Abstract
In hepatocellular carcinoma (HCC), the hypoxic tumor microenvironment can drive enhance tumor malignancy and recurrence. The microRNA (miRNA) miR-196-5p has been shown to modulate the progression of several cancer types, but its roles in HCC remain uncertain. In the present report we observed significant miR-196-5p downregulation in HCC tissues and cells, and we found that the expression of this miRNA significantly impaired the proliferation and metastatic potential of HCC in vitro and in vivo. We identified high-mobility group AT-hook 2 (HMGA2) as a miR-196-5p target gene that was associated with the ability of miR-196-5p to modulate the progression of HCC. Expression of miR-196-5p and HMGA2 were correlated with the clinical characteristics and poor outcomes in patients with HCC. Finally, we found that hypoxic conditions were linked with reduced miR-196-5p expression in the context of HCC. Together these results highlight the role for miR-196-5p as an inhibitor of the proliferation and metastasis of HCC via the targeting of HMGA2, with this novel hypoxia/miR-196-5p/HMGA2 pathway serving as a potential target for future therapeutic intervention.
Electronic supplementary material
The online version of this article (10.1007/s12672-019-00370-5) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, miR-196-5p, HMGA2, Biomarker, Hypoxia
Introduction
Hepatocellular carcinoma (HCC) remains the leading form of liver cancer in addition to being among the most common cancers globally [1–3]. HCC cells often grow rapidly, inducing the angiogenic development of new blood vessels. However, this local microvasculature often becomes disorganized and is insufficient to provide the oxygen needed for tumor cells to grow normally, leading to intratumoral hypoxia [4]. Hypoxic tumor in turn often becomes more aggressive, metastatic, and radio/chemoresistant [5]. As such, the thorough characterization of hypoxia-induced signaling pathways is vital in order to identify potential therapeutic targets for treating such hypoxic tumors. Hypoxia-induced factor (HIF) is a key transcription factor associated with tumor cell detection of and responses to a hypoxic environment [6]. HIF regulates a wide range of processes including tumor cell proliferation, angiogenesis, invasion, and metastasis via regulating an array of target genes [7].
In addition to directly regulating particular genes, HIF has been shown to further regulate the expression of particular microRNAs (miRNAs), which are short RNA molecules that lack coding potential [8], and which negatively regulate the expression of specific genes in a wide range of contexts [9–13]. miRNAs play vital regulatory roles in cancer, with certain miRNAs promoting or suppressing oncogenesis [14]. The miR-196 family of miRNAs is encoded in the HOX gene cluster [15], with reported dysregulation in many cancers [16]. These miRNAs reportedly drive the metastasis, growth, and therapeutic resistance of lung, pancreatic, oral, colorectal, and gastric cancers [17–23]. In breast cancer and melanoma, however, there is evidence that miR-196 may instead serve to suppress tumor growth [24, 25]. As such, it is clear that miR-196-5p plays a context-dependent role in human cancers, and the relevance and roles of miR-196-5p in HCC remain unknown.
In the present report, we observed significant downregulation of miR-196-5p in HCC, with such downregulation being linked to clinicopathological features and a poorer patient prognosis. We found that this mRNA was capable of impairing HCC progression via suppression of its target gene high-mobility group AT-hook 2 (HMGA2). We further found that miR-196-5p was hypoxia-inducible in HCC cells. Together, these findings indicate that the reduced expression of miR-196-5p can enhance the aggressive growth of HCC, potentially being a biomarker of a poorer patient prognosis.
Materials and Methods
Clinical Samples
In total, we obtained 186 pairs of HCC and adjacent normal tissue samples from the Eastern Hepatobiliary Surgery Hospital (Shanghai, China). Samples were frozen immediately upon collection and were later used for both histological and quantitative real-time polymerase chain reaction (qRT-PCR) analyses. Micrometastases were detected via microscopic examination and identification of small tumors adjacent to the primary tumor border. The tumor-node-metastasis (TNM) classification system (6th edition) generated by the International Union against Cancer was used for tumor staging. The Institutional Review Board of the Eastern Hepatobiliary Surgery Hospital approved this study, with all patients giving written informed consent and all data being deidentified.
Cell Culture and Transfection
Both normal hepatocyte (THLE-3) and HCC (HCCLM3, SMMC7721, HepG2, Huh7, and Hep3B) cell lines were obtained from the Shanghai Institute of Life Sciences Cell Resource Center (Shanghai, China) and applied short tandem repeat (STR) for semiannually after the first recovery. Cells were grown using DMEM (HyClone, CA, USA) containing 10% FBS and penicillin/streptomycin (Gibco, CA, USA) in a 5%CO2 37 °C incubator. To induce hypoxia, cells were instead incubated at 37 °C in a 1% O2 environment for indicated periods of time. Lipofectamine 2000 was used to transfect miR-196-5p mimics, inhibitors, and controls (Genecopoeia. Guangzhou, China) into cells based on provided protocols. pUC-HMGA2 plasmids (GenePharma, Shanghai, China) were cloned into pcDNA3.1 vector (Invitrogen, CA, USA), yielding HMGA2-pcDNA3.1 vector. These vectors of siRNAs specific for HMGA2 were also transfected into cells via Lipofectamine 2000.
qRT-PCR
qRT-PCR was conducted as in previous studies [26]. Expression levels of miR-196-5p were normalized to those of U6, while GAPDH was used for mRNA normalization. Primers used were as follows: HMGA2: forward: 5′-GGCGGTGAAGGAGATGAAC-3′ and reverse: 5′-TGATGAGGAAATCCACGATAGAG-3′; GAPDH: forward: 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse: 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
Western Blotting
Lysates were prepared in 1× sodium dodecyl sulfate buffer, after which SDS-PAGE was used to separate equivalent protein amounts followed by transfer to nitrocellulose membranes that were then probed using antibodies against HMGA2, HIF1α, or GAPDH (Abcam, CA, USA). Next, secondary antibodies conjugated to IRDye 700 or IRDye 800 were used to detect protein, after which an Odyssey infrared scanner (Li-Cor) was used for visualization. GAPDH served as a loading control.
Immunofluorescence Analysis
Immunohistochemistry and immunofluorescence were conducted as in previous reports [27].
CCK8 Assay
After growth for the indicated amount of time, cell supernatants were removed, and a 100 μL solution of DMEM supplemented with 10 μL CCK8 was added per well for 2 h at 37 °C, after which absorbance at 450 nm was assessed.
Colony Formation Assay
A total of 100 cells were added to a 1-cm plate and allowed to grow for 7 days, after which time methanol was used to fix colonies. Colonies were then washed and stained using crystal violet (Sigma-Aldrich, Dorset), after which they were counted.
Wound Healing and Transwell Tests
Wound healing and transwell assays were conducted as in previous reports [26].
