Abstract
Phosphatase and tensin homologue (PTEN) is a known tumour suppressor. To explore the role of Pten in ovarian tumorigenesis, we used transgenic (Tg) SOX2. Cre and AMH. Cre mouse models to direct global Pten haploinsufficiency (Pten +/−) or ovary-specific granulosa cell (GC) Pten disruption (Pten GC). Pten mutant models were combined with progressively rising Tg-follicle-stimulating hormone (TgFSH) levels to study the tumorigenic potential of combined genetic/endocrine modification in vivo. Global Pten +/− mice exhibited grossly detectable tumours in multiple organs including uterine and mammary tissue and displayed reduced survival. Despite extra-ovarian tumorigenesis, Pten +/− females had no detectable ovarian tumours, although elevated corpus luteum numbers increased ovary size and estrous cycling was altered. Combined TgFSH/Pten +/−mice also had no ovarian tumours, but early survival was reduced in the presence of TgFSH. Ovary-specific Pten GC ± TgFSH females exhibited no detectable ovarian or uterine tumours, and corpus luteum numbers and estrous cycling remained unchanged. The non-tumorigenic ovarian phenotypes in Pten +/− and Pten GC ± TgFSH mice support the proposal that multi-hit genetic mutations (including ovarian and extra-ovarian tissue) initiate ovarian tumours. Our findings suggest that elevated FSH may reduce early cancer survival; however, the ovary remains remarkably resistant to Pten-induced tumorigenic changes even in the presence of uterine and reproductive cancers.
Keywords: Granulosa Cell, Corpus Luteum, Premature Ovarian Failure, Granulosa Cell Tumour, Pten Mutation
Introduction
Phosphatase and tensin homologue (PTEN) is one of the most commonly lost tumour suppressors in human cancers, and somatic PTEN mutations have been found in ovarian cancers [1]. PTEN acts as a key negative feedback regulator of the phosphoinositide 3-kinase (PI3K) signalling pathway [2]. During tumorigenesis, PTEN mutations that upregulate PI3K signalling cause increased cell proliferation rates and decreased cell death [3, 4]. Pten mutations in humans and mice cause tumours in selected tissues suggesting that PTEN acts in a tissue-specific manner to regulate PI3K pathways and normal cellular growth [2, 5]. Loss of heterozygosity on chromosomes containing Pten has been reported to occur frequently in ovarian cancer [6–10], suggesting the involvement of tumour suppressor gene inactivation in ovarian tumour development.
Follicle-stimulating hormone (FSH) is synthesised and secreted by gonadotrophs of the anterior pituitary gland [11]. FSH sustains ovarian follicle development and steroid production by binding the FSH receptor localised to follicular granulosa cells (GCs). The FSH receptor is also found in ovarian surface epithelium, although its function there remains unknown [12]. FSH actions inhibit apoptosis [13] and promote GC proliferation and differentiation [14–16], as well as stimulate production of steroid and protein hormones [17]. An early sign of reproductive ageing in women is a rising serum FSH levels; however, it is not known whether this contributes to the decline in female fertility with age [18]. Previously, we developed a double transgenic (Tg) FSH mouse model that exhibited progressively rising serum levels of bioactive heterodimeric human FSH independent of follicle depletion, which also displayed premature infertility [19]. Development and progression of ovarian cancer has previously been attributed to repetitive ovulatory trauma (‘incessant ovulation hypothesis’) and high-circulating concentrations of gonadotropins [20], as well as the ‘excessive gonadotropin’ hypothesis [21], which proposes elevated gonadotropin (and estradiol) levels as a contributing factor in ovarian cancer risk. The activated FSH receptor signals via cAMP and protein kinase A [22], as well as the PI3K/AKT pathway [23], which can regulate many aspects of cell function including cell cycle progression and arrest, DNA repair and apoptosis [24, 25].
Conditional disruption of Pten in mouse models is expected to advance our understanding of its specific role and the pathophysiology of ovarian tumorigenesis. Homozygous loss of murine Pten is embryonic lethal, whereas heterozygous Pten +/− mice are viable but develop tumours in multiple tissues [26]. Although ovarian cancer was absent in Pten +/− females, no detailed ovarian analysis was reported [26]. Transgenic Cre-loxP strategies designed to study the local role of Pten in ovarian tumorigenesis have produced contrasting results. For example, the use of Tg. Amhr2. Cre mice to modify Pten in ovarian GCs led to 7 % of females developing ovarian tumours of GC origin between 7 weeks and 7 months of age [27]. In contrast, Tg. Cyp19. Cre-mediated disruption of Pten in GCs did not cause ovarian tumour formation [28], although ovaries had increased proliferation of mutated GCs and corpora lutea (CL) that persisted at least twice of long as controls. The precise cause of these different ovarian phenotypes remains to be determined, but may reflect different cell types and tissues targeted by the supposed GC-specific Tg promoters [29].
Here, we investigated the selective role of global Pten (heterozygous) haploinsufficiency via the SOX2 gene promoter [30] or ovarian Pten disruption using a GC-specific Tg. Cre directed via the AMH gene promoter [31]. Furthermore, we combined both Pten disruption models with our Tg model overexpressing circulating FSH activity (Tg.FSH) and steroids (testosterone) [32]. Our global Tg.SOX2. Cre-induced Pten (Pten +/− ) disruption model and granulosa cell-specific Tg.AMH. Cre-induced Pten disruption model (Pten GC−/− and Pten GC+/−) combined with Tg.FSH provides a unique experimental in vivo platform to determine the impact of multiple factors (genetic and gonadotropin) proposed to contribute to ovarian tumorigenesis.
Materials and Methods
Genetic Mouse Models
All animal procedures were approved by the Animal Welfare Committee of the Sydney Local Health District and performed in accordance to the National Health and Medical Research Council code of practice for care and use of animals and the NSW Animal Research Act (1985).
Global Pten+/− Mice
To generate global heterozygous Pten +/− females, Tg.SOX2. Cre mice that display Cre activity detectable in all tissues [30] were mated with Pten-floxed mice; Pten exon 5 was flanked by loxP sites as previously described [33, 34]. SOX2-Cre promoter is a universal deleter that is expressed from embryonic day 6.5 [30].
