Abstract
Purpose
The gastrointestinal system is the most commonly affected organ, followed by the lungs, in patients with primary immunodeficiency disease (PID). Hence, it is common for children with PIDs to present with gastrointestinal symptoms. We aimed to analyze the clinical and histopathological findings of patients who were initially admitted to pediatric gastroenterology/hepatology clinics and subsequently diagnosed with PIDs to identify the clinical clues for PIDs.
Methods
The demographic, laboratory, and histopathological findings, treatment modality, and outcomes of patients initially admitted to the pediatric gastroenterology/hepatology unit and subsequently diagnosed with PIDs were recorded.
Results
The study included 24 patients (58.3% male; median age [range]: 29 [0.5–204] months). Common clinical presentations included chronic diarrhea (n=8), colitis (n=6), acute hepatitis (n=4), and acute liver failure (n=2). The association of autoimmune diseases, development of malignant diseases, and severe progression of viral diseases was observed in 20.8%, 8.3%, and 16.6% of the patients, respectively. Antibody deficiency was predominantly diagnosed in 29.2% of patients, combined immunodeficiency in 20.8%, immune dysregulation in 12.5%, defects in intrinsic and innate immunity in 4.2%, autoinflammatory disorders in 8.3%, and congenital defects of phagocytes in 4.2%. Five patients remained unclassified (20.8%).
Conclusion
Patients with PIDs may initially experience gastrointestinal or liver problems. It is recommended that the association of autoimmune or malignant diseases or severe progression of viral diseases provide pediatric gastroenterologists some suspicion of PIDs. After screening using basic laboratory tests, genetic analysis is mandatory for a definitive diagnosis.
Keywords: Primary immunodeficiency, Gastrointestinal involvement, Genetic testing, Chronic diarrhea, Liver involvement, Colitis
INTRODUCTION
Primary immunodeficiency diseases (PIDs) are a heterogeneous group of inherited disorders characterized by poor or absent function in one or more components of the immune system, which predispose affected individuals to increased frequency and severity of infections, autoimmune diseases, and chronic inflammations [1]. PIDs have a wide spectrum of clinical manifestations associated with all organ systems. After the lungs, the gastrointestinal system is most commonly affected because it has one of the largest lymphoid organs. Hence, patients with PIDs commonly present with gastrointestinal symptoms or mimic gastrointestinal disease [2,3]. Hepatic involvement (25%) ranges from mild enzyme elevation to acute liver failure (ALF), sclerosing cholangitis, autoimmune hepatitis, and cirrhosis, which may also be initial manifestations of PIDs [3,4,5].
Although the number of patients diagnosed with PID is increasing, physicians still know little about these disorders. Many patients are referred late to specialized centers; thus, they suffer from complications due to chronic infections and irreversible end-organ damage before a definitive diagnosis is made. Therefore, pediatric gastroenterologists and hepatologists emphasize the need for early diagnosis of PIDs, since many patients may initially be admitted for gastrointestinal symptoms/findings. Therefore, we aimed to analyze the clinical findings of patients who were initially admitted to pediatric gastroenterology/hepatology clinics and subsequently diagnosed with PIDs, and to identify clinical clues for PIDs in these patients.
MATERIALS AND METHODS
The study included patients admitted to the pediatric gastroenterology/hepatology unit who were initially and subsequently diagnosed with PIDs over a 10-year period in Karadeniz Technical University, Farabi Hospital, Department of Pediatric Gastroenterology Hepatology and Nutrition unit. Demographic features, laboratory and histopathological findings, treatments, and patient outcomes were recorded.
PID was diagnosed according to a previously defined criteria [1,6]. Patients with familial Mediterranean fever or secondary immunodeficiency were excluded. Hypogammaglobulinemia is defined as a marked decrease in IgG and IgA levels, with or without low IgM levels, according to age [7]. Colitis was defined as the presence of bloody diarrhea with/without mucous, fever, abdominal pain, and compatible laboratory and histopathological findings [8].
Whole exome sequencing was performed using a TruSeq Exome Enrichment Kit (Illumina) on an Illumina HiSeq 2500 sequencer as paired-end 100-125 base-pair reads. On average, over 95% of the exons were covered at >30x.
This study was approved by the Karadeniz Technical University Scientific Research Ethics Committee (2020-115). All participants provided written informed consent prior to participation in the study. The study was conducted in accordance with the principles of the Declaration of Helsinki.
RESULTS
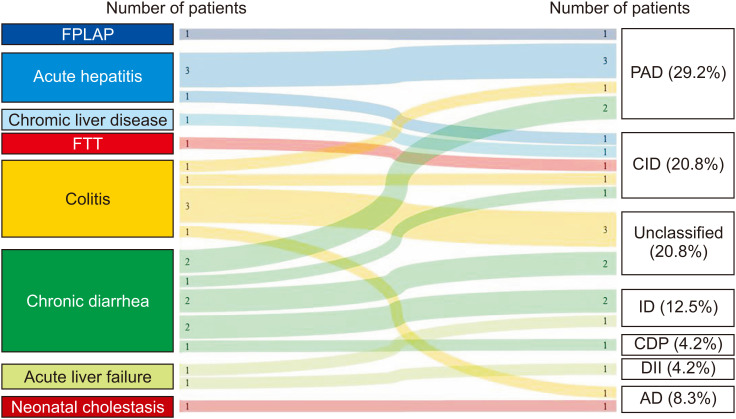
The study included 24 patients (58.3% male, median age [range]: 29 [0.5–204] months) from 23 families (Table 1). The final diagnoses of the patients are shown in Fig. 1. The median time between initial admission and diagnosis of PIDs was 6 months (1 week–60 months).