Luciferase Reporter Assay
The putative miR-196-5p binding site in the HMGA2 3′UTR, or a mutated version thereof, was synthesized and added to the pGL3 vector (Promega, WI, USA), yielding the wtHMGA2-3′UTR and mtHMGA2-3′UTR constructs, respectively. Next, 24-well plates were seeded with SMMC7721 cells (1 × 105) in serum-free OptiMEM media (Life Technologies), after which these constructs were co-transfected into cells along with miR-196-5p mimics or controls (50 nM) through use of Fugene (Promega). Following a 48-h incubation, cells were collected and a dual-luciferase reporter assay system (Promega) was used to measure luciferase activity, with Renilla luciferase being used for normalization. Firefly luciferase activity was normalized to Renilla luciferase activity.
Animal Studies
The Institutional Animal Care and Use Committee of the Second Military Medical University approved all animal studies. These studies utilized male athymic BALB/cnude mice (4–5 weeks old) for modelling subcutaneous tumor growth, as in previous reports [27].
Statistical Analysis
Data are means ± SEM. SPSS v13.0 (SPSS, IL, USA), and GraphPad Prism 5 (GraphPad Software, Inc, CA, USA) were used for all statistical testing. Results were compared via two-tailed Student’s t tests, ANOVAs, Kaplan-Meier analyses with log-rank tests, chi-squared tests, or Spearman’s rank correlation test as appropriate. P < 0.05 was the significance threshold. Biological replicates (inter-assay) and technical replicates (intra-assay) were used for all in vitro assays that were at least in triplicate.
Results
Decreased Expression of miR-196-5p in HCC Tissues Correlated with More Aggressive Disease
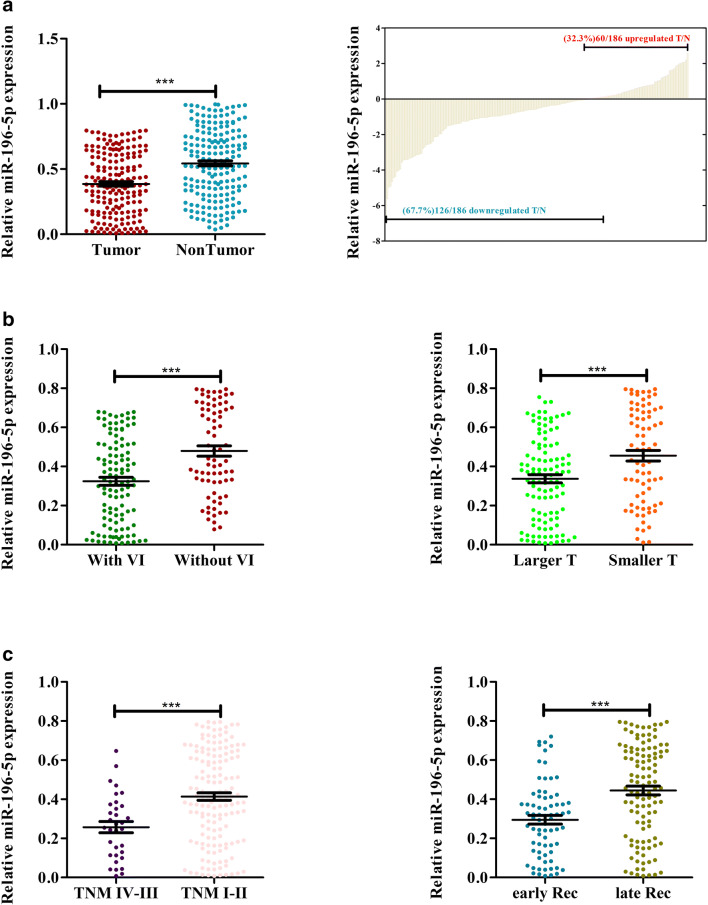
We first began by assessing the potential clinical relevance of miR-196-5p in HCC via measuring its relative expression levels in 186 pairs of HCC tumor tissue and adjacent healthy tissue via qRT-PCR. This analysis indicated that there was significantly lower miR-196-5p expression in HCC tumors relative to normal tissue (Fig. 1a, p < 0.001). Indeed, this miRNA was downregulated in 67.7% (126/186) of these HCC tissue samples relative to their matched normal control samples (Fig. 1b). In addition, patients with larger tumors (> 5 cm), vascular invasion, more advanced disease (stage III–IV), and early recurrence exhibited further reduced miR-196-5p expression (Fig. 1c–f).
Fig. 1.
miR-196-5p expression is reduced in HCC. a, b Expression of miR-196-5p was measured in HCC tumors and adjacent healthy tissue, with U6 used for normalization. c miR-196-5p expression in patients with or without vascular invasion. d The expression of miR-196-5p in large and small HCC tissues. e The expression of miR-196-5p in HCC tissues of TNM stages III–IV vs. I–II. The expression of miR-196-5p in HCC tumors with early and late recurrence (*p < 0.05, **p < 0.01, ***p < 0.001) Abbreviations: VI, vascular invasion; T, tumor size; Rec, recurrence
We next further assessed the link between miR-196-5p expression and clinicopathologic characteristics via separating these 186 patients according to median miR-196-5p levels into miR-196-5p-high and miR-196-5p-low subgroups. Lower expression of this miRNA was significantly associated with higher levels of alpha-fetoprotein (AFP) levels (≥ 20 μg/L) (p = 0.028), larger tumors (≥ 5 cm) (p = 0.037), having multiple tumors (n ≥ 2) (p < 0.001), vascular invasion (p = 0.036), a more advanced TNM stage (p < 0.001), more frequent recurrence (p = 0.004), and death (p < 0.001) (Table 1). In a multivariate analysis, the expression of miR-196-5p, tumor size, and tumor number were all identified as factors that independently predicted HCC patient recurrence-free survival (RFS) and overall survival (OS) (Table 2 and 3). Together, these results thus show a direct correlation between reduced miR-196-5p expression and HCC disease progression.
Table 1.
Clinical characteristics of study participants (n = 186)
| Feature | miR-196-5p | P value | |
|---|---|---|---|
| High | Low | ||
| Age, year | 0.437 | ||
| ≥55 | 59 | 65 | |
| <55 | 34 | 28 | |
| Gender | 0.282 | ||
| Male | 78 | 83 | |
| Female | 15 | 10 | |
| HBsAg | 0.809 | ||
| Positive | 84 | 83 | |
| Negative | 9 | 10 | |
| HBeAg | 0.216 | ||
| Positive | 69 | 76 | |
| Negative | 24 | 17 | |
| AFP, μg/L | 0.028 | ||
| ≥20 | 63 | 76 | |
| <20 | 30 | 17 | |
| Tumor size, cm | 0.037 | ||
| ≥5 | 48 | 62 | |
| <5 | 45 | 31 | |
| Tumor number | < 0.001 | ||
| Single | 86 | 60 | |
| Multiple | 7 | 33 | |
| Vascular invasion | 0.036 | ||
| Present | 49 | 63 | |
| Absent | 44 | 30 | |
| Hepatitis B virus DNA,IU/mL | 0.237 | ||
| ≥1.0°× 103 | 45 | 37 | |
| <1.0°× 103 | 48 | 56 | |
| Tumor differentiation | 0.226 | ||
| I–II | 7 | 12 | |
| III–IV | 86 | 81 | |
| TNM tumor stage | < 0.001 | ||
| I–II | 86 | 67 | |
| III–IV | 7 | 26 | |
The median miR-196-5p expression level served as the cut-off value to differentiate high and low-expression groups (those above and below the 50th percentile, respectively; n = 93 each). The correlation between miR-196-5p expression and patient clinical features was assessed via chi-squared tests. P < 0.05 was the significance threshold
Table 2.
