Abstract
Background
Chronic kidney disease (CKD) is a long‐term condition that occurs as a result of damage to the kidneys. Early recognition of CKD is becoming increasingly common due to widespread laboratory estimated glomerular filtration rate (eGFR) reporting, raised clinical awareness, and international adoption of the Kidney Disease Improving Global Outcomes (KDIGO) classifications. Early recognition and management of CKD affords the opportunity to prepare for progressive kidney impairment and impending kidney replacement therapy and for intervention to reduce the risk of progression and cardiovascular disease. Angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) are two classes of antihypertensive drugs that act on the renin‐angiotensin‐aldosterone system. Beneficial effects of ACEi and ARB on kidney outcomes and survival in people with a wide range of severity of kidney impairment have been reported; however, their effectiveness in the subgroup of people with early CKD (stage 1 to 3) is less certain.
This is an update of a review that was last published in 2011.
Objectives
To evaluate the benefits and harms of ACEi and ARB or both in the management of people with early (stage 1 to 3) CKD who do not have diabetes mellitus (DM).
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 6 July 2023 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and Embase, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) reporting the effect of ACEi or ARB in people with early (stage 1 to 3) CKD who did not have DM were selected for inclusion. Only studies of at least four weeks duration were selected. Authors independently assessed the retrieved titles and abstracts and, where necessary, the full text to determine which satisfied the inclusion criteria.
Data collection and analysis
Data extraction was carried out by two authors independently, using a standard data extraction form. The methodological quality of included studies was assessed using the Cochrane risk of bias tool. Data entry was carried out by one author and cross‐checked by another. When more than one study reported similar outcomes, data were pooled using the random‐effects model. Heterogeneity was analysed using a Chi² test and the I² test. Results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach
Main results
Six studies randomising 9379 participants with CKD stages 1 to 3 (without DM) met our inclusion criteria. Participants were adults with hypertension; 79% were male from China, Europe, Japan, and the USA. Treatment periods ranged from 12 weeks to three years. Overall, studies were judged to be at unclear or high risk of bias across all domains, and the quality of the evidence was poor, with GRADE rated as low or very low certainty.
In low certainty evidence, ACEi (benazepril 10 mg or trandolapril 2 mg) compared to placebo may make little or no difference to death (any cause) (2 studies, 8873 participants): RR 2.00, 95% CI 0.26 to 15.37; I² = 76%), total cardiovascular events (2 studies, 8873 participants): RR 0.97, 95% CI 0.90 to 1.05; I² = 0%), cardiovascular‐related death (2 studies, 8873 participants): RR 1.73, 95% CI 0.26 to 11.66; I² = 54%), stroke (2 studies, 8873 participants): RR 0.76, 95% CI 0.56 to 1.03; I² = 0%), myocardial infarction (2 studies, 8873 participants): RR 1.00, 95% CI 0.84 to 1.20; I² = 0%), and adverse events (2 studies, 8873 participants): RR 1.33, 95% CI 1.26 to 1.41; I² = 0%).
It is uncertain whether ACEi (benazepril 10 mg or trandolapril 2 mg) compared to placebo reduces congestive heart failure (1 study, 8290 participants): RR 0.75, 95% CI 0.59 to 0.95) or transient ischaemic attack (1 study, 583 participants): RR 0.94, 95% CI 0.06 to 15.01; I² = 0%) because the certainty of the evidence is very low.
It is uncertain whether ARB (losartan 50 mg) compared to placebo (1 study, 226 participants) reduces: death (any‐cause) (no events), adverse events (RR 19.34, 95% CI 1.14 to 328.30), eGFR rate of decline (MD 5.00 mL/min/1.73 m2, 95% CI 3.03 to 6.97), presence of proteinuria (MD ‐0.65 g/24 hours, 95% CI ‐0.78 to ‐0.52), systolic blood pressure (MD ‐0.80 mm Hg, 95% CI ‐3.89 to 2.29), or diastolic blood pressure (MD ‐1.10 mm Hg, 95% CI ‐3.29 to 1.09) because the certainty of the evidence is very low.
It is uncertain whether ACEi (enalapril 20 mg, perindopril 2 mg or trandolapril 1 mg) compared to ARB (olmesartan 20 mg, losartan 25 mg or candesartan 4 mg) (1 study, 26 participants) reduces: proteinuria (MD ‐0.40, 95% CI ‐0.60 to ‐0.20), systolic blood pressure (MD ‐3.00 mm Hg, 95% CI ‐6.08 to 0.08) or diastolic blood pressure (MD ‐1.00 mm Hg, 95% CI ‐3.31 to 1.31) because the certainty of the evidence is very low.
Authors' conclusions
There is currently insufficient evidence to determine the effectiveness of ACEi or ARB in patients with stage 1 to 3 CKD who do not have DM. The available evidence is overall of very low certainty and high risk of bias. We have identified an area of large uncertainty for a group of patients who account for most of those diagnosed as having CKD.
Keywords: Adult; Female; Humans; Male; Angiotensin Receptor Antagonists; Angiotensin Receptor Antagonists/adverse effects; Angiotensin-Converting Enzyme Inhibitors; Angiotensin-Converting Enzyme Inhibitors/adverse effects; Diabetes Mellitus; Diabetes Mellitus/drug therapy; Losartan; Losartan/therapeutic use; Proteinuria; Renal Insufficiency, Chronic; Renal Insufficiency, Chronic/complications; Renal Insufficiency, Chronic/drug therapy
Plain language summary
Blood pressure lowering medication for adults with early stages of chronic kidney disease (without diabetes)
What is the issue?
Chronic kidney disease (CKD) is a long‐term condition that occurs when the kidneys are damaged. It is important to diagnose and treat CKD in the early stages to prevent or delay the more serious stages of CKD (dialysis or transplant). People with CKD are at risk of cardiovascular disease (heart and lung disease). It is reported that blood pressure‐lowering medications can reduce or delay cardiovascular problems in adults with CKD (across early stages, dialysis, and transplant). However, we are less certain about these benefits in adults with early CKD (stages 1 to 3 only) who do not have diabetes.
We wanted to discover whether taking blood pressure‐lowering medications is better or worse than placebo, and if so, which type of blood pressure‐lowering medication works best.
What did we do?
We explored the evidence about the effect of blood pressure‐lowering medications on reducing patients' chance of death, cardiovascular disease and side effects or improving kidney function in people with early CKD (without diabetes). We found six poor‐quality studies. The evidence is current to 6 July 2023.
What did we find?
We found six studies randomising a total of 9379 patients who have early CKD (stages 1 to 3 only, without diabetes). Patients were 18 to 75 years old, 79% were males, most had high blood pressure and were from China, Europe, Japan, and the USA.
All six studies were considered to be at a high risk of bias. This was due to poorly described trial methods, not all of the patients were kept blind to their treatments, and there were high numbers of participants dropping out. Five out of the six studies were funded by the drug manufacturer or by an agency with a commercial interest in the results of the studies, one study did not declare their source of funding.
1. Benazepril or trandolapril may reduce the chance of death, having a stroke, myocardial infarction, and side effects, but may or may not reduce the chance of congestive heart failure or transient ischaemic attack.
2. Losartan may or may not reduce the chance of death, side effects or the presence of proteinuria and may not improve kidney function (eGFR) or blood pressure.
3. Enalapril, perindopril, or trandolapril may or may not be any better than olmesartan, losartan, or candesartan for proteinuria and blood pressure.
Conclusions
There is not enough evidence to know whether blood pressure‐lowering medications work well in patients with early CKD (stages 1 to 3) who do not have diabetes. We also do not know which type of blood pressure‐lowering medication is better than another.
The quality and certainty of the evidence is considered to be very low. This is due to a high risk of bias in the studies, poorly conducted methods, and too little data from too few patients. We have identified an area of large uncertainty for further research.
Summary of findings
Summary of findings 1. Angiotensin‐converting enzyme inhibitors versus placebo for early (stage 1 to 3) non‐diabetic chronic kidney disease.
| ACEi versus placebo for early (stage 1 to 3) non‐diabetic CKD | |||||
|
Patient or population: adults with early (stage 1 to 3) non‐diabetic CKD Settings: hospitals Intervention: ACEi (benazepril 10 mg or trandolapril 2 mg) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | ACEi | ||||
|
Death (any cause) Follow‐up: 3 to 4.8 years |
CKD Stage 2 and 3 |
RR 2.00 (0.26 to 15.37) |
8873 (2) | ⊕⊕⊝⊝ low1,2 | |
| 76 per 1000 | 152 per 1000 (20 to 1000) | ||||
|
CVD events: total (fatal and non‐fatal) Follow‐up: 3 to 4.8 years |
CKD Stage 2 and 3 |
RR 0.97 (0.90 to 1.05) |
8873 (2) | ⊕⊕⊝⊝ low1,2 | |
| 214 per 1000 | 207 per 1000 (192 to 224) | ||||
|
Adverse events: number reporting an adverse event Follow‐up: 3.6 years |
CKD Stage 2 and 3 |
RR 1.33 (1.26 to 1.41) |
8873 (2) | ⊕⊕⊝⊝ low1,2 | |
| 304 per 1000 | 405 per 1000 (383 to 429) | ||||
|
Kidney failure progression: doubling of SCr (mg/dL) Follow‐up: not available |
No data | No data | No data | No data | No data3 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: angiotensin‐converting enzyme inhibitor; CKD: chronic kidney disease; CI: confidence interval; CVD: cardiovascular disease; RR: risk ratio; SCr: serum creatinine | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded once (‐1) for serious limitations in the execution (due to risk of bias)
2 Downgraded once (‐1) for indirectness, likely related to variability within subgroups due to historical definitions of CKD stages
3 No meta‐analysed data from included studies. No GRADE rating for this outcome
Summary of findings 2. Angiotensin receptor blockers versus placebo for early (stage 1 to 3) non‐diabetic chronic kidney disease.
| ARB versus placebo for early (stage 1 to 3) non‐diabetic CKD | |||||
|
Patient or population: adults with early (stage 1 to 3) non‐diabetic CKD Settings: hospital Intervention: ARB (losartan 50 mg) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | ARB | ||||
|
Death (any cause) Follow‐up: 12 months |
CKD Stage 3 | Not estimable | 226 (1) | No data1 | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| CVD events | No data | No data | No data | No data | No data1 |
|
Adverse events (number reporting an adverse event) Follow‐up: 12 months |
CKD Stage 3 |
RR 19.34 (1.14 to 328.30) |
226 (1) | ⊕⊝⊝⊝ very low2,3 | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
|
Kidney failure progression: eGFR (mL/min/1.73m²) Follow‐up: 12 months |
CKD Stage 3 | ‐‐ | 226 (1) | ⊕⊝⊝⊝ very low2,3 | |
| The mean eGFR was 5 mL/min/1.73 m² higher in the ARB group (3.03 higher to 6.97 higher) compared to the placebo group | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARB: angiotensin receptor blockers; CI: confidence interval; CKD: chronic kidney disease; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No meta‐analysed data from included studies. No GRADE rating for this outcome
2 Downgraded once (‐1) for serious limitations in the execution (due to risk of bias)
3 Downgraded once (‐1) for sparse data: one study only
Summary of findings 3. Angiotensin‐converting enzyme inhibitors versus angiotensin receptor blockers for early (stage 1 to 3) non‐diabetic chronic kidney disease.
| ACEi versus ARB for early (stage 1 to 3) non‐diabetic CKD | |||||
|
Patient or population: adults with early (stage 1 to 3) non‐diabetic CKD Settings: hospital Intervention A: ACEi (enalapril 20 mg, perindopril 2 mg, trandolapril 1 mg) Intervention B: ARB (olmesartan 20 mg, losartan 25 mg, candesartan 4 mg) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| ARB | ACEi | ||||
| Death (any cause) | No data | No data | No data | No data | No data1 |
| CVD events | No data | No data | No data | No data | No data1 |
| Adverse events | No data | No data | No data | No data | No data1 |
| Kidney failure progression | No data | No data | No data | No data | No data1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: angiotensin‐converting enzyme inhibitor; ARB: angiotensin II receptor blockers; CI: Confidence interval; CVD: cardiovascular disease. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No meta‐analysed data from included studies. No GRADE rating for this outcome
Background
Description of the condition
Chronic kidney disease (CKD) is a long‐term condition that occurs as a result of damage to the kidneys. Prevalence estimates for CKD vary substantially. Several large, high‐quality, population‐based screening studies have reported the prevalence of CKD stage 3 to 5 disease to be around 3.8% to 4.7%; more than 95% of people with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m² have stage 3 disease (Coresh 2005; Drey 2003; Hallan 2006). In most epidemiological studies, the GFR is estimated from serum creatinine (SCr) measurements using an equation; several equations exist, and this contributes to the variation in prevalence reported in these studies. The prevalence of stage 1 and 2 disease is based on microalbuminuria (albumin:creatinine ratio (ACR) of 17 to 250 mg/g for men or 25 to 355 mg/g for women) or macroalbuminuria (ACR > 250 mg/g for men or > 355 mg/g for women) has been reported to be as high as 11% of the population (CDC 2007). The prevalence of CKD increases with age (Coresh 2005; Hallan 2006; Imai 2007; John 2004). An ageing population, escalating prevalence of diabetes mellitus (DM) (one of the major risk factors for CKD), and increasing recognition are contributing to a reported increase in the prevalence of early CKD and its growing recognition as a major public health problem.
In 2002, the US Kidney Disease Outcomes Quality Initiative (KDOQI) proposed a classification for CKD that has been widely adopted internationally (Levey 2003). In 2012, the 2002 KDOQI CKD guidelines were updated by The KDIGO CKD Guideline Development Work Group to the KDIGO 2012 CKD classifications (Stevens 2013). See Appendix 1 for full details of the KDIGO 2012 CKD classifications.
Early CKD, whilst often asymptomatic, is an important health issue and has implications for individuals and health services. Progressive deterioration of kidney function can result in end‐stage kidney disease (ESKD) and the need for kidney replacement therapy (KRT) in the form of dialysis or transplantation. The rate of progression of CKD may be influenced by secondary factors such as age, race, intraglomerular haemodynamic factors, hypertension and proteinuria. ESKD has risen globally over the last two decades at a high cost to individuals, their carers and families, and health services. However, the proportion of people with early CKD who progress to ESKD is low (Daly 2007; Hallan 2006). A greater risk for people with early CKD is cardiovascular disease (CVD). One study that followed patients over a period of five years, reported that 3.1% of people with CKD progressed to requiring KRT, whereas 24.9% died before reaching dialysis, probably as a result of CVD (Daly 2007).
Early recognition and management of CKD affords the opportunity not only to prepare for progressive kidney impairment and impending KRT (CHOICE Study 2001; Dogan 2005; Khan 2007; Kinchen 2002) but also for intervening to reduce the risk of progression and CVD.
Description of the intervention
Angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) are two classes of antihypertensive drugs that act on the renin‐angiotensin‐aldosterone system (RAAS). Both drug classes have been widely recommended in guidelines for the management of CKD, particularly in patients with evidence of proteinuria, and have been reported to provide both cardioprotective and renoprotective effects. Beneficial effects of ACEi and ARB on kidney outcomes and survival in people with diabetic kidney disease (DKD) have been reported (Strippoli 2006). For patients with moderate to severe CKD without diabetes, there was evidence of benefit in terms of kidney outcomes whether or not proteinuria was present (Jafar 2003a). The evidence for cardioprotective effects, particularly in patients with CKD without diabetes, is less consistent (ASCOT‐BPLA Study 2005; HOPE Study 1996; Strippoli 2006).
To date, reviews have combined evidence from study participants with a wide range of severity of kidney impairment, but the subgroup of those with early CKD (stage 1 to 3) has not been presented separately.
How the intervention might work
As kidneys become damaged and begin to lose nephrons, patients experience systemic hypertension, proteinuria and a progressive decline in eGFR (Metcalfe 2007). Common pathological changes are observed regardless of the underlying causes and include early kidney inflammation; tubulointerstitial injury, and glomerulosclerosis (Ruster 2006). The pathophysiology of progressive kidney function loss involves complex haemodynamic, endocrine, and inflammatory factors (Metcalfe 2007; Ruster 2006).
ACEi and ARB both act to inhibit the RAAS endocrine system. ACEi mode of action includes blocking the conversion of inactive angiotensin I to active angiotensin II at the level of the enzyme needed for its conversion. ARB works at a later stage in the RAAS system and selectively blocks the type I subtype, which is a receptor for angiotensin II (Kumar 2002). Once thought of as a systemic endocrine system important in mediating vascular tone, RAAS is now understood to be complex, operating both systemically and locally within the kidney. Products of the RAAS are understood to impact a wide range of kidney, as well as haemodynamic, factors that contribute to the progression of CKD (Kshirsagar 2000a; Kumar 2002; Ruster 2006).
The action of ACEi and ARB extends beyond simple blood pressure (BP) control and may reflect effects on the complex RAAS pathways. The ability of both of these drugs to inhibit the RAAS at different points means that they have the potential to moderate the functional and structural changes that occur in progressive kidney insufficiency (Giatras 1997a; Jafar 2003a; Kshirsagar 2000a).
Why it is important to do this review
Early recognition of CKD is becoming increasingly common due to widespread laboratory reporting of eGFR, raised clinical awareness, and international adoption of the KDIGO classification. The high prevalence of early CKD means that many individuals and clinicians are faced with choices about management. Another Cochrane review (Strippoli 2006) has reviewed the evidence of effectiveness in people with diabetic CKD and demonstrated that ACEi and ARB play a core role in the management and prevention of DKD, where proteinuria is a key feature. For people without diabetes, particularly those with normal or mild to moderate kidney function impairment where proteinuria may or may not be present, the role of ACEi and ARB is less certain. This is an update of a review last published in 2011 (Sharma 2011) that seeks to summarise the evidence in relation to the benefits and harms of ACEi and ARB for patients with early (stage 1 to 3) CKD without diabetes.
Objectives
To evaluate the benefits and harms of ACEi and ARB in the management of people with early (stage 1 to 3) CKD without DM.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (studies in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the effect of ACEi or ARB were included. The first period of randomised cross‐over studies was considered for inclusion. Only studies of at least four weeks duration were included to show that any effects of RAAS therapy occur beyond haemodynamic changes at the introduction of new antihypertensive therapies.
Types of participants
Inclusion criteria
All adults (18 years or over) with early CKD without DM, with no restriction to gender or race, were considered. Early CKD was defined as KDIGO stages 1 to 3 (Stevens 2013; Appendix 1). We included studies that measured GFR by any method (excretion of iohexol, inulin or similar marker; estimated from 24‐hour urine collection; or estimated from SCr using a recognised equation).
Studies defining CKD based on SCr or other thresholds of eGFR were included in the review if the results had been presented separately for those with KDIGO stages 1 to 3. Studies in general populations that included people with DM were kept in the review if the results were presented separately for those with and without DM. If the results were not presented separately, and less than 30% of the study population had DM, the study was included, and the effect on the outcomes was assessed with a sensitivity analysis. The same process was adopted for study populations that included people with specific renal pathologies (e.g. immunoglobulin A (IgA) nephropathy, lupus nephritis, polycystic kidney disease).
Exclusion criteria
Any studies of patients with a diagnosis of DM (type I or II) were excluded. Because the prognosis for people with specific kidney diagnoses cannot be generalised to the wider population with CKD, studies restricted to patients with a single specific kidney diagnosis (e.g. IgA nephropathy, lupus nephritis, polycystic kidney disease) were excluded.
Types of interventions
All ACEi and ARB or combinations were included as outlined below:
Treatment with ACEi versus placebo
Treatment with ARB versus placebo
Treatment with ACEi plus ARB versus placebo
Treatment with ACEi versus ARB
The ACEi class includes the following:
Benazepril
Captopril
Cilazapril
Delapril
Enalapril maleate
Fosinopril sodium
Imidapril hydrochloride
Lisinopril
Moexipril hydrochloride
Perindopril erbumine
Quinapril
Ramipril
Spirapril
Trandolapril
Zofenopril
The ARB class includes the following:
Candesartan
Eprosartan
Irbesartan
Losartan
Olmesartan
Telmisartan
Valsartan
Azilsartan
Any dose and dosing regimen were included in the review. Combination preparations with medicines other than ACEi and ARB were not included. Only oral preparations were included.
As they are licensed, new drugs will be added to the review in subsequent updates.
Types of outcome measures
Primary outcomes
Death (any cause)
CVD events, including total CVD events (fatal and non‐fatal), death, stroke, myocardial infarction (MI), cerebrovascular accident, congestive heart failure, and transient ischaemic attack (TIA))
ESKD including KRT
Secondary outcomes
Quality of life (QoL) measured by a visual analogue scale, such as SF‐36 and KDQoL
Adverse events including but not limited to: allergic reactions, cough, headache, hyperkalaemia, hypotension, angioedema, and acute kidney injury (AKI)
Kidney failure progression defined by: eGFR, doubling of SCr, progression of CKD stage
Proteinuria and albuminuria including: progression of microalbuminuria to macroalbuminuria, regression of macroalbuminuria to microalbuminuria, progression of normo‐albuminuria to microalbuminuria, regression of microalbuminuria to normo‐albuminuria measured by urinary protein:creatinine ratio (UPCR) (mg/mmol); urinary total protein excretion (g/24 hours); urinary ACR (mg/mmol); urinary albumin excretion (µg/min)
BP (mm Hg) including: total BP, systolic (S) BP or diastolic (D) BP (reported as mean change from baseline or percentage reaching study‐specific target)
Costs: total healthcare costs
Hospital admission rates
Falls
Fatigue
Dementia
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 6 July 2023 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals and the proceedings and abstracts from major kidney and transplant conferences
Searching the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 2 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were not searched.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies potentially relevant to the review. The titles and abstracts were screened independently by two authors who discarded the studies that did not meet the inclusion criteria, although studies and reviews that might include relevant data or information on trials were retained initially. Two authors independently assessed the abstracts and, whenever necessary, the full text of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out by the same authors independently using a standard data extraction form. Studies reported in non‐English language journals we planned to translate before assessment; none of the included studies required translation. Where more than one publication of one study existed, reports were grouped together, and all relevant data was included. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancy between published versions was to be highlighted. Where further information was required from the original investigator, attempts were made for additional data requested by written correspondence, and any relevant information obtained in this manner was to be included in the review. Disagreements were resolved in consultation with a third author.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2022) (see Appendix 3).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. death, cardiovascular morbidity, adverse events), results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. SCr), the mean difference (MD) was used. According to the protocol, the standardised mean difference (SMD) was to be used where different scales had been applied. In instances where change from baseline data (change scores) were reported, the difference in mean change scores was to be used. It was planned that if standard deviations (SDs) for change scores were not available, missing data were not to be imputed. Data were presented in tabular form, and where appropriate, final score data and change from baseline were incorporated into meta‐analyses. If adjustment had been undertaken to account for baseline values, these data were to be reported. Time‐to‐event data (e.g. survival, time to ESKD) were to be analysed as a dichotomous variable where data were presented for all participants up to a specified time period. Alternatively, hazard ratios (and 95% CI) were to be used, with the application of the proportional hazard assumption, for the purpose of comparison and meta‐analysis (Higgins 2022).
Unit of analysis issues
We did not anticipate that there would be any non‐standard designs, such as cross‐over studies and cluster‐RCTs, but multiple arm studies could be identified. Here, all intervention groups relevant to the review were included. It was planned that if there were several relevant comparisons, all independent comparisons were to be included. It was also planned that if a comparator group overlapped (such as a single placebo arm), either comparison groups would be combined (if appropriate), or only the most important comparison would be selected for meta‐analysis.
Dealing with missing data
Intention‐to‐treat was the primary analysis sought from studies. Missing outcomes data and the implications were discussed, but the imputation of missing data was not undertaken. Attempts to contact the original authors for additional outcome data were made. No such data were obtained.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test or a CI for I²) (Higgins 2022).
Assessment of reporting biases
It was planned that funnel plots were to be assessed for evidence of publication bias and to plot effect estimates against study size where data permitted.
Data synthesis
Data were pooled using the random‐effects model, but the fixed‐effects model was also analysed to ensure the robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Where data were available, subgroup analysis was undertaken to explore possible sources of heterogeneity related to the following characteristics:
CKD stage
Presence of microalbuminuria/proteinuria.
The remaining characteristics were planned; however, no such data were available:
Age and sex
Comorbidities (CVD, hypertension)
Distribution of underlying renal pathologies
Interventions (heterogeneity in treatments could be related to prior agent(s) used and the agent, dose and duration of therapy)
Study quality.
Adverse effects were tabulated and assessed with descriptive techniques because they were likely to be different for the various agents used. Where possible, the risk difference (RD) with 95% CI was to be calculated for each adverse effect, compared with either no treatment or another agent.
It was planned that QoL measures would be tabulated and reported descriptively, or if data permitted, meta‐analysis would be undertaken as described.
Sensitivity analysis
It was planned to undertake sensitivity analyses to explore the robustness of findings to key decisions in the review process. These were to be determined as the review process took place(Higgins 2022).
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2022a).
The 'Summary of findings' tables also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011a; GRADE 2011b). The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2022b). See Appendix 4 for details on how GRADE was assessed and applied to the evidence found in this review.
We presented the following outcomes, where data were available, in the 'Summary of findings' tables:
Death (any cause)
CVD events
Adverse events
Kidney failure progression: doubling of SCr
Results
Description of studies
The following section contains broad descriptions of the studies considered in this review. For further details on each individual study please see the characteristics of studies table Characteristics of included studies and Characteristics of excluded studies.
Results of the search
A search of the Cochrane Kidney and Transplant Specialised Register of Studies on 6 July 2023 identified 244 new reports. A full‐text assessment identified 28 new studies, of which two new studies (2 reports) were included (Espinel 2013; Shen 2012) and 26 (112 reports) were excluded. Seven potential studies (9 reports) were identified prior to publication and will be assessed in a future update of this review.
A total of six studies (45 reports, 9379 participants, Figure 1) were included, and 73 studies were excluded in this review.
1.
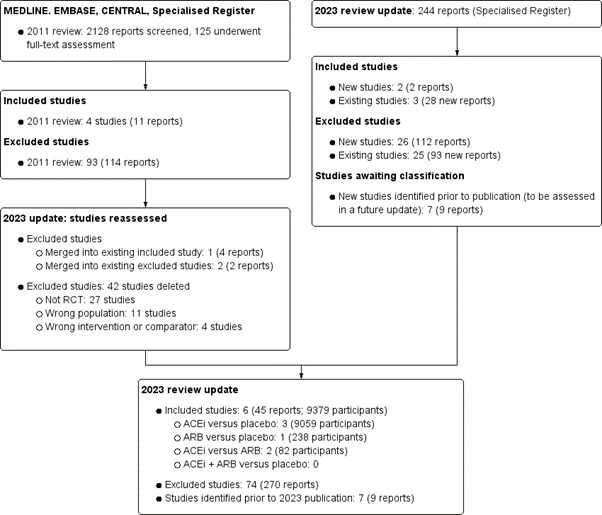
Flow diagram
Included studies
Six studies (9379 randomised participants) met our inclusion criteria (Characteristics of included studies).
Of these, three were single‐centre studies (Espinel 2013; Matsuda 2003; Shen 2012), and three were multi‐centre studies (AIPRI 1996; PEACE 2004; REIN 1991), and all took place in a hospital setting. Studies were undertaken in Canada, China, France, Germany, Italy, Japan, Puerto Rico, Spain and the USA.
Sample sizes ranged from 30 participants (Espinel 2013) to 8290 participants (PEACE 2004).
Participants were adults with CKD stages G1 and G2 (Matsuda 2003), G2 (Espinel 2013; PEACE 2004), and G3b (AIPRI 1996; Shen 2012). REIN 1991 included participants with CKD stages 1 to 4.
Five studies compared two parallel arms, and one study was a cross‐over which did not report separate data for the first phase of the trial (Espinel 2013). Three studies compared ACEi to placebo (AIPRI 1996; PEACE 2004; REIN 1991), one study compared ARB to placebo (Shen 2012), and two studies compared ACEi to ARB (Espinel 2013; Matsuda 2003). Treatment periods ranged from 12 weeks (Espinel 2013) to 3 years (AIPRI 1996).
Excluded studies
For this update, we reassessed all previously excluded studies. One study was merged with an existing included study (REIN 1991), and two studies were merged with existing excluded studies (Ihle 1996; Kanno 2006). We deleted 42 studies: 27 studies were not randomised, 11 studies were the wrong or mixed population, and four studies used the wrong intervention or comparator.
For the 2023 search, we excluded 26 new studies (112 reports) and identified 93 new reports of 25 already excluded studies. In total, we have excluded 74 studies (270 reports).
Studies awaiting classification
Seven potential studies (9 reports) were identified prior to publication and will be assessed in a future update of this review.
Ongoing studies
There are no ongoing studies.
Risk of bias in included studies
See Figure 2 and Figure 3 for a graphical summary of the risk of bias assessment within each study.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Most studies were characterised by an unclear to high risk of bias across the domains, mostly pertaining to a lack of sufficient detail provided regarding methodology, lack of blinding, or industry funding. Using funnel plots to detect publication bias was not feasible because of the small number of studies included in this review.
Allocation
Random sequence generation
Two studies were judged to have an unclear risk of bias due to a lack of information provided on randomisation methods within the text, although they were stated to be randomised (AIPRI 1996; Matsuda 2003).
Four studies were judged to have a low risk of bias for providing an adequate description of how their randomisation methods were undertaken, such as permuted blocks on a 1 to 1 basis, sealed envelopes, or a computerised random number generator (Espinel 2013; PEACE 2004; REIN 1991; Shen 2012).
Allocation concealment
Two studies were open‐label and judged to have a high risk of bias (Matsuda 2003; Shen 2012).
Two studies were judged to have an unclear risk of bias due to a lack of information provided on how allocation was concealed for the intervention arms (AIPRI 1996; PEACE 2004).
Two studies judged to have a low risk of bias; they reported that only a third party knew the treatment allocations (Espinel 2013; REIN 1991).
Blinding
Blinding of participants and personnel (performance bias)
Two studies were open‐label and judged to have a high risk of bias (Matsuda 2003; Shen 2012).
Two studies were judged to have an unclear risk of bias due to not providing any details as to how any blinding of personnel took place (AIPRI 1996; PEACE 2004).
Two studies judged to have a low risk of bias for describing that only a third part knew the treatment groups (Espinel 2013; REIN 1991).
Blinding of outcome assessors (detection bias)
Two studies were open‐label and judged to have a high risk of bias (Matsuda 2003; Shen 2012).
Four studies were judged to have an unclear risk of bias due to not providing any details as to how any blinding of the outcome assessors took place (AIPRI 1996; Espinel 2013; PEACE 2004; REIN 1991).
Incomplete outcome data
Three studies were judged to have a high risk of bias due to a high attrition rate (43%) or missing data on withdrawals and completers (Espinel 2013; Matsuda 2003; REIN 1991).
Three studies were judged to have an unclear risk of bias due to low attrition (6%) but with some unclear details of completers or moderate attrition (23% to 25%) (AIPRI 1996; PEACE 2004; Shen 2012).
Selective reporting
Five studies were judged to have an unclear risk of bias due to no information being available on trial registration or an a priori published protocol (AIPRI 1996; Matsuda 2003; PEACE 2004; REIN 1991; Shen 2012).
One study was judged to have a low risk of bias due to an available pre‐registered trial registration number (Espinel 2013).
Other potential sources of bias
Five studies were judged to have a high risk of bias due to pharmaceutical industry funding (AIPRI 1996; Espinel 2013; PEACE 2004; REIN 1991), or traditional Chinese medicine (TMC) industry funding (Shen 2012).
One study was judged to have an unclear risk of bias as it did not report the sources of funding (Matsuda 2003).
Four studies did not report if there were any conflicts of interest (AIPRI 1996; Matsuda 2003; REIN 1991; Shen 2012). Of the two studies that did report their conflicts of interest, PEACE 2004 reported financial collaboration with the pharmaceutical industry.
Effects of interventions
See: Table 1; Table 2; Table 3
Angiotensin‐converting enzyme inhibitors versus placebo
Three studies compared an ACEi with placebo, in people who have CKD stage 2 or 3, over a 36 to 63‐month treatment period.
AIPRI 1996: benazepril 10 mg/day
PEACE 2004: trandolapril 2 mg/day
REIN 1991: ramipril 1.25 mg/day titrated to 5 mg
REIN 1991 investigated participants with CKD stages 1 to 4 with no separate data reported for the participants with early CKD stages 1 to 3. The results have been reported narratively.
See Table 1.
Primary outcomes
Death (any cause)
ACEi may make little or no difference in reducing the number of deaths (any cause) at 4.8 years (median follow‐up) compared to placebo (Analysis 1.1 (2 studies, 8873 participants): RR 2.00, 95% CI 0.26 to 15.37; I² = 76%; low certainty evidence).
1.1. Analysis.

