Abstract
Background
Metabolomics approaches have been widely used to define the consumption of foods but have less often been used to study exposure to dietary supplements.
Objectives
This study aimed to identify dietary supplements associated with metabolite levels and to examine whether these metabolites predicted incident diabetes risk.
Methods
We studied 3972 participants from a prospective cohort study of 18–74-y-old Hispanic/Latino adults. At a baseline examination, we ascertained use of dietary supplements using recall methods and concurrently, a serum metabolomic panel. After adjustment for potential confounders, we identified dietary supplements associated with metabolites. We then examined the association of these metabolites with incident diabetes at the 6-y study examination.
Results
We observed a total of 110 dietary supplement–metabolite associations that met the criteria for statistical significance adjusted for age, sex, field center, Hispanic/Latino background, body mass index, diet, smoking, physical activity, and number of medications (adjusted P < 0.05). This included 13 metabolites uniquely associated with only one dietary supplement ingredient. Vitamin C had the most associated metabolites (n = 15), including positive associations with oxalate, tartronate, threonate, and isocitrate, which were each in turn protective for the risk of incident diabetes. Vitamin C was also associated with higher N-acetylvaline level, which was an unfavorable diabetes risk factor. Other findings related to branched chain amino acid related compounds including α-hydroxyisovalerate and 2-hydroxy-3-methylvalerate, which were inversely associated with thiamine or riboflavin intake and also predicted higher diabetes risk. Vitamin B12 had an inverse association with γ-glutamylvaline, levels of which were positively associated with the risk of diabetes.
Conclusions
Our data point to potential metabolite changes associated with vitamin C and B vitamins, which may have favorable metabolic effects. Knowledge of blood metabolites that can be modified by dietary supplement intake may aid understanding the health effects of dietary supplements and identify potential biological mediators.
Keywords: diabetes mellitus, dietary supplements, metabolomics, Hispanic, Latino, epidemiology, risk factors, diet, vitamins, minerals
Introduction
Metabolomics provides a promising approach to measure the intake and exposure of diet [1], although the method has been more widely applied to foods than to dietary supplements. Dietary supplements may be useful to correct nutritional deficiencies [2], and their widespread use is largely driven by claims that they may reduce the risk or sequelae of a wide range of diseases. However, the risks and benefits, and even the content [3] of commonly used dietary supplements are poorly understood. Randomized clinical trials (RCTs) provide no clear evidence that dietary supplements reduce the risk of mortality, cardiovascular disease, asthma, or cancer [[4], [5], [6], [7], [8], [9]]. Meanwhile, some data from RCTs and observational studies have suggested harms, for example, linking calcium [10] and vitamin A supplements [4,10] with incident cancer, and β-carotene supplements with increased mortality [4]. Identification of blood metabolites that are altered by dietary supplement intake may provide new opportunities to understand the health effects of dietary supplements. For example, the assessment of dietary supplement use is time-consuming and prone to misclassification, so it would be useful to know which metabolites in circulation provide information about exposure to dietary supplement ingredients.
We conducted analyses of the community-based Hispanic Community Health Study/Study of Latinos (HCHS/SOL) epidemiological cohort study of 18–74-y-old adults. The goal was to identify serum metabolomic features that were associated with the dosage of dietary supplements including vitamins, minerals, fatty acids (FAs), and amino acids (AAs). We also addressed the hypothesis that metabolites having evidence of being modified by dietary supplement intake were associated with incident diabetes, which may have implications for which dietary supplement ingredients have a potential role in diabetes prevention and treatment.
Methods
Study setting
HCHS/SOL is a population-based prospective cohort study conducted at 4 urban US field centers (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA), which were selected to provide diversity in national background and behaviors such as diet. Of the 16,415 participants aged 18 to 74 who during 2008–2011 completed a baseline examination (Visit 1), n = 11,623 completed a follow-up examination (Visit 2) approximately 6 y later during 2014–2017. Protocols (available at https://sites.cscc.unc.edu/hchs/protocols-and-manuals) were approved by institutional review boards at all study institutions, and participants provided informed consent.
Data collection
At Visits 1 and 2, participants completed a standardized battery of questionnaires (including demographics, medical history, and health behaviors), a medication inventory, anthropometry including height, weight, and body circumferences, and seated blood pressures. We computed waist-to-hip ratio based on measured body circumferences and BMI as weight in kilograms divided by height in meters squared. Participants were asked to arrive at the study center after fasting for at least 8 h, having consumed only water and usual medications. Venous blood samples were drawn in the fasting state and again 2 h after a 75-g glucose load. Specimens were centrifuged, separated and frozen at −70°C on site, and then sent to a central laboratory for clinical laboratory tests and long-term storage. Assisted by Nutrition Data Systems for Research (NDSR) software, we calculated the usual intake of foods and nutrients by averaging 2 24-h recalls, first during Visit 1 and again by telephone at least 6 wk later [11]. During the first diet recall, we also asked about the use of dietary supplements during the 30 d preceding the interview.