Table 1. Demographic and clinical findings of the patients.
| Parameters | Value (n=24) | |
|---|---|---|
| Sex, male | 14 (58.3) | |
| Age (mo) | 29 (0.5–204) | |
| Consanguinity | 6 (25.0)* | |
| Sibling death | 3 (12.5)* | |
| Recurrent pulmonary infections | 7 (29.2) | |
| Physical findings | ||
| Severe malnutrition | 5 (20.8) | |
| Hepatosplenomegaly | 10 (41.7) | |
| Skin findings | 6 (25.0) | |
| Atypical facial appearance | 4 (16.7) | |
| Initial symptoms | ||
| Colitis | 6 (25.0) | |
| Chronic diarrhea | 8 (33.3) | |
| Acute hepatitis | 4 (16.7) | |
| Acute liver failure | 2 (8.3) | |
| Failure to thrive | 1 (4.2) | |
| FPIAP | 1 (4.2) | |
| Neonatal cholestasis | 1 (4.2) | |
| Chronic liver disease | 1 (4.2) | |
Values are presented as number (%) or median (range).
FPIAP: food protein induced proctocolitis.
*Expressed among 23 families.
Fig. 1. Sankey diagram that shows the initial symptoms and final diagnosis of patients in the study group.
FPIAP: food protein induced allergic proctocolitis, FTT: failure to thrive, PAD: predominantly antibody deficiency, CID: combined immunodeficiencies, ID: immune dysregulation, CDP: congenital defects of phagocyte, DII: defects in intrinsic and innate immunity, AD: autoinflammatory disorders.
Four patients with primary antibody deficiency (PAD) had transient hypogammaglobulinaemia during infancy. Two patients were admitted with acute hepatitis (Patients #1 and #2), one with food protein-induced allergic proctocolitis (Patient #3), and the other with colitis (Patient #4). The etiological investigation for acute hepatitis was non-diagnostic in two patients, and hepatitis improved spontaneously on follow-up. Histopathological examination of the patient with food protein-induced allergic proctocolitis revealed severe eosinophilic cytopathy and plasma cell infiltration. Specific IgE for food allergens was negative, and the patient’s condition improved with the elimination of cow’s milk. Patients with colitis have recurrent pulmonary infections, bloody diarrhea, and hypogammaglobulinemia. Histopathological examination revealed lymphonodular hyperplasia and focal active colitis with mixed-cell infiltration. Hypogammaglobulinemia improved in all patients during the follow-up period.
Three patients had selective IgA deficiency, from which, two patients had chronic diarrhea (Patients #5 and #6). Laboratory and endoscopic interventions revealed giardiasis in one patient and celiac disease (CD) in another. Another patient was admitted with acute hepatitis (Patient #7), and laboratory and histopathological examinations revealed ANA-positive type-1 autoimmune hepatitis.
Two patients with combined immunodeficiencies (CID) had syndromic features. One patient (Patient #8) was admitted with early onset diarrhea, woolly hair, and hypogammaglobulinemia. A definite diagnosis of trichohepatoenteric syndrome (THES) was made based on the homozygous mutation c.2122C>T(p.Gln708) in TTC37 gene. The other patient (Patient #9) was admitted with failure to thrive, an atypical facial appearance, and pigmented lesions. Laboratory examinations revealed hypogammaglobulinemia. He developed autoimmune thyroiditis two years later and non-Hodgkin lymphoma (NHL) three years later. Whole-exome sequencing revealed pathogenic variants of c.2643G>A (p.W881) in BLM, suggestive of Bloom syndrome.
The clinical presentations of the other three patients with combined immunodeficiency were colitis (Patient #10), acute hepatitis (Patient #11), and chronic liver disease (Patient #12). Patients with colitis exhibit Crohn-like colitis, hypogammaglobulinemia, hypoalbuminemia, and lymphopenia. Despite intensive treatment, the patient died of disseminated cytomegalovirus (CMV) disease. CD55 deficiency was ruled out by sequence analysis. Genetic analysis of immunodeficiencies was nondiagnostic. Another patient was admitted with acute hepatitis due to CMV infection, and later developed disseminated CMV disease. There was no response to ganciclovir; hence, treatment with foscarnet was initiated. Lymphocyte flow cytometry revealed a low T-CD4+ cell count and absence of HLA-DR expression. Genetic analysis of RFXANK revealed homozygous c.634C>T (p.R212X) mutations, suggesting an MHC class II deficiency. Hematopoietic stem cell transplantation was then performed. Patients with chronic liver disease were admitted at 12 months of age with hepatosplenomegaly and high liver enzyme levels. There was cholestasis in the neonatal period, and a diagnostic workup revealed a CMV infection. On admission, laboratory examination results were non-diagnostic, and liver biopsy revealed chronic hepatitis, grade 3 fibrosis. During follow-up, she developed mucocutaneous candidiasis 6 months later, miliary tuberculosis 1 year later, and Hodgkin’s lymphoma 2 years later. Whole-exome sequencing results were non-diagnostic.