Comparison 1: ACEi versus placebo, Outcome 1: Death (any cause)
Long‐term follow‐up
AIPRI 1996 reported no difference in the number of deaths between ACEi and placebo at 6.6 years follow‐up (benazepril 10 mg: 25 in 300; placebo: 23 in 283). AIPRI 1996 also reported during the extended follow‐up, 64% of those patients randomised to benazepril continued on an ACEi, and 61% in the placebo arm started treatment with an ACEi.
For participants with CKD stages 1 to 4, REIN 1991 reported no difference in the number of deaths at 63 months between ACEi and placebo for participants with CKD stages 1 to 4 (ramipril: 1 in 99; placebo: 0 in 87).
Cardiovascular events: total events
ACEi may make little or no difference in reducing the number of total cardiovascular events compared to placebo (Analysis 1.2 (2 studies, 8873 participants): RR 0.97, 95% CI 0.90 to 1.05; I² = 0%; low certainty evidence).
1.2. Analysis.

Comparison 1: ACEi versus placebo, Outcome 2: Cardiovascualar disease events: total (fatal and non‐fatal)
Cardiovascular disease events: death
ACEi may make little or no difference in reducing the number of cardiovascular‐related deaths compared to placebo at 4.8 years (median follow‐up) (Analysis 1.3 (2 studies, 8873 participants): RR 1.73, 95% CI 0.26 to 11.66; I² = 54%; low certainty evidence).
1.3. Analysis.

Comparison 1: ACEi versus placebo, Outcome 3: Cardiovascular disease events: death
For participants with CKD stages 1 to 4, REIN 1991 reported ACEi compared to placebo made no difference to CVD‐related deaths at 63 months (ramipril: 0 in 99; placebo: 0 in 87).
Cardiovascular disease events: stroke
ACEi may make little or no difference in reducing the number of strokes compared to placebo at 4.8 years (median follow‐up) (Analysis 1.4 (2 studies, 8873 participants): RR 0.76, 95% CI 0.56 to 1.03; I² = 0%; low certainty evidence).
1.4. Analysis.

Comparison 1: ACEi versus placebo, Outcome 4: Cardiovascular disease events: stroke
For participants with CKD stages 1 to 4, REIN 1991 reported ACEI compared to placebo made no difference to stroke at 63 months (ramipril: 1 in 99; placebo: 0 in 87).
Cardiovascular disease events: myocardial infarction
ACEi may make little or no difference in reducing the number of MIs compared to placebo at 4.8 years (median follow‐up) (Analysis 1.5 (2 studies, 8873 participants): RR 1.00, 95% CI 0.84 to 1.20; I² = 0%; low certainty evidence).
1.5. Analysis.

Comparison 1: ACEi versus placebo, Outcome 5: Cardiovascular disease events: myocardial infarction
Cardiovascular disease events: congestive heart failure
PEACE 2004 reported ACEi may reduce the number of congestive heart failures compared to placebo at 4.8 years (median follow‐up) (Analysis 1.6 (1 study, 8290 participants): RR 0.75, 95% CI 0.59 to 0.95; low certainty evidence).
1.6. Analysis.

Comparison 1: ACEi versus placebo, Outcome 6: Cardiovascular disease events: congestive heart failure
For participants with CKD stages 1 to 4, REIN 1991 reported ACEi compared to placebo made no difference to congestive heart failure at 63 months (ramipril: 1 in 99; placebo: 0 in 87).
Cardiovascular disease events: transient ischaemic attack
It is uncertain whether ACEi makes a difference in reducing the number of TIAs compared to placebo at 4.8 years (median follow‐up) (Analysis 1.7 (1 study, 583 participants): RR 0.94, 95% CI 0.06 to 15.01; very low certainty evidence).
1.7. Analysis.

Comparison 1: ACEi versus placebo, Outcome 7: Cardiovascular disease events: transient ischaemic attack
End‐stage kidney disease
For participants whose baseline eGFR was > 45 mL/min/1.73 m², REIN 1991 reported no difference in the progression to KRT between ACEi and placebo at 63 months (3 in 101 participants ‐ it was not clear from which groups these participants came).
Secondary outcomes
Adverse events
ACEi may make little or no difference in reducing the number of adverse events compared to placebo at 4.8 years (median follow‐up) (Analysis 1.8 (2 studies, 8873 participants): RR 1.33, 95% CI 1.26 to 1.41; I² = 0%; low certainty evidence). Types of adverse events are summarised in Table 4 and Table 5.
1.8. Analysis.

Comparison 1: ACEi versus placebo, Outcome 8: Adverse events (number reporting an adverse event)
1. Withdrawals and adverse events for angiotensin‐converting enzyme inhibitors versus placebo.
| AIPRI 1996 |
ACEi (benazepril) (N = 300) |
Placebo (N = 283) |
| Withdrawals due to adverse events | ||
| Total adverse events | 35 | 23 |
| Cancer | 8 | 5 |
| Dry cough | 1 | 2 |
| Hyperkalaemia | 5 | 3 |
| Hypertensive crisis | 0 | 4 |
| Hypotension | 3 | 3 |
| Local or systemic allergic reaction | 3 | 3 |
| Other | 15 | 7 |
| Withdrawals due to other events | ||
| Total | 18 | 22 |
| Lack of cooperation | 13 | 15 |
| Violation of protocol | 5 | 7 |
ACEi ‐ angiotensin‐converting enzyme inhibitors
2. Number and types of adverse events for angiotensin‐converting enzyme inhibitors versus placebo.
| PEACE 2004 |
ACEi (trandolapril) (N = 4158) |
Placebo (N = 4132) |
| Number of events reported per adversity | ||
| Cough | 1625 (39.1%) | 1136 (27.5%) (P < 0.01) |
| Syncope | 119 (4.8%) | 161 (3.9%) (P = 0.04) |
| Angioedema | 8 (< 1%) | 5 (< 1%) |
ACEi ‐ angiotensin‐converting enzyme inhibitors
PEACE 2004 and REIN 1991 reported adverse events and withdrawals for those who were randomised at study level, but not for the relevant subgroups that were of interest for this review
Kidney failure progression: change in eGFR
For patients whose baseline GFR was > 45 mL/min/1.73m², REIN 1991 reported no difference in the mean eGFR at 27 months between ACEi and placebo (ramipril: 0.19 mL/min/1.73 m² (standard error (SE) 0.07) in 99 participants; placebo: 0.34 mL/min/1.73 m² (SE 0.09) in 87 participants).
Kidney failure progression: doubling of serum creatinine
AIPRI 1996 reported a composite outcome of the combined total of participants 'experiencing doubling of SCr (mg/dL) or the need for dialysis' for 31 in 300 participants receiving an ACEi, compared with 57 in 283 participants receiving placebo, at three years.
Proteinuria and albuminuria
AIPRI 1996 observed a difference in urinary protein between ACEi and placebo at 36 months (benazepril: 29% decrease in urinary protein in 300 participants; placebo: 9% increase in urinary protein in 283 participants). The difference between the two treatments was not reported.
Systolic blood pressure
AIPRI 1996 reported the mean SBP at 6, 12, 24, and 36 months (benazepril: decrease by 4.5 to 8.0 mm Hg in 300 participants; placebo: increase of 1.0 to 3.7 mm Hg in 283 participants).
Diastolic blood pressure
AIPRI 1996 reported the mean DBP at 6, 12, 24, and 36 months (benazepril: decrease by 3.5 to 5.0 mm Hg in 300 participants; placebo: increase of 0.2 to 1.5 mm Hg in 283 participants).
AIPRI 1996 reported uncontrolled hypertension (benazepril: decrease by 28% to 18% in 300 participants; placebo: increase of 27% to 32% in 283 participants).
No data were reported for the remaining primary or secondary outcomes.
Angiotensin receptor blockers versus placebo
Shen 2012 compared an ARB (losartan 50 mg/day) with a placebo in people with stage 3 CKD, over a 12‐month treatment period.
See Table 2.
Primary outcomes
Death (any cause)
Shen 2012 reported that no patients died during the 12‐month study period (Analysis 2.1 (226 participants; very low certainty evidence).
2.1. Analysis.

Comparison 2: ARB versus placebo, Outcome 1: Death (any cause) at 12 months
Secondary outcomes
Adverse events
Shen 2012 reported nine adverse events in 119 participants receiving ARB, and no adverse events in 119 participants receiving placebo (Analysis 2.2 (1 study, 226 participants): RR 19.34, 95% CI 1.14 to 328.30; very low certainty evidence). The types of mild adverse events are summarised in Table 6.
2.2. Analysis.

Comparison 2: ARB versus placebo, Outcome 2: Adverse events (number reporting an adverse event)
3. Adverse events number and types angiotensin receptor blockers versus placebo.
| Shen 2012 | ARB (losartan) (N = 199) | Placebo (N = 119) |
| Number of patients reporting an adverse event at 12 months | 9 | 0 |
| Number of adverse events reported at 12 months | 7 | 0 |
| Types and number of adverse events | ||
| Cough | 3 | 0 |
| Hypotension | 0 | 0 |
| Mild dizziness | 6 | 0 |
ARB ‐ angiotensin receptor blockers
Kidney failure progression: eGFR rate of decline
Shen 2012 reported a difference in mean eGFR favouring ARB compared to placebo at 12 months (Analysis 2.3 (1 study, 226 participants): MD 5.00 mL/min/1.73 m², 95% CI 3.03 to 6.97; very low certainty evidence).
2.3. Analysis.

Comparison 2: ARB versus placebo, Outcome 3: Kidney failure progression at 12 months: eGFR
Kidney failure progression: doubling of serum creatinine
Shen 2012 did not report a doubling of SCr but reported that a difference was found between the mean (± SD) SCr (mg/dL) favouring ARB compared to placebo at 12 months (losartan: 1.48 ± 0.27 to 1.51 ± 0.32 in 112 participants; placebo: 1.49 ± 0.28 to 1.69 ± 0.30 in 114 participants).
Presence of proteinuria
Shen 2012 reported a decrease in mean proteinuria with ARB (losartan 50 mg/day) compared to placebo at 12 months (Analysis 2.4 (1 study, 226 participants): MD ‐0.65 g/24 hours, 95% CI ‐0.78 to ‐0.52; very low certainty evidence).
2.4. Analysis.

Comparison 2: ARB versus placebo, Outcome 4: Proteinuria at 12 months
Systolic blood pressure
Shen 2012 reported no difference in the mean SBP between ARB and placebo at 12 months (Analysis 2.5 (1 study, 226 participants): MD ‐0.80 mm Hg, 95% CI ‐3.89 to 2.29; very low certainty evidence).
2.5. Analysis.