Metabolomic profiling
For a random sample of 3972 participants (approximately one-quarter of the cohort), metabolomic profiling on Visit 1 serum samples was performed using the discoveryHD4 platform (Metabolon) (Supplemental Figure 1). Metabolites were quantified using an untargeted liquid chromatography-mass spectrometry (MS)-based metabolomic quantification protocol that captured information for a total of 1136 metabolites, including 782 metabolites with known structural identities and 354 unknown metabolites. Serum samples were extracted with methanol and subjected to non-targeted MS analysis using ultra-performance liquid chromatography (UPLC)-MS/MS, as described [12]. Briefly, all methods utilized a Waters ACQUITY UPLC and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. One aliquot was analyzed using acidic positive ion conditions, chromatographically optimized for more hydrophilic compounds. In this method, the extract was gradient eluted from a C18 column (Waters UPLC BEH C18-2.1x100 mm, 1.7 μm) using water and methanol, containing 0.05% perfluoropentanoic acid and 0.1% formic acid. Another aliquot was also analyzed using acidic positive ion conditions, and it was chromatographically optimized for more hydrophobic compounds. In this method, the extract was gradient eluted from the same aforementioned C18 column using methanol, acetonitrile, water, 0.05% perfluoropentanoic acid, and 0.01% formic acid and was operated at an overall higher organic content. The third aliquot was analyzed using basic negative ion optimized conditions using a separate dedicated C18 column. The basic extracts were gradient eluted from the column using methanol and water with 6.5 mM ammonium bicarbonate at pH 8. The fourth aliquot was analyzed via negative ionization following elution from a HILIC column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 μm) using a gradient consisting of water and acetonitrile with 10 mM ammonium formate, pH 10.8. The MS analysis alternated between MS and data-dependent MSn (sequential mass spectrometry) scans using dynamic exclusion. The scan range varied slightly between methods but covered 70 to 1000 molecular weight. Metabolites were identified by automated comparison of the ion features in the experimental samples to a reference library of chemical standard entries that included retention time, molecular weight, preferred adducts, and in-source fragments as well as associated MS spectra and curated by visual inspection for quality control using software developed at Metabolon [13]. Identification of known chemical entities (listed in Supplemental Table 1) was based on comparison to metabolomic library entries of purified standards. Peaks were quantified using area-under-the-curve. Raw area counts for each metabolite in each sample were normalized to correct for variation resulting from instrument inter-day tuning differences by the median value for each run-day, therefore, setting the medians to 1.0 for each run.
Definition of exposure to dietary supplement ingredients
We defined exposure to dietary supplement ingredients based on the Dietary Supplement Assessment Module of the NDSR software developed by the Nutrition Coordinating Center (NCC) at the University of Minnesota, which utilized the most currently available NHANES Supplement Database with enhancements from NCC [14]. NCC provided standardized training and certification to data collection staff, monitored staff performance in an ongoing fashion, and provided other quality assurance procedures for the dietary and supplement intake assessment. At the time of the field center clinic visit, staff interviewed participants in Spanish or English, according to their preference, about their recalled dietary supplement use in the 30 d prior to the study visit. The dietary supplements from the 30-d supplement interview were recorded in the Dietary Supplement Assessment Module of the NDSR. Information for supplements that did not match to a product coded in the Dietary Supplement Assessment Module was updated from the product label (obtained from the manufacturer when possible), and all the ingredients listed on the label of the container were entered into the database, including the amounts and units. If the product had a long list of ingredients, the dietary interviewer included at least the first 3 ingredients. The dietary supplement interview data consisted of ∼2700 different products representing ∼15,000 entries. The present study used information on the use of vitamins, minerals, FAs, and AAs.
For each dietary supplement ingredient included in the analysis (except biotin, boron, and chromium), we also captured intake from dietary sources using the 24-h diet recalls, using the mean of intakes reported at the 2 recalls.
Statistical analyses
The main analysis examined the cross-sectional association between reported dosages of dietary supplement ingredients (independent variable) and levels of blood metabolites (dependent variable). We limited this analysis to supplement users only, modeling the inverse normal transformed metabolite level compared with the z-score normalized, log-transformed supplement dosage level (Supplemental Figure 2) as a linear independent variable. Missing metabolite values were imputed to half of the minimum detected value prior to restricting to the analytic subset. Linear regressions used to model this association (Supplemental Table 2) were first examined in unadjusted analyses (model 1), then in age, sex, field center, and Hispanic/Latino background adjusted models (model 2), and next with additional adjustment for BMI, Alternate Healthy Eating Index-2010 (AHEI-2010) as a measure of diet quality [15], current smoking, average daily minutes of moderate-to-vigorous physical activity calculated from the Global Physical Activity Questionnaire [16], and number of medications taken within the last 4 wk (model 3). After adjusting the P value for multiple comparisons using a false discovery rate adjustment, we used the P < 0.05 criterion to identify metabolites associated with dietary supplement ingredient dosage [17].
Some dietary supplement ingredients were also commonly consumed in foods. This might have confounded the association of metabolite levels with dietary supplement intake in these instances. Through sensitivity analyses, we considered the dietary intake of that ingredient from nonsupplement (e.g., food) sources as an additional predictor of metabolite levels. This was done by extending model 3 to a fourth model (model 4), which included, as an additional independent variable, the amount of the target vitamin, mineral, FA, or AA consumed from foods. That is, a variable for intake from foods was included in the model in addition to the variable on the amount of the target vitamin, mineral, FA, or AA ingested in the form of supplements.
To relate dietary supplements with levels of metabolites, model 3 served as the source of the main study results (Supplemental Table 3). Supplemental Tables 4–31 contain the results of model 1, model 2, and model 4. In order to determine whether geographic location may have influenced metabolite levels, we replicated the principal findings of this study after excluding each of the 4 HCHS/SOL field centers in turn.
Next, in a prospective analysis, we related baseline metabolite levels with the risk of incident diabetes. This analysis was performed in the overall group, including both dietary supplement users and nonusers, in order to reflect the full range of metabolite values observed in the population. This was performed among 2010 participants, after excluding those who had prevalent diabetes (n = 804) or cardiovascular disease or cancer (n = 261) at baseline, and those who died during follow-up or for other reasons did not attend Visit 2 (n = 895). We estimated the relative risk of incident diabetes (n = 224 cases observed) associated with baseline levels of metabolites using multivariable survey Poisson regression models with adjustment for demo-socioeconomic characteristics, lifestyle behaviors, diet quality, medication use, and family history of diabetes. Participants were classified as having diabetes if they reported use of antidiabetic medications, or met the following American Diabetes Association criteria: 1) fasting plasma glucose ≥126 mg/dL; 2) 2-h oral-glucose-tolerance test plasma glucose ≥200 mg/dL; or 3) hemoglobin A1c ≥6.5%. Based on these criteria, participants free of diabetes at baseline (Visit 1) who were identified as having diabetes at Visit 2 were deemed to be incident cases of diabetes [18]. Finally, after excluding 1058 participants who were users of antihypertensive medications (n = 675), lipid-lowering treatments (n = 456), or diabetes medications (n = 447), we assessed each dietary supplement-associated metabolite for its association with metabolic traits in a cross-sectional analysis. We computed partial Spearman’s rank correlations, adjusted for age, sex, and Hispanic/Latino background, between metabolite concentrations and BMI, waist-to-hip ratio, glycemic traits including fasting glucose, hemoglobin A1c, 2-h glucose, fasting insulin, and Homeostatic Model Assessment for Insulin Resistance [19], lipid levels including low density-lipoprotein cholesterol, high density-lipoprotein cholesterol, total cholesterol, and triglycerides, and systolic and diastolic blood pressure levels.
