Abstract
Background
Punding is a stereotyped behavior characterized by an intense fascination with a complex, excessive, non‐goal oriented, repetitive activity affecting individuals with Parkinson's disease (PD) on dopamine replacement therapy (DRT).
Objectives
In 2010, we published the first review focused on the pathophysiology of punding. This study aims to systematically review the literature of the past decade on punding in PD, particularly focusing on the clinical features, underlying pathophysiological mechanisms, and treatment.
Methods
Following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines, we searched PubMed, Embase, and APA PsycInfo for articles published between July 1, 2010 and March 19, 2022. The search strategy included: (punding) AND (parkinson*).
Results
Of 256 studies identified, 29 were eligible for inclusion with 19 original research articles and 10 case reports. This review confirmed that predictors of punding in PD are higher doses of DRT, younger age, male sex, and increasing disease severity. We also found an association between punding and psychiatric and/or cognitive symptoms. Neuroimaging studies have showed that punding in PD is associated with a disconnection between midbrain, limbic and white matter tracts projecting to the frontal cortices and a breakdown of the connectivity among the crucial nodes of the reward circuit. Low‐frequency repetitive transcranial magnetic stimulation on the dorsolateral prefrontal cortex has been shown to produce a transient beneficial effect in PD patients with punding.
Conclusion
In conclusion, although the clinical features of punding have been established, in the past 12 years, we gained a better understanding of the pathophysiological mechanisms of punding, mainly thanks to magnetic resonance imaging techniques.
Keywords: punding, Parkinson's disease, impulsive compulsive behaviors, pathophysiology, neuroimaging
Parkinson's disease (PD) is the second most common degenerative disease of the central nervous system. It is caused by a loss of nigral dopaminergic neurons and is characterized clinically by cardinal motor symptoms, such as bradykinesia, tremor, rigidity, and postural instability. 1 PD patients can also be affected by non‐motor symptoms, which include psychiatric and behavioral disorders that can be as debilitating as the motor symptoms. 2 PD patients are most commonly treated with dopamine replacement therapy (DRT) such as the dopamine precursor levodopa or dopamine agonists (DA). However, there has also been increasing evidence of DRT‐related complex behaviors found in PD patients. 3 These are categorized as impulse compulsive behaviors (ICBs) that include impulse control disorders (ICDs), dopamine dysregulation syndrome (DDS), hobbyism, and punding behaviors. The relationship between hobbyism and punding is unclear at the moment and might both represent the same neurobiological entity.
Punding is a stereotyped behavior characterized by an intense fascination with a complex, excessive, non‐goal oriented, repetitive activity. 4 , 5 Unlike ICBs, punding is not driven by pleasure, anxiety, or obsessions. 6 Patients exhibit behaviors that appear to be more peculiar and less stressful. However, any interruption or disruption of the activity often leads to irritation, anxiety, and frustration. 7 Patients with punding can recognize that time and money spent on their behaviors are excessive and inappropriate; however, they are not able to stop the behavior, which can lead to devastating psychosocial consequences with a significant impact on their quality of life. 4 , 5
DRT in individuals with PD, cocaine or amphetamine abuse are the most common causes of punding. 3 Punding was first described in PD in a patient treated with levodopa by Friedman in 1994. 8 The prevalence of reported punding in PD varies between 1.4% 9 and 14%. 4 However, because of the lack of knowledge about the condition among clinicians and patients’ reluctance to discuss these behaviors, a widespread under‐recognition of punding exists until now. 10 , 11 Punding is significantly associated with poorer disease‐related quality of life in PD patients. 12
Although the link between high doses of DRT and punding has been shown, not all patients taking these dopaminergic drugs develop this condition. 3 Therefore, it is suggested to be arising from a complex interaction of habits, basal ganglia dysfunction, frontal lobe degeneration, and dopaminergic treatment. 3 Because the underlying pathophysiological mechanism of punding is not fully established, developing an effective treatment to manage these individuals has been difficult. 11
In 2010, we published the first review focused on the pathophysiology of punding. 3 This study aims to review the punding literature of the following 12 years, once again focusing on clinical features, underlying pathophysiological mechanisms, and treatment.
Methods
This systematic review follows the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 13 No protocol was published or registered before conducting the search.
Search Strategy and Information Sources
We searched PubMed, Embase, and APA PsycInfo within the last 12 years starting from July 1, 2010 until March 19, 2022 for articles published since our previous review. 3 The search strategy included: (punding) AND (parkinson*) with articles limited to English language applied throughout all the three databases. Further studies were identified from reference lists of included studies and review articles.
Eligibility Criteria
Inclusion Criteria
Articles were included if they were: (1) peer‐reviewed journal articles; and (2) focusing on the pathophysiological mechanism, clinical features, and treatment. We included articles that use both clinical and animal models. Additionally, relevant case reports of punding in PD were included.
Exclusion Criteria
The exclusion criteria were as follows: articles not written in English, conference abstracts, reviews, book chapters, full text unavailable, and duplicated studies.
Study Selection
All identified references through electronic database searches were exported to the Rayyan QCRI tool (http://rayyan.qcri.org; Rayyan Systems, Cambridge, MA), and duplicate results were removed. All identified articles were assessed for eligibility using the title, abstract, or full text, as necessary.
Data Collection and Synthesis
Data were extracted using a standardized template generated in Microsoft Excel. For group‐level studies, data extracted included name of authors, year of publication, study design, groups and respective sample size, mean age, sex, outcome measure method, intervention, and main findings of the studies. For individual cases, data extracted include sex, age at presentation of punding, type and dose of medications used, occurrence of dyskinesia and presence of co‐morbid psychopathology, including DDS, psychosis, and ICDs. This review uses a narrative synthesis approach.
Results
Study Selection and Characteristics
The search identified 256 studies, of which 118 duplicates were removed. The remaining 138 studies were assessed for eligibility, and 57 studies were excluded by title and abstract screening. The eligibility of the remaining 81 studies was assessed by a full‐text review, resulting in 29 studies of which 19 original research articles and 10 case reports being included in this review. The flow of the study selection process is shown in the PRISMA flow diagram in Fig. 1.
FIG. 1.