Univariate and multivariate analyses of recurrence-free survival (RFS)
| Variables | Univariate analysis of RFS | Multivariate analysis of RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (years) ≥ 55 vs < 55 | 1.334 | 0.906–1.964 | 0.144 | – | – | – |
| Gender female vs male | 0.607 | 0.325–1.132 | 0.117 | – | – | – |
| HBsAg positive vs negative | 1.129 | 0.605–2.106 | 0.704 | – | – | – |
| HBeAg positive vs negative | 1.231 | 0.776–1.952 | 0.378 | – | – | – |
| AFP, ng/ml ≥ 20 vs < 20 | 1.207 | 0.766–1.877 | 0.403 | – | – | – |
| HBV DNA, IU/ml ≥ 1000 vs < 1000 | 1.203 | 1.205–2.206 | 0.036 | 1.077 | 0.738–1.521 | 0.166 |
| Tumor size, cm ≥ 5 vs < 5 | 1.646 | 1.110–2.441 | 0.013* | 1.502 | 1.005–2.237 | 0.047* |
| Tumor number multiple vs single | 2.467 | 1.596–3.814 | < 0.001* | 1.935 | 1.226–3.055 | 0.005* |
| Vascular invasion present vs absent | 1.037 | 0.706–1.525 | 0.852 | – | – | – |
| Tumor differentiation III–IV vs I–II | 0.994 | 0.533–1.856 | 0.986 | – | – | – |
| miR-196-5p level high vs low | 0.48 | 0.327–0.705 | < 0.001* | 0.551 | 0.369–0.821 | 0.003* |
HR hazard ratio, 95% CI 95% confidence interval
*P value < 0.05
Table 3.
Univariate and multivariate analyses of overall survival (OS)
| Variables | Univariate analysis of OS | Multivariate analysis of OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (years) ≥ 55 vs < 55 | 1.268 | 0.807–1.993 | 0.303 | – | – | – |
| Gender female vs male | 0.761 | 0.380–1.525 | 0.442 | – | – | – |
| HBsAg positive vs negative | 1.003 | 0.483–2.084 | 0.994 | – | – | – |
| HBeAg positive vs negative | 1.262 | 0.753–2.116 | 0.376 | – | – | – |
| AFP, ng/ml ≥ 20 vs < 20 | 1.663 | 1.397–2.332 | 0.038 | 0.863 | 0.483–1.582 | 0.394 |
| HBV DNA, IU/ml ≥ 1000 vs < 1000 | 0.988 | 0.634–1.541 | 0.058 | – | – | – |
| Tumor size, cm ≥5 vs < 5 | 1.814 | 1.128–2.917 | 0.014* | 1.628 | 1.006–2.635 | 0.047* |
| Tumor number multiple vs single | 2.886 | 1.796–4.640 | <0.001* | 2.105 | 1.279–3.465 | 0.003* |
| Vascular invasion present vs absent | 0.868 | 0.556–1.356 | 0.534 | – | – | – |
| Tumor differentiation III–IV vs I–II | 0.827 | 0.381–1.799 | 0.633 | – | – | – |
| miR-196-5p level high vs low | 0.369 | 0.232–0.589 | <0.001* | 0.437 | 0.268–0.712 | 0.001* |
HR hazard ratio, 95% CI 95% confidence interval
*P value < 0.05
HCC Cell Proliferation is Suppressed by miR-196-5p In Vitro and In Vivo
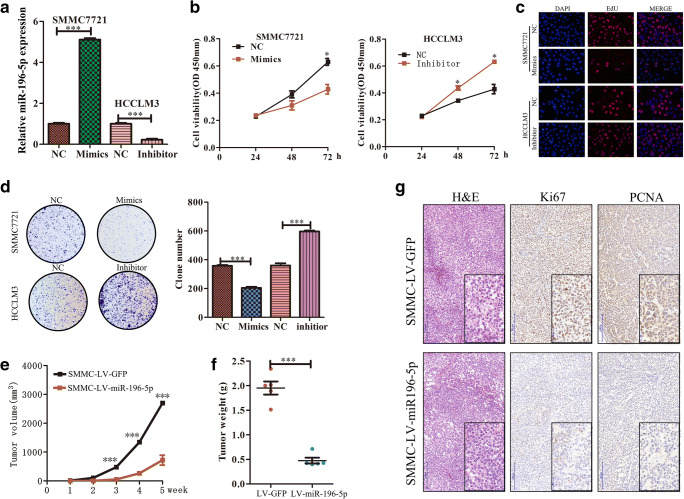
To further gauge the functional relevance of miR-196-5p in the progression of HCC, we next assessed the expression levels of this miRNA in the HCCLM3, Hep3B, HepG2, Huh7, and SMMC7721 HCC cell lines relative to a normal liver cell line (THLE-3). These analyses demonstrated that expression of miR-196-5p was significantly lower in the HCC cell lines relative to THLE-3 controls (Fig. S1). As the expression of miR-196-5p in HCCLM3 cells was relatively high, while the expression of SMMC7721 was relatively low among the five HCC cell lines, the SMMC7721 and HCCLM3 lines were therefore utilized for subsequence studies of the effects of increasing or reducing the expression of this miRNA. These cells were transfected with constructs designed to modulate miR-196-5p expression, with qRT-PCR used to confirm transfection efficiency (p < 0.001, Fig. 2a). Subsequent CCK8, EdU incorporation, and colony formation assays revealed that overexpression of miR-196-5p impaired the ability of SMMC7721 cells to proliferate and form colonies (Fig. 2b–d), whereas knocking down this miRNA had the opposite effect (Fig. 2b–d). To better gauge the relevance of this miRNA in vivo, we generated a murine xenograft model in which mice were subcutaneously implanted with SMMC7721 cells stably overexpressing miR-196-5p. These miR-196-5p-overexpressing tumors grew significantly more slowly than did control tumors (Fig. 2e, f). Consistent with this, this SMMC-LV-miR196-5p xenograft exhibited reduced PCNA and Ki67 staining relative to control tumors (Fig. 2h). These results therefore indicate that miR-196-5p is able to suppress the proliferation and growth of HCC tumors.