Missing values were present in fewer than 5% for all variables, and a complete-case analysis approach was used. Analyses were performed using the R software version 4.1.0.
Results
Participant characteristics
Average age was 42.8 y (SD, 14.0) among nonusers of dietary supplements and 49.6 y (12.6) among those who used any dietary supplement (Table 1). Compared with nonusers, users of dietary supplements were more likely to be female (63.2% compared with 52.3%), had lower smoking prevalence (current smoking, 16.8% compared with 25.5%), were more often insured (54.0% compared with 46.6%), and used more medications (average of 2.29 compared with 1.51). Other lifestyle behaviors and socioeconomic characteristics were similar between users and nonusers of dietary supplements.
TABLE 1.
Characteristics of the Hispanic Community Health Study/Study of Latinos participants
| Nonusers of supplements |
Users of any supplement |
|
|---|---|---|
| n = 2177 | n = 1573 | |
| Age (y) | 42.8 (14.0) | 49.6 (12.6) |
| Female sex | 1138 (52.3%) | 994 (63.2%) |
| Hispanic background | ||
| Dominican | 200 (9.2%) | 145 (9.2%) |
| Central/South American | 333 (15.3%) | 272 (17.3%) |
| Cuban | 401 (18.4%) | 261 (16.6%) |
| Mexican | 814 (37.4%) | 571 (36.3%) |
| Puerto-Rican | 351 (16.1%) | 273 (17.4%) |
| Other or more than one background | 70 (3.2%) | 50 (3.2%) |
| Field center | ||
| Bronx | 528 (24.3%) | 367 (23.3%) |
| Chicago | 570 (26.2%) | 327 (20.8%) |
| Miami | 619 (28.4%) | 481 (30.6%) |
| San Diego | 460 (21.1%) | 398 (25.3%) |
| Insured | ||
| No | 1135 (52.1%) | 707 (44.9%) |
| Yes | 1014 (46.6%) | 849 (54.0%) |
| Missing | 28 (1.3%) | 17 (1.1%) |
| Education | ||
| Less than 9th grade | 495 (22.7%) | 373 (23.7%) |
| 9th grade to less than high school | 422 (19.4%) | 274 (17.4%) |
| High school graduate | 542 (24.9%) | 337 (21.4%) |
| At least some college | 705 (32.4%) | 579 (36.8%) |
| Meets physical activity guidelines for moderate-to-vigorous physical activity1 | ||
| No | 762 (35.0%) | 585 (37.2%) |
| Yes | 1404 (64.5%) | 985 (62.6%) |
| Alternate Healthy Eating Index | 47.8 (7.53) | 49.8 (7.69) |
| Cigarette use | ||
| Never smoked | 1225 (56.3%) | 963 (61.2%) |
| Former smoker | 395 (18.1%) | 344 (21.9%) |
| Current smoker | 556 (25.5%) | 265 (16.8%) |
| Body mass index, kg/m2 | 29.9 (6.20) | 29.5 (5.75) |
| Body mass index categories | ||
| Underweight (below 18.5) | 17 (0.8%) | 12 (0.8%) |
| Normal body weight (18.5–25) | 423 (19.4%) | 334 (21.2%) |
| Overweight (25–30) | 779 (35.8%) | 593 (37.7%) |
| Obese (30 or greater) | 950 (43.6%) | 629 (40.0%) |
| Diabetes | ||
| No | 1801 (82.7%) | 1258 (80.0%) |
| Yes | 376 (17.3%) | 315 (20.0%) |
| Hypertension | ||
| No | 1641 (75.4%) | 1064 (67.6%) |
| Yes | 536 (24.6%) | 509 (32.4%) |
| Dyslipidemia | ||
| No | 1299 (59.7%) | 984 (62.6%) |
| Yes | 877 (40.3%) | 588 (37.4%) |
| Coronary heart disease, including myocardial infarction or angina pectoris | ||
| No | 2023 (92.9%) | 1460 (92.8%) |
| Yes | 154 (7.1%) | 111 (7.1%) |
| Cancer | ||
| No | 2111 (97.0%) | 1500 (95.4%) |
| Yes | 64 (2.9%) | 70 (4.5%) |
| Number of medications | 1.51 (2.61) | 2.29 (3.00) |
Values are mean (standard deviation) or n (%). Except where otherwise indicated, missing values were present in <1% of individuals.
Meeting the 2008 PA Guidelines for Americans were defined as having at least 150 min/wk moderate-intensity activity, 75 min/wk vigorous-intensity activity, or ≥150 min/wk for a combination of the 2.
Correlates of dietary supplement exposure
Correlation analysis revealed the clustering structure among dietary supplement ingredients, which reflected both the simultaneous use of multiple dietary supplement products and the composition of combined dietary supplement formulations (Figure 1). We observed a cluster of 4 FA dietary supplement categories (total PUFA, ω-3 FA, PUFA 20:5n–3, and PUFA 22:6n–3), an inositol-choline cluster, a lutein-lycopene cluster, a copper-chromium cluster, a riboflavin-thiamin-vitamin B6 cluster, and a broad cluster of 20 dietary supplement ingredients commonly found in multivitamins. Dietary supplement exposure had little correlation with the intake of vitamins, minerals, and trace metals from foods (Figure 1). Correlations of dietary supplement exposure with demographics, health behaviors, BMI, and number of prescription medications were modest, although they reflected a propensity for greater dietary supplement exposure among older individuals and males (except for calcium, which had higher consumption by females) (Figure 1).
