Abstract
Incidentally discovered adrenal masses are common and the clinical evaluation and surveillance aims to diagnose hormone excess and malignancy. Adrenocortical cancer (ACC) is a very rare malignancy. This study aims to define the imaging characteristics of adrenal tumors preceding the diagnosis of ACC. Patients with prior (>5 months) adrenal tumors (<6 cm) subsequently diagnosed with ACC were identified in a large registry at a tertiary referral center. Retrospective chart and image review for patient characteristics and initial, interval, and diagnostic imaging characteristics (size, homogeneity, borders, density, growth rate, etc.) was conducted. Twenty patients with a diagnosis of ACC and a prior adrenal tumor were identified among 422 patients with ACC. Of these, 17 patients were initially imaged with CT and 3 with MR. Only 2 of the 20 patients had initial imaging characteristics suggestive of a benign lesion. Of initial tumors, 25 % were <2 cm in size. Surveillance led to the diagnosis of ACC within 24 months in 50 % of patients. The growth pattern was variable with some lesions showing long-term stability (up to 8 years) in size. In conclusion, antecedent lesions in patients with a diagnosis of ACC are often indeterminate by imaging criteria and can be small. Surveillance over 2 years detected only 50 % of ACCs. Current practice and guidelines are insufficient in diagnosing ACCs. Given the rarity of ACC, the increased risk and health care costs of additional evaluation may not be warranted.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-015-0225-2) contains supplementary material, which is available to authorized users.
Keywords: Congenital Adrenal Hyperplasia, Hounsfield Unit, Initial Imaging, Adrenal Tumor, Adrenal Mass
Introduction
Adrenal tumors are often discovered when cross-sectional imaging is performed for unrelated indications (1, 2). These incidentally discovered adrenal tumors are a significant health concern due to the deleterious effects of excess hormone production and possible malignancy. Further evaluation and surveillance incur both additional cost and health risk. Among these risks are exposure to ionizing radiation from CT or PET scans and complications from invasive procedures such as biopsy or surgery (3, 4).
The goal of the initial evaluation of an incidentally discovered adrenal nodule is to (1) determine the functional status by biochemical testing and (2) establish the likelihood of malignancy by imaging (1, 2). Biochemical and clinical evaluation will diagnose hormone-secreting tumors, and treatment can be instituted.
Benign adrenal adenomas are common, being reported with a prevalence of 2–9 %, depending on the population studied (1, 2, 5). In contrast, adrenocortical carcinoma (ACC) is exceedingly rare (6). The risk of ACC had been estimated to be as high as 2–6 % for lesions 4–6 cm in diameter and <2 % for those less than 4 cm (7). However, a more recent meta-analysis estimates the fraction of ACCs among incidentally discovered adrenal masses to be only 1.4 % (5). A review of 973 patients with 1049 incidentally discovered adrenal masses found no malignancies, though follow-up was as short as 1 year in some patients (8).
Evidence of metastatic disease is regarded as definitive for the diagnosis of malignant disease. Several characteristics have been reported to increase the suspicion for malignant adrenal tumors on cross-sectional imaging. These include heterogeneity, areas of necrosis or hemorrhage, calcification, irregular borders, invasion into adjacent structures, size >4–6 cm, decreased wash-out and irregular enhancement on contrast-enhanced CT scan, T2 intense signal on MRI, and Hounsfield units >10 on unenhanced CT scan (9). Size has been found to be the most valuable criteria, and a recent study has shown that HU >10 is sensitive but not specific in determining the diagnosis of ACC (10, 11).
Above a size threshold of 6 cm, consensus guidelines (e.g., AACE, ACR, AME, NIH) are consistent in recommending surgery, although this is not mandated if all aspects of imaging characteristics are entirely benign (12–14). There is a lack of consensus for smaller lesions; recommendations typically involve initial radiographic evaluation as well as surveillance due to concern for the possibility of ACC. Given the rarity of this malignancy, the benefits, frequency, or duration of serial imaging have not been established and the antecedent imaging appearance of lesions prior to development of ACC is unknown. The current study aims to retrospectively analyze the radiographic characteristics of adrenal tumors in patients later diagnosed with ACC.
