Abstract
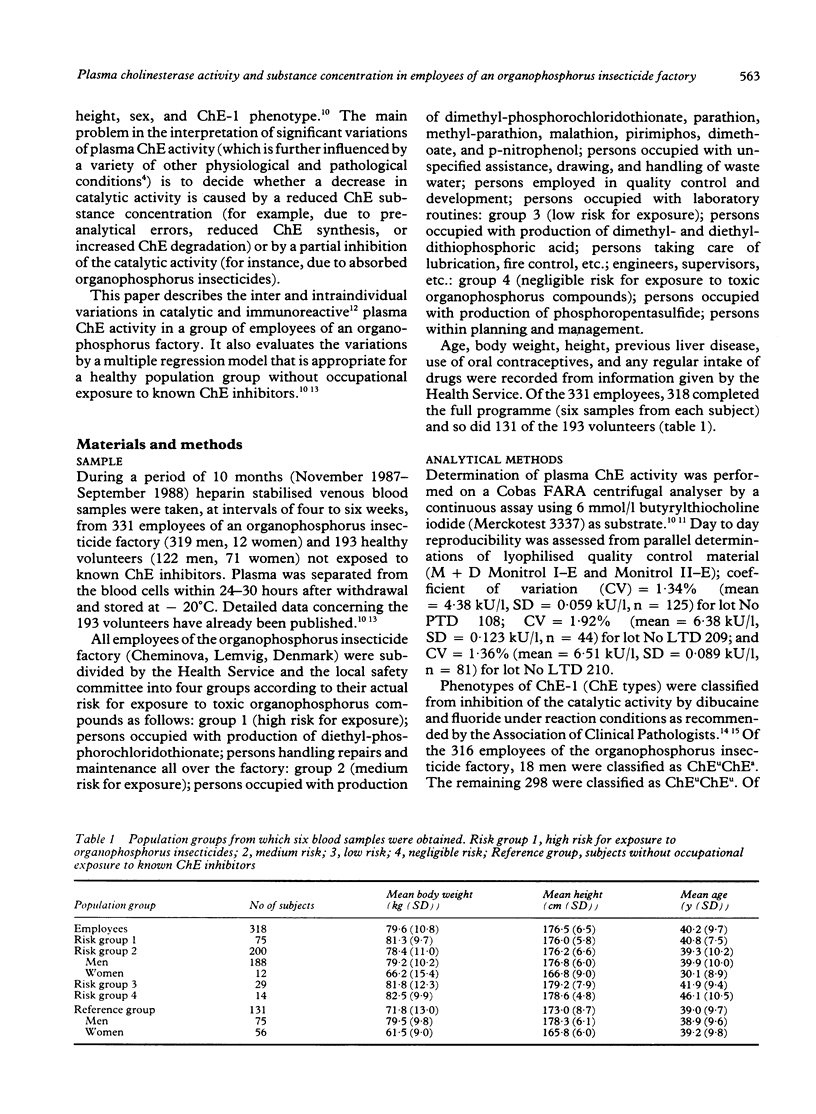
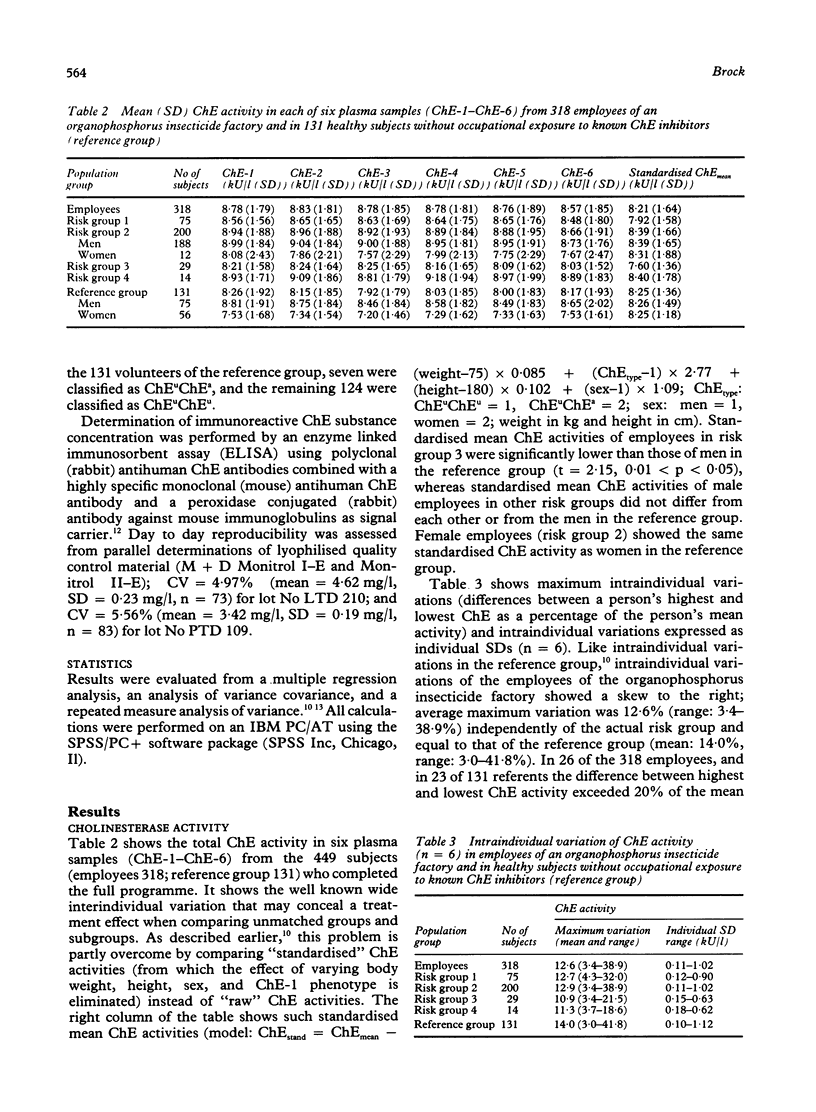
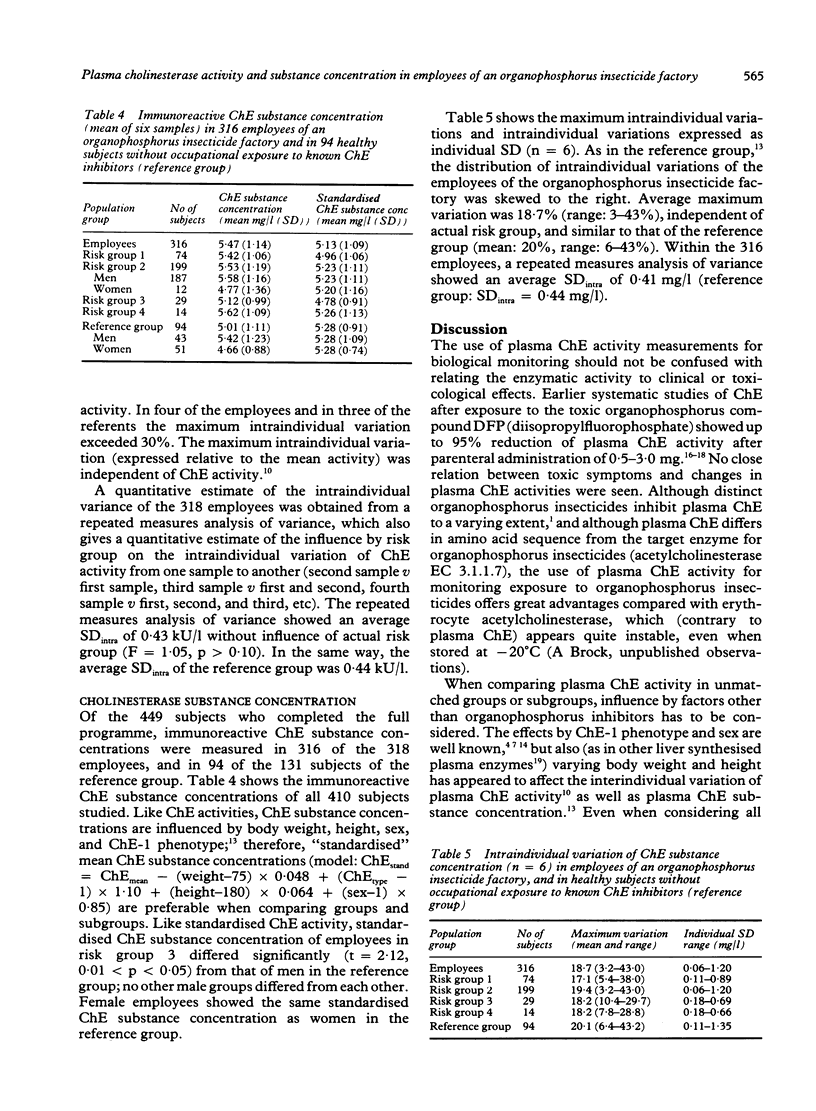
During a period of 10 months, inter and intraindividual variations in plasma cholinesterase (ChE) activity were studied in 331 employees of an organophosphorus insecticide factory, and in 193 healthy volunteers without occupational exposure to known ChE inhibitors. Repeated (n = 6) measurements of ChE activity and ChE substance concentration were performed in 410 subjects. The study showed substantial intraindividual variations of ChE activity and ChE substance concentration (up to 40%) in the employees and in the reference group. When effects due to sex, ChE-1 phenotype, body weight, and height were considered, one subgroup of employees of the organophosphorus insecticide factory showed a significantly lower average ChE activity than other subgroups; as ChE substance concentrations were found to be proportionally decreased, it was concluded that the low ChE activity was unrelated to occupational exposure. A combined determination of ChE activity and ChE substance concentration is recommended as a rational diagnostic tool when an unexpected decrease of plasma ChE activity is registered in people joining organophosphorus insecticide health surveillance programmes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock A. Additional electrophoretic components of cholinesterase in plasma: a phenomenon of no importance to the total plasma cholinesterase activity. J Clin Chem Clin Biochem. 1989 Jul;27(7):429–431. [PubMed] [Google Scholar]