Comparison 2: ARB versus placebo, Outcome 5: Systolic blood pressure at 12 months
Diastolic blood pressure
Shen 2012 reported no difference in the mean DBP between ARB and placebo at 12 months (Analysis 2.6 (1 study, 226 participants): MD ‐1.10 mm Hg, 95% CI ‐3.29 to 1.09; very low certainty evidence).
2.6. Analysis.

Comparison 2: ARB versus placebo, Outcome 6: Diastolic blood pressure at 12 months
No data were reported for the remaining primary or secondary outcomes.
Angiotensin‐converting enzyme inhibitors plus angiotensin receptor blockers versus placebo
No studies compared ACEi plus ARB with placebo.
Angiotensin‐converting enzyme inhibitors versus angiotensin receptor blockers
Two studies compared an ACEi (Espinel 2013: enalapril 20 mg; Matsuda 2003: perindopril 2 mg or trandolapril 1 mg), with an ARB (Espinel 2013: olmesartan 20 mg; Matsuda 2003: losartan 25 mg or candesartan 4 mg) in people with CKD stage G1, G2, or G3, over a 12 to 48 week treatment period.
Espinel 2013 investigated participants with CKD stage 2, and Matsuda 2003 stratified by baseline proteinuria level; those with mild (< 1 g/day) and moderate (> 1 g/day) proteinuria levels to classify approximately as CKD stage G1 and G2. Espinel 2013 conducted a two‐arm cross‐over study but did not report separate data for the first phase of the trial.
See Table 3.
Primary outcomes
No data were reported on the primary outcomes of death (any cause), CVD events, or ESKD.
Secondary outcomes
Kidney failure progression: eGFR
Matsuda 2003 reported "no effect" on creatinine clearance (mL/min/1.73 m²) when comparing ACEi to ARB over 48 weeks (52 participants total).
Proteinuria
Subgroup analysis: low proteinuria group < 1.0 g/day
Matsuda 2003 reported "no significant change" in mean proteinuria (g/day) when comparing ACEi to ARB at 12 and 48 weeks (26 participants total).
Subgroup analysis: high proteinuria group > 1.0 g/day
It is uncertain whether ACEi makes a difference to mean proteinuria compared to placebo at 12 weeks (Analysis 3.1 (1 study, 26 participants): MD ‐0.50 g/24 hours, 95% CI ‐0.81 to ‐0.19; very low certainty evidence) or at 48 weeks (Analysis 3.2 (1 study, 26 participants): MD ‐0.40 g/24 hours, 95% CI ‐0.60 to ‐0.20; very low certainty evidence).
3.1. Analysis.

Comparison 3: ACEi versus ARB, Outcome 1: Proteinuria at 12 weeks
3.2. Analysis.

Comparison 3: ACEi versus ARB, Outcome 2: Proteinuria at 48 weeks
Systolic blood pressure
Subgroup analysis systolic blood pressure: low proteinuria group < 1.0 g/day
It is uncertain whether ACEi makes a difference to SBP compared to placebo at 12 weeks (Analysis 3.3.1 (1 study, 26 participants): MD ‐2.00 mm Hg, 95% CI ‐4.31 to 0.31; very low certainty evidence).
3.3. Analysis.

Comparison 3: ACEi versus ARB, Outcome 3: Systolic blood pressure at 12 weeks
Matsuda 2003 found no observed differences in change of SBP when comparing ACEi to ARB at 24 weeks (ACEi: 132 ± 4 mm Hg, N = 13; ARB: no values reported, N = 13) and 48 weeks (ACEi: 131 ± 4 mm Hg, N = 13; ARB: no values reported, N = 13).
Subgroup analysis systolic blood pressure: high proteinuria group > 1.0 g/day
It is uncertain whether ACEi makes a difference to SBP compared to placebo at 12 weeks (Analysis 3.3.2 (1 study, 26 participants): MD ‐3.00 mm Hg, 95% CI ‐6.08 to 0.08; very low certainty evidence).
Matsuda 2003 found no observed differences in change of SBP when comparing ACEi to ARB at 24 weeks (ACEi: 120 ± 3 mm Hg, N = 14; ARB: no values reported, N = 12) and 48 weeks (ACEi: 124 ± 3 mm Hg, N = 14; ARB: no values reported, N = 12).
Diastolic blood pressure
Subgroup analysis diastolic blood pressure: low proteinuria group < 1.0 g/day
It is uncertain whether ACEi makes a difference to DBP compared to placebo at 12 weeks (Analysis 3.4.1 (1 study, 26 participants): MD 5.00 mm Hg, 95% CI 2.57 to 7.43; very low certainty evidence).
3.4. Analysis.