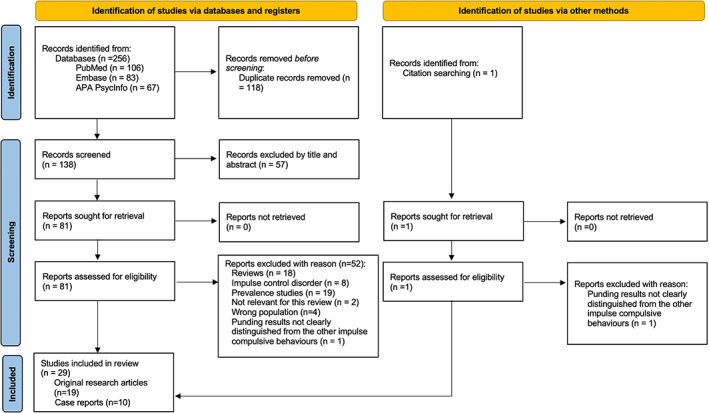
Preferred reporting items for systematic reviews and meta‐analyses flow diagram of the study selection process.
Among the 19 included original research articles, 18 are human studies and one animal study. The human studies included participants with idiopathic PD with punding and other ICBs, PD controls without any ICBs, and healthy controls.
Clinical Features of Punding
Nine studies examined the clinical features of punding in PD. Study characteristics and summary of the findings of these studies are presented in Table 1. The mean age of participants ranged from 52.6 to 77 years. The proportion of males with punding exceeded that of females (64% vs. 36%). Two studies reported that punding was significantly more common in patients treated with DA. 14 , 18 One study found that the risk factors for developing punding were higher doses of levodopa and younger age at the baseline. 17 Another study found that increasing disease severity and younger age were predictors of hobbyism/punding. 18 In a case–control study, a higher frequency and increased severity of punding/hobbyism was found in the parkin‐PD group compared to non‐mutated PD group. 19 Male and female patients engaged differently in hobbyism and punding showing different preferences for specific compulsive activities. 15 Males were more likely to be involved in activities such as repairing, dismantling, putting together, and work on projects, whereas females were involved in cleaning, tidying, and sorting objects. Compared to males, only a few female patients disclosed any ICBs to a clinician, and they only addressed certain symptoms such as compulsive gambling, buying, and eating, but not hobbyism and punding. 15
TABLE 1.
Study characteristics and summary of findings of studies on clinical features of punding in PD
| Author, year | Study design | Groups | Sample size | Mean age | Sex, male (%) | Outcome measure methods | Main findings |
|---|---|---|---|---|---|---|---|
| Morgante et al, 2016 19 | Case–control | Parkin‐PD | 22 | 52.6 ± 10.9 | 13 (59.1) |
Semi‐structured interview QUIP‐RS |
‐ A higher frequency of punding/hobbyism in the parkin‐PD compared to PD‐NM (P = 0.01) ‐ Total and partial QUIP‐RS scores for punding/hobbyism higher in parkin‐PD compared to PD‐NM (P = 0.03) |
| PD‐NM | 26 | 55.1 ± 8.5 | 17 (65.4) | ||||
| Pettorruso et al, 2016 14 | Case–control | PD + punding | 25 | 65 ± 8.9 | 15 (60.0) | Psychiatric evaluation |
‐Punding behaviors were associated with psychiatric comorbidity (ie, psychosis, bipolar disorder) and with addictive behaviors (pathological gambling, DDS) ‐Significantly more punding patients were in treatment with DA agonists |
| PD controls | 130 | 67.2 ± 9.4 | 67 (51.5) | ||||
| Callesen and Damholdt, 2017 15 | Cross‐sectional study | Idiopathic PD | 490 | 70.9 ± 9.3 | 303 (61.8) |
Demographic and clinical questionnaire QUIP‐RS |
‐ Most ICBs were hobbyism and punding (n = 135 [27.5%]), with punding in 10.8% ‐Male and female patients show different preferences for specific compulsive activities ‐Compared to males, only 12.1% female patients disclosed ICBs to a clinician, and none about hobbyism and punding ‐Punding was associated with advanced motor symptoms |
| Aoki et al, 2019 16 | Case–control | P‐H only group | 25 | 73.6 ± 9.1 | 11 (44.0) | Measurement of trunk forward and lateral flexion angles | ‐Significantly higher values were seen in the P‐H only group compared with non‐ICB group for the angle of forward flexion of the trunk |
| Non‐ICB | 44 | 72.2 ± 9.0 | 17 (38.6) | ||||
| Marković et al, 2020 17 | Prospective cohort study | PD‐ICB | 21 | 60.8 ± 11.2 | 16 (76.2) | Clinical, psychiatric, and neuropsychological evaluations | ‐Risk factors for developing punding are higher doses of levodopa and younger age at the baseline |
| PD‐no ICB | 85 | 61.5 ± 9.2 | 42 (49.4) | ||||
| HCs | 112 | 59.6 ± 11.4 | 53 (47.3) | ||||
| Martinez‐Martin et al, 2020 18 | Cross‐sectional study | PD‐D | 85 | 77.7 ± 5.2 | 51 (60.0) |
Clinical Impression of Severity Index for PD SEND‐PD HY MMSE |
‐Hobbyism‐punding more common in PD‐D compared with PD‐ND (32.9% vs. 10.6%, P < 0.001) ‐Hobbyism‐punding symptoms more common in those treated with a DA compared with non‐DA‐treated patients ‐ Other predictors of hobbyism‐punding were increasing disease severity and younger age |
| PD‐ND | 444 | 69.1 ± 9.9 | 256 (57.6) | ||||
| Santos‐García et al, 2021 20 | Cross‐sectional study | ICBs | 104 | 59.7 ± 9.4 | 64 (61.5) |
BDI‐II QUIP‐RS |
‐Depression was more frequent in patients with hobbyism‐punding than in those without (69% [29/42] vs. 49.4% [282/571]; P = 0.010) |
| Non ICBs | 509 | 63 ± 8.9 | 303 (59.5) | ||||
| Hinkle et al, 2021 21 | Multi‐center observational study | Idiopathic PD | 484 | 61.5 ± 9.8 | 318 (65.7) |
QUIP‐RS Neurocognitive assessments |
‐Punding was reported in 51 (10.5%) participants as a cumulative prevalence (unique cases) ‐Worse performance on the Letter Number Sequencing test of attention (OR = 0.87; 95% CI = 0.78–0.97; Holm‐P = 0.022) was significantly associated with punding ‐Attention subscale of the MoCA was significantly associated with punding (OR = 0.59; 95% CI = 0.45–0.77; Holm‐P < 0.001) ‐ADL dysfunction was a statistically significant predictor of punding (OR = 1.55; 95% CI = 1.20–2.00; P < 0.001), but not hobbyism |
| Barbosa et al, 2021 22 | Case–control | Punding (PD + pu) | 21 | 59.5 ± 16 | 15 (71.4) |
QUIP‐RS Structured interview Clinical and neuropsychological assessments |