Three patients belonged to the immune dysregulation (ID) group. One patient was admitted with ALF (Patient #13), one with early onset diarrhea (Patient #14), and one with chronic diarrhea (Patient #15). The ALF was also associated with EBV infection. Laboratory examination revealed anemia, thrombocytopenia, leukocytosis, and hypogammaglobulinemia. The patient died despite receiving intensive care. Two years later, his twin sister was admitted to our hospital with septic shock and died. Genetic analysis revealed a hemizygote, c.137+5G>A(IVS1+5G>A) in SH2D1A. Patients with early onset diarrhea had hyponatremia, pancytopenia, hepatosplenomegaly, and cranial MR findings compatible with hemophagocytosis. Whole exome sequencing revealed a c.902+5G>A (IVS10+5G>A) homozygous mutation in STXBP2, indicating familial hemophagocytic lymphohistiocytosis type 5 (FHL-5). She underwent hematopoietic stem cell transplantation but died due to septicemia during the previous transplantation period. The patient with chronic diarrhea presented with eczematous lesions, elevated IgE levels, and food allergies (wheat and legumes). Endoscopic and histopathological examinations revealed focal active duodenitis, ileitis, and eosinophilic pancreatitis. Whole-exome sequencing revealed c.1190G>A(p.Arg397Gln) in FOXP3 gene.
A patient with defects in intrinsic and innate immunity (Patient #16) was followed-up in the endocrinology department due to suspected metabolic bone disease and was subsequently admitted with ALF. On follow-up, she developed recurrent ALF attacks, and laboratory examinations revealed hypogammaglobulinemia. Whole exome sequencing revealed a homozygous c.1556T>A (p.Val519Glu) mutation on NBAS gene.
One patient (Patient #17) with an autoinflammatory disorder (AD) was hospitalized for hepatosplenomegaly, cholestasis, and pancytopenia during the neonatal period. The laboratory parameters were not diagnostic. On follow-up, cholestasis improved; however, he developed autoimmune hemolytic anemia, chronic arthritis, and chronic urticaria. The immunodeficiency panel revealed a c.4175G>A (p.Arg1392Gln) heterozygous mutation in NLRP1. Another patient (Patient #18) was admitted with bloody diarrhea, hypogammaglobulinemia, and recurrent pulmonary infections. Histopathological examination revealed lymphonodular hyperplasia, ileitis, and colitis without plasma cells. Genetic analysis revealed a heterozygous c.259C>T mutation in TNFAIP3, indicating an A20 deficiency.
Patients with congenital defects of phagocytes (Patient #19) exhibited growth retardation, chronic diarrhea, cardiomegaly, neutropenia, myopathy, and lactic acidosis. Additionally, the sweat chloride test results were positive. Detailed family history revealed Barth syndrome (BS) in close relatives. Genetic analysis of the BS showed the exon 1 mutation c51.G> C (p.Trp17X) in TAZ. CFTR gene analysis revealed a compound heterozygous mutation (Table 2).
Table 2. Detailed clinical findings, genetic study results, and patient outcomes of the patients (n=19).
| Patient no. | A/S | GIS/liver manifestations | Physical findings | Histopathological findings | Additional manifestations | Final diagnosis (group/type) | Genetic study | Treatment modality | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 36/M | Acute hepatitis | - | ND | - | PAD/THI | ND | Supportive | Alive |
| #2 | 6/M | Acute hepatitis | - | ND | - | PAD/THI | ND | Supportive | Alive |
| #3 | 2/M | FPIAP | - | Cryptitis with severe eosinophilic and plasma cell infiltration | - | PAD/THI | ND | Cow’s milk elimination | Alive |
| #4 | 42/F | Colitis | - | LNH and focal active colitis with mixt cell infiltration | - | PAD/THI | ND | Steroids in the active phase and with azathioprine in remission | Alive |
| #5 | 204/M | Chronic diarrhea | - | Giardia trophozoites in duodenal biopsy | - | PAD/SIgAD | ND | Antiparasitic treatment | Alive |
| #6 | 146/F | Chronic diarrhea | - | Total villous atrophy | CD | PAD/SIgAD | ND | Gluten-free diet | Alive |
| #7 | 36/F | Acute hepatitis | - | Portal inflammation and piecemeal necrosis | Type 1 AIH | PAD/SIgAD | ND | Steroids in the active phase and with azathioprine in remission | Alive |
| #8 | 3/F | Chronic diarrhea | Wooly hair | Normal colonoscopy/histopathology | - | CID (syndromic)/THES | WES/homozygous mutation of c.2122C>T (p.Gln708) in TTC37 gene | Periodic IVIG/Infliximab and TPN | Died (six months after admission) |
| #9 | 18/M | Failure to thrive | Atypical facial appearance | Normal upper endoscopy/histopathology | AIT, NHL | CID (syndromic)/Bloom syndrome | WES/pathogenic variants of c.2643G>A (p.W881) in BLM gene | Periodic IVIG | Alive |
| Pigmentated skin lesions | |||||||||
| #10 | 180/F | Colitis | - | Active colitis and ileitis | CMV disease, PLE | CID | Targeted gene for immune deficiency – nondiagnostic | Steroids and ganciclovir | Died (two months after admission) |
| #11 | 4/F | Acute hepatitis | Maculopapular rash | ND | Disseminated CMV disease | CID/MHC class II deficiency | WES/homozygote c.634C>T (p.R212X) mutation in RFXANK gene | HSCT | Alive |
