Abstract
Urinary steroid profiling (USP) was studied using high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) methods in 108 patients with adrenocortical adenoma (ACA) and in 31 patients with adrenocortical carcinoma (ACC). Thirteen ACC and Cushing’s syndrome (ACC-CS) patients had two types of USP as well as 18 ACC patients without hypercortisolism. These four types differed by androgen and glucocorticoid secretion of the adrenal cortex. Fifteen main ACC features were observed by GC-MS. Urinary excretion of dehydroepiandrosterone (DHEA) was increased in 67.7 % of ACC patients and tetrahydro-11-deoxycortisol (THS) in 74.2 %. By combination of the following parameters: THS >900 μg/24 h and/or DHEA >1500 μg/24 h with ratios of 3α,16,20-pregnentriol/3β,16,20-pregnentriol (3α,16,20dP3/3β,16,20dP3) less than 6.0 and 3α,17,20dP3/3β,17,20dP3 less than 9.0 and the detection of “non-classical” 5-en-pregnens, not found in ACA and healthy persons, 100 % sensitivity and specificity of ACC and ACA differential diagnosis were achieved. Features of 21-hydroxylase and 11β-hydroxylase deficiency were observed by GC-MS in 32.2 and 61.3 % of the ACC patients, respectively. Additional features for ACC-CS diagnostic were increased urinary excretion of 6β-hydroxycortisol, 18-hydroxycorticosterone, the sum (UFF + UFE) obtained by HPLC, tetrahydrocorticosterone, and the sum (THF + THE + allo-THF) obtained by GC-MS.
Keywords: Congenital Adrenal Hyperplasia, Primary Hyperaldosteronism, Adrenal Tumor, Adrenal Mass, Adrenocortical Carcinoma
Introduction
Adrenocortical carcinoma (ACC) is a malignant tumor, which can occur at any age. The ACC prevalence in population is 0.5–2 cases per 1,000,000 persons a year; the incidence of ACC in patients with adrenal incidentalomas is 1.2–12 %. When adrenal tumor is found, it is necessary to identify whether this adrenal mass is malignant and/or hormonally active [1–4]. Routine clinical practice does not always allow detection adrenal mass malignancy using such visual diagnostic methods as ultrasound, CT, MRI, and PET. Unfortunately, density, size, accumulation, and washout of contrast agents on enhanced CT, fine-needle aspiration biopsy of adrenal masses are not absolute indications for adrenalectomy [5–7]. About 60 % of patients with ACC are known to have clinical signs of excessive steroid hormones production, necessitating hormonal examination [4, 8, 9]. It should be noted that classical tests can result in Cushing’s syndrome (CS), primary hyperaldosteronism (PHA), and pheochromocytoma diagnosis [1, 2, 10, 11]. One of the adrenal gland tumor malignancies is high blood dehydroepiandrosterone-sulfate (DHEA-S) level [12]. Some experts consider urinary steroid profiling (USP) to be the most significant factor for adrenal carcinoma diagnosis [4, 13–16]. Others showed high-performance liquid chromatography (HPLC) to be of great value in adrenal tumor differential diagnosis [13, 17]. Gas chromatography-mass spectrometry (GC-MS) showed increased adrenal steroidogenesis precursor level in 85 % of patients with ACC, and it was suggested to be a more sensitive and specific method for differential diagnosis between benign and malignant adrenocortical tumors [4, 13, 15, 16]. The authors note that patients with ACC without any clinical signs of steroid excessive secretion may have enhanced steroid precursor production due to steroidogenesis enzyme inhibition [4, 15, 16]. All patients with suspected ACC are recommended to undergo steroid profiling examination in biological fluids by chromatographic methods to reveal signs of adrenal gland tumor malignancy. The authors stress the necessity of further searching for the most informative ACC biochemical markers [4, 13, 15, 16].
Materials and Methods
In 2014–2015, the 24-h urinary samples for evaluation of adrenal tumor were taken from patients in the Federal State Budget Institution of Higher Education “North-Western State Medical University named after I.I Mechnikov” under the Ministry of Public Health of the Russian Federation (Saint Petersburg, Russia), Leningrad Regional Clinical Hospital (Saint Petersburg, Russia), Saint Petersburg Multiprofile Centre of Ministry of Healthcare of Russian Federation (Saint Petersburg, Russia). One hundred thirty-nine patients with different adrenocortical masses (56 men and 83 women) and 25 healthy persons (9 men and 16 women) were examined. Diagnosis of ACC was confirmed histologically in 31 patients according to L.M. Weiss scale (Table 1). The following parameters were determined by immunoassay: blood adrenocorticotropic hormone (ACTH), cortisol (C) at 9 a.m. and 9 p.m., DHEA-S, 17-hydroxyprogesterone (17-HP), aldosterone (ALD) and renin levels, and free cortisol in saliva (SFC) at 11 p.m. Pheochromocytoma was excluded due to metanephrine and normetanephrine blood levels. To diagnose Cushing’s syndrome (CS), suppression test with 1 mg dexamethasone (DST) was carried out, and saline infusion test for diagnostic primary hyperaldosteronism (PHA) was used. USP was investigated by GC-MS and 66 steroids were identified. The investigations were performed using the equipment of Resource Center Scientific Park, Saint Petersburg State University “Chemical Analysis and Materials Research Centre.” USP was obtained by using gas chromatography-mass spectrometer SHIMADZU GCMS-QP2010 ULTRA. The procedure of sample preparation was optimized and included three main steps: conjugate hydrolysis using sulfatase from Helix pomatia, analyte liquid extraction, and their subsequent derivatization. Methoxyamine and trimethylsilylimidazole were used as derivatizing agents. Urine free cortisol (UFF), free cortisone (UFE), 6β-hydroxycortisol (6β-OHF), and 18-hydroxycorticosterone (U18-OHB) were determined by HPLC with UV-diode array detection. Statistical analysis was performed using Statistica for Windows 7 software (StatSoft Inc., USA). Results are presented as median, lower, and upper quartiles (ME/LQ-UQ), and comparison was made by the Mann-Whitney criteria. The p values <0.05 were considered to be significantly important. Figures are presented using software Prism 6.0 (GraphPad Software, La Jolla, USA).