GC-Specific Pten Mutant Mice
To target GC Pten mutation (denoted Pten GC), Tg.AMH. Cre mice were crossbred with Pten-floxed mice [35]. Tg.AMH. Cre mice exhibit GC-specific Cre expression, with no detectable uterine or pituitary Cre activity [36, 37]. Cross breeding regimes generated Tg.AMH. Cre:Pten flox/flox (denoted Pten GC−/− or Pten GC), Tg.AMH. Cre:Pten flox/wt (denoted Pten GC+/−) and non-Tg control groups.
TgFSH Mice
Our TgFSH model (previously referred to as TgFSHH for high FSH levels [32]) exhibits pituitary-independent FSH expression driven via the rat insulin II gene promote [32, 38]. TgFSH mice were crossbred with Tg.AMH. Cre and Tg.SOX2. Cre mice then combined with the Pten-floxed background.
Age-matched Tg and non-TgFSH female littermates used in experiments were housed under controlled conditions (12 h light-dark cycle) with ad lib access to food and water. Mice weights and general well-being were monitored weekly over the period of 3–12 months of age.
Genotyping
Genomic DNA samples isolated from toe, tail tip or ovary tissue by lysis using proteinase K as previously described [39] were used for PCR genotyping to detect floxed, Tg. Cre-mediated mutant or wild type mouse Pten [40] all using previously described primers and conditions. Mice containing Tg.AMH. Cre, Tg.SOX2. Cre and TgFSH were detected using PCR conditions as previously described, with actin providing an internal sample control [31, 38]. Primer pairs and expected amplicon product sizes for genotyping are listed in Table 1.
Table 1.
Genotyping by PCR analysis confirmed the presence of specific gene mutations and transgenes in experimental mice, using primer pair sequences and expected amplicon product sizes described in the references shown
| Target gene | Forward primer | Reverse primer | PCR product size | Source |
|---|---|---|---|---|
| Pten (floxed/Wt) | 5′-TCC CAG AGT TCAT ACC AGG A − 3’ | 5′-AAT CTG TGC ATG AAG GGA AC-3’ | Wt—500 bp Floxed—650 bp | [40] |
| Pten ∆ | 5′-TCC CAG AGT TCAT ACC AGG A − 3’ | 5′-GCA ATG GCC AGT ACT AGT GAA C-3’ | 300 bp | [40] |
| TgCre | 5′-CTG ACC GTA CAC CAA AAT TTG CCT G-3′ | 5′-GAT AAT CGC GAA CAT CTT CAG GTT C-3′ | 600 bp | [31] |
| TgFSH | 5′-AAT GCT CAG CCA AGG ACA AAG A-3′ | 5′-AAC TTA ATG AAA CCG GCC TAA T-3′ | 213 bp | [38] |
Estrous Cycle Analysis
Estrous cycle analysis was performed at 6 months of age and again just prior to sacrifice of mice at 12 months of age. Vaginal samples (in 20 uL of sterile 0.9 % saline) were obtained daily at 08:00–09:00 am over 12 days and smeared onto glass slides and stained with 0.05 % Trypan Blue for microscopic classification into estrous stages [19]. All control TgFSH and PtenGC mice were collected at diestrus stage (if they were cycling), and Pten+/− mice were collected at diestrus (when possible) but due to health deterioration due to tumour burden with non-cycling mice, this was not always possible.
Serum Collection, Tissue Processing and Hormone Analysis
Mice were examined weekly for general health (activity, body weight, fur condition and palpable tumour development) and at 12 months of age mice, or when health significantly decreased, were weighed and blood collected by cardiac exsanguination under ketamine/xylazine anaesthesia. Blood was allowed to clot at room temperature for 20 min before centrifugation at 5000 rpm for 5 min, then serum was collected and stored at −80 °C until assay. Serum TgFSH levels were measured by species-specific (human) FSH immunoassay as described previously [38, 41]. Serum testosterone (T), estradiol (E2), dihydrotestosterone (DHT), adione and progesterone (P4) levels were measured in extracts of 100-uL serum by liquid chromatography tandem mass spectrometry (LC-MS/MS; [42]) as modified for mouse samples [43]. The lowest limits of quantification (defined as detectable with a coefficient of variation <20 %) were 0.025 ng/ml for testosterone, 5 pg/ml for E2, 0.1 ng/ml for DHT, 0.05 ng/ml for adione and 0.1 ng/ml for P4.
Ovaries, uteri, pituitary and any detectable tumour tissues were removed and weighed, and tail tip or toe were removed and immediately frozen (liquid N2) for DNA or RNA (pituitary, tail, toe, one ovary), steroid assay (liquid chromatography tandem mass spectrometry, LC-MS/MS) or fixed in 4 % paraformaldehyde (ovary, uterus, tumours) overnight and transferred to 70 % ethanol for histological analysis.
Ovarian Histology and Corpora Lutea Quantification
One fixed ovary from each mouse was embedded in paraffin, sectioned at 8 μm and every 10th section stained with haematoxylin and eosin (H&E). Total corpus luteum counts were undertaken by light microscopy using a ×40 oil objective and the Stereo Investigator software (MicroBrightfield, Williston, VT).
Tumour Histology and Classification
Histological H&E-stained 8-μm tissue sections were examined using light microscopy by a board-certified veterinary anatomic pathologist. Tumours were classified as per Guides in Toxicologic Pathology for Proliferative Lesions in Rats for the reproductive tract [44] and mammary gland [45].
Statistical Analysis
Statistical analysis was performed using the NCSS (NCSS Statistical Software) software. Statistical differences were tested by one-way or two-way ANOVA (to assess the effect of genotype (G), FSH status, and genotype × FSH status interaction) with post hoc test using Fisher’s least significant difference multiple-comparison test. Main effects of genotype (G) and FSH status are reported when two-way ANOVA was utilised, and interaction results are omitted if not significant. Survival data was analysed by Kaplan Meier analysis, and percentage/proportional data was analysed by z test. P ≤ 0.05 was considered statistically significant.
