Abstract
Background
Restless legs syndrome (RLS) is a complex sensorimotor disorder occurring with a typical circadian fashion. Association with additional features, like alexithymia and nocturnal compulsive behaviors further complicates the framework.
Objectives
To assess interoception in RLS.
Methods
A total of 25 RLS patients and 28 controls underwent the heartbeat tracking task (interoceptive accuracy [IAC]). RLS symptoms’ frequency, disturbance and duration, nocturnal behaviors, interoceptive awareness (IAW), alexithymia, depressive and anxiety symptoms were also collected.
Results
RLS patients showed significant lower IAC (P = 0.0003) and IAW (P = 0.012), and reported more nocturnal eating behaviors (P < 0.001). IAC positively correlated with IAW (R = 0.32), and negatively correlated with age (R = −0.58). Nocturnal eating behavior negatively correlated with IAC (R = −0.44) and IAW (R = −0.50).
Conclusions
RLS patients presented reduced interoceptive abilities correlating with higher nocturnal eating behaviors. Future studies are needed to explore the role of interoception in RLS pathophysiology, also in relation to other sensorimotor aspects.
Keywords: restless legs syndrome, interoception, accuracy, nocturnal eating habits, alexithymia
Restless leg syndrome (RLS), also known as Willis‐Ekbom disease, is a common neurological sensorimotor disorder, characterized by an urge to move the legs or other body parts because of unpleasant sensations. 1 Symptoms worsen toward evening and at rest, are temporarily relieved by movement, 2 and interfere with sleep, quality of life, and mood. Women and the elderly are more affected, with a prevalence of up to 13% in occidental countries. 1 RLS can be primary or associated with other medical conditions. 1 , 3
The pathophysiology of RLS is still an open issue, involving genetic and environmental interactions. Brain iron deficiency 4 and central dopaminergic pathways’ dysfunction 5 play a pivotal role but glutamate, GABA, adenosine, and opioid pathways are also implicated, 1 , 4 leading to a network disorder that involves cortical, subcortical, spinal, and peripheral nerve generators. 6 Neuroimaging studies reported the involvement of both sensory and motor structures along with impaired sensorimotor integration. 7 , 8 , 9 RLS is, therefore, a complex condition where abnormal sensorimotor processing, related to altered neurochemical states likely triggered by iron deficiency, would occur as a circadian sensorimotor disorder. 10
Associations with nocturnal behaviors like night‐eating syndrome (NES), nocturnal smoking syndrome (NSS), sleep‐related eating disorder (SRED) have been described in RLS, 11 , 12 , 13 , 14 , 15 , 16 along with the association with neuroticism and harm avoidance personality traits. 12 , 17 Anxiety, depression, obsessive–compulsive traits, and alexithymia have been also reported in these patients. 12 , 17 , 18 Lower cardiac interoception accuracy (ie, the ability to perceive visceral internal body sensations) might be associated to higher level of alexithymia, poor sleep quality, and eating disorders. 19 , 20 , 21 Considering these premises and the peculiar sensory symptoms that RLS patients frequently have difficulty to describe, we aimed at investigating interoception accuracy 22 , 23 , 24 and awareness. 25
Methods
Participants
A total of 28 RLS patients with a clinical primary RLS diagnosis, according to latest international criteria 2 were consecutively recruited from the sleep disorder outpatient clinic of Verona University Hospital. A total of 34 healthy individuals matched for age, gender, body mass index (BMI), and smoking rate were tested as control group. Inclusion criteria were age >18 years and detection of at least one heartbeat during the 3‐min heart rate baseline (see task description below). 26 Exclusion criteria were concurrent neurological, cardiologic, psychiatric or medical conditions, and treatment with medications affecting cardiac function. Four participants were excluded because of technical issues; four for not detecting any heartbeat during baseline evaluation, and one for having cardiac disease. Finally, we included 25 RLS patients and 28 healthy controls (HCs). All signed a consent form; the local institution review board approved the protocol (Prog. 3049CESC).
Procedure
Participants underwent neurological examination and history taking with a sleep expert, including age, educational level, onset and disease duration, pharmacological treatments, comorbid conditions, body weight and height, smoking rate, and self‐administered questionnaires (Table 1).
