Abstract
Inducible nitric oxide synthase (iNOS) is expressed when cells are induced or stimulated by proinflammatory cytokines and/or bacterial lipopolysaccharide (LPS). iNOS is a downstream gene of the NF-κB pathway. Our previous studies demonstrated that five Nfkb genes are expressed in mouse taste epithelium and taste organoids. However, it is unclear whether activation of the NF-κB pathway could induce iNOS gene expression and increase nitric oxide (NO) production in taste buds. In this study, we investigated the expression of iNOS mRNA and protein after LPS stimulation.
Our results showed that a subset of taste bud cells and taste neurons express iNOS proteins after LPS stimulation. In addition, isolated mouse taste epithelium can release NO after exposure to LPS ex vivo. In taste behavioral tests, the NO donor nitroprusside enhanced mouse aversive responses to salty, bitter, and sour taste compounds. The enhanced aversive responses were especially strong for salty taste. In conclusion, our results suggest that iNOS and NO may play a role in the inflammation-associated taste disturbances.
Keywords: Inflammation, lipopolysaccharide, nitric oxide, Inducible nitric oxide synthase, taste buds, taste disturbances
1. Introduction
Nitric oxide (NO), the smallest cellular signaling molecule, participates in diverse physiological functions, such as vasodilation, neural transmission, and immune responses. It is produced by three isoforms of nitric oxide synthase (NOS). Neuronal NOS (nNOS) and endothelial NOS (eNOS) are constitutively expressed in specific neurons and endothelial cells, respectively (Ally et al., 2020; Cyr et al., 2020; Pereira et al., 2022). However, inducible NOS (iNOS) is not constantly present in cells and is induced when the cells are stimulated by proinflammatory cytokines and/or bacterial lipopolysaccharide (LPS) (Pautz et al., 2010; Farlik et al., 2010). iNOS could generate significant amounts of NO (micromolar range), which may result in cytotoxicity or help to defend against invading pathogens (Vannini et al., 2015).
iNOS is a downstream gene of the NF-κB pathway. Its induction is mostly through transcriptional activation by NF-κB and IRF-1 (Cinelli et al. 2020). It is well known that macrophages express iNOS gene upon LPS stimulation (Förstermann and Sessa 2012). Our previous studies demonstrated that five Nfkb genes (RelA, RelB, c-Rel, NF-κB1, and NF-κB2) and IRF-1 are expressed in mouse taste epithelium and/or taste organoids, and RelA, RelB, and Nfkb1 are abundantly expressed (Feng et al., 2020; Wang et al., 2007). However, it is unclear whether the iNOS gene could be induced in the taste buds when NF-κB and IRF-1 pathways are activated. Besides, it is still unknown whether induction of iNOS could lead to an increase in NO production and alter taste-related behaviors.
Inflammation has profound effects on energy metabolism and food intake. Lipopolysaccharide-induced systemic inflammation leads to anorexia and reduced food intake. Taste sensing is an important mechanism governing food preference and intake. Patients with viral infections (e.g., COVID-19) and other diseases with underlying inflammation often experience taste alterations, including taste loss and taste distortion (i.e., dysgeusia) (Schiffman 1983; Bromley and Doty 2003; Pribitkin et al., 2003; Parma et al., 2020; Doyle et al., 2021). Inflammatory stimuli, such as LPS and inflammatory cytokines, were shown to alter electrophysiological, neural, and behavioral responses to taste compounds (Phillips and Hill 1996; Feng et al., 2015; Kumarhia et al. 2016; Dantzer and Kelley 2007). However, the mechanisms of inflammation-associated taste disturbances, especially taste distortion, remain poorly understood.
NO is a biological messenger molecule that is synthesized in high levels in the brain and has been reported to play a role in the modulation of food intake (Morley and Flood, 1991, 1992, 1994; Morley et al., 1995). Its roles in the peripheral taste system, however, are largely unclear. The expression of nNOS and eNOS were detected in rat taste buds (Kretz et al., 1998), but whether they play any roles in taste signaling is unknown. In some insects, NO appears to modulate sodium and sugar taste responses. Schuppe et al. (2007) showed that in locusts NO decreased the frequency of action potentials induced by NaCl through a cyclic guanosine monophosphate (cGMP)-independent pathway while increasing the frequency of action potentials stimulated by sucrose. A recent report by Cano et al. (2017) showed that activation of the NO/soluble guanylate cyclase (sGC)/cGMP pathway is involved in modulating preference and avoidance behavior to sodium taste solutions in a blood-sucking insect model.
In this study, we investigated the expression of iNOS mRNA and protein after LPS stimulation. We found that a subset of taste bud cells and taste neurons express iNOS proteins after LPS stimulation. Furthermore, we showed that the mouse taste epithelium can release NO after exposure to LPS ex vivo. In taste behavioral tests, the NO donor nitroprusside enhances aversive responses to salty, bitter, and sour taste compounds. Our results suggest that iNOS and NO may play a role in the inflammation-associated taste disturbances.