Results
Genetic Mouse Models
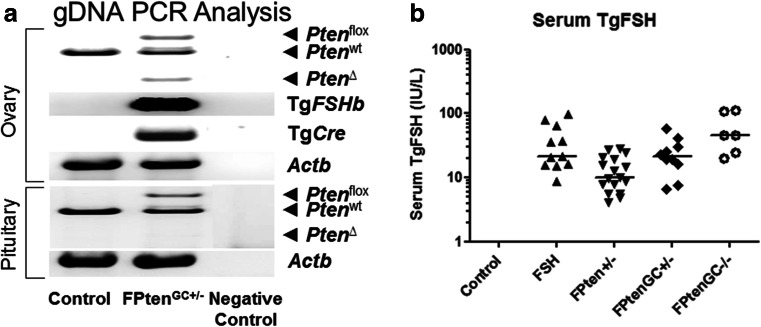
Genotyping confirmed the presence of Cre-mediated Pten mutations and transgenes in experimental mice, with groups designated as shown in Table 2. Whole ovary genotyping detected the presence of floxed Pten and Tg.AMH. Cre-loxP-mediated Pten disruption, 650 and 300 bp PCR products, respectively, as shown in Fig. 1. In contrast, pituitary (Fig. 1) and toe (not shown) had no detectable Pten deletion, consistent with ovary-specific Cre-mediated gene disruption described elsewhere [37, 46]. Genotyping control animals produced the expected 500 bp PCR product for normal Pten. Transgenic Cre and TgFSH (β-subunit) screening produced the expected PCR products, and the beta-actin PCR product confirmed the presence of genomic DNA in all samples.
Table 2.
Study group genotypes, descriptions of genetic modifications and abbreviations of genotype, where ‘+’ indicates presence of Pten mutations or TgFSH overexpression and ‘−’ indicates the absence of Pten mutations or TgFSH overexpression
| Abbreviation | TgFSH | PtenGC+/− | PtenGC−/− | Pten+/− | |
|---|---|---|---|---|---|
| AMH. Cre model (ovary specific) | |||||
| Non-Tg control | C | − | − | − | − |
| Non-Tg PtenGC+/− | PtenGC+/− | − | + | − | − |
| Non-Tg PtenGC−/− | PtenGC−/− | − | − | + | − |
| TgFSH control | F | + | − | − | − |
| TgFSH PtenGC+/− | FPtenGC+/− | + | + | − | − |
| TgFSH PtenGC−/− | FPtenGC−/− | + | − | + | − |
| Sox2. Cre model (global) | |||||
| Non-Tg Pten+/− | Pten+/− | − | − | − | + |
| TgFSH Pten+/− | FPten+/− | + | − | − | + |
Fig. 1.
a Whole ovary genotyping showed the presence of floxed Pten and the Tg.AMH. Cre-loxP-mediated Pten deletion. In contrast, pituitary had no detectable Pten deletion. Genotyping wild type animals produced the expected PCR products for normal Pten introns. Transgenic Cre and Tg.FSH (β-subunit) screening produced the expected PCR products, and the beta-actin PCR product confirmed the presence of genomic DNA in all samples. b Serum human (Tg)FSH levels were measured using a species-specific immunoassay. TgFSH levels were significantly increased in all Tg females when compared to non-Tg controls (two-way ANOVA G, p < 0.001; FSH, p < 0.001; GxFSH, p < 0.001). Data presented as raw data plotted with median
TgFSH Expression
As expected, serum TgFSH levels were not detectable in non-Tg control females (Fig. 1). Serum TgFSH levels were 41.0 ± 13.6 IU/L (N = 12) in aged control TgFSH alone (F) mice and equivalent to levels found in FPten GC+/− (29.1 ± 6.3 IU/L, N = 6) and FPten GC−/− (71.0 ± 22.6 IU/L, N = 4) females (Fig. 1). In comparison, lower circulating TgFSH levels were found in FPten +/− mice compared to control TgFSH (15.6 ± 2.1 IU/L, N = 12, one-way ANOVA p < 0.05).
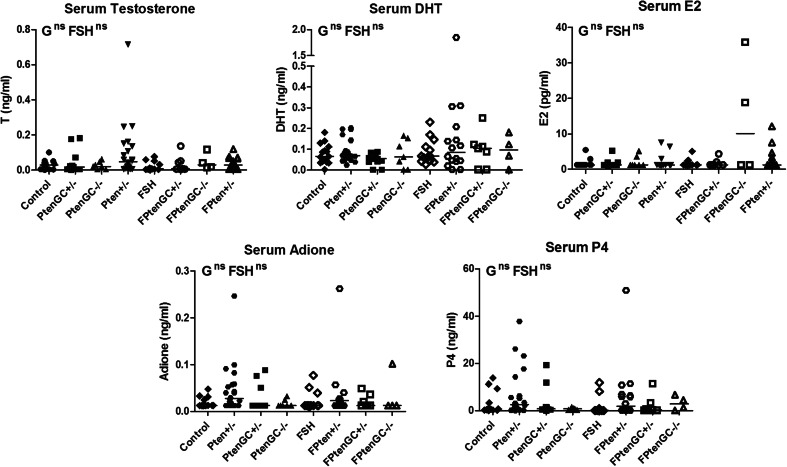
Effect of Genotype on Serum Steroid Levels
Serum steroid analysis found no significant differences according to G or FSH p < 0.05 (by two-way ANOVA) in testosterone (T), dihydrotestosterone (DHT), estradiol (E2), adione and progesterone (P4) (Fig. 2).
Fig. 2.
Analysis of serum steroids found no significant differences according to G or FSH p < 0.05 by two-way ANOVA. N > 4 mice per genotype
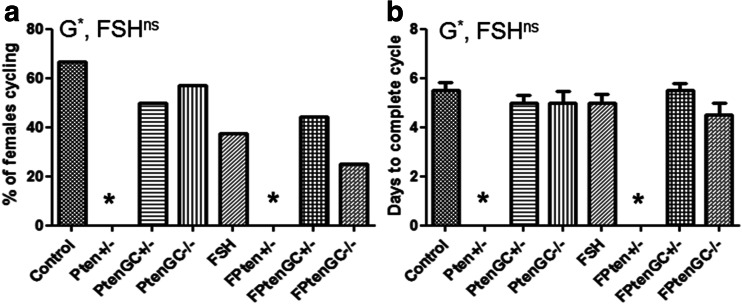
Effects of Pten Mutation and TgFSH on Estrous Cycles
The percentage of aged female mice able to cycle was not affected by the presence of TgFSH (Fig. 3). However, the Pten +/− genotype (regardless of the presence of TgFSH) completely removed the ability of aged mice to complete an estrous cycle over 12 days (one-way ANOVA, p < 0.001) (Fig. 3a). Pten +/− females typically displayed only the metestrus and diestrus stages for the entirety of the smearing period. In contrast, cycle length was found to be normal (two-way ANOVA G, p = 0.74; FSH, p = 1.00; one-way ANOVA p = 0.61) in Pten GC mutant mice (Fig. 3b).