TABLE 1.
Demographic data and self‐reported questionnaires
| RLS (N = 25) | HC (N = 28) | P‐values | |
|---|---|---|---|
| Age, years (± SD) a | 62.04 (± 16.70) | 54.50 (± 14.47) | 0.087 |
| Women, no. (%) b | 15 (60) | 16 (57) | 1 |
| Years of education, median (IQR) a | 11 (8–13) | 13 (9.5–13.75) | 0.37 |
| BMI, median (IQR) a | 25.15 (23.39–26.78) | 24.18 (22.37–26.40) | 0.29 |
| Smokers, no. (%) b | 5 (20) | 5 (18) | 1 |
| Heart rate, median (IQR) a , c | 70.5 (60.5–74.25) | 70 (60–82) | 0.70 |
| Mean symptoms’ duration, years (± SD) | 7.14 (± 8.19) | – | |
| IRLSSS (0–40), mean (± SD) d | 22.67 (± 5.59) | – | |
| BDI‐II (0–63), median (IQR) a , e | 5 (3–8) | 4 (1–9.75) | 0.37 |
| BAI (0–63), median (IQR) a , d | 10 (5–13.25) | 7.5 (6–12.75) | 1 |
| TAS‐20 (20–100), median (IQR) a , e | 43.5 (34.75–53.25) | 36.5 (35–40) | 0.055 |
| MAIA (0–160), median (IQR) a , e | 80.5 (71.75–96) | 94.5 (84.25–103) | 0.012* |
| NEQ (0–52), median (IQR) a , f | 9 (5.25–12.75) | 3 (1.75–5) | <0.001* |
Abbreviations: RLS, restless legs syndrome; HC, healthy controls; no., number; SD, standard deviation; IQR, interquartile range; BMI, body mass index; IRLSSS, International RLS Severity Scale; BDI‐II, Beck Depression Inventory; BAI, Beck Anxiety Inventory; TAS‐20, Toronto Alexithymia Scale; MAIA, Multidimensional Assessment of Interoceptive Awareness; NEQ, Night Eating Questionnaire.
P < 0.05.
Two sample independent t‐test, or Wilcoxon Mann–Whitney test.
χ2 test, or Fisher's exact test.
Four missing information.
One missing information.
Three missing information.
Seven missing information.
Heartbeat Tracking Task
The experimental procedure was conducted in a soft‐lighted, sound‐attenuated room before 2 pm. 20 Participants were comfortably seated, gently resting their wrists on the armrests, wearing a belt over their chests to measure their heartbeat. They underwent the Schandry's heartbeat perception task, 22 to evaluate interoception accuracy (IAC). Heart rate was recorded with a Polar wrist‐worn (model V800) wireless connected to the chest belt. Before starting the task, we recorded the heart rate at baseline for 3 min to also avoid confounding factors; participants were instructed to sit quietly, stay relaxed with their eyes closed, and listen to their heartbeat. They concentrated on their heartbeats, without taking their pulse or attempting any other physical manipulations. After the 3 min, participants were asked if they had detected their heartbeat at least once. If so, they were instructed to count their heartbeats silently during three different counting phases, lasting for 25, 35, and 45 s, each separated by 20‐s rest periods. The phases’ order was randomized between participants of each group. The experimenter provided “start” and “stop” signals. After each stop signal, participants verbally reported the counted heartbeats’ number, without being aware about the counting phases’ length, nor about their performance.
Self‐Administered Questionnaires
Patients completed the International RLS Severity Scale (IRLSSS), 27 composed of 10 items on symptoms’ frequency, intensity, and disturbance degree on a 5‐point scale, ranging from 0 (no RLS) to 40 (very severe RLS). All participants also completed the Multidimensional Assessment of Interoceptive Awareness (MAIA) questionnaire (0–160); higher scores mean better interoceptive awareness (IAW). 25 Information regarding nocturnal smoking and eating behaviors were collected through the Nocturnal Smoking Questionnaire (NSQ), 16 where participants need to meet the first criteria to qualify for nocturnal smoking, and the Night Eating Questionnaire (NEQ), 28 with 13 questions on a 5‐point scale (0–52), and two additional questions, one to evaluate SRED presence, and one on the disorder duration. Depressive and anxiety symptoms were collected through the Beck Inventories, both composed of 21 items on a 4‐point scale (0–63, higher scores, worse symptoms). 29 , 30 The Toronto Alexithymia Scale (TAS‐20) 31 measures alexithymia, with 20 items on a 5‐point scale (20–100). Higher scores indicate less emotion awareness, perception and expression.
