Abstract
Adrenocortical carcinoma (ACC) is a rare malignancy with limited data to guide the management of metastatic disease. The optimal treatment strategies and outcomes of patients with metastatic ACC remain areas of active interest. We retrospectively reviewed patients with ACC who were treated with systemic therapy between January 1997 and October 2016 at The Ohio State University Comprehensive Cancer Center. Kaplan-Meier and Cox proportional hazards regression models were used for survival analysis. We identified 65 patients diagnosed with ACC during the given time period, and 36 patients received systemic therapy for distant metastatic disease. Median age at diagnosis was 50 (range 28–87). Median overall survival (OS) from time of diagnosis of ACC was 27 months (95% CI 19.6–39.3), and median OS from time of systemic treatment for metastatic disease was 18.7 months (95% CI 9.3–26.0). Clinical characteristics at time of initiation of systemic therapy were assessed, and presence of bone metastases (p = 0.66), ascites (p = 0.19), lung metastases (p = 0.12), liver metastases (p = 0.47), as well as hormonal activity of tumor (p = 0.19), were not prognostic for survival. Six patients with liver metastases treated with systemic therapy who received liver-directed therapy with either transarterial chemoembolization (TACE) or selective internal radiation therapy (SIRT) had longer survival than those who did not (p = 0.011). Our data expands the knowledge of clinical characteristics and outcomes of patients with ACC and suggests a possible role for incorporating liver-directed therapies for patients with hepatic metastases.
Electronic supplementary material
The online version of this article (10.1007/s12672-019-00367-0) contains supplementary material, which is available to authorized users.
Keywords: Adrenal cortical carcinoma (ACC), Chemotherapy, Trans-arterial chemoembolization (TACE), Selective internal radiation therapy (SIRT)
Introduction
Adrenal carcinoma (ACC) is a rare tumor with an incidence reported to be approximately 0.72 per million cases per year, accounting for 0.2% of all cancer deaths in the USA [1, 2]. Although ACC can develop at any age, there appears to be a bimodal age distribution [3, 4], with disease peaks before the age of five and in the fourth to fifth decade of life [5]. In general, the level of aggressiveness and pace of disease progression are more rapid in adults than in children. Although most cases of adult ACC appear to be sporadic, some are associated with hereditary cancer syndromes such as Li-Fraumeni syndrome associated with inactivating mutations of TP53 on chromosome 17p, Beckwith-Wiedemann syndrome associated with abnormalities on chromosome 11p15, and Multiple Endocrine Neoplasia type 1 (MEN1) associated with inactivating mutations of the MEN1 gene on chromosome 11. ACC may be functional, causing Cushing syndrome and/or virilization, or nonfunctional, presenting as an abdominal mass or as an incidental finding. Clinical studies support surgical resection as the mainstay of treatment, either with curative or palliative intention [6]. While adjuvant treatment with oral mitotane appears to improve recurrence-free survival in select patients after curative resection [7, 8], the benefit of adjuvant radiation remains controversial [9–12]. However, two large recent studies have suggested that select patients with resected ACC may benefit from adjuvant radiation including patients with positive margins [13, 14]. Identifying patients at high risk for disease recurrence after curative surgery is an area of active research, with one of the largest studies conducted in ACC showing that Ki-67% is an independent prognostic factor for recurrence [15]. Systemic chemotherapy is generally reserved for advanced unresectable tumors. Cisplatin and etoposide in combination with doxorubicin and mitotane (EDPM) have demonstrated efficacy in adrenocortical carcinoma and is an accepted palliative treatment option. Even with optimal treatment, median overall survival is poor—reported between 12–15 months in the landmark FIRM-ACT study [16]. Predictive and prognostic criteria have largely been based on pathological findings, including the Weiss score and Helsinki score [17–20]. In 2018, the first international guidelines were published to guide treatment decisions for patients with this rare cancer and emphasized the need for multidisciplinary expert team care and enrollment in clinical trials and registries [21]. The prognostic value of clinical characteristics at the time of treatment initiation and the efficacy of liver-directed therapies such as transarterial chemoembolization (TACE) or selective internal radiation therapy (SIRT) in patients with hepatic metastatic disease is unclear [22–24]. In this study, we report the clinical characteristics and outcomes of patients with ACC treated at our center over the course of nearly 20 years.