2. Material and methods
2.1. Animals
C57BL/6 mice were purchased from either the Jackson Laboratory (Bar Harbor, ME, USA) or the Experimental Animal Center of Chongqing Medical University (Certificate number SYXK, 2012–0001, Chongqing, Sichuan, China). All mice were housed and bred in a climate-controlled environment at either the animal facility of Southwest University or the Monell Chemical Senses Center. All animal experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH Publications No. 8023), and approved by experimental animal ethics committee of School of Pharmaceutical Sciences & School of Chinese Medicine, Southwest University and the Institutional Animal Care and Use Committee at the Monell Chemical Senses Center.
2.2. Reagents
Rabbit polyclonal antibodies to iNOS and Mouse monoclonal antibodies against iNOS were purchased from Abcam (Cambridge, UK). Rabbit polyclonal antibodies against PLC-β2 (sc-206) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An affinity-purified goat polyclonal antibody against carbonic anhydrase 4 (CA4, AF2414) was purchased from R&D Systems. An affinity-purified rabbit polyclonal antibody against P2X3 was purchased from Sigma-Aldrich (AB5895). DyLight 649 (or DyLight 488)-conjugated donkey anti-rabbit, anti-mouse, or anti-goat antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Collagenase A and dispase II were from Roche Applied Science (Indianapolis, IN). LPS (from E. coli O111:B4) was purchased from Sigma (St. Louis, MO). Nitric Oxide Assay Kit for NO determination was purchased from R&D Systems (Minneapolis, USA). Superscript III Reverse Transcriptase Reagent Kit was purchased from Invitrogen. Protector RNase Inhibitor and recombinant RNase-free DNase I were from Roche Applied Science. PowerUP SYBR Green Master Mix or Power SYBR Green master Mix for real-time PCR was obtained from Thermo Fisher (Waltham, MA, USA). RAW 264.7 cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA).
2.3. LPS treatments in vivo and in vitro
For in vivo experiments, C57BL/6 mice, both male and female, were injected with 2.5 mg/kg LPS (i.p.). At 2, 6, 24, and 48 h after LPS injection (n=3 for each time point), mice were euthanized and then the spleen and lingual epithelia were isolated as described before (Feng et al., 2020) and immediately put into the lysis buffer for RNA preparation (see below).
For in vitro experiments, 1 μg/ml LPS was added directly into the culture medium. Mature taste organoids from early passages that had been cultured for more than 12 days were treated with LPS. RAW 264.7 is a mouse monocyte/macrophage cell line established from ascites of a tumor induced in a male mouse by intraperitoneal injection of Abelson Leukaemia Virus. RAW 264.7 is widely used in the research of inflammation, immunity, apoptosis, and tumor. In the present study, RAW 264.7 cells were used as an in vitro immune cell model to evaluate its response to LPS stimulation. RAW 264.7 cells were cultured in DMEM with 10% fetal bovine serum (FBS). For iNOS gene expression analysis, organoids or RAW 264.7 cells were collected at 1, 2, 3, 6, 12, 24, 48 and 72 h after adding LPS. RNAs were isolated as described below. For secreted NO analysis, cell culture medium was collected at 3, 6, 9, 12, 24, and 48 h after adding LPS.
For NO secretion by native lingual epithelia, tongue epithelium was peeled off as described above. The epithelial sections containing circumvallate and foliate taste buds were cut off and cultured together as the taste epithelium group, while the nontaste epithelium (from the intermolar eminence section) was cultured as the nontaste epithelium group. Before LPS treatment, the taste epithelium and nontaste epithelium were cultured in DMEM with 10% FBS for 30 min. After adding LPS (1 μg/ml), culture medium was collected at 3 and 6h.
2.4. Gene expression analyses by quantitative RT-PCR (qRT-PCR)
Total RNA was prepared from mouse tongue taste epithelium, cultured taste organoids, and RAW 264.7 cells using Absolutely RNA Microprep Kit (Agilent) with DNase I (RNase-free) treatment. cDNA was synthesized using Superscript III reverse transcriptase following the manufacturer recommended protocol. Real-time PCR with gene specific primers was set up using Power SYBR Green master Mix (Thermo Fisher) and run on StepOnePlus Real-Time PCR System equipment (Thermo Fisher). The iNOS gene specific primers used were: 5’-TCAACTGCAAGAGAACGGAGA-3’ and 5’-TGAGAACAGCACAAGGGGTTT-3’. Relative quantification of gene expression was performed based on the 2−ΔΔCt method (Wang et al., 2007). β-Actin was used as the endogenous control gene for these analyses. The β-Actin gene specific primers used were: 5’- GATTACTGCTCTGGCTCCTA-3’ and 5’- ATCGTACTCCTGCTTGCTGA-3’.