| #12 | 13/F | CLD | HSMG | Chronic hepatitis with severe fibrosis (grade 3) | CMV disease, Mucocutaneous candidiasis, military tuberculosis, HL | CID | WES/non-diagnostic | HSCT planning | Alive |
| #13 | 22/M | ALF | HSMG | ND | - | ID/X-linked lymphoproliferative disease. | Targeted gene analysis/hemizygote c.137+5G>A (IVS1+5G>A) in SH2D1A gene in twin sister | Supportive | Died (two days after admission) |
| #14 | 6/F | Chronic diarrhea | Pancytopenia, Hyponatremia, HSMG | ND | - | ID/FHL-5 | WES/homozygote c.902+5G>A (IVS10+5G>A) mutation on STXBP2 gene | HSCT | Died (six months after HSTC) |
| #15 | 90/M | Chronic diarrhea | Eczematous skin lesions | Gastritis, focal active duodenitis, ileitis and eosinophil dominant pancolitis | Food allergy | ID/FOXP3 deficiency (IPEX) | WES/c.1190G>A (p.Arg397Gln) in FOXP3 gene | HSCT planning | Alive |
| #16 | 8/F | Recurrent ALF | Metabolic bone diseases and dysmorphic appearance | - | - | DIII/NBAS deficiency | WES/homozygote c.1556T>A mutation on NBAS gene | Periodic IVIG | Alive |
| #17 | 0.5/M | Neonatal cholestasis | HSMG, localized lymphadenopathies | Normal bone marrow aspiration | AIHA, chronic arthritis | AD/NLRP1 deficiency | WES/p.Arg1392Gln in NRP1 gene | IL-1 receptor blockers | Alive |
| #18 | 192/M | Colitis | - | LNH, ileitis and colitis without plasma cells | - | AD/A20 deficiency | WES/heterozygote c.259C>T mutation in TNFAIP3 gene | Periodic IVIG/mesalamine and HSCT planning | Alive |
| #19 | 6/M | Chronic diarrhea | Cardiomyopathy, sweat chloride test positivity | ND | Cystic fibrosis | CDP/Barth syndrome | Targeted gene analysis/exon 1 mutation c51.G> C (p.Trp17X) in TAZ gene and compound heterozygote mutation in CFTR gene | Supportive | Died (nine hours after admission) |
A: age at the time of diagnosis (months), S: sex, GIS: gastrointestinal system, M: male, F: female, FPIAP: food protein induced allergic proctocolitis, CLD: chronic liver disease, ALF: acute liver failure, HSMG: hepatosplenomegaly, ND: not done, LNH: lymphonodular hyperplasia, CD: celiac disease, AIT: autoimmune thyroiditis, AIH: autoimmune hepatitis, NHL: non-Hodgkin lymphoma, CMV: cytomegalovirus, PLE: protein-losing enteropathy, HL: Hodgkin lymphoma, AIHA: autoimmune hemolytic anemia, PAD: predominantly antibody deficiency, THI: transient hypogammaglobulinemia of infancy, SIgAD: selective IgA deficiency, CID: combined immunodeficiencies, THES: tricho-hepato-enteric syndrome, MHC: major histocompatibility complex, ID: immune dysregulation, FHL-5: familial hemophagocytic histiocytosis type 5, DIII: defects in intrinsic and innate immunity, NBAS: Neuroblastoma amplified sequence, AD: autoinflammatory disorders, CDP: congenital defects of phagocyte, WES: whole exome sequencing, TPN: total parenteral nutrition, IVIG: intravenous globulin, HSCT: human stem cell transplantation.
There were five patients in the unclassified group. The demographic, clinical, and laboratory findings of the patients are shown in Table 3.
Table 3. Demographic, clinical and laboratory findings of patients with PIDs in the unclassified group.
| Patient no. | A/S | GIS/hepatic manifestation | Additional findings | Endoscopic and Histopathological findings | Immunological workup | Genetic study | Final diagnosis | Treatment |
|---|---|---|---|---|---|---|---|---|
| #20 | 168/M | Colitis | Chronic urticaria | Aphthous colonic ulcers and focal active colitis | High IgE (850 IU/mL), eosinophilia, positive vaccine response, normal flow cytometry, low C3 levels (0.82 g/L, N:>0.9 g/L) | WES/nondiagnostic | Unclassified ID | Steroids in the active phase and with azathioprine in remission |
| #21 | 167/F | Colitis | - | LNH and ileitis in terminal ileum | High IgE (>3,000 IU/mL), atopy, normal flow cytometry, positive vaccine response, normal immunoglobulin levels | DOCK8 nondiagnostic | Unclassified ID | Steroids in the active phase and with azathioprine in remission |
| #22 | 16/M | Chronic diarrhea | AIHA, RPI | Normal endoscopy and colonoscopy | Hypogammaglobulinemia, no response to vaccination, normal flow cytometry | WES/nondiagnostic | Unclassified AD | Periodic IVIG |
| #23 | 102/M | Chronic diarrhea | Sibling of patient #23 | Normal endoscopy, colonoscopy, video capsule endoscopy | Hypogammaglobulinemia, no response to vaccination, normal flow cytometry | ND | Unclassified AD | Periodic IVIG |
| #24 | 120/M | Colitis | - | Non-caseating granulomas in terminal ileum | High IgE (>3,000 IU/mL), eosinophilia, normal flow cytometry, positive vaccine response, normal immunoglobulin levels | WES/nondiagnostic | Unclassified ID | Steroids in the active phase and with azathioprine in remission |
PIDs: primary immunodeficiency diseases, A: age at the time of diagnosis (months), S: sex, GIS: gastrointestinal system, M: male, F: female, AIHA: autoimmune hemolytic anemia, RPI: recurrent pulmonary infections, LNH: lymphonodular hyperplasia, WES: whole exome sequencing, ID: immune dysregulation, AD: antibody deficiency, ND: not done, IVIG: intravenous globulin.