Methods and Materials
We retrospectively reviewed patients with ACC who were diagnosed between January 1997 and October 2016 at The Ohio State University Comprehensive Cancer Center. Clinical data was extracted from the medical records, including patient demographics, presenting symptoms, co-existing disease, tissue biopsy result, histology, germline and/or somatic genetic test results, treatment modalities, and survival. Staging was done per the European Network for the Study of Adrenal Tumors (ENSAT) staging system [25]. The protocol was approved by The Ohio State University Institutional Review Board (#2016C0136). As all research involved materials (data, documents, and records) that were collected solely for non-research purposes (i.e., medical treatment or diagnosis) and therefore represented only minimal risk research, permission was obtained for a waiver of assent.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics. Categorical data were summarized as frequency and percentage and continuous variables as medians and ranges. Overall survivals (OS) from time of diagnosis of ACC or from time of systemic treatment for metastatic to death from any cause were calculated. Patients who were still alive were censored at the date of last follow-up. For the survival outcomes, Kaplan-Meier method was used to estimate the median survival with 95% confidence interval. The associations between the survival outcomes and the categorical variables were studied using log-rank test. Cox-regression models were used to study the associations between the survival outcomes and the continuous variables. Fisher’s exact test was used to study the association between categorical outcomes. p values < 0.05 were considered statistically significant. Statistical analysis was performed using SAS version 9.4 for Windows (SAS Institute, Cary, NC).
Results
Patient Characteristics
Patient characteristics are detailed in Table 1. A total of 65 patients were identified with diagnosis of ACC treated at our institution from 1997 through 2016. Fifteen patients had surgical resection for ACC between 1997 and 2006, prior to the availability of electronic records and therefore had limited clinical follow-up data available. Median overall survival (OS) from time of first diagnosis of ACC for the entire cohort was 27 months (95% CI 19.6–39.3, Supplemental Fig. 1).
Table 1.
Patient characteristics
| Characteristic of patient sample (N = 65) | N |
|---|---|
| Number of patients | 65 |
| Age at primary tumor diagnosis (median, range) | 50 (28–87) |
| Sex | |
| Male | 25 (38%) |
| Female | 40 (62%) |
| ENSAT stage at diagnosis | |
| I | 4 (6%) |
| II | 17 (26%) |
| III | 18 (28%) |
| IV | 25 (38%) |
| Unknown | 1 (2%) |
| Characteristics of patients receiving systemic therapy for metastatic disease (N = 36) | |
| Referral to tertiary institution | 16 (44%) |
| Age at systemic treatment (median, range) | 50 (27-79) |
| Sex | |
| Male | 18 (50%) |
| Female | 18 (50%) |
| ENSAT Stage at Diagnosis | |
| I | 1 (3%) |
| II | 8 (22%) |
| III | 7 (19%) |
| IV | 19 (53%) |
| Unknown | 1 (3%) |
Tumor Characteristics and Survival
For patients who underwent surgical resection and had tumor pathology available for review (N = 46), tumor size was not associated with OS from time of diagnosis of ACC (HR 1.07, 95% CI 1.00–1.14, p = 0.059). Tumor weight was available for 32 patients and did not have a significant association with OS from diagnosis (HR 1.00, p = 0.10). Twenty-seven patients had sufficient data to derive a modified Weiss score, and no significant association between OS from diagnosis and Weiss score was observed (HR 1.28, 95% CI 0.94–1.76, p = 0.12). There was no significant association between adjuvant mitotane and recurrence risk (p = 0.60) or survival (p = 0.50); however, only 14 patients received adjuvant mitotane.
Systemic Therapy for Metastatic Disease and Survival
A total of 36 patients received systemic therapy for metastatic disease (Table 2, Fig. 1). The median OS from time of treatment initiation for metastatic disease was 18.7 months (95% CI 9.3–26.0). The median duration of first-line therapy was 2.7 months (95% CI 1.7–3.9). Clinical characteristics at time of initiation of systemic therapy were assessed, and presence or absence of bone metastases (p = 0.66), ascites (p = 0.19), lung metastases (p = 0.12), liver metastases (p = 0.47), and hormonal activity of tumor (p = 0.19) were not prognostic for survival (Table 3). Whether EDP chemotherapy was given as first-line therapy (n = 21) or beyond (n = 14) did not impact OS from time of treatment initiation for metastatic disease (p = 0.34). Regimens utilized for treatment of metastatic ACC in the first-line setting included EDPM/Berruti regimen in 21/36, single-agent mitotane in 9/36, and clinical trial in 6/36 patients (with IMC-A12 in three patients, IMC-A12 with temsirolimus in one patient, AT101 in one patient, and bevacizumab and sunitinib in one patient). Twenty-three patients received second-line systemic therapy, which included EDPM in 8/23, mitotane alone in one patient, gemcitabine and capecitabine in one patient, cisplatin/etoposide with mitotane in one patient, cisplatin/etoposide alone in one patient, and clinical trial in 11/23 patients: nivolumab in three patients, an oral BH3-mimetic anti-apoptotic compound (AT101) in four patients, an antibody to insulin-like growth factor receptor (IMC-A12, cixutumumab) in two patients, thalidomide in one patient, and docetaxel and the hypoxia-activated prodrug TH302 in one patient. Additional treatments used beyond the second-line setting included trifluridine/tipiracil in one patient on clinical trial; capecitabine and gemcitabine in one patient; streptozocin single-agent therapy in one patient; and combination 5FU, bevacizumab, and streptozocin in one patient followed by clinical trial with AT-101 and then sunitinib at time of progression.