Statistical Analysis
Descriptive statistics included frequencies for categorical variables and means and standard deviations or median and interquartile range for continuous variables. Comparisons between groups were performed by χ2 or Fisher's exact test for categorical variables and independent t test or Mann–Whitney test for continuous variables.
IAC was calculated as the mean score of three heartbeat perception intervals according to the formula 32 :
The IAC score vary between 0 and 1: higher scores indicate smaller differences between recorded and perceived heartbeats (i.e., more accuracy, or higher IAC).
Because most variables were not normally distributed (Shapiro–Wilk test), non‐parametric analyses were used to compare demographic and psychometric characteristics of the two groups. Correlations were conducted using Spearman bivariate correlations. When necessary, Bonferroni correction was applied. All analyses were performed with Rstudio software (Version 1.3.1093 2009–2020 Rstudio, PBC).
Results
Age, gender, BMI, heart rate, and smokers’ rate did not differ between groups (Table 1). RLS patients reported moderate to severe symptoms’ severity levels (IRLSSS: 22.67 ± 5.59), and of 18 patients investigated (72%), none reported nocturnal smoking (nor did the HC), three (16.7%) reported conscious nocturnal eating (only one scoring >25, indicating disorder presence), and one reported not always aware nocturnal eating (Table 1). All patients were under dopamine‐agonist therapy, one was also under antiepileptics and opioids, whereas controls were not under therapy with a direct cardiac effect. The two groups did not differ for anxiety and depressive symptoms, nor for alexithymia, although a trend emerged (W = 411; P = 0.055; Table 1). Moreover, the two groups significantly differ at MAIA and NEQ. RLS patients revealed lower IAW (W = 182.5; P = 0.012), and reported more nocturnal eating behaviors (W = 447; P < 0.001).
Interoceptive Accuracy
RLS patients showed a significant lower IAC (W = 132.5; P = 0.0001); median for RLS patients: 0.30 (IQR = 0.23–0.36); median for HC = 0.62 (IQR = 0.43–0.75); Fig. 1.
FIG. 1.
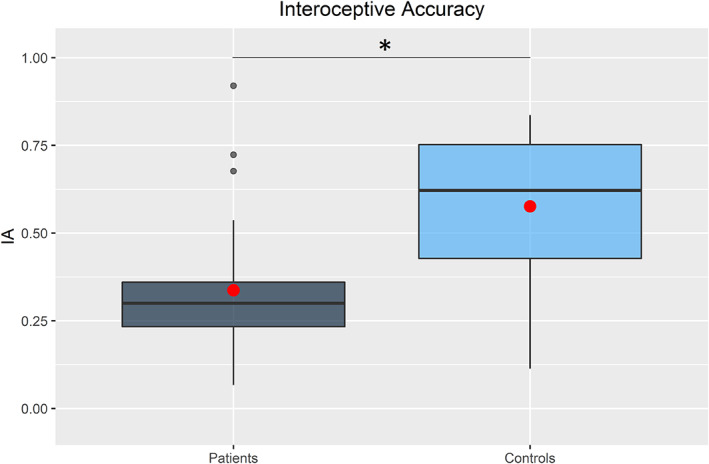
Interoceptive accuracy. Interoceptive accuracy boxplot for restless legs syndrome patients (dark gray) and HC (light blue). Data represent mean scores of three heartbeat perception intervals for each participant (1 = perfect accuracy). The horizontal black lines represent median values. The red dots represent mean values. *P < 0.05.
Correlations
IAC positively correlated with IAW (R = 0.32, P = 0.024) and negatively correlated with NEQ (R = −0.44, P = 0.003). Furthermore, IAC negatively correlated with age (R = −0.58, P < 0.001). No other correlations were significant.