Table 1.
Demographic and clinical characteristics of adrenal tumor patients
| Patients with adrenocortical adenomas | Patients with adrenocortical carcinoma (n = 31) | ||
|---|---|---|---|
| Hormonally non-active adenomas (n = 52) | Cushing’s syndrome (n = 44) | ||
| Age (years), median (range) | 55 (50–61) | 48 (21–54) | 43 (33–57) |
| Sex (male, female) | 17, 35 | 18, 26 | 8, 23 |
| Tumor size, median (range) | 33 (23–45) mm | 30 (25–42) mm | 91 (72–110) mm |
| Weiss scorea, median (range) | 0 | 0 | 5 (4–9) |
| Native density | 10/2–18 | 14/5–21 | 30/24–40 HU |
| Surgical removal of adrenal tumor | 43/52 (82.7 %) | 39/44 (88.6 %) | 31/31 (100 %) |
aScores of 4 and above are indications of malignancy
Results
According to classical tests based on immunoassay, 108 adrenocortical adenoma (ACA) patients were divided into the following groups: 44 patients had CS, 12 had PHA, and 52 had hormonally non-active adenomas (HNA). They had no malignant score (MS) due to L.M. Weiss scale by histological analysis of postoperative material. Diagnosis of ACA in 15 patients was established by biochemical and imaging studies as well as by clinical follow-up (12 months). Patients with ACC were divided into two groups: 18 patients with ACC and without hypercortisolism (group 1) and 13 ACC patients and CS (ACC-CS) (group 2). Results of hormonal evaluation in patients from groups 1 and 2 were compared with those of HNA patients, and ACC-CS patients were additionally compared with CS. Patients with PHA were not included into the groups of comparison.
Blood levels of 17-HP, ALD, C at 9 a.m. and C after the DST (less than 50 nmol/l), and SFC (less 10 nmol/l) in HNA patients and those with ACC (group 1) were not different compared with healthy persons. However, blood 17-HP level was increased at 9 a.m. (10.2/3.7–14.3 nmol/l, p = 0.03) and after the ACTH test (32.6/19.8–37.8 nmol/l, p = 0.04) in seven HNA patients. Non-classical form of congenital adrenal hyperplasia (CAH) due to 21-hydroxylase (21-H) deficiency was confirmed by genetic analysis in five HNA patients. Patients with CS and ACC-CS had increased SFC (>20 nmol/l). ACC-CS patients had cortisol blood concentration at 9 a.m. (1228/1014–1457 nmol/l, p < 0.001) and after the DST (918/488–1085 nmol/l, p < 0.05), which is higher in comparison with CS patients (618/523–761 and 113/73–380 nmol/l, respectively) and ACC patients (group 1; p < 0.0004). Thus, immunoassay revealed CS both in ACA and in ACC groups. Blood DHEA-S level in patients with ACC (19.8/3.0–47.7 μmol/l, p < 0.01) was higher than in patients with HNA (1.9/1.4–2.7 μmol/l).
According to HPLC data, patients of both ACC groups had an increased 6β-OHF urinary excretion, and U18-OHB was increased in ACC-CS patients in comparison with HNA (Table 2). Urinary excretion of UFF, UFE, and 6β-OHF was higher in ACC-CS patients compared with HNA and CS patients (Table 2). Urinary excretion of UFF (p = 0.0009), UFE (p = 0.005), 6β-OHF (p = 0.01), and U18-OHB (p = 0.02) was also higher in ACC-CS patients than in ACC patients (group 1). 6β-OHF >900 μg/24 h, U18-OHB >75 μg/24 h, and the sum of (UFF + UFE) >700 μg/24 h were obtained in ACC-CS patients, while 6β-OHF >300 μg/24 h was obtained in ACC patients (group 1) by HPLC.
Table 2.