FIGURE 1.
Correlations of daily dose from supplements with intake from diet and participant characteristics, among users only. Rows represent the intake of ingredients from dietary supplements. Columns indicate the intake of ingredients from dietary supplements (green label), intake from diet (orange label), and participant characteristics. Color represents the strength and direction of the correlation coefficient.
Association of dietary supplement exposure with serum metabolites
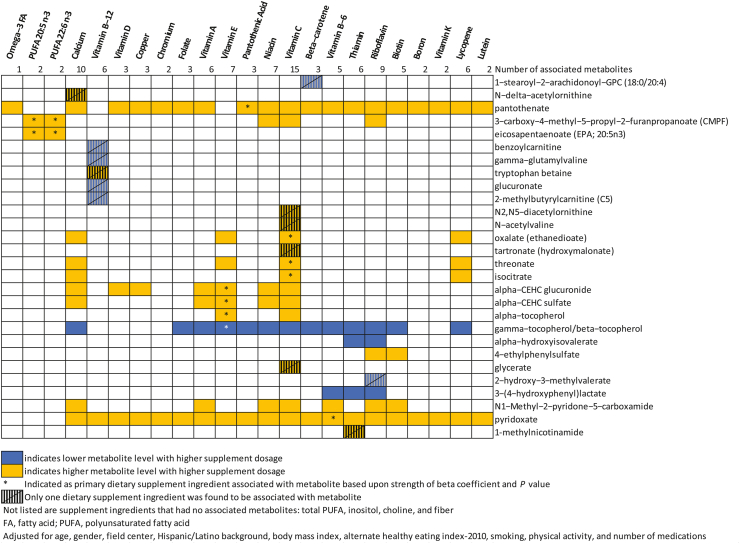
In total, 782 known metabolites were analyzed. These included 177 AA-related metabolites, 25 cofactors and vitamins, 315 lipid metabolites, 22 carbohydrates, 34 nucleotides, 32 peptides, 10 energy-related metabolites, and 167 metabolites from xenobiotics. In linear regression analyses relating each metabolite with 27 dietary supplement ingredients (Table 2), we observed a total of 110 dietary supplement–metabolite associations that met FDR-adjusted P < 0.05 criteria after adjustment (model 3) for age, sex, field center, Hispanic/Latino background, BMI, AHEI-2010 score, current smoking, minutes of moderate-to-vigorous physical activity, and number of prescription medications (Supplemental Table 3). For all but 24 of these 110 significant results, higher metabolite levels were observed with higher consumed dose of the dietary supplement. The associations that achieved statistical significance involved 28 metabolites in all. Of 27 dietary supplement ingredients studied, 23 had at least one statistically significant metabolite association (Figure 2).
TABLE 2.
Dietary supplement ingredients used by Hispanic Community Health Study/Study of Latinos participants
| Supplement ingredient | Users, n | Daily dose, median (min, max) |
|---|---|---|
| Non-vitamin, non-mineral | ||
| PUFA 22:6n–3 (docosahexaenoic acid) (g) | 342 | 0.12 (0.004, 1.5) |
| PUFA 20:5n–3 (eicosapentaenoic acid) (g) | 340 | 0.18 (0.006, 1.86) |
| Lycopene (μg) | 338 | 300 (10, 5300) |
| ω-3 fatty acids in supplements (mg) | 206 | 500 (12, 3200) |
| Total dietary fiber (g) | 113 | 0.8 (0.017, 14.452) |
| Total PUFA (g) | 83 | 0.5 (0.033, 10) |
| Inositol (g) | 74 | 0.0115 (0.001, 0.313) |
| Choline (mg) | 72 | 10.975 (0.01, 576) |
| Mineral | ||
| Calcium (mg) | 985 | 200 (0.8, 4238) |
| Copper (mg) | 610 | 1 (0.001, 10) |
| Chromium (μg) | 567 | 60 (0.7, 600) |
| Boron (μg) | 451 | 125 (2, 3000) |
| Vitamin | ||
| Vitamin C (ascorbic acid) (mg) | 861 | 63.333 (0.35, 5050) |
| Vitamin E (IU) | 838 | 30 (0.033, 1333.333) |
| Vitamin D (calciferol) (μg) | 830 | 8.333 (0.05, 2043.222) |
| Synthetic folate (folic acid) (μg) | 759 | 320 (2.083, 160000) |
| Vitamin B-12 (cobalamin) (μg) | 759 | 6.667 (0.035, 100012.5) |
| Vitamin B-6 (pyridoxine, pyridoxyl, and pyridoxamine) (mg) | 742 | 2 (0.012, 600) |
| Thiamine (vitamin B-1) (mg) | 723 | 1.5 (0.009, 220) |
| Riboflavin (vitamin B-2) (mg) | 723 | 1.7 (0.01, 300) |
| Niacin (vitamin B-3) (mg) | 720 | 15.77 (0.117, 1020) |
| Pantothenic acid (mg) | 691 | 8.333 (0.031, 215) |
| Total vitamin A activity (IU) | 679 | 2500 (29.167, 103500) |
| Biotin (μg) | 633 | 30 (0.333, 5030) |
| Vitamin K (phylloquinone) (μg) | 499 | 20 (0.222, 400) |
| Lutein and/or zeaxanthin (μg) | 323 | 250 (8.333, 10000) |
| β-Carotene (provitamin A carotenoid) (μg) | 160 | 406 (10, 5100) |
Abbreviations: IU, international unit
FIGURE 2.
Summary of associations between the intake of dietary supplement ingredients and blood metabolites.