Overall, the association of autoimmune diseases, development of malignant diseases during follow-up, and severe progression of viral diseases was observed in 20.8%, 8.3%, and 16.6% of patients, respectively (Table 4). Five of the 24 patients died during the follow-up period.
Table 4. Frequency of some clinical and laboratory parameters in our patient group that suggest the primary immunodeficiencies.
| Parameters | Value (n=24) |
|---|---|
| Hypogammaglobulinemia | 12 (50.0) |
| Selective IgA deficiency | 3 (12.5) |
| Severe viral infections | 4 (16.7) |
| Chronic infections | 1 (4.2) |
| Autoimmune manifestations | 5 (20.8) |
| Malign disease | 2 (8.3) |
Values are presented as number (%).
DISCUSSION
We report 24 patients who were admitted to the pediatric gastroenterology department with common gastrointestinal and hepatic symptoms and were subsequently diagnosed with PIDs.
Eight patients were admitted because of chronic diarrhea. Chronic diarrhea may be the initial manifestation of PIDs. This may be related to (i) infectious diarrhea related to underlying host defense impairment or (ii) autoimmunity, such as CD, in patients with selective IgA deficiency. The prevalence of CD in patients with selective IgA deficiency is reportedly 10–30%. The prevalence of selective IgA deficiency in patients with CD is reportedly 2–3%, which is 10–18-fold higher than that in the general population [9]. Additionally, patients with THES, immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, FHL-5, or BS may present with chronic diarrhea [10,11,12].
Two patients with selective IgA deficiency (Patients #5 and #6) presented with chronic diarrhea associated with giardiasis and CD. Additionally, two patients (Patients #22 and #23) in the unclassified AD group had chronic diarrhea. Etiological investigations were non-diagnostic for chronic diarrhea. The two patients were siblings; one had recurrent pulmonary infections and autoimmune hemolytic anemia. Both patients had hypogammaglobulinemia and did not respond to vaccination, but the flow cytometry results were normal. Endoscopic and histopathological findings were normal, excluding gastrointestinal loss. Whole-exome sequencing was non-diagnostic.
THES should be suspected in all infants with neonatal-onset diarrhea, hair abnormalities, intrauterine growth retardation, or hypogammaglobulinemia. Approximately 69% of patients had mutations in TTC37 gene, which encodes subunits of the putative human superkiller complex involved in RNA degradation. The mechanism through which defects in the mRNA degradation system lead to THES-associated symptoms remains unclear [13]. IPEX is an X-linked recessive condition that primarily affects males, and is caused by loss-of-function mutations in the FOXP3 gene located on the X chromosome. Mutations in the FOXP3 gene lead to defects in its DNA-binding domain. This impairs the normal function of regulatory T cells, leading to abnormal immune responses that result in autoimmune manifestations, such as enteropathy. Watery diarrhea generally begins in the first year of life [14]. Chronic diarrhea may be the initial manifestation of FHL-5. Pagel et al. [15] reported that 37.8% of patients with FHL-5 had chronic diarrhea. Gastrointestinal symptoms develop before classical symptoms, particularly in patients without exon-15 splice-site mutations. Defective Munc18-2 expression in epithelial cells may disturb the regulation of secretory pathways in intestinal cells [15]. BS is an X-linked disorder characterized by neutropenia, myopathy, and developmental delays caused by mutations in the TAZ gene. This impairs phospholipid metabolism and disrupts mitochondrial biogenesis. Infectious diarrhea due to neutropenia and fat malabsorption owing to impaired lipid metabolism may lead to chronic diarrhea in BS [16]. Chronic diarrhea generally develops within the first few years of life in patients with monogenic disorders. Only patients with IPEX syndrome developed chronic diarrhea later. Similar to our case, some cases of milder forms of late-onset enteropathy without endocrinopathy have been reported [17].
Patients with PIDs have a higher risk of inflammatory bowel disease (IBD)-like intestinal inflammation than immunocompetent individuals. PIDs should be considered in all patients with early onset colitis, positive family history, and unresponsiveness to conventional treatments [3,18,19]. Additionally, approximately 50 genetic variants have been defined for the monogenic IBD-like phenotype, which is associated with the impairment of the intestinal epithelial layer and innate and adaptive immune functions [20]. Akkelle et al. [21] reported the endoscopic findings in 45 children with PIDs and found that 26% had IBD-like colitis. In our study, 24% of the patients had colitis.