2.5. Immunohistochemistry
Tissue preparation and immunofluorescent staining procedures were described previously (Feng et al., 2015; Wang et al., 2007). All mice used for this procedure were 4–6 months old. Briefly, excised mouse tongue tissues were fixed in freshly prepared 4% paraformaldehyde in phosphate-buffered saline (PBS) for 1 h on ice and then cryoprotected in 20% sucrose/PBS solution at 4°C overnight and embedded in mounting medium. Tissues were sliced into 10-μm-thick sections using a LEICA CM1900 cryostat. Purified Rabbit polyclonal (or mouse monoclonal) antibodies against iNOS (see Reagents) were used to detect the expression of iNOS in taste tissues. Control experiment for iNOS immunostaining was conducted. In control experiment, primary antibodies against iNOS were omitted in the procedure. To investigate what types of taste bud cells express iNOS, double immunostaining was carried out using rabbit or goat antibodies against taste-cell-type markers and rabbit (or mouse) antibodies against iNOS. Antibodies to the following taste-cell-type or taste neuron markers were used: PLC-β2 (1:500), CA4 (1:500), and P2X3 (1:300). DyLight 649 (or DyLight 488)-conjugated donkey anti-rabbit, anti-mouse or anti-goat secondary antibodies were used. Fluorescent images were acquired using Olympus FV1200 laser scanning confocal microscopy.
2.6. NO measurements
The Griess reaction was performed to measure the concentration of nitrite in the medium as an indicator of NO production. Nitric Oxide Assay Kit for NO determination was purchased from R&D Systems (Minneapolis, USA). Culture media from LPS-treated or control samples were either diluted or used directly for measurement. All incubation and washing steps were performed following the manufacturer recommended protocols. The optical density values were measured using a FlexStation 3 instrument. NO levels were determined by comparison with the standard curve.
2.7. Taste preference tests
Two-bottle preference tests were conducted as previously described (Wang et al., 2009; Feng et al., 2015). Briefly, male C57BL/6 mice were individually caged. Only male mice were used for this experiment to reduce variations in behavioral data. For the first 2 days, mice were familiarized with the two drinking bottles, both containing deionized water. For the following days, mice were presented with two drinking bottles: one contained deionized water and the other a taste solution with or without the NO donor nitroprusside. The positions of the bottles were switched after 24 h to minimize positional effect. The volume of consumed liquid from each bottle was recorded at 24 and 48 h. Each concentration of a taste compound was tested for 48 h. The taste compounds were tested in the following order: NaCl (75, 150 and 300 mM), quinine hydrochloride (QHCl) ( 0.01, 0.03, and 0.1 mM), Saccharin (3, 30, and 300 mM), and citric acid (1, 10, and 30 mM). Each mouse in the experiment was tested with all the above listed compounds. Between the testing of two different compounds, mice received deionized water in both drinking tubes for at least three days. During the experiment mice had free access to food. Taste preference scores were calculated for each animal by dividing the volume of consumed taste solution during the 48 h test period by the total volume of fluid intake during the same 48 h test period (i.e. preference score = intake of taste solution/(intake of taste solution + intake of water)). The average preference scores were then calculated for each group. By this calculation formula, preference scores between 0.5 and 1 indicate that mice prefer the taste solution over water, whereas preference scores between 0 and 0.5 indicate that mice avoid the taste solution. 10 male mice (4–8 months old) in each group were used.
2.8. Data analyses
Data are expressed as mean ± standard deviation (SD). For statistical analyses, repeated measures two-way ANOVA with post hoc t tests were performed using SPSS, version 22.0. P-values < 0.05 were considered significant.
3. Results
3.1. LPS strongly induces iNOS mRNA and protein expression in mouse taste tissues
iNOS, the enzyme encoded by the Nos2 gene and responsible for NO production during infection, is synthesized de novo by various types of cells in response to the recognition of microbial molecular patterns, such as LPS. To determine whether the taste tissues could express iNOS during infection, the expression of the iNOS gene and protein in taste tissues were analyzed after LPS stimulation.
In lingual epithelia (i.e., fungiform, cirumvallate, and foliate) from LPS-treated mice, the iNOS mRNA expression increased rapidly after administration of LPS (i.p., 2.5 mg/kg), peaked at 2h, and then returned to pre-stimulation levels (Fig.1A–B). LPS stimulation experiment was also conducted on lingual organoids, a model established in our labs for studying molecular mechanisms of cytokine responses in taste buds. Similar to taste tissues, the expression of iNOS mRNA in cultured organoids peaks at 2h after LPS stimulation (1 μg/ml), and then decreased in the subsequent 72 hours (Fig.1C).
Figure 1.

LPS-induced iNOS mRNA expression. qRT-PCR analysis of iNOS mRNA levels in mouse fungiform (A), circumvallate and foliate epithelium (B), cultured taste organoids (C), cultured RAW 264.7 cells (D), and mouse spleen (E) at various time points after LPS treatments (2.5 mg/kg i.p. for mice and 1 μg/ml in culture medium for organoid and RAW 264.7 cells). Relative quantification was performed using β-actin as the endogenous control. iNOS mRNA levels in control samples (CT, treated with buffer only) were set to 1. Values are presented as means ± SD (n = 3 per time point).