No correlations emerged between IRLSSS and Beck Anxiety Inventory (BAI), Beck Depression Inventory (BDI‐II), TAS‐20, MAIA, and NEQ. Heart rate and interoceptive measures (IAC and IAW) did not correlate. NEQ and MAIA negatively correlated (R = −0.50, P = 0.001), whereas NEQ did not correlate with TAS‐20 total score.
Discussion
We explored interoception in RLS patients through IAC (heartbeat tracking task), IAW (MAIA score), and their relation. We also explored alexithymia, depressive and anxiety symptoms, and compulsory nocturnal behaviors. As expected, IAC was significantly reduced in RLS patients. We also found lower IAW, and IAW and IAC positively correlate, partly in line with previous findings, 25 showing a correspondence between subjective and objective interoceptive abilities. Indeed, the different interoceptive constructs and dimensions are known to be dissociable, partly related, and predicted by one another. 33 Last, IAC negatively correlated with age, confirming previous results in healthy population,34 but not related to symptoms’ severity (IRLSSS). Interoception is a complex concept and a precise direct measure of interoceptive signal is often difficult to obtain, 35 and different factors might influence the task performance, like time estimation, 26 personal beliefs, and attention on visceral sensations. 33 Nevertheless, given that some participants were honest in reporting not perceiving their heartbeat at baseline (and excluded from the task); we feel that this was not our case, underling the clarity of the instructions presented.
Conversely to previous studies, 12 , 17 , 18 our patients did not differ to controls with regard to anxiety or depressive symptoms, nor for alexithymia scores, although in such a case a trend toward a difference emerged. Probably those differences might be related to the smaller sampler of our cohort. 17 , 18 Alternatively, we may infer that interoception is an intrinsic RLS signature, stronger than other features, such as alexithymia, depression, or anxiety. Interestingly, no significant correlations between IAC and symptoms’ severity or duration, nor with other questionnaires, were reported, except for NEQ, which was also found correlating with IAW. Indeed, four patients described nocturnal eating behaviors, and one without total awareness, as reported also in a larger case–control study, 15 but these behaviors were not related to alexithymia. 36 Those findings underline a strict relation between interoception and compulsory behaviors that need to be further explored.
Altogether, those results further enlarge the complex framework of RLS pathophysiology highlighting that RLS patients have an overall reduced ability in interoception. Interoception may have an important role in well‐being. 34 Whether impaired interoception represents a clinical signature of the disorder or a related consequence of the disrupted sleep quality and distress related to symptoms remains an open issue. However, because altered circadian rhythm is known to influence interoceptive awareness, 20 the experiment was carried out during the morning, at the lowest of symptoms’ presentation, and no correlations with IRLSSS emerged. Therefore, interoception could be a specific RLS feature, given also that severe anxiety and depression levels were not found in our cohort. Our patients were treated with dopamine‐agonists for RLS symptoms; however, none of these treatments has been reported to affect interoception. Of course, we cannot ultimately exclude whether pharmacological treatment has somehow influenced our results. Considering the RLS complexity, a more detailed psychological, emotional, and cognitive investigation is required to better comprehend those intertwined aspects of the disorder. Future studies should also assess different interoceptive aspects, for instance through gastric interoception 37 or thermosensation, 38 and its relation to exteroceptive perception.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.S.: 1B, 1C, 2A, 2B, 3A.
G.C.: 1C, 2C, 3B.
G.P.M.: 1C, 2C, 3B.
M.L.: 1C, 2C, 3B.
M.T.: 1B, 2C, 3B.
E.A.: 1A, 1B, 1C, 2C, 3A.
Disclosures
Funding Sources and Conflicts of Interest: No specific funding was received for this work and the authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: All authors declare that there are no additional disclosures to report.
Ethical Compliance Statement: The local institution review board of Verona University approved the protocol (Prog. 3049CESC), all patients gave written consent. All authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Relevant disclosures and conflict of interest are listed at the end of this article.