One of our patients had Crohn’s-like colitis (Patient #10) and was subsequently diagnosed with CID. Despite intensive treatment, the patient died of disseminated CMV disease. IBD-like colitis is common in patients with CID with ICOS and CD3γ, IL-21, and IL-21R deficiencies; however, genetic analysis for immune deficiencies was non-diagnostic in our patient [3].
A 20 is a major anti-apoptotic protein in the intestinal epithelium, and its elevated expression is associated with caspase-3 activation, which causes barrier deterioration and intestinal inflammation. It is characterized by early onset systemic inflammation and autoimmune features. IBD-like diseases and colonic perforations are common [22]. Our patient had hypogammaglobulinemia, colitis, ileitis, and lymphonodular hyperplasia without plasma cells, and whole exome sequencing revealed a heterozygous c.259C>T mutation in TNFAIP3.
Three patients had colitis in the unclassified ID group (Patients #20, #21, and #24). They exhibited an IBD-like phenotype, high IgE levels, and no evidence of T- or B-cell deficiencies. Interestingly, two of them had localized terminal ileal diseases. High IgE levels in IBD-like colitis are common in the IPEX syndromes [14]. However, neither patient had other clinical, laboratory, or histopathological findings of IPEX; milder forms could not be ruled out. Although the IBD-like phenotype is uncommon in hyper-IgE syndromes, DOCK8 deficiency was ruled out in one case (Patient #21). Additionally, high IgE levels and Crohn-like colitis may be observed in patients with chronic granulomatous diseases. Although we did not perform NBT and/or genetic testing, the absence of other clinical findings and pigmented macrophages ruled out chronic granulomatous disease. Additionally, one case had chronic urticaria and hypocomplementemia, and monogenic disorders were ruled out using whole-exome sequencing (Patient #20).
One of our patients (Patient #4) with colitis had transient hypogammaglobulinemia during infancy. Hypogammaglobulinemia has been reported in adult patients with IBD and is mainly correlated with hypoalbuminemia, age, and disease [23]. However, our patient did not exhibit hypoalbuminemia. An association between gastrointestinal problems, including chronic diarrhea and gastrointestinal infections, has been reported in up to 10% of patients with transient hypogammaglobulinemia in infancy [22,24]. Upon initial admission, common variable immunodeficiency (CVID) was suspected, but the immunoglobulin levels returned to normal during follow-up. IBD-like colitis associated with transient hypogammaglobulinemia in infancy has not been previously reported. An imbalance between T and B cells results in an improper immune response that may cause colitis. Another patient with transient hypogammaglobulinemia in infancy presented with food protein-induced allergic proctocolitis (Patient #3). Food allergies have been reported in 5.9% of patients with transient hypogammaglobulinemia in infancy [25]. Food allergies, especially cow’s milk allergies, are common in infants and may coexist with antibody production defects [26]. Hypogammaglobulinemia may be related to the leakage of immunoglobulins through the inflamed mucosa, and normalization of immunoglobulin levels was observed with improvement of symptoms after exclusion of the allergens or treatment of colitis in two of our patients. Although we did not study stool alpha-1-antitrypsin in our patients, neither had hypoalbuminemia on initial admission, which may exclude gastrointestinal loss. However, the coincidence of transient hypogammaglobulinemia in infancy with colitis and food protein-induced allergic proctocolitis in our cases cannot be ruled out.
Liver involvement is common in patients with PID mainly related with infections and/or immune dysregulation [4,5]. In an Italian study, 36.6% of children with various types of PIDs had liver abnormalities [27]. Life-threatening hepatic complications have been reported in approximately 50% of patients with X-linked hyper-IgM syndrome–CD40 ligand deficiency. Approximately 10% of CVID patients present with liver damage [4,5]. In our study, liver involvement was the initial manifestation in eight patients (33.3%). Acute hepatitis was found in two patients with transient hypogammaglobulinemia of infancy (Patients #1 and #2). No etiological factors were found, and the hepatitis improved on follow-up, suggesting a non-specific viral infection. One patient had disseminated CMV infection-related acute hepatitis and was subsequently diagnosed with MHC Class II deficiency. Severe viral infections, such as CMV and herpes simplex, are hallmarks of MHC Class II deficiency in the first year of life and are associated with poor prognosis [28]. Another patient with selective IgA deficiency also had autoimmune hepatitis type-1 (Patient #7). The prevalence of autoimmune hepatitis among patients with selective IgA deficiency has been reported to be 1.6%, and this association is more common in patients with anti-LKM1-positive [29]. ALF related to EBV infection is a common feature of XLP1 (60%) and is associated with high mortality. Affected boys are reported as being clinically well until EBV infection, when they mount dysregulated and exaggerated immune responses with the proliferation of cytotoxic T cells, EBV-infected B cells, and macrophages in tissues throughout the body. Dysregulated cytokine release results in extensive parenchymal damage, which is prominent in the liver and causing ALF [30]. In our patient, we confirmed the diagnosis of XLP1 by genetic analysis of the twin sister who was admitted with septic shock. NBAS deficiency is a novel disease associated with recurrent ALF, precipitated by vomiting and fever. Multisystemic involvement such as short stature, skeletal dysplasia, optic atrophy, cardiomyopathy, and epilepsy may be observed. CID, characterized by hypogammaglobulinemia, low T cells, and near-absent B cells, along with other clinical findings, has been reported in patients with NBAS deficiency [31]. Our patient (Patient #16) had recurrent ALF, severe metabolic bone disease, dysmorphic features, and hypogammaglobulinemia. One of our patients with chronic liver disease was diagnosed with CID during follow-up (Patient #12). She also presented with hepatosplenomegaly, chronic hepatitis, and severe fibrosis. Upon follow-up, the patient developed mucocutaneous candidiasis, miliary tuberculosis, and HL. Whole exome sequencing was not performed. Similar cases have been reported by Rodrigues et al. [4] but most were associated with sclerosing cholangitis. One of our patients (Patient #17) was initially admitted with neonatal cholestasis and was subsequently diagnosed with NLRP1 deficiency based on clinical findings and genetic analyses. Although the association of liver diseases such as drug-induced liver injury, non-alcoholic steatohepatitis, endotoxin-induced liver injury, and cholestasis with NLRP1 deficiency has been reported, the association with neonatal cholestasis in our patient might be coincidental [32].
