Abstract
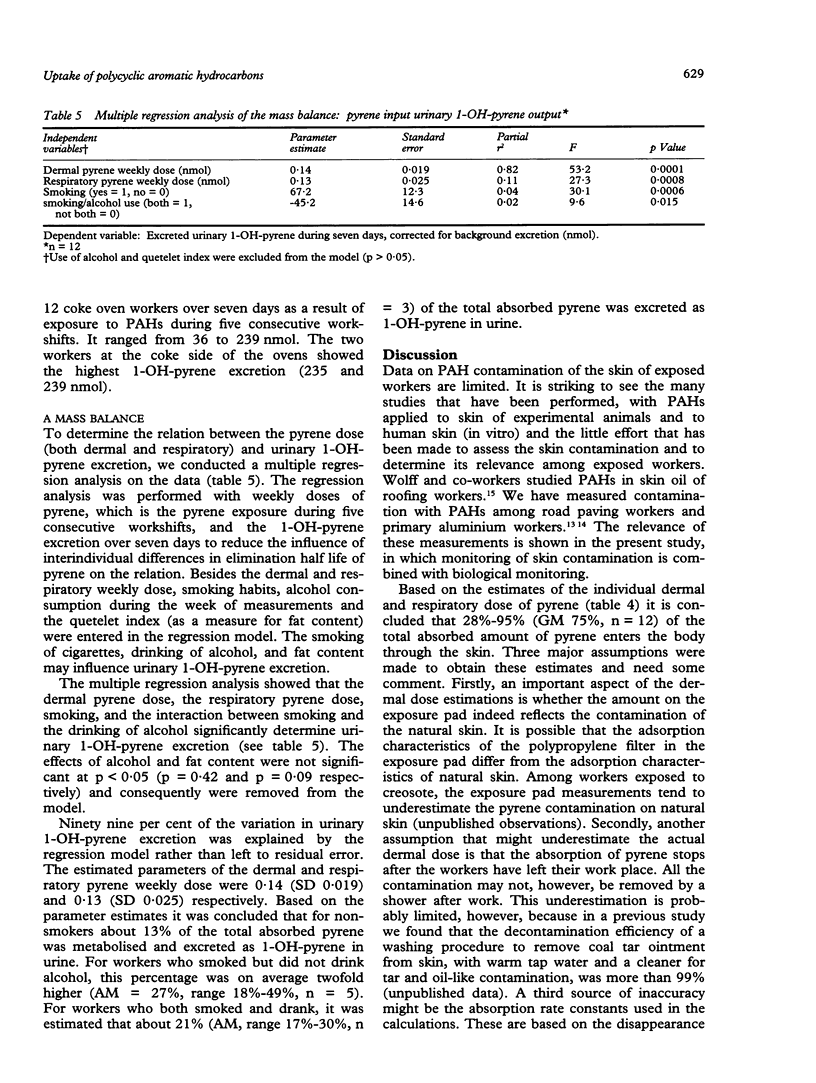
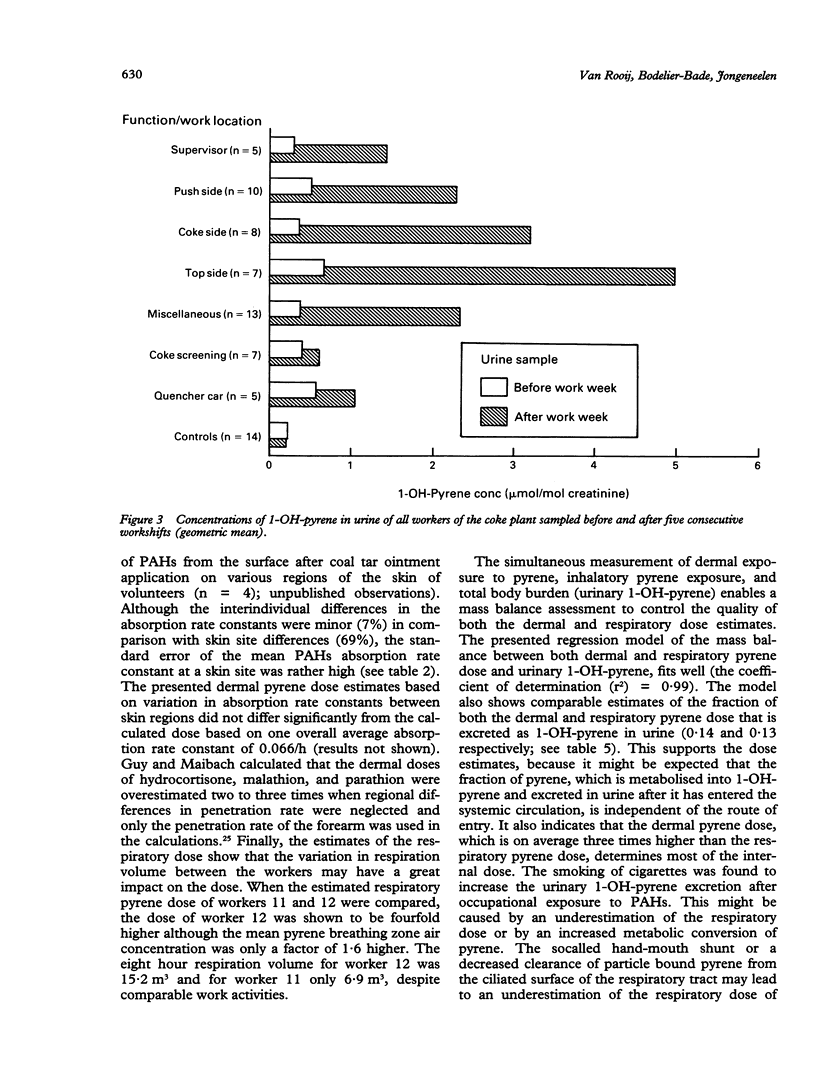
Twelve workers from a coke plant in The Netherlands participated in an intensive skin monitoring programme combined with personal air sampling and biological monitoring during five consecutive eight hour workshifts. The purpose of the study was to make a quantitative assessment of both the dermal and respiratory intake of polycyclic aromatic hydrocarbons (PAHs). Pyrene was used as a marker compound for both dermal and respiratory exposure to PAHs. The biological measure for the internal exposure to PAHs was urinary 1-OH-pyrene concentration. Measurements on exposure pads at six skin sites showed that mean total skin contamination of the 12 workers ranged between 21 and 166 micrograms pyrene a day. The dermal uptake of pyrene ranged between 4 and 34 micrograms/day, which was about 20% of the pyrene contamination on skin. The mean concentration of total pyrene in the breathing zone air of the 12 coke oven workers ranged from 0.1 to 5.4 micrograms/m3. The mean respiratory uptake of pyrene varied between 0.5 and 32.2 micrograms/day. Based on the estimates of the dermal and respiratory pyrene uptake it is concluded that an average 75% (range 28%-95%, n = 12) of the total absorbed amount of pyrene enters the body through the skin. Because of the difference in the pyrene:benzo(a)pyrene ratio between the air samples and the skin contamination samples, the dermal uptake of benzo(a)pyrene was also estimated. This was about 51% of the total absorbed amount (range 8%-92%, n = 12). The total excreted amount of urinary 1-OH-pyrene as a result of exposure to PAHs during the five consecutive workshifts varied between 36 and 239 nmol. A multiple regression model of the mass balance between pyrene dose (both dermal and respiratory) and 1-OH-pyrene excretion confirmed the relevance of the dermal exposure route. The variation in urinary 1-OH-pyrene excretion was determined more by the dermal pyrene dose than by the respiratory dose. The model showed an estimate of the percentage of the absorbed amount of pyrene that is metabolised and excreted as 1-OH-pyrene in urine. For the 12 workers this percentage varied between 13% and 49% depending on smoking habits and consumption of alcohol. The results of this study indicate that among coke oven workers, the skin is the main route of uptake of PAHs. Preventive measures to reduce exposure to PAHs should be focused more on the reduction of dermal contamination by PAHs than on the reduction of inhaled dose.