References
- 1. Manconi M, Garcia‐Borreguero D, Schormair B, Videnovic A, Berger K, Ferri R, Dauvilliers Y. Restless legs syndrome. Nat Rev Dis Primers 2021;7:80. [DOI] [PubMed] [Google Scholar]
- 2. Allen RP, Picchietti DL, Garcia‐Borreguero D, et al. Restless legs syndrome/Willis‐Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria‐history, rationale, description, and significance. Sleep Med 2014;15:860–873. [DOI] [PubMed] [Google Scholar]
- 3. Trenkwalder C, Allen R, Högl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology 2016;86:1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanza G, Ferri R. The neurophysiology of hyperarousal in restless legs syndrome: hints for a role of glutamate/GABA. Adv Pharmacol 2019;84:101–119. [DOI] [PubMed] [Google Scholar]
- 5. Rizzo G, Plazzi G. Neuroimaging applications in restless legs syndrome. In: Politis M, ed. Imaging in Movement Disorders: Imaging Applications in Non‐Parkinsonian and Other Movement Disorders. International Review of Neurobiology 143. Cambridge, MA: Academic Press Books, Elsevier; 2018:31–64. [DOI] [PubMed] [Google Scholar]
- 6. Lanza G, Lanuzza B, Aricò D, et al. Impaired short‐term plasticity in restless legs syndrome: a pilot rTMS study. Sleep Med 2018;46:1–4. [DOI] [PubMed] [Google Scholar]
- 7. Tuovinen N, Stefani A, Mitterling T, et al. Functional connectivity and topology in patients with restless legs syndrome: a case‐control resting‐state functional magnetic resonance imaging study. Eur J Neurol 2021;28:448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Paiva JPQ, Magalhāes SC, Moura LM, et al. Sensorimotor white matter projections and disease severity in primary restless legs syndrome/Willis‐Ekbom disease: a multimodal DTI analysis. Sleep Med 2020;73:106–116. [DOI] [PubMed] [Google Scholar]
- 9. Stefani A, Mitterling T, Heidbreder A, et al. Multimodal magnetic resonance imaging reveals alterations of sensorimotor circuits in restless legs syndrome. Sleep 2019;42:zsz171. [DOI] [PubMed] [Google Scholar]
- 10. Antelmi E, Rocchi L, Latorre A, Belvisi D, Magrinelli F, Bhatia KP, Tinazzi M. Restless legs syndrome: known knowns and known unknowns. Brain Sci 2022;12:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howell MJ. Restless eating, restless legs, and sleep related eating disorder. Curr Obes Rep 2014;3:108–113. [DOI] [PubMed] [Google Scholar]
- 12. Marconi S, Scarlatti F, Rizzo G, et al. Is nocturnal eating in restless legs syndrome linked to a specific psychopathological profile? A pilot study. J Neural Transm 2015;122:1563–1571. [DOI] [PubMed] [Google Scholar]
- 13. Nigam G, Babu SS, Peter S, Chindrippu S, Mittal GK. Familial RLS with nocturnal eating–case report and review of literature. Ann Clin Case Rep 2022;7:2075. [Google Scholar]
- 14. Antelmi E, Vinai P, Pizza F, Marcatelli M, Speciale M, Provini F. Nocturnal eating is part of the clinical spectrum of restless legs syndrome and an underestimated risk factor for increased body mass index. Sleep Med 2014;15:168–172. [DOI] [PubMed] [Google Scholar]
- 15. Provini F, Antelmi E, Vignatelli L, et al. Association of Restless Legs Syndrome with nocturnal eating: a case‐control study. Mov Dis 2009;24:871–877. [DOI] [PubMed] [Google Scholar]
- 16. Provini F, Antelmi E, Vignatelli L, et al. Increased prevalence of nocturnal smoking in restless legs syndrome (RLS). Sleep Med 2010;11:218–220. [DOI] [PubMed] [Google Scholar]
- 17. Winkelman JW, Finn I, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Winsconsin sleep cohort. Sleep Med 2006;7:545–552. [DOI] [PubMed] [Google Scholar]
- 18. Yilmaz O, Şengül Y, Şengül HS, Parlakkaya FB, Öztürk A. Investigation of alexithymia and levels of anxiety and depression among patients with restless legs syndrome. Neuropsychiatry Dis Treat 2018;14:2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quadt L, Critchley HD, Garfinkel SN. The neurobiology of interoception in health and disease. Ann N Y Acad Sci 2018;1428:112–128. [DOI] [PubMed] [Google Scholar]