Comparison 3: ACEi versus ARB, Outcome 4: Diastolic blood pressure at 12 weeks
Matsuda 2003 reported no difference in the change of DBP when comparing ACEi to ARB at 24 weeks (ACEi: 80 ± 3, N = 13; ARB: no values reported, N = 13) and 48 weeks (ACEi: 74 ± 4, N = 13; ARB: no values reported, N = 13).
Subgroup analysis diastolic blood pressure: high proteinuria group > 1.0 g/day
It is uncertain whether ACEi makes a difference to DBP compared to placebo at 12 weeks (Analysis 3.4.2 (1 study, 26 participants): MD ‐1.00 mm Hg, 95% CI ‐3.31 to 1.31; very low certainty evidence).
Matsuda 2003 reported no difference in the change of DBP when comparing ACEi to ARB at 24 and 48 weeks (no values reported for either treatment group).
No data were reported for the remaining secondary outcomes.
Discussion
Summary of main results
Six studies (9379 randomised participants) were included in this review, with sample sizes ranging from 30 to 8290 participants. Three studies were single‐centre, and three were multi‐centre, all taking place in hospital settings. Study participants were adults with CKD stages G1 to G3 from nine countries. Treatment periods ranged from 12 weeks to three years. Overall, studies were mostly judged to be at either unclear or high risk of bias across all domains.
Low certainty evidence found ACEi (benazepril 10 mg or trandolapril 2 mg) compared to placebo made little or no difference to death (any cause), total cardiovascular events, cardiovascular‐related death, stroke, MI, and adverse events. Very low certainty evidence found ACEi (benazepril 10 mg or trandolapril 2 mg) compared to placebo had uncertain effects on congestive heart failure and TIA.
Based on very low certainty evidence, ARB (losartan 50 mg) had uncertain effects on death (any cause), adverse events, eGFR rate of decline, presence of proteinuria, SBP, and DBP compared to placebo.
Based on very low certainty evidence, ACEi (enalapril 20 mg, perindopril 2 mg or trandolapril 1 mg) (olmesartan 20 mg, losartan 25 mg or candesartan 4 mg) had uncertain effects on proteinuria, SBP and DBP, compared to ARB.
There is very limited data to ascertain the benefits and harms of the use of ACEi or ARBs in adults with early (stages 1 to 3) non‐diabetic CKD.
Overall completeness and applicability of evidence
Despite the growing international emphasis on the early detection and management of CKD and the recognition that stage 1 to 3 CKD accounts for most people with evidence of kidney impairment, we found very few studies that reported the effectiveness of ACEi or ARB in this population.
This review highlights the striking lack of studies in people with stage 1 to 3 CKD, which is the population group that accounts for most people identified as having CKD. Identification of CKD stages 1 and 2 requires evidence of kidney damage, such as confirmation of proteinuria, haematuria or structural abnormality. This requirement may make identifying people to participate in studies more challenging, and even more so for investigators looking into single, specific kidney diagnoses.
The two major issues around the applicability of the evidence to clinical practice and patients were:
definition of CKD, and
the underlying pathologies that caused the kidney damage.
The patient groups included in the studies varied considerably, particularly in terms of comorbidities and severity of proteinuria. This made it difficult to draw any overall conclusions. Each of the six included studies differed in their definitions of CKD in terms of eGFR cut‐off levels and measures of eGFR. These differences are particularly important when identifying a cohort of people with stage 3 CKD. Invariably, participants in each study differed in regard to their reported degrees of kidney impairment.
Of the four studies that reported data for death (any cause) and CVD events, two defined their CKD groups based on single eGFR assays (AIPRI 1996; PEACE 2004) and therefore were prone to classification bias because of the chance of including people with AKI. Only two studies based the definition of CKD on an eGFR using standard equations (REIN 1991; Shen 2012), which is the method most widely adopted in clinical practice, but likely to underestimate eGFR in those with true eGFR level of around 60 mL/min/1.73 m².
The included patient population comprised mainly of hypertensive patients with varying renal pathologies, and 4% (AIPRI 1996) to 19% (PEACE 2004) had DM at baseline. The high proportion of people with DM, and the inclusion of only people with existing CVD in PEACE 2004, make it difficult to draw conclusions for the wider population with early CKD. PEACE 2004 reported a reduction in death (any cause) consistent with other studies of ACEi in populations with CVD; however, the investigators did not report the statistically significant benefits in kidney outcomes that were observed in other studies conducted among diabetic study participants only. The proportions of patients with CVD and specifically reported kidney diagnoses varied from study to study.
We identified a number of significant gaps in the evidence. No studies were identified that compared an ACEi combined with ARB versus placebo. Only some of the many preparations of ACEi and ARB drugs available were investigated in the studies, and none compared one preparation with another. Intervention features such as compliance, timing, dosing or intensity could not be elicited because of poor reporting in studies. Although most of the included studies reported on some measure of kidney function over time, death and ESKD, none reported QoL, healthcare costs, admissions to hospital, falls, fatigue, or dementia.
Quality of the evidence
Overall, the quality of the evidence was poor. Across all treatment comparisons, studies were downgraded primarily for serious limitations in the study design and execution, whereby studies were mostly judged to be at unclear or high risk of bias across all domains. Studies were also downgraded due to some uncertainty about the directness of the evidence or sparse data. The small number of studies, their relatively small size, and low event rates for some of the primary outcomes and single study data meant that the evidence was of very low certainty. Findings should therefore be viewed with caution.
Potential biases in the review process
This review was conducted as per the protocol following pre‐specified inclusion criteria and included comprehensive literature searches to find all relevant studies. Two authors screened all titles, abstracts and full papers to avoid selection bias. There was no arbitration required during the selection process, data extraction or quality assessment that needed a third author.
We found very few studies reporting outcomes for the group of patients of interest in this review (stage 1 to 3 CKD). We excluded studies of single specific kidney diagnoses, and it is acknowledged that some of these may include patients with evidence of isolated proteinuria (stage 1 CKD). A large number of studies conducted in people with CKD were excluded because the authors did not report their findings in subgroups that were relevant to this study. In addition, studies of the use of ACEi and ARB drugs in hypertensive patients may also include people with stage 1 to 3 CKD. Before asking authors to consider re‐analysing data into specified subgroups, we considered it appropriate to establish what data were available in the published literature.
Agreements and disagreements with other studies or reviews
We did not find any other published reviews that matched our inclusion criteria. Two systematic reviews reported that ACEi was found to have little benefit over placebo or other antihypertensive drugs in reducing death (any cause) among patients with CKD or hypertension (P = 0.12) (Jafar 2001) or those with DM (Strippoli 2006). Jafar 2001 reported ACEi to be effective in reducing mean BP, protein excretion, and the risk of kidney progression, compared with placebo or other antihypertensive drugs. The 11 included studies in Jafar 2001 used different measures of CKD. Baseline mean SCr was > 155 µmol/L in all but two studies (where it was 124 µmol/L and 88 µmol/L). In general, these studies included participants with greater kidney impairment than we have included here. The findings reported by Jafar 2001 were not stratified by CKD stage, and therefore, they could not be compared directly.
In Strippoli 2006, the meta‐analysis of 21 studies of 7295 patients with DM and CKD, reported no survival benefit was observed compared with placebo (RR 0.91, 95% CI 0.71 to 1.17), except when treated with maximum tolerable doses (RR 0.78, 95% CI 0.61 to 0.098). Similarly, in people with DM and CKD, ACEi produced a 31% risk reduction of ESKD compared with placebo (RR 0.60, 95% CI 0.39 to 0.93). These outcomes were found to be similar for ARB (RR 0.78, 95% CI 0.67 to 0.91). However, the findings were not reported separately by stage.
One systematic review of patients with CKD (119 studies, 64,768 patients; Xie 2016) reported that the odds of kidney failure were reduced by 39% (OR 0.61, 95% credible interval 0.47 to 0.79) with ACEi and 30% (OR 0.70, 95% credible interval 0.52 to 0.89) with ARB compared to placebo. Odds reductions for major cardiovascular events were also reported for ACEi (OR 0.82, 95% credible interval 0.71 to 0.92) and ARB (OR 0.76, 95% credible interval 0.62 to 0.89) compared to placebo. However, these findings were not stratified by CKD stage.
Authors' conclusions
Implications for practice.
With increased recognition of the importance and impact of CKD, the diagnosis and labelling of people with early (stage 1 to 3) CKD is now common. Drugs in the ACEi and ARB families are the cornerstone for the management of CKD. This review highlights that there have been very few studies that report on the effectiveness of ACEi and ARB drugs for patients with early CKD without those studies investigating single, specific kidney diagnoses. Despite the fact that people with early CKD are at greater risk of death (any cause) and cardiovascular events than progression to ESKD (Daly 2007; Hallan 2006; Sharma 2010), we found very few studies that considered the management of stage 1 to 3 CKD to reduce cardiovascular risk, death (any cause) or kidney disease progression.
Our review demonstrated that there is currently insufficient evidence to determine the effectiveness of ACEi or ARB treatment for patients with stage 1 to 3 CKD who do not have DM. Studies have been conducted among patients with single, specific kidney diagnoses, but we have not included these here. Based on evidence from studies of specific kidney conditions, current clinical guidelines (Levey 2003; NICE Guideline 2008; SIGN Guideline 2008) and practice supports the use of ACEi and ARB drugs for patients with proteinuric kidney disease. We have not found sufficient evidence to support any change in current practice.
Implications for research.
Despite the growing international emphasis on the early detection and management of CKD and the recognition that stage 1 to 3 CKD accounts for most people with evidence of kidney impairment, we found very few studies that reported the effectiveness of ACEi or ARB in this population.
We have identified an area of significant uncertainty for a group of patients who account for most of those labelled as having CKD. We identified more than 50 studies where relevant data may exist, but the subgroups of patients that are necessary to derive supported evidence were not presented in the original publications (see Characteristics of excluded studies). These studies may provide relevant subgroup or individual patient data for analysis of patients with stage 1 to 3 CKD. In addition, studies of ACEi (such as HOPE Study 1996; PART 2 (MacMahon 2000); PROGRESS Collaborative Group 2001; SCAT Study 2000) and ARB (such as LIFE (Lindholm 2002); SCOPE (Lithell 2003)) conducted in people with hypertension or CVD may also include people who meet the criteria of CKD stage 1 to 3 that would potentially enable subgroup or individual patient data analysis.
There is a need for further RCTs to be conducted that focus on patients with stage 1 to 3 CKD. These studies should feature designs that are adequate in size and duration, include quality of life outcomes and establish recruitment criteria to ensure that the study population can be generalised to the wider community.
What's new
| Date | Event | Description |
|---|---|---|
| 19 July 2023 | New citation required but conclusions have not changed | New studies added but no changes to conclusions |
| 19 July 2023 | New search has been performed | Thirty‐one new studies identified (2 included, 25 excluded, 4 ongoing/to be classified) |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 14 January 2020 | Amended | Search strategies amended |
| 30 August 2018 | Amended | Search strategies amended |
| 5 December 2014 | Amended | Updated for authors |
Notes
Changes from the last version (Sharma 2010) of this review: we have updated the data according to the newest classification of CKD stage for each study according to the definitions within the KDIGO Clinical Practice Guidelines (Stevens 2013; Appendix 1). Therefore, some subgroups have been changed by stages, and where some subgroups include stages 3 and 4 and do not report separate data, the data have been removed from the meta‐analyses and are reported narratively within the text.
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this review.
We wish to acknowledge the authors of the previous version of this review: Pawana Sharma, Rachel C Blackburn, Claire L Parke, Keith McCullough, Angharad Marks, and Corri Black.
Appendices
Appendix 1. Classification of CKD stages ‐ KDIGO 2012
The Kidney Disease: Improving Global Outcomes (KDIGO) organization developed clinical practice guidelines in 2012 by which the CKD classification was updated by The KDIGO CKD Guideline Development Work Group (Stevens 2013)
| Persistent Albuminuria Categoria Description and Range |
||||||
| A1 | A2 | A3 | ||||
| Normal to mildly increased | Moderately increased | Severely increased | ||||
| ACR < 30 mg/g | ACR of 30 ‐ 300 mg/g | ACR > 300 mg/g | ||||
| eGFR categories (mL/min/1.73m²) Description and range |
G1 | Normal or high* | ≥ 90 | 1 if CKD | 1 | 2 |
| G2 | Mildly decreased* | 60 ‐ 89 | 1 if CKD | 1 | 2 | |
| G3a | Mildly to moderately decreased | 45 ‐ 59 | 1 | 2 | 3 | |
| G3b | Moderately to severely decreased | 30 ‐ 44 | 2 | 3 | 3 | |
| G4 | Severely decreased | 15 ‐ 29 | 3 | 3 | 4+ | |
| G5 | Kidney failure | < 15 | 4+ | 4+ | 4+ | |
* In the absence of evidence of kidney damage, neither eGFR category G1 nor G2 fulfil the criteria for CKD (Stevens 2013).
Classification is based on two markers: evidence of kidney damage (such as the presence of microalbuminuria, proteinuria or structural abnormality); and the sustained impairment of estimated glomerular filtration rate (eGFR) for at least three months. Normal eGFR in young adults is around 100 to 120 mL/min/1.73 m².
Early CKD is described as stages 1 to 3 of the KDIGO 2012 classification. At these stages, a patient may have no outward symptoms or signs of illness and only testing such as dipstick urine measurement for proteinuria/haematuria or blood test may detect the presence of a kidney abnormality.
Appendix 2. Electronic search strategies
| Databases | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 3. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 4. GRADE Approach (Grades of Recommendation, Assessment, Development and Evaluation)
The GRADE approach assesses the certainty of a body of evidence, rating it in one of four grades (Guyatt 2008):
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate; the true effect is likely to be close the estimate of effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; or
very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We decreased the certainty of evidence if there was (GRADE 2011c):
serious (‐1) or very serious (‐2) limitation in the study design or execution (risk of bias);
important inconsistency of results (‐1);
some (‐1) or major (‐2) uncertainty about the directness of evidence;
imprecise or sparse data (‐1) or serious imprecision (‐2); or
high probability of publication bias (‐1).
We increased the certainty of evidence if there was (GRADE 2011b):
a large magnitude of effect (direct evidence, relative risk (RR) = 2 – 5 or RR = 0.5 – 0.2 with no plausible confounders) (+1); very large with RR > 5 or RR < 0.2 and no serious problems with risk of bias or precision; more likely to rate up if effect is rapid and out of keeping with prior trajectory; usually supported by indirect evidence (+2);
evidence of a dose response gradient (+1); or
all plausible residual confounders or biases would reduce a demonstrated effect, or suggest a spurious effect when results show no effect (+1).
Data and analyses
Comparison 1. ACEi versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death (any cause) | 2 | 8873 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.26, 15.37] |
| 1.1.1 CKD stage G2 | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.03] |
| 1.1.2 CKD stage G3b | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 7.55 [0.95, 59.96] |
| 1.2 Cardiovascualar disease events: total (fatal and non‐fatal) | 2 | 8873 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 1.2.1 CKD stage G2 | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 1.2.2 CKD stage G3b | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.50, 2.06] |
| 1.3 Cardiovascular disease events: death | 2 | 8873 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.26, 11.66] |
| 1.3.1 CKD stage G2 | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.19] |
| 1.3.2 CKD stage G3b | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 8.49 [0.46, 157.01] |
| 1.4 Cardiovascular disease events: stroke | 2 | 8873 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.56, 1.03] |
| 1.4.1 CKD stage G2 | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.04] |
| 1.4.2 CKD stage G3b | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.11, 3.74] |
| 1.5 Cardiovascular disease events: myocardial infarction | 2 | 8873 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.20] |
| 1.5.1 CKD stage G2 | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.20] |
| 1.5.2 CKD stage G3b | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.13, 6.65] |
| 1.6 Cardiovascular disease events: congestive heart failure | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 1.6.1 CKD stage G2 | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 1.7 Cardiovascular disease events: transient ischaemic attack | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 15.01] |
| 1.7.1 CKD stage G3b | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 15.01] |
| 1.8 Adverse events (number reporting an adverse event) | 2 | 8873 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.26, 1.41] |
| 1.8.1 CKD stage G2 | 1 | 8290 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.26, 1.42] |
| 1.8.2 CKD stage G3b | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.82, 1.74] |
Comparison 2. ARB versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Death (any cause) at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Adverse events (number reporting an adverse event) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Kidney failure progression at 12 months: eGFR | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Proteinuria at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Systolic blood pressure at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.6 Diastolic blood pressure at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. ACEi versus ARB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proteinuria at 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.2 Proteinuria at 48 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Systolic blood pressure at 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.1 Low proteinuria group (< 1.0 g/day) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.2 High proteinuria group (> 1.0 g/day) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4 Diastolic blood pressure at 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4.1 Low proteinuria group (< 1.0 g/day) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4.2 High proteinuria group (> 1.0 g/day) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AIPRI 1996.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline charactertistics
|
|
| Interventions | Screening period
Intervention group (ACEi)
Control group
Co‐interventions
Follow‐up details
|
|
| Outcomes | All outcomes reported by this study (at 36 months)
Open‐label follow‐up at median of 6.6 years for death and kidney failure progression (ESKD reported only as part of composite kidney progression endpoint) |
|
| Definition of CKD | CKD Stage G3b
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients in each group were then randomly assigned to receive … benazepril or placebo". Comment: insufficient details were provided regarding how the randomisation process was carried out |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details are provided regarding methods on how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "We conducted a prospective, double‐blind, randomised study…". Quote: "Each patient was examined by a physician, who was unaware of the group assignment" (also the person who took measurements) Comment: although the study states to be double‐blind, there is insufficient information provided regarding how blinding was carried out and ensured throughout the trial for either the participants or study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Each patient was examined by a physician, who was unaware of the group assignment" (also the person who took measurements) Comment: although the study states to be double‐blind, there is insufficient information provided regarding how blinding was carried out and ensured throughout the trial for either the participants or study personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: all participants were accounted for from start to end of trial. No missing outcome data. Attrition was moderate at 23% (23% benazepril, 22% placebo) due to adverse events, lack of cooperation, or protocol violation |
| Selective reporting (reporting bias) | Unclear risk |
Comment: all outcomes that were planned in the methods were planned in the results (main publication). However, no trial registration number was provided and no a priori published protocol reported |
| Other bias | High risk |
Comment: pharmaceutical industry funding for this trial, study does not state whether conflicts of interest exist for the authors |
Espinel 2013.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Phase 1 Intervention group 1 (ACEi)
Intervention group 2 (ARB)
Phase 2 Intervention group 1 (ACEi)
Intervention group 2 (ARB)
Co‐interventions
Follow‐up details
|
|
| Outcomes | All outcomes reported by this study
|
|
| Definition of CKD | eGFR between 30 to 60 mL/min/1.73m² | |
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…simple randomization using a computerised random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "This was achieved with the independent contribution of pharmacists who were also responsible for dispensing the drugs in numbered bottles to conceal the allocation sequence…and storage of the allocation list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Health care providers and participants were blinded to the drug assignment" Quote: "This was achieved with the independent contribution of pharmacists who were also responsible for dispensing the drugs in numbered bottles to conceal the allocation sequence…and storage of the allocation list" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no explicit description of who took outcome assessments (and whether they are different to the study personnel), and whether they were completely blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: all participants were accounted for from start to end of trial. No missing outcome data Attrition: in this crossover study, 43% of patients withdrew after phase one. Participants who developed hyperkalaemia were also withdrawn from each phase |
| Selective reporting (reporting bias) | Low risk |
Comment: all outcomes planned in the methods were reported in the results. No a priori published protocol but trial registration provided with methods matching the present publication |
| Other bias | High risk |
Comment: no other known sources of bias were identified |
Matsuda 2003.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Intervention group 1 (ACEi)
Intervention group 2 (ARB)
Co‐interventions
|
|
| Outcomes | All outcomes reported in this study (at 12, 24, 48 weeks)
|
|
| Definition of CKD |
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to ACE‐I and ARB‐treated groups" Comment: insufficient information provided for methods of randomisation used |
| Allocation concealment (selection bias) | High risk | Comment: open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no description of any blinding, assumed open‐label to all participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of any blinding, assumed open‐label to all outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no follow‐up data was provided. Withdrawals or dropouts were not reported, attrition rate unknown. Unclear if all participants were accounted for from start to end of trial |
| Selective reporting (reporting bias) | Unclear risk |
Comment: all outcomes that were planned in the methods were reported in the results (main publication). However, no trial registration number was provided, and no a priori published protocol reported |
| Other bias | Unclear risk |
Comment: Funding sources and conflicts of interest have not been declared. There is insufficient information to assess whether any other important risk of bias exists |
PEACE 2004.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
Co‐morbidities (n, %)
Differences between groups at baseline
|
|
| Interventions | Intervention group (ACEi)
Control group
Co‐interventions
|
|
| Outcomes | All outcomes reported in this study (at minimum 3.5 years after recruitment, median 4.8 years)
Not presented for relevant subgroups
|
|
| Definition of CKD | CKD Stage G2
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with the use of permuted blocks, stratified according to clinical site" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details are provided regarding allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients… remained blinded to the treatment assignments" Comment: methods used to ensure blinding are not described in detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Investigators and staff members remained blinded to the treatment assignments" Comment: does not clearly indicate that the outcome assessors were also blinded to drug assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The percentage of patients assigned to trandolapril or placebo who withdrew from therapy and were not taking an open label ACE inhibitor was 25.5% and 8.3% respectively" Comment: 10/8290 with missing baseline creatinine excluded. Intention to treat analysis. All participants were accounted for from start to end of the study |
| Selective reporting (reporting bias) | Unclear risk |
Comment: all outcomes that were planned in the methods were reported in the results |
| Other bias | High risk |
Comment: pharmaceutical industry involvement |
REIN 1991.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Pre‐randomisation/screening (all participants)
Intervention group (ACEi)
Control group
Co‐interventions
|
|
| Outcomes | All outcomes reported in this study
|
|
| Definition of CKD |
|
|
| Notes | Additional informatoin
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients in each centre were randomly assigned 1.25 mg capsules of ramipril or placebo (identical appearance) on a 1 to 1 basis within each stratum" Quote: "A sequence of patients’ numbers was randomly assigned to each study centre, and the study medication randomly assigned to patient numbers in advance" Quote: "Randomisation code prepared by Hoechst Clinical Research Institute" |