Urinary corticosteroid excretion in patients with adrenocortical carcinoma without cortisol and its metabolite hypersecretion (ACC) and in patients with adrenocortical carcinoma and Cushing’s syndrome (ACC-CS) by high-performance liquid chromatography
| Name of steroids | Median/lower and upper quartiles (μg/24 h) | |||
|---|---|---|---|---|
| Adrenocortical adenoma | Adrenocortical carcinoma | |||
| Hormonally non-active adenomas (n = 52) | Cushing’s syndrome (n = 44) | ACC (n = 18) | ACC-CS (n = 13) | |
| Free cortisol (UFF) | 23/16–35 | 108/42–234** | 19/12–21 | 496/381–892** p = 0.02 |
| Free cortisone (UFE) | 60/48–82 | 133/86–231** | 45/30–60 | 552/342–592** p = 0.004 |
| 6β-Hydroxycortisol (6β-OHF) | 114/37–266 | 173/51–295 | 414/303–450* | 1490/967–2500** p = 0.02 |
| 18-Hydroxycorticosterone | 24/13–44 | 35/30–57 | 38/31–521 | 32/75–336** |
| 6βOHF/UFF ratio | 4.7/1.6–8.2 | 1.4/0.8–2.2** | 23/11–36* | 3.7/1.2–5.2 |
*p < 0.05, **p < 0.001—comparison of each group of patients with hormonally non-active adenomas; p—comparison of ACC-CS with CS patients
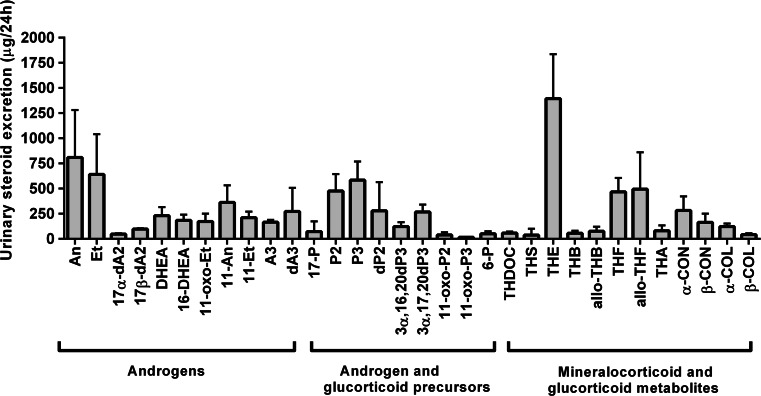
GC-MS data of healthy persons are presented in Fig. 1. Common ACC signs in ACC-CS patients and ACC patients without hypercortisolism were received by analysis and statistical processing of USP data obtained by GC-MS. Urinary excretions of androgens, glucocorticoid and androgen precursor metabolites, and tetrahydro-11-deoxycorticosterone (THDOC) were increased in patients of both ACC groups (Table 3). It is necessary to note that urinary DHEA, 17β-androstendiol (17βdA2), and 16-DHEA excretion were increased in ACC-CS patients in comparison with CS patients (Table 3). Among the patients with ACC, 5-en-pregnens (“non-classical”) were identified: 3β,16,20-pregnentriol (3β,16,20dP3), 3β,17,20dP3 (dP3), 16-pregnenolon (16dP), 21dP, 11dP3, 21-pregnendiol (21dP2), and the ratios of 3α,16,20dP3/3β,16,20dP3 <6.0 and 3α,17,20dP3/3β,17,20dP3 <9 were obtained (Table 4). These non-classical 5-en-pregnens and ratios were not found in ACA patients without MS and in healthy persons and are important ACC steroid biomarkers. Thus, 27 common ACC features were obtained by GC-MS in ACC-CS patients and in ACC patients without hypercortisolism.
Fig. 1.
Excretion of urinary steroids in healthy persons. Androgens: An androsterone, Et etiocholanolone, 17α-dA2 17α-androstendiol, DHEA dehydroepiandrosterone, 16-DHEA 16-hydroxy-DHEA, 11oxo-Et 11-oxo-etiocholanolone, 11-An 11-hydroxy-androsteron, 11-Et 11-hydroxy-etiocholanolone, A3 3,11,17-androstantriol, dA3 androstenetriol. Androgen and glucocorticoid precursors: 17-P 17-hydroxy-pregnanolone, P pregnanolone, P2 pregnanediol, P3 pregnanetriol, dP2 pregnenediol, 3α,17,20dP3 3α,17,20-pregnenetriol, 3α,16,20dP3 3α,16,20-pregnenetriol, 11-oxo-P2 11-oxo-pregnandiol, 11-oxo-P3 11-oxo-pregnantriol, 6-P 6-hydroxy-pregnanolone. Mineralocorticoid and glucocorticoid metabolites: THDOC tetrahydro-11-deoxycorticosterone, THS tetrahydro-11-deoxycortisol, THE tetrahydrocortisone, THB tetrahydrocorticosterone, THF tetrahydrocortisol, THA tetrahydro-11-dehydrocorticosterone, α-CON α-cortolon, α-COL α-cortol
Table 3.