Table 2.
Association with clinical characteristics and survival for patients treated with metastatic disease
| Characteristic | N | Association with OS |
|---|---|---|
| Systemic therapy for metastatic disease | 36 | |
| Bone metastasis | 9 (25%) | p = 0.66 |
| Lung metastasis | 25 (69%) | p = 0.12 |
| Liver metastasis | 23 (64%) | p = 0.47 |
| Ascites | 4 (11%) | p = 0.19 |
| Cushing syndrome at time of diagnosis | 14 (40%) | p = 0.19 |
| TACE/regional liver-directed therapy | 6 (17%) | p = 0.011 |
OS, overall survival; TACE, transarterial chemoeombolization
Fig. 1.
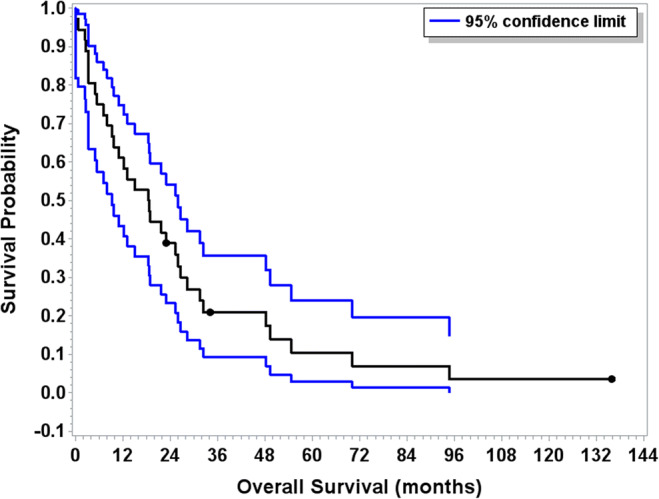
Overall survival for patients treated with systemic therapy for metastatic disease (N = 36). Blue bars indicate 95% confidence interval. The median OS from time of treatment initiation for metastatic disease was 18.7 months (95% CI 9.3–26.0)
Table 3.
First- and second-line therapies utilized in the treatment in patients with ACC
| First line (N = 36) | Second line (N = 23) | |
|---|---|---|
| EDPM | 21/36 | 8/23 |
| Mitotane (single agent) | 9/36 | 1/23 |
| Gemcitabine and capecitabine | – | 1/23 |
| Cisplatin and etoposide (+/−mitotane) | – | 2/23 |
| Clinical trial | 6/36 | 11/23 |
ACC, adrenal cortical carcinoma; EDPM, mitotane plus etoposide, doxorubicin, and cisplatin
Maximum SUV on FDG-PET and Survival
Twenty-six patients had fluorodeoxyglucose positron emission tomography (FDG-PET) performed as part of their care, and all patients had PET-avid lesions. The maximum SUV uptake ranged from 4.1–51.6, with median SUV of 12.4. There was no association between maximum SUV uptake and OS from time of systemic treatment in 20 patients who received systemic therapy (HR 1.02, 95% CI 0.98–1.06, p = 0.431).
Liver-Directed Therapy and Survival
Twenty-three patients had liver metastases while receiving treatment for systemic disease. Six patients received liver-directed regional therapies, including TACE (n = 2), SIRT (n = 3), or microwave ablation (n = 1). Five patients had imaging performed after TACE or SIRT, with near-complete resolution of liver lesions noted in one case. Median OS from time of treatment initiation for metastatic disease was longer for patients treated with TACE or SIRT compared to those who were not (32.4 months (95% CI 17.1–70.2) vs 9.9 months (95% CI 3.3–18.8); (p = 0.011), Fig. 2).