Meanwhile, to compare the difference of iNOS expression between immune cells and taste tissues, iNOS expression in mouse spleen and the mouse macrophage cell line RAW 264.7 were also determined after LPS stimulation. As shown in Fig. 1D, expression of the iNOS gene in RAW 264.7 cells increased 6h after LPS addition (1 μg/ml), peaked at 24h, and then decreased. Expression of iNOS mRNA in mice spleen increased 2h after administration of LPS (i.p., 2.5 mg/kg), peaked at 6h, and then decreased (Fig. 1E). Taken together, both the taste tissues and lingual organoids express iNOS mRNA when stimulated with LPS, and the induction of iNOS mRNA expression is more rapid in the taste tissues than in the immune organ spleen and RAW 264.7 cells.
We further examined iNOS protein expression in circumvallate taste buds by immunohistochemistry. Mice treated with PBS showed no detectable iNOS protein expression in circumvallate taste buds (Fig.2), which is consistent with the very low level iNOS mRNA expression as shown by RT-PCR experiments (Fig. 1). Following LPS stimulation, iNOS immunofluorescence signals in circumvallate taste buds became detectable from 3 to 24 hours (Fig.2). However, compared to iNOS mRNA induction time course, which peaked at 2h, iNOS protein induction was delayed, which may reflect the lagging protein expression and accumulation.
Figure 2.
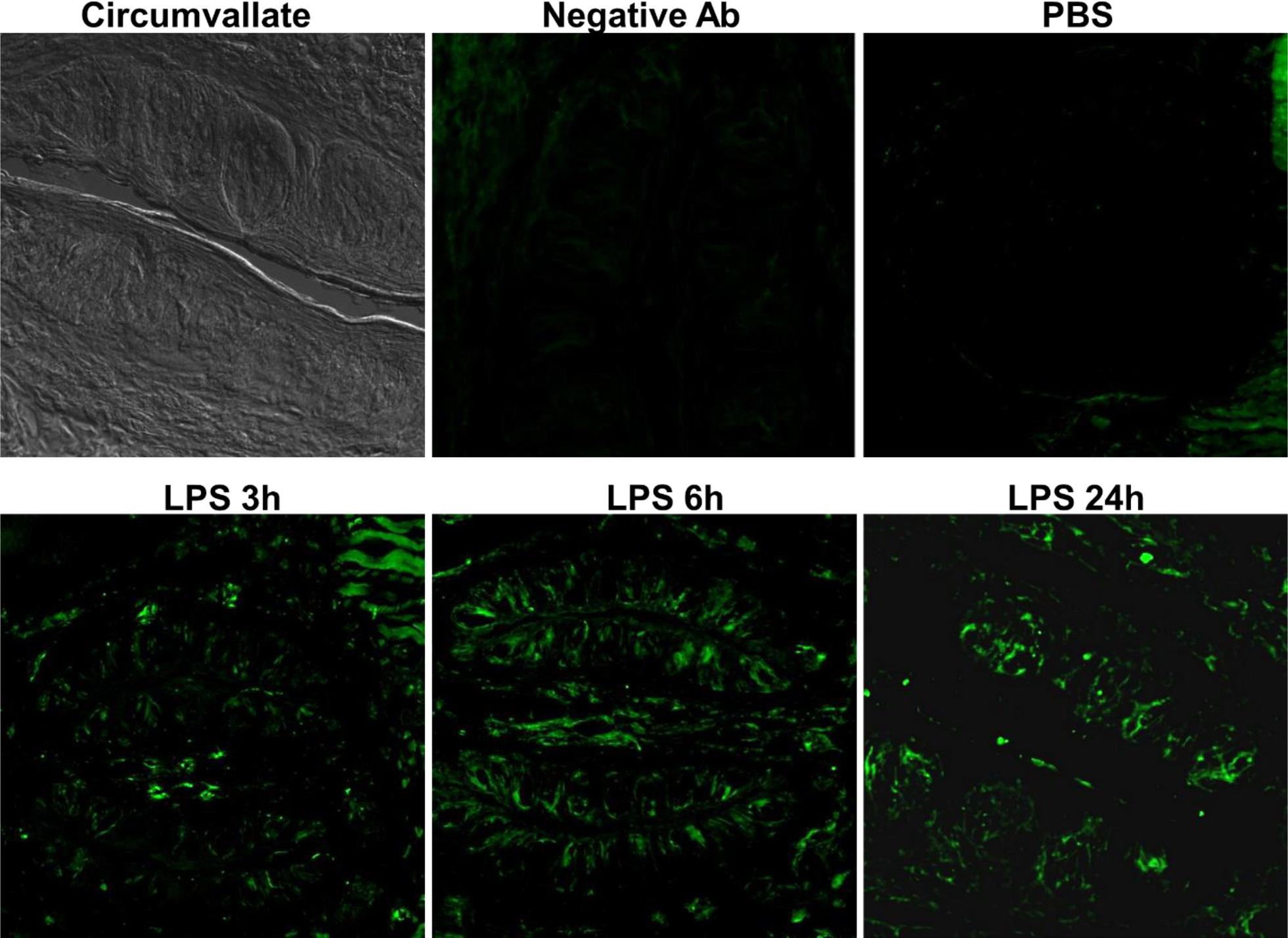
LPS induces iNOS protein expression in circumvallate taste buds. Circumvallate: a bright-field image of a circumvallate section. Negative Ab: mice treated with LPS (2.5 mg/kg i.p.) and immunostained with a nonspecific antibody. PBS: mice treated with PBS (vehicle control) and stained with antibodies against iNOS (green). LPS 3h, 6h, and 24h: mice treated with LPS (2.5 mg/kg i.p.) for 3h, 6h, and 24h and stained with antibodies against iNOS (green).
