Abstract
Adrenocortical carcinoma (ACC) has a dismal prognosis in advanced stages. Despite treatment with the adrenal toxicant mitotane and/or aggressive chemotherapy, tumor control is often short-lived. Here, we examine trofosfamide as a salvage treatment of ACC in an observational cohort study within the German ACC registry. Response defined as progression-free survival (PFS) at first tumor evaluation was assessed by RECIST 1.1 or clinically, and PFS and overall survival (OS) were estimated by the Kaplan-Meier method. Twenty-seven patients (11 males; median age 46.9 years) progressing after mitotane and three (median, range 0–5) other systemic treatments were evaluated for safety. Trofosfamide (150 mg/day) was administered as monotherapy (n = 13) or in combination with mitotane (n = 14). Overall tolerability was good with only mild adverse events. Six patients did not meet criteria for response assessment. Of the 21 patients, 8 patients had clinically progressive disease (3 deaths from ACC); among the 13 patients evaluable by RECIST 1.1, best response to treatment was stable disease (SD, n = 3) or progressive disease (n = 10). Hence, predefined response criteria were met in 3/21 patients (14 %). Median PFS was 84 days (95 % confidence interval 74–95) and median OS survival 198 days (95 % CI 89–307). One prolonged disease stabilization (best response by RECIST 1.1 −26 %) was observed for 479 days. In conclusion, trofosfamide is overall well tolerated but disease stabilization is rather rare. Accordingly, it may be used in selected cases of ACC not amenable to other treatment options such as clinical trials.
Keywords: Adrenocortical Carcinoma, Mitotane, Tumor Assessment, Tumor Drug Resistance, Potential Adverse Drug Effect
Introduction
Adrenocortical carcinoma (ACC) is an orphan malignancy with a dismal prognosis [1–4]. In advanced disease, the adrenostatic agent mitotane is a cornerstone of medical therapy [5–8]. Mitotane is virtually always associated with adverse effects. Among these, central nervous symptoms such as dizziness, fatigue, depression, or even stroke-like symptoms and psychosis are most bothersome [9]. Toxicity and the induction of microsomal liver enzymes that may lead to serious drug interaction [10–14] render the treatment with mitotane potentially dangerous [15]. Therefore, frequent drug monitoring [13] is necessary. Nevertheless, mitotane can be considered a molecular targeted therapy since we recently demonstrated this drug to inhibit sterol-O-acyl transferase 1 (SOAT1) which leads to accumulation of cholesterol and fatty acids in the adrenal cortical cell. These toxic lipids then induce the endoplasmic reticulum stress response resulting in inhibition of steroid hormone synthesis and apoptosis [16].
Importantly, mitotane is often only temporarily effective and additional treatment options are required. The first phase III clinical trial in ACC [17] established combination chemotherapy with etoposide, doxorubicin, and cisplatin in addition to mitotane [18] as standard of care for the treatment of advanced disease. Despite intensive treatment, tumor stabilization is often short-lived and progression is frequent. Over the last few years, several cytotoxic [19] and molecular targeted therapies have been evaluated as potential alternatives on a compassionate use basis [20, 21] or in phase II clinical trials [22, 23] but failed to reach significant improvement. These disappointing results may have been due—at least in part—to unrecognized drug interaction with mitotane [11]. Targeting the IGF1 receptor with linsitinib in a phase III randomized trial likewise failed to achieve significant tumor response in the majority of patients [24] despite few cases with remarkable response. In the absence of established treatments, salvage therapies combining low toxicity with oral administration are warranted. Such metronomic therapies have been suggested in ACC [25, 26] but solid evidence is lacking with respect to choice of drug and the regimen to apply.
Trofosfamide belongs to the class of oxazaphosphorines such as cyclophosphamide and ifosfamide. These prodrugs are metabolically activated in the liver to yield daughter compounds that form alkyl adducts with DNA [27, 28]. Trofosfamide has been successfully used as a palliative oral treatment of non-Hodgkin lymphoma [29] and demonstrated activity as a metronomic therapy of soft tissue sarcoma [30] and gynecological cancers [31]. Overall, an acceptable tolerability of trofosfamide even after multiple previous regimens and in advanced tumor stages has been reported, making it an obvious candidate drug for salvage treatment of ACC.
Here, we retrospectively evaluated patients treated with trofosfamide on a compassionate use basis to determine its efficacy and tolerability in patients with refractory ACC.