- Brock A., Brock V. Plasma cholinesterase activity in a healthy population group with no occupational exposure to known cholinesterase inhibitors: relative influence of some factors related to normal inter- and intra-individual variations. Scand J Clin Lab Invest. 1990 Jun;50(4):401–408. doi: 10.3109/00365519009091598. [DOI] [PubMed] [Google Scholar]
- Brock A. Immunoreactive plasma cholinesterase (EC 3.1.1.8) substance concentration, compared with cholinesterase activity concentration and albumin: inter- and intra-individual variations in a healthy population group. J Clin Chem Clin Biochem. 1990 Nov;28(11):851–856. doi: 10.1515/cclm.1990.28.11.851. [DOI] [PubMed] [Google Scholar]
- Brock A. Plasma cholinesterase genetic variants phenotyped using a Cobas-Fara centrifugal analyser. J Clin Chem Clin Biochem. 1988 Dec;26(12):873–875. [PubMed] [Google Scholar]
- Fraser C. G. Desirable performance standards for clinical chemistry tests. Adv Clin Chem. 1983;23:299–339. doi: 10.1016/s0065-2423(08)60403-5. [DOI] [PubMed] [Google Scholar]
- Fraser C. G. The application of theoretical goals based on biological variation data in proficiency testing. Arch Pathol Lab Med. 1988 Apr;112(4):404–415. [PubMed] [Google Scholar]
- Harris E. K., Yasaka T. On the calculation of a "reference change" for comparing two consecutive measurements. Clin Chem. 1983 Jan;29(1):25–30. [PubMed] [Google Scholar]
- Lepage L., Schiele F., Gueguen R., Siest G. Total cholinesterase in plasma: biological variations and reference limits. Clin Chem. 1985 Apr;31(4):546–550. [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987 Jan 15;262(2):549–557. [PubMed] [Google Scholar]
