Abstract
Mitotane is the only drug approved for treatment of the orphan disease adrenocortical carcinoma (ACC) and was recently shown to be the first clinically used drug acting through endoplasmic reticulum (ER)-stress induced by toxic lipids. Since mitotane has limited clinical activity as monotherapy, we here study the potential of activating ER-stress through alternative pathways. The single reliable NCI-H295 cell culture model for ACC was used to study the impact MG132, bortezomib (BTZ) and carfilzomib (CFZ) on mRNA and protein expression of ER-stress markers, cell viability and steroid hormone secretion. We found all proteasome inhibitors alone to trigger expression of mRNA (spliced X-box protein 1, XBP1) and protein markers indicative of the inositol-requiring enzyme 1 (IRE1) dependent pathway of ER-stress but not phosphorylation of eukaryotic initiation factor 2α (eIF2α), a marker of the PRKR-like endoplasmic reticulum kinase (PERK)-dependent pathway. Whereas mitotane alone activated both pathways, combination of BTZ and CFZ with low-dose mitotane blocked mitotane-induced eIF2α phosphorylation but increased XBP1-mRNA splicing indicating that proteasome inhibitors can commit signalling towards a single ER-stress pathway in ACC cells. By applying the median effect model of drug combinations using cell viability as a read out, we determined significant drug synergism between mitotane and both BTZ and CFZ. In conclusion, combination of mitotane with activators of ER-stress through the unfolded protein response is synergistic in an ACC cell culture model. Since proteasome inhibitors are readily available clinically, they are attractive candidates to study for ACC treatment in clinical trials in combination with mitotane.
Keywords: Adrenal cancer, Unfolded protein response, Synergism, Toxic lipids
Introduction
Adrenocortical carcinoma (ACC) is an orphan malignant disease which frequently recurs even after complete resection and has a high risk of metastatic spread.
Overall survival is poor in advanced stages and therapeutic options are limited [1, 2]. Tumour-related steroid hormone excess often leads to diagnosis of ACC [3] while also conferring increased morbidity and entailing a particularly dismal prognosis [4]. Mitotane (1,1 dichloro-2(o-chlorophenyl)-2-(p-chloro-phenyl) ethane, o,p’-DDD) is a derivative and former by-product of industrially produced pesticide DDT and was discovered more than 60 years ago to induce adrenocortical atrophy in dogs [5–7]. It has since then been used for treatment of ACC in humans and animals [8]. In advanced disease, mitotane monotherapy induces objective tumour response in about every fifth patient [9–11] with non-resectable ACC and is a cornerstone of most combination therapies [12]. Adjuvant mitotane treatment is advocated in most centres [13] but remains controversial [14]. Retrospective studies and a small prospective clinical trial have shown that mitotane serum concentrations above 14 mg/l are associated with an improved treatment response [9, 15–17]. The likelihood and severity of adverse effects such as adrenal insufficiency, nausea, diarrhoea and dizziness [18] increases with higher concentrations. Therefore, serum concentrations between 14 and 20 mg/l (43–62 μM) are aimed at clinically [19].
Mitotane has unfavourable pharmacokinetic properties. Even with high oral doses of mitotane plasma concentrations >14 mg/l are achieved in only half of patients [20]. On the other hand, mitotane has a very long terminal elimination half-life of up to 159 days [21].
The molecular underpinnings of mitotane adrenocortical toxicity were ill-defined until our laboratory identified sterol-O-acyltransferase 1 (SOAT1) as a key target of mitotane [22]. SOAT1 catalyses esterification of cholesterol and activated fatty acids to form cholesteryl esters which form a storage pool for steroid hormone synthesis in steroidogenic cells. We have shown that SOAT1 inhibition leads to accumulation of toxic lipids, in particular free cholesterol, which results in activation of endoplasmic reticulum stress of adrenocortical cells. ER-stress constitutes a major adaptive response of cells to unmet needs of biosynthesis and excess macromolecules. Impaired lipid homeostasis is increasingly acknowledged to activate this pathway [23, 24], but the unfolded protein response is the paradigmatic pathway of ER-stress activation [25]. Bortezomib and carfilzomib are proteasome inhibitors approved for treatment of multiple myeloma [26] that indirectly activate ER-stress and consecutively apoptosis through accumulation of unfolded proteins.
Our hypothesis was that, due to pathway convergence, combined treatment with mitotane and inhibitors of the proteasome would lead to enhanced efficacy. In this study, we investigated the potential of such combinatorial treatment in the adrenocortical carcinoma reference cell line NCI-H295R. We show that targeting the unfolded protein response activates additional signalling pathways of ER-stress, which leads to synergistic anti-proliferative activity of the combination regimens compared to monotherapy. This opens new perspectives for combination treatment in future clinical trials.
Materials and Methods
Chemicals
Mitotane was from ISP chemical products and diluted in ethanol; bortezomib from Millenium Pharmaceuticals and carfilzomib from Selleckchem were diluted in DMSO. All buffers and solvents from Merck (Darmstadt, Germany) unless otherwise stated.
Cell Culture
Adherent variant NCI-H295R cells were obtained from ATCC and cell line identity ascertained by short tandem repeat profiling in collaboration with Sabine Herterich from the Department of Clinical Chemistry and Laboratory Medicine, University Hospital Würzburg. Cells were cultured as described [22]. In short Ham’s F12 medium supplemented with 2.5 % Corning Nu-Serum (Fisher Scientific, Schwerte, Germany) , 5.2 ng/ml sodium selenite, 100 μg/ml transferrin and 5 μg/ml insulin was used and the cells were cultured in flasks in a humid atmosphere at 37 °C and 5 % CO2.
Cortisol Measurements
106 NCI-H295 cells/well were incubated with medium containing increasing concentrations of active substances or with the equivalent amount of solvent as negative control for indicated time periods. Cortisol was measured in the supernatant with an Immulite2000® Analyser (Siemens Healthcare Diagnostics). The cells in each well were subsequently lysed and the amount of protein was measured using a BCA assay (Sigma Aldrich) according to the manufacturer’s instructions. To compensate for cell toxicity during treatment, the cortisol values were normalized to the amount of protein in each experiment.
WST-1 Assay
Viability testing using WST1 reagent was performed according to the manufacturer’s protocol (Roche) by employing a Victor2 multi-plate reader (Perkin-Elmer).