Subjects and Methods
Patients
Patients and clinical parameters such as sex, age at diagnosis, tumor size, evidence of hormone excess, and tumor stage according to the European Network for the Study of Adrenal Tumors (ENSAT) classification [32], date of documented irresectability, Weiss score [33], Ki67 index [34], presence and number of distant metastasis, concomitant treatment with mitotane, and follow-up information were retrieved from the German ACC Registry and the ENSAT Registry (www.ensat.org/registry[35]). These studies were approved by the ethics committee of the University of Würzburg (approval number 86/03 and 88/11), and written informed consent was obtained from all patients. The following inclusion criteria were applied in this observational cohort study: histologically confirmed adrenocortical carcinoma, previous mitotane treatment with or without additional lines of therapies, documented irresectable disease, and documented oral treatment with trofosfamide.
Treatment and Treatment Evaluation
The following criteria were defined for evaluation of response: documented treatment with trofosfamide for ≥30 days. Dosage and dose adjustments were at the discretion of the treating physician according to the label use of trofosfamide.
Time interval from staging to initiation of trofosfamide is ≤56 days. Time interval between radiologic tumor assessments is ≤112 days. Response to treatment was assessed radiologically from initiation of therapy until tumor progression using the response evaluation criteria in solid tumors (RECIST) 1.1 [36] or clinically. All imaging studies were individually reviewed in a blinded fashion by two experienced radiologists (W.S., A.H.). Potential adverse drug effects (AE) were retrieved from patient records, retrospectively graded according to the National Cancer Institute common toxicity criteria (CTC) version 4.0 and relatedness to treatment assessed. In uncertain cases, the physician who originally supervised the treatment was contacted to clarify potential adverse events. Adverse drug effects at least considered possibly treatment-related are reported in this study.
Statistical Analysis
Response analysis was per protocol according to the inclusion criteria defined above. Progression-free survival was defined as the time interval between the beginning of trofosfamide treatment and the date at which disease progression was documented at imaging, clinically or death of any cause. Overall survival was calculated as the time between start of trofosfamide and death of any cause or last follow-up. Survival curves were constructed using the Kaplan-Meier method. Continuous variables are presented as the median and range, Kaplan-Meier estimates as median and—where appropriate—95 % confidence interval unless otherwise stated. SPSS Version 23 was used for statistical calculations.
Results
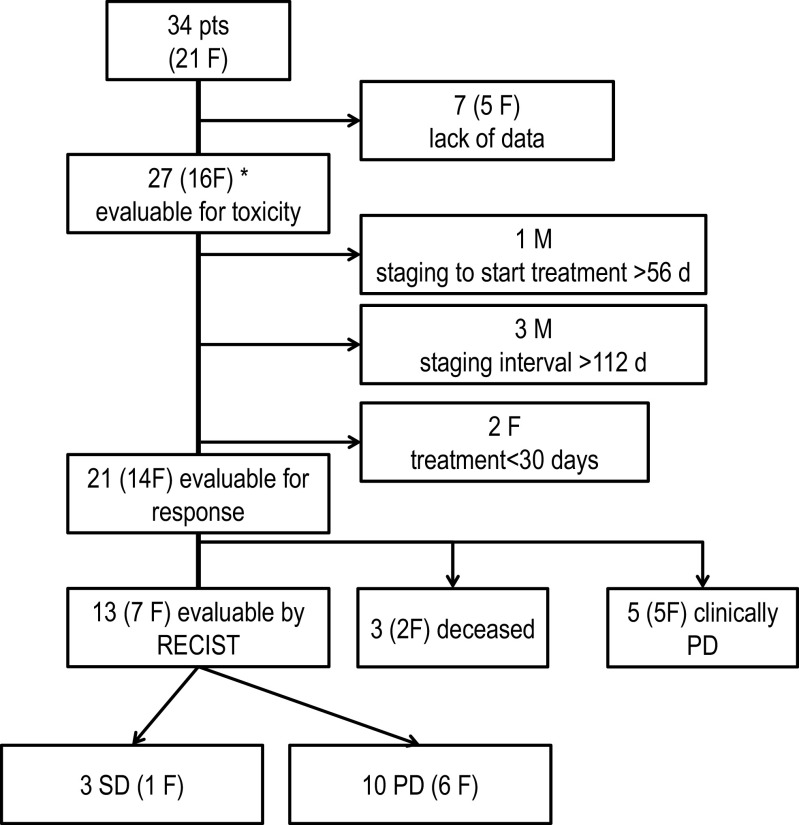
Thirty-four patients from three German centers who had undergone treatment with trofosfamide were identified. Of those, seven had to be excluded due to lack of data. Finally, 27 patients treated between 2003 and 2012 were evaluable for safety out of whom 21 fulfilled criteria for assessment of response (see Fig. 1).
Fig. 1.