Fig. 3.
a Estrous cycle analysis was performed just prior to collection of mice at 12 months of age. Follicular Pten mutation alone or combined with Tg.FSH had no effect on the ability females to exhibit estrous cycling; however, the presence of a global Pten mutation removed the ability of the mice to complete an estrous cycle. b Cycle length was significantly altered in the global Pten mutant mice. *p < 0.05. N = 7–12 per genotype
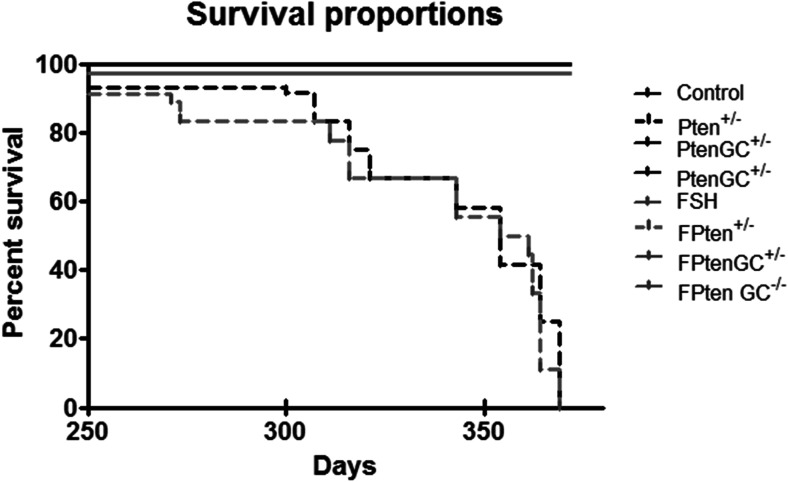
Effects of Pten Mutations and TgFSH on Female Survival
Global Pten +/− mutant mice exhibited a significantly reduced lifespan (log-rank (Mantel-Cox) Test p < 0.005) with none surviving to 12 months, compared to control females which all survived to 12 months of age (Fig. 4). In contrast, no deaths were observed before 1 year of age in the ovary-specific Pten GC-mutated females.
Fig. 4.
Pten +/− ± TgFSH mice had accelerated tumour formation and significantly decreased survival rate when compared to controls (p < 0.05). Black line represents controls, Pten GC+/− and Pten GC−/− and grey line TgFSH, FPten GC+/− and FPten GC−/− mice (N = 7–12 mice per genotype), black broken line represents Pten +/− (N = 31) and grey broken line represents FPten +/− (N = 27)
Effects of Genotype on Ovarian Weight, Morphology, and Corpora Lutea Numbers
Global Pten +/− ovary weights were increased (Pten +/− vs controls one-way ANOVA p < 0.05; two-way ANOVA Pten effect p = 0.42; FSH p = 1.00 (Fig. 5a)), and a significant increase in corpora lutea (CL) numbers was observed in these mice (Fig. 5b) (one-way ANOVA, p < 0.001; two-way ANOVA, Pten effect p < 0.05; FSH p = 1.00). FPten +/− mice ovary weights and CL numbers remained unchanged (ovary weights: FPten +/− vs controls two-way ANOVA Pten effect p = 0.42; FSH p = 1.00; CL numbers: one-way ANOVA, p = 0.68; two-way ANOVA, Pten effect p < 0.05; FSH p = 1.00). Ovary weights remained unchanged among other genotypes when compared to controls (PtenGC+/− p = 1.000, PtenGC−/− p = 0.100, FSH p = 0.99, FPtenGC+/− p = 0.98 and FPtenGC−/− p = 0.98), and total CL of Pten GC mutation mice remained unchanged (PtenGC+/− p = 1.000, PtenGC−/− p = 0.99, FSH p = 1.000, FPtenGC+/− p = 1.000 and FPtenGC−/− p = 1.000) (Fig. 5a). No tumorigenic changes were observed in any ovarian morphology in the presence of Pten +/− or Pten GC mutations with or without TgFSH (Fig. 5c).
Fig. 5.
a Global Pten +/− ovary weights were increased (Pten +/− vs controls one-way ANOVA p < 0.05; two-way ANOVA Pten effect p = 0.42; FSH p = 1.00). Ovary weights remained unchanged among other genotypes when compared to controls (PtenGC+/− p = 1.000, PtenGC−/− p = 0.100, FSH p = 0.99, FPtenGC+/− p = 0.98 and FPtenGC−/− p = 0.98). b Total numbers of corpora lutea were significantly increased in 12-month-old Pten +/− (one-way ANOVA, p < 0.001; two-way ANOVA, Pten effect p < 0.05; FSH p = 1.00) but remained unchanged in FPten +/− genotype (one-way ANOVA, p = 0.68; two-way ANOVA, Pten effect p < 0.05; FSH p = 1.00) compared to Tg and non-Tg controls. Pten +/− was significantly increased when compared to FPten +/−. Pten GC mice CL numbers were not significantly different from controls (PtenGC+/− p = 1.000, PtenGC−/− p = 0.99, FSH p = 1.000, FPtenGC+/− p = 1.000 and FPtenGC−/− p = 1.000). Genotype abbreviations defined in the text shown in bar graph by small checks, C; large checks, Pten +/−; horizontal stripes, Pten GC+/−; vertical stripes, Pten GC−/−; left diagonal lines, F; right diagonal lines; FPten +/−; cross hatch, FPten GC+/−; brick pattern, FPten GC−/−. Data shown as mean ± SEM, N = 7–12 per genotype. Asterisk indicates significant difference via one-way ANOVA compared to C mice. c No tumorigenic changes were observed in any ovarian morphology in the presence of Pten +/− or Pten GC mutations with or without TgFSH. (4× magnification). Asterisk indicates corpus luteum and circumflex accent indicates haemorrhagic cyst
Effects of Genotype on Uterine Weight and Morphology
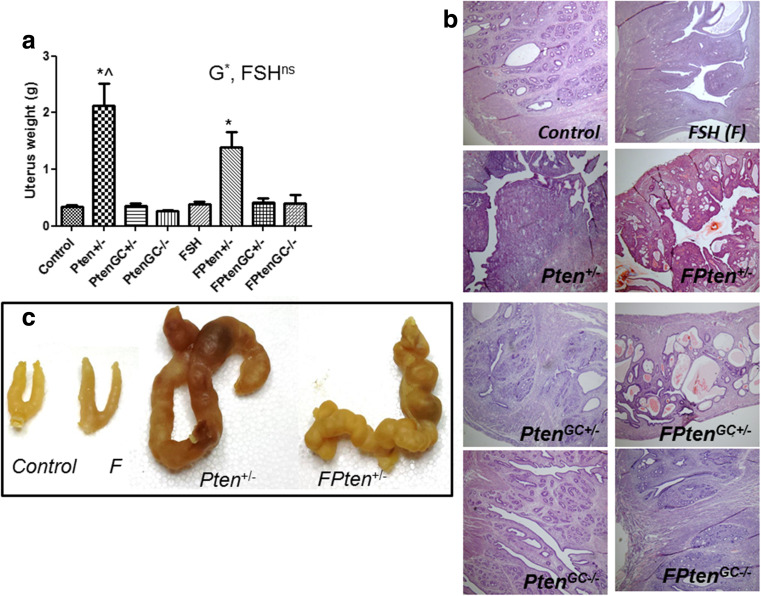
Global Pten +/− resulted in a significantly increased uteri weights in both Tg and non-TgFSH mice (two-way ANOVA Pten effect, p < 0.001; FSH, p = 1.00) (Fig. 6a), which was due to the presence of uterine tumours (Fig. 7d, e). No tumorigenic changes were observed in uterine morphology of Pten GC mice with or without TgFSH (Fig. 6b and c), which all had normal-sized uteri.