Proteasome Activity Assay
Specific proteasome inhibition by mitotane was measured using a commercially available Proteasome Activity Assay kit (ab107921, Abcam) according to manufacturer’s instructions. In short, 2 × 10 [6] NCI-H295R cells were seeded per well and treated with 5, 10, 25, 50 and 100 μM mitotane or with diluent for 6 h. The cells were then lysed in 0.5 % Igepal CA630 and the soluble material was incubated with proteasome substrate, incubated further 30 min at 37 °C and output measured in a microplate reader (Perkin-Elmer) at Ex/Em 350/440 nm. The values were normalized to maximum inhibition values achieved under the same conditions by adding 100 μM of the proteasome inhibitor MG132.
Quantitative Real Time PCR (qRT-PCR) and Immunoblotting
qRT-PCR of XBP1 mRNA splicing and immunoblotting of NCI-H295 cell lysate was carried out as described [22]. GRP78 was detected using sc-1050 goat polyclonal antibody (Santa Cruz) diluted 1:150.
Assessment of Drug Interaction
For assessment of drug interaction parameters, the median effect model proposed by Chou et al. [27] was applied using Compusyn Software (Version 1.0, downloaded from www.combosyn.com). Cell viability measured by WST-1 testing was used as a read-out. To determine cooperativity index and dose-reduction index, viability was normalized using Prism Version 6.0 (GraphPad) and the following informative drug concentrations employed: bortezomib between 15 and 100 nM, carfilzomib between 1 and 50 μM and mitotane concentration between 5 and 25 μM.
Statistical Analysis
Prism 6.0 software (GraphPad) was used for all statistical analyses. The Fisher’s exact test or the Chi-square test was used to investigate dichotomic variables, a two-way RM ANOVA test followed by Tukey correction for multiple comparisons was used to test continuous variables. P values <0.05 were considered as statistically significant. Single drug concentrations inducing 50 % (EC50) of an observed effect (cell viability, cortisol production) were determined by applying curve fit nonlinear regression analyses.
Results
Proteasome Inhibition in NCI-H295R Cells
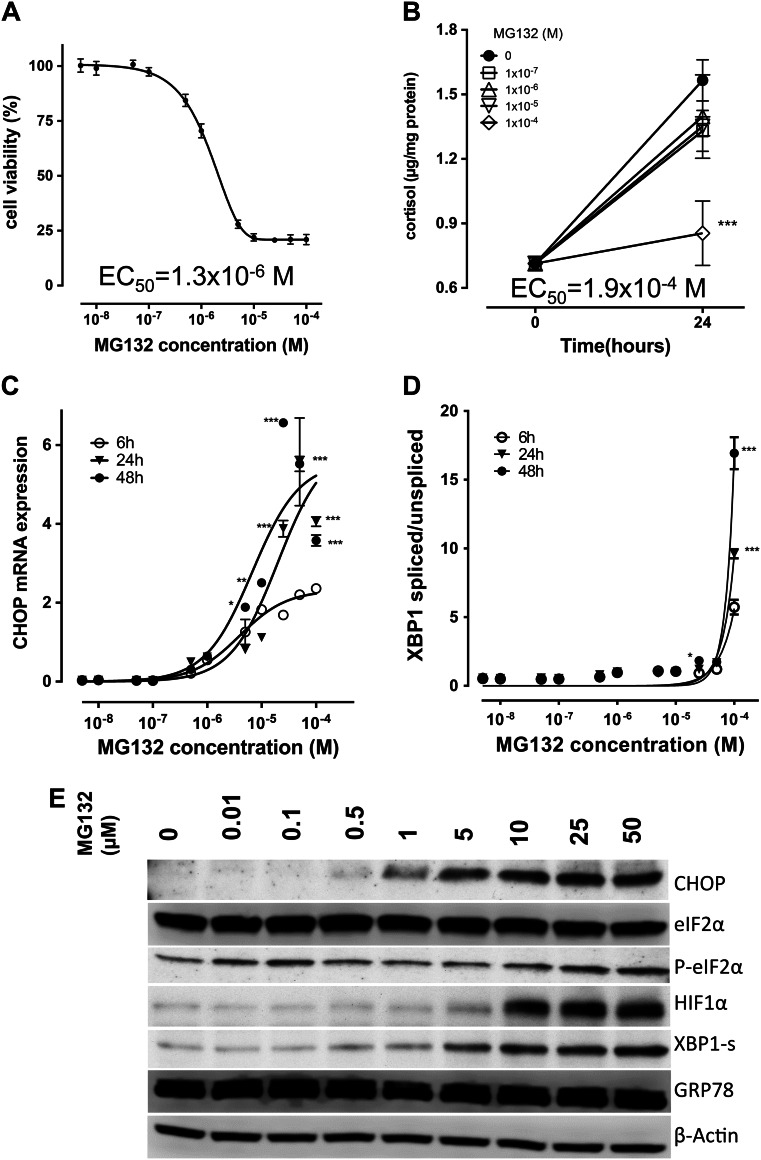
To determine the impact of proteasome inhibition alone on NCI-H295 cells, we studied the model compound MG132. Cell viability was decreased in a dose-dependent manner with an EC50 after 48 h of 1.3 μM (Fig. 1a). In contrast, MG132 did not exhibit a profound impact on cortisol production with an EC50 for cortisol inhibition after 24 h of 0.2 mM (Fig. 1b). To account for impaired cell viability (EC50 after 24 h 0.03 mM), the cortisol values were normalized to total cell protein of the same experiment.
Fig. 1.
Effect of the model proteasome inhibitor MG132 on adrenocortical tumour cells. a, MG132 concentration dependent decrease of cell viability after 48 h. b, Influence of MG132 treatment on cortisol production. MG132 leads to a concentration and time dependent increase in CHOP mRNA c and XBP1 mRNA splicing d in NCI-H295R cells. e, Concentration dependent changes induced by MG132 treatment of NCI-H295 cells. M, molar concentration; CHOP, C/EBP homologous protein; eIF2α, eukaryotic translation initiation factor 2 A; P-eIF2α, phosphorylated eIF2α; HIF1α, hypoxia inducible factor 1; XBP1-s, X-Box binding protein 1, encoded by the spliced mRNA; GRP78, 78 kDa glucose-regulated protein; *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to reference treatment; experiments were performed in biological triplicates
To elucidate the mechanisms underlying MG132-dependent reduction of cell viability, we assessed markers of ER-stress and found MG132 to strongly induce C/EBP homologous protein (CHOP) mRNA expression (Fig. 1c). Further, we found MG132 to induce splicing of X-box binding protein (XBP1)-mRNA up to 29.2 ± 2.0-fold (from 0.58 ± 0.07 to 16.93 ± 1.16) compared to untreated cells at 100 μM MG132 after 48 h (Fig. 1d), pointing towards activation of the inositol-requiring enzyme 1 (IRE1)-dependent ER-stress pathway.