Patient disposition (CONSORT diagram)
Patient Characteristics and Treatment
Twenty-seven patients (11 males) with a median age of 46.9 years (range 17.0–78.6) were included in the analysis (Table 1). Tumor stage at diagnosis and other clinical characteristics are given in Table 1. Of note, the median time intervals between (a) primary diagnosis and (b) diagnosis of irresectability to initiation of trofosfamide were 41.0 (range 0.8–69.7) and 25.3 months (range 0–121), respectively. All patients had previously received mitotane and 13 subjects (48 %) continued with mitotane during trofosfamide treatment. All but one patient had received additional lines of systemic treatment in addition to mitotane, with a median number of three regimens (range 0–5). Median duration of treatment with trofosfamide in all 27 patients was 91 days (range 4–766 days). Twenty-five patients received 150 mg trofosfamide daily as 50-mg tablets. One patient (PID2) received 50 mg. In a second case (PID 8), treatment was started with 300 mg for 2 days and then reduced to 100-mg daily dose.
Table 1.
Clinical characteristics of the patients in this study
| Characteristic | Number of patients or median (range) |
|---|---|
| Number of patients | 27 |
| Sex | |
| Males | 11 |
| Females | 16 |
| Age at initial diagnosis (years) | |
| Median (range) | 46.9 (17.0–78.6) |
| Age at treatment initiation with trofosfamide (years) | |
| Median (range) | 49.9 (22.8–78.7) |
| Interval between initial diagnosis and treatment initiation with trofosfamide (months) | |
| Median (range) | 40.9 (0.8–69.7) |
| Interval between the diagnosis of irresectable ACC and starting trofosfamide (months) | |
| Median (range) | 21.9 (0.8–62.5) |
| ENSAT tumor stage at initial diagnosis | |
| I | 1 |
| II | 11 |
| III | 7 |
| IV | 8 |
| Overt clinical hormone excess at initial diagnosis | |
| Glucocorticoid excess only | 1 |
| Androgen excess only | 2 |
| Glucocorticoid + androgen excess | 6 |
| Glucocorticoid + mineralocorticoid excess | 2 |
| None | 16 |
| Histopathological workup | |
| Ki67 index available | 23 |
| Median (range) | 20 (2–60) |
| Weiss score available | 18 |
| Median (range) | 6 (3–8) |
| Sum of diameter of target lesions (RECIST; mm) | |
| Median (range) | 163 (9–326) |
| Number of target lesions (RECIST) | |
| Median (range) | 4 (1–5) |
| Sites of target lesions (RECIST) | |
| Local recurrence | 8 |
| Liver | 16 |
| Lung | 15 |
| Abdominal lymph nodes | 14 |
| Peritoneum | 6 |
| Skin and soft tissue | 4 |
| Mediastinum | 5 |
| Other | 2 |
| Therapies prior to treatment with trofosfamide | |
| Surgery | 27 |
| Mitotane | 27 |
| Continued at the time of treatment initiation with trofosfamidea | 13 |
| Cytotoxic chemotherapy | |
| Streptozotocin | 22 |
| Etoposide, doxorubicin, and cisplatin | 19 |
| Other platinum containing regimen | 4 |
| Gemcitabine and capecitabine | 17 |
| Thalidomide | 3 |
| Other | 5 |
| Targeted therapy | |
| Linsitinib | 5 |
| Sunitinib | 3 |
| Radiotherapy | 12 |
| Chemoembolization | 4 |
| 131I-Iodometomidate | 1 |
aOne of these patients (PID27) started mitotane during trofosfamide treatment
The median RECIST diameter at baseline (n = 25) was 165 mm (9–326). Sites of tumor localizations were liver (n = 16; 64 %), lung (n = 15; 60 %), abdominal lymph nodes (n = 14; 56 %), peritoneum (n = 6; 24 %), and soft tissue (n = 4; 16 %).
Treatment Emergent Adverse Events
Seventeen of 27 patients experienced a total of 26 adverse events (AEs) emerging from treatment with trofosfamide (Table 2). One patient had grade 3 stroke and one patient with grade 4 pulmonary embolism each on the fifth day of trofosfamide judged to be possibly treatment related. In both cases, trofosfamide was stopped. Chart review could not further clarify relatedness of these AEs to trofosfamide treatment. The short time interval between initiation of trofosfamide and AE onset in our opinion renders the likelihood that trofosfamide treatment contributed to their appearance rather low, but we cannot exclude a relation to treatment with trofosfamide. However, all other adverse events were grade 1/2 such as dyspnea, loss of appetite, fatigue, and muscle weakness. In general, though, treatment was very well tolerated.
Table 2.