Common features of adrenocortical carcinoma in patients with adrenocortical carcinoma without cortisol and its metabolite hypersecretion (ACC) and in patients with adrenocortical carcinoma and Cushing’s syndrome (ACC-CS) by gas chromatography-mass spectrometry
| Name of steroids | Median/lower and upper quartiles (μg/24 h) | |||
|---|---|---|---|---|
| Adrenocortical adenomas | Patients with adrenocortical carcinoma | |||
| Hormonally non-active adenomas (n = 52) | Cushing’s syndrome (n = 44) | ACC (n = 18) | ACC-CS (n = 13) | |
| Androgens | ||||
| Etiocholanolone | 240/148–440 | 279/129–412 | 1464/554–2476*** | 723/365–8215* p = 0.005 |
| Androstendiol-17β | 58/37–91 | 75/25–115 | 705/348–1673*** | 1010/54–3142 p = 0.03 |
| Dehydroepiandrosterone (DHEA) | 40/32–55 | 11/9–14* | 3407/776–11171*** | 3283/20–10235 p = 0.005 |
| 16-Hydroxy-DHEA | 150/36–212 | 153/65–269 | 1851/953–9837* | 2113/245–7379 p = 0.02 |
| 11-Hydroxy-etiocholanolone | 227/73–377 | 313/136–701 | 672/214–968* | 1720/839–2229*** p = 0.02 |
| Androstenetriol | 133/41–177 | 118/51–234 | 1630/492–4462* | 1322/299–4248* p = 0.003 |
| 16-Oxo-androstendiol | 27/14–40 | 32/23–56 | 533/387–659* | 1232/504–2189* p = 0.005 |
| Androgen and glucocorticoid precursor and their metabolites | ||||
| 17-Hydroxy-pregnanolone | 172/70–185 | 86/48–109 | 355/275–1237* | 1253/696–3139** p = 0.002 |
| Pregnanediol (P2) | 228/186–495 | 483/181–628* | 2356/1097–3528*** | 3278/2803–6864*** p = 0.001 |
| Pregnanetriol (P3) | 458/283–705 | 523/256–745 | 1195/739–2200** | 3167/1612–5479*** p = 0.002 |
| 11-Oxo-pregnanetriol | 37/33–46 | 66/36–101 | 150/99–227* | 305/165–721* p = 0.04 |
| Pregnenediol | 430/181–558 | 386/236–688 | 2530/1540–3214*** | 3669/2176–5981*** p = 0.006 |
| 5-Pregnen,3α,16α,20α-triol | 75/51–149 | 121/83–208 | 957/306–1299*** | 2130/1184–9722*** p = 0.02 |
| 5-Pregnen,3α,17α,20α-triol | 146/91–314 | 168/118–266 | 1554/1112–2576*** | 2489/524–11235*** p = 0.002 |
| 6-Hydroxy-pregnanolone | 33/15–55 | 19/15–43 | 113/27–210* | 198/102–312* p = 0.03 |
| Tetrahydro-11-deoxycortisol (THS) | 93/49–171 | 411/100–539 | 858/131–1355* | 1081/691–3732*** p = 0.04 |
| Hexahydro-11-deoxycortisol (HHS) | 75/27–143 | 52/25–183 | 272/132–1370* | 622/160–8165* p = 0.02 |
| 21-Deoxy-tetrahydrocortisol | 48/22–54 | 131/110–217 | 192/80–203* | 1036/881–1258** p = 0.02 |
| Mineralocorticoid metabolite | ||||
| Tetrahydro-11-deoxycorticosterone | 54/14–74 | 66/35–89 | 110/88–168* | 176/148–205* p = 0.04 |
*p < 0.05, **p < 0.001, ***p < 0.0001—comparison of each group of patients with hormonally non-active adenomas; p—comparison of ACC-CS with ACA-CS
Table 4.
Urinary excretion of “non–classical” 5-en-pregnenes in patients with adrenocortical carcinoma without cortisol and its metabolite hypersecretion (ACC) and in patients with adrenocortical carcinoma and Cushing’s syndrome (ACC-CS) by gas chromatography-mass spectrometry
| Name of 5-en-pregnenes | Median/lower and upper quartiles (μg/24 h) | |
|---|---|---|
| ACC (n = 18) | ACC-CS (n = 13) | |
| 3β,17,20-pregnentriol (3βdP3) | 351/151–989 | 1326/188–2433 |
| 3β,16,20-pregnentriol (3β,16,20dP3) | 461/222–929 | 1343/300–4204 |
| 16-hydroxypregnenolone | 392/120–581 | 548/240–1628 |
| 21-hydroxypregnenolone | 172/68–303 | 1102/414–2495 |
| 11-hydroxypregnentriol | 138/28–879 | 794/350–1408 |
| 21-hydroxypregnendiol | 971/329–2195 | 655/157–1200 |
| Ratios | ||
| 3α,16,20dP3/3β,16,20dP3 | 1.9/1.2–2.9 | 2.4/0.9–2.9 |
| 3α,17,20dP3/3β,17,20dP3 | 5.0/2.9–8.2 | 4.4/1.8–7.7 |
According to GC-MS data, ACC-CS patients showed increased urinary glucocorticoid metabolite excretion compared with HNA patients and CS patients (Table 5). The following urinary glucocorticoid metabolites: tetrahydrocortisone (THE), tetrahydrocortisol (THF), tetrahydrocorticosterone (THB), cortolones and cortols, dihydrocortisone, dihydrocortisol, cortisone, and cortisol were also increased in ACC-CS patients in comparison with ACC (group 1) (p < 0.02). GC-MS data show that THB >1000 μg/24 h and the sum of (THE + THF + allo-THF) >10,000 μg/24 h can serve additional signs of ACC-CS patients.
Table 5.