3.2. LPS-induced iNOS is expressed in subsets of taste bud cells
To determine whether iNOS is expressed in subsets of taste bud cells, two well-established taste receptor cell markers, PLC-β2 and CA4, were applied to identify types II and III taste cells, respectively. As shown in Figs. 3 and 4, iNOS partially overlaps with PLC-β2 and CA4 in the circumvallate sections, suggesting that iNOS is expressed in subsets of types II and III taste bud cells.
Figure 3.

Mouse circumvallate sections immunostained with antibodies against iNOS (green) and Phospholipase C-β2 (PLC-β2, red) after LPS stimulation (2.5 mg/kg i.p.). Enlarged view: colocalization of iNOS protein (green) and PLC-β2 (red). Green arrow: iNOS protein positive expression. Yellow arrow: colocalization of iNOS protein and PLC-β2.
Figure 4.

Mouse circumvallate sections immunostained with antibodies against iNOS (green) and carbonic anhydrase 4 (CA4, red) after LPS stimulation (2.5 mg/kg i.p.). Enlarged view: colocalization of iNOS protein (green) and CA4 (red). Green arrow: iNOS protein positive expression. Yellow arrow: colocalization of iNOS protein and CA4.
3.3. iNOS is also found in some P2X3-positive taste neurons.
The ionotropic purinergic receptor, P2X3, is widely expressed on many sensory afferents, including sensory nerve fibers that innervate gustatory papillae and taste buds. P2X3 was used as a marker for taste neurons to further determine whether iNOS is expressed in taste neurons. As shown in Fig. 5, immunostaining signals of iNOS partially overlap with the signals of P2X3 in sections of circumvallate papillae, suggesting that iNOS is also expressed in some P2X3-positive taste neurons.
Figure 5.

Mouse circumvallate sections immunostained with antibodies against iNOS (green) and P2X3 (P2X3, red) after LPS stimulation (2.5 mg/kg i.p.). White arrows: colocalization of iNOS protein and P2X3.
3.4. LPS induces the level of NO in taste buds.
After verifying the expression of iNOS in taste buds and taste neurons, we then examined NO production after LPS stimulation in lingual epithelium and compared it to NO production in RAW 264.7 cells. As shown in Fig. 6A, LPS significantly induced NO production in circumvallate and foliate epithelium 6 hours after stimulation. While in RAW 264.7 cells, the accumulation of NO was observed from 6 to 48 hours.
Figure 6.

Induction of NO production in isolated lingual epithelia and RAW 264.7 cells at various time points after LPS treatments (1 μg/ml in culture medium). Values are presented as means ± SD (n = 3 per group). *p < 0.05 versus the nontaste epithelium group.
3.5. NO enhances aversive responses to salty, bitter, and sour taste compounds.
Although the above results showed that LPS could induce iNOS expression and NO production in a subset of taste bud cells and taste nerves, the role of NO in the peripheral taste system remains unclear. In this part, we further investigated whether NO affects taste-related behaviors. Only male mice were used for this experiment to minimize variations in taste behavioral responses. Nitroprusside is a NO donor that has been used by various studies to evaluate the effects of NO (Cano et al. 2017, Trevlopoulou et al. 2016). We first evaluate the preference of nitroprusside (0.01, 0.1, or 1 mM) vs water via two-bottle preference tests. As shown in Fig. 7A, mice did not show significant preference or aversion to 0.01 or 0.1 mM nitroprusside compared to water (p > 0.05, water intake vs intake of 0.01 or 0.1 mM nitroprusside), whereas mice did show reduced preference to 1 mM nitroprusside compared to water (p = 0.001, water intake vs. intake of 1 mM nitroprusside). Based on this analysis, we then applied 0.1 mM nitroprusside in the next set of experiments. In the 48 h two-bottle preference tests, mice were given free access to water and taste solutions with or without nitroprusside (0.1 mM). The volumes of the consumed water and taste solutions were recorded and preference scores for the taste solutions were calculated. As shown in Fig. 7B-1, mice strongly avoided all concentrations of NaCl tested when NaCl was co-administrated with nitroprusside. The effect of nitroprusside was most striking for 75mM NaCl, as mice did not avoid 75mM NaCl without nitroprusside but strongly avoided the solution when nitroprusside was co-administrated. These results indicate that NO enhances the aversive response to salty taste. For the bitter taste test (Fig. 7B-2), co-administration of quinine and nitroprusside enhanced the aversive response to bitter taste only when quinine was at higher concentrations (0.03 and 0.1 mM). For the sour taste test (Fig. 7B-3), mice displayed significantly enhanced avoidance behavior when nitroprusside was co-administrated with citric acid solutions. In contrast, nitroprusside co-administration has no significant effect on sweet taste preference (Fig. 7B-4). Together, these results suggest that NO enhances the aversive taste responses to salty, bitter, and sour taste compounds, and this effect is particularly robust for salty taste.