Methods
This is a single-center retrospective analysis of patients with ACC in a tertiary referral center with a specialized endocrine oncology service. ACC patients were identified in the Michigan Endocrine Oncology Repository (MEOR, HUM00024461). Medical records were reviewed to identify patients with a diagnosis of an adrenal tumor of less than 6 cm in size predating the diagnosis of histologically confirmed ACC by at least 5 months. As all patients were initially evaluated and followed outside the University of Michigan, initial images were obtained from the initial imaging facilities. All pathological specimens were reviewed by an experienced endocrine pathologist at the University of Michigan to confirm the diagnosis of ACC. Images were reviewed by the study team including two experienced radiologists with expertise in adrenal imaging. Average growth rates were calculated for the largest diameter from initial imaging to final diagnosis of ACC.
Results
Patient Characteristics
Among 422 patients within the MEOR, 20 had an adrenal mass less than 6 cm in diameter that was diagnosed more than 5 months prior to the final ACC diagnosis (Table 1). The interval of 5 months was chosen because the minimum recommendation for repeat imaging of adrenal lesions is 6 months following initial identification. The chosen time frame ensures the inclusion of patients that had their follow-up imaging marginally earlier. The cut-off of 6 cm was used as this is the maximum diameter considered for benign lesions in at least some of the current guidelines. All patients had initially been evaluated and followed outside the University of Michigan Health Care System. The reason for initial imaging was unrelated to endocrine or local tumor-related symptoms in all cases. Eleven patients were female, and 9 were male. Nineteen patients were Caucasian and one was African-American. Mean age at initial imaging was 47.1 ± 16.8 years (mean ± SD) and 50.2 ± 16.1 years (mean ± SD) at the time of diagnostic imaging. The mean lead time to diagnosis of ACC was 44.1 ± 39.5 months (mean ± SD), range (6.0 to 131.0) with 5 of 20 ACCs (25 %) diagnosed within 1 year, and 10 (50 %) being diagnosed within 2 years.
Table 1.
Patient and imaging characteristics
| Patients and tumor demographics | |
|---|---|
| Gender | |
| Male | 9 |
| Female | 11 |
| Race | |
| Caucasian | 19 |
| African-American | 1 |
| Mean age at initial imaging (years ± SD, range) | 47.1 ± 16.8 (12–81) |
| Mean size of initial lesions (cm ± SD (range)) | 3.2 ± 1.3 (1.1–5.3) |
| Mean size at ACC diagnosis (cm ± SD (range)) | 6.9 ± 3.1 (3.1–16) |
| Lesion appearance at initial imaging | |
| Heterogeneous | 12 |
| Homogeneous | 8 |
| Mean lead time to ACC diagnosis (months ± SD (range)) | 44.1 ± 39.5 (6.0–131.0) |
| <12 months (N (%)) | 5 (25) |
| <24 months (N (%)) | 10 (50) |
| Mean growth rate (cm/year ± SD (range)) | 1.9 ± 1.7 (0.2–6.0) |
| <1 cm/year (N (%)) | 7 (35) |
| Lesions with a latent period of stability (N (%)) | 6 (30) |
| Latent period range (years) | 0.6–8 |
| Hormone secretion at time of ACC diagnosis (N (%)) | 8 (40) |
Initial and final sizes, hormone work-up
Initial Lesion Characteristics
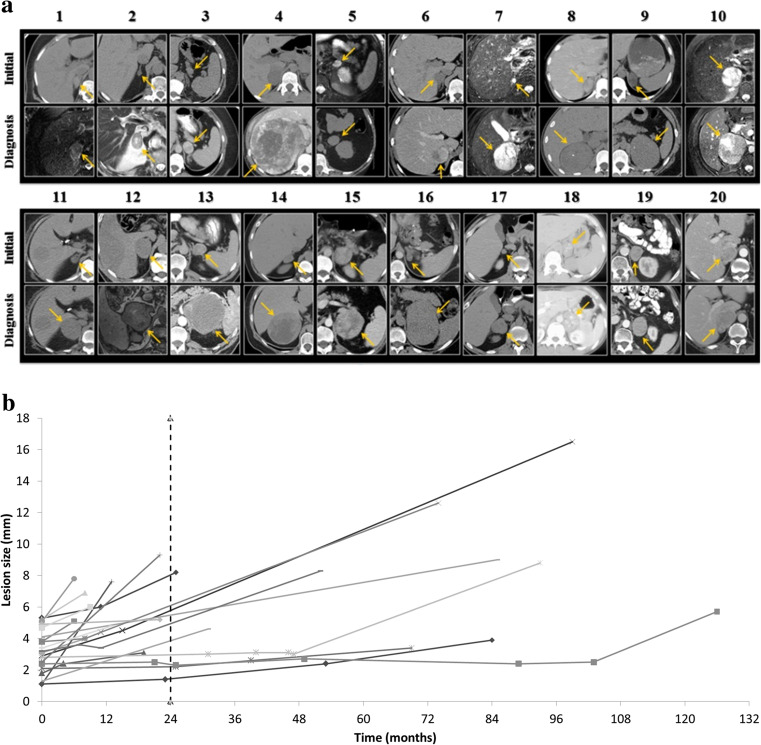
The initial imaging study in 17 of 20 patients was a CT scan. Twelve lesions were heterogeneous in appearance on initial imaging with a median size of 3.7 cm (2.1–5.3), compared to a median size of 1.7 cm (1.1–4.5) for 8 homogeneous lesions. In all cases, where an initial unenhanced CT was available, the attenuation exceeded 11 Hounsfield units (HU) (12–67 HU). Three patients initially underwent MR imaging; all adrenal lesions demonstrated hyperintense signal intensity on T2-weighted sequences (Table 1, Fig. 1a).