We identified 13 metabolites that were uniquely associated with only one dietary supplement ingredient. This included 4 vitamin C-related metabolites (N-acetylvaline, N2,N5-diacetylornithine, glycerate tartronate [hydroxymalonate], all positive associations), and 5 vitamin B-12 related metabolites (tryptophan betaine having a positive association, and γ-glutamylvaline, benzoylcarnitine, glucuronate, and 2-methylbutyrylcarnitine [C5] having inverse associations). Five out of 6 metabolites associated with vitamin B-12 had no other dietary supplement associations, suggesting that this set of metabolites correlated with relative specificity to vitamin B-12 exposure. In addition, we found one metabolite associated uniquely with intake levels for β-carotene (1-stearoyl-2-arachidonoyl-GPC [18:0/20:4], inverse), calcium (N-delta-acetylornithine, positive), riboflavin (2-hydroxy-3-methylvalerate, inverse), and thiamine (1-methylnicotinamide, positive).
Among 28 metabolites associated with one or more dietary supplement ingredients, 15 had associations with 2 or more dietary supplement ingredients. The common co-occurrence of dietary supplement ingredients (for instance, in combination tablets or multivitamins) precluded multivariable adjustment to isolate independent associations with metabolites. However, by comparing the magnitude of standardized β coefficients and P values, we could, in many cases, identify dietary supplements that likely drove the association: vitamin E – (α-CEHC-glucoronide, α-CEHC-sulfate, α-tocopherol, and γ-tocopherol/β-tocopherol); vitamin C – (threonate, oxalate, and isocitrate); pantothenic acid – pantothenate; and vitamin B-6 – pyridoxate. Both PUFA 20:5n–3 and PUFA 22:6n–3 were, with similar β coefficients and P values, associated with the level of eicosapentaenoate (EPA; 20:5n–3) and 3-carboxy-4-methyl-5-propyl-2-furanpropanoate, and they appeared to be the main dietary supplement correlates of these metabolites.
Using a “leave-one-out” approach, we found that the results changed little when we excluded one of the HCHS/SOL field centers, suggesting no geographic variability in the described phenomena (Supplemental Figure 4).
Incorporation of data on the intake of foods
In sensitivity analyses, we additionally adjusted for nutrients consumed from foods (model 4) to examine whether this altered the observed association of dietary supplements with metabolite levels. This produced nearly the same conclusions as the main findings (model 3) described above (Supplemental Table 4). In almost all cases, after further adjustment for food intake of the ingredient contained in dietary supplements, the association between dietary supplements and metabolites remained significant at adjusted P below 0.05. Even in the 9 instances when the P value crossed above the criterion level of P < 0.05 after adjustment for food intake, the β coefficients associating supplement dosages with metabolite levels were changed little. In about half of the associations analyzed in this way (48 of 101 analyses), the metabolite had statistically significant associations with the ingredient (eg, vitamin) measured both from foods and from dietary supplements. In all but one of these 48 instances, the direction of association between ingredients from food or dietary supplement sources and metabolites was the same. The exception was vitamin K – pyridoxate, β = 0.48, P = 6 × 10-6 for supplement sources and β = –0.04, P = 0.01 for food sources.
Association of dietary supplement-associated metabolites with a risk of incident diabetes
Of 28 dietary supplement-associated metabolites, 13 were independent predictors of the risk of incident diabetes after adjustment for age, sex, study field center, Hispanic/Latino background, education, annual household income, cigarette and alcohol use, physical activity, AHEI-2010 score, lipid-lowering medication use, antihypertensive medication use, and family history of diabetes (Table 3). The phosphatidylcholine pathway metabolite that was inversely associated with β-carotene exposure, 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4), predicted a ∼40% increased risk in diabetes risk per SD increase. Multiple metabolites positively associated with vitamin C use were protective against diabetes (RR < 1, P < 0.05). However, the vitamin C-related metabolite (positively associated) N-acetylvaline had an RRdiabetes per SD of 1.36 (95% confidence interval [CI]: 1.11, 1.66). Also associated with a higher risk of diabetes were branched chain AA-related compounds, including α-hydroxyisovalerate, which was reduced with a higher doses of both riboflavin and thiamine, and 2-hydroxy-3-methylvalerate, which was reduced with higher riboflavin dose (RRs per SD, 1.76 [95% CI: 1.46, 2.12] and 1.87 [95% CI: 1.53, 2.28], respectively). The level of 3,4-hydroxyphenylacetate, which was another metabolite inversely associated with a dose of B vitamins (riboflavin, thiamin, vitamin B-6), had a positive association with incident diabetes. γ-Glutamylvaline, inversely associated with vitamin B-12 exposure, had a diabetes RR per SD of 1.60 (95% CI: 1.30, 1.97), whereas a second metabolite inversely associated with vitamin B-12, glucuronate, had a borderline-significant association with higher diabetes risk (P = 0.06).
TABLE 3.