Figure 7.

NO enhances the aversive responses to salty, bitter and sour taste compounds. (A) Preference scores of NO donor solutions vs. water. (B-1) Mice avoided all concentrations of NaCl tested when NaCl was co-administrated with nitroprusside. (B-2) Nitroprusside enhanced the aversive response to bitter taste when quinine was at higher concentrations (0.03 and 0.1 mM). (B-3) Nitroprusside enhanced the aversive response to sour taste. (B-4) Nitroprusside co-administration had no significant effect on sweet taste preference. Values are presented as means ± SD (n = 10 per group). *p < 0.05, **p < 0.01 versus the tastant administrated alone group.
4. Discussion
In the present study, we found that iNOS gene and its protein are induced in taste buds and taste neurons after LPS stimulation. NO can also be detected in isolated lingual epithelium after LPS treatment. We also evaluated the effects of NO on taste-related behavior. Our results showed that NO enhances the aversive responses to salty, bitter, and sour taste compounds, and this effect is especially strong for NaCl. These results suggest that iNOS and NO induction may contribute to taste disorders, such as taste distortion, in inflammatory diseases.
It has been reported that nNOS was expressed in the basal and apical parts of taste cells, while eNOS was expressed in taste buds and lingual epithelium in rat (Kretz et al. 1998). Later, Zaccone et al. (2002), reported that antibodies against nNOS revealed a positive immunoreaction in the cell body of taste receptor cells, basal cells and intragemmal nerve fibers in frog (Zaccone et al., 2002). In the same study by Kretz et al. (1998), iNOS did not show detectable expression in taste buds of unstimulated rats in immunostaining experiments, which is consistent with our results (Fig. 2, PBS). It is well known that iNOS is not constantly expressed in cells and is only expressed when the cells are induced or stimulated, primarily by proinflammatory cytokines and/or LPS. Indeed, our results showed that iNOS mRNA and protein were strongly induced in taste buds after LPS stimulation in vitro and in vivo (Figs. 1 and 2). Our results also showed that iNOS is induced in subsets of taste bud cells, including types II and III taste receptor cells, and taste neurons. How this expression pattern of iNOS relates to the altered taste responses to aversive compounds by NO is unclear at this point and will be investigated in the future.
The effect of NO on taste signaling is poorly understood. Murata et al. reported that in blowfly NO, which may be produced by intrinsic NOS in sugar receptor cells, participates in the transduction cascade of these cells (Murata et al. 2004). Later, Newland and Yates (2008) showed that NO modulates behavioral responses to NaCl via a cGMP/PKG-independent pathway while modulating the responses to sucrose via a NO-cGMP/PKG-dependent pathway in locusts (Newland and Yates 2008). Cano et al. found that activation of NO/sGC/cGMP cascade changed the appetitive responses to low-salt diets into aversive response in the blood-sucking insect R. prolixus (Cano et al. 2017). Interestingly, our results showed that mice administrated with the NO donor nitroprusside together with salty, bitter, and sour solutions showed enhanced aversive responses. In particular, mice strongly avoided 75mM NaCl with nitroprusside but was neutral to 75mM NaCl (Fig. 7B-1). This result is consistent with the report by Cano et al. (2017) and suggest that NO may play a role in aversive taste responses in rodents.
The lateral hypothalamus is a key brain area in the regulation of feeding behavior. And the role of NO in regulating feeding behavior in the hypothalamus has been solidified over the past decade. The series of research by the Morley group demonstrated that L-arginine, which results in NO synthesis, increased food intake in mice while the inhibitor of NO synthesis, L-NG -nitro arginine (L-NO Arg) inhibited food intake in food deprived mice (Morley and Flood,1991, 1992, 1994; Morley et al., 1995). Later, they further found that NO inhibitor L-NAME was more effective at decreasing food intake in 12- and 24-month-old mice than in 3-month-old months, which suggested that NO may play an increasingly important role in the feeding drive with advancing age (Morley et al., 1996). Besides, Stricker demonstrated that L-NAME produced a dose-dependent decrease in food intake in the obese rats, which indicate a role of nitric oxide in the expression of hyperphagia (Stricker et al., 1996). Meanwhile, Calapai et al. reported inhibition of NO formation accompanies reduction of ethanol intake and suggest a possible role for NO in ethanol self-administration (Calapai et al., 1996). Similarly, Tack et al. further demonstrated that NO synthase inhibition in human dose dependently inhibited gastric accommodation to a meal, suggesting involvement of a nitrergic pathway (Tack et al., 2002). In addition, research has shown that some neuropeptides such as ghrelin, neuropeptide Y, leptin and orexin regulate food consumption through affecting NO levels in the hypothalamus (Morley et al., 1999; Gaskin et al., 2003; Mannucci et al., 2005; Yang et al., 2007). Above all, the production of endogenous NO in hypothalamus has been reported to increase food intake, while the inhibition of NO production has negative effects on feeding behaviors (Morley et al., 2011). Compared to the present study, it is interesting to note that NO in the peripheral taste system may play a different role in food intake. NO in taste buds appears to enhance aversive responses to salty, bitter, and sour compounds, while increased NO levels in the hypothalamus may stimulate food intake. Since iNOS is only induced under inflammatory conditions, NO produced by iNOS in taste buds may interfere with normal taste signaling and contribute to reduced food intake under disease conditions.