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjøorseth A., Bjørseth O., Fjeldstad P. E. Polycyclic aromatic hydrocarbons in the work atmosphere. II. Determination in a coke plant. Scand J Work Environ Health. 1978 Sep;4(3):224–236. doi: 10.5271/sjweh.2703. [DOI] [PubMed] [Google Scholar]
- Braszczyńska Z., Linscheid D., Osińska R. Ocena wpływu nowych technologii w przemyśle koksowniczym na stezenia wielopierścieniowych weglowodorów aromatycznych w powietrzu otaczajacym stanowiska pracy. Med Pr. 1978;29(5):357–364. [PubMed] [Google Scholar]
- Buckley T. J., Lioy P. J. An examination of the time course from human dietary exposure to polycyclic aromatic hydrocarbons to urinary elimination of 1-hydroxypyrene. Br J Ind Med. 1992 Feb;49(2):113–124. doi: 10.1136/oem.49.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman J. K., Bhave N. A., Hirom P. C., Millburn P. Metabolism and excretion of benzo[a]pyrene in the rabbit. Xenobiotica. 1982 Jun;12(6):397–404. doi: 10.3109/00498258209052481. [DOI] [PubMed] [Google Scholar]
- Clonfero E., Zordan M., Venier P., Paleologo M., Levis A. G., Cottica D., Pozzoli L., Jongeneelen F. J., Bos R. P., Anzion R. B. Biological monitoring of human exposure to coal tar. Urinary excretion of total polycyclic aromatic hydrocarbons, 1-hydroxypyrene and mutagens in psoriatic patients. Int Arch Occup Environ Health. 1989;61(6):363–368. doi: 10.1007/BF00381025. [DOI] [PubMed] [Google Scholar]
- Foth H., Kahl R., Kahl G. F. Pharmacokinetics of low doses of benzo[a]pyrene in the rat. Food Chem Toxicol. 1988 Jan;26(1):45–51. doi: 10.1016/0278-6915(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Henderson P. T. Determination of hydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr. 1987 Jan 23;413:227–232. doi: 10.1016/0378-4347(87)80230-x. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Anzion R. B., Scheepers P. T., Bos R. P., Henderson P. T., Nijenhuis E. H., Veenstra S. J., Brouns R. M., Winkes A. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann Occup Hyg. 1988;32(1):35–43. doi: 10.1093/annhyg/32.1.35. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J. Biological exposure limit for occupational exposure to coal tar pitch volatiles at cokeovens. Int Arch Occup Environ Health. 1992;63(8):511–516. doi: 10.1007/BF00386338. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., Scheepers P. T., Groenendijk A., Van Aerts L. A., Anzion R. B., Bos R. P., Veenstra S. J. Airborne concentrations, skin contamination, and urinary metabolite excretion of polycyclic aromatic hydrocarbons among paving workers exposed to coal tar derived road tars. Am Ind Hyg Assoc J. 1988 Dec;49(12):600–607. doi: 10.1080/15298668891380312. [DOI] [PubMed] [Google Scholar]
- Jongeneelen F. J., van Leeuwen F. E., Oosterink S., Anzion R. B., van der Loop F., Bos R. P., van Veen H. G. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990 Jul;47(7):454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt G., Sollenberg J. Polycyclic aromatic hydrocarbons in the occupational environment: with special reference to benzo[a]pyrene measurements in Swedish industry. Scand J Work Environ Health. 1982 Mar;8(1):1–19. doi: 10.5271/sjweh.2503. [DOI] [PubMed] [Google Scholar]
- Popendorf W. J., Leffingwell J. T. Regulating OP pesticide residues for farmworker protection. Residue Rev. 1982;82:125–201. doi: 10.1007/978-1-4612-5709-7_3. [DOI] [PubMed] [Google Scholar]
- Randerath E., Mittal D., Randerath K. Tissue distribution of covalent DNA damage in mice treated dermally with cigarette 'tar': preference for lung and heart DNA. Carcinogenesis. 1988 Jan;9(1):75–80. doi: 10.1093/carcin/9.1.75. [DOI] [PubMed] [Google Scholar]
- Schoket B., Hewer A., Grover P. L., Phillips D. H. Covalent binding of components of coal-tar, creosote and bitumen to the DNA of the skin and lungs of mice following topical application. Carcinogenesis. 1988 Jul;9(7):1253–1258. doi: 10.1093/carcin/9.7.1253. [DOI] [PubMed] [Google Scholar]
- Vanrooij J. G., Bodelier-Bade M. M., De Looff A. J., Dijkmans A. P., Jongeneelen F. J. Dermal exposure to polycyclic aromatic hydrocarbons among primary aluminium workers. Med Lav. 1992 Sep-Oct;83(5):519–529. [PubMed] [Google Scholar]
- Zhao Z. H., Quan W. Y., Tian D. H. Urinary 1-hydroxypyrene as an indicator of human exposure to ambient polycyclic aromatic hydrocarbons in a coal-burning environment. Sci Total Environ. 1990 Mar;92:145–154. doi: 10.1016/0048-9697(90)90326-p. [DOI] [PubMed] [Google Scholar]