Treatment-emergent adverse events
| Category | Grade 1 or 2 (n) | Grade 3 or 4 (n) |
|---|---|---|
| Cardiovascular | ||
| pulmonary embolism | 1 | |
| stroke | 1 | |
| Pulmonary | ||
| Dyspnea | 3 | |
| Renal system and electrolytes | ||
| Hypokalemia | 1 | |
| Infection | ||
| Lung | 1 | |
| Gastrointestinal | ||
| Dry mouth | 1 | |
| Nausea | 1 | |
| Pain | ||
| Abdominal pain | 2 | |
| General | ||
| Malaise | 2 | |
| Loss of appetite | 3 | |
| Fatigue | 3 | |
| Edema limb | 2 | |
| Neuromuscular | ||
| Muscle weakness | 3 | |
| Numbness foot | 1 | |
| Memory impairment | 1 | |
Treatment Response
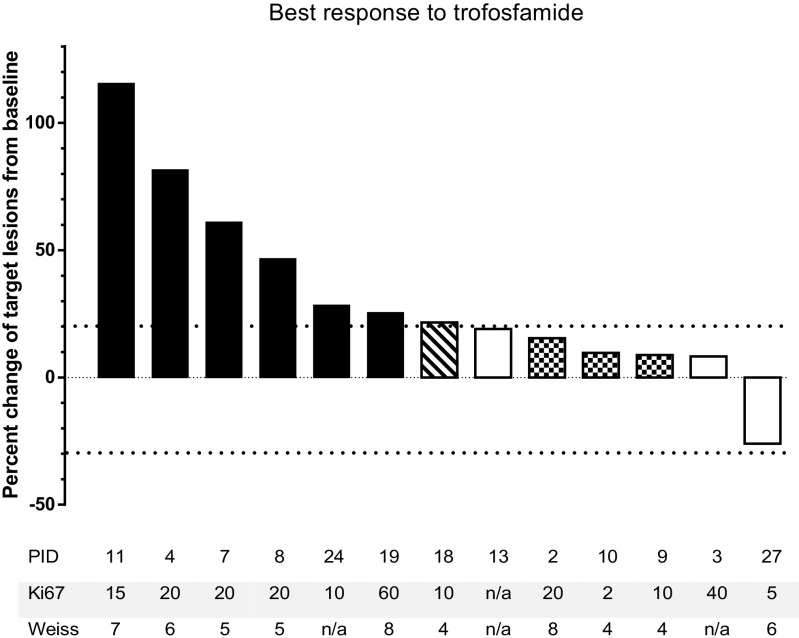
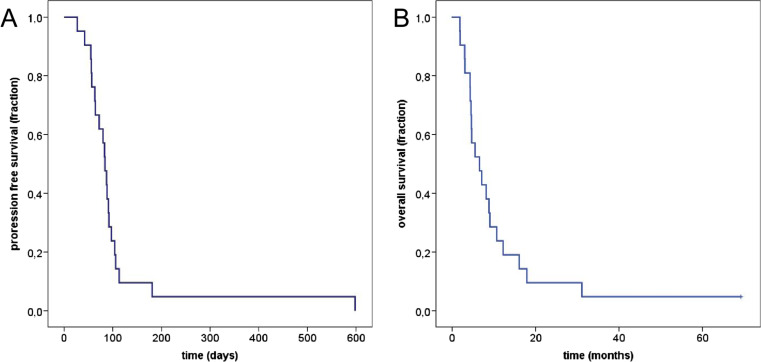
Of the 21 patients evaluable for response, three died due to advanced ACC prior to first tumor assessment and five had clinically clearly progressive disease leading to discontinuation of treatment. Thirteen patients were evaluated by RECIST 1.1. Ten patients (77 %) exhibited progressive disease at first tumor assessment, whereas three individuals (23 %) had stable disease. The median change of tumor size from baseline to the time of best response to therapy was +22 % (range −26 to +115 %). Four patients were judged as progressive disease by RECIST 1.1 due to new metastases, including one with new malignant ascites and pleural effusion and another with peritoneal metastases. The individual best response to treatment with trofosfamide is shown in Fig. 2. Hence, response defined as progression-free survival at first tumor assessment was found in three of 21 patients evaluated per protocol (14 %). The median progression-free survival in this cohort was 84 days (95 % confidence interval (CI) 74–94 days, Fig. 3a), and the median overall survival was 198 days (95 % CI 89–307, Fig. 3b).
Fig. 2.
Waterfall plot of RECIST sum diameter at best response in patients with ACC treated with trofosfamide. Black bars: PD of target lesions, white bars: SD according to RECIST, squared bars: SD of target lesions, new tumor lesions, overall PD; dashed bar: new tumor lesions. Ki67: Ki 67 index (%), Weiss: Weiss score
Fig. 3.