Excretion of glucocorticoid steroids in patients with adrenocortical adenoma, in patients with adrenocortical carcinoma without hypersecretion of cortisol and its metabolites (ACC), and in patients with adrenocortical carcinoma and Cushing’s syndrome (ACC-CS) by gas chromatography-mass spectrometry
| Name of steroids | Median/lower and upper quartiles (μg/24 h) | |||
|---|---|---|---|---|
| Patients with adrenocortical adenoma | Patients with adrenocortical carcinoma | |||
| Hormonally non-active adenomas (n = 52) | Cushing’s syndrome (n = 44) | ACC (n = 18) | ACC-CS (n = 13) | |
| Tetrahydrocortisone | 1705/1334–2632 | 3061/2154–3834** | 1501/1104–2289 | 4946/4272–8336*** p = 0.01 |
| Tetrahydrocorticosterone | 99/70–203 | 178/117–363* | 234/114–401 | 583/361–1090** p = 0.03 |
| Tetrahydrocortisol (THF) | 679/399–867 | 1898/1500–2910** | 932/557–1260 | 5739/4717–6889*** p = 0.004 |
| Allo-THF | 569/215–1069 | 1060/624–1810* | 424/234–1027 | 1515/915–2078* |
| α + β-Cortolones | 655/406–1028 | 1167/606–1650* | 563/484–880 | 3016/2056–3856*** p = 0.04 |
| α + β-Cortols | 91/54–151 | 217/87–373* | 212/141–487 | 593/315–2065* p = 0.04 |
| Hexahydrocorticosterone | 194/97–346 | 239/132–369 | 334/86–419 | 582/145–722* |
| Dihydrocortisone | 32/14–38 | 35/28–97 | 88/29–101 | 101/88–135* |
| Dihydrocortisol | 17/9–25 | 61/29–101* | 29/15–35 | 201/138–265* |
| Cortisone | 37/13–58 | 48/24–98 | 47/12–55 | 133/110–235* |
| Cortisol | 29/13–47 | 105/71–154* | 25/15–35 | 1162/471–1217 ** p = 0.02 |
*p < 0.05, **p < 0.001, ***p < 0.0001—comparison of each group of patients with hormonally non-active adenomas; p—comparison of ACC-CS patients with CS patients
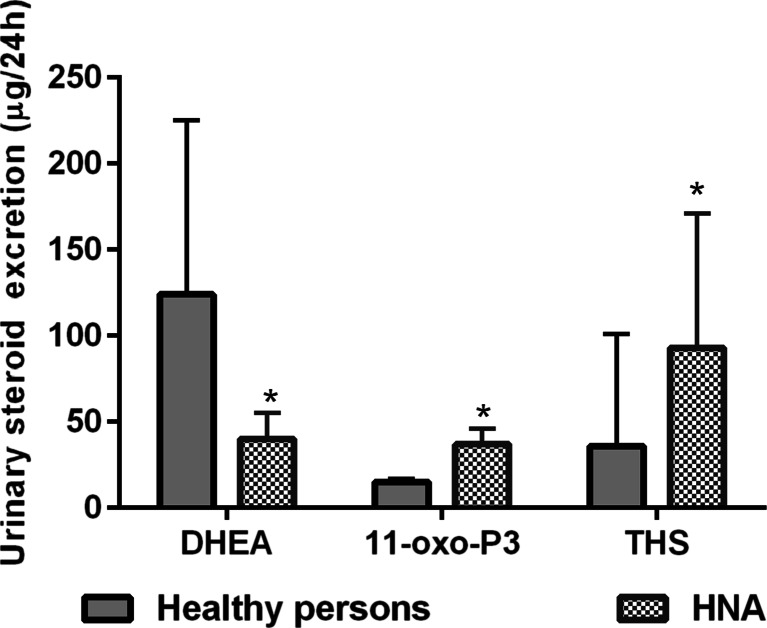
USP can reveal adrenal steroidogenesis enzyme deficiency in ACA and ACC patients. Increased urinary 11-oxo-pregnantriol (11-oxo-P3) excretion and decreased ratio of (THE + THF + allo-THF)/11-oxo-P3 compared with healthy persons may indicate the deficiency of 21-H, increased urinary THS excretion, and decreased ratio of (THF + allo-THF + THE)/THS-11β-hydroxylase (11β-H) deficiency in HNA patients (Fig. 2, Table 6). Five HNA patients in our study had non-classical form of CAH due to 21-H deficiency, confirmed by genetic analysis and the ACTH test. They had a ratio of (THE + THF + allo-THF)/11-oxo-P3 <30 (15/5–30). Increased P3, 11-oxo-P3, and 21-deoxy-THF urinary excretion was observed in patients of both ACC groups (Table 3). Decreased (THE + THF + allo-THF)/P3 and (THE + THF + allo-THF)/11-oxo-P3 ratios were received in ACC patients (group 1), while the ACC-CS patients only had a decreased ratio of (THE + THF + allo-THF)/11-oxo-P3 (Table 6). Ten ACC patients had thresholds (THE + THF + allo-THF)/P3 <4 (2.1/1.4–3.6) and (THE + THF + allo-THF)/11-oxo-P3 <30 (21/11–25), which may indicate 21-H deficiency. Patients of both ACC groups had elevated THS and hexahydro-11-deoxycortisol (HHS) urinary excretion and reduced (THF + allo-THF + THE)/THS ratio (Tables 3 and 6). Nineteen patients with ACC had the ratio of (THF + allo-THF + THE)/THS <20 (5.4/2.3–16.1), which may indicate 11β-H deficiency. This threshold ratio was less than the upper quartile of statistical data of ACC patients (Table 6). Features of 21-H and 11β-hydroxylase deficiency were observed by GC-MS in 32.2 and 61.3 % of the ACC patients, respectively.
Fig. 2.
Urinary excretion of dehydroepiandrosterone (DHEA), 11-oxo-pregnanetriol (11-oxo-P3), and tetrahydro-11-deoxycortisol (THS) in healthy persons and patients with hormonally non-active adenomas (HNA). *p < 0.05—comparison with healthy persons
Table 6.