Fig. 2.
miR-196-5p suppresses the proliferation of HCC tumors in vitro and in vivo. a SMMC cells transfected with the miRNA mimic exhibited significantly higher miR-196-5p expression than those transfected with NC control, while transfection of HCCLM3 cells with a miR-196-5p inhibitor significantly reduced expression of this miRNA. b Overexpression of miR-196-5p impaired the proliferation of SMMC7721 cells, whereas inhibition of this miRNA enhanced the proliferation of HCCLM3 cells, n = 3. c EdU incorporation was used to assess the proliferation of SMMC-7721 and HCCCLM3 cells, n = 3. d SMMC colony formation ability was suppressed upon overexpression of miR-196-5p, whereas inhibition of this miRNA in HCCLM3 cells had the opposite effect. Tumor growth (e), and weight (f). g H&E-stained tumor sections from xenografy mouse models, with Ki67 and PCNA expression assessed in both groups. Original magnification, n = 5, × 200, × 400 (*p < 0.05, **p < 0.01, ***p < 0.001)
miR-196-5p Suppresses the Migration and Invasion Abilities of HCC Cells In Vitro and In Vivo
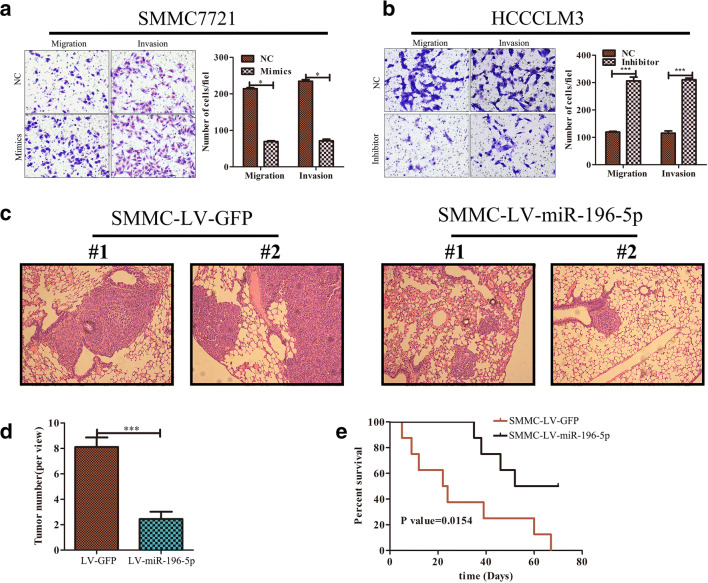
We next assessed the ability of miR-196-5p to influence HCC metastasis through wound healing and transwell-based assays. These tests revealed that overexpressing miR-196-5p impaired the migration and invasive potential of SMMC7721 cells (Fig. S2A and Fig. 3a), while knocking down this miRNA in HCCLM3 cells had the opposite effect (Fig. S2B and Fig. 3b). We additionally used a murine model of lung metastasis, sacrificing mice 70 days after intravenous injection with WT or miR-196-5p-overepxressing SMMC cells and assessing microsmetastatic lung lesions via H&E staining. This analysis revealed that overexpression of this miRNA was associated with a reduced number of lung metastases (Fig. 3c, d). In addition, survival rates of mice injected with SMMC-LV-miR-196-5p cells were higher than control animals (p < 0.05, Fig. 3e). These results thus together indicate that miR-196-5p plays a key role in regulating the metastatic potential of HCC cells.
Fig. 3.
miR-196-5p expression disrupts the migration and invasion abilities of HCC cells. a, b Transwell assays revealed that SMMC cell invasion potential was impaired upon miR-196-5p overexpression, whereas downregulation of this miRNA in HCCLM3 cells had the opposite effect, n = 5. c Murine lung tissue samples were collected and H&E-stained following the intravenous injection of 1x106 SMMC-7721 cells (Scale bar = 500 μm). d Lung metastatic nodule counts (n = 9). e Murine survival curves in mice following injection with SMMC-LV-GFP or SMMC-LV-miR-196-5p cells. A two-sided log-rank test was used to assess significance (*p < 0.05, **p < 0.01, ***p < 0.001).
miR-196-5p Targets HMGA2 in HCC
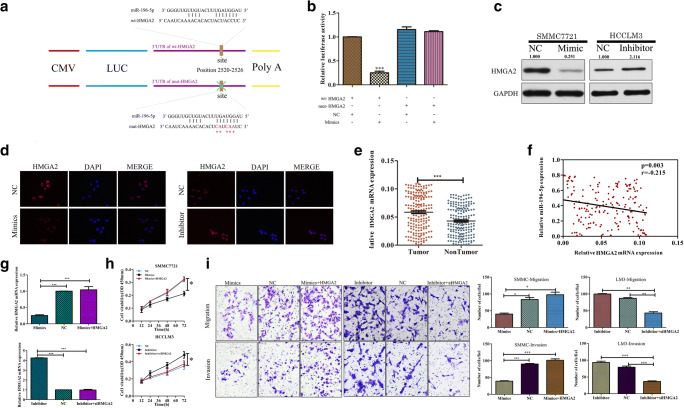
We next sought to identify candidate miR-196-5p target genes relevant to HCC progression using the TargetScan, miRanda, and miRBase applications. Through these analyses, we identified a putative miR-196-5p binding site in the 3′-UTR of HMGA2 (Fig. 4a). We then conducted luciferase reporter assays to confirm the specificity of this binding site via cloning this region of the HMGA2 3′-UTR into luciferase reporter plasmids and generating addition plasmids in which this site had been mutated. We found that miR-196-5p overexpression suppressed WT but not mutated luciferase reporter activity (Fig. 4b; p < 0.001). Western blotting and qRT-PCR further confirmed that miR-196-5p expression was inversely correlated with mRNA and protein levels of HMGA2 (Fig. S3A and B, Fig. 4c). Immunofluorescent staining further demonstrated that overexpression and inhibition of miR-196-5p decreased and increased HMGA2 protein levels, respectively (Fig. 4d). We further found in our xenograft mouse model system that animals bearing miR-196-5p-overexpressing tumors exhibited lower HMGA2 expression than did those bearing control tumors (Fig. S3C, D). We further found that HMGA2 expression was significantly elevated in patient HCC samples relative to adjacent control tissue expression (p < 0.001, Fig. 4e), and in tumor tissues, the expression of HMGA2 was negatively correlated with that of miR-196-5p (p = 0.003, r = − 0.215; Fig. 4f). These results thus demonstrate that HMGA2 is a direct miR-196-5p target in the context of HCC.