Kaplan-Meier plots (n = 21) of a progression-free survival and b overall survival of ACC patients treated with trofosfamide
Among the patients with stable disease, one female patient (PID 3) had the first tumor assessment after 56 days and had progressive disease with new metastases in liver, bone, and peritoneum after another 57 days. This patient continued on mitotane and was on therapeutic mitotane plasma concentrations prior to (17.3 mg/l) and during trofosfamide treatment (day 8 16.2 mg/l, day 43 22.8 mg/l) to control steroid hormone excess. The patient had received mitotane as part of chemotherapy regimens with both streptozotocin and etoposide, doxorubicin, and cisplatin (EDP), and tumor progression was evident at restaging despite documented mitotane plasma concentrations >14 mg/l.
A second patient (PID13) had stable disease at the first evaluation after 73 days with an increase of target lesions by 19 % and non-significant progression of lung metastases (non-target lesions). At second tumor assessment after 183 days from baseline, target lesions had progressed by 40 %. Due to some potential clinical benefit, this patient continued treatment for an overall duration of 299 days and died after 325 days. Patient 13 had started with mitotane 14 months before and continued during trofosfamide treatment. Mitotane plasma concentration at initiation of trofosfamide was 21.6 mg/l. Progression was radiologically documented at restaging examinations every 2 to 3 months during previous treatment with mitotane in combination with EDP, gemcitabine/capecitabine, streptozotocin, and thalidomide.
The third patient (PID 27) had stable disease after 79 days. The best response to treatment was a decrease of target lesions by 26 % after 479 days. This individual had discontinued mitotane 5 months before trofosfamide, and the mitotane plasma concentration was documented to be undetectable at treatment initiation with trofosfamide. Mitotane was reintroduced 249 days after start with trofosfamide and >14 mg/l 353 days
The subject experienced progressive disease with a new liver lesion after 598 days and continued treatment with trofosfamide for an overall time period of 766 days in the absence of alternative treatment options. Upon further progression, the patient received stereotactic radiation therapy of pulmonary lesions, while liver metastases were treated with stereotactic radiation therapy and radiofrequency ablation. Since all tumor sites were stable after reinitiation of gemcitabine and capecitabine, remaining tumor lesions were resected and the patient is now tumor-free 5.8 years after start of trofosfamide.
No histopathological and clinical differences were observed between patients experiencing disease stabilization and progressive disease, respectively.
Discussion
Mitotane is considered a standard treatment of unresectable ACC despite lack of prospective efficacy data and unavailability of factors predicting treatment response. Objective response rates to mitotane monotherapy of approximately 25 % [4, 37] have been observed in ten series, but most of them were retrospective and rather small studies. Platin-based chemotherapy regimens such as etoposide, doxorubicin, cisplatin plus mitotane are considered standard regimens according to the results of the first randomized multicenter phase III trial [17], in which significantly longer progression-free survival was observed in comparison to streptozotocin and mitotane. However, objective tumor response has been observed in only 23 % and has usually been rather short-lived. Hence, treatment of ACC refractory to standard treatment remains a challenge. Gemcitabine combined with capecitabine has been evaluated in a phase II clinical trial [19] and is commonly used by many centers. Therapies targeting angioneogenesis [22, 23] or insulin-like growth factor receptor I [24] have not found their way into clinical routine, potentially due to drug interactions with previous or concomitant treatment with mitotane [11]. No markers of treatment response have been identified rendering choice of treatment for an individual patient difficult.
In the case series reported here, we investigated the potential of continuous oral trofosfamide treatment for treatment of ACC. Among the 21 patients evaluable for treatment response, 3 patients had documented stable disease. In the latter subset of patients, time to progression ranged from 113 to 598 days. In the most remarkable case, criteria for partial response were marginally missed and later, multimodality of treatment led to a prolonged period of tumor-free survival.
Although two of the three patients with stable disease at first tumor evaluation received concomitant mitotane treatment, both had documented disease progression during mitotane, and hence, mitotane likely did not contribute significantly to the observed disease stabilization. The one patient with prolonged tumor response (PID 27) had undetectable mitotane plasma concentration at baseline and stable disease at two subsequent tumor assessments before mitotane was reintroduced based on clinical considerations. Hence, we consider trofosfamide to have by itself moderate antitumoral efficacy in ACC.
Despite a small proportion of only 3 out of 21 patients (14 %) who achieved disease stabilization according to RECIST, tumor growth of target lesions was comparably small with 22 % (but new metastases were detected in 3 patients with stable target lesions).