‐Punding patients showed higher levels of anxiety, non‐motor symptoms and motor symptoms, and lower Frontal Assessment Battery scores ‐The hobbyism group exhibited similar levels of anxiety and motor fluctuations to the punding group |
| Hobbyism (PD + h) | 26 | 56.0 ± 11.7 | 23 (88.5) | ||||
| PD controls | 25 | 59.8 ± 9 | 18 (72.0) |
Abbreviations: PD, Parkinson's disease; Parkin‐PD, PD with parkin mutation; PD‐NM, Parkinson's disease–negative for mutations; QUIP‐RS, Questionnaire for Impulsive‐Compulsive Disorders in Parkinson's Disease‐Rating Scale; DDS, dopamine dysregulation syndrome; DA, dopamine agonist; ICB, impulsive‐compulsive behavior; P‐H, punding or hobbyism; HC, healthy controls; PD‐D, PD with dementia; SEND‐PD, Scale for Evaluation of Neuropsychiatric Disorders in Parkinson's Disease; HY, Hoehn and Yahr scale; MMSE, Mini‐Mental State Examination; BDI‐II, Beck Depression Inventory‐II; OR, odds ratio; CI, confidence interval; MoCA, Montreal Cognitive Assessment; ADL, activities of daily living.
Psychiatric comorbidities and cognitive impairments were common clinical features in PD patients with punding. One study found that punding was associated with psychiatric comorbidity (ie, psychosis, bipolar disorder) and with addictive behaviors (pathological gambling, DDS). 14 Another study found that depression was more frequent in patients with hobbyism/punding than in those without. 20 PD patients with punding showed higher levels of anxiety in a different study. 22
For the role of cognition, hobbyism/punding was significantly more common in PD with dementia compared with PD without dementia, 18 whereas another study found lower Frontal Assessment Battery scores in these patients compared to controls. 22 Finally, an association between punding and a worse performance on the Letter Number Sequencing test of attention and the attention subscale of the Montreal Cognitive Assessment was found in another study. 21
Pathophysiological Mechanisms of Punding
Five studies studied the underlying pathophysiology of punding in PD, four conducted in humans and one in the animal model. Table 2 displays the human studies’ characteristics and summary of findings. A study screened PD patients who underwent subthalamic nucleus deep brain stimulation (STN DBS) using a structured interview of patients and relatives. 23 The study found five patients with new onset of punding after STN DBS. These patients only differed significantly from the nonpunders in terms of shorter follow up since DBS implantation.
TABLE 2.
Study characteristics and summary of findings of studies in humans elucidating the underlying pathophysiological mechanisms of punding in PD
| Author, year | Study design | Groups | Sample size | mean age | Sex, male | Outcome measure methods | Main findings |
|---|---|---|---|---|---|---|---|
| Pallanti et al, 2010 23 | Cross‐sectional study | Punders | 5 | 63.8 ± 6.6 | 3 (60.0) |
Clinical interview Questionnaires OCI SDS |
‐Punder and nonpunder groups only differed statistically by the length of time from DBS implantation (punders = 3.20 ± 1.30 years, nonpunders = 5.16 ± 3.06 years, P = 0.047). ‐The decrease in LEDD (nonpunders = 31.78%, and punders =18.78%, not statistically significant) |
| Nonpunders | 19 | 66.7 ± 5.8 | 9 (47.4) | ||||
| Yoo et al, 2015 24 | Case–control | PD‐punder | 10 | 66.8 ± 6.8 | 6 (60.0) |
Comprehensive neuropsychological tests Voxel‐based and ROIs‐based cortical thickness analysis using MRI |
‐Severe cognitive deficits in the color Stroop task ‐ Significant cortical thinning in the dorsolateral prefrontal area ‐ Cortical thinning was localized in the prefrontal cortices, extending into orbitofrontal area ‐ Punding severity was correlated with LEDD |
| PD‐nonpunder | 43 | 67.1 ± 6.6 | 25 (58.1) | ||||
| Markovic et al, 2017 25 | Case–control | PD‐punding | 22 | 63.1 ± 9.2 | 19 (86.4) | Structural and resting‐state functional MRI |
‐Higher functional connectivity of habenula and amygdala with thalamus and striatum bilaterally, and lower connectivity between bilateral habenula and left frontal and precentral cortices in PD‐punding compared to PD no‐ICB and HC ‐Cortical thinning of the left superior frontal and precentral gyri and right middle temporal gyrus and isthmus cingulate in PD‐punding compared to HC, and of the right inferior frontal gyrus compared to both controls and PD–no ICB patients |
| PD no‐ICB | 30 | 63.9 ± 6.6 | 21 (70.0) | ||||
| HC | 30 | 63.0 ± 9.1 | 21 (70.0) | ||||
| Canu et al, 2017 26 | Case–control | PD‐punding | 21 | 63.8 ± 8.8 | 18 (85.7) |
Clinical, cognitive and psychopathological evaluations Diffusion tensor MRI metrics of the main white matter tracts |
‐White matter microstructural alterations of the left pedunculopontine tract and splenium of the corpus callosum ‐Damage to the right pedunculopontine tract and uncinate fasciculus, genu of the corpus callosum, and left parahippocampal tract relative to controls ‐Greater damage of the genu of the corpus callosum and the left pedunculopontine tract was found in PD punding compared with patients with no ICB |
| PD no‐ICB | 28 | 63.6 ± 6.5 | 19 (67.9) | ||||
| HC | 28 | 61.9 ± 8.3 | 19 (67.9) |
Abbreviations: PD, Parkinson's disease; OCI, Obsessive–Compulsive Inventory; SDS, Sheehan Disability Scale; DBS, deep brain stimulation; LEDD, levodopa equivalent daily dose; ROIs, regions‐of‐interest; MRI, magnetic resonance imaging; ICB, impulsive compulsive behavior; HC, healthy controls.