| Allocation concealment (selection bias) | Low risk | Quote: "The study medication was packed and supplied with a label showing the patient’s number" Quote: "Each patient was given only the study medication carrying his or her number. A sequence of patients’ numbers was randomly assigned to each study centre, and the study medication randomly assigned to patient numbers in advance" Quote: "Each centre received a set of randomisation envelopes, one for each patient number. Copies of these envelopes were held at the coordinating centre (Mario Negri Institute for Pharmacological Research, Bergamo, Italy)" Quote: "The randomisation envelopes could be opened only if it was necessary to know for medical reasons which medication the patient was receiving" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study medication was packed and supplied with a label showing the patient’s number" Quote: "Each patient was given only the study medication carrying his or her number" Quote: "The randomisation envelopes could be opened only if it was necessary to know for medical reasons which medication the patient was receiving" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Outcome measures (were) assessed centrally (at Mario Negri Institute for Pharmacological Research, Bergamo, Italy)" Comment: authors do not give explicit details that the outcome assessors were kept blind to the treatment groups. These were conducted where the envelopes were also kept. Only moderate concern due to objective laboratory outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no clear description of attrition and final group numbers. Adverse events and events leading to withdrawal are unclear to whom actually dropped out Comment: 'Low' proteinuria subgroup: 175/186 had at least 3 eGFR estimates but all 186 included in main analysis |
| Selective reporting (reporting bias) | Unclear risk |
Comment: all outcomes that were planned in the methods were reported in the results. No access to a trial registration or a priori protocol (unable to assess) |
| Other bias | High risk |
Comment: trial in 'High' proteinuria group stopped early due to apparent benefit. Long‐term outcomes based on open‐label follow‐up. Industry funding Conflicts of interest/disclosures: not reported Funding declared: "This study was supported by a grant from Hoechst Marion Roussel Clinical Research Institute, Frankfurt am Main, Germany" |
Shen 2012.
| Study characteristics | ||
| Methods | Study design
Time frame
|
|
| Participants | Study characteristics
Baseline characteristics
|
|
| Interventions | Screening period
Intervention group (ARB)
Control group
Collection days
Co‐interventions
|
|
| Outcomes | All outcomes reported by this study
Time points
|
|
| Definition of CKD |
|
|
| Notes | Additional information
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients had an equal probability of assignment to administration of losartan 50 mg (losartan group) or matching placebo (placebo group) by a random number generator and sealed envelopes" |
| Allocation concealment (selection bias) | High risk | Quote: "Allocation numbers were provided in a sealed envelope which were assigned to patients in a consecutive order". Comment: no clear indication whether allocation to treatment groups remained concealed and if so, how was this carried out |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study. Allocation of treatment groups was not concealed from participants or study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The second limitation is investigator bias due to lack of blinding. It is well known that blinding of study subjects and investigators reduces bias. This study was an open‐label study, and as a result, there is a possibility of bias in the recording of the results, as patients on active drug are more likely to receive more attention and encouragement" Comment: open‐label study. Allocation of treatment groups was not concealed from outcome assessors. The authors acknowledge this potential risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. Losartan group (7/119; 5.9%) and placebo group (5/119; 4.2%) Comment: attrition was low (losartan 6%, placebo 3%). ITT analysis was difficult as mean data were provided for completers only |
| Selective reporting (reporting bias) | Unclear risk |
Comment: all outcomes planned in the methods were reported in the results in the main article. No access to original protocol or trial registration, therefore unable to assess |
| Other bias | High risk |
Comment: TCM industry funding. Conflicts not declared. There is insufficient information to assess whether any other important risk of bias exists |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ARB ‐ angiotensin receptor blocker; BMI ‐ body mass index; BP ‐ blood pressure; CCB ‐ calcium channel blocker; CHF ‐ congestive heart failure; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CVA ‐ cerebrovascular accident; CVD ‐ cardiovascular disease; DBP ‐ diastolic blood pressure; DKD ‐ diabetic kidney disease; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; F/M ‐ female/male; FSGS ‐ focal segmental glomerulosclerosis; GI ‐ gastrointestinal; HbA1c% ‐ glycated haemoglobin A1c; IgAN ‐ Immunoglobulin A nephropathy; IQR ‐ interquartile range; ITT ‐ intention‐to‐treat; KRT ‐ kidney replacement therapy; MDRD ‐ Modification of Diet in Renal Disease; MI ‐ myocardial infarction; NKF: National Kidney Foundation; NSAIDs ‐ nonsteroidal anti‐inflammatory drugs; QoL ‐ quality of life; RCT randomised controlled trial; SBP ‐ systolic blood pressure; SCr ‐ serum creatinine SD ‐ standard deviation; TCM ‐ Traditional Chinese Medicine; UACR ‐ urinary albumin:creatinine ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AASK Pilot 1996 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Acone 2003 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Agarwal 2001 | Participants: all participants had DM |
| Aranda 2005 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Bakris 2000 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Bianchi 1992 | Outcomes: results are presented for a subgroup with CrCl < 25 mL/min (not CKD stages 1 to 3) |
| Bilic 2011 | Participants: > 30% had GN |
| Campbell 2003 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Cinotti 2001 | Comparator: ACEi compared to another antihypertensive, not to placebo or no treatment Outcomes: results not presented for CKD stages 1 to 3 |
| COOPERATE 2003 | Withdrawn from publication 2009. The Lancet has published a retraction of COOPERATE (Combination treatment of angiotensin‐II receptor blocker and angiotensin‐converting‐enzyme inhibitor in non‐diabetic renal disease) and reported that its lead author, Naoyuki Nakao, appears to have engaged in serious scientific misconduct |
| Crowe 2003 | Comparator: comparison of different doses of ACEi, not compared to placebo or no treatment |
| Deuber 2003b | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Dyadyk 1997 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Elung‐Jensen 2003 | Participants: study included only people with severe CKD (not stages 1 to 3) |
| Erley 1994 | Comparison: long vesus short acting treatment, not compared to placebo or no treatment arm |
| Esnault 2005 | Participants: > 30% of study participants had DKD Outcomes: results not presented separately for those participants without diabetes |
| Esnault 2010 | Comparators: dose comparison of the same ACEi/ARB combination therapy, not to placebo or no treatment |
| Faulhaber 1997 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Fliser 1995 | Participants: only included people with primary renal pathology, not early CKD stages 1 to 3 |
| Frimodt‐Moller 2010 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Garg 2005 | Participants: included 64% with DM; results are not presented separately for non‐diabetics |
| Greenbaum 2000 | Participants: only included people with CrCl < 30, not classified as early CKD stages 1 to 3 |
| Hannedouche 2001 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Hemmelder 1996 | Intervention: single bolus IV therapy |
| Homma 2004 | Comparator: ARB monotherapy compared to ARB/amlodipine and ARB/ACEi combination therapy. Results for combination therapy groups were not reported separately; not compared to placebo or no treatment |
| HOPE 1996 | Participants: > 30% had DM Outcomes: results not presented separately for CKD stages 1 to 3 for non‐diabetic participants |
| Hou 2006 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Ihle 1996 | Outcomes: results not reported separately for stages 1 to 3 |
| Kamper 1990 | Participants: > 30% had DM Outcomes: data not presented separately for non‐diabetics; results not presented for CKD stages 1 to 3 |
| Kanno 2006 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Keilani 1993 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Kim 2000 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Kincaid‐Smith 2002 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Klein 2003 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Lee 2011a | Participants: > 30% of patients with IgAN (not early CKD stages 1 to 3) |
| LIRICO 2007 | Participants: participants had DM |
| MacGregor 2003 | Comparator: ACEi compared to a CCB; not compared to placebo or no treatment |
| Marcantoni 1998 | Participants: unclear from the abstract if study participants had early CKD stages 1 to 3 as it is not clearly described (no full‐text publication) |
| Meier 2011 | Participants: DM and CKD stage 4 |
| Minetti 1995 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Mitchell 1997 | Comparator: ACEi compared with another ACEi treatment; not compared to placebo or no treatment |
| NEPHROS 2001 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| ONTARGET 2008 | Participants: > 30% with DM; atherosclerotic vascular disease or with DM with end‐organ damage |
| Papadogiannakis 2006 | Population: patients with hypertensive nephrosclerosis, stage of CKD not reported Outcomes: only reports plasminogen activator inhibitor‐1 levels |
| Perico 1997 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Plum 1998 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Plum 2000 | Comparator: ACEi compared to ARB; not compared to placebo or no treatment |
| Praga 2003a | Comparator: ARB compared to CCB; not compared to placebo or no treatment |
| PROGRESS 1995 | Participants: not early CKD stages 1 to 3 Study design: post hoc analysis of PROGRESS trial (stroke patients) |
| PUTS 1992 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| ROAD 2007 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Ruilope 2000 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Rump 1999 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Rutkowski 2004 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| SAVE 1991 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Scaglione 2005 | Comparator: ACEi compared to ARB compared to ACEi/ARB combination therapy, not to placebo or no treatment |
| Schmieder 2005 | Comparator: compares dosed of ACEi; not compared to placebo or no treatment |
| Schulz 1999 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Shand 2000 | Participants: population consists of renal parenchymal disease, not early CKD stage 1 to 3 |
| Shoda 2006 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| SMART 2009 | Participants: > 30% with DM Outcomes: results not reported separately for stages CKD 1 to 3 or non‐diabetic participants |
| Takagi 1990 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Toto 1996 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| TRANSCEND 2009 | Participants: > 30% with DM |
| Tylicki 2007 | Comparator: ARB/ACEi combination therapy compared to ARB/ACEi/diuretic combination therapy; not compared to placebo or no treatment |
| Tylicki 2008a | Comparator: dose comparisons of the same ACEi/ARB, not to placebo or no treatment |
| VALERIA 2008 | Participants: > 30% with DM Outcomes: results not reported separately for CKD stages 1 to 3 or non‐diabetic participants |
| van der Wouden 2009 | Comparison: dosing comparisons of the same ACEi/ARB, not to placebo or no treatment |
| Vogt 2008 | Comparator: ARB monotherapy compared to addition of low‐sodium diet and diuretic to ARB therapy, not to placebo or no treatment |
| Wang 2013a | Comparator: dosing comparisons of the same ACEi/ARB, not to placebo or no treatment |
| Yanagi 2013 | Participants: DM and CKD stages 4 and 5 |
| Yasuda 2013 | Participants: DM and advanced CKD stages 4 and 5 |
| Yildiz 1999 | Outcomes: results not reported separately for CKD stages 1 to 3 |
| Yilmaz 2007a | Outcomes: results not reported separately for CKD stages 1 to 3 |
ACEi: angiotensin‐converting enzyme inhibitors; ARB: angiotensin receptor blockers; CCB: calcium channel blocker; CKD: chronic kidney disease; CrCL: creatinine clearance; DKD: diabetic kidney disease; DM: diabetes mellitus; GN: glomerulonephritis; IgAN: IgA nephropathy; IV: intravenous
Characteristics of studies awaiting classification [ordered by study ID]
NCT04736329.
| Methods | To be determined |
| Participants | To be determined |
| Interventions | To be determined |
| Outcomes | To be determined |
| Notes |
NCT05402397.
| Methods | To be determined |
| Participants | To be determined |
| Interventions | To be determined |
| Outcomes | To be determined |
| Notes |
Osipova 2021.
| Methods | To be determined |
| Participants | To be determined |
| Interventions | To be determined |
| Outcomes | To be determined |
| Notes |
Segura 2003.
| Methods | To be determined |
| Participants | To be determined |
| Interventions | To be determined |
| Outcomes | To be determined |
| Notes |
Shoji 2001.
| Methods | To be determined |
| Participants | To be determined |
| Interventions | To be determined |
| Outcomes | To be determined |
| Notes |
Suzuki 2003.
| Methods | To be determined |
| Participants | To be determined |
| Interventions | To be determined |
| Outcomes | To be determined |
| Notes |
TCTR20220426002.
| Methods | To be determined |
| Participants | To be determined |
| Interventions | To be determined |
| Outcomes | To be determined |
| Notes |
Differences between protocol and review
Modifications were made to the background to clarify terminology around eGFR and to provide context with what is known with regard to diabetic kidney disease.
Changes from the last version (Sharma 2010) of this review: additional outcomes were added to address the SONG 2017 patient‐centred outcomes. These were planned a priori to study selection and data extraction.
Contributions of authors
Wrote the original protocol and conducted tasks for the 2011 review: CB, RB, CP, PS, KMcC
Contributed to the new methods for this update: TC, CT
Study selection: TC, CT, DT, BC
Extract data from studies: TC, CT, DT, BC
Enter data into RevMan: TC, CT, DT, BC
Carry out the analysis: TC, CT, DT, BC
Interpret the analysis: TC, DT
Wrote and contributed to the final review: TC, CT, DT, BC, GS
Sources of support
Internal sources
-
University of Aberdeen, UK
Funding of core staff salaries and support facilities
External sources
No sources of support provided
Declarations of interest
TC: no relevant interests were disclosed
CT: no relevant interests were disclosed
DT: no relevant interests were disclosed
BC: no relevant interests were disclosed
GS: no relevant interests were disclosed
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AIPRI 1996 {published data only}
- Apperloo AJ, Rensma PL. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curren CG. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):597. [MEDLINE: ] [PubMed] [Google Scholar]
- Hogan TJ, Elliott WJ, Seto AH, Bakris GL. Antihypertensive treatment with and without benazepril in patients with chronic renal insufficiency: a US economic evaluation. Pharmacoeconomics 2002;20(1):37-47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Carbarns IR, Maschio G, Mann JF, Ponticelli C, Ritz E, et al. Long-term progression of chronic renal insufficiency in the AIPRI extension study. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Kidney International - Supplement 1997;52(63):S63-6. [MEDLINE: ] [PubMed] [Google Scholar]
- Locatelli F, Del Vecchio L, Andrulli S, Marai P, Tentori F. The role of underlying nephropathy in the progression of renal disease. Kidney International - Supplement 2000;57(75):S49-55. [MEDLINE: ] [PubMed] [Google Scholar]
- Mann JF, Maschio G, Alberti D, AIPRI investigators. Perspectives of ACE inhibition in progressive renal failure [abstract no: 103]. Nephrology 1997;3(Suppl 1):S39. [CENTRAL: CN-00461248] [Google Scholar]
- Maschio G, Alberti D, Janin G, Locatelli F, Mann J, Motolese M, et al. The use of benazepril in the treatment of progressive renal disease [abstract]. In: 13th International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:65. [CENTRAL: CN-00550723]
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. New England Journal of Medicine 1996;334(15):939-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maschio G, Alberti D, Locatelli F, Mann JF, Motolese M, Ponticelli C, et al. Angiotensin-converting enzyme inhibitors and kidney protection: the AIPRI trial. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study Group. Journal of Cardiovascular Pharmacology 1999;33 Suppl 1:S16-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maschio G. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596-7. [CENTRAL: CN-00550722] [DOI] [PubMed] [Google Scholar]
- Pedersen EB. Reduced progression, increased mortality in chronic renal disease after treatment with the angiotensin-converting enzyme inhibitor benazepril [Nedsat progressionshastighed ved kronisk nyresygdom efter behandling med den angiotensinkonverterende enzymhaemmer benazepril, men ogsa oget mortalitet]. Ugeskrift for Laeger 1996;158(41):5798-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Hout BA, Simeon GP, McDonnell J, Mann JF. Economic evaluation of benazepril in chronic renal insufficiency. Kidney International - Supplement 1997;52(63):S159-62. [MEDLINE: ] [PubMed] [Google Scholar]
Espinel 2013 {published data only}
- Espinel E, Joven J, Gil I, Sune P, Renedo B, Fort J, et al. Risk of hyperkalemia in patients with moderate chronic kidney disease initiating angiotensin converting enzyme inhibitors or angiotensin receptor blockers: a randomized study. BMC Research Notes 2013;6:306. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuda 2003 {published data only}
- Matsuda H, Hayashi K, Homma K, Yoshioka K, Kanda T, Takamatsu I, et al. Differing anti-proteinuric action of candesartan and losartan in chronic renal disease. Hypertension Research - Clinical & Experimental 2003;26(11):875-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Matsuda H, Hayashi K, Saruta T. Distinct time courses of renal protective action of angiotensin receptor antagonists and ACE inhibitors in chronic renal disease. Journal of Human Hypertension 2003;17(4):271-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PEACE 2004 {published data only}
- Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. New England Journal of Medicine 2004;351(20):2058-68. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer MA, Domanski M, Rosenberg Y, Verter J, Geller N, Albert P, et al. Prevention of events with angiotensin-converting enzyme inhibition (the PEACE study design). Prevention of Events with Angiotensin-Converting Enzyme Inhibition. American Journal of Cardiology 1998;82(3A):25-30H. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solomon SD, Rice MM, Jablonski A, Jose P, Domanski M, Sabatine M, et al. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation 2006;114(1):26-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
REIN 1991 {published data only}
- Brunetti M, Pagano E, Tammuzzo L, Benini R, Remuzzi G, Ruggenenti P. In chronic proteinuric nephropathies ramipril therapy is either life and cost-saving [abstract no: A0309]. Journal of the American Society of Nephrology 2000;11(Sept):57A. [CENTRAL: CN-00550419] [Google Scholar]
- Hebert LA. Renoprotective therapy: how good can it get? Kidney International 2000;57(1):343-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kramer BK, Schweda F. Ramipril in non-diabetic renal failure (REIN study). Ramipril Efficiency in Nephropathy study. Lancet 1997;350(9079):736. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Del Vecchio L, Andrulli S. REIN follow-up trial. Ramipril Efficacy in Nephropathy. Lancet 1998;352(9145):2020-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mallamaci F, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, et al. ACE inhibition is renoprotective among obese patients with proteinuria. Journal of the American Society of Nephrology 2011;22(6):1122-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnino G. REIN follow-up trial. Ramipril Efficacy in Nephropathy. Lancet 1998;352(9145):2021-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perna A, Benini R, Remuzzi G, Ruggenenti P. In chronic nephropathies dihydropyridine calcium channel blockers (CCBs) have an adverse effect on renal function that is prevented by ace inhibition or tight blood pressure control [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):78A. [CENTRAL: CN-00447169] [DOI] [PubMed] [Google Scholar]
- Porter AM. Ramipril in non-diabetic renal failure (REIN study) Ramipril Efficiency in Nephropathy Study. Lancet 1997;350(9079):736. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997;349(9069):1857-63. [MEDLINE: ] [PubMed] [Google Scholar]
- Remuzzi G, Tognoni G, Ruggenenti P, Perna A, Maggiorre Q, Pizzarelli F, et al. A long term, randomized clinical trial to evaluate the effects of ramipril on the evolution of renal function in chronic nephropathies. Journal of Nephrology 1991;4(3):193-202. [EMBASE: 22157068] [Google Scholar]
- Ruggenenti P, Gaspari F, Perna A, Remuzzi G. Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes [Erratum in: BMJ 1998 Nov 28;317(7171):1491]. BMJ 1998;316(7130):504-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P, Pagano E, Tammuzzo L, Benini R, Garattini L, Remuzzi G. Ramipril prolongs life and is cost effective in chronic proteinuric nephropathies. Kidney International 2001;59(1):286-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Benini R, Bertani T, Zoccali C, Maggiore Q, et al. In chronic nephropathies prolonged ACE inhibition can induce remission: dynamics of time-dependent changes in GFR. Investigators of the GISEN Group. Gruppo Italiano Studi Epidemiologici in Nefrologia. Journal of the American Society of Nephrology 1999;10(5):997-1006. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Benini R, Remuzzi G. Effects of dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibition, and blood pressure control on chronic, nondiabetic nephropathies. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Journal of the American Society of Nephrology 1998;9(11):2096-101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Gherardi G, Benini R, Remuzzi G. Chronic proteinuric nephropathies: outcomes and response to treatment in a prospective cohort of 352 patients with different patterns of renal injury. American Journal of Kidney Diseases 2000;35(6):1155-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999;354(9176):359-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 1998;352(9136):1252-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Gherardi G, Gaspari F, Remuzzi G, GISEN Group. Deny ACE inhibitors to proteniuric patients with severe renal insufficiency: is it justified? [abstract no: A0436]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):83A. [CENTRAL: CN-00447505] [Google Scholar]
- Ruggenenti P, Perna A, Mosconi L, Matalone M, Gaspari F, Garini G, et al. The angiotensin-converting-enzyme (ACE) inhibitor ramipril slows the rate of GFR decline (GFR) and the progression to end stage renal failure (ESRF) in proteinuric, non-diabetic chronic renal diseases [abstract no: A0696]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):147A. [CENTRAL: CN-00447506] [Google Scholar]
- Ruggenenti P, Perna A, Mosconi L, Matalone M, Pisoni R, Gaspari F, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Kidney International - Supplement 1997;52(63):S54-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Mosconi L, Matalone M, Pisoni R, Remuzzi G. The REIN study: in non-diabetic chronic nephropathies urinary protein excretion rate accurately predicts progression to end stage renal failure [abstract no: 105]. Nephrology 1997;3(Suppl 1):S39. [CENTRAL: CN-00461630] [Google Scholar]
- Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Kidney International 1998;53(5):1209-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Remuzzi G, Gruppo Italiano di Studi Epidemiologici in Nefrologia. ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. Journal of the American Society of Nephrology 2001;12(12):2832-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Remuzzi G, GISEN Group Investigators. Retarding progression of chronic renal disease: the neglected issue of residual proteinuria. Kidney International 2003;63(6):2254-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Zoccali C, Gherardi G, Benini R, Testa A, et al. Chronic proteinuric nephropathies. II. Outcomes and response to treatment in a prospective cohort of 352 patients: differences between women and men in relation to the ACE gene polymorphism. Gruppo Italiano di Studi Epidemologici in Nefrologia (Gisen). Journal of the American Society of Nephrology 2000;11(1):88-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schadlich PK, Brecht JG, Brunetti M, Pagano E, Rangoonwala B, Huppertz E. Cost effectiveness of ramipril in patients with non-diabetic nephropathy and hypertension: economic evaluation of Ramipril Efficacy in Nephropathy (REIN) Study for Germany from the perspective of statutory health insurance. Pharmacoeconomics 2001;19((5 Pt 1)):497-512. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shen 2012 {published data only}
- Shen PC, He LQ, Yang XJ, Cao HX. Renal protection of losartan 50 mg in normotensive Chinese patients with nondiabetic chronic kidney disease. Journal of Investigative Medicine 2012;60(7):1041-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AASK Pilot 1996 {published data only}
- Breyer JA, Toto RD, Hall WD, Norris KC, Massry SG, AASK Pilot Investigators. Baseline characteristics of patients entering the pilot African-American Study of Kidney Disease (Pilot-AASK) [abstract]. Journal of the American Society of Nephrology 1994;5(3):557. [CENTRAL: CN-00550486] [Google Scholar]
- Coresh J, Toto RD, Kirk KA, Whelton PK, Massry S, Jones C, et al. Creatinine clearance as a measure of GFR in screenees for the African-American Study of Kidney Disease and Hypertension pilot study. American Journal of Kidney Diseases 1998;32(1):32-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hall WD, Kusek JW, Kirk KA, Appel LJ, Schulman G, Agodoa LY, et al. Short-term effects of blood pressure control and antihypertensive drug regimen on glomerular filtration rate: the African-American Study of Kidney Disease and Hypertension Pilot Study. American Journal of Kidney Diseases 1997;29(5):720-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kusek JW, Lee JY, Smith DE, Milligan S, Faulkner M, Cornell CE, et al. Effect of blood pressure control and antihypertensive drug regimen on quality of life: the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Controlled Clinical Trials 1996;17(4 Suppl):40-6S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lee JY, Greene PG, Douglas M, Grim C, Kirk KA, Kusek JW, et al. Determinants of minority participant adherence in clinical trials: experience of the African American Study of Kidney Disease and Hypertension (AASK) pilot study [abstract]. Journal of the American Society of Nephrology 1995;6(3):395. [CENTRAL: CN-00484778] [Google Scholar]
- Lee JY, Smith DE, Cornell CE, Faulkner M, Kopple J, Kusek JW, et al. Quality of life in the African American Study of Kidney Disease and Hypertension (AASK) pilot study [abstract no: 1522]. Journal of the American Society of Nephrology 1995;6(3):644. [CENTRAL: CN-00484779] [Google Scholar]
- Rahman M, Phillips R, Gassman J, Greene T, Wright J Jr, Appel L. Can nocturnal blood pressure (BP) be lowered in African Americans with hypertensive chronic kidney (CKD)? Results from the ABPM pilot study of the African American Study of Kidney Disease and Hypertension (AASK) [abstract no: TH-FC041]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):10A. [Google Scholar]
- Toto R, Coresh J, Jones C, Kirk K, AASK Pilot Study Investigators. Serum creatinine is more precise than creatinine clearance for estimating GFR in African Americans (AA): results from the African American Study of Kidney Disease and Hypertension (AASK) pilot study [abstract no: PO1123]. Journal of the American Society of Nephrology 1995;6(3):406. [CENTRAL: CN-00486179] [DOI] [PubMed] [Google Scholar]
- Whelton P, Lee J, Kopple J, Faulkner M, Charleston J, AASK Pilot Investigators. Recruitment in the African American Study of Kidney Disease and hypertension (AASK) pilot study [abstract]. Journal of the American Society of Nephrology 1994;5(3):570. [CENTRAL: CN-00550379] [Google Scholar]
- Wright JT, Kusek JW, Toto RD, Lee JY, Agodoa LY, Kirk KA, et al. Design and baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Controlled Clinical Trials 1996;17(4 Suppl):3-16S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Acone 2003 {published data only}