Fig. 6.
a Relative uterus weights remained unchanged between genotypes in 12–month-old females from all Pten GC genotypes, with or without Tg.FSH expression; however, both Tg and non-Tg Pten+/− females had significantly increased uteri weights due to uterine tumorigenesis (one-way ANOVA p < 0.001; two-way ANOVA G, p < 0.001; FSH, p = 1.00). b Analysis of uteri histology of H&E-stained sections showed that no macroscopic tumours were present in 12-month-old PtenGC females of any genotypes examined (4× magnification); however, some Pten+/− (±TgFSH) females developed uterine tumours. c Typical gross uteri appearance from the indicated genotypes. Genotype abbreviations defined in the text shown in bar graph by small checks, C; large checks, Pten +/−; horizontal stripes, Pten GC+/−; vertical stripes, Pten GC−/−; left diagonal lines, F; right diagonal lines; FPten +/−; cross hatch, FPten GC+/−; brick pattern, FPten GC−/−. Data shown as mean ± SEM, N = 7–12 per genotype. Asterisk indicates significant difference via two-way ANOVA compared to C and F mice and circumflex accent indicates significant differentce to FPten+/−
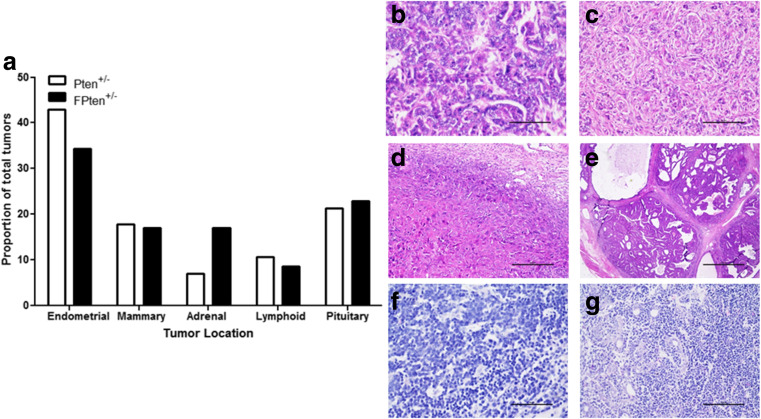
Fig. 7.
Incidence of tissue tumours in Pten +/− ± TgFSH females. a Proportion of commonly found tumours. b–g Haematoxylin and eosin-stained tissue sections showing typical mammary adenocarcinoma (b), mammary fibroadenoma (c), endometrial choriocarcinoma (d), endometrial adenocarcinoma (e), lymphocarcoma (f) and lymphosarcoma invading mammary adenoma (g)
Global Pten mice developed a variety of neoplastic, pre-neoplastic benign and malignant cancers with only approximately 35 % of mice retaining grossly normal uteri at termination. Of the Pten +/− mice, 13 % displayed metaplasia and 23 % had dysplastic endometrium, 10 % exhibited polyps, 6 % had endometrial adenomas and 16 % had endometrial choriocarcinomas. Similarly, the FPten +/− mice displayed hyperplasia (7 %) and dysplasia (22 %) of endometrial tissue, endometrial polyps (11 %), endometrial adenocarcinoma (4 %) and choriocarcinoma (15 %) (Fig. 7 and Table 3). No neoplastic, pre-neoplastic benign and malignant tumours were observed in control and Pten GC mice.
Table 3.
Tumorigenesis in Pten +/− ± TgFSH females. Summary of tumour types and incidence (number affected in whole group and shown as %) in the indicated tissues of Pten +/− and FPten +/− mice.
| Pten+/− (%) | FPten+/− (%) | |
|---|---|---|
| Endometrium | ||
| NAD | 10/31 (32) | 10/27 (37) |
| Polyps | 3/31 (10) | 3/27 (11) |
| Metaplasia | 4/31 (13) | 0/27 (0) |
| Hyperplasia | 0/31 (0) | 2/27 (7) |
| Dysplasia | 7/31 (23) | 6/27 (22) |
| Endometrial adenoma | 2/31 (6) | 1/27 (4) |
| Endometrial adenocarcinoma | 0/31 (0) | 1/27 (4) |
| Endometrial choriocarcinoma | 5/31 (16) | 4/27 (15) |
| Mammary | ||
| NAD | 26/31(84) | 21/27 (78) |
| Mammary adenoma | 1/31 (3) | 3/27 (11) |
| Mammary adenocarcinoma | 3/31 (10) | 2/27 (7) |
| Fibroadenoma | 1/31 (3) | 1/27 (4) |
| Adrenal | ||
| NAD | 30/31 (97) | 21/27 (78) |
| Adrenocortical nodular hyperplasia | 1/31 (3) | 6/27 (22) |
| Lymph | ||
| NAD | 28/31 (90) | 24/27 (89) |
| Lymphosarcoma | 3/31 (10) | 3/27 (11) |
| Pituitary | ||
| NAD | 25/31 (81) | 19/27 (70) |
| Pituitary adenoma | 6/31 (19) | 8/27 (30) |
NAD no abnormalities detected
Effects of Genotype on Overall Tumour Development
The presence of a global Pten +/− mutation produced tumours in a variety of tissues including the uterus, kidney, mammary and lymph nodes (Fig. 7). However, TgFSH global Pten +/− (FPten +/−) mice developed palpable tumours from 6 months of age and non-TgFSH global Pten +/− mice from 9 months onwards (data not shown). No significant difference was observed in proportion of tumours per location between Pten +/− and FPten +/− mice (Fig. 7). Cancer incidence also remained consistent between both genotypes for specific cancer types (Table 3.). Pten GC mice remained tumour free.