Features of 21-hydroxylase and 11β-hydroxylase deficiency in patients with adrenocortical carcinoma without hypersecretion of cortisol and its metabolites (ACC) and in patients with adrenocortical carcinoma and Cushing’s syndrome (ACC-CS) by gas chromatography-mass spectrometry
| Rations | Median/lower and upper quartiles | |||
|---|---|---|---|---|
| Healthy persons (n = 25) | Hormonally non-active adenomas (n = 52) | Patients with adrenocortical carcinoma | ||
| ACC(n = 18) | ACC-CS (n = 13) | |||
| (THE + THF + allo-THF)/P3 | 6/5–8 | 7/5–11 | 2.7/1.5–3.6* p = 0.001 | 4.4/2.1–9.5 |
| (THE + THF + allo-THF)/11-oxo-P3 | 169/135–219 | 97/75–114* | 23/19–40* | 56/18–75* |
| (THE + THF + allo-THF)/THS | 138/47–160 | 45/27–71* | 5.4/2.3–16.1* p = 0.001 | 16/3–29* p = 0.04 |
*p < 0.05—comparison with healthy persons; p—comparison with hormonally non-active adenomas
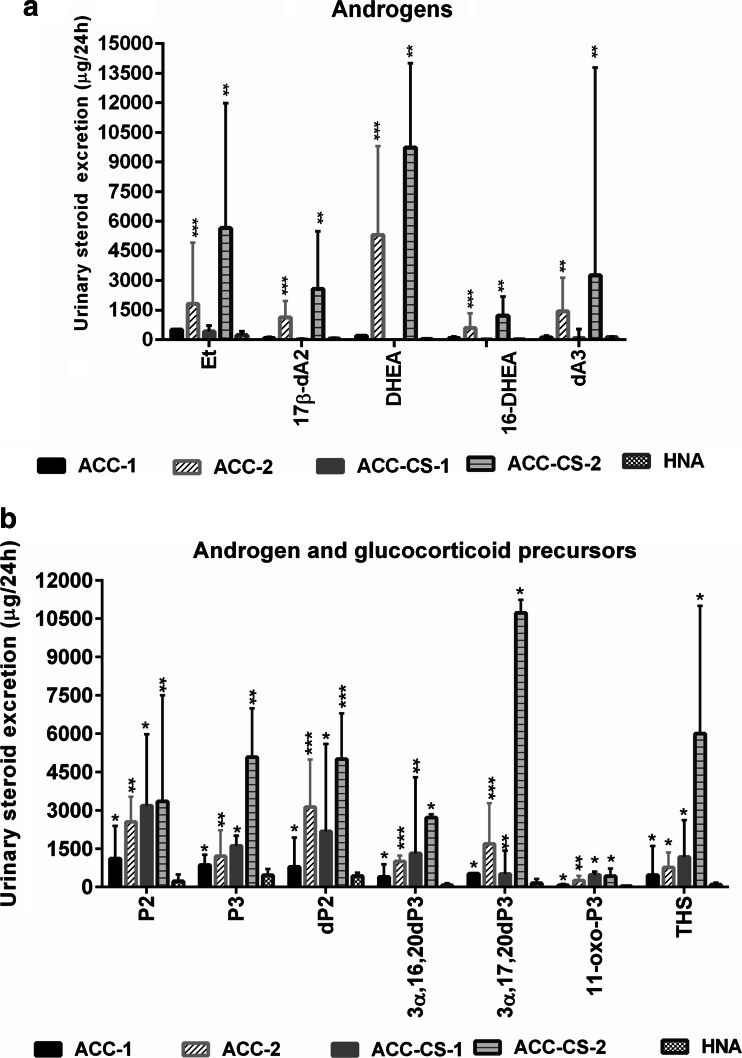
Summarizing GC-MS data, four types of USP in ACC patients were obtained: two types of USP in ACC patients (group 1) and two types of USP in ACC-CS patients. These four types differed in androgen and glucocorticoid secretion of the adrenal cortex. Increased urinary excretion of etiocholanolone, DHEA, 17β-dA2, 16-DHEA, and androstenetriol was observed in 13 persons with ACC (group 1) and in 8 persons with ACC-CS (Fig. 3). Increased urinary excretions of THS, P2, P3, dP2, 11-oxo-P3, 3αdP3, and 3α,16,20dP3 and the ratios of 3α,16,20dP3/3β,16,20dP3 <6.0 and 3αdP3/3βdP3 <9.0 were ACC signs found in all four ACC patient groups by GC-MS (Figs. 3 and 4). Detection of non-classical 5-en-pregnens (3β,16,20dP3, 3β,17,20dP3, 16dP, 21dP, 21dP2, 11dP3) was also an important sign of ACC and found in all four ACC groups. Thus, four USP types and 15 main ACC laboratory features were observed by GC-MS.
Fig. 3.