At protein level (Fig. 1e), MG132 treatment was confirmed to increase both CHOP and spliced XBP1 (BBP1-s) proteins. Phosphorylation of eukaryotic initiation factor 2α (eIF2α) indicative of PERK (protein kinase R-like endoplasmic reticulum kinase)-dependent ER-stress activation did not show dose-dependent changes. Total eIF2α and ER-stress sensor BiP/GRP78 (binding immunoglobulin protein/78 kDa glucose-regulated protein) protein expression remained unchanged. As a positive control for proteasome inhibition, high-turnover protein hypoxia-inducible factor 1a (HIF1A) showed dose-dependent accumulation.
Impact of clinically Used Proteasome Inhibitors in NCI-H295 Cells
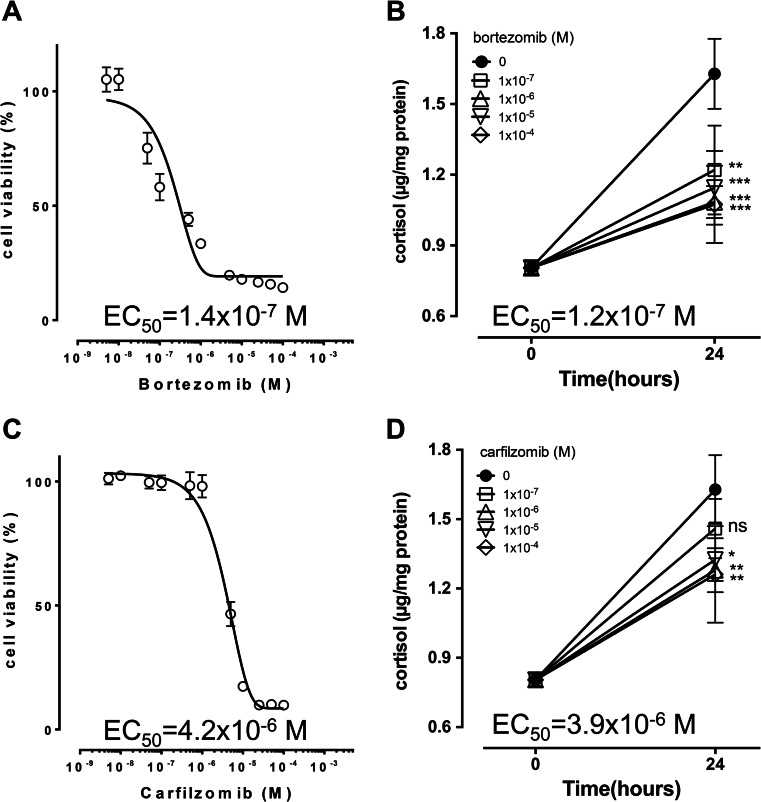
To explore the potential clinical application of proteasome inhibition in ACC, we next examined the efficacy of bortezomib and carfilzomib in NCI-H295R cells. Bortezomib has an average EC50 in different cell lines of 7 nM (e.g., human myeloma cell lines U266 and RPMI8226 EC50 = 3 and 30 nM, respectively [28]), and reached an average maximum plasma concentration of 1.32 μM (0.3–3.4 μM) in multiple myeloma patients [29]. Carfilzomib has similar EC50 values with bortezomib, both in myeloma cell lines (5 nM for ANBL-6 and 10 nM in MM1S cells) [30] and in myeloma patients (average maximum plasma concentration of 0.5 μM (0.1–1 μM) [31]).
As a single drug, bortezomib reduced cell viability at 48 h with an EC50 of 0.14 μM (Fig. 2a) and was alone sufficient to reduce cortisol production in cell culture supernatant within 24 h at already at 0.1 μM (Fig. 2b) with a calculated EC50 of 0.12 μM. Carfilzomib treatment alone reduced cell viability at 48 h with an EC50 of 4.2 μM (Fig. 2c) and reduced cortisol production in cell culture supernatant within 24 h significantly above 1 μM with an apparent EC50 of 3.9 μM (Fig. 2d). EC50 of mitotane in the same cells was 18.1 μM for cell viability and 19.1 μM for cortisol production at 24 h.
Fig. 2.
Effect of proteasome inhibitors bortezomib and carfilzomib on cell viability and cortisol secretion in adrenocortical tumour cells. Bortezomib (a and b) and carfilzomib (c and d) induce concentration dependent decrease in cell viability (a and c) and inhibition of cortisol production (b and d) after 48 and 24 h, respectively, in NCI-H295R cells. Apparent EC50 values are indicated. M, molar concentration; *, p < 0.05; **, p < 0.01 compared to reference treatment; experiments were performed in biological triplicates
Effects of Bortezomib and Mitotane on ER-Stress Activation
We next investigated whether and how mitotane and proteasome inhibitors bortezomib and carfilzomib impact on ER-stress markers in NCI-H295 cells. To this end, concentrations of both mitotane and proteasome inhibitors below and above their respective EC50 values for cell viability were analysed alone and in combination for XBP1-mRNA splicing, expression of CHOP protein, and phosphorylation of eIF2α. HIF1α served as control for proteasome inhibition.
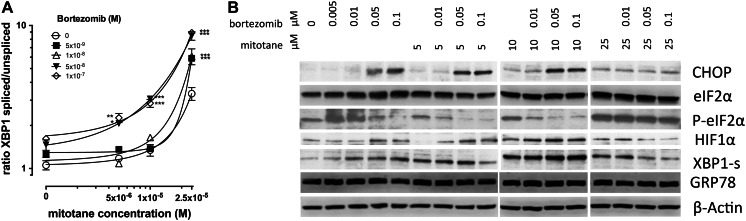
Splicing of XBP1-mRNA increased 1.2 ± 0.1, 1.2 ± 0.1, 1.4 ± 0.1 and 1.5 ± 0.1-fold compared to untreated cells with 5, 10, 50 and 100 nM bortezomib, respectively for 24 h (Fig. 3a), which was associated with a concomitant dose-dependent increase of XBP1-s protein expression (Fig. 3b). We found ER-stress marker CHOP protein to be induced by bortezomib at concentrations of 50 nM and higher without a dose-dependent increase of expression at higher concentrations (not shown) indicating complete activation of CHOP protein expression. Surprisingly, we consistently demonstrated bortezomib-dependent decrease of eIF2α-phosphorylation (Fig. 3b) with bortezomib alone whereas total eIF2α-protein remained unchanged.
Fig. 3.