Discussion
We have determined the selective role of global or GC-specific Pten mutations in ovarian tumorigenesis. In vivo effects of global Pten haploinsufficiency and ovarian GC-specific Pten mutation were compared using established Tg.SOX2. Cre and Tg.AMH. Cre mouse lines, respectively. In addition, our Pten mutant models were combined with elevated circulating FSH expression to investigate an age-related endocrine change which may promote ovarian cancer development. Our findings show that ovarian tumours failed to develop in the presence of Pten disruption, regardless of rising TgFSH levels in ageing mice.
Ageing Pten +/− females exhibited increased ovarian weights due to a fivefold increase in corpora lutea (CL) numbers relative to normal females. Earlier work using Tg. Cyp19. Cre mice to selectively disrupt follicular Pten also reported elevated CL numbers [28], and GC-specific Pten mutations were shown to hyperactivate the Pi3K pathway and increase follicle growth and survival, as well as prolong the lifespan of CL with disrupted luteolysis [27, 28]. Pten was proposed to normally have a negative impact on GC proliferation and promote conditions favouring growing follicle atresia, which constrains the number of oocytes to be ovulated [28]. Higher CL numbers in our global Pten +/− model resembled the persistent CL reported in Tg. Cyp19. Cre-induced Pten mutant ovaries and may indicate that extra-follicular Pten haploinsufficiency (ie. beyond GC-specific Pten disruption) has no additional impact upon the regulation of late follicular and CL dynamics. In contrast, ovaries of global Pten +/− females did not display the premature loss of follicles that was reported after targeted oocyte-specific Pten loss [47]. Selective homozygous loss of Pten in early follicle oocytes [47], but not in oocytes of later stage (primary and beyond) follicles [48], caused premature activation of the primordial follicle pool and premature ovarian failure. Our findings show that the global Pten +/− mutation caused aberrant CL accumulation but does not cause premature ovarian failure.
Intriguingly, Pten GC females exhibited normal ovarian size, morphology and CL numbers, contrasting with the global Pten +/− ovarian phenotype. We previously showed that Tg.AMH. Cre provided ovarian GC-specific Cre activity more strongly detected in antral follicles, weaker in large preovulatory follicles and absent in CL [37]. In comparison, Tg. Cyp19. Cre predominantly drives Cre activity in GCs of growing antral to large preovulatory follicles, but not in earlier primordial-primary follicles nor theca cells and oocytes [28]. Therefore, it is likely that Cre activity largely targeted to antral follicles GCs explains the inability of Tg.AMH. Cre-mediated Pten disruption to cause the extended CL longevity associated with either global (Tg.SOX2. Cre-mediated) or GC-specific Tg. Cyp19. Cre-induced Pten loss.
In the presence of TgFSH, ovarian weights of global Pten +/− and ovary-specific Pten GC females were equivalent to controls. However, many TgFSH/Pten +/−and TgFSH/Pten GC ovaries displayed haemorrhagic and fluid-filled ovarian cysts, which were also observed in >6-month-old females expressing TgFSH alone. Similar follicular-derived cysts were present in an independent TgFSH mouse line expressing supraphysiological levels of circulating FSH [49]. We found no ovarian cysts in a separate TgFSH mouse line expressing more moderate levels of elevated FSH activity ([19, 32] denoted TgFSHm), which suggests a threshold level of progressively rising FSH in ageing TgFSHH line females caused the cystic phenotype in our current studies. No ovarian cancer was present in any Pten mutant females with or without TgFSH expression.
All Pten +/− females examined failed to complete a normal estrous cycle (typically 4–5 days) during 12 days of screening. Most were in metestrus-diestrus stages, perhaps indicative of a prolonged luteal-like phase associated with the accumulation of CL. This abnormal or non-cycling effect appears attributed to the Pten mutation alone as it was not further affected or rescued by TgFSH expression. Undetermined uterine and/or vaginal defects may also contribute to the absence of estrous cycling in this model as it is based upon vaginal cytology. In contrast, normal estrous cycling was observed in older Pten GC mutant females, suggesting that the defect in Pten +/− females lies beyond the role of GCs in growing antral to early preovulatory follicles. Serum androgen, estradiol and progesterone levels remained normal in global and GC-specific Pten mutant females, although levels during different stages of the estrous cycle were not determined.
In the present study, Pten haploinsufficiency induced spontaneous tumours of various histological origins in ageing mice. All global Pten mutant mice were collected due to significant tumour burden before reaching 12 months of age, highlighting the critical role of PTEN in tumour suppression. TgFSH/Pten +/− females developed palpable tumours 3 months earlier than Pten +/− females (6 vs 9 months of age), and TgFSH appeared to exacerbate the survival of Pten +/− 6–8-month-old females. However, the declining survival curves of older Pten +/− and TgFSH/Pten +/− females were similar. Reduced survival of global Pten +/− females was associated with the gross and histological detection of tumours in many tissues, including uterine, mammary, adrenal, pituitary and lymphoid tissues, consistent with the variety of tumours previously observed in heterozygous Pten knock-out mice [26]. The significantly larger uteri observed in Pten +/− (±TgFSH) females reflected a range of pre-neoplastic and tumorigenic morphological aberrations, which included endometrial metaplasia, hyperplasia and dysplasia, benign cancers such as endometrial polyps and adenomas, as well as malignant cancers such as endometrial adenomas and choriocarcinomas. Despite the emergence of widespread tumorigenesis and uterine cancers, we did not observe ovarian tumours in any Pten +/− mutant females, suggesting that any metastatic uterine-derived ovarian cancer requires additional genetic or trophic modifiers.