Fig. 1.
a All tumors (yellow arrows), initial imaging (upper panels) and final imaging (lower panels) at time of diagnosis. b Growth of tumors from initial tumor to final size (considering intermediates where available). Dashed line shows 24 month mark, which is the recommended follow-up by several guidelines
The overall mean size of the initial lesion was 3.2 ± 1.3 cm (median 3.4, range 1.1–5.3). Six of 20 lesions were greater than 4 cm on initial imaging and had a lead time range of 6–96 months to ACC diagnosis. Three of these were identified as ACCs within 12 months. Nine tumors were between 2 and 4 cm in diameter on initial imaging with a lead time range to diagnosis of 6–131 months, of which 2 were identified within 12 months. Five tumors were less than 2 cm on initial imaging and had a lead time range of 13–84 months.
All five lesions less than 2 cm in size were homogeneous. In retrospective review, one or more findings suggesting a benign diagnosis (homogeneity, attenuation <10 HU, regular margins, signal drop in and out-of-phase MR imaging, low signal intensity in T2-weighted images) were present in only two patients. However, both of these nodules were only evaluated by contrast-enhanced CT and no unenhanced HU are available, leaving the possibility that they would have been possible to diagnose as not clearly benign lesions based on the HU criteria. Two other lesions had non-contrast enhancement >10 HU and one tumor was bright on T2-weighted MRI image. Therefore, none of these lesions had a full work-up identifying them as benign tumors.
Rate of Growth
The mean increase in the largest diameter was 1.9 ± 1.7 cm per year, range (0.2 to 6.0) (Fig. 1b). Seven of 20 lesions had an average growth rate of less than 1 cm per year (see Supplementary Figure 1). A variety of growth patterns were observed, including rapid growth (exceeding 2 cm/year) in 5 patients, slower but steady growth in 9 patients, and a period of stability followed by growth in 6 patients. Of these 6 cases where a latent period of relative stability was observed, minimal growth was observed from a range of 7 months to 2 years in 4 patients, and more than 4 years of stability was observed in the remaining 2 patients. Lesions exhibiting a latent period had an initial mean size of 2.6 ± 0.82 cm (median 2.7, range 1.4–3.6). When lesions exhibiting a period of stability were excluded from the analysis, the mean increase in largest diameter was 2.4 ± 1.8 cm per year, range (0.69 to 6.0).
Clinical Evaluation
At the time of final ACC diagnosis, eight of 20 patients (40 %) had clinical or biochemical evidence of excess hormone secretion (cortisol 4, androgen 5, aldosterone 2). Three of the eight functional lesions produced hormones in combination. Unfortunately, initial endocrine work-up is largely unavailable for all patients. In 2 patients, an initial concern of hormone excess was raised but not confirmed (one congenital adrenal hyperplasia, one Cushing’s syndrome). Final pathology evaluation and clinical parameters are available in supplementary tables 1 and 2. There were no obvious differences when compared to ACCs without prior imaging available. Clinical and pathological parameters fall well into the usually reported ranges for ACC and are not different than that found in the overall collective.
Discussion
Our findings raise several concerns regarding the management of incidentally identified adrenal lesions. The initial management differed from guideline recommendations in the majority of patients. This may be due to the infrequency with which ACCs are encountered in community practices. Of the 6 patients with lesions greater than 4 cm in diameter, surgery could have been recommended at least for 4 patients with lesions with a heterogeneous appearance. The remaining 2 lesions exceeding 4 cm in size were homogeneous but had attenuation values well above 10 HU, a trigger for an adrenal protocol CT study with thin slices and delayed enhanced images to assess washout characteristics or further MRI evaluation (15–17). These studies could have been applied to 6 additional smaller lesions, including one with a stability period of 2 years.