Fig. 2.
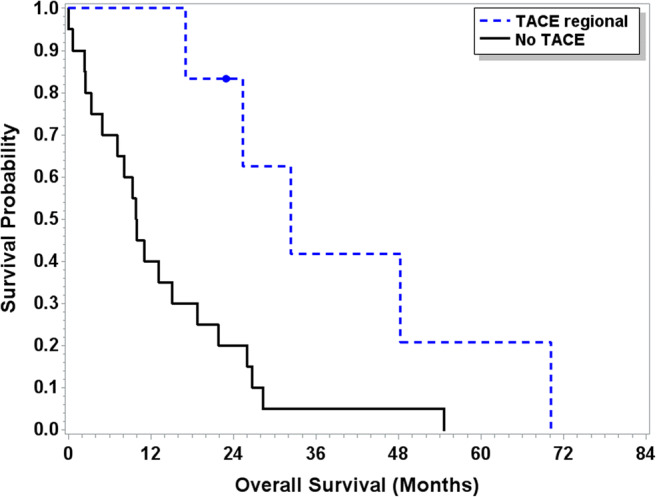
Overall survival patients with liver metastases treated with regional therapy (N = 23). Median OS from time of treatment initiation for metastatic disease was longer for patients treated with TACE or SIRT (N = 6) compared to those who were not (N = 17) (32.4 months (95% CI 17.1–70.2) vs 9.9 months (95% CI 3.3–18.8); (p = 0.011))
Somatic and Germline Genetics
Five patients had somatic next-generation sequencing mutational analysis performed on tumor specimens. One patient was found to have the following mutations: TP53 F134C, EGFR P848L, DAXX A47fs*92, and KDM5C V833fs*21. Another patient had mutations reported in ATM V2808 fs*49, RICTOR amplification, DNMT3A F384fs*11, and CTNNB1 W25*. A third patient had CDKN2A loss and was the only patient to have tumor mutational burden reported (low, 3 Muts/Mb). Two other patients were found to have TP53 alterations (V281del in one and splice site 782+1G>A in another). Seven patients underwent germline testing for mutational analysis, with three patients having no known pathogenic mutations. One patient was identified who had the c.942+3A>T intronic mutation in the MSH2 gene as well as a variant of uncertain significance (VUS) in PDGFRA (c.1475A>C). Three other patients were found to have VUS in various genes: MSH2 (c.138C>G) in one patient; POLD1 VUS in one patient; and BMPR1A gene (c.1318A>G), BRCA1 gene (c.3649T>C), PALB2 gene (c.2474G>C), and TSC2 gene (c.2866G>A) in the last patient.
Discussion
ACC is a rare tumor, and therefore, data regarding optimal management and clinical outcomes from rigorous prospective studies are limited. This study adds to the current body of literature by reporting the experience of 65 patients treated at our tertiary care institution, including a detailed account of the therapeutic strategies used in 36 patients with metastatic disease. The median survival for patients with metastatic disease treated in the FIRM-ACT study ranged from 12–15 months [16]. Here, we observed a median survival of 18.7 months for patients with metastatic disease from the time of treatment initiation. In our study, five patients’ surgical specimens were evaluated for somatic mutations, which showed genetic abnormalities in ATM, RICTOR, CTNNB1, DNMT3A, CTNNB1, TP53, EGFR, DAXX, KDM5C, and CDKN2A. Incorporating next-generation sequencing technology and liquid biopsies to clinical practice may help identify potential therapeutic targets.
For the patients in our study who underwent surgery for localized disease and had follow-up data available, the median OS of 35.9 months (95% CI 23.1–71.0) is lower than in prior studies perhaps due to the small numbers available for follow-up. Surgical resection is the only curative treatment for ACC and has been the main focus of prior research, specifically the identification of prognostic markers such as Ki-67 [8, 15, 26], the role of adjuvant mitotane [8] and radiation [11], and the role of surgery for recurrent or oligometastatic disease [27]. Outcomes from surgery vary, with reported median 5-year overall survival rates ranging from 27 [28] to 58% in longitudinal studies [29].