Combined effects of bortezomib and mitotane treatment on markers of ER-stress in adrenocortical tumour cells. Concomitant treatment for 24 h with different concentrations of bortezomib and mitotane result in a concentration dependent increase in XBP1 mRNA splicing (a) and specific changes in expression of marker proteins (b), compared to treatment with the two substances alone. M, molar concentration; μM, concentration in micromoles/litre; CHOP, C/EBP homologous protein; eIF2α, eukaryotic translation initiation factor 2 A; P-eIF2α, phosphorylated eIF2α; HIF1α, hypoxia inducible factor 1; XBP1-s, X-Box binding protein 1, encoded by the spliced mRNA; GRP78, 78 kDa glucose-regulated protein; ***, p < 0.001 compared to reference treatment; experiments were performed in biological triplicates
Mitotane alone triggered splicing of XBP1 mRNA (Fig. 3a) resulting in expression of XBP1-s (Fig. 3b) protein. CHOP expression was increased at protein level with 5 and 10 μM mitotane and no further significant increase was observed at 25 μM (Fig. 3b). We found dose dependent increase eIF2α phosphorylation up to 25 μM mitotane (Fig. 3b).
Bortezomib in combination with mitotane induced a dose dependent increase of XBP1 mRNA splicing (Fig. 3a) whereas XBP1-s protein showed only marginal change (Fig. 3b). eIF2α phosphorylation decreased in a bortezomib dose dependent manner at 5 and 10 μM mitotane similar to our observation with bortezomib alone. At 25 μM mitotane, strong phosphorylation of eIF2α was observed which was not significantly decreased upon bortezomib co-treatment.
Combined Effects of Carfilzomib and Mitotane on ER-Stress Activation
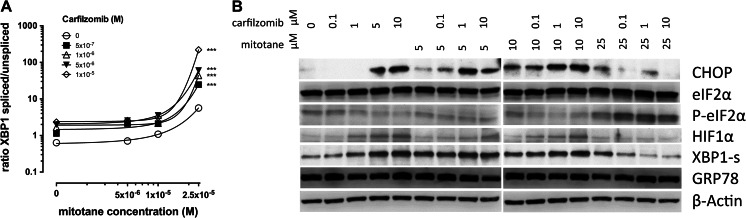
Carfilzomib treatment alone triggered XBP1 mRNA splicing 1.8 ± 0.1, 2.6 ± 0.1, 3.1 ± 0.3 and 3.7 ± 0.1-fold at 0.1, 1, 5 and 10 μM for 24 h, respectively (Fig. 4a), compared to mock treated cells which was associated with a concomitant dose-dependent increase of XBP1-s protein expression (Fig. 4b). CHOP protein expression was strongly induced (Fig. 4b) at concentrations of 5 μM and above. Phosphorylation of eIF2α was slightly decreased with carfilzomib alone (Fig. 4b).
Fig. 4.
Combined effects of carfilzomib and mitotane treatment on adrenocortical tumour cells. Concomitant treatment for 24 h with different concentrations of carfilzomib and mitotane result in a concentration dependent increase in XBP1 mRNA splicing (a) and to specific changes in expression of different proteins (b), compared to treatment with the two substances alone. M, molar concentration; μM, concentration in micromoles/litre; CHOP, C/EBP homologous protein; eIF2α, eukaryotic translation initiation factor 2 A; P-eIF2α, phosphorylated eIF2α; HIF1α, hypoxia inducible factor 1; XBP1-s, X-Box binding protein 1, encoded by the spliced mRNA; GRP78, 78 kDa glucose-regulated protein; ***, p < 0.001 compared to reference treatment; experiments were performed in biological triplicates
Combination with mitotane led to increased XBP1 mRNA splicing (Fig. 4a), XBP1-s and CHOP protein expression (Fig. 4b) in a carfilzomib dose-dependent manner at mitotane concentrations of 5 and 10 μM. Phosphorylation of eIF2α was decreased similar to treatment with carfilzomib alone.
At 25 μM mitotane, XBP1 mRNA was increased with increasing carfilzomib (Fig. 4a) doses which did not translate into increase of XBP1-s and CHOP protein (Fig. 4b). Strong phosphorylation of eIF2α was observed at 25 μM mitotane, which was unchanged upon co-treatment with carfilzomib.
Synergistic Activity of Proteasome Inhibitors with Mitotane
Using a proteasome activity assay, we found no significant inhibition of proteasome activity by mitotane when proteasome activity was normalized to account for cell death due to mitotane treatment (data not shown) suggesting that mitotane induced ER-stress has no direct impact on proteasome activity.
To determine to which extent activation of alternative ER-stress pathways by proteasome inhibitors and mitotane translates into cytotoxic effects, we used cell viability measured by WST-1 assay as a read-out. We applied bortezomib, carfilzomib and mitotane at concentrations in the range of their respective EC50 for NCI-H295R cells and determined drug synergy using algorithms developed by Chou et al. [27]. Cooperativity indices (CI) were determined using Compusyn software with CI-values below 1 indicating additivity, and values between 0.3 and 0.7 being considered synergistic.
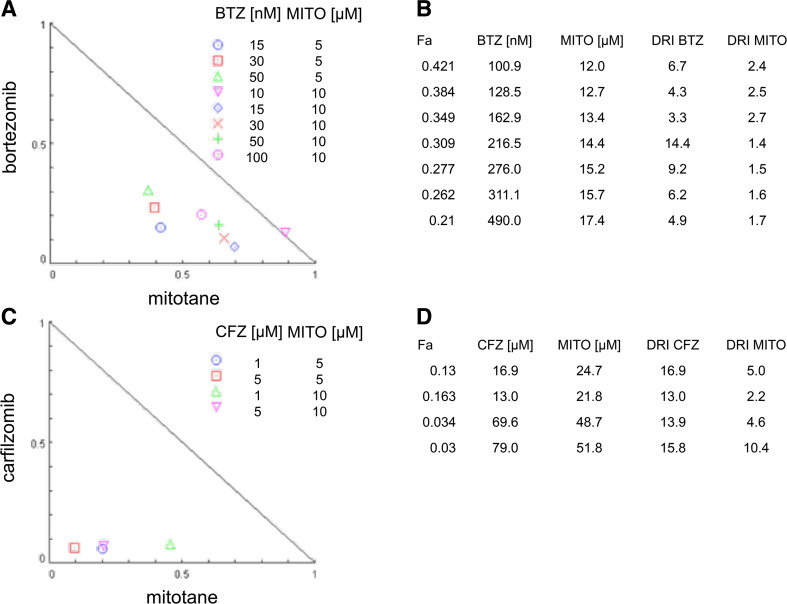
In a concentration range of mitotane that alone did not impact on cell viability (5 μM, 10 μM), we found synergism with bortezomib, as exemplified by a CI of 0.56 for 15 nM bortezomib (ten times less than the average maximum concentration reached in serum of the multiple myeloma patients) and 5 μM Mitotane (ten times less than the therapeutically relevant concentration). Cooperativity was less pronounced at higher relative efficacies of both drugs (Fig. 5a). This synergism translated into dose-reduction indices (DRI) for bortezomib between 3.3 and 14.4 but also mitotane where DRI between 1.4 and 2.7 were calculated (Fig. 5b).
