Abstract
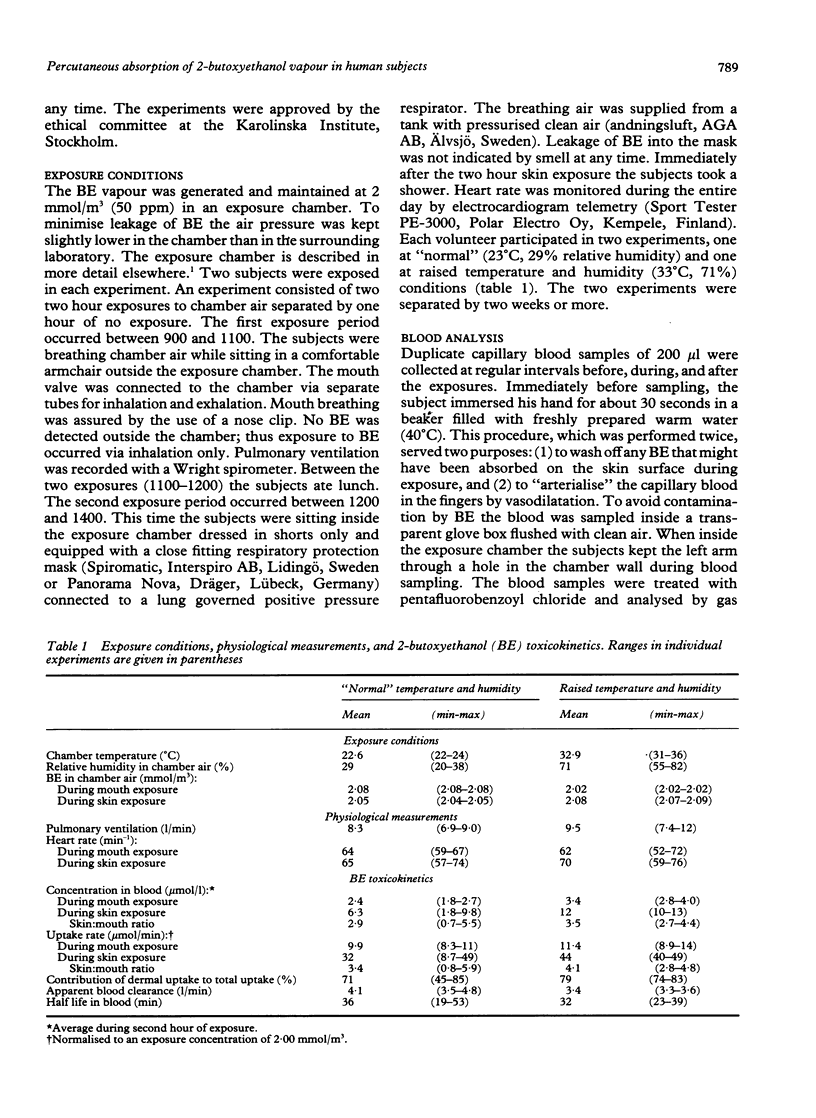
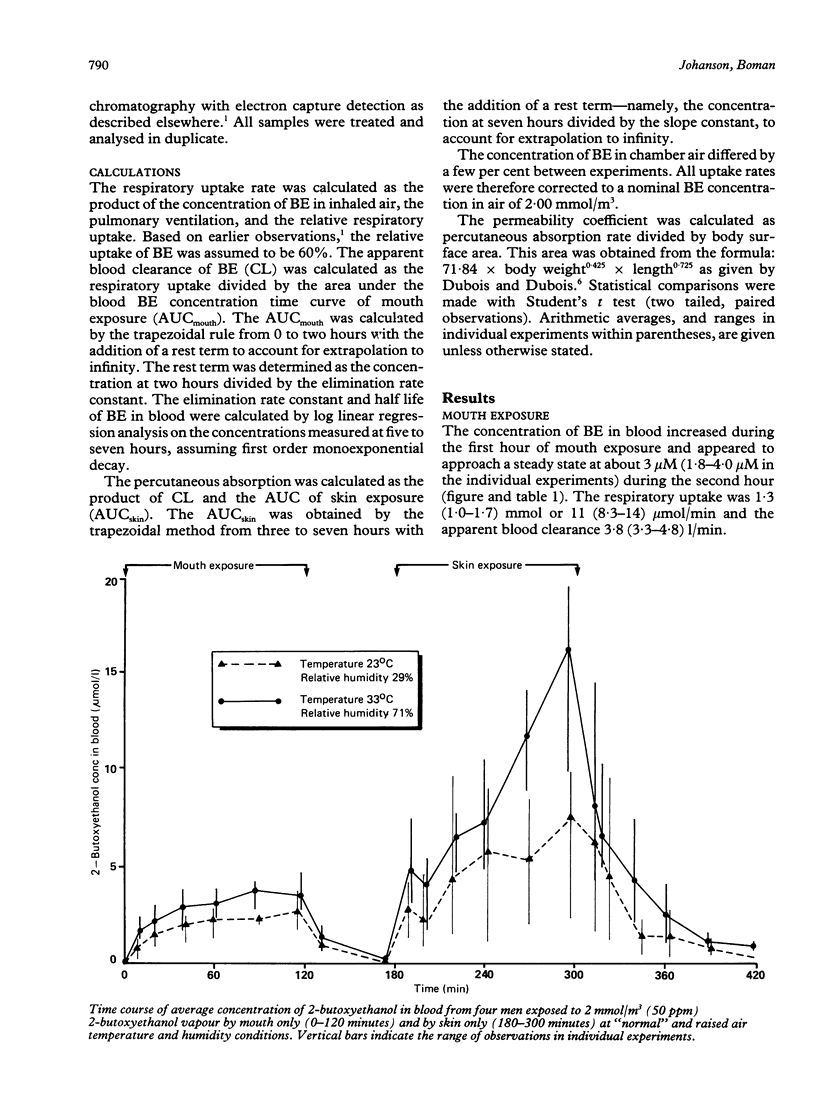
Four male volunteers were exposed at rest for two periods of two hours, separated by a one hour exposure free interval, to 50 ppm 2-butoxyethanol (BE) vapour generated in an exposure chamber. During the first two hour period the men were exposed by mouth only via a respiratory valve connected by tubes to the exposure chamber. During the second exposure period the men were exposed by skin only while sitting inside the exposure chamber, naked except for shorts, and wearing a respiratory protection mask supplied with compressed air. Capillary blood samples were collected at regular intervals and analysed for BE by a gas chromatographic method. Two experiments separated by at least two weeks were carried out with each volunteer, one at "normal" (23 degrees C, 29% relative humidity) and one at raised (33 degrees C, 71% relative humidity) air temperature and humidity in the chamber. The average concentration in blood and the calculated rate of uptake of BE were about three to four times higher during dermal exposure than during inhalation exposure. These experiments suggest that dermal uptake of BE accounts for about 75% (45-85% in individual experiments) of the total uptake during whole body exposure to BE vapour. Thus it appears that the use of a respiratory protection mask will not protect efficiently against exposure to BE vapours. A tendency towards increased percutaneous absorption rate was seen in the raised temperature and humidity condition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dugard P. H., Walker M., Mawdsley S. J., Scott R. C. Absorption of some glycol ethers through human skin in vitro. Environ Health Perspect. 1984 Aug;57:193–197. doi: 10.1289/ehp.8457193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeseneken D., Veulemans H., Masschelein R. Respiratory uptake and elimination of ethylene glycol monoethyl ether after experimental human exposure. Br J Ind Med. 1986 Aug;43(8):544–549. doi: 10.1136/oem.43.8.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeseneken D., Veulemans H., Masschelein R., Van Vlem E. Experimental human exposure to ethylene glycol monomethyl ether. Int Arch Occup Environ Health. 1989;61(4):243–247. doi: 10.1007/BF00381421. [DOI] [PubMed] [Google Scholar]
- Hursh J. B., Clarkson T. W., Miles E. F., Goldsmith L. A. Percutaneous absorption of mercury vapor by man. Arch Environ Health. 1989 Mar-Apr;44(2):120–127. doi: 10.1080/00039896.1989.9934385. [DOI] [PubMed] [Google Scholar]