- Acone D, Cillo F, Giordano G. Long term effects of combined therapy with ace-inhibitor and angiotensin II receptor antagonist on non-diabetic nephropathies [abstract no: T265]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):365. [CENTRAL: CN-00444102] [Google Scholar]
Agarwal 2001 {published data only}
- Agarwal R, Siva S, Dunn SR, Sharma K. Add-on angiotensin II receptor blockade lowers urinary transforming growth factor-beta levels. American Journal of Kidney Diseases 2002;39(3):486-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Agarwal R. Add-on angiotensin receptor blockade with maximized ACE inhibition. Kidney International 2001;59(6):2282-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Agarwal R. Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. American Journal of Physiology - Renal Physiology 2003;284(4):F863-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aranda 2005 {published data only}
- Aranda P, Segura J, Ruilope LM, Aranda FJ, Frutos MA, Lopez V, et al. Long-term renoprotective effects of standard versus high doses of telmisartan in hypertensive nondiabetic nephropathies. American Journal of Kidney Diseases 2005;46(6):1074-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakris 2000 {published data only}
- Bakris GL, Siomos M, Bolton WK, Hebert l, Agerwall R, Catanzaro D, et al. Differential effects of valsartan (V) and lisinopril (L) on potassium [K+] homeostasis in hypertensive patients with nephropathy [abstract no: A0349]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):68A. [CENTRAL: CN-00550414] [Google Scholar]
- Bakris GL, Siomos M, Richardson D, Janssen I, Bolton WK, Hebert L, et al. ACE inhibition or angiotensin receptor blockade: impact on potassium in renal failure. VAL-K Study Group. Kidney International 2000;58(5):2084-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bianchi 1992 {published data only}
- Bianchi S, Mazzoni A, Bigazzi R, Baldari G, Campese VM. Effect of converting enzyme inhibitors (CEI) on renal function in hypertensive patients with chronic renal failure (CRF) [abstract]. Journal of the American Society of Nephrology 1992;3(3):280. [CENTRAL: CN-00602026] [Google Scholar]
Bilic 2011 {published data only}
- Bilic M, Horvatic I, Trkes V, Galesic K. Effects of ramipril and valsartan on proteinuria and renal function in patients with non-diabetic proteinuria [abstract no: SAP089]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi262. [CENTRAL: CN-00756495] [Google Scholar]
- Bilic M, Munjas-Samarin R, Ljubanovic D, Horvatic I, Galesic K. Effects of ramipril and valsartan on proteinuria and renal function in patients with nondiabetic proteinuria. Collegium Antropologicum 2011;35(4):1061-6. [MEDLINE: ] [PubMed] [Google Scholar]
Campbell 2003 {published data only}
- Campbell R, Sangalli F, Perticucci E, Aros C, Viscarra C, Perna A, et al. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney International 2003;63(3):1094-103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perticucci E, Campbell R, Perna A, Ferrari S, Cattaneo D, Ruggenenti P, et al. ACE inhibitors (ACEI), angiotensin II antagonists (ATA) or their combination: how to best renoprotect patients with non diabetic chronic nephropathies? [abstract no: A0432]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):82A. [CENTRAL: CN-00447182] [Google Scholar]
Cinotti 2001 {published data only}
- Cinotti G, Zucchelli P. Effect of lisinopril on the progression of non-diabetic chronic renal failure [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A94. [CENTRAL: CN-00261351] [DOI] [PubMed] [Google Scholar]
- Cinotti GA, Zucchelli PC, Collaborative Study Group. Effect of lisinopril on the progression of renal insufficiency in mild proteinuric non-diabetic nephropathies. Nephrology Dialysis Transplantation 2001;16(5):961-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
COOPERATE 2003 {published data only}
- Nakao N, Baba M, Seno H, Kasuga H, Toriyama T, Kawahara H, et al. Baseline b-adrenergic blockade-based differences for the effect of renin-angiotensin blockade for the treatment of non-diabetic hypertensive patients with chronic kidney failure: a COOPERATE-Carvedilol substudy [abstract no: F-PO621]. Journal of the American Society of Nephrology 2004;15(Oct):203A. [Google Scholar]
- Nakao N, Hasegawa H, Fujimori A, Fukagawa M. Baseline proteinuria level and an relative nephroprotective efficacy of combination therapy of angiotensin-converting-enzyme inhibitor and angiotensin-II receptor blocker: subgroup analysis of the COOPERATE Trial [abstract no: F-PO191]. Journal of the American Society of Nephrology 2006;17(Abstracts):377A. [Google Scholar]
- Nakao N, Seno H, Kasuga H, Toriyama T, Kawahara H, Fukagawa M. Effects of combination treatment with losartan and trandolapril on office and ambulatory blood pressures in non-diabetic renal disease: a COOPERATE-ABP substudy. American Journal of Nephrology 2004;24(5):543-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nakao N, Takada M, Nakagawa T, Sanaka T, Kayano T. Combination therapy of ace inhibitor and A-II receptor antagonist more powerfully retard progression of non-diabetic renal failure than monotherapy of each drug - a multicenter, three year, double-blind, randomized trial in Japan (COOPERATE) [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A90. [Google Scholar]
- Nakao N, Takeshi U, Yoshida A, Tadaka M, Kimura G. Is small-dose combination of angiotensin receptor blocker and angiotensin-converting enzyme inhibitor still beneficial in management of CRF?: A six-by-five factorial design prospective study [abstract no: M430]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):134-5. [CENTRAL: CN-00446902] [Google Scholar]
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial [Erratum in: Lancet. 2003 Apr 5;361(9364):1230] [Retraction in: Lancet. 2009 Oct 10;374(9697):1226; PMID: 19819378]. Lancet 2003;361(9352):117-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crowe 2003 {published data only}
- Crowe A, Howse M, Kemp G, Vinjamuir S, Williams P. The antiproteinuric effect of losartan and its relationship to systemic blood pressure and renal haemodynamics in patients with chronic renal failure [abstract no: M111]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):81. [CENTRAL: CN-00550437] [Google Scholar]
- Crowe AV, Howse M, Vinjamuri S, Kemp GJ, Williams PS. The antiproteinuric effect of losartan and its relationship to systemic blood pressure and renal haemodynamics in patients with chronic renal failure [abstract no: A2486]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):481A. [CENTRAL: CN-00550445] [Google Scholar]
- Crowe AV, Howse M, Vinjamuri S, Kemp GJ, Williams PS. The antiproteinuric effect of losartan is systemic blood pressure dependent. Nephrology Dialysis Transplantation 2003;18(10):2160-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deuber 2003b {published data only}
- Deuber HJ. Improvement of proteinuria by candesartan, eprosartan, irbesartan, losartan [abstract no: T267]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):365-6. [CENTRAL: CN-00445086] [Google Scholar]
Dyadyk 1997 {published data only}
- Dyadyk AI, Bagriy AE, Lebed IA, Yarovaya NF, Schukina EV, Taradin GG. ACE inhibitors captopril and enalapril induce regression of left ventricular hypertrophy in hypertensive patients with chronic renal failure. Nephrology Dialysis Transplantation 1997;12(5):945-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elung‐Jensen 2003 {published data only}
- Elung-Jensen T, Heisterberg J, Kamper A, Sonne J, Strandgaard S. Renoprotective effect of high or low dosage of enalapril in progressive chronic nephropathy [abstract no: T181]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):239-40. [CENTRAL: CN-00550546] [Google Scholar]
- Elung-Jensen T, Heisterberg J, Kamper AL, Sonne J, Strandgaard S. Blood pressure response to conventional and low-dose enalapril in chronic renal failure. British Journal of Clinical Pharmacology 2003;55(2):139-46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elung-Jensen T, Heisterberg J, Sonne J, Strandgaard S, Kamper AL. Enalapril dosage in progressive chronic nephropathy: a randomised, controlled trial. European Journal of Clinical Pharmacology 2005;61(2):87-96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flemming TG, Heisterberg J, Kamper AL, Sonne J, Strandgaard S. Short-term blood pressure response to conventional or low dose enalapril in chronic renal failure [abstract no: A2491]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):482A. [Google Scholar]
Erley 1994 {published data only}
- Erley CM, Komini E, Nicaeus T, Braun N, Risler T. Short versus long-acting ace-inhibitors: reduction of proteinuria and changes of renal function in patients with glomerulonephritis and mild renal insufficiency [abstract]. Journal of the American Society of Nephrology 1992;3(3):310. [CENTRAL: CN-00460706] [DOI] [PubMed] [Google Scholar]
- Erley CM, Komini E, Nicaeus T, Braun N, Wolf S, Risler T. Reduction of proteinuria and changes of renal function in patients with glomerulonephritis and mild renal insufficiency: short versus long-acting ace-inhibitors [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:85. [DOI: ] [DOI] [PubMed]
- Erley CM, Komini E, Nicaeus T, Braun N, Wolf S, Risler T. The effect of angiotensin-converting enzyme inhibitors on proteinuria in chronic glomerulonephritis [Einfluss von Angiotensin-Converting-Enzym-Hemmern auf die Proteinurie bei chronischer Glomerulonephritis]. Deutsche Medizinische Wochenschrift 1994;119(4):89-95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Erley CM, Wolf S, Komini E, Nicaeus T, Braun N, Risler T. Reduction of proteinuria and changes of renal function in patients with glomerulonephritis and mild renal insufficiency. Short versus long-acting angiotensin-converting enzyme inhibitors. Contributions to Nephrology 1994;106:68-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esnault 2005 {published data only}
- Ekhlas Eid A, Nguyen J, Delcroix C, Moutel M, Esnault VL. Effect of diuretics after dual blockade of renin-angiotensin-aldosterone system on severe proteinuria [abstract no: T022]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:434-5. [CENTRAL: CN-00509172]
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. Journal of the American Society of Nephrology 2005;16(2):474-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Esnault VL, Ekhlas A, Nguyen JM, Delcroix C, Moutel MG. Angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB) and diuretics for refractory proteinuria [abstract no: SU-PO1030]. Journal of the American Society of Nephrology 2003;14(Nov):762A. [CENTRAL: CN-00583888] [Google Scholar]
Esnault 2010 {published data only}
- Esnault VL, Ekhlas A, Nguyen J. An increase in diuretic dosage on top of ACEI and ARB dual blockade better decrease proteinuria than up titration of combined ACEI and ARB [abstract no: SA-PO1097]. Journal of the American Society of Nephrology 2006;17(Abstracts):804A. [CENTRAL: CN-00790625] [Google Scholar]
- Esnault VL, Ekhlas A, Nguyen JM, Moranne O. Diuretic uptitration with half dose combined ACEI + ARB better decreases proteinuria than combined ACEI + ARB uptitration. Nephrology Dialysis Transplantation 2010;25(7):2218-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Faulhaber 1997 {published data only}
- Faulhaber HD, Mann JF, Stein G, Jansa U. Tolerability of valsartan and effects on renal function in treated hypertensive patients with stable renal insufficiency [abstract no: A1450]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):315A. [CENTRAL: CN-00445300] [Google Scholar]
Fliser 1995 {published data only}
- Fliser D, Rett K, Hubinger A, Ritz E. Influence of ACE inhibition on glucose tolerance in patients with stable chronic renal failure. Nephrology Dialysis Transplantation 1995;10(5):643-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Fliser D, Rett K, Ritz E. Influence of ACE inhibition on glucose tolerance in patients with stable chronic renal failure [abstract]. Nephrology Dialysis Transplantation 1994;9(7):960. [CENTRAL: CN-00261009] [PubMed] [Google Scholar]
Frimodt‐Moller 2010 {published data only}
- Frimodt-Moller M, Hoj Nielson A, Strandgaard S, Kamper AL. Feasibility of combined treatment with enalapril and candesartan in advanced chronic kidney disease. Nephrology Dialysis Transplantation 2010;25(3):842-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frimodt-Moller M, Kamper A, Strandgaard S, Kreiner S, Nielsen AH. Beneficial effects on arterial stiffness and pulse-wave reflection of combined enalapril and candesartan in chronic kidney disease [abstract no: SA-PO2371]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):653A. [Google Scholar]
- Frimodt-Moller M, Kamper AL, Strandgaard S, Kreiner S, Nielsen AH. Beneficial effects on arterial stiffness and pulse-wave reflection of combined enalapril and candesartan in chronic kidney disease--a randomized trial. PLoS ONE [Electronic Resource] 2012;7(7):e41757. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimodt-Moller M, Nielsen AH, Strandgaard S, Kamper AL. Feasibility of dual blockade of the renin-angiotensin system (RAS) in chronic kidney disease (CKD) stage 3-5 [abstract no: F-PO1928]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):546A. [CENTRAL: CN-00747273] [Google Scholar]
- Lyngso C, Frimodt-Moller M, Rix M, Kamper AL, Strandgaard S, Nielsen AH, et al. Lack of association between serum osteoprotegerin, osteopontin and fetuin-a levels and the longitudinal changes in arterial stiffness in CKD patients undergoing renin-angiotensin system bloackade [abstract no: SA-PO600]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):762A. [Google Scholar]
Garg 2005 {published data only}
- Garg JP, Ellis R, Elliott WJ, Hasabou N, Chua D, Chertow GM, et al. Angiotensin receptor blockade and arterial compliance in chronic kidney disease: a pilot study. American Journal of Nephrology 2005;25(4):393-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Greenbaum 2000 {published data only}
- Greenbaum R, Zucchelli P, Caspi A, Nouriel H, Paz R, Sclarovsky S, et al. Comparison of the pharmacokinetics of fosinoprilat with enalaprilat and lisinopril in patients with congestive heart failure and chronic renal insufficiency. British Journal of Clinical Pharmacology 2000;49(1):23-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannedouche 2001 {published data only}
- Hannedouche T, Chanard J, Baumelou B, French Collaborative Telmisartan Study Group. Evaluation of the safety and efficacy of telmisartan and enalapril, with the potential addition of frusemide, in moderate-renal failure patients with mild-to-moderate hypertension. Journal of the Renin-Angiotensin-Aldosterone System 2001;2(4):246-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hemmelder 1996 {published data only}
- Hemmelder MH, Zeeuw D, Gansevoort RT, Jong PE. Blood pressure reduction initiates the antiproteinuric effect of ACE inhibition. Kidney International 1996;49(1):174-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hemmelder MH, Zeeuw D, Gansevoort RT, Jong PE. Role of systemic and renal hemodynamics in the antiproteinuric effect of acute ace-inhibition (ACEi) [abstract]. Journal of the American Society of Nephrology 1994;5(3):331. [CENTRAL: CN-00583317] [Google Scholar]
Homma 2004 {published data only}
- Homma K, Hayashi K, Kanda T, Yoshioka K, Takamatsu I, Tatematsu S, et al. Beneficial action of candesartan cilexetil plus amlodipine or ACE inhibitors in chronic nondiabetic renal disease. Journal of Human Hypertension 2004;18(12):879-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOPE 1996 {published data only}
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators [Erratum in: Lancet 2000 Sep 2;356(9232):860]. Lancet 2000;355(9200):253-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Gerstein HC, Bosch J, Pogue J, Taylor DW, Zinman B, Yusuf S. Rationale and design of a large study to evaluate the renal and cardiovascular effects of an ACE inhibitor and vitamin E in high-risk patients with diabetes. The MICRO-HOPE Study. Microalbuminuria, cardiovascular, and renal outcomes. Heart Outcomes Prevention Evaluation. Diabetes Care 1996;19(11):1225-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Pogue J, Mann JF, Lonn E, Dagenais GR, McQueen M, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 2005;48(9):1749-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerstein HC. Diabetes and the HOPE study: implications for macrovascular and microvascular disease. International Journal of Clinical Practice. Supplement 2001;(117):8-12. [MEDLINE: ] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. New England Journal of Medicine 2000;342(3):154-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. [Erratum in: 2000 May 4;342(18):1376] [Erratum in: N Engl J Med 2000 Mar 9;342(10):748]. New England Journal of Medicine 2000;342(3):145-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoogwerf BJ, Young JB. The HOPE study. Ramipril lowered cardiovascular risk, but vitamin E did not. Cleveland Clinic Journal of Medicine 2000;67(4):287-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lamy A, Yusuf S, Pogue J, Gafni A, Heart Outcomes Prevention Evaluation Investigators. Cost implications of the use of ramipril in high-risk patients based on the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 2003;107(7):960-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Gerstein HC, Pogue J, Yi Q, Hoogwerf BJ, Bosch J, et al. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk diabetic patients [abstract no: 1051-29]. Journal of the American College of Cardiology 2002;39(Suppl 2):291A. [CENTRAL: CN-00455135] [Google Scholar]
- Lonn E, Mathew J, Pogue J, Johnstone D, Danisa K, Bosch J, et al. Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high-risk patients. European Journal of Cardiovascular Prevention & Rehabilitation 2003;10(6):420-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Roccaforte R, Yi Q, Bosch J, Probstfield J, Sleight P, et al. Ramipril prevents major cardiovascular events in high-risk women: results of the HOPE trial [abstract no: 1051-28]. Journal of the American College of Cardiology 2002;39(5 Suppl 2):290-1A. [CENTRAL: CN-00455136] [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, et al. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care 2002;25(11):1919-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency (RI) as predicator of cardiovascular (CV) outcomes and impact of ramipril: the HOPE study [abstract no: A0836]. Journal of the American Society of Nephrology 2000;11(Sept):156A. [CENTRAL: CN-00671781] [Google Scholar]
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Annals of Internal Medicine 2001;134(8):629-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Yi QL, Franke J, Lonn EM, Hoogwerf BJ, et al. Progression of renal insufficiency in type 2 diabetes with and without microalbuminuria: results of the Heart Outcomes and Prevention Evaluation (HOPE) randomized study. American Journal of Kidney Diseases 2003;42(5):936-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Yi QL, Lonn EM, Hoogwerf BJ, Rashkow A, et al. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. Journal of the American Society of Nephrology 2003;14(3):641-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, et al. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Kidney International 2004;65(4):1375-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Yi QL, Sleight P, Dagenais GR, Gerstein HC, Lonn EM, et al. Serum potassium, cardiovascular risk, and effects of an ACE inhibitor: results of the HOPE study. Clinical Nephrology 2005;63(3):181-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001;104(14):1615-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McQueen MJ, Lonn E, Gerstein HC, Bosch J, Yusuf S. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scandinavian Journal of Clinical & Laboratory Investigation Supplement 2005;240:143-56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sleight P, Yusuf S, Pogue J, Tsuyuki R, Diaz R, Probstfield J, et al. Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet 2001;358(9299):2130-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smieja M, Gnarpe J, Lonn E, Gnarpe H, Olsson G, Yi Q, et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2003;107(2):251-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The HOPE (Heart Outcomes Prevention Evaluation) Study: the design of a large, simple randomized trial of an angiotensin-converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. The HOPE study investigators. Canadian Journal of Cardiology 1996;12(2):127-37. [MEDLINE: ] [PubMed] [Google Scholar]
- Veres A, Fust G, Smieja M, McQueen M, Horvath A, Yi Q, et al. Relationship of anti-60 kDa heat shock protein and anti-cholesterol antibodies to cardiovascular events. Circulation 2002;106(22):2775-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hou 2006 {published data only}
- Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. New England Journal of Medicine 2006;354(2):131-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ihle 1996 {published data only}
- Ihle B, Becker G, Whitworth J, Shahinfar S, Cnaan A, Kincaid-Smith P. The effect of angiotensin converting enzyme inhibition (ACEi) on progression of chronic renal insufficiency (CRI) [abstract]. Journal of the American Society of Nephrology 1992;3(3):284. [CENTRAL: CN-00460980] [Google Scholar]
- Ihle BU, Whitworth JA, Shahinfar S, Cnaan A, Kincaid-Smith PS, Becker GJ. Angiotensin-converting enzyme inhibition in nondiabetic progressive renal insufficiency: a controlled double-blind trial. American Journal of Kidney Diseases 1996;27(4):489-95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kamper 1990 {published data only}
- Kamper A, Strandgaard S, Leyssac PP. Late outcome of a controlled trial of enalapril treatment in progressive chronic renal failure [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):251. [CENTRAL: CN-00484580] [Google Scholar]
- Kamper A, Strandgaard S, Leyssac PP. Randomised controlled trial of enalapril in progressive chronic renal failure [abstract]. Nephrology Dialysis Transplantation 1990;5(8):672-3. [PubMed] [Google Scholar]
- Kamper A, Strandgaard S, Leyssac PP. Randomised controlled trial of enalapril in progressive chronic renal failure [abstract]. Nephrology Dialysis Transplantation 1990;5(9):829. [CENTRAL: CN-00260487] [PubMed] [Google Scholar]
- Kamper A, Strandgaard S, Leyssac PP. The validity of doubling of plasma creatinine (Pcrea) as a marker of progression of chronic renal failure [abstract no: A0375]. Journal of the American Society of Nephrology 1996;7(9):1321. [CENTRAL: CN-00626067] [Google Scholar]
- Kamper AL, Holstein-Rathlou NH, Leyssac PP, Strandgaard S. The influence of angiotensin-converting enzyme inhibition on renal tubular function in progressive chronic nephropathy. American Journal of Kidney Diseases 1996;28(6):822-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kamper AL, Nielsen OJ. Effect of enalapril on haemoglobin and serum erythropoietin in patients with chronic nephropathy. Scandinavian Journal of Clinical & Laboratory Investigation 1990;50(6):611-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kamper AL, Strandgaard S, Leyssac PP. Angiotensin I converting enzyme inhibitor enalapril in treatment of progressive chronic nephropathy. An open randomized controlled trial [Angiotensin I konverteringshaemmeren enalapril til behandling af progredierende kronisk nefropati. En aben randomiseret kontrolleret undersogelse]. Ugeskrift for Laeger 1993;155(31):2406-9. [MEDLINE: ] [PubMed] [Google Scholar]
- Kamper AL, Strandgaard S, Leyssac PP. Effect of enalapril on the progression of chronic renal failure. American Journal of Hypertension 1992;5(7):423-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kamper AL, Strandgaard S, Leyssac PP. Late outcome of a controlled trial of enalapril treatment in progressive chronic renal failure. Hard end-points and influence of proteinuria. Nephrology Dialysis Transplantation 1995;10(7):1182-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Kamper AL, Strandgaard S, Leyssac PP. Randomized controlled trial of enalapril in progressive chronic renal failure [abstract]. In: 11th International Congress of Nephrology; 1990 Jul 15-20; Tokyo, Japan. 1990:9A. [CENTRAL: CN-00583728]
Kanno 2006 {published data only}
- Kanno Y, Takenaka T, Nakamura T, Suzuki H. Add-on angiotensin receptor blocker in patients who have proteinuric chronic kidney diseases and are treated with angiotensin-converting enzyme inhibitors. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(4):730-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kanno Y, Takenaka T, Nakamura T, Suzuki H. Add-on angiotensin receptor blocker in patients with chronic kidney diseases treated with angiotensin converting enzyme inhibitors [abstract no: SA-FC104]. Journal of the American Society of Nephrology 2005;16:105A. [CENTRAL: CN-00626068] [DOI] [PubMed] [Google Scholar]
Keilani 1993 {published data only}
- Keilani T, Schlueter WA, Levin ML, Batlle DC. Improvement of lipid abnormalities associated with proteinuria using fosinopril, an angiotensin-converting enzyme inhibitor. Annals of Internal Medicine 1993;118(4):246-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schlueter W, Keilani T, Batlle DC. Metabolic effects of converting enzyme inhibitors: focus on the reduction of cholesterol and lipoprotein(a) by fosinopril. American Journal of Cardiology 1993;72(20):37-44H. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2000 {published data only}
- Kim HJ, Han SW. No more aggravation of hyperkalemia by the combined therapy of angiotensin II receptor antagonist and angiotensin converting enzyme inhibitor in renal diseases than either therapy alone [abstract no: FP.8.4]. In: 13th Asian Colloquium in Nephrology; 2000 Nov 23-25; Bali, Indonesia. 2000. [CENTRAL: CN-00615853]
Kincaid‐Smith 2002 {published data only}
- Kincaid-Smith P, Fairley K, Packham D. Comparison of the effect of 50% increase in angiotensin converting enzyme inhibitor (ACEI) with a combination of candesartan and ACEI on proteinuria and blood pressure in chronic renal disease [abstract no: A0392]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):75A. [CENTRAL: CN-00446096] [Google Scholar]
- Kincaid-Smith P, Fairley K, Packham D. Comparison of the effect of 50% increase in angiotensin converting enzyme inhibitor (ACEI) with a combination of candesartan and ACEI on proteinuria and blood pressure in chronic renal disease [abstract no: P99]. Nephrology 2002;7(Suppl 3):A25. [CENTRAL: CN-00792336] [Google Scholar]
- Kincaid-Smith P, Fairley K, Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrology Dialysis Transplantation 2002;17(4):597-601. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kincaid-Smith P, Fairley K. A fall in urine protein with combined angiotensin converting enzyme inhibitor (ACEI) and aII receptor antagonist (AIIRA) treatment predicts a good outcome at three years in patients with chronic renal disease with continuing reduction in urine protein levels. Diabetic and obese patients with a poor response to combined ACEI and AIIRA treatment have a poor outcome [abstract no: M419]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):131. [CENTRAL: CN-00446094] [Google Scholar]
- Kincaid-Smith PS, Fairley KF, Packham D. Randomised controlled crossover study of the effect on proteinuria of adding an angiotensin 11-receptor antagonist (ARA) to an angiotensin converting enzyme inhibitor (ACEi) [abstract no: A1825]. Journal of the American Society of Nephrology 2000;11(Sept):349A. [CENTRAL: CN-00626048] [Google Scholar]
- Kincaid-Smith PS, Fairley KF. A fall in urine protein with combined angiotensin converting enzyme inhibitor (ACEI) and AII receptor antagonist (AIIRA) treatment predicts a good outcome at three years in patients with chronic renal disease (mean serum creatinine level of 0.18 mmols/litre mean urinary protein level of 2.3 grams/24 hours). Diabetic and obese patients with poor response to combined ACEI and AIIRA treatment have a poor outcome [abstract no: PUB059]. Journal of the American Society of Nephrology 2003;14(Nov):786A. [Google Scholar]
Klein 2003 {published data only}
- Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. Journal of the American Society of Nephrology 2003;14(2):425-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein IH, Oey PL, Ligtenberg G, Koomans HA, Blankestijn PJ. Enalapril and losartan are equally effective in reducing sympathetic hyperactivity [abstract no: A1826]. Journal of the American Society of Nephrology 2000;11(Sept):349A. [CENTRAL: CN-00626052] [DOI] [PubMed] [Google Scholar]
Lee 2011a {published data only}