Fig. 5.
Drug synergism between mitotane and proteasome inhibitors. Normalized isobolograms (a and c) and corresponding dose-reduction index (DRI) tables (b and d) for combination treatment with bortezomib and mitotane (a and b) and carfilzomib and mitotane (c and d). The diagonal line represents a cooperativity index of 1 indicating additive drug interaction of the combined treatment. Drug combinations below the curve indicate synergism, the area over the curve antagonism. Fa, fraction affected; BTZ, bortezomib; CFZ, carfilzomib; MITO, mitotane; DRI, dose-reduction index for the combination treatment; [nM], concentration range in nanomoles/litre and [μM], concentration range in micromoles/litre where the synergistic effect is observed
Similarly, combination of carfilzomib with mitotane resulted in CI between 0.16 and 0.53, indicating relevant synergism between the two drugs (Fig. 5c). Depending on the fractional activity of both drugs, DRI for carfilzomib between 13.0 and 16.9 were calculated with a higher DRI for mitotane between 2.2 and 10.4 (Fig. 5d) compared to bortezomib.
Overall, these data demonstrate that —with cell viability as a read-out—mitotane and proteasome inhibitors exhibit significant synergism.
Discussion
In this study, we examined the impact of two types of ER-stress inducing drugs to study their individual and combined impact on signal transduction pathways in the single available hormone producing adrenocortical carcinoma cell line. We reasoned that proteasome inhibitors might be re-purposed for the treatment of adrenocortical carcinoma in combination with mitotane.
Previously, our group provided evidence that a key mechanism of mitotane activity involves perturbation of lipid homeostasis in adrenocortical carcinoma cells by interfering with cholesterol esterification through inhibition of sterol-O-acyltransferase (SOAT1) [22]. Accumulation of toxic lipids, in particular free cholesterol, was shown to be causative of triggering endoplasmic reticulum stress since experimental reduction of cholesterol synthesis alleviated the toxic effects of mitotane. In contrast, non-steroidogenic cell lines were far less susceptible to SOAT1 inhibition since they express low SOAT1 levels. We have further demonstrated that—at clinically effective concentrations—mitotane is capable of activating both the IRE1 and PERK-dependent pathways of ER-stress [22]. Mitotane is the first drug in clinical use for which the mechanism of lipotoxic ER-stress has been demonstrated as a central mechanism of action.
In contrast, proteasome inhibitors have been established as prototypic drugs that induce ER-stress through activation of the unfolded protein response (UPR) and are clinically used for treatment of multiple myeloma [32].
As a proof of principle, we first investigated the impact of proteasome inhibition using the generic proteasome inhibitor MG132. As expected, we found reduced proteasomal degradation of the client protein hypoxia inducible factor 1α (HIF1A). Impaired proteasomal degradation, which is known to result in accumulation of unfolded proteins, led to activated ER-stress as shown by expression of CHOP mRNA and CHOP protein. Increased XBP1-mRNA splicing provided evidence of IRE1 pathway activation. Strikingly, phosphorylation of eIF2α indicative of PERK-dependent ER-stress, was only marginally changed. Our previous experiments had provided evidence that experimental induction of ER-stress alone is sufficient to decrease steroid hormone synthesis in NCI-H295 cells. Since MG132 was capable to strongly reduce cortisol secretion and also cell viability, we further pursued investigation of proteasome inhibitors using two therapeutically relevant drugs.
The impact of bortezomib, the first proteasome inhibitors used for treatment of multiple myeloma, on viability of NCI-H295 cells had been examined previously and found to trigger apoptosis as measured by caspase 3/7 assay [33]. Carfilzomib, a second-generation proteasome inhibitor, has been more recently approved for treatment of multiple myeloma and was also investigated. We recapitulated the experiments performed with MG132 and confirmed that individually both bortezomib and carfilzomib activate ER-stress through the IRE1 pathway in ACC cells whereas PERK-dependent ER-stress was marginally if at all activated as indicated by constant phosphorylation levels of eIF2α.
IRE1-induced XBP1-mRNA splicing has been previously shown to be of key importance in mediating bortezomib effects in multiple myeloma [34]. However, in different cellular contexts, inhibition of the proteasome has been shown to trigger eIF2α phosphorylation as well [35–37]. We have previously demonstrated that mitotane treatment alone is capable of strongly activating both PERK-dependent eIF2α-phosphorylation and IRE1-induced XBP1 mRNA splicing, indicating that both pathways are present and functional in NCI-H295 cells [22]. It is currently unclear, why in this steroidogenic cell line, proteasome inhibitors preferentially trigger the IRE1 dependent pathway but lead to a down-regulation of PERK-dependent eIF2α phosphorylation. One explanation may be that downstream effects of XBP1, a highly active transcription factor of ER-stress responsive genes, partially overlap with the consequences of eIF2α-phosporylation. We have already shown by using gene expression microarrays that mitotane induced ER-stress leads to reduced expression of several genes involved in lipid homeostasis such as SCD encoding stearoyl-coA-desaturase, an enzyme catalysing fatty acid desaturation, and SQLE encoding squalene epoxidase that catalyses the first step of cholesterol synthesis. In hepatocytes, it has been observed that transcription of lipid-related genes is XBP1 dependent [38, 39] whereas the individual contribution of ER-stress pathways in the adrenal cortex has not been investigated.
Since no significant inhibition of proteasome activity by mitotane alone was observed, mitotane induced ER-stress appears to depend primarily on lipotoxicity, which may explain the different molecular pattern of ER-stress response.
In combination with low-dose mitotane, both bortezomib and carfilzomib treatment decreased mitotane induced eIF2α phosphorylation. This is remarkable and—to our knowledge—has not been described previously. It is conceivable that in a cellular context characterized by high turnover of lipids for hormone synthesis, strong activation of the IRE1 response alleviates basal activity of the PERK-dependent pathway. Hence, combined treatment with proteasome inhibitors commits ER-stress towards the IRE1-dependent pathway. This mechanism however can be overcome through mitotane concentrations beyond its effective EC50 as indicated by strong eIF2α-phosphorylation irrespective of proteasome inhibitors’ concentration at 25 μM mitotane.