5. Conclusions
The present study found that iNOS mRNA and protein are expressed in subsets of taste receptor cells and taste neurons and NO can be detected in isolated lingual epithelium after LPS stimulation. Furthermore, NO was found to enhance aversive responses to salty, bitter, and sour taste compounds. iNOS and NO induced in taste buds under inflammatory conditions may contribute to taste disturbances in some diseases.
Acknowledgements
This research was in-part supported by National Institutes of Health/National Institute of Deafness and Other Communication Disorders grants R56DC016921 (HW) and P30DC011735 (RFM). SF was supported by grants from National Natural Science Foundation of China (NSFC) (NSFC-81903817 to SF) and Fundamental Research Funds for the Central Universities (XDJK2019C113 to SF).
Footnotes
Conflict of Interest statement
All authors declare that there are no conflicts of interest.
References
- Ally A, Powell I, Ally MM, Chaitoff K, Nauli SM, 2020. Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states. Nitric Oxide. 102, 52–73. 10.1016/j.niox.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SM, Doty RL, 2003. Clinical disorders affecting taste: evaluation and management. In Handbook of Olfaction and Gustation. 2nd Edition. Edited by Doty RL. New York: Marcel Dekker, Inc. pp, 935–957. [Google Scholar]
- Cano A, Pontes G, Sfara V, Anfossi D, Barrozo RB, 2017. Nitric oxide contributes to high-salt perception in a blood-sucking insect model. Sci Rep. 7, 15551. 10.1038/s41598-017-15861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G, Mazzaglia G, Sautebin L, Costantino G, Marciano MC, Cuzzocrea S, Di Rosa M, Caputi AP, 1996. Inhibition of nitric oxide formation reduces voluntary ethanol consumption in the rat. Psychopharmacology (Berl). 125, 398–401. 10.1007/BF02246024. [DOI] [PubMed] [Google Scholar]
- Cinelli MA, Do HT, Miley GP, Silverman RB, 2020. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med Res Rev. 40, 158–189. 10.1002/med.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS, 2020. Nitric Oxide and Endothelial Dysfunction. Crit Care Clin. 36, 307–321. 10.1016/j.ccc.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW, 2007. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 21, 153–160. 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle ME, Appleton A, Liu QR, Yao Q, Mazucanti CH, Egan JM, 2021. Human Type II Taste Cells Express Angiotensin-Converting Enzyme 2 and Are Infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Am J Pathol. 191, 1511–1519. 10.1016/j.ajpath.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlik M, Reutterer B, Schindler C, Greten F, Vogl C, Muller M, Decker T, 2010. Nonconventional initiation complex assembly by STAT and NF-kappaB transcription factors regulates nitric oxide synthase expression. Immunity. 33, 25–34. 10.1016/j.immuni.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Jyotaki M, Kim A, Chai J, Simon N, Zhou M, Bachmanov AA, Huang L, Wang H, 2015. Regulation of bitter taste responses by tumor necrosis factor. Brain Behav Immun. 49, 32–42. 10.1016/j.bbi.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Achoute L, Margolskee RF, Jiang PH, Wang H, 2020. Lipopolysaccharide-Induced Inflammatory Cytokine Expression in Taste Organoids. Chem Senses. 45, 187–194. 10.1093/chemse/bjaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U, Sessa WC, 2012. Nitric oxide synthases: regulation and function. Eur Heart J. 33, 829–837. 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin FS, Farr SA, Banks WA, Kumar VB, Morley JE, 2003. Ghrelin-induced feeding is dependent on nitric oxide. Peptides. 24, 913–918. 10.1016/s0196-9781(03)00160-8. [DOI] [PubMed] [Google Scholar]
- Kretz O, Bock R, Lindemann B, 1998. Occurrence of nitric oxide synthase in taste buds of the rat vallate papilla. Histochem J. 30, 293–299. 10.1023/A:1003220125067. [DOI] [PubMed] [Google Scholar]
- Kumarhia D, He L, McCluskey LP, 2016. Inflammatory stimuli acutely modulate peripheral taste function. J Neurophysiol. 115, 2964–2975. 10.1152/jn.01104.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci C, Catania MA, Adamo EB, Bellomo M, Caputi AP, Calapai G, 2005. Long-term effects of high doses of nicotine on feeding behavior and brain nitric oxide synthase activity in female mice. J Pharmacol Sci. 98, 232–238. 10.1254/jphs.fpe05001x. [DOI] [PubMed] [Google Scholar]
- Morley JE, Flood JF, 1991. Evidence that nitric oxide modulates food intake in mice. Life Sci. 49, 707–711. 10.1016/0024-3205(91)90102-h. [DOI] [PubMed] [Google Scholar]