One magnetic resonance imaging (MRI) study that used voxel‐based and regions‐of‐interest (ROIs)‐based cortical thickness analysis and found that punders had significant thinning in the dorsolateral prefrontal cortex (DLPFC) relative to controls and that cortical thinning in PD‐punders relative to PD‐nonpunders was localized in the prefrontal cortices, extending into the orbitofrontal area. 24 Another study that used structural and resting‐state functional MRI showed the specific brain structural and functional alterations affecting the reward network in punding PD patients. 25 This study showed cortical thinning of the right inferior frontal cortex, a reduced functional connectivity between habenula and frontal cortices, and an enhanced functional connectivity of both habenula and amygdala with the striatum and thalamus when comparing PD‐punding to PD‐nonpunders and healthy controls.
Finally, another study used diffusion tensor MRI to assess the integrity of the main white matter tracts and showed that PD‐punding patients compared with PD‐nonpunders have a distinctive pattern of white matter damage, affecting tracts of critical importance for orchestrating goal‐oriented behaviors and the reward processing. 26 In particular, they were characterized by a greater damage to the genu of the corpus callosum, the parahippocampal and uncinated tracts compared to controls.
Kwan et al 27 conducted the only animal study on 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐lesioned common marmosets. They included six marmosets consisting of 50% males, and performed behavioral analysis after administering levodopa. Following the administration of levodopa, these animals exhibited stereotypical behavior similar to punding behaviors seen in PD patients.
Treatment of Punding
There are five studies investigating the treatment of punding in PD (Table 3). A prospective open‐label study on 10 PD‐punders was conducted to test the validity of a multistep algorithm for medical management of punding. 28 The study found that reduction of levodopa therapy was efficacious in two patients, amantadine was effective in controlling punding in four cases, and quetiapine only had mild efficacy in two cases.
TABLE 3.
Study characteristics and summary of findings of studies on treatment of punding in PD
| Author, year | Study design | Groups | Sample size | mean age (SD or range) | Sex, male | Outcome measure methods | Intervention | Main findings |
|---|---|---|---|---|---|---|---|---|
| Fasano et al, 2011 28 | Prospective open‐label study | PD‐punders | 10 | 61.1 ± 12.5 | 7 (70.0) | Clinical evaluations (assessment of side‐effects, UPDRS‐III, and PRS‐II) | Multistep algorithm for medical management of punding |
‐Reduction of levodopa therapy was efficacious in 2 cases. ‐Amantadine was effective in controlling punding in 4 cases. ‐Quetiapine was mildly efficacious in 2 cases. |
| Ávila et al, 2011 29 | Prospective cohort study | PD patients with ICBs | 25 | 74.0 ± 6.7 | 19 (76.0) |
Structures clinical interview (included information on any intervention for ICBs) Questionnaires UPDRS HY |
Increase or decrease of PD pharmacotherapy, use of psychotropic medication, psychosocial treatment. |
‐Of the 11 patients with punding, these symptoms remained unchanged in 9 patients (81.82%) independently of changes in dopaminergic drugs. ‐Only 2 (18.18%) patients reported experiencing a full or partial remission of their punding symptoms over time; both had discontinued or decreased their doses of DA and had modified the psychiatric treatment. |
| Lhommée et al, 2012 30 | Prospective cohort study | PD patients | 63 | 57.8 ± 7.2 | 40 (63.5) |
Ardouin scale MINI MDRS UPDRS III |
STN DBS | ‐1 patient diagnosed with punding at preoperative assessment showed clear improvement of the symptoms at 1‐year assessment after surgery. |
| Catalán et al, 2013 31 | Prospective cohort study | PD patients | 8 | 67.9 ± 10.9 | 7 (87.5) | Motor complications and behavioral disorders assessments | JLI | ‐ Of the 5 patients with punding, symptoms improved in all but disappeared completely in only 1. |
| Nardone et al, 2014 32 | Randomized control trial | PD‐Punders | 4 | 65.8 (62–70) | 3 (75.0) |
PRS OCI‐distress subscale scores HAM‐A HAM‐D |
rTMS and sham stimulation | LF‐rTMS produced a transient beneficial effect in PD patients with punding. |
| Healthy controls | 8 | 65.2 (59–71) | 7 (77.8) |
Abbreviations: PD, Parkinson's disease; SD, standard deviation; UPDRS‐III, Unified Parkinson's Disease Rating Scale motor score; PRS, Punding Rating Scale; ICB, impulse compulsive behavior; HY, Hoehn and Yahr scale; DA, dopamine agonists; MINI, Mini International Neuropsychiatric Interview; MDRS, Mattis Dementia Rating Scale; STN, subthalamic nucleus; DBS, deep brain stimulation; JLI, jejunal levodopa infusion; PRS, Punding Rating Scale; OCI, Obsessive–Compulsive Inventory; HAM‐A, Hamilton Anxiety Scale; HAM‐D, Hamilton Depression Rating Scale; rTMS, repetitive transcranial magnetic stimulation; LF, low frequency.
In a 1‐year follow‐up study, information was collected on the effect of changes on parkinsonian and psychiatric medications, as well as psychosocial treatment in patients with ICBs. 29 When the 11 patients with punding were analyzed separately, the symptoms remained unchanged in nine patients independently of the changes in dopaminergic drugs, and only two patients reported a full or partial remission of their punding symptoms over time; both had discontinued or decreased their doses of DA and had modified the psychiatric treatment (ie either the introduction of antidepressants, benzodiazepine, or quetiapine or their doses increase.).
One study assessed the effect of jejunal levodopa infusion (JLI) on behavioral symptoms in PD patients with motor complications and severe impulsivity and DDS. 31 Punding was present in five patients and improved in all, but disappeared completely only in one after ~25 weeks of infusion treatment. Another study assessed the effects of repetitive transcranial magnetic stimulation (rTMS) over the DLPFC on PD‐related punding. 32 The study showed that low‐frequency (LF)‐rTMS produced a transient (12 h) beneficial effect in PD patients with punding in absence of motor impairment.