- MUNKNER T., MATZKE J., VIDEBAEK A. Cholinesterase activity of human plasma after intramuscular diisopropyl fluorophosphonate (DFP). Acta Pharmacol Toxicol (Copenh) 1961;18:170–174. doi: 10.1111/j.1600-0773.1961.tb00327.x. [DOI] [PubMed] [Google Scholar]
- McGuire M. C., Nogueira C. P., Bartels C. F., Lightstone H., Hajra A., Van der Spek A. F., Lockridge O., La Du B. N. Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase. Proc Natl Acad Sci U S A. 1989 Feb;86(3):953–957. doi: 10.1073/pnas.86.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses G. C., Tuckerman J. F., Henderson A. R. Biological variance of cholinesterase and 5'-nucleotidase in serum of healthy persons. Clin Chem. 1986 Jan;32(1 Pt 1):175–177. [PubMed] [Google Scholar]
- Robinson D., Whitehead T. P. Effect of body mass and other factors on serum liver enzyme levels in men attending for well population screening. Ann Clin Biochem. 1989 Sep;26(Pt 5):393–400. doi: 10.1177/000456328902600503. [DOI] [PubMed] [Google Scholar]
- Sidell F. R., Kaminskis A. Temporal intrapersonal physiological variability of cholinesterase activity in human plasma and erythrocytes. Clin Chem. 1975 Dec;21(13):1961–1963. [PubMed] [Google Scholar]
- Silk E., King J., Whittaker M. Scientific Review No. 5. Assay of cholinesterase in clinical chemistry. Ann Clin Biochem. 1979 Mar;16(2):57–75. doi: 10.1177/000456327901600114. [DOI] [PubMed] [Google Scholar]
- Verberk M. M. Incipient cholinesterase inhibition in volunteers ingesting monocrotophos or mevinphos for one month. Toxicol Appl Pharmacol. 1977 Nov;42(2):345–350. doi: 10.1016/0041-008x(77)90011-4. [DOI] [PubMed] [Google Scholar]