The presence of pigmented skin lesions, atypical facial appearance, and hypogammaglobulinemia suggested immunodeficiency, and whole-exome sequencing revealed Bloom syndrome (Patient #9). Upon follow-up, the patient developed autoimmune thyroiditis and NHL. Feeding problems and growth deficiencies are common in children with Bloom syndrome. Some patients require gastrostomy tube placement, with an increase in weight, but not linear growth [33].
This study has some limitations. The study only included patients admitted to our pediatric gastroenterology unit; hence, patients with gastrointestinal or liver manifestations who were initially admitted to other units were not included in the study. This may have caused some differences, especially in the demographic and clinical findings, when compared with those in the literature.
We report 24 patients who were admitted with common gastrointestinal and liver problems and were subsequently diagnosed with PIDs. The association of autoimmune diseases, development of malignant diseases, or severe progression of viral diseases may give physicians/pediatric gastroenterologists some suspicion of PIDs.
Footnotes
Funding: None.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Raje N, Dinakar C. Overview of immunodeficiency disorders. Immunol Allergy Clin North Am. 2015;35:599–623. doi: 10.1016/j.iac.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40:66–81. doi: 10.1007/s10875-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartono S, Ippoliti MR, Mastroianni M, Torres R, Rider NL. Gastrointestinal disorders associated with primary immunodeficiency diseases. Clin Rev Allergy Immunol. 2019;57:145–165. doi: 10.1007/s12016-018-8689-9. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues F, Davies EG, Harrison P, McLauchlin J, Karani J, Portmann B, et al. Liver disease in children with primary immunodeficiencies. J Pediatr. 2004;145:333–339. doi: 10.1016/j.jpeds.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Hadzic N. In: Concise Pediatric and Adolescent Hepatology. Dhawan A, editor. Karger; 2012. Immune deficiency-related liver disease; pp. 64–75. [Google Scholar]
- 6.ESID Registry - Working definitions for clinical diagnosis of PID. [Internet] ESID; 2019. [cited 2021 Jan 28]. Available from: https://esid.org/Working-Parties/Registry-Workingy-Party/Diagnosis-criteria. [Google Scholar]
- 7.Aksu G, Genel F, Koturoğlu G, Kurugöl Z, Kütükçüler N. Serum immunoglobulin (IgG, IgM, IgA) and IgG subclass concentrations in healthy children: a study using nephelometric technique. Turk J Pediatr. 2006;48:19–24. [PubMed] [Google Scholar]
- 8.Jessurun J. The differential diagnosis of acute colitis: clues to a specific diagnosis. Surg Pathol Clin. 2017;10:863–885. doi: 10.1016/j.path.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Guerrerio AL, Frischmeyer-Guerrerio PA, Lederman HM, Oliva-Hemker M. Recognizing gastrointestinal and hepatic manifestations of primary immunodeficiency diseases. J Pediatr Gastroenterol Nutr. 2010;51:548–555. doi: 10.1097/MPG.0b013e3181efe56b. [DOI] [PubMed] [Google Scholar]
- 10.Schwimmer D, Glover S. Primary immunodeficiency and the gut. Gastroenterol Clin North Am. 2019;48:199–220. doi: 10.1016/j.gtc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Lee WI, Chen CC, Jaing TH, Ou LS, Hsueh C, Huang JL. A nationwide study of severe and protracted diarrhoea in patients with primary immunodeficiency diseases. Sci Rep. 2017;7:3669. doi: 10.1038/s41598-017-03967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malesza IJ, Malesza M, Krela-Kaźmierczak I, Zielińska A, Souto EB, Dobrowolska A, et al. Primary humoral immune deficiencies: overlooked mimickers of chronic immune-mediated gastrointestinal diseases in adults. Int J Mol Sci. 2020;21:5223. doi: 10.3390/ijms21155223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourgeois P, Esteve C, Chaix C, Béroud C, Lévy N, et al. THES clinical consortium. Tricho-Hepato-Enteric Syndrome mutation update: mutations spectrum of TTC37 and SKIV2L, clinical analysis and future prospects. Hum Mutat. 2018;39:774–789. doi: 10.1002/humu.23418. [DOI] [PubMed] [Google Scholar]
- 14.Jamee M, Zaki-Dizaji M, Lo B, Abolhassani H, Aghamahdi F, Mosavian M, et al. Clinical, immunological, and genetic features in patients with immune dysregulation, polyendocrinopathy, enteropathy, x-linked (IPEX) and IPEX-like syndrome. J Allergy Clin Immunol Pract. 2020;8:2747–60.e7. doi: 10.1016/j.jaip.2020.04.070. [DOI] [PubMed] [Google Scholar]