- Lee YJ, Cho S, Kim SR, Jang HR, Lee JE, Huh W, et al. Effect of losartan on proteinuria and urinary angiotensinogen excretion in non-diabetic patients with chronic kidney disease. Postgraduate Medical Journal 2011;87(1032):664-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LIRICO 2007 {published data only}
- Maione A, Nicolucci A, Craig JC, Tognoni G, Moschetta A, Palasciano G, et al. Angiotensin converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in microalbuminuric patients with one or more cardiovascular risk factors. Protocol of the Long-term Impact of RAS Inhibition on Cardiorenal Outcomes randomized trial (LIRICO) [ACE-inibizione, antagonismo recettoriale e terapia combinata in soggetti microalbuminurici con fattori di rischio cardiovascolare. Protocollo dello studio randomizzato multicentrico ''Long-term impact of RAS inhibition on cardiorenal outcomes-LIRICO"]. Giornale Italiano di Nefrologia 2007;24(5):446-56. [MEDLINE: ] [PubMed] [Google Scholar]
- Maione A, Nicolucci A, Craig JC, Tognoni G, Moschetta A, Palasciano G, et al. Protocol of the Long-term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) randomized trial. Journal of Nephrology 2007;20(6):646-55. [MEDLINE: ] [PubMed] [Google Scholar]
- Saglimbene V, Palmer SC, Ruospo M, Natale P, Maione A, Nicolucci A, et al. The Long-Term Impact of Renin-Angiotensin System (RAS) Inhibition on Cardiorenal Outcomes (LIRICO): A randomized, controlled trial. Journal of the American Society of Nephrology 2018;29(12):2890-99. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strippoli GF, Palmer S, Saglimbene VM, Ruospo M, Hegbrant JB, Craig JC. ACE inhibitor, ARB, or combined therapy in patients with diabetes and microalbuminuria: the long-term impact of RAS inhibition on cardiorenal outcomes (LIRICO) randomized clinical trial [abstract no: SA-OR115]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):103. [EMBASE: 633700766] [Google Scholar]
MacGregor 2003 {published data only}
- MacGregor MS, Deighan CJ, Rodger RS, Boulton-Jones JM. A prospective open-label randomised trial of quinapril and/or amlodipine in progressive non-diabetic renal failure. Nephron 2005;101(3):c139-49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- MacGregor MS, Deighan CJ, Rodger RS, Boulton-Jones JM. A prospective open-label randomized trial of quinapril and/or amlodipine in progressive non-diabetic renal failure [abstract no: SA-PO667]. Journal of the American Society of Nephrology 2003;14(Nov):443A. [CENTRAL: CN-00583942] [Google Scholar]
- MacGregor MS, Deighan CJ, Rodger RS, Boulton-Jones JM. Effect of ACE inhibition on hemoglobin in chronic renal failure: a randomized controlled trial [abstract no: SA-PO666]. Journal of the American Society of Nephrology 2003;14:443A. [CENTRAL: CN-00583943] [Google Scholar]
Marcantoni 1998 {published data only}
- Marcantoni C, Oldrizzi L, Rugiu C, Maschio G, AIPRI Study Group. Antihypertensive therapy and progression of renal failure in hypertensive nephropathy (HN) [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A150. [Google Scholar]
- Marcantoni C, Oldrizzi L, Rugiu C, Maschio G, AIPRI Study Group. Antihypertensive therapy and progression of renal failure in hypertensive nephropathy (HN) [abstract no: S124]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):75A. [CENTRAL: CN-00446593] [Google Scholar]
Meier 2011 {published data only}
- Meier P, Maillard M, Meier R, Burnier M. Antiproteinuric effect of losartan: is a higher dose as effective as an association with an ACE inhibitor? Results of a randomised triple-crossover study in proteinuric patients [abstract no: F-PO1920]. Journal of the American Society of Nephrology 2008;19:543A.
- Meier P, Maillard M, Tremblay S, Meier R, Burnier M. Comparative 24h blood pressure effects of increasing doses of losartan and the association of losartan and an ACE inhibitor in proteinuric patients [abstract no: SA-PO2249]. Journal of the American Society of Nephrology 2008;19:617A.
- Meier P, Maillard MP, Meier JR, Tremblay S, Gauthier T, Burnier M. Combining blockers of the renin-angiotensin system or increasing the dose of an angiotensin II receptor antagonist in proteinuric patients: a randomized triple-crossover study. Journal of Hypertension 2011;29(6):1228-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Minetti 1995 {published data only}
- Guidi EP, Minetti EE, Cozzi MG. Acute and long-term effects of 2 different ACE inhibitors on renal hemodynamics [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):619A. [CENTRAL: CN-00626080] [Google Scholar]
- Minetti EE, Cozzi MG, Guidi E. Long versus short-term effects of two different ACE inhibitors on renal haemodynamics: preliminary results from an ongoing trial. Nephrology Dialysis Transplantation 1995;10 Suppl 6:18-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitchell 1997 {published data only}
- Mitchell HC, Smith RD, Cutler RE, Sica D, Videen J, Thompsen-Bell S, et al. Racial differences in the renal response to blood pressure lowering during chronic angiotensin-converting enzyme inhibition: a prospective double-blind randomized comparison of fosinopril and lisinopril in older hypertensive patients with chronic renal insufficiency. American Journal of Kidney Diseases 1997;29(6):897-906. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NEPHROS 2001 {published data only}
- Bader BD, Risler T. The NEPHROS study - effects of an ACE inhibitor, a calcium channel blocker, or their combination on progression of renal impairment [abstract]. Deutsche Medizinische Wochenschrift 1999;124(Suppl 3):S112. [CENTRAL: CN-00382241] [Google Scholar]
- Herlitz H, Harris K, Risler T, Boner G, Bernheim J, Chanard J, et al. The effects of an ACE inhibitor and a calcium antagonist on the progression of renal disease: the Nephros Study. Nephrology Dialysis Transplantation 2001;16(11):2158-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ONTARGET 2008 {published data only}
- Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurology 2011;10(1):43-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, Clase CM, Mente A, Mann JF, Sleight P, et al. Albuminuria and rapid loss of GFR and risk of new hip and pelvic fractures. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(2):233-40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, O'Donnell M, Mann JF, Anderson C, Fagard R, et al. Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Archives of Internal Medicine 2011;171(2):142-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bohm M, Baumhakel M, Teo K, Sleight P, Probstfield J, Gao P, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation 2010;121(12):1439-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bohm M, Schumacher H, Laufs U, Sleight P, Schmieder R, Unger T, et al. Effects of nonpersistence with medication on outcomes in high-risk patients with cardiovascular disease. American Heart Journal 2013;166(2):306-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Clase CM, Barzilay J, Gao P, Smyth A, Schmieder RE, Tobe S, et al. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney International 2017;91(3):683-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cukierman-Yaffe T, Gerstein HC, Anderson C, Zhao F, Sleight P, Hilbrich L, et al. Glucose intolerance and diabetes as risk factors for cognitive impairment in people at high cardiovascular risk: results from the ONTARGET/TRANSCEND research programme. Diabetes Research & Clinical Practice 2009;83(3):387-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dans AL, Teo K, Gao P, Chen JH, Jae-Hyung K, Yusoff K, et al. In a subgroup of high-risk Asians, telmisartan was non-inferior to ramipril and better tolerated in the prevention of cardiovascular events. PLoS ONE [Electronic Resource] 2010;5(12):e13694. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan M, Mente A, Teo KK, Gao P, Sleight P, Dagenais G, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 2012;126(23):2705-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dunkler D, Dehghan M, Teo KK, Heinze G, Gao P, Kohl M, et al. Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Internal Medicine 2013;173(18):1682-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallieni M, Cozzolino M, Brancaccio D, Giovannini M. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2019. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heerspink HJ, Gao P, Zeeuw D, Clase C, Dagenais GR, Sleight P, et al. The effect of ramipril and telmisartan on serum potassium and its association with cardiovascular and renal events: results from the ONTARGET trial. European Journal of Preventive Cardiology 2014;21(3):299-309. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kappert K, Bohm M, Schmieder R, Schumacher H, Teo K, Yusuf S, et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 2012;126(8):934-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clinical Research in Cardiology 2014;103(2):149-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, Bilo G, Gao P, Fagard R, Redon J, et al. Ambulatory blood pressure values in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET). Hypertension 2012;60(6):1400-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Schumacher H, Bohm M, Mann JF, Redon J, Facchetti R, et al. Visit-to-visit blood pressure variability and renal outcomes: results from ONTARGET and TRANSCEND trials. Journal of Hypertension 2020;38(10):2050-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Schumacher H, Redon J, Verdecchia P, Schmieder R, Jennings G, et al. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET). Circulation 2011;124(16):1727-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Ryden L, et al. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. Journal of Hypertension 2013;31(2):414-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372(9638):547-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen MM, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both in people at high vascular risk: results from the ONTARGET trial [abstract no: F-FC209]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):48A. [CENTRAL: CN-00747294] [Google Scholar]
- Narayanan RM, Chi LS, Choo LL, Ying YL, Fang SC. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2020. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nonoguchi H, Nanami M, Hasuike Y, Kuragano T, Nakanishi T. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2019-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. New England Journal of Medicine 2008;358(15):1547-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Redon J, Mancia G, Sleight P, Schumacher H, Gao P, Pogue J, et al. Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial). Journal of the American College of Cardiology 2012;59(1):74-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruilope LM, Redon J, Schmieder R. Cardiovascular risk reduction by reversing endothelial dysfunction: ARBs, ACE inhibitors, or both? Expectations from the ONTARGET Trial Programme. Vascular Health & Risk Management 2007;3(1):1-9. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Schmieder RE, Mann JF, Dyal L, Pogue J, Copland I, Teo KK, et al. ONTARGET: cardiovascular outcome in patients at high cardiovascular risk and with chronic kidney disease (CKD) [abstract no: TH-PO886]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):309A. [CENTRAL: CN-00747295] [Google Scholar]
- Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. Journal of Hypertension 2009;27(7):1360-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sleight P. Clinical evidence from ONTARGET: the value of an angiotensin II receptor blocker and an angiotensin-converting enzyme inhibitor. Journal of Hypertension - Supplement 2009;27(5):S23-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. American Heart Journal 2004;148(1):52-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, et al. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011;123(10):1098-107. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Dagenais G, Healey J, Gao P, Dans AL, Chazova I, et al. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. Journal of Hypertension 2012;30(5):1004-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Sleight P, Mancia G, Fagard R, Trimarco B, Schmieder RE, et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation 2009;120(14):1380-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wetzels JF. Renal outcomes in the ONTARGET study. Lancet 2008;372(9655):2020. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yu LT, Zhu J, Tan HQ, Wang GG, Teo KK, Liu LS. Telmisartan, ramipril, or both in high-risk Chinese patients: analysis of ONTARGET China data. Chinese Medical Journal 2011;124(12):1763-8. [MEDLINE: ] [PubMed] [Google Scholar]
Papadogiannakis 2006 {published data only}
- Papadogiannakis A, Xydakis D, Sfakianaki M, Zouridakis A, Kostakis K, Korsavas K, et al. Effect of angiotensin converting enzyme inhibitors and angiotensin Type I receptor antagonists on plasminogen activator inhibitor - 1 levels in patients with hypertensive nephrosclerosis [abstract no: MP127]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv341. [Google Scholar]
Perico 1997 {published data only}
- Perico N, Spormann D, Peruzzi E, Bodin F, Sioufi A, Bertocchi F. Efficacy and tolerability of valsartan compared with lisinopril in patients with hypertension and renal insufficiency. Clinical Drug Investigation 1997;14(4):252-9. [EMBASE: 27477552] [Google Scholar]
- Perico N, Spormann D, Peruzzi E, Sioufi A, Bertocchi F, Remuzzi G. Valsartan, a new angiotensin II receptor antagonist for the treatment of hypertension: efficacy and safety compared to lisinopril in patients with stable renal insufficiency [abstract no: A1474]. Journal of the American Society of Nephrology 1997;9(Program & Abstracts):320A. [CENTRAL: CN-00447166] [Google Scholar]
Plum 1998 {published data only}
- Plum J, Bunten B, Nemeth R, Grabensee B. Effects of the angiotensin II antagonist valsartan on blood pressure, proteinuria, and renal hemodynamics in patients with chronic renal failure and hypertension. Journal of the American Society of Nephrology 1998;9(12):2223-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Plum J, Bunten B, Nemeth R, Grabensee B. Treatment with the angiotensin II antagonist valsartan in patients with chronic renal failure and hypertension. Nephrology Dialysis Transplantation 1999;14 Suppl 4:25-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plum 2000 {published data only}
- Plum J, Bockenhoff U, Bockenhoff A, Grabensee B. Combined treatment with an angiotensin II receptor antagonist and an ACE inhibitor in patients with proteinuria hypertension [abstract no: P3-8]. Deutsche Medizinische Wochenschrift 2000;125(Suppl 3):S49. [CENTRAL: CN-00383974] [Google Scholar]
Praga 2003a {published data only}
- Ghosh AK, Ghosh K, Praga M. Anti-proteinuric effect of losartan: statistical vs clinical significance (multiple letters). Nephrology Dialysis Transplantation 2004;19(3):747-8. [EMBASE: 38263144] [DOI] [PubMed] [Google Scholar]
- Praga M, Andrade CF, Arias M, Poveda R, Mora J, Prat MV, et al. Antiproteinuric effect of losartan in nondiabetic proteinuric renal diseases: a double blind, randomized clinical trial [abstract no: F-FC013]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):3A. [CENTRAL: CN-00420850] [Google Scholar]
- Praga M, Andrade CF, Luno J, Arias M, Poveda R, Mora J, et al. Antiproteinuric efficacy of losartan in comparison with amlodipine in non-diabetic proteinuric renal diseases: a double-blind, randomized clinical trial. Nephrology Dialysis Transplantation 2003;18(9):1806-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PROGRESS 1995 {published data only}
- Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. Journal of Hypertension 2006;24(6):1201-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Arima H, Tzourio C, Butcher K, Anderson C, Bousser MG, Lees KR, et al. Prior events predict cerebrovascular and coronary outcomes in the PROGRESS trial. Stroke 2006;37(6):1497-502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berthet K, Neal BC, Chalmers JP, MacMahon SW, Bousser MG, Colman SA, et al. Reductions in the risks of recurrent stroke in patients with and without diabetes: the PROGRESS Trial. Blood Pressure 2004;13(1):7-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chapman N, Huxley R, Anderson C, Bousser MG, Chalmers J, Colman S, et al. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 2004;35(1):116-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Neal B, MacMahon S. PROGRESS (perindopril protection against recurrent stroke study): rationale and design. PROGRESS Management Committee [corrected] [Erratum in: J Hypertens 1996 Apr;14(4):535]. Journal of Hypertension 1995;13(12 Pt 2):1869-73. [MEDLINE: ] [PubMed] [Google Scholar]
- Ninomiya T, Perkovic V, Gallagher M, Jardine M, Cass A, Arima H, et al. Lower blood pressure and risk of recurrent stroke in patients with chronic kidney disease: PROGRESS trial. Kidney International 2008;73(8):963-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Perkovic V, Gallagher M, Jardine M, Cass A, Neal B, et al. The efficacy of lower blood pressure goals on the prevention of stroke in chronic kidney disease: data from the PROGRESS study [abstract no: 1122]. Nephrology 2007;12(Suppl 2):A31. [Google Scholar]
- Ninomiya T, Perkovic V, Gallagher M, Jardine M, Craig J, Cass A, et al. Lower blood pressure goals and the risk of stroke in chronic kidney disease: data from the PROGRESS study [abstract no: F-FC048]. Journal of the American Society of Nephrology 2007;18(Abstracts):11A. [DOI] [PubMed] [Google Scholar]
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack [Erratum in: Lancet 2001 Nov 3;358(9292):1556] [Erratum in: Lancet 2002 Jun 15;359(9323):2120]. Lancet 2001;358(9287):1033-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perkovic V, Algert C, Arima H, Gallagher M, Cass A, Neal B, et al. Predictive ability of different measures of kidney function: data from the PROGRESS study [abstract no: TH-PO401]. Journal of the American Society of Nephrology 2006;17(Abstracts):192A. [CENTRAL: CN-00601995] [Google Scholar]
- Perkovic V, Algert C, Arima H, Gallagher M, Cass A, Neal B, et al. Reductions in the risks of vascular events and stroke in patients with and without chronic kidney disease; new data from the PROGRESS trial [abstract no: 1571]. Nephrology 2006;11(Suppl 2):A21. [CENTRAL: CN-00583826] [Google Scholar]
- Perkovic V, Algert C, Arima H, Gallagher M, Cass A, Neal B, et al. The cardiovascular effects of perindopril based therapy in CKD: data from the PROGRESS study [abstract no: SA-PO1122]. Journal of the American Society of Nephrology 2006;17(Abstracts):809A. [CENTRAL: CN-00601994] [DOI] [PubMed] [Google Scholar]
- Perkovic V, Algert C, Arima H, Gallagher M, Cass A, Neal B, et al. The predictive ability of different measures of kidney function: new data from the PROGRESS study [abstract no: 2542]. Nephrology 2006;11(Suppl 2):A46. [CENTRAL: CN-00583830] [Google Scholar]
- Perkovic V, Ninomiya T, Arima H, Gallagher M, Jardine M, Cass A, et al. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the PROGRESS study. Journal of the American Society of Nephrology 2007;18(10):2766-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PUTS 1992 {published data only}
- Bonner G, Lederle RM, Scholze J, Stumpe KO. Therapeutic safety of perindopril in the treatment of mild hypertension with concomitant nephropathy. Arzneimittel-Forschung 1993;43(8):852-5. [MEDLINE: ] [PubMed] [Google Scholar]
- Stumpe KO, Overlack A. A new trial of the efficacy, tolerability, and safety of angiotensin-converting enzyme inhibition in mild systemic hypertension with concomitant diseases and therapies. Perindopril Therapeutic Safety Study Group (PUTS). American Journal of Cardiology 1993;71(17):32-7E. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stumpe KO, Overlack A. Angiotensin-converting enzyme inhibition in mild hypertension with concomitant diseases and therapies: an efficacy, safety, and compatibility study of novel design, the Perindopril Therapeutic Safety Study. American Journal of Medicine 1992;92(4B):98-101S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ROAD 2007 {published data only}
- Hou F, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, et al. Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. Journal of the American Society of Nephrology 2007;18(6):1889-98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hou FF, Xie D, Zhang X. Renoprotection of optimal antiproteinuric doses of benazepril and losartan in chronic renal insufficiency [abstract no: SA-PO1118]. Journal of the American Society of Nephrology 2006;17(Abstracts):808A. [CENTRAL: CN-00602014] [DOI] [PubMed] [Google Scholar]
- Xie D, Hou FF, Fu BL, Zhang X, Liang M. High level of proteinuria during treatment with renin-angiotensin inhibitors is a strong predictor of renal outcome in nondiabetic kidney disease. Journal of Clinical Pharmacology 2011;51(7):1025-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruilope 2000 {published data only}
- Ruilope LM, Aldigier JC, Ponticelli C, Oddou-Stock P, Botteri F, Mann JF. Safety of the combination of valsartan (V) and benazepril (BZ) in patients with chronic renal disease (CRD) [abstract no: A0361]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):75A. [CENTRAL: CN-00447507] [Google Scholar]
- Ruilope LM, Aldigier JC, Ponticelli C, Oddou-Stock P, Botteri F, Mann JF. Safety of the combination of valsartan and benazepril in patients with chronic renal disease. European Group for the Investigation of Valsartan in Chronic Renal Disease. Journal of Hypertension 2000;18(1):89-95. [MEDLINE: ] [PubMed] [Google Scholar]
Rump 1999 {published data only}
- Rump LC, Baron A, Dendorfer A, Oberhauser V, Andre M. Angiotensin II receptor blockade in comparison to ACE inhibition in patients with chronic kidney failure [Angiotensin II-rezeptorblockade im vergleich zu ACE-hemmung bei patienten mit chronischem nierenversagen]. Journal fur Hypertonie 1999;3(4):25-33. [EMBASE: 30014539] [Google Scholar]
Rutkowski 2004 {published data only}
- Renke M, Tylicki L, Ruthkowski P, Wonjnarowski K, Lysiak-Szydlowska W, Rutkowski B. Low-dose dual blockade of the renin-angiotensin system improves tubular status in nondiabetic proteinuric patients [abstract no: MP093]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:260. [CENTRAL: CN-00509434]
- Renke M, Tylicki L, Rutkowski P, Wojnarowski K, Lysiak-Szydlowska W, Rutkowski B. Low-dose dual blockade of the renin-angiotensin system improves tubular status in non-diabetic proteinuric patients. Scandinavian Journal of Urology & Nephrology 2005;39(6):511-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rutkowski P, Tylicki L, Renke M, Korejwo G, Zdrojewski Z, Rutkowski B. Low-dose dual blockade of the renin-angiotensin system in patients with primary glomerulonephritis. American Journal of Kidney Diseases 2004;43(2):260-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SAVE 1991 {published data only}
- Hager WD, Davis BR, Riba A, Moye LA, Wun CC, Rouleau JL, et al. Absence of a deleterious effect of calcium channel blockers in patients with left ventricular dysfunction after myocardial infarction: The SAVE Study Experience. SAVE Investigators. Survival and Ventricular Enlargement. American Heart Journal 1998;135(3):406-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jose P, Skali H, Anavekar N, Tomson C, Krumholz HM, Rouleau JL, et al. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. Journal of the American Society of Nephrology 2006;17(10):2886-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lamas GA, Pfeffer MA, Hamm P, Wertheimer J, Rouleau JL, Braunwald E. Do the results of randomized clinical trials of cardiovascular drugs influence medical practice? The SAVE Investigators. New England Journal of Medicine 1992;327(4):241-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation 1997;96(12):4254-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Moye LA, Pfeffer MA, Braunwald E. Rationale, design and baseline characteristics of the survival and ventricular enlargement trial. SAVE Investigators. American Journal of Cardiology 1991;68(14):70-9D. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. New England Journal of Medicine 1992;327(10):669-77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Moye LA, Braunwald E, Basta L, Brown EJ, Cuddy TE, et al. Selection bias in the use of thrombolytic therapy in acute myocardial infarction. The SAVE Investigators. JAMA 1991;266(4):528-32. [MEDLINE: ] [PubMed] [Google Scholar]
- Rouleau JL, Moye LA, Pfeffer MA, Arnold JM, Bernstein V, Cuddy TE, et al. A comparison of management patterns after acute myocardial infarction in Canada and the United States. The SAVE investigators. New England Journal of Medicine 1993;328(11):779-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rutherford JD, Pfeffer MA, Moye LA, Davis BR, Flaker GC, Kowey PR, et al. Effects of captopril on ischemic events after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. SAVE Investigators. Circulation 1994;90(4):1731-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tokmakova MP, Skali H, Kenchaiah S, Braunwald E, Rouleau JL, Packer M, et al. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation 2004;110(24):3667-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Urena PE, Lamas GA, Mitchell G, Flaker GC, Smith SC, Wackers FJ, et al. Ejection fraction by radionuclide ventriculography and contrast left ventriculogram. A tale of two techniques. SAVE Investigators. Survival and Ventricular Enlargement. Journal of the American College of Cardiology 1999;33(1):180-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vantrimpont P, Rouleau JL, Wun CC, Ciampi A, Klein M, Sussex B, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. Journal of the American College of Cardiology 1997;29(2):229-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Scaglione 2005 {published data only}
- Scaglione R, Argano C, Corrao S, Di Chiara T, Licata A, Licata G. Transforming growth factor beta1 and additional renoprotective effect of combination ACE inhibitor and angiotensin II receptor blocker in hypertensive subjects with minor renal abnormalities: a 24-week randomized controlled trial. Journal of Hypertension 2005;23(3):657-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmieder 2005 {published data only}
- Fleischmann EH, Klingbeil AU, Schneider MP, Delles C, Schmieder RE. The effect of treatment with ultra high dose candesartan (64 mg) on proteinuria: interim results of a double-blind, randomized, multi-center study [abstract no: P1782]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):133-4. [CENTRAL: CN-00445352] [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Delles C, Veelken R. Additive effect of ultra high dose (64 mg) candesartan on proteinuria: a double blind randomized study [abstract no: W-PO20003]. Nephrology 2005;10(Suppl 1):A244. [CENTRAL: CN-00602110] [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Delles C, Veelken R. Dose-response effect of angiotensin receptor blocker candesartan on proteinuria: a double blind, randomized study [abstract no: SP149]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v69. [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Veelken R, Delles C. Additional antiproteinuric effect of ultra high dose (64mg) candesartan: a double blind, randomized study. [abstract no: F-P0243]. Journal of the American Society of Nephrology 2004;15(Oct):119A. [CENTRAL: CN-00602112] [DOI] [PubMed] [Google Scholar]
- Schmieder RE, Klingbeil AU, Fleischmann EH, Veelken R, Delles C. Additional antiproteinuric effect of ultrahigh dose candesartan: a double-blind, randomized, prospective study. Journal of the American Society of Nephrology 2005;16(10):3038-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1999 {published data only}
- Schulz E, Bech JN, Pedersen EB, Muller GA. A randomized, double-blind, parallel study of the safety and antihypertensive efficacy of losartan compared to captopril in patients with mild to moderate hypertension and impaired renal function [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A69. [CENTRAL: CN-00384185] [DOI] [PubMed] [Google Scholar]
- Schulz E, Bech JN, Pedersen EB, Muller GA. A randomized, double-blind, parallel study on the safety and antihypertensive efficacy of losartan compared to captopril in patients with mild to moderate hypertension and impaired renal function. Nephrology Dialysis Transplantation 1999;14 Suppl 4:27-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schulz E, Bech JN, Pederson EB, Muller GA. A randomized, double-blind, parallel study of the safety and antihypertensive efficacy of losartan compared to captopril in patients with mild to moderate hypertension and impaired renal function [abstract]. Deutsche Medizinische Wochenschrift 1999;124(Suppl 3):S107. [DOI] [PubMed] [Google Scholar]