Systemic treatment for patients with metastatic disease often includes palliative mitotane given in combination with etoposide, doxorubicin, and cisplatin based on a large randomized, controlled, open-label trial [16]. There is no standard treatment for disease refractory to first-line combination chemotherapy, with several studies showing an overall low efficacy rate associated with gemcitabine-based therapy [30, 31]. In our study, the median OS of 18.7 months from start of treatment for metastatic disease compares favorably to published data from randomized clinical trials [16], as well as trials with larger patient numbers collected from databases [26]. This may be due in part to availability of—and a high rate of participation in—clinical trials and multi-modality and multi-disciplinary care. In our study, the presence of ascites, bone metastases, liver metastases, functional tumor, and choice of first-line systemic regimen were not associated with OS. There was no association between maximum SUV uptake on PET imaging and OS which is consistent with the literature [32]. These findings should be considered in the context of prior larger studies including the previously documented associated between hormonal activity of ACC and worse clinical outcomes [33, 34].
Liver-directed therapies such as TACE and SIRT have limited data in the treatment in ACC [22–24]. These therapies have traditionally been better evaluated as part of the treatment of patients with hepatocellular carcinoma [35] as well as liver metastases from colorectal cancer [36] and neuroendocrine tumors [37–39]. In our study, six patients with liver metastases who received liver-directed therapy with either TACE or SIRT had significantly prolonged survival compared to 17 patients who did not (p = 0.011). This may be reflective of the natural underlying biology in those patients, relatively low extra-hepatic metastatic burden, or due to the effect of local therapy with TACE or SIRT. Due to the small number of patients, this would need to be evaluated in larger prospective trials; however, the outcomes seen in these patients deserves further investigation.
Recent genomic characterization has shed light on possible treatment options but thus far, these are all investigative [40]. Interestingly, one study has shown a higher tumor mutational burden in metastatic ACC lesions compared to the primary tumors [41]. Despite this, the response rate to single-agent immunotherapy in trials is low [42]. Other promising therapies include insulin growth factor targeting therapies which have been investigated in early phase clinical trials [43] but despite early promise, IGF-1R directed treatment did not improve survival compared to placebo in a placebo-controlled phase 3 trial [44]. This reflects the ongoing need for novel therapies as evidenced by the large number of patient in our study who received treatment on a clinical trial.
There are several limitations to our study, including the small sample size, that all patients were treated at a single institution, and the lack of known prognostic measures such as Ki-67% and Weiss Criteria for many patients The significant amount of patients treated on clinical trial may also introduce a bias toward patients with better performance status, preserved organ function, and lack of significant comorbidities. Finally, the small number of patients with genetic testing or who underwent liver-directed therapies limits the generalizability of our findings in these populations.
Conclusion
Despite advances in the treatment of ACC and availability of clinical trials, the median survival of under 2 years from start of systemic therapy for metastatic disease shows the ongoing need for novel therapies for patients with this rare cancer. The clinical benefit seen in six patients who received liver-directed therapy with TACE or SIRT should be investigated in prospective studies.
Electronic supplementary material
(DOCX 36 kb)
Acknowledgments
We would like to thank our patients and their families, as well as the clinical investigators and research teams, who participate in trials for adrenal cortical carcinoma.
Compliance with Ethical Standards
Conflict of Interest
The authors confirm that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ng L, Libertino JM. Adrenocortical carcinoma: diagnosis, evaluation and treatment. J Urol. 2003;169(1):5–11. doi: 10.1016/S0022-5347(05)64023-2. [DOI] [PubMed] [Google Scholar]