Guidelines from the AME, ACR, and AACE/AAES recommend consideration of operative management for lesions <4 cm in the presence of concerning imaging characteristics (12–14). Of 14 patients with lesions smaller than 4 cm, 8 had a heterogeneous appearance and 1 was bright on T2-weighted MR imaging, which could have led to the consideration of surgery as initial management in 9 (64 %) patients. Therefore, initial surgical therapy would have been an option for more than half of patients. However, considering the high prevalence of incidental adrenal tumors and the low prevalence of ACC, surgical resection of all lesions >2 cm with indeterminate features will likely cause significant overtreatment and treatment-associated morbidity. Therefore, new diagnostic tools with increased specificity for ACC are needed in the initial work-up of incidentally discovered masses.
Although a variety of surveillance recommendations have been proposed, there is consensus that growth >1 cm/year is an indication for surgery. However, slower growth may be falsely reassuring. In this series, 7 (35 %) of 20 patients had average growth rates <1 cm per year. Moreover, the presence of a latent period in 6 (30 %) of the patients in our series, ranging from 7 months to 8 years, was interpreted as suggestive of a benign nature. The growth pattern was very variable. One lesion had a period of stability for the initial 2 years of surveillance, eventually undergoing surgery 7 years after the initial identification. Another had accelerated growth, from 1.1 cm on initial imaging to 7.6 cm only 13 months later. Differences in initial imaging characteristics were not discernable in lesions exhibiting a latent phase compared with those that grew more steadily. Currently recommended strategies do not mandate surveillance beyond 2 years; however, in our series, the time to diagnosis exceeded this timeframe in 10 (50 %) patients (12–14). Therefore, the current approach of limited surveillance will identify only half of all ACCs. Furthermore, this finding shows that not all lesions stable over 1–2 years can confidently be diagnosed as benign (particularly those with indeterminate imaging features), as suggested by many guidelines (12–14).
Alarmingly, 5 (25 %) of the patients in our series had lesions smaller than 2 cm on initial imaging. Such lesions would potentially be excluded from surveillance by guidelines advocating follow-up only of tumors exceeding 2 cm in diameter. However, none of these lesions had a complete imaging and biochemical work-up identifying them as clearly benign. Particularly, the two homogeneous lesions, only evaluated on contrast-enhanced CT scan, should have been evaluated with an unenhanced CT scan. It has recently been shown that the criterion of <10 HU is a good discriminator for adenomas vs. ACCs (11).
The low incidence of ACC has left many gaps in our understanding of its antecedent history. A limitation of this study is the retrospective nature including the often incomplete work-up of adrenal nodules not in accordance with suggested guidelines and lack of proof for a pathogenic connection between initial lesions and the later diagnosed malignant tumor. Another concern is the non-standardized follow-up of the patients in this study, exclusively at institutions other than our own center. Indeed, at our center, it is standard to complete all imaging and biochemical work-up in accordance with guidelines to gather as much information as possible to judge about the biological behavior of adrenal tumors. Imaging cannot determine whether the initial lesions represent adenomatous precursors or small ACCs. Therefore, three main explanations for the observed findings can be proposed: (1) The initial adrenal lesions can be benign lesions unrelated to the later development of ACC, (2) the initial nodules might represent benign tumors that progress to malignant tumors, or (3) the initial tumors represent small ACCs, which were failed to be diagnosed as ACCs either because of incomplete work-up or insufficient criteria to determine the biological behavior. Regardless of any of these possibilities, progression of adrenal lesions to an overt malignancy is the main reason to recommend imaging surveillance and this study shows a significant time interval between initial imaging and final ACC diagnosis, often outside the recommended range of follow-up imaging. Unfortunately, the rarity of ACC and the high prevalence of benign adrenal lesions preclude a prospective study to gather evidence for more general recommendations in follow-up.
In view of the wide variability in progression, this study raises the concern that current strategies for surveillance may not be effective in the early identification of malignant adrenal tumors arising in patients with incidentally discovered adrenal masses. Aggressive responses to such evidence could involve a combination of lower operative thresholds and more frequent and prolonged surveillance. Other imaging modalities such as fluorodeoxyglucose (FDG)-PET scans have been suggested to be employed in the differential diagnosis of benign adrenal tumors and ACC. Unfortunately, these studies included only a small number of ACCs or evaluated very late stage disease (16, 17). Furthermore, 10 % of adenomas are FDG-avid giving FDG-PET a low positive predictive value for diagnosing the very rare ACC (15).