Shand 2000 {published data only}
- Shand B, Lynn K, Robson R. Comparative study of losartan and enalapril on haemorrheology in patients with renal disease and hypertension [abstract]. New Zealand Medical Journal 1998;111(1067 Suppl):165. [CENTRAL: CN-00319474] [Google Scholar]
- Shand BI, Bailey RR, Lynn KL, Smith AH, Robson RA. Comparative study between losartan and enalapril on renal function, blood pressure and haematology in patients with renal disease and hypertension [abstract no: P1782]. Nephrology 1997;3(Suppl 1):S517. [CENTRAL: CN-00461715] [Google Scholar]
- Shand BI, McGregor DO, Agarwal M, Lynn KL, Lewis J, Elder PA. Comparative study of the effects of enalapril and losartan on glomerular size selectivity in patients with proteinuria [abstract no: 22] [Comparative study of the effects of enalapril and losartan on glomerular size selectivity in patients with proteinuria [abstract no: 22]]. Nephrology 2000;5(1-2 Suppl):A8. [Google Scholar]
- Shand BI. Haemorheological effects of losartan and enalapril in patients with renal parenchymal disease and hypertension. Journal of Human Hypertension 2000;14(5):305-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shoda 2006 {published data only}
- Shoda J, Kanno Y, Suzuki H. A five-year comparison of the renal protective effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with non-diabetic nephropathy. Internal Medicine 2006;45(4):193-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shoda J, Kanno Y, Suzuki H. Angiotensin converting enzyme inhibitor (ACE) versus angiotensin receptor blocker (ARB): a five-year comparison of renal survival and effects on blood pressure, creatinine clearance, urinary protein excretion [abstract no: F-PO293]. Journal of the American Society of Nephrology 2004;15(Oct):130a. [Google Scholar]
SMART 2009 {published data only}
- Burgess E, Muirhead N, Rene de Cotret P, Chiu A, Pichette V, Tobe S, et al. Supramaximal dose of candesartan in proteinuric renal disease. Journal of the American Society of Nephrology 2009;20(4):893-900. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess ED, Muirhead N, Rene de Cotret P. A double-blind randomized controlled trial of high dose candesartan cilexetil in proteinuric renal disease - results from SMART (Supra Maximal Atacand Renal Trial) [abstract no: SA-FC104]. Journal of the American Society of Nephrology 2007;18(Abstracts):57A. [Google Scholar]
- Muirhead N, Burgess E, Rene de Cotret P, SMART Investigators. A randomised controlled trial of high dose candesartan in the treatment of proteinuric renal disease: design and baseline characteristics [abstract no: SA-PO261]. Journal of the American Society of Nephrology 2004;15(Oct):357A-8A. [CENTRAL: CN-00550711] [Google Scholar]
Takagi 1990 {published data only}
- Takagi N, Tokita Y, Takeda K, Ueda S, Abe Y, Yabana M, et al. Effect of long-term administration of angiotensin converting enzyme inhibitors on renal function in hypertensive patients associated with renal failure [abstract]. In: 11th International Congress of Nephrology; 1990 Jul 15-20; Tokyo, Japan. 1990:321A. [CENTRAL: CN-00583223]
Toto 1996 {published data only}
- Smith R, Fenves A, Mulcahy W, Toto R. Rampril reduces proteinuria in normotensive non-diabetic renal disease: a prospective double-blind randomized trial [abstract]. Journal of the American Society of Nephrology 1994;5(3):341. [Google Scholar]
- Toto RD, Adams-Huet B, Fenves AZ, Mitchell HC, Mulcahy W, Smith RD. Effect of ramipril on blood pressure and protein excretion rate in normotensive nondiabetic patients with proteinuria. American Journal of Kidney Diseases 1996;28(6):832-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TRANSCEND 2009 {published data only}
- Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurology 2011;10(1):43-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, Clase CM, Mente A, Mann JF, Sleight P, et al. Albuminuria and rapid loss of GFR and risk of new hip and pelvic fractures. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(2):233-40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, O'Donnell M, Mann JF, Anderson C, Fagard R, et al. Albuminuria and decline in cognitive function: the ONTARGET/TRANSCEND studies. Archives of Internal Medicine 2011;171(2):142-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Gao P, Ryden L, Schumacher H, Probstfield J, Commerford P, et al. Effects of telmisartan on glucose levels in people at high risk for cardiovascular disease but free from diabetes: the TRANSCEND study. Diabetes Care 2011;34(9):1902-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm M, Baumhakel M, Teo K, Sleight P, Probstfield J, Gao P, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation 2010;121(12):1439-46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Clase CM, Barzilay J, Gao P, Smyth A, Schmieder RE, Tobe S, et al. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney International 2017;91(3):683-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cukierman-Yaffe T, Gerstein HC, Anderson C, Zhao F, Sleight P, Hilbrich L, et al. Glucose intolerance and diabetes as risk factors for cognitive impairment in people at high cardiovascular risk: results from the ONTARGET/TRANSCEND research programme. Diabetes Research & Clinical Practice 2009;83(3):387-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dans AL, Teo K, Gao P, Chen JH, Jae-Hyung K, Yusoff K, et al. In a subgroup of high-risk Asians, telmisartan was non-inferior to ramipril and better tolerated in the prevention of cardiovascular events. PLoS ONE [Electronic Resource] 2010;5(12):e13694. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan M, Mente A, Teo KK, Gao P, Sleight P, Dagenais G, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 2012;126(23):2705-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation 2007;115(11):1371-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kappert K, Bohm M, Schmieder R, Schumacher H, Teo K, Yusuf S, et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 2012;126(8):934-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mancia G, Schumacher H, Bohm M, Mann JF, Redon J, Facchetti R, et al. Visit-to-visit blood pressure variability and renal outcomes: results from ONTARGET and TRANSCEND trials. Journal of Hypertension 2020;38(10):2050-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann J, Schmieder R, Dyal L, Schumacher H, Dagenais G, Teo K, et al. Renal outcomes with telmisartan in 5926 people at high vascular risk, results of the TRANSCEND study, a randomised placebo controlled trial [abstract no: SA777]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Mann JF, Schmieder RE, Dyal L, McQueen MJ, Schumacher H, Pogue J, et al. Effect of telmisartan on renal outcomes: a randomized trial [summary for patients in Ann Intern Med. 2009 Jul 7;151(1):I28; PMID: 19451555]. Annals of Internal Medicine 2009;151(1):1-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shiffman D, Pare G, Louie JZ, Rowland CM, Devlin JJ, McQueen M. A gene variant in the CERS2 gene is associated with worsening albuminuria in diabetic patients of ONTARGET and TRANSCEND [abstract no: SA-PO351]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):707A. [Google Scholar]
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators, Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial [Erratum in: Lancet. 2008 Oct 18;372(9647):1384]. Lancet 2008;372(9644):1174-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. American Heart Journal 2004;148(1):52-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, et al. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011;123(10):1098-107. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Dagenais G, Healey J, Gao P, Dans AL, Chazova I, et al. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. Journal of Hypertension 2012;30(5):1004-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Sleight P, Mancia G, Fagard R, Trimarco B, Schmieder RE, et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation 2009;120(14):1380-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tylicki 2007 {published data only}
- Larczynski W, Tylicki L, Rutkowski P, Renke M, Rutkowski B. The triple renin-angiotensin-aldosterone system blockade - new promising nephroprotective strategy in patients with proteinuric nondiabetic renal disease [abstract no: SA-PO81]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi259. [CENTRAL: CN-00653742] [Google Scholar]
- Tylicki L, Rutkowski P, Renke M, Larczynski W, Aleksandrowicz E, Lysiak-Szydlowska W, et al. Triple pharmacological blockade of the renin-angiotensin-aldosterone system in nondiabetic CKD: an open-label crossover randomized controlled trial. American Journal of Kidney Diseases 2008;52(3):486-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tylicki L, Rutkowski P, Renke M, Rutkowski B. Addition of aldosterone receptor blocker to dual renin-angiotensin-aldosterone blockade leads to limitation of tubulointerstitial injury of kidney. Kidney International 2007;72(9):1164-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tylicki 2008a {published data only}
- Larczynski W, Tylicki L, Renke M, Rutkowski P, Rutkowski B. The influence of very high dosage of cilazapril on proteinuria during dual renin-angiotensin-aldosteron system blockade in patients with proteinuric nondiabetic renal disease [abstract no: MP106]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv334. [CENTRAL: CN-00615893] [Google Scholar]
- Renke M, Tylicki L, Knap N, Rutkowski P, Neuwelt A, Petranyuk A, et al. High-dose angiotensin-converting enzyme inhibitor attenuates oxidative stress in patients with chronic kidney disease. Nephrology Dialysis Transplantation 2009;24(2):689-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tylicki L, Renke M, Rutkowski P, Larczynski W, Aleksandrowicz E, Lysiak-Szydlowska W, et al. Dual blockade of the renin-angiotensin-aldosterone system with high-dose angiotensin-converting enzyme inhibitor for nephroprotection: an open, controlled, randomized study. Scandinavian Journal of Urology & Nephrology 2008;42(4):381-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VALERIA 2008 {published data only}
- Menne J, Farsang C, Deak L, Klebs S, Meier M, Handrock R, et al. Valsartan in combination with lisinopril versus the respective high dose monotherapies in hypertensive patients with microalbuminuria: the VALERIA trial. Journal of Hypertension 2008;26(9):1860-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van der Wouden 2009 {published data only}
- Van Der Wouden EA, Vogt L, Henning RH, Navis GJ, Zeeuw D, Hemmelder MH. Changing the trandolapril dosage regimen does not affect the proteinuria-lowering effect of ACE inhibition in non-diabetic renal disease [Wijziging doseerregime trandolapril heeft geen effect op proteinurieverlagend effect van ACE-remming bij niet-diabetische nierziekte]. Pharmaceutisch Weekblad 2009;144(38):156-9. [EMBASE: 355415651] [Google Scholar]
- Vogt L, Van Der Wouden EA, Navis GJ, Zeeuw D, Hemmelder MH. Optimal dosing time for the long-acting ace inhibitor trandolapril in non-diabetic kidney disease. Journal of Hypertension 2010;28(Suppl A):e387. [EMBASE: 70215411] [Google Scholar]
- Wouden EA, Vogt L, Navis GJ, Zeeuw D, Hemmelder MH. Altered dosing of trandolapril does not overcome nocturnal resistance to the antiproteinuric effect of ACE inhibition in non-diabetic renal disease [abstract no: SA-PO1036]. Journal of the American Society of Nephrology 2005;16:783A. [CENTRAL: CN-00790458] [Google Scholar]
Vogt 2008 {published data only}
- Gant CM, Laverman GD, Vogt L, Heerspink HJL, Hemmelder MH, Navis G, et al. Lower renal function is associated with higher aldosterone and high blood pressure in chronic kidney disease, irrespective of RAAS inhibition [abstract no: FR-PO726]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):532A. [Google Scholar]
- Gant CM, Laverman GD, Vogt L, Slagman MC, Heerspink HJ, Waanders F, et al. Renoprotective RAAS inhibition does not affect the association between worse renal function and higher plasma aldosterone levels. BMC Nephrology 2017;18(1):370. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikken JA, Waanders F, Dallinga-Thie GM, Dikkeschei LD, Vogt L, Navis GJ, et al. Antiproteinuric therapy decreases LDL-cholesterol as well as HDL-cholesterol in non-diabetic proteinuric patients: relationships with cholesteryl ester transfer protein mass and adiponectin. Expert Opinion on Therapeutic Targets 2009;13(5):497-504. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mahmoodi BK, Mulder AB, Waanders F, Spronk HM, Mulder R, Slagman MC, et al. The impact of antiproteinuric therapy on the prothrombotic state in patients with overt proteinuria. Journal of Thrombosis & Haemostasis 2011;9(12):2416-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Slagman MC, Kwakernaak AJ, Yazdani S, Laverman GD, den Born J, Titze J, et al. Vascular endothelial growth factor C levels are modulated by dietary salt intake in proteinuric chronic kidney disease patients and in healthy subjects. Nephrology Dialysis Transplantation 2012;27(3):978-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Slagman MC, Nguyen TQ, Waanders F, Vogt L, Hemmelder MH, Laverman GD, et al. Effects of antiproteinuric intervention on elevated connective tissue growth factor (CTGF/CCN-2) plasma and urine levels in nondiabetic nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(8):1845-50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagman MC, Sinkeler SJ, Hemmelder MH, Waanders F, Vogt L, Kluin-Nelemans HC, et al. Erythropoietin is reduced by combination of diuretic therapy and RAAS blockade in proteinuric renal patients with preserved renal function. Nephrology Dialysis Transplantation 2010;25(10):3256-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Slagman MC, Waanders F, Vogt L, Damman K, Hemmelder M, Navis G, et al. Elevated N-terminal pro-brain natriuretic peptide levels predict an enhanced anti-hypertensive and anti-proteinuric benefit of dietary sodium restriction and diuretics, but not angiotensin receptor blockade, in proteinuric renal patients. Nephrology Dialysis Transplantation 2012;27(3):983-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vogt L, Waanders F, Boomsma F, Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. Journal of the American Society of Nephrology 2008;19(5):999-1007. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt L, Zeeuw D, Waanders F, Navis G. Independent and added effects of low sodium and diuretic on renoprotective effect of AII antagonist in non-diabetic proteinuric patients [abstract no: TH-FC171]. Journal of the American Society of Nephrology 2005;16:37A. [CENTRAL: CN-00644129] [Google Scholar]
- Waanders F, Vaidya VS, Goor H, Leuvenink H, Damman K, Hamming I, et al. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. American Journal of Kidney Diseases 2009;53(1):16-25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waanders F, Vogt L, Zeeuw D, Navis G. Specific antiproteinuric benefit of sodium depletion in non-diabetic patients resistant to RAS-blockade [abstract no: SA-PO922]. Journal of the American Society of Nephrology 2007;18(Abstracts):546A. [Google Scholar]
- Wouda R, Waanders F, De Zeeuw D, Navis G, Vogt L. Efficacy of the angiotensin receptor blocker losartan during high potassium intake in CKD patients [abstract no: FP158]. Nephrology Dialysis Transplantation 2019;34(Suppl 1):A96-7. [EMBASE: 631306750] [Google Scholar]
- Wouda RD, Waanders F, De Zeeuw D, Navis G, Vogt L. Diminished efficacy of the angiotensin receptor blocker losartan during high potassium intake in CKD patients [abstract no: TH-OR056]. Journal of the American Society of Nephrology 2019;30(Abstract Suppl):15. [EMBASE: 633767617] [Google Scholar]
- Wouda RD, Waanders F, Zeeuw D, Navis G, Vogt L, K+ Consortium. Diminished antiproteinuric effect of the angiotensin receptor blocker losartan during high potassium intake in patients with CKD. Clinical Kidney Journal 2021;14(10):2170-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013a {published data only}
- Wang C, Zhang J, Liu X, Li CC, Ye ZC, Peng H, et al. Effect of valsartan with bedtime dosing on chronic kidney disease patients with nondipping blood pressure pattern. Journal of Clinical Hypertension 2013;15(1):48-54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yanagi 2013 {published data only}
- Yanagi M, Tamura K, Fujikawa T, Wakui H, Kanaoka T, Ohsawa M, et al. The angiotensin II type 1 receptor blocker olmesartan preferentially improves nocturnal hypertension and proteinuria in chronic kidney disease. Hypertension Research - Clinical & Experimental 2013;36(3):262-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi M, Toya Y, Fujikawa T, Kobori H, Umemura S. Beneficial effects of ARB olmesartan on ambulatory blood pressure profile and renal function in hypertensive patients with CKD [abstract no: F-PO1694]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):501A. [Google Scholar]
Yasuda 2013 {published data only}
- Uehara K, Yasuda T, Shibagaki Y, Kimura K. Estimated glomerular filtration rate variability independently predicts renal prognosis in advanced chronic kidney disease patients. Nephron 2015;130(4):256-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yasuda T, Endoh M, Suzuki D, Yoshimura A, Ideura T, Tamura K, et al. Effects of valsartan on progression of kidney disease in Japanese hypertensive patients with advanced, predialysis, chronic kidney disease: Kanagawa Valsartan Trial (KVT). Hypertension Research - Clinical & Experimental 2013;36(3):240-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yildiz 1999 {published data only}
- Yildiz A, Azamir M, Erkoc R, Kayacan SM, Tukek T, Gorcin B, et al. The comparison of enalapril and losartan treatment in patients with moderate chronic renal failure [abstract]. In: 36th Congress. European Renal Association. European Dialysis and Transplantation Association; 1999 Sept 5-8; Madrid, Spain. 1999:162. [CENTRAL: CN-00486535]
Yilmaz 2007a {published data only}
- Yilmaz MI, Saglam M, Sonmez A, Caglar K, Cakir E, Kurt Y, et al. Improving proteinuria, endothelial functions and asymmetric dimethylarginine levels in chronic kidney disease: ramipril versus valsartan. Blood Purification 2007;25(4):327-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT04736329 {published data only}
- Telmisartan versus enalapril in heart failure with reduced ejection fraction patients with moderately impaired kidney functions. www.clinicaltrials.gov/show/NCT04736329 2021. [DOI] [PMC free article] [PubMed]
NCT05402397 {published data only}
- Losartan and uric acid metabolism in children with proteinuric nephropathies. www.clinicaltrials.gov/show/NCT05402397 2022.
Osipova 2021 {published data only}
- Osipova OA, Gosteva EV, Belousova ON, Golivets TP, Chefranova J, Lykov Y et al. Effect of angiotensin II receptor blocker therapy on markers of fibrosis and immune inflammation in hypertensive patients with chronic kidney disease after ischemic stroke. Cardiovascular Therapy & Prevention (Russian Federation) 2021;20(7):121‐8. [DOI: 10.15829/1728-8800-2021-3078] [DOI] [Google Scholar]
Segura 2003 {published data only}
- Segna J, Camp C, Praga M, Oddou P, Mann J, Ruilope LM. Relevance of BP control and angiotensin II blockade in controlling proteinuria [abstract no: A0417]. Journal of the American Society of Nephrology 2000;11(Sept):77a. [Google Scholar]
- Segura J, Praga M, Campo C, Rodicio JL, Ruilope LM. Combination is better than monotherapy with ACE inhibitor or angiotensin receptor antagonist at recommended doses. Journal of the Renin-Angiotensin-Aldosterone System 2003;4(1):43-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shoji 2001 {published data only}
- Shoji T, Suzuki A, Takabatake Y, Oka K, Togawa M, Okada N, Imai E, Tsubakishara Y. The impact of the combination of losartan and enalapril on erythropoiesis in Japanese patients with chronic glomerulonephritis (cgn) [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):411a. [Google Scholar]
- Shoji T, Tomida K, Furumatsu Y, Kaneko T, Togawa M, Okada N, Imai E, Tsubakihar Y. The long term influence of the combination of losartan and enalapril on erythropoiesis in Japanese patients with chronic glomerulonephritis. [abstract no: SU-PO1080]. Journal of the American Society of Nephrology 2003;14(Nov):773a. [Google Scholar]
Suzuki 2003 {published data only}
- Suzuki H, Saitama Candesartan Study Group. Effects of candesartan on renal and cardiovascular outcomes in hypertensive patients with renal impairment [abstract no: SU-PO633]. Journal of the American Society of Nephrology 2003;14(Nov):672-3a. [Google Scholar]
TCTR20220426002 {published data only}
- Comparison of efficacy in renoprotection between azilsartan medoxomil and enalapril: a randomized, open-labeled controlled trial. www.trialsearch.who.int/Trial2.aspx?TrialID=TCTR20220426002 2022.
Additional references
ASCOT‐BPLA Study 2005
- Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366(9489):895-906. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CDC 2007
- Centers for Disease Control and Prevention (CDC). Prevalence of chronic kidney disease and associated risk factors - United States, 1999-2004. MMWR - Morbidity & Mortality Weekly Report 2007;56(8):161-5. [MEDLINE: ] [PubMed] [Google Scholar]
CHOICE Study 2001
- Astor BC, Eustace JA, Powe NR, Klag MJ, Sadler JH, Fink NE, et al. The timing of nephrologist referral and arteriovenous access use: the CHOICE study. American Journal of Kidney Diseases 2001;38(3):494-501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coresh 2005
- Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. Journal of the American Society of Nephrology 2005;16(1):180-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Daly 2007
- Daly C. Is early chronic kidney disease an important risk factor for cardiovascular disease? A background paper prepared for the UK Consensus Conference on early chronic kidney disease. Nephrology Dialysis Transplantation 2007;22 Suppl 9:ix19-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dogan 2005
- Dogan E, Erkoc R, Sayarlioglu H, Durmus A, Topal C. Effects of late referral to a nephrologist in patients with chronic renal failure. Nephrology 2005;10(5):516-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Drey 2003
- Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. American Journal of Kidney Diseases 2003;42(4):677-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giatras 1997a
- Giatras I, Lau J, Levey AS. Effect of angiotensin converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Annals of Internal Medicine 1997;127(5):337-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2011b
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2011c
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hallan 2006
- Hallan SI, Dahl K, Oien CM, Grootendorst DC, Aasberg A, Holmen J, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ 2006;333(7577):1047. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
HOPE Study 1996
- Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients [Erratum in: 2000 May 4;342(18):1376] [Erratum in: N Engl J Med 2000 Mar 9;342(10):748]. New England Journal of Medicine 2000;342(3):145-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Imai 2007
- Imai E, Horio M, Iseki K, Yamagata K, Watanabe T, Hara S, et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clinical & Experimental Nephrology 2007;11(2):156-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jafar 2001
- Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Annals of Internal Medicine 2001;135(2):73-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jafar 2003a
- Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Jong PE, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria and angiotensin-converting enzyme inhibition: proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Annals of Internal Medicine 2003;139(4):244-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
John 2004
- John R, Webb M, Young A, Stevens PE. Unreferred chronic kidney disease: a longitudinal study. American Journal of Kidney Diseases 2004;43(5):825-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khan 2007
- Khan S, Amedia CA Jr. Economic burden of chronic kidney disease. Journal of Evaluation in Clinical Practice 2007;14(3):422-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kinchen 2002
- Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Annals of Internal Medicine 2002;137(6):479-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kshirsagar 2000a
- Kshirsagar AV, Joy MS, Hogan SL, Falk RJ, Colindres RE. Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: a systematic overview of randomized placebo-controlled trials. American Journal of Kidney Diseases 2000;35(4):695-707. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumar 2002
- Kumar PJ, Clark ML. Kumar & Clark clinical medicine. 5th edition. Edinburgh: WB Saunders, 2002. [ISBN: 0702025798] [Google Scholar]
Levey 2003
- Levey AS, Coresh J, Balk M, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Annals of Internal Medicine 2003;139(2):137-47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lindholm 2002
- Lindholm L, Ibsen H, Dahlof B, Devereux RB, Beevers G, Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359(9311):1004-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lithell 2003
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. Journal of Hypertension 2003;21(5):875-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacMahon 2000
- MacMahon S, Sharpe N, Gamble G, Clague A, Mhurchu CN, Clark T, et al. Randomized, placebo-controlled trial of the angiotensin converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. PART-2 Collaborative Research Group. Prevention of Atherosclerosis with Ramipril. Journal of the American College of Cardiology 2000;36(2):438-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Metcalfe 2007
- Metcalfe W. How does early chronic kidney disease progress? A background paper prepared for the UK Consensus Conference on early chronic kidney disease. Nephrology Dialysis Transplantation 2007;22 Suppl 9:ix26-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NICE Guideline 2008
- National Institute for Health and Clinical Excellence. Early identification and management of chronic kidney disease in adults in primary and secondary care. 2008. http://guidance.nice.org.uk/CG73 (accessed 17 January 2022). [PubMed]
PROGRESS Collaborative Group 2001
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358(9287):1033-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruster 2006
- Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. Journal of the American Society of Nephrology 2006;17(11):2985-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SCAT Study 2000
- Teo KK, Burton JR, Buller CE, Plante S, Catellier D, Tymchak W, et al. Long-term effects of cholesterol lowering and angiotensin-converting enzyme inhibition on coronary atherosclerosis: the Simvastatin/Enalapril Coronary Atherosclerosis Trial (SCAT). Circulation 2000;102(15):1748-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sharma 2010
- Sharma P, McCullough K, Scotland G, McNamee P, Prescott G, MacLeod A, et al. Does stage-3 chronic kidney disease matter? A systematic literature review. British Journal of General Practice 2010;60(575):e266-76. [EMBASE: 360210152] [Google Scholar]
SIGN Guideline 2008
- Scottish Intercollegiate Guidelines Network. Diagnosis and management of chronic kidney disease. 2008. www.sign.ac.uk/guidelines/fulltext/103/index.html (last accessed August 2010).
SONG 2017
- SONG. The SONG Handbook Version 1.0, 2017. songinitiative.org/reports-and-publications/ (accessed 17 January 2022).
Stevens 2013
- Stevens PE, Levin A, Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of Internal Medicine 2013;158(11):825-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strippoli 2006
- Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD006257. [DOI: 10.1002/14651858.CD006257] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2016
- Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. American Journal of Kidney Diseases 2016;67(5):728-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Blackburn 2009
- Blackburn RC, Parke CL, Sharma P, McCullough K, Black C. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1‐3) non‐diabetic chronic kidney disease. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007751. [DOI: 10.1002/14651858.CD007751] [DOI] [PubMed] [Google Scholar]
Sharma 2011
- Sharma P, Blackburn RC, Parke CL, McCullough K, Marks A, Black C. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD007751. [DOI: 10.1002/14651858.CD007751.pub2] [DOI] [PubMed] [Google Scholar]