- 2.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30(5):872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 3.Wasserman JD, Zambetti GP, Malkin D. Towards an understanding of the role of P53 in adrenocortical carcinogenesis. Mol Cell Endocrinol. 2012;351(1):101–110. doi: 10.1016/j.mce.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wooten MD, King DK. Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer. 1993;72(11):3145–3155. doi: 10.1002/1097-0142(19931201)72:11<3145::aid-cncr2820721105>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113(11):3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 6.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Grisanti S, Pulzer A, Claps M, Daffara F, Loli P, Mannelli M, Boscaro M, Arvat E, Tiberio G, Hahner S, Zaggia B, Porpiglia F, Volante M, Fassnacht M, Terzolo M. Long-term outcomes of adjuvant mitotane therapy in patients with radically resected adrenocortical carcinoma. J Clin Endocrinol Metab. 2017;102(4):1358–1365. doi: 10.1210/jc.2016-2894. [DOI] [PubMed] [Google Scholar]
- 9.Sabolch A, Feng M, Griffith K, Hammer G, Doherty G, Ben-Josef E. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2011;80(5):1477–1484. doi: 10.1016/j.ijrobp.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Habra MA, Ejaz S, Feng L, Das P, Deniz F, Grubbs EG, Phan A, Waguespack SG, Ayala-Ramirez M, Jimenez C, Perrier ND, Lee JE, Vassilopoulou-Sellin R. A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):192–197. doi: 10.1210/jc.2012-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassnacht M, Hahner S, Polat B, Koschker A-C, Kenn W, Flentje M, Allolio B. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metabol. 2006;91(11):4501–4504. doi: 10.1210/jc.2006-1007. [DOI] [PubMed] [Google Scholar]
- 12.Srougi V, Bessa J, Tanno FY, Ferreira AM, Hoff AO, Bezerra JE, et al. Adjuvant radiotherapy for the primary treatment of adrenocortical carcinoma: are we offering the best? Int Braz J Urol. 2017;43(5):841–848. doi: 10.1590/S1677-5538.IBJU.2017.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharzai LA, Green MD, Griffith KA, Else T, Mayo CS, Hesseltine E, Spratt DE, Ben-Josef E, Sabolch A, Miller BS, Worden F, Giordano TJ, Hammer GD, Jolly S. Adjuvant radiation improves recurrence-free survival and overall survival in adrenocortical carcinoma. J Clin Endocrinol Metab. 2019;104:3743–3750. doi: 10.1210/jc.2019-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson DW, Chang SC, Bandera BC, Fischer TD, Wollman R, Goldfarb M. Adjuvant radiation is associated with improved survival for select patients with non-metastatic adrenocortical carcinoma. Ann Surg Oncol. 2018;25(7):2060–2066. doi: 10.1245/s10434-018-6510-x. [DOI] [PubMed] [Google Scholar]
- 15.Beuschlein F, Weigel J, Saeger W, Kroiss M, Wild V, Daffara F, Libé R, Ardito A, al Ghuzlan A, Quinkler M, Oßwald A, Ronchi CL, de Krijger R, Feelders RA, Waldmann J, Willenberg HS, Deutschbein T, Stell A, Reincke M, Papotti M, Baudin E, Tissier F, Haak HR, Loli P, Terzolo M, Allolio B, Müller HH, Fassnacht M. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100(3):841–849. doi: 10.1210/jc.2014-3182. [DOI] [PubMed] [Google Scholar]
- 16.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 17.Aubert S, Wacrenier A, Leroy X, Devos P, Carnaille B, Proye C, Wemeau JL, Lecomte-Houcke M, Leteurtre E. Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol. 2002;26(12):1612–1619. doi: 10.1097/00000478-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Duregon E, Cappellesso R, Maffeis V, Zaggia B, Ventura L, Berruti A, Terzolo M, Fassina A, Volante M, Papotti M. Validation of the prognostic role of the "Helsinki Score" in 225 cases of adrenocortical carcinoma. Hum Pathol. 2017;62:1–7. doi: 10.1016/j.humpath.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Weiss LM, Medeiros LJ, Vickery AL., Jr Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1989;13(3):202–206. doi: 10.1097/00000478-198903000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–g46. doi: 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 22.Cazejust J, De Baere T, Auperin A, Deschamps F, Hechelhammer L, Abdel-Rehim M, et al. Transcatheter arterial chemoembolization for liver metastases in patients with adrenocortical carcinoma. J Vasc Interv Radiol. 2010;21(10):1527–1532. doi: 10.1016/j.jvir.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Ripley RT, Kemp CD, Davis JL, Langan RC, Royal RE, Libutti SK, Steinberg SM, Wood BJ, Kammula US, Fojo T, Avital I. Liver resection and ablation for metastatic adrenocortical carcinoma. Ann Surg Oncol. 2011;18(7):1972–1979. doi: 10.1245/s10434-011-1564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soga H, Takenaka A, Ooba T, Nakano Y, Miyake H, Takeda M, Tanaka K, Hara I, Fujisawa M. A twelve-year experience with adrenal cortical carcinoma in a single institution: long-term survival after surgical treatment and transcatheter arterial embolization. Urol Int. 2009;82(2):222–226. doi: 10.1159/000200804. [DOI] [PubMed] [Google Scholar]