Case Reports of Punding in PD
The 10 case reports with a total of 11 PD patients with punding are presented in Table 4. Of these, 82% were male, with an age ranging from 43 to 71 years. Along with punding, patients were reported to have developed dyskinesia in 7 (64%), psychosis in 5 (45%), ICDs in 8 (73%), DDS in 3 (27%). The patients were reported to have been involved in classic punding activities such as woodworking, fixing furniture, painting, playing musical instruments, absorbed in puzzles, picking wild berries, and collecting and stockpiling purses and other miscellaneous items of litter. Punding was reported as a cause of death in one patient after sustaining a head and neck trauma while fixing a sofa bed. 37 Most of these patients developed punding after the introduction or increase of DA and/or levodopa, or with surreptitious intake of levodopa. Treatment‐wise, the reduction or discontinuation of the DA and/or levodopa, the introduction of clozapine or IJL resulted in complete relief or improvement in seven patients (64%). 33 , 34 , 35 , 38 , 39 , 40
TABLE 4.
Case review of punding in PD
| Author, year | Dysk | DDS | Psyc | ICD | Sex | Age | Punding type | Medication (daily dose in mg) when punding began | Trigger event | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Todorova et al, 2015 33 | + | + | + | HS | M | NA | NA |
LD (NA) CBG (NA) PMX (NA) RPL (NA) |
LD abuse, introduction of PMX and RPL | JLI introduction, DAs and oral LD discontinuation | ↑↑ |
| Hirao et al, 2019 34 | − | − | − | HS | M | 71 | Absorbed in puzzles |
LD (NA) RPL (NA) SLG (NA) |
SLG introduction | SLG discontinuation | ↑↑ |
| Guedes et al, 2016 35 | + | − | + | PG | M | 58 | Collecting miscellaneous items of litter and assembling parts of broken machinery |
LD (600) PMX (4.5) |
NA | Quetiapine introduction (400 mg). LD reduction (350 mg), and PMX discontinuation | ↑↑ |
| Salomone et al, 2015 36 | + | + | − | − | M | 71 | Building wood constructions, tidying up repetitively, analyzing and cataloging objects, and tinkering with home equipment | LCIG infusion (1476) |
LCIG therapy introduction coinciding with abrupt conversion of PMX to LD. Covert oral medication abuse of extra tablet of LD‐methyl‐ester 125 mg pills |
NA | NA |
| Köksal and Köksal, 2014 37 | + | − | − | + | M | 71 | Fixing furniture and electronic devices |
LD (750), PMX (12), AMN (NA) RSG (NA) |
LD and PMX abuse, and stopped taking AMN. | Quetiapine introduction (400 mg), and PMX reduction | = |
| Aquino et al, 2013 38 | − | − | + | − | F | 51 | Collecting purses, diaries with repetitive phone numbers and notes |
LD/BZ (500) PMX (1.5) AMN (200) |
LD/BZ introduction | Reduction of LD/BZ to 300 mg and PMX to 1 mg, and AMN discontinuation | ↑ |
| Vitale et al, 2013 39 | − | − | − | − | M | 68 | Playing percussion musical instruments |
LD/CD (300) PMX (3) |
PMX introduction | Quetiapine introduction (50 mg), and PMX discontinuation | ↑↑ |
| Hardwick et al, 2013 40 | + | − | + | HS | M | 64 | Organizing and reorganizing CDs |
LD (625) Entacapone (600) Venlafaxine (75) |
NA | Clozapine introduction (50 mg) | ↑↑ |
| Hardwick et al, 2013 40 | + | − | − | DB | M | 43 | Wood‐working, fishing, painting, sanding surfboards, weaving, and sculpting |
RSG (1) Clonazepam (1) Escitalopram (10) RPL (6) |
Treatment with RPL triggered the onset of punding and worsened after switching to LD | Clozapine introduction (12.5 mg), escitalopram increased (40 mg), and LD/CD‐IR changed to LD/CD‐CR (150 mg) | ↑↑ |
| Paviour and Marion, 2012 41 | + | + | + | CS | F | 59 | Purchased a new computer and spent increasing amount of time on researching her condition | JLI (Duodopa) (NA) | Kept the Duodopa pump running longer by infusing for 24 h/day over a 2‐week period and surreptitious self‐administration of LD | Duodopa infusion discontinued and oral LD dose introduced and increased to LEDD 4–500 mg, and quetiapine introduction | NA |
| Joutsa et al, 2012 42 | − | − | − | PG | M | 55 | Picking wild berries |
SLG (10) CBG (2) LD/CD (200/50) |
Increasing LD/CD and SLG doses. | NA | NA |
Abbreviations: PD, Parkinson's disease; Dysk, dyskinesias; DDS, dopamine dysregulation syndrome; Psyc, psychosis; ICD, impulse control disorder; HS, hypersexuality; M, male; NA, not available; LD, levodopa; CBG, cabergoline; PMX, pramipexole; RPL, ropinirole; JLI, jejunal levodopa infusion; DA, dopamine agonists; ↑↑, complete relief; SLG, selegiline; PG, pathological gambling; LCIG, levodopa‐carbidopa intestinal gel; AMN, amantadine; RSG, rasagiline; =, unchanged; F, female; LD/BZ, levodopa/benserazide; ↑, improvement; LD/CD, levodopa/carbidopa; DB, drinking beer; IR, immediate release; CR, controlled release; CS, compulsive shopping; LEDD, levodopa equivalent daily dose; CBG, cabergoline.
Discussion
Following our initial review of 2010, 3 this systematic review focused on summarizing the findings of articles published within the past 12 years on the clinical features, pathophysiological mechanism, and treatment of punding in PD.
