Google Scholar]
- Piotrowski J. K., Trojanowska B., Mogilnicka E. M. Excretion kinetics and variability of urinary mercury in workers exposed to mercury vapour. Int Arch Occup Environ Health. 1975 Sep 19;35(3-4):245–246. doi: 10.1007/BF01837099. [DOI] [PubMed] [Google Scholar]
- Planas-Bohne F. The effect of 2,3-dimercaptorpropane-1-sulfonate and dimercaptosuccinic acid on the distribution and excretion of mercuric chloride in rats. Toxicology. 1981;19(3):275–278. doi: 10.1016/0300-483x(81)90138-4. [DOI] [PubMed] [Google Scholar]
- Roels H., Abdeladim S., Ceulemans E., Lauwerys R. Relationships between the concentrations of mercury in air and in blood or urine in workers exposed to mercury vapour. Ann Occup Hyg. 1987;31(2):135–145. doi: 10.1093/annhyg/31.2.135. [DOI] [PubMed] [Google Scholar]
- Roels H., Gennart J. P., Lauwerys R., Buchet J. P., Malchaire J., Bernard A. Surveillance of workers exposed to mercury vapour:validation of a previously proposed biological threshold limit value for mercury concentration in urine. Am J Ind Med. 1985;7(1):45–71. doi: 10.1002/ajim.4700070106. [DOI] [PubMed] [Google Scholar]
- Tucker S. M., Boyd P. J., Thompson A. E., Price R. G. Automated assay of N-acetyl-beta-glucosaminidase in normal and pathological human urine. Clin Chim Acta. 1975 Jul 23;62(2):333–339. doi: 10.1016/0009-8981(75)90245-4. [DOI] [PubMed] [Google Scholar]
- Wang S. C., Ting K. S., Wu C. C. Chelating therapy with Na-DMS in occupational lead and mercury intoxications. Chin Med J. 1965 Jul;84(7):437–439. [PubMed] [Google Scholar]