Urinary steroid excretion in patients with adrenocortical carcinoma without cortisol hypersecretion (ACC), in patients with adrenocortical carcinoma and Cushing’s syndrome (ACC-CS), and in patients with hormonally non-active adenomas (HNA). ACC-1—patients with ACC without cortisol and androgen hypersecretion (n = 5); ACC-2—patients with ACC and androgen hypersecretion (n = 13); ACC-CS-1—patients with ACC-CS and without androgen hypersecretion (n = 5); ACC-CS-2—patients with ACC-CS and androgen hypersecretion (n = 8). a Urinary androgen excretion: Et etiocholanolone, 17β-dA2 17β-androstendiol, DHEA dehydroepiandrosterone, 16-DHEA 16-OH-dehydroepiandrosterone, dA3 androstenetriol. b Urinary androgen and glucocorticoid precursor excretion (common ACC signs found in all four ACC patient groups): P2 pregnanediol, P3 pregnanetriol, dP2 pregnenediol, 3α,17,20dP3 3α,17,20-pregnenetriol, 3α,16,20dP3 3α,16,20-pregnenetriol, 11-oxo-P3 11-oxo-pregnanetriol, THS tetrahydro-11-deoxycortisol. *p < 0.05, **p < 0.01, ***p < 0.001—comparison with hormonally non-active adenomas
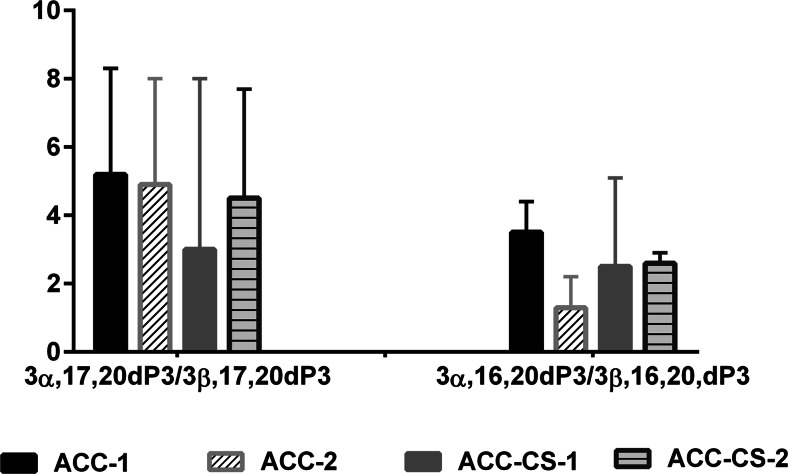
Fig. 4.
The ratios of 3α,16,20-pregnenetriol/3β,16,20-pregnenetriol and 3α,17,20-pregnenetriol/3β,17,20-pregnenetriol in adrenocortical carcinoma patients without cortisol hypersecretion (ACC) and in adrenocortical carcinoma with Cushing’s syndrome (ACC-CS) patients. ACC-1—patients with ACC without cortisol and androgen hypersecretion; ACC-2—patients with ACC and androgen hypersecretion; ACC-CS-1—patients with ACC-CS and without androgen hypersecretion; ACC-CS-2—patients with ACC-CS and androgen hypersecretion
It is necessary to note that DHEA was increased in 67.7 % of patients and made up to 5443/1595–14902 μg/24 h, while THS was increased in 74.2 % (1355/905–3732 μg/24 h) ACC patients. Only the combination of THS >900 μg/24 h and/or DHEA >1500 μg/24 h, with ratios 3α,16,20dP3/3β,16,20dP3 <6.0 and 3α,17,20dP3/3β,17,20dP3 <9.0, and detection of non-classical 5-en-pregnens had 100 % sensitivity and specificity for differential ACC and ACA diagnosis in the present study.
Discussion
The determination of adrenal tumor malignancy is mainly based on CT characteristics, which have high sensitivity but low specificity [5–7]. Recently, USP examinations with GC-MS become of special importance in ACA and ACC differential diagnostics [4, 13, 15, 16]. The present study confirms the great importance of urinary androgens and glucocorticoid precursors, DHEA, and THS determination by GC-MS for differentiation of ACC and ACA. Unlike other researchers, we tried to determine the most informative features of ACC in pre-operative period in combination of HPLC and GC-MS data for the evaluation of diagnostic accuracy of ACC. All patients were examined by HPLC and GC-MS methods. HPLC and GC-MS are not opposed to each other but complement each other’s data. The HPLC method with UV-diode array detection is more available and expressive compared with GC-MS because it does not require derivatization and hydrolysis steps, enables to determine free forms of steroids, and is not detected by GC-MS in our modifications. Additional ACC biomarkers were determined by HPLC: increased 6β-OHF, U18-OHB, and sum (UFF + UFE) excretions. In our earlier studies, we obtained referent 11-deoxycortisol, 11-deoxycorticosterone, 18-OHB, and corticosterone blood levels by HPLC for diagnostic of ACC-CS [13]. In the present study, all patients with ACC were divided into two groups—ACC-CS and ACC without hypercortisolism on the basis immunoassay data. Moreover, results of patients with ACC were compared with data of healthy persons and HNA; ACC-CS was additionally compared with CS. This approach made it possible to find 27 ACC common features by GC-MS and 6β-OHF—by HPLC both in patients with ACC-CS and in patients with ACC without hypercortisolism. It also promotes detection of additional ACC signs in ACC-CS patients by GC-MS and HPLC. Dividing UPS of ACC patients into four groups, we revealed the 15 most important features of ACC by GC-MS: increased urinary excretion P2, P3, 11-oxo-P3, dP2, 3α,17,20dP3, and 3α,16,20dP3; the detection of non-classical 5-en-pregnens; and the ratios 3α,16,20dP3/3β, 16,20dP3 <6.0, and 3α,17,20,dP3/3β,17,20dP3 <9.0. Specific ACC USP was obtained for group with hypercortisolism, as well as for the group without cortisol and its metabolite hypersecretion. The increased urinary excretion of THS in 74.2 % and DHEA and its metabolites in 67.7 % of the ACC patients indicates the importance of these parameters for ACC diagnostics as was noted by other researchers earlier [4, 16]. However, only the combination of increased urinary THS and/or DHEA secretion with detection of non-classical 5-en-pregnens and ratios 3α,16,20dP3/3β,16,20dP3 <6.0 and 3α,17,20dP3/3β,17,20dP3 <9.0 reaches 100 % sensitivity and specificity in differential diagnosis of ACA and ACC. (THE + THF + allo-THF)/THS <20 for 11β-H deficiency, (THE + THF + allo-THF)/11-oxo-P3 <30, and (THE + THF + allo-THF)/P3 <4 for 21-H deficiency thresholds were suggested in this study. We observed the same thresholds in HNA patients and patients with non-classical form of CAH due to 21-H deficiency, confirmed by genetic analysis and the ACTH test. This allows to suggest the importance of 21-H deficiency as one of the causes of ACA and ACC. Features of 21-H and 11β-H deficiency were observed by GC-MS in 32.2 and 61.3 % of the ACC patients, respectively, which show the important role of these enzymes in ACC pathophysiology.