No tumours were detected in the ovary-specific Pten GC model and age-matched control females, which was equivalent to the absence of tumorigenesis in Tg. Cyp19. Cre/Pten GC females [28]. In contrast, another model using Tg. Amhr2. Cre to target ovarian Pten disruption found approximately 7 % of females developed very aggressive and metastatic GC tumours by 7 months of age [27]. However, Tg. Amhr2. Cre expression is not exclusively directed to ovarian GCs and is also reported to target ovarian surface epithelial cells [29], as well as fallopian and uterine tissues [50]. For instance, Tg. Amhr2. Cre-mediated mutation of both Pten and oncogenic Kras G12D did not induce GC tumours (due to GC cell cycle arrest), but produced aggressive ovarian surface epithelial cell tumours [29]. Disrupting Pten, Foxo1 and Foxo3 in granulosa cells leads to granulosa cell tumour formation, again confirming that GC is highly resistant to transformation by Pten alone [51]. Likewise, combined extra-ovarian Pten, Brca1/2 and Tp53 mutations driven by Tg. Pax. Cre (which targets fallopian and uterine, but not ovarian cells) lead to the development of high-grade ovarian serous carcinoma [52]. Therefore, extra-follicular Pten disruption using Tg. Amhr2. Cre may initiate ovarian tumour development. It is also noteworthy that Tg. Amhr2. Cre/Pten ovaries did not exhibit an accumulation of CL, contrasting with the elevated CL observed in our current global Pten +/− model or found in Tg. Cyp19. Cre/Pten GC ovaries [28]. These variable results may reflect distinct functional differences of the GCs (or other tissues) targeted by Cre activity in these different Tg lines (summarised in Table 4). It is also possible that altered follicle dynamics and elevated CL numbers may inhibit the initiation of GC tumours in the global Pten +/− and Tg. Cyp19. Cre/Pten GC females. While it is possible that low penetrance may explain the lack of ovarian cancers in these Pten models, the total numbers of PtenGC mutant (N = 48) and Pten+/− mutant mice (N = 58) would be expected to cover most low penetrance, especially the reported 7 % penetrance [27]. The overall resistance of ovaries to tumour formation in murine models of isolated Pten mutation supports the concept that tumorigenic effects of Pten depletion are cell type specific within the ovary and that granulosa cells may possess mechanisms to contest oncogenesis [53].
Table 4.
Murine models of Pten mutations relating to ovarian tumorigenesis. Summary of Tg promoters used to drive Cre expression for Pten-floxed modification, ovarian and uterine phenotypes and other tissue-specific effects. All models targeted exon 5 of Pten
| Promoter | Cre activity | Ovarian phenotype | Uterine phenotype | Other phenotypes |
|---|---|---|---|---|
| Amhr2 [27] |
Few granulosa cells (early follicles) Ovarian surface epithelial cells Fallopian and uterine tissue |
• 7 % Females had aggressive, anaplastic granulosa cell tumours with pulmonary metastases • Normal follicle development and growth, ovulation and formation and function of corpora lutea (CL) |
• Devoid of uterine defects | • Mice failed to carry pregnancies to term or had small litters, suggested to be due to an extra-ovarian defect |
| Cyp19 [28] |
Granulosa cells (antral and preovulatory follicles) Most luteal cells (CL) |
• No ovarian tumour formation at 12 months of age • Increased ovulation and ovarian volume due to accumulation of CLs |
• No reported uterine abnormality. | • Fertile and no extra-ovarian abnormalities detected |
| Pax [51] | Fallopian and uterine tissue | • High-grade serous ovarian cancer development | • Endometrial hyperplasia, dysplasia and carcinomas observed | • Some high-grade serous carcinomas originated in fallopian tube and metastasized to the ovary |
| AMH | Granulosa cells (small-large antral, some preovulatory follicles) |
• No ovarian tumours at 12 months of age • No ovarian histological or functional anomalies |
• Normal uterine phenotype | • No abnormalities detected |
| SOX2 | Global heterozygous disruption |
• No ovarian tumours • Increased ovarian volume due to accumulation of CLs |
• Endometrial polyps, metaplasia, hyperplasia, dysplasia, adenoma, adenocarcinoma and choriocarcinoma |
• Mammary, lymphatic, adrenal and pituitary abnormalities • Disrupted estrous cycling |
The absence of ovarian tumorigenesis in our global (Tg.SOX2. Cre) and Tg.AMH. Cre-driven Pten disruption models supports an emerging multi-hit mechanism for induction of cancer in the ovary. Models with concurrent genetic mutations have reported that Pten mutation greatly increased the incidence of GC tumours when combined with Tg. Amhr2. Cre-induced mutation of Ctnnb1 [27] or ovarian serous carcinoma when combined with Brac1/2 and Tp53 mutations [52]. Murine models of Pten mutations relating to ovarian tumorigenesis are summarised in Table 4.
In summary, our findings indicate that the ovary is highly resistant to tumorigenic changes due to selective global or GC-specific Pten disruption and rising FSH levels. The absence of any detectable ovarian tumours, despite global Pten haploinsufficiency inducing a range of uterine tumours, supports an emerging view of a multi-hit genetic mechanism to produce either intrinsic or uterine-derived ovarian tumorigenesis.
Acknowledgments
We thank Mamdouh Khalil and the staff of the ANZAC animal facilities.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by National Health and Medical Research Council (Australia) Project Grant (APP1008160).