Our review confirmed that predictors of punding in PD are higher doses of DRT, younger age, male sex, and increasing disease severity. Four studies in particular reported a significant association with a higher level of DRT and punding in PD. 14 , 17 , 18 , 24 These findings support the hypothesis that punding could involve the alteration in the dopaminergic transmission that is exacerbated by DRT, and it may reflect an underlying pathological condition predisposing to both punding and psychiatric comorbidities. 3 An association between punding and psychiatric comorbidities as well as cognitive impairments was indeed also found. A possible explanation for this association could be the underlying frontal lobe dysfunctions, 22 which has been supported by neuroimaging studies showing prefrontal cortical thinning in PD patients with punding. 24 , 25
We found a higher proportion of males with punding compared to females in the included studies. This is most likely related to the sex‐related susceptibility of the brain, but could be also because of the reluctance of female patients to report punding behaviors with their neurologist, as found in one study. 15 Punding behavior may be masked by patients engaging in activities that are similar to previous occupations or habits. 3 Patients underestimate the presence and severity of ICBs, which shows the importance of assessing them with the caregiver. 43
Although the exact pathophysiology of punding has not yet been established, it is suggested that neural plastic changes in the dorsal and ventral striatal structures because of chronic intermittent stimulation by dopaminergic medications and impaired reward mechanisms play a role. 3 , 11 , 44 Additionally, it has been suggested that projections from the frontal cortex to the striatum inhibit the dopamine‐dependent induction of the striatal stereotypies. 3 These hypotheses regarding the pathophysiological mechanism are supported by the findings from the MRI studies included in this review.
In the past decade, MRI studies have indeed expanded our understanding of the neural substrates underlying punding in PD. A study combining MRI with neuropsychological tests demonstrated that PD patients with punding performed poorly on cognitive tasks in frontal executive functions and showed severe cortical thinning in the dorsolateral prefrontal and orbitofrontal areas. 24 These findings suggest that prefrontal modulation may be an essential component in the development of punding behavior in patients with PD, as initially hypothesized based on occurrence of hoarding in humans after frontal lobe lesion. 3 Another MRI study found that punding in PD is associated with a white matter disconnection between midbrain, limbic system, and projections to the frontal cortices. 26 Finally, a breakdown of the connectivity among the crucial nodes of the reward circuit (ie, habenula, amygdala, basal ganglia, and frontal cortex) has been reported as a possible contributor to punding in PD. 25
The study by Pallanti et al 23 found few patients with new onset of punding after functional neurosurgery suggesting that punding might be induced by STN DBS. However, the punders remained on levodopa and DA after surgery and the decrease in dose was lower than in the nonpunder group, possibly arguing that DBS had a negative impact on striatal‐frontal connections, therefore, representing a risk factor rather than a cause. In fact, in the study by Lhommée et al, 30 one patient diagnosed with punding at preoperative assessment had clear improvement in punding at 1‐year assessment after STN DBS. However, this could also be because of a 73% reduction of the DRT post‐surgery in this cohort. Based on the small sample sizes of these studies it is not possible to draw firm conclusions on the role of STN DBS on the pathophysiology or treatment of punding.
In this review, we identified five studies investigating the treatment of punding in PD. The study by Fasano et al 28 based on a possible proposed algorithm for treating punding, recommended that following adjustment of dopaminergic medications and entacapone, patients should receive a trial with amantadine 100 to 300 mg. If punding continues then quetiapine 200 mg or clozapine 50 mg is recommended. The study did not test the use of clozapine because of the requirement of monitoring white blood cells. However, in the case series by Hardwick et al, 40 a trial of clozapine in two punding patients resulted in complete relief. It is important to note that in certain cases, patients might need psychiatric medical treatment in addition to reduction or discontinuation of DA and levodopa as found in the study by Avila et al. 29
One study that assessed the effect of JLI on ICBs in PD patients also enrolled five punding patients who improved. 31 The small sample size prevent us to make any conclusions, but it is likely that this resulted from a non‐pulsatile dopaminergic stimulation combined with the reduction or discontinuation of other oral anti‐PD therapies. 45 Not surprising high doses of levodopa in JLI can also trigger these behavioral abnormalities in predisposed PD patients, as seen in patients receiving 24 h infusion. 46
Another study assessing the effects of rTMS on DLPFC showed that LF‐rTMS produced a transient beneficial effect in PD patients with punding without enhancing motor impairment. 32 This study builds on the previous hypothesis that disruption of the reciprocal loops between the striatum and structures in the prefrontal cortex following dopamine depletion may predispose patients with PD to punding behaviors. However, because of the transient effect and a small sample size, it is unclear at the moment the real impact of this treatment strategy.
In summary, the findings from our review suggest that the following strategies could be considered to treat patients with punding on an individual basis. These include reducing or discontinuing the use of DA and/or levodopa, introducing amantadine, treating with JLI, and using antidepressants or quetiapine to improve comorbid psychiatric problems. A low dose of clozapine may be tried with caution because of the risk of significant adverse effects in those not responding to previous treatments. Currently, the effects of STN DBS and rTMS on punding are not established and further studies are needed.
Clearly we need specific treatments for punding, possibly tested in animal model. MPTP‐lesioned common marmosets, displaying stereotypical behavior similar to punding behaviors following levodopa administration could be a useful pre‐clinical model to further study the underlying pathophysiology and aid the development of such therapies. 27
Limitations
Although this systematic review provides a broad overview of punding in PD, it should be viewed within the context of certain limitations. The review excluded articles not written in English, conference abstracts, and book chapters may have led to the omission of relevant results. A comprehensive statistical analysis could not be performed because of the variability and non‐comparability of the included studies. Because our study included various observational and interventional studies with differing methodologies, it was not feasible to conduct a risk of bias and quality assessment. This is because instruments designed for these assessments are tailored to specific study designs, making it challenging to provide a comprehensive assessment using a single or even two tools.
Another limitation of our study was that we only included the search terms “punding” and “Parkinson,” and did not include related terms like “ICD,” “DRT,” “DDS,” and “hedonistic homeostatic dysregulation syndrome.” This may have led us to overlook some relevant articles that were published using those terms. It is worth noting that in recent years, the understanding of punding has improved, and the use of terms has become more accurate. For example, it is no longer believed to be a type of ICD.