Overall, this study suffers from considerable heterogeneity due to its retrospective nature. This results in divergent previous therapies and—most notably—different trajectories of disease progression. However, molecular or histopathological markers reflecting tumor biology and aggressiveness are not defined. Most patients in this study were heavily pretreated likely resulting in tumor drug resistance. Moreover, disease assessment was based on RECIST in 13 of 21 cases only. Although this may result in discrepancies between the clinical judgments at the time of treatment, we chose this as a means to more uniformly assess disease response in this study.
Importantly, treatment was overall very well tolerated. In this retrospective study, no grade III/IV hematologic adverse events were observed. All other adverse events were tolerable and manageable. In the two cases excluded from response analysis for treatment <30 days, we considered early serious adverse events to be unrelated to trofosfamide treatment due to the short time interval between treatment initiation and onset of the adverse event. However, we can certainly not completely exclude that these thromboembolic events were really unrelated to trofosfamide. Thus, in case trofosfamide is considered for a given patient, one should discuss the individual thromboembolic risk. Obviously, the retrospective nature of the study is likely to underestimate the true prevalence and severity of adverse drug reactions.
It is noteworthy that chemical derivatization of the phosphoramidate ifosfamide yielded the hypoxia-activated prodrug TH-302 (evofosfamide) that is activated at low intratumoral oxygen potential through P450 cytochrome reductase (POR) [38, 39] and has been successfully used in phase II clinical trials of advanced pancreatic cancer [40] and soft tissue sarcoma [41]. Hence, alkylating chemotherapeutic agents that were thought to be overcome in the future by more molecular targeted approaches potentially will gain new interest in the future, particularly in tumors which are otherwise difficult to treat and prone to tumor drug resistance.
In conclusion, trofosfamide in advanced ACC showed only limited overall efficacy. However, 1 of 21 patients had a long-term benefit. The durable disease stabilization in one case studied here supports antitumoral efficacy of trofosfamide monotherapy in a subset of ACC patients. Yet, markers of treatment response to cytotoxic chemotherapy have not been established in ACC. Absence of response markers and the small proportion of patients with stable disease leads us to not pursue trofosfamide within prospective clinical trials. Nevertheless, we would use trofosfamide in patients who likely would not tolerate aggressive cytotoxic drug regimens or for whom treatment within an early phase clinical trial is unavailable.
As a salvage treatment, trofosfamide combines the advantage of oral administration with overall very good tolerability.
Acknowledgments
This research was supported by grants from the Deutsche Forschungsgemeinschaft (grant FA 466/4-1 to Martin Fassnacht and KR 4371/1-1 to Matthias Kroiss), the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement 259735 to Martin Fassnacht, and a grant from the Interdisciplinary Center for Clinical Research (IZKF) of the University of Würzburg (grant Z-2/57) to Timo Deutschbein.
Abbreviations
- ACC
Adrenocortical carcinoma
- CT
Computed tomography
- CTC
Common toxicity criteria
- ECOG
Eastern Cooperative Oncology Group
- ENSAT
European Network for the Study of Adrenal Tumours
- OS
Overall survival
- PFS
Progression-free survival
- RECIST
Response evaluation criteria in solid tumors
Compliance with Ethical Standards
Grants
These studies were approved by the ethics committee of the University of Würzburg (approval number 86/03 and 88/11), and written informed consent was obtained from all patients.
Conflict of interest
The authors declare that they have no conflict of interest
References
- 1.Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 2.Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–1878. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4551–4564. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 4.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35:282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, Laudat MH, Louvel A, Chapuis Y, Blondeau P, Bonnin A, Bricaire H. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 6.Haak HR, Hermans J, van de Velde CJ, Lentjes EG, Goslings BM, Fleuren GJ, Krans HM. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baudin E, Pellegriti G, Bonnay M, Penfornis A, Laplanche A, Vassal G, Schlumberger M. Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o, p'DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer. 2001;92:1385–1392. doi: 10.1002/1097-0142(20010915)92:6<1385::AID-CNCR1461>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Kerkhofs TM, Baudin E, Terzolo M, Allolio B, Chadarevian R, Mueller HH, Skogseid B, Leboulleux S, Mantero F, Haak HR, Fassnacht M. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4759–4767. doi: 10.1210/jc.2013-2281. [DOI] [PubMed] [Google Scholar]