- Morley JE, Flood JF, 1992. Competitive antagonism of nitric oxide synthetase causes weight loss in mice. Life Sci. 51, 1285–1289. 10.1016/0024-3205(92)90018-k. [DOI] [PubMed] [Google Scholar]
- Morley JE, Flood JF, 1994. Effect of competitive antagonism of NO synthetase on weight and food intake in obese and diabetic mice. Am J Physiol. 266, R164–8. 10.1152/ajpregu.1994.266.1.R164. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Suarez MD, Flood JF, 1995. Nitric oxide synthase inhibition and food intake: effects on motivation to eat and in female mice. Pharmacol Biochem Behav. 50, 369–373. 10.1016/0091-3057(94)00276-o. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kumar VB, Mattammal MB, Farr S, Morley PM, Flood JF, 1996. Inhibition of feeding by a nitric oxide synthase inhibitor: effects of aging. Eur J Pharmacol. 311, 15–19. 10.1016/0014-2999(96)00546-8. [DOI] [PubMed] [Google Scholar]
- Morley JE, Alshaher MM, Farr SA, Flood JF, Kumar VB, 1999. Leptin and neuropeptide Y (NPY) modulate nitric oxide synthase: further evidence for a role of nitric oxide in feeding. Peptides. 20, 595–600. 10.1016/s0196-9781(99)00012-1. [DOI] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Sell RL, Hileman SM, Banks WA, 2011. Nitric oxide is a central component in neuropeptide regulation of appetite. Peptides. 32, 776–780. 10.1016/j.peptides.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Murata Y, Mashiko M, Suzuki H, Ozaki M, Amakawa T, Nakamura T, 2004. Intrinsic nitric oxide regulates the taste response of the sugar receptor cell in the blowfly, Phormia regina. Chem Senses. 29, 75–81. 10.1093/chemse/bjh007. [DOI] [PubMed] [Google Scholar]
- Newland PL, Yates P, 2008. Nitric oxide modulates salt and sugar responses via different signaling pathways. Chem Senses. 33, 347–356. 10.1093/chemse/bjm094. [DOI] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, et al. , 2020. More than smell - COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 45, 609–622. 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H, 2010. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 23, 75–93. 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Pereira BP, do Vale GT, Ceron CS, 2022. The role of nitric oxide in renovascular hypertension: from the pathophysiology to the treatment. Naunyn Schmiedebergs Arch Pharmacol. 395, 121–131. 10.1007/s00210-021-02186-z. [DOI] [PubMed] [Google Scholar]
- Phillips LM, Hill DL, 1996. Novel regulation of peripheral gustatory function by the immune system. Am J Physiol. 271, R857–862. 10.1152/ajpregu.1996.271.4.R857. [DOI] [PubMed] [Google Scholar]
- Pribitkin E, Rosentha MD, Cowart BJ, 2003. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann Otol Rhinol Laryngol. 112, 971–978. 10.1177/000348940311201110. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, 1983. Taste and smell in disease (first of two parts). N Engl J Med 308, 1275–1279. 10.1056/NEJM198305263082107. [DOI] [PubMed] [Google Scholar]
- Schuppe H, Cuttle M, Newland PL, 2007. Nitric oxide modulates sodium taste via a cGMP-independent pathway. Dev Neurobiol. 67, 219–232. 10.1002/dneu.20343. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Beck B, Burlet C, 1996. Nitric oxide mediates hyperphagia of obese Zucker rats: relation to specific changes in the microstructure of feeding behavior. Life Sci. 58, PL9–15. 10.1016/0024-3205(95)02260-0. [DOI] [PubMed] [Google Scholar]
- Tack J, Demedts I, Meulemans A, Schuurkes J, Janssens J, 2002. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut. 51, 219–224. 10.1136/gut.51.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevlopoulou A, Touzlatzi N, Pitsikas N, 2016. The nitric oxide donor sodium nitroprusside attenuates recognition memory deficits and social withdrawal produced by the NMDA receptor antagonist ketamine and induces anxiolytic-like behaviour in rats. Psychopharmacology (Berl). 233, 1045–1054. 10.1007/s00213-015-4181-x. [DOI] [PubMed] [Google Scholar]
- Vannini F, Kashfi K, Nath N, 2015. The dual role of iNOS in cancer. Redox Biol. 6, 334–343. 10.1016/j.redox.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L, 2007. Inflammation activates the interferon signaling pathways in taste bud cells. J Neurosci. 27, 10703–10713. 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Denbow DM, 2007. Interaction of leptin and nitric oxide on food intake in broilers and Leghorns. Physiol Behav. 92, 651–657. 10.1016/j.physbeh.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Zaccone G, Crescimanno C, Lo Cascio P, Mauceri A, Fasulo S, Sbarbati A, 2002. Immunohistochemical investigation of the nitrergic system in the taste organ of the frog, Rana esculenta. Chem Senses. 27, 825–830. 10.1093/chemse/27.9.825. [DOI] [PubMed] [Google Scholar]