- 15.Pagel J, Beutel K, Lehmberg K, Koch F, Maul-Pavicic A, Rohlfs AK, et al. Distinct mutations in STXBP2 are associated with variable clinical presentations in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL5) Blood. 2012;119:6016–6024. doi: 10.1182/blood-2011-12-398958. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds S. Successful management of Barth syndrome: a systematic review highlighting the importance of a flexible and multidisciplinary approach. J Multidiscip Healthc. 2015;8:345–358. doi: 10.2147/JMDH.S54802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge T, Wang Y, Che Y, Xiao Y, Zhang T. Atypical late-onset immune dysregulation, polyendocrinopathy, enteropathy, x-Linked syndrome with intractable diarrhea: a case report. Front Pediatr. 2017;5:267. doi: 10.3389/fped.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelsen JR, Sullivan KE. Inflammatory bowel disease in primary immunodeficiencies. Curr Allergy Asthma Rep. 2017;17:57. doi: 10.1007/s11882-017-0724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tegtmeyer D, Seidl M, Gerner P, Baumann U, Klemann C. Inflammatory bowel disease caused by primary immunodeficiencies-Clinical presentations, review of literature, and proposal of a rational diagnostic algorithm. Pediatr Allergy Immunol. 2017;28:412–429. doi: 10.1111/pai.12734. [DOI] [PubMed] [Google Scholar]
- 20.Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. COLORS in IBD Study Group and NEOPICS. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007. doi: 10.1053/j.gastro.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akkelle BS, Tutar E, Volkan B, Sengul OK, Ozen A, Celikel CA, et al. Gastrointestinal manifestations in children with primary immunodeficiencies: single center: 12 years experience. Dig Dis. 2019;37:45–52. doi: 10.1159/000492569. [DOI] [PubMed] [Google Scholar]
- 22.Takagi M, Ogata S, Ueno H, Yoshida K, Yeh T, Hoshino A, et al. Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2017;139:1914–1922. doi: 10.1016/j.jaci.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Rai T, Wu X, Shen B. Frequency and risk factors of low immunoglobulin levels in patients with inflammatory bowel disease. Gastroenterol Rep (Oxf) 2015;3:115–121. doi: 10.1093/gastro/gou082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yorulmaz A, Artaç H, Reisli İ. Evaluation of patient follow-up with transient hypogammaglobulinemia in infancy diagnosis. J Contemp Med. 2019;9:15–20. [Google Scholar]
- 25.Karaca NE, Aksu G, Gulez N, Yildiz B, Azarsiz E, Kutukculer N. New laboratory findings in Turkish patients with transient hypogammaglobulinemia of infancy. Iran J Allergy Asthma Immunol. 2010;9:237–243. [PubMed] [Google Scholar]
- 26.Bezrodnik L, Raccio AC, Canil LM, Rey MA, Carabajal PC, Fossati CA, et al. Hypogammaglobulinaemia secondary to cow-milk allergy in children under 2 years of age. Immunology. 2007;122:140–146. doi: 10.1111/j.1365-2567.2007.02628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore M, Ammendola R, Gaetaniello L, De Felice C, Iorio R, Vegnente A, et al. Chronic unexplained liver disease in children with primary immunodeficiency syndromes. J Clin Gastroenterol. 1998;26:187–192. doi: 10.1097/00004836-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Hanna S, Etzioni A. MHC class I and II deficiencies. J Allergy Clin Immunol. 2014;134:269–275. doi: 10.1016/j.jaci.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Odineal DD, Gershwin ME. The epidemiology and clinical manifestations of autoimmunity in selective IgA deficiency. Clin Rev Allergy Immunol. 2020;58:107–133. doi: 10.1007/s12016-019-08756-7. [DOI] [PubMed] [Google Scholar]
- 30.Gaspar HB, Sharifi R, Gilmour KC, Thrasher AJ. X-linked lymphoproliferative disease: clinical, diagnostic and molecular perspective. Br J Haematol. 2002;119:585–595. doi: 10.1046/j.1365-2141.2002.03851.x. [DOI] [PubMed] [Google Scholar]
- 31.Khoreva A, Pomerantseva E, Belova N, Povolotskaya I, Konovalov F, Kaimonov V, et al. Complex multisystem phenotype with immunodeficiency associated with NBAS mutations: peports of three patients and review of the literature. Front Pediatr. 2020;8:577. doi: 10.3389/fped.2020.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 33.Cunniff C, Bassetti JA, Ellis NA. Bloom’s syndrome: clinical spectrum, molecular pathogenesis, and cancer oredisposition. Mol Syndromol. 2017;8:4–23. doi: 10.1159/000452082. [DOI] [PMC free article] [PubMed] [Google Scholar]