Association of dietary supplement-related metabolites with the risk of incident diabetes
| Metabolite | Primary supplements associated with metabolite (direction of association) | RR for incident diabetes (95% CI) | P value |
|---|---|---|---|
| 1-Stearoyl-2-arachidonoyl-GPC (18:0/20:4) | Β-carotene (-) | 1.41 (1.15–1.74) | 1.18E-03 |
| N-Δ-acetylornithine | Calcium (+) | 0.91 (0.78–1.08) | 0.29 |
| Pantothenate | Pantothenic acid (+) | 1.15 (0.96–1.37) | 0.12 |
| 3-Carboxy-4-methyl-5−propyl−2−furanpropanoate | PUFA 20:5n–3/PUFA 22:6n–3 (+) | 0.91 (0.75–1.10) | 0.33 |
| Eicosapentaenoate (20:5n–3) | PUFA 20:5n–3/PUFA 22:6n–3 (+) | 1.12 (0.90–1.38) | 0.32 |
| Benzoylcarnitine | Vitamin B-12 (-) | 0.86 (0.67–1.09) | 0.21 |
| γ−Glutamylvaline | Vitamin B-12 (-) | 1.60 (1.30–1.97) | 1.12E-05 |
| Tryptophan betaine | Vitamin B-12 (+) | 1.00 (0.85–1.16) | 0.96 |
| Glucuronate | Vitamin B-12 (-) | 1.21 (0.99–1.49) | 0.06 |
| 2-Methylbutyrylcarnitine (C5) | Vitamin B-12 (-) | 1.17 (0.92–1.48) | 0.20 |
| N2,N5-diacetylornithine | Vitamin C (+) | 1.12 (0.91–1.39) | 0.27 |
| N-Acetylvaline | Vitamin C (+) | 1.36 (1.11–1.66) | 2.69E-03 |
| Oxalate (ethanedioate) | Vitamin C (+) | 0.68 (0.57–0.80) | 1.06E-05 |
| Tartronate (hydroxymalonate) | Vitamin C (+) | 0.80 (0.66–0.98) | 0.03 |
| Threonate | Vitamin C (+) | 0.76 (0.64–0.91) | 3.44E-03 |
| Isocitrate | Vitamin C (+) | 0.71 (0.60–0.85) | 1.64E-04 |
| α-CEHC glucuronide | Vitamin E (+) | 0.70 (0.54–0.91) | 0.01 |
| α-CEHC sulfate | Vitamin E (+) | 0.81 (0.67–0.99) | 0.04 |
| α-Tocopherol | Vitamin E (+) | 1.12 (0.91–1.39) | 0.29 |
| γ-Tocopherol/β-tocopherol | Vitamin E (-) | 1.59 (1.32–1.91) | 1.02E-06 |
| 1-Methylnicotinamide | Thiamine (+) | 0.90 (0.72–1.12) | 0.34 |
| 2-Hydroxy-3-methylvalerate | Riboflavin (-) | 1.87 (1.53–2.28) | 1.84E-09 |
| α-Hydroxyisovalerate | NA1 | 1.76 (1.46–2.12) | 3.73E-09 |
| 4-Ehylphenylsulfate | NA | 0.86 (0.74–1.00) | 0.05 |
| Glycerate | NA | 0.90 (0.74–1.08) | 0.25 |
| 3−(4−hydroxyphenyl)lactate | NA | 1.77 (1.43–2.18) | 1.43E-07 |
| N1−Methyl−2−pyridone−5−carboxamide | NA | 1.06 (0.87–1.29) | 0.58 |
| Pyridoxate | NA | 0.93 (0.77–1.13) | 0.47 |
RR is adjusted for age, sex, study field center, Hispanic/Latino background, education, annual household income, cigarette and alcohol use, physical activity, Alternative Healthy Eating Index-2010 score, lipid-lowering medication use, antihypertensive medication use, and family history of diabetes.
NA, not applicable due to the fact that no single supplement ingredient appeared to be driving the association with metabolite.
Association of dietary supplement-related metabolites with metabolic traits
The 28 metabolites that were significantly associated with dietary supplement exposure had additional cross-sectional associations with multiple cardiometabolic risk variables related to lipid, adiposity and inflammation related traits (Supplemental Figure 3). α-Tocopherol, which was positively associated with vitamin E exposure but unrelated to incident diabetes risk, had a particularly strong association with higher total cholesterol, low density-lipoprotein cholesterol, and triglyceride levels.
Discussion
Our observational study identified serum metabolites that appeared to be altered by the intake of dietary supplements. Among 27 dietary supplements examined, 7 had at least one associated metabolite that was specific to that dietary supplement and not others, including fish oils (PUFA 20:5n–3/PUFA 22:6n–3), calcium, vitamin B-12, vitamin C, β-carotene, riboflavin, and thiamine. Those associated with the broadest signals of altered metabolites included vitamin C (15 metabolites, 4 of which were unique to vitamin C) and calcium (10 metabolites, 1 unique). Vitamin B-12 had the most distinctive signature of metabolites, such that 6 metabolites were statistically significant, and 5 of the 6 were uniquely associated with vitamin B-12. The potential implication is that blood metabolites may be a surrogate measure of exposure to dietary supplements, which is something that can be more definitively tested using interventional designs. Furthermore, because of the limitations and complexity of dietary supplement recalls, our results could provide a population-based method to further study the effects of dietary supplements through the use of metabolomics as a substitute or addition to diet supplement recall. As an example, we examined the association with incident diabetes. Several metabolites that appeared to be influenced by dietary supplements, including those associated with the use of vitamin C, vitamin E, β-carotene, and B complex vitamins (riboflavin and vitamin B-12), were in turn associated with the risk of incident diabetes over 6-y follow-up.
Some of the dietary supplements we examined have been tested as chemoprevention for diabetes using RCT designs, which provides a basis to compare the present results. The Women’s Antioxidant Cardiovascular Study randomized women at high cardiovascular risk to receive 500 mg/d synthetic vitamin C or placebo and observed a trend toward reduced diabetes risk in the vitamin C group (RR: 0.89, 95% CI: 0.78, 1.02; P = 0.09) [20]. In contrast, the Supplementation with Antioxidant Vitamins and Minerals study found no effects of vitamin C on fasting plasma glucose among nondiabetic adults, using a more modest dose of vitamin C taken as part of a combination regimen (120 mg vitamin C, 30 mg vitamin E, 6 mg β-carotene, 100 μg selenium, and 20 mg zinc). Summarizing prior studies on vitamin C effects in diabetic patients, a meta-analysis of 28 RCTs found that vitamin C produced statistically significant decreases compared with placebo in fasting and postprandial glucose levels and hemoglobin A1c [21]. In our study, a higher dose of vitamin C was associated with higher levels of oxalate, tartronate, threonate, and isocitrate, and each of these vitamin C-related metabolites had a protective association with diabetes risk. Intake of vitamin C supplements was also associated with a higher serum level of N-acetylvaline, which was an adverse risk factor for diabetes in our study and was also previously identified as the lead metabolite for prediction of mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study [22]. Thus, the metabolites associated with vitamin C intake appeared to have potentially both favorable and adverse health implications.