Fig. 4.
miR-196-5p targets HMGA2 to modulate HCC biology. The wild-type (wt) and mutated (mt) versions of the putative miR-196-5p binding site in the HMGA2 3′UTR are shown and were generated. b WT construct luciferase activity was markedly suppressed by miR-196-5p overexpression, whereas the mutant construct activity was unaffected in SMMC7721 cells. c Overexpression and inhibition of miR-196-5p expression markedly reduced and increased HMGA2 protein levels in SMMC and HCCLM3 cells, respectively. d HMGA2 expression as assessed via immunofluorescent microscopy. (Original magnification, × 400). e Comparison of HMGA2 expression in HCC tumor samples and adjacent controls, with GAPDH used for normalization. f A negative correlation between expression of HMGA2 and miR-196-5p in HCC tumors. (g, upper) Measurement of HMGA2 expression in SMMC7721 cells transfected with miR-196-5p mimics with or without an HMGA2 or control vector. (g, lower) Measurement of HMGA2 expression in cells expressing miR-196-5p inhibitors with or without siHMGA2 or control vectors. h CCK8 assays were conducted using SMMC7721 cells overexpressing miR-196-5p that were or were not transfected with HMGA2 or control vectors, as well as using HCCLM3 cells transfected using a miR-196-5p inhibitor with or without an siHMGA2 or control vector. i Transwell assays revealed that overexpression of HMGA2 was able to reverse the ability of miR-196-5p to inhibit HCC migration and invasion in SMMC cells, whereas knockdown of HMGA2 had the opposite effect in LM3 cells (*p < 0.05, **p < 0.01, ***p < 0.001)
miR-196-5p Suppresses HMGA2 Expression to Inhibit HCC Progression
To assess whether HMGA2 downregulation is a mechanism whereby miR-196-5p suppresses HCC cell proliferation, we conducted further analyses of HCC cells in which HMGA2 was overexpressed or knocked down (p < 0.01, Fig. 4g). We found that overexpressing HMGA2 overcame the ability of miR-196-5p to inhibit SMMC cell proliferation (Fig. 4h), migration, and invasion (p < 0.01, Fig. 4i). In contrast, knocking down HMGA2 in HCCLM3 cells in which miR-196-5p had been knocked down reduced their proliferation (Fig. 4h), migration, and invasion (p < 0.01, Fig. 4). This thus suggests that miR-196-5p targeting of HMGA2 has a direct impact on HCC progression.
The Combined Measurement of miR-196-5p and HMGA2 Enhances Prognostic Accuracy
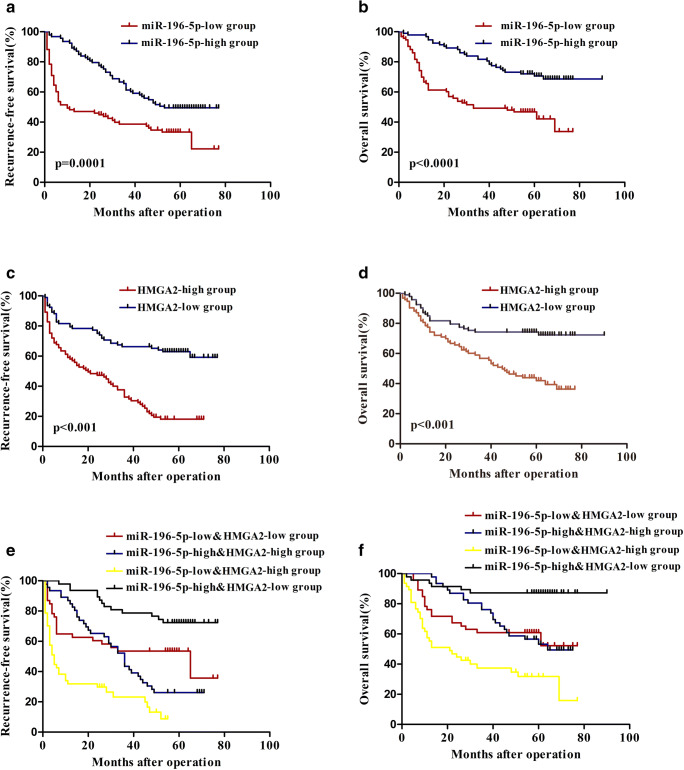
We next assessed the potential combined value of miR-196-5p and HMGA2 as prognostic biomarkers in HCC patients. In the 186 HCC patient samples processed via RT-PCR (Fig. 1a and Fig. 4e), the median expression levels of HMGA2 and miR-196-5p in HCC tumor samples were used as cut-offs to differentiate patients into high- and low-expression groups. Relative to individuals with high levels of miR-196-5p expression, those with lower expression of this miRNA had a lower RFS (p = 0.001, Fig. 5a) and OS (p < 0.001, Fig. 5b). Consistent with this, patients expressing high levels of HMGA2 had markedly poorer RFS and OS than did those with lower expression of this gene (p < 0.001, Fig. 5c, d). Those patients with both low miR-196-5p levels and high HMGA2 levels had the poorest OS and RFS of all patient subgroups, whereas individuals with high miR-196-5p and low HMGA2 expression had the most favorable survival outcomes (Fig. 5e, f). This thus suggests that combined assessment of miR-196-5p and HMGA2 offered better prognostic accuracy than did examination of either marker individually.
Fig. 5.
Clinical significance of miR-196-5p and HMGA2 expression in HCC patients. a, b The 186 HCC patient samples were grouped high (n = 93) and low (n = 93) groups based on median miR-196-5p expression in HCC. a RFS and b OS were compared between patients suffering from HCC that had high or low miR-196-5p expression levels. c, d The 186 HCC patient samples were grouped high (n = 93) and low (n = 93) groups based on median HMG1 expression in HCC. c RFS and d OS were compared between patients suffering from HCC that had high or low HMGA2 expression levels. e RFS and f OS were compared in HCC patient subgroups four subgroups of HCC patients (subgroup I: high miR-196-5p/low HMGA2, n = 41 ; subgroup II: low miR-196-5p/low HMGA2, n = 52; subgroup III: high miR-196-5p/high HMGA2, n = 52; subgroup IV: low miR-196-5p/high HMGA2, n = 41). The median expression levels of HMGA2 and miR-196-5p in HCC tumor samples were used as cut-offs to differentiate patients into high- and low-expression groups.