Conclusion and Future Directions
In this review, we synthesized the results from recent studies on punding in PD to understand the underlying pathophysiological mechanism, relevant clinical features, and treatment. In fact, although the clinical features of punding have been established, in the past 12 years we gained a better understanding of the pathophysiological mechanisms of punding, mainly thanks to MRI techniques. Future studies should consider using MRI along with clinical features in prospective cohort to further study the roles the frontal cortex, dorsal and ventral striatal structures, DRT, and impaired reward mechanisms in the physiopathology of punding in PD. Interestingly, punding might be seen as an early involvement of the orbital cortex (stage 4 according to Braak et al), 47 therefore, representing a biomarker of pre‐dementia in PD. Future studies should consider investigating the therapeutic potential of rTMS in large cohorts undergoing periodic sessions, as routinely done nowadays for depression. Finally, more studies using validated pre‐clinical model are needed to further study the underlying pathophysiology and—more important—develop therapies for punding.
Together, these studies will help the development of effective treatment for these patients, hopefully resulting in an improvement of their quality of life.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
R.R.: 1A, 1B, 1C, 2A, 2B, 3A.
A.F.: 1A, 1B, 2A, 2C, 3B.
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board and patient consent was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This study was partly funded by the University of Toronto and University Health Network Chair in Neuromodulation to A.F. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: R.R. is employed at University Health Network and declares that there are no additional disclosures to report. A.F. reports the following disclosures: consultancies with AbbVie, Abbott, Boston Scientific, Ipsen, Medtronic, and Sunovion; served on advisory boards for AbbVie, Boston Scientific, Ceregate, Inbrain, and Ipsen; honoraria received from AbbVie, Abbott, American Academy of Neurology, Boston Scientific, Brainlab, Ipsen, Medtronic, Merz, Movement Disorders Society, Sunovion, Paladin Labs, and UCB; royalties from Springer; and research grants from AbbVie, Boston Scientific, Dystonia Medical Research Foundation, University of Toronto, The Michael J. Fox Foundation, Medtronic, and MSA coalition.
Supporting information
Data S1. Search strategy and results.
Relevant disclosures and conflict of interest are listed at the end of this article.
References
- 1. Rodriguez‐Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol 2009;8(12):1128–1139. [DOI] [PubMed] [Google Scholar]
- 2. Park A, Stacy M. Dopamine‐induced nonmotor symptoms of Parkinson's disease. Parkinsons Dis 2011;2011:485063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fasano A, Petrovic I. Insights into pathophysiology of punding reveal possible treatment strategies. Mol Psychiatry 2010;15(6):560–573. [DOI] [PubMed] [Google Scholar]
- 4. Evans AH, Katzenschlager R, Paviour D, O'Sullivan JD, Appel S, Lawrence AD, Lees AJ. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov Disord 2004;19(4):397–405. [DOI] [PubMed] [Google Scholar]
- 5. Voon V. Repetition, repetition, and repetition: compulsive and punding behaviors in Parkinson's disease. Mov Disord 2004;19(4):367–370. [DOI] [PubMed] [Google Scholar]
- 6. Zhang G, Zhang Z, Liu L, Yang J, Huang J, Xiong N, Wang T. Impulsive and compulsive behaviors in Parkinson's disease. Front Aging Neurosci 2014;6:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fullana MA, Mataix‐Cols D, Caspi A, et al. Obsessions and compulsions in the community: prevalence, interference, help‐seeking, developmental stability, and co‐occurring psychiatric conditions. Am J Psychiatry 2009;166(3):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedman JH. Punding on levodopa. Biol Psychiatry 1994;36(5):350–351. [DOI] [PubMed] [Google Scholar]
- 9. Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson's disease. Mov Disord 2007;22(8):1179–1181. [DOI] [PubMed] [Google Scholar]
- 10. O'Sullivan SS, Evans AH, Lees AJ. Punding in Parkinson's disease. Pract Neurol 2007;7(6):397–399. [DOI] [PubMed] [Google Scholar]
- 11. Spencer AH, Rickards H, Fasano A, Cavanna AE. The prevalence and clinical characteristics of punding in Parkinson's disease. Mov Disord 2011;26(4):578–586. [DOI] [PubMed] [Google Scholar]
- 12. Lawrence AJ, Blackwell AD, Barker RA, Spagnolo F, Clark L, Aitken MRF, Sahakian BJ. Predictors of punding in Parkinson's disease: results from a questionnaire survey. Mov Disord 2007;22(16):2339–2345. [DOI] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pettorruso M, Fasano A, De Risio L, et al. Punding in non‐demented Parkinson's disease patients: relationship with psychiatric and addiction spectrum comorbidity. J Neurol Sci 2016;362:344–347. [DOI] [PubMed] [Google Scholar]
- 15. Callesen MB, Damholdt MF. Phenomenology and gender characteristics of hobbyism and punding in Parkinson's disease: a self‐report study. Basal Ganglia 2017;9:1–6. [Google Scholar]
- 16. Aoki R, Shiraishi M, Mikami K, Kamo T. Deterioration of postural deformity in Parkinson's disease patients with punding and hobbyism. J Clin Neurosci 2019;69:179–183. [DOI] [PubMed] [Google Scholar]
- 17. Marković V, Stanković I, Petrović I, et al. Dynamics of impulsive‐compulsive behaviors in early Parkinson's disease: a prospective study. J Neurol 2020;267(4):1127–1136. [DOI] [PubMed] [Google Scholar]
- 18. Martinez‐Martin P, Wan YM, Ray Chaudhuri K, Schrag AE, Weintraub D. Impulse control and related behaviors in Parkinson's disease with dementia. Eur J Neurol 2020;27(6):944–950. [DOI] [PubMed] [Google Scholar]
- 19. Morgante F, Fasano A, Ginevrino M, et al. Impulsive‐compulsive behaviors in parkin‐associated Parkinson disease. Neurology 2016;87(14):1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santos‐García D, de Deus FT, Cores Bartolomé C, et al. Depression is associated with impulse‐compulsive behaviors in Parkinson's disease. J Affect Disord 2021;280(Pt B):77–89. [DOI] [PubMed] [Google Scholar]