Other factors apparently affected by dietary supplement intake included α-hydroxyisovalerate and 2-hydroxy-3-methylvalerate, 2 compounds related to branched chain AAs that are known to be involved in insulin resistance and diabetes [23]. Both were inversely associated with riboflavin intake, and the former also was inversely associated with thiamine intake. Both of these metabolites had a strong positive association with the risk of diabetes (P∼10-9 for each) in our study. They have previously been associated with increased risk of breast cancer [24], elevated BMI [25], fatty liver [26], high blood pressure [27], and insulin resistance [28].
Vitamin B-12 has some prior RCT evidence for glucose-lowering effects when used in diabetic patients [29]. Only one relevant primary diabetes prevention trial is available, which showed that combined folic acid, vitamin B-6, and vitamin B-12 were neutral with regard to diabetes incidence [30]. γ-Glutamylvaline, which has been previously associated with liver fat content [31] and weight gain [32] and here was shown to predict diabetes incidence, was among the metabolites that appeared to be affected (lowered) by vitamin B-12 intake.
Several dietary supplements that we examined here have been studied in large RCTs, which found that they failed to reduce the incidence of diabetes. These include β-carotene [20,33,34] and vitamin E [20]. Each of these dietary supplements was associated with metabolite features that seemed to be favorable with regard to diabetes risk (for β-carotene, reductions in 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4), and for vitamin E, as previously shown [24], increases in α-CEHC glucuronide and α-CEHC sulfate). This demonstrates the imperative to avoid extrapolating from surrogate biomarkers of metabolic health to clinical risks and benefits.
Although we used a detailed approach to collect dietary supplement use, inaccuracies in the recall and recording of dietary supplement ingredients and the doses consumed are inevitable, and the data on supplement use were not validated by home inspection. In addition, some products are known to have discrepancies between their labeled and actual content. We only obtained metabolomics from serum, while other samples such as urine may provide additional useful information on relevant metabolites. The metabolite panel was not designed in an optimal way to meet the goal of identifying in a comprehensive way blood metabolites that change in response to dietary supplement intake. For instance, we lacked measurements such as circulating vitamin levels or well-established biomarkers of vitamin exposure (eg, pyridoxal 5′-phosphate as marker of vitamin B-6). In our correlative study, the links among dietary supplement use, metabolomic profiles, and incident diabetes are only hypothesis generating. On the other hand, the strengths of our study included a large community-based population sample and detailed data collection methods that captured an array of confounding behaviors and clinical variables including diet. Furthermore, using dosage information and limiting analyses to users only, we were able to examine metabolite levels across the range of low- to high-dose exposure, which limits indication biases that may have biased comparisons between users and nonusers. Nevertheless, it is possible that the effects of diet supplements on metabolites may vary by individual-level variables such as genetics or dietary habits, which could be addressed in future replication studies among non-Hispanic cohorts.
In summary, our study is among the first to apply a nutritional metabolomics approach to identifying metabolites that are affected by dietary supplement exposure. Several metabolites were nonspecific markers of dietary supplement use, reflecting either polyexposure to multiple concurrently used dietary supplements or else shared metabolic or effector pathways of dietary supplement ingredients. For other metabolites, we could identify a unique or a leading dietary supplement exposure that appeared to drive a positive or inverse association between dietary supplement dose and metabolite level. Our findings provide some potential hypotheses pertaining to the relationship among commonly consumed dietary supplements, their related metabolites, and diabetes risk.
Acknowledgments
We thank Dr. Sarah Alver for her invaluable assistance with data analysis that contributed to this work for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.02.021.
Author contribution
The authors’ responsibilities were as follows – RCK, JSW, QQ, HG: designed the research (project conception, development of overall research plan, and study oversight); JSW, AC, YH: conducted research (hands-on conduct of the data collection), analyzed data, or performed statistical analysis; BY, EB, YM, LVH, MDG, MD, KF, QQ, RCK: provided essential materials (constructs, databases, etc., necessary for the research); RCK, KF, HG: wrote the paper; RCK: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Funding
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01-HC-65233), University of Miami (HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I/N01-HC-65236 Northwestern Univ), and San Diego State University (HHSN268201300005I/N01-HC-65237). The following Institutes/Centers/Offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements. Additional funding was provided through R01DK119268 and R01HL060712. Dr. Yu was in part supported by R01HL141824. The funders had no role in the design or interpretation of this study.
Author disclosures
The authors report no conflicts of interest.