Despite the retrospective kind of our study, the findings suggest the importance of using GC-MS and HPLC for differential ACC and ACA diagnostics, which, in combination with visualizing methods, could improve the accuracy of revealing ACC on preoperative stage. The presented results demonstrate the importance of the extensive hormonal survey in patients with adrenal masses both with and without obvious clinical symptoms of hormonal activity. To further assess of sensitivity and specificity of this method, we are planning to investigate USP in patients with ACC 1 year after adrenalectomy; this will also promote the active use of USP by chromatographic methods in practice. We hope that USP determination will be included in the guideline for diagnostics and management of adrenal masses.
Acknowledgments
Our group is very thankful to Dr. N.F. Taylor from King’s College School of Medicine in London (Department of Clinical Biochemistry) for his cooperation and invaluable assistance during the implementation of GC-MS in the clinic of our university. Many thanks also are due to the European Network for the Study of Adrenal Tumors (ENS@T) for giving an opportunity for the short visit to King’s College School of Medicine, London, to study this new method (GC-MS).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
For this type of study, formal consent is not required.
References
- 1.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–4564. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 2.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reibetanz J, Kroiss M, Deutschbein T, Fenske W, Gasser M, Jurowich C, Germer CT, Allolio B, Fassnacht M. German adrenocortical carcinoma registry. Surgical therapy results and follow-up treatment. Chirurg. 2012;83(6):528–535. doi: 10.1007/s00104-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 4.Arlt W, Biehl M, Taylor AE, Hahner S, Libé R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775–3784. doi: 10.1210/jc.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JK, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the clinically inapparent adrenal mass (“incidentaloma”) Ann Intern Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 6.Hamrahian AH, Ioachimescu AG, Remer EM, Motta-Ramirez G, Bogabathina H, Levin HS, Reddy S, Gill IS, Siperstein A, Bravo EL. Clinical utility of noncontrast computed tomography attenuation value (Hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J Clin Endocrinol Metab. 2005;90:871–87. doi: 10.1210/jc.2004-1627. [DOI] [PubMed] [Google Scholar]
- 7.Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95:4106–4113. doi: 10.1210/jc.2010-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald JG, Matthew S, Auchus RJ. Steroid profiling by gas chromatography–mass spectrometry and high performance liquid chromatography–mass spectrometry for adrenal diseases. Horm Cancer. 2011;2:324–332. doi: 10.1007/s12672-011-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B, German Adrenocortical Carcinoma Registry Group; European Network for the Study of Adrenal Tumors Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115(2):243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 10.Velikanova LI, Shafigullina ZR, Vorokhobina NV, Silnitsky PA, Bessonova Ye A. Diagnostic value of high performance liquid chromatography of corticosteroids in diseases of the pituitary-system. Probl Endokrinol. 2005;51(6):9–12. doi: 10.14341/probl20055169-11. [DOI] [PubMed] [Google Scholar]
- 11.Nieman LK, Biller BMR, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terzolo M, Ali A, Osella G, Reimondo G, Pia A, Peretti P, Paccotti P, Angeli A. The value of dehydroepiandrosterone sulfate measurement in the differentiation between benign and malignant adrenal masses. Eur J Endocrinol. 2000;142(6):611–617. doi: 10.1530/eje.0.1420611. [DOI] [PubMed] [Google Scholar]
- 13.Shafigullina ZR, Velikanova LI, Vorokhobina NV, Lisitsin AA, Kukhianidze EA, Strelnikova EA, Povarov VG, Taylor NF. The diagnostical importance of steroid profiles of biological fluids of patients with Cushing’s syndrome. Probl Endokrinol. 2015;61(4):4–8. doi: 10.14341/probl20156144-8. [DOI] [Google Scholar]
- 14.Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS) J Steroid Biochem Mol Biol. 2010;121(3-5):496–504. doi: 10.1016/j.jsbmb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stigliano A, Chiodini I, Giordano R, Faggiano A, Canu L, Della Casa S, Loli P, Luconi M, Mantero F, Terzolo M. Management of adrenocortical carcinoma consensus statement of the Italian Society of Endocrinology (SIE) J Endocrinol Investig. 2015;39(1):103–121. doi: 10.1007/s40618-015-0349-9. [DOI] [PubMed] [Google Scholar]
- 16.Kerkhofs TM, Kerstens MN, Kema IP, Willems TP, Haak HR. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm Cancer. 2015;6(4):168–75. doi: 10.1007/s12672-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velikanova LI, Shafigullina ZR, Vorokhobina NV, Grigoryan K, Lisitsin AA, Obedkova EV (2015) Differential diagnostics of adrenocortical incidentalomas with different laboratory technologies. Herald of the Northwestern State Medical University named after I.I. Mechnikov 7(4):52–57, in Russ