- 9.Daffara F, De Francia S, Reimondo G, Zaggia B, Aroasio E, Porpiglia F, Volante M, Termine A, Di Carlo F, Dogliotti L, Angeli A, Berruti A, Terzolo M. Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer. 2008;15:1043–1053. doi: 10.1677/ERC-08-0103. [DOI] [PubMed] [Google Scholar]
- 10.van Erp NP, Guchelaar HJ, Ploeger BA, Romijn JA, Hartigh J, Gelderblom H. Mitotane has a strong and a durable inducing effect on CYP3A4 activity. Eur J Endocrinol. 2011;164:621–626. doi: 10.1530/EJE-10-0956. [DOI] [PubMed] [Google Scholar]
- 11.Kroiss M, Quinkler M, Lutz WK, Allolio B, Fassnacht M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol (Oxf) 2011;75:585–591. doi: 10.1111/j.1365-2265.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 12.Chortis V, Taylor AE, Schneider P, Tomlinson JW, Hughes BA, O'Neil DM, Libe R, Allolio B, Bertagna X, Bertherat J, Beuschlein F, Fassnacht M, Karavitaki N, Mannelli M, Mantero F, Opocher G, Porfiri E, Quinkler M, Sherlock M, Terzolo M, Nightingale P, Shackleton CH, Stewart PM, Hahner S, Arlt W. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5alpha-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98:161–171. doi: 10.1210/jc.2012-2851. [DOI] [PubMed] [Google Scholar]
- 13.Hermsen IG, Fassnacht M, Terzolo M, Houterman S, den Hartigh J, Leboulleux S, Daffara F, Berruti A, Chadarevian R, Schlumberger M, Allolio B, Haak HR, Baudin E. Plasma concentrations of o, p'DDD, o, p'DDA, and o, p'DDE as predictors of tumor response to mitotane in adrenocortical carcinoma: results of a retrospective ENS@T multicenter study. J Clin Endocrinol Metab. 2011;96:1844–1851. doi: 10.1210/jc.2010-2676. [DOI] [PubMed] [Google Scholar]
- 14.Theile D, Haefeli WE, Weiss J. Effects of adrenolytic mitotane on drug elimination pathways assessed in vitro. Endocrine. 2015;49:842–853. doi: 10.1007/s12020-014-0517-2. [DOI] [PubMed] [Google Scholar]
- 15.Terzolo M, Zaggia B, Allasino B, De Francia S. Practical treatment using mitotane for adrenocortical carcinoma. Curr Opin Endocrinol Diabetes Obes. 2014;21:159–165. doi: 10.1097/MED.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 16.Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, Matysik S, Eckhardt C, Gardill F, Gehl A, Kendl S, Weigand I, Bala M, Ronchi CL, Deutschbein T, Schmitz G, Rosenwald A, Allolio B, Fassnacht M, Kroiss M. Mitotane inhibits sterol-o-acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156:3895–3908. doi: 10.1210/en.2015-1367. [DOI] [PubMed] [Google Scholar]
- 17.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardiere C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Muller HH, Skogseid B, Group F-AS Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 18.Berruti A, Terzolo M, Sperone P, Pia A, Casa SD, Gross DJ, Carnaghi C, Casali P, Porpiglia F, Mantero F, Reimondo G, Angeli A, Dogliotti L. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 19.Sperone P, Ferrero A, Daffara F, Priola A, Zaggia B, Volante M, Santini D, Vincenzi B, Badalamenti G, Intrivici C, Del Buono S, De Francia S, Kalomirakis E, Ratti R, Angeli A, Dogliotti L, Papotti M, Terzolo M, Berruti A. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: a multicenter phase II study. Endocr Relat Cancer. 2010;17:445–453. doi: 10.1677/ERC-09-0281. [DOI] [PubMed] [Google Scholar]
- 20.Quinkler M, Hahner S, Wortmann S, Johanssen S, Adam P, Ritter C, Strasburger C, Allolio B, Fassnacht M. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J Clin Endocrinol Metab. 2008;93:2057–2062. doi: 10.1210/jc.2007-2564. [DOI] [PubMed] [Google Scholar]
- 21.Wortmann S, Quinkler M, Ritter C, Kroiss M, Johanssen S, Hahner S, Allolio B, Fassnacht M. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162:349–356. doi: 10.1530/EJE-09-0804. [DOI] [PubMed] [Google Scholar]
- 22.Kroiss M, Quinkler M, Johanssen S, van Erp NP, Lankheet N, Pollinger A, Laubner K, Strasburger CJ, Hahner S, Muller HH, Allolio B, Fassnacht M. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97:3495–3503. doi: 10.1210/jc.2012-1419. [DOI] [PubMed] [Google Scholar]