In conclusion, co-treatment with proteasome inhibitors may be a useful addition to mitotane at low concentrations of mitotane. Importantly, relatively high mitotane serum concentrations above 14 mg/l are associated with a higher likelihood of tumour response [9, 15, 17, 40, 41] but are reached within 3 months of treatment only in about half of patients [20, 42] and are never reached in some. Hence, there is frequently a time lag between treatment initiation with mitotane and onset of anti-tumoural effects. Therefore, combination treatment with a synergistic drug would be a major treatment advance and would potentially counteract cellular mechanisms of resistance to mitotane.
Drug synergism is widely searched after in many pharmacological contexts but oftentimes the term is not sufficiently substantiated by experimental data. Several methods to describe drug interactions have been developed in the past [43], among which the methods of Loewe [44] and Bliss [45] have been applied widely to determine drug additivity. An appealing method relies on the median effect equation as a unifying theorem of drug-drug interaction which can be applied in multiple experimental settings and has been elaborated by Chou and co-workers [27]. Of note, the EC50 of, e.g., mitotane and bortezomib differ by nearly three orders of magnitude rendering the Bliss method uninformative. By using cell viability after 48 h as relevant a read-out, we found that the differential capacities of proteasome inhibitors and mitotane to activate ER-stress through alternative pathways translate into drug synergism as demonstrated by cooperativity indices of ≪1. These experiments were conducted at mitotane concentrations that alone are insufficient to induce cell death in vitro since mitotane alone is capable of impairing cell viability completely above 25 μM after 48 h. The absence of synergism at higher concentrations of mitotane likely is a result from maximal activation of ER-stress through both the PERK and IRE1 dependent pathways with subsequently saturation of transducers such as CHOP. It is important to note that both carfilzomib and bortezomib both exhibit this synergism, indicating a class effect. The proteasome inhibitors concentrations at which drug synergism is observed are lower or equal than those achieved clinically [46, 47]. Unfortunately, both drugs are eliminated rapidly from the blood stream through tissue redistribution and elimination [48, 49]. While this increased clearance in part explains the good tolerability of proteasome inhibitors it would be more difficult to keep the clinically relevant concentrations for longer periods of time, especially for carfilzomib. However, although DRI are less impressive for mitotane, they may be clinically more important given the otherwise extremely long time required to achieve therapeutic plasma mitotane concentrations.
The conclusions of our study are based entirely on in vitro experiments performed in only one cell line. While this is a drawback, one has to bear in mind that NCI-H295 cells are the only human cell-line model available for the study of steroidogenic adrenocortical tumours. Whereas the cell line SW13 was derived from a cancer of the adrenal cortex, it lacks steroidogenic factor 1 expression, a reliable marker for cells of steroidogenic origin, and steroid hormone production [50, 51] thus limiting its usefulness as an adrenocortical tumour model. Our results in the NCI-H295 cell line model are consistent within a broad array of methods applied and robust, and are the first step in exploiting drug synergisms based on convergent activation of ER-stress in the treatment of adrenocortical cancer.
It is evident that validation of these in vitro results in an animal model is extremely desirable. However, two published studies using NCI-H295 xenografts in nude mice failed to demonstrate significant anti-tumoural activity of mitotane compared to control treatment on established tumours regardless of the means of administration and despite measurable mitotane concentration in plasma in one study [52, 53]. The reason for this is unknown but may depend on lipoprotein metabolism [54, 55]. At variance, a spontaneous mouse tumour model of ACC is unavailable [56]. On the other hand, with respect to the overall good tolerability of proteasome inhibitors in humans, it appears justified to conduct a phase I/II study of combination treatment in ACC patients treated with mitotane in a palliative setting. To account for the in vivo metabolism of bortezomib through CYP3A4, an enzyme that is strongly induced by mitotane [57–60], we would recommend to prioritise investigation of carfilzomib, as this drug undergoes elimination without involvement of the cytochrome P450 system.
Acknowledgments
The authors are grateful to Sabine Herterich for conducting STR analyses for cell line authentication, and Martina Zink for supporting the study with technical skills.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft DFG (grant numbers KR-43,741/1–1 to M.K. and FA 466/4-1 to M.F.) and a fellowship of the Comprehensive Cancer Center Mainfranken to M.K.
Footnotes
Matthias Kroiss and Silviu Sbiera contributed equally to this manuscript
References
- 1.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4551–4564. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 2.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35:282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johanssen S, Hahner S, Saeger W, Quinkler M, Beuschlein F, Dralle H, Haaf M, Kroiss M, Jurowich C, Langer P, Oelkers W, Spahn M, Willenberg HS, Mader U, Allolio B, Fassnacht M. Deficits in the management of patients with adrenocortical carcinoma in Germany. Dtsch Arztebl Int. 2010;107:885–891. doi: 10.3238/arztebl.2010.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berruti A, Fassnacht M, Haak H, Else T, Baudin E, Sperone P, Kroiss M, Kerkhofs T, Williams AR, Ardito A, Leboulleux S, Volante M, Deutschbein T, Feelders R, Ronchi C, Grisanti S, Gelderblom H, Porpiglia F, Papotti M, Hammer GD, Allolio B, Terzolo M. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol. 2014;65:832–838. doi: 10.1016/j.eururo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Nelson AA, Woodard G. Adrenal cortical atrophy and liver damage produced in dogs by feeding 2,2-bis-(parachloro-phenyl)-1,1-dichloroethane. Fed Proc. 1948;7:277. [PubMed] [Google Scholar]
- 6.Vilar O, Tullner WW. Effects of o,p’ DDD on histology and 17-hydroxycorticosteroid output of the dog adrenal cortex. Endocrinology. 1959;65:80–86. doi: 10.1210/endo-65-1-80. [DOI] [PubMed] [Google Scholar]
- 7.Hart MM, Reagan RL, Adamson RH. The effect of isomers of DDD on the ACTH-induced steroid output, histology and ultrastructure of the dog adrenal cortex. Toxicol Appl Pharmacol. 1973;24:101–113. doi: 10.1016/0041-008X(73)90185-3. [DOI] [PubMed] [Google Scholar]
- 8.Bergenstal D, Lipsett M, Moy R, Hertz R. Regression of adrenal cancer and suppression of adrenal function in men by o,p-DDD. Trans Am Physicians. 1959;72:341. [Google Scholar]
- 9.Hermsen IG, Fassnacht M, Terzolo M, Houterman S, den Hartigh J, Leboulleux S, Daffara F, Berruti A, Chadarevian R, Schlumberger M, Allolio B, Haak HR, Baudin E. Plasma concentrations of o,p’DDD, o,p’DDA, and o,p’DDE as predictors of tumor response to mitotane in adrenocortical carcinoma: results of a retrospective ENS@T multicenter study. J Clin Endocrinol Metab. 2011;96:1844–1851. doi: 10.1210/jc.2010-2676. [DOI] [PubMed] [Google Scholar]