Hypoxic Conditions Drive miR-196-5p Downregulation in HCC
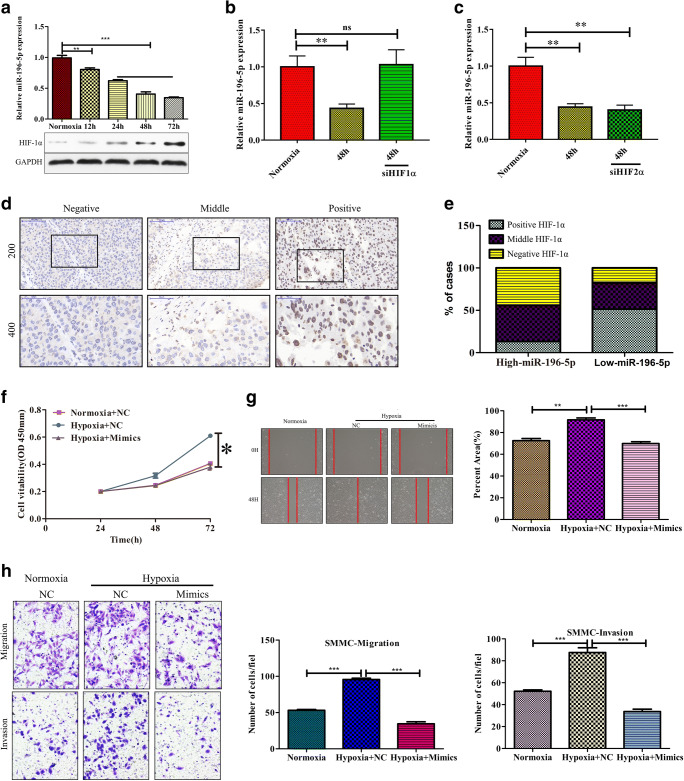
Through regulation of HIF-1ɑ, hypoxia can modulate gene expression [28], and as such, we sought to determine whether miR-196-5p expression was influenced by hypoxia in the context of HCC. When SMMC cells were grown in a hypoxic 1% oxygen environment, they exhibited both elevated HIF-1α expression and a gradual decline in expression of miR-196-5p (Fig. 6a; p < 0.01 for 12 h, p < 0.001 for 24 h, 48 h, and 72 h). Hypoxia can regulate gene expression in a HIF-1ɑ–dependent and –independent fashion [29]. To assess whether hypoxia-induced downregulation of miR-196-5p was HIF-1ɑ dependent, we used siRNA to knock down HIF-1ɑ in SMMC7721 cells, which prevented this hypoxia-induced loss of miR-196-5p expression (Fig. 6b, p < 0.05). We further assessed whether knocking down HIF-2ɑ had a similar impact, but found that doing so did not alter downregulation of miR-196-5p in response to hypoxic conditions (Fig. 6c). To additionally confirm that HIF-1ɑ mediates reduced miR-196-5p expression under hypoxic conditions, we assessed protein levels of HIF-1ɑ in HCC patient samples, revealing that samples with higher levels of miR-196-5p expression exhibited a reduced frequency of HIF-1ɑ positivity relative to samples with low miR-196-5p levels (Fig. 6d, e). This suggests that hypoxia can reduce miR-196-5p expression in an HIF-1ɑ–dependent fashion.
Fig. 6.
Hypoxia drives reduced miR-196-5p expression in HCC. a Levels of miR-196-5p expression under normoxic and hypoxic conditions. b HIF-1ɑ was knocked down using a specific siRNA in SMMC cells, reducing hypoxia-induced miR-196-5p downregulation at 48 h. c siRNA-mediated HIF-2ɑ knockdown failed to alter hypoxia-induced miR-196-5p downregulation at 48 h. d Representative HIF1α staining results. e IHC HIF-1α staining patters in tumors with high and low miR-196-5p expression. f SMMC7221 cells transfected with or without miR-196-5p mimics were cultured under normoxic or hypoxic conditions, after which a CCK8 assay was conducted. g SMMC7221 cells transfected with or without miR-196-5p mimics were cultured under normoxic or hypoxic conditions, after which a wound healing assay was conducted. h SMMC7221 cells transfected with or without miR-196-5p mimics were cultured under normoxic or hypoxic conditions, after which a Transwell assay was conducted (*p < 0.05, **p < 0.01, ***p < 0.001)
miR-196-5p is a Key Factor Influencing the Effects of Hypoxia on HCC
Hypoxia/HIF-1ɑ signaling is known to be a key mediator of cancer cell progression [30–34]. Given that hypoxia reduced the levels of miR-196-5p in HCC, we sought to assess whether such downregulation was necessary in order to mediate the influence of hypoxia on HCC cellular proliferation. We found that miR-196-5p overexpression reduced the ability of hypoxia to enhance SMMC7221 cell proliferation, invasion, and migration (Fig. 6e–h). These results thus confirm that miR-196-5p has the potential to suppress hypoxia/HIF-1ɑ signaling-mediated promotion of HCC progression.
Discussion
In order to develop more effective treatments for HCC, it is vital that the molecular mechanisms underlying this disease be better understood [6, 35]. miRNAs are well known to regulate a diverse array of cellular processes, including cancer-related processes such as proliferation and metastasis [36, 37]. The roles of particular miRNAs in HCC are still incompletely elucidated. However, in this study, we clarified the relevance and expression of miR-196-5p in HCC, revealing it to be downregulated in HCC tumor tissues, with such downregulation being correlated with signs of more advanced disease such as larger tumors, increased metastasis, tumor recurrence, and more advanced TNM stage, indicating that miR-196-5p may act as a suppressor of HCC progression.
Multiple reports have found miRNAs to be key regulators of tumor proliferation and metastasis in human cancer [38–41]. Through in vitro and in vivo studies, we determined that overexpression of miR-196-5p led to the impaired proliferation and metastasis of HCC cells, whereas knocking down this miRNA had the opposite effect. HMGA proteins are small, highly charged proteins that have three DNA-binding domains and an acidic C terminus. While this proteins lack the ability to serve as transcription factors directly, HMGA proteins can interact with other proteins in the nucleus and can alter chromatin structure, thereby regulating the transcription of certain genes [42, 43]. HMGA2 is an HMGA family protein that can bind to AT-rich B-form DNA, and which is known to be expressed at higher levels in a variety of tumors derived from epithelial and mesenchymal cells [44]. In this report, we determined that HMGA2 was directly regulated by miR-196-5p in HCC cells and that altering the expression of HMGA2 was sufficient to reverse the effects of miR-196-5p overexpression/inhibition on HCC cell metastasis and proliferation. This thus suggests that the miR-196-5p/HMGA2 axis is a key pathway regulating HCC progression.
We further found that decreased miR-196-5p expression was associated with poorer clinical findings and decreased survival in HCC patients. Importantly, we found that combined assessment of both miR-196-5p and HMGA2 expression was a more reliable predictor of HCC patient prognosis, suggesting that these two prognostic biomarkers of HCC patient outcomes are better than either marker individually.
The hypoxic environment within tumors is known to be a key factor governing tumor proliferation and metastasis [45–47], in addition to driving altered miRNA expression [48, 49]. We found that miR-196-5p expression was markedly reduced by hypoxic conditions, and overexpressing this miRNA reversed the effects of hypoxia on HCC proliferation and invasion, suggesting that hypoxia-mediated downregulation of miR-196-5p is a key mechanism whereby hypoxia drives enhanced tumor progression. However, hypoxia is likely just one factor influencing the observed reduction in miR-196-5p expression, with other factors such as epigenetic changes further downregulating this miRNA in HCC. Further future explorations of the mechanisms whereby hypoxia regulates miR-196-5p expression are thus warranted. Some study limitations should be noted: (1) only one cell line model was utilized for analysis of the down- or upregulation of the miR-196-5p; (2) the diagnostic/prognostic use of combined with mIR-196 and HMGA2 should be determined in the further; (3) we did not determine the biology effect of miR-196-5p on normal cells (CTHLE-3); (4) we did not explore whether knockdown of mIR-196 would increase xenograft growth in vivo. These factors now warrant further investigation.