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Obata K, et al. Frequent PTEN/MMAC mutations in Endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58(10):2095–2097. [PubMed] [Google Scholar]
- 2.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci. 1999;96(8):4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki A, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8(21):1169–1178. doi: 10.1016/S0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 4.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/S0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 5.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, et al. Allele loss at the c-Ha-ras1 locus in human ovarian cancer. Cancer Res. 1989;49(5):1220–1222. [PubMed] [Google Scholar]
- 7.Ehlen T, Dubeau L. Loss of heterozygosity on chromosomal segments 3p, 6q and 11p in human ovarian carcinomas. Oncogene. 1990;5(2):219–223. [PubMed] [Google Scholar]
- 8.Lee JH, et al. Frequent loss of heterozygosity on chromosomes 6q, 11, and 17 in human ovarian carcinomas. Cancer Res. 1990;50(9):2724–2728. [PubMed] [Google Scholar]
- 9.Russell S, et al. Allele loss from chromosome 17 in ovarian cancer. Oncogene. 1990;5(10):1581–1583. [PubMed] [Google Scholar]
- 10.Eccles D, et al. Allele losses on chromosome 17 in human epithelial ovarian carcinoma. Oncogene. 1990;5(10):1599–1601. [PubMed] [Google Scholar]
- 11.Carroll RS, et al. In vivo regulation of FSH synthesis by inhibin and activin. Endocrinology. 1991;129(6):3299–3304. doi: 10.1210/endo-129-6-3299. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, et al. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am J Pathol. 1996;148(1):47. [PMC free article] [PubMed] [Google Scholar]
- 13.Chun SY, et al. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137(4):1447–1456. doi: 10.1210/endo.137.4.8625923. [DOI] [PubMed] [Google Scholar]
- 14.Monniaux D, Pisselet C. Control of proliferation and differentiation of ovine granulosa cells by insulin-like growth factor-I and follicle-stimulating hormone in vitro. Biol Reprod. 1992;46(1):109–119. doi: 10.1095/biolreprod46.1.109. [DOI] [PubMed] [Google Scholar]
- 15.Rao MC, Midgley AR, Richards JS. Hormonal regulation of ovarian cellular proliferation. Cell. 1978;14(1):71–78. doi: 10.1016/0092-8674(78)90302-1. [DOI] [PubMed] [Google Scholar]
- 16.Richards J, et al. Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res. 1994;50:223–254. doi: 10.1016/b978-0-12-571150-0.50014-7. [DOI] [PubMed] [Google Scholar]
- 17.UI M, et al. An insulin-like growth factor-binding protein in ovarian follicular fluid blocks follicle-stimulating hormone-stimulated steroid production by ovarian granulosa cells*. Endocrinology. 1989;125(2):912–916. doi: 10.1210/endo-125-2-912. [DOI] [PubMed] [Google Scholar]
- 18.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 19.McTavish KJ, et al. Rising follicle-stimulating hormone levels with age accelerate female reproductive failure. Endocrinology. 2007;148(9):4432–4439. doi: 10.1210/en.2007-0046. [DOI] [PubMed] [Google Scholar]
- 20.Fathalla MF. Incessant ovulation? A factor in ovarian neoplasia? Lancet. 1971;298(7716):163. doi: 10.1016/S0140-6736(71)92335-X. [DOI] [PubMed] [Google Scholar]
- 21.Cramer D, Welch WR. Determinants of ovarian cancer rsik II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–721. [PubMed] [Google Scholar]
- 22.Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase a. Cell Signal. 2006;18(9):1351–1359. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunzicker-Dunn ME, et al. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc Natl Acad Sci U S A. 2012;109(44):8. doi: 10.1073/pnas.1205661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Viciana P, et al. Activation of phosphoinositide 3-kinase by interaction with ras and by point mutation. EMBO J. 1996;15(10):2442. [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, et al. Binding of ras to phosphoinositide 3-kinase p110α is required for ras-driven tumorigenesis in mice. Cell. 2007;129(5):957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 26.Podsypanina K, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci. 1999;96(4):1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lague M-N, et al. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29(11):2062–2072. doi: 10.1093/carcin/bgn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan H-Y, et al. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22(9):2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan H-Y, et al. Cell type–specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res. 2009;69(16):6463–6472. doi: 10.1158/0008-5472.CAN-08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi S, et al. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Supplement(0)):S97–S101. doi: 10.1016/S0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 31.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23(24):5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allan CM, et al. Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci U S A. 2010;107(52):22629–22634. doi: 10.1073/pnas.1012141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignarajan S, et al. Loss of PTEN stabilizes the lipid modifying enzyme cytosolic phospholipase a(2)alpha via AKT in prostate cancer cells. Oncotarget. 2014;5(15):6289–6299. doi: 10.18632/oncotarget.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesche R, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene . Genesis. 2002;32(2):148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 35.Lim P, et al. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology. 2009;150(10):4755–4765. doi: 10.1210/en.2009-0416. [DOI] [PubMed] [Google Scholar]
- 36.Lécureuil C, et al. Sertoli and granulosa cell-specific cre recombinase activity in transgenic mice. Genesis. 2002;33(3):114–118. doi: 10.1002/gene.10100. [DOI] [PubMed] [Google Scholar]
- 37.Walters KA, et al. Targeted loss of androgen receptor signaling in murine granulosa cells of Preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87(6):151. doi: 10.1095/biolreprod.112.102012. [DOI] [PubMed] [Google Scholar]
- 38.Allan CM, et al. A novel transgenic model to characterize the specific effects of follicle-stimulating hormone on gonadal physiology in the absence of luteinizing hormone actions. Endocrinology. 2001;142(6):2213–2220. doi: 10.1210/endo.142.6.8092. [DOI] [PubMed] [Google Scholar]
- 39.Singh J, O’Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136(12):5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- 40.Byun, D.-S., et al. (2011) Intestinal epithelial-specific PTEN inactivation results in tumor formation. 301: G856-G864 [DOI] [PMC free article] [PubMed]
- 41.Jimenez M, et al. Validation of an ultrasensitive and specific immunofluorometric assay for mouse follicle-stimulating hormone. Biol Reprod. 2005;72(1):78–85. doi: 10.1095/biolreprod.104.033654. [DOI] [PubMed] [Google Scholar]
- 42.Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta. 2009;409(1):78–84. doi: 10.1016/j.cca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 43.McNamara KM, et al. Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2010;121(3–5):611–618. doi: 10.1016/j.jsbmb.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Dixon D et al. (1999) Proliferative lesions of the ovary, uterus, vagina, cervix and oviduct in rats. Guides for Toxicologic Pathology:1
- 45.Rudmann D, et al. Proliferative and nonproliferative lesions of the rat and mouse mammary, Zymbal’s, preputial, and clitoral glands. Toxicol Pathol. 2012;40(6 suppl):7S–39S. doi: 10.1177/0192623312454242. [DOI] [PubMed] [Google Scholar]
- 46.Upton DH, et al. Granulosa cell-specific Brca1 loss alone or combined with Trp53 haploinsufficiency and transgenic FSH expression fails to induce ovarian tumors. Horm Cancer. 2015;6(4):142–152. doi: 10.1007/s12672-015-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddy P, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 48.Jagarlamudi K, et al. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One. 2009;4(7):0006186. doi: 10.1371/journal.pone.0006186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar TR, et al. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13(6):851–865. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- 50.Garson K, et al. Technical challenges and limitations of current mouse models of ovarian cancer. J Ovarian Res. 2012;5(1):39. doi: 10.1186/1757-2215-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, et al. FOXO1/3 and PTEN depletion in granulosa cells promotes ovarian granulosa cell tumor development. Mol Endocrinol. 2015;29(7):1006–1024. doi: 10.1210/me.2015-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perets R, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24(6):751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courtois-Cox S, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10(6):459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]