In addition to imaging characterization, there might be additional benefit from annual biochemical surveillance. At the time of diagnosis of ACC, at least 40 % of tumors had become overtly hormone-producing. Therefore, thorough biochemical surveillance would have at least diagnosed these tumors. Future methods, such as mass spectrometry analysis of urine or serum steroids, might even display better diagnostic parameters (18).
Significant public health implications exist for both broadening the indications for adrenalectomy with attendant risks requiring specialized expertise for optimal performance, and increased cross-sectional imaging, given emerging evidence that diagnostic doses of ionizing radiation may contribute to risk of malignancy and the significant cost of alternatives such as MRI (3, 5, 19, 20). In light of the high prevalence of incidentalomas, consequences of such measures would be compounded and therefore should be considered judiciously.
Certainly, new modes to determine the differential diagnosis of benign and malignant adrenal masses are needed.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Example of patient followed with serial imaging. (DOCX 458 kb)
(DOCX 24 kb)
(DOCX 15 kb)
Acknowledgments
RL and TE were sponsored by the T32-DK007245.
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
T. M. Nogueira and R. Lirov contributed equally to this work.
References
- 1.Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol. 2002;179:559–568. doi: 10.2214/ajr.179.3.1790559. [DOI] [PubMed] [Google Scholar]
- 2.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–340. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 3.Park HS, Roman SA, Sosa JA. Outcomes from 3144 adrenalectomies in the United States: which matters more, surgeon volume or specialty? Arch Surg. 2009;144:1060–1067. doi: 10.1001/archsurg.2009.191. [DOI] [PubMed] [Google Scholar]
- 4.Williams AR, Hammer GD, Else T. Transcutaneous biopsy of adrenocortical carcinoma is rarely helpful in diagnosis, potentially harmful, but does not affect patient outcome. Eur J Endocrinol Eur Fed Endoc Soc. 2014;170:829–835. doi: 10.1530/EJE-13-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cawood TJ, Hunt PJ, O’Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol Eur Fed Endoc Soc. 2009;161:513–527. doi: 10.1530/EJE-09-0234. [DOI] [PubMed] [Google Scholar]
- 6.Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35:282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85:637–644. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 8.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190:1163–1168. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 9.Bharwani N, Rockall AG, Sahdev A, et al. Adrenocortical carcinoma: the range of appearances on CT and MRI. AJR Am J Roentgenol. 2011;196:W706–714. doi: 10.2214/AJR.10.5540. [DOI] [PubMed] [Google Scholar]
- 10.Hussain S, Belldegrun A, Seltzer SE, Richie JP, Gittes RF, Abrams HL. Differentiation of malignant from benign adrenal masses: predictive indices on computed tomography. AJR Am J Roentgenol. 1985;144:61–65. doi: 10.2214/ajr.144.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Petersenn S, Richter PA, Broemel T, et al. Computed tomography criteria for discrimination of adrenal adenomas and adrenocortical carcinomas: analysis of the German ACC registry. Eur J Endocrinol Eur Fed Endoc Soc. 2015;172:415–422. doi: 10.1530/EJE-14-0916. [DOI] [PubMed] [Google Scholar]
- 12.Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol JACR. 2010;7:754–773. doi: 10.1016/j.jacr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Terzolo M, Stigliano A, Chiodini I, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol Eur Fed Endoc Soc. 2011;164:851–870. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 14.Zeiger MA, Thompson GB, Duh QY, et al. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endoc Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2009;15(Suppl 1):1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 15.Caoili EM, Korobkin M, Brown RK, Mackie G, Shulkin BL. Differentiating adrenal adenomas from nonadenomas using (18)F-FDG PET/CT: quantitative and qualitative evaluation. Acad Radiol. 2007;14:468–475. doi: 10.1016/j.acra.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Leboulleux S, Dromain C, Bonniaud G, et al. Diagnostic and prognostic value of 18-fluorodeoxyglucose positron emission tomography in adrenocortical carcinoma: a prospective comparison with computed tomography. J Clin Endocrinol Metab. 2006;91:920–925. doi: 10.1210/jc.2005-1540. [DOI] [PubMed] [Google Scholar]
- 17.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med Off Publ Soc Nucl Med. 2006;47:32–37. [PubMed] [Google Scholar]
- 18.Arlt W, Biehl M, Taylor AE, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96:3775–3784. doi: 10.1210/jc.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 20.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example of patient followed with serial imaging. (DOCX 458 kb)
(DOCX 24 kb)
(DOCX 15 kb)