- 10.Hahner S, Fassnacht M. Mitotane for adrenocortical carcinoma treatment. Curr Opin Investig Drugs. 2005;6:386. [PubMed] [Google Scholar]
- 11.Igaz P, Tombol Z, Szabo PM, Liko I, Racz K. Steroid biosynthesis inhibitors in the therapy of hypercortisolism: theory and practice. Curr Med Chem. 2008;15:2734–2747. doi: 10.2174/092986708786242921. [DOI] [PubMed] [Google Scholar]
- 12.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardiere C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Muller HH, Skogseid B, Group F-AS. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 13.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Fojo T. Adjuvant mitotane for adrenocortical cancer—a recurring controversy. J Clin Endocrinol Metab. 2008;93:3730–3732. doi: 10.1210/jc.2008-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baudin E, Pellegriti G, Bonnay M, Penfornis A, Laplanche A, Vassal G, Schlumberger M. Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p’DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer. 2001;92:1385–1392. doi: 10.1002/1097-0142(20010915)92:6<1385::AID-CNCR1461>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Malandrino P, Al Ghuzlan A, Castaing M, Young J, Caillou B, Travagli JP, Elias D, de Baere T, Dromain C, Paci A, Chanson P, Schlumberger M, Leboulleux S, and Baudin E (2010) Prognostic markers of survival after combined mitotane- and platinum-based chemotherapy in metastatic adrenocortical carcinoma (ACC). Endocr Relat Cancer. [DOI] [PubMed]
- 17.Terzolo M, Baudin AE, Ardito A, Kroiss M, Leboulleux S, Daffara F, Perotti P, Feelders RA, deVries JH, Zaggia B, De Francia S, Volante M, Haak HR, Allolio B, Al Ghuzlan A, Fassnacht M, Berruti A. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol. 2013;169:263–270. doi: 10.1530/EJE-13-0242. [DOI] [PubMed] [Google Scholar]
- 18.Daffara F, De Francia S, Reimondo G, Zaggia B, Aroasio E, Porpiglia F, Volante M, Termine A, Di Carlo F, Dogliotti L, Angeli A, Berruti A, Terzolo M. Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer. 2008;15:1043–1053. doi: 10.1677/ERC-08-0103. [DOI] [PubMed] [Google Scholar]
- 19.Terzolo M, Zaggia B, Allasino B, De Francia S. Practical treatment using mitotane for adrenocortical carcinoma. Curr Opin Endocrinol Diabetes Obes. 2014;21:159–165. doi: 10.1097/MED.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 20.Kerkhofs TM, Baudin E, Terzolo M, Allolio B, Chadarevian R, Mueller HH, Skogseid B, Leboulleux S, Mantero F, Haak HR, Fassnacht M. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4759–4767. doi: 10.1210/jc.2013-2281. [DOI] [PubMed] [Google Scholar]
- 21.Moolenaar AJ, van Slooten H, van Seters AP, Smeenk D. Blood levels of o,p’-DDD following administration in various vehicles after a single dose and during long-term treatment. Cancer Chemother Pharmacol. 1981;7:51–54. doi: 10.1007/BF00258213. [DOI] [PubMed] [Google Scholar]
- 22.Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, Matysik S, Eckhardt C, Gardill F, Gehl A, Kendl S, Weigand I, Bala M, Ronchi CL, Deutschbein T, Schmitz G, Rosenwald A, Allolio B, Fassnacht M, Kroiss M. Mitotane inhibits Sterol-O-Acyl Transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156:3895–3908. doi: 10.1210/en.2015-1367. [DOI] [PubMed] [Google Scholar]
- 23.Xue J, Wei J, Dong X, Zhu C, Li Y, Song A, Liu Z. ABCG1 deficiency promotes endothelial apoptosis by endoplasmic reticulum stress-dependent pathway. J Physiol Sci. 2013;63:435–444. doi: 10.1007/s12576-013-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou NS, Gutschmidt A, Choi DY, Pather K, Shi X, Watts JL, Hoppe T, Taubert S. Activation of the endoplasmic reticulum unfolded protein response by lipid disequilibrium without disturbed proteostasis in vivo. Proc Natl Acad Sci U S A. 2014;111:E2271–E2280. doi: 10.1073/pnas.1318262111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 26.Kubiczkova L, Pour L, Sedlarikova L, Hajek R, Sevcikova S. Proteasome inhibitors—molecular basis and current perspectives in multiple myeloma. J Cell Mol Med. 2014;18:947–961. doi: 10.1111/jcmm.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 28.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 29.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, Bradley S, Gobburu JV, Goheer A, Lee SL, Leighton J, Liang CY, Lostritto RT, McGuinn WD, Morse DE, Rahman A, Rosario LA, Verbois SL, Williams G, Wang YC, Pazdur R. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 30.Ashley JD, Stefanick JF, Schroeder VA, Suckow MA, Alves NJ, Suzuki R, Kikuchi S, Hideshima T, Anderson KC, Kiziltepe T, Bilgicer B. Liposomal carfilzomib nanoparticles effectively target multiple myeloma cells and demonstrate enhanced efficacy in vivo. J Control Release. 2014;196:113–121. doi: 10.1016/j.jconrel.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Redic K. Carfilzomib: a novel agent for multiple myeloma. J Pharm Pharmacol. 2013;65:1095–1106. doi: 10.1111/jphp.12072. [DOI] [PubMed] [Google Scholar]
- 32.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–959. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilubol N, Zhang L, Shen M, Zhang YQ, He M, Austin CP, Kebebew E. Four clinically utilized drugs were identified and validated for treatment of adrenocortical cancer using quantitative high-throughput screening. J Transl Med. 2012;10:198. doi: 10.1186/1479-5876-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, Chung KC, Tiedemann RE. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013;24:289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280:14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- 36.Raven JF, Baltzis D, Wang S, Mounir Z, Papadakis AI, Gao HQ, Koromilas AE. PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2alpha phosphorylation. J Biol Chem. 2008;283:3097–3108. doi: 10.1074/jbc.M709677200. [DOI] [PubMed] [Google Scholar]
- 37.Yerlikaya A, Kimball SR, Stanley BA. Phosphorylation of eIF2alpha in response to 26S proteasome inhibition is mediated by the haem-regulated inhibitor (HRI) kinase. Biochem J. 2008;412:579–588. doi: 10.1042/BJ20080324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen S, Jumaa H, Webster NJ. Splicing factor SRSF3 is crucial for hepatocyte differentiation and metabolic function. Nat Commun. 2013;4:1336. doi: 10.1038/ncomms2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piperi C, Adamopoulos C, Papavassiliou AG. XBP1: a pivotal transcriptional regulator of glucose and lipid metabolism. Trends Endocrinol Metab. 2016;27:119–122. doi: 10.1016/j.tem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Haak HR, Hermans J, van de Velde CJ, Lentjes EG, Goslings BM, Fleuren GJ, Krans HM. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malandrino P, Al Ghuzlan A, Castaing M, Young J, Caillou B, Travagli JP, Elias D, de Baere T, Dromain C, Paci A, Chanson P, Schlumberger M, Leboulleux S, Baudin E. Prognostic markers of survival after combined mitotane- and platinum-based chemotherapy in metastatic adrenocortical carcinoma. Endocr Relat Cancer. 2010;17:797–807. doi: 10.1677/ERC-09-0341. [DOI] [PubMed] [Google Scholar]
- 42.Kerkhofs TM, Derijks LJ, Ettaieb H, den Hartigh J, Neef K, Gelderblom H, Guchelaar HJ, Haak HR. Development of a pharmacokinetic model of mitotane: toward personalized dosing in adrenocortical carcinoma. Ther Drug Monit. 2015;37:58–65. doi: 10.1097/FTD.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 43.Breitinger H-G (2012) Drug synergy - mechanisms and methods of analysis. Toxicity and Drug Testing ISBN: 978–953–51-0004-1: 143–166.