- 20. Ewing DL, Manassei M, Gould van Praag C, Philippides AO, Critchley HD, Garfinkel SN. Sleep and the heart: interoceptive differences linked to poor experiential sleep quality in anxiety and depression. Biol Psychol 2017;127:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mason TB, Engwall A, Mead MP, Irish LA. Sleep and eating disorders among adults enrolled in a commercial weight loss program: associations with self‐report and objective sleep measures. Eat Weight Disord 2019;24:307–312. [DOI] [PubMed] [Google Scholar]
- 22. Schandry R. Heart beat perception and emotional experience. Psychophysiology 1981;18:483–488. [DOI] [PubMed] [Google Scholar]
- 23. Ricciardi L, Ferrazzano G, Demartini B, et al. Know thyself: exploring interoceptive sensitivity in Parkinson's disease. J Neurol Sci 2016;364:110–115. [DOI] [PubMed] [Google Scholar]
- 24. Ferrazzano G, Berardelli I, Conte A, Suppa A, Fabbrini G, Berardelli A. Interoceptive sensitivity in patients with cervical dystonia. Parkinsonism Rel Dis 2017;44:129–132. [DOI] [PubMed] [Google Scholar]
- 25. Calì G, Ambrosini E, Picconi L, Mehling WE, Committeri G. Investigating the relationship between interoceptive accuracy, interoceptive awareness, and emotional susceptibility. Front Psychol 2015;6:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Desmedt O, Corneille O, Luminet O, Murphy J, Bird G, Maurage P. Contribution of time estimation and knowledge to heartbeat counting task performance under original and adapted instructions. Biol Psychol 2020;154:107904. [DOI] [PubMed] [Google Scholar]
- 27. Walters AS, LeBrocq C, Dhar A, et al. Validation of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med 2003;4:121–132. [DOI] [PubMed] [Google Scholar]
- 28. Vinai P, Ferri R, Ferini‐Strambi L, et al. Defining the borders between sleep‐related eating disorder and night eating syndrome. Sleep Med 2012;13:686–690. [DOI] [PubMed] [Google Scholar]
- 29. Ghisi M, Flebus G, Montano A, Sanavio E, Sica C. Beck Depression Inventory‐Second Edition. Adattamento italiano: manuale [manual of Italian adaptation]. Florence, Italy: Giunti Organizzazioni Speciali; 2006:79. [Google Scholar]
- 30. Sica C, Coradeschi D, Ghisi M, Sanavio E. Beck Anxiety Inventory. Adattamento italiano: manuale [manual of Italian adaptation]. Florence, Italy: Giunti Organizzazioni Speciali; 2006:45. [Google Scholar]
- 31. Bressi C, Taylor G, Parker J, et al. Cross validation of the factor structure of the 20‐item Toronto Alexithymia Scale: an Italian multicentre study. J Psychosom Res 1996;41(6):551–559. [DOI] [PubMed] [Google Scholar]
- 32. Damasio AR. Descartes' Error: Emotion, Reason and the Human Brain. New York (Grosset/Putnam): Avon Books; 1994. [Google Scholar]
- 33. Forkmann T, Scherer A, Meessen J, Michal M, Schächinger H, Vögele C, Schulz A. Making sense of what you sense: disentangling interoceptive awareness, sensibility and accuracy. Int J Psychophysiol 2016;109:71–80. [DOI] [PubMed] [Google Scholar]
- 34. Murphy J, Geary H, Millgate E, Catmur C, Bird G. Direct and indirect effects of age on interoceptive accuracy and awareness across the adult lifespan. Psychon Bull Rev 2018;25:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quigley KS, Kanoski S, Grill WM, Barrett LF, Tsakiris M. Functions of interoception: from energy regulation to experience of the self. Trends Neuro 2021;44:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vinai P, Provini F, Antelmi E, et al. Alexithymia is not related to severity of night eating behavior: a useful distinction from other eating disorders. Eat Behav 2015;17:94–98. [DOI] [PubMed] [Google Scholar]
- 37. Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS One 2012;7(5):e36646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crucianelli L, Enmalm A, Ehrsson HH. Interoception as independent cardiac, thermosensory, nociceptive, and affective touch perceptual submodalities. Biol Psychol 2022;172:108355. [DOI] [PubMed] [Google Scholar]