- 21. Hinkle JT, Perepezko K, Mills KA, Pontone GM. Attentional dysfunction and the punding spectrum in Parkinson's disease. Parkinsonism Relat Disord 2021;84:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barbosa P, O'Sullivan SS, Joyce E, Lees AJ, Warner TT, Djamshidian A. Neuropsychiatric features of Punding and Hobbyism in Parkinson's disease. Mov Disord Clin Pract 2021;9(1):82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pallanti S, Bernardi S, Raglione LM, Marini P, Ammannati F, Sorbi S, Ramat S. Complex repetitive behavior: punding after bilateral subthalamic nucleus stimulation in Parkinson's disease. Parkinsonism Relat Disord 2010;16(6):376–380. [DOI] [PubMed] [Google Scholar]
- 24. Yoo HS, Yun HJ, Chung SJ, Sunwoo MK, Lee JM, Sohn YH, Lee PH. Patterns of neuropsychological profile and cortical thinning in Parkinson's disease with Punding. PLoS One 2015;10(7):e0134468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Markovic V, Agosta F, Canu E, et al. Role of habenula and amygdala dysfunction in Parkinson disease patients with punding. Neurology 2017;88(23):2207–2215. [DOI] [PubMed] [Google Scholar]
- 26. Canu E, Agosta F, Markovic V, et al. White matter tract alterations in Parkinson's disease patients with punding. Parkinsonism Relat Disord 2017;43:85–91. [DOI] [PubMed] [Google Scholar]
- 27. Kwan C, Nuara SG, Gourdon JC, Huot P. Further characterisation of psychosis‐like behaviours induced by L‐DOPA in the MPTP‐lesioned marmoset. Naunyn Schmiedebergs Arch Pharmacol 2021;394(8):1685–1692. [DOI] [PubMed] [Google Scholar]
- 28. Fasano A, Ricciardi L, Pettorruso M, Bentivoglio AR. Management of punding in Parkinson's disease: an open‐label prospective study. J Neurol 2011;258(4):656–660. [DOI] [PubMed] [Google Scholar]
- 29. Ávila A, Cardona X, Martín‐Baranera M, Bello J, Sastre F. Impulsive and compulsive behaviors in Parkinson's disease: a one‐year follow‐up study. J Neurol Sci 2011;310(1–2):197–201. [DOI] [PubMed] [Google Scholar]
- 30. Lhommée E, Klinger H, Thobois S, et al. Subthalamic stimulation in Parkinson's disease: restoring the balance of motivated behaviours. Brain 2012;135(Pt 5):1463–1477. [DOI] [PubMed] [Google Scholar]
- 31. Catalán MJ, de Pablo‐Fernández E, Villanueva C, Fernández‐Diez S, Lapeña‐Montero T, García‐Ramos R, López‐Valdés E. Levodopa infusion improves impulsivity and dopamine dysregulation syndrome in Parkinson's disease. Mov Disord 2013;28(14):2007–2010. [DOI] [PubMed] [Google Scholar]
- 32. Nardone R, De Blasi P, Höller Y, et al. Repetitive transcranial magnetic stimulation transiently reduces punding in Parkinson's disease: a preliminary study. J Neural Transm 2014;121(3):267–274. [DOI] [PubMed] [Google Scholar]
- 33. Todorova A, Bitsara C, Teleg E, Chaudhuri K. Impulse control dysfunction and dopamine agonist withdrawal syndrome in genetic Parkinson's: a case report. Basal Ganglia 2015;5(2–3):57–58. [Google Scholar]
- 34. Hirao K, Kaneko Y, Hirose D, et al. Patient with Parkinson's disease presenting with impulse control disorders following treatment with selegiline. Int Psychogeriatr 2019;31(9):1375–1376. [DOI] [PubMed] [Google Scholar]
- 35. Guedes BF, Gonçalves MR, Cury RG. Psychosis and concurrent impulse control disorder in Parkinson's disease: a review based on a case report. Dement Neuropsychol 2016;10(2):148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salomone G, Marano M, di Biase L, Melgari JM, Di Lazzaro V. Dopamine dysregulation syndrome and punding in levodopa‐carbidopa intestinal gel (LCIG) infusion: a serious but preventable complication. Parkinsonism Relat Disord 2015;21(9):1124–1125. [DOI] [PubMed] [Google Scholar]
- 37. Köksal A, Köksal NH. Punding as a cause of death in a patient with Parkinson's disease. J Neuropsychiatry Clin Neurosci 2014;26(2):E15. [DOI] [PubMed] [Google Scholar]
- 38. Aquino CC, Celso de Castro P, Doná F, Medeiros L, Silva SMCA, Borges V, Ferraz HB. Reduction in Parkinson's disease therapy improved punding but not feeling of presence. J Neuropsychiatry Clin Neurosci 2013;25(3):E43–E44. [DOI] [PubMed] [Google Scholar]
- 39. Vitale C, Trojano L, Barone P, et al. Compulsive drumming induced by dopamine agonists in Parkinson's disease: another aspect of punding. Behav Neurol 2013;27(4):559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardwick A, Ward H, Hassan A, Romrell J, Okun MS. Clozapine as a potential treatment for refractory impulsive, compulsive, and punding behaviors in Parkinson's disease. Neurocase 2013;19(6):587–591. [DOI] [PubMed] [Google Scholar]
- 41. Paviour DC, Marion MH. PINK1: pumps, paraesthesia, punding and psychosis. J Neurol 2012;259(6):1241–1242. [DOI] [PubMed] [Google Scholar]
- 42. Joutsa J, Martikainen K, Kaasinen V. Parallel appearance of compulsive behaviors and artistic creativity in Parkinson's disease. Case Rep Neurol 2012;4(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baumann‐Vogel H, Valko PO, Eisele G, Baumann CR. Impulse control disorders in Parkinson's disease: don't set your mind at rest by self‐assessments. Eur J Neurol 2015;22(4):603–609. [DOI] [PubMed] [Google Scholar]
- 44. Miwa H, Morita S, Nakanishi I, Kondo T. Stereotyped behaviors or punding after quetiapine administration in Parkinson's disease. Parkinsonism Relat Disord 2004;10(3):177–180. [DOI] [PubMed] [Google Scholar]
- 45. Fasano A, Gurevich T, Jech R, et al. Concomitant medication usage with levodopa‐carbidopa intestinal gel: results from the COSMOS study. Mov Disord 2021;36(8):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cannas A, Solla P, Marrosu MG, Marrosu F. Dopamine dysregulation syndrome in Parkinson's disease patients on duodenal levodopa infusion. Mov Disord 2013;28(6):840–841. [DOI] [PubMed] [Google Scholar]
- 47. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search strategy and results.