In summary, these results clearly demonstrate that in HCC miR-196-5p can target HMGA2 expression and thereby serve as a tumor suppressor. These results offer new insights into the mechanisms of HCC metastasis in a hypoxic environment and suggest that this novel hypoxia/miR-196-5p/HMGA2 pathway may be viable target for future therapeutic intervention.
Electronic Supplementary Material
(PNG 121 kb)
(PNG 3342 kb)
(PNG 6869 kb)
Funding Information
The study was funded by the National Natural Science Foundation of China (NSFC81672350, 81872225); the National Key Basic Research Program of China (grant no. 2014CB542102); The Shanghai Health and Family Planning Commission Foundation (grant no. 20164Y0189); The National Human Genetic Resources Sharing Service Platform (grant no. 2005DKA21300); The Science Fund for Creative Research Groups, NSFC, China (grant no. 81521091); the NewtritionTM Asia Research Grant by BASF and National Natural Science Foundation of China (Grant No. 81672350, 81872225); and The State Key Infection Disease Project of China (grant no. 2017ZX10203208).
Compliance with Ethical Standards
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s12672-023-00659-6"
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Zheng, Feng-rui Bi, Yuan Yang and Yong-gang Hong contributed equally to this work.
Change history
4/24/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s12672-023-00659-6
Contributor Information
Wei-ping Zhou, Email: ehphwp@163.com.
Li-hua Song, Email: lihuas@sjtu.edu.cn.
Hong-Li Yan, Email: hongliyan@smmu.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Mcglynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19(2):223–238. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xuan M, Franklin DA, Jiahong D, Yanping Z. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 2011;74(24):7161–7167. doi: 10.1158/0008-5472.CAN-14-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3(1):83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 6.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2005;25(15):6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352(6282):175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bersani F, Lingua MF, Morena D, Foglizzo V, Miretti S, Lanzetti L, Carrà G, Morotti A, Ala U, Provero P, Chiarle R, Singer S, Ladanyi M, Tuschl T, Ponzetto C, Taulli R. Deep sequencing reveals a novel miR-22 regulatory network with therapeutic potential in rhabdomyosarcoma. Cancer Res. 2016;76(20):6095–6106. doi: 10.1158/0008-5472.CAN-16-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Ippolito E, Plantamura I, Bongiovanni L, Casalini P, Baroni S, Piovan C, Orlandi R, Gualeni AV, Gloghini A, Rossini A, Cresta S, Tessari A, de Braud F, di Leva G, Tripodo C, Iorio MV. miR-9 and miR-200 Regulate PDGFRβ-mediated endothelial differentiation of tumor cells in triple-negative breast cancer. Cancer Res. 2016;76(18):5562–5572. doi: 10.1158/0008-5472.CAN-16-0140. [DOI] [PubMed] [Google Scholar]
- 10.Orso F, Quirico L, Virga F, Penna E, Dettori D, Cimino D, Coppo R, Grassi E, Elia AR, Brusa D, Deaglio S, Brizzi MF, Stadler MB, Provero P, Caselle M, Taverna D. miR-214 and miR-148b targeting inhibits dissemination of melanoma and breast cancer. Cancer Res. 2016;76(17):5151–5162. doi: 10.1158/0008-5472.CAN-15-1322. [DOI] [PubMed] [Google Scholar]
- 11.Chen YF, Yang CC, Kao SY, Liu CJ, Lin SC, Chang KW. MicroRNA-211 enhances the oncogenicity of carcinogen-induced oral carcinoma by repressing TCF12 and increasing antioxidant activity. Cancer Res. 2016;76(16):4872–4886. doi: 10.1158/0008-5472.CAN-15-1664. [DOI] [PubMed] [Google Scholar]
- 12.Xue J, Zhou A, Wu Y, Morris SA, Lin K, Amin S, et al. miR-182-5p induced by stat3 activation promotes glioma tumorigenesis. Cancer Res. 2016;76(14):4293. doi: 10.1158/0008-5472.CAN-15-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen-Ping X, Min Y, Qian-Qian L, Wei-Ping Z, Wen-Ming C, Yuan Y, et al. Perturbation of MicroRNA-370/Lin-28 homolog A/nuclear factor kappa B regulatory circuit contributes to the development of hepatocellular carcinoma. Hepatology. 2013;58(6):1977–1991. doi: 10.1002/hep.26541. [DOI] [PubMed] [Google Scholar]
- 14.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2007;302(1):1–12. doi: 10.1056/ENEJMicm020631. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Zhang Y, Zhang L, Weakley SM, Yao Q. MicroRNA-196: critical roles and clinical applications in development and cancer. J Cell Mol Med. 2011;15(1):14–23. doi: 10.1111/j.1582-4934.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller DW, Anja-Katrin B. MicroRNA miR-196a controls melanoma-associated genes by regulating HOX-C8 expression. Int J Cancer. 2011;129(5):1064–1074. doi: 10.1002/ijc.25768. [DOI] [PubMed] [Google Scholar]
- 17.Andrea T, Amemiya CT, Chang-Bae K, Stadler PF. Evolution of microRNAs located within Hox gene clusters. J Exp Zool B Mol Dev Evol. 2005;304(1):75–85. doi: 10.1002/jez.b.21021. [DOI] [PubMed] [Google Scholar]
- 18.Yae-Eun S, Nina R, Joop GK, Katherine L, Teresa Guerrero U, Jessica B, et al. MicroRNA-196a promotes an oncogenic effect in head and neck cancer cells by suppressing annexin A1 and enhancing radioresistance. Int J Cancer. 2015;137(5):1021–1034. doi: 10.1002/ijc.29397. [DOI] [PubMed] [Google Scholar]
- 19.Lu YC, Chang JT, Liao CT, Kang CJ, Huang SF, Chen IH, et al. OncomiR-196 promotes an invasive phenotype in oral cancer through the NME4-JNK-TIMP1-MMP signaling pathway. Mol Cancer. 2014;13(1):218. doi: 10.1186/1476-4598-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popovic R, Riesbeck L, Cs CA, Zhang J, Achille N, Erfurth F, et al. Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood. 2009;113(14):3314. doi: 10.1182/blood-2008-04-154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo-Wang T, Yu-Lun L, Chew-Wun W, Ling-Yueh H, Sung-Chou L, Wen-Ching C, et al. Aberrant expression of miR-196a in gastric cancers and correlation with recurrence. Genes Chromosomes Cancer. 2012;51(4):394–401. doi: 10.1002/gcc.21924. [DOI] [PubMed] [Google Scholar]
- 22.Sun M, Liu XH, Li JH, Yang JS, Zhang EB, Yin DD, Liu ZL, Zhou J, Ding Y, Li SQ, Wang ZX, Cao XF, de W. MiR-196a is upregulated in gastric cancer and promotes cell proliferation by downregulating p27(kip1) Mol Cancer Ther. 2012;11(4):842–852. doi: 10.1158/1535-7163.MCT-11-1015. [DOI] [PubMed] [Google Scholar]