- 44.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 45.Bliss CI. The toxicity of poisons applied jointly. Ann Appl Biol. 1939;26:585–615. doi: 10.1111/j.1744-7348.1939.tb06990.x. [DOI] [Google Scholar]
- 46.Osawa T, Naito T, Kaneko T, Mino Y, Ohnishi K, Yamada H, Kawakami J. Blood distribution of bortezomib and its kinetics in multiple myeloma patients. Clin Biochem. 2014;47:54–59. doi: 10.1016/j.clinbiochem.2014.06.077. [DOI] [PubMed] [Google Scholar]
- 47.Wang M, Martin T, Bensinger W, Alsina M, Siegel DS, Kavalerchik E, Huang M, Orlowski RZ, Niesvizky R. Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122:3122–3128. doi: 10.1182/blood-2013-07-511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, Pien CS, Millikan RE, SM T, Pagliaro L, Kim J, Adams J, Elliott P, Esseltine D, Petrusich A, Dieringer P, Perez C, Logothetis CJ. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 49.Papadopoulos KP, Siegel DS, Vesole DH, Lee P, Rosen ST, Zojwalla N, Holahan JR, Lee S, Wang Z, Badros A. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33:732–739. doi: 10.1200/JCO.2013.52.3522. [DOI] [PubMed] [Google Scholar]
- 50.Leibovitz A, WM MC, III, Johnston D, McCoy CE, Stinson JC. New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J Natl Cancer Inst. 1973;51:691–697. [PubMed] [Google Scholar]
- 51.Sbiera S, Schmull S, Assie G, Voelker HU, Kraus L, Beyer M, Ragazzon B, Beuschlein F, Willenberg HS, Hahner S, Saeger W, Bertherat J, Allolio B, Fassnacht M. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab. 2010;95:E161–E171. doi: 10.1210/jc.2010-0653. [DOI] [PubMed] [Google Scholar]
- 52.Lindhe O, Skogseid B. Mitotane effects in a H295R xenograft model of adjuvant treatment of adrenocortical cancer. Horm Metab Res. 2010;42:725–730. doi: 10.1055/s-0030-1261923. [DOI] [PubMed] [Google Scholar]
- 53.Doghman M, Lalli E. Lack of long-lasting effects of mitotane adjuvant therapy in a mouse xenograft model of adrenocortical carcinoma. Mol Cell Endocrinol. 2013;381:66–69. doi: 10.1016/j.mce.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Hescot S, Seck A, Guerin M, Cockenpot F, Huby T, Broutin S, Young J, Paci A, Baudin E, Lombes M. Lipoprotein-free mitotane exerts high cytotoxic activity in adrenocortical carcinoma. J Clin Endocrinol Metab. 2015;100:2890–2898. doi: 10.1210/JC.2015-2080. [DOI] [PubMed] [Google Scholar]
- 55.Kroiss M, Plonne D, Kendl S, Schirmer D, Ronchi CL, Schirbel A, Zink M, Lapa C, Klinker H, Fassnacht M, Heinz W, Sbiera S. Association of mitotane with chylomicrons and serum lipoproteins: practical implications for treatment of adrenocortical carcinoma. Eur J Endocrinol. 2016;174:343–353. doi: 10.1530/EJE-15-0946. [DOI] [PubMed] [Google Scholar]
- 56.Hantel C, Beuschlein F. Mouse models of adrenal tumorigenesis. Best Pract Res Clin Endocrinol Metab. 2010;24:865–875. doi: 10.1016/j.beem.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 57.van Erp NP, Guchelaar HJ, Ploeger BA, Romijn JA, Hartigh J, Gelderblom H. Mitotane has a strong and a durable inducing effect on CYP3A4 activity. Eur J Endocrinol. 2011;164:621–626. doi: 10.1530/EJE-10-0956. [DOI] [PubMed] [Google Scholar]
- 58.Kroiss M, Quinkler M, Lutz WK, Allolio B, Fassnacht M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol. 2011;75:585–591. doi: 10.1111/j.1365-2265.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 59.Kroiss M, Quinkler M, Johanssen S, van Erp NP, Lankheet N, Pollinger A, Laubner K, Strasburger CJ, Hahner S, Muller HH, Allolio B, Fassnacht M. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97:3495–3503. doi: 10.1210/jc.2012-1419. [DOI] [PubMed] [Google Scholar]
- 60.Chortis V, Taylor AE, Schneider P, Tomlinson JW, Hughes BA, O’Neil DM, Libe R, Allolio B, Bertagna X, Bertherat J, Beuschlein F, Fassnacht M, Karavitaki N, Mannelli M, Mantero F, Opocher G, Porfiri E, Quinkler M, Sherlock M, Terzolo M, Nightingale P, Shackleton CH, Stewart PM, Hahner S, Arlt W. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5alpha-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98:161–171. doi: 10.1210/jc.2012-2851. [DOI] [PubMed] [Google Scholar]