- 25.Lughezzani G, Sun M, Perrotte P, Jeldres C, Alasker A, Isbarn H, Budäus L, Shariat SF, Guazzoni G, Montorsi F, Karakiewicz PI. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer. 2010;46(4):713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Chen SS, Gao WC, Bai L, Luo L, Zheng XG, Luo Y. Prognostic factors of adrenocortical carcinoma: an analysis of the surveillance epidemiology and end results (Seer) database. Asian Pac J Cancer Prev. 2017;18(10):2817–2823. doi: 10.22034/APJCP.2017.18.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baur J, Buntemeyer TO, Megerle F, Deutschbein T, Spitzweg C, Quinkler M, et al. Outcome after resection of adrenocortical carcinoma liver metastases: a retrospective study. BMC Cancer. 2017;17(1):522. doi: 10.1186/s12885-017-3506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran TB, Postlewait LM, Maithel SK, Prescott JD, Wang TS, Glenn J, Phay JE, Keplinger K, Fields RC, Jin LX, Weber SM, Salem A, Sicklick JK, Gad S, Yopp AC, Mansour JC, Duh QY, Seiser N, Solorzano CC, Kiernan CM, Votanopoulos KI, Levine EA, Hatzaras I, Shenoy R, Pawlik TM, Norton JA, Poultsides GA. Actual 10-year survivors following resection of adrenocortical carcinoma. J Surg Oncol. 2016;114(8):971–976. doi: 10.1002/jso.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margonis GA, Kim Y, Prescott JD, Tran TB, Postlewait LM, Maithel SK, et al. Adrenocortical carcinoma: impact of surgical margin status on long-term outcomes. Ann Surg Oncol. 2016;23(1):134–141. doi: 10.1245/s10434-015-4803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henning JEK, Deutschbein T, Altieri B, Steinhauer S, Kircher S, Sbiera S, Wild V, Schlötelburg W, Kroiss M, Perotti P, Rosenwald A, Berruti A, Fassnacht M, Ronchi CL. Gemcitabine-based chemotherapy in adrenocortical carcinoma: a multicenter study of efficacy and predictive factors. J Clin Endocrinol Metab. 2017;102(11):4323–4332. doi: 10.1210/jc.2017-01624. [DOI] [PubMed] [Google Scholar]
- 31.Sperone P, Ferrero A, Daffara F, Priola A, Zaggia B, Volante M, Santini D, Vincenzi B, Badalamenti G, Intrivici C, del Buono S, de Francia S, Kalomirakis E, Ratti R, Angeli A, Dogliotti L, Papotti M, Terzolo M, Berruti A. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: a multicenter phase II study. Endocr Relat Cancer. 2010;17(2):445–453. doi: 10.1677/ERC-09-0281. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi S, Balachandran A, Habra MA, Phan AT, Bassett RL, Jr, Macapinlac HA, et al. Impact of (1)(8)F-Fdg Pet/Ct on the management of adrenocortical carcinoma: analysis of 106 patients. Eur J Nucl Med Mol Imaging. 2014;41(11):2066–2073. doi: 10.1007/s00259-014-2834-3. [DOI] [PubMed] [Google Scholar]
- 33.Ayala-Ramirez M, Jasim S, Feng L, Ejaz S, Deniz F, Busaidy N, Waguespack SG, Naing A, Sircar K, Wood CG, Pagliaro L, Jimenez C, Vassilopoulou-Sellin R, Habra MA. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169(6):891–899. doi: 10.1530/EJE-13-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berruti A, Fassnacht M, Haak H, Else T, Baudin E, Sperone P, Kroiss M, Kerkhofs T, Williams AR, Ardito A, Leboulleux S, Volante M, Deutschbein T, Feelders R, Ronchi C, Grisanti S, Gelderblom H, Porpiglia F, Papotti M, Hammer GD, Allolio B, Terzolo M. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol. 2014;65(4):832–838. doi: 10.1016/j.eururo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Kolligs FT, Bilbao JI, Jakobs T, Inarrairaegui M, Nagel JM, Rodriguez M, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35(6):1715–1721. doi: 10.1111/liv.12750. [DOI] [PubMed] [Google Scholar]
- 36.Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is Yttrium-90 Radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann Surg Oncol. 2015;22(3):794–802. doi: 10.1245/s10434-014-4164-x. [DOI] [PubMed] [Google Scholar]