- Johanson G., Boman A., Dynésius B. Percutaneous absorption of 2-butoxyethanol in man. Scand J Work Environ Health. 1988 Apr;14(2):101–109. doi: 10.5271/sjweh.1947. [DOI] [PubMed] [Google Scholar]
- Johanson G., Dynésius B. Liquid/air partition coefficients of six commonly used glycol ethers. Br J Ind Med. 1988 Aug;45(8):561–564. doi: 10.1136/oem.45.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson G., Fernström P. Influence of water on the percutaneous absorption of 2-butoxyethanol in guinea pigs. Scand J Work Environ Health. 1988 Apr;14(2):95–100. doi: 10.5271/sjweh.1941. [DOI] [PubMed] [Google Scholar]
- Johanson G., Fernström P. Percutaneous uptake rate of 2-butoxyethanol in the guinea pig. Scand J Work Environ Health. 1986 Oct;12(5):499–503. doi: 10.5271/sjweh.2108. [DOI] [PubMed] [Google Scholar]
- Johanson G., Kronborg H., Näslund P. H., Byfält Nordqvist M. Toxicokinetics of inhaled 2-butoxyethanol (ethylene glycol monobutyl ether) in man. Scand J Work Environ Health. 1986 Dec;12(6):594–602. doi: 10.5271/sjweh.2097. [DOI] [PubMed] [Google Scholar]
- McDougal J. N., Jepson G. W., Clewell H. J., 3rd, Andersen M. E. Dermal absorption of dihalomethane vapors. Toxicol Appl Pharmacol. 1985 Jun 15;79(1):150–158. doi: 10.1016/0041-008x(85)90377-1. [DOI] [PubMed] [Google Scholar]
- McDougal J. N., Jepson G. W., Clewell H. J., 3rd, Gargas M. L., Andersen M. E. Dermal absorption of organic chemical vapors in rats and humans. Fundam Appl Toxicol. 1990 Feb;14(2):299–308. doi: 10.1016/0272-0590(90)90209-3. [DOI] [PubMed] [Google Scholar]
- Riihimäki V., Pfäffli P. Percutaneous absorption of solvent vapors in man. Scand J Work Environ Health. 1978 Mar;4(1):73–85. doi: 10.5271/sjweh.2721. [DOI] [PubMed] [Google Scholar]
- Smith R. L. Review of glycol ether and glycol ether ester solvents used in the coating industry. Environ Health Perspect. 1984 Aug;57:1–4. doi: 10.1289/ehp.84571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta H. Skin absorption of organic solvent vapors in nude mice in vivo. Ind Health. 1989;27(2):37–47. doi: 10.2486/indhealth.27.37. [DOI] [PubMed] [Google Scholar]
- Wieczorek H. Evaluation of low exposure to styrene. II. Dermal absorption of styrene vapours in humans under experimental conditions. Int Arch Occup Environ Health. 1985;57(1):71–75. doi: 10.1007/BF00383547. [DOI] [PubMed] [Google Scholar]