Data availability
Data from this article can be obtained by contacting the author, and use of data from the HCHS/SOL cohort is governed by the policies of the collaborative study as described at https://sites.cscc.unc.edu/hchs/publications-pub
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Maruvada P., Lampe J.W., Wishart D.S., Barupal D., Chester D.N., Dodd D., et al. Perspective: dietary biomarkers of intake and exposure-exploration with omics approaches. Adv. Nutr. 2020;11(2):200–215. doi: 10.1093/advances/nmz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietary Guidelines for Americans Dietary Guidelines 2020–2025. [Internet] 2020 https://dietaryguidelines.gov/ [cited December 10, 2021]. Available from: [Google Scholar]
- 3.Dwyer J.T., Coates P.M., Smith M.J. Dietary supplements: regulatory challenges and research resources. Nutrients. 2018;10(1):41. doi: 10.3390/nu10010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwingshackl L., Boeing H., Stelmach-Mardas M., Gottschald M., Dietrich S., Hoffmann G., et al. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a systematic review and meta-analysis of primary prevention trials. Adv. Nutr. 2017;8(1):27–39. doi: 10.3945/an.116.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortmann S.P., Burda B.U., Senger C.A., Lin J.S., Whitlock E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013;159(12):824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- 6.Khan S.U., Khan M.U., Riaz H., Valavoor S., Zhao D., Vaughan L., et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann. Intern. Med. 2019;171(3):190–198. doi: 10.7326/M19-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alsharairi N.A. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: a review of the literature. Nutrients. 2019;11(4):725. doi: 10.3390/nu11040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jolliffe D.A., Greenberg L., Hooper R.L., Griffiths C.J., Camargo C.A., Jr., Kerley C.P., et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2017;5(11):881–890. doi: 10.1016/S2213-2600(17)30306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forno E., Bacharier L.B., Phipatanakul W., Guilbert T.W., Cabana M.D., Ross K., et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA. 2020;324(8):752–760. doi: 10.1001/jama.2020.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F., Du M., Blumberg J.B., Ho Chui K.K., Ruan M., Rogers G., et al. Association among dietary supplement use, nutrient intake, and mortality among U.S. adults: a cohort study. Ann. Intern. Med. 2019;170(9):604–613. doi: 10.7326/M18-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siega-Riz A.M., Sotres-Alvarez D., Ayala G.X., Ginsberg M., Himes J.H., Liu K., et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am. J. Clin. Nutr. 2014;99(6):1487–1498. doi: 10.3945/ajcn.113.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans A.M., Bridgewater B.R., Liu Q., Mitchell M.W., Robinson R.J., Dai H., et al. High-resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4:2. doi: 10.4172/2153-0769.1000132. [DOI] [Google Scholar]
- 13.Dehaven C.D., Evans A.M., Dai H., Lawton K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010;2(1):9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harnack L., Stevens M., Van Heel N., Schakel S., Dwyer J.T., Himes J. A computer-based approach for assessing dietary supplement use in conjunction with dietary recalls. J. Food Compost. Anal. 2008;21(Suppl 1):S78–S82. doi: 10.1016/j.jfca.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., McCullough M.L., Wang M., et al. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull F.C., Maslin T.S., Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J. Phys. Act. Health. 2009;6(6):790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 18.Chen G.C., Chai J.C., Yu B., Michelotti G.A., Grove M.L., Fretts A.M., et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Am. J. Clin. Nutr. 2020;112(1):57–65. doi: 10.1093/ajcn/nqaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Song Y., Cook N.R., Albert C.M., Van Denburgh M., Manson J.E. Effects of vitamins C and E and β-carotene on the risk of type 2 diabetes in women at a high risk of cardiovascular disease: a randomised controlled trial. Am. J. Clin. Nutr. 2009;90(2):429–437. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason S.A., Keske M.A., Wadley G.D. Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2021;44(2):618–630. doi: 10.2337/dc20-1893. [DOI] [PubMed] [Google Scholar]
- 22.Huang J., Weinstein S.J., Moore S.C., Derkach A., Hua X., Liao L.M., et al. Serum metabolomic profiling of all-cause mortality: a prospective analysis in the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study cohort. Am. J. Epidemiol. 2018;187(8):1721–1732. doi: 10.1093/aje/kwy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newgard C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15(5):606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Playdon M.C., Ziegler R.G., Sampson J.N., Stolzenberg-Solomon R., Thompson H.J., Irwin M.L., et al. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017;106(2):637–649. doi: 10.3945/ajcn.116.150912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore S.C., Matthews C.E., Sampson J.N., Stolzenberg-Solomon R.Z., Zheng W., Cai Q., et al. Human metabolic correlates of body mass index. Metabolomics. 2014;10(2):259–269. doi: 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sha W., da Costa K.A., Fischer L.M., Milburn M.V., Lawton K.A., Berger A., et al. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J. 2010;24(8):2962–2975. doi: 10.1096/fj.09-154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menni C., Graham D., Kastenmüller G., Alharbi N.H., Alsanosi S.M., McBride M., et al. Metabolomic identification of a novel pathway of blood pressure regulation involving hexadecanedioate. Hypertension. 2015;66(2):422–429. doi: 10.1161/HYPERTENSIONAHA.115.05544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lustgarten M.S., Price L.L., Chale A., Phillips E.M., Fielding R.A. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(6):717–724. doi: 10.1093/gerona/glt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satapathy S., Bandyopadhyay D., Patro B.K., Khan S., Naik S. Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: a multi-arm randomized controlled clinical trial, Complement. Ther. Med. 2020;53 doi: 10.1016/j.ctim.2020.102526. [DOI] [PubMed] [Google Scholar]
- 30.Song Y., Cook N.R., Albert C.M., Van Denburgh M., Manson J.E. Effect of homocysteine-lowering treatment with folic acid and B vitamins on the risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes. 2009;58(8):1921–1928. doi: 10.2337/db09-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch M., Freitag-Wolf S., Schlesinger S., Borggrefe J., Hov J.R., Jensen M.K., et al. Serum metabolomic profiling highlights pathways associated with liver fat content in a general population sample. Eur. J. Clin. Nutr. 2017;71(8):995–1001. doi: 10.1038/ejcn.2017.43. [DOI] [PubMed] [Google Scholar]
- 32.Menni C., Migaud M., Kastenmüller G., Pallister T., Zierer J., Peters A., et al. Metabolomic profiling of long-term weight change: role of oxidative stress and urate levels in weight gain. Obesity (Silver Spring) 2017;25(9):1618–1624. doi: 10.1002/oby.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataja-Tuomola M., Sundell J.R., Männistö S., Virtanen M.J., Kontto J., Albanes D., et al. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51(1):47–53. doi: 10.1007/s00125-007-0864-0. [DOI] [PubMed] [Google Scholar]
- 34.Liu S., Ajani U., Chae C., Hennekens C., Buring J.E., Manson J.E. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA. 1999;282(11):1073–1075. doi: 10.1001/jama.282.11.1073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this article can be obtained by contacting the author, and use of data from the HCHS/SOL cohort is governed by the policies of the collaborative study as described at https://sites.cscc.unc.edu/hchs/publications-pub