- 23.Berruti A, Sperone P, Ferrero A, Germano A, Ardito A, Priola AM, De Francia S, Volante M, Daffara F, Generali D, Leboulleux S, Perotti P, Baudin E, Papotti M, Terzolo M. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166:451–458. doi: 10.1530/EJE-11-0918. [DOI] [PubMed] [Google Scholar]
- 24.Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16:426–435. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- 25.Berruti A, Sperone P, Bellini E, Daffara F, Perotti P, Ardito A, Saini A, Terzolo M. Metronomic therapy concepts in the management of adrenocortical carcinoma. Horm Cancer. 2011;2:378–384. doi: 10.1007/s12672-011-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrero A, Sperone P, Ardito A, Rossi G, Del Buono S, Priola AM, Bracarda S, Taberna E, Terzolo M, Berruti A. Metronomic chemotherapy may be active in heavily pre-treated patients with metastatic adreno-cortical carcinoma. J Endocrinol Investig. 2013;36:148–152. doi: 10.3275/8334. [DOI] [PubMed] [Google Scholar]
- 27.Latz D, Nassar N, Frank R. Trofosfamide in the palliative treatment of cancer: a review of the literature. Oncol Res Treat. 2004;27:572–576. doi: 10.1159/000081342. [DOI] [PubMed] [Google Scholar]
- 28.Giraud B, Hebert G, Deroussent A, Veal GJ, Vassal G, Paci A. Oxazaphosphorines: new therapeutic strategies for an old class of drugs. Expert Opin Drug Metab Toxicol. 2010;6:919–938. doi: 10.1517/17425255.2010.487861. [DOI] [PubMed] [Google Scholar]
- 29.Salminen E, Nikkanen V, Lindholm L. Palliative chemotherapy in non-Hodgkin's lymphoma. Oncology. 1997;54:108–111. doi: 10.1159/000227672. [DOI] [PubMed] [Google Scholar]
- 30.Blomqvist C, Wiklund T, Pajunen M, Virolainen M, Elomaa I. Oral trofosfamide: an active drug in the treatment of soft-tissue sarcoma. Cancer Chemother Pharmacol. 1995;36:263–265. doi: 10.1007/BF00685858. [DOI] [PubMed] [Google Scholar]
- 31.Gunsilius E, Gierlich T, Mross K, Gastl G, Unger C. Palliative chemotherapy in pretreated patients with advanced cancer: oral trofosfamide is effective in ovarian carcinoma. Cancer Investig. 2001;19:808–811. doi: 10.1081/CNV-100107742. [DOI] [PubMed] [Google Scholar]
- 32.Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 33.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8:163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Libe R, Borget I, Ronchi CL, Zaggia B, Kroiss M, Kerkhofs T, Bertherat J, Volante M, Quinkler M, Chabre O, Bala M, Tabarin A, Beuschlein F, Vezzosi D, Deutschbein T, Borson-Chazot F, Hermsen I, Stell A, Fottner C, Leboulleux S, Hahner S, Mannelli M, Berruti A, Haak H, Terzolo M, Fassnacht M, Baudin E, network E Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): a European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol. 2015;26:2119–2125. doi: 10.1093/annonc/mdv329. [DOI] [PubMed] [Google Scholar]
- 35.Fassnacht M, Johanssen S, Fenske W, Weismann D, Agha A, Beuschlein F, Führer D, Jurowich C, Quinkler M, Petersenn S, Spahn M, Hahner S, Allolio B (2010) Improved survival in patients with stage II adrenocortical carcinoma followed prospectively by specialized centers. J Clin Endocrinol Metab 95(11):4925–32. doi:10.1210/jc.2010-0803 [DOI] [PubMed]
- 36.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 37.Hahner S, Fassnacht M. Mitotane for adrenocortical carcinoma treatment. Curr Opin Investig Drugs. 2005;6:386. [PubMed] [Google Scholar]
- 38.Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, Duan JX, Cai X, Mowday AM, Guise CP, Maroz A, Anderson RF, Patterson AV, Stachelek GC, Glazer PM, Matteucci MD, Hart CP. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012;11:740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- 39.Hunter FW, Young RJ, Shalev Z, Vellanki RN, Wang J, Gu Y, Joshi N, Sreebhavan S, Weinreb I, Goldstein DP, Moffat J, Ketela T, Brown KR, Koritzinsky M, Solomon B, Rischin D, Wilson WR, Wouters BG. Identification of P450 oxidoreductase as a major determinant of sensitivity to hypoxia-activated prodrugs. Cancer Res. 2015;75:4211–4223. doi: 10.1158/0008-5472.CAN-15-1107. [DOI] [PubMed] [Google Scholar]
- 40.Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J, Jr, Chiorean EG, Rosen PJ, Ulrich B, Dragovich T, Del Prete SA, Rarick M, Eng C, Kroll S, Ryan DP. Randomized phase II Trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2015;33:1475–1481. doi: 10.1200/JCO.2014.55.7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chawla SP, Cranmer LD, Van Tine BA, Reed DR, Okuno SH, Butrynski JE, Adkins DR, Hendifar AE, Kroll S, Ganjoo KN. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J Clin Oncol. 2014;32:3299–3306. doi: 10.1200/JCO.2013.54.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]