- 23.Liu CJ, Tsai MM, Tu HF, Lui MT, Cheng HW, Lin SC. miR-196a Overexpression and miR-196a2 gene polymorphism are prognostic predictors of oral carcinomas. Ann Surg Oncol. 2013;20(3):S406–SS14. doi: 10.1245/s10434-012-2618-6. [DOI] [PubMed] [Google Scholar]
- 24.Simone B, Mueller DW, Tanja R, Anja-Katrin B. MicroRNA miR-196a is a central regulator of HOX-B7 and BMP4 expression in malignant melanoma. Cell Mol Life Sci. 2010;67(20):3535–3548. doi: 10.1007/s00018-010-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yong L, Zhang M, Chen H, Zheng D, Ganapathy V, Thangaraju M, et al. Ratio of miR-196s to HOXC8 mRNA correlates with breast cancer cell migration and metastasis. Cancer Res. 2010;70(20):7894–7904. doi: 10.1158/0008-5472.CAN-10-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, Wang SB, Wang YZ, Yang Y, Yang N, Zhou WP, Yang GS, Sun SH. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Ji-Hang Y, Fu Y, Bi-Feng C, Zhi L, Xi-Song H, Wei-Ping Z, et al. The histone deacetylase 4/SP1/microrna-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology. 2011;54(6):2025–2035. doi: 10.1002/hep.24606. [DOI] [PubMed] [Google Scholar]
- 28.Ji-Won L, Seong-Hui B, Joo-Won J, Se-Hee K, Kyu-Won K. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 29.Jacques P, Frédéric D, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 30.Mcintyre A, Hulikova A, Ledaki I, Snell C, Singleton D, Steers G, et al. Disrupting hypoxia-induced bicarbonate transport acidifies tumor cells and suppresses tumor growth. Cancer Res. 2016;76(13):0008-5472.CAN-15-1862. doi: 10.1158/0008-5472.CAN-15-1862. [DOI] [PubMed] [Google Scholar]
- 31.Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, Sun X, Li GG, Hu QD, Fu QH, Liang TB. Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016;76(4):818–830. doi: 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- 32.Muhammad Zaeem N, Bassam J, Shijun H, Wu JC, Fabio M, Vincenzo B, et al. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. 2015;75(18):3771–3787. doi: 10.1158/0008-5472.CAN-15-0405. [DOI] [PubMed] [Google Scholar]
- 33.Yee KM, Vuvi N, Robert L, Darnay BG, Galina K, Mena A, et al. Hypoxia-induced SUMOylation of E3 ligase HAF determines specific activation of HIF2 in clear-cell renal cell carcinoma. Cancer Res. 2015;75(2):316–329. doi: 10.1158/0008-5472.CAN-13-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 35.Choi E, Choi E, Hwang KC. MicroRNAs as novel regulators of stem cell fate. World J Stem Cells. 2013;5(4):172–187. doi: 10.4252/wjsc.v5.i4.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Victor A. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 37.Pang MF, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC, Nelson CM. Tissue stiffness and hypoxia modulate the integrin-linked Kinase ILK to control breast cancer stem-like cells. Cancer Res. 2016;76(18):5277–5287. doi: 10.1158/0008-5472.CAN-16-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di MM, Regondi V, Sandri M, Iorio MV, Zanetti A, Tagliabue E, et al. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017;384:94–100. doi: 10.1016/j.canlet.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Zhang C, Chen R, Xiong H, Qiu F, Liu S, Zhang M, Wang F, Wang Y, Zhou X, Xiao G, Wang X, Jiang Q. Disrupting MALAT1/miR-200c sponge decreases invasion and migration in endometrioid endometrial carcinoma. Cancer Lett. 2016;383(1):28–40. doi: 10.1016/j.canlet.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Gilam A, Conde J, Weissglas-Volkov D, Oliva N, Friedman E, Artzi N, et al. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat Commun. 2016;7:12868. doi: 10.1038/ncomms12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seviour EG, Sehgal V, Mishra D, Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Lee JS, Sood AK, Kim MP, Mills GB, Ram PT. Targeting KRas-dependent tumour growth, circulating tumour cells and metastasis in vivo by clinically significant miR-193a-3p. Oncogene. 2016;36(10):1339–1350. doi: 10.1038/onc.2016.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reeves R, Beckerbauer L. HMGI/Y proteins: flexible regulators of transcription and chromatin structure. BBA - Gene Struct Express. 2001;1519(1):13–29. doi: 10.1016/S0167-4781(01)00215-9. [DOI] [PubMed] [Google Scholar]
- 43.Kloth L, Gottlieb A, Helmke B, Wosniok W, Löning T, Burchardt K, Belge G, Günther K, Bullerdiek J. HMGA2 expression distinguishes between different types of postpubertal testicular germ cell tumour. J Pathol Clin Res. 2015;1(4):239–251. doi: 10.1002/cjp2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenmakers EF, Wanschura S, Mols R, Bullerdiek J, Berghe H, Van Den VWJ, De V. Recurrent rearrangements in the high mobility group protein gene, HMGI-C, in benign mesenchymal tumours. Nat Genet. 1995;10(4):436. doi: 10.1038/ng0895-436. [DOI] [PubMed] [Google Scholar]
- 45.Wallace EM, Rizzi JP, Han G, Wehn PM, Cao Z, Du X, et al. A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 2016;76(18):5491–5500. doi: 10.1158/0008-5472.CAN-16-0473. [DOI] [PubMed] [Google Scholar]
- 46.Chowdhury R, Leung IKH, Tian YM, Abboud MI, Ge W, Domene C, et al. Structural basis for oxygen degradation domain selectivity of the HIF prolyl hydroxylases. Nat Commun. 2016;7:12673. doi: 10.1038/ncomms12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calinescu AA, Yadav VN, Carballo E, Kadiyala P, Tran D, Zamler D, Doherty R, Srikanth M, Lowenstein PR, Castro MG. Survival and proliferation of neural progenitor derived glioblastomas under hypoxic stress is controlled by a CXCL12/CXCR4 autocrine positive feedback mechanism. Clin Cancer Res. 2017;23(5):1250–1262. doi: 10.1158/1078-0432.CCR-15-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dou C, Liu Z, Xu M, Jia Y, Wang Y, Li Q, Yang W, Zheng X, Tu K, Liu Q. miR-187-3p inhibits the metastasis and epithelial–mesenchymal transition of hepatocellular carcinoma by targeting S100A4. Cancer Lett. 2016;381(2):380–390. doi: 10.1016/j.canlet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Teng S, Cheng T, Wu K. miR-1236 regulates hypoxia-induced epithelial–mesenchymal transition and cell migration/invasion through repressing SENP1 and HDAC3. Cancer Lett. 2016;378(1):59–67. doi: 10.1016/j.canlet.2016.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 121 kb)
(PNG 3342 kb)
(PNG 6869 kb)