- 37.Devcic Z, Rosenberg J, Braat AJ, Techasith T, Banerjee A, Sze DY, et al. The Efficacy of hepatic 90y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med. 2014;55(9):1404–1410. doi: 10.2967/jnumed.113.135855. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, Murthy R, Rose S, Warner RRP, Liu D, Palmedo H, Overton C, Jones B, Salem R. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31(3):271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 39.Shirley LA, McNally M, Chokshi R, Jones N, Tassone P, Guy G, et al. Transarterial chemoembolization is ineffective for neuroendocrine tumors metastatic to the caudate lobe: a single institution review. World J Surg Oncol. 2015;13:167. doi: 10.1186/s12957-015-0551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, Kim S, Assie G, Morozova O, Akbani R, Shih J, Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ, Shao L, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida MQ, Fragoso MCBV, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE, Asa SL, Bertherat J, Fassnacht M, Wheeler DA, Hammer GD, Giordano TJ, Verhaak RGW, Zheng S, Verhaak RGW, Giordano TJ, Hammer GD, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, Kim S, Assié G, Morozova O, Akbani R, Shih J, Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida M, Barisson Fragoso MC, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE, Asa SL, Bertherat J, Fassnacht M, Wheeler DA, Benz C, Ally A, Balasundaram M, Bowlby R, Brooks D, Butterfield YSN, Carlsen R, Dhalla N, Guin R, Holt RA, Jones SJM, Kasaian K, Lee D, Li HI, Lim L, Ma Y, Marra MA, Mayo M, Moore RA, Mungall AJ, Mungall K, Sadeghi S, Schein JE, Sipahimalani P, Tam A, Thiessen N, Park PJ, Kroiss M, Gao J, Sander C, Schultz N, Jones CD, Kucherlapati R, Mieczkowski PA, Parker JS, Perou CM, Tan D, Veluvolu U, Wilkerson MD, Hayes DN, Ladanyi M, Quinkler M, Auman JT, Latronico AC, Mendonca BB, Sibony M, Sanborn Z, Bellair M, Buhay C, Covington K, Dahdouli M, Dinh H, Doddapaneni H, Downs B, Drummond J, Gibbs R, Hale W, Han Y, Hawes A, Hu J, Kakkar N, Kalra D, Khan Z, Kovar C, Lee S, Lewis L, Morgan M, Morton D, Muzny D, Santibanez J, Xi L, Dousset B, Groussin L, Libé R, Chin L, Reynolds S, Shmulevich I, Chudamani S, Liu J, Lolla L, Wu Y, Yeh JJ, Balu S, Bodenheimer T, Hoyle AP, Jefferys SR, Meng S, Mose LE, Shi Y, Simons JV, Soloway MG, Wu J, Zhang W, Mills Shaw KR, Demchok JA, Felau I, Sheth M, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Zhang J(J), Davidsen T, Crawford C, Hutter CM, Sofia HJ, Roach J, Bshara W, Gaudioso C, Morrison C, Soon P, Alonso S, Baboud J, Pihl T, Raman R, Sun Q, Wan Y, Naresh R, Arachchi H, Beroukhim R, Carter SL, Cho J, Frazer S, Gabriel SB, Getz G, Heiman DI, Kim J, Lawrence MS, Lin P, Noble MS, Saksena G, Schumacher SE, Sougnez C, Voet D, Zhang H, Bowen J, Coppens S, Gastier-Foster JM, Gerken M, Helsel C, Leraas KM, Lichtenberg TM, Ramirez NC, Wise L, Zmuda E, Baylin S, Herman JG, LoBello J, Watanabe A, Haussler D, Radenbaugh A, Rao A, Zhu J, Bartsch DK, Sbiera S, Allolio B, Deutschbein T, Ronchi C, Raymond VM, Vinco M, Shao L, Amble L, Bootwalla MS, Lai PH, van den Berg DJ, Weisenberger DJ, Robinson B, Ju Z, Kim H, Ling S, Liu W, Lu Y, Mills GB, Sircar K, Wang Q, Yoshihara K, Laird PW, Fan Y, Wang W, Shinbrot E, Reincke M, Weinstein JN, Meier S, Defreitas T. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;29(5):723–736. doi: 10.1016/j.ccell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gara SK, Lack J, Zhang L, Harris E, Cam M, Kebebew E. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat Commun. 2018;9(1):4172–4172. doi: 10.1038/s41467-018-06366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tourneau L, Christophe CH, Zarwan C, Wong DJ, Bauer S, Claus R, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the Javelin Solid Tumor Trial. J Immunother Cancer. 2018;6(1):111–111. doi: 10.1186/s40425-018-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beuschlein F, Jakoby J, Mentz S, Zambetti G, Jung S, Reincke M, Süss R, Hantel C. Igf1-R Inhibition and liposomal doxorubicin: progress in preclinical evaluation for the treatment of adrenocortical carcinoma. Mol Cell Endocrinol. 2016;428:82–88. doi: 10.1016/j.mce.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD. Linsitinib (Osi-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–435